Introduction
Low back pain (LBP) is a major threat to our health
and society due to the huge socio-economic burdens and the high
incidence of disability and 60-80% of the population suffer from
LBP at some point in their lives. LBP is exhausting our limited
medical resources (1,2). However, our understanding of
pathophysiology of LBP remains limited.
A widely recognized contributor to LBP is
intervertebral disc (IVD) degeneration (IDD). The degree of disc
degeneration positively correlates with the severity of LBP
(3,4). The structure of degenerative discs
is characterized by a loss of water and proteoglycans in nucleus
pulposus (NP), annulus fibrosus (AF) tears and cartilage endplate
(CEP) calcification. The etiological factors of IDD are involved in
aging, infection, diabetes, trauma and genetic predisposition
(5-10). Mechanical stress is one of the
major causes of IDD (11-13). Spine resists multidirectional
mechanical loadings in daily life, which contributes to maintaining
the structure and function of discs. However, when mechanical
stress is overloaded, it accelerates the initiation and progression
of IDD. As also, disc degeneration causes the disturbed stress
distribution in discs, which generates mechanical stress
concentration. As a result, a vicious circle is formed between
mechanical loadings and disc structure to promote the process of
IDD (12).
In consideration of that the pathological process of
IDD is mediated by the phenotypic shift of disc cells from an
extracellular matrix (ECM) anabolic phenotype to a catabolic and
pro-inflammatory phenotype (14,15), the effects of mechanical stress on
the structure and function of IVDs are proposed to depend on the
relationship between mechanical behavior and disc cell functions
that is known as the mechanobiology of disc cells (12,13). Flexercell tension system (Flexcell
International Corp., Hillsborough, NC, USA), a system that applies
cyclic mechanical tension (CMT) on cells in vitro, is widely
used to investigate the mechanobiology of disc cells. CMT has been
reported to regulate the matrix metabolism, cytokines production,
cytoskeleton organization and apoptosis of disc cells (16-19). Elucidating the mechanobiology of
disc cells in detail contributes to understanding the roles of
mechanical stress in the pathogenesis of IDD in depth.
Disc cell senescence is a new hallmark of disc
degeneration (20,21). The growth of senescent disc cells
is irreversibly arrested. As a consequence, the decrease in the
number of viable and functional disc cells in IVDs caused by cell
death can not be compensated by disc cell proliferation.
Furthermore, the senescence-associated secretory phenotype (SASP)
of disc cells is characterized by an increased secretion of
pro-inflammatory cytokines, ECM proteases and chemokines. Thus,
senescent disc cells promote matrix degradation of discs and induce
the pro-inflammatory cytokine storm in the micro-environment of
discs, which accelerates the establishment and progression of IDD
(22,23). The triggers of disc cell
senescence involve telomere erosion, oxidative stress, cytokines
and DNA damage (24-26). Interestingly, abnormal mechanical
loadings caused by prolonged upright posture have been found to
promote disc cell senescence in rat IVDs, and then to accelerate
the progression of IDD (27,28), suggesting that mechanical stress
is a crucial trigger of disc cell senescence. Revealing the role of
mechanical stress in disc cell senescence benefits our
understanding of the pathogenesis of IDD.
Focusing on NP cells, they are exposed to various
mechanical stresses, including compression, shear stress,
hydrostatic pressure as well as tension (29,30). However, there have been no studies
investigating the effect of mechanical stress on NP cell senescence
so far. Therefore, in this study, we applied a physiological CMT
(5% elongation, 5% CMT) and an unphysiological CMT (20% elongation,
20% CMT) to rat NP cells using a FX-5000T Flexercell tension plus
system. CMT with 20% elongation is regarded as unphysiological due
to that the physiological limit of IVD area change caused by
mechanical stress is known as 15% (16,19,31). Senescence-associated
β-galactosidase (SA-β-gal) staining and BrdU incorporation were
performed to investigate NP cell senescence after CMT stimulation.
The DNA damage and redox state of NP cells were evaluated after CMT
application. Moreover, we also examined the activation of
senescence-associated molecular pathways in NP cells subjected to
CMT. This study elucidated the role of mechanical stress in the
senescence of NP cells, providing a novel insight into the causes
and molecular mechanism of disc cell senescence.
Materials and methods
Ethics statement
This study was approved by the Ethics Committee of
Xinqiao Hospital. All protocols were in accordance with the ethical
standards set by the Declaration of Helsinki.
Antibodies
The mouse monoclonal anti-rat
glyceraldehyde-3-phosphate dehydrogenase (GAPDH, sc-47724), p53
(sc-126), p21 (sc-6246), p16 (sc-1661) and the rabbit polyclonal
anti-rat retinoblastoma protein (Rb, sc-50) antibody were purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The
rabbit monoclonal rabbit anti-rat phospho-histone γ-H2A.X (Ser139)
antibody was obtained from Cell Signaling Technology (#9718;
Danvers, MA, USA). The donkey polyclonal anti-mouse IgG Alexa
Fluor® 647-conjugated secondary antibody (AP192SA6) and
the goat polyclonal goat anti-rabbit IgG (H+L) Alexa
Fluor® 647-conjugated secondary antibody (AP187SA6) were
purchased from Merck Millipore (Billerica, MA, USA). The goat
polyclonal anti-mouse IgG (H+L) horseradish peroxidase
(HRP)-conjugated secondary antibody (ZB2305) and the goat
anti-rabbit IgG (H+L) HRP-conjugated secondary antibody (ZB2301)
were purchased from ZSGB-BIO (Beijing, China).
Isolation and culture of rat NP
cells
Caudal spines were aseptically excised from adult
(3-month-old) male Sprague-Dawley rats (Laboratory Animal Research
Center of Daping Hospital, Chongqing, China) after sacrificed by
peritoneal injection of excessive pentobarbital sodium. The
gelatinous NP tissues were separated from caudal discs (C1-C10),
and then, were digested in Dulbecco's modified Eagle's medium
(DMEM)/F-12 medium (Invitrogen, Carlsbad, CA, USA) containing 0.2%
type II collagenase (Sigma, St. Louis, MO, USA) for 2 h at 37°C.
After being passed through a 70 µm cell mesh to remove
tissue debris, the single-cell suspension was centrifuged at 100 ×
g for 5 h and the supernatant was removed. The cellular pellet was
resuspended in DMEM/F12 medium containing 10% fetal bovine serum
(FBS) and 1% penicillin/streptomycin (Invitrogen). Isolated NP
cells were plated in 25 cm2 culture flasks (Corning,
Inc., Corning, NY, USA) at 37°C and 5% CO2. The medium
was replaced twice a week. When confluent, the cells were
subcultured. The cells at the second passage were used in the
experiments.
Application of CMT on cultured NP
cells
NP cells were seeded on a 6-well flexible silicone
membrane BioFlex™ plates coated with collagen type I (Flexcell
International Corp., McKeesport, PA, USA) at a density of
2×105 cells/well. After reaching 70-80% confluence, the
cells were starved with serum-free DMEM/F12 for 24 h for
synchronization and then stretched using a FX-5000T Flexercell
tension plus system (Flexcell International Corp.) in DMEM/F12
medium containing 10% FBS at 37°C and 5% CO2. CMT with
5% elongation and the CMT with 20% elongation at a frequency of 1
Hz for 6, 12, 24 or 48 h were delivered as per the protocol. The
cells cultured in the same plates under the same conditions were
kept static to serve as the control. The morphology of cells was
observed and imaged using a phase contrast microscope (Olympus,
Tokyo, Japan).
SA-β-gal staining
The activity of SA-β-gal in NP cells was stained
using a SA-β-gal staining kit (#9860; Cell Signaling Technology)
according to the protocol provided by the manufacturer. Briefly, NP
cells cultured in BioFlex™ plates were washed using
phosphate-buffered saline (PBS) and fixed with 2% formaldehyde for
25 min at room temperature. After rinsing with PBS, the cells were
incubated with the staining solution containing X-gel (1 mg/ml) at
37°C for 12 h. Then, the mean percentage of SA-β-gal-positive cells
in nine random fields per well was determined using a
phase-contrast micro-scope (×200 magnification; Olympus).
BrdU incorporation assay and DNA damage
assay
For BrdU incorporation assay, NP cells were
incubated with BrdU (1 µg/ml; BD Biosciences, San Jose, CA,
USA) at 37°C and 5% CO2 for 2 h after cyclic stretch,
and then, were fixed with 70% of ethanol. For DNA damage assay, NP
cells were washed using PBS and then were fixed with 4%
paraformaldehyde for 30 min after cyclic stretch. The rounded
silicon membranes were separated from the BioFlex™ plates, and were
cut into minor sectors. Next, the sectorial membranes were attached
to the bottom of culture dishes. After rinsing with PBS, NP cells
on the sectorial membranes were incubated with 1 ml HCl (2 mol/l)
at room temperature for 30 min for BrdU incorporation assay. After
permeabilization and antigen blocking, cells in culture dishes were
incubated with a mouse monoclonal anti-rat BrdU (B8434, 1:500
dilution; Sigma) and the primary antibody against histone γ-H2A.X
(1:400, a DNA damage marker) overnight at 4°C. After washing, the
cells were incubated with the Alexa Fluor 647 dye-conjugated
secondary antibodies (AP192SA6, 1:400 dilution and AP187SA6, 1:400
dilution) respectively in the dark and then stained with DAPI (0.1
mg/ml; Sigma). Cells without incubation with primary antibodies
served as the negative control. Images in three random fields were
obtained using a confocal microscope (×200 magnification; Lecia,
Weltzlar, Germany). The mean percentage of BrdU-positive cells was
calculated. The mean integrated density (nuclear area × mean gray
value, MID) of γ-H2A.X-expressing cells was analyzed using ImageJ
software (National Institutes of Health, Bethesda, MD, USA).
Reactive oxygen species (ROS)
measurement
The ROS production of NP cells was measured using
2′,7′-dichlorfluorescein-diacetate (DCFH-DA) (D6883; Sigma).
DCFH-DA was oxidized by ROS to generate the highly fluorescent
dichlorofluorescein (DCF). After CMT application, NP cells were
isolated with trypsin and were centrifuged at 100 × g for 5 min.
Next, the cells were resuspended using PBS containing
H2DCF-DA (25 µM) and were incubated at 37°C and
5% CO2 for 30 min. After incubation, the cells were
washed with serum-free DMEM/F12 medium three times. The mean
fluorescence intensity (MFI) was analyzed using a flow cytometer
(Beckman-Coulter, Pasadena, CA, USA).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from stretched and static
control NP cells using 1 ml TRIzol reagent (Takara Bio, Shiga,
Japan). RNA quality and quantity were determined using a NanoDrop
ND-1000 spectrophotometer (Thermo Scientific, Waltham, MA, USA).
One microgram RNA was reverse transcribed using a Prime Script RT
Reagent kit (Takara Bio) according to the manufacturer's protocols.
Real-time quantitative PCR was performed in triplicate on a ViiA™ 7
Real-Time PCR system (Applied Biosystems, Thermo Scientific) with
SYBR® Premix Ex Taq™ II (Takara Bio). The 20 µl
reaction mixtures (10 µl SYBR, 6 µl H2O,
0.4 µl ROX, 0.8 µl forward primer, 0.8 µl
reverse primer and 2 µl cDNA) was amplified under the
following conditions: 95°C for 30 sec, followed by 40 cycles of
95°C for 5 sec and 60°C for 30 sec. PCR products were subjected to
melting curve analysis. Relative mRNA expression was calculated
using the 2−ΔΔCt method (32). GAPDH was the internal reference
gene. We measured the relative expression of p53, p21, p16, Rb,
methionine sulfoxide reductase A (MsrbA), Msrb1 and Msrb2 in NP
cells. Mean Ct values were normalized to that of GAPDH. The primers
of genes investigated in this study are listed in Table I.
 | Table IPrimer sequences used in the
real-time PCR analysis. |
Table I
Primer sequences used in the
real-time PCR analysis.
| Target gene | Forward primer | Reverse primer |
|---|
| p53 |
GGGAATCTTCTGGGACGGGACA |
CTGGTGGGCAGTGCTCTCTTTG |
| p21 |
CTGCCTGGTTCCTTGCCACTTC |
GCTCTGGACGGTACGCTTAGGT |
| p16 |
CGTCGTGCGGTATTTGCGGTAT |
GCGTTGCCAGAAGTGAAGCCA |
| Rb |
AGCAGCCTCAGCCTTCCATACT |
TGTTCTGGCTCTGGGTGGTCAG |
| MsrA |
GGCAATGACTGTGGCACGCA |
CCTCTCGGATGTCGGTGGTGAT |
| MsrB1 |
TCCTGTGGCAAGTGTGGCAATG |
TGACTGAGGCTGGAGTGGTTGG |
| MsrB2 |
AGCAAGGCAGACTGGCAGAAGA |
GGGCTATCACAGCACACGCAAT |
Western blot analysis
Total proteins were extracted from NP cells using a
protein extraction reagent (Thermo Fisher Scientific). Protein
concentration was quantified using BCA method (Beyotime, Shanghai,
China). Cell lysates mixed with loading buffer (Invitrogen) were
electrophoresed on 10% (w/v) sodium dodecyl sulfate-polyacrylamide
(SDS) gels and transferred to polyvinylidenefluoride membranes
(Millipore). The membranes were blocked using 5% milk proteins in
Tris-buffered saline containing 0.1% Triton X-100 (TBST) at 37°C
for 1 h and then incubated with primary antibodies against GAPDH
(1:1,000 dilution), p53 (1:700 dilution), p21 (1:500 dilution) and
Rb (1:700 dilution) overnight at 4°C, followed by incubation with
the HRP-conjugated secondary antibodies (ZB2301, 1:400 dilution and
ZB2305, 1:400 dilution) respectively at 37°C for 1 h. Proteins were
detected using ECL Western Blotting Detection Reagent (Thermo
Scientific).
Statistical analysis
All measurements were performed in three replicates
at least. Data are presented as mean ± standard error of the mean
(SEM). For comparisons between two independent groups, the
two-tailed Student's t-test was used. Differences between three or
more groups were statistically tested by one-way ANOVA and least
significant difference (LSD) multiple comparisons. The data of
RT-qPCR assays were statistically tested using Kruskal-Wallis
nonparametric analysis and Mann-Whitney U post-hoc tests as
described previously (33,34).
Data were analyzed using GraphPad Prism 6 (GraphPad Software Inc.,
La Jolla, CA, USA) and SPSS version 22.0 software programs
(International Business Machines Corp., Amonk, NY, USA). P<0.05
was considered to indicate statistical significance.
Results
Morphology of NP cells after CMT
application
With the duration of 20% CMT increasing, NP cells
gradually aligned in a certain direction. The morphology of NP
cells changed from a polygonal morphology into a spindle-like
morphology (Fig. 1B). However,
these changes were not obvious in NP cells subjected to 5% CMT
(Fig. 1A). Furthermore, NP cells
attached well on the silicon membrane after the application of 5%
CMT and 20% CMT for 48 h (Fig.
1).
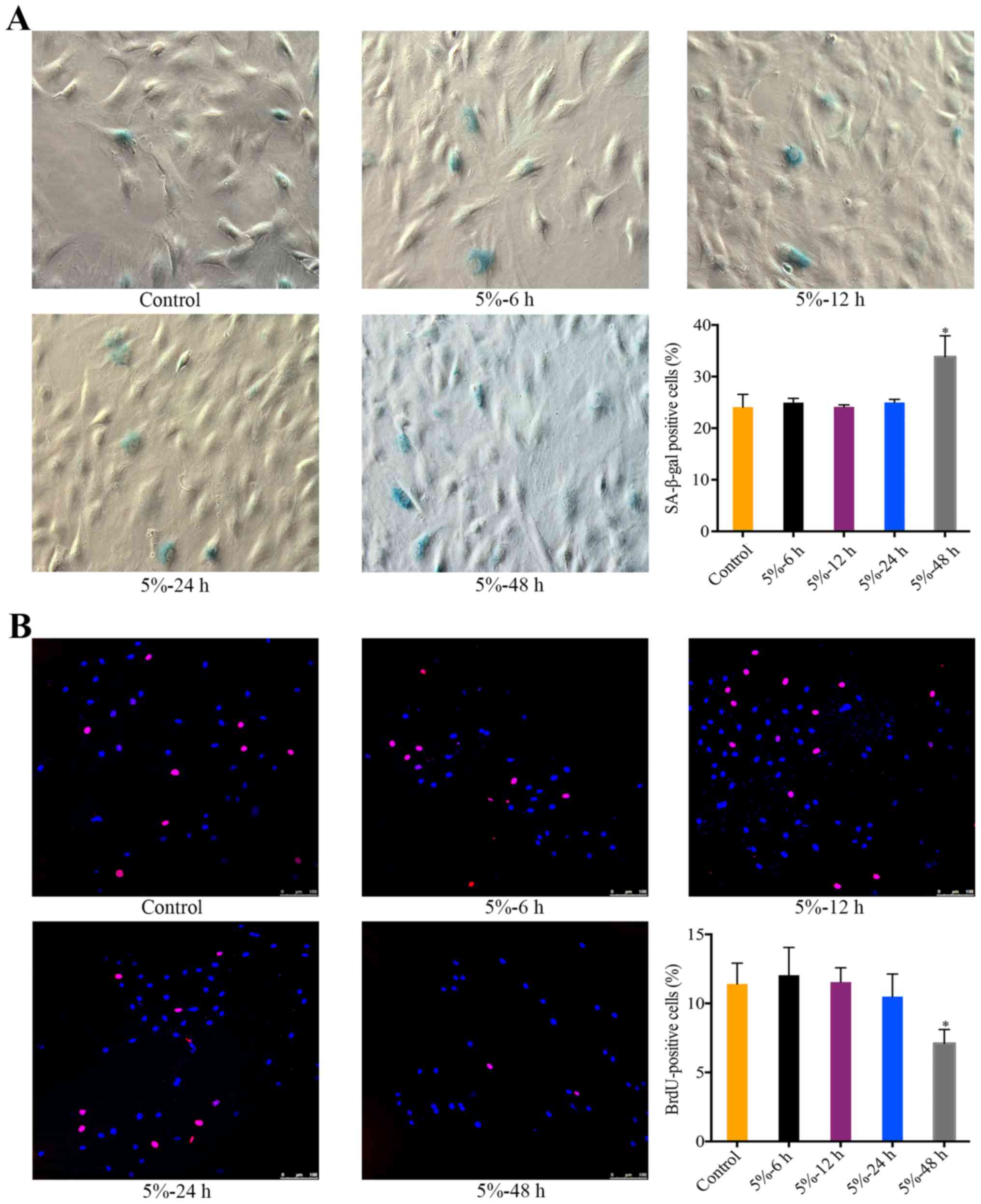
The effect of 5% CMT on the senescence of
NP cells
There was a significant increase in the percentage
of SA-β-gal-positive NP cells after the application of 5% CMT for
48 h (P<0.05). During 6 to 24 h, the percentage of
SA-β-gal-positive cells was stable and not significantly different
from the control (Fig. 2A). The
effect of 5% CMT on the cell cycle of NP cells was analyzed by
performing BrdU incorporation assays. Consistent with the results
of SA-β-gal staining, the percentage of BrdU-positive cells showed
slight changes without statistical significance after 5% CMT
application for 6 to 24 h (Fig.
2B). The percentage of BrdU-positive cells was significantly
higher than that in the control after 5% CMT application for 48 h
(P<0.05) (Fig. 2B).
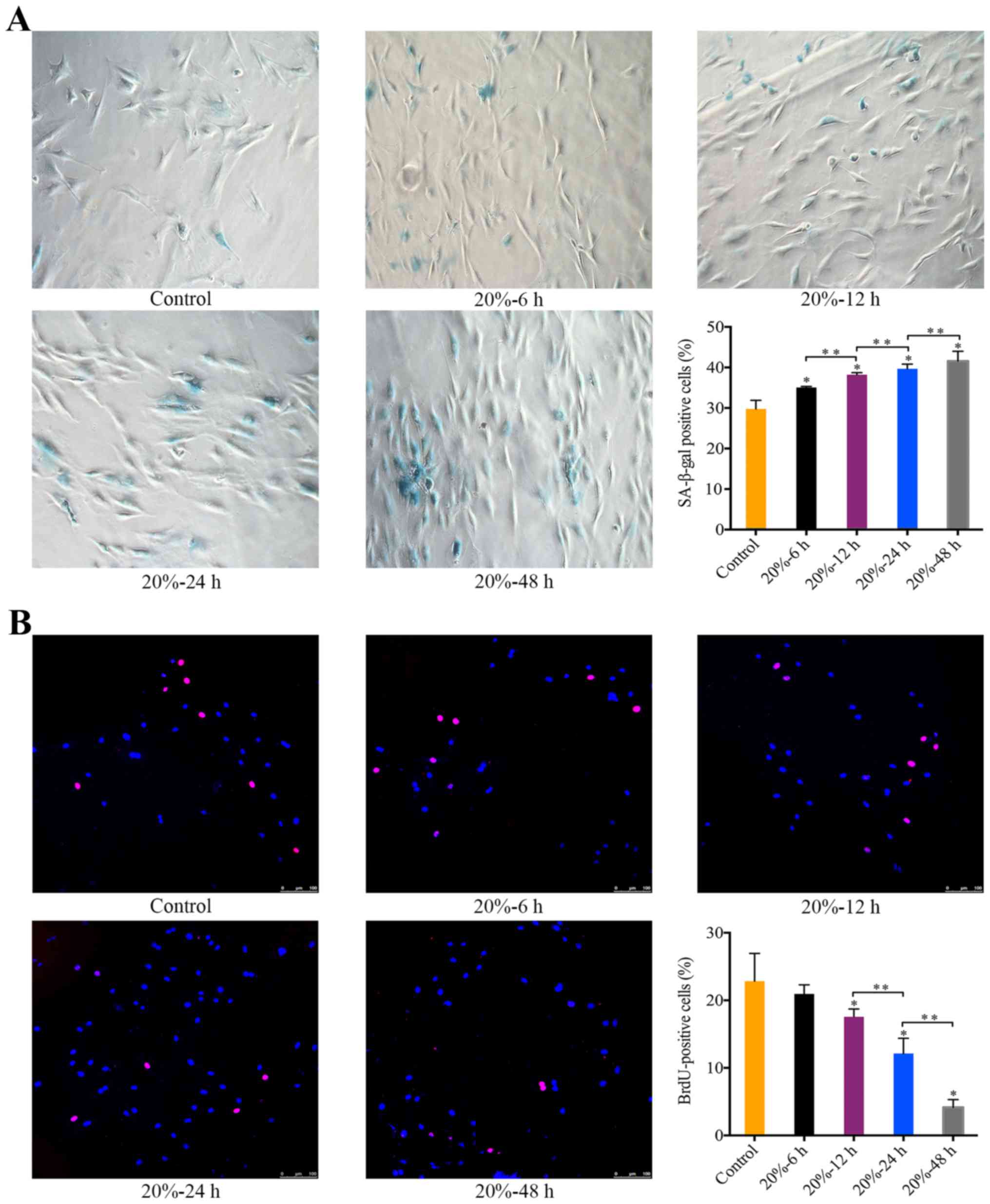
The effect of 20% CMT on the senescence
of NP cells
Exposure of NP cells to 20% CMT resulted in a
significant increase in the percentage of SA-β-gal-positive cells
in a duration-dependent manner (P<0.05) (Fig. 3A). Starting at 12 h after the
application of 20% CMT, the percentage of BrdU-positive NP cells
gradually declined with the duration of 20% CMT increasing
(P<0.05) (Fig. 3B). The
results suggest that the premature senescence of NP cells subjected
to 20% CMT is more prominent than that subjected to 5% CMT. Thus,
we assessed the molecular mechanism of the mechanical
stress-induced premature senescence of NP cells under 20% CMT.
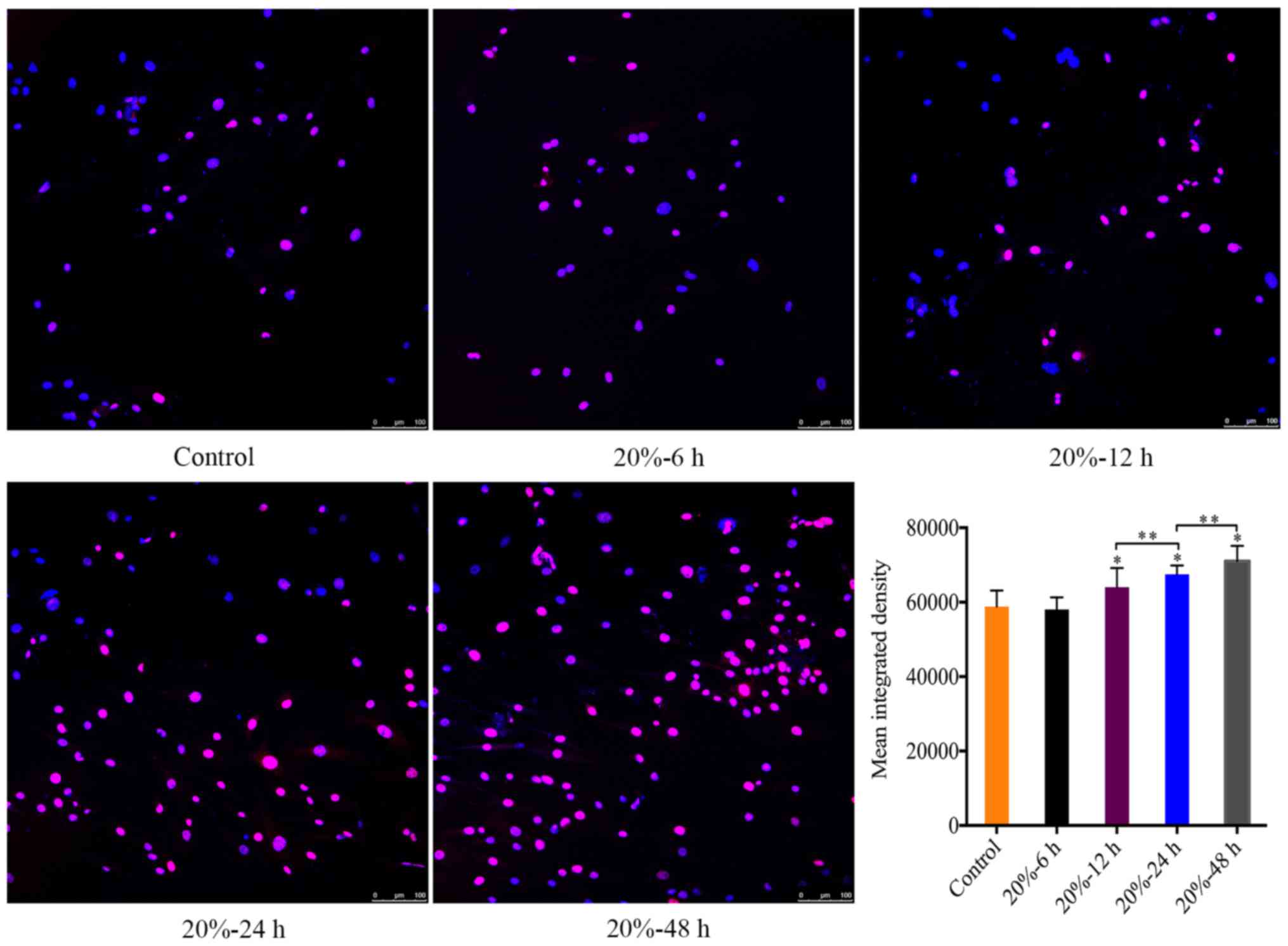
CMT reinforced the DNA damage of NP
cells
In consideration of that DNA damage is an internal
trigger of NP cell senescence (23), we investigated the expression of
γ-HAX foci in the nuclei of NP cells subjected to 20% CMT using
immunofluorescence assays, which revealed the DNA damage in the
nuclei of NP cells. The MID of γ-H2A.X-positive cells was
calculated using ImageJ. The MID of γ-HAX-positive cells gradually
increased (P<0.05) (Fig. 4)
with the duration of CMT increasing since the application of CMT
for 12 h.
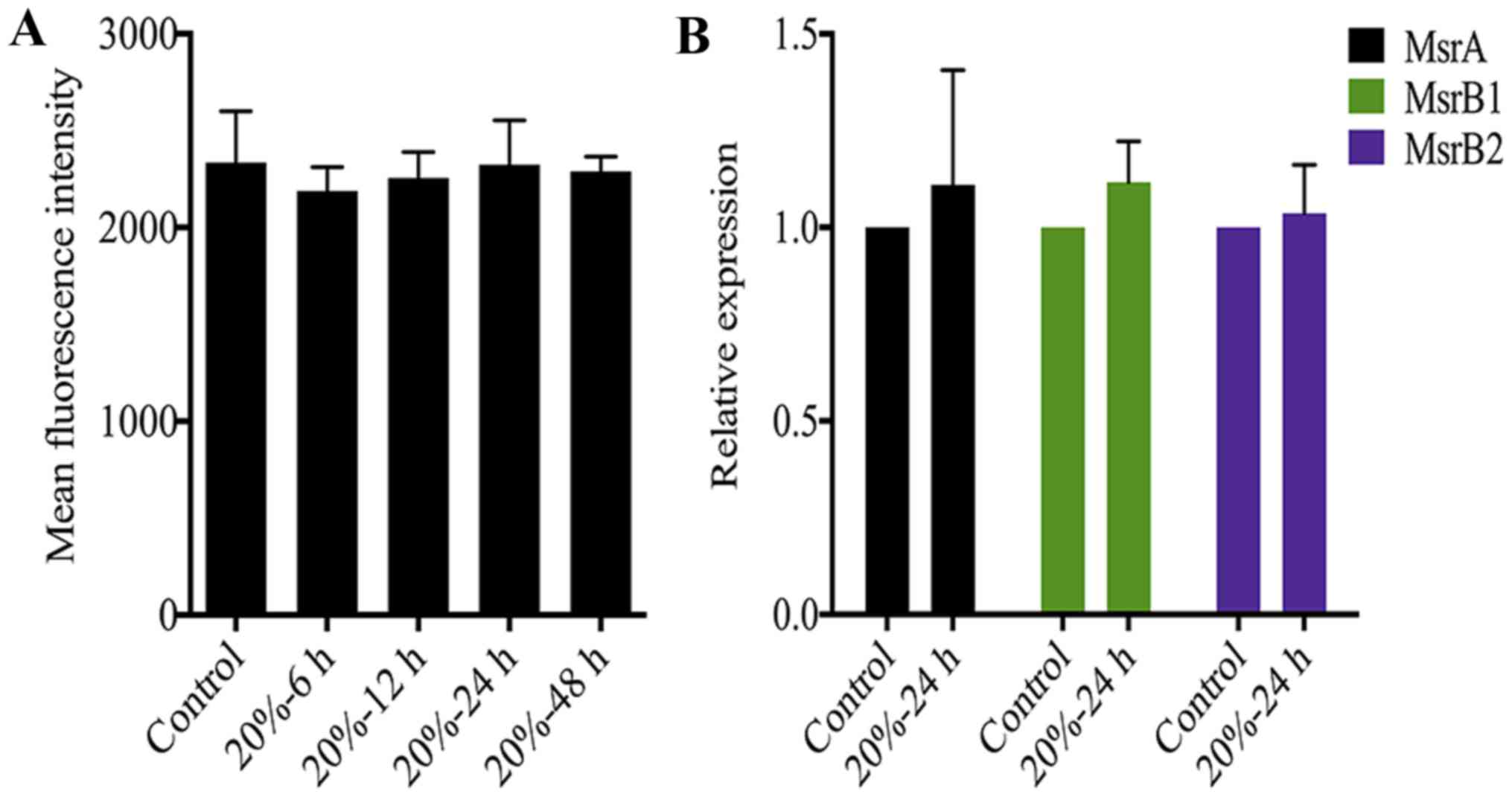
The redox state of NP cells is not
affected by CMT
Oxidative stress caused by excessive ROS generation
is also an essential trigger of NP cell senescence (23,24). A previous study demonstrated that
the DNA damage of senescent fibroblasts is partially ROS-dependent
(35). Therefore, we measured ROS
levels in NP cells after 20% CMT application. Notably, CMT had
little effect on the ROS production of NP cells although the
duration of 20% CMT increased to 48 h (Fig. 5A). On the other hand, Msr is
responsible for repairing the oxidative damage of proteins through
the reduction of methionine residues in proteins, and is a newly
identified oxidative stress marker of disc cells (36). However, the results of RT-qPCR
analysis showed that the expression of MsrA, MsrB1 and MsrB2 in NP
cells were not regulated by 20% CMT significantly, suggesting that
the intracellular redox state of NP cells is not affected by CMT
(Fig. 5B). Oxidative stress is
possibly not involved in mediating the inductive effect of CMT on
the DNA damage and premature senescence of NP cells.
CMT activates the p53-p21-RB signaling
pathway in NP cells
Concerning the molecular mechanism of NP cell
senescence, the p53-p21-Rb pathway and the p16-RB pathway are
essential molecular pathways that induce the cell cycle arrest of
disc cells (25,37). Nevertheless, their roles in the
CMT-induced premature senescence of NP cell were unclear.
Therefore, PCR analysis and western blot analysis were performed to
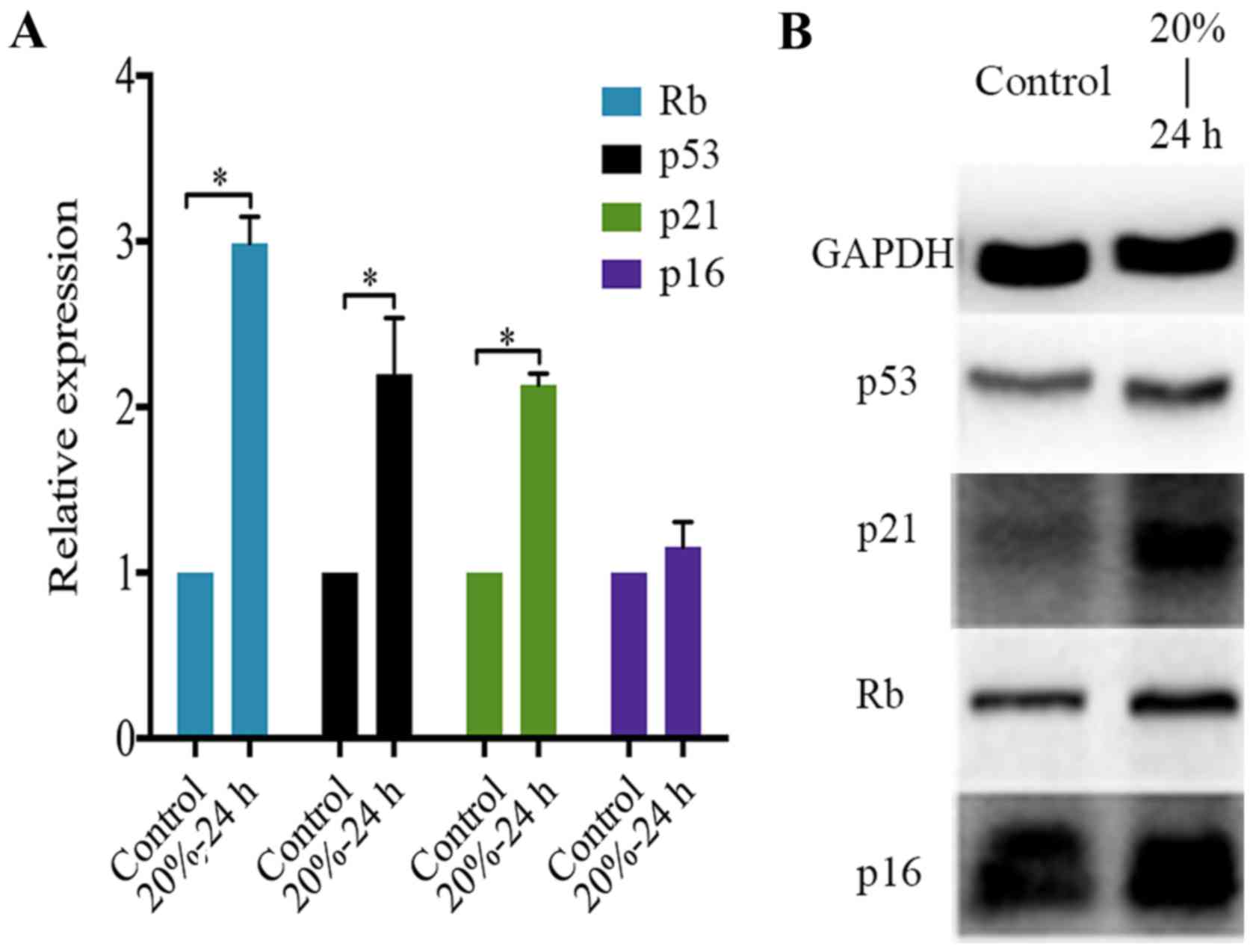
determine the expression of p53, p21, p16 and Rb in NP cells
subjected to 20% CMT for 24 h. The result showed that CMT
significantly upregulated the expression of p53, p21 and Rb rather
than p16 in NP cells (P<0.05) (Fig. 6A). Consistent with PCR analysis,
western blot analysis confirmed the upregulation of p53, p21 and Rb
in NP cells induced by the application of 20% CMT for 24 h. The
expression of p16 in NP cells was prominent and was not regulated
by CMT (Fig. 6B).
Discussion
To our knowledge, this is the first study
investigating the effect of CMT on the senescence of NP cells. Disc
cell senescence is a newly identified cellular event during the
process of IDD (22,23). The accumulation of senescent disc
cells in degenerative discs has been identified in previous
studies. The number of senescent disc cells in degenerative IVDs is
positively correlated with the severity of disc degeneration
(20,21,38). Therefore, elucidating the roles of
disc cell senescence in the initiation and progression of IDD
contributes to understating the pathogenesis of IDD better.
Although IVD is a tissue with low cell density, disc cells are
homeostasis maintenance cells in discs and regulate the ECM
homeostasis of discs to maintain the structure and function of IVDs
(39). Senescent disc cells are
replication exhausted, resulting in a decrease in the number of
viable and functional disc cells due to apoptosis or cell death. On
the other hand, senescent disc cells secrete various
pro-inflammatory cytokines, ECM proteases and chemokines. Excessive
matrix degradation enzymes reinforce ECM catabolism in discs
(15). Pro-inflammatory cytokines
and chemokines arouse inflammatory response, nociception and
neovascularization in discs, which is strongly associated with low
back pain (14,40,41). Thus, disc cell senescence is
recognized as a new hallmark of IDD (22,23). However, the causes and molecular
mechanisms of disc cell senescence are very complicated and involve
telomere uncapping, aging, oxidative stress, nutrition deprivation
and various molecular signaling pathways.
Herein, we investigated the effect of CMT on rat NP
cell senescence using a Flexercell tension system. It showed that
the percentage of SA-β-gal-positive cells in NP cells significantly
increases after the application of CMT. CMT significantly
suppressed the BrdU incorporation of NP cells, suggesting cell
cycle arrest induced by CMT. The results indicate that CMT induces
the premature senescence of NP cells. Notably, the pro-senescent
effect of CMT was magnitude-dependent. CMT with 20% elongation
caused strong morphological changes of NP cells and induced
premature senescence of NP cells starting at 12 h post-stimulation.
However, CMT with 5% elongation induced premature senescence of NP
cells starting at 48 h post-stimulation and showed little effect on
the morphology of NP cells, suggesting that unphysiological
mechanical stress accelerates the premature senescence of NP cells
more prominently than physiological mechanical stress. Moreover,
the pro-senescent effect of CMT was also duration-dependent. The
number of senescent NP cells increased with the duration of CMT
increasing. The results may explain the high risk of degeneration
in discs with abnormal mechanical loadings. Prolonged mechanical
stress with unphysiological magnitude induces the premature
senescence of disc cells and consequently causes a decrease in the
number of viable and functional disc cells, impairing the
structural and functional homeostasis of IVDs to accelerate the
initiation and progression of IDD. Therefore, keeping a healthy
posture to reduce prolonged abnormal mechanical stress on IVDs is
beneficial to retard disc cell senescence and to prevent or delay
IDD.
CMT showed a direct genotoxic effect on NP cells. It
markedly exacerbated the DNA damage of NP cells, which is revealed
by the formation of γ-H2A.X foci in the nuclei of NP cells. The MID
of γ-H2A.X foci in NP cell nuclei significantly increased in a
duration-dependent manner starting at 12 h after 20% CMT
application. DNA damage response activated by DNA damage is crucial
to the cell cycle arrest of senescent cells, suggesting that DNA
damage is involved in the CMT-induced premature senescence of NP
cells. Moreover, ROS induce oxidative damage to biological
macromolecules such as proteins, lipids and DNA. The DNA damage of
senescent fibroblasts has been reported to be partially
ROS-dependent (35). ROS are also
an essential mediator of cell senescence and result in the
stress-induced premature senescence (SIPS) (24,42). Notably, mechanical stress has been
reported to induce SIPS of chondrocytes by increasing the
intracellular ROS production (43). Therefore, we investigated the ROS
production of NP cells subjected to CMT. CMT had little effect on
the ROS production of NP cells, on the other hand, Msr is an enzyme
repairing the oxidative damage of proteins through the reduction of
methionine residues in proteins. The upregulation of Msr is
recognized as a marker of oxidative stress in disc cells (36). However, the expression of MsrA,
MsrB1 and MsrB2 in NP cells was not regulated by CMT.
In conclusion, the above results suggest that the
DNA damage of NP cells induced by CMT is not associated with ROS
overproduction. ROS are probably not involved in the CMT-induced
premature senescence of NP cells. The findings remind us that
preventing the excessive DNA damage of NP cells caused by CMT is a
promising approach to suppress the premature senescence of NP
cells. Antioxidant application may be ineffective to delay the
CMT-induced premature senescence of NP cells. Furthermore, the
mechanism under-lying the CMT-induced DNA damage of NP cells needs
to be elucidated further.
The p53-p21-Rb and p16-Rb are two central molecular
pathways executing disc cell senescence. Generally, the p53-p21-Rb
pathway is activated by telomere erosion, decreased telomerase
activity and DNA damage to induce the replicative senescence of
disc cells. The formation of γ-HAX foci at the site of DNA damage
assembles DNA repair proteins, ATM and ATR and cell cycle
checkpoint proteins Chk1 and Chk2, which leads to the activation of
the p53-p21-Rb signaling cascade. Besides, the p16-Rb pathway is
activated by various stimuli, including oxidative stress,
pro-inflammatory cytokines and high glucose, to mediate the SIPS of
disc cells (22,23). However, the molecular signaling
pathways of cell senescence depend on differences in cause, cell
type and species. Thus, we investigated the signaling pathways that
mediate the CMT-induced premature senescence of NP cells. In
contrast to our previous knowledge, CMT upregulated the expression
of p53, p21 and Rb rather than p16 in NP cells, which was
consistent with the enhanced DNA damage of NP cells induced by CMT.
The p53-p21-p16 pathway mediated the pro-senescent effect of CMT on
NP cells. Notably, p16 was prominently expressed in NP cells. It
may be caused by stress resulting from the elastic silicone
membrane coated with collagen type I. The external stresses induced
the upregulation of p16 in NP cells. It also could explain why the
percentage of BrdU-positive NP cells was relatively low even in the
control group.
There are some limitations to the current study.
First one is that CMT generated by the Flexercell tension system
in vitro does not perfectly reflect the mechanical stress to
which NP cells are subjected in vivo. Recent studies have
used a custom-made external loading device to apply mechanical
tension to the IVD of rabbit in vivo (44,45). Therefore, we can use an external
loading device to apply mechanical tension to the discs of animals,
and then investigate the effect of mechanical tension on senescence
of NP cells in vivo, which will test our hypothesis further
in depth. Secondly, the biological responses of disc cells to
mechanical stress vary according to disc cell type as well as the
magnitude, frequency and duration of mechanical stress (11). In the present study, we found that
the effect of CMT on NP cell senescence is duration-dependent and
magnitude-dependent. However, the effect of frequency remains
unknown. Besides, taking the heterogeneity of NP cells (46-48) and the inherent biomechanical,
biochemical and mechanobiological differences between rat and human
NP cells into account (49,50), further studies based on human NP
cells must be performed to translate the results from rats to
humans.
In conclusion, prolonged exposure of unphysiological
CMT dramatically induces the premature senescence of NP cells. CMT
reinforces DNA damage of NP cells rather than disturbs the redox
homeostasis of NP cells to result in oxidative stress in NP cells.
Furthermore, the p53-p21-Rb pathway is activated to mediate the
CMT-induced premature senescence of NP cells. The results are
beneficial to understanding the mechanism of disc cell senescence
and the mechanobiology of disc cells further. It suggests that
prolonged mechanical stress with unphysiological magnitude induces
the premature senescence of disc cells and consequently decreases
the number of viable and functional cells in IVDs. Eventually,
mechanical stress impairs the structural and functional homeostasis
of IVDs and accelerates the process of IDD. It explains the high
risk of IDD in persons with prolonged unphysiological mechanical
loadings on the spine. Preventing the pro-senescent effects of
mechanical stresses on disc cells is a promising approach to delay
the process of IDD.
Acknowledgments
Not applicable.
Abbreviations:
|
IVD
|
intervertebral disc
|
|
IDD
|
intervertebral disc degeneration
|
|
NP
|
nucleus pulposus
|
|
AF
|
annulus fibrosus
|
|
SA-β-Gal
|
senescence-associated
β-galactosidase
|
|
ECM
|
extracellular matrix
|
|
DDR
|
DNA damage response
|
|
SIPS
|
stress-induced premature
senescence
|
|
Rb
|
retinoblastoma protein
|
|
CMT
|
cyclic mechanical tension
|
|
LBP
|
low back pain
|
|
CEP
|
cartilage endplate
|
|
ROS
|
reactive oxygen species
|
|
Msr
|
methionine sulfoxide reductase
|
|
FBS
|
fetal bovine serum
|
|
PBS
|
phosphate-buffered saline
|
Notes
[1]
Funding
The design of the study and collection, analysis,
and interpretation of data and in writing the manuscript study were
supported by the National Natural Science Foundation of China
(grant nos. 81672215, 81572186, 81271982, 81472076 and
81401801).
[2] Authors'
contributions
The authors' contributions were as follows: CF,
conception and design, acquisition of data, analysis and
interpretation of data, and manuscript writing; MY, acquisition of
data and provision of study material or patients; YZ, acquisition
of data and analysis and interpretation of data; ML, acquisition of
data and analysis and interpretation of data; BH, conception and
design and final approval of the version to be published; HL, given
final approval of the version to be published, conception and
design, financial support, and administrative support; YZ, final
approval of the version to be published, conception and design,
financial support, and administrative support. All authors read and
approved the final manuscript.
[3] Availability
of data and material
All data generated or analyzed during this study are
included in this published article.
[4] Ethics
approval and consent to participate
This research was approved by the Ethics Committee
of Xinqiao Hospital. All procedures described in this study were in
accordance with the standards set forth in the eighth edition of
Guide for the Care and Use of Laboratory Animals published by the
National Academy of Sciences (Washington, DC, USA).
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Vos T, Flaxman AD, Naghavi M, Lozano R,
Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V,
et al: Years lived with disability (YLDs) for 1160 sequelae of 289
diseases and injuries 1990-2010: A systematic analysis for the
Global Burden of Disease Study 2010. Lancet. 380:2163–2196. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hong J, Reed C, Novick D and Happich M:
Costs associated with treatment of chronic low back pain: An
analysis of the UK General Practice Research Database. Spine.
38:75–82. 2013. View Article : Google Scholar
|
|
3
|
Cheung KM, Karppinen J, Chan D, Ho DW,
Song YQ, Sham P, Cheah KS, Leong JC and Luk KD: Prevalence and
pattern of lumbar magnetic resonance imaging changes in a
population study of one thousand forty-three individuals. Spine.
34:934–940. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takatalo J, Karppinen J, Niinimäki J,
Taimela S, Näyhä S, Mutanen P, Sequeiros RB, Kyllönen E and
Tervonen O: Does lumbar disc degeneration on magnetic resonance
imaging associate with low back symptom severity in young Finnish
adults? Spine. 36:2180–2189. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roberts S, Evans H, Trivedi J and Menage
J: Histology and pathology of the human intervertebral disc. J Bone
Joint Surg Am. 88(Suppl 2): 10–14. 2006.PubMed/NCBI
|
|
6
|
Kanayama M, Togawa D, Takahashi C, Terai T
and Hashimoto T: Cross-sectional magnetic resonance imaging study
of lumbar disc degeneration in 200 healthy individuals. J Neurosurg
Spine. 11:501–507. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Battié MC, Videman T, Kaprio J, Gibbons
LE, Gill K, Manninen H, Saarela J and Peltonen L: The Twin Spine
Study: Contributions to a changing view of disc degeneration. Spine
J. 9:47–59. 2009. View Article : Google Scholar
|
|
8
|
Wang D, Nasto LA, Roughley P, Leme AS,
Houghton AM, Usas A, Sowa G, Lee J, Niedernhofer L, Shapiro S, et
al: Spine degeneration in a murine model of chronic human tobacco
smokers. Osteoarthritis and cartilage/OARS. Osteoarthritis Res Soc.
20:896–905. 2012. View Article : Google Scholar
|
|
9
|
Stirling A, Worthington T, Rafiq M,
Lambert PA and Elliott TS: Association between sciatica and
Propionibacterium acnes. Lancet. 357:2024–2025. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park EY and Park JB: Dose- and
time-dependent effect of high glucose concentration on viability of
notochordal cells and expression of matrix degrading and fibrotic
enzymes. Int Orthop. 37:1179–1186. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Neidlinger-Wilke C1, Galbusera F,
Pratsinis H, Mavrogonatou E, Mietsch A, Kletsas D and Wilke HJ:
Mechanical loading of the intervertebral disc: from the macroscopic
to the cellular level. Eur Spine J. 23(Suppl 3): S333–S343. 2014.
View Article : Google Scholar
|
|
12
|
Vergroesen PP, Kingma I, Emanuel KS,
Hoogendoorn RJ, Welting TJ, van Royen BJ, van Dieën JH and Smit TH:
Mechanics and biology in intervertebral disc degeneration: A
vicious circle. Osteoarthritis and cartilage/OARS. Osteoarthritis
Res Soc. 23:1057–1070. 2015. View Article : Google Scholar
|
|
13
|
Setton LA and Chen J: Mechanobiology of
the intervertebral disc and relevance to disc degeneration. J Bone
Joint Surg Am. 88(Suppl 2): 52–57. 2006.PubMed/NCBI
|
|
14
|
Risbud MV and Shapiro IM: Role of
cytokines in intervertebral disc degeneration: Pain and disc
content. Nat Rev Rheumatol. 10:44–56. 2014. View Article : Google Scholar
|
|
15
|
Vo NV, Hartman RA, Yurube T, Jacobs LJ,
Sowa GA and Kang JD: Expression and regulation of
metalloproteinases and their inhibitors in intervertebral disc
aging and degeneration. Spine J. 13:331–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miyamoto H, Doita M, Nishida K, Yamamoto
T, Sumi M and Kurosaka M: Effects of cyclic mechanical stress on
the production of inflammatory agents by nucleus pulposus and
anulus fibrosus derived cells in vitro. Spine. 31:4–9. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gilbert HT, Hoyland JA and Millward-Sadler
SJ: The response of human anulus fibrosus cells to cyclic tensile
strain is frequency-dependent and altered with disc degeneration.
Arthritis Rheum. 62:3385–3394. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li S, Jia X, Duance VC and Blain EJ: The
effects of cyclic tensile strain on the organisation and expression
of cytoskeletal elements in bovine intervertebral disc cells: An in
vitro study. Eur Cell Mater. 21:508–522. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang YH, Zhao CQ, Jiang LS and Dai LY:
Cyclic stretch-induced apoptosis in rat annulus fibrosus cells is
mediated in part by endoplasmic reticulum stress through nitric
oxide production. Eur Spine J. 20:1233–1243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roberts S, Evans EH, Kletsas D, Jaffray DC
and Eisenstein SM: Senescence in human intervertebral discs. Eur
Spine J. 15(Suppl 3): S312–S316. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Le Maitre CL, Freemont AJ and Hoyland JA:
Accelerated cellular senescence in degenerate intervertebral discs:
A possible role in the pathogenesis of intervertebral disc
degeneration. Arthritis Res Ther. 9:R452007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang F, Cai F, Shi R, Wang XH and Wu XT:
Aging and age related stresses: A senescence mechanism of
intervertebral disc degeneration. Osteoarthritis and
cartilage/OARS. Osteoarthritis Res Soc. 24:398–408. 2016.
View Article : Google Scholar
|
|
23
|
Feng C, Liu H, Yang M, Zhang Y, Huang B
and Zhou Y: Disc cell senescence in intervertebral disc
degeneration: Causes and molecular pathways. Cell Cycle.
15:1674–1684. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dimozi A, Mavrogonatou E, Sklirou A and
Kletsas D: Oxidative stress inhibits the proliferation, induces
premature senescence and promotes a catabolic phenotype in human
nucleus pulposus intervertebral disc cells. Eur Cell Mater.
30:89–103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jeong SW, Lee JS and Kim KW: In vitro
lifespan and senescence mechanisms of human nucleus pulposus
chondrocytes. Spine J. 14:499–504. 2014. View Article : Google Scholar
|
|
26
|
Purmessur D, Walter BA, Roughley PJ,
Laudier DM, Hecht AC and Iatridis J: A role for TNFα in
intervertebral disc degeneration: A non-recoverable catabolic
shift. Biochem Biophys Res Commun. 433:151–156. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xing QJ, Liang QQ, Bian Q, Ding DF, Cui
XJ, Shi Q and Wang YJ: Leg amputation accelerates senescence of rat
lumbar intervertebral discs. Spine. 35:E1253–E1261. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liang QQ, Zhou Q, Zhang M, Hou W, Cui XJ,
Li CG, Li TF, Shi Q and Wang YJ: Prolonged upright posture induces
degenerative changes in intervertebral discs in rat lumbar spine.
Spine. 33:2052–2058. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
O'Connell GD, Johannessen W, Vresilovic EJ
and Elliott DM: Human internal disc strains in axial compression
measured noninvasively using magnetic resonance imaging. Spine.
32:2860–2868. 2007. View Article : Google Scholar
|
|
30
|
Nerurkar NL, Elliott DM and Mauck RL:
Mechanical design criteria for intervertebral disc tissue
engineering. J Biomech. 43:1017–1030. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Broberg KB: On the mechanical behaviour of
intervertebral discs. Spine. 8:151–165. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
33
|
Hiyama A, Sakai D, Risbud MV, Tanaka M,
Arai F, Abe K and Mochida J: Enhancement of intervertebral disc
cell senescence by WNT/β-catenin signaling-induced matrix
metalloproteinase expression. Arthritis Rheum. 62:3036–3047. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Keorochana G, Johnson JS, Taghavi CE, Liao
JC, Lee KB, Yoo JH, Ngo SS and Wang JC: The effect of needle size
inducing degeneration in the rat caudal disc: Evaluation using
radiograph, magnetic resonance imaging, histology, and
immunohistochemistry. Spine J. 10:1014–1023. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jun JI and Lau LF: The matricellular
protein CCN1 induces fibroblast senescence and restricts fibrosis
in cutaneous wound healing. Nat Cell Biol. 12:676–685. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gruber HE, Watts JA, Hoelscher GL, Bethea
SF, Ingram JA, Zinchenko NS and Hanley EN Jr: Mitochondrial gene
expression in the human annulus: In vivo data from annulus cells
and selectively harvested senescent annulus cells. Spine J.
11:782–791. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim KW, Chung HN, Ha KY, Lee JS and Kim
YY: Senescence mechanisms of nucleus pulposus chondrocytes in human
inter-vertebral discs. Spine J. 9:658–666. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gruber HE, Ingram JA, Norton HJ and Hanley
EN Jr: Senescence in cells of the aging and degenerating
intervertebral disc: Immunolocalization of senescence-associated
beta-galactosidase in human and sand rat discs. Spine. 32:321–327.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sakai D and Andersson GB: Stem cell
therapy for intervertebral disc regeneration: Obstacles and
solutions. Nat Rev Rheumatol. 11:243–256. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Binch AL, Cole AA, Breakwell LM, Michael
AL, Chiverton N, Cross AK and Le Maitre CL: Expression and
regulation of neurotrophic and angiogenic factors during human
intervertebral disc degeneration. Arthritis Res Ther. 16:4162014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Binch AL, Cole AA, Breakwell LM, Michael
AL, Chiverton N, Creemers LB, Cross AK and Le Maitre CL: Nerves are
more abundant than blood vessels in the degenerate human
intervertebral disc. Arthritis Res Ther. 17:3702015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Khan IM, Gilbert SJ, Caterson B, Sandell
LJ and Archer CW: Oxidative stress induces expression of
osteoarthritis markers procollagen IIA and 3B3(-) in adult bovine
articular cartilage. Osteoarthritis and cartilage/OARS.
Osteoarthritis Res Soc. 16:698–707. 2008. View Article : Google Scholar
|
|
43
|
Martin JA, Brown TD, Heiner AD and
Buckwalter JA: Chondrocyte senescence, joint loading and
osteoarthritis. Clin Orthop Relat Res. 427(Suppl): S96–S103. 2004.
View Article : Google Scholar
|
|
44
|
Xu HG, Zheng Q, Song JX, Li J, Wang H, Liu
P, Wang J, Wang C and Zhang X: Intermittent cyclic mechanical
tension promotes endplate cartilage degeneration via canonical Wnt
signaling pathway and E-cadherin/beta-catenin complex cross-talk.
Osteoarthritis and cartilage/OARS. Osteoarthritis Res Soc.
24:158–168. 2016. View Article : Google Scholar
|
|
45
|
Xiao L, Xu HG, Wang H, Liu P, Liu C, Shen
X, Zhang T and Xu YM: Intermittent cyclic mechanical tension
promotes degeneration of endplate cartilage via the nuclear
factor-κB signaling pathway: An in vivo study. Orthop Surg.
8:393–399. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hunter CJ, Matyas JR and Duncan NA:
Cytomorphology of notochordal and chondrocytic cells from the
nucleus pulposus: A species comparison. J Anat. 205:357–362. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rufai A, Benjamin M and Ralphs JR: The
development of fibro-cartilage in the rat intervertebral disc. Anat
Embryol (Berl). 192:53–62. 1995. View Article : Google Scholar
|
|
48
|
Stevens JW, Kurriger GL, Carter AS and
Maynard JA: CD44 expression in the developing and growing rat
intervertebral disc. Dev Dyn. 219:381–390. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Alini M, Eisenstein SM, Ito K, Little C,
Kettler AA, Masuda K, Melrose J, Ralphs J, Stokes I and Wilke HJ:
Are animal models useful for studying human disc
disorders/degeneration? Eur Spine J. 17:2–19. 2008. View Article : Google Scholar
|
|
50
|
Daly C, Ghosh P, Jenkin G, Oehme D and
Goldschlager T: A Review of animal models of intervertebral disc
degeneration: Pathophysiology, regeneration, and translation to the
clinic. BioMed Res Int. 2016:59521652016. View Article : Google Scholar : PubMed/NCBI
|