Introduction
Diabetes mellitus (DM) is a metabolic disorder that
constitutes a major global health problem (1). It has been estimated that by 2030,
significant alterations in the nutrition and lifestyle in
developing countries within Asia and the Middle East will lead to a
marked increase in the prevalence of type 2 DM (T2DM) worldwide
(2,3). Cardiovascular disease (CVD) is the
leading cause of morbidity and mortality as a result of diabetes,
accounting for up to 80% of premature mortality cases in diabetes
patients (4,5). Endothelial damage is critical in the
development of CVD, and the inhibition of endothelial cell
migration and angiogenesis are limiting processes in the repair of
the endothelium (6,7). However, the mechanism of endothelial
damage is currently unclear.
Increasing evidence has verified that non-coding
RNAs serve an important role in the regulation of cell metabolism
(8,9). Circular RNAs (circRNAs) are a
subclass of endogenous non-coding RNAs, mainly consisting of exonic
transcripts generated through the process of back-splicing
(10). Recently, it has been
reported that circRNAs are widely expressed in human cells and
function as vital regulators in the process of transcriptional and
post-transcriptional gene expression (11). Zhao et al (12) observed that the expression of
hsa_circ_0054633 was significantly increased in T2DM. Therefore,
hsa_circ_0054633 presents a potential diagnostic ability for
pre-diabetes and T2DM. However, the regulating effect of
hsa_circ_0054633 in diabetes remains unclear.
In diabetes, the roles of long non-coding RNA
(lncRNA) and microRNA (miRNA) have been systematically expanded
(13). Our previous studies
revealed that the lncRNA MALAT1 regulates renal tubular epithelial
pyroptosis by modulating the miR-23c that targets ELAVL1 in
diabetic nephropathy (14).
circRNAs that have yet to be extensively studied may constitute a
novel reservoir of therapeutic targets and biomarkers (15). It has been reported that circRNAs
are miRNA sponges, which may serve as a novel class of biomarkers
in diabetes (16). Yang et
al (17) identified that
miR-218 was upregulated in podocytes treated with a high glucose
concentration and promoted high glucose-induced apoptosis in these
cells by targeting heme oxygenase-1 (HO-1). Zhang et al
(18) also observed that miR-218
expression promoted angiogenesis by targeting roundabout 1 (ROBO1).
ROBO1, also known as protein SAX3, is the receptor of slit guidance
ligand 1 (SLIT1) and SLIT2, which are associated with cell
migration. In addition, several studies have demonstrated that
ROBO1 functions as a promoter of cell proliferation (19). It has also been observed that the
expression of miR-218 has an inhibitory effect on cell
proliferation and metastasis (19,20). These previous findings suggested
that the expression of miR-218 suppressed cell proliferation and
metastasis by targeting ROBO1. Furthermore, a previous study
identified that miR-218 accelerated high glucose-induced podocyte
apoptosis through directly down-regulating HO-1 (17). HO-1, an antioxidant and
cytoprotective enzyme, has been reported to have a protective
effect against high glucose-induced cell toxicity, including
oxidative stress and inflammation. Additionally, the expression of
HO-1 offered protection against high glucose-induced apoptosis in
endothelial cells and promoted angiogenesis (21).
In the present study, bioinformatics analysis
(circnet.mbc.nctu.edu.tw/) identified
that hsa_circ_0054633 targets miR-218 and inhibits miR-218
expression. It was observed that the expression of hsa_circ_0054633
was able to regulate the high glucose-induced dysfunction including
proliferation, migration and angiopoiesis suppression in
endothelial cells by targeting miR-218. It was also demonstrated
that downregulating the expression of miR-218 inhibited the high
glucose-induced endothelial cell dysfunction by relieving the
targeting of ROBO1 and HO-1. Furthermore, hsa_circ_0054633
functions as an miR-218 sponge, leading to the subsequent
upregulation of HO-1 and ROBO1.
Materials and methods
Cell culture and high glucose
treatment
Human umbilical vein endothelial cells (HUVECs) were
obtained from the American Type Culture Collection (Manassas, VA,
USA) and cultured in Dulbecco’s modified Eagle’s medium
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) at 37°C in a humidified atmosphere
containing 5% CO2. For high sugar analysis, HUVECs were
assigned to the normal glucose-treated (NG; 5.5 mmol/l; control) or
high glucose-treated (HG; 33.3 mmol/l) groups, and the treatments
were performed as previously reported (22). Following incubation for 48 h,
HUVECs were trypsinized prior to collected by centrifugation (2,000
× g for 5 min) at room temperature, and then subjected to
proliferation, migration and angiogenesis analyses.
Cell mimic transfection or inhibitor
treatment
For miR-218 overexpression, miR-218 mimics or a
corresponding miR-negative control (NC) were purchased from
GenePharma Co., Ltd. (Shanghai, China). Next, HUVECs were
transfected with miR-218 mimic or miR-NC at a final concentration
of 50 nM using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer’s protocol.
For miR-218 expression inhibition, HUVECs was pretreated with 5 nM
miR-218 inhibitor (Invitrogen; Thermo Fisher Scientific, Inc.).
Following transfection with miR-218 mimics or treatment with
miR-218 inhibitor, cells were incubated at 37°C with 5%
CO2 for 48 h, and then used for miR-218 expression
analysis or other experiments. For hsa_circ_0054633 downregulation,
small interfering RNA (siRNA) against hsa_circ_0054633 (5′-CCC AGA
ACA UGA CAA AUU AUU-3′) or a NC (5′-GGG ACU UUC AGA CAA AUU AUU-3′)
were constructed (GenePharma Co., Ltd.) and transfected into HUVECs
for 48 h prior to further experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was isolated from cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and the RNA
concentration was determined by Multiskan Spectrum (Thermo Fisher
Scientific, Inc.) at optical density 260/230. The RNA was then
reverse transcribed into cDNA using the DyNAmo cDNA Synthesis kit
(Pierce; Thermo Fisher Scientific, Inc.). qPCR was then performed
on an HT7900 Real-Time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.) using the following specific primers:
Hsa_circ_0054633 (NM_021629.3) forward, 5′-CCA ATA TTG TAT AAC TAG
CTC CTC-3′, and reverse, 5′-GCA CTT TAT TAG ATT ACA GTA TC-3′;
miR-218 (NR_029631.1) forward, 5′-GCG CTT GTG CTT GAT CTA A-3′, and
reverse, 5′-GTG CAG GGT CCG AGG T-3′; U6 forward, 5′-CTC GCT TCG
GCA GCA CA-3′, and reverse, 5′-AAC GCT TCA CGA ATT TGC GT-3′; HO-1
(NC_000022.11) forward, 5′-CTC AAA CCT CCA AAA GCC-3′, and reverse,
5′-TCA AAA ACC ACC CCA ACC C-3′; ROBO1 (NC_000003) forward, 5′-GGA
AGA AGA CGA AGC CGA CAT-3′, and reverse, 5′-TCT CCA GGT CCC CAA CAC
TG-3′. PCR products were detected using the SYBR Green PCR Master
Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
thermocycling conditions were as follows: 95°C for 30 sec, followed
by 40 cycles of 95°C for 30 sec, 60°C for 20 sec, 70°C for 1 min,
with a final extension step at 70°C for 5 min. Relative mRNA levels
were calculated following normalization to the U6 mRNA levels using
the comparative cycle threshold (2−ΔΔCq) method
(23).
Flow cytometry
Flow cytometry was used to determine the rate of
apoptosis in HUVECs. Apoptotic cells were differentiated from
viable or necrotic cells by the combined application of Annexin V
(AV)-FITC and propidium iodide (PI) (both Gibco; Thermo Fisher
Scientific, Inc.). Briefly, cells were washed twice and adjusted to
a concentration of 1×106 cells/ml with cold D-Hanks
buffer (Gibco; Thermo Fisher Scientific, Inc.). Next, AV-FITC (10
μl) and PI (10 μl) were added to 100 μl of
cell suspension and incubated for 15 min at room temperature in the
dark. Finally, 400 μl binding buffer was added to each
sample without washing, and the samples were analyzed using flow
cytometry. Each experiment was performed in triplicate.
Tube formation assay
In vitro neovascularization assays were
performed in human fibrin matrices. Briefly, transfected HUVECs
with/without sihsa_circ_0054633 were pretreated with different
concentrations of glucose (5.5 or 33 mM) for 48 h and then plated
(2×105 cells) on Matrigel. Next, serum-starved HUVECs
(2×105 cells) were seeded onto 24-well plates coated
with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) in
endothelial basal medium and incubated at 37°C with 5%
CO2 for 16 h. Tubular structures of HUVECs in the
Matrigel were analyzed by phase-contrast microscopy. To quantify
the length of newly formed tubes, six random phase-contrast
photomicrographs were captured per well.
Cell proliferation assays
The study next attempted to determine the effects of
hsa_circ_0054633 on high glucose-induced HUVEC proliferation.
Briefly, HUVECs transfected with/without siRNA against
hsa_circ_0054633 or with/without high glucose treatment were seeded
onto six 96-well plates at a density of 2×103 cells per
well. Following incubation at 37°C with 5% CO2 for 48 h,
a cell counting kit-8 (CCK-8) assay (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) was conducted according to the
manufacturer’s protocol. Briefly, DMEM (900 μl) and CCK8
(100 μl) were added to each well and incubated for 1.5 h at
37°C. Subsequently, the absorption of the cells was measured at a
wavelength of 450 nm by an enzyme-linked immunosorbent assay reader
(Thermo Fisher Scientific, Inc.). Data represent at least three
independent experiments with three replications each time.
Boyden chamber assay
The migratory ability of HUVECs was evaluated with
Transwell chambers (8 μm; BD Biosciences). Briefly, cells
suspended in 100 μl medium without serum were seeded into
the upper chamber at a density of (5–10)
×104 cells/well, while the lower chamber was filled with
20% FBS to induce the migration or invasion of HUVECs through the
membrane. For the invasion assay, Matrigel (1:6 dilution; BD
Biosciences) was also added to the upper chamber. Subsequent to
incubation at 37°C with 5% CO2 for 24 h, cells that had
migrated or invaded across the Transwell membrane were stained with
crystal violet (MedChem Express, Shanghai, China) and counted under
an optical microscope.
Western blot analysis
Total protein was extracted from the cells by lysis
buffer (Gibco; Thermo Fisher Scientific, Inc.) containing protease
inhibitors, and the cell lysate was centrifuged at 13,400 × g at
4°C for 5 min. The protein concentration was then examined by a
bicinchoninic acid kit (Pierce; Thermo Fisher Scientific, Inc.).
Next, protein samples (40 μg) were separated by
SDS-polyacrylamide gel electrophoresis and transferred onto
polyvinylidene difluoride membranes. Subsequent to blocking in 5%
fat-free milk for 2 h at 4°C, the membranes were incubated at 4°C
overnight with the following primary antibodies: Anti-ROBO1 (cat.
no. sc-25672), anti-vascular endothelial growth factor (VEGF; cat.
no. sc-4570), anti-HO-1 (cat. no. sc-120745) and anti-GAPDH (cat.
no. sc-293335) (all purchased from Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA). All the antibodies were diluted to 1:1,000 with
PBS prior to use. The membranes were then incubated for 1 h at room
temperature with horseradish peroxidase-conjugated secondary
antibodies (cat. no. sc-324565; Santa Cruz Biotechnology, Inc.).
The blots were visualized using an Enhanced Chemiluminescence
Detection kit obtained from GE Healthcare Life Sciences (Amersham;
Little Chalfont, UK). The intensity of the protein bands was
quantified with the Quantity One software 5.0 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and normalized against the
loading controls.
Bioinformatics analysis
In order to identify the association among
circRNA-0054633, miR-218, HO-1 and ROBO1, bioinformatics analysis
(circnet.mbc.nctu.edu.tw/) was performed
in the current study. The BioEdit 3.0 software (Ionis
Pharmaceuticals, Inc., Carlsbad, CA, USA) was used for comparison
of the interaction between miR-218 and circRNA. In addition, the
database TargetScan (targetscan.org/) was used to search for potential
interactions between miR-218 and the 3′-untranslated region
(3′-UTR) of HO-1 or ROBO1.
Dual-luciferase reporter assay
A luciferase reporter assay was used to detect the
direct binding of circRNA-0054633, miR-218, ROBO1 and HO-1. The
firefly luciferase reporter assay was conducted using a pGL3
control vector (Promega Corporation, Madison, WI, USA). Initially,
the wild-type and mutated 3′-UTR of ROBO1, wild-type and mutated
3′-UTR of HO-1 and wild-type and mutated circRNA-0054633 sequence
containing putative miR-218 target sites or mutated 3′-UTR were
constructed by replacing a number of binding sites of miR-218 with
other nucleotides. These sequences were synthesized and subcloned
into the pGL3 control vector. For the reporter assay, 0.1 mg pGL3
vectors containing wild-type or mutated 3′-UTR of HO-1 were
co-transfected with pre-miR-218 or pre-miR-control into 293 cells
(American Type Culture Collection) using Lipofectamine®
2000. Subsequent to transfection and incubation at 37°C with 5%
CO2 for 48 h, cells were harvested by centrifugation
(2,000 × g for 5 min) at room temperature, and the luciferase
activity was analyzed with the Dual-Luciferase Reporter Assay
System (Promega Corporation).
Statistical analysis
Results are expressed as the mean ± standard
deviation. Statistically significant differences were evaluated by
one-way analysis of variance followed by the Tukey’s test. Groups
were compared using GraphPad Prism software, version 5.0 (GraphPad
Software, Inc., La Jolla, CA, USA). A value of P<0.05 denotes a
statistically significant difference.
Results
Regulation effect of circRNA-0054633 on
high glucose-induced endothelial cell dysfunction
In a previous study, microarray analysis of the
expression profiles of circRNAs in the peripheral blood of T2DM
patients demonstrated that hsa_circ_0054633 was upregulated
(12). However, the regulation
mechanism remains unclear. Bioinformatics analysis identified that
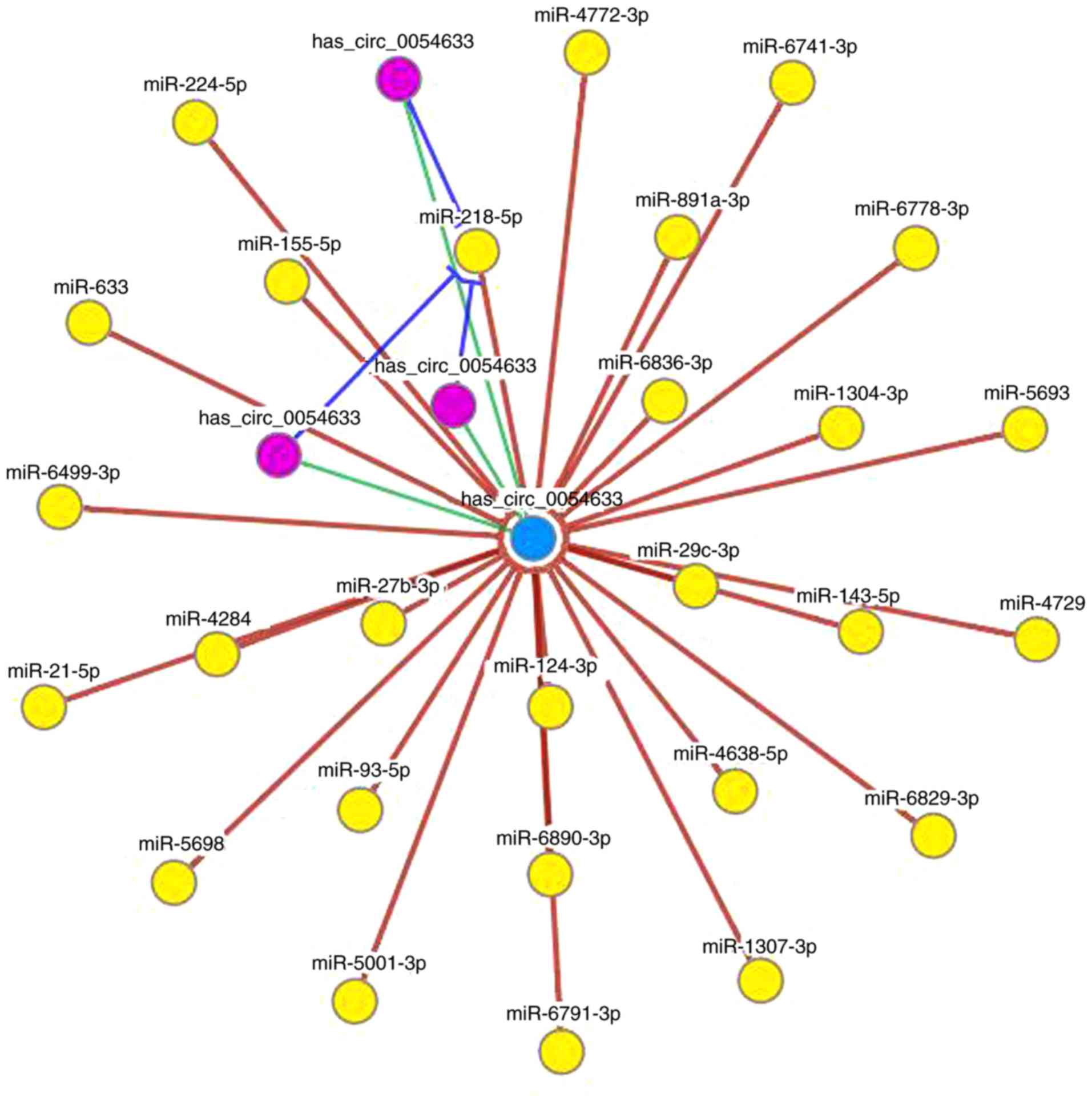
hsa_circ_0054633 targets miR-218 (Fig. 1). In this network, the
circRNA/miRNA interaction was predicted, and the results revealed
that miR-218 was one of the most likely predicted targets.
In order to identify the effect of hsa_circ_0054633
on endothelial cell proliferation, migration and angiopoiesis,
siRNA against hsa_circ_0054633 was constructed or NC and
transfected into HUVECs. In addition, to identify the effect of a
high-glucose environment on the HUVECs, cells were also treated
with different concentrations of glucose, namely 5.5 and 33 mmol/l,
corresponding to the NG and HG groups. After 48 h of transfection,
the expression of hsa_circ_0054633 was measured by RT-qPCR. The
results revealed that the expression of hsa_circ_0054633 was
significantly decreased in the siRNA-transfected cells compared
with that in the NC-transfected cells in the NG and HG groups
(Fig. 2A). Furthermore, high
glucose treatment significantly increased the expression of
hsa_circ_0054633 in the NC + HG cells, as compared with the NC + NG
group. However, following transfection with siRNA against
hsa_circ_0054633, the expression of hsa_circ_0054633 decreased
significantly, even upon high glucose treatment (Fig. 2A).
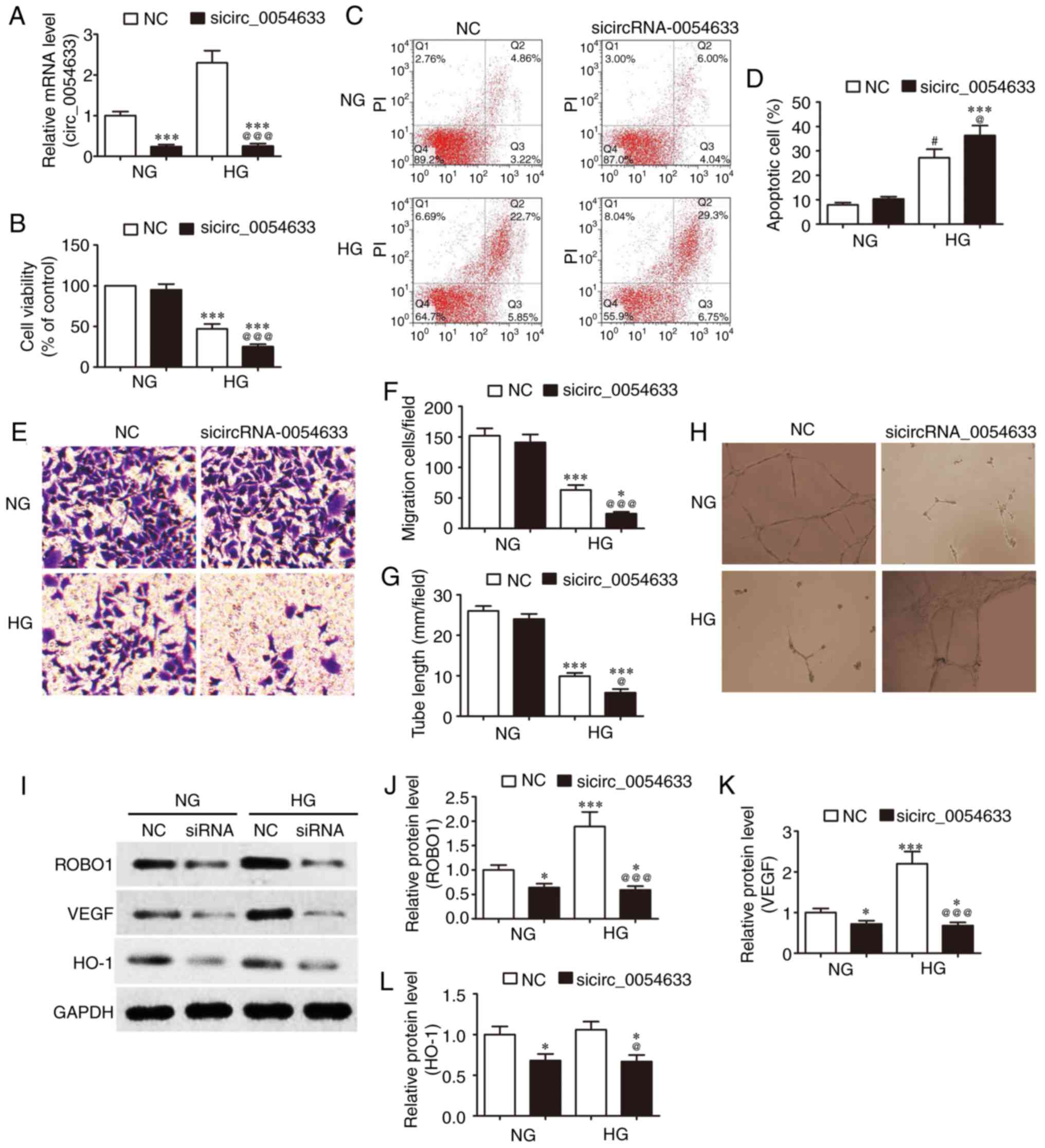 | Figure 2Downregulation of circRNA-0054633
expression increased the high glucose-induced endothelial cell
dysfunction. HUVECs transfected with/without siRNA against
hsa_circ_0054633 were treated with a normal (5.5 mM) or high (33
mM) concentration of glucose for 48 h. (A) hsa_circ_0054633
expression was analyzed by reverse transcription-quantitative
polymerase chain reaction. (B) Cell viability was detected by a
cell counting kit-8 assay after 48 h. (C) Flow cytometry graphs and
(D) apoptotic cell percentage of HUVECs stained with
Annexin-V-FITC/PI. (E) Crystal violet staining of migrating cells
and (F) migration rate graph. Downregulating the expression of
circRNA-0054633 aggravated the inhibitory effect of HG on the
migration of HUVECs. (G) Effect of circRNA-0054633 on tube
formation ability and (H) phase-contrast images. At 16 h after the
addition of pretreated HUVECs to the Matrigel, images were acquired
and analyzed in Image-Pro Plus for quantification of the tube
length. (I) Western blots, and quantified protein expression levels
of (J) ROBO1, (K) VEGF and (L) HO-1 in HUVECs. Data are represented
as the mean ± standard deviation. *P<0.05 and
***P<0.001 vs. NC + NG; @P<0.05 and
@@@P<0.001 vs. NC + HG. hsa_circ_0054633, human
circular RNA-0054633; HUVECs, human umbilical vein endothelial
cells; NC, normal control; NG, normal glucose; HG, high glucose;
ROBO1, roundabout 1; HO-1, heme oxygenase-1; VEGF, vascular
endothelial growth factor. |
The proliferation (Fig. 2B) and apoptosis (Fig. 2C and D) of HUVECs were detected by
a CCK-8 assay. Flow cytometry demonstrated that in a HG environment
(33 mM) the proliferative activity of HUVECs in the HG groups were
significantly inhibited (Fig.
2B), and the apoptotic ratio was markedly increased compared
with the NG concentration (5.5 mM; Fig. 2C). In addition, downregulation of
the expression of hsa_circ_0054633 further depressed the
proliferative activity and increased the apoptotic ratio.
Subsequently, the migration of HUVECs was detected by a Transwell
assay. The results demonstrated that downregulation of the
expression of hsa_circ_0054633 further depressed the high
glucose-induced migration (Fig. 2E
and F). To further assess the angiogenic function of
hsa_circ_0054633, an in vitro capillary tube formation assay
was employed in HUVECs. The results revealed that high glucose
treatment significantly suppressed the tube formation, and this
phenomenon was aggravated following the downregulation of
hsa_circ_0054633 expression (Fig. 2G
and H). Furthermore, western blot analysis was conducted and
demonstrated that high glucose treatment increased the expression
levels of ROBO1 and VEGF. However, the downregulation of
hsa_circ_0054633 expression significantly decreased the ROBO1 and
VEGF expression levels. The expression of HO-1 was not
significantly altered as a result of high glucose treatment;
however, it decreased following the downregulation of
hsa_circ_0054633 expression (Fig.
2I-L).
Interaction between hsa_circ_0054633 and
miR-218
Next, the study aimed to determine the interaction
between miR-218 and hsa_circ_0054633. Initially, a bioinformatic
comparison was performed between miR-218 and hsa_circ_0054633.
Overlap analysis indicated that the binding sites of miR-218 were
more broadly conserved to hsa_circ_0054633. A mutated version of
hsa_circ_0054633 was generated, in which 10 complementary
nucleotides in the binding site were altered. This mutated
construct was fused to the luciferase coding region and
co-transfected into 293 cells along with miR-218 mimics (Fig. 3A). The results demonstrated that
miR-218 expression in 293 cells was significantly increased
subsequent to transfection with miR-218 mimics (Fig. 3B). Determination of the relative
luciferase activity revealed that, when the wild-type
hsa_circ_0054633 was co-transfected with miR-218 mimics,
hsa_circ_0054633 expression was significantly decreased compared
with that upon co-transfection with control miRNA. However, this
effect was not observed when the mutant hsa_circ_0054633 construct
was used for co-transfection, indicating the specific targeting and
suppression of miR-218 by hsa_circ_0054633 (Fig. 3C). Taken together, the
dual-luciferase reporter analysis suggested that hsa_circ_0054633
interacted with miR-218. To further identify whether decreased
hsa_circ_0054633 expression is able to remove this inhibitory
effect, siRNA against hsa_circ_0054633 was transfected into HUVECs.
RT-qPCR analysis demonstrated that decreased hsa_circ_0054633
expression significantly increased miR-218 expression, suggesting
that the regulation effect of hsa_circ_0054633 on HUVEC
proliferation, migration and angiopoiesis is mediated by miR-218
(Fig. 3D).
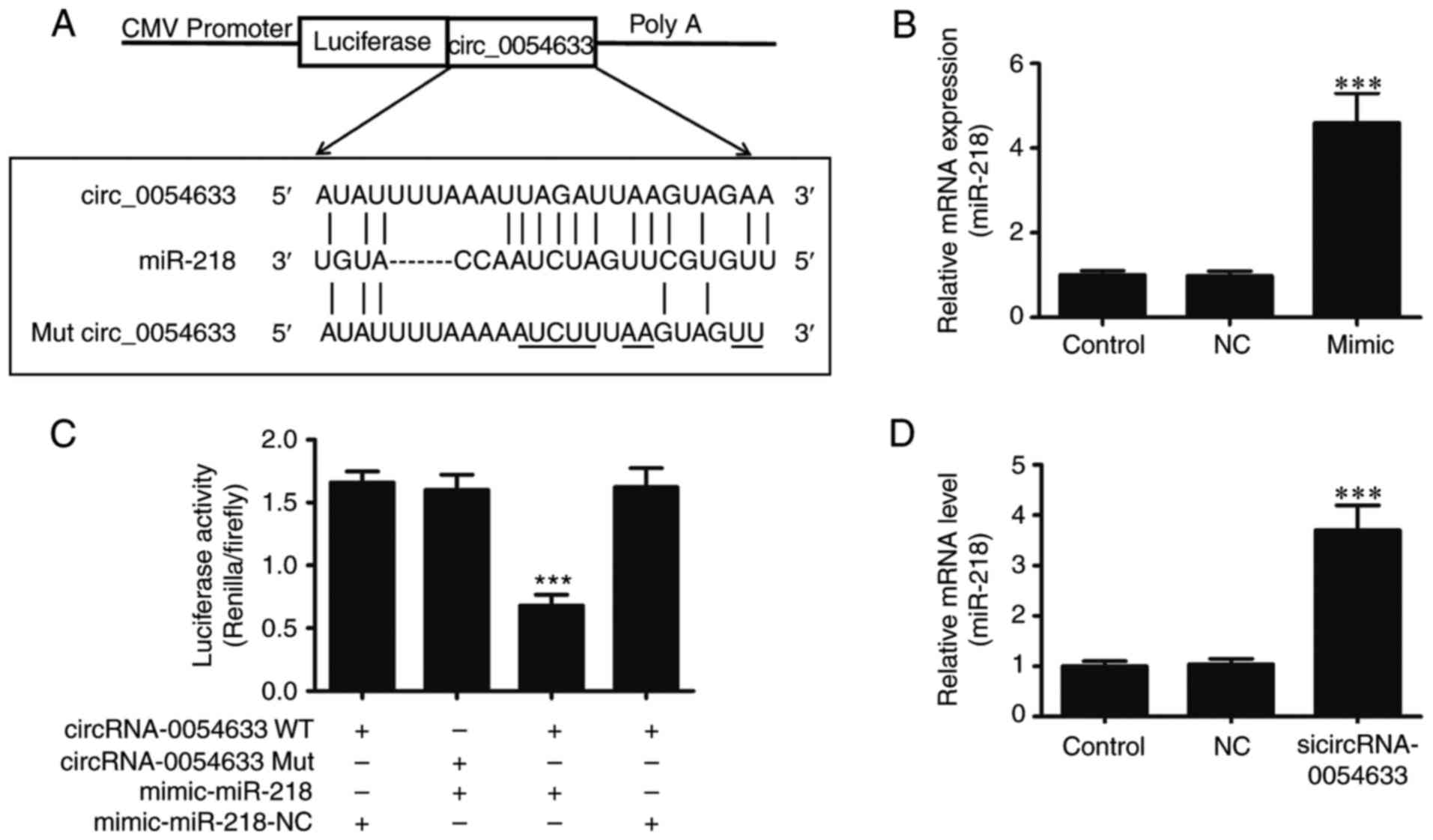 | Figure 3hsa_circ_0054633 is a target of
miR-218. (A) Sequence alignment between miR-218 and
hsa_circ_0054633. Complementary bases between the sequences are
underlined. The sequence of the mutant circRNA-0054633 construct is
also shown. (B) RT-qPCR detection demonstrated the expression of
miR-218 in 293 cells following transfection with miR-218 mimics or
NC, and in the untransfected control. (C) Dual-luciferase reporter
assay of 293 cells co-transfected with circRNA-0054633-WT or
circRNA-0054633-Mut, and with miR-218 mimic or miR-NC. (D) miR-218
expression in HUVECs was measured by RT-qPCR following transfection
with siRNA against circRNA-0054633 for 48 h. The results revealed
that downregulating the expression of circRNA-0054633 significantly
increased miR-218 expression. Data are represented as the mean ±
standard deviation from six individual experiments.
***P<0.001 vs. control group. hsa_circ_0054633, human
circular RNA-0054633; HUVECs, human umbilical vein endothelial
cells; miR, microRNA; siRNA, small interfering RNA; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction; NC,
normal control; WT, wild-type; Mut, mutated. |
Regulation effect of miR-218 on high
glucose-induced endo- thelial cell dysfunction
To identify the effect of miR-218 on endothelial
cell proliferation, migration and angiopoiesis, HUVECs were
pretreated with miR-218-specific inhibitors for 48 h. Next, the
expression of miR-218 was measured by RT-qPCR. The results
demonstrated that the expression of miR-218 was significantly
decreased compared with the NC group (Fig. 4A). In order to identify the effect
of miR-218 on high glucose-induced HUVEC proliferation, migration
and angiopoiesis, HUVECs pretreated with/without miR-218 inhibitor
were co-treated with different concentrations of glucose (5.5 and
33 mM) for 48 h. Subsequently, the proliferation (Fig. 4B) and apoptosis (Fig. 4C and D) of HUVECs were detected by
CCK-8 assay and flow cytometry. The results identified that
downregulation of the expression of miR-218 reversed the
suppressive effect of high glucose on the proliferative activity
and decreased the apoptotic ratio. In addition, the migration of
HUVECs was detected by Transwell assay, and it was observed that
downregulation of the expression of miR-218 suppressed the high
glucose-induced migration (Fig. 4E
and F). To further assess the angiogenic function of miR-218,
an in vitro capillary tube formation assay was conducted.
This assay indicated that high glucose treatment significantly
suppressed the tube formation and that this phenomenon was reversed
following downregulation of the expression of miR-218 (Fig. 4G and H). Western blot analysis
also revealed that high glucose increased the expression levels of
ROBO1 and VEGF, while the downregulation of miR-218 expression
further increased these levels. By contrast, the expression of HO-1
was not significantly altered by high glucose treatment, whereas it
increased subsequent to the downregulation of miR-218 expression
(Fig. 4I-L).
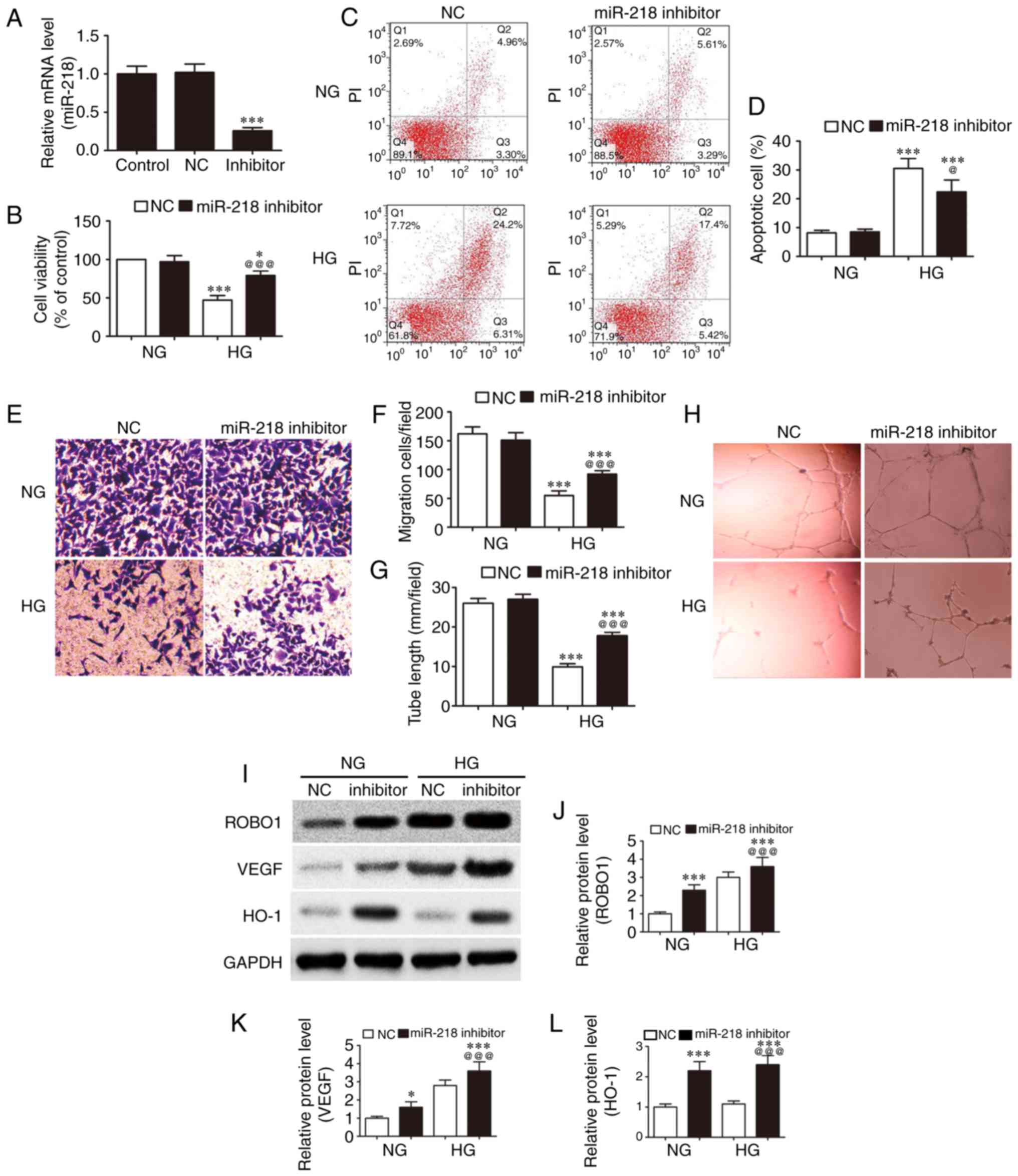 | Figure 4Downregulation of miR-218 expression
inhibits the high glucose-induced endothelial cell dysfunction.
HUVECs were treated with/without miR-218 inhibitor, followed by
normal (5.5 mM) or high (33 mM) concentration of glucose for 48 h.
(A) miR-218 expression was analyzed by reverse
transcription-quantitative polymerase chain reaction.
***P<0.001 vs. control group. (B) Cell viability was
detected by cell counting kit-8 after 48 h. (C) Apoptotic
percentage of HUVECs and (D) flow cytometry graphs. (E) Crystal
violet staining of migrating cells and (F) migration rate, measured
by a Transwell assay. (G) Effect of miR-218 on tube formation
ability and (H) phase-contrast images. At 16 h after addition of
pretreated HUVECs to the Matrigel, images were acquired and
analyzed by Image-Pro Plus for quantification of the tube length.
(I) Western blots, and quantified protein levels of (J) ROBO1, (K)
VEGF and (L) HO-1. Data are represented as the mean ± standard
deviation. *P<0.05 and ***P<0.001 vs.
NC + NG; @P<0.05 and @@@P<0.001 vs. NC
+ HG. HUVECs, human umbilical vein endothelial cells; NC, normal
control; NG, normal glucose; HG, high glucose; ROBO1, roundabout 1;
HO-1, heme oxygenase-1; VEGF, vascular endothelial growth
factor. |
Interaction of miR-218, HO-1, and
ROBO1
Previous studies have demonstrated that miR-218
targets HO-1 and ROBO1, and that the expression of miR-218 promotes
high glucose-induced cell apoptosis (17,18). Furthermore, the expression of HO-1
or ROBO1 promotes cell survival. In order to identify whether
miR-218 regulates high glucose-induced endothelial dysfunction by
targeting HO-1 and ROBO1, the TargetScan databases was used to
search any potential interactions between miR-218 and the 3′-UTR of
HO-1 or ROBO1. Subsequently, the wild-type and mutant 3′-UTR
sequences of HO-1 and ROBO1 were cloned into luciferase reporter
vectors (Fig. 5A and B). The
luciferase assay revealed that miR-218 suppressed the luciferase
activities of HO-1 and ROBO1 containing a wild-type 3′-UTR;
however, it did not suppress the activities of ROBO1 and HO-1
containing a mutant 3′-UTR (Fig. 5C
and D). RT-PCR analysis also demonstrated that inhibiting
miR-218 expression significantly increased the expression levels of
ROBO1 and HO-1 (Fig. 5E and F).
These findings suggested that miR-218 regulates the high
glucose-induced endothelial dysfunction by targeting HO-1 and
ROBO1.
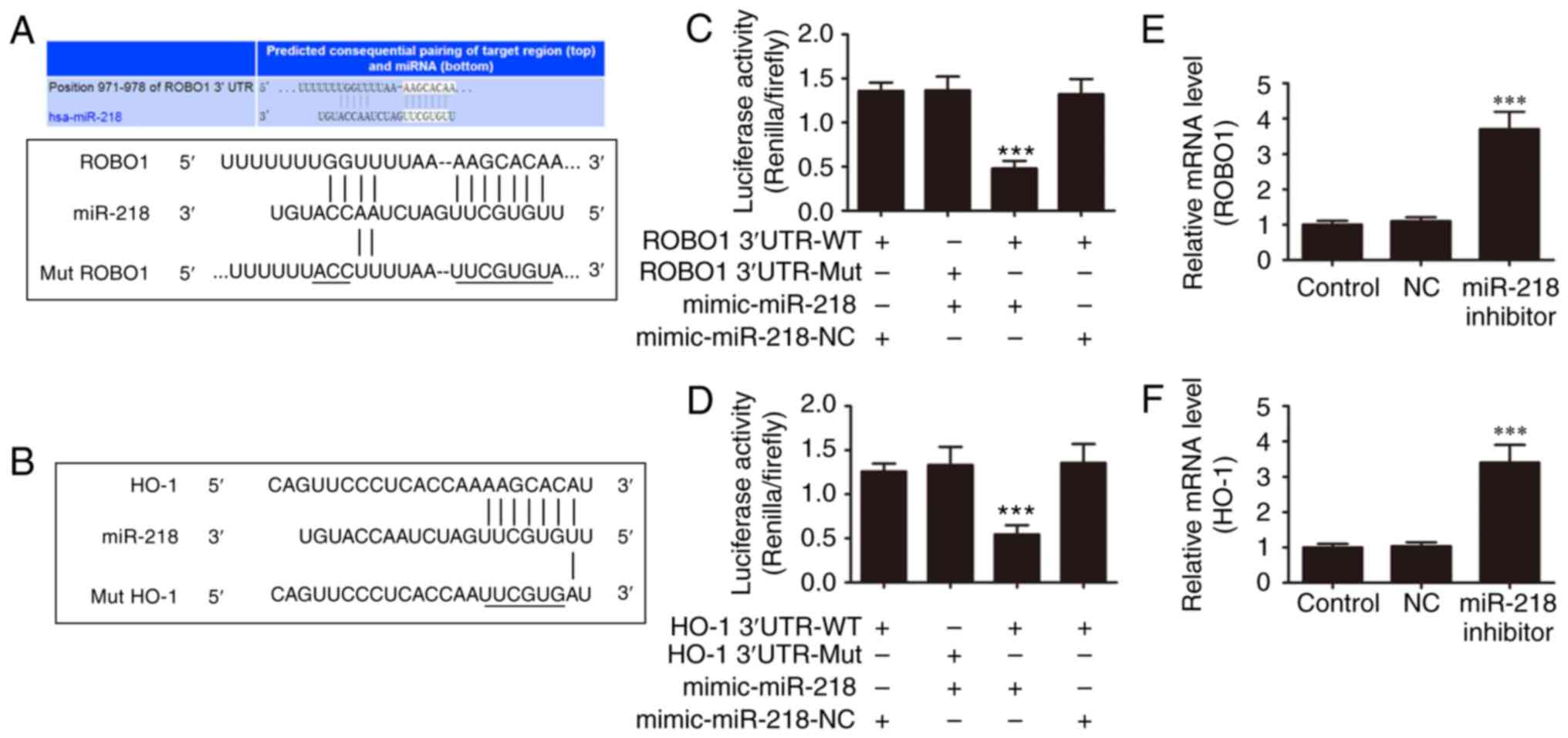 | Figure 5miR-218 targets ROBO1 and HO-1. (A)
Sequence alignment between miR-218 and the 3′-UTR of human ROBO1.
Complementary bases between the sequences are shown as underlined.
The sequence of the mutant ROBO1 construct is also shown. (B)
Sequence alignment between miR-218 and the 3′-UTR of human HO-1.
Complementary bases between the sequences are underlined. The
sequence of the mutant HO-1 construct is also shown. (C)
Dual-luciferase reporter assay of 293 cells co-transfected with
ROBO1 3′-UTR-WT or 3′-UTR-Mut, and with miR-218 mimic or miR-NC.
(D) Dual-luciferase reporter assay of 293 cells co-transfected with
HO-1 3′-UTR-WT or 3′-UTR-Mut, and with miR-218 mimic or miR-NC. (E)
RT-qPCR was used to analyze the mRNA expression of ROBO1 in HUVECs
following treatment with miR-218 inhibitor for 48 h. (F) RT-qPCR
analysis was performed to examine the mRNA expression of HO-1 in
HUVECs following treatment with miR-218 inhibitor for 48 h. Data
are represented as the mean ± standard deviation.
***P<0.001 vs. control group. miR, microRNA; ROBO1,
roundabout 1; HO-1, heme oxygenase-1; HUVECs, human umbilical vein
endothelial cells; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; UTR, untranslated region; NC, normal
control; WT, wild-type; Mut, mutated. |
Discussion
The expression of circRNAs in plants has been well
established for decades, thus, these are not a novel RNA subtype
(24). However, in spite of
increasing knowledge, in-depth details on the functions of circRNAs
in multiple human diseases remain poorly understood, particularly
in diabetic angiopathy. Numerous circRNAs have been identified to
be involved in a regulation effect in stress environments,
including the circRNAs cZNF292, cAFF1 and cDENND4C (25). Vascular endothelial cell (VEC)
dysfunction is considered pivotal to pathogenesis and the initial
step for a series of CVDs, including atherosclerosis, thrombus
formation, hypertension and diabetic cardiovascular complications
(26). Thus, the functional study
of VECs is critical for the identification of novel therapies for
CVD (27). However, the role of
circRNAs in VEC function remains to be elucidated. Previously,
hsa_circ_0054633 expression was observed to be significantly
increased in T2DM, following the analysis of the circRNA expression
profiles in this disease. This suggests that hsa_circ_0054633 may
present a certain diagnostic capability for pre-diabetes and T2DM
(12). However, the potential
regulatory mechanism of circRNAs in high glucose-induced HUVECs
remains unclear.
circRNAs, a family of naturally occurring endogenous
noncoding RNAs, has been reported to have diverse functions and
widespread distribution. circRNAs are single-stranded RNA molecules
with a length of ~100 nucleotides that form a circle through
covalent binding (28–30). They are highly represented in the
eukaryotic transcriptome, while they are also abundant in exosomes.
A large number of circRNAs have been identified to date, while
certain circRNAs have been confirmed to function as miRNA sponges
in mammalian cells through bioinformatics analysis and
high-throughput RNA sequencing (29–31). In addition, a previous study has
demonstrated that circRNAs function as an miRNA sponge that can
mediated cell metabolism (32).
In the present study, bioinformatics analysis
identified that hsa_circ_0054633 targets miR-218, and this was then
verified by dual-luciferase analysis. The study then further
assessed whether hsa_circ_0054633 is able to function as an miR-218
sponge to regulate the circRNA-miRNA-mRNA network. hsa_
circ_0054633 expression was knocked down in HUVECs, and the results
revealed that downregulation of hsa_circ_0054633 expression
significantly increased miR-218 expression. The present study also
demonstrated that hsa_circ_0054633 expression in HUVECs was
increased following exposure to high glucose. In addition,
hsa_circ_0054633 expression suppressed the injury effects of high
glucose to HUVECs by targeting miR-218. Numerous studies have
illustrated that the expression of miR-218 inhibits the cell
proliferation, migration and angiogenesis (20,33–38). The current study also identified
that the expression of miR-218 was able to inhibit the high
glucose-induced HUVEC proliferation, migration and angiogenesis by
targeting ROBO1 and HO-1. A previous study verified that the high
expression levels of ROBO1 and HO-1 were able to significantly
increase cell migration, proliferation and angiogenesis (17,18,39). Herein, it was also observed that
the expression of VEGF was increased in high glucose conditions.
However, angiogenesis was suppressed upon decreased
hsa_circ_0054633 expression in normal conditions, whereas
overexpression of hsa_circ_0054633 or inhibition of miR-218
increased angiogenesis. These observations suggested that the
overexpression of hsa_circ_0054633 increased HO-1 expression by
targeting miR-218, which resulted in eventually reducing the
apoptosis of HUVECs. There is already evidence showing that VEGF
receptor 2 becomes inactive and no longer responds to VEGF
stimulation with the increase of cellular
H2O2 (32),
which indicates that hsa_circ_0054633 expression may also suppress
cellular oxidative stress.
In conclusion, the present study observed that high
glucose treatment was able to induce the increase of
hsa_circ_0054633 and miR-218 expression levels. However, increased
hsa_circ_0054633 expression was unable to entirely inhibit miR-218
expression. Furthermore, overexpression of hsa_ circ_0054633 may
effectively reverse high glucose-induced cell proliferation,
migration and angiogenesis inhibition by targeting the
miR-218/ROBO1 and miR-218/HO-1 signals.
Acknowledgments
Not applicable.
References
|
1
|
Zatalia SR and Sanusi H: The role of
antioxidants in the pathophysiology, complications, and management
of diabetes mellitus. Acta Med Indones. 45:141–147. 2013.PubMed/NCBI
|
|
2
|
Ghassemi H, Harrison G and Mohammad K: An
accelerated nutrition transition in Iran. Public Health Nutr.
5:149–155. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hossain P, Kawar B and El Nahas M: Obesity
and diabetes in the developing world-a growing challenge. N Engl J
Med. 356:213–215. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huxley R, Barzi F and Woodward M: Excess
risk of fatal coronary heart disease associated with diabetes in
men and women: Meta-analysis of 37 prospective cohort studies. BMJ.
332:73–78. 2006. View Article : Google Scholar :
|
|
5
|
McFarlane SI, Salifu MO, Makaryus J and
Sowers JR: Anemia and cardiovascular disease in diabetic
nephropathy. Curr Diab Rep. 6:213–218. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seetharam D, Mineo C, Gormley AK, Gibson
LL, Vongpatanasin W, Chambliss KL, Hahner LD, Cummings ML, Kitchens
RL, Marcel YL, et al: High-density lipoprotein promotes endothelial
cell migration and reendothelialization via scavenger receptor-B
type I. Circ Res. 98:63–72. 2006. View Article : Google Scholar
|
|
7
|
Li Y, Zhao M, He D, Zhao X, Zhang W, Wei
L, Huang E, Ji L, Zhang M, Willard B, et al: HDL in diabetic
nephropathy has less effect in endothelial repairing than diabetes
without complications. Lipids Health Dis. 15:762016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shang C, Lang B, Ao CN and Meng L: Long
non-coding RNA tumor suppressor candidate 7 advances chemotherapy
sensitivity of endometrial carcinoma through targeted silencing of
miR-23b. Tumour Biol. 39:1010428317707883. 2017. View Article : Google Scholar
|
|
9
|
Iparraguirre L, Muñoz-Culla M,
Prada-Luengo I, Castillo-Triviño T, Olascoaga J and Otaegui D:
Circular RNA profiling reveals that circular RNAs from ANXA2 can be
used as new biomarkers for multiple sclerosis. Hum Mol Genet.
26:3564–3572. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yao T, Chen Q, Fu L and Guo J: Circular
RNAs: Biogenesis, properties, roles, and their relationships with
liver diseases. Hepatol Res. 47:497–504. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hou LD and Zhang J: Circular RNAs: An
emerging type of RNA in cancer. Int J Immunopathol Pharmacol.
30:1–6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao Z, Li X, Jian D, Hao P, Rao L and Li
M: Hsa_circ_0054633 in peripheral blood can be used as a diagnostic
biomarker of pre-diabetes and type 2 diabetes mellitus. Acta
Diabetol. 54:237–245. 2017. View Article : Google Scholar :
|
|
13
|
Yan B, Yao J, Liu JY, Li XM, Wang XQ, Li
YJ, Tao ZF, Song YC, Chen Q and Jiang Q: lncRNA-MIAT regulates
microvascular dysfunction by functioning as a competing endogenous
RNA. Circ Res. 116:1143–1156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li X, Zeng L, Cao C, Lu C, Lian W, Han J,
Zhang X, Zhang J, Tang T and Li M: Long noncoding RNA MALAT1
regulates renal tubular epithelial pyroptosis by modulated miR-23c
targeting of ELAVL1 in diabetic nephropathy. Exp Cell Res.
350:327–335. 2017. View Article : Google Scholar
|
|
15
|
Kumar L, Shamsuzzama, Haque R, Baghel T
and Nazir A: Circular RNAs: The emerging class of non-coding RNAs
and their potential role in human neurodegenerative diseases. Mol
Neurobiol. 54:7224–7234. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kulcheski FR, Christoff AP and Margis R:
Circular RNAs are miRNA sponges and can be used as a new class of
biomarker. J Biotechnol. 238:42–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang H, Wang Q and Li S: microRNA-218
promotes high glucose-induced apoptosis in podocytes by targeting
heme oxygenase-1. Biochem Biophys Res Commun. 471:582–588. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang X, Dong J, He Y, Zhao M, Liu Z, Wang
N, Jiang M, Zhang Z, Liu G, Liu H, et al: miR-218 inhibited tumor
angiogenesis by targeting ROBO1 in gastric cancer. Gene. 615:42–49.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Zhou Y, Fei X, Chen X, Chen R, Zhu
Z and Chen Y: Integrative bioinformatics analysis identifies ROBO1
as a potential therapeutic target modified by miR-218 in
hepatocellular carcinoma. Oncotarget. 8:61327–61337.
2017.PubMed/NCBI
|
|
20
|
Fang M, Du H, Han B, Xia G, Shi X, Zhang
F, Fu Q and Zhang T: Hypoxia-inducible microRNA-218 inhibits
trophoblast invasion by targeting LASP1: Implications for
preeclampsia development. Int J Biochem Cell Biol. 87:95–103. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mahmoud AM, Zaki AR, Hassan ME and
Mostafa-Hedeab G: Commiphora molmol resin attenuates
diethylnitrosamine/phenobarbital-induced hepatocarcinogenesis by
modulating oxidative stress, inflammation, angiogenesis and
Nrf2/ARE/HO-1 signaling. Chem Biol Interact. 270:41–50. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhong ZY and Tang Y: Upregulation of
periostin prevents high glucose-induced mitochondrial apoptosis in
human umbilical vein endothelial cells via activation of Nrf2/HO-1
signaling. Cell Physiol Biochem. 39:71–80. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
24
|
Szabo L and Salzman J: Detecting circular
RNAs: Bioinformatic and experimental challenges. Nat Rev Genet.
17:679–692. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boeckel JN, Jaé N, Heumüller AW, Chen W,
Boon RA, Stellos K, Zeiher AM, John D, Uchida S and Dimmeler S:
Identification and characterization of hypoxia-regulated
endothelial circular RNA. Circ Res. 117:884–890. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shin HJ, Kim SN, Chung H, Kim TE and Kim
HC: Intravitreal anti-vascular endothelial growth factor therapy
and retinal nerve fiber layer loss in eyes with age-related macular
degeneration: A meta-analysis. Invest Ophthalmol Vis Sci.
57:1798–1806. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aragona CO, Imbalzano E, Mamone F, Cairo
V, Lo Gullo A, D’Ascola A, Sardo MA, Scuruchi M, Basile G, Saitta A
and Mandraffino G: Endothelial progenitor cells for diagnosis and
prognosis in cardiovascular disease. Stem Cells Int.
2016:80437922016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen LL and Yang L: Regulation of circRNA
biogenesis. RNA Biol. 12:381–388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen I, Chen CY and Chuang TJ: Biogenesis,
identification, and function of exonic circular RNAs. Wiley
Interdiscip Rev RNA. 6:563–579. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu D and Xu AD: Mini review: Circular RNAs
as potential clinical biomarkers for disorders in the central
nervous system. Front Genet. 7:532016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kang DH, Lee DJ, Lee KW, Park YS, Lee JY,
Lee SH, Koh YJ, Koh GY, Choi C, Yu DY, et al: Peroxiredoxin II is
an essential antioxidant enzyme that prevents the oxidative
inactivation of VEGF receptor-2 in vascular endothelial cells. Mol
Cell. 44:545–558. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zeng XJ, Wu YH, Luo M, Cong PG and Yu H:
Inhibition of pulmonary carcinoma proliferation or metastasis of
miR-218 via down-regulating CDCP1 expression. Eur Rev Med Pharmacol
Sci. 21:1502–1508. 2017.PubMed/NCBI
|
|
34
|
Shi ZM, Wang L, Shen H, Jiang CF, Ge X, Li
DM, Wen YY, Sun HR, Pan MH, Li W, et al: Downregulation of miR-218
contributes to epithelial-mesenchymal transition and tumor
metastasis in lung cancer by targeting Slug/ZEB2 signaling.
Oncogene. 36:2577–2588. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang LL, Wang L, Wang XY, Shang D, Yin SJ,
Sun LL and Ji HB: microRNA-218 inhibits the proliferation,
migration, and invasion and promotes apoptosis of gastric cancer
cells by targeting LASP1. Tumour Biol. 37:15241–15252. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Han S, Kong YC, Sun B, Han QH, Chen Y and
Wang YC: microRNA-218 inhibits oxygen-induced retinal
neovascularization via reducing the expression of roundabout 1.
Chin Med J. 129:709–715. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kong Y, Sun B, Han Q, Han S, Wang Y and
Chen Y: Slit-miR-218-Robo axis regulates retinal
neovascularization. Int J Mol Med. 38:19472016. View Article : Google Scholar :
|
|
38
|
Amin ND, Bai G, Klug JR, Bonanomi D,
Pankratz MT, Gifford WD, Hinckley CA, Sternfeld MJ, Driscoll SP,
Dominguez B, et al: Loss of motoneuron-specific microRNA-218 causes
systemic neuromuscular failure. Science. 350:1525–1529. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Guerrero-Cazares H, Lavell E, Chen L,
Schiapparelli P, Lara-Velazquez M, Capilla-Gonzalez V, Clements AC,
Drummond G, Noiman L, Thaler K, et al: Brief report: Robo1
regulates the migration of human subventricular zone neural
progenitor cells during development. Stem Cells. 35:1860–1865.
2017. View Article : Google Scholar : PubMed/NCBI
|