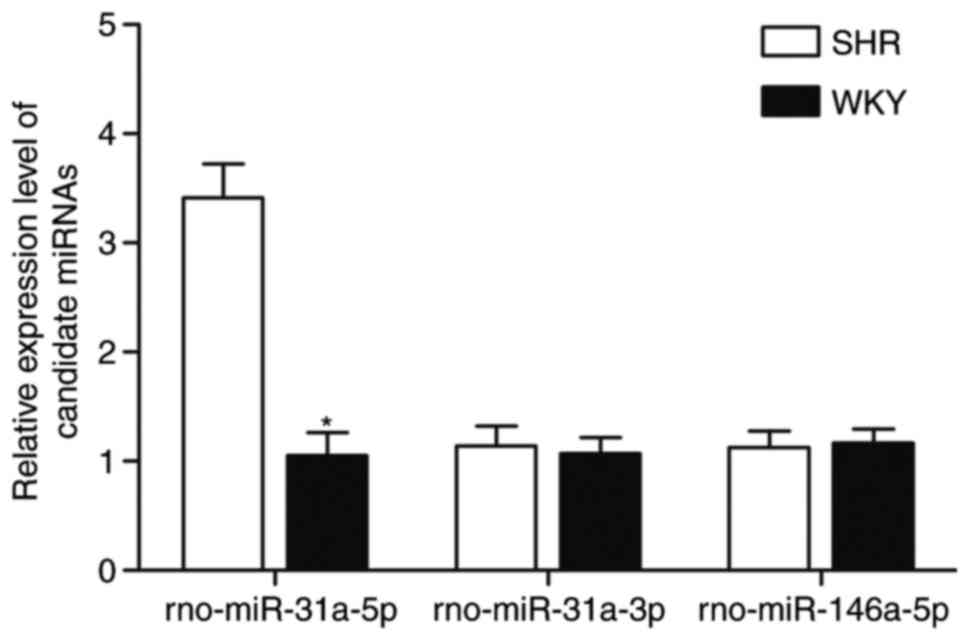
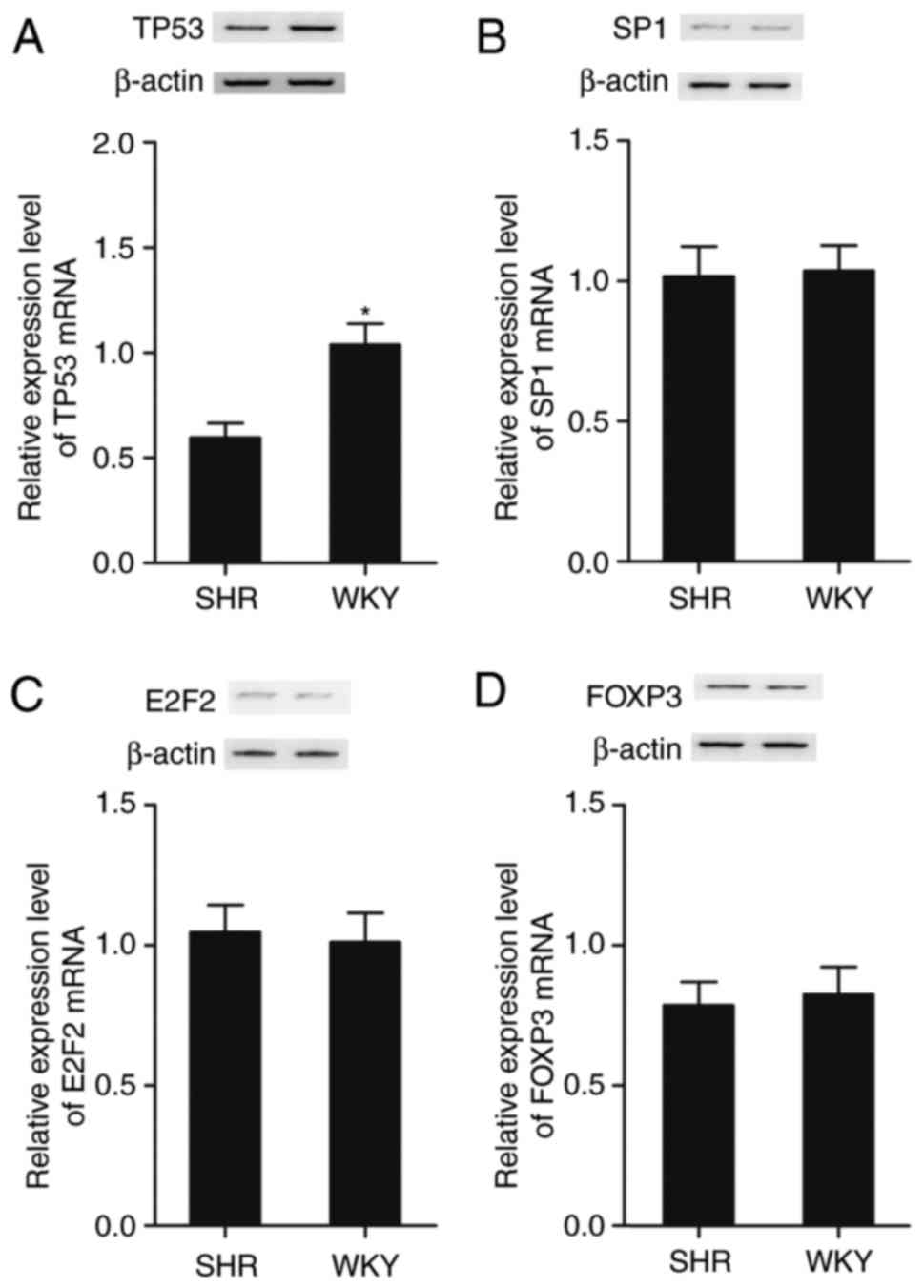
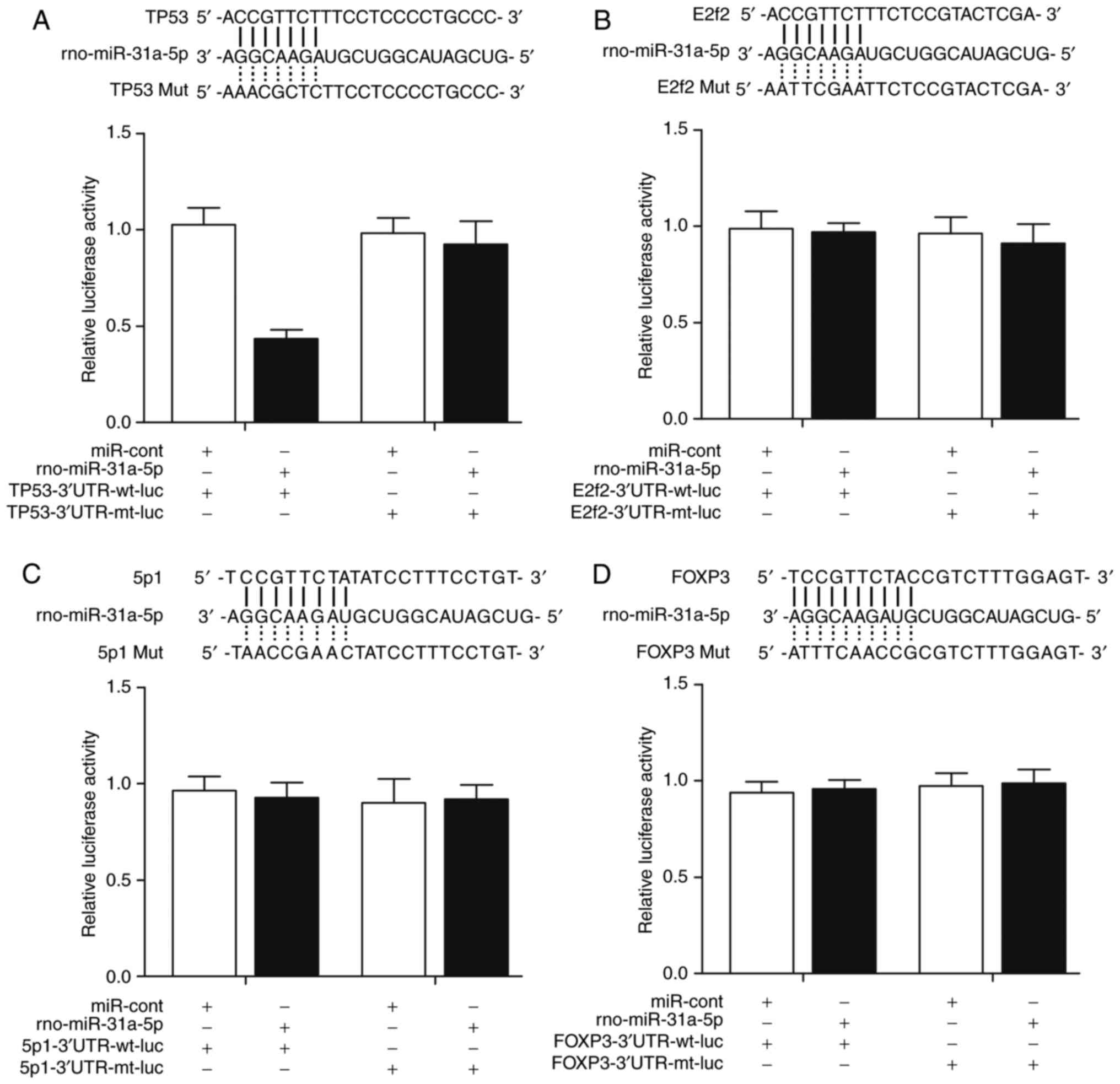
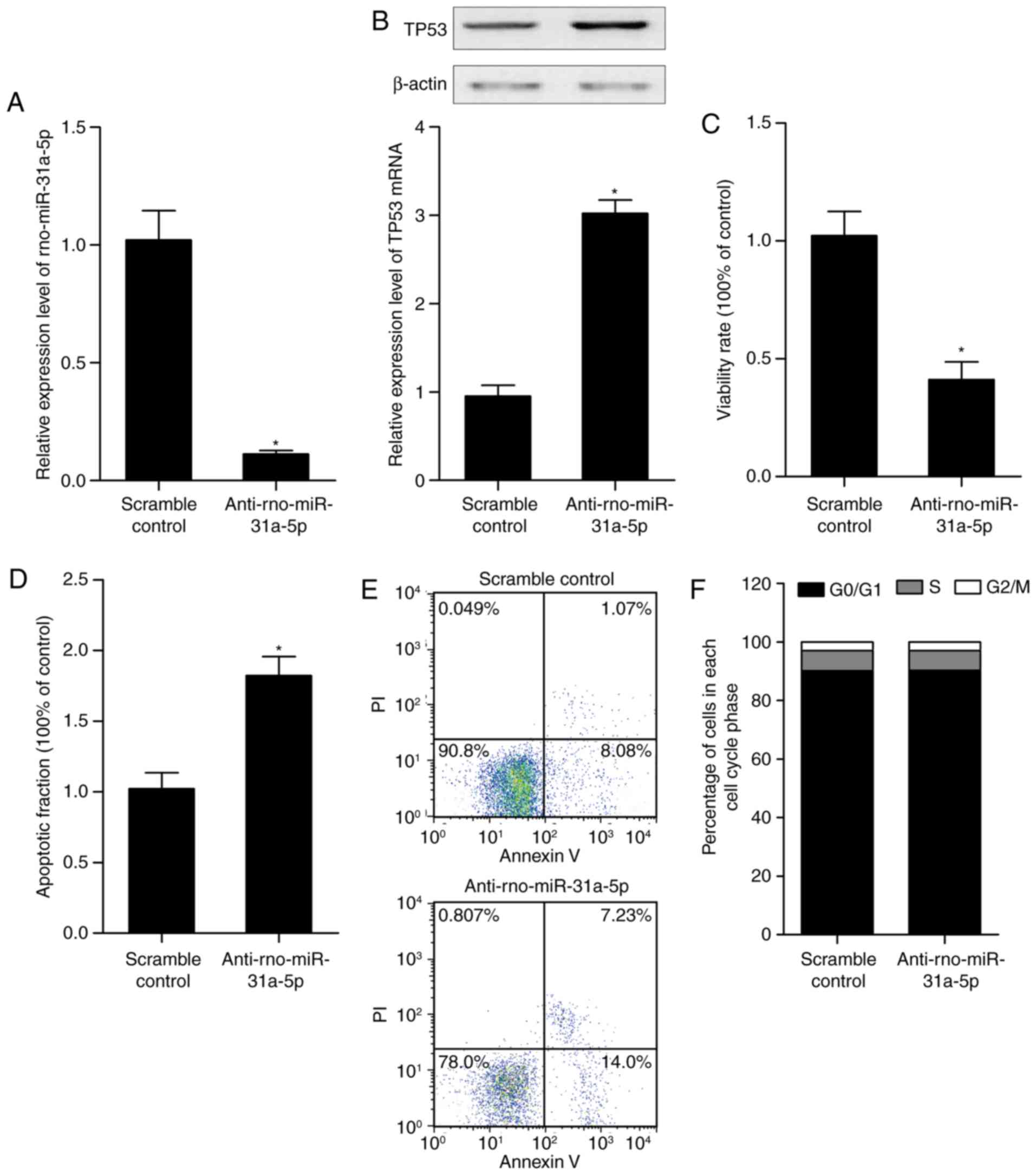
|
1
|
Nwankwo T, Yoon SS, Burt V and Gu Q:
Hypertension among adults in the United States: National Health and
Nutrition Examination Survey, 2011–2012. NCHS Data Brief. 1–8.
2013.
|
|
2
|
Chiong M, Cartes-Saavedra B,
Norambuena-Soto I, Mondaca-Ruff D, Morales PE, Garcia-Miguel M and
Mellado R: Mitochondrial metabolism and the control of vascular
smooth muscle cell proliferation. Front Cell Dev Biol. 2:722014.
View Article : Google Scholar
|
|
3
|
Ragolia L, Palaia T, Paric E and Maesaka
JK: Prostaglandin D2 synthase inhibits the exaggerated growth
phenotype of spontaneously hypertensive rat vascular smooth muscle
cells. J Biol Chem. 278:22175–22181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng GQ, Zhang GH, Xu XW, Wang JW, Yu LB,
Zhang YN, Huang JW, Li YL, Braudt-Rauf PW and Xia ZL: Association
of telomere length with chromosomal damage among Chinese workers
exposed to Vinyl Chloride monomer. J Occup Environ Med.
59:e252–e256. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pacurari M, Kafoury R, Tchounwou PB and
Ndebele K: The Renin-Angiotensin-aldosterone system in vascular
inflammation and remodeling. Int J Inflam. 2014:6893602014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mercer J, Figg N, Stoneman V, Braganza D
and Bennett MR: Endogenous p53 protects vascular smooth muscle
cells from apoptosis and reduces atherosclerosis in ApoE knockout
mice. Circ Res. 96:667–674. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Giannakakou P, Sackett DL, Ward Y, Webster
KR, Blagosklonny MV and Fojo T: p53 is associated with cellular
microtubules and is transported to the nucleus by dynein. Nat Cell
Biol. 2:709–717. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krones-Herzig A, Adamson E and Mercola D:
Early growth response 1 protein, an upstream gatekeeper of the p53
tumor suppressor, controls replicative senescence. Proc Natl Acad
Sci USA. 100:3233–3238. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mizuno S, Bogaard HJ, Kraskauskas D,
Alhussaini A, Gomez-Arroyo J, Voelkel NF and Ishizaki T: p53 Gene
deficiency promotes hypoxia-induced pulmonary hypertension and
vascular remodeling in mice. Am J Physiol Lung Cell Mol Physiol.
300:L753–L761. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sempere LF, Freemantle S, Pitha-Rowe I,
Moss E, Dmitrovsky E and Ambros V: Expression profiling of
mammalian microRNAs uncovers a subset of brain-expressed microRNAs
with possible roles in murine and human neuronal differentiation.
Genome Biol. 5:R132004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pan ZW, Lu YJ and Yang BF: MicroRNAs: A
novel class of potential therapeutic targets for cardiovascular
diseases. Acta Pharmacol Sin. 31:1–9. 2010. View Article : Google Scholar
|
|
13
|
Zapolska-Downar D, Siennicka A,
Chelstowski K, Widecka K, Goracy I, Halasa M, Machalinski B and
Naruszewicz M: Is there an association between
angiotensin-converting enzyme gene polymorphism and functional
activation of monocytes and macrophage in young patients with
essential hypertension. J Hypertens. 24:1565–1573. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kontaraki JE, Marketou ME, Parthenakis FI,
Maragkoudakis S, Zacharis EA, Petousis S, Kochiadakis GE and Vardas
PE: Hypertrophic and antihypertrophic microRNA levels in peripheral
blood mononuclear cells and their relationship to left ventricular
hypertrophy in patients with essential hypertension. J Am Soc
Hypertens. 9:802–810. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Muller DN, Kvakan H and Luft FC:
Immune-related effects in hypertension and target-organ damage.
Curr Opin Nephrol Hypertens. 20:113–117. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Palao T, Sward K, Jongejan A, Moerland PD,
de Vos J, van Weert A, Arribas SM, Groma G, vanBavel E and Bakker
EN: Gene expression and microrna expression analysis in small
arteries of spontaneously hypertensive rats. Evidence for ER
stress. PLoS One. 10:e01370272015. View Article : Google Scholar
|
|
17
|
Jacquin S, Rincheval V, Mignotte B,
Richard S, Humbert M, Mercier O, Londono-Vallejo A, Fadel E and
Eddahibi S: Inactivation of p53 is sufficient to induce development
of pulmonary hypertension in rats. PLoS One. 10:e01319402015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cai L, Li J, Zhang X, Lu Y, Wang J, Lyu X,
Chen Y, Liu J, Cai H, Wang Y, et al: Gold nano-particles (AuNPs)
carrying anti-EBV-miR-BART7-3p inhibit growth of EBV-positive
nasopharyngeal carcinoma. Oncotarget. 6:7838–7850. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Kim BM and Choi MY: Non-canonical
microRNAs miR-320 and miR-702 promote proliferation in
Dgcr8-deficient embryonic stem cells. Biochem Biophys Res Commun.
426:183–189. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dong Z, Zhong Z, Yang L, Wang S and Gong
Z: MicroRNA-31 inhibits cisplatin-induced apoptosis in non-small
cell lung cancer cells by regulating the drug transporter ABCB9.
Cancer Lett. 343:249–257. 2014. View Article : Google Scholar
|
|
22
|
Joshi SR, Dhagia V, Gairhe S, Edwards JG,
McMurtry IF and Gupte SA: MicroRNA-140 is elevated and mitofusin-1
is downregulated in the right ventricle of the
Sugen5416/hypoxia/normoxia model of pulmonary arterial
hypertension. Am J Physiol Heart Circ Physiol. 311:H689–H698. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alzoubi A, Toba M, Abe K, O'Neill KD,
Rocic P, Fagan KA, McMurtry IF and Oka M: Dehydroepiandrosterone
restores right ventricular structure and function in rats with
severe pulmonary arterial hypertension. Am J Physiol Heart Circ
Physiol. 304:H1708–H1718. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu X, Cheng Y, Chen X, Yang J, Xu L and
Zhang C: MicroRNA-31 regulated by the extracellular regulated
kinase is involved in vascular smooth muscle cell growth via large
tumor suppressor homolog 2. J Biol Chem. 286:42371–42380. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pedrioli DM, Karpanen T, Dabouras V,
Jurisic G, van de Hoek G, Shin JW, Marino D, Kalin RE, Leidel S,
Cinelli P, et al: MiR-31 functions as a negative regulator of
lymphatic vascular lineage-specific differentiation in vitro and
vascular development in vivo. Mol Cell Biol. 30:3620–3634. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shi B, Guo Y, Wang J and Gao W: Altered
expression of microRNAs in the myocardium of rats with acute
myocardial infarction. BMC Cardiovasc Disord. 10:112010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Men M, Yang W, Zheng H and Xue S:
MiR-31 downregulation protects against cardiac ischemia/reperfusion
injury by targeting protein kinase C epsilon (PKCepsilon) directly.
Cell Physiol Biochem. 36:179–190. 2015. View Article : Google Scholar
|
|
28
|
Huang CY, Kuo CH, Pai PY, Ho TJ, Lin YM,
Chen RJ, Tsai FJ, Vijaya Padma V, Kuo WW and Huang CY: Inhibition
of HSF2 SUMOylation via MEL18 upregulates IGF-IIR and leads to
hypertension-induced cardiac hypertrophy. Int J Cardiol.
S0167–5273:33565–33569. 2017.
|
|
29
|
Ueno T, Takagi H, Fukuda N, Takahashi A,
Yao EH, Mitsumata M, Hiraoka-Yamamoto J, Ikeda K, Matsumoto K and
Yamori Y: Cardiovascular remodeling and metabolic abnormalities in
SHRSP.Z-Lepr(fa)/IzmDmcr rats as a new model of metabolic syndrome.
Hypertens Res. 31:1021–1031. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cannell IG, Merrick KA, Morandell S, Zhu
CQ, Braun CJ, Grant RA, Cameron ER, Tsao MS, Hemann MT and Yaffe
MB: A pleiotropic Rna-binding protein controls distinct cell cycle
checkpoints to drive resistance of p53-defective tumors to
chemotherapy. Cancer Cell. 28:623–637. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Muller P, Ceskova P and Vojtesek B: Hsp90
is essential for restoring cellular functions of
temperature-sensitive p53 mutant protein but not for stabilization
and activation of wild-type p53: Implications for cancer therapy. J
Biol Chem. 280:6682–6691. 2005. View Article : Google Scholar
|
|
32
|
Zilfou JT and Lowe SW: Tumor suppressive
functions of p53. Cold Spring Harb Perspect Biol. 1:a0018832009.
View Article : Google Scholar :
|
|
33
|
Rodriguez-Campos A, Ruiz-Enriquez P,
Faraudo S and Badimon L: Mitogen-induced p53 downregulation
precedes vascular smooth muscle cell migration from healthy tunica
media and proliferation. Arterioscler Thromb Vasc Biol. 21:214–219.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vousden KH and Prives C: Blinded by the
light: The growing complexity of p53. Cell. 137:413–431. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Laptenko O and Prives C: Transcriptional
regulation by p53: One protein, many possibilities. Cell Death
Differ. 13:951–961. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tang X, Milyavsky M, Shats I, Erez N,
Goldfinger N and Rotter V: Activated p53 suppresses the histone
methyltransferase EZH2 gene. Oncogene. 23:5759–5769. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Carcagno AL, Marazita MC, Ogara MF, Ceruti
JM, Sonzogni SV, Scassa ME, Giono LE and Canepa ET: E2F1-mediated
upregulation of p19INK4d determines its periodic expression during
cell cycle and regulates cellular proliferation. PLoS One.
6:e219382011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Creighton CJ, Fountain MD, Yu Z, Nagaraja
AK, Zhu H, Khan M, Olokpa E, Zariff A, Gunaratne PH, Matzuk MM, et
al: Molecular profiling uncovers a p53-associated role for
microRNA-31 in inhibiting the proliferation of serous ovarian
carcinomas and other cancers. Cancer Res. 70:1906–1915. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang Y, Tarapore RS, Jarmel MH, Tetreault
MP and Katz JP: p53 mutation alters the effect of the esophageal
tumor suppressor KLF5 on keratinocyte proliferation. Cell Cycle.
11:4033–4039. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ning Z, Zhu H, Li F, Liu Q, Liu G, Tan T,
Zhang B, Chen S, Li G, Huang D, et al: Tumor suppression by miR-31
in esophageal carcinoma is p21-dependent. Genes Cancer. 5:436–444.
2014.
|