Introduction
Atopic dermatitis (AD) is a chronic inflammatory
skin disorder and persistent inflammatory skin disease accompanied
by eczematous lesions and severe itching. It is caused by
environmental and genetic factors, including microbial infection
and environmental pollutants (1-3).
AD results from a combination of severe pruritus, epidermal barrier
abnormalities, imbalanced immune responses and genetic
predisposition (4,5). The diagnosis of AD is based on the
following clinical phenotypes: Erythematous papules, eczematous
skin, and severe pruritus (6,7).
Chemokines are a large group of small cytokines
produced by various types of cell, and are separated into the CX3C,
CXC, CC, and C subfamilies based on NH2-terminal
cysteine-motifs. On the basis of the categorization of these
subfamilies, a methodical nomenclature system of the chemokine
ligands has been devised in previous studies (8). The main function of chemokines is
the control of inflammatory cell recruitment, including
macrophages, T cells, eosinophils and the trafficking of dendritic
cells (DC) at sites of inflammation and infection (9). The thymus and activation-regulated
chemokines (TARC/CCL17) are CC chemokines that are constitutively
expressed and produced by monocyte-derived keratinocytes and
dendritic cells (10,11). Macrophage-derived chemokines
(MDC/CCL22) are also the specific ligands for C-C chemokine
receptor type 4 (CCR4). MDC/CCL22 is constitutively produced by
epithelial cells, B cells, keratinocytes, macrophages and dendritic
cells (10,12). MDC and TARC may serve an important
function in increasing the incidence of certain skin diseases,
including AD. Previous researchers have reported that serum
concentrations of MDC and TARC are positively correlated with
disease gravity in patients with AD patients (13). Considering all these factors, MDC
and TARC may be involved in the pathogenesis of AD.
Tumor necrosis factor (TNF) is a pro-inflammatory
cytokine with multiple biological functions, including cytostatic
and cytotoxic effects, differentiation and proliferation in various
types of tumor cell. The antitumor effects of TNF are enhanced by
interferons (IFNs) (14). The
nuclear transcription factor, nuclear factor (NF)-κB has been
reported to be an anti-apoptotic factor that serves a major
function in cell survival during apoptosis induced by cytokines,
including TNF-α/IFN-γ, and various chemotherapeutic agents
(15,16). In addition, the phosphorylation of
the Janus kinase/signal transducers and activators of transcription
(JAK/STAT) pathway has been reported to be an important
anti-inflammatory factor. JAK activation stimulates cell
differentiation, proliferation, cell migration and apoptosis.
Kimura et al (14)
reported that a combination of TNF-α and IFN-γ treatment resulted
in an upregulated immune response in murine fibroblasts (14). In addition, this TNF-α/IFN-γ
combined treatment also increased the in vitro and in
vivo induction of human inflammation (17,18). Based on these observations, our
group hypothesized that the synergism of atopic dermatitis and
immune responses results in the downregulation of
TNF-α/IFN-γ-induced cell survival mechanisms.
Rhododendron album Blume (RA) is a species of
plant that belongs to the family Ericaceae. It is primarily found
in humid primeval forests at high altitudes throughout western and
central Java. RA is 1-2 m tall, and the leaves (5-12 cm long, and
2-3 cm wide) are stiff, with a brown, scaly underside. The corolla
is widely campanulate, and is pale yellow in colour with scattered
brown scales. RA is endemic to Java and is rare in the wild.
However, RA is grown widely in nurseries throughout the world,
particularly in the United States. Certain plants belonging to the
genus Rhododendron have documented anti-cancer (19,20) and antioxidant functions (21). Therefore, RA may also exert
anti-atopic or anti-inflammatory activity. The present study
focused on the anti-inflammatory effects of RA methanol (MeOH)
extract (RAME) (22). At present,
there are no data on the anti-inflammatory activity of this plant.
Therefore, the purpose of the present study was to evaluate the
anti-inflammatory and anti-atopic dermatitis activity of RAME in
HaCaT keratinocyte cells.
Materials and methods
Preparation of RA extract
The plant species was collected from Cantigi in
Indonesia in 2008, and it was identified by the Center for
Pharmaceutical and Medical Technology, Agency for Assessment and
Application of Technology (Tangerang, Indonesia) and verified by
Herbarium Bogoriense (Indonesian Institute of Sciences, Bogor,
Indonesia). A voucher specimen (KRIB 0019989) has been deposited in
the herbarium of the Korea Research Institute of Bioscience and
Biotechnology, and also in the Center for Pharmaceutical and
Medical Technology and Herbarium Bogoriense. The RA plant material
was treated with MeOH and sonicated for 15 min, at 36.5 kHz using
Ultrasonic Cleaning Bath (Branson Ultrasonics; Emerson Electric
Co., St. Louis, MO, USA) and incubated for 2 h at room temperature.
This procedure was repeated 30 times for 3 days to produce an
extract. The methanol extract (360 mg) was suspended in distilled
water, the same amount of n-hexane was added and mixed, and then
the n-hexane soluble fraction and the water-soluble fraction were
separated. This was performed three times, followed by cotton wool
filtration and concentration under reduced pressure to obtain an
n-hexane fraction (yield, 17.2 mg). Then, the n-hexane fraction was
removed and the same amount of chloroform was added to the
remaining water layer. A chloroform fraction (yield, 21.0 mg) was
obtained in the same manner. The same amount of ethyl acetate was
added to the remaining water later, and an ethyl acetate fraction
(yield, 14.0 mg) was obtained in the same manner. Finally, an
equivalent amount of butanol was added to the water layer, and a
butanol fraction (yield, 47.3 mg) was obtained in the same manner.
The remaining water layer was then concentrated to obtain a water
fraction (yield, 235.5 mg).
Cell culture
The human keratinocyte HaCaT cell line (ATCC;
American Type Culture Collection, Manassas, VA, USA) was cultured
in high glucose Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS) and antibiotics (100
U/ml penicillin and 100 μg/ml streptomycin) at 37°C in a
humidified 5% CO2 incubator. Prior to further
association studies, the growth medium was changed to serum-free
medium. Cells in the control group were treated with dimethyl
sulfoxide (DMSO, 0.1% per 100 μl) alone. DMEM, FBS,
penicillin, streptomycin, and PBS (pH 7.4) were purchased from
Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Recombinant TNF-α (10 ng/ml) was purchased from Invitrogen; Thermo
Fisher Scientific, Inc. and IFN-γ (10 ng/ml) was purchased from
Merck KGaA (Darmstadt, Germany). The HaCaT cells were pretreated
with RAME (2.5, 5, 10, 20 μg/ml) for 1 h and subsequently
treated with TNF-α (10 ng/ml) and IFN-γ (10 ng/ml), and incubated
for 24 h at 37°C. In another set of cultures, the cells were
co-incubated with 5 μM Bay11-7082, a nuclear factor of κ
light polypeptide gene enhancer in B-cells inhibitor, α (IκB-α)
inhibitor, 10 μM SB203580, a p38 inhibitor, and 10 μM
SP600125, a c-Jun N-terminal kinase (JNK) inhibitor (Calbiochem;
EMD Millipore, Billerica, MA, USA) for 1 h at 37°C in a humidified
5% CO2 incubator.
Cell viability
To confirm the effect of RAME on HaCaT cells, the
reduction of MTT (Amresco, Inc., Solon, OH, USA) by viable cells
was measured. The cells were plated in 96-well culture plates (SPL
Life Sciences, Pocheon, Korea) at a density of 1×104
cells/well, and allowed to bind for 6 h. Cells were allowed to
attach for 6 h, and the culture was continued for 24 h following
addition of RAME (2.5, 5, 10, 20 μg/ml) at 37°C. The cells
were cultured with 0.5 mg/ml MTT solution. After 4 h of incubation
at 37°C in 5% CO2, the supernatant was removed and DMSO
was added. Absorbance was measured at 570 nm using a microplate
reader (Tecan Group, Ltd., Männedorf, Switzerland). The percentage
of viable cells was calculated by taking the optical density of the
cells following a particular treatment and dividing that number
with the optical density of untreated control cells, followed by
multiplication by 100.
Interleukin (IL)-6 and IL-8
production
The culture medium was collected and the production
of cytokines (IL-6 and IL-8) in the supernatant was measured using
ELISA kits (IL-6; cat. no. 555220, BD Biosciences, San Jose, CA,
USA; IL-8; cat. no. DY208, R&D Systems, Inc., Minneapolis, MN,
USA) according to the manufacturers' protocols, as previously
described (23,24).
TARC and MDC production
The culture medium of the cells was harvested, and
chemokine production (TARC and MDC) in the supernatant was measured
using ELISA kits (cat nos. TARC cat. no. DY364 and MDC cat. no.
DY336; R&D Systems, Inc.) according to the manufacturer's
protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted using TRIzol reagent (Thermo
Fisher Scientific, Inc.) for RT-qPCR analysis. cDNA was synthesized
using the AMPIGENE® cDNA Synthesis kit (cat. no.
ENZ-KIT106; Enzo Life Sciences, Inc., Farmingdale, NY, USA). qPCR
was performed using SYBR Green PCR Master Mix (KAPA
SYBR® FAST qPCR kits; Kapa Biosystems, Inc., Wilmington,
MA, USA). Reactions were run in a CFX96 Real-Time PCR System
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) using the following
thermocycler conditions: Stage 1, 50°C for 2 min and 95°C for 10
min; stage 2, 95°C for 15 sec and 60°C for 1 min. Stage 2 was
repeated for 40 cycles. Relative mRNA levels were calculated using
the comparative threshold cycle 2−ΔΔCq method and
normalized to that of GAPDH (25). The primer sequences used in the
present study are listed in Table
I.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene | Primer
sequencesa (5′-3′) | Fragment size
(bp) |
|---|
| TARC | | 222 |
| Forward |
CACGCAGCTCGAGGGACCAATGTG | |
| Reverse |
TCAAGACCTCTCAAGGCTTTGCAGG | |
| MDC | | 362 |
| Forward |
AGGACAGAGCATGGCTCGCCTACAGA | |
| Reverse |
TAATGGCAGGGAGGTAGGGCTCCTGA | |
| IL-6 | | 124 |
| Forward |
GACAGCCACTCACCTCTTCA | |
| Reverse |
AGTGCCTCTTTGCTGCTTTC | |
| IL-8 | | 299 |
| Forward |
ATGACTTCCAAGCTGGCCGTGGCT | |
| Reverse |
TTATGAATTCTCAGCCCTCTTCAAAAA | |
| β-actin | | 250 |
| Forward |
CATGTACGTTGCTATCCAGGC | |
| Reverse |
CTCCTTAATGTCACGCACGAT | |
Sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and western blot analysis
Briefly, the HaCaT cells were washed with cold PBS
and then lysed in RIPA buffer (ELPIS Biotech, Inc., Daejeon, Korea)
on ice for 20 min with a strong vortex. The cell lysate was
centrifuged at 10,000 × g for 10 min at 4°C and protein
concentrations were measured using the bicinchoninic acid method.
Sample proteins (20 μg) were analyzed on 10% SDS-PADE and
electrophoretically transferred to a polyvinylidene difluoride
(PVDF) membrane, following which the PVDF membrane was blocked in
5% non-fat dry milk in Tris-buffered saline (TBS, 20 mM Tris, 0.2 M
NaCl, pH 7.5) containing 0.05% Tween-20 (TBS/T) for 1 h at room
temperature. The PVDF membrane was incubated with primary
antibodies for overnight at 4°C. Following washing three times with
TBS/T, the membranes were incubated for 1 h at room temperature
with horseradish peroxidase (HRP)-conjugated secondary antibodies,
diluted 1:5,000 with 5% skim milk in TBS/T. The membranes were then
visualized using SuperSignal West Pico Chemiluminescent Substrate
(cat. no. 32106; Pierce; Thermo Fisher Scientific, Inc.). The
primary antibodies used were as follows: anti-NF-κB p65 (cat. no.
sc-8242; 1:1,000), anti-JNK (cat. no. sc-474; 1:1,000),
anti-extracellular signal-regulated kinase (ERK; cat. no. sc-154;
1:1,000), anti-p38 (cat. no. sc-7149; 1:1,000), anti-p-p38 (cat.
no. sc-7973; 1:1,000), anti-phosphorylated (p-) JAK1 (cat. no.
sc-16773; 1:1,000), anti-JAK1 (cat. no. sc-376996; 1:1,000),
anti-p-STAT1 (cat. no. sc-8394; 1:1,000), anti-STAT1 (cat. no.
sc-464; 1:1,000; all from Santa Cruz Biotechnology, Inc., Dallas,
TX, USA), anti-p-ERK 1/2 (cat. no. 9101; 1:1,000), anti-p-IκB-α
(cat. no. 2859; 1:1,000), anti-IκB-α (cat. no. 9242; 1:1,000),
anti-NF-κB p-p65 (cat. no. 3033; 1:1,000; all from Cell Signaling
Technology, Inc., Danvers, MA, USA), anti-p-JNK (cat. no.
KAP-SA011; 1:1,000; Enzo Life Sciences, Inc.), and anti-β-actin
(cat. no. 4967; 1:1,000; Cell Signaling Technology, Inc.). The
secondary antibodies used were as follows: HRP-conjugated goat
anti-rabbit IgG (cat. no. sc-2030; 1:5,000 in 5% skim milk; Santa
Cruz Biotechnology, Inc.; used for detection of NF-κB p65, JNK,
ERK, p38, p-p38, p-JNK, p-ERK, p-IκB-α, IκB-α, NF-κB p-p65,
β-actin) and HRP-conjugated goat anti-mouse IgG (cat. no. sc-2005;
1:5,000 in 5% skim milk; Santa Cruz Biotechnology, Inc.; used for
detection of JAK1, STAT1, p-JAK1, p-STAT1). Each protein was
detected using an Enhanced Chemiluminescence detection system using
ImageQuant LAS4000 (GE Healthcare Life Sciences, Little Chalfont,
UK). Western blot bands were quantified using ImageJ software
(version 1.6.0; National Institutes of Health, Bethesda, MD,
USA).
Luciferase assay
HaCaT cells were transfected with 0.1 μg
pGL4.32 (luc2P/NF-κB-RE/Hygro, Promega Corporation, Madison, WI,
USA) plasmids, using Lipofectamine 2000 transfection reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Then, 20 h after transfection, the cells
were stimulated with TNF-α/IFN-γ (10 ng/ml) for 24 h at 37°C,
harvested, and then assessed for luciferase activity using the
ONE-Glo™ luciferase reporter assay system (Promega Corporation)
according to the manufacturer's protocol. Normalization was
performed by comparison with Renilla luciferase
activity.
Immunocytochemistry (ICC)
HaCaT cells were cultured in Lab-Tek™ chamber slides
(Thermo Fisher Scientific, Inc.) and immobilized in ethanol at 4°C
for 15 min. Slides were washed three times with PBS and blocked
with 2% (w/v) bovine serum albumin (BSA100, Bovogen Biologicals Pty
Ltd., Melbourne, Australia) in PBS for an additional 1 h at room
temperature. Slides were then incubated with anti-NF-κB p65 subunit
(cat. no. sc-8242; rabbit polyclonal IgG; 1:200, Santa Cruz
Biotechnology) and anti-STAT1 (cat. no. sc-464; mouse monoclonal
IgG; 1:100, Santa Cruz Biotechnology, Inc.) antibodies for 24 h at
4°C. Following washing with PBS three times to remove excess
primary antibody, the slides were further incubated with Alexa
Fluor 488-conjugated goat anti-rabbit IgG (cat. no. A-11034;
1:1,000; Invitrogen; Thermo Fisher Scientific, Inc.) or Texas Red
conjugated goat anti-mouse IgG secondary antibodies (cat. no.
A-11003; 1:1,000; Invitrogen; Thermo Fisher Scientific, Inc.) for 2
h at room temperature, washed with PBS, and stained with Gold
Antifade reagent containing DAPI (Invitrogen; Thermo Fisher
Scientific, Inc.) for 5 min prior to the position and
quantification of ProLong nuclei. Subsequently, the slides were
coverslipped and visualized using confocal laser scanning
microscopy (LSM 510 m; Carl Zeiss AG, Oberkochen, Germany). All
samples were quantified from the images obtained, which were taken
under the same exposure conditions.
Statistical analysis
The data represent the mean ± standard error of the
mean. Statistical differences among groups were determined by
one-way analysis of variance with repeated measures followed by
Student-Newman–Keuls testing in SPSS 14.0 software (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Cell viability
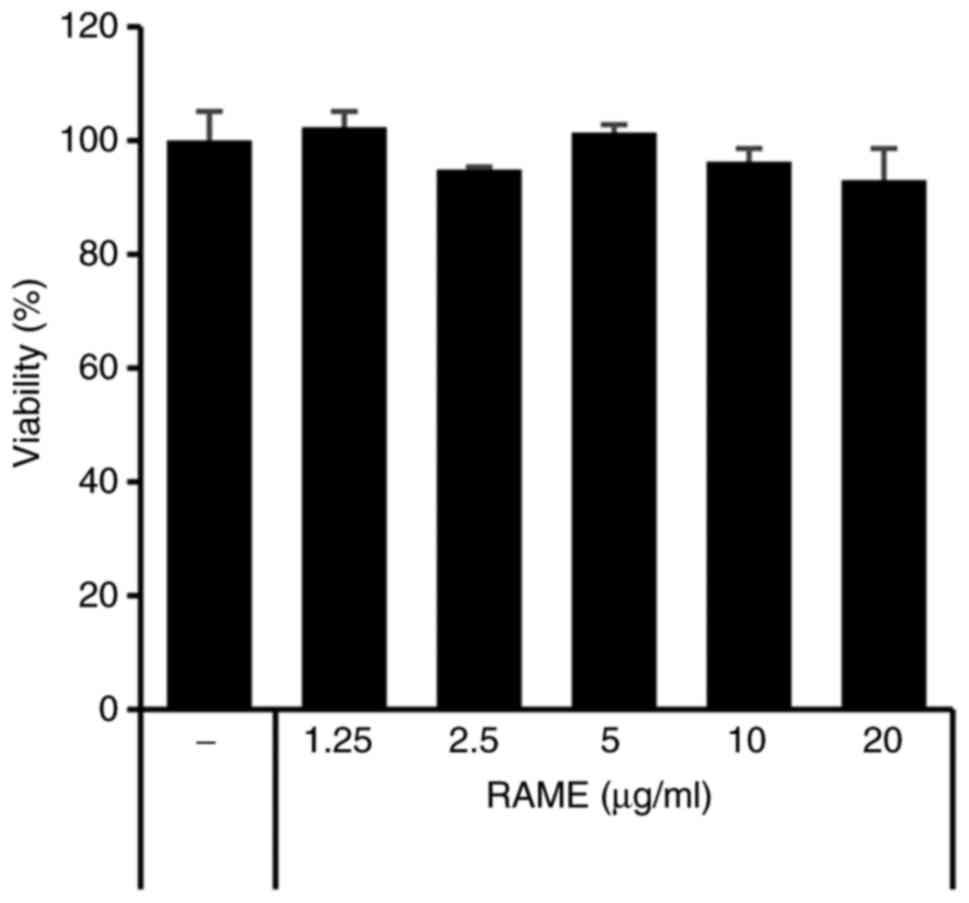
The effect of RAME on cell viability was confirmed
using HaCaT cells and an MTT assay. As presented in Fig. 1, RAME did not result in a
significant cytotoxic effect, failing to affect cell viability even
at a relatively high concentration of 20 μg/ml for a
treatment period of 24 h. Therefore, RAME was experimentally
confirmed to not show toxicity, even when HaCaT cells were treated
with 20 μg/ml. In addition, the effect of RAME and solvent
fraction (n-hexane, chloroform, ethyl acetate, butanol, water
layer) on cell viability was confirmed using HaCaT cells and MTT
assays. RAME and solvent fraction did not affect cell viability,
even at a relatively high concentration of 20 μg/ml for a 24
h treatment period (data not shown).
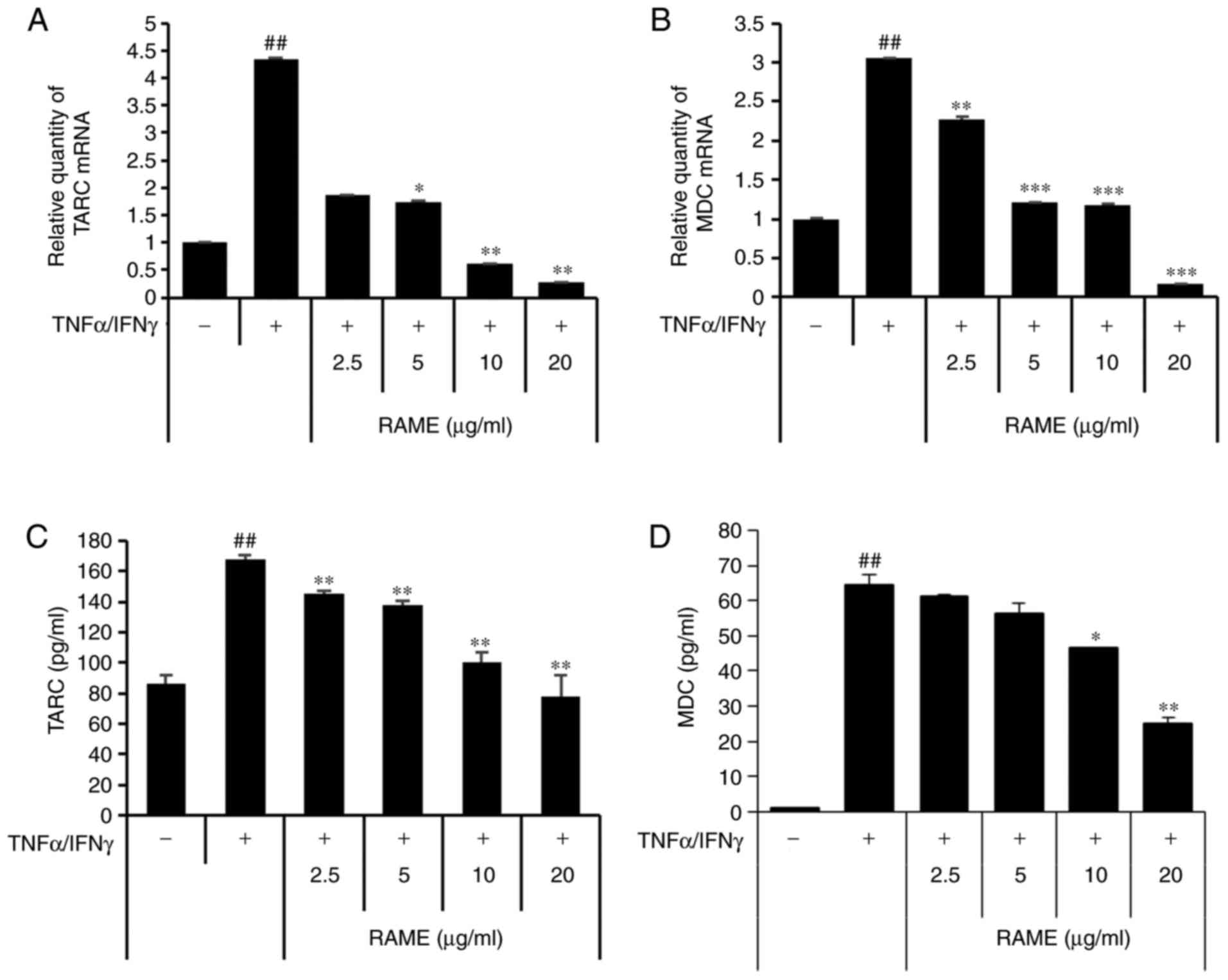
Treatment with RAME inhibits
TNF-α/IFN-γ-induced TARC and MDC expression in HaCaT cells
Next, the effect of suppressing TARC and MDC
production by RAME in HaCaT cells stimulated with TNF-α/IFN-γ was
investigated using ELISA and RT-qPCR (Fig. 2). TARC and MDC were significantly
increased in the group treated with TNF-α/IFN-γ compared with the
untreated group. In addition, when HaCaT cells were treated with
TNF-α/IFN-γ following RAME treatment, TARC and MDC mRNA expression
decreased in a concentration-dependent manner, compared with the
group treated with TNF-α/IFN-γ. Furthermore, RAME treatment
inhibited the expression of TARC and MDC protein in the HaCaT cells
stimulated with TNF-α/IFN-γ.
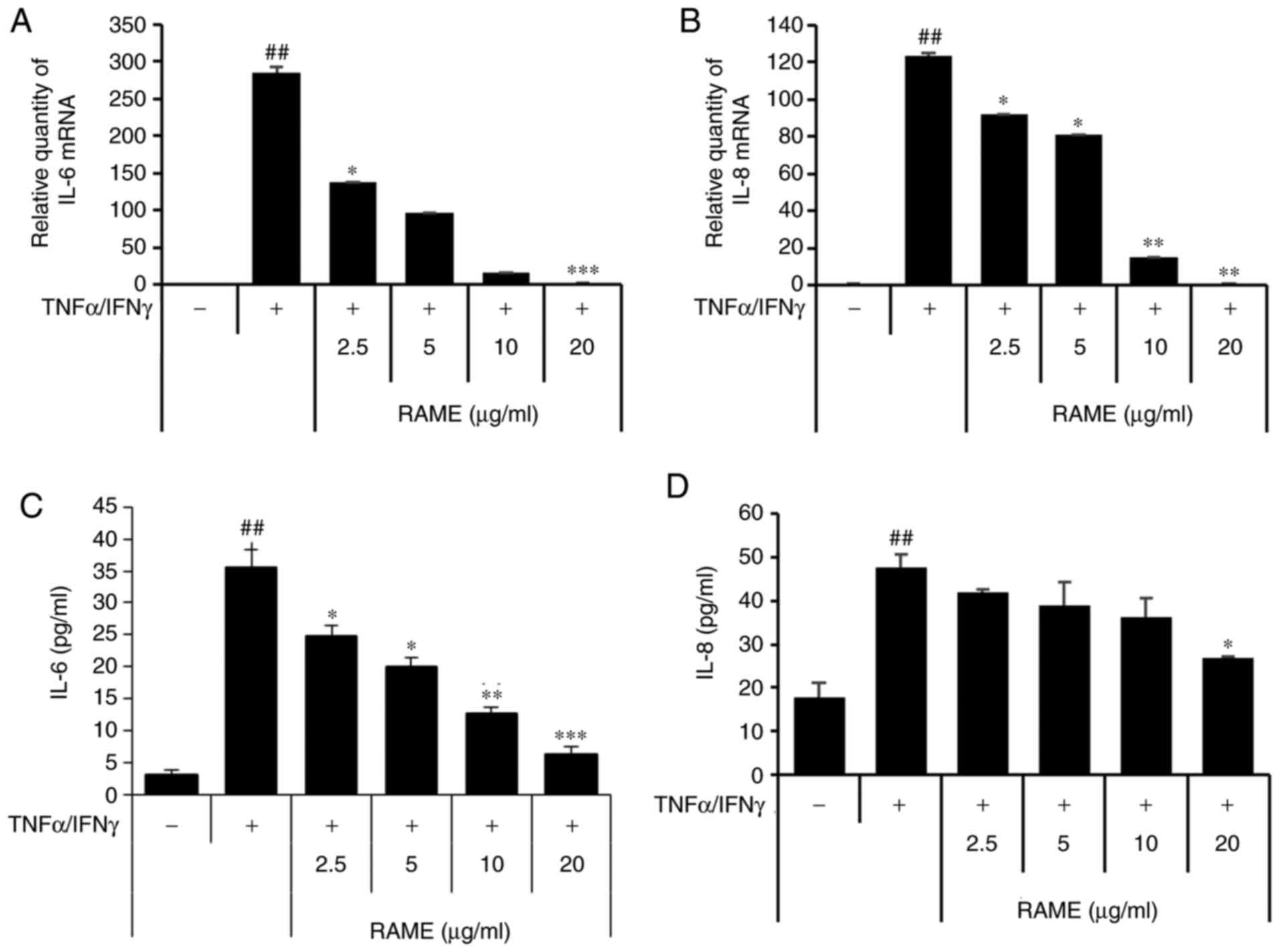
Treatment with RAME inhibits
TNF-α/IFN-γ-induced IL-8 and IL-6 expression in HaCaT cells
Next, the effect of inhibiting the production of
IL-6 and IL-8 through RAME treatment was investigated in HaCaT
cells stimulated with TNF-α/IFN-γ using ELISA and RT-qPCR (Fig. 3). The results revealed that IL-6
and IL-8 were significantly increased in the group treated with
TNF-α/IFN-γ compared with the untreated group. In addition, when
HaCaT cells were treated with TNF-α/IFN-γ following RAME treatment,
IL-6 and IL-8 mRNA expression decreased in a
concentration-dependent manner compared with the group treated with
TNF-α/IFN-γ. Furthermore, RAME treatment inhibited the expression
of IL-6 and IL-8 protein in the HaCaT cells stimulated with
TNF-α/IFN-γ.
Treatment with RAME inhibits the
activation of mitogen activated protein kinases (MAPKs) in HaCaT
cells
TNF-α/IFN-γ activate JNK, ERK and p38 expression
(24). Therefore, the effect of
RAME on JNK, ERK and p38 protein expression in HaCaT cells was
investigated (Fig. 4). HaCaT
cells were treated with TNF-α/IFN-γ and RAME was added after 2 h.
Phosphorylation changes to JNK, ERK, and p38, which are MAPKs that
are known to be important in the atopy pathway, were confirmed. The
results revealed an increase in the phosphorylation of JNK, ERK,
and p38 in HaCaT cells stimulated for 1 h with TNF-α/IFN-γ,
compared with unstimulated HaCaT cells. Furthermore, this
experiment confirmed that when the cells were pretreated with RAME,
the phos-phorylation of JNK, ERK, and p38 was markedly suppressed
as compared with the group treated with TNF-α/IFN-γ alone.
Consequently, RAME exerted an inhibitory effect on the expression
of p-JNK, p-ERK, and p-p38 following TNF-α/IFN-γ stimulation.
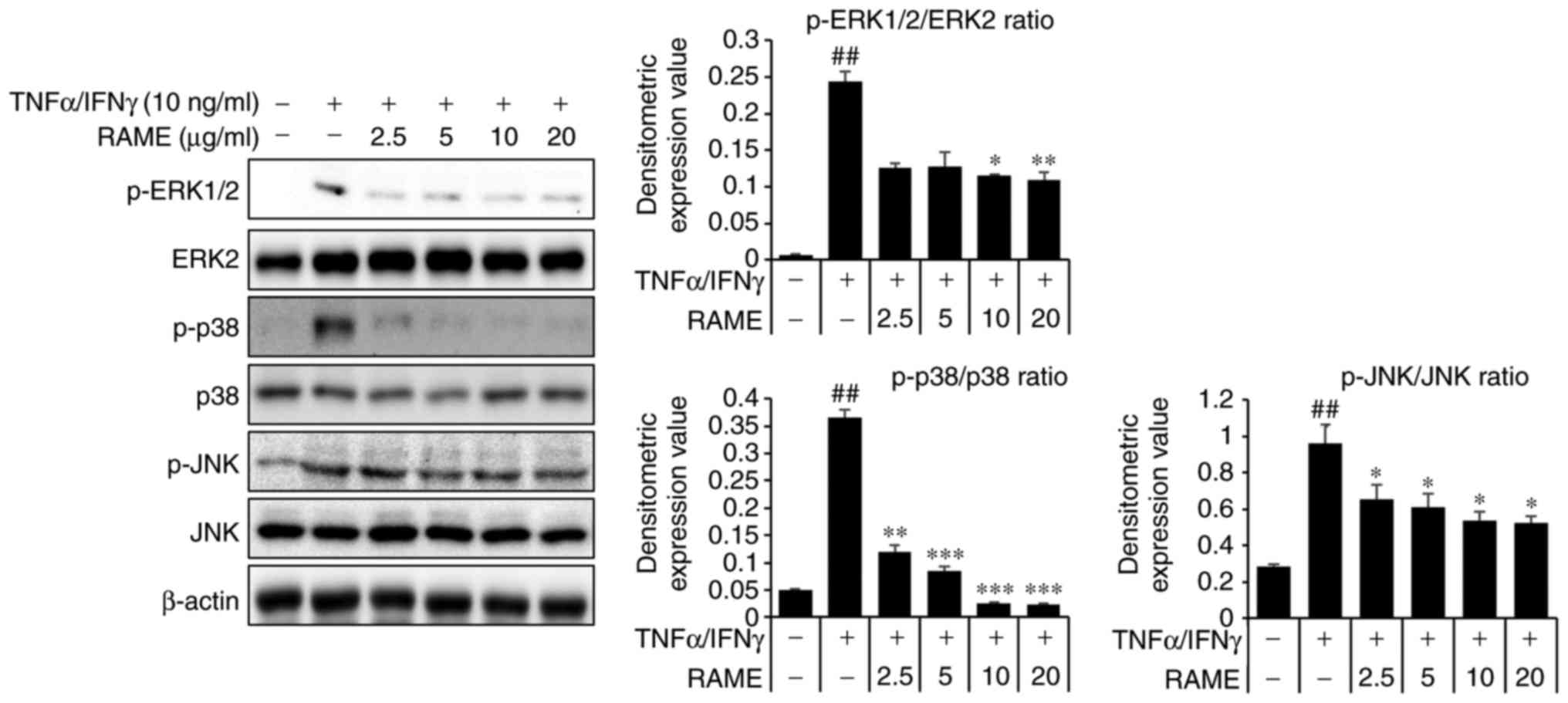 | Figure 4Effect of RAME treatment on
TNF-α/IFN-γ induced MAPKs in HaCaT cells. Cells were pretreated
with 2.5, 5.0, 10.0, and 20.0 μg/ml RAME for 1 h and then
exposed to TNF-α and IFN-γ (each 10 ng/ml) for 2 h. Cell extracts
were prepared and MAPK activation was analyzed by western blotting,
using specific antibodies. ##P<0.01 vs. the negative
control; *P<0.05, **P<0.01 and
***P<0.001 vs. TNF-α/IFN-γ stimulated cells. RAME,
Rhododendron album Blume methanol extract; TNF-α, tumor
necrosis factor-α; IFN-γ, interferon-γ; MAPK, mitogen-activated
protein kinase; p-, phosphorylated; ERK, extracellular
signal-regulated kinase; JNK, c-Jun N-terminal kinase. |
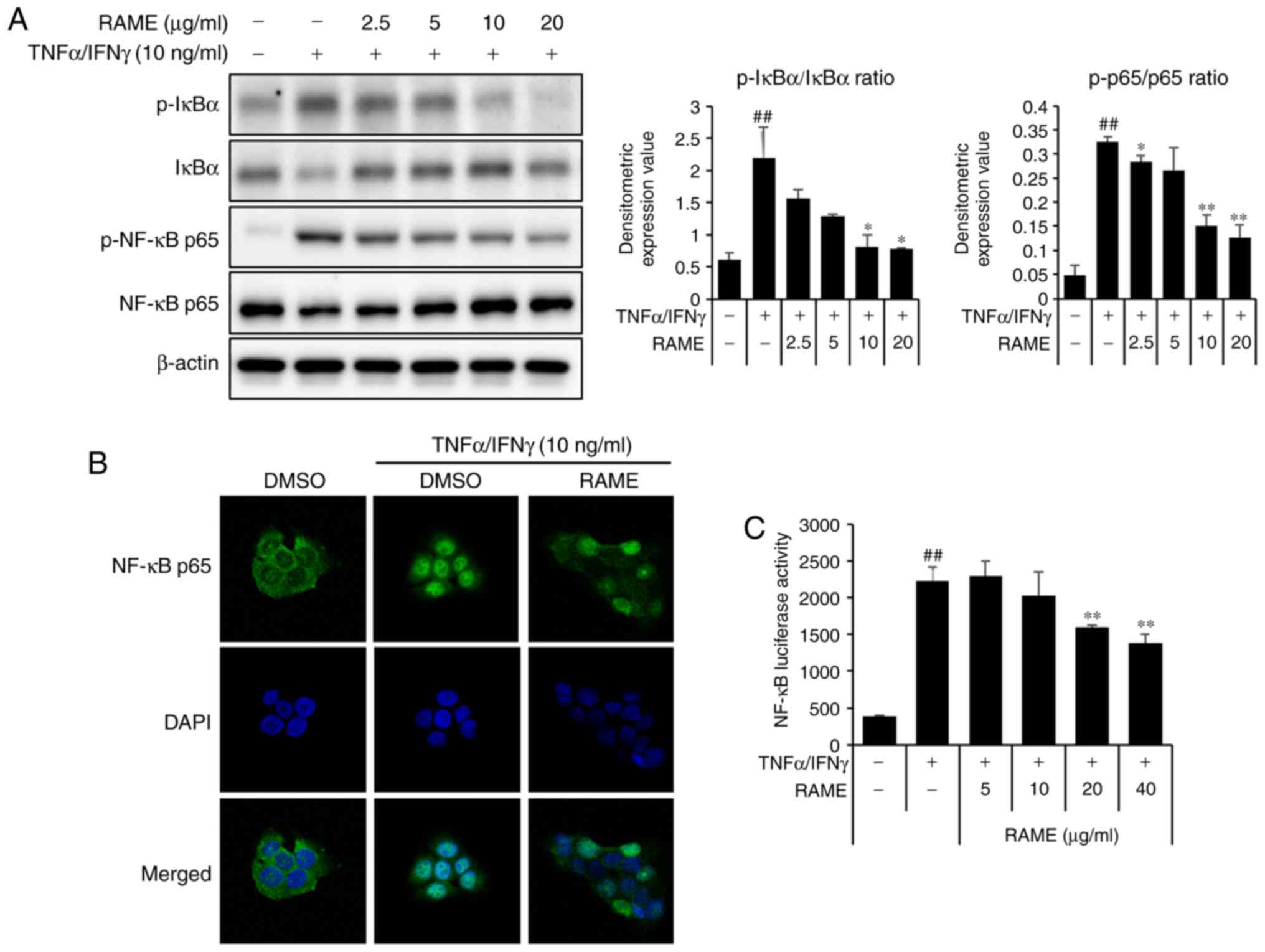
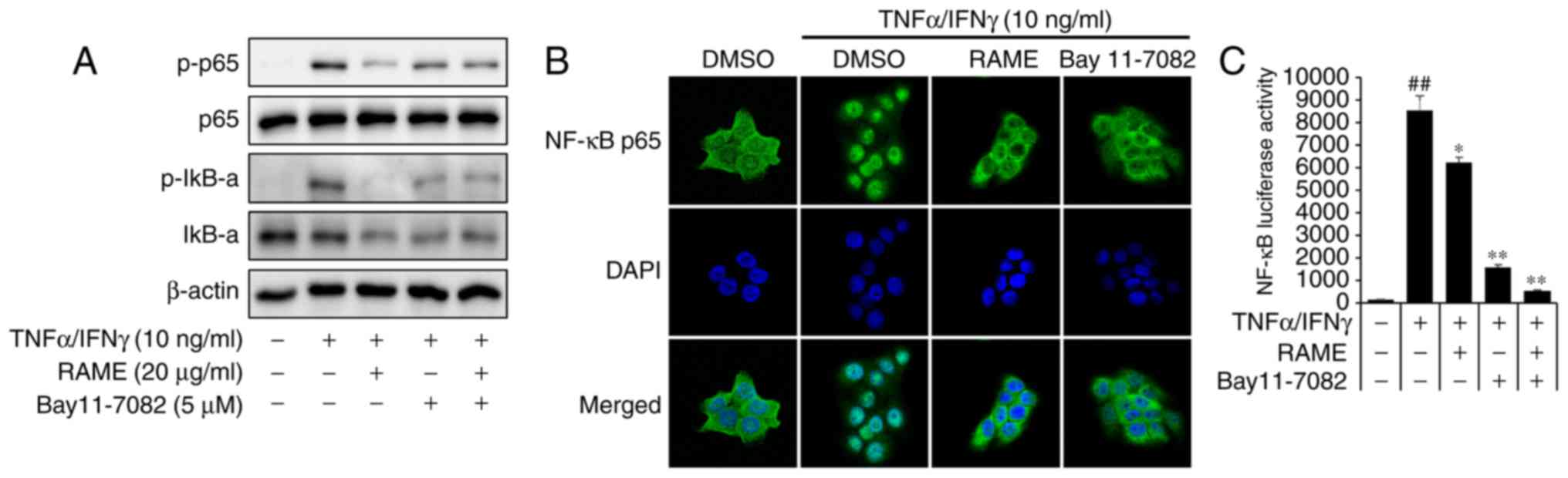
RAME inhibited the activation of NF-κB in
HaCaT cells
NF-κB signaling is critical in skin inflammatory AD
responses induced by MDC and TARC (26). To examine whether RAME
downregulated TNF-α/IFN-γ-mediated NF-κB activation in the
keratinocyte cells, the expression of associated proteins was
examined by western blot analysis using specific antibodies, ICC,
and luciferase assays. TNF-α/IFN-γ significantly increased the
phosphorylation of NF-κB p65 at 1 h. RAME decreased the
phosphorylation of NF-κB p65 in TNF-α/IFN-γ-induced HaCaT cells
(Fig. 5A). TNF-α/IFN-γ-induced
nuclear translocation of p65 was inhibited by treatment with RAME
at 10 μg/ml (Fig. 5B).
Furthermore, the inhibitory effect of RAME on
TNF-α/IFN-γ-stimulated nuclear translocation of NF-κB p65 was also
observed during ICC analysis (Fig.
5C). These results revealed that RAME inhibited the activation
of NF-κB in HaCaT cells.
RAME and Bay11-7082 inhibited the
activation of NF-κB in HaCaT cells
The p65 subunit is an important element of activated
NF-κB. Activation of NF-κB in TNF-α/IFN-γ induced HaCaT cells was
studied by measuring the phosphorylation of NF-κB subunit p65 by
western blot analysis. Pretreatment with RAME and Bay11-7082
decreased the levels of p-IκB-α and p-p65 compared with the
TNF-α/IFN-γ treatment only, while the TNF-α/IFN-γ treatment group
increased the levels of p-IκB-α and p-p65 compared with the
negative control (Fig. 6A).
Stimulation of HaCaT cells with TNF-α/IFN-γ induced nuclear
translocation of p65 NF-κB and degradation of IκB-α.
NF-κB-dependent gene reporter analysis was used to confirm the
inhibitory effect of RAME and Bay11-7082 on NF-κB activation. RAME
and Bay11-7082 suppressed TNF-α/IFN-γ-stimulated nuclear
translocation of p65 NF-κB (Fig.
6B). As presented in Fig. 6C,
the TNF-α/IFN-γ stimulated NF-κB promoter activity in HaCaT cells
was significantly reduced by RAME and Bay11-7082 inhibitors. In
addition, stimulation of HaCaT cells with TNF-α/IFN-γ induced the
expression of chemokines and pro-inflammatory cytokines. RAME and
Bay11-7082 treatment suppressed TNF-α/IFN-γ-stimulated expression
of TARC, MDC, IL-6, and IL-8 (Fig.
7A-D). As presented in Fig.
7, the expression of TNF-α/IFN-γ stimulated chemokines (TARC
and MDC) and pro-inflammatory cytokines (IL-6 and IL-8) in HaCaT
cells was significantly decreased by treatment with RAME and
Bay11-7082 inhibitors.
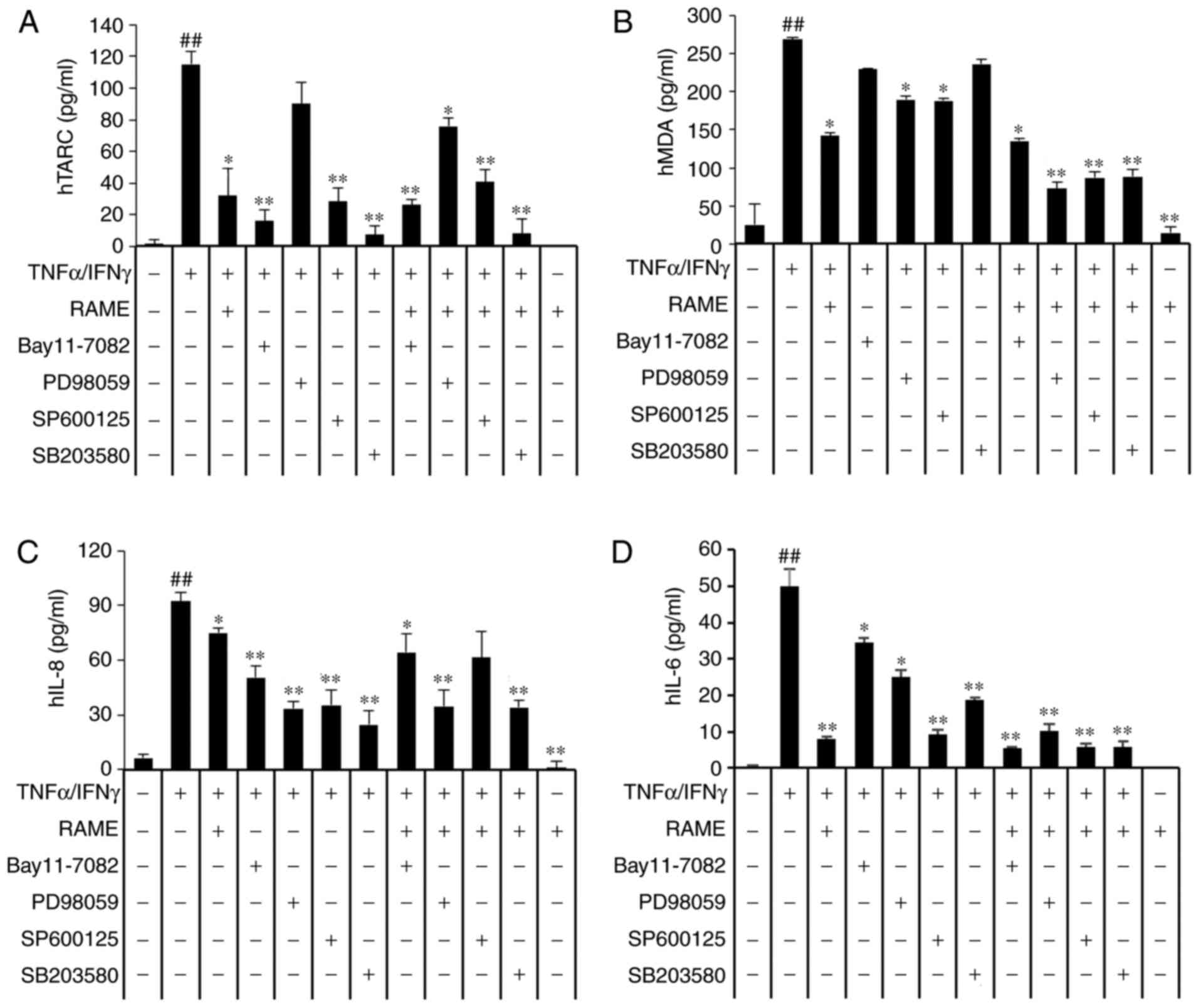 | Figure 7Effects of mitogen-activated protein
kinase inhibitors on the expression of chemokines and
pro-inflammatory cytokines. HaCaT cells were pretreated with 20
μg/ml RAME, and 5 μM Bay11-7082, 10 μM
SB203580, 10 μM PD98059 and 10 μM SP600125 for 1 h
followed by incubation with 10 ng/ml TNF-α/IFN-γ for 18 h. (A) TARC
(B) MDC (C) IL-8 and (D) IL-6 levels were measured by ELISA. Data
are presented as the mean ± standard error of the mean (n=3).
##P<0.01 vs. the the normal control group,
*P<0.05 and **P<0.01 vs the TNF-α/IFN-γ
stimulated cells. RAME, Rhododendron album Blume methanol
extract; TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ; TARC,
thymus- and activation-regulated chemokine; MDC, macrophage-derived
chemokine; IL, interleukin. |
RAME and inhibitors inhibit the
expression of chemokines and cytokines in TNF-α/IFN-γ-stimulated
HaCaT cells
The translocation of NF-κB into the nucleus was
reduced in RAME-treated HaCaT cells (Fig. 5). The MAPKs may be activated by
TNF-α/IFN-γ, a key stimulator of the skin inflammation response in
keratinocytes, as well as multiple other types of cell. As
presented in Fig. 4, RAME
markedly decreased the expression levels of p-p38, p-ERK, and p-JNK
in TNF-α/IFN-γ-induced HaCaT cells compared with untreated cells.
These data suggested that RAME suppresses the inflammatory response
via partial regulation of NF-κB and MAPK signaling. To assess the
function of NF-κB and MAPKs in the production of
TNF-α/IFN-γ-induced inflammatory mediators, the effects of
selective NF-κB and MAPK inhibitors on TNF-α/IFN-γ-induced
chemokine and cytokine production were investigated. ELISA analysis
revealed that Bay11-7082 (IκB-α inhibitor) SP600125 (JNK
inhibitor), PD98059 (ERK inhibitor) and SB203580 (p38 inhibitor)
markedly inhibited TARC, MDC, IL-6, and IL-8 expression,
respectively. Furthermore, treatment with RAME and inhibitors were
demonstrated to markedly inhibit TNF-α/IFN-γ-mediated expression of
TARC (Fig. 7A), MDC (Fig. 7B), IL-8 (Fig. 7C), and IL-6 (Fig. 7D). These results suggested that
RAME-mediated inactivation of NF-κB p65, JNK, ERK, and p38 MAPK
may, at least in part, be responsible for the suppression of TARC,
MDC, IL-8 and IL-6 in HaCaT cells. In addition, TNF-α/IFN-γ
stimulation induced an increase in the activation of NF-κB and
MAPKs, while treatment with RAME inhibited this effect.
Treatment with RAME inhibits the
activation of JAK/STAT in HaCaT cells
The JAK/STAT pathway is important to the immune
system. To investigate whether RAME treatment resulted in the
downregulation of TNF-α/IFN-γ-mediated JAK/STAT activation in HaCaT
cells, western blot analysis and immunocytochemistry using specific
antibodies were utilized (Fig.
8). RAME pre-treatment 1 h prior to TNF-α/IFN-γ exposure in the
HaCaT cells. TNF-α/IFN-γ increased the phosphorylation of JAK/STAT
signaling for 1 h. As a result, RAME was revealed to inhibit the
phosphorylation of the JAK/STAT pathway in TNF-α/IFN-γ-stimulated
HaCAT cells (Fig. 8A). In
addition, RAME treatment was revealed to decrease translocation of
STAT1 in HaCaT cells by immunocytochemistry (Fig. 8B). These results revealed that
RAME inhibited the activation of JAK/STAT in HaCaT cells.
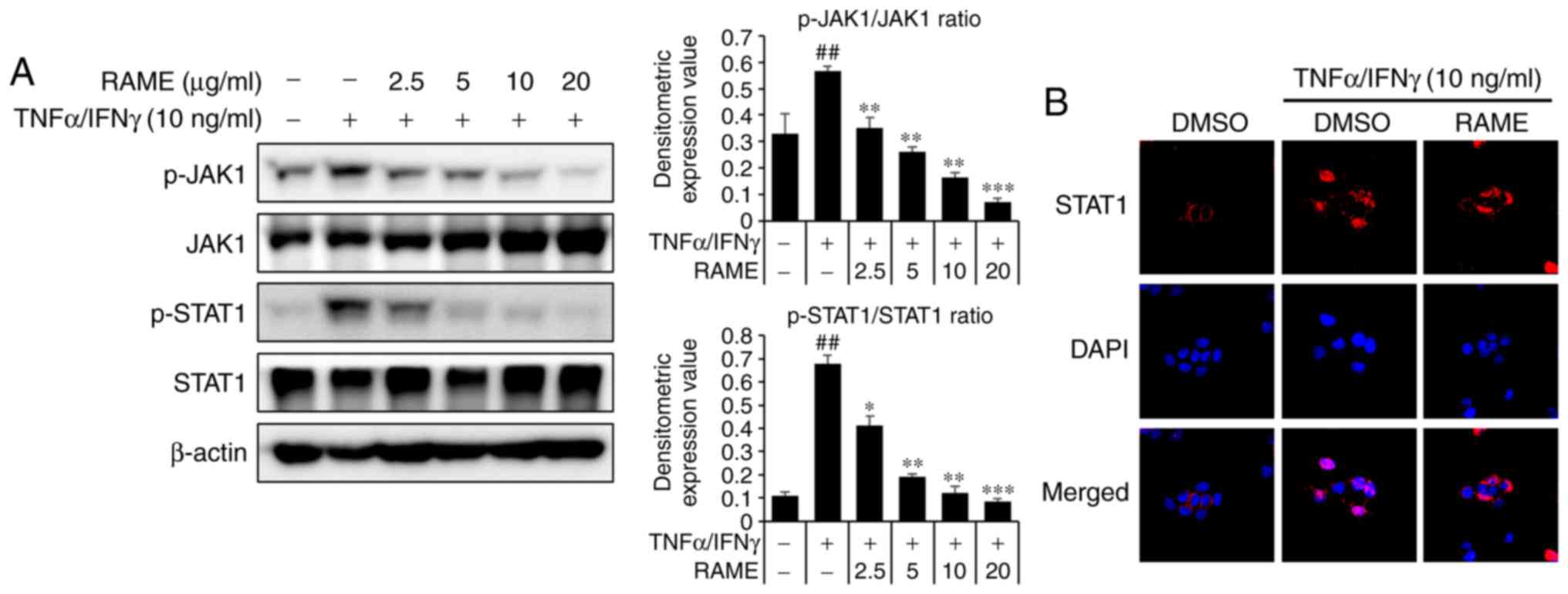 | Figure 8Effect of RAME on TNF-α/IFN-γ induced
NF-κB activation in HaCaT cells. (A) The phosphorylation of JAK1,
JAK2 and STAT1 was analyzed using western blotting. (B) Cell
localization of STAT1 was determined by immunocytochemistry. Using
DAPI (blue), the nuclei were visualized and observed at ×400
magnification. Data are presented as the mean ± standard error of
the mean of three samples. ##P<0.01 vs. the negative
control; *P<0.05, **P<0.01 and
***P<0.001 vs. TNF-α/IFN-γ stimulated cells. RAME,
Rhododendron album Blume methanol extract; TNF-α, tumor
necrosis factor-α; IFN-γ, interferon-γ; NF-κB, nuclear factor-κB;
JAK, Janus kinase; STAT, signal transducers and activators of
transcription; p-, phosphorylated. |
Discussion
Traditional medicine using natural herbs may help to
prevent and treat several immune-related diseases, including AD,
atopic inflammation and allergy (27). The present study revealed that
RAME treatment reduced the production of various inflammatory and
allergy-mediated chemokines and cytokines in TNF-α/IFN-γ-induced
HaCaT cells, through inactivation of NF-κB and MAPKs.
Regulation of cytokine production is central to the
pathogenesis of allergic disorders. Cho et al (27) observed increased levels of IL-6,
IL-8, IL-10, IL-23 and TGF-β expression in patients with AD
compared with healthy individuals. In the present study, RAME
decreased IL-8 and IL-6 gene expression in HaCaT cells (Fig. 3A-D). These data suggested that
RAME exerted anti-inflammatory effects by suppressing the
production of inflammatory chemokines and cytokines. In addition,
chemokines and their receptors serve an important function in AD by
regulating the onset and worsening of inflammation in response to
allergens (28,29). Giuliani et al and Chen
et al (30,31) have demonstrated that serum levels
of MDC and TARC are increased in AD, and that this increase is
positively correlated with the illness severity in AD. To the best
of our knowledge, the present study is the first to demonstrate
that RAME inhibits TNF-α/IFN-γ-induced TARC and MDC expression in
HaCaT keratinocyte cells (Fig.
2A-D).
NF-κB represents part of an important
pro-inflammatory signaling pathway. NF-κB regulates the expression
of several asthma, inflammation, allergy and immune-associated
genes through the degradation of IκB (30-32). Therefore, RAME may have an
anti-allergic effect based on a decrease in activated NF-κB levels
(Fig. 5A-C). The IκB-α inhibitor
Bay11-7082 (33), which prevents
the phosphorylation and activation of p65, was used because
specific inhibitor constructs that reduce p65 and IκB-α levels in
HaCaT cells were not identified (Fig.
6A-C). The effect of the NF-κB specific inhibitor Bay11-7082
was investigated and revealed to significantly inhibit the
activation of NF-κB. This result seems to be due to the mechanisms
underlying the anti-inflammatory effects of RAME, which are caused
by the suppression of NF-κB transcriptional activity. The MAPK
cascade is one of the signaling pathways involved in immune
responses (24,34,35). The MAPK signaling pathway
regulates multiple cellular processes, including gene expression,
cell death and survival, cell motility and cell proliferation
(36,37). MAPKs include three major
subfamilies, namely p42/p44 ERKs, p38 MAPKs, and JNKs. The
inhibition of MAPKs has been reported to reduce the synthesis of
pro-inflammatory cytokines and their intracellular signaling
pathways, and inhibit the activation of NF-κB (38,39). TNF-α/IFN-γ stimulation of HaCaT
cells was confirmed to activate three major MAPK modules, including
ERK, JNK and p38. Treatment with RAME, the p38 MAPK inhibitor
SB203580, the ERK inhibitor PD98059 and the JNK inhibitor SP600125
reduced TNF-α/IFN-γ-activated expression of pro-inflammatory
cytokine (IL-6 and IL-8) and chemokines (TARC and MDC) to baseline
values. Furthermore, these inhibitors and RAME reduced
pro-inflammatory cytokine (IL-6 and IL-8) and chemokine (TARC and
MDC) expression by ~50% (Fig. 7).
These results suggested that the inhibition of JNK, ERK and p38
MAPK by RAME treatment reduces the production of pro-inflammatory
cytokines and chemo-kines in HaCaT cells (Fig. 4). The fact that RAME reduces
TNF-α/IFN-γ-induced MAPK, MDC, and TARC mRNA expression again
suggested that RAME has anti-allergic and anti-inflammatory
effects.
In mammals, the JAK/STAT pathway is the main
signaling mechanism for a wide array of growth factors and
cytokines. JAK/STAT signaling is one of a number of pleiotropic
cascades used to transduce signals for development in animals and
humans. The results of the present study suggested that the RAME
may have an anti-allergic effect based on the decrease in activated
JAK/STAT (Fig. 8A and B).
In conclusion, the present study confirmed that RAME
inhibits the expression of AD-associated chemokines and cytokines
in TNF-α/IFN-γ-induced keratinocytes. These effects were considered
to be associated with the suppression of NF-κB activation, These
experimental results provide a scientific basis for the use of RAME
to treat AD. Additional experiments to test the in vitro
effects of RAME on AD are currently in progress in our
laboratory.
Acknowledgments
Not applicable.
Notes
[1]
Funding
The present study was supported by the Bio &
Medical Technology Development Program of the National Research
Foundation and funded by the Korean government (MSIT; grant no.
NRF-2016K1A1A8A01939075) and the Korea Research Institute of
Bioscience and Biotechnology Research Initiative Program of the
Republic of Korea (grant no. KGM1221814).
[2] Availability
of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
JWP analyzed the data and wrote the manuscript. HSL
and YRL conducted the in vitro experiments. JHP, OKK, JHK,
IP and PY prepared the Rhododendron album Blume, and
analyzed and edited the manuscript. SC, SRO and KSA designed the
study and edited the manuscript. All authors critically revised the
article and have consented to the final version of the
manuscript.
[4] Ethics
approval and consent to participate
Not applicable.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Brandt EB and Sivaprasad U: Th2 cytokines
and atopic dermatitis. J Clin Cell Immunol. 2:pii. 1102011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abramovits W: Atopic dermatitis. J Am Acad
Dermatol. 53(1 Suppl 1): S86–S93. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Portugal-Cohen M, Horev L, Ruffer C,
Schlippe G, Voss W, Ma'or Z, Oron M, Soroka Y, Frušić-Zlotkin M,
Milner Y and Kohen R: Non-invasive skin biomarkers quantification
of psoriasis and atopic dermatitis: Cytokines, antioxidants and
psoriatic skin auto-fluorescence. Biomed Pharmacother. 66:293–299.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hoffjan S and Stemmler S: On the role of
the epidermal differentiation complex in ichthyosis vulgaris,
atopic dermatitis and psoriasis. Br J Dermatol. 157:441–449. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakatsuji T, Chen TH, Two AM, Chun KA,
Narala S, Geha RS, Hata TR and Gallo RL: Staphylococcus aureus
exploits epidermal barrier defects in atopic dermatitis to trigger
cytokine expression. J Invest Dermatol. 136:2192–2200. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leung DY, Boguniewicz M, Howell MD, Nomura
I and Hamid QA: New insights into atopic dermatitis. J Clin Invest.
113:651–657. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang IJ, Lee DU and Shin HM: Inhibitory
effect of valencene on the development of atopic dermatitis-like
skin lesions in nc/nga mice. Evid Based Complement Alternat Med.
2016:93708932016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zlotnik A and Yoshie O: Chemokines: A new
classification system and their role in immunity. Immunity.
12:121–127. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kang GJ, Han SC, Yi EJ, Kang HK and Yoo
ES: The inhibitory effect of premature citrus unshiu extract on
atopic dermatitis in vitro and in vivo. Toxicol Res. 27:173–180.
2011. View Article : Google Scholar
|
|
10
|
Vestergaard C, Yoneyama H, Murai M,
Nakamura K, Tamaki K, Terashima Y, Imai T, Yoshie O, Irimura T,
Mizutani H and Matsushima K: Overproduction of Th2-specific
chemokines in NC/Nga mice exhibiting atopic dermatitis-like
lesions. J Clin Invest. 104:1097–1105. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Horikawa T, Nakayama T, Hikita I, Yamada
H, Fujisawa R, Bito T, Harada S, Fukunaga A, Chantry D, Gray PW, et
al: IFN-gamma-inducible expression of thymus and
activation-regulated chemokine/CCL17 and macrophage-derived
chemokine/CCL22 in epidermal keratinocytes and their roles in
atopic dermatitis. Int Immunol. 14:767–773. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sebastiani S, Albanesi C, De PO, Puddu P,
Cavani A and Girolomoni G: The role of chemokines in allergic
contact dermatitis. Arch Dermatol Res. 293:552–559. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shimada Y, Takehara K and Sato S: Both Th2
and Th1 chemokines (TARC/CCL17, MDC/CCL22, and Mig/CXCL9) are
elevated in sera from patients with atopic dermatitis. J Dermatol
Sci. 34:201–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kimura M, Haisa M, Uetsuka H, Takaoka M,
Ohkawa T, Kawashima R, Yamatsuji T, Gunduz M, Kaneda Y, Tanaka N
and Naomoto Y: TNF combined with IFN-alpha accelerates
NF-kappaB-mediated apoptosis through enhancement of Fas expression
in colon cancer cells. Cell Death Differ. 10:718–728. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rath PC: Relationship between constitutive
nuclear factor-kappaB (NF-kappaB) and inhibitor kappaB-alpha
(IkappaB-alpha) in an interferon-alpha-sensitive human Burkitt
lymphoma cell line. Biochim Biophys Acta. 1741:253–263. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baeuerle PA and Baichwal VR: NF-kappa B as
a frequent target for immunosuppressive and anti-inflammatory
molecules. Adv Immunol. 65:111–137. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ortis F, Pirot P, Naamane N, Kreins AY,
Rasschaert J, Moore F, Théâtre E, Verhaeghe C, Magnusson NE,
Chariot A, et al: Induction of nuclear factor-kappaB and its
downstream genes by TNF-alpha and IL-1beta has a pro-apoptotic role
in pancreatic beta cells. Diabetologia. 51:1213–1225. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao ZH, Yin WD, Zheng QY, Feng SL, Xu GL
and Zhang KQ: Caspase-3 is involved in IFN-γ- and TNF-α-mediated
MIN6 cells apoptosis via NF-κB/Bcl-2 pathway. Cell Biochem Biophys.
67:1239–1248. 2013. View Article : Google Scholar
|
|
19
|
Ali S, Nisar M, Qaisar M, Khan A and Khan
AA: Evaluation of the cytotoxic potential of a new pentacyclic
triterpene from Rhododendron arboreum stem bark. Pharm Biol.
55:1927–1930. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li FR, Yu FX, Yao ST, Si YH, Zhang W and
Gao LL: Hyperin extracted from Manchurian rhododendron leaf induces
apoptosis in human endometrial cancer cells through a mitochondrial
pathway. Asian Pac J Cancer Prev. 13:3653–3656. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu YL, Lin LC, Tung YT, Ho ST, Chen YL,
Lin CC and Wu JH: Rhododendron oldhamii leaf extract improves fatty
liver syndrome by increasing lipid oxidation and decreasing the
lipogenesis pathway in mice. Int J Med Sci. 14:862–870. 2017.
View Article : Google Scholar :
|
|
22
|
Park JW, Kwon OK, Kim JH, Oh SR, Kim JH,
Paik JH, Marwoto B, Widjhati R, Juniarti F, Irawan D and Ahn KS:
Rhododendron album Blume inhibits iNOS and COX-2 expression in
LPS-stimulated RAW264.7 cells through the downregulation of NF- κB
signaling. Int J Mol Med. 35:987–994. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee JW, Park JW, Kwon OK, Lee HJ, Jeong
HG, Kim JH, Oh SR and Ahn KS: NPS2143 Inhibits MUC5AC and
proinflammatory mediators in cigarette smoke extract
(CSE)-stimulated human airway epithelial cells. Inflammation.
40:184–194. 2017. View Article : Google Scholar
|
|
24
|
Ko JW, Park JW, Shin NR, Kim JH, Cho YK,
Shin DH, Kim JC, Lee IC, Oh SR, Ahn KS and Shin IS: Copper oxide
nanoparticle induces inflammatory response and mucus production via
MAPK signaling in human bronchial epithelial cells. Environ Toxicol
Pharmacol. 43:21–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
26
|
Shibata S, Maeda S, Kondo N, Chimura N,
Inoue A and Fukata T: Identification of the signaling pathway of
TNF-α-induced CCL17/TARC transcription in a canine keratinocyte
cell line. Vet Immunol Immunopathol. 139:90–98. 2011. View Article : Google Scholar
|
|
27
|
Cho BO, Che DN, Yin HH, Shin JY and Jang
SI: Diospyros lotus leaf and grapefruit stem extract
synergistically ameliorate atopic dermatitis-like skin lesion in
mice by suppressing infiltration of mast cells in skin lesions.
Biomed Pharmacother. 89:819–826. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Homey B, Steinhoff M, Ruzicka T and Leung
DY: Cytokines and chemokines orchestrate atopic skin inflammation.
J Allergy Clin Immunol. 118:178–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nedoszytko B, Sokołowska-Wojdyło M,
Ruckemann-Dziurdzińska K, Roszkiewicz J and Nowicki RJ: Chemokines
and cytokines network in the pathogenesis of the inflammatory skin
diseases: Atopic dermatitis, psoriasis and skin mastocytosis.
Postepy Dermatol Alergol. 31:84–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Giuliani C, Napolitano G, Bucci I, Montani
V and Monaco F: Nf-κB transcription factor: Role in the
pathogenesis of inflammatory, autoimmune, and neoplastic diseases
and therapy implications. Clin Ter. 152:249–253. 2001.In Italian.
PubMed/NCBI
|
|
31
|
Chen Y, Xian Y, Lai Z, Loo S, Chan WY and
Lin ZX: Anti-inflammatory and anti-allergic effects and underlying
mechanisms of Huang-Lian-Jie-Du extract: Implication for atopic
dermatitis treatment. J Ethnopharmacol. 185:41–52. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Park JW, Kim YJ, Shin IS, Kwon OK, Hong
JM, Shin NR, Oh SR, Ha UH, Kim JH and Ahn KS: Type III secretion
system of Pseudomonas aeruginosa affects matrix metalloproteinase
12 (MMP-12) and MMP-13 Expression via nuclear factor κB signaling
in human carcinoma epithelial cells and a pneumonia mouse model. J
Infect Dis. 214:962–969. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park JW, Shin IS, Ha UH, Oh SR, Kim JH and
Ahn KS: Pathophysiological changes induced by Pseudomonas
aeruginosa infection are involved in MMP-12 and MMP-13 upregulation
in human carcinoma epithelial cells and a pneumonia mouse model.
Infect Immun. 83:4791–4799. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim HH, Kim SW, Kim DS, Oh HM, Rho MC and
Kim SH: Vigna angularis inhibits mast cell-mediated allergic
inflammation. Int J Mol Med. 32:736–742. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Park JW, Lee IC, Shin NR, Jeon CM, Kwon
OK, Ko JW, Kim JC, Oh SR, Shin IS and Ahn KS: Copper oxide
nanoparticles aggravate airway inflammation and mucus production in
asthmatic mice via MAPK signaling. Nanotoxicology. 10:445–452.
2016. View Article : Google Scholar
|
|
36
|
Jeong YH, Oh YC, Cho WK, Lee B and Ma JY:
Anti-inflammatory effects of melandrii herba ethanol extract via
inhibition of NF-κB and MAPK signaling pathways and induction of
HO-1 in RAW 264.7 cells and mouse primary macrophages. Molecules.
21:pii. E8182016. View Article : Google Scholar
|
|
37
|
Zheng G, Shen Z, Chen H, Liu J, Jiang K,
Fan L, Jia L and Shao J: Metapristone suppresses non-small cell
lung cancer proliferation and metastasis via modulating
RAS/RAF/MEK/MAPK signaling pathway. Biomed Pharmacother.
90:437–445. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao S, Sun Y, Li X, Wang J, Yan L, Zhang
Z, Wang D, Dai J, He J and Wang S: Scutellarin inhibits
RANKL-mediated osteoclastogenesis and titanium particle-induced
osteolysis via suppression of NF-κB and MAPK signaling pathway. Int
Immunopharmacol. 40:458–465. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Park JW, Kwon OK, Yuniato P, Marwoto B,
Lee J, Oh SR, Kim JH and Ahn KS: Amelioration of an LPS-induced
inflammatory response using a methanolic extract of Lagerstroemia
ovalifolia to suppress the activation of NF-κB in RAW264.7
macrophages. Int J Mol Med. 38:482–490. 2016. View Article : Google Scholar : PubMed/NCBI
|