Introduction
Asthma is one of the most frequent chronic
respiratory diseases worldwide and its prevalence has increased
over the last decade (1). This
disease is generally characterized by a chronic inflammatory
response in all airways, which leads to increased airway
mucus-secreting cells and increased mucus secretion, and
contributes to the formation of mucus plugs that occlude asthmatic
airways, potentially leading to possibly fatal asthma attacks
(2,3). Although current pharmacotherapies
are effective for most patients, asthma has yet to be controlled in
a systematic and satisfactory manner, and no specific treatment is
currently available for mucus hypersecretion (4).
Curcumin, a polyphenol, is the major active
component of turmeric. Various studies have documented the
therapeutic benefits of curcumin in managing various disorders due
to its anti-inflammatory, anti-oxidant and anti-viral properties
(5–7). Curcumin was previously reported to
effectively control the occurrence and development of asthma by
reducing inflammation, inhibiting airway remodeling and maintaining
structural integrity (8).
Furthermore, Heo et al (9)
revealed that curcumin suppressed endothelial growth factor
(EGF)-induced mucin 5AC (MUC5AC) production in human airway
epithelial cells, which indicated that it may be a promising agent
for treating mucin overproduction. However, the underlying
mechanisms have remained to be elucidated.
MUC5AC expression is induced by various stimuli,
including interleukin-13 (IL-13), tumor necrosis factor-α, EGF,
neutrophil elastase and bacterial products (10–13). These stimuli are principally
mediated via the EGF receptor (EGFR) signaling pathway, including
the downstream c-Jun-N-terminal kinase, extracellular
signal-regulated kinase or AKT, which has been identified as a key
effector for mucin production (14–16). According to Heo et al
(9), curcumin inhibited MUC5AC
protein production that was induced by EGF, indicating that the
EGFR-mediated signaling pathway may be a target for curcumin;
however, the exact mechanism remains elusive.
In the present study, the effect of curcumin on
MUC5AC expression in NCI-H292 cells was investigated and its role
in regulating the EGFR signaling pathway was explored.
Materials and methods
Cell culture
The NCI-H292 human lung mucoepidermoid carcinoma
cell line (The Cell Bank of Type Culture Collection of Chinese
Academy of Sciences, Shanghai, China) was maintained in RPMI-1640
medium supplemented with 10% fetal bovine serum, penicillin and
streptomycin (all from Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Cells were treated with curcumin (20 μM at 37°C for 0,
1, 3, 24 and 48 h or 10 and 30 min; 10 μM at 37°C for 48 h;
Selleck Chemicals, Houston, TX, USA), EGF (50 ng/ml at 37°C for 0,
3, 24 and 48 h, or 10 and 30 min; PeproTech, Inc., Rocky Hill, NJ,
USA), PI3K inhibitor LY294002 (25 μM at 37°C for 30 min or
48 h) and a STAT3 inhibitor STATTIC (10 μM at 37°C for 48 h)
(both from Selleck Chemicals).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated using TRIzol reagent (Thermo
Fisher Scientific, Inc.) and first-strand complementary DNAs were
prepared using a random hexamer primer according to the
instructions included in the First-Strand Synthesis kit (cat. no.
04897030001; Roche Diagnostics, Basel, Switzerland). PCR was
performed using specific forward and reverse primers (MUC5AC
forward, 5′-GCTTCCTGCTCCGAGATGT-3′ and reverse,
5′-AAGACGCAGCCCTCATAGAA-3′; β-actin forward,
5′-CCAACCGCGAGAAGATGA-3′ and reverse, 5′-CCAGAGGCGTACAGGGATAG-3′;
EGFR forward, 5′-AGGCACGAGTAACAAGCTCAC-3′ and reverse,
5′-ATGAGGACATAACCAGCCACC-3′). Real-time PCR was performed using
Universal Master Mix (Roche Diagnostics) on an ABI Real-Time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
conditions for the amplifications were as follows: 10 min at 95°C,
followed by 45 two-step cycles at 95°C for 15 sec and 60°C for 1
min. The relative expression levels of the MUC5AC and EGFR were
normalized against β-actin. PCR results were quantified by using
the 2−ΔΔCq method (17).
Western blot analysis
The cells were washed once in PBS and dissolved in
cell lysis reagents (Cell Signaling Technology, Inc., Danvers, MA,
USA). The lysis buffer constituted 20 mM Tris-HCl (pH 7.5), 150 mM
NaCl, 1 mM Na2 EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium
pyrophosphate, 1 mM β-glycerophosphate, 1 mM
Na3VO4 and 1 μg/ml leupeptin. The
supernatants were harvested after centrifugation at 13,400 × g for
15 min in 4°C. The protein concentrations were determined by the
bicinchoninic acid assay (Beyotime Institute of Biotechnology,
Jiangsu, China). The total isolated protein was separated by 6%
SDS-PAGE for MUC5AC detection as described by Ruchaud-Sparagano
et al (18) or 10%
SDS-PAGE for the other target proteins, followed by transfer to a
polyvinylidene difluoride (PVDF) membrane (EMD Millipore,
Billerica, MA, USA). The PVDF membranes were blocked with 5% bovine
serum albumin (Sangon Biotech, Co., Ltd., Shanghai, China), washed
twice with Tris-buffered saline containing Tween-20 (TBST), and
then incubated with the antibodies separately overnight at 4°C. The
antibodies to β-actin (1:1,000; cat. no. 4967), phosphorylated
(p)-EGFR (Tyr1045 or Tyr1068) (1:1,000; cat. nos. 2237 or 3777,
respectively), EGFR (1:1,000; cat. no. 4267), AKT (1:1,000; cat.
no. 4691), p-AKT (Ser473) (1:1,000; cat. no. 4060), STAT3 (1:1,000;
cat. no. 4904) and p-STAT3 (1:1,000; cat. no. 9145) were all
purchased from Cell Signaling Technology, Inc. and the antibodies
to MUC5AC (1:1,000; cat. no. sc-21701), were obtained from Santa
Cruz Biotechnology Inc. (Dallas, TX, USA). The membranes were then
washed with TBST three times, followed by incubation with
horseradish peroxidase-conjugated anti-rabbit immunoglobulin G
(1:1,000; cat. no. 7074S; Cell Signaling Technology, Inc.) as the
secondary antibody for 2 h at room temperature. Finally,
immunoreactive bands were detected by enhanced chemiluminescence
reagent (Bio-Rad Laboratories, Inc., Hercules, CA, USA) by
luminometer (Shanghai PeiQing Science & Technology, Co., Ltd.,
Shanghai, China). Protein levels were quantified using ImageJ
version 1.51 software (National Institutes of Health, Bethesda, MD,
USA).
Nuclear protein extraction
NCI-H292 cells were harvested and nuclear protein
fractions were isolated using a NE-PER Nuclear and Cytoplasmic
Extraction Kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions.
Immunocytochemistry
Cells were seeded in 96-well plates at a density of
1×104 cells/well and treated with curcumin, EGF,
STATTIC, or LY294002. Cells were fixed on slides using 4%
paraformaldehyde for 2 min at room temperature. The cells were
permeabilized three times for 5 min with 0.1% Triton X-100 in PBS
and blocked with blocking buffer (10% normal goat serum purchased
from Hangzhou Sijiqing Biological Engineering Materials Co., Ltd.,
Hangzhou, China, 0.1% Triton X-100) for 30 min at room temperature.
After blocking, the cells were washed with PBS and incubated for 2
h at 37°C with biotin-labeled anti-human MUC5AC (1:200; cat. no.
Ab79082; Abcam, Cambridge, UK). Subsequently, the cells were washed
three times with PBS, incubated for 30 min with horseradish
peroxidase-streptavidin (1:1,000; cat. no. 43-8323; Thermo Fisher
Scientific, Inc.), and washed with PBS for another three times. The
sections were visualized using diaminobenzidine solution (Fuzhou
Maixin Biotech Co., Ltd., Fuzhou, China) for 1 min at room
temperature.
Statistical analysis
SPSS version 21.0 software (IBM Corp., Armonk, NY,
USA) was used for all data analyses. Values are expressed as the
mean ± standard deviation. Statistical significance was determined
by one-way analysis of variance followed by the Dunnett's method or
the least-significant differences test. P<0.05 was considered to
indicate a statistically significant difference.
Results
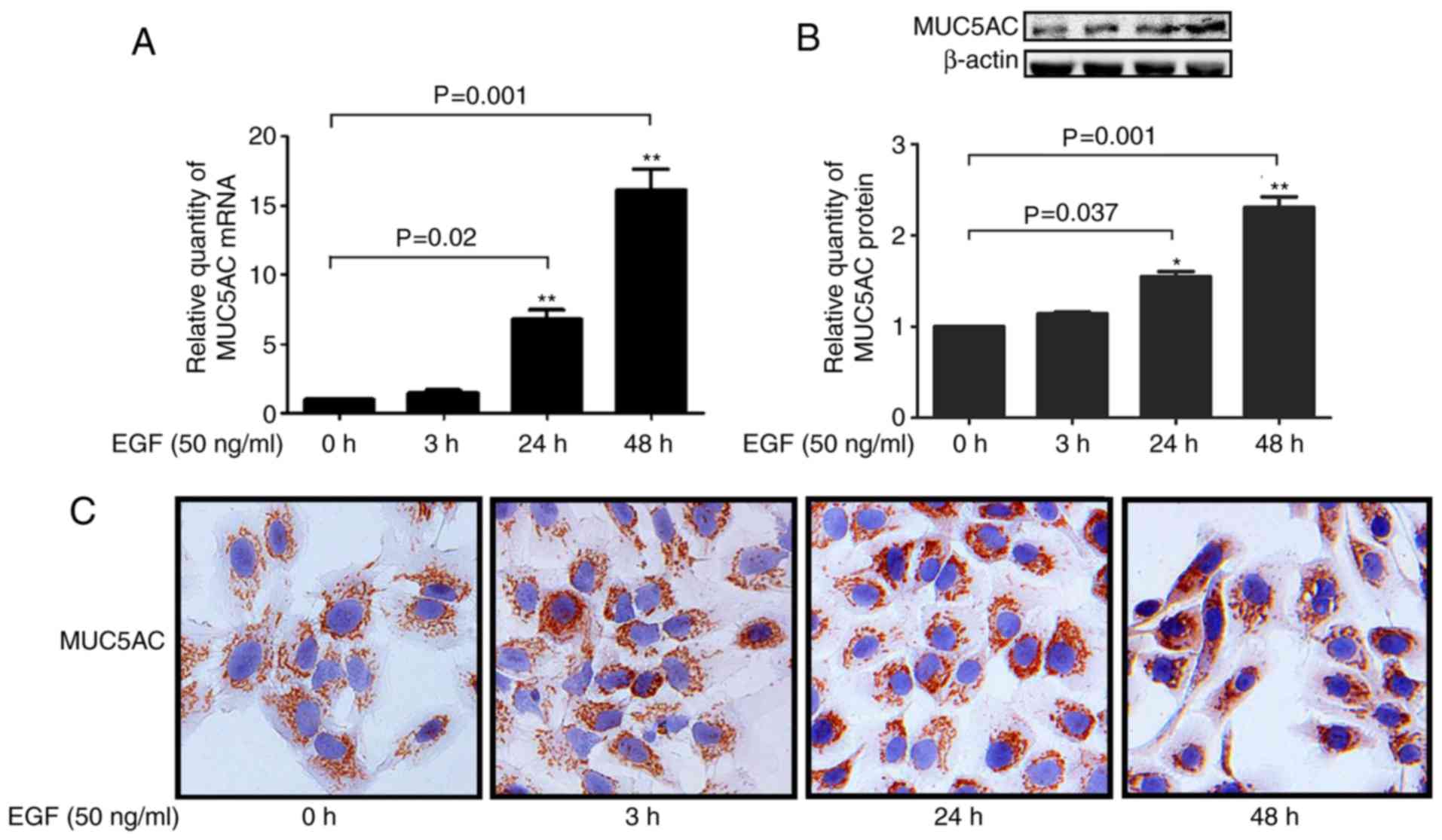
Effect of EGF on MUC5AC expression
NCI-H292 cells were used to evaluate the effect of
EGF on MUC5AC expression in airway cells. NCI-H292 cells were used
as an in vitro model for mucin production, as this cell line
shares key components of mucin signaling pathways (19). EGF-stimulated H292 cells displayed
upregulated expression of MUC5AC mRNA in a time-dependent manner
(Fig. 1A), and EGF treatment
significantly increased MUC5AC protein expression as detected by
western blot analysis and immunohistochemistry (Fig. 1B and C), which is consistent with
the upregulation at the mRNA level.
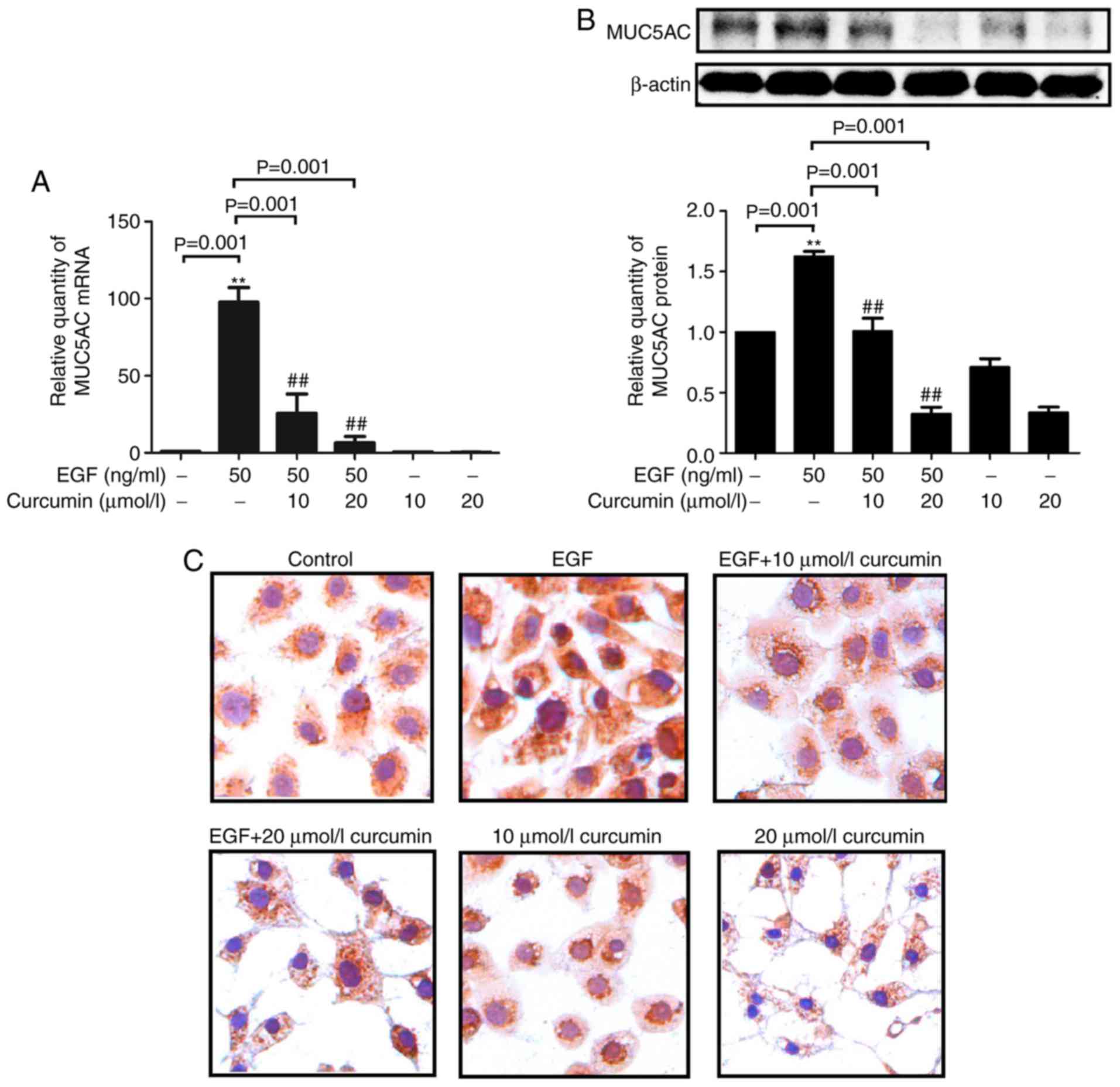
Curcumin inhibits EGF-induced expression
of MUC5AC
EGF treatment slightly increased MUC5AC expression
after 3 h, but the expression was markedly upregulated at 24 and 48
h (Fig. 1). NCI-H292 cells were
then treated with curcumin (10 and 20 μM) plus EGF (50
ng/ml) for 48 h. Curcumin treatment markedly decreased MUC5AC mRNA
expression in a dose-dependent manner in EGF-stimulated H292 cells
(Fig. 2A). In addition, MUC5AC
protein expression was greatly reduced in the curcumin-treated,
EGF-stimulated H292 cells compared with that in the
curcumin-untreated, EGF-stimulated H292 cells, which is consistent
with the down-regulation of MUC5AC mRNA observed (Fig. 2B). Decreases of EGF-induced MUC5AC
protein expression by curcumin were also confirmed by
immunocytochemistry (Fig.
2C).
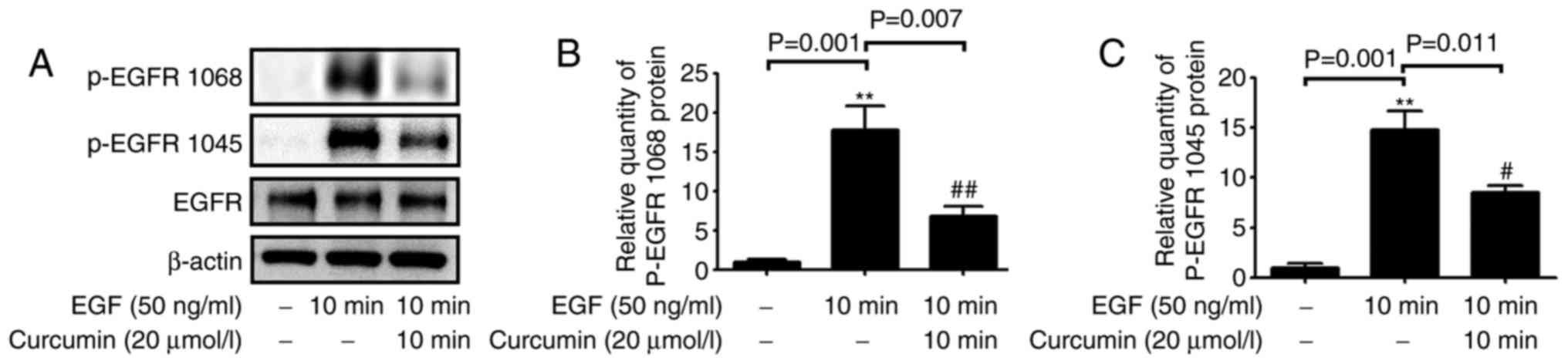
Curcumin inhibits EGF-induced activation
of EGFR phosphorylation
In order to explore the effect of curcumin on the
EGFR signaling pathway, activation of EGFR in NCI-H292 cells was
investigated after treatment with EGF and curcumin. The results
indicated that curcumin treatment inhibited EGFR phosphorylation at
Tyr1045 and Tyr1068 in EGF-stimulated cells (Fig. 3).
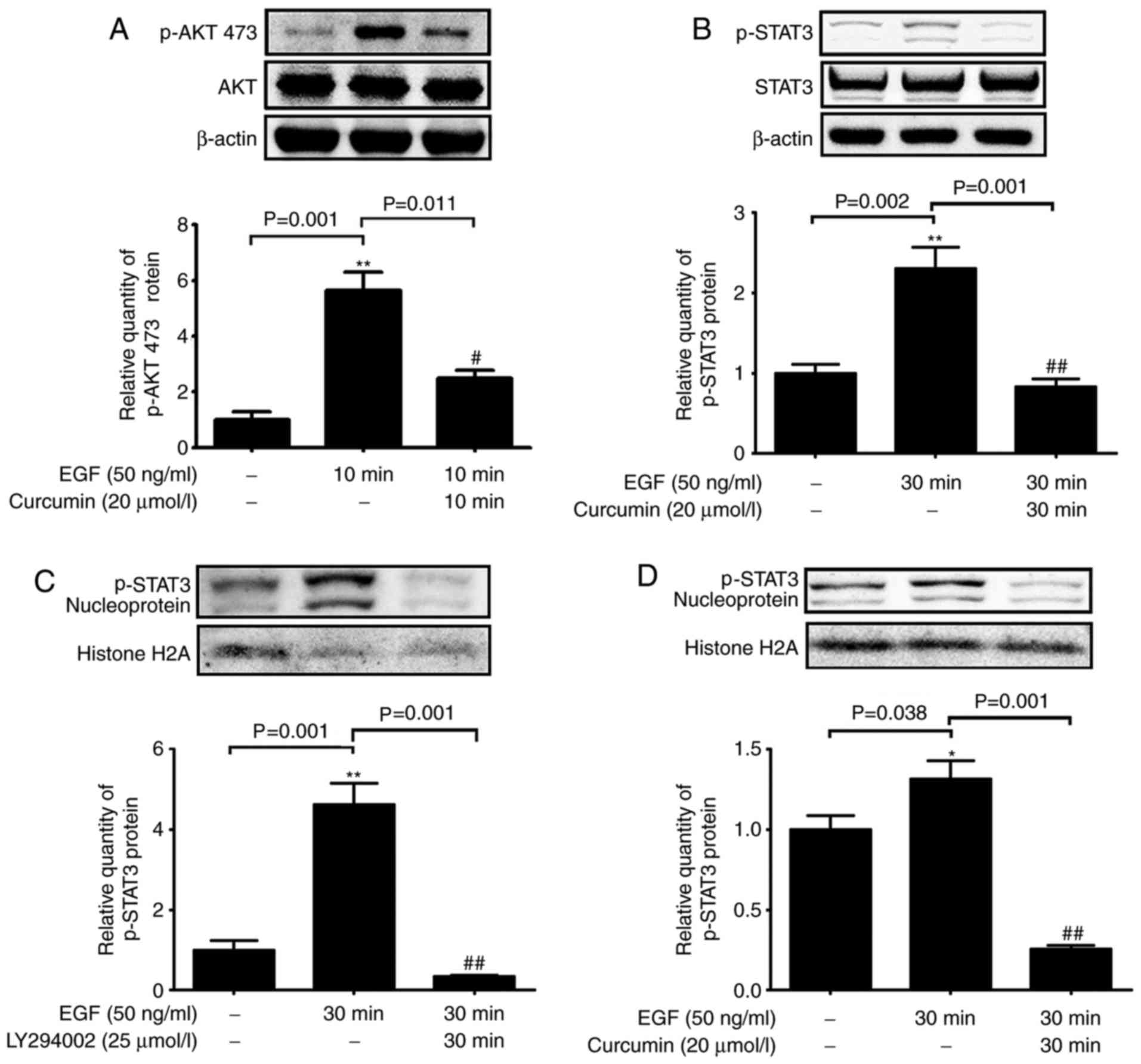
The AKT-STAT3 pathway is responsible for
the inhibitory effect of curcumin on EGF-induced MUC5AC
expression
The AKT-STAT3 pathway is a downstream target of EGFR
signaling (20); thus, the
phosphorylation status of AKT and STAT3 was determined in NCI-H292
cells treated with EGF and curcumin. EGF-stimulated NCI-H292 cells
displayed elevated levels of p-AKT-Ser473 and p-STAT3; however,
simultaneous curcumin treatment reduced this phosphorylation
(Fig. 4A and B). In addition,
EGF-induced increases in STAT3 phosphorylation in the nuclear
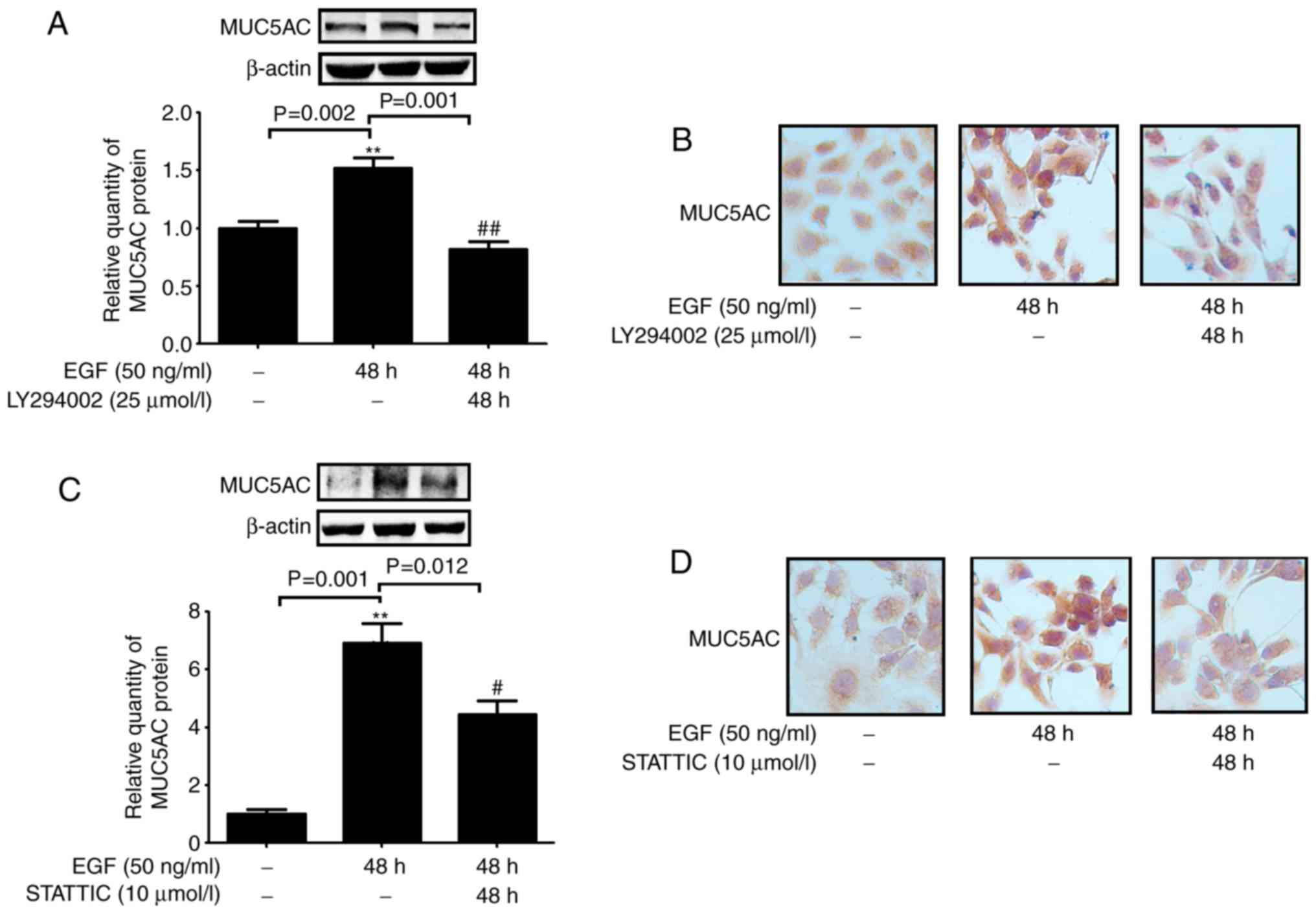
fraction were inhibited by curcumin and an PI3K inhibitor (Fig. 4C and D). In addition, NCI-H292
cells treated with an PI3K inhibitor (LY294002) and a STAT3
inhibitor (STATTIC) exhibited a significant reduction in MUC5AC
expression compared with that in EGF-stimulated cells (Fig. 5). These results indicated that
curcumin attenuated MUC5AC expression by inhibiting the
PI3K/AKT/STAT3 signaling pathway.
Curcumin inhibits the expression of
MUC5AC and EGFR under basal conditions
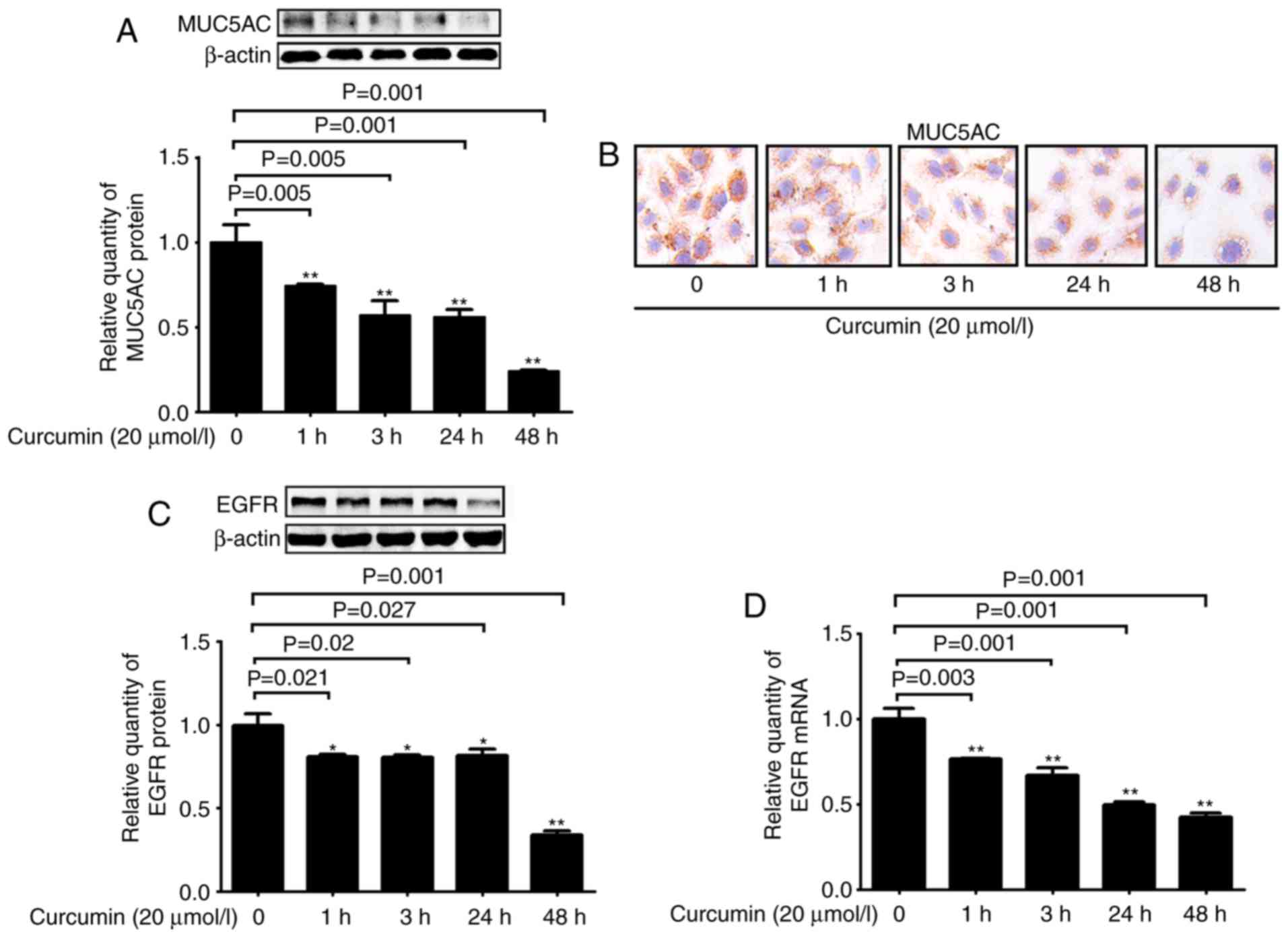
The effect of curcumin on MUC5AC expression under
basal conditions was next investigated. Treatment with curcumin
significantly decreased MUC5AC expression in a time-dependent
manner at the protein level (Fig. 6A
and B). In addition, the expression of EGFR was also decreased
after curcumin treatment (Fig. 6C and
D). These results suggested that downregulation of EGFR
expression was involved in the inhibitory effects of curcumin.
Discussion
Mucus hypersecretion is an independent risk factor
for asthma that has an important impact on its occurrence,
development and prognosis (21).
It has been indicated that curcumin, a naturally occurring dietary
polyphenol, has beneficial effects against the acute allergic
asthma response (22). However,
the potential role of curcumin in reducing mucus hypersecretion in
asthma has remained to be fully investigated. The present study
investigated the possible mechanism of action of curcumin in
reducing airway mucus hypersecretion. Assessment of changes in
p-EGFR, p-AKT, p-STAT3 levels and EGFR expression under basal and
EGF-stimulated conditions revealed that curcumin displays
inhibitory effects on MUC5AC expression under such conditions by
interfering with EGFR signaling in H292 cells.
Activation of the EGFR signaling pathway has been
reported to have a critical role in mucin regulation (23–25). EGF binds to the extracellular
domain of EGFR, which results in receptor dimerization and
phosphorylation of the intracellular domains. Activated EGFR
activates downstream molecules, including AKT, which in turn
activate MUC5AC gene expression. Therefore, increased EGFR kinase
activity is critical for constitutive mucin production (26,27). In the present study, the AKT/STAT3
pathway was assessed as a downstream target of EGFR signaling, and
was identified to be responsible for the inhibitory effect of
curcumin on EGF-induced MUC5AC expression. More importantly, EGF
treatment of NCI-H292 cells increased MUC5AC, p-AKT and p-STAT3
levels, whereas co-treatment with the PI3K inhibitor LY294002 or
curcumin reduced MUC5AC, p-AKT and p-STAT3 levels. These results
implied that EGF induced crosstalk between the EGFR/STAT3 and
EGFR/AKT pathways in regulating MUC5AC expression, whereas curcumin
may interfere with this signaling cascade. However further loss-and
gain-of function experiments should be conducted to fully
demonstrate this mechanism.
In the present study, curcumin significantly
inhibited EGFR phosphorylation in NCI-H292 cells. Using biomimetic
membrane models, Starok et al (28) observed that curcumin is inserted
into the lipid bilayer and may affect receptor dimerization, which
in turn affects EGFR activation. However, additional study is
required to confirm this mechanism. In the present study, curcumin
altered total EGFR expression in NCI-H292 cells, which indicated
that inhibition of EGFR expression partially reflected the
molecular mechanism by which curcumin regulates EGFR
phosphorylation. The regulatory mechanisms of EGFR expression by
curcumin have been investigated by several groups (29,30). In the present study, changes in
EGFR phosphorylation occurred earlier than the decrease in EGFR
mRNA levels when the cells were treated with curcumin, suggesting
that curcumin regulates EGFR at the transcriptional and
post-translational levels. This represents a novel mechanism of
action of curcumin in human bronchial epithelial cells, which may
be applied for treating mucin overproduction.
Curcumin has also been reported to decrease
IL-1β-induced MUC5AC expression, which suggests that curcumin may
interfere with other signaling pathways in addition to EGFR/AKT
signaling (31). Furthermore,
Song et al (32)
demonstrated that curcumin also decreases the transcription of
MUC2, another important human mucin gene. In a mouse model,
curcumin was administered through the nasal route, suggesting a
novel and efficient delivery method (33). Thus, curcumin therapy may be a
practical treatment for mucus hypersecretion. However, evidence for
the use of curcumin in asthma remains sparse and has mostly been
obtained using either in vitro or animal models, as the
clinical translation of curcumin has been hampered due to its poor
bioavailability (34). Further
studies should be performed to improve the pharmacological effects
of curcumin.
The present study confirmed that curcumin suppresses
MUC5AC production in human bronchial epithelial cells under basal
and EGF-stimulated conditions. This inhibition was accompanied by
decreased activation of the EGFR/AKT/STAT3 signaling and reduced
EGFR expression, which indicated that curcumin may have a dual role
in interfering with the EGFR signaling pathway and inhibiting mucin
expression in human airway epithelial cells.
Acknowledgments
Not applicable.
References
|
1
|
Alhassan S, Hattab Y, Bajwa O, Bihler E
and Singh AC: Asthma. Crit Care Nurs Q. 39:110–123. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeVries A and Vercelli D: Epigenetic
mechanisms in asthma. Ann Am Thorac Soc. 13(Suppl 1): S48–S50.
2016.PubMed/NCBI
|
|
3
|
Evans CM, Kim K, Tuvim MJ and Dickey BF:
Mucus hypersecretion in asthma: Causes and effects. Curr Opin Pulm
Med. 15:4–11. 2009. View Article : Google Scholar :
|
|
4
|
Lai HY and Rogers DF: Mucus hypersecretion
in asthma: Intracellular signalling pathways as targets for
pharmacotherapy. Curr Opin Allergy Clin Immunol. 10:67–76. 2010.
View Article : Google Scholar
|
|
5
|
Gokce EC, Kahveci R, Gokce A, Sargon MF,
Kisa U, Aksoy N, Cemil B and Erdogan B: Curcumin attenuates
inflammation, oxidative stress, and ultrastructural damage induced
by spinal cord ischemia-reperfusion injury in rats. J Stroke
Cerebrovasc Dis. 25:1196–1207. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bayomi SM, El-Kashef HA, El-Ashmawy MB,
Nasr MN, El-Sherbeny MA, Abdel-Aziz NI, El-Sayed MA, Suddek GM,
El-Messery SM and Ghaly MA: Synthesis and biological evaluation of
new curcumin analogues as antioxidant and antitumor agents:
Molecular modeling study. Eur J Med Chem. 101:584–594. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jeong EH, Vaidya B, Cho SY, Park MA,
Kaewintajuk K, Kim SR, Oh MJ, Choi JS, Kwon J and Kim D:
Identification of regulators of the early stage of viral
hemorrhagic septicemia virus infection during curcumin treatment.
Fish Shellfish Immunol. 45:184–193. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Narumoto O, Matsuo Y, Sakaguchi M, Shoji S
and Yamashita N, Schubert D, Abe K, Horiguchi K, Nagase T and
Yamashita N: Suppressive effects of a pyrazole derivative of
curcumin on airway inflammation and remodeling. Exp Mol Pathol.
93:18–25. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Heo HJ, Lee SY, Lee MN, Lee HJ, Seok JH
and Lee CJ: Genistein and curcumin suppress epidermal growth
factor-induced MUC5AC mucin production and gene expression from
human airway epithelial cells. Phytother Res. 23:1458–1461. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yan F, Li W, Zhou H, Wu Y, Ying S, Chen Z
and Shen H: Interleukin-13-induced MUC5AC expression is regulated
by a PI3K-NFAT3 pathway in mouse tracheal epithelial cells. Biochem
Biophys Res Commun. 446:49–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sikder MA, Lee HJ, Mia MZ, Park SH, Ryu J,
Kim JH, Min SY, Hong JH, Seok JH and Lee CJ: Inhibition of
TNF-α-induced MUC5AC mucin gene expression and production by
wogonin through the inactivation of NF-κB signaling in airway
epithelial cells. Phytother Res. 28:62–68. 2014. View Article : Google Scholar
|
|
12
|
Kim HJ, Park SH, Park SY, Moon UY, Lee BD,
Yoon SH, Lee JG, Baek SJ and Yoon JH: Epigallocatechin-3-gallate
inhibits interleukin-1beta-induced MUC5AC gene expression and
MUC5AC secretion in normal human nasal epithelial cells. J Nutri
Biochem. 19:536–544. 2008. View Article : Google Scholar
|
|
13
|
Wang W, Xu X, Zheng M and Wan L:
Lipopolysaccharides induces MUC5AC overproduction in human nasal
epithelium. Eur Arch Otorhinolaryngol. 270:541–547. 2013.
View Article : Google Scholar
|
|
14
|
Song S, Byrd JC, Guha S, Liu KF, Koul D
and Bresalier RS: Induction of MUC5AC mucin by conjugated bile
acids in the esophagus involves the phosphatidylinositol
3-kinase/protein kinase C/activator protein-1 pathway. Cancer.
117:2386–2397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nie YC, Wu H, Li PB, Xie LM, Luo YL, Shen
JG and Su WW: Naringin attenuates EGF-induced MUC5AC secretion in
A549 cells by suppressing the cooperative activities of MAPKs-AP-1
and IKKs-IκB-NF-κB signaling pathways. Eur J Pharmacol.
690:207–213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Navidshad B, Liang JB and Jahromi MF:
Correlation coefficients between different methods of expressing
bacterial quantification using real time PCR. Int J Mol Sci.
13:2119–2132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Ruchaud-Sparagano MH, Westley BR and May
FE: The trefoil protein TFF1 is bound to MUC5AC in human gastric
mucosa. Cell Mol Life Sci. 61:1946–1954. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kanai K, Koarai A, Shishikura Y, Sugiura
H, Ichikawa T, Kikuchi T, Akamatsu K, Hirano T, Nakanishi M,
Matsunaga K, et al: Cigarette smoke augments MUC5AC production via
the TLR3-EGFR pathway in airway epithelial cells. Respir Investig.
53:137–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abdelhamed S, Ogura K, Yokoyama S, Saiki I
and Hayakawa Y: AKT-STAT3 pathway as a downstream target of EGFR
signaling to regulate PD-L1 expression on NSCLC cells. J Cancer.
7:1579–1586. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang J, Li Q, Zhou XD, Kolosov VP and
Perelman JM: Naringenin attenuates mucous hypersecretion by
modulating reactive oxygen species production and inhibiting NF-κB
activity via EGFR-PI3K-Akt/ERK MAPKinase signaling in human airway
epithelial cells. Mol Cell Biochem. 351:29–40. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rahman I and Chung S: Dietary polyphenols,
deacetylases and chromatin remodeling in inflammation. World Rev
Nutr Diet. 101:84–94. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lv S, Dai C, Liu Y, Sun B, Shi R, Han M,
Bian R and Wang R: Cell surface protein C23 affects EGF-EGFR
induced activation of ERK and PI3K-AKT pathways. J Mol Neurosci.
55:519–524. 2015. View Article : Google Scholar
|
|
24
|
Duan H, Qu L and Shou C: Activation of
EGFR-PI3K-AKT signaling is required for Mycoplasma
hyorhinis-promoted gastric cancer cell migration. Cancer Cell Int.
14:1352014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu Q, Chen X, Fang X, Chen Q and Hu C:
Caveolin-1 aggravates cigarette smoke extract-induced MUC5AC
secretion in human airway epithelial cells. Int J Mol Med.
35:1435–1442. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhen G, Park SW, Nguyenvu LT, Rodriguez
MW, Barbeau R, Paquet AC and Erle DJ: IL-13 and epidermal growth
factor receptor have critical but distinct roles in epithelial cell
mucin production. Am J Respirat Cell Mol Biol. 36:244–253. 2007.
View Article : Google Scholar
|
|
27
|
Le Cras TD, Acciani TH, Mushaben EM,
Kramer EL, Pastura PA, Hardie WD, Korfhagen TR, Sivaprasad U,
Ericksen M, Gibson AM, et al: Epithelial EGF receptor signaling
mediates airway hyperreactivity and remodeling in a mouse model of
chronic asthma. Am J Physiol Lung Cell Mol Physiol. 300:L414–L421.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Starok M, Preira P, Vayssade M, Haupt K,
Salomé L and Rossi C: EGFR inhibition by curcumin in cancer cells:
A dual mode of action. Biomacromolecules. 16:1634–1642. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang AP, Zhou DH, Meng XL, Zhang AP,
Zhang C, Li XT and Feng Q: Down-regulation of epidermal growth
factor receptor by curcumin-induced UBE1L in human bronchial
epithelial cells. J Nutr Biochem. 25:241–249. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee JY, Lee YM, Chang GC, Yu SL, Hsieh WY,
Chen JJ, Chen HW and Yang PC: Curcumin induces EGFR degradation in
lung adenocarcinoma and modulates p38 activation in intestine: The
versatile adjuvant for gefitinib therapy. PLoS One. 6:e237562011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang JH, Song KJ, Kim HJ, Kim JH, Kim NH
and Kim KS: Dietary polyphenols affect MUC5AC expression and
ciliary movement in respiratory cells and nasal mucosa. Am J Rhinol
Allergy. 24:e59–e62. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Song S, Byrd JC, Koo JS and Bresalier RS:
Bile acids induce MUC2 overexpression in human colon carcinoma
cells. Cancer. 103:1606–1614. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Subhashini, Chauhan PS and Singh R:
Ovalbumin-induced allergic inflammation lead to structural
alterations in mouse model and protective effects of intranasal
curcumin: A comparative study. Allergol Immunopathol (Madr).
44:246–256. 2016. View Article : Google Scholar
|
|
34
|
Lelli D, Sahebkar A, Johnston TP and
Pedone C: Curcumin use in pulmonary diseases: State of the art and
future perspectives. Pharmacol Res. 115:133–148. 2017. View Article : Google Scholar
|