Introduction
Tumor metastasis is one of the major causes of
cancer-related deaths in patients with malignancies, so it is
critical to understand the molecular mechanisms of effective
therapeutic strategies to treat cancer. In recent years, a large
and growing body of literature has investigated the involvement of
the epithelial-mesenchymal transition (EMT) in the initiation of
metastasis (1,2). Through EMT, which is a developmental
process, epithelial cells lose their adhesion properties and
acquire mesenchymal features allowing them to migrate and invade
(3,4). In the tumor microenvironment,
several molecules are involved in the EMT process. The transforming
growth factor-β (TGF-β) and hypoxia pathways are the best
characterized and most frequently used signaling pathways in the
EMT process (5–7). Transcription factors whose
overexpression induces the EMT process in a variety of cancer cell
lines include members of the Twist, Snail, Zeb and FoxC families
(8).
MicroRNAs (miRNAs) are small, non-coding RNAs
(containing ~22 nucleotides) that regulate gene expression
post-transcriptionally by binding to complementary sequences within
target mRNAs (9,10). miRNAs have recently been
identified as a class of factors that are involved in the EMT-MET
switch during malignant tumor progression and metastasis. The
miR-200 family of miRNAs cooperatively regulate the expression of
the E-cadherin transcriptional repressors ZEB1/2 which is
implicated in the EMT and tumor metastasis (11). Actually, our previous study
revealed that the expression of miR-214 was elevated in lung
adenocarcinoma (LAD) and correlated positively with LAD metastasis
and EMT by targeting the suppressor of fused homolog (SUFU).
Indeed this was the first evidence to demonstrate that the
expression of miR-214 by LAD cells contributes to the EMT and
metastasis in LAD (12).
Additionally, miRNAs can also be regulated by some molecules. For
instance, the tumor suppressor p53 targets miR-34a and
miR-215 to repress SNAIL and ZEB2 expression (13). However, the mechanisms upstream of
the miRNAs remain largely unknown.
TWIST1, a basic-helix-loop-helix (bHLH)
transcription factor, is one of the most important EMT-inducer
prototypes (14). TWIST1 binds to
the E-box elements of E-cadherin and represses its expression,
whereas it promotes N-cadherin expression by binding to the E-box
elements of N-cadherin (15). In
addition, TWIST1 regulates the expression of miRNAs. For instance,
TWIST1 upregulates miR-10b to promote breast cancer metastasis
(16). miR-223, which is directly
induced by TWIST1, could promote migration and invasion in gastric
cancer cells (17). More
importantly, TWIST1 have been shown to drive the expression of a
7.9-kb noncoding RNA transcript that encodes a miR-199a and miR-214
cluster (18). As expressed
above, we hold the hypothesis that the miR-214 expression is
elevated by TWIST1 during the EMT process in LAD cells.
In this study, our data demonstrated that TWIST1 was
highly expressed by EMT and high expression of TWIST1 upregulates
miR-214 to promote the EMT process and metastasis in LAD.
Materials and methods
Cell culture and patient samples
The human LAD cell lines A549 and NCI-H1650 were
obtained from the American Type Culture Collection (ATCC, Manassas,
VA, USA). These cell lines were last tested and authenticated by
short tandem repeat profiling in September, 2014. The cell lines
were maintained in Dulbecco's modified Eagle's medium (DMEM) with
10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100
µg/ml streptomycin, and were incubated at 5% CO2
at 37°C. In experiments designed to induce EMT by hypoxia, the
cells were cultured under normoxic conditions (21% O2)
or hypoxic conditions (0.5% O2) for 24 h, as previously
detailed in the literature (6,7).
To induce the EMT with TGF-β or paclitaxel, cells were treated with
TGF-β (5 ng/ml) for 7 days (6) or
with paclitaxel (5 ng/ml) for 2 days (19), and then the cells were collected
for further study. LAD tissue samples and their corresponding
paracancerous tissue samples were collected by surgical resection
at Daping Hospital (Chongqing, China) from August, 2011 to
September, 2013. Fresh primary and metastatic LAD tissue samples
were collected at Xinqiao Hospital (Chongqing, China) from August,
2012 to September, 2014. This study was approved by the
Institutional Review Board of the Third Military Medical
University, and informed consent was obtained from each
patient.
RNA extraction and real-time PCR
miRNA expression levels were measured using the
qRT-PCR miRNA kit and qRT-PCR primer sets, according to the
manufacturer's instructions (Ribobio, Guangzhou, China). Primers
for the human E-cadherin, vimentin, SUFU, TWIST1 and
β-actin (ACTB) are listed in Table I. Real-time PCR was performed in
an ABI 7500 Prism Sequence Detection system (Applied Biosystems,
Foster City, CA, USA) using a SYBR-Green kit (Takara, Tokyo,
Japan), and the relative changes in expression were quantified.
Each experiment was repeated at least three times.
 | Table IPrimers for selected genes. |
Table I
Primers for selected genes.
| Gene name | Primers
| Product (bp) |
|---|
| Sense | Antisense |
|---|
| E-cadherin |
GTCTGTCATGGAAGGTGCT |
TACGACGTTAGCCTCGTTC | 320 |
| Vimentin |
CCACGAAGAGGAAATCCAGG |
CAGAGAGGTCAGCAAACTTGG | 188 |
| SUFU |
GCCTGAGTGATCTCTATGGTGA |
TCTCTCTTCAGACGAAAGGTCAA | 100 |
| TWIST1 |
GTCCGCAGTCTTACGAGGAG |
GCTTGAGGGTCTGAATCTTGCT | 156 |
| β-actin |
GAGCTACGAGCTGCCTGACG |
GTAGTTTCGTGGATGCCACAG | 120 |
RNA interference
LAD cells were stably infected with the pre-microRNA
expression construct known as the lenti-miR expression plasmid,
which contained the full-length miR-214 in the H1-MCS-CMV-EGFP
vector (GeneChem Inc., Shanghai, China; vector information:
http://www.genechem.com.cn/Zaiti.aspx?zt=GV259). The
sh-miR-214 sequence (ACTGCCTG TCTGTGCCTGCTGT) was cloned into the
H1-MCS-CMV-EGFP (vector information: http://www.genechem.com.cn/Zaiti.aspx?zt=GV159) to
generate the H1-MCS-CMV-EGFP-sh-miR-214 (both from GeneChem Inc.).
For the knockdown of TWIST1, TWIST1-specific shRNAs (shRNA1,
GCUGAGCAAGAUUCAGACCTT; shRNA2, GGUCUGAAUCUUGCUCAGCTT) were packaged
into the lentivirus from GeneChem Inc. A non-targeting sequence,
purchased from GeneChem Inc. was used as a lentivirus negative
control. An infection efficiency >80% was verified by
fluorescent microscopy and confirmed for TWIST1 and miR-214
expression.
Migration and invasion assay
The migration assay was performed using 24-well
culture inserts with a porous (pore size 8.0 µm)
polycarbonate membrane (Millipore, Billerica, MA, USA). For the
Matrigel invasion assay, the filters were pre-coated with 30
µl of Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) for
3 h. The migration and invasion assays were performed following our
previously published protocol (20). Briefly, 2×104 cells in
200 µl of serum-free medium were added to the upper chamber,
and 800 µl of medium with 5% serum was added to the lower
chamber. The plates were incubated for 24 h at 37°C in 5%
CO2. Cells that did not migrate or invade through the
pores were removed with a cotton swab. Cells on the lower surface
of the membrane were examined and counted under a microscope. Each
experiment was repeated at least three times.
Flow cytometry sorting
Dissociated LAD cells, which were stably infected
with the pre-microRNA expression, were counted and transferred to a
5-ml tube, washed twice with phosphate-buffered saline (PBS),
counted and resuspended in PBS at 1×106 cell/100
µl. The samples were then suspended in 500 µl of PBS.
The cells were routinely sorted twice and they were analyzed for
GFP purity, which was typically >80%. The data were analyzed
with the CellQuest software (BD Biosciences).
Immunofluorescence
The immunofluorescence analysis was performed on
8-µm-thick frozen sections of tissue, which were fixed with
ice-cold 4% paraformaldehyde for 15 min and blocked with normal
serum for 20 min at room temperature before being incubated with
one or more specific antibodies against vimentin (1:200),
E-cadherin (1:200), SUFU (1:200) or TWIST1 (1:200) (all from Abcam,
Cambridge, UK) overnight and in the dark at 4°C. After three
washes, the slides were stained with FITC-conjugated anti-rabbit
antibodies or Cy3-conjugated anti-mouse antibodies (1:500; Abcam).
The nuclei were counterstained with 4′,6-diamidino-2-phenylindole
(DAPI). Stained cells were visualized with an Olympus confocal
microscope (Olympus Corp., Tokyo, Japan). All the experiments were
repeated at least three times.
Western blot analysis
All the cell lysates were prepared and western blot
analysis was performed as previously described (20). The following antibodies were used:
E-cadherin (1:500), vimentin (1:500) (both from Abcam), SUFU
(1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA),
TWIST1 (1:200; BD Biosciences), and β-actin (1:400; Boster, Wuhan,
China).
Statistical analyses
The data are presented as the means ± SD. The data
were statistically analyzed by the Student's t-test or a one-way
ANOVA test. A difference was considered to be statistically
significant at P<0.05. All the statistical analyses were
performed with the SPSS 13.0 software (IBM Corp., Armonk, NY,
USA).
Results
TWIST1 is a poor prognostic indicator in
LAD and positively correlates with miR-214 expression
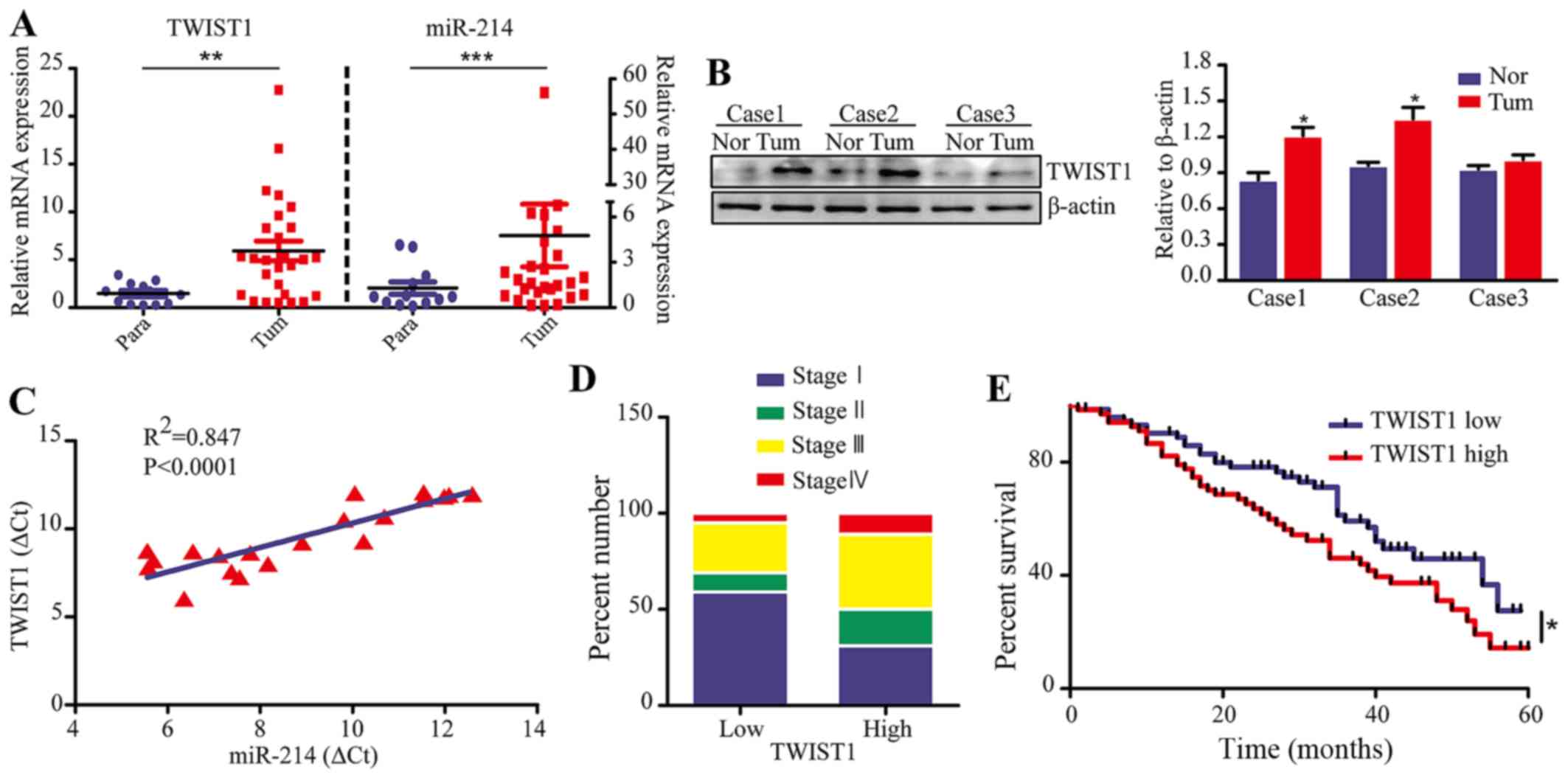
We assessed the expression of TWIST1 and
miR-214 in 22 primary and 13 paracancerous LAD tissue samples by
quantitative real-time PCR (qRT-PCR) analysis. The results revealed
that the TWIST1 expression was significantly higher in tumor
tissues compared with paracancerous tissues (P<0.01), which is
the same trend as observed with the expression of miR-214 (Fig. 1A). Additionally, western blot
analysis showed that TWIST1 expression was higher in tumor tissues
compared to non-tumor tissue (Fig.
1B). More importantly, we found a positive correlation between
miR-214 expression and TWIST1 expression in 20 cases of clinical
LAD tissue (P<0.0001, R2=0.847) (Fig. 1C). To investigate the potential
roles of TWIST1 in LAD, we analyzed the correlation between
the TWIST1 levels and the clinical pathological parameters
in LAD patients (gene expression data were obtained from the NCBI
GEO datasets, GPL3877). The results revealed that patients with the
most advanced stage cancer (stage III and IV) had high
TWIST1 expression (>50%). Conversely, patients with early
stage cancer (stage I) had low TWIST1 expression (>50%)
(Fig. 1D). In addition, the
expression of TWIST1 was significantly correlated with
recurrence and final stage of LAD patients (Table II). However, there was no
significant correlation between TWIST1 expression and age,
sex, tobacco history or vital statistics (all P>0.05).
Comparison of the survival curves of LAD patients revealed that
TWIST1-positive (high expression) patients had significantly
poorer survival than TWIST1-negative (low expression)
patients (P<0.05) (Fig. 1E).
Further evaluation of these findings by univariate analysis
revealed that the TWIST1 status was an independent
prognostic factor for survival. In fact, TWIST1-positive
patients were more likely to suffer from relapse than
TWIST1-negative patients [hazard ratio (HR), 2.057; 95% confidence
interval (95% CI), 1.030–4.111]. On the other hand, no association
was found between the patient's prognosis and sex, age or tobacco
history. Additionally, multivariate Cox regression analyses
confirmed that higher expression of TWIST1 was indeed an
independent prognostic factor of survival of LAD patients (HR,
2.201; 95% CI, 1.192–4.062), whereas sex, age or tobacco history
were not independent prognostic factors (Table III). Collectively, our findings
indicate a positive correlation between the expression of miR-214
and TWIST1, and TWIST1 was an important prognostic factor of
LAD patients.
 | Table IIAssociation between TWIST1 expression
in LAD patients' characteristics. |
Table II
Association between TWIST1 expression
in LAD patients' characteristics.
| TWIST1
|
|---|
| Total | High | Low | P-value |
|---|
| Age | | | | |
| ≥60 | 84 | 45 | 39 | |
| <60 | 16 | 5 | 11 | 0.101 |
| Sex | | | | |
| Male | 50 | 30 | 22 | |
| Female | 48 | 20 | 28 | 0.109 |
| Tobacco
history | | | | |
| Yes | 83 | 44 | 39 | |
| No | 17 | 6 | 11 | 0.183 |
| Vital
statistics | | | | |
| Alive | 43 | 27 | 16 | |
| Death | 57 | 23 | 34 | 0.026 |
| Recurrence | | | | |
| Yes | 36 | 23 | 13 | |
| No | 64 | 27 | 37 | 0.037a |
| Final stage | | | | |
| I | 53 | 19 | 34 | |
| II | 18 | 13 | 5 | |
| III | 27 | 18 | 9 | |
| IV | 2 | 2 | 0 | 0.005b |
 | Table IIIUnivariate and multivariate analysis
of variables with overall survival. |
Table III
Univariate and multivariate analysis
of variables with overall survival.
| Variables | Univariate
| Multivariate
|
|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex (female vs.
male) | 1.480
(0.773–2.759) | 0.243 | | |
| Age (≥60 vs.
<60) | 0.516
(0.233–1.145) | 0.104 | | |
| Tobacco history (no
vs. yes) | 1.316
(0.487–3.553) | 0.588 | | |
| TWIST1 (low vs.
high) | 2.057
(1.030–4.111) | 0.041a | 2.201
(1.192–4.062) | 0.012a |
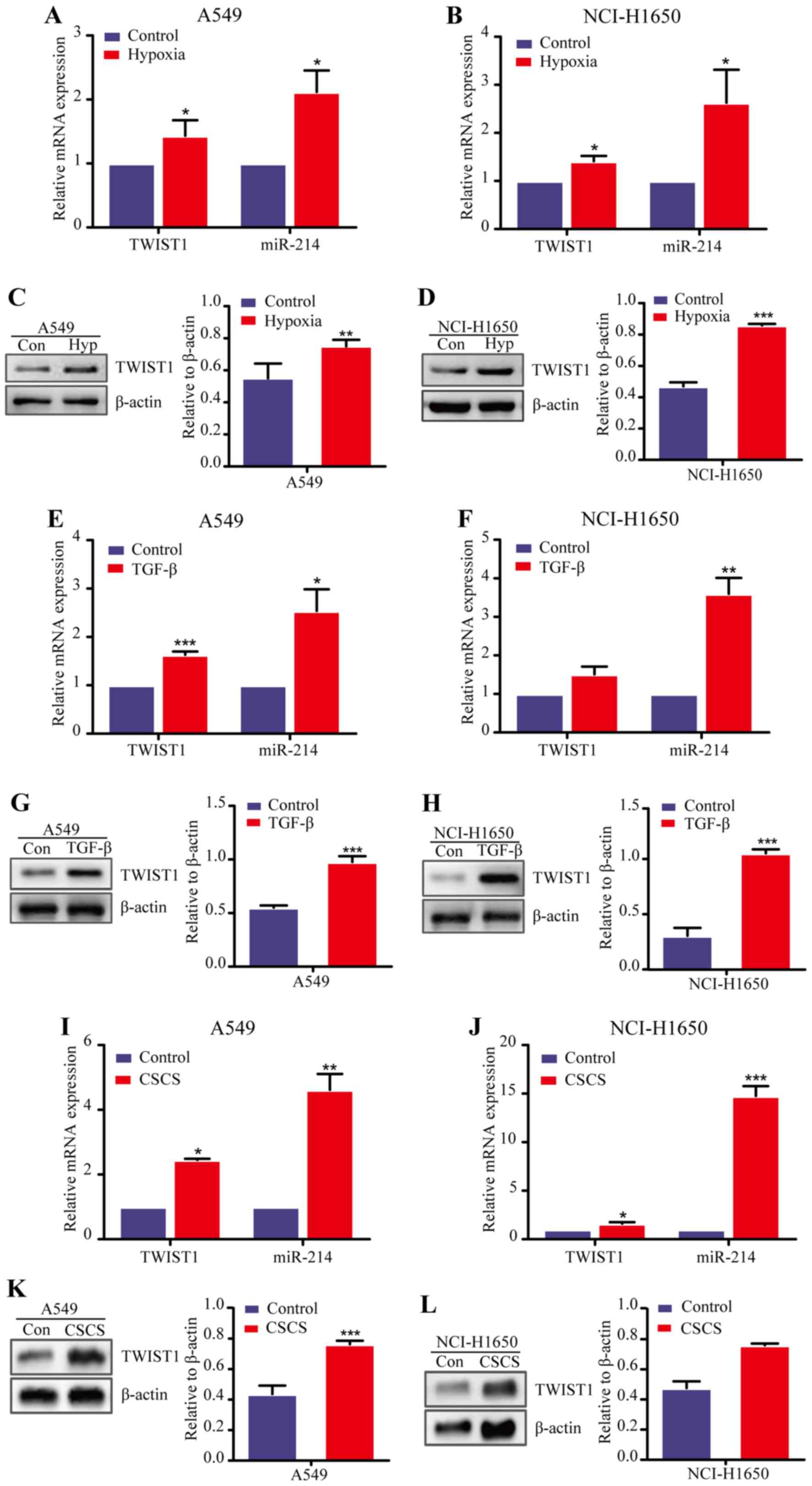
TWIST1 expression is increased in the EMT
of LAD cells
Our previous studies revealed the important role
played by miR-214 in the EMT process and metastasis of LAD cells,
as well as the positive correlation between the expression of
miR-214 and TWIST1. Accordingly, we further investigated
whether TWIST1 promoted LAD metastasis through the EMT process.
Previous studies described that the role of TGF-β and hypoxia in
the EMT process were identified as the best characterized inducers
of EMT, and some researchers indicated that cancer stem cells
(CSCs) displayed an epithelial-mesenchymal transition phenotype
(21). Thus, we first established
a hypoxia-induced EMT model using two LAD cell lines (A549 and
NCI-H1650) and used qRT-PCR analysis to measure the expression of
TWIST1 and miR-214. We found that both the TWIST1 and
miR-214 were upregulated in the two LAD cell lines after hypoxic
(0.5% O2) induction for 24 h compared with normoxia
condition (21% O2) (Fig.
2A and B). In addition, western blot analysis confirmed that
TWIST1 expression was significantly increased at the protein level
in A549 and NCI-H1650 cells after exposure to hypoxia (Fig. 2C and D). Similar results were also
consistently obtained in the TGF-β-induced EMT model and CSCs
(12,22) (Fig.
2E–L). Together, these data demonstrate that the TWIST1
expression was increased in the EMT cells of LAD in parallel with
an increased expression of miR-214, suggesting that TWIST1 may
activate the EMT process in LAD in association with the elevated
expression of miR-214.
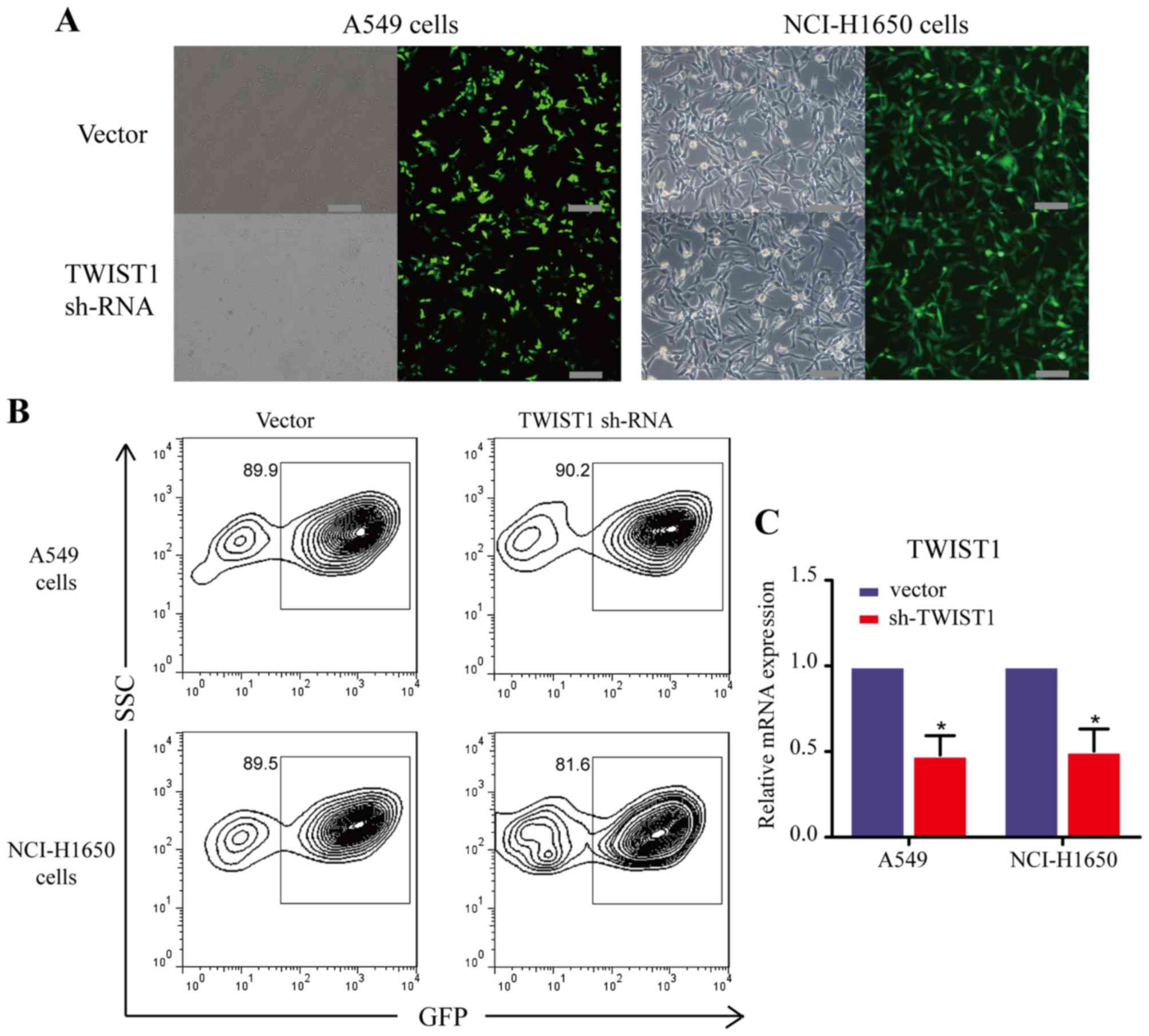
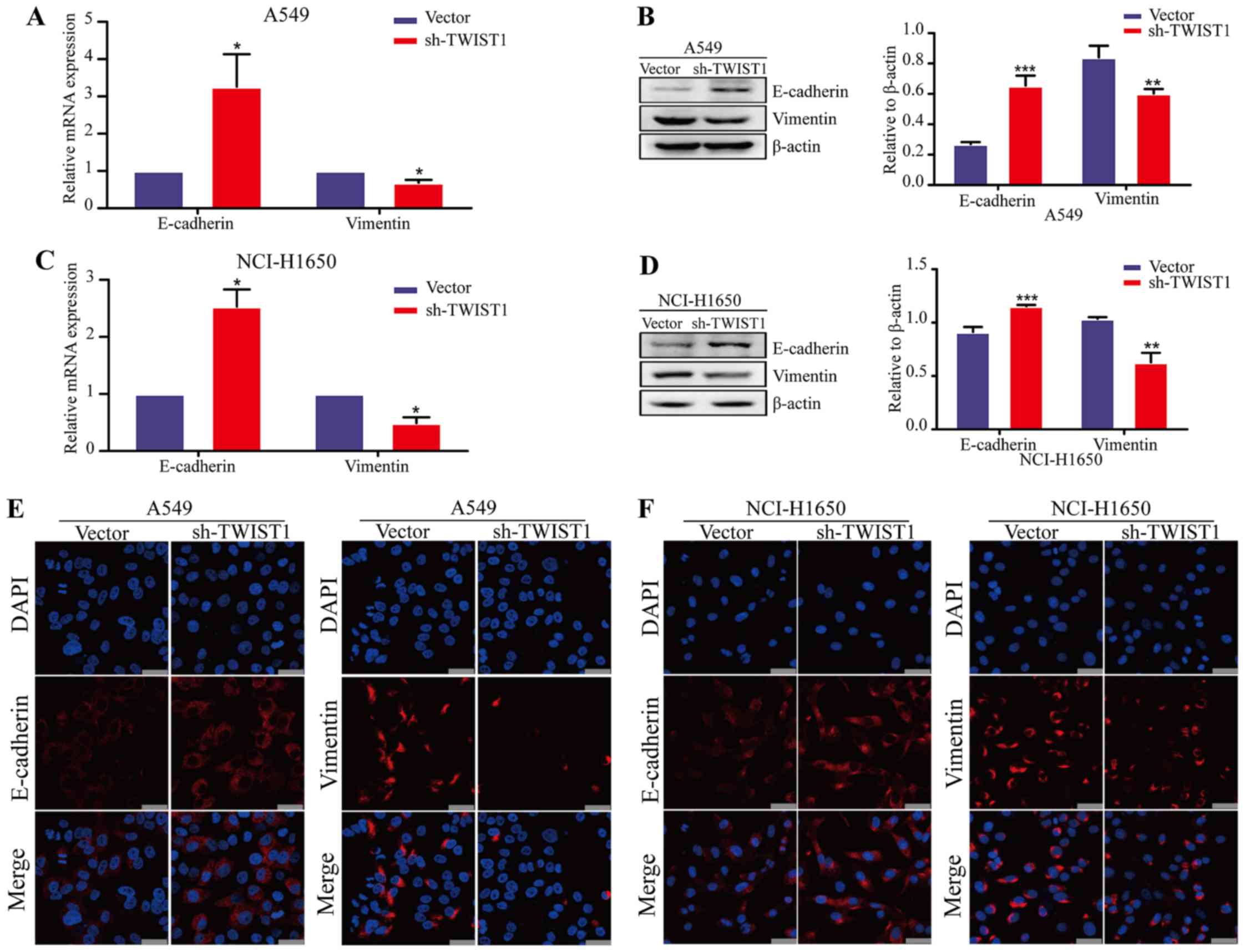
Sh-TWIST1 impaires the EMT process in LAD
cells
As described above, we found that TWIST1 expression
was increased in the EMT of LAD cells. Considering these findings,
we focused on TWIST1 for further pro-EMT functional studies.
Accordingly, we employed a loss-of-function approach by using shRNA
in A549 and NCI-H1650 cells to investigate the role of TWIST1 in
the EMT process. We first generated LAD cell lines with stable
TWIST1 downregulation (A549 and NCI-H1650) using lentivirus
transfection (Fig. 3).
Fluorescent microscopy (Fig. 3A),
flow cytometry (Fig. 3B) and
qRT-PCR (Fig. 3C) analyses
demonstrated that, as intended, the expression of TWIST1 was
successfully downregulated in the NCI-H1650 and A549 cells. Indeed,
the epithelial marker E-cadherin was significantly increased,
whereas the mesenchymal marker vimentin was significantly decreased
at both the gene and protein levels after the loss of expression of
TWIST1 in LAD cells (Fig. 4A–D).
Moreover, similar results were confirmed in LAD cell lines by an
immunofluorescence approach (Fig. 4E
and F). Taken together, these data indicate that sh-TWIST1
could weaken the EMT process in LAD cells.
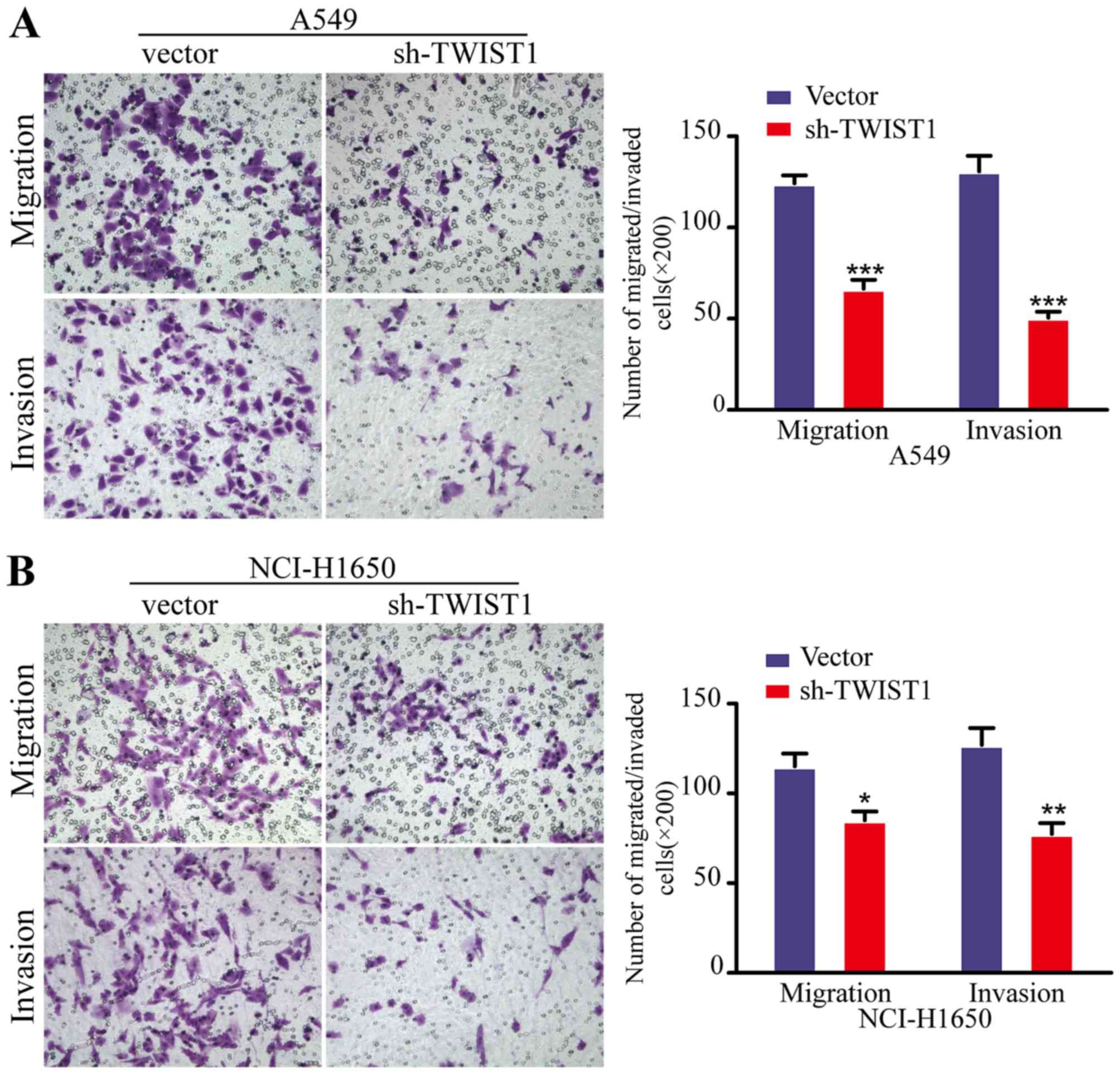
Sh-TWIST1 impairs LAD cell migration and
invasion in vitro
The EMT process is one of the key initiation steps
in metastatic progression, which provides cancer cells with
motility, invasion and migration properties. Accordingly, we next
employed the Boyden chamber migration/invasion technique to
investigate the function of TWIST1 in metastasis of LAD cells in
vitro. As anticipated, the downregulation of TWIST1 by shRNA
dramatically decreased the migratory and invasive abilities of both
the A549 and NCI-H1650 cells (Fig.
5), compared with cells transfected with the control vector.
Collectively, these data suggested that sh-TWIST1 significantly
moderated LAD cell metastasis in vitro.
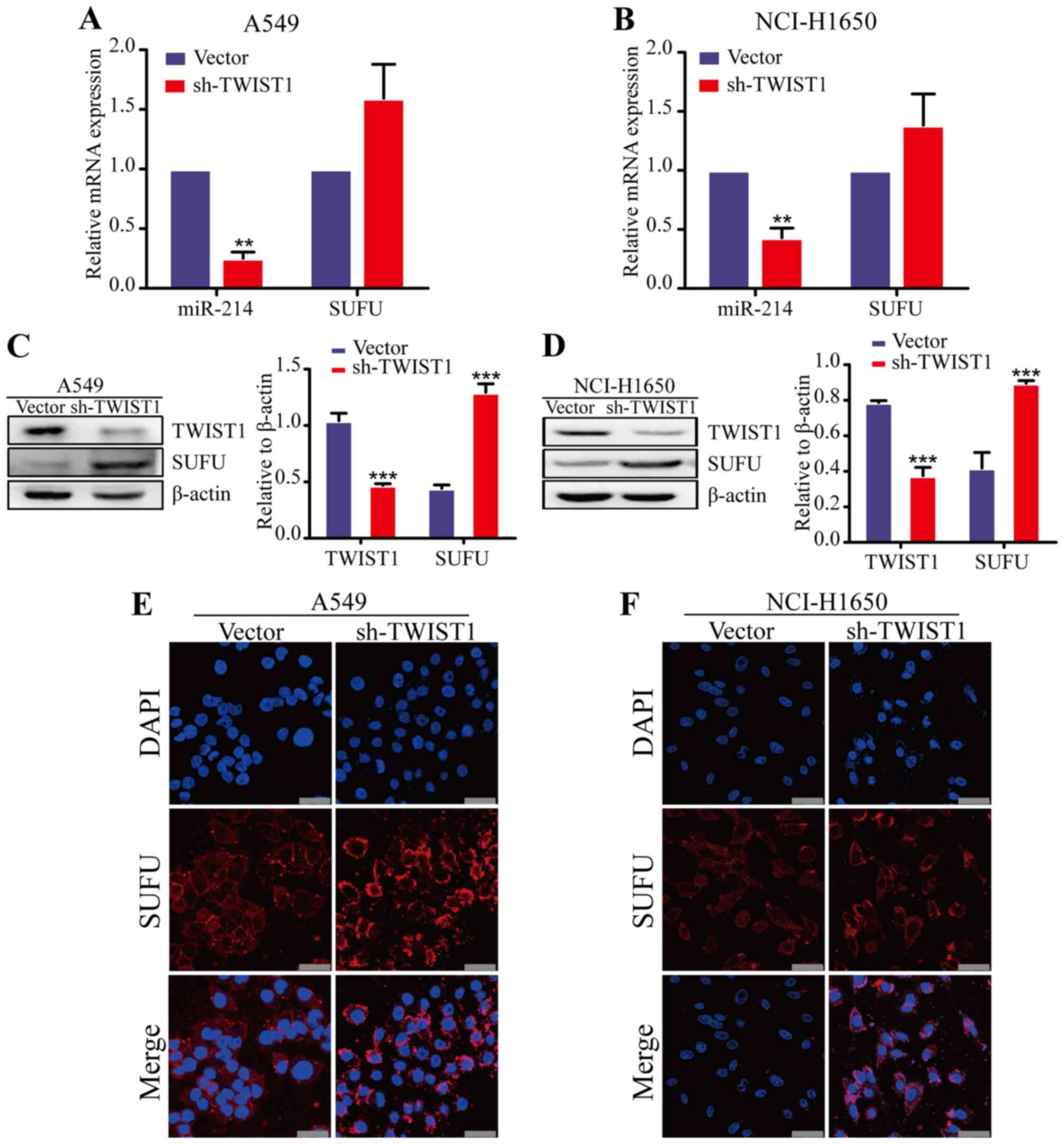
TWIST1 upregulates miR-214 expression in
LAD
Previous research revealed that TWIST1 drives the
expression of a 7.9-kb noncoding RNA transcript that encodes the
miR-199a and miR-214 cluster (18). Thus, we hypothesized that miR-214
may be upregulated by TWIST1 in LAD cells. To prove this hypothesis
we analyze the effects of TWIST1 on miR-214 expression. The results
of the qRT-PCR analysis showed that the miR-214 expression was
decreased in TWIST1 knockdown LAD cells, compared with the control
vector group (Fig. 6A and B). Our
previous studies identified SUFU as a direct target of
miR-214 in LAD cells (12). To
determine whether TWIST1 inhibition, mimicking miR-214 expression,
is sufficient to regulate SUFU expression, we examined the
SUFU expression in TWIST1 knockdown LAD cells. TWIST1 knockdown
increased SUFU levels in the two LAD cell lines (A549 and
NCI-H1650) by qRT-PCR, western blot and immunofluorescence analyses
(Fig. 6C–F). These data are
consistent with our hypothesis that TWIST1 induces miR-214 to
promote the EMT process and metastatic progression in LAD.
Discussion
LAD is the most common form of lung cancer.
Approximately 90% of LAD patients develop distant metastasis at the
advanced stage (23). A better
understanding of the molecular mechanisms underlying distant
metastasis is necessary to facilitate the development of effective
therapeutic strategies for LAD patients. While TWIST1 is known for
its ability to induce EMT and tumor progression (24–26), to our knowledge, little is known
about TWIST1 being a negative prognostic factor in LAD. In this
study, TWIST1 was found to be increased and correlated with
recurrence and final stage in LAD patients. Moreover, LAD patients
with high expression of TWIST1 have a poor survival rate.
Univariate and multivariate analyses revealed that TWIST1 was an
independent prognostic factor for overall survival of patients with
LAD. Thus, our findings establish a previously unrecognized and
important role for TWIST1 in the recurrence of different stages of
LAD patients and malignant progression.
TWIST1 functions as a negative regulator of
epithelial gene expression and a positive regulator of mesenchymal
gene expression, leading to induction of the EMT (15,27). In this study, TWIST1 was found to
be upregulated in two LAD cell lines after hypoxic induction.
Additionally, similar results were also consistently obtained in
the TGF-β-induced EMT model and CSCs. Moreover, downregulation of
TWIST1 in LAD cells upregulated E-cadherin, downregulated vimentin
and impaired LAD cells migration and invasion. These data indicate
that TWIST1 contribute to the EMT process and metastasis of LAD
cells.
Prior studies have noted that TWIST1 regulates the
expression of several miRNAs, which leads to induction of EMT.
Here, for the first time, we report a positive correlation between
miR-214 expression and TWIST1 expression in clinical LAD tissue
samples. We also demonstrated that knockdown of TWIST1 expression
in LAD not only represses the expression of miR-214, but also leads
to an increased expression of the target gene SUFU in two
different cell lines. These data indicate that TWIST1 levels
clearly upregulate miR-214 expression and as a result enhance the
induction of EMT and metastasis in LAD. Nevertheless, Li et
al (28) reported that in
human intrahepatic cholangiocarcinoma cells miR-214 represses
TWIST1 expression by targeting the 3′UTR of TWIST1, and
downregulation of miR-214 promotes the EMT by directly targeting
the TWIST1. In this study, we performed experiments in two
different LAD cell lines and provide strong evidence with clinical
samples. Moreover, we also demonstrated that miR-214 enhanced the
EMT of LAD cells, an important process involved in metastatic
progression, supporting a promoting function of miR-214 in LAD
metastasis. However, it may be explained by the following reasons:
i) both findings indicate an intricate interaction network between
miRNAs and TWIST1, which is involved in different stages of cancer
progression through regulating the EMT process. Thus, while the
high level of TWIST1 upregulates miR-214 expression, miR-214 may
further impair TWIST1 as a feedback mechanism. ii) The contrasting
results may be related to the use of different cell lines, since
the same miRNA could perform different functions through distinct
pathways in a way that was dependent on the tissue or cell type
(29). iii) A single gene has
complex functions, it can play a dual role in distinct mechanisms
to regulate tumor growth depending on the specific situation.
However, this is no way to fully define the function of one gene.
For instance, a review of the literature reveals that TWIST1
overexpression in mammary epithelial and cancer cell lines has been
shown to promote tumor stemness (25), and miR-214 enhances the stemness
and self-renewal of cancer stem-like cells (CSLCs) in lung
adeno-carcinomas by targeting CTNNBIP1 (22). This indicates that TWIST1 may play
a critical role in CSLCs self-renewal and stemness by upregulating
miR-214 expression.
Collectively, this study has significant
implications for understanding the underlying mechanisms of how
TWIST1 elevates the expression of miR-214 to contribute EMT, tumor
metastasis and poor clinical outcomes in LAD. The knowledge on
crosstalk between miR-214 and TWIST1 provides new potential
diagnostic and therapeutic strategies in LAD treatment.
Acknowledgments
Not applicable.
References
|
1
|
Thiery JP and Lim CT: Tumor dissemination:
An EMT affair. Cancer Cell. 23:272–273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brabletz T: EMT and MET in metastasis:
Where are the cancer stem cells? Cancer Cell. 22:699–701. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gunaratne A, Thai BL and Di Guglielmo GM:
Atypical protein kinase C phosphorylates Par6 and facilitates
transforming growth factor β-induced epithelial-to-mesenchymal
transition. Mol Cell Biol. 33:874–886. 2013. View Article : Google Scholar :
|
|
6
|
Mak P, Leav I, Pursell B, Bae D, Yang X,
Taglienti CA, Gouvin LM, Sharma VM and Mercurio AM: ERbeta impedes
prostate cancer EMT by destabilizing HIF-1alpha and inhibiting
VEGF-mediated snail nuclear localization: Implications for Gleason
grading. Cancer Cell. 17:319–332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cooke VG, LeBleu VS, Keskin D, Khan Z,
O'Connell JT, Teng Y, Duncan MB, Xie L, Maeda G, Vong S, et al:
Pericyte depletion results in hypoxia-associated
epithelial-to-mesenchymal transition and metastasis mediated by met
signaling pathway. Cancer Cell. 21:66–81. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Puisieux A, Brabletz T and Caramel J:
Oncogenic roles of EMT-inducing transcription factors. Nat Cell
Biol. 16:488–494. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bartel DP and Bartel DP: MicroRNAs:
Genomics, biogenesis, mechanism, and function. Cell. 116:281–297.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gregory PA, Bert AG, Paterson EL, Barry
SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y and Goodall GJ:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Long H, Wang Z, Chen J, Xiang T, Li Q,
Diao X and Zhu B: microRNA-214 promotes epithelial-mesenchymal
transition and metastasis in lung adenocarcinoma by targeting the
suppressor-of-fused protein (Sufu). Oncotarget. 6:38705–38718.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Georges SA, Biery MC, Kim SY, Schelter JM,
Guo J, Chang AN, Jackson AL, Carleton MO, Linsley PS, Cleary MA, et
al: Coordinated regulation of cell cycle transcripts by
p53-inducible microRNAs, miR-192 and miR-215. Cancer Res.
68:10105–10112. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ansieau S, Morel AP, Hinkal G, Bastid J
and Puisieux A: TWISTing an embryonic transcription factor into an
oncoprotein. Oncogene. 29:3173–3184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khanbabaei H, Teimoori A and Mohammadi M:
The interplay between microRNAs and Twist1 transcription factor: A
systematic review. Tumour Biol. 37:7007–7019. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li X, Xu F, Chang C, Byon J,
Papayannopoulou T, Deeg HJ and Marcondes AM: Transcriptional
regulation of miR-10a/b by TWIST-1 in myelodysplastic syndromes.
Haematologica. 98:414–419. 2013. View Article : Google Scholar :
|
|
17
|
Li X, Zhang Y, Zhang H, Liu X, Gong T, Li
M, Sun L, Ji G, Shi Y, Han Z, et al: miRNA-223 promotes gastric
cancer invasion and metastasis by targeting tumor suppressor
EPB41L3. Mol Cancer Res. 9:824–833. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee YB, Bantounas I, Lee DY, Phylactou L,
Caldwell MA and Uney JB: Twist-1 regulates the miR-199a/214 cluster
during development. Nucleic Acids Res. 37:123–128. 2009. View Article : Google Scholar :
|
|
19
|
Kajiyama H, Shibata K, Terauchi M,
Yamashita M, Ino K, Nawa A and Kikkawa F: Chemoresistance to
paclitaxel induces epithelial-mesenchymal transition and enhances
metastatic potential for epithelial ovarian carcinoma cells. Int J
Oncol. 31:277–283. 2007.PubMed/NCBI
|
|
20
|
Long H, Xie R, Xiang T, Zhao Z, Lin S,
Liang Z, Chen Z and Zhu B: Autocrine CCL5 signaling promotes
invasion and migration of CD133+ ovarian cancer
stem-like cells via NF-κB-mediated MMP-9 upregulation. Stem Cells.
30:2309–2319. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schieber MS and Chandel NS: ROS links
glucose metabolism to breast cancer stem cell and EMT phenotype.
Cancer Cell. 23:265–267. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qi W, Chen J, Cheng X, Huang J, Xiang T,
Li Q, Long H and Zhu B: Targeting the Wnt-regulatory protein
CTNNBIP1 by microRNA-214 enhances the stemness and self-renewal of
cancer stem-like cells in lung adenocarcinomas. Stem Cells.
33:3423–3436. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reck M, Popat S, Reinmuth N, De Ruysscher
D, Kerr KM and Peters S; ESMO Guidelines Working Group: Metastatic
non-small-cell lung cancer (NSCLC): ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol.
25(Suppl 3): iii27–iii39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen ZF and Behringer RR: Twist is
required in head mesenchyme for cranial neural tube morphogenesis.
Genes Dev. 9:686–699. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morel AP, Hinkal GW, Thomas C, Fauvet F,
Courtois-Cox S, Wierinckx A, Devouassoux-Shisheboran M, Treilleux
I, Tissier A, Gras B, et al: EMT inducers catalyze malignant
transformation of mammary epithelial cells and drive tumorigenesis
towards claudin-low tumors in transgenic mice. PLoS Genet.
8:e10027232012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fan Q, Qiu MT, Zhu Z, Zhou JH, Chen L,
Zhou Y, Gu W, Wang LH, Li ZN, Xu Y, et al: Twist induces
epithelial-mesen-chymal transition in cervical carcinogenesis by
regulating the TGF-β/Smad3 signaling pathway. Oncol Rep.
34:1787–1794. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li B, Han Q, Zhu Y, Yu Y, Wang J and Jiang
X: Downregulation of miR-214 contributes to intrahepatic
cholangiocarcinoma metastasis by targeting twist. FEBS J.
279:2393–2398. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Contreras J and Rao DS: MicroRNAs in
inflammation and immune responses. Leukemia. 26:404–413. 2012.
View Article : Google Scholar
|