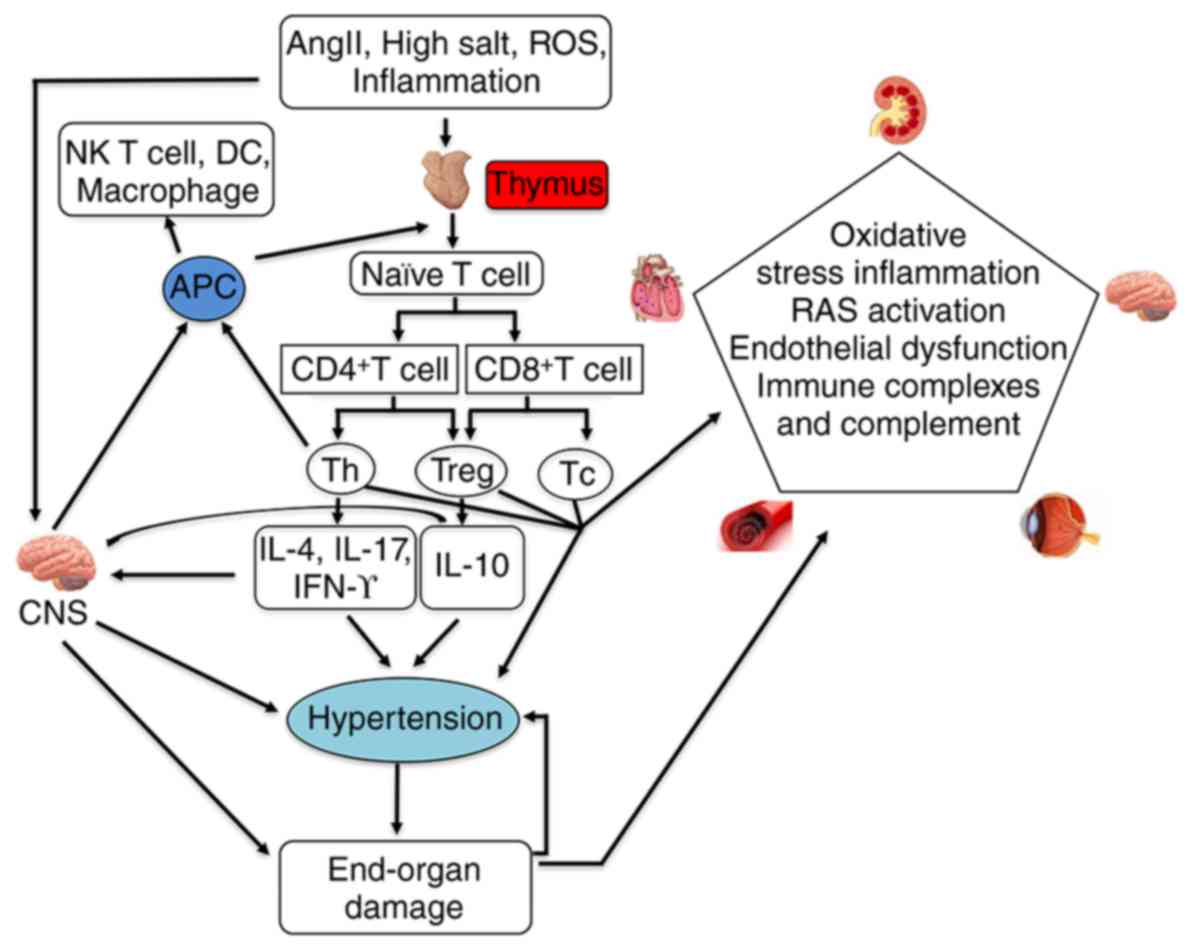
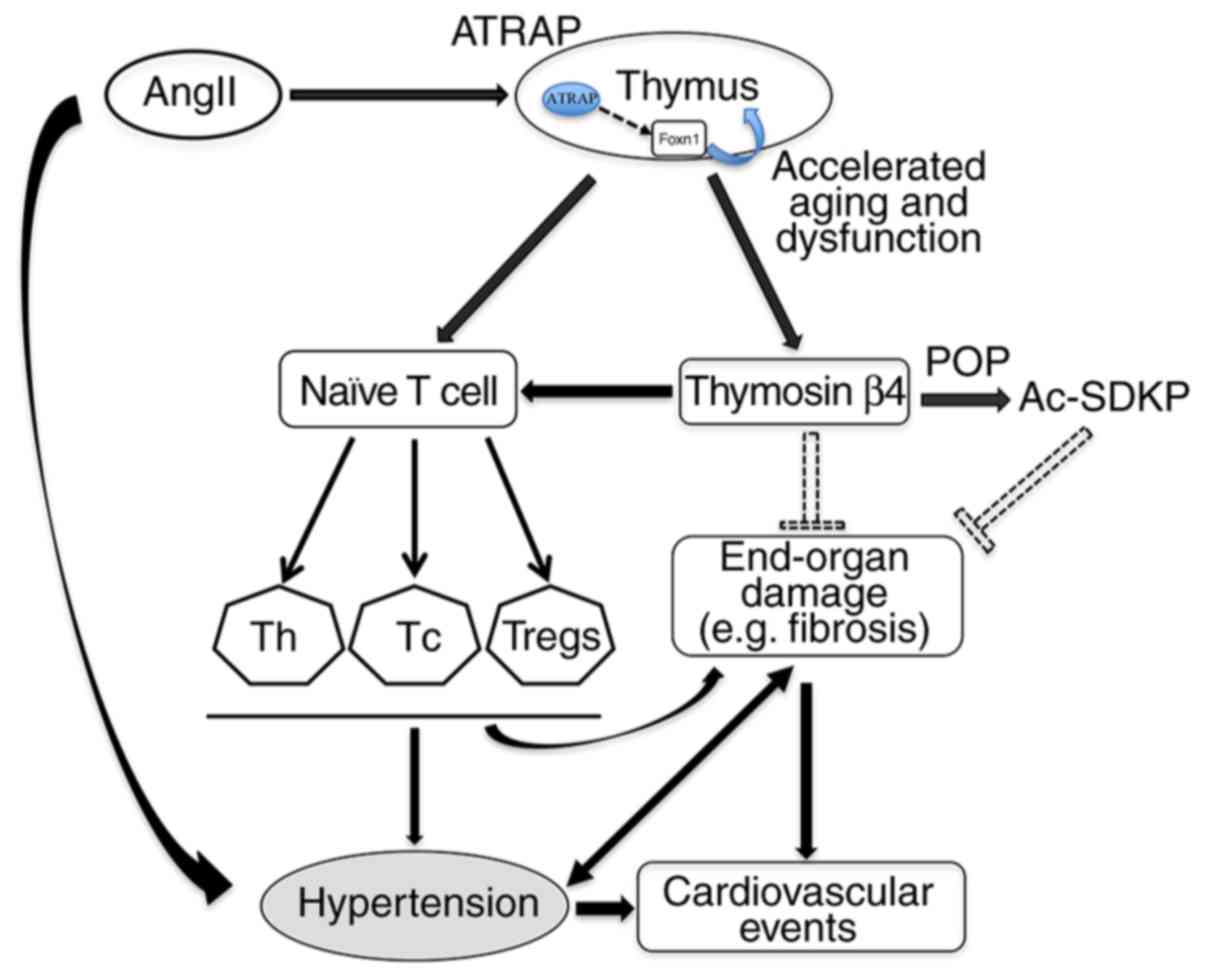
|
1
|
Cavasin MA, Liao TD, Yang XP, Yang JJ and
Carretero OA: Decreased endogenous levels of Ac-SDKP promote organ
fibrosis. Hypertension. 50:130–136. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Neutel JM, Giles TD, Punzi H, Weiss RJ, Li
H and Finck A: Long-term safety of nebivolol and valsartan
combination therapy in patients with hypertension: An open-label,
single-arm, multicenter study. J Am Soc Hypertens. 8:915–920. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Safaeian L, Hajhashemi V, Haghjoo
Javanmard S and Sanaye Naderi H: The effect of protocatechuic acid
on blood pressure and oxidative stress in glucocorticoid-induced
hypertension in rat. Iran J Pharm Res. 15(Suppl): S83–S91.
2016.
|
|
4
|
Chmielewski V, Drupt F and Morfin R:
Dexamethasone-induced apoptosis of mouse thymocytes: Prevention by
native 7alpha-hydroxysteroids. Immunol Cell Biol. 78:238–246. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Walters SN, Webster KE, Daley S and Grey
ST: A role for intrathymic B cells in the generation of natural
regulatory T cells. J Immunol. 193:170–176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lynch HE, Goldberg GL, Chidgey A, Van den
Brink MR, Boyd R and Sempowski GD: Thymic involution and immune
reconstitution. Trends Immunol. 30:366–373. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Murray JM, Kaufmann GR, Hodgkin PD, Lewin
SR, Kelleher AD, Davenport MP and Zaunders JJ: Naive T cells are
maintained by thymic output in early ages but by proliferation
without phenotypic change after age twenty. Immunol Cell Biol.
81:487–495. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ruan L, Zhang Z, Mu L, Burnley P, Wang L,
Coder B, Zhuge Q and Su DM: Biological significance of FoxN1
gain-of-function mutations during T and B lymphopoiesis in juvenile
mice. Cell Death Dis. 5:e14572014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fukuda S, Tsuchikura S and Iida H:
Age-related changes in blood pressure, hematological values,
concentrations of serum biochemical constituents and weights of
organs in the SHR/Izm, SHRSP/Izm and WKY/Izm. Exp Anim. 53:67–72.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodriguez-Iturbe B and Johnson RJ: The
role of renal microvascular disease and interstitial inflammation
in salt-sensitive hypertension. Hypertens Res. 33:975–980. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Svendsen UG: The importance of thymus in
the pathogenesis of the chronic phase of hypertension in mice
following partial infarction of the kidney. Acta Pathol Microbiol
Scand A. 85:539–547. 1977.PubMed/NCBI
|
|
12
|
Svendsen UG: The effect of penicillamine
on blood pressure and vascular disease in mice with infarct-kidney
hypertension. Scand J Rheumatol. 8:81–86. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Svendsen UG: The role of thymus for the
development and prognosis of hypertension and hypertensive vascular
disease in mice following renal infarction. Acta Pathol Microbiol
Scand A. 84:235–243. 1976.PubMed/NCBI
|
|
14
|
Ba D, Takeichi N, Kodama T and Kobayashi
H: Restoration of T cell depression and suppression of blood
pressure in spontaneously hypertensive rats (SHR) by thymus grafts
or thymus extracts. J Immunol. 128:1211–1216. 1982.PubMed/NCBI
|
|
15
|
Bento-de-Souza L, Victor JR,
Bento-de-Souza LC, Arrais-Santos M, Rangel-Santos AC, Pereira-Costa
É, Raniero-Fernandes E, Seixas-Duarte MI, Oliveira-Filho JB and
Silva Duarte AJ: Constitutive expression of genes encoding notch
receptors and ligands in developing lymphocytes, nTreg cells and
dendritic cells in the human thymus. Results Immunol. 6:15–20.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Plum J, De Smedt M, Leclercq G, Taghon T,
Kerre T and Vandekerckhove B: Human intrathymic development: A
selective approach. Semin Immunopathol. 30:411–423. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu Z, Lancaster JN and Ehrlich LI: The
contribution of chemokines and migration to the induction of
central tolerance in the Thymus. Front Immunol. 6:3982015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu Z, Lancaster JN, Sasiponganan C and
Ehrlich LI: CCR4 promotes medullary entry and thymocyte-dendritic
cell interactions required for central tolerance. J Exp Med.
212:1947–1965. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Love PE and Bhandoola A: Signal
integration and crosstalk during thymocyte migration and
emigration. Nat Rev Immunol. 11:469–477. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Richards DM, Delacher M, Goldfarb Y,
Kägebein D, Hofer AC, Abramson J and Feuerer M: Treg cell
differentiation: From Thymus to peripheral tissue. Prog Mol Biol
Transl Sci. 136:175–205. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abbas AK, Benoist C, Bluestone JA,
Campbell DJ, Ghosh S, Hori S, Jiang S, Kuchroo VK, Mathis D,
Roncarolo MG, et al: Regulatory T cells: Recommendations to
simplify the nomenclature. Nat Immunol. 14:307–308. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bod L, Douguet L, Auffray C, Lengagne R,
Bekkat F, Rondeau E, Molinier-Frenkel V, Castellano F, Richard Y
and Prévost-Blondel A: IL-4-induced gene 1: A negative immune
checkpoint controlling B cell differentiation and activation. J
Immunol. 200:1027–1038. 2018. View Article : Google Scholar
|
|
23
|
McLeod JJ, Baker B and Ryan JJ: Mast cell
production and response to IL-4 and IL-13. Cytokine. 75:57–61.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang X, Voskens CJ, Sallin M, Maniar A,
Montes CL, Zhang Y, Lin W, Li G, Burch E, Tan M, et al: CD137
promotes proliferation and survival of human B cells. J Immunol.
184:787–795. 2010. View Article : Google Scholar
|
|
25
|
Nilsson G and Nilsson K: Effects of
interleukin (IL)-13 on immediate-early response gene expression,
phenotype and differentiation of human mast cells. Comparison with
IL-4. Eur J Immunol. 25:870–873. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Groves AM, Johnston CJ, Misra RS, Williams
JP and Finkelstein JN: Effects of IL-4 on pulmonary fibrosis and
the accumulation and phenotype of macrophage subpopulations
following thoracic irradiation. Int J Radiat Biol. 92:754–765.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Francos-Quijorna I, Amo-Aparicio J,
Martinez-Muriana A and López-Vales R: IL-4 drives microglia and
macrophages toward a phenotype conducive for tissue repair and
functional recovery after spinal cord injury. Glia. 64:2079–2092.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Czimmerer Z, Varga T, Kiss M, Vázquez CO,
Doan-Xuan QM, Rückerl D, Tattikota SG, Yan X, Nagy ZS, Daniel B, et
al: The IL-4/STAT6 signaling axis establishes a conserved microRNA
signature in human and mouse macrophages regulating cell survival
via miR-342-3p. Genome Med. 8:632016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miossec P, Korn T and Kuchroo VK:
Interleukin-17 and type 17 helper T cells. N Engl J Med.
361:888–898. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Veldhoen M, Hocking RJ, Atkins CJ,
Locksley RM and Stockinger B: TGFbeta in the context of an
inflammatory cytokine milieu supports de novo differentiation of
IL-17-producing T cells. Immunity. 24:179–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mangan PR, Harrington LE, O'Quinn DB,
Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR and
Weaver CT: Transforming growth factor-beta induces development of
the T(H)17 lineage. Nature. 441:231–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ivanov II, McKenzie BS, Zhou L, Tadokoro
CE, Lepelley A, Lafaille JJ, Cua DJ and Littman DR: The orphan
nuclear receptor RORgammat directs the differentiation program of
proinflammatory IL-17+ T helper cells. Cell.
126:1121–1133. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kolls JK and Lindén A: Interleukin-17
family members and inflammation. Immunity. 21:467–476. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mazidi M, Penson P, Gluba-Brzozka A, Rysz
J and Banach M: Relationship between long noncoding RNAs and
physiological risk factors of cardiovascular disease. J Clin
Lipidol. 11:617–623. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kaur J: A comprehensive review on
metabolic syndrome. Cardiol Res Pract. 2014:9431622014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ahbap E, Sakaci T, Kara E, Sahutoglu T,
Koc Y, Basturk T, Sevinc M, Akgol C, Hasbal B, Isleem M, et al:
Serum uric acid levels and inflammatory markers with respect to
dipping status: A retrospective analysis of hypertensive patients
with or without chronic kidney disease. Clin Exp Hypertens.
38:555–563. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Virdis A, Dell'Agnello U and Taddei S:
Impact of inflammation on vascular disease in hypertension.
Maturitas. 78:179–183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Taddei S, Caraccio N, Virdis A, Dardano A,
Versari D, Ghiadoni L, Ferrannini E, Salvetti A and Monzani F:
Low-grade systemic inflammation causes endothelial dysfunction in
patients with Hashimoto's thyroiditis. J Clin Endocrinol Metab.
91:5076–5082. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mirsaeidi M, Omar HR, Ebrahimi G and
Campos M: The association between ESR and CRP and systemic
hypertension in sarcoidosis. Int J Hypertens. 2016:24025152016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hezel M, Peleli M, Liu M, Zollbrecht C,
Jensen BL, Checa A, Giulietti A, Wheelock CE, Lundberg JO,
Weitzberg E and Carlström M: Dietary nitrate improves age-related
hypertension and metabolic abnormalities in rats via modulation of
angiotensin II receptor signaling and inhibition of superoxide
generation. Free Radic Biol Med. 99:87–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Victorio JA, Clerici SP, Palacios R,
Alonso MJ, Vassallo DV, Jaffe IZ, Rossoni LV and Davel AP:
Spironolactone prevents endothelial nitric oxide synthase
uncoupling and vascular dysfunction induced by β-adrenergic
overstimulation: Role of perivascular adipose tissue. Hypertension.
68:726–735. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Goto K, Fujii K, Onaka U, Abe I and
Fujishima M: Renin-angiotensin system blockade improves endothelial
dysfunction in hypertension. Hypertension. 36:575–580. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cheng ZJ, Vaskonen T, Tikkanen I, Nurminen
K, Ruskoaho H, Vapaatalo H, Muller D, Park JK, Luft FC and Mervaala
EM: Endothelial dysfunction and salt-sensitive hypertension in
spontaneously diabetic Goto-Kakizaki rats. Hypertension.
37:433–439. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
McIntyre M, Bohr DF and Dominiczak AF:
Endothelial function in hypertension: The role of superoxide anion.
Hypertension. 34:539–545. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Virdis A, Ghiadoni L, Plantinga Y, Taddei
S and Salvetti A: C-reactive protein and hypertension: Is there a
causal relationship. Curr Pharm Des. 13:1693–1698. 2007. View Article : Google Scholar
|
|
46
|
Bautista LE, Vera LM, Arenas IA and
Gamarra G: Independent association between inflammatory markers
(C-reactive protein, interleukin-6, and TNF-alpha) and essential
hypertension. J Hum Hypertens. 19:149–154. 2005. View Article : Google Scholar
|
|
47
|
Lakoski SG, Cushman M, Palmas W,
Blumenthal R, D'Agostino RB Jr and Herrington DM: The relationship
between blood pressure and C-reactive protein in the multi-ethnic
study of atherosclerosis (MESA). J Am Coll Cardiol. 46:1869–1874.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Schiffrin EL: Immune mechanisms in
hypertension and vascular injury. Clin Sci (Lond). 126:267–274.
2014. View Article : Google Scholar
|
|
49
|
Blake GJ, Rifai N, Buring JE and Ridker
PM: Blood pressure, C-reactive protein, and risk of future
cardiovascular events. Circulation. 108:2993–2999. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bermudez EA, Rifai N, Buring J, Manson JE
and Ridker PM: Interrelationships among circulating interleukin-6,
C-reactive protein, and traditional cardiovascular risk factors in
women. Arterioscler Thromb Vasc Biol. 22:1668–1673. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chae CU, Lee RT, Rifai N and Ridker PM:
Blood pressure and inflammation in apparently healthy men.
Hypertension. 38:399–403. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yamada S, Gotoh T, Nakashima Y, Kayaba K,
Ishikawa S, Nago N, Nakamura Y, Itoh Y and Kajii E: Distribution of
serum C-reactive protein and its association with atherosclerotic
risk factors in a Japanese population: Jichi medical school cohort
study. Am J Epidemiol. 153:1183–1190. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ford ES and Giles WH: Serum C-reactive
protein and fibrinogen concentrations and self-reported angina
pectoris and myocardial infarction: Findings from national health
and nutrition examination survey III. J Clin Epidemiol. 53:95–102.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rohde LE, Hennekens CH and Ridker PM:
Survey of C-reactive protein and cardiovascular risk factors in
apparently healthy men. Am J Cardiol. 84:1018–1022. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Venugopal SK, Devaraj S, Yuhanna I, Shaul
P and Jialal I: Demonstration that C-reactive protein decreases
eNOS expression and bioactivity in human aortic endothelial cells.
Circulation. 106:1439–1441. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Verma S, Li SH, Badiwala MV, Weisel RD,
Fedak PW, Li RK, Dhillon B and Mickle DA: Endothelin antagonism and
interleukin-6 inhibition attenuate the proatherogenic effects of
C-reactive protein. Circulation. 105:1890–1896. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Verma S, Wang CH, Li SH, Dumont AS, Fedak
PW, Badiwala MV, Dhillon B, Weisel RD, Li RK, Mickle DA and Stewart
DJ: A self-fulfilling prophecy: C-reactive protein attenuates
nitric oxide production and inhibits angiogenesis. Circulation.
106:913–919. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Romero JC and Reckelhoff JF:
State-of-the-Art lecture. Role of angiotensin and oxidative stress
in essential hypertension. Hypertension. 34:943–949. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mervaala E, Müller DN, Schmidt F, Park JK,
Gross V, Bader M, Breu V, Ganten D, Haller H and Luft FC: Blood
pressure-independent effects in rats with human renin and
angiotensinogen genes. Hypertension. 35:587–594. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Muller DN, Dechend R, Mervaala EM, Park
JK, Schmidt F, Fiebeler A, Theuer J, Breu V, Ganten D, Haller H and
Luft FC: NF-kappaB inhibition ameliorates angiotensin II-induced
inflammatory damage in rats. Hypertension. 35:193–201. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Müller DN, Mervaala EM, Dechend R,
Fiebeler A, Park JK, Schmidt F, Theuer J, Breu V, Mackman N, Luther
T, et al: Angiotensin II (AT(1)) receptor blockade reduces vascular
tissue factor in angiotensin II-induced cardiac vasculopathy. Am J
Pathol. 157:111–122. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ji Q, Cheng G, Ma N, Huang Y, Lin Y, Zhou
Q, Que B, Dong J, Zhou Y and Nie S: Circulating Th1, Th2, and Th17
levels in hypertensive patients. Dis Markers. 2017:71462902017.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
McMaster WG, Kirabo A, Madhur MS and
Harrison DG: Inflammation, immunity, and hypertensive end-organ
damage. Circ Res. 116:1022–1033. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Madhur MS, Lob HE, McCann LA, Iwakura Y,
Blinder Y, Guzik TJ and Harrison DG: Interleukin 17 promotes
angiotensin II-induced hypertension and vascular dysfunction.
Hypertension. 55:500–507. 2010. View Article : Google Scholar :
|
|
65
|
De Ciuceis C, Rossini C, La Boria E,
Porteri E, Petroboni B, Gavazzi A, Sarkar A, Rosei EA and Rizzoni
D: Immune mechanisms in hypertension. High Blood Press Cardiovasc
Prev. 21:227–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Dai X, Huang S, He Z, Wu F, Ding R, Chen
Y, Liang C and Wu Z: Dysfunction of the thymus in mice with
hypertension. Exp Ther Med. 13:1386–1392. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ebringer A and Doyle AE: Raised serum IgG
levels in hypertension. Br Med J. 2:146–148. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Leibowitz A and Schiffrin EL: Immune
mechanisms in hypertension. Curr Hypertens Rep. 13:465–472. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lin J, Yang L, Silva HM, Trzeciak A, Choi
Y, Schwab SR, Dustin ML and Lafaille JJ: Increased generation of
Foxp3(+) regulatory T cells by manipulating antigen presentation in
the thymus. Nat Commun. 7:105622016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wing K and Sakaguchi S: Regulatory T cells
exert checks and balances on self tolerance and autoimmunity. Nat
Immunol. 11:7–13. 2010. View Article : Google Scholar
|
|
71
|
Mellanby RJ, Thomas DC and Lamb J: Role of
regulatory T-cells in autoimmunity. Clin Sci (Lond). 116:639–649.
2009. View Article : Google Scholar
|
|
72
|
Piccirillo CA, d'Hennezel E, Sgouroudis E
and Yurchenko E: CD4+Foxp3+ regulatory T
cells in the control of autoimmunity: In vivo veritas. Curr Opin
Immunol. 20:655–662. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Takeichi N, Suzuki K and Kobayashi H:
Characterization of immunological depression in spontaneously
hypertensive rats. Eur J Immunol. 11:483–487. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Olsen F: Transfer of arterial hypertension
by splenic cells from DOCA-salt hypertensive and renal hypertensive
rats to normotensive recipients. Acta Pathol Microbiol Scand C.
88:1–5. 1980.PubMed/NCBI
|
|
75
|
Takeichi N, Suzuki K, Okayasu T and
Kobayashi H: Immunological depression in spontaneously hypertensive
rats. Clin Exp Immunol. 40:120–126. 1980.PubMed/NCBI
|
|
76
|
Svendsen UG: Evidence for an initial,
thymus independent and a chronic, thymus dependent phase of DOCA
and salt hypertension in mice. Acta Pathol Microbiol Scand A.
84:523–528. 1976.PubMed/NCBI
|
|
77
|
Marvar PJ, Vinh A, Thabet S, Lob HE, Geem
D, Ressler KJ and Harrison DG: T lymphocytes and vascular
inflammation contribute to stress-dependent hypertension. Biol
Psychiatry. 71:774–782. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Harrison DG, Guzik TJ, Lob HE, Madhur MS,
Marvar PJ, Thabet SR, Vinh A and Weyand CM: Inflammation, immunity,
and hypertension. Hypertension. 57:132–140. 2011. View Article : Google Scholar
|
|
79
|
Muller DN, Kvakan H and Luft FC:
Immune-related effects in hypertension and target-organ damage.
Curr Opin Nephrol Hypertens. 20:113–117. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Calame DG, Mueller-Ortiz SL and Wetsel RA:
Innate and adaptive immunologic functions of complement in the host
response to Listeria monocytogenes infection. Immunobiology.
221:1407–1417. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
D'Alincourt Salazar M, Manuel ER, Tsai W,
D'Apuzzo M, Goldstein L, Blazar BR and Diamond DJ: Evaluation of
innate and adaptive immunity contributing to the antitumor effects
of PD1 blockade in an orthotopic murine model of pancreatic cancer.
Oncoimmunology. 5:e11601842016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Weyd H: More than just innate affairs-on
the role of annexins in adaptive immunity. Biol Chem.
397:1017–1029. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Kvakan H, Luft FC and Muller DN: Role of
the immune system in hypertensive target organ damage. Trends
Cardiovasc Med. 19:242–246. 2009. View Article : Google Scholar
|
|
84
|
Vergaro G, Prud'homme M, Fazal L, Merval
R, Passino C, Emdin M, Samuel JL, Cohen Solal A and Delcayre C:
Inhibition of Galectin-3 pathway prevents isoproterenol-induced
left ventricular dysfunction and fibrosis in mice. Hypertension.
67:606–612. 2016.PubMed/NCBI
|
|
85
|
Wenzel U, Turner JE, Krebs C, Kurts C,
Harrison DG and Ehmke H: Immune mechanisms in arterial
hypertension. J Am Soc Nephrol. 27:677–686. 2016. View Article : Google Scholar :
|
|
86
|
Harrison DG: The immune system in
hypertension. Trans Am Clin Climatol Assoc. 125:130–140.
2014.PubMed/NCBI
|
|
87
|
Kossmann S, Hu H, Steven S, Schönfelder T,
Fraccarollo D, Mikhed Y, Brahler M, Knorr M, Brandt M, Karbach SH,
et al: Inflammatory monocytes determine endothelial nitric-oxide
synthase uncoupling and nitro-oxidative stress induced by
angiotensin II. J Biol Chem. 289:27540–27550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Liu J, Yang F, Yang XP, Jankowski M and
Pagano PJ: NAD(P)H oxidase mediates angiotensin II-induced vascular
macrophage infiltration and medial hypertrophy. Arterioscler Thromb
Vasc Biol. 23:776–782. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wenzel P, Knorr M, Kossmann S, Stratmann
J, Hausding M, Schuhmacher S, Karbach SH, Schwenk M, Yogev N,
Schulz E, et al: Lysozyme M-positive monocytes mediate angiotensin
II-induced arterial hypertension and vascular dysfunction.
Circulation. 124:1370–1381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ko EA, Amiri F, Pandey NR, Javeshghani D,
Leibovitz E, Touyz RM and Schiffrin EL: Resistance artery
remodeling in deoxycorticosterone acetate-salt hypertension is
dependent on vascular inflammation: Evidence from m-CSF-deficient
mice. Am J Physiol Heart Circ Physiol. 292:H1789–H1795. 2007.
View Article : Google Scholar
|
|
91
|
De Ciuceis C, Amiri F, Brassard P,
Endemann DH, Touyz RM and Schiffrin EL: Reduced vascular
remodeling, endothelial dysfunction, and oxidative stress in
resistance arteries of angiotensin II-infused macrophage
colony-stimulating factor-deficient mice: Evidence for a role in
inflammation in angiotensin-induced vascular injury. Arterioscler
Thromb Vasc Biol. 25:2106–2113. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Abais-Battad JM, Rudemiller NP and Mattson
DL: Hypertension and immunity: Mechanisms of T cell activation and
pathways of hypertension. Curr Opin Nephrol Hypertens. 24:470–474.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Schiffrin EL: The immune system: Role in
hypertension. Can J Cardiol. 29:543–548. 2013. View Article : Google Scholar
|
|
94
|
Verlohren S, Muller DN, Luft FC and
Dechend R: Immunology in hypertension, preeclampsia, and
target-organ damage. Hypertension. 54:439–443. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Idris-Khodja N, Mian MO, Paradis P and
Schiffrin EL: Dual opposing roles of adaptive immunity in
hypertension. Eur Heart J. 35:1238–1244. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Guzik TJ, Hoch NE, Brown KA, McCann LA,
Rahman A, Dikalov S, Goronzy J, Weyand C and Harrison DG: Role of
the T cell in the genesis of angiotensin II induced hypertension
and vascular dysfunction. J Exp Med. 204:2449–2460. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zhang W and Victor RG: Calcineurin
inhibitors cause renal afferent activation in rats: A novel
mechanism of cyclosporine-induced hypertension. Am J Hypertens.
13:999–1004. 2000. View Article : Google Scholar
|
|
98
|
Rodríguez-Iturbe B, Pons H, Quiroz Y,
Gordon K, Rincón J, Chávez M, Parra G, Herrera-Acosta J,
Gómez-Garre D, Largo R, et al: Mycophenolate mofetil prevents
salt-sensitive hypertension resulting from angiotensin II exposure.
Kidney Int. 59:2222–2232. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Crowley SD, Frey CW, Gould SK, Griffiths
R, Ruiz P, Burchette JL, Howell DN, Makhanova N, Yan M, Kim HS, et
al: Stimulation of lymphocyte responses by angiotensin II promotes
kidney injury in hypertension. Am J Physiol Renal Physiol.
295:F515–F524. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wei Z, Spizzo I, Diep H, Drummond GR,
Widdop RE and Vinh A: Differential phenotypes of
tissue-infiltrating T cells during angiotensin II-induced
hypertension in mice. PLoS One. 9:e1148952014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Rosenthal AS: Regulation of the immune
response-role of the macrophage. N Engl J Med. 303:1153–1156. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Gordon S: The role of the macrophage in
immune regulation. Res Immunol. 149:685–688. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Lam RS, O'Brien-Simpson NM, Holden JA,
Lenzo JC and Fong SB: Reynolds EC. Unprimed, M1 and M2 macrophages
differentially interact with porphyromonas gingivalis. PLoS One.
11:e01586292016. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Mellman I and Steinman RM: Dendritic
cells: Specialized and regulated antigen processing machines. Cell.
106:255–258. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Goldschneider I and Cone RE: A central
role for peripheral dendritic cells in the induction of acquired
thymic tolerance. Trends Immunol. 24:77–81. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Oh J and Shin JS: The role of dendritic
cells in central tolerance. Immune Netw. 15:111–120. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Gelosa P, Pignieri A, Gianazza E, Criniti
S, Guerrini U, Cappellini MD, Banfi C, Tremoli E and Sironi L:
Altered iron homeostasis in an animal model of hypertensive
nephropathy: Stroke-prone rats. J Hypertens. 31:2259–2269. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Singh MV, Chapleau MW, Harwani SC and
Abboud FM: The immune system and hypertension. Immunol Res.
59:243–253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Rudemiller N, Lund H, Jacob HJ, Geurts AM
and Mattson DL: PhysGen Knockout Program: CD247 modulates blood
pressure by altering T-lymphocyte infiltration in the kidney.
Hypertension. 63:559–564. 2014. View Article : Google Scholar
|
|
110
|
Luft FC, Dechend R and Muller DN: Immune
mechanisms in angiotensin II-induced target-organ damage. Ann Med.
44(Suppl 1): S49–S54. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Rodríguez-Iturbe B, Franco M, Tapia E,
Quiroz Y and Johnson RJ: Renal inflammation, autoimmunity and
salt-sensitive hypertension. Clin Exp Pharmacol Physiol. 39:96–103.
2012. View Article : Google Scholar
|
|
112
|
Moon JY: Recent update of
renin-angiotensin-aldosterone system in the pathogenesis of
hypertension. Electrolyte Blood Press. 11:41–45. 2013. View Article : Google Scholar
|
|
113
|
White FN and Grollman A: Autoimmune
factors associated with infarction of the kidney. Nephron.
1:93–102. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Rodríguez-Iturbe B, Quiroz Y, Ferrebuz A,
Parra G and Vaziri ND: Evolution of renal interstitial inflammation
and NF-kappaB activation in spontaneously hypertensive rats. Am J
Nephrol. 24:587–594. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Rodríguez-Iturbe B, Ferrebuz A, Vanegas V,
Quiroz Y, Mezzano S and Vaziri ND: Early and sustained inhibition
of nuclear factor-kappaB prevents hypertension in spontaneously
hypertensive rats. J Pharmacol Exp Ther. 315:51–57. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Marvar PJ, Gordon FJ and Harrison DG:
Blood pressure control: Salt gets under your skin. Nat Med.
15:487–488. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Crowley SD, Song YS, Lin EE, Griffiths R,
Kim HS and Ruiz P: Lymphocyte responses exacerbate angiotensin
II-dependent hypertension. Am J Physiol Regul Integr Comp Physiol.
298:R1089–R1097. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
De Miguel C, Das S, Lund H and Mattson DL:
T lymphocytes mediate hypertension and kidney damage in Dahl
salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol.
298:R1136–R1142. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Yang T, Zollbrecht C, Winerdal ME, Zhuge
Z, Zhang XM, Terrando N, Checa A, Sällström J, Wheelock CE,
Winqvist O, et al: Genetic abrogation of adenosine A3 receptor
prevents uninephrectomy and high salt-induced hypertension. J Am
Heart Assoc. 5:e0038682016. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Dong L, Nordlohne J, Ge S, Hertel B, Melk
A, Rong S, Haller H and von Vietinghoff S: T Cell CX3CR1 Mediates
excess atherosclerotic inflammation in renal impairment. J Am Soc
Nephrol. 27:1753–1764. 2016. View Article : Google Scholar :
|
|
121
|
Lucchini M, Fifer WP, Sahni R and
Signorini MG: Novel heart rate parameters for the assessment of
autonomic nervous system function in premature infants. Physiol
Meas. 37:1436–1446. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Reijman S, Bakermans-Kranenburg MJ,
Hiraoka R, Crouch JL, Milner JS, Alink LR and van IJzendoorn MH:
Baseline functioning and stress reactivity in maltreating parents
and at-risk adults: Review and meta-analyses of autonomic nervous
system studies. Child Maltreat. 1077559516659937. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Olofsson PS, Rosas-Ballina M, Levine YA
and Tracey KJ: Rethinking inflammation: Neural circuits in the
regulation of immunity. Immunol Rev. 248:188–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Marvar PJ, Thabet SR, Guzik TJ, Lob HE,
McCann LA, Weyand C, Gordon FJ and Harrison DG: Central and
peripheral mechanisms of T-lymphocyte activation and vascular
inflammation produced by angiotensin II-induced hypertension. Circ
Res. 107:263–270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Ganta CK, Lu N, Helwig BG, Blecha F, Ganta
RR, Zheng L, Ross CR, Musch TI, Fels RJ and Kenney MJ: Central
angiotensin II-enhanced splenic cytokine gene expression is
mediated by the sympathetic nervous system. Am J Physiol Heart Circ
Physiol. 289:H1683–H1691. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Borovikova LV, Ivanova S, Zhang M, Yang H,
Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW and Tracey
KJ: Vagus nerve stimulation attenuates the systemic inflammatory
response to endotoxin. Nature. 405:458–462. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Ader R, Felten D and Cohen N: Interactions
between the brain and the immune system. Annu Rev Pharmacol
Toxicol. 30:561–602. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Bulloch K and Pomerantz W: Autonomic
nervous system innervation of thymic-related lymphoid tissue in
wildtype and nude mice. J Comp Neurol. 228:57–68. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Nance DM, Hopkins DA and Bieger D:
Re-investigation of the innervation of the thymus gland in mice and
rats. Brain Behav Immun. 1:134–147. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Tollefson L and Bulloch K: Dual-label
retrograde transport: CNS innervation of the mouse thymus distinct
from other mediastinum viscera. J Neurosci Res. 25:20–28. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Roggero E, Besedovsky HO and del Rey A:
The role of the sympathetic nervous system in the thymus in health
and disease. Neuroimmunomodulation. 18:339–349. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Winklewski PJ, Radkowski M and Demkow U:
Relevance of immune-sympathetic nervous system interplay for the
development of hypertension. Adv Exp Med Biol. 884:37–43. 2016.
View Article : Google Scholar
|
|
133
|
Pongratz G and Straub RH: The sympathetic
nervous response in inflammation. Arthritis Res Ther. 16:5042014.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Fisher JP and Paton JF: The sympathetic
nervous system and blood pressure in humans: Implications for
hypertension. J Hum Hypertens. 26:463–475. 2012. View Article : Google Scholar
|
|
135
|
Cupić V, Colić M, Jandrić D, Milojković B
and Varagić VM: Xylazine, an alpha 2-adrenergic agonist, induces
apoptosis of rat thymocytes and a thymocyte hybridoma line in
vitro. Methods Find Exp Clin Pharmacol. 25:5–10. 2003. View Article : Google Scholar
|
|
136
|
Trotter RN, Stornetta RL, Guyenet PG and
Roberts MR: Transneuronal mapping of the CNS network controlling
sympathetic outflow to the rat thymus. Auton Neurosci. 131:9–20.
2007. View Article : Google Scholar
|
|
137
|
Elenkov IJ, Wilder RL, Chrousos GP and
Vizi ES: The sympathetic nerve-an integrative interface between two
supersystems: The brain and the immune system. Pharmacol Rev.
52:595–638. 2000.PubMed/NCBI
|
|
138
|
Vizi ES, Orsó E, Osipenko ON, Haskó G and
Elenkov IJ: Neurochemical, electrophysiological and
immunocytochemical evidence for a noradrenergic link between the
sympathetic nervous system and thymocytes. Neuroscience.
68:1263–1276. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
ThyagaRajan S, Madden KS, Teruya B,
Stevens SY and Felten DL: Bellinger DL. Age-associated alterations
in sympathetic noradrenergic innervation of primary and secondary
lymphoid organs in female Fischer 344 rats. J Neuroimmunol.
233:54–64. 2011. View Article : Google Scholar :
|
|
140
|
Leposavić G, Ugresić N,
Pejcić-Karapetrović B and Mićić M: Castration of sexually immature
rats affects sympathetic innervation of the adult thymus.
Neuroimmunomodulation. 7:59–67. 2000. View Article : Google Scholar
|
|
141
|
Leposavić G, Mićić M, Ugresić N, Bogojević
M and Isaković K: Components of sympathetic innervation of the rat
thymus during late fetal and postnatal development:
Histofluorescence and biochemical study. Sympathetic innervation of
the rat thymus Thymus. 19:77–87. 1992.
|
|
142
|
Vink EE, Boer A, Verloop WL, Spiering W,
Voskuil M, Vonken E, Hoogduin JM, Leiner T, Bots ML and Blankestijn
PJ: The effect of renal denervation on kidney oxygenation as
determined by BOLD MRI in patients with hypertension. Eur Radiol.
25:1984–1992. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Abboud FM, Harwani SC and Chapleau MW:
Autonomic neural regulation of the immune system: Implications for
hypertension and cardiovascular disease. Hypertension. 59:755–762.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Heran BS, Galm BP and Wright JM: Blood
pressure lowering efficacy of alpha blockers for primary
hypertension. Cochrane Database Syst Rev. CD004643. 2012.
|
|
145
|
Esler M: The sympathetic nervous system
through the ages: From Thomas Willis to resistant hypertension. Exp
Physiol. 96:611–622. 2011.PubMed/NCBI
|
|
146
|
Frishman WH: Saunders E. β-adrenergic
blockers. J Clin Hypertens (Greenwich). 13:649–653. 2011.
View Article : Google Scholar
|
|
147
|
Abboud FM: The Walter B. Cannon Memorial
Award Lecture, 2009. Physiology in perspective: The wisdom of the
body. In search of autonomic balance: The good, the bad, and the
ugly. Am J Physiol Regul Integr Comp Physiol. 298:R1449–R1467.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Zucker IH, Hackley JF, Cornish KG, Hiser
BA, Anderson NR, Kieval R, Irwin ED, Serdar DJ, Peuler JD and
Rossing MA: Chronic baroreceptor activation enhances survival in
dogs with pacing-induced heart failure. Hypertension. 50:904–910.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Li M, Zheng C, Sato T, Kawada T, Sugimachi
M and Sunagawa K: Vagal nerve stimulation markedly improves
long-term survival after chronic heart failure in rats.
Circulation. 109:120–124. 2004. View Article : Google Scholar
|
|
150
|
Hering D and Schlaich M: The role of
central nervous system mechanisms in resistant hypertension. Curr
Hypertens Rep. 17:582015. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Brody MJ, Varner KJ and Vasquez EC: Lewis
SJ. Central nervous system and the pathogenesis of hypertension.
Sites and mechanisms. Hypertension. 18(Suppl 5): pp. III7–III12.
1991, View Article : Google Scholar
|
|
152
|
Zubcevic J, Waki H, Raizada MK and Paton
JF: Autonomic-immune-vascular interaction: An emerging concept for
neurogenic hypertension. Hypertension. 57:1026–1033. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Raizada MK and Paton JF: Recent advances
in the renin-angiotensin system: Angiotensin-converting enzyme 2
and (pro) renin receptor. Exp Physiol. 93:517–518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Wyss JM: The role of the sympathetic
nervous system in hypertension. Curr Opin Nephrol Hypertens.
2:265–273. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Felder RB, Francis J, Zhang ZH, Wei SG,
Weiss RM and Johnson AK: Heart failure and the brain: New
perspectives. Am J Physiol Regul Integr Comp Physiol.
284:R259–R276. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Eikelis N, Hering D, Marusic P, Walton A,
Lambert E, Krum H, Lambert G, Esler M and Schlaich M: [Op.7d.10].
The effect of renal denervation on adipokines in patients with
resistant hypertension. J Hypertens. 34(Suppl 2): e972016.
View Article : Google Scholar
|
|
157
|
Rosa J, Widimsky P, Waldauf P, Lambert L,
Zelinka T, Taborsky M, Branny M, Tousek P, Petrak O, Curila K, et
al: [Op.7d.09] the role of adding spironolactone and renal
denervation in true resistant hypertension. One-year outcomes of
randomized prague-15 study. J Hypertens. 34(Suppl 2): e96–e97.
2016. View Article : Google Scholar
|
|
158
|
Rosa J, Widimský P, Waldauf P, Lambert L,
Zelinka T, Táborský M, Branny M, Toušek P, Petrák O, Čurila K, et
al: Role of adding spironolactone and renal denervation in true
resistant hypertension: One-year outcomes of randomized PRAGUE-15
study. Hypertension. 67:397–403. 2016.
|
|
159
|
Calhoun DA: Spironolactone versus renal
nerve denervation for treatment of uncontrolled resistant
hypertension. J Hypertens. 34:1701–1703. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Qi XY, Cheng B, Li YL and Wang YF: Renal
denervation, adjusted drugs, or combined therapy for resistant
hypertension: A meta-regression. Medicine (Baltimore).
95:e39392016. View Article : Google Scholar
|
|
161
|
Esler M: Renal denervation for treatment
of drug-resistant hypertension. Trends Cardiovasc Med. 25:107–115.
2015. View Article : Google Scholar
|
|
162
|
Esler MD, Krum H, Schlaich M, Schmieder
RE, Böhm M and Sobotka PA: Symplicity HTN-2 Investigators: Renal
sympathetic denervation for treatment of drug-resistant
hypertension: One-year results from the Symplicity HTN-2
randomized, controlled trial. Circulation. 126:2976–2982. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Fink GD and Osborn JW: Renal nerves: Time
for reassessment of their role in hypertension. Am J Hypertens.
27:1245–1247. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Kandzari DE, Bhatt DL, Brar S, Devireddy
CM, Esler M, Fahy M, Flack JM, Katzen BT, Lea J, Lee DP, et al:
Predictors of blood pressure response in the SYMPLICITY HTN-3
trial. Eur Heart J. 36:219–227. 2015. View Article : Google Scholar :
|
|
165
|
Schlaich MP, Esler MD, Fink GD, Osborn JW
and Euler DE: Targeting the sympathetic nervous system: Critical
issues in patient selection, efficacy, and safety of renal
denervation. Hypertension. 63:426–432. 2014. View Article : Google Scholar
|
|
166
|
Krum H, Schlaich M, Whitbourn R, Sobotka
PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar
S, et al: Catheter-based renal sympathetic denervation for
resistant hypertension: A multicentre safety and proof-of-principle
cohort study. Lancet. 373:1275–1281. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Symplicity HTN-2 Investigators; Esler MD,
Krum H, Sobotka PA, Schlaich MP, Schmieder RE and Böhm M: Renal
sympathetic denervation in patients with treatment-resistant
hypertension (The Symplicity HTN-2 Trial): A randomised controlled
trial. Lancet. 376:1903–1909. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Symplicity HTN-1 Investigators:
Catheter-based renal sympathetic denervation for resistant
hypertension: Durability of blood pressure reduction out to 24
months. Hypertension. 57:911–917. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Worthley SG, Tsioufis CP, Worthley MI,
Sinhal A, Chew DP, Meredith IT, Malaiapan Y and Papademetriou V:
Safety and efficacy of a multi-electrode renal sympathetic
denervation system in resistant hypertension: The EnligHTN I trial.
Eur Heart J. 34:2132–2140. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Guo J, Feng Y, Barnes P, Huang FF, Idell S
and Su DM: Shams H. Deletion of FoxN1 in the thymic medullary
epithelium reduces peripheral T cell responses to infection and
mimics changes of aging. PLoS One. 7:e346812012. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Chidgey A, Dudakov J, Seach N and Boyd R:
Impact of niche aging on thymic regeneration and immune
reconstitution. Semin Immunol. 19:331–340. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Taub DD and Longo DL: Insights into thymic
aging and regeneration. Immunol Rev. 205:72–93. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Fry TJ and Mackall CL: Current concepts of
thymic aging. Springer Semin Immunopathol. 24:7–22. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Zook EC, Krishack PA, Zhang S, Zeleznik-Le
NJ, Firulli AB and Witte PL: Le PT. Overexpression of Foxn1
attenuates age-associated thymic involution and prevents the
expansion of peripheral CD4 memory T cells. Blood. 118:5723–5731.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Swain S, Clise-Dwyer K and Haynes L:
Homeostasis and the age-associated defect of CD4 T cells. Semin
Immunol. 17:370–377. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Haynes BF, Markert ML, Sempowski GD, Patel
DD and Hale LP: The role of the thymus in immune reconstitution in
aging, bone marrow transplantation, and HIV-1 infection. Annu Rev
Immunol. 18:529–560. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Stutman O and Good RA: Duration of thymic
function. Ser Haematol. 7:505–523. 1974.PubMed/NCBI
|
|
178
|
Thoman ML: The pattern of T lymphocyte
differentiation is altered during thymic involution. Mech Ageing
Dev. 82:155–170. 1995. View Article : Google Scholar
|
|
179
|
Ortman CL, Dittmar KA, Witte PL and Le PT:
Molecular characterization of the mouse involuted thymus:
Aberrations in expression of transcription regulators in thymocyte
and epithelial compartments. Int Immunol. 14:813–822. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Bredenkamp N, Nowell CS and Blackburn CC:
Regeneration of the aged thymus by a single transcription factor.
Development. 141:1627–1637. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Žuklys S, Handel A, Zhanybekova S, Govani
F, Keller M, Maio S, Mayer CE, Teh HY, Hafen K, Gallone G, et al:
Foxn1 regulates key target genes essential for T cell development
in postnatal thymic epithelial cells. Nat Immunol. 17:1206–1215.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Kim J, Wang S, Hyun J, Choi SS, Cha H and
Ock M: Jung Y. Hepatic stellate cells express thymosin Beta 4 in
chronically damaged liver. PLoS One. 10:e01227582015. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Paulussen M, Landuyt B, Schoofs L, Luyten
W and Arckens L: Thymosin beta 4 mRNA and peptide expression in
phagocytic cells of different mouse tissues. Peptides.
30:1822–1832. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Low TL and Goldstein AL: Chemical
characterization of thymosin beta 4. J Biol Chem. 257:1000–1006.
1982.PubMed/NCBI
|
|
185
|
Low TL, Hu SK and Goldstein AL: Complete
amino acid sequence of bovine thymosin beta 4: A thymic hormone
that induces terminal deoxynucleotidyl transferase activity in
thymocyte populations. Proc Natl Acad Sci USA. 78:1162–1166. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Dedova IV, Nikolaeva OP, Safer D, De La,
Cruz EM and dos Remedios CG: Thymosin beta4 induces a
conformational change in actin monomers. Biophys J. 90:985–992.
2006. View Article : Google Scholar
|
|
187
|
Ballweber E, Hannappel E, Huff T, Stephan
H, Haener M, Taschner N, Stoffler D, Aebi U and Mannherz HG:
Polymerisation of chemically cross-linked actin: Thymosin beta(4)
complex to filamentous actin: Alteration in helical parameters and
visualisation of thymosin beta(4) binding on F-actin. J Mol Biol.
315:613–625. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Safer D, Elzinga M and Nachmias VT:
Thymosin beta 4 and Fx, an actin-sequestering peptide, are
indistinguishable. J Biol Chem. 266:4029–4032. 1991.PubMed/NCBI
|
|
189
|
Pearse G: Normal structure, function and
histology of the thymus. Toxicol Pathol. 34:504–514. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Bock-Marquette I, Saxena A, White MD,
Dimaio JM and Srivastava D: Thymosin beta4 activates
integrin-linked kinase and promotes cardiac cell migration,
survival and cardiac repair. Nature. 432:466–472. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Sosne G, Szliter EA, Barrett R, Kernacki
KA, Kleinman H and Hazlett LD: Thymosin beta 4 promotes corneal
wound healing and decreases inflammation in vivo following alkali
injury. Exp Eye Res. 74:293–299. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Malinda KM, Sidhu GS, Mani H, Banaudha K,
Maheshwari RK, Goldstein AL and Kleinman HK: Thymosin beta4
accelerates wound healing. J Invest Dermatol. 113:364–368. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Goldstein AL, Hannappel E and Kleinman HK:
Thymosin beta4: Actin-sequestering protein moonlights to repair
injured tissues. Trends Mol Med. 11:421–429. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Xu BJ, Shyr Y, Liang X, Ma LJ, Donnert EM,
Roberts JD, Zhang X, Kon V, Brown NJ, Caprioli RM and Fogo AB:
Proteomic patterns and prediction of glomerulosclerosis and its
mechanisms. J Am Soc Nephrol. 16:2967–2975. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Vasilopoulou E, Winyard PJ, Riley PR and
Long DA: The role of thymosin-β4 in kidney disease. Expert Opin
Biol Ther. 15(Suppl 1): S187–S190. 2015. View Article : Google Scholar
|
|
196
|
Zuo Y, Chun B, Potthoff SA, Kazi N, Brolin
TJ, Orhan D, Yang HC, Ma LJ, Kon V, Myöhänen T, et al: Thymosin β4
and its degradation product, Ac-SDKP, are novel reparative factors
in renal fibrosis. Kidney Int. 84:1166–1175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Ghosh AK, Murphy SB and Kishore R: Vaughan
DE. Global gene expression profiling in PAI-1 knockout murine heart
and kidney: Molecular basis of cardiac-selective fibrosis. PLoS
One. 8:e638252013. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Ma LJ and Fogo AB: PAI-1 and kidney
fibrosis. Front Biosci (Landmark Ed). 14:2028–2041. 2009.
View Article : Google Scholar
|
|
199
|
Smart N, Risebro CA, Melville AA, Moses K,
Schwartz RJ, Chien KR and Riley PR: Thymosin beta4 induces adult
epicardial progenitor mobilization and neovascularization. Nature.
445:177–182. 2007. View Article : Google Scholar
|
|
200
|
Grant DS, Rose W, Yaen C, Goldstein A,
Martinez J and Kleinman H: Thymosin beta4 enhances endothelial cell
differentiation and angiogenesis. Angiogenesis. 3:125–135. 1999.
View Article : Google Scholar
|
|
201
|
Liao TD, Yang XP, D'Ambrosio M, Zhang Y,
Rhaleb NE and Carretero OA: N-acetyl-seryl-aspartyl-lysyl-proline
attenuates renal injury and dysfunction in hypertensive rats with
reduced renal mass: Council for high blood pressure research.
Hypertension. 55:459–467. 2010. View Article : Google Scholar
|
|
202
|
Cavasin MA, Rhaleb NE, Yang XP and
Carretero OA: Prolyl oligopeptidase is involved in release of the
antifibrotic peptide Ac-SDKP. Hypertension. 43:1140–1145. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Myöhänen TT, Tenorio-Laranga J, Jokinen B,
Vázquez-Sánchez R, Moreno-Baylach MJ, García-Horsman JA and
Männistö PT: Prolyl oligopeptidase induces angiogenesis both in
vitro and in vivo in a novel regulatory manner. Br J Pharmacol.
163:1666–1678. 2011. View Article : Google Scholar :
|
|
204
|
García-Horsman JA, Männistö PT and
Venäläinen JI: On the role of prolyl oligopeptidase in health and
disease. Neuropeptides. 41:1–24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Myöhänen TT, García-Horsman JA,
Tenorio-Laranga J and Männistö PT: Issues about the physiological
functions of prolyl oligopeptidase based on its discordant spatial
association with substrates and inconsistencies among mRNA, protein
levels, and enzymatic activity. J Histochem Cytochem. 57:831–848.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Shibuya K, Kanasaki K, Isono M, Sato H,
Omata M, Sugimoto T, Araki S, Isshiki K, Kashiwagi A, Haneda M and
Koya D: N-ace tyl-seryl-aspartyl-lysyl-proline prevents renal
insufficiency and mesangial matrix expansion in diabetic db/db
mice. Diabetes. 54:838–845. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Cingolani OH, Yang XP, Liu YH, Villanueva
M, Rhaleb NE and Carretero OA: Reduction of cardiac fibrosis
decreases systolic performance without affecting diastolic function
in hypertensive rats. Hypertension. 43:1067–1073. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Yang F, Yang XP, Liu YH, Xu J, Cingolani
O, Rhaleb NE and Carretero OA: Ac-SDKP reverses inflammation and
fibrosis in rats with heart failure after myocardial infarction.
Hypertension. 43:229–236. 2004. View Article : Google Scholar
|
|
209
|
Peng H, Carretero OA, Brigstock DR,
Oja-Tebbe N and Rhaleb NE: Ac-SDKP reverses cardiac fibrosis in
rats with renovascular hypertension. Hypertension. 42:1164–1170.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Pradelles P, Frobert Y, Creminon C, Liozon
E, Massé A and Frindel E: Negative regulator of pluripotent
hematopoietic stem cell proliferation in human white blood cells
and plasma as analysed by enzyme immunoassay. Biochem Biophys Res
Commun. 170:986–993. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Pradelles P, Frobert Y, Créminon C,
Ivonine H and Frindel E: Distribution of a negative regulator of
haematopoietic stem cell proliferation (AcSDKP) and thymosin beta 4
in mouse tissues. FEBS Lett. 289:171–175. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Hrenak J, Paulis L and Simko F:
N-acetyl-seryl-aspartyl-lysy l-proline (Ac-SDKP): Potential target
molecule in research of heart, kidney and brain. Curr Pharm Des.
21:5135–5143. 2015. View Article : Google Scholar
|
|
213
|
Worou ME, Liao TD, D'Ambrosio M, Nakagawa
P, Janic B, Peterson EL, Rhaleb NE and Carretero OA: Renal
protective effect of N-acetyl-seryl-aspartyl-lysyl-proline in dahl
salt-sensitive rats. Hypertension. 66:816–822. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Omata M, Taniguchi H, Koya D, Kanasaki K,
Sho R, Kato Y, Kojima R, Haneda M and Inomata N:
N-acetyl-seryl-aspartyl-lysyl-proline ameliorates the progression
of renal dysfunction and fibrosis in WKY rats with established
anti-glomerular basement membrane nephritis. J Am Soc Nephrol.
17:674–685. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Lopez-Ilasaca M, Liu X, Tamura K and Dzau
VJ: The angiotensin II type I receptor-associated protein, ATRAP,
is a transmembrane protein and a modulator of angiotensin II
signaling. Mol Biol Cell. 14:5038–5050. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Daviet L, Lehtonen JY, Tamura K, Griese
DP, Horiuchi M and Dzau VJ: Cloning and characterization of ATRAP,
a novel protein that interacts with the angiotensin II type 1
receptor. J Biol Chem. 274:17058–17062. 1999. View Article : Google Scholar : PubMed/NCBI
|