Introduction
Human skin is divided into epidermal, dermal and
fatty subcutaneous tissues. The epidermis, which is comprised
mainly of keratinocytes, is classified into multiple layers, the
stratum corneum, granular layer, spinous layer and basal layer,
based on the differentiation stage of the keratinocytes (1). Keratinocytes are known to have an
important role in inflammation (2). Indeed, many inflammation-related
skin diseases, such as allergic contact dermatitis, psoriasis and
atopic dermatitis, are highly associated with the function of
keratinocytes and cytokines (3).
Keratinocytes are also a major source of inflammatory mediators,
including members of the tumor necrosis factor (TNF)-α and
interleukin (IL) families (2).
Overproduction of pro-inflammatory mediators may lead to an
abnormal inflammatory response. For this reason, potential
anti-inflammatory agents can be used to suppress pro-inflammatory
mediators (4).
Multiple studies have discovered anti-inflammatory
and wound healing activities in substances extracted from plants
(5,6). The effects of Ashwagandha
(Withania Somnifera) leaves and roots, one of the most
popular herbal treatments in the traditional Ayurvedic medicine in
India, are considered to include enhancements in physical strength,
energy, resistance to various stresses, and immunity. Since the
roots of the Ashwagandha plant are also known to be beneficial in
treating ulcers, leucoderma and scabies, they have also been
applied topically to heal skin sores and reduce swelling (7). These findings suggest that one or
more components of Ashwagandha have active physiological effects on
the skin. Studies on topical treatment with Ashwagandha organic and
aqueous extracts have confirmed their chemopreventive effects on
skin cancer (8) and their melanin
regulatory effects (9). In
addition, Ashwagandha, in the form of a paste of boiled leaves and
roots, is considered to have wound-healing abilities in Ayurveda
(10).
It has been determined that the main organic solvent
extract of Ashwagandha is the water-insoluble Withaferin-A, and
that Withaferin-A exhibits anti-inflammatory, antiangiogenesis,
antimetastasis and anticancer activities (11). In Ayurveda, however, most herbal
remedies are applied as a hot-water extract. Whether the healing
effects of Withaferin-A would be observed by applying a hot-water
extract of Ashwagandha (ASH-WEX) to skin remains largely
unexplored.
Recently, a report determined that ASH-WEX had
physiological activities and effects that were different than those
of Withaferin-A, and were unrelated to Withaferin-A (12). Bhat et al (13) also reported that Ashwagandha tea
increased natural killer-cell activity, possibly due to various
effects of the components of the tea in cytokine secretion. The
present study hypothesized that ASH-WEX may modulate the expression
of cytokines when used as a topical treatment for skin. Thus, the
anti-inflammatory effects and the intracellular mechanisms of
ASH-WEX were investigated in vitro and in vivo.
Materials and methods
Materials
Ashwagandha roots were a kind gift from Dr Reiji
Nishio (Toray Industries, Inc., Tokyo, Japan). Lipopolysaccharide
(LPS) and MTT were purchased from Wako Pure Chemical Industries,
Ltd. (Osaka, Japan). Primary antibodies for p38 (cat. no. 9212S),
phosphorylated (P)-p38 (cat. no. 9211S), c-Jun N-terminal kinase
(JNK) (cat. no. 9252S), P-JNK (cat. no. 9251S), extracellular
signal-regulated kinase (ERK) (cat. no. 9102S), P-ERK (cat. no.
9101S), nuclear factor (NF)-κB, p65 (cat. no. 4764S), histone H3
(cat. no. 9715) and β-actin (cat. no. 4967S) were purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA). TNF-α
polyclonal antibody (bs-2081R) was purchased from Bioss Antibodies,
Inc. (Woburn, MA, USA). TGF-β1 polyclonal antibody (cat. no.
5559-100) was purchased from BioVision, Inc. (Milpitas, CA, USA).
Horseradish peroxidase-conjugated goat anti-rabbit IgG (cat. no.
7074) (Cell Signaling Technology, Inc.) was used as a secondary
antibody. Medatomidine hydrochloride and atipamezole hydrochloride
were purchased from Wako Pure Chemical Industries, Ltd. Vetorphale
was purchased from Meiji Seika Kaisha, Ltd. (Tokyo, Japan) and
midazolam was purchased from Sandoz (Tokyo, Japan).
Extraction of ASH-WEX
Several root pieces from different Ashwagandha roots
were cut and powdered with ceramic mortar, and then added to Milli
Q water (5 g powder in 50 ml water). After 2 h of heating with an
electric heater (Tokyo Technological Laboratories, Tokyo, Japan) at
100°C, the extract was passed through a paper filter (GE Healthcare
Life Sciences, Little Chalfont, UK). Following centrifugation at
180 × g for 5 min, the supernatants were filtered with a 0.2
μm filter (Sartorius AG, Goettinggen, Germany) and stored at
−80°C until use. The final concentration of ASH-WEX was 100 mg/ml.
ASH-WEX was diluted with Dulbecco's modified Eagle medium (DMEM;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) or Milli Q prior to
its use in the treatment of HaCaT cells or mouse skin,
respectively.
Cell culture
The human keratinocyte cell line HaCaT was provided
by Professor P. Boukamp and Dr N. Fusenig (German Cancer Research
Center, Heidelberg, Germany) (14) and cultured in DMEM supplemented
with 10% fetal bovine serum (FBS; Biofill, Victoria, Australia),
100 U/ml penicillin and 100 μg/ml streptomycin at 37°C in a
humidified chamber under an atmosphere of 95% air and 5%
CO2.
Animals
A total of 18 six-week-old male C57BL/6J mice
(weight, ~20 g) were purchased from Tokyo Laboratory Animals
Science Co., Ltd. (Tokyo, Japan). The mice were given ad libitum
access to standard chow and water, and housed in our departmental
animal room under specific pathogen-free conditions. All animals
were kept in a controlled environment for 1 week prior to
experiments and all experimental procedures were approved by the
Tokyo Denki University Animal Care and Use Committee in accordance
with their Ethical and Animal Experiment Regulations.
Cell viability assay
Cell viability was evaluated by MTT assay. HaCaT
cells (1×104 cells/well) were cultured in 96-well plates
for 24 h (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
then treated with different concentrations of ASH-WEX for 24 h.
Cells were then incubated with 10 μl of 5 mg/ml MTT for 1 h
at 37°C. Finally, the supernatants were discarded and DMSO (100
μl/well) was added to dissolve MTT formation. The absorbance
was measured with a microplate reader (Awareness Technology, Palm
City, FL, USA) at 570 nm.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) in
vitro
HaCaT cells (1×106 cells/dish) were
incubated in a dish (diameter, 100 mm) for 24 h and then treated
with ASH-WEX (0.25 or 1,5 mg/ml) for 24 h, and total RNA was
isolated by TRIzol (Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. PrimeScript Reverse Transcriptase
(Takara Bio, Inc., Kusatsu, Japan) was used for the reverse
transcription reaction. In brief, 2.5 μl of Milli Q, 2
μl of 5X PrimeScript buffer, 2 μl of 2.5 mM dNTP, 2
μl of 2.5 μM Oligo dT (Sigma-Aldrich; Merck KGaA),
0.25 μl of PrimeScript, 0.25 μl of recombinant RNase
inhibitor and 1 μl of isolated RNA (1 μg/μl)
were combined as a single sample, incubated at 42°C for 30 min and
70°C for 15 min, and placed in short-term storage at 4°C in a
thermal cycler (ASTEC, Shime, Japan). Reverse
transcriptase-generated complementary DNA encoding IL-1β, IL-6,
IL-8, IL-12, TNF-α, and transforming growth factor (TGF)-β1 genes
were amplified by PCR using the following specific primers: IL-1β
forward, GCTGAGGAAGATGCTGGTTCand reverse, TCCATATCCTGTCCCTGGAG;
IL-6 forward, CCTGAACCTTCCAAAGATGGC and reverse,
TTCACCAGGCAAGTCTCCTCA; IL-8 forward, GTCCTTGTTCCACTGTGCCTand
reverse, GCTTCCACATGTCCTCACAA; IL-12 forward,
GATGGCCCTGTGCCTTAGTAand reverse, TCCATATCCTGTCCCTGGAG; TNF-α
forward, TCCTTCAGACACCCTCAACC and reverse, TCCTTCAGACACCCTCAACC;
TGF-β forward, CCCTGGACACCAACTATTGC and reverse,
GTCCAGGCTCCAAATGTAGG; and GAPDH forward, ATCATCAGCAATGCCTCCTG and
reverse, GTGCTTCACCACCTTCTTGA. In brief, a reaction solution [12.5
μl of 2X GeneAce SYBR qPCR Mix α (Wako Pure Chemical
Industries, Ltd.), 0.5 μl of each primer, Milli Q, and 2.5
μl of cDNA sample] was dispensed into optical 96-well
reaction plates (Applied Biosystems; Thermo Fisher Scientific,
Inc.), and set in an ABI PRISM 7000 (Applied Biosystems; Thermo
Fisher Scientific, Inc.). Then, amplification consisted of 1 cycle
at 95°C for 10 min, followed by 45 cycles at 95°C for 30 sec and
60°C for 1 min, with a final dissociation stage at 95°C for 15 sec,
60°C for 20 min, and 95°C for 15 sec. Relative fold changes in mRNA
expression were calculated using the 2−ΔΔCq method,
according to Livak and Schmittgen (15).
Western blot analysis
HaCaT cells (1×106 cells/dish) were
incubated in a dish (diameter, 100 mm) for 24 h and then pretreated
with ASH-WEX (5 mg/ml) for 20 h, followed by 4 h incubation with
LPS (1 μg/ml). To examine the mitogen-activated protein
kinase (MAPK) pathway, total proteins were extracted with lysis
buffer (50 mM HEPES, 150 mM NaCl, 10% glycerol, 1% Triton-X 100,
1.5 mM MgCl2, 1 mM EGTA, 1 mM sodium orthovanadate, 1%
protease inhibitor cocktail; pH 7.5) and western blot was performed
as previously described (16). To
investigate the NF-κB translocation to the nucleus, the nuclear
fractions were prepared as previously described (17) with slight modifications. HaCaT
cells (1×106 cells/dish) were incubated a dish
(diameter, 100 mm) for 24 h and then pretreated with ASH-WEX for 20
h, followed by 4 h incubation with LPS (1 μg/ml). After the
cells were collected and washed with PBS, the pellets were
resuspended in 100 μl of buffer A [10 mM HEPES, 10 mM KCl,
0.1 mM EDTA, 0.4% (v/v) NP-40, 1% protease inhibitor; pH 7.9] and
incubated on ice for 10 min. Following centrifugation at 15,000 × g
for 3 min, the supernatant was collected as a cytosolic fraction.
The remaining pellets were washed with buffer A, resuspended in 60
μl of buffer B (20 mM HEPES, 0.4 M NaCl, 1 mM EDTA, 10%
glycerin, 1% protease inhibitor; pH 7.9) and stirred at 4°C for 2
h. The supernatants were collected as a nucleic fraction following
centrifugation at 15,000 × g at 4°C for 3 min. Protein
concentrations were determined using a BCA protein assay kit
(Thermo Fisher Scientific, Inc.). For each sample, 20 μg of
protein was subjected to 12% polyacrylamide gel electrophoresis,
and then transferred to a polyvinylidene difluoride membrane
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The membranes were
incubated in blocking solution (3% non-fat skimmed milk) for 1 h,
and then probed with primary antibodies (1:1,000) at 4°C overnight.
After washing with 0.1% Tween 20-PBS, the membrane was incubated
with secondary antibody (1:2,000) for 1 h at room temperature.
Finally, the membranes were visualized with enhanced
chemiluminescence reagents (Pierce ECL Western Blotting Substrate;
Thermo Fisher Scientific, Inc.) following exposure for 1 min, and
observed with an Image Quant LAS-4000 system (GE Healthcare Life
Sciences). The relative density of bands was quantified using
ImageJ software (National Institutes of Health, Bethesda, MD,
USA).
RNA isolation, RT-qPCR and wound-healing
effect of ASH-WEX in vivo
The anti-inflammatory effect of ASH-WEX in
vivo was investigated using 7-week-old male C57BL/6J mice. The
back of each mouse was shaved under anesthesia (mixed anesthesia of
0.75-mg/kg bw medatomidine hydrochloride, 4-mg/kg bw midazolam, and
5-mg/kg bw vetorphale in physiological saline and antagonist of
0.75-mg/kg bw atipamezole hydrochloride in physiological saline)
(18), and a 0.5-cm diameter
wound area was excised on both sides of the back using a dermal
punch (Nipro, Osaka, Japan), for initiation of an inflammatory
response. Each mouse was topically treated with Milli Q on one side
(20 μl; control side) and with ASH-WEX on the other side (20
μl; 10 mg/ml; treatment side). Following treatment, two
types of tape (Tegaderm Roll 16006S, 3M Health Care, Tokyo, Japan;
Dressing tape, Kyowa, Osaka, Japan) were placed at the sites, in
order to avoid direct contact with the wound and to prevent
evaporation. The tapes were peeled off gently and changed following
topical treatment. Each mouse was housed separately, and treated
every 24 h. The day when the wounds were made and treated with
ASH-WEX or Milli Q was designated as day 0. The wounds were scanned
and photographed prior to treatment and every day, until day 11.
Wound closure was calculated as follows: wound closure of Day n=Day
n wound area/Day 0 wound area. The weight of each mouse was
measured every day following treatment, with a Table Top High
Precision Balance (A&D Company, San Jose, CA, USA). Relative
wound closure, expressed as the wound size ratio of day n/day 0,
was quantified using ImageJ software.
To investigate cytokine expression, mice were
sacrificed on day 5, and a 1×1 cm area of injured skin was removed
and used for RNA extraction. Total RNA was isolated by TRIzol
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocols. Following reverse transcription, RT-qPCR was performed,
as aforementioned. The following primers were used: TNF-α forward,
CCACCACGCTCTTCTGTCTA and reverse, CACTTGGTGGTTTGCTACGA;TGF-β
forward, TTGCTTCAGCTCCACAGAGA and reverse, TGGTTGTAGAGGGCAAGGAC;
and GAPDH forward, AACTTTGGCATTGTGGAAGG and reverse,
ACACATTGGGGGTAGGAACA.
Hematoxylin and eosin (H&E)
staining
Seven-week-old male C57BL/6J mice were treated with
ASH-WEX or Milli Q as described above. On day 5, six mice were
sacrificed and a 1×1 cm area including the entire wound and
surrounding unwounded skin were collected for H&E staining.
Mouse skin samples were fixed in 4% paraformaldehyde at 4°C for 72
h. The samples were dehydrated and embedded in paraffin and skin
sections were cut at a thickness of 5 μm. Following
deparaffinization and rehydration, the samples were stained with
H&E and the epidermal and dermal thicknesses were measured by
ImageJ. Digital images were captured with an Olympus BX51
microscope (Olympus Corporation, Tokyo, Japan) and DP714 digital
camera (Olympus Corporation).
Statistical analysis
The Student's t-test was used to compare paired
groups. One-way analysis of variance was used for multi-group
analysis, followed by Bonferroni test as a post hoc test (Prism 7,
ver. 7.0d, GraphPad Software, Inc., La Jolla, CA, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Low toxicity effect of ASH-WEX on HaCaT
cells
Cells of the human keratinocyte line HaCaT have
features similar to normal human cells, and thus are widely applied
in inflammatory skin-related research. In the present study, HaCaT
cells were used to investigate the anti-inflammatory effects of
ASH-WEX. To determine whether ASH-WEX may have a toxic effect on
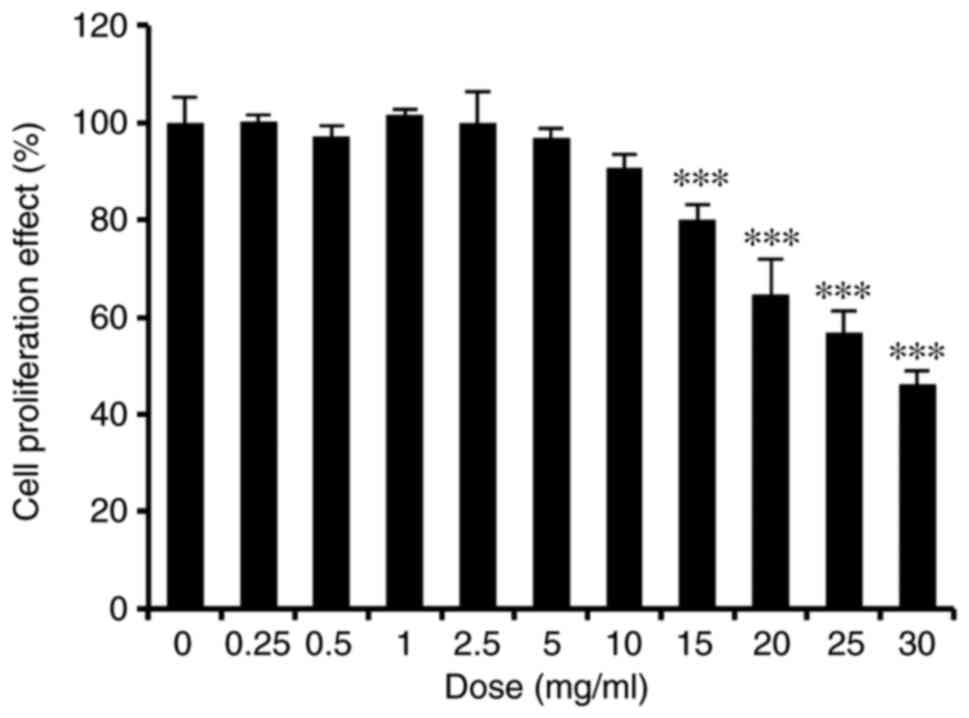
HaCaT cells, an MTT assay was performed. The results demonstrated
that ASH-WEX was not toxic to HaCaT cells up to a dose of 10 mg/ml
(Fig. 1).
ASH-WEX treatment downregulates
inflammatory cytokines, while it upregulates anti-inflammatory
cytokines
Cytokines released from keratinocytes have vital
roles in many inflammation-related skin diseases (19). In order to examine whether ASH-WEX
might regulate the cytokine expression in keratinocytes, the
expression levels of several cytokines were examined in HaCaT cells
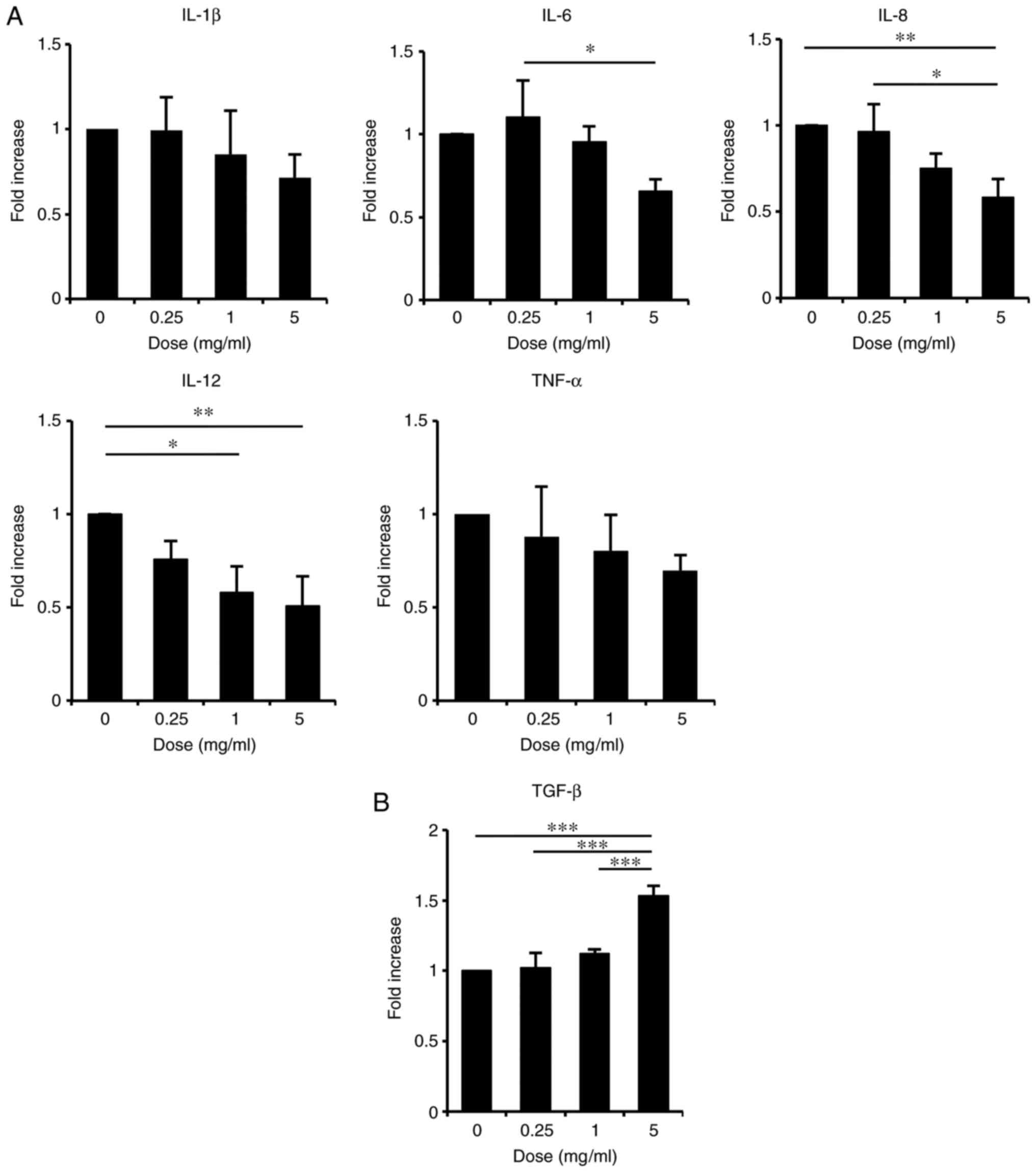
treated with ASH-WEX by RT-qPCR. The results demonstrated that,
when cells were treated with ASH-WEX at concentrations <5 mg/ml
which were non-toxic in HaCaT cells (Fig. 1), the mRNA expression levels of
the inflammatory cytokines IL-8, IL-6 and IL-12 were significantly
decreased in a dose-dependent manner (Fig. 2A). The mRNA levels of TNF-α and
IL-1β also appeared decreased, although this effect was less
significant (Fig. 2A). By
contrast, treatment with ASH-WEX at concentrations <5 mg/ml
significantly increased the mRNA expression levels of the
anti-inflammatory cytokine TGF-β1 in a dose-dependent manner
(Fig. 2B). These results
indicated that ASH-WEX might have an anti-inflammatory effect on
keratinocytes.
HaCaT cells possess Toll-like receptor (TLR) 2 and
TLR4 (20), and the expression of
pro-inflammatory cytokines, including IL-8, IL-6, TNF-α, IL-1β and
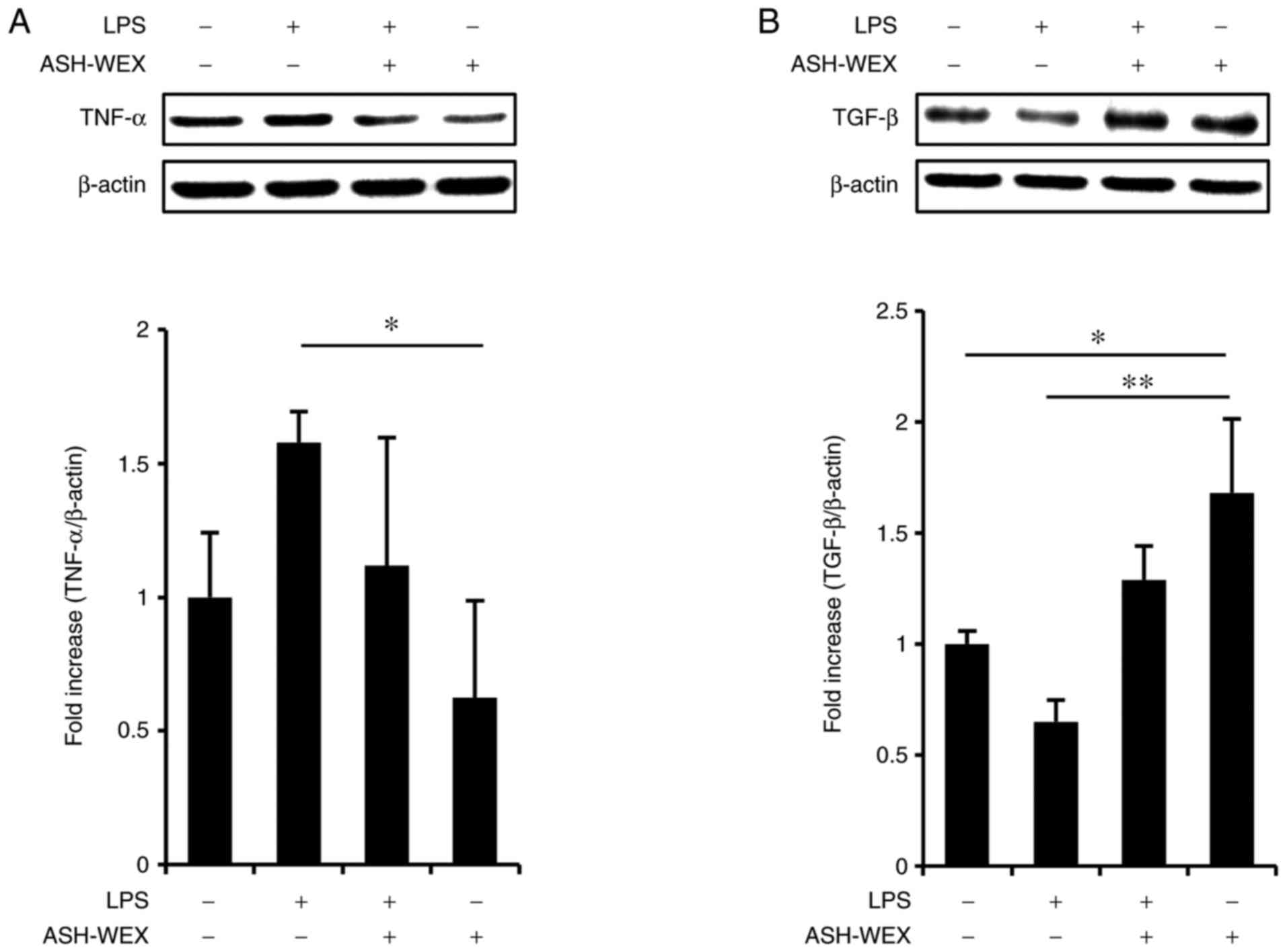
IL-12, are modulated by TLR signaling (21). Therefore the TLR4 agonist, LPS,
was used as a model of inflammation and the effects of ASH-WEX
cytokine production were investigated at the protein level by
western blotting. The results demonstrated that treatment with 5
mg/ml ASH-WEX inhibited the LPS-induced TNF-α upregulation
(Fig. 3A), but significantly
increased TGF-β1 levels (Fig.
3B). These results were in accordance with the mRNA results
(Fig. 2).
ASH-WEX treatment inhibits the MAPK/NF-κB
pathways
The present data suggested that ASH-WEX might have
anti-inflammatory properties by suppressing the expression of
inflammatory cytokines. It is well stablished that the NF-κB and
MAPK signaling pathways are strongly associated with inflammatory
cytokine expression in HaCaT cells (22,23). LPS stimulation activates NF-κB by
modulating TLR4 (24). LPS can
also activate the MAPK pathway in HaCaT cells (22). To determine whether ASH-WEX
suppressed the LPS-induced MAPK and NF-κB pathways, a western blot
analysis was performed. The results revealed that treatment with 5
mg/ml ASH-WEX inhibited the LPS-induced phosphorylation of p38
(Fig. 4A) and JNK (Fig. 4B), but upregulated the
phosphorylation of ERK (Fig. 4C).
Next, it was investigated whether ASH-WEX inhibited LPS-induced
nuclear transduction of NF-κB, and the results demonstrated that
treatment with 5 mg/ml ASH-WEX inhibited the LPS-induced nuclear
translocation of p65 (Fig. 4D),
which is a significant mediator of the NF-κB signaling pathway.
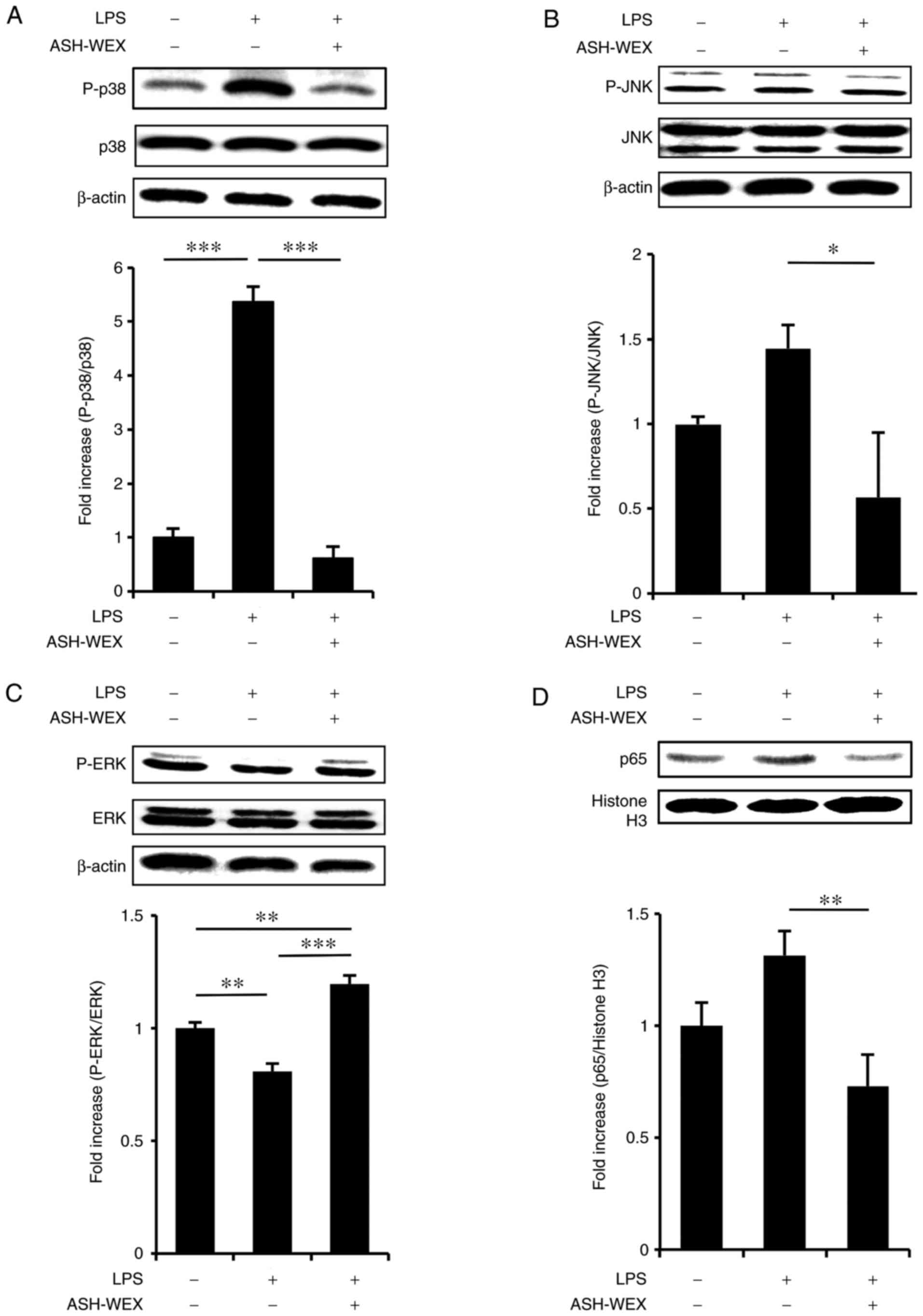 | Figure 4ASH-WEX treatment attenuates the
LPS-induced inflammatory response signaling. HaCaT cells
(1×105 cells) were treated with 5 mg/ml of ASH-WEX for
20 h and then with LPS (1 μg/ml) for 4 h. (A) The levels of
p38 MAPK phosphorylation, (B) JNK phosphorylation, and (C) ERK
phosphorylation were measured by western blotting. β-actin was used
as internal control. (D) The protein levels of p65 NF-κB in the
nuclear fraction of the treated cells were determined by western
blotting. Histone H3 was used as internal control. The relative
density of the bands was estimated and the mean ± standard
deviation of three independent experiments is presented as
quantified data. *P<0.05, **P<0.01 and
***P<0.001 with comparisons indicated by lines.
ASH-WEX, Ashwagandha root water extract; LPS, lipopolysaccharide;
MAPK, mitogen-activated protein kinase; JNK, c-Jun N-terminal
kinase; ERK, extracellular signal-regulated kinase; NF-κB, nuclear
factor-κB; P-, phosphorylated. |
Effect of ASH-WEX on cytokine expression
and wound-closure effect in vivo
Finally, the anti-inflammatory effect of ASH-WEX was
investigated in vivo. It is well known that external
stimuli, such as wounds, initiate inflammation in the skin
(25). In the present study,
wounded mouse skin was employed as an in vivo inflammation
model in order to investigate the anti-inflammatory effects of
ASH-WEX. On each mouse, ASH-WEX was topically applied to a wound on
the left side of the back skin and solvent Milli Q was topically
applied to a wound at the right side of the back skin every 24 h
until day 5. Thereafter, the skin was removed and used to assay the
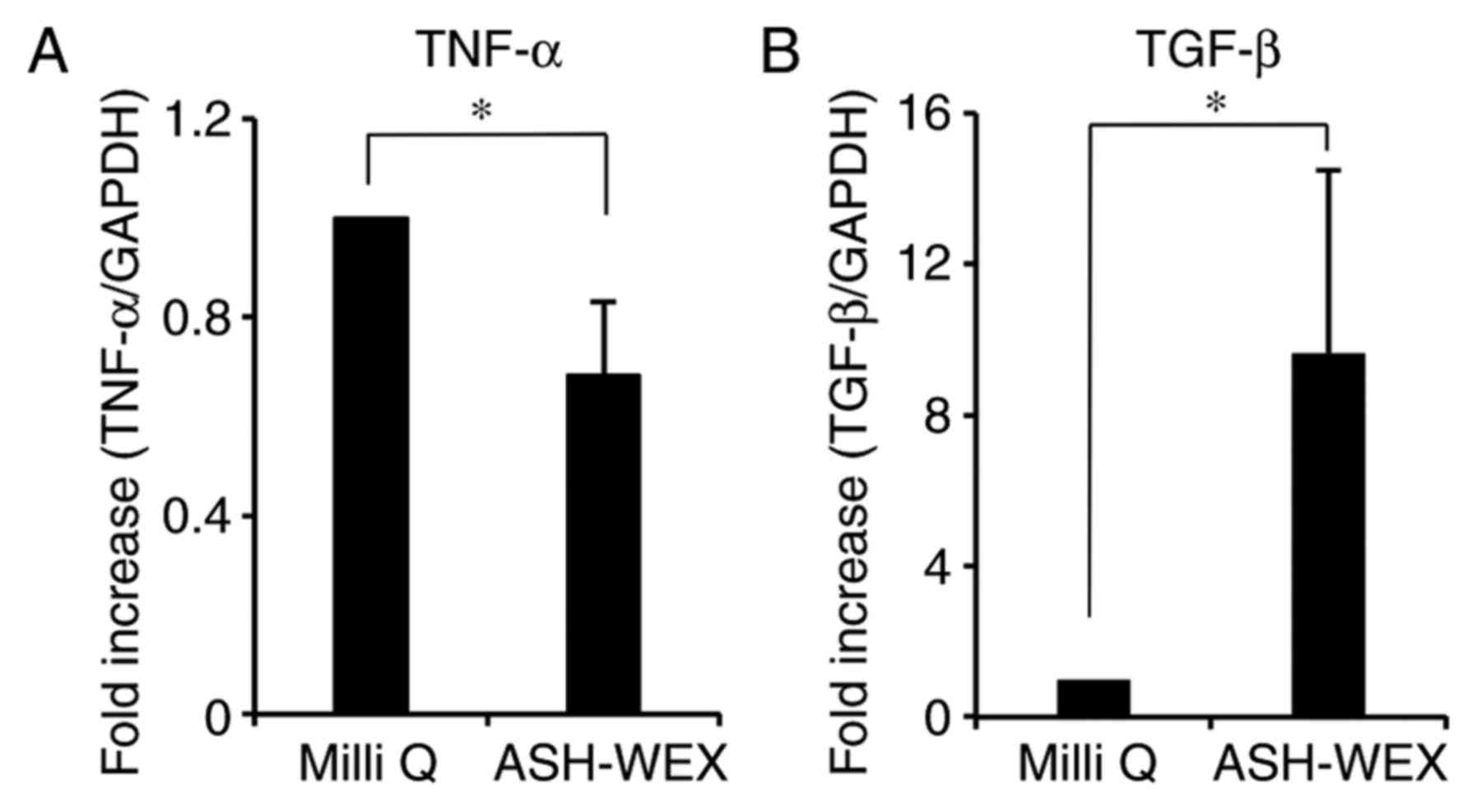
mRNA expression levels of cytokines by RT-qPCR. The results
demonstrated that ASH-WEX treatment inhibited the mRNA expression
of the pro-inflammatory cytokine TNF-α, while it increased the mRNA
expression of the anti-inflammatory cytokine TGF-β1 (Fig. 5). These results further
demonstrated that ASH-WEX may regulate the inflammatory response
associated with wound closure (26).
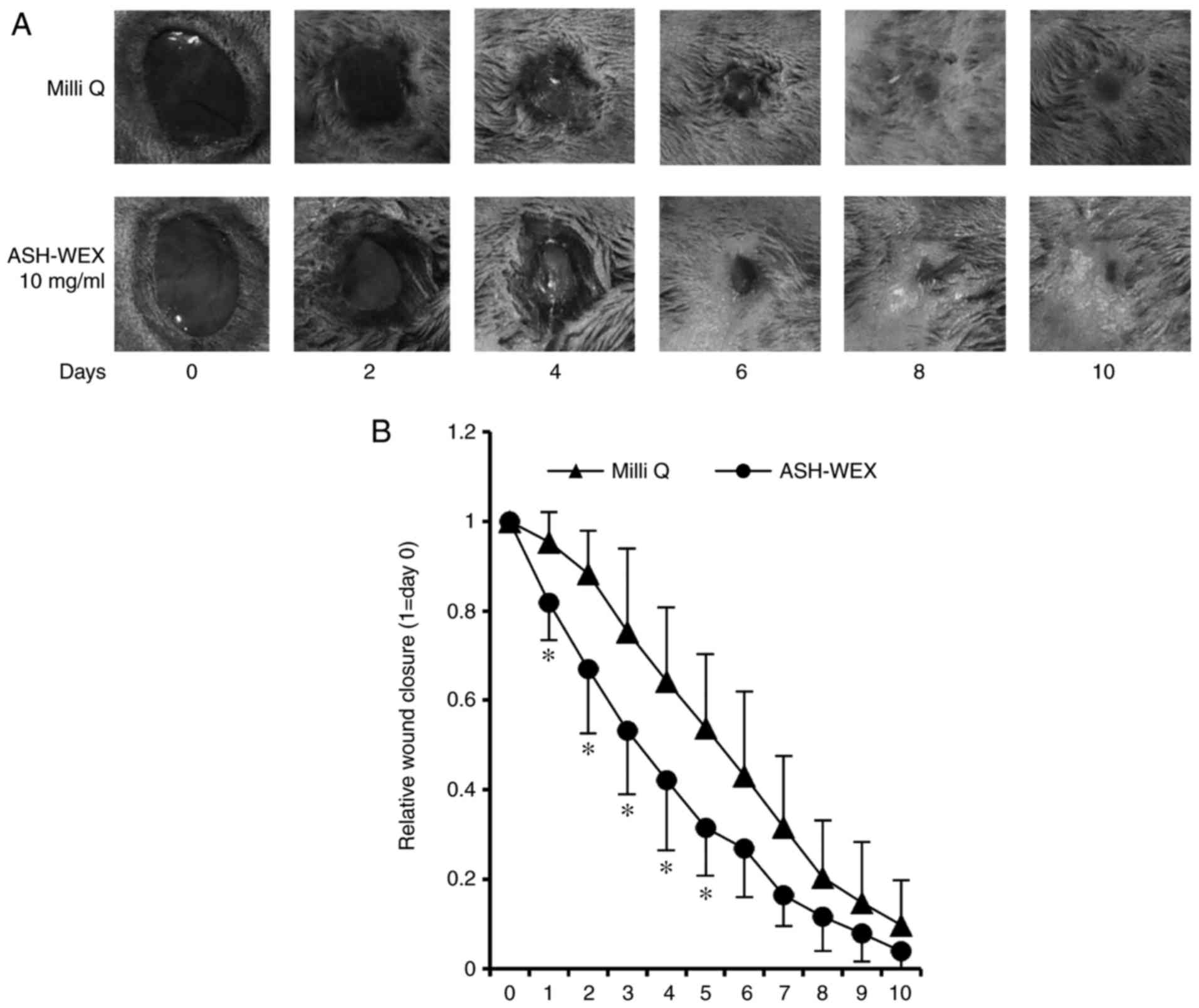
Finally, a wound-healing assay was performed in
vivo to examine the properties of ASH-WEX. The results
demonstrated that, on days 1-5, the ASH-WEX treatment side
exhibited a significantly decreased wound area compared with the
control Milli Q- treated side (Fig.
6). These results suggest that ASH-WEX treatment accelerated
the speed of wound-closure in the early stage, which is the
inflammation phase of wound healing. In addition, the
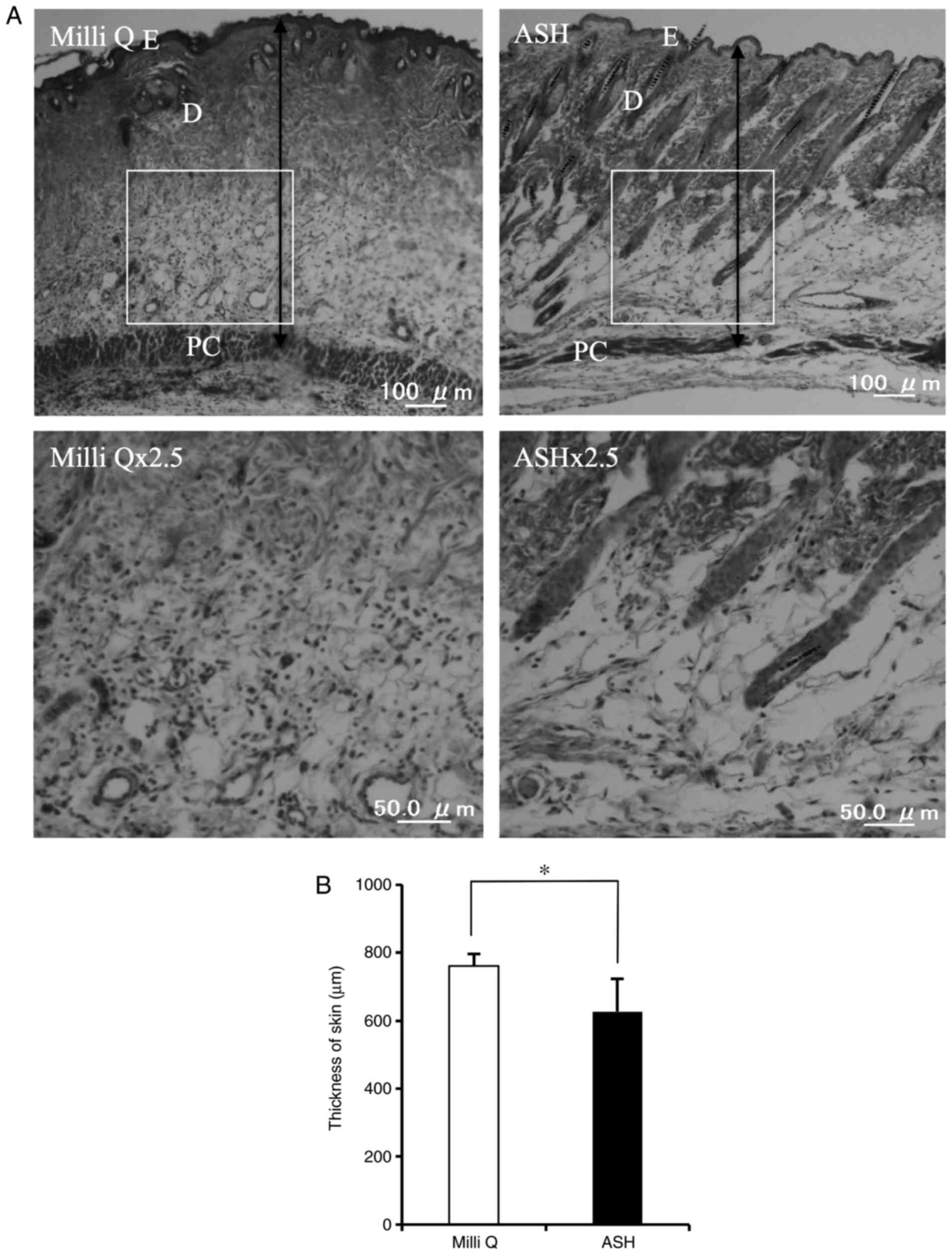
ASH-WEX-treated skin displayed a thinner epidermis and thinner
dermis compared with the control skin (Fig. 7). Furthermore, less aggregation of
immune cells was observed in the ASH-WEX-treated skin compared with
the control-treated skin (Fig.
7). Of note, ASH-WEX treatment did not affect the weight of the
mice (data not shown).
Discussion
The present study investigated the anti-inflammatory
and cytokine modulatory effect of ASH-WEX in vitro. HaCaT
cells were used to examine the cytokine modulatory effect of
ASH-WEX, and the results revealed that ASH-WEX treatment inhibited
the MAPK and NF-κB pathways to decrease the expression of
pro-inflammatory cytokines and increase the expression of
anti-inflammatory cytokines. Finally, ASH-WEX treatment was
demonstrated to also exert an anti-inflammatory effect in
vivo.
The present results indicated that ASH-WEX treatment
resulted in a decrease of pro-inflammatory cytokine expression in
HaCaT cells at both the mRNA and protein level. To investigate the
mechanism underlying these effects, the MAPK pathway and its
downstream pathway NF-κB were investigated, which are strongly
related to pro-inflammatory cytokine release in monocytes (27). Ultraviolet electromagnetic
radiation (UV) and bacteria can activate the MAPK and NF-κB
pathways (4,28). Tang et al (4) confirmed that the anti-inflammatory
effect of glycolic acid is due to its ability to inhibit
UVB-induced mRNA expression of inflammatory cytokines and NF-κB
nuclear translocation in HaCaT cells. In addition, LPS can activate
NF-κB and MAPK signaling in HaCaT cells (20,21). In the present study, LPS-treated
HaCaT cells were used to determine whether ASH-WEX suppressed the
LPS-induced MAPK and NF-κB pathways. The results demonstrated that
ASH-WEX indeed inhibited the LPS-induced phosphorylation of the
MAPKs p38 and JNK, as well as the nuclear translocation of p65
NF-κB. These results are in agreement with the study of Wang et
al (29), in which the
anti-inflammatory agent Baicalein inhibited LPS-induced p38 MAPK
and NF-κB activation. There has been at least one report that
Ashwagandha roots exert an antibacterial effect (30). Based on the present results,
ASH-WEX may also have antibacterial activity.
Multiple plants possess wound-healing activity due
to the anti-inflammatory effects of active components in their
extracts (31). It is well known
that external stimuli, such as wounds, initiate inflammation in the
skin. Indeed, one of the main and early phases of wound healing is
the inflammatory phase, in which many pro-inflammatory mediators,
such as TNF-α, are released in the wound area to initiate
inflammation (32). This
inflammatory response is indispensable for wound repair. However,
an excessive or prolonged inflammation phase will delay wound
healing (33). TGF-β1 serves a
vital role in wound healing, and increased levels of TGF-β1 signal
the transition from inflammatory phase to proliferation phase,
which is another essential stage of wound healing (34). In the present study, at days 1-5
following ASH-WEX treatment, the size of the wound decreased
significantly compared with the control-treated wound. Furthermore,
histological analysis revealed that the immune cells were less
aggregated in the ASH-WEX-treated skin compared with the control
skin. These data suggested that the control side remained in the
inflammatory phase, while the ASH-WEX-treated side rapidly
progressed to the proliferation phase. Ghlissi et al
(35) suggested that the
wound-healing activity of Artemisia campestris aqueous
extract in vivo may be due to the reduction of the
inflammatory phase, and the rapid transition to the proliferation
phase by its anti-inflammatory effect. Collectively, these findings
suggest that ASH-WEX may be a promising wound-healing agent, and
thus the wound-healing activity of ASH-WEX warrants further
investigation.
Both the organic and the aqueous extract of
Ashwagandha have anti-inflammatory activity (36,37). Previous phytochemical studies have
confirmed that Ashwagandha contains alkaloids, flavonols,
withanolides, glycowithanolides, steroidal lactones, sterols and
phenolic acids (7,38-40). The most bioactive ingredients from
the root include sitoindosides (VII, VIII, IX, X) withanine,
withananine, and ashwagandhanolide (41). However, most of the bioactive
components of Ashwagandha have been isolated from its organic layer
extracts. There are few studies about the bioactive ingredients of
ASH-WEX. One of the existing reports on this subject indicates that
the main component of ASH-WEX is Withaferin-A (42), but, when the solubility of
purified Withaferin-A in water was tested by heating for 2 h, the
same condition used to extract ASH-WEX, the Withaferin-A powder
remained in the solution. Another study reported that 5
withanolides and one glyco-withanolide were the active constituents
of ASH-WEX (43). Withanolides
have anti-inflammatory activities (44). To the best of our knowledge, the
anti-inflammatory effects of withanolides on HaCaT cells remain
unclear. Recently, a study reported that triethylene glycol (TEG)
was present in the aqueous extract of Ashwagandha, and particularly
concentrated in the leaves (12).
In the present study, therefore, we tested whether TEG modulates
the levels of cytokine expression in HaCaT cells and revealed that
TEG suppresses the LPS-induced expression of the inflammatory
cytokines TNF-α, IL-1β, IL-6 and IL-8 (data not shown). TEG also
inhibited LPS-induced nuclear transduction of NF-κB (data not
shown). From this result, TEG might be one of the bioactive
components of ASH-WEX that induce anti-inflammation. However, the
anti-inflammatory effects of TEG and its wound-healing activities
require further investigation.
In conclusion, the present study demonstrated that
ASH-WEX had an anti-inflammatory effect, which it exerted by
suppressing the NF-κB and MAPK pathways, and by modulating the
cytokine expression in HaCaT cells. ASH-WEX treatment increased the
mRNA expression of the anti-inflammatory cytokine TGF-β1 and
decreased the mRNA expression of the pro-inflammatory cytokine
TNF-α in vivo. Thus, ASH-WEX could be a promising
anti-inflammatory agent for skin, as well as a potential
wound-healing therapeutic agent.
Abbreviations:
|
TNF
|
tumor necrosis factor
|
|
IL
|
interleukin
|
|
ASH-WEX
|
Ashwagandha root water extract
|
|
LPS
|
lipopolysaccharide
|
|
JNK
|
c-Jun N-terminal kinase
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
NF-κB
|
nuclear factor-κB
|
|
TGF
|
transforming growth factor
|
|
PCR
|
polymerase chain reaction
|
|
MAPK
|
mitogen-activated protein kinase
|
Acknowledgments
We are grateful to Mr. Daiki Yagi for technical
assistance.
References
|
1
|
McGrath JA, Eady RAJ and Pope FM: Anatomy
and organization of human skin. Burns T, Breathnach S, Cox N and
Griffiths C: Rook's Textbook of Dermatology. 7th edition. Blackwell
Publishing Inc; Malden: pp. 3.1–3.84. 2004
|
|
2
|
Juránová J, Franková J and Ulrichová J:
The role of keratinocytes in inflammation. J Appl Biomed.
15:169–179. 2017. View Article : Google Scholar
|
|
3
|
Albanesi C, Scarponi C, Giustizieri ML and
Girolomoni G: Keratinocytes in inflammatory skin diseases. Curr
Drug Targets Inflamm Allergy. 4:329–334. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tang SC, Liao PY, Hung SJ, Ge JS, Chen SM,
Lai JC, Hsiao YP and Yang JH: Topical application of glycolic acid
suppresses the UVB induced IL-6, IL-8, MCP-1 and COX-2 inflammation
by modulating NF-κB signaling pathway in keratinocytes and mice
skin. J Dermatol Sci. 86:238–248. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim M, Lee HJ, Randy A, Yun JH, Oh SR and
Nho CW: Stellera chamaejasme and its constituents induce cutaneous
wound healing and anti-inflammatory activities. Sci Rep.
7:424902017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ghlissi Z, Kallel R, Sila A, Harrabi B,
Atheymen R, Zeghal K, Bougatef A and Sahnoun A: Globularia alypum
methanolic extract improves burn wound healing process and
inflammation in rats and possesses antibacterial and antioxidant
activities. Biomed Pharmacother. 84:1488–1495. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kurian A and Asha Sankar M: Medicinal
Plants. Peter KV: New India Publishing; New Delhi: pp. 61–68.
2007
|
|
8
|
Prakash J, Gupta SK and Dinda AK: Withania
somnifera root extract prevents DMBA induced squamous cell
carcinoma of skin in swiss albino mice. Nutr Cancer. 42:91–97.
2002. View Article : Google Scholar
|
|
9
|
Imokawa G and Ishida K: Inhibitors of
intracellular signaling pathways that lead to stimulated epidermal
pigmentation: Perspective of anti-pigmenting agents. Int J Mol Sci.
15:8293–8315. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sharma Beena: Health awareness in women
with the use of traditional medicinal plants in district raipur of
Chhattisgarh. J Ecobiotech. 3:15–17. 2011.
|
|
11
|
Kanika P, Singh RB and Dinesh KP:
Pharmacological and analytical aspects of withaferin A: A concise
report of current scientific literature. Asian Pac J Reprod.
2:238–243. 2013. View Article : Google Scholar
|
|
12
|
Wadhwa R, Singh R, Gao R, Shah N, Widodo
N, Nakamoto T, Ishida Y, Terao K and Kaul SC: Water extract of
Ashwagandha leaves has anticancer activity: Identification of an
active component and its mechanism of action. PLoS One.
8:e771892013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bhat J, Damle A, Vaishnav PP, Albers R,
Joshi M and Banerjee G: In vivo enhancement of natural killer cell
activity through tea fortified with Ayurvedic herbs. Phytother Res.
24:129–135. 2010. View
Article : Google Scholar
|
|
14
|
Boukamp P, Petrussevska RT, Breitkreutz D,
Hornung J, Markham A and Fusenig NE: Normal keratinization in a
spontaneously immortalized aneuploid human keratinocyte cell line.
J Cell Biol. 106:761–771. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Suita H, Shinomiya T and Nagahara Y:
Caspase-6 induces 7A6 antigen localization to mitochondria during
FAS-induced apoptosis of Jurkat cells. Anticancer Res.
37:1697–1704. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tomar D, Sripada L, Prajapati P and Singh
R, Singh AK and Singh R: Nucleo-cytoplasmic trafficking of TRIM8, a
novel oncogene, is involved in positive regulation of TNF induced
NF-κB pathway. PLoS One. 7:e486622012. View Article : Google Scholar
|
|
18
|
Kawai S, Takagi Y, Kaneko S and Kurosawa
T: Effect of three types of mixed anesthetic agents alternate to
ketamine in mice. Exp Anim. 60:481–487. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hänel KH, Cornelissen C, Lüscher B and
Baron JM: Cytokines and the skin barrier. Int J Mol Sci.
14:6720–6745. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pivarcsi A, Koreck A, Bodai L, Széll M,
Szeg C, Belso N, Kenderessy-Szabó N, Bata-Csörgo Z, Dobozy A and
Kemény A: Differentiation-regulated expression of Toll-like
receptors 2 and 4 in HaCaT keratinocytes. Arch Dermatol Res.
296:120–124. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Su SB, Silver PB, Grajewski RS, Agarwal
RK, Tang J, Chan CC and Caspi RR: Essential role of the MyD88
pathway, but nonessential roles of TLRs 2, 4, and 9, in the
adjuvant effect promoting Th1-mediated autoimmunity. J Immunol.
175:6303–6310. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lv F, Yu Y, Wang G, Hu J, Zhang B, You W
and Wang J: Mechanisms by which the N-terminal 24 amino acids of
the p55 regulatory subunit of phosphatidylinositol 3-kinase affect
endotoxin-induced cytokine release in human keratinocytes. Mol Med
Rep. 11:3753–3759. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Zhang H, Du G, Wang Y, Cao T, Luo
Q, Chen J, Chen F and Tang G: Total glucosides of paeony (TGP)
inhibits the production of inflammatory cytokines in oral lichen
planus by suppressing the NF-κB signaling pathway. Int
Immunopharmacol. 36:67–72. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ge Y, Xu Y, Sun W, Man Z, Zhu L, Xia X,
Zhao L, Zhao Y and Wang X: The molecular mechanisms of the effect
of Dexamethasone and Cyclosporin A on TLR4/NF-κB signaling pathway
activation in oral lichen planus. Gene. 508:157–164. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gurtner GC, Werner S, Barrandon Y and
Longaker MT: Wound repair and regeneration. Nature. 453:314–321.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
César I, Paggiaro AO, Conduta JL, Aldunate
B, Herson MR, Altran SC, Mathor MB and Ferreira MC: Role of
keratinocytes in wound contraction: An impact assessment using a
model of collagen matrix populated with fibroblasts. Revista
Brasileira de Cirurgia Plástica. 26:402–406. 2011. View Article : Google Scholar
|
|
27
|
Muthusamy V and Piva TJ: The UV response
of the skin: A review of the MAPK, NF-kappaB and TNF-alpha signal
transduction pathways. Arch Dermatol Res. 302:5–17. 2010.
View Article : Google Scholar
|
|
28
|
Chung WO and Dale BA: Innate immune
response of oral and foreskin keratinocytes: Utilization of
different signaling pathways by various bacterial species. Infect
Immun. 72:352–358. 2004. View Article : Google Scholar :
|
|
29
|
Wang J, Luo H, Yang L and Li Y: Baicalein
induces apoptosis and reduces inflammation in LPS-stimulated
keratinocytes by blocking the activation of NF-κB: Implications for
alleviating oral lichen planus. Cell Mol Biol (Noisy-le-grand).
62:55–60. 2016.
|
|
30
|
Girish KS, Machiah KD, Ushanandini S,
Harish Kumar K, Nagaraju S, Govindappa M, Vedavathi M and Kemparaju
K: Antimicrobial properties of a non-toxic glycoprotein (WSG) from
Withania somnifera (Ashwagandha). J Basic Microbiol. 46:365–374.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Budovsky A, Yarmolinsky L and Ben-Shabat
S: Effect of medicinal plants on wound healing. Wound Repair Regen.
23:171–183. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Spiekstra SW, Breetveld M, Rustemeyer T,
Scheper RJ and Gibbs S: Wound-healing factors secreted by epidermal
keratinocytes and dermal fibroblasts in skin substitutes. Wound
Repair Regen. 15:708–717. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamanaka O, Kitano-Izutani A, Tomoyose K
and Reinach PS: Pathobiology of wound healing after glaucoma
filtration surgery. BMC Ophthalmol. 15(Suppl 1): 1572015.
View Article : Google Scholar
|
|
34
|
Zhang F, Wang H, Wang X, Jiang G, Liu H,
Zhang G, Wang H, Fang R, Bu X, Cai S and Du J: TGF-β induces
M2-like macrophage polarization via SNAIL-mediated suppression of a
pro-inflammatory phenotype. Oncotarget. 7:52294–52306.
2016.PubMed/NCBI
|
|
35
|
Ghlissi Z, Sayari N, Kallel R, Bougatef A
and Sahnoun Z: Antioxidant, antibacterial, anti-inflammatory and
wound healing effects of Artemisia campestris aqueous extract in
rat. Biomed Pharmacother. 84:115–122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gupta A and Singh S: Evaluation of
anti-inflammatory effect of Withania somnifera root on
collagen-induced arthritis in rats. Pharm Biol. 52:308–320. 2014.
View Article : Google Scholar
|
|
37
|
Pawar P, Gilda S, Sharma S, Jagtap S,
Paradkar A, Mahadik K, Ranjekar P and Harsulkar A: Rectal gel
application of Withania somnifera root extract expounds
anti-inflammatory and muco-restorative activity in TNBS-induced
inflammatory bowel disease. BMC Complement Altern Med. 11:342011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Singh P, Guleri R, Singh V, Kaur G,
Kataria H, Singh B, Kaur G, Kaul SC, Wadhwa R and Pati PK:
Biotechnological interventions in Withania somnifera (L.) Dunal.
Biotechnol Genet Eng Rev. 31:1–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Trivedi MK, Panda P, Sethi KK and Jana S:
Metabolite profiling in Withania somnifera roots hydroalcoholic
extract using LC/MS, GC/MS and NMR spectroscopy. Chem Biodivers.
14:2017. View Article : Google Scholar
|
|
40
|
Alam N, Hossain M, Khalil MI, Moniruzzaman
M, Sulaiman SA and Gan SH: High catechin concentrations detected in
Withania somnifera (ashwagandha) by high performance liquid
chromatography analysis. BMC Complement Altern Med. 11:652011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mill BA, Khazir J, Mir NA, Hasan TU and
Koul S: Botanical, chemical and pharmacological review of Withania
somnifera (Indian Ginseng): An ayurvedic medicinal plant. Indian J
Drugs Dis. 1:147–160. 2012.
|
|
42
|
Halder B, Singh S and Thakur SS: Withania
somnifera root extract has potent cytotoxic effect against human
nalignant nelanoma cells. PLoS One. 10:e01374982015. View Article : Google Scholar
|
|
43
|
Bani S, Gautam M, Sheikh FA, Khan B, Satti
NK, Suri KA, Qazi GN and Patwardhan B: Selective Th1 up-regulating
activity of Withania somnifera aqueous extract in an experimental
system using flow cytometry. J Ethnopharmacol. 107:107–115. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sun GY, Li R, Cui J, Hannink M, Gu Z,
Fritsche KL, Lubahn DB and Simonyi A: Withania somnifera and its
withanolides attenuate oxidative and inflammatory responses and
up-regulate antioxidant responses in BV-2 microglial cells.
Neuromolecular Med. 18:241–252. 2016. View Article : Google Scholar : PubMed/NCBI
|