Introduction
High blood pressure (HBP) is a risk factor for
cardiovascular disorders, including chronic kidney disease,
congestive heart failure, myocardial infarction and stroke, and is
a leading contributor to rates of mortality and morbidity in the
world. Substantial progress has been made in the treatment of HBP
via the application of α-adrenoreceptor antagonists, β-blockers,
calcium channel blockers, renin-angiotensin system inhibitors and
diuretics. Despite the availability of a variety of blood pressure
drugs, a larger number of patients with HBP are unable to get their
blood pressure under control. It was estimated that only 51.9% of
patients with HBP in the United States had their blood pressure
under control (<140/90 mm Hg) between 2011 and 2012 (1).
As a basic composition of vascular walls, vascular
smooth muscle cells (VSMCs) have a well-differentiated contractile
phenotype, which is important to maintain vascular tone (2). The increased proliferation of VSMCs
is associated with HBP (3,4).
Vascular inflammation is considered to be involved in vascular
remodeling in a variety of cardiovascular disorders, including
atherosclerosis and HBP (5).
The tumor inhibitor protein p53 (protein product of
TP53, also known as p53) is known to be involved in the development
of restenosis and atherosclerosis, VSMC growth and cell death
(6). A previous study reported
that elevated nuclear translocation of p53 in cancer cells was
observed in response to therapy with microtubule stabilizing agents
(MTSAs) (7). It is widely known
that the microtubule stabilization triggered by MTSA is the primary
mechanism attributable to the elevation in the association of p53
with microtubules and its nuclear export, which is associated with
the apoptotic pathway dependent on p53 (7). Due to the tumor inhibitory functions
of p53, this protein has attracted attention from the pulmonary
arterial hypertension community (8). Mizuno et al revealed that
mice with p53 knockout formed more serious pulmonary hypertension
in response to chronic hypoxia than wild-type mice (9).
As small and non-coding RNA molecules, microRNAs
(miRNAs) consist of ~22 nucleotides and binding to their target
mRNAs to suppress translation, which have a key regulatory role in
eukaryotic genes, particularly in cell proliferation,
differentiation and apoptosis (10). Often, miRNAs can bind to the 3′
untranslated region (3′UTR) of the mRNAs of target genes in an
imperfect or perfect complementary manner, leading to translational
repression or mRNA degradation (11). Increasing data have revealed that
dysregulated miRNAs are associated with cardiovascular disorders,
including vascular atherosclerosis, heart failure and cardiac
hypertrophy (12). Baseline gene
expression levels of miRNA-26b, miRNA-499, miRNA-208b, miRNA-21,
miRNA-133a and miRNA-1 have been determined in peripheral blood
mononuclear cells (PBMCs), cells identified to be important in the
pathophysiology of target organ injury (13). These miRNAs were selected as they
have a different expression profile in HBP, and have been
associated with heart and vascular remodeling (14). The expression of miRNAs in the
PBMCs of patients has been investigated, as PBMCs are of important
in the cardiovascular complications of HBP (15).
A previous study demonstrated the differential
expression of miR-31a-5p in the smooth muscle cells collected from
an animal model of primary hypertension, compared with the control,
and it has been reported that dysregulated p53 is associated with
the molecular mechanism of smooth muscle cell apoptosis (16–18). The present study performed a
search on an online miRNA database and found that miR-31a-5p
virtually targets p53. In the present study, miR-31a-5p was found
to target p53, and the association of p53 and miR-31a-5p in the
occurrence of primary hypertension was confirmed.
Materials and methods
Animals
All experiments were performed in 28 male adult
spontaneously hypertensive rat (SHR; 16 rats) and normotensive
Wistar-Kyoto (WKY; 12 rats) rats (15–16 weeks old, weighing 430±40
g) following the institutional guidelines that comply with the
recommendations in the Guide for the Care and Use of Laboratory
Animals published by the US National Institutes of Health (8th
edition, 2011). All procedures of experiments were approved by the
Experimental Animal Care and Use Committee of China Medical
University (Shenyang, China). All rats were housed at room
temperature (23±2°C) with a 12 h-12 h light/dark cycle, and were
provided with a rodent chow diet and drinking water throughout the
experiment. Gold nanoparticles (AuNPs) with miR-31a-5p were used
for treatment of the rats.
Isolation and culture of pulmonary artery
smooth muscle cells (PASMCs)
The PASMCs were isolated from tissue samples derived
from the rats; forceps were utilized to mince the tissue samples,
and 4 mg/ml dispase (Sigma-Aldrich; EMD Millipore, Bedford, MA,
USA) was used to digest the tissues for 30 min at 37°C, and
subjected to additional incubation for another 5 h. A 40 μm
cell strainer (BD Falcon, Bedford, MA, USA) was utilized to filter
the dissociated cell suspension. Centrifugation was performed for
15 min at 107.3 × g at 4°C to yield the cells. DMEM with 10% fetal
bovine serum (FBS; HyClone; GE Healthcare Life Sciences, Logan, UT,
USA), 100 μg/ml streptomycin and 100 U/ml penicillin was
utilized to incubate the cells under a humidified atmosphere with
5% CO2/95% air at 37°C. The medium was replaced at 2-day
intervals until the cells were cloned. The cells at passage three
were used in subsequent experiments.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) was utilized to isolate total RNA from the tissue
samples, and the mirVana™ PARIS™ kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.) was utilized to extract total RNA from the
PASMCs, based on the manufacturer's protocol. Subsequently, 8%
denaturing polyacrylamide gels were utilized to monitor RNA
integrity. TaqMan miRNA assays was performed to perform RT-qPCR
analysis, and a high-capacity cDNA archive kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.) was utilized to reverse transcribe
miRNA to cDNA according to the manufacturer's protocol. A NanoDrop
instrument (NanoDrop; Thermo Fisher Scientific, Inc., Wilmington,
DE, USA) was utilized to determine the concentrations of RNA, and
U6 served as a control to normalize the expression of miR-31a-5p.
SYBR-Green-based detection systems (Applied Biosystems; Thermo
Fisher Scientific, Inc.) were utilized to perform the PCR
amplifications on a GeneAmp PCR 9700 Thermocycler (Applied
Biosystems; Thermo Fisher Scientific, Inc.) in accordance with the
manufacturer's protocol, using Standard Taq Reaction Buffer (10X, 5
μl), dNTPs (10 mM, 1 μl), Forward Primer (10
μM, 1 μl), Reverse Primer (10 μM, 1
μl), Template DNA (10 ng), Taq DNA Polymerase (0.25
μl) and nuclease-free water (50 μl). The primer
sequences used were as follows: RT primer,
5′GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACCAGCTA-3′;
forward, 5′-GGGAGGCAAGATGCTGGCA-3′ and reverse,
5′-CAGTGCGTGTCGTGGAGT-3′. The thermocycling conditions were as
follows: 95°C for 30 sec, 55°C for 30–60 sec, 72°C for 30–60 sec
for 30 cycles, and 72°C for 5 min. Melting curve analysis was
utilized to confirm the lack of primer dimers and specificity of
amplification. The 2−ΔΔCq method (19) was utilized to analyze the
expression of TP53 mRNA and miR-31a-5p. All experiments were run
three times.
Cell culture and transfection
Dulbecco's modified Eagle's medium (DMEM;
Invitrogen; Thermo Fisher Scientific, Inc.) containing 10% FBS, 100
mg/ml streptomycin and 100 U/ml penicillin was utilized to culture
the PASMCs under a humidified atmosphere of 5% CO2 at
37°C. Lipofectamine 2000 (Thermo Fisher Scientific, Inc.) was
utilized to perform transient transfections of miR-31a-5p mimic,
miRNA control, and TP53 small interfering (si)RNA. The sequences
were as follows: miR-31a-5p mimic, 5′-AGGCAAGAUGCUGGCAUAGCUG-3′;
miRNA control, 5′-CAGCUAUGCCAGCAUACUUGCCU-3′. The TP53 siRNA
sequence was as follows: 5′-TTTTGGGACTTGAGGCATCTG-3′. All
experiments were performed in triplicate.
Cell proliferation assay
The PASMCs were seeded into 24-well plates at a
final concentration of 2×103 cells per well. An MTT
assay was performed to evaluate cell viability 24, 48 and 72 h
post-MTT addition. An ELISA reader (ELX-800 type; Bio-Tek
Instruments, Inc., Winooski, VT, USA) was used to measure the
optical density of each well at 570 nm to quantify cell
proliferation. Each experiment was repeated three times.
Luciferase assay
RT-PCR was performed to amplify the p53 3′UTR
containing the putative or mutated binding site of miR-31a-5p. The
thermocycling conditions were as follows: 25°C for 10 min, 42°C for
50 min, 70°C for 15 min and 37°C for 20 min. The PCR products were
then inserted into the hR-luc luciferase coding sequence
downstream, which was located in the pmir-RB-REPORT™ vector
(Guangzhou RiboBio Co., Ltd., Guangzhou, China) following standard
protocol. Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.) was utilized to co-transfect the cells with the luciferase
constructs and miR-31a-5p mimic or negative control. At 48 h
post-transfection, a Dual Luciferase Reporter Assay system (Promega
Corporation, Madison, WI, USA) was utilized to measure the
luciferase activity of Renilla and Firefly based on the
manufacturer's protocol. The Renilla luciferase activity was
normalized to Firefly luciferase activity. Three independent
experiments were performed.
Western blot analysis
RIPA buffer (Sigma-Aldrich; EMD Millipore) was
utilized to extract protein from the cells at 48 h
post-transfection following the standard protocol. The lysates were
centrifuged at 13,000 g for 15 min at 4°C to collect the upper
supernatant. The bicinchoninic acid method was utilized to measure
the protein concentration. SDS-polyacrylamide gels (12.5%;
Invitrogen; Thermo Fisher Scientific, Inc.) were utilized to
electrophorese 30 μg of the extracted protein, which were
then blotted onto polyvinylidene difluoride membranes (EMD
Millipore, Bedford), followed by blocking with 5% non-fat milk.
Specific primary antibodies against p53 (cat. no. 9282T; 1:5,000;
Cell Signaling Technology, Inc., Beverly, MA, USA) and against
β-actin (cat. no. 4967S; 1:80,000, Sigma; EMD Millipore) were added
for incubation with the membrane for 12 h at 4°C, and TBST buffer
was utilized to wash the membrane three times, following which
corresponding horseradish peroxidase-labeled mouse IgG secondary
antibody (1:1,000; cat. no. HAF007; R&D Systems, Inc.,
Minneapolis, MN, USA) was added for incubation with the membrane
for 60 min at 4°C. The chemiluminescent reagents (GE Healthcare
Life Sciences) and X-ray films (Denville Scientific, Holliston, MA,
USA) were used to visualize the protein bands in accordance with
the manufacturer's protocol. All assays were performed three
times.
Analysis of apoptosis
At 48 h post-transfection, the PASMCs were
harvested, and PBS was utilized to wash cells. A FITC-Annexin
V/Propidium Iodide Apoptosis Detection kit (BestBio, Shanghai,
China) was used to resuspend and stain the cells according to the
manufacturer's protocol. Flow cytometry (BD FACSCanto II; BD
Biosciences, San Jose, USA) was used to analyze cell apoptosis.
Each experiment was performed three times.
Statistical analysis
The results are shown as the mean ± standard
deviation. Two-way analysis of variance or Student's t-test was
used to perform all relative analyses using Statistical package for
the Social Sciences for Windows Version 12.0 (SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference. GraphPad Prism Version 5.0 (GraphPad
Software, Inc., La Jolla, CA, USA) was used to generate all
graphs.
Results
Identifying candidate miRNAs involved in
hypertension
The SHR model is a genetic animal model for
essential hypertension, which shows elevated blood pressure
compared with normotensive WKY rats. RT-qPCR analysis was used to
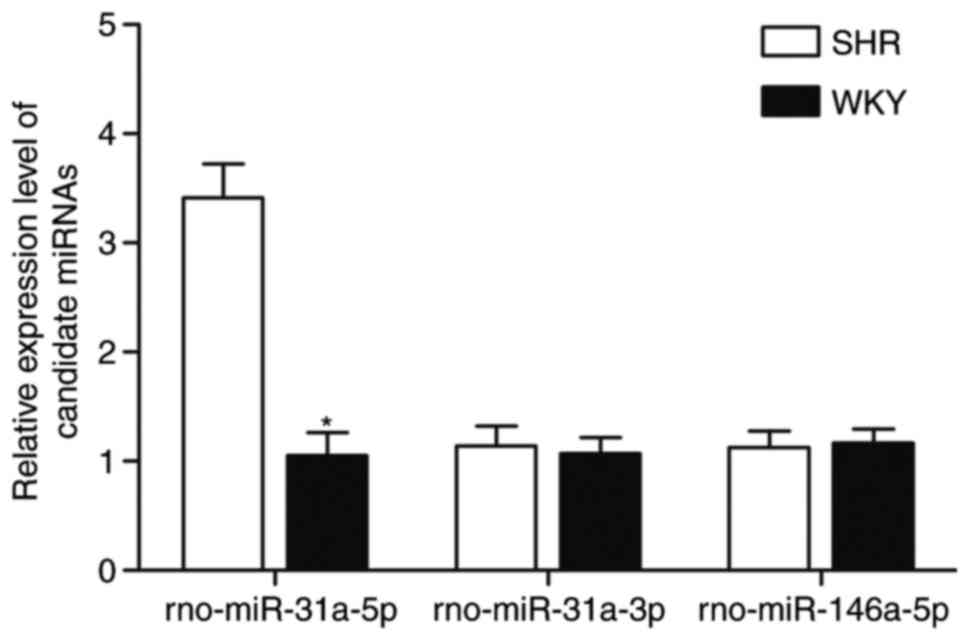
compare the expression of the candidate miRNAs (rno-miR-31a-5p,
rno-miR-31a-3p and rno-miR-146a-5p) between the SHR and WKY rats,
as shown in Fig. 1. Only
rno-miR-31a-5p showed differential expression in the SHR rats in
comparison with that in WKY rats, whereas no significant difference
in levels of rno-miR-31a-3p and rno-miR-146a-5p were observed
between the SHR and WKY rats.
Identifying candidate target genes of
rno-miR-31a-5p
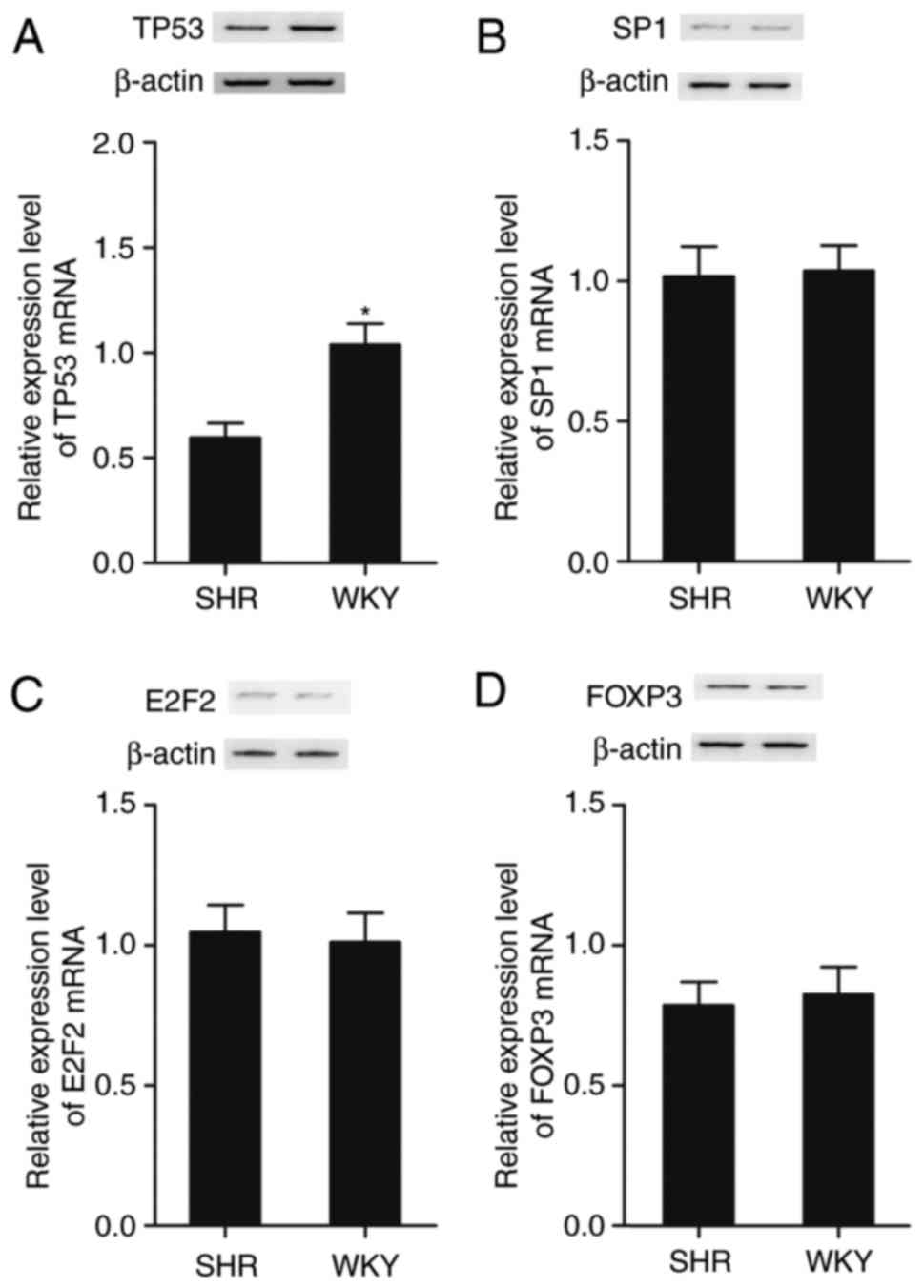
The PASMCs were collected from the SHR and WKY rats,
and RT-qPCR analysis and western blot analysis were performed to
examine candidate target genes, including p53, specificity protein
1 (SP1), E2F transcription factor 2 (E2F2) and forkhead box P3
(FOXP3), which were identified by searching the online miRNA
database, (www.mirdb.org). The expression of these
candidate target genes were determined and compared between cells
collected from the SHR and WKY rats. As shown in Fig. 2, only the mRNA and protein
(Fig. 2A) levels of p53 were
decreased in the SHR group, compared with those in the WKY group,
whereas the mRNA and protein levels of SP1 (Fig. 2B), E2F2 (Fig. 2C) and FOXP3 (Fig. 2D) were comparable between the SHR
and WKY groups.
p53 is a candidate target gene of
miR-31a-5p
To further validate p53 as a direct target of
rno-miR-31a-5p, vectors were constructed containing wild-type or
mutant 3′UTR of candidate genes, including p53 (Fig. 3A), SP1 (Fig. 3B), E2F2 (Fig. 3C) and FOXP3 (Fig. 3D), all of which had a
complementary binding site of miR-31a-5p located in their 3′UTR.
Subsequently, a luciferase assay was performed by transfecting the
rat PASMCs with the above-mentioned constructs together with
rno-miR-31a-5p. As shown in Fig.
3A, miR-31a-5p markedly reduced the luciferase activity of the
Luc-wild-p53-3′UTR relative to that in the control, whereas mutated
putative miR-31a-5p binding located on p53-3′UTR was found to be
refractile to this inhibitory effect. By contrast, there was no
significant difference in the luciferase activity of either
wild-type or mutant SP1 3′UTR (Fig.
3B), wild-type or mutant E2F2 3′UTR (Fig. 3C), or wild-type or mutant FOXP3
3′UTR (Fig. 3D) in the
miR-31-5p-overexpressing cells, compared with that in the scramble
control, confirming that miR-31-5p directly and negatively
regulated the expression of p53, but not the expression of SP1,
E2F2 or FOXP3.
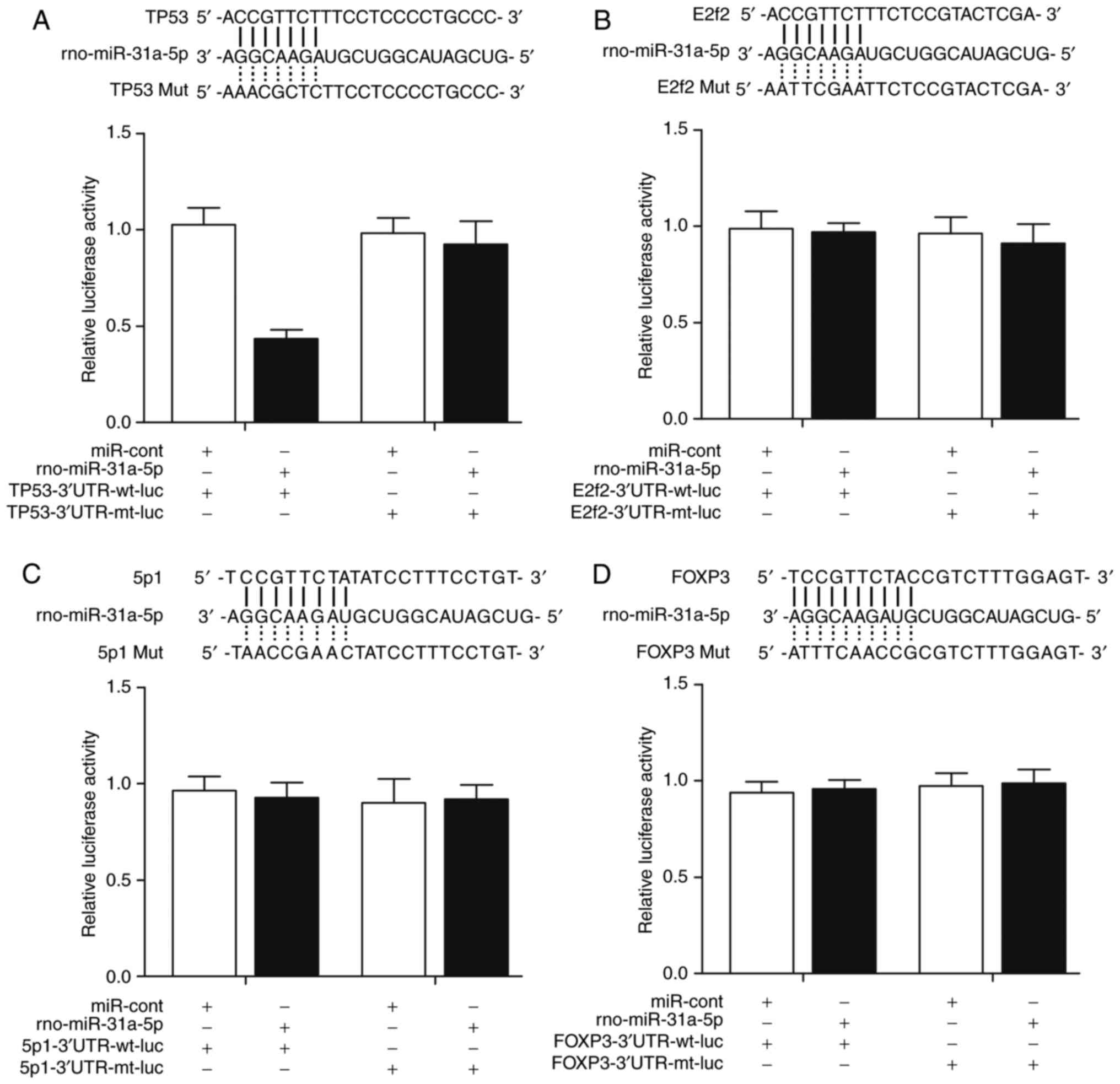 | Figure 3(A) Computational analysis showed
that there were candidate seed sequences of miR-31a-5p in the 3′UTR
of TP53. miR-31a-5p appeared to attenuate the luciferase activity
of the wild-type TP53 3′UTR. (B) Computational analysis showed that
there were candidate seed sequences of miR-31a-5p in the 3′UTR of
SP1, and that miR-31a-5p had no effect on the luciferase activity
of wild-type or mutant SP1 3′UTR. (C) Computational analysis showed
that there were candidate seed sequences of miR-31a-5p in the 3′UTR
of E2F2, and that miR-31a-5p had no effect on luciferase activity
of wild-type or mutant E2F2 3′UTR. (D) Computational analysis
showed that there were candidate seed sequences of miR-31a-5p in
the 3′UTR of FOXP3, and that miR-31a-5p had no effect on luciferase
activity of wild-type or mutant FOXP3 3′UTR. miR, microRNA; SP1,
specificity protein 1; E2F2, E2F transcription factor 2; FOXP3,
forkhead box P3; 3′UTR, 3′ untranslated region; mut/mt, mutant; wt,
wild-type; cont, control; luc, luciferase. |
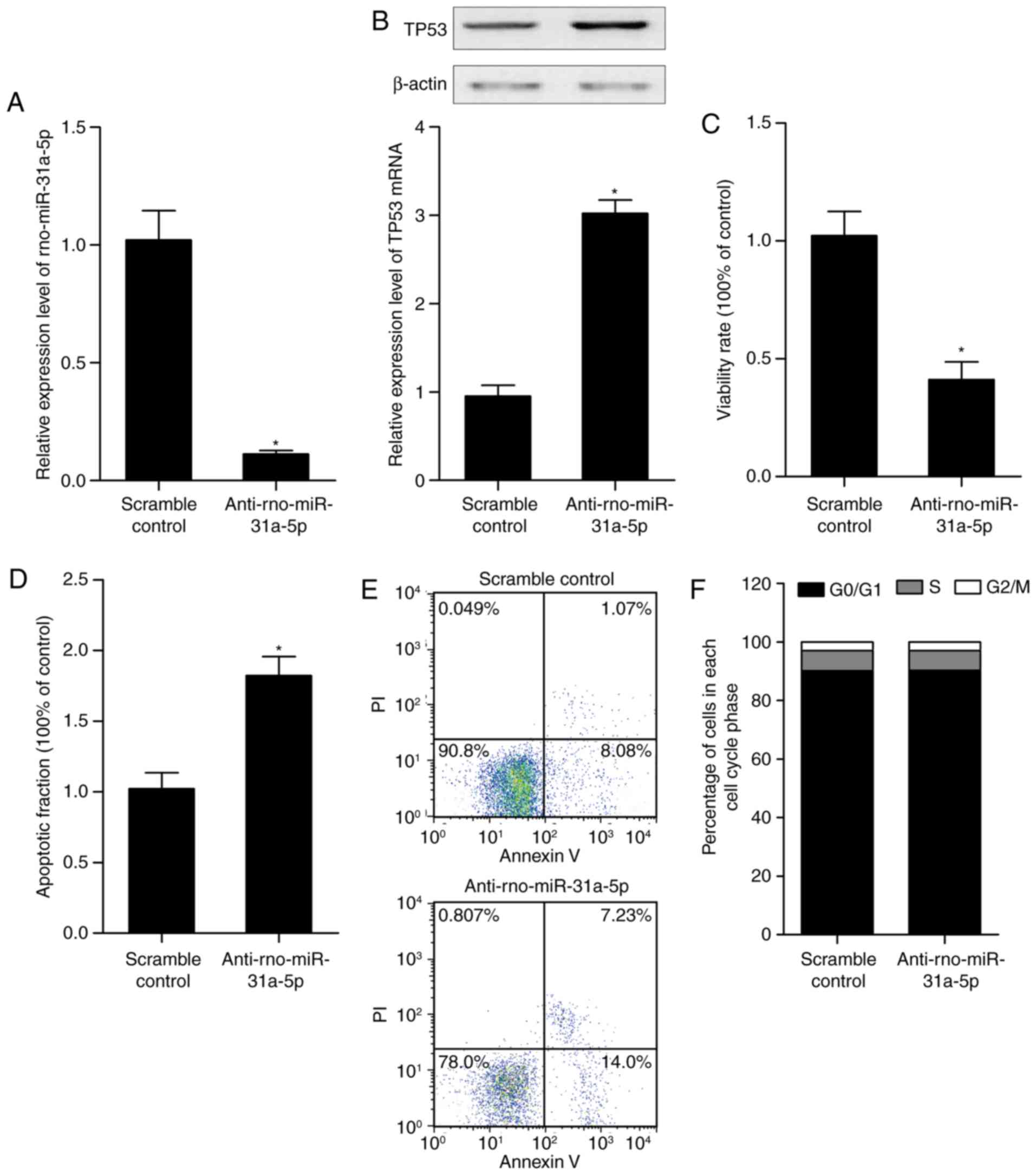
Alteration of the expression of
rno-miR-31a-5p affects the expression of p53, and the proliferation
and apoptosis of PASMCs of the SHR model
AuNPs containing scramble control or
anti-rno-miR-31a-5p were used to treat the SHR rats, from which
PASMCs were collected and the level of miR-31a-5p, expression of
p53, and growth and apoptotic rates of the PASMCs were examined. As
shown in Fig. 4A,
anti-rno-miR-31a-5p treatment suppressed the expression of
rno-miR-31a-5p, compared with that in the scramble control. The
downregulation of rno-miR-31a-5p upregulated the expression of p53
(Fig. 4B), and suppressed the
proliferation of PASMCs (Fig. 4C)
by promoting the apoptosis of the PASMCs (Fig. 4D and E), however, it did not
affect the cell cycle status (Fig.
4F).
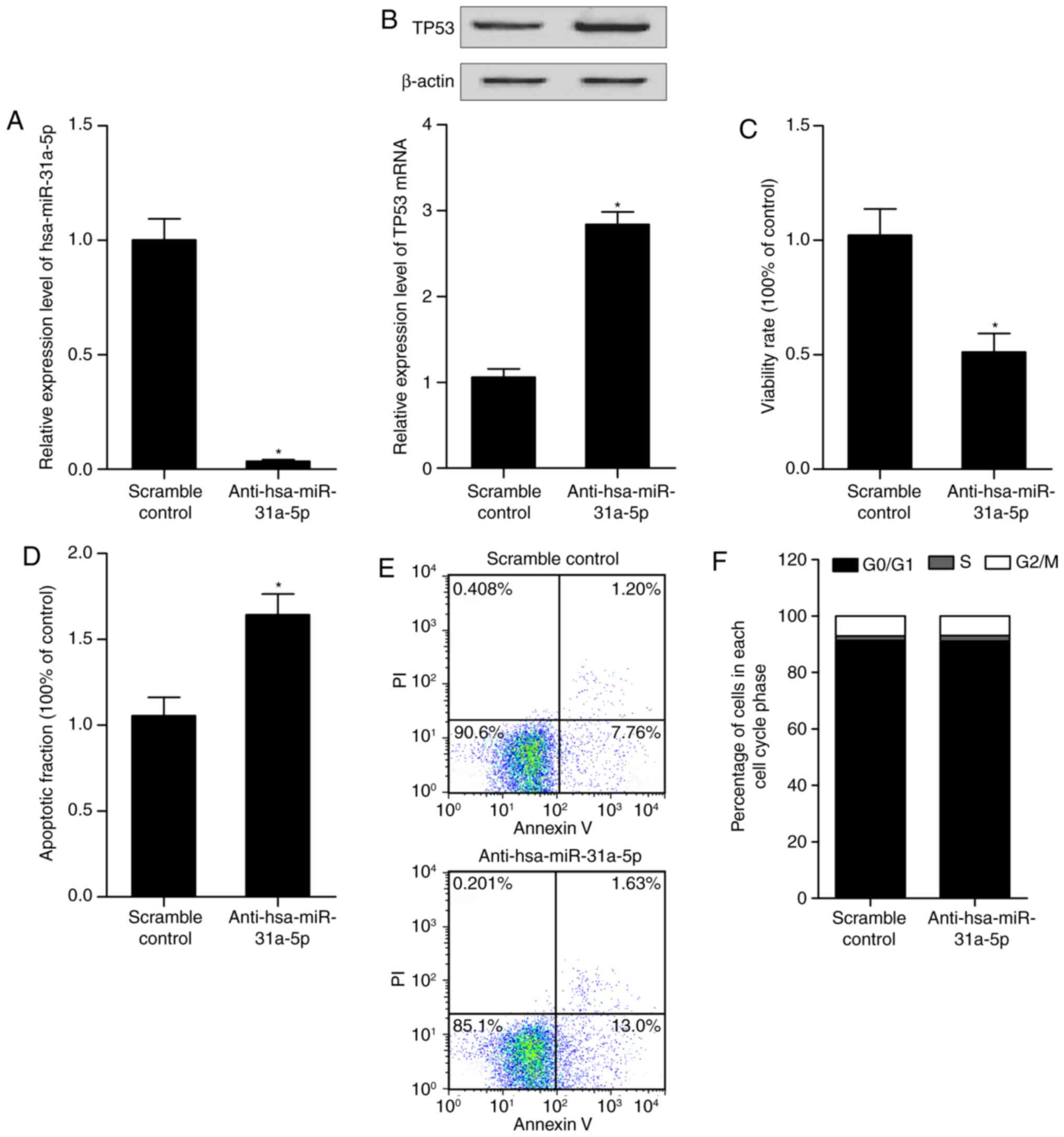
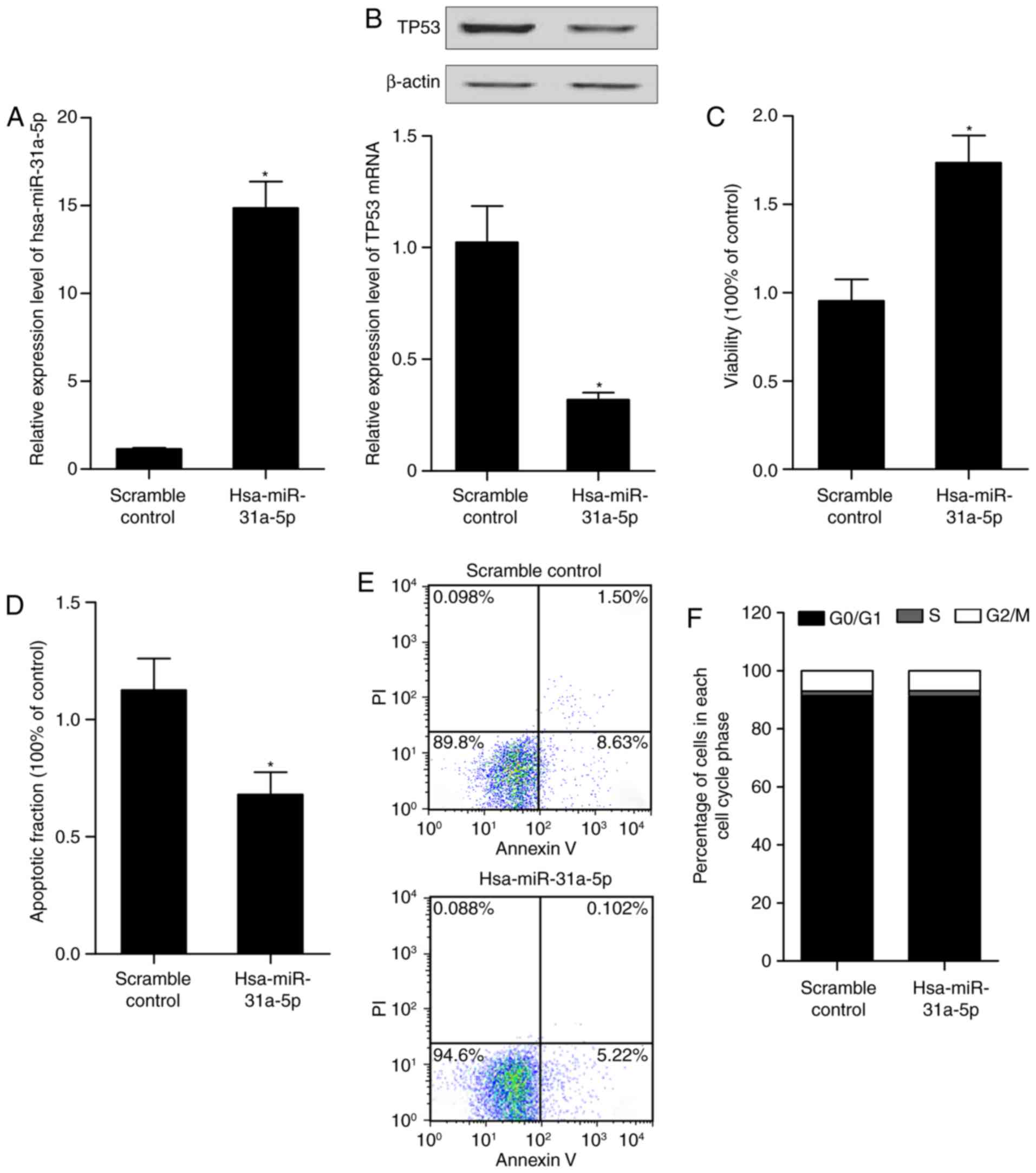
Alteration of has-miR-31a-5p affects the
expression of p53, and proliferation and apoptosis of PASMCs human
arterial smooth muscle cells
To further examine the role of miR-31-5p in the
control of cell proliferation and apoptosis in PASMCs, the cells
were transfected with the hsa-miR-31a-5p mimic, anti-hsa-miR-31a-5p
mimic or the scramble controls. RT-qPCR analysis, western blot
analysis, an MTT assay and flow cytometry were performed to
determine the levels of miR-31-5p and p53, and the proliferation
and apoptosis of the differently treated cells. As shown in
Fig. 5, the anti-hsa-miR-31a-5p
mimic (Fig. 5A) reduced the level
of miR-31a-5p. The mRNA and protein levels of p53 in the cells were
upregulated subsequent to transfect with the anti-hsa-miR-31a-5p
mimic (Fig. 5B). The
downregulation of miR-31a-5p by transfection with the
anti-hsa-miR-31a-5p mimic inhibited the viability of arterial
smooth muscle cells (Fig. 5C) and
promoted the apoptosis of arterial smooth muscle cells (Fig. 5D and E), but did not affect the
cell cycle status (Fig. 5F). The
hsa-miR-31a-5p mimic (Fig. 6A)
increased the level of miR-31a-5p. The mRNA and protein levels of
p53 in the cells were downregulated following transfection with the
hsa-miR-31a-5p mimic (Fig. 6B).
The overexpression of miR-31a-5p significantly promoted the growth
of the arterial smooth muscle cells (Fig. 6C) and significantly inhibited the
apoptosis of the cells (Fig. 6D and
E), but did not affect the cell cycle status (Fig. 6F). Collectively, these findings
indicated that miR-31a-5p accelerated the proliferation of arterial
smooth muscle cells and inhibited apoptosis via targeting p53.
Discussion
It has been shown that miRNAs, which modulate the
proliferation or migration of endothelial progenitor cells and
embryonic/mesenchymal stem cells (miR-702, miR-221/222 and miR-31),
and reduce stem cell apoptosis and cancer (miR-31 and miR-702), are
increased in hypertrophic RV, compared with those in controls
according to unbiased quantitative miR microarray analysis
(20,21). It was previously revealed that
only three miRs (miR-31a-5p, miR-31a-3p and miR-208b) were
increased in the LV of PAH rats (22). The levels of C-kit and miR-31 were
elevated in hypertrophic RV of PAH rats, compared with those in
control rats, and miR-31 was elevated in the RV of PAH rats, which
acts as a compensatory mechanism to decrease the reduction in
capillary density, which is associated with the failing hearts
(23). In the present study,
candidate miRNAs (rno-miR-31a-5p, rno-miR-31a-3p and
rno-miR-146a-5p) were investigated by comparing the expression
levels between PASMCs from SHR and WKY rats, and it was revealed
that only rno-miR-31a-5p was significantly upregulated in the SHR
rats, compared with the WKY rats. Previously, studies have shown
that miR-31 is involved in VSMC proliferation, angiogenesis and
tumor metastasis, although miR-31 was found to be the most
increased miRNA following acute myocardial infarction in rats
(24–26). miR-31-induced cardioprotection was
eliminated when the activation of nuclear factor (NF)-κB was
suppressed by Adv-dnIκBα during the ischemia/reperfusion (I/R)
process, revealing that diverse mechanisms may be involved in the
miR-31/PKCε signaling-induced and I/R-induced activation of NF-κB
in cardiac myocytes (27).
Patients with primary hypertension, a hereditary
polygenic disease, eventually develop complications, including
nephrosclerosis, cardiovascular remodeling and stroke. These
complications are practical targets for the treatment of underlying
HBP with blood pressure drugs. SHR rats, a genetic animal model for
underlying hypertension, indicates poorer growth of cardiovascular
organs compared with normotensive WKY rats (28). SHR-derived VSMCs in culture
exhibit accelerated entry into the S phase of the cell cycle,
aberrant contact suppression, a higher specific growth rate, and
nonspecific hyperproliferation responding to a variety of growth
factors, compared with cells from WKY rats (29). In the present study, candidate
genes of miR-31a-5p were investigated using an online miRNA
database, and four genes were found, including p53, SP1, E2F2 and
FOXP3, which may be target genes of miR-31a-5p with a complementary
binding site of miR-31a-5p located in their 3′UTR respectively. It
was then found that only the mRNA and protein levels of p53 were
differential in the SHR group, compared with those in the WKY
group, whereas the mRNA and protein levels of SP1, E2F2 and FOXP3
in the SHR group did not differ significantly compared with those
in the WKY group, and this regulatory association was further
confirmed by the results of the luciferase assay.
p53, a tumor inhibitor, is a key transcription
factor that modulates several cellular processes. It can suppress
cell proliferation by triggering cell cycle arrest in the G1, G2
and S phases of the cell cycle (30); its expression is associated with
elevated cell apoptosis in vitro and in vivo
(31). p53 predominantly acts as
a transcription factor, which can induce various anti-proliferative
programs by the inhibition or activation of critical genes or
effects (32). For VSMCs, the
downregulation of p53 occurs prior to VSMC migration and
proliferation (33). A
hypermethylation status has been observed in p53 promoter region
when treatment by Hcy, which indicates a causative function for
VSMC proliferation (17).
Therefore, p53 is considered a potent negative modulator of cell
proliferation, including that of VSMCs. The p53 tumor inhibitor
protein is stimulated in response to various cellular stresses,
including nucleotide depletion, oncogene activation and DNA damage,
with p21 and MDM2 being the most well-known examples of these
targets (34). Indirect
p53-mediated suppression is also involved via stimulation of its
direct transcriptional target, known as p21 (35). Representing the Ink4a/Cip1 family
of cyclin-dependent kinase (CDK) inhibitors, p21 suppressors bind
to and suppress CDK4 and CDK6/cyclin D complexes to trigger
cell-cycle arrest, leading to the activation and de-phosphorylation
of retinoblastoma pocket proteins, which act together with E2F
transcription factors to inhibit genes associated with the cell
cycle, including hTERT, EZH2 and CHK1, which are p53-inhibition
targets modulated by p21 (36,37). In the present study, PASMCs were
collected from SHR rats treated with AuNPs containing scramble
control or anti-rno-miR-31a-5p mimic, and it was revealed that
anti-rno-miR-31a-5p suppressed the expression of miR-31a-5p,
compared with the scramble control, and attenuated
miR-31a-5p-inhibited PASMC growth, but markedly increased apoptosis
of the PASMCs. Finally, RT-qPCR analysis, western blot analysis, an
MTT assay, and flow cytometry were used to determine the levels of
miR-31-5p and p53, and the proliferation and apoptosis of cells
transfected with the hsa-miR-31a-5p mimic and anti-hsa-miR-31a-5p
mimic. It was revealed that miR-31a-5p markedly suppressed the
expression of p53, and miR-31a-5p accelerated the proliferation of
PASMCs but inhibited apoptosis.
The effects of miR-31 have been associated with the
status of p53. miR-31 acts as an inhibitor only in tumor cells that
harbor mutant p53, which indicates miR-31 as a target for therapy
in patients with p53-deficient tumors (38). Of note, the p53 mutation is an
early indicator in esophageal squamous cell cancer (ESCC);
additionally, alterations in the p53 status may contribute to
context-dependent effects of several molecules, which include
microRNAs, including miR-31 (38,39). It has been shown that the
inhibitory role of miR-31 in ESCC relies on a deficiency of p21 in
addition to modulation by p53 (40).
In conclusion, the findings of the present study
demonstrated that the deregulation of miR-31a-5p was associated
with the risk of hypertension by suppressing the apoptosis of
arterial smooth muscle cells. It was found that p53, a well-known
tumor suppressor, was a direct target gene of miR-31a-5p, which was
important in the apoptosis of arterial smooth muscle cells and was
involved in the pathogenesis of hypertension. Therefore, miR-31a-5p
may be a novel therapeutic strategy for the treatment of
hypertension.
References
|
1
|
Nwankwo T, Yoon SS, Burt V and Gu Q:
Hypertension among adults in the United States: National Health and
Nutrition Examination Survey, 2011–2012. NCHS Data Brief. 1–8.
2013.
|
|
2
|
Chiong M, Cartes-Saavedra B,
Norambuena-Soto I, Mondaca-Ruff D, Morales PE, Garcia-Miguel M and
Mellado R: Mitochondrial metabolism and the control of vascular
smooth muscle cell proliferation. Front Cell Dev Biol. 2:722014.
View Article : Google Scholar
|
|
3
|
Ragolia L, Palaia T, Paric E and Maesaka
JK: Prostaglandin D2 synthase inhibits the exaggerated growth
phenotype of spontaneously hypertensive rat vascular smooth muscle
cells. J Biol Chem. 278:22175–22181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng GQ, Zhang GH, Xu XW, Wang JW, Yu LB,
Zhang YN, Huang JW, Li YL, Braudt-Rauf PW and Xia ZL: Association
of telomere length with chromosomal damage among Chinese workers
exposed to Vinyl Chloride monomer. J Occup Environ Med.
59:e252–e256. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pacurari M, Kafoury R, Tchounwou PB and
Ndebele K: The Renin-Angiotensin-aldosterone system in vascular
inflammation and remodeling. Int J Inflam. 2014:6893602014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mercer J, Figg N, Stoneman V, Braganza D
and Bennett MR: Endogenous p53 protects vascular smooth muscle
cells from apoptosis and reduces atherosclerosis in ApoE knockout
mice. Circ Res. 96:667–674. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Giannakakou P, Sackett DL, Ward Y, Webster
KR, Blagosklonny MV and Fojo T: p53 is associated with cellular
microtubules and is transported to the nucleus by dynein. Nat Cell
Biol. 2:709–717. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krones-Herzig A, Adamson E and Mercola D:
Early growth response 1 protein, an upstream gatekeeper of the p53
tumor suppressor, controls replicative senescence. Proc Natl Acad
Sci USA. 100:3233–3238. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mizuno S, Bogaard HJ, Kraskauskas D,
Alhussaini A, Gomez-Arroyo J, Voelkel NF and Ishizaki T: p53 Gene
deficiency promotes hypoxia-induced pulmonary hypertension and
vascular remodeling in mice. Am J Physiol Lung Cell Mol Physiol.
300:L753–L761. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sempere LF, Freemantle S, Pitha-Rowe I,
Moss E, Dmitrovsky E and Ambros V: Expression profiling of
mammalian microRNAs uncovers a subset of brain-expressed microRNAs
with possible roles in murine and human neuronal differentiation.
Genome Biol. 5:R132004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pan ZW, Lu YJ and Yang BF: MicroRNAs: A
novel class of potential therapeutic targets for cardiovascular
diseases. Acta Pharmacol Sin. 31:1–9. 2010. View Article : Google Scholar
|
|
13
|
Zapolska-Downar D, Siennicka A,
Chelstowski K, Widecka K, Goracy I, Halasa M, Machalinski B and
Naruszewicz M: Is there an association between
angiotensin-converting enzyme gene polymorphism and functional
activation of monocytes and macrophage in young patients with
essential hypertension. J Hypertens. 24:1565–1573. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kontaraki JE, Marketou ME, Parthenakis FI,
Maragkoudakis S, Zacharis EA, Petousis S, Kochiadakis GE and Vardas
PE: Hypertrophic and antihypertrophic microRNA levels in peripheral
blood mononuclear cells and their relationship to left ventricular
hypertrophy in patients with essential hypertension. J Am Soc
Hypertens. 9:802–810. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Muller DN, Kvakan H and Luft FC:
Immune-related effects in hypertension and target-organ damage.
Curr Opin Nephrol Hypertens. 20:113–117. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Palao T, Sward K, Jongejan A, Moerland PD,
de Vos J, van Weert A, Arribas SM, Groma G, vanBavel E and Bakker
EN: Gene expression and microrna expression analysis in small
arteries of spontaneously hypertensive rats. Evidence for ER
stress. PLoS One. 10:e01370272015. View Article : Google Scholar
|
|
17
|
Jacquin S, Rincheval V, Mignotte B,
Richard S, Humbert M, Mercier O, Londono-Vallejo A, Fadel E and
Eddahibi S: Inactivation of p53 is sufficient to induce development
of pulmonary hypertension in rats. PLoS One. 10:e01319402015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cai L, Li J, Zhang X, Lu Y, Wang J, Lyu X,
Chen Y, Liu J, Cai H, Wang Y, et al: Gold nano-particles (AuNPs)
carrying anti-EBV-miR-BART7-3p inhibit growth of EBV-positive
nasopharyngeal carcinoma. Oncotarget. 6:7838–7850. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Kim BM and Choi MY: Non-canonical
microRNAs miR-320 and miR-702 promote proliferation in
Dgcr8-deficient embryonic stem cells. Biochem Biophys Res Commun.
426:183–189. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dong Z, Zhong Z, Yang L, Wang S and Gong
Z: MicroRNA-31 inhibits cisplatin-induced apoptosis in non-small
cell lung cancer cells by regulating the drug transporter ABCB9.
Cancer Lett. 343:249–257. 2014. View Article : Google Scholar
|
|
22
|
Joshi SR, Dhagia V, Gairhe S, Edwards JG,
McMurtry IF and Gupte SA: MicroRNA-140 is elevated and mitofusin-1
is downregulated in the right ventricle of the
Sugen5416/hypoxia/normoxia model of pulmonary arterial
hypertension. Am J Physiol Heart Circ Physiol. 311:H689–H698. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alzoubi A, Toba M, Abe K, O'Neill KD,
Rocic P, Fagan KA, McMurtry IF and Oka M: Dehydroepiandrosterone
restores right ventricular structure and function in rats with
severe pulmonary arterial hypertension. Am J Physiol Heart Circ
Physiol. 304:H1708–H1718. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu X, Cheng Y, Chen X, Yang J, Xu L and
Zhang C: MicroRNA-31 regulated by the extracellular regulated
kinase is involved in vascular smooth muscle cell growth via large
tumor suppressor homolog 2. J Biol Chem. 286:42371–42380. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pedrioli DM, Karpanen T, Dabouras V,
Jurisic G, van de Hoek G, Shin JW, Marino D, Kalin RE, Leidel S,
Cinelli P, et al: MiR-31 functions as a negative regulator of
lymphatic vascular lineage-specific differentiation in vitro and
vascular development in vivo. Mol Cell Biol. 30:3620–3634. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shi B, Guo Y, Wang J and Gao W: Altered
expression of microRNAs in the myocardium of rats with acute
myocardial infarction. BMC Cardiovasc Disord. 10:112010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Men M, Yang W, Zheng H and Xue S:
MiR-31 downregulation protects against cardiac ischemia/reperfusion
injury by targeting protein kinase C epsilon (PKCepsilon) directly.
Cell Physiol Biochem. 36:179–190. 2015. View Article : Google Scholar
|
|
28
|
Huang CY, Kuo CH, Pai PY, Ho TJ, Lin YM,
Chen RJ, Tsai FJ, Vijaya Padma V, Kuo WW and Huang CY: Inhibition
of HSF2 SUMOylation via MEL18 upregulates IGF-IIR and leads to
hypertension-induced cardiac hypertrophy. Int J Cardiol.
S0167–5273:33565–33569. 2017.
|
|
29
|
Ueno T, Takagi H, Fukuda N, Takahashi A,
Yao EH, Mitsumata M, Hiraoka-Yamamoto J, Ikeda K, Matsumoto K and
Yamori Y: Cardiovascular remodeling and metabolic abnormalities in
SHRSP.Z-Lepr(fa)/IzmDmcr rats as a new model of metabolic syndrome.
Hypertens Res. 31:1021–1031. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cannell IG, Merrick KA, Morandell S, Zhu
CQ, Braun CJ, Grant RA, Cameron ER, Tsao MS, Hemann MT and Yaffe
MB: A pleiotropic Rna-binding protein controls distinct cell cycle
checkpoints to drive resistance of p53-defective tumors to
chemotherapy. Cancer Cell. 28:623–637. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Muller P, Ceskova P and Vojtesek B: Hsp90
is essential for restoring cellular functions of
temperature-sensitive p53 mutant protein but not for stabilization
and activation of wild-type p53: Implications for cancer therapy. J
Biol Chem. 280:6682–6691. 2005. View Article : Google Scholar
|
|
32
|
Zilfou JT and Lowe SW: Tumor suppressive
functions of p53. Cold Spring Harb Perspect Biol. 1:a0018832009.
View Article : Google Scholar :
|
|
33
|
Rodriguez-Campos A, Ruiz-Enriquez P,
Faraudo S and Badimon L: Mitogen-induced p53 downregulation
precedes vascular smooth muscle cell migration from healthy tunica
media and proliferation. Arterioscler Thromb Vasc Biol. 21:214–219.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vousden KH and Prives C: Blinded by the
light: The growing complexity of p53. Cell. 137:413–431. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Laptenko O and Prives C: Transcriptional
regulation by p53: One protein, many possibilities. Cell Death
Differ. 13:951–961. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tang X, Milyavsky M, Shats I, Erez N,
Goldfinger N and Rotter V: Activated p53 suppresses the histone
methyltransferase EZH2 gene. Oncogene. 23:5759–5769. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Carcagno AL, Marazita MC, Ogara MF, Ceruti
JM, Sonzogni SV, Scassa ME, Giono LE and Canepa ET: E2F1-mediated
upregulation of p19INK4d determines its periodic expression during
cell cycle and regulates cellular proliferation. PLoS One.
6:e219382011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Creighton CJ, Fountain MD, Yu Z, Nagaraja
AK, Zhu H, Khan M, Olokpa E, Zariff A, Gunaratne PH, Matzuk MM, et
al: Molecular profiling uncovers a p53-associated role for
microRNA-31 in inhibiting the proliferation of serous ovarian
carcinomas and other cancers. Cancer Res. 70:1906–1915. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang Y, Tarapore RS, Jarmel MH, Tetreault
MP and Katz JP: p53 mutation alters the effect of the esophageal
tumor suppressor KLF5 on keratinocyte proliferation. Cell Cycle.
11:4033–4039. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ning Z, Zhu H, Li F, Liu Q, Liu G, Tan T,
Zhang B, Chen S, Li G, Huang D, et al: Tumor suppression by miR-31
in esophageal carcinoma is p21-dependent. Genes Cancer. 5:436–444.
2014.
|