Introduction
The incidence of pelvic floor dysfunctional disease
(PFD) in aging population is increasing because of prolonged life
span after menopause (1,2). Older women with PFD have various
complications and it affects the quality of life significantly. The
occurrence of PFD is closely related with elasticity, toughness,
and functional changes of the connective tissue of the pelvic
support tissue (3). Normal pelvic
structure support and function depend on the pelvic floor
connective tissues. It has previously been stated that an
alteration in the extracellular matrix proteins of the supporting
ligament has been implicated in the PFD (4).
Therapeutic use of mesenchymal stem cells (MSC) is
one potential solution, which has already been employed in clinical
trials, including the treatment of human myocardial infarction,
osteogenesis imperfecta, and graft versus host disease (5). Historically, MSC were first isolated
from bone marrow mesenchymal stem cells (BMSCs) (6) and bone marrow is the current gold
standard tissue source for therapy. BMSCs come from the mesoderm
with multilineage capacity potential and generate a variety of cell
types (7), such as osteoblasts,
adipocytes, myoblasts, chondrocytes and neurons (8). After injury, endogenous or exogenous
BMSCs migrate into lesion for repair. Increasing studies reported
that in the process of ligament injury repair, different
pluripotent stem cells are released from bone marrow mesenchyme.
These stem cells are similar to the surrounding ligament cells,
move to and accumulate in the injury sites (9,10).
Mesenchymal stem cells also display several anti-inflammatory
properties, presenting the possibility for allogenic
transplantation (11). However,
the underlying regulatory mechanisms for triggering differentiation
are only partially understood.
A wound healing reaction is orchestrated by a
variety of signals derived from endogenous soluble factors. It is
widely accepted that transforming growth factor-β (TGF-β) is one of
the most critical growth factors regulating cellular responses in
the process of wound healing or tissue inflammation (12). TGF-β is known as a stimulator for
extracellular matrix proteins production in fibroblasts and
mediates the response of fibroblasts to mechanical stress (13). With the employment of a co-culture
system, our previous studies have demonstrated that mechanical
stretch could indirectly promote the synthesis of TGF-β expression
and increase expression of collagen I and II, elastin, LOX, and
fibulin-5 during the process of BMSCs differentiation (14). It was also found that TGF-β1 and
MAPK pathways are involved in the differentiation of BMSCs to
pelvic ligament fibroblasts stimulated by mechanic stretch.
However, it is still not clearly illustrated whether other
molecules are implicated in this process and the underlying
mechanisms.
Peroxisome proliferator-activated receptors (PPARs)
are ligand-activated transcription factors belonging to the nuclear
hormone receptor superfamily. PPARs consist of three members:
PPAR-α, -β or -γ, which are involved in modulation of metabolism,
immune responses and cell proliferation, i.e., fibrogenic reaction
or cell proliferation during wound healing (15,16). It is well known that PPAR-γ is
mainly related to regulation of adipocyte differentiation and
fatty-acid uptake and storage (17–20). Previous studies showed that
thiazolidinedione, one of PPAR-γ synthetic agonists, benefits
patients with diabetes type II (21,22). In addition, PPAR-γ was found to
possess a new action in fibrosis through activation of PPAR-γ then
reduce fibrosis in several organs, such as heart and lung (23). Furthermore, PPAR-γ has been
reported to participate in the differentiation of fibroblast
(24,25). However, the underlying mechanisms
and whether PPAR-γ is involved in the regulation of BMSCs
differentiation into ligament fibroblasts are still unclear.
Therefore, the present study explored the effects of
PPAR-γ in BMSC differentiation to ligament fibroblasts. Our data
showed that PPAR-γ expression was significantly decreased in
ligament fibroblasts and in the differentiation of BMSCs to
fibroblasts induced by cyclic mechanical stretch in vitro.
Additionally, downregulation of PPAR-γ by shRNA contributed to the
differentiation of BMSCs. Furthermore, upregulation of PPAR-γ by
natural ligand and PPAR-γ overexpression plasmids attenuated BMSCs
differentiation to fibroblasts in vitro. The present study
suggests that PPAR-γ negatively regulates the differentiation of
BMSCs in a co-culture system. This will help us to further
understand the differentiation potential of BMSCs and
characteristics in order to develop a new therapy for pelvic organ
prolapse (POP).
Materials and methods
Experimental animals
Sprague-Dawley (SD) rats were purchased from
Experimental Animal Center of Fourth Military Medical University
(Xi'an, China). All studies were conducted in accordance with the
standards of humane animal care described in the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals, using protocols approved by Zhengzhou University
Institutional Animal Care and Research Advisory Committee.
Viral production and purification
The lentiviral vector encoding PPAR-γ shRNA or
control shRNA lentiviral particles was generated by co-transfection
of 293T cells with the PPAR-γ-shRNA plasmid vector (sc-156077-V) or
the control shRNA Lentiviral Particles (sc-108080) (both from Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Cell debris was
removed through 0.22 μm filtration and concentrated by
ultracentrifuge at 17,000 rpm, 4°C, for 1 h. The bottom pellets
containing the lentiviral vector were re-suspended with DMEM medium
and diluted to the concentration at 1×108 TCID50/ml. The
titer was determined by the measurement of the cytosolic p24
protein, 1 pg of p24 reading was assigned as 10 tissue culture
infective dosage (TCID50) for freshly isolated lentiviral
vectors.
Rat BMSC preparation
Isolation, culture and passage of rat BMSCs from
7-day old SD rats were prepared as previously described (14). Briefly, under sterile conditions,
syringe was used to rinse out bone marrow. Then the bone marrow
cells were isolated with pre-filled Percoll separation medium. The
interface layer of mononuclear cells was flushed two times with
cold phosphate-buffered saline (PBS) and maintained in LG-DMEM.
These primary cells were subcultured at 80–90% confluence. BMSCs
are characterized by the phenotypes as positive for CD44 and CD90,
but negative for CD34 and CD45 in flow cytometry analysis. BMSCs of
passage 3 to passage 5 were used in the experiments.
Rat pelvic ligament fibroblasts and
fibroblast traction injury model
Rat pelvic ligament fibroblasts were prepared as
described and maintained in growth medium. The medium was replaced
with fresh medium every other day and the fourth passage of
fibroblasts cells was collected for immunohistochemistry staining.
Passage 3 fibroblasts (3×105/membrane) were seeded onto
gelatin-coated silicone membrane and cultured for 24 h. Then 10% of
the load transformation was exerted on cells with 1 Hz horizontal
stretch stimulation for different duration under 5% CO2
and 37°C conditions. Cells were fixed in paraformaldehyde, and then
stained with DAPI (5 μg/ml). Cell morphology was examined
under a confocal microscope (Olympus, Tokyo, Japan). BMSCs were
seeded into lower chamber while ligament fibroblasts in the upper
chamber of 6-well plate. After indirect co-culture for different
duration or other treatments, cells were collected for real-time
RT-PCR and western blot assay.
Lentiviral transfection of SD rat
BMSCs
Confluent BMSCs (70–80%) of passage 3–5 were
collected after digestion and were centrifuged at 168 × g for 5
min. Then the cell pellet was re-suspended and were seeded into a
6-well plate at a density of 1×105 cells/well for
overnight incubation. Followed by replacement with 1 ml of freshly
prepared BMSC complete medium, the cells were then transfected with
lentivirus, respectively. Eight hours after the transduction, the
BMSC complete medium was replaced and transduction was continued
for different duration. After treatment, cells were collected to
perform real-time RT-PCR and western blot analysis.
TGF-β1 enzyme-linked immunosorbent assay
(ELISA)
All samples were analyzed for TGF-β1 by using a
commercially available ELISA kit (R&D Systems, Minneapolis, MN,
USA). Representative samples were tested for the presence of active
TGF-β1, but the concentration was under the detection limit of the
assay. Accordingly, all samples were activated by acidification to
separate TGF-β1 from its binding proteins, allowing for measurement
of total TGF-β1. For activation of samples containing fetal bovine
serum (FBS), 2.5 M acetic acid-10 M urea were used, and 1 M HCl was
used for micro-dialysis samples (as recommended by the supplier).
Activated samples were neutralized by use of 2.7 M NaOH-1 M HEPES
for samples with FBS and 1.2 M NaOH-0.5 M HEPES for microdialysis
(as recommended by the supplier). Samples were loaded on ELISA
plates immediately after neutralization. All samples were measured
in duplicate, and samples obtained from each subject were measured
in the same assay.
Immunofluorescence and high content
screening
BMSCs with or without treatments were fixed in 4%
paraformaldehyde for 30 min. Then cells were rinsed twice with PBS,
incubated with 0.5% Triton X-100 at room temperature for 15 min,
blocked with 2% BSA for 1 h, and incubated overnight with primary
antibodies to collagen I (1:200), collagen III (1:200) and PPAR-γ
(1:250) (all from Santa Cruz Biotechnology, Inc.), followed by
secondary antibody conjugated with Cy3 (1:500; Abcam, Shanghai,
China) for 1 h. These cells were stained with 10 μg/ml DAPI
for 5 min. For negative controls, cells were stained with the same
process. The plates were imaged on a Thermo Scientific Cell Insight
personal cell imaging (PCI) platform (Cellomics; Thermo Fisher
Scientific Inc., Waltham, MA, USA) using the Thermo Scientific
Cellomics iDEV software. High content analysis was performed as
described previously. Briefly, 3,000 cells in 36 fields were
automatically examined by the software. The fluorescence intensity
was analyzed using Cellomics Cell Health Profiling BioApplication
software.
Real-time PCR
Quantitative RT-PCR was carried out using real-time
PCR with the SYBR Green reporter. RNA was isolated using TRIzol RNA
extraction kit. OD260 nm was used to determine RNA yield. RNA was
subsequently reverse transcribed to cDNA. Three-stage program
parameters were provided by the manufacturer as follows: 2 min at
94°C, 30 sec at 60°C, and 60 sec at 55°C, then 45 cycles total.
Specificity of the produced amplification product was confirmed by
examination of dissociation reaction plots. Each sample was tested
in triplicate, and samples obtained from three independent
experiments were used for analysis of relative gene expression. The
following primers for real-time PCR were designed by Takara Co.
(Dalian, China): rat PPAR-γ, forward, 5′-CATTTTTCAAGGGTGCCAGT-3′
and reverse, 3′-GAGGCCAGCATGGTGTAGAT-5′; rat glyceraldehyde
3-phosphate dehydrogenase (GAPDH), forward,
5′-CCTTCCGTGTTCCTACCC-3′ and reverse, 5′-CAA
CCTGGTCCTCAGTGTAG-3′.
Western blot assay
Protein was extracted in 200 μl RIPA lysis
buffer. Equal amounts of proteins were loaded on 10% Tris-glycine
gels after being denatured. The proteins were then transferred to
PVDF membranes. The membranes were blocked with 5% milk in PBS-T
(0.1% Tween-20), followed by incubation with primary antibodies
against PPAR-γ and GAPDH at 4°C overnight. The membranes were
washed with PBS-T and incubated for 1 h at room temperature with
horseradish peroxidase-conjugated secondary antibodies. Following
washing, the specific proteins were detected using a
chemiluminescent protein detection kit. Each experiment was
repeated in triplicate. GAPDH was used as the internal control. The
densities of the bands were analyzed using Quantity One (Bio-Rad,
Hercules, CA, USA).
Statistical analysis
Data were presented as mean ± SD. Statistical
significance was analyzed by one-way ANOVA using the GraphPad
Presim 6.0 software. A value of P<0.05 was considered
significant.
Results
PPAR-γ is downregulated in rat ligament
fibroblasts and BMSCs after mechanical stretch
Recent studies report that PPAR-γ expression is
decreased in fibroblasts in in vitro and in vivo
studies (26,27). In order to examine PPAR-γ
expression in fibroblasts and BMSCs after direct mechanical
stretch, a custom-made mechanical device was used to apply cyclic
uniaxial strains to fibroblasts and BMSCs. Our previous study
reported that the expression of collagen type I and III, main ECM
proteins, were increased in a dose-dependent manner in both
fibroblasts and BMSCs (28). In
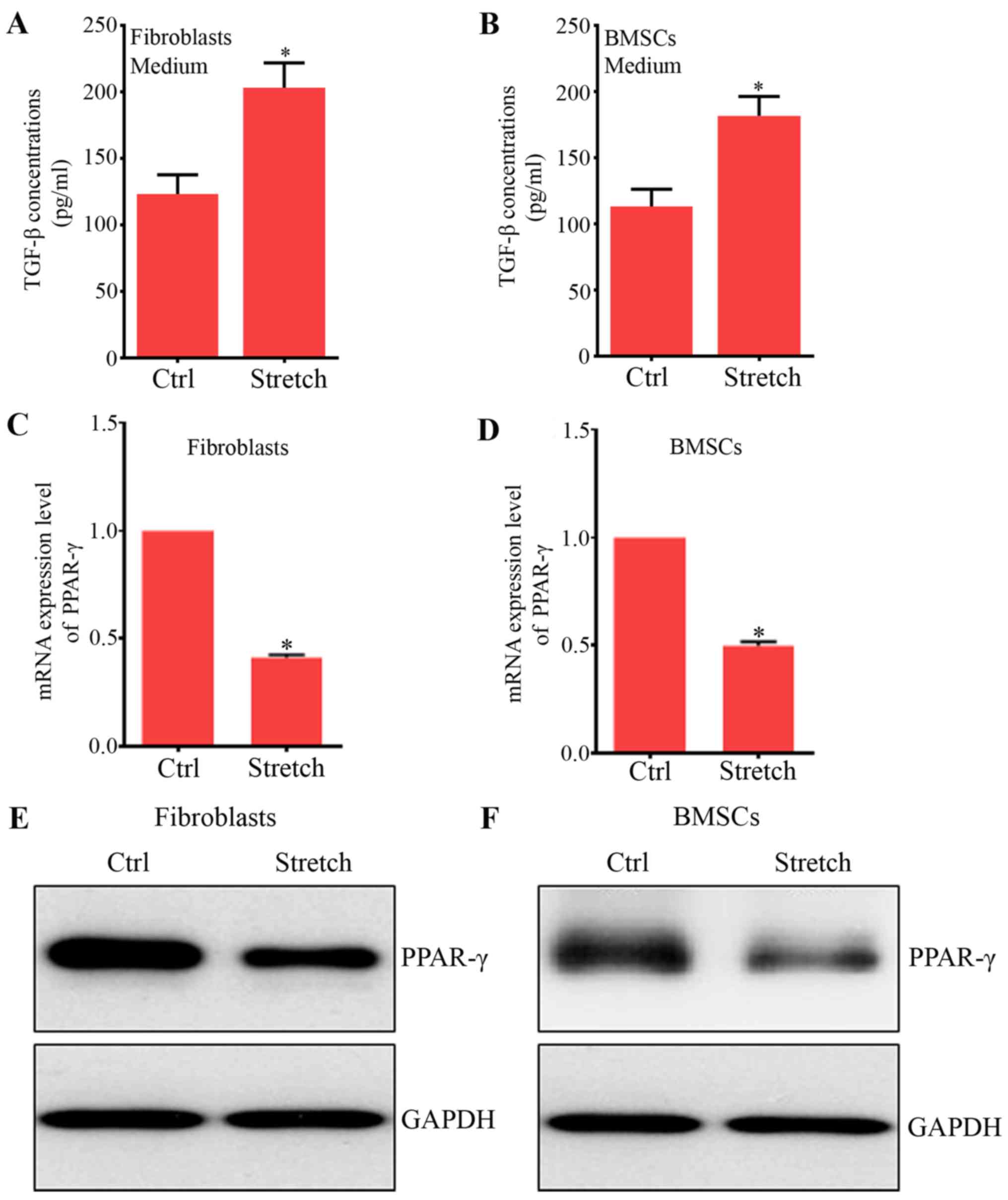
addition, a similar and significant increase was found in the
protein levels of TGF-β1 in fibroblasts (Fig. 1A) and BMSCs (Fig. 1B), key growth factors contributing
to BMSC differentiation into vascular smooth muscle cells (29), ligament or tendon fibroblast cells
(30). These findings of
increased TGF-β1 and ECM proteins were consistent with other
studies (31,32). Furthermore, our results showed
that PPAR-γ mRNA and protein expression were significantly reduced
by mechanical stretch compared with their control groups,
respectively (Fig. 1C–F). The
expression of PPAR-γ was reduced in fibroblast due to mechanical
stretch (33,34). Also, the mechanical stretch could
inhibit adipocyte differentiation (35,36). Therefore, these results indicated
that PPAR-γ may play an essential role in the effects of mechanical
forces on BMSC differentiation to ligament fibroblasts.
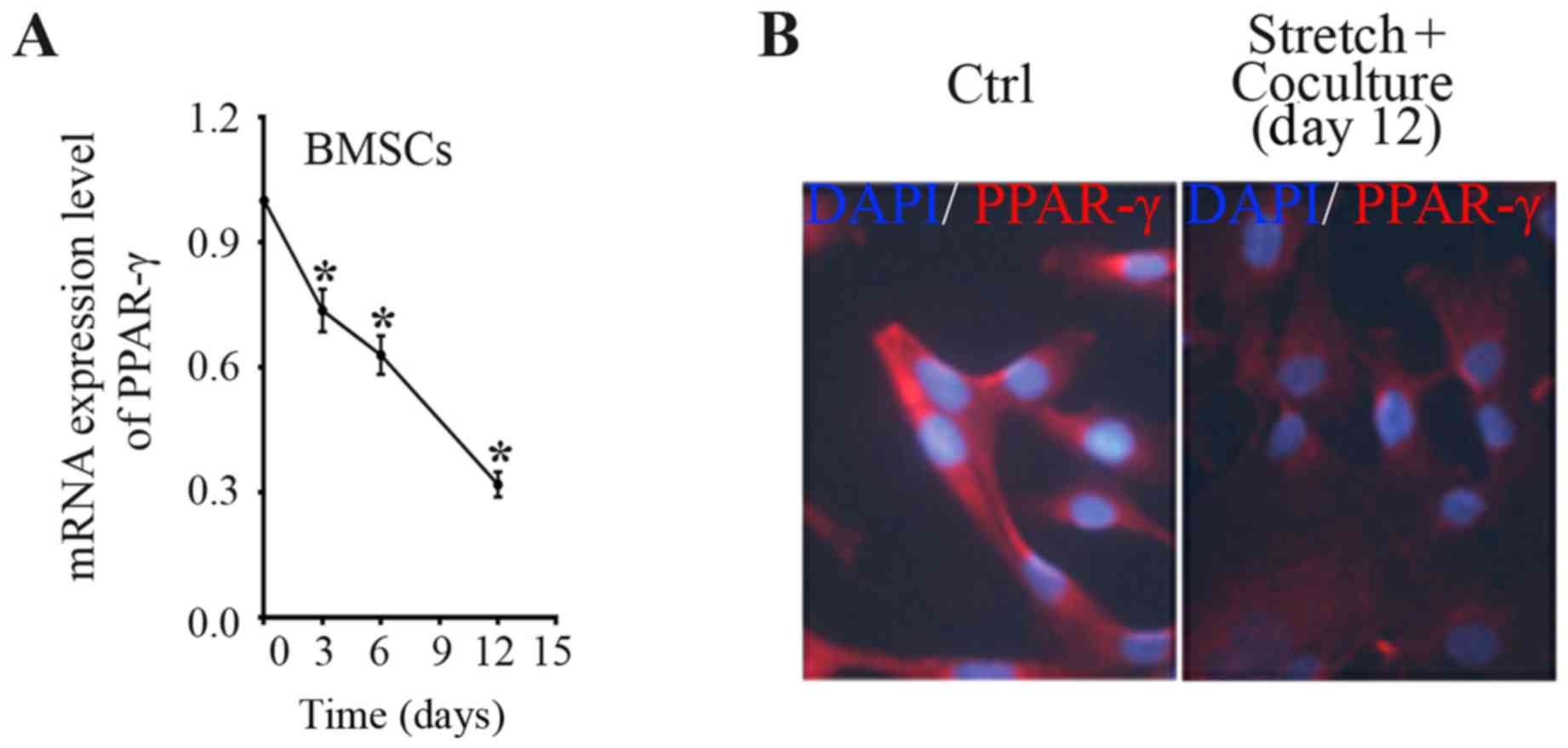
PPAR-γ expression reduced by indirect
co-culture system with mechanical stretching
The above preliminary data displayed that mechanical
stress significantly and directly reduced PPAR-γ expression in
BMSCs with a time-dependent manner. However, whether indirect
co-culture system with mechanically stretched ligament fibroblasts
can regulate PPAR-γ expression in the BMSCs is uncertain.
Therefore, in the present study, indirect co-culture system was
employed to determine the PPAR-γ expression in BMSCs induced by
stretched fibroblasts. With the use of indirect co-culture system,
an approximate 26% decrease of PPAR-γ mRNA level in BMSCs was
acquired after 3 days of co-culture with mechanical stretched
fibroblasts, a 37% decrease after co-culture for 6 days, and a 68%
decrease after 12 days as compared with the time-point day 0
(Fig. 2A). The data indicate that
co-culture with mechanically stretched fibroblasts reduced the
PPAR-γ expression level in a time-dependent manner. In accordance,
immune-fluorescence staining showed that PPAR-γ staining degree was
weakened in BMSCs after mechanical stimulation for 12 days compared
to BMSCs of control group (Fig.
2B).
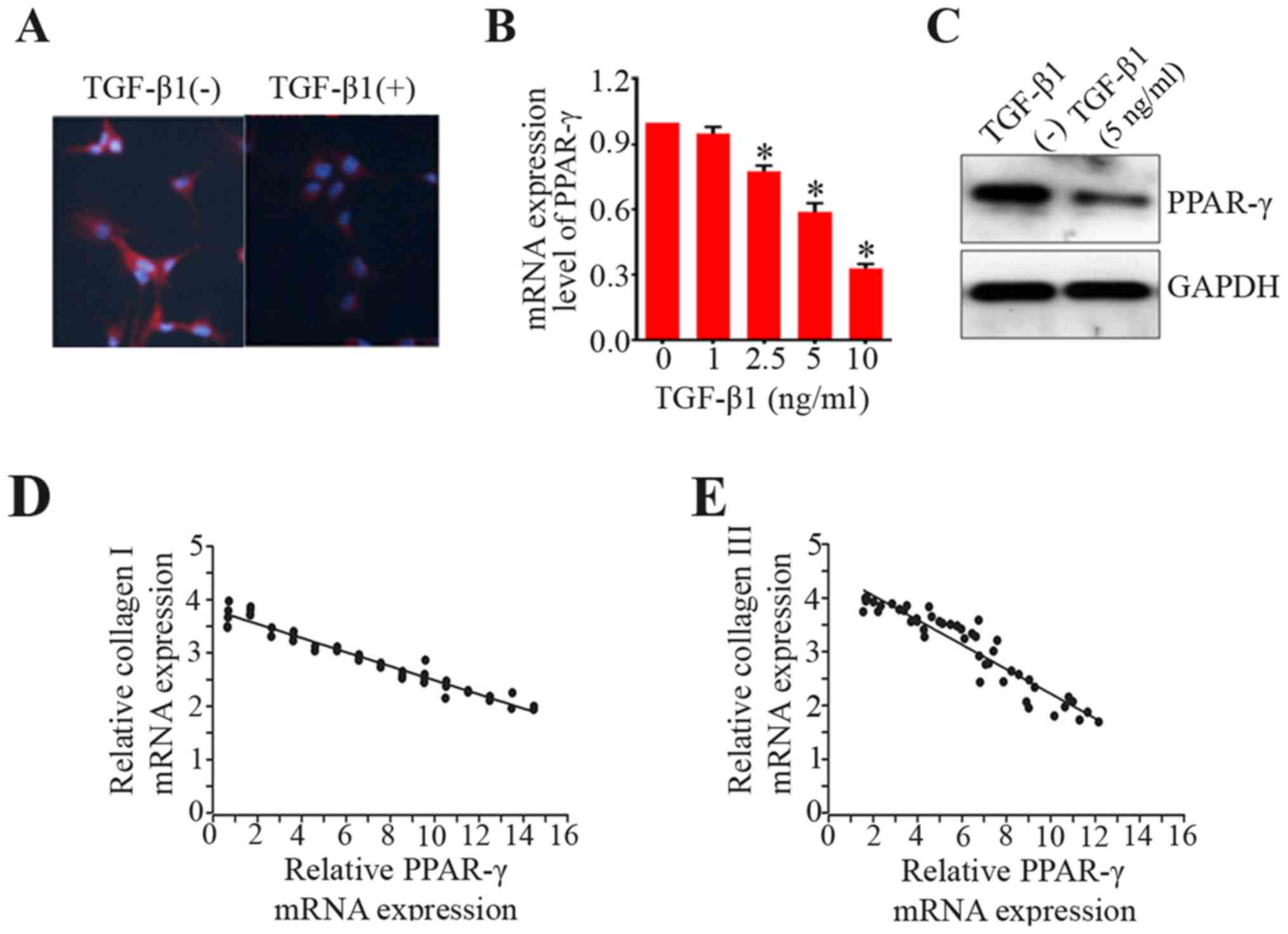
PPAR-γ has negative correlation with
collagen type I and III
Increased levels of collagen I and III are
indicators of BMSCs differentiation to fibroblasts, because they
are usually described as the common characteristics of ligament
fibroblasts (37). Given that
PPAR-γ had negative correlation with these collagen type I and III,
we then explored the function of PPAR-γ in the differentiation of
BMSCs. Here, we employed immunostaining to examine PPAR-γ
expression in the process of BMSC differentiation to ligament
fibroblasts. The results of immunofluorescence staining showed that
the degree of PPAR-γ staining was much weaker in the BMSC
differentiation induced by TGF-β1 only as compared with control
group (Fig. 3A). Furthermore, the
PPAR-γ mRNA level was dramatically reduced during differentiation
process activated by TGF-β1 in a dose-dependent manner (Fig. 3B). The protein expression of
PPAR-γ was also decreased (Fig.
3C). Additionally, the data showed that there were negative
relationships between PPAR-γ mRNA expression and collagen I or III
in BMSCs (Fig. 3D and E). These
findings uncover the roles of PPAR-γ in the differentiation of
BMSCs to ligament fibroblasts.
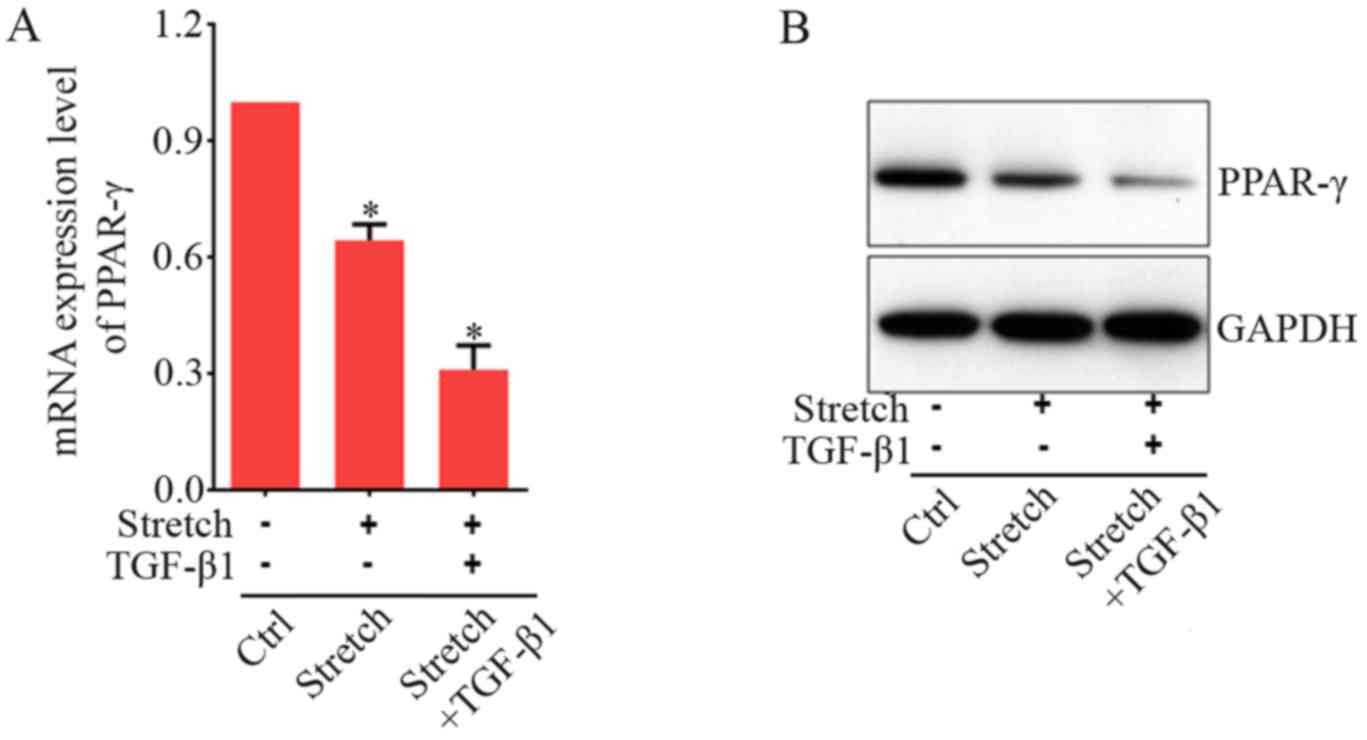
Synergistic effects of TGF-β1 and
indirect co-culture with mechanically stretched fibroblasts on
PPAR-γ expression
To study whether growth factor and co-culture with
strained fibroblasts are synergistic, we investigated their effects
on the PPAR-γ mRNA expression in BMSCs. TGF-β1 is a paracrine
growth factor-mediating cell response to mechanical strain. This
factor then downregulates PPAR-γ mRNA expression in fibroblasts
(38). Our above data showed that
direct mechanical stretch significantly increased TGF-β1 expression
in fibroblasts and BMSCs (Fig. 1A and
B). Therefore, we hypothesized that mechanical stretch signal
indirectly reduced PPAR-γ expression in BMSCs mediated via TGF-β1,
released from strained fibroblasts an addition of exogenous factor
should synergistically increase the effects of mechanical stretch
on the PPAR-γ expression. As shown in Fig. 4A, without addition of TGF-β1, the
expression level of PPAR-γ was reduced by indirect mechanical
stimulation to ~63% less than control group. As shown in the above
(Fig. 4A), addition of TGF-β1 at
5 ng/ml significantly decreased PPAR-γ expression levels as
compared to those without TGF-β1 treatment. With the application of
indirect mechanical stretch, an extra PPAR-γ expression level
decrease was acquired after addition of TGF-β1 (Fig. 4B). These results indicated that
the effect of growth factor and indirect co-culture with strained
fibroblasts appeared to be synergistic on the induction of PPAR-γ
expression in BMSCs.
PPAR-γ negatively modulates the
differentiation of BMSCs toward fibroblasts induced by indirect
cocultred with stretched fibroblasts
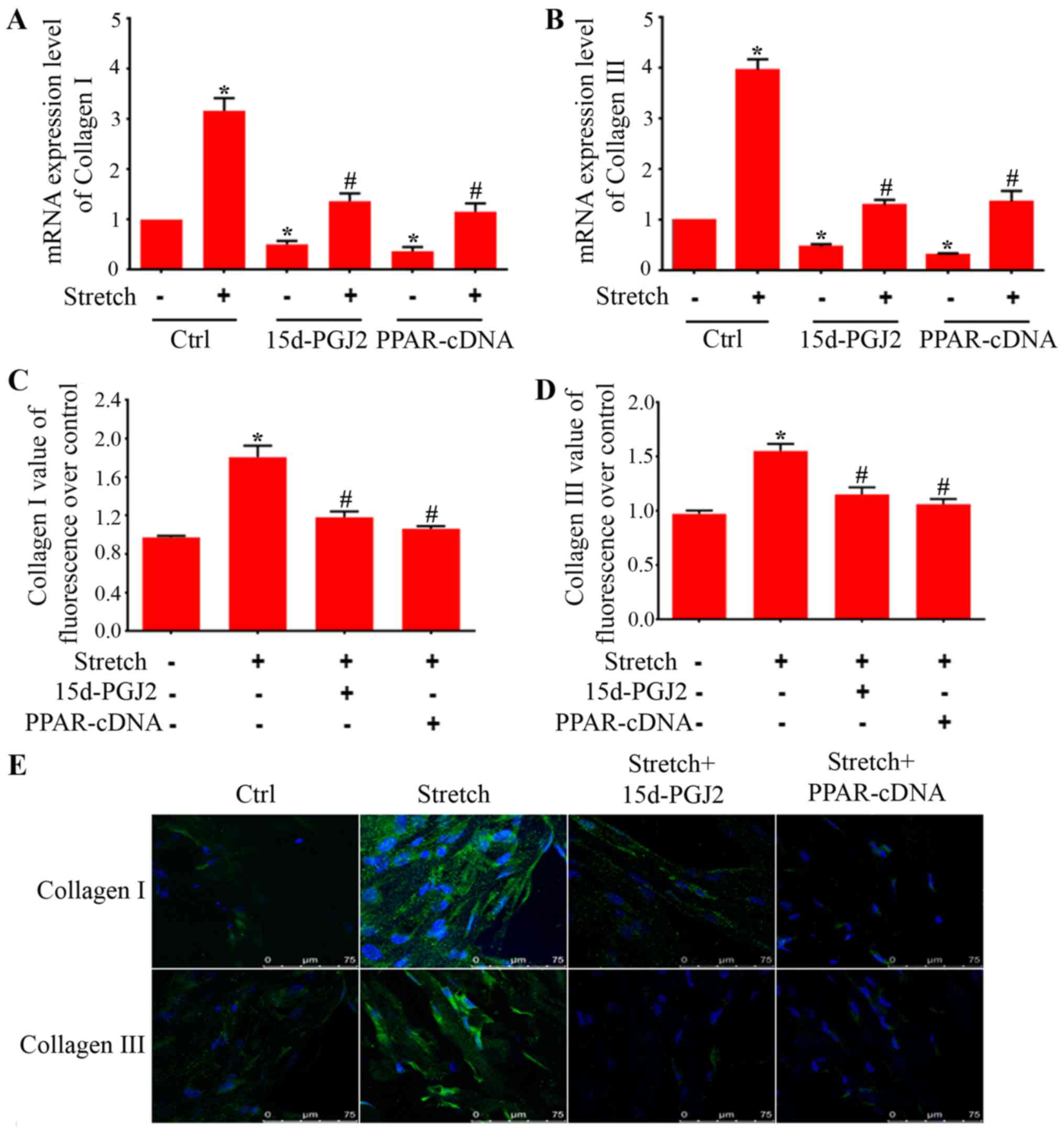
Upregulation of collagen I and III is an essential
characteristic during BMSC differentiation to fibroblasts.
Therefore, to further seek the characteristic effects of PPAR-γ on
the expression of collagen I and III in BMSC differentiation, we
employed endogenous ligand (15d-PGJ2) and PPAR-γ overexpression
plasmids. As showed in Fig. 5A and
B, endogenous ligand and overexpression plasmids were able to
attenuate the increases of collagen I and III mRNA expression in
BMSCs treated with indirect mechanical stretch. Transfection of
BMSCs with PPAR-γ shRNA decreased the mRNA expression of PPAR-γ
around 75% (Fig. 5F).
Furthermore, western blot analysis showed that PPAR-γ protein was
also significantly reduced (Fig.
5G). The real-time PCR showed that PPAR-γ shRNA increased
collagen I and III mRNA expression as compared with scrambled
control plasmids (Fig. 5H and
I).
To further confirm the effects of PPAR-γ in BMSC
differentiation, we employed immunohistochemistry staining for
collagen I and III to determine morphological changes in BMSCs. As
shown in Fig. 5E, the BMSCs
without treatment displayed very weak expression of collagen I and
III. Indirect mechanical stretch treatment stimulated formation of
thick bundles of actin filaments and dramatically increased the
expression of collagen I and III in BMSCs. However, treatment with
ligand of PPAR-γ and overexpression plasmid markedly attenuated
collagen I and III expression in BMSCs induced after exposure to
indirect mechanical stretch. In addition, our immunostaining assay
showed that treatment with 15d-PGJ2 and PPAR-γ expression plasmids
reduced ECM protein levels of collagen I (Fig. 5C) and collagen III (Fig. 5D) in differentiated BMSCs
triggered by mechanical forces.
Discussion
PFD is a distressing morbidity that affects the
quality of women's life in developed and developing countries
(39). Pelvic organ support is
maintained by complex interactions between levator ani muscles and
connective tissues of the urethra, vaginal wall and rectum. Major
ani muscle defects is a key factor of POP (40). In the past decade, the field of
stem cell biology has undergone a remarkable evolution. Stem cells
have a great potential to develop into many different specialized
cells in the body. There is increasing evidence suggesting that
BMSCs can be used as a new revolutionary cell-based therapy to
repair ligament, tendon and cartilage (41).
Differentiation of BMSCs toward fibroblasts is an
essential event for ligament tissue engineering. Our previous study
reported that the BMSCs with positive expression of CD90 and CD44
differentiate towards fibroblast triggered by indirect co-culture
with stretched ligament fibroblasts. Indirect mechanical stretch
also changes cell morphology and stimulates expression of collagen
I and II, elastin, Lox and fibulin-5 in BMSCs (42). However, further studies needed to
be performed to explore the underlying mechanisms. In the present
study, we explored the potential role of PPAR-γ in the
differentiation of primary rat BMSCs to fibroblasts. We found
PPAR-γ negatively regulated BMSC differentiation toward
fibroblasts. It has been reported that expression of PPAR-γ was
reduced in fibroblasts (33).
Differentiation of BMSCs was accompanied by the decrease of PPAR-γ
expression. Activated PPAR-γ by specific ligands were able to
attenuate BMSC differentiation in vitro. These results
critically revealed a mechanism underlying differentiation of BMSCs
toward fibroblasts.
There is accumulating evidence demonstrating that
PPAR-γ expression was significantly decreased in the fibroblasts
during the formation of fibrosis (43). However, activation of PPAR-γ has
the capacity to reduce the fibrosis (43). In this study, the expression
levels of PPAR-γ were dramatically reduced in BMSCs during
differentiation to fibroblasts, similar to the report that PPAR-γ
expression was downregulated in the differentiation of bone
marrow-derived mesenchymal stem cells into myofibroblasts (34). Additionally, the relationships
between the mRNA expression level of PPAR-γ and other ECM proteins,
such as collagen I and III, were negative, which are similar to a
report by other researchers (44). Furthermore, PPAR-γ was negatively
regulated by TGF-β1, which can mediate fibrosis (45). These results indicate that PPAR-γ
may play an important role in BMSC differentiation to
fibroblasts.
Previous studies reported that PPAR-γ was
significantly decreased in the differentiation of various
fibroblasts (38). In the present
study, the expression level of PPAR-γ was significantly
downregulated in BMSC differentiation toward fibroblasts induced by
TGF-β1 only and indirect co-culture with strained fibroblasts. In
addition, the downregulation of PPAR-γ by specific shRNA had the
capacity of promoting the differentiation of BMSCs. BMSCs retain
their growth potential and multipotency to differentiate toward a
variety of mesenchymal cells, such as fibroblasts and adipocytes
(46). Therefore, it was
hypothesized that in our study the downregulated expression of
PPAR-γ related to fat-formation may contribute to the
differentiation of BMSCs to fibroblasts because of the decreased
potential to differentiate to adipocytes. Previous study reported
that PPAR-γ expression could be decreased by TGF-β1 via smad
binding with the PPAR-γ gene promoter to reduce the promoter
activity of PPAR-γ gene or through increasing the binding of
histone deacetylase 1 (HDAC1) and decreasing the levels of
acetylated histone3 (AcH3) at the PPAR-γ promoter (25,38). However, the detailed underlying
mechanisms of PPAR-γ expression involved in the BMSC
differentiation to fibroblasts is still unclear and should be
explored in further study.
The effects of PPAR-γ require prior activation by
ligands. In this study, our data showed that natural endogenous
ligand of PPAR-γ was able to inhibit BMSC differentiation to
fibroblasts induced by TGF-β1 and indirect co-culture in
vitro. Moreover, this natural ligand also attenuate expression
of collagen type I and III. Therefore, these results evidently
support that PPAR-γ is significantly downregulated in the process
of BMSC differentiation to fibroblasts. Our previous result
indicated that TGF-β1 and MAPK/ERK pathway participates in the
differentiation of BMSCs to fibroblasts. In the present study,
PPAR-γ negatively regulates BMSC differentiation to fibroblasts.
There are several studies demonstrating that PPAR-γ is involved in
TGF-β1/Smad and MAPK signaling pathway (47–49). Recent studies have reported that
the PPAR-γ agonist was able to activate PPAR-γ to regulate ECM
protein expression through targeting the TGF-β1/Smad3 and MAPK
signaling pathways (49,50). Therefore, the PPAR-γ, TGF-β1/Smad
and MAPK signaling pathways may play important roles in
transcriptional regulation of collagen and expression of other ECM
proteins in the process of BMSC differentiation to fibroblasts.
In conclusion, our results demonstrate that PPAR-γ
negatively regulate differentiation of BMSC to fibroblasts in
vitro. Downregulation of PPAR-γ expression occurs in the
differentiation of BMSCs to fibroblasts, and increase of PPAR-γ by
agonists restrains BMSC differentiation in vitro. However,
this study suggests investigation of these molecular mechanisms
underlying the effects of PPAR-γ on BMSC differentiation to
fibroblasts are warranted.
Acknowledgments
We would like to thank all the other staff in the
Laboratory Animal Center of Zhengzhou University for their
assistance in the experiments.
References
|
1
|
Choi KH and Hong JY: Management of pelvic
organ prolapse. Korean J Urol. 55:693–702. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giarenis I and Robinson D: Prevention and
management of pelvic organ prolapse. F1000Prime Rep. 6:772014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wiegersma M, Panman CM, Kollen BJ, Berger
MY, Lisman-Van Leeuwen Y and Dekker JH: Effect of pelvic floor
muscle training compared with watchful waiting in older women with
symptomatic mild pelvic organ prolapse: Randomised controlled trial
in primary care. BMJ. 349:g73782014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aznal SS, Meng FG, Nalliah S, Tay A,
Chinniah K and Jamli MF: Biochemical evaluation of the supporting
structure of pelvic organs in selected numbers of premenopausal and
postmenopausal Malaysian women. Indian J Pathol Microbiol.
55:450–455. 2012. View Article : Google Scholar
|
|
5
|
Wei X, Yang X, Han ZP, Qu FF, Shao L and
Shi YF: Mesenchymal stem cells: A new trend for cell therapy. Acta
Pharmacol Sin. 34:747–754. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Via AG, Frizziero A and Oliva F:
Biological properties of mesenchymal stem cells from different
sources. Muscles Ligaments Tendons J. 2:154–162. 2012.
|
|
7
|
Pelosi E, Castelli G and Testa U: Human
umbilical cord is a unique and safe source of various types of stem
cells suitable for treatment of hematological diseases and for
regenerative medicine. Blood Cells Mol Dis. 49:20–28. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marion NW and Mao JJ: Mesenchymal stem
cells and tissue engineering. Methods Enzymol. 420:339–361. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miller MD, Nichols T and Butler CA:
Patella fracture and proximal patellar tendon rupture following
arthroscopic anterior cruciate ligament reconstruction.
Arthroscopy. 15:640–643. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Omoto M, Miyashita H, Shimmura S, Higa K,
Kawakita T, Yoshida S, McGrogan M, Shimazaki J and Tsubota K: The
use of human mesenchymal stem cell-derived feeder cells for the
cultivation of transplantable epithelial sheets. Invest Ophthalmol
Vis Sci. 50:2109–2115. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li J, Lu S, Yang S, Xing W, Feng J, Li W,
Zhao Q, Wu H, Ge M, Ma F, et al: Impaired immunomodulatory ability
of bone marrow mesenchymal stem cells on CD4(+) T cells in aplastic
anemia. Results Immunol. 2:142–147. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pakyari M, Farrokhi A, Maharlooei MK and
Ghahary A: Critical role of transforming growth factor beta in
different phases of wound healing. Adv Wound Care (New Rochelle).
2:215–224. 2013. View Article : Google Scholar
|
|
13
|
Horiguchi M, Ota M and Rifkin DB: Matrix
control of transforming growth factor-β function. J Biochem.
152:321–329. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bing Z, Linlin L, Jianguo Y, Shenshen R,
Ruifang R and Xi Z: Effect of mechanical stretch on the expressions
of elastin, LOX and Fibulin-5 in rat BMSCs with ligament
fibroblasts co-culture. Mol Biol Rep. 39:6077–6085. 2012.
View Article : Google Scholar
|
|
15
|
Evans RM: The steroid and thyroid hormone
receptor super-family. Science. 240:889–895. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kota BP, Huang TH and Roufogalis BD: An
overview on biological mechanisms of PPARs. Pharmacol Res.
51:85–94. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Knouff C and Auwerx J: Peroxisome
proliferator-activated receptor-gamma calls for activation in
moderation: Lessons from genetics and pharmacology. Endocr Rev.
25:899–918. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lehrke M and Lazar MA: The many faces of
PPARgamma. Cell. 123:993–999. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Szanto A and Nagy L: The many faces of
PPARgamma: Anti-inflammatory by any means. Immunobiology.
213:789–803. 2008. View Article : Google Scholar
|
|
20
|
Rosen ED and Spiegelman BM: PPARgamma: A
nuclear regulator of metabolism, differentiation, and cell growth.
J Biol Chem. 276:37731–37734. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Imano E, Kanda T, Nakatani Y, Nishida T,
Arai K, Motomura M, Kajimoto Y, Yamasaki Y and Hori M: Effect of
troglitazone on microalbuminuria in patients with incipient
diabetic nephropathy. Diabetes Care. 21:2135–2139. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bakris GL, Ruilope LM, McMorn SO, Weston
WM, Heise MA, Freed MI and Porter LE: Rosiglitazone reduces
microalbuminuria and blood pressure independently of glycemia in
type 2 diabetes patients with microalbuminuria. J Hypertens.
24:2047–2055. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Deng YL, Xiong XZ and Cheng NS: Organ
fibrosis inhibited by blocking transforming growth factor-β
signaling via peroxisome proliferator-activated receptor γ
agonists. Hepatobiliary Pancreat Dis Int. 11:467–478. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gonzalez EG, Selvi E, Balistreri E,
Akhmetshina A, Palumbo K, Lorenzini S, Lazzerini PE, Montilli C,
Capecchi PL, Lucattelli M, et al: Synthetic cannabinoid ajulemic
acid exerts potent antifibrotic effects in experimental models of
systemic sclerosis. Ann Rheum Dis. 71:1545–1551. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng S and Chen A: Disruption of
transforming growth factor-beta signaling by curcumin induces gene
expression of peroxisome proliferator-activated receptor-gamma in
rat hepatic stellate cells. Am J Physiol Gastrointest Liver
Physiol. 292:G113–G123. 2007. View Article : Google Scholar
|
|
26
|
Ibarra-Lara ML, Sánchez-Aguilar M, Soria
E, Torres-Narváez JC, Del Valle-MondragóL, Cervantes-Pérez LG,
Pérez-Severiano F, Ramírez-Ortega MC, Pastelín-Hernández G,
Oidor-Chan VH, et al: Peroxisome proliferator-activated receptors
(PPAR) downregulate the expression of pro-inflammatory molecules in
an experimental model of myocardial infarction. Can J Physiol
Pharmacol. 94:634–642. 2016. View Article : Google Scholar
|
|
27
|
Ravingerová T, Adameová A, Kelly T,
Antonopoulou E, Pancza D, Ondrejcáková M, Khandelwal VK, Carnická S
and Lazou A: Changes in PPAR gene expression and myocardial
tolerance to ischaemia: Relevance to pleiotropic effects of
statins. Can J Physiol Pharmacol. 87:1028–1036. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ren CC, Ren RF, Zhao B, Zhang X and Jiang
YJ: Study on oriented differentiation of bone marrow mesenchymal
stem cells by fibroblast in rat uterine ligament with mechanical
stretch. Zhonghua Fu Chan Ke Za Zhi. 46:527–532. 2011.In Chinese.
PubMed/NCBI
|
|
29
|
Guo X and Chen SY: Transforming growth
factor-β and smooth muscle differentiation. World J Biol Chem.
3:41–52. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sahoo S, Ang LT, Cho-Hong Goh J and Toh
SL: Bioactive nanofibers for fibroblastic differentiation of
mesenchymal precursor cells for ligament/tendon tissue engineering
applications. Differentiation. 79:102–110. 2010. View Article : Google Scholar
|
|
31
|
Gutierrez JA and Perr HA: Mechanical
stretch modulates TGF-beta1 and alpha1(I) collagen expression in
fetal human intestinal smooth muscle cells. Am J Physiol.
277:G1074–G1080. 1999.PubMed/NCBI
|
|
32
|
Lindahl GE, Chambers RC, Papakrivopoulou
J, Dawson SJ, Jacobsen MC, Bishop JE and Laurent GJ: Activation of
fibroblast procollagen alpha 1(I) transcription by mechanical
strain is transforming growth factor-beta-dependent and involves
increased binding of CCAAT-binding factor (CBF/NF-Y) at the
proximal promoter. J Biol Chem. 277:6153–6161. 2002. View Article : Google Scholar
|
|
33
|
David V, Martin A, Lafage-Proust MH,
Malaval L, Peyroche S, Jones DB, Vico L and Guignandon A:
Mechanical loading downregulates peroxisome proliferator-activated
receptor gamma in bone marrow stromal cells and favors
osteoblastogenesis at the expense of adipogenesis. Endocrinology.
148:2553–2562. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jia S, Liu X, Li W, Xie J, Yang L and Li
L: Peroxisome proliferator-activated receptor gamma negatively
regulates the differentiation of bone marrow-derived mesenchymal
stem cells toward myofibroblasts in liver fibrogenesis. Cell
Physiol Biochem. 37:2085–2100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tanabe Y, Koga M, Saito M, Matsunaga Y and
Nakayama K: Inhibition of adipocyte differentiation by mechanical
stretching through ERK-mediated downregulation of PPARgamma2. J
Cell Sci. 117:3605–3614. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tanabe Y and Nakayama K: Mechanical
stretching inhibits adipocyte differentiation of 3T3-L1 cells: The
molecular mechanism and pharmacological regulation. Nihon
Yakurigaku Zasshi. 124:337–344. 2004.In Japanese. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Takayama S, Murakami S, Miki Y, Ikezawa K,
Tasaka S, Terashima A, Asano T and Okada H: Effects of basic
fibroblast growth factor on human periodontal ligament cells. J
Periodontal Res. 32:667–675. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gong K, Chen YF, Li P, Lucas JA, Hage FG,
Yang Q, Nozell SE, Oparil S and Xing D: Transforming growth
factor-β inhibits myocardial PPARγ expression in pressure
overload-induced cardiac fibrosis and remodeling in mice. J
Hypertens. 29:1810–1819. 2011.PubMed/NCBI
|
|
39
|
Ewies AA, Al-Azzawi F and Thompson J:
Changes in extracellular matrix proteins in the cardinal ligaments
of post-menopausal women with or without prolapse: A computerized
immunohistomorphometric analysis. Hum Reprod. 18:2189–2195. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Boreham MK, Wai CY, Miller RT, Schaffer JI
and Word RA: Morphometric properties of the posterior vaginal wall
in women with pelvic organ prolapse. Am J Obstet Gynecol.
187:1501–1509. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kon E, Filardo G, Roffi A, Andriolo L and
Marcacci M: New trends for knee cartilage regeneration: From
cell-free scaffolds to mesenchymal stem cells. Curr Rev
Musculoskelet Med. 5:236–243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee IC, Wang JH, Lee YT and Young TH: The
differentiation of mesenchymal stem cells by mechanical stress
or/and co-culture system. Biochem Biophys Res Commun. 352:147–152.
2007. View Article : Google Scholar
|
|
43
|
Meng Z, Yu XH, Chen J, Li L and Li S:
Curcumin attenuates cardiac fibrosis in spontaneously hypertensive
rats through PPAR-γ activation. Acta Pharmacol Sin. 35:1247–1256.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Miyahara T, Schrum L, Rippe R, Xiong S,
Yee HF Jr, Motomura K, Anania FA, Willson TM and Tsukamoto H:
Peroxisome proliferator-activated receptors and hepatic stellate
cell activation. J Biol Chem. 275:35715–35722. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wei J, Ghosh AK, Sargent JL, Komura K, Wu
M, Huang QQ, Jain M, Whitfield ML, Feghali-Bostwick C and Varga J:
PPARγ downregulation by TGFß in fibroblast and impaired expression
and function in systemic sclerosis: A novel mechanism for
progressive fibrogenesis. PLoS One. 5:e137782010. View Article : Google Scholar
|
|
46
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tontonoz P, Hu E, Graves RA, Budavari AI
and Spiegelman BM: mPPAR gamma 2: Tissue–specific regulator of an
adipocyte enhancer. Genes Dev. 8:1224–1234. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jeon KI, Kulkarni A, Woeller CF, Phipps
RP, Sime PJ, Hindman HB and Huxlin KR: Inhibitory effects of PPARγ
ligands on TGF-β1-induced corneal myofibroblast transformation. Am
J Pathol. 184:1429–1445. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Guo B, Koya D, Isono M, Sugimoto T,
Kashiwagi A and Haneda M: Peroxisome proliferator-activated
receptor-gamma ligands inhibit TGF-beta 1-induced fibronectin
expression in glomerular mesangial cells. Diabetes. 53:200–208.
2004. View Article : Google Scholar
|
|
50
|
Liu Y, Dai B, Xu C, Fu L, Hua Z and Mei C:
Rosiglitazone inhibits transforming growth factor-β1 mediated
fibrogenesis in ADPKD cyst-lining epithelial cells. PLoS One.
6:e289152011. View Article : Google Scholar
|