Hypertension is characterized by the elevation of
arterial pressure, and can be complicated by damage and metabolic
changes in the heart, blood vessels, brain, kidney, retina and
other target organs. It is a multifactorial disease and various
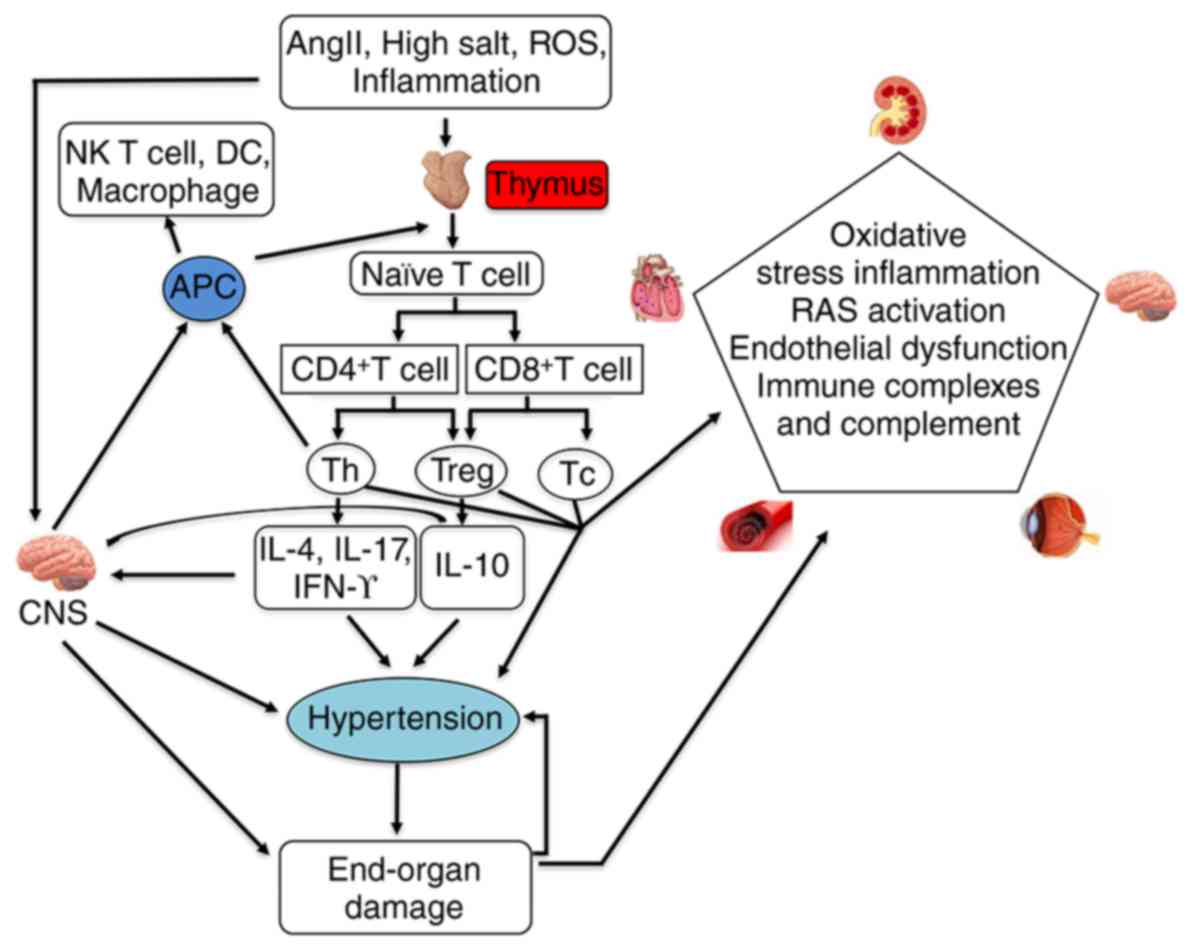
immune cells and factors have been shown to be involved (Fig. 1) (1). There have been increases in the
incidence and mortality rates of patients with heart and
cerebrovascular disease; therefore, reducing the incidence and
mortality rates of heart and cerebrovascular disease in patients
with hypertension is the ultimate goal of antihypertensive therapy.
However, even when blood pressure is under control, organ damage
and abnormal metabolism may not be completely resolved, which
suggests that other mechanisms may be involved in, or contribute
to, the complex pathological processes of hypertension and may not
be eliminated by current drug strategies. Antihypertensive therapy
requires the establishment of blood pressure control (2). Therefore, it is of clinical and
practical significance to investigate the mechanism of
hypertension.
The thymus, as a key organ in T lymphocyte
ontogenesis, has been shown to be crucial in optimizing immune
system function throughout life (3–7),
therefore, the pathological processes of high blood pressure are
considered to be closely associated with the thymus. Studies have
revealed that the thymus exhibits constant atrophy or hypofunction
with age (8). Fukuda et al
(9) suggested that the values of
thymus weights were lower in Spontaneously hypertensive rats (SHR),
compared with those in Wistar Kyoto (WKY) rats, when they
investigated age-related changing in hematological values, serum
biochemical constituents, and weights of various organs in both
genders of SHR/Izm, Stroke-prone SHR and WKY/Izm rat strains. A
previous study by Svendsen et al found that the
salt-dependent phase of deoxycorticosterone acetate salt
hypertension did not develop and the decreased perivascular
infiltration of immune cells following renal infarction was not
present in athymic 'nude' mice. However, if the thymus gland was
transplanted into these athymic mice, then the capacity for
developing salt-driven hypertension was restored (10–13). Ba et al showed that the
thymus transplanted from neonatal normotensive Wistar rats to the
prehypertensive SHR strain delayed the onset of hypertension from 5
to 32 weeks and decreased blood pressure in hypertensive adults; it
is known that the SHR strain has normal blood pressure at birth and
gradually develops high blood pressure from ~5 weeks of age,
reaching maximal levels at ~15–20 weeks of age (14). Therefore, the thymus may be
involved in the process of hypertension. However, the mechanism of
thymus function in the process of hypertension remains to be fully
elucidated. The purpose of this review was to summarize and discuss
advances in our knowledge of hypertensive vascular disease by the
effect of thymus function on hypertension, with a particular focus
on the mechanism underlying the effect of thymus function on
hypertension.
The thymus is known to be essential in T cell
development and maturation. The thymus is where the T cell
repertoire is generated, and where T cells undergo positive and
negative selection, leading to a wide functional MHC-restricted
naïve T cell receptor αβ repertoire (15,16). In the development of T cells, they
migrate within distinct thymus microenvironments, where they
interact with stromal cells to provide signals crucial to the
survival, proliferation, differentiation and selection of
thymocytes (17–19). Naïve T cells can differentiate
into helper T cells (Th), regulatory T cells (Tregs) and cytotoxic
T cells. The generation and maturation of the specific T cell
lineage involves specific and complex processes within the thymus,
and several signaling pathways are involved in these processes. If
thymocytes respond spontaneously to these antigens, they undergo
negative selection, through apoptosis, or into Treg lineages
(17). It is now well established
that Tregs are produced via two main pathways in vivo. The
majority of functionally mature Treg cells are produced in the
thymus, where recognition of self-antigen by certain clones leads
to their deviation into the thymus-derived forkhead box
(Fox)p3+ Treg cell lineage (20,21). Th cells can secrete interleukin
(IL)-4, IL-17 and interferon (IFN)-γ. In addition, Tregs can
secrete IL-10. IL-4 regulates the proliferation of activated
B-cells and mast cells (22–25). In the absence of vascular tissue,
the presence of IL4 promotes the substitution of activated
macrophages into M2 cells and inhibits the activation of classical
activated macrophage M1 cells. Increased macrophage repair (M2)
combined with the secretion of IL-10 and transforming growth factor
(TGF)-β results in a reduction of pathological inflammation
(26–28). IL-17 is involved in the induction
and regulation of pro-inflammatory responses. IL-17 induces the
production of other cytokines, [IL-6, TGF-β, tumor necrosis factor
(TNF)-α, granulocyte colony-stimulating factor,
granulocyte-macrophage colony-stimulating factor and IL-1β],
chemokines (IL-8, growth regulated oncogene-α and monocyte
chemoattractant protein-1), and prostaglandin, including
prostaglandin E2, from fibroblasts, endothelial cells and several
other cell types (29–33). All of these cytokines, chemokines
and inflammatory cells are involved in the inflammatory procedure
(34,35). By contrast, these factors and
cytokines also promote the inflammatory response. Therefore,
changes in thymus function can affect the inflammatory
response.
Low-grade inflammation has been shown to be crucial
in the pathogenesis of hypertension and involved in several
processes that promote the development of blood pressure (36–39). Inflammatory factors in the process
of inflammation can cause endothelial damage and activate the renin
system, and studies have demonstrated that activation of the
intrarenal renin-angiotensin system (RAS) and endothelial
dysfunction are important in the development of hypertension
(40–42). Nitric oxide (NO) and superoxide
may cause endothelial dysfunction in hypertension, and the balance
between them may be more important than the absolute levels of
either alone (43,44). Other cross-sectional studies have
shown a correlation between C-reactive protein (CRP), TNF and IL-6
and essential hypertension (37,45–47). Elevation of the serum
concentrations of CRP and cytokines demonstrates that low-grade
inflammation is present in hypertension (48,49). The association between CRP and
systemic hypertension has been established in multiple
cross-sectional studies, particularly following the emergence of
the high sensitivity CRP assays capable of detecting levels that
were earlier considered to be normal (39,50–54). Higher levels of CRP may contribute
to the development of systemic hypertension by reducing the
production of NO in endothelial cells, increasing the production of
endothelin 1 and leading to vasoconstriction (39,55–57).
Evidence suggests that oxidative stress and
angiotensin II (Ang II) are critical in the pathogenesis of
hypertension and vascular endothelial dysfunction (43,44,58). Studies have shown that Ang II
induces severe inflammation and activates redox-sensitive genes via
the activation of nuclear factor (NF)-κB, independent of blood
pressure in double transgenic rats harboring human renin and human
angiotensinogen genes (43,59–61). It is well known that these cells,
inflammatory factors and oxidative stress are involved in the
process of target organ damage, as mentioned above (62–64). The thymus may be involved in the
process of hypertension and target organ damage by regulating the
inflammatory reaction.
In 1970, Ebringer and Doyle found that serum
immunoglobulin levels were significantly increased in 30% of
patients with hypertension, and with the rapid development of
clinical immunology, increasing evidence has shown that
hypertension is always accompanied with immune dysfunction and
there are immune factors involved in the complications of
hypertension (65–67). Several studies have shown that, in
hypertension, T cells can cause high blood pressure, vascular
disorders and kidney disease, and the possible mechanisms include
the release of cytokines directly affecting vascular and renal
function or indirectly stimulating cells to release cytokines
(68). The thymus is an important
and essential site for the generation and maturation of T cells
in vivo, as this microenvironment induces and supports
lineage commitment, differentiation and the survival of
thymus-seeding cells.
T cells and their subsets are involved, either
directly or through the secretion of certain factors, in the
process of hypertension. The selection of one of the T cells
subsets, Tregs, in the thymus is essential for preventing
autoimmune diseases (69). Tregs
of the CD4+CD25+FOXP3+ phenotype
are generated in the thymus, and are critical for the maintenance
of immune homeostasis and the suppression of naturally occurring
self-reactive T cells (70–72). Previously, it was shown that
changes in the immune system are important in the SHR model and
other models of hypertension; T cell activation and vascular
inflammation may contribute to the formation of high blood pressure
(48,73–76).
There is emerging evidence suggesting that the
immune response is significant in the pathogenesis of hypertension
(77–79). The classical immune system is
considered to consist of two parts: Innate and adaptive immunity
(77,80–82). Innate immune responses mediated by
macrophages are triggered through toll-like receptors. In animal
models of hypertension, the infiltration of inflammatory cells from
the innate immune system, including dendritic cells (DCs), natural
killer cells and predominantly monocytes/macrophages has been
documented in the perivascular fat and adventitia of blood vessels,
and in target organs including the kidney and heart (77,83). Various studies have suggested a
role for macrophages in the pathogenesis of hypertension and
vascular damage (84–87). Macrophages generate superoxide
when NADPH oxidase is activated by Ang II or mineralocorticoids,
and this may lead to vascular wall remodeling and contribute to
blood pressure (BP) elevation (49,88). Therefore, the role of
monocytes/macrophages has been expanded (89–91).
Adaptive immune responses are characterized by
activated lymphocytes, and interact with innate immunity in the
pathophysiology of cardiovascular disease and hypertension
(92–94). Several studies have confirmed that
T cells and their subsets, including effector T lymphocytes and
Treg cells, are involved in the process of hypertension (95–97). Rodriguez-Iturbe and Johnson
(98) showed that T lymphocytes
contribute to renal changes and BP elevation in rodents, which
others have since confirmed and extended (99). It has been reported that
T-effector cells that mediate, in part, the pressor effects of Ang
II are predominantly CD8+ rather than CD4+
(100). Studies have shown that
immune cells are involved in target organ damage caused by
hypertension (63,96). Possible mechanisms for their
involvement in the process of hypertension have been investigated
previously. The change in thymus function can affect the function
of macrophages and B cells. The monocyte-macrophage system is
crucial in innate immunity and in the initiation of the adaptive
immune response (101–103). Plasma cells derived from B cells
are involved in the humoral immune response. In addition, DCs are
significant in establishing self-tolerance and inducing
antigen-specific immunity through their ability to present
self-antigens to developing T cells in the thymus (104–106). Therefore, the thymus may be
involved in the process of hypertension via the immune system.
Inflammation is associated with several hypertensive
models in the kidney, including two-kidney-one-clip hypertension
and salt-sensitive hypertension (107). T lymphocytes and macrophages
infiltrate the kidneys in various models of hypertension (108–111). Changes of thymus function can
also affect the function of macrophages and T lymphocytes;
therefore, changes in thymus function can affect kidney function.
The effects of RAS activation on kidney function and its role in
hypertension have been investigated extensively (112). Here discusses the role of immune
cells, induced by changes in thymus function in the kidney, in
hypertension.
As early as 1964, the injection of kidney extract in
normal rat hypertension confirmed the role of autoimmunity in the
renal infarct model (113). It
was observed that the infiltration of immune-cells and increased
activity of NF-κB in the kidney occurred at a prehypertensive age
and progressively increased with age in the SHR model, which was
inhibited by a broad-spectrum inhibitor, phyrrolidine
dithiocarbamate (114,115). Other studies have shown that
hypertension induced by Ang II or high salt can lead to the
activation of T cells and the subsequent entry of activated T cells
into the peripheral blood vessels and kidney (96,116). Studies have also shown that the
cells which accumulate in the kidneys and blood vessel release
pro-inflammatory cytokines, and promote vasoconstriction and sodium
retention, leading to high BP (64,96,117,118).
The ANS, which is composed of sympathetic and
parasympathetic (vagal) innervation of the heart and predominantly
sympathetic innervation of the vascular system, controls and
regulates the secretion and activity of various organs, blood
vessels, smooth muscles and glands, and is involved in the
endocrine regulation of glucose, fat and fluid, electrolyte
metabolism, body temperature, sleep and BP (121,122). There is evidence that the ANS is
key in regulating the immune system (123–127), and there is substantial evidence
that the thymus receives dense sympathetic innervation, which
originates from postganglionic neurons in the upper paravertebral
ganglia of the sympathetic chain, particularly the superior
cervical and stellate ganglia (128–131). The importance of the ANS has
been recognized to be of main clinical and therapeutic significance
in the progression of chronic cardiovascular disease, primarily as
a result of changes in the immune system (132–134). Previous studies have shown that
thymocytes and thymic epithelial cells express functional
adrenergic receptors (135,136). Norepinephrine (NE) released from
the sympathetic nervous system (SNS) can influence immune responses
and innervate the thymus (137,138). Thymus involution is associated
with increased noradrenergic nerve fiber density and NE
concentration, accompanied with immunosuppression in male rats and
mice (139). Several studies
have also shown that the effect of the SNS on thymic cell
maturation and development is the outcome of multiple interactions
between sympathetic and other neurotransmitters and the endocrine
system, and may also depend on the immunological status of the
host, under physiological conditions (22,131,140,141).
The SNS is also activated in hypertension,
influencing renal perfusion and oxygenation (142). In the majority of studies in
humans and animal models of hypertension, drugs and interventions
can reduce BP and prolong survival rates by activating the
parasympathetic nervous system (vagal), by blocking or inhibiting
the SNS and the RAS (143–149). Early studies have shown that
experimental lesioning of specific circumventricular organs of the
forebrain, including the subfornical organ, the anteroventral third
ventricle region involving the inferior aspects of the lateral
terminalis, prevents the formation of several forms of experimental
high BP (150,151). Increasing evidence indicates
that the cardiovascular damage caused by overstimulation of the SNS
and RAS, their α- and β-adrenergic receptors, and Ang II AT1
receptors is mediated through proinflammatory activation of the
immune system (152–155).
Resistant hypertension refers to the case of patient
BP remaining >140/90 mmHg following the use of a variety of
antihypertensive drugs (156–158). Traditional treatments are not
effective, therefore, it is necessary to develop novel therapeutic
approaches, and highly selective renal denervation (RDN) is one of
these approaches (159–161). RDN, a catheter-based approach
developed to disrupt the renal sympathetic nerves using
radiofrequency energy, is a promising therapy for resistant
hypertension (142). Studies
have shown that the efficacy of RDN in different models of
hypertension requires examination as a method that matches the
causal mechanisms of the hypertension (161–165). Reported for the first time in
2009, the ablation of renal artery denervation technology, as a
sympathetic nerve and RAS activity non-drug block technique, has
been successfully applied to the clinical treatment of resistant
hypertension (166). In a
relatively small number of patients, the first clinical study
showed that this technique appeared to be safe and effective
(166–168). Systolic BP and diastolic BP were
reduced by 22/11 and 27/17 mmHg at 6 and 12 months post-RDN,
respectively, and no serious adverse events had occurred at the
follow-up at 1 year. Worthley et al (169) adopted a single electrode
radiofrequency ablation catheter for RDN treatment in a
prospective, multicenter, nonrandomized cohort study, which showed
that the patient BP was also significantly reduced at 6 months by
26/10 mmHg, compared with preoperative BP. The early stage of the
preliminary results of transcatheter renal artery ablation
treatment technology show it can safely and effectively reduce BP
levels in patients with resistant hypertension. Consequently, it is
suggested that RDN decreases sympathetic activity and may
potentially improve renal oxygenation, resulting in altered sodium
handling by the kidneys and a decrease in peripheral vascular
resistance, thereby removing the trigger for hypertension.
Taken together, the ANS may be involved in the
process of hypertension by regulating the function of the
thymus.
The thymus is the main immune organ capable of
generating T cells throughout life, and is crucial for the
development, selection and maintenance of peripheral T cells. It is
well documented that aging leads to an increase in infection and
mortality rate, which has a negative impact on the immune response.
Aging reduces immune function, partly due to thymic involution
leading to a marked loss of progenitors, epithelial cells and
differentiating thymocytes, causing a decline in the production of
naïve T cells by the thymus (170–176).
The thymus transcription factor forkhead box N1
(Foxn1) is the most important factor for complete physiological
function of the thymus (174,177–179). With atrophy of the thymus, the
expression of the thymus aging-associated gene Foxn1 decreases,
which leads to the downregulation of Foxn1 with age. Increasing the
expression of Foxn1 can improve the function of the thymus, and
even promote regeneration of the thymus by increasing the
expression of Foxn1 (180).
Žuklys et al (181)
reported that Foxn1 regulates the expression of genes involved in
antigen processing and thymocyte selection, in addition to the
transcriptional control of genes involved in the attraction and
lineage commitment of T cell precursors. Therefore, there is
evidence to suggest that the thymus Foxn1 may be involved in the
process of high BP. In previous studies, the atrophy of thymus
organs in hypertensive mice has been confirmed, however, the
specific change in thymus function remains to be fully
elucidated.
Thymosin β4 (Tβ4), a peptide of 43 amino acids first
identified by extraction from the calf thymus, is the most abundant
member of the highly conserved β-thymosin family (182,183). It is part of the thymosin
fraction 5, a partially purified thymic preparation, which is
involved in thymus-dependent lymphocyte regulation (184,185). The functions of Tβ4 include the
involvement of thymus-dependent lymphocyte maturation and a variety
of cellular processes, including cell migration, chemotaxis,
maintenance of cell shape and cell division (186–189). It is well documented that Tβ4
can prevent inflammation and fibrosis, promoting healing in the
eye, skin, and heart, and can control cell morphogenesis and
motility by regulating the dynamics of the actin cytoskeleton
(190–193). In addition, Tβ4 can promote
repair and reduce late fibrosis in kidney injury, and is increased
in vascular, tubulointerstitial and myocardial fibrosis (194–199). Tβ4 can also enhance endothelial
cell differentiation and angiogenesis (200).
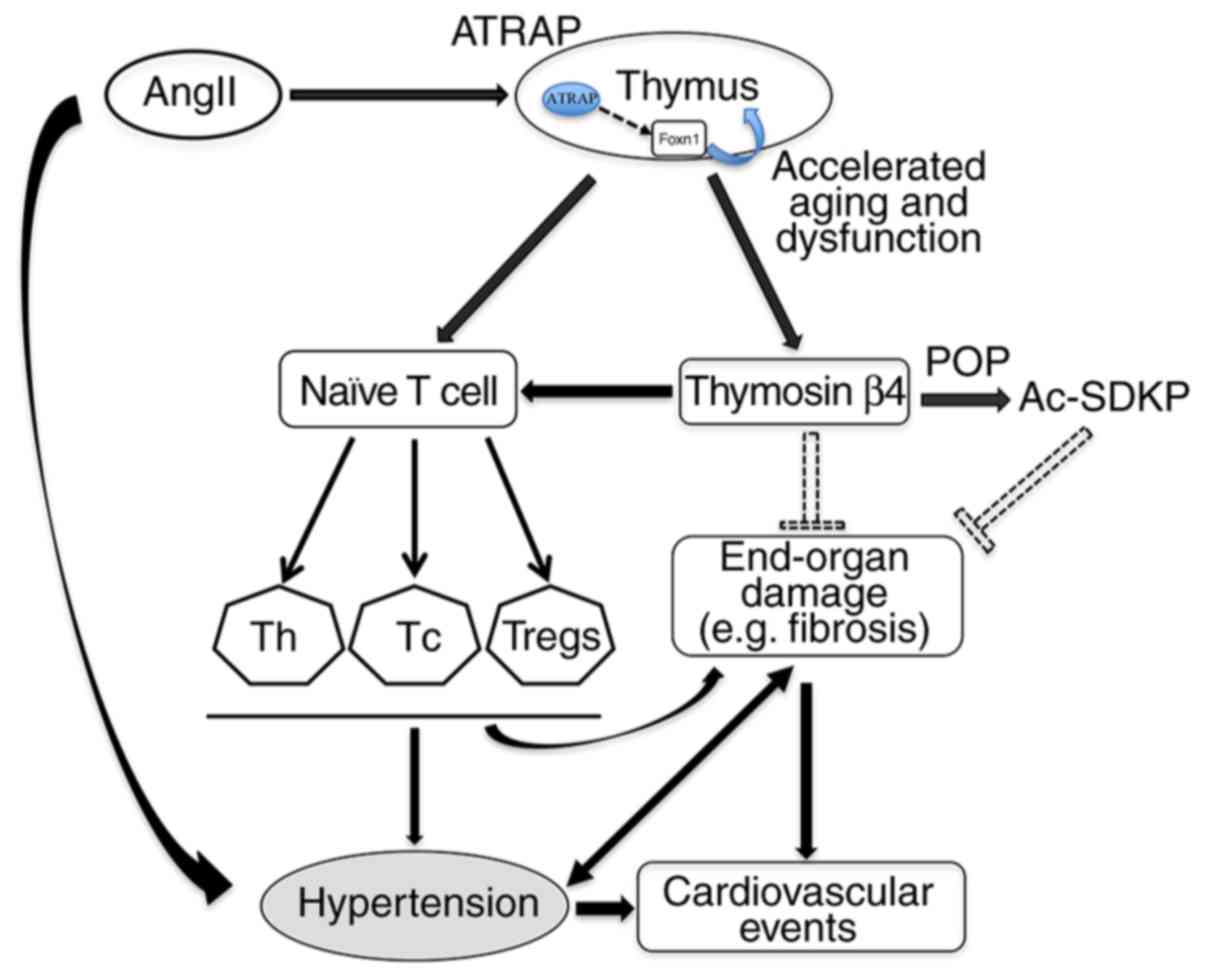
According to the above findings and previous
studies, it appears that the expression of Ang II type 1
receptor-associated protein (ATRAP) is downregulated, due to the
increase of Ang II, resulting in a decline in function of the
thymus and a decrease in expression of the thymus transcription
factor Foxn1, causing an imbalance of T cell subsets. ATRAP is a
transmembrane protein localized in intracellular trafficking
vesicles and the plasma membrane (215,216). In addition, the secretion of
thymosin prevents target organ damage and fibrosis, which slows or
reverses the process of hypertension. Tβ4 is decomposed into
Ac-SDKP under the action of POP, which can reduce the damage to the
target organ, ameliorating or improving BP. Based on the above, a
proposed mechanism of thymic function involved in the process of
hypertension has been hypothesized (Fig. 2). The change in thymus function
provides a novel target for the treatment of hypertension.
Inflammatory and immune system mechanisms are
crucial in the pathophysiology of hypertension and cardiovascular
disease. T lymphocytes mature in the thymus and are important in
the inflammatory response and the immune response, which can induce
hypertension. The important mechanism for regulating the
inflammatory response involves tissue and circulating leukocytes
and macrophages. T lymphocytes are involved in the pathogenesis of
hypertensive vascular remodeling. An imbalance between Tregs and T
effector lymphocytes may be the cause of elevated BP and the
progression of vascular damage.
T lymphocytes and macrophages infiltrate the kidneys
in various models of hypertension. The aggregation of inflammatory
factors, complement and immune response in the kidney and renal
vascular injury can cause hypertension. The ANS is involved in the
process of hypertension by modulating the immune response.
Based on previous studies, the changes in thymus
function appear to have an effect on the process of hypertension.
The proposed mechanism underlying the involvement of the thymus in
the process of hypertension is as follows: Ang II may affect the
function of the thymus and expression of the thymus transcription
factor Foxn1 through the downregulated expression of ATRAP, and
then affect the balance of T lymphocytes, which causes endothelial
dysfunction and target organ damage, including fibrosis, thereby
leading to hypertension.
In conclusion, novel data increasingly suggests the
potential for novel targets involved in thymus function for
therapeutic intervention to modify the course and reduce events in
cardiovascular disease and hypertension, as evidence has
increasingly implicated thymus-related mechanisms. Further
investigations on the changes of thymus function are likely to
assist in the development of novel therapeutic targets that may
improve outcomes in hypertension and cardiovascular disease, and
assist in identifying novel approaches for the treatment of
hypertension and vascular disease.
Not applicable.
|
1
|
Cavasin MA, Liao TD, Yang XP, Yang JJ and
Carretero OA: Decreased endogenous levels of Ac-SDKP promote organ
fibrosis. Hypertension. 50:130–136. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Neutel JM, Giles TD, Punzi H, Weiss RJ, Li
H and Finck A: Long-term safety of nebivolol and valsartan
combination therapy in patients with hypertension: An open-label,
single-arm, multicenter study. J Am Soc Hypertens. 8:915–920. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Safaeian L, Hajhashemi V, Haghjoo
Javanmard S and Sanaye Naderi H: The effect of protocatechuic acid
on blood pressure and oxidative stress in glucocorticoid-induced
hypertension in rat. Iran J Pharm Res. 15(Suppl): S83–S91.
2016.
|
|
4
|
Chmielewski V, Drupt F and Morfin R:
Dexamethasone-induced apoptosis of mouse thymocytes: Prevention by
native 7alpha-hydroxysteroids. Immunol Cell Biol. 78:238–246. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Walters SN, Webster KE, Daley S and Grey
ST: A role for intrathymic B cells in the generation of natural
regulatory T cells. J Immunol. 193:170–176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lynch HE, Goldberg GL, Chidgey A, Van den
Brink MR, Boyd R and Sempowski GD: Thymic involution and immune
reconstitution. Trends Immunol. 30:366–373. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Murray JM, Kaufmann GR, Hodgkin PD, Lewin
SR, Kelleher AD, Davenport MP and Zaunders JJ: Naive T cells are
maintained by thymic output in early ages but by proliferation
without phenotypic change after age twenty. Immunol Cell Biol.
81:487–495. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ruan L, Zhang Z, Mu L, Burnley P, Wang L,
Coder B, Zhuge Q and Su DM: Biological significance of FoxN1
gain-of-function mutations during T and B lymphopoiesis in juvenile
mice. Cell Death Dis. 5:e14572014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fukuda S, Tsuchikura S and Iida H:
Age-related changes in blood pressure, hematological values,
concentrations of serum biochemical constituents and weights of
organs in the SHR/Izm, SHRSP/Izm and WKY/Izm. Exp Anim. 53:67–72.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodriguez-Iturbe B and Johnson RJ: The
role of renal microvascular disease and interstitial inflammation
in salt-sensitive hypertension. Hypertens Res. 33:975–980. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Svendsen UG: The importance of thymus in
the pathogenesis of the chronic phase of hypertension in mice
following partial infarction of the kidney. Acta Pathol Microbiol
Scand A. 85:539–547. 1977.PubMed/NCBI
|
|
12
|
Svendsen UG: The effect of penicillamine
on blood pressure and vascular disease in mice with infarct-kidney
hypertension. Scand J Rheumatol. 8:81–86. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Svendsen UG: The role of thymus for the
development and prognosis of hypertension and hypertensive vascular
disease in mice following renal infarction. Acta Pathol Microbiol
Scand A. 84:235–243. 1976.PubMed/NCBI
|
|
14
|
Ba D, Takeichi N, Kodama T and Kobayashi
H: Restoration of T cell depression and suppression of blood
pressure in spontaneously hypertensive rats (SHR) by thymus grafts
or thymus extracts. J Immunol. 128:1211–1216. 1982.PubMed/NCBI
|
|
15
|
Bento-de-Souza L, Victor JR,
Bento-de-Souza LC, Arrais-Santos M, Rangel-Santos AC, Pereira-Costa
É, Raniero-Fernandes E, Seixas-Duarte MI, Oliveira-Filho JB and
Silva Duarte AJ: Constitutive expression of genes encoding notch
receptors and ligands in developing lymphocytes, nTreg cells and
dendritic cells in the human thymus. Results Immunol. 6:15–20.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Plum J, De Smedt M, Leclercq G, Taghon T,
Kerre T and Vandekerckhove B: Human intrathymic development: A
selective approach. Semin Immunopathol. 30:411–423. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu Z, Lancaster JN and Ehrlich LI: The
contribution of chemokines and migration to the induction of
central tolerance in the Thymus. Front Immunol. 6:3982015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu Z, Lancaster JN, Sasiponganan C and
Ehrlich LI: CCR4 promotes medullary entry and thymocyte-dendritic
cell interactions required for central tolerance. J Exp Med.
212:1947–1965. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Love PE and Bhandoola A: Signal
integration and crosstalk during thymocyte migration and
emigration. Nat Rev Immunol. 11:469–477. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Richards DM, Delacher M, Goldfarb Y,
Kägebein D, Hofer AC, Abramson J and Feuerer M: Treg cell
differentiation: From Thymus to peripheral tissue. Prog Mol Biol
Transl Sci. 136:175–205. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abbas AK, Benoist C, Bluestone JA,
Campbell DJ, Ghosh S, Hori S, Jiang S, Kuchroo VK, Mathis D,
Roncarolo MG, et al: Regulatory T cells: Recommendations to
simplify the nomenclature. Nat Immunol. 14:307–308. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bod L, Douguet L, Auffray C, Lengagne R,
Bekkat F, Rondeau E, Molinier-Frenkel V, Castellano F, Richard Y
and Prévost-Blondel A: IL-4-induced gene 1: A negative immune
checkpoint controlling B cell differentiation and activation. J
Immunol. 200:1027–1038. 2018. View Article : Google Scholar
|
|
23
|
McLeod JJ, Baker B and Ryan JJ: Mast cell
production and response to IL-4 and IL-13. Cytokine. 75:57–61.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang X, Voskens CJ, Sallin M, Maniar A,
Montes CL, Zhang Y, Lin W, Li G, Burch E, Tan M, et al: CD137
promotes proliferation and survival of human B cells. J Immunol.
184:787–795. 2010. View Article : Google Scholar
|
|
25
|
Nilsson G and Nilsson K: Effects of
interleukin (IL)-13 on immediate-early response gene expression,
phenotype and differentiation of human mast cells. Comparison with
IL-4. Eur J Immunol. 25:870–873. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Groves AM, Johnston CJ, Misra RS, Williams
JP and Finkelstein JN: Effects of IL-4 on pulmonary fibrosis and
the accumulation and phenotype of macrophage subpopulations
following thoracic irradiation. Int J Radiat Biol. 92:754–765.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Francos-Quijorna I, Amo-Aparicio J,
Martinez-Muriana A and López-Vales R: IL-4 drives microglia and
macrophages toward a phenotype conducive for tissue repair and
functional recovery after spinal cord injury. Glia. 64:2079–2092.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Czimmerer Z, Varga T, Kiss M, Vázquez CO,
Doan-Xuan QM, Rückerl D, Tattikota SG, Yan X, Nagy ZS, Daniel B, et
al: The IL-4/STAT6 signaling axis establishes a conserved microRNA
signature in human and mouse macrophages regulating cell survival
via miR-342-3p. Genome Med. 8:632016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miossec P, Korn T and Kuchroo VK:
Interleukin-17 and type 17 helper T cells. N Engl J Med.
361:888–898. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Veldhoen M, Hocking RJ, Atkins CJ,
Locksley RM and Stockinger B: TGFbeta in the context of an
inflammatory cytokine milieu supports de novo differentiation of
IL-17-producing T cells. Immunity. 24:179–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mangan PR, Harrington LE, O'Quinn DB,
Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR and
Weaver CT: Transforming growth factor-beta induces development of
the T(H)17 lineage. Nature. 441:231–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ivanov II, McKenzie BS, Zhou L, Tadokoro
CE, Lepelley A, Lafaille JJ, Cua DJ and Littman DR: The orphan
nuclear receptor RORgammat directs the differentiation program of
proinflammatory IL-17+ T helper cells. Cell.
126:1121–1133. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kolls JK and Lindén A: Interleukin-17
family members and inflammation. Immunity. 21:467–476. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mazidi M, Penson P, Gluba-Brzozka A, Rysz
J and Banach M: Relationship between long noncoding RNAs and
physiological risk factors of cardiovascular disease. J Clin
Lipidol. 11:617–623. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kaur J: A comprehensive review on
metabolic syndrome. Cardiol Res Pract. 2014:9431622014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ahbap E, Sakaci T, Kara E, Sahutoglu T,
Koc Y, Basturk T, Sevinc M, Akgol C, Hasbal B, Isleem M, et al:
Serum uric acid levels and inflammatory markers with respect to
dipping status: A retrospective analysis of hypertensive patients
with or without chronic kidney disease. Clin Exp Hypertens.
38:555–563. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Virdis A, Dell'Agnello U and Taddei S:
Impact of inflammation on vascular disease in hypertension.
Maturitas. 78:179–183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Taddei S, Caraccio N, Virdis A, Dardano A,
Versari D, Ghiadoni L, Ferrannini E, Salvetti A and Monzani F:
Low-grade systemic inflammation causes endothelial dysfunction in
patients with Hashimoto's thyroiditis. J Clin Endocrinol Metab.
91:5076–5082. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mirsaeidi M, Omar HR, Ebrahimi G and
Campos M: The association between ESR and CRP and systemic
hypertension in sarcoidosis. Int J Hypertens. 2016:24025152016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hezel M, Peleli M, Liu M, Zollbrecht C,
Jensen BL, Checa A, Giulietti A, Wheelock CE, Lundberg JO,
Weitzberg E and Carlström M: Dietary nitrate improves age-related
hypertension and metabolic abnormalities in rats via modulation of
angiotensin II receptor signaling and inhibition of superoxide
generation. Free Radic Biol Med. 99:87–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Victorio JA, Clerici SP, Palacios R,
Alonso MJ, Vassallo DV, Jaffe IZ, Rossoni LV and Davel AP:
Spironolactone prevents endothelial nitric oxide synthase
uncoupling and vascular dysfunction induced by β-adrenergic
overstimulation: Role of perivascular adipose tissue. Hypertension.
68:726–735. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Goto K, Fujii K, Onaka U, Abe I and
Fujishima M: Renin-angiotensin system blockade improves endothelial
dysfunction in hypertension. Hypertension. 36:575–580. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cheng ZJ, Vaskonen T, Tikkanen I, Nurminen
K, Ruskoaho H, Vapaatalo H, Muller D, Park JK, Luft FC and Mervaala
EM: Endothelial dysfunction and salt-sensitive hypertension in
spontaneously diabetic Goto-Kakizaki rats. Hypertension.
37:433–439. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
McIntyre M, Bohr DF and Dominiczak AF:
Endothelial function in hypertension: The role of superoxide anion.
Hypertension. 34:539–545. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Virdis A, Ghiadoni L, Plantinga Y, Taddei
S and Salvetti A: C-reactive protein and hypertension: Is there a
causal relationship. Curr Pharm Des. 13:1693–1698. 2007. View Article : Google Scholar
|
|
46
|
Bautista LE, Vera LM, Arenas IA and
Gamarra G: Independent association between inflammatory markers
(C-reactive protein, interleukin-6, and TNF-alpha) and essential
hypertension. J Hum Hypertens. 19:149–154. 2005. View Article : Google Scholar
|
|
47
|
Lakoski SG, Cushman M, Palmas W,
Blumenthal R, D'Agostino RB Jr and Herrington DM: The relationship
between blood pressure and C-reactive protein in the multi-ethnic
study of atherosclerosis (MESA). J Am Coll Cardiol. 46:1869–1874.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Schiffrin EL: Immune mechanisms in
hypertension and vascular injury. Clin Sci (Lond). 126:267–274.
2014. View Article : Google Scholar
|
|
49
|
Blake GJ, Rifai N, Buring JE and Ridker
PM: Blood pressure, C-reactive protein, and risk of future
cardiovascular events. Circulation. 108:2993–2999. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bermudez EA, Rifai N, Buring J, Manson JE
and Ridker PM: Interrelationships among circulating interleukin-6,
C-reactive protein, and traditional cardiovascular risk factors in
women. Arterioscler Thromb Vasc Biol. 22:1668–1673. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chae CU, Lee RT, Rifai N and Ridker PM:
Blood pressure and inflammation in apparently healthy men.
Hypertension. 38:399–403. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yamada S, Gotoh T, Nakashima Y, Kayaba K,
Ishikawa S, Nago N, Nakamura Y, Itoh Y and Kajii E: Distribution of
serum C-reactive protein and its association with atherosclerotic
risk factors in a Japanese population: Jichi medical school cohort
study. Am J Epidemiol. 153:1183–1190. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ford ES and Giles WH: Serum C-reactive
protein and fibrinogen concentrations and self-reported angina
pectoris and myocardial infarction: Findings from national health
and nutrition examination survey III. J Clin Epidemiol. 53:95–102.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rohde LE, Hennekens CH and Ridker PM:
Survey of C-reactive protein and cardiovascular risk factors in
apparently healthy men. Am J Cardiol. 84:1018–1022. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Venugopal SK, Devaraj S, Yuhanna I, Shaul
P and Jialal I: Demonstration that C-reactive protein decreases
eNOS expression and bioactivity in human aortic endothelial cells.
Circulation. 106:1439–1441. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Verma S, Li SH, Badiwala MV, Weisel RD,
Fedak PW, Li RK, Dhillon B and Mickle DA: Endothelin antagonism and
interleukin-6 inhibition attenuate the proatherogenic effects of
C-reactive protein. Circulation. 105:1890–1896. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Verma S, Wang CH, Li SH, Dumont AS, Fedak
PW, Badiwala MV, Dhillon B, Weisel RD, Li RK, Mickle DA and Stewart
DJ: A self-fulfilling prophecy: C-reactive protein attenuates
nitric oxide production and inhibits angiogenesis. Circulation.
106:913–919. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Romero JC and Reckelhoff JF:
State-of-the-Art lecture. Role of angiotensin and oxidative stress
in essential hypertension. Hypertension. 34:943–949. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mervaala E, Müller DN, Schmidt F, Park JK,
Gross V, Bader M, Breu V, Ganten D, Haller H and Luft FC: Blood
pressure-independent effects in rats with human renin and
angiotensinogen genes. Hypertension. 35:587–594. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Muller DN, Dechend R, Mervaala EM, Park
JK, Schmidt F, Fiebeler A, Theuer J, Breu V, Ganten D, Haller H and
Luft FC: NF-kappaB inhibition ameliorates angiotensin II-induced
inflammatory damage in rats. Hypertension. 35:193–201. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Müller DN, Mervaala EM, Dechend R,
Fiebeler A, Park JK, Schmidt F, Theuer J, Breu V, Mackman N, Luther
T, et al: Angiotensin II (AT(1)) receptor blockade reduces vascular
tissue factor in angiotensin II-induced cardiac vasculopathy. Am J
Pathol. 157:111–122. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ji Q, Cheng G, Ma N, Huang Y, Lin Y, Zhou
Q, Que B, Dong J, Zhou Y and Nie S: Circulating Th1, Th2, and Th17
levels in hypertensive patients. Dis Markers. 2017:71462902017.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
McMaster WG, Kirabo A, Madhur MS and
Harrison DG: Inflammation, immunity, and hypertensive end-organ
damage. Circ Res. 116:1022–1033. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Madhur MS, Lob HE, McCann LA, Iwakura Y,
Blinder Y, Guzik TJ and Harrison DG: Interleukin 17 promotes
angiotensin II-induced hypertension and vascular dysfunction.
Hypertension. 55:500–507. 2010. View Article : Google Scholar :
|
|
65
|
De Ciuceis C, Rossini C, La Boria E,
Porteri E, Petroboni B, Gavazzi A, Sarkar A, Rosei EA and Rizzoni
D: Immune mechanisms in hypertension. High Blood Press Cardiovasc
Prev. 21:227–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Dai X, Huang S, He Z, Wu F, Ding R, Chen
Y, Liang C and Wu Z: Dysfunction of the thymus in mice with
hypertension. Exp Ther Med. 13:1386–1392. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ebringer A and Doyle AE: Raised serum IgG
levels in hypertension. Br Med J. 2:146–148. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Leibowitz A and Schiffrin EL: Immune
mechanisms in hypertension. Curr Hypertens Rep. 13:465–472. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lin J, Yang L, Silva HM, Trzeciak A, Choi
Y, Schwab SR, Dustin ML and Lafaille JJ: Increased generation of
Foxp3(+) regulatory T cells by manipulating antigen presentation in
the thymus. Nat Commun. 7:105622016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wing K and Sakaguchi S: Regulatory T cells
exert checks and balances on self tolerance and autoimmunity. Nat
Immunol. 11:7–13. 2010. View Article : Google Scholar
|
|
71
|
Mellanby RJ, Thomas DC and Lamb J: Role of
regulatory T-cells in autoimmunity. Clin Sci (Lond). 116:639–649.
2009. View Article : Google Scholar
|
|
72
|
Piccirillo CA, d'Hennezel E, Sgouroudis E
and Yurchenko E: CD4+Foxp3+ regulatory T
cells in the control of autoimmunity: In vivo veritas. Curr Opin
Immunol. 20:655–662. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Takeichi N, Suzuki K and Kobayashi H:
Characterization of immunological depression in spontaneously
hypertensive rats. Eur J Immunol. 11:483–487. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Olsen F: Transfer of arterial hypertension
by splenic cells from DOCA-salt hypertensive and renal hypertensive
rats to normotensive recipients. Acta Pathol Microbiol Scand C.
88:1–5. 1980.PubMed/NCBI
|
|
75
|
Takeichi N, Suzuki K, Okayasu T and
Kobayashi H: Immunological depression in spontaneously hypertensive
rats. Clin Exp Immunol. 40:120–126. 1980.PubMed/NCBI
|
|
76
|
Svendsen UG: Evidence for an initial,
thymus independent and a chronic, thymus dependent phase of DOCA
and salt hypertension in mice. Acta Pathol Microbiol Scand A.
84:523–528. 1976.PubMed/NCBI
|
|
77
|
Marvar PJ, Vinh A, Thabet S, Lob HE, Geem
D, Ressler KJ and Harrison DG: T lymphocytes and vascular
inflammation contribute to stress-dependent hypertension. Biol
Psychiatry. 71:774–782. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Harrison DG, Guzik TJ, Lob HE, Madhur MS,
Marvar PJ, Thabet SR, Vinh A and Weyand CM: Inflammation, immunity,
and hypertension. Hypertension. 57:132–140. 2011. View Article : Google Scholar
|
|
79
|
Muller DN, Kvakan H and Luft FC:
Immune-related effects in hypertension and target-organ damage.
Curr Opin Nephrol Hypertens. 20:113–117. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Calame DG, Mueller-Ortiz SL and Wetsel RA:
Innate and adaptive immunologic functions of complement in the host
response to Listeria monocytogenes infection. Immunobiology.
221:1407–1417. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
D'Alincourt Salazar M, Manuel ER, Tsai W,
D'Apuzzo M, Goldstein L, Blazar BR and Diamond DJ: Evaluation of
innate and adaptive immunity contributing to the antitumor effects
of PD1 blockade in an orthotopic murine model of pancreatic cancer.
Oncoimmunology. 5:e11601842016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Weyd H: More than just innate affairs-on
the role of annexins in adaptive immunity. Biol Chem.
397:1017–1029. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Kvakan H, Luft FC and Muller DN: Role of
the immune system in hypertensive target organ damage. Trends
Cardiovasc Med. 19:242–246. 2009. View Article : Google Scholar
|
|
84
|
Vergaro G, Prud'homme M, Fazal L, Merval
R, Passino C, Emdin M, Samuel JL, Cohen Solal A and Delcayre C:
Inhibition of Galectin-3 pathway prevents isoproterenol-induced
left ventricular dysfunction and fibrosis in mice. Hypertension.
67:606–612. 2016.PubMed/NCBI
|
|
85
|
Wenzel U, Turner JE, Krebs C, Kurts C,
Harrison DG and Ehmke H: Immune mechanisms in arterial
hypertension. J Am Soc Nephrol. 27:677–686. 2016. View Article : Google Scholar :
|
|
86
|
Harrison DG: The immune system in
hypertension. Trans Am Clin Climatol Assoc. 125:130–140.
2014.PubMed/NCBI
|
|
87
|
Kossmann S, Hu H, Steven S, Schönfelder T,
Fraccarollo D, Mikhed Y, Brahler M, Knorr M, Brandt M, Karbach SH,
et al: Inflammatory monocytes determine endothelial nitric-oxide
synthase uncoupling and nitro-oxidative stress induced by
angiotensin II. J Biol Chem. 289:27540–27550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Liu J, Yang F, Yang XP, Jankowski M and
Pagano PJ: NAD(P)H oxidase mediates angiotensin II-induced vascular
macrophage infiltration and medial hypertrophy. Arterioscler Thromb
Vasc Biol. 23:776–782. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wenzel P, Knorr M, Kossmann S, Stratmann
J, Hausding M, Schuhmacher S, Karbach SH, Schwenk M, Yogev N,
Schulz E, et al: Lysozyme M-positive monocytes mediate angiotensin
II-induced arterial hypertension and vascular dysfunction.
Circulation. 124:1370–1381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ko EA, Amiri F, Pandey NR, Javeshghani D,
Leibovitz E, Touyz RM and Schiffrin EL: Resistance artery
remodeling in deoxycorticosterone acetate-salt hypertension is
dependent on vascular inflammation: Evidence from m-CSF-deficient
mice. Am J Physiol Heart Circ Physiol. 292:H1789–H1795. 2007.
View Article : Google Scholar
|
|
91
|
De Ciuceis C, Amiri F, Brassard P,
Endemann DH, Touyz RM and Schiffrin EL: Reduced vascular
remodeling, endothelial dysfunction, and oxidative stress in
resistance arteries of angiotensin II-infused macrophage
colony-stimulating factor-deficient mice: Evidence for a role in
inflammation in angiotensin-induced vascular injury. Arterioscler
Thromb Vasc Biol. 25:2106–2113. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Abais-Battad JM, Rudemiller NP and Mattson
DL: Hypertension and immunity: Mechanisms of T cell activation and
pathways of hypertension. Curr Opin Nephrol Hypertens. 24:470–474.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Schiffrin EL: The immune system: Role in
hypertension. Can J Cardiol. 29:543–548. 2013. View Article : Google Scholar
|
|
94
|
Verlohren S, Muller DN, Luft FC and
Dechend R: Immunology in hypertension, preeclampsia, and
target-organ damage. Hypertension. 54:439–443. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Idris-Khodja N, Mian MO, Paradis P and
Schiffrin EL: Dual opposing roles of adaptive immunity in
hypertension. Eur Heart J. 35:1238–1244. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Guzik TJ, Hoch NE, Brown KA, McCann LA,
Rahman A, Dikalov S, Goronzy J, Weyand C and Harrison DG: Role of
the T cell in the genesis of angiotensin II induced hypertension
and vascular dysfunction. J Exp Med. 204:2449–2460. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zhang W and Victor RG: Calcineurin
inhibitors cause renal afferent activation in rats: A novel
mechanism of cyclosporine-induced hypertension. Am J Hypertens.
13:999–1004. 2000. View Article : Google Scholar
|
|
98
|
Rodríguez-Iturbe B, Pons H, Quiroz Y,
Gordon K, Rincón J, Chávez M, Parra G, Herrera-Acosta J,
Gómez-Garre D, Largo R, et al: Mycophenolate mofetil prevents
salt-sensitive hypertension resulting from angiotensin II exposure.
Kidney Int. 59:2222–2232. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Crowley SD, Frey CW, Gould SK, Griffiths
R, Ruiz P, Burchette JL, Howell DN, Makhanova N, Yan M, Kim HS, et
al: Stimulation of lymphocyte responses by angiotensin II promotes
kidney injury in hypertension. Am J Physiol Renal Physiol.
295:F515–F524. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wei Z, Spizzo I, Diep H, Drummond GR,
Widdop RE and Vinh A: Differential phenotypes of
tissue-infiltrating T cells during angiotensin II-induced
hypertension in mice. PLoS One. 9:e1148952014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Rosenthal AS: Regulation of the immune
response-role of the macrophage. N Engl J Med. 303:1153–1156. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Gordon S: The role of the macrophage in
immune regulation. Res Immunol. 149:685–688. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Lam RS, O'Brien-Simpson NM, Holden JA,
Lenzo JC and Fong SB: Reynolds EC. Unprimed, M1 and M2 macrophages
differentially interact with porphyromonas gingivalis. PLoS One.
11:e01586292016. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Mellman I and Steinman RM: Dendritic
cells: Specialized and regulated antigen processing machines. Cell.
106:255–258. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Goldschneider I and Cone RE: A central
role for peripheral dendritic cells in the induction of acquired
thymic tolerance. Trends Immunol. 24:77–81. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Oh J and Shin JS: The role of dendritic
cells in central tolerance. Immune Netw. 15:111–120. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Gelosa P, Pignieri A, Gianazza E, Criniti
S, Guerrini U, Cappellini MD, Banfi C, Tremoli E and Sironi L:
Altered iron homeostasis in an animal model of hypertensive
nephropathy: Stroke-prone rats. J Hypertens. 31:2259–2269. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Singh MV, Chapleau MW, Harwani SC and
Abboud FM: The immune system and hypertension. Immunol Res.
59:243–253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Rudemiller N, Lund H, Jacob HJ, Geurts AM
and Mattson DL: PhysGen Knockout Program: CD247 modulates blood
pressure by altering T-lymphocyte infiltration in the kidney.
Hypertension. 63:559–564. 2014. View Article : Google Scholar
|
|
110
|
Luft FC, Dechend R and Muller DN: Immune
mechanisms in angiotensin II-induced target-organ damage. Ann Med.
44(Suppl 1): S49–S54. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Rodríguez-Iturbe B, Franco M, Tapia E,
Quiroz Y and Johnson RJ: Renal inflammation, autoimmunity and
salt-sensitive hypertension. Clin Exp Pharmacol Physiol. 39:96–103.
2012. View Article : Google Scholar
|
|
112
|
Moon JY: Recent update of
renin-angiotensin-aldosterone system in the pathogenesis of
hypertension. Electrolyte Blood Press. 11:41–45. 2013. View Article : Google Scholar
|
|
113
|
White FN and Grollman A: Autoimmune
factors associated with infarction of the kidney. Nephron.
1:93–102. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Rodríguez-Iturbe B, Quiroz Y, Ferrebuz A,
Parra G and Vaziri ND: Evolution of renal interstitial inflammation
and NF-kappaB activation in spontaneously hypertensive rats. Am J
Nephrol. 24:587–594. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Rodríguez-Iturbe B, Ferrebuz A, Vanegas V,
Quiroz Y, Mezzano S and Vaziri ND: Early and sustained inhibition
of nuclear factor-kappaB prevents hypertension in spontaneously
hypertensive rats. J Pharmacol Exp Ther. 315:51–57. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Marvar PJ, Gordon FJ and Harrison DG:
Blood pressure control: Salt gets under your skin. Nat Med.
15:487–488. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Crowley SD, Song YS, Lin EE, Griffiths R,
Kim HS and Ruiz P: Lymphocyte responses exacerbate angiotensin
II-dependent hypertension. Am J Physiol Regul Integr Comp Physiol.
298:R1089–R1097. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
De Miguel C, Das S, Lund H and Mattson DL:
T lymphocytes mediate hypertension and kidney damage in Dahl
salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol.
298:R1136–R1142. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Yang T, Zollbrecht C, Winerdal ME, Zhuge
Z, Zhang XM, Terrando N, Checa A, Sällström J, Wheelock CE,
Winqvist O, et al: Genetic abrogation of adenosine A3 receptor
prevents uninephrectomy and high salt-induced hypertension. J Am
Heart Assoc. 5:e0038682016. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Dong L, Nordlohne J, Ge S, Hertel B, Melk
A, Rong S, Haller H and von Vietinghoff S: T Cell CX3CR1 Mediates
excess atherosclerotic inflammation in renal impairment. J Am Soc
Nephrol. 27:1753–1764. 2016. View Article : Google Scholar :
|
|
121
|
Lucchini M, Fifer WP, Sahni R and
Signorini MG: Novel heart rate parameters for the assessment of
autonomic nervous system function in premature infants. Physiol
Meas. 37:1436–1446. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Reijman S, Bakermans-Kranenburg MJ,
Hiraoka R, Crouch JL, Milner JS, Alink LR and van IJzendoorn MH:
Baseline functioning and stress reactivity in maltreating parents
and at-risk adults: Review and meta-analyses of autonomic nervous
system studies. Child Maltreat. 1077559516659937. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Olofsson PS, Rosas-Ballina M, Levine YA
and Tracey KJ: Rethinking inflammation: Neural circuits in the
regulation of immunity. Immunol Rev. 248:188–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Marvar PJ, Thabet SR, Guzik TJ, Lob HE,
McCann LA, Weyand C, Gordon FJ and Harrison DG: Central and
peripheral mechanisms of T-lymphocyte activation and vascular
inflammation produced by angiotensin II-induced hypertension. Circ
Res. 107:263–270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Ganta CK, Lu N, Helwig BG, Blecha F, Ganta
RR, Zheng L, Ross CR, Musch TI, Fels RJ and Kenney MJ: Central
angiotensin II-enhanced splenic cytokine gene expression is
mediated by the sympathetic nervous system. Am J Physiol Heart Circ
Physiol. 289:H1683–H1691. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Borovikova LV, Ivanova S, Zhang M, Yang H,
Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW and Tracey
KJ: Vagus nerve stimulation attenuates the systemic inflammatory
response to endotoxin. Nature. 405:458–462. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Ader R, Felten D and Cohen N: Interactions
between the brain and the immune system. Annu Rev Pharmacol
Toxicol. 30:561–602. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Bulloch K and Pomerantz W: Autonomic
nervous system innervation of thymic-related lymphoid tissue in
wildtype and nude mice. J Comp Neurol. 228:57–68. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Nance DM, Hopkins DA and Bieger D:
Re-investigation of the innervation of the thymus gland in mice and
rats. Brain Behav Immun. 1:134–147. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Tollefson L and Bulloch K: Dual-label
retrograde transport: CNS innervation of the mouse thymus distinct
from other mediastinum viscera. J Neurosci Res. 25:20–28. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Roggero E, Besedovsky HO and del Rey A:
The role of the sympathetic nervous system in the thymus in health
and disease. Neuroimmunomodulation. 18:339–349. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Winklewski PJ, Radkowski M and Demkow U:
Relevance of immune-sympathetic nervous system interplay for the
development of hypertension. Adv Exp Med Biol. 884:37–43. 2016.
View Article : Google Scholar
|
|
133
|
Pongratz G and Straub RH: The sympathetic
nervous response in inflammation. Arthritis Res Ther. 16:5042014.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Fisher JP and Paton JF: The sympathetic
nervous system and blood pressure in humans: Implications for
hypertension. J Hum Hypertens. 26:463–475. 2012. View Article : Google Scholar
|
|
135
|
Cupić V, Colić M, Jandrić D, Milojković B
and Varagić VM: Xylazine, an alpha 2-adrenergic agonist, induces
apoptosis of rat thymocytes and a thymocyte hybridoma line in
vitro. Methods Find Exp Clin Pharmacol. 25:5–10. 2003. View Article : Google Scholar
|
|
136
|
Trotter RN, Stornetta RL, Guyenet PG and
Roberts MR: Transneuronal mapping of the CNS network controlling
sympathetic outflow to the rat thymus. Auton Neurosci. 131:9–20.
2007. View Article : Google Scholar
|
|
137
|
Elenkov IJ, Wilder RL, Chrousos GP and
Vizi ES: The sympathetic nerve-an integrative interface between two
supersystems: The brain and the immune system. Pharmacol Rev.
52:595–638. 2000.PubMed/NCBI
|
|
138
|
Vizi ES, Orsó E, Osipenko ON, Haskó G and
Elenkov IJ: Neurochemical, electrophysiological and
immunocytochemical evidence for a noradrenergic link between the
sympathetic nervous system and thymocytes. Neuroscience.
68:1263–1276. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
ThyagaRajan S, Madden KS, Teruya B,
Stevens SY and Felten DL: Bellinger DL. Age-associated alterations
in sympathetic noradrenergic innervation of primary and secondary
lymphoid organs in female Fischer 344 rats. J Neuroimmunol.
233:54–64. 2011. View Article : Google Scholar :
|
|
140
|
Leposavić G, Ugresić N,
Pejcić-Karapetrović B and Mićić M: Castration of sexually immature
rats affects sympathetic innervation of the adult thymus.
Neuroimmunomodulation. 7:59–67. 2000. View Article : Google Scholar
|
|
141
|
Leposavić G, Mićić M, Ugresić N, Bogojević
M and Isaković K: Components of sympathetic innervation of the rat
thymus during late fetal and postnatal development:
Histofluorescence and biochemical study. Sympathetic innervation of
the rat thymus Thymus. 19:77–87. 1992.
|
|
142
|
Vink EE, Boer A, Verloop WL, Spiering W,
Voskuil M, Vonken E, Hoogduin JM, Leiner T, Bots ML and Blankestijn
PJ: The effect of renal denervation on kidney oxygenation as
determined by BOLD MRI in patients with hypertension. Eur Radiol.
25:1984–1992. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Abboud FM, Harwani SC and Chapleau MW:
Autonomic neural regulation of the immune system: Implications for
hypertension and cardiovascular disease. Hypertension. 59:755–762.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Heran BS, Galm BP and Wright JM: Blood
pressure lowering efficacy of alpha blockers for primary
hypertension. Cochrane Database Syst Rev. CD004643. 2012.
|
|
145
|
Esler M: The sympathetic nervous system
through the ages: From Thomas Willis to resistant hypertension. Exp
Physiol. 96:611–622. 2011.PubMed/NCBI
|
|
146
|
Frishman WH: Saunders E. β-adrenergic
blockers. J Clin Hypertens (Greenwich). 13:649–653. 2011.
View Article : Google Scholar
|
|
147
|
Abboud FM: The Walter B. Cannon Memorial
Award Lecture, 2009. Physiology in perspective: The wisdom of the
body. In search of autonomic balance: The good, the bad, and the
ugly. Am J Physiol Regul Integr Comp Physiol. 298:R1449–R1467.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Zucker IH, Hackley JF, Cornish KG, Hiser
BA, Anderson NR, Kieval R, Irwin ED, Serdar DJ, Peuler JD and
Rossing MA: Chronic baroreceptor activation enhances survival in
dogs with pacing-induced heart failure. Hypertension. 50:904–910.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Li M, Zheng C, Sato T, Kawada T, Sugimachi
M and Sunagawa K: Vagal nerve stimulation markedly improves
long-term survival after chronic heart failure in rats.
Circulation. 109:120–124. 2004. View Article : Google Scholar
|
|
150
|
Hering D and Schlaich M: The role of
central nervous system mechanisms in resistant hypertension. Curr
Hypertens Rep. 17:582015. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Brody MJ, Varner KJ and Vasquez EC: Lewis
SJ. Central nervous system and the pathogenesis of hypertension.
Sites and mechanisms. Hypertension. 18(Suppl 5): pp. III7–III12.
1991, View Article : Google Scholar
|
|
152
|
Zubcevic J, Waki H, Raizada MK and Paton
JF: Autonomic-immune-vascular interaction: An emerging concept for
neurogenic hypertension. Hypertension. 57:1026–1033. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Raizada MK and Paton JF: Recent advances
in the renin-angiotensin system: Angiotensin-converting enzyme 2
and (pro) renin receptor. Exp Physiol. 93:517–518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Wyss JM: The role of the sympathetic
nervous system in hypertension. Curr Opin Nephrol Hypertens.
2:265–273. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Felder RB, Francis J, Zhang ZH, Wei SG,
Weiss RM and Johnson AK: Heart failure and the brain: New
perspectives. Am J Physiol Regul Integr Comp Physiol.
284:R259–R276. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Eikelis N, Hering D, Marusic P, Walton A,
Lambert E, Krum H, Lambert G, Esler M and Schlaich M: [Op.7d.10].
The effect of renal denervation on adipokines in patients with
resistant hypertension. J Hypertens. 34(Suppl 2): e972016.
View Article : Google Scholar
|
|
157
|
Rosa J, Widimsky P, Waldauf P, Lambert L,
Zelinka T, Taborsky M, Branny M, Tousek P, Petrak O, Curila K, et
al: [Op.7d.09] the role of adding spironolactone and renal
denervation in true resistant hypertension. One-year outcomes of
randomized prague-15 study. J Hypertens. 34(Suppl 2): e96–e97.
2016. View Article : Google Scholar
|
|
158
|
Rosa J, Widimský P, Waldauf P, Lambert L,
Zelinka T, Táborský M, Branny M, Toušek P, Petrák O, Čurila K, et
al: Role of adding spironolactone and renal denervation in true
resistant hypertension: One-year outcomes of randomized PRAGUE-15
study. Hypertension. 67:397–403. 2016.
|
|
159
|
Calhoun DA: Spironolactone versus renal
nerve denervation for treatment of uncontrolled resistant
hypertension. J Hypertens. 34:1701–1703. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Qi XY, Cheng B, Li YL and Wang YF: Renal
denervation, adjusted drugs, or combined therapy for resistant
hypertension: A meta-regression. Medicine (Baltimore).
95:e39392016. View Article : Google Scholar
|
|
161
|
Esler M: Renal denervation for treatment
of drug-resistant hypertension. Trends Cardiovasc Med. 25:107–115.
2015. View Article : Google Scholar
|
|
162
|
Esler MD, Krum H, Schlaich M, Schmieder
RE, Böhm M and Sobotka PA: Symplicity HTN-2 Investigators: Renal
sympathetic denervation for treatment of drug-resistant
hypertension: One-year results from the Symplicity HTN-2
randomized, controlled trial. Circulation. 126:2976–2982. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Fink GD and Osborn JW: Renal nerves: Time
for reassessment of their role in hypertension. Am J Hypertens.
27:1245–1247. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Kandzari DE, Bhatt DL, Brar S, Devireddy
CM, Esler M, Fahy M, Flack JM, Katzen BT, Lea J, Lee DP, et al:
Predictors of blood pressure response in the SYMPLICITY HTN-3
trial. Eur Heart J. 36:219–227. 2015. View Article : Google Scholar :
|
|
165
|
Schlaich MP, Esler MD, Fink GD, Osborn JW
and Euler DE: Targeting the sympathetic nervous system: Critical
issues in patient selection, efficacy, and safety of renal
denervation. Hypertension. 63:426–432. 2014. View Article : Google Scholar
|
|
166
|
Krum H, Schlaich M, Whitbourn R, Sobotka
PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar
S, et al: Catheter-based renal sympathetic denervation for
resistant hypertension: A multicentre safety and proof-of-principle
cohort study. Lancet. 373:1275–1281. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Symplicity HTN-2 Investigators; Esler MD,
Krum H, Sobotka PA, Schlaich MP, Schmieder RE and Böhm M: Renal
sympathetic denervation in patients with treatment-resistant
hypertension (The Symplicity HTN-2 Trial): A randomised controlled
trial. Lancet. 376:1903–1909. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Symplicity HTN-1 Investigators:
Catheter-based renal sympathetic denervation for resistant
hypertension: Durability of blood pressure reduction out to 24
months. Hypertension. 57:911–917. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Worthley SG, Tsioufis CP, Worthley MI,
Sinhal A, Chew DP, Meredith IT, Malaiapan Y and Papademetriou V:
Safety and efficacy of a multi-electrode renal sympathetic
denervation system in resistant hypertension: The EnligHTN I trial.
Eur Heart J. 34:2132–2140. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Guo J, Feng Y, Barnes P, Huang FF, Idell S
and Su DM: Shams H. Deletion of FoxN1 in the thymic medullary
epithelium reduces peripheral T cell responses to infection and
mimics changes of aging. PLoS One. 7:e346812012. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Chidgey A, Dudakov J, Seach N and Boyd R:
Impact of niche aging on thymic regeneration and immune
reconstitution. Semin Immunol. 19:331–340. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Taub DD and Longo DL: Insights into thymic
aging and regeneration. Immunol Rev. 205:72–93. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Fry TJ and Mackall CL: Current concepts of
thymic aging. Springer Semin Immunopathol. 24:7–22. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Zook EC, Krishack PA, Zhang S, Zeleznik-Le
NJ, Firulli AB and Witte PL: Le PT. Overexpression of Foxn1
attenuates age-associated thymic involution and prevents the
expansion of peripheral CD4 memory T cells. Blood. 118:5723–5731.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Swain S, Clise-Dwyer K and Haynes L:
Homeostasis and the age-associated defect of CD4 T cells. Semin
Immunol. 17:370–377. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Haynes BF, Markert ML, Sempowski GD, Patel
DD and Hale LP: The role of the thymus in immune reconstitution in
aging, bone marrow transplantation, and HIV-1 infection. Annu Rev
Immunol. 18:529–560. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Stutman O and Good RA: Duration of thymic
function. Ser Haematol. 7:505–523. 1974.PubMed/NCBI
|
|
178
|
Thoman ML: The pattern of T lymphocyte
differentiation is altered during thymic involution. Mech Ageing
Dev. 82:155–170. 1995. View Article : Google Scholar
|
|
179
|
Ortman CL, Dittmar KA, Witte PL and Le PT:
Molecular characterization of the mouse involuted thymus:
Aberrations in expression of transcription regulators in thymocyte
and epithelial compartments. Int Immunol. 14:813–822. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Bredenkamp N, Nowell CS and Blackburn CC:
Regeneration of the aged thymus by a single transcription factor.
Development. 141:1627–1637. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Žuklys S, Handel A, Zhanybekova S, Govani
F, Keller M, Maio S, Mayer CE, Teh HY, Hafen K, Gallone G, et al:
Foxn1 regulates key target genes essential for T cell development
in postnatal thymic epithelial cells. Nat Immunol. 17:1206–1215.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Kim J, Wang S, Hyun J, Choi SS, Cha H and
Ock M: Jung Y. Hepatic stellate cells express thymosin Beta 4 in
chronically damaged liver. PLoS One. 10:e01227582015. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Paulussen M, Landuyt B, Schoofs L, Luyten
W and Arckens L: Thymosin beta 4 mRNA and peptide expression in
phagocytic cells of different mouse tissues. Peptides.
30:1822–1832. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Low TL and Goldstein AL: Chemical
characterization of thymosin beta 4. J Biol Chem. 257:1000–1006.
1982.PubMed/NCBI
|
|
185
|
Low TL, Hu SK and Goldstein AL: Complete
amino acid sequence of bovine thymosin beta 4: A thymic hormone
that induces terminal deoxynucleotidyl transferase activity in
thymocyte populations. Proc Natl Acad Sci USA. 78:1162–1166. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Dedova IV, Nikolaeva OP, Safer D, De La,
Cruz EM and dos Remedios CG: Thymosin beta4 induces a
conformational change in actin monomers. Biophys J. 90:985–992.
2006. View Article : Google Scholar
|
|
187
|
Ballweber E, Hannappel E, Huff T, Stephan
H, Haener M, Taschner N, Stoffler D, Aebi U and Mannherz HG:
Polymerisation of chemically cross-linked actin: Thymosin beta(4)
complex to filamentous actin: Alteration in helical parameters and
visualisation of thymosin beta(4) binding on F-actin. J Mol Biol.
315:613–625. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Safer D, Elzinga M and Nachmias VT:
Thymosin beta 4 and Fx, an actin-sequestering peptide, are
indistinguishable. J Biol Chem. 266:4029–4032. 1991.PubMed/NCBI
|
|
189
|
Pearse G: Normal structure, function and
histology of the thymus. Toxicol Pathol. 34:504–514. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Bock-Marquette I, Saxena A, White MD,
Dimaio JM and Srivastava D: Thymosin beta4 activates
integrin-linked kinase and promotes cardiac cell migration,
survival and cardiac repair. Nature. 432:466–472. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Sosne G, Szliter EA, Barrett R, Kernacki
KA, Kleinman H and Hazlett LD: Thymosin beta 4 promotes corneal
wound healing and decreases inflammation in vivo following alkali
injury. Exp Eye Res. 74:293–299. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Malinda KM, Sidhu GS, Mani H, Banaudha K,
Maheshwari RK, Goldstein AL and Kleinman HK: Thymosin beta4
accelerates wound healing. J Invest Dermatol. 113:364–368. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Goldstein AL, Hannappel E and Kleinman HK:
Thymosin beta4: Actin-sequestering protein moonlights to repair
injured tissues. Trends Mol Med. 11:421–429. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Xu BJ, Shyr Y, Liang X, Ma LJ, Donnert EM,
Roberts JD, Zhang X, Kon V, Brown NJ, Caprioli RM and Fogo AB:
Proteomic patterns and prediction of glomerulosclerosis and its
mechanisms. J Am Soc Nephrol. 16:2967–2975. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Vasilopoulou E, Winyard PJ, Riley PR and
Long DA: The role of thymosin-β4 in kidney disease. Expert Opin
Biol Ther. 15(Suppl 1): S187–S190. 2015. View Article : Google Scholar
|
|
196
|
Zuo Y, Chun B, Potthoff SA, Kazi N, Brolin
TJ, Orhan D, Yang HC, Ma LJ, Kon V, Myöhänen T, et al: Thymosin β4
and its degradation product, Ac-SDKP, are novel reparative factors
in renal fibrosis. Kidney Int. 84:1166–1175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Ghosh AK, Murphy SB and Kishore R: Vaughan
DE. Global gene expression profiling in PAI-1 knockout murine heart
and kidney: Molecular basis of cardiac-selective fibrosis. PLoS
One. 8:e638252013. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Ma LJ and Fogo AB: PAI-1 and kidney
fibrosis. Front Biosci (Landmark Ed). 14:2028–2041. 2009.
View Article : Google Scholar
|
|
199
|
Smart N, Risebro CA, Melville AA, Moses K,
Schwartz RJ, Chien KR and Riley PR: Thymosin beta4 induces adult
epicardial progenitor mobilization and neovascularization. Nature.
445:177–182. 2007. View Article : Google Scholar
|
|
200
|
Grant DS, Rose W, Yaen C, Goldstein A,
Martinez J and Kleinman H: Thymosin beta4 enhances endothelial cell
differentiation and angiogenesis. Angiogenesis. 3:125–135. 1999.
View Article : Google Scholar
|
|
201
|
Liao TD, Yang XP, D'Ambrosio M, Zhang Y,
Rhaleb NE and Carretero OA: N-acetyl-seryl-aspartyl-lysyl-proline
attenuates renal injury and dysfunction in hypertensive rats with
reduced renal mass: Council for high blood pressure research.
Hypertension. 55:459–467. 2010. View Article : Google Scholar
|
|
202
|
Cavasin MA, Rhaleb NE, Yang XP and
Carretero OA: Prolyl oligopeptidase is involved in release of the
antifibrotic peptide Ac-SDKP. Hypertension. 43:1140–1145. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Myöhänen TT, Tenorio-Laranga J, Jokinen B,
Vázquez-Sánchez R, Moreno-Baylach MJ, García-Horsman JA and
Männistö PT: Prolyl oligopeptidase induces angiogenesis both in
vitro and in vivo in a novel regulatory manner. Br J Pharmacol.
163:1666–1678. 2011. View Article : Google Scholar :
|
|
204
|
García-Horsman JA, Männistö PT and
Venäläinen JI: On the role of prolyl oligopeptidase in health and
disease. Neuropeptides. 41:1–24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Myöhänen TT, García-Horsman JA,
Tenorio-Laranga J and Männistö PT: Issues about the physiological
functions of prolyl oligopeptidase based on its discordant spatial
association with substrates and inconsistencies among mRNA, protein
levels, and enzymatic activity. J Histochem Cytochem. 57:831–848.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Shibuya K, Kanasaki K, Isono M, Sato H,
Omata M, Sugimoto T, Araki S, Isshiki K, Kashiwagi A, Haneda M and
Koya D: N-ace tyl-seryl-aspartyl-lysyl-proline prevents renal
insufficiency and mesangial matrix expansion in diabetic db/db
mice. Diabetes. 54:838–845. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Cingolani OH, Yang XP, Liu YH, Villanueva
M, Rhaleb NE and Carretero OA: Reduction of cardiac fibrosis
decreases systolic performance without affecting diastolic function
in hypertensive rats. Hypertension. 43:1067–1073. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Yang F, Yang XP, Liu YH, Xu J, Cingolani
O, Rhaleb NE and Carretero OA: Ac-SDKP reverses inflammation and
fibrosis in rats with heart failure after myocardial infarction.
Hypertension. 43:229–236. 2004. View Article : Google Scholar
|
|
209
|
Peng H, Carretero OA, Brigstock DR,
Oja-Tebbe N and Rhaleb NE: Ac-SDKP reverses cardiac fibrosis in
rats with renovascular hypertension. Hypertension. 42:1164–1170.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Pradelles P, Frobert Y, Creminon C, Liozon
E, Massé A and Frindel E: Negative regulator of pluripotent
hematopoietic stem cell proliferation in human white blood cells
and plasma as analysed by enzyme immunoassay. Biochem Biophys Res
Commun. 170:986–993. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Pradelles P, Frobert Y, Créminon C,
Ivonine H and Frindel E: Distribution of a negative regulator of
haematopoietic stem cell proliferation (AcSDKP) and thymosin beta 4
in mouse tissues. FEBS Lett. 289:171–175. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Hrenak J, Paulis L and Simko F:
N-acetyl-seryl-aspartyl-lysy l-proline (Ac-SDKP): Potential target
molecule in research of heart, kidney and brain. Curr Pharm Des.
21:5135–5143. 2015. View Article : Google Scholar
|
|
213
|
Worou ME, Liao TD, D'Ambrosio M, Nakagawa
P, Janic B, Peterson EL, Rhaleb NE and Carretero OA: Renal
protective effect of N-acetyl-seryl-aspartyl-lysyl-proline in dahl
salt-sensitive rats. Hypertension. 66:816–822. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Omata M, Taniguchi H, Koya D, Kanasaki K,
Sho R, Kato Y, Kojima R, Haneda M and Inomata N:
N-acetyl-seryl-aspartyl-lysyl-proline ameliorates the progression
of renal dysfunction and fibrosis in WKY rats with established
anti-glomerular basement membrane nephritis. J Am Soc Nephrol.
17:674–685. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Lopez-Ilasaca M, Liu X, Tamura K and Dzau
VJ: The angiotensin II type I receptor-associated protein, ATRAP,
is a transmembrane protein and a modulator of angiotensin II
signaling. Mol Biol Cell. 14:5038–5050. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Daviet L, Lehtonen JY, Tamura K, Griese
DP, Horiuchi M and Dzau VJ: Cloning and characterization of ATRAP,
a novel protein that interacts with the angiotensin II type 1
receptor. J Biol Chem. 274:17058–17062. 1999. View Article : Google Scholar : PubMed/NCBI
|