Introduction
Rheumatoid arthritis (RA) is an autoimmune disease,
which causes chronic inflammation of the joints and other areas of
the body (1). The damaged of
tissues in the body by its own immune system are termed autoimmune
diseases (2). RA is characterized
by inflammation of the tissue around the joints, and this disease
can also cause inflammation and injury in other organs in the body
(3). Chronic inflammation of RA
can cause permanent joint destruction and deformity, which begins
with the proliferation of synovial macrophages and fibroblasts
(4). The treatment of RA involves
a combination of patient education, rest and exercise, joint
protection, medications and occasionally surgery (5). For pharmacologic treatment,
non-steroidal anti-inflammatory drugs are commonly used to reduce
joint pain. Disease-modifying anti-rheumatic drugs, including
methotrexate and sulfasalazine, are initiated to slow disease
progression as early as possible. In addition, novel drugs,
including transforming growth factor-α (TNF-α) inhibitors,
interleukin-6 (IL-6) inhibitors and Janus kinase (JAK) inhibitors
have been introduced (6).
However, there is no cure for RA. Early RA treatment leads to an
improved prognosis. The fibroblast-like synoviocytes (FLS) is the
most prominent cell type in the rheumatoid joint and is central
role to the propagation of RA (7). It has been reported that the FLS is
directly responsible for cartilage destruction, and drives
inflammation and autoimmunity (7). Therefore, treatment designed to
inhibit the proliferation of RA-FLS may provide a reasonably
efficient approach for curing RA.
Polygonum cuspidatum, also known as
Reynoutria japonica Houtt and Huzhang in China, is a
perennial herb plant belonging to the family Polygonaceae (8). Polygonum cuspidatum is widely
distributed in southern China and Japan, and is a traditional and
well-known Chinese medicinal herb (8). It has long been used in folk
medicine for the treatment of inflammation, infection, jaundice,
skin burns and hyper-lipemia (8,9).
As demonstrated by extensive reports, the root of Polygonum
cuspidatum has wide pharmacological effects, including
anti-shock, anti-inflammatory, antioxidant, anticancer and
hepatoprotective effects (10–14). Currently, >67 compounds have
been isolated and identified from this plant, including quinones,
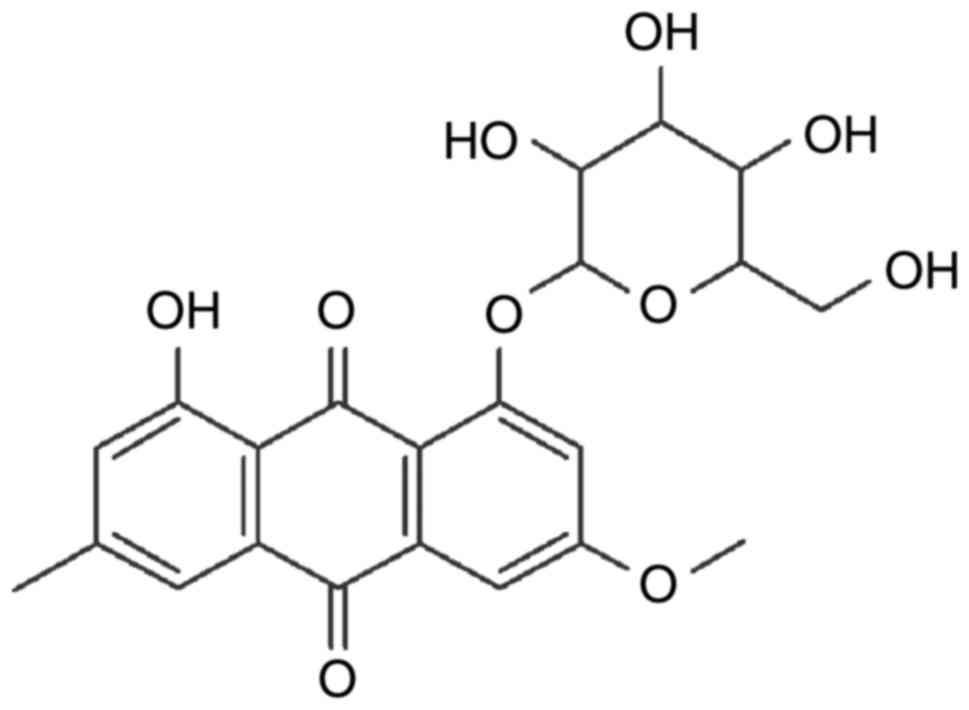
stilbenes, flavonoids, counmarins and ligans (8). Physcion 8-O-β-glucopyranoside (POGD)
is an anthraquinonesone (Fig. 1)
isolated from the root of Polygonum cuspidatum. It has been
reported that POGD has significant anti-proliferative activity on
A549 cell lines by inducing cell cycle arrest and apoptosis
(9). The aim of the present study
was to increase current understanding of the anti-proliferative and
anti-inflammatory potency of POGD in MH7A RA-derived
fibroblast-like synoviocytes.
Materials and methods
Herbal medicines
Polygonum cuspidatum was purchased from the
traditional Chinese Medicine Store of Shandong Province (Shandong,
China). A voucher specimen of P. cuspidatum was deposited at
the Department of Pharmacy, Shandong Zibo Central Hospital
(Shandong, China).
Cells and culture
The MH7A RA-derived fibroblast-like synoviocyte cell
line was obtained from the Type Culture Collection of the Chinese
Academy of Sciences (Shanghai, China). The MH7A cells were cultured
in RPMI-1640 medium with 10% fetal bovine serum (FBS), 1%
penicillin and 1% streptomycin in a 5% CO2 humidified
atmosphere at 37°C.
Chemicals and reagents
Interleukin-1β (IL-1β), interleukin-6 (IL-6),
interleukin-8 (IL-8), interleukin-10 (IL-10), interleukin-12
(IL-12) and interleukin-17A (IL-17A) enzyme-linked immunosorbent
assay (ELISA) kits were purchased from Invitrogen; Thermo Fisher
Scientific, Inc. (Waltham, MA USA); the Dulbecco's modified Eagle's
medium (DMEM), FBS and trypsinase were from Gibco; Thermo Fisher
Scientific, Inc. The Cell Counting Kit-8, BCA protein assay reagent
and goat-anti-rabbit/rat horseradish-peroxidase-conjugated (HRP)
secondary antibodies were purchased from Beyotime Institute of
Biotechnology (Haimen, Jiangshu province, China); the tumor
necrosis factor-α (TNF-α), dimethyl sulfoxide (DMSO),
dexamethasone, complete Freund's adjuvant (CFA) and incomplete
Freund's adjuvant (IFA) were purchased from Sigma (Shanghai,
China); EMD Millipore (Billerica, MA, USA); TGF-β1 (cat. no.
sc-130348), small mothers against decapentaplegic (Smad)4 (cat. no.
sc-7154), Smad7 (cat. no. sc-11392), Histone H1 (cat. no. sc-8030)
and β-actin (cat. no. sc-47778) antibodies were purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA); c-Jun
N-terminal kinase (JNK; cat. no. ab179461), phosphorylated [(p-)JNK
(cat. no. ab124956)], p-P38 (cat. no. ab4822), P38 (cat. no.
ab170099), p-extracellular signal-regulated kinase (ERK)1/2 (cat.
no. ab223500), ERK1/2 (cat. no. ab17942), nuclear factor (NF)-κB
p65 (cat. no. ab32536), and inhibitor of NF-κB (IκB; cat. no.
ab32518) antibodies were purchased from Abcam (Cambridge, MA, USA).
Chicken type II collagen (CII) was purchased from Xi'an Herb King
Biotechnology Co., Ltd. (Xi'an, Shanxi, China). All other reagents
used were of analytically pure grade.
Isolation and preparation of POGD
The preparation of POGD was performed according to a
previously described method with minor modification (9). Briefly, the comminuted herbal
medicines were extracted with 8X 75% aqueous ethanol by reflux
three times. The total extracts were evaporated under a vacuum to
remove the ethanol. The residual extract solution was then orderly
extracted with ethyl acetate and n-butanol, and the n-butanol
fraction was evaporated under a vacuum to obtain the dried
n-butanol extracts. Subsequently, the n-butanol extracts were
isolated by silica gel (200-300 mesh) column chromatography eluting
with chloroform: Methanol (20:1, 15:1, 10:1, 7:1, 5:1, 2:1 and
1:1). Based on the thin layer chromatography assay, the similar
fractions of the sub-fractions of n-butanol extracts were combined
to obtain six sub-fractions (Fra. 1-Fra. 6). Subsequently, using a
series of repeated silica gel column chromatography and Sephadex
LH-20 chromatography, the target compound was isolated from
fractions 4.
Identification of the POGD
The POGD was identified based on the 1H
NMR and 13C NMR experiments, and the spectral data were
as follows: 1H-NMR (600 MHz, DMSO-d6) δ: 11.32 (1H, s, 1-OH), 7.42
(1H, d, J=1.6 Hz, H-4), 7.25 (1H, d, J=2.8 Hz, H-5), 7.12 (1H, d,
J=2.8 Hz, H-7), 6.98 (H, s, H-2), 5.09 (1H, d, J=7.4 Hz, H-1′),
3.14 (3H, s, 6-OCH3), 2.37 (3H, s, 3-CH3); 13C-NMR (150 MHz,
DMSO-d6) δ: 187.9 (C-9), 183.6 (C-10), 165.7 (C-6), 163.2 (C-1),
162.6 (C-8), 148.3 (C-3), 138.0 (C-10a), 133.5 (C-4a), 125.6 (C-2),
120.7 (C-4), 115.9 (C-8a), 114.7 (C-9a), 109.8 (C-7), 109.8 (C-5),
102.3 (C-1′), 78.8 (C-3′), 77.9 (C-5′), 74.7 (C-2′), 70.9 (C-4′),
62.1 (C-6′), 50.1 (6-OCH3), 22.9 (3-CH3). These data were similar
to the previously reported data (9).
Cell viability assay using Cell-Counting
Kit-8 (CCK-8)
The effects of POGD on cellular viability were
determined using CCK-8 according to the manufacturer's protocol.
The MH7A cells (5×103 cells/well) were seeded in 96-well
plates and incubated with different concentrations of POGD (4, 8,
16, 32, 64, 128 and 256 μg/ml) for 12, 24, 36, 48 or 72 h.
The CCK-8 solution was then added to each well and incubated for
another 1 h in the incubator. Finally, the optical density (OD)
value was read at 450 nm using a 96-well plate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The results were calculated
as a percentage of the DMSO-treated control cells.
Determination of cell cytokine levels by
ELISA
The MH7A cells (5×105 cells/well) were
treated with TNF-α (10 ng/ml) for 12 h at 37°C in a 5%
CO2 humidified atmosphere prior to the assay. The cell
supernatant was removed via pippetting and cells were washed twice
with PBS. The cells were then exposed to increased concentrations
of POGD (8, 16 and 32 μg/ml) for another 24 h. Following
treatment, the cell supernatant was collected, and the levels of
IL-1β, IL-6, IL-8, IL-10, IL-12, and IL-17 cytokines were
determined using ELISA assay kits according to the manufacturer's
protocol (R&D Systems, Inc., Minneapolis, MN, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis of the mRNA expression
levels of matrix metalloproteinase (MMP)-2, MMP-3, MMP-9,
cyclooxygenase-2 (COX-2) and vascular endothelial growth factor
(VEGF) in MH7A cells
The effects of POGD on the mRNA expression levels of
MMP-2, MMP-3, MMP-9, COX-2 and VEGF were determined by RT-qPCR
analysis. The MH7A cells (3×105 cells/well) were seeded
in 6-well plates and incubated with either increasing
concentrations of POGD (15, 30 and 60 μg/ml) or vehicle
(DMSO) in the presence of 10 ng/ml TNF-α for 72 h at 37°C.
Subsequently, the cells were collected and the total RNA was
extracted using an RNAiso Plus kit (Takara Biotechnology Co., Ltd.,
Dalian, China). Total RNA was then used to synthesize cDNAs of
MMP-2, MMP-3, MMP-9, COX-2, VEGF and β-actin using PrimeScript™ RT
reagent kits (Takara Biotechnology Co., Ltd.). The synthesized
cDNAs were amplified using SYBR Green mixture on a CFX96 Touch
Real-Time PCR Detection system (Bio-Rad Laboratories, Inc.). Each
10 μl reaction mixture contained 5 μl of SYBR Green
mixture, 1 μl of 2 μM forward and reverse primer
mixture (Table I), 1 μl of
cDNA, and 3 μl of ddH2O. The PCR conditions were 95°C for 10
min, followed by 39 cycles of 95°C for 15 sec and 58°C for 60 sec.
The primers used for RT-qPCR analysis are shown in Table I. The relative mRNA expression
levels of TNF-α, IL-6 and IL-1β were measured using the 2−ΔΔ
Cq relative quantitative analysis method (15) and all samples were analyzed in
triplicate.
 | Table IPrimers used for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I
Primers used for reverse
transcription-quantitative polymerase chain reaction analysis.
| Author, year | Gene | Primer sequence
(5′-3′) | Product size
(bp) | (Refs.) |
|---|
| Pu et al,
2016 | MMP-2 | Forward:
TGGCAAGTACGGCTTCTGTC | 179 | (16) |
| Reverse:
TTCTTGTCGCGGTCGTAGTC | | |
| Tran et al,
2005 | MMP-3 | Forward:
CAGGCTTTCCCAAGCAAATA | 129 | (17) |
| Reverse:
TTGCATTTGGGTCAAACTCC | | |
| Pu et al,
2016 | MMP-9 | Forward:
TGCGCTACCACCTCGAACTT | 200 | (16) |
| Reverse:
GATGCCATTGACGTCGTCCT | | |
| Ospelt et
al, 2008 | COX-2 | Forward:
TTCAAATGAGATTGTGGGAAAT | 305 | (18) |
| Reverse:
AGATCATCTCTGCCTGAGTATCTT | | |
| Pu et al,
2016 | VEGF | Forward:
CGGCGAAGAGAAGAGACACA | 196 | (16) |
| Reverse:
GGAGGAAGGTCAACCACTCA | | |
| Pu et al,
2016 | GAPDH | Forward:
GAGTCAACGGATTTGGTCGT | 185 | (16) |
| Reverse:
GACAAGCTTCCCGTTCTCAG | | |
Western blot analysis
The effects of POGD on the protein expression levels
of TGF-β1, Smad4, Smad2, Smad7, ERK1/2, P-ERK1/2, JNK, P-JNK, P-38,
P-P38, NF-KB p65 (N), NF-KB p65 (C) and IKB were measured in MH7A
cells using western blot analysis. Following treatment with either
grade concentrations of POGD (15, 30 and 60 μg/ml) or
vehicle (DMSO) in the presence of 10 ng/ml TNF-α for 72 h, the
cells were collected and total protein was extracted using western
blot & IP cell lysis buffer (Sangon Biotech Co., Ltd.,
Shanghai, China). Subsequently, the concentration of total protein
was determined using BCA protein assay reagent (Sangon Biotech Co.,
Ltd.). Total protein (40 μg) in each sample was separated
using SDS-PAGE (12% gel) and blotted onto a polyvinylidene
difluoride filter membrane (PVDF). Subsequently, the protein on the
PVDF was probed with anti-TGF-β1 (1:3,000), Smad4 (1:1,000), Smad2
(1:1,000), Smad7 (1:1,000), ERK1/2 (1:1,000), P-ERK1/2 (1:1,000),
JNK (1:1,000), P-JNK (1:1,000), P-38 (1:1,000), P-P38 (1:1,000),
NF-KB p65 (1:500), IκB (1:500), β-actin (1:3,000) and Histone H1
(1:1,000) antibodies at 4°C for 12 h, followed by incubation with
corresponding HRP-conjugated secondary antibodies for 2 h at 37°C.
Finally, the immunoreactive bands were visualized with
ECL-detecting reagents and the OD values were analyzed using ImageJ
software (version 1.48; National Institutes of Health, Bethesda,
MD, USA). To normalize for protein loading, the expression levels
of TGF-β1, Smad4, Smad2, Smad7, ERK1/2, P-ERK1/2, JNK, P-JNK, P-38,
P-P38, NF-KB p65 (N), NF-KB p65 (C) and IKB were determined as a
relative value to that of β-actin, and the expression level of p65
(N) was determined as a relative value to that of Histone H1.
Determination of the anti-arthritic
effects of POGD in vivo
60 rats weighing 220±20 g with 30 male and 30 female
were purchased from the Shanghai Laboratory Animal Centre
(Shanghai, China). Each animal was housed under standard conditions
(21±1°C, 50-10% relative humidity, 12 h light/dark cycle) and had
free access to food and water. A total of 60 rats were randomly
divided into the following six groups (n=10): i) normal, healthy
rats treated with saline (10 ml/kg); ii) control, collagen-induced
arthritis (CIA) rats treated with saline (10 ml/kg); iii)
dexamethasone, CIA rats treated with dexamethasone (positive drug,
0.05 mg/kg); iv) POGD (20 mg/kg), CIA rats treated with POGD at a
dose of 20 mg/kg; v) POGD (40 mg/kg), CIA rats treated with POGD at
a dose of 40 mg/kg; and vi) POGD (60 mg/kg), CIA rats treated with
POGD at a dose of 60 mg/kg. The experimental protocols were
approved by the Animal Care and Use Committee of Dezhou People's
Hospital (Shandong, China).
To evaluate the anti-arthritic effects of POGD in
vivo, a CIA animal model was established as previously reported
with minor modifications (16).
In brief, CII in 0.1 mM acetic acid (4 mg/ml) was emulsified with
an equal volume of CFA. The rats were initially immunized by a
subcutaneous injection of CII emulsion at the base of the tail (100
μl/rat). After 14 days, the CII emulsion with an equal
volume of IFA (100 μl/rat) was administered via subcutaneous
injection at the same location, and the rats were administered
orally with saline, dexamethasone and POGD for 21 days. During the
experiment, the paw volumes and arthritic indices of the rats were
measured every 3 days. The arthritic indices were recorded using
the following ordinal scale: 0, unaffected, 1, one type of joint
affected, 2, two types of joint affected, 3, three types of joint
affected, 4, three types of joint affected plus maximal erythema
and swelling. At the end of the experiment, blood samples were
collected using an orbital blood sampling protocol and centrifuged
for 15 min (1,800 × g, 4°C). The serum levels of TNF-α, IL-1β,
IL-6, IL-8 and IL-17A were determined using commercial ELISA kits
according to the manufacturer's protocols.
Statistical analysis
All results are expressed as the mean ± standard
deviation of three independent experiments performed in triplicate.
Statistical analyses were performed using the SPSS 19.0 software
package (IBM SPSS, Armonk, NY, USA) and one-way analysis of
variance with Dunnett's test was used to compare the means between
two groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of POGD on MH7A cell
viability
The effect of POGD on MH7A cell viability was
determined using CCK-8. As shown in Fig. 2A, POGD exerted marked inhibitory
effects on MH7A cell growth in a concentration-dependent manner
with IC50 values of 49.76 μg/ml. In addition,
POGD inhibited MH7A cell proliferation in time-dependent manner in
72 h (Fig. 2B).
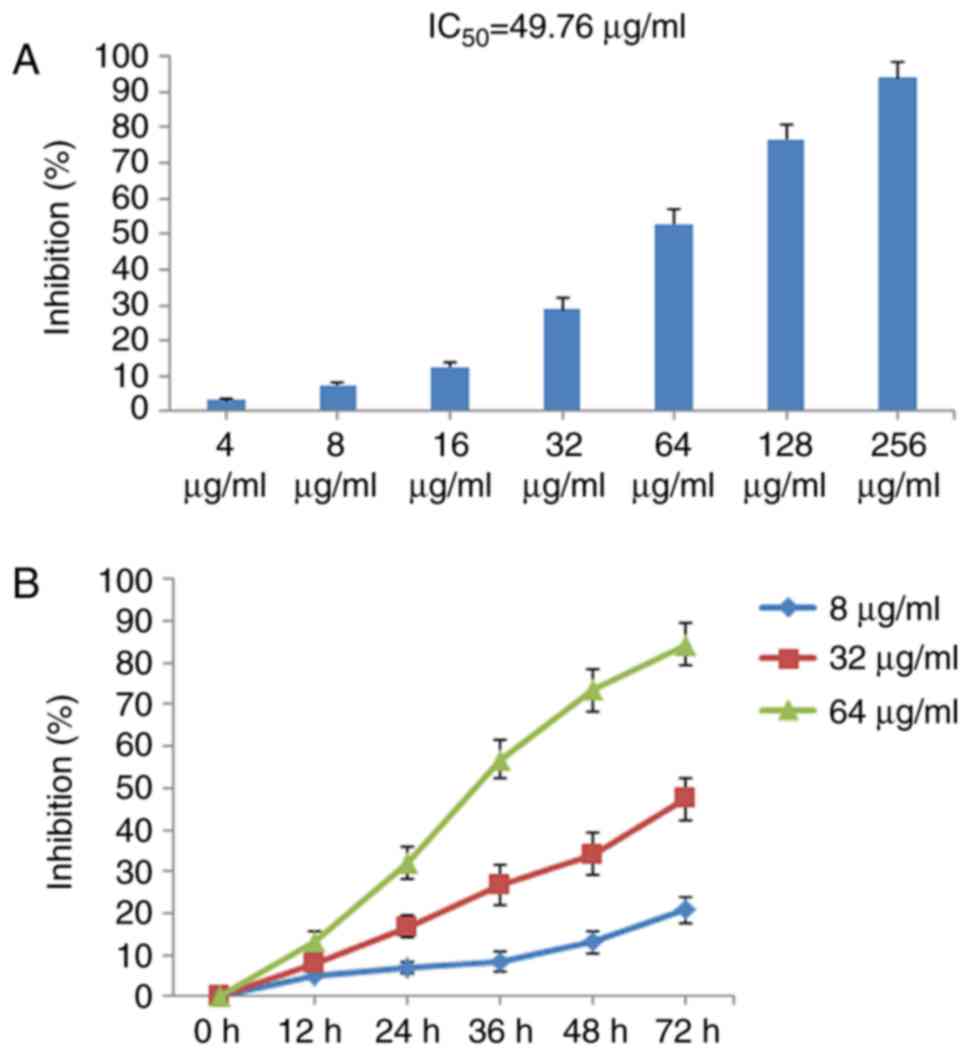 | Figure 2Inhibitory effects of POGD on the
proliferation of MH7A cells. (A) Cells were treated with POGD (4,
8, 16, 32, 64, 128 and 256 μg/ml) for 36 h; a CCK-8 assay
was performed to determine the cell percentage proliferation
inhibition (n=4), and IC50 value of POGD was calculated.
(B) Cells were treated with POGD (8, 32 and 64 μg/ml) for
12, 24, 36, 48 and 72 h, respectively; a CCK-8 assay was performed
to determine the percentage cell proliferation inhibition (n=4).
POGD, physcion 8-O-β-glucopyranoside; CCK-8, Cell Counting
Kit-8. |
Effects of POGD on levels of IL-1β, IL-6,
IL-8, IL-10, IL-12 and IL-17A in TNF-α-stimulated MH7A cells
To verify the anti-inflammatory effects of POGD in
MH7A cells, the levels of inflammatory cytokines IL-1β, IL-6, IL-8,
IL-10, IL-12 and IL-17A were determined by ELISA in TNF-α-induced
MH7A cells. As shown in Fig. 3,
with the exception of IL-10, the levels of these inflammatory
cytokines were significantly reduced, compared with those in the
TNF-α group (P<0.01, by POGD at concentrations of 8, 16 and 32
μg/ml.
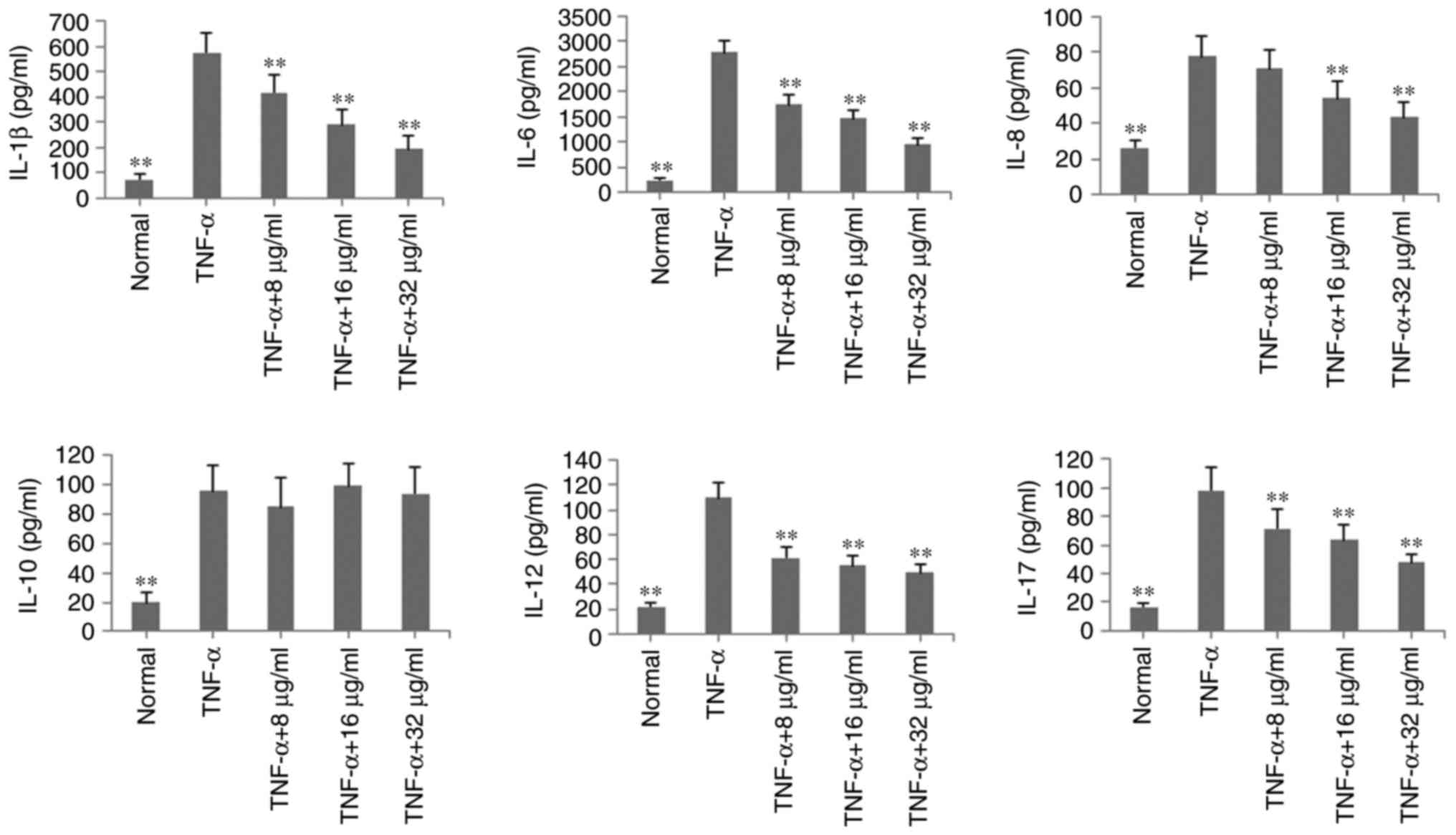 | Figure 3Inhibitory effects of POGD on levels
of IL-1β, IL-6, IL-8, IL-10, IL-12 and IL-17A in TNF-α-stimulated
MH7A cells. Cells were pretreated with TNF-α (10 ng/ml) for 12 h,
following which the cells were exposed to POGD (8, 16 and 32
μg/ml) for another 24 h; enzyme-linked immunosorbent assays
were performed to determine the levels of cytokines in cell
supernatants (n=4). **P<0.01, vs. control (TNF-α
group). POGD, physcion 8-O-β-glucopyranoside; IL, interleukin;
TNF-α, tumor necrosis factor-α. |
Effects of POGD on mRNA expression levels
of MMP-2, MMP-3, MMP-9, VEGF and COX-2 in TNF-α-stimulated MH7A
cells
In the TNF-α-induced MH7A cells, the mRNA expression
levels of MMP-2, MMP-3, MMP-9, VEGF and COX-2 were determined using
RT-qPCR analysis. Following treatment with POGD for 24 h, the mRNA
expression levels of MMP-2, MMP-3, MMP-9, VEGF and COX-2 were
significantly decreased, compared with those in the TNF-α group
(P<0.01; Fig. 4).
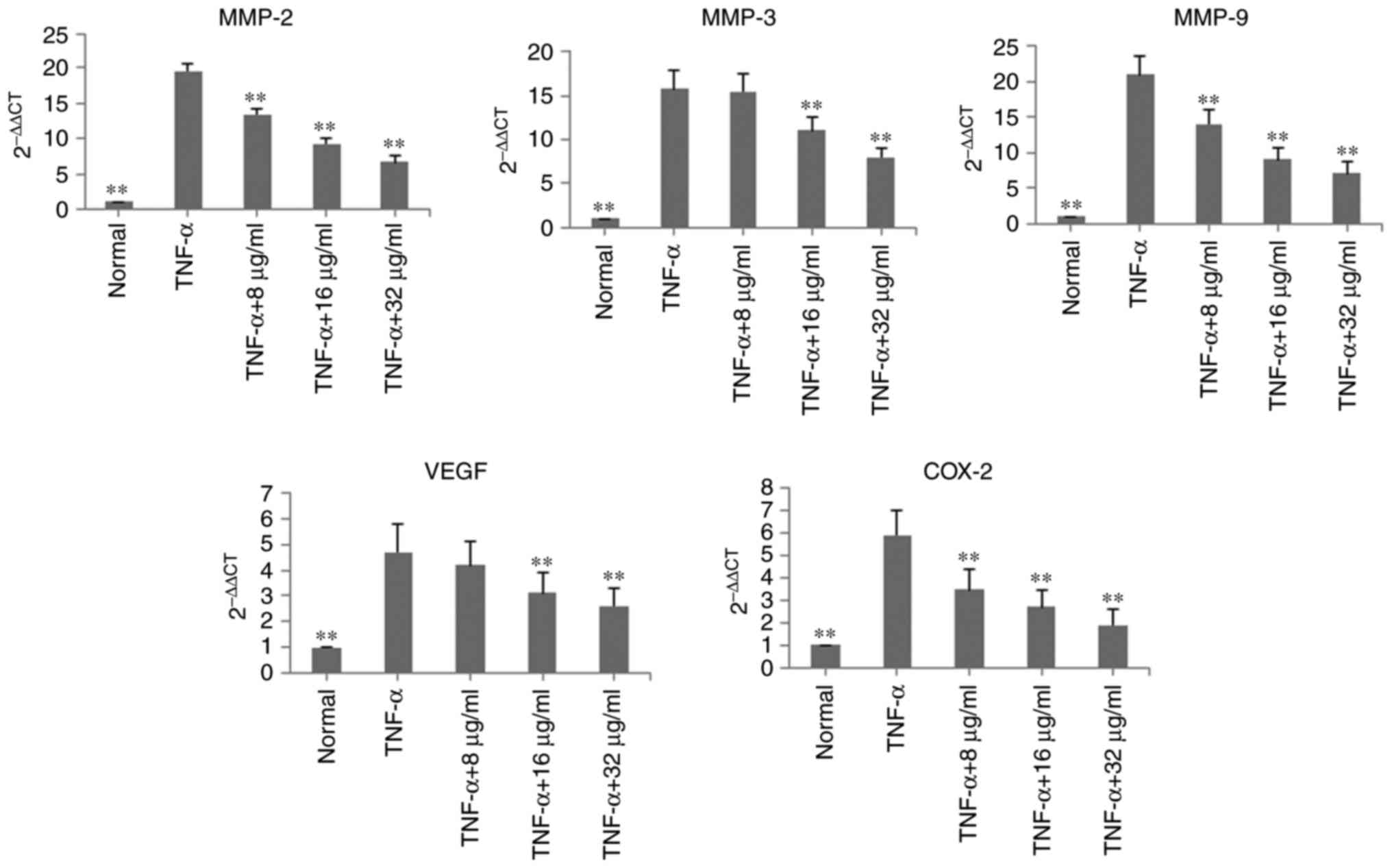 | Figure 4Inhibitory effects of POGD on mRNA
expression levels of MMP-2, MMP-3, MMP-9, VEGF and COX-2 in
TNF-α-stimulated MH7A cells. Cells were pretreated with TNF-α (10
ng/ml) for 12 h, following which the cells were exposed to POGD (8,
16 and 32 μg/ml) for another 24 h; reverse
transcription-quantitative polymerase chain reaction assays were
performed to determine the mRNA expression levels of MMP-2, MMP-3,
MMP-9, VEGF and COX-2 (n=4). **P<0.01, vs. control
(TNF-α group). POGD, physcion 8-O-β-glucopyranoside; MMP, matrix
metalloproteinase; VEGF, vascular endothelial factor; COX-2,
cyclooxygenase 2; TNF-α, tumor necrosis factor-α. |
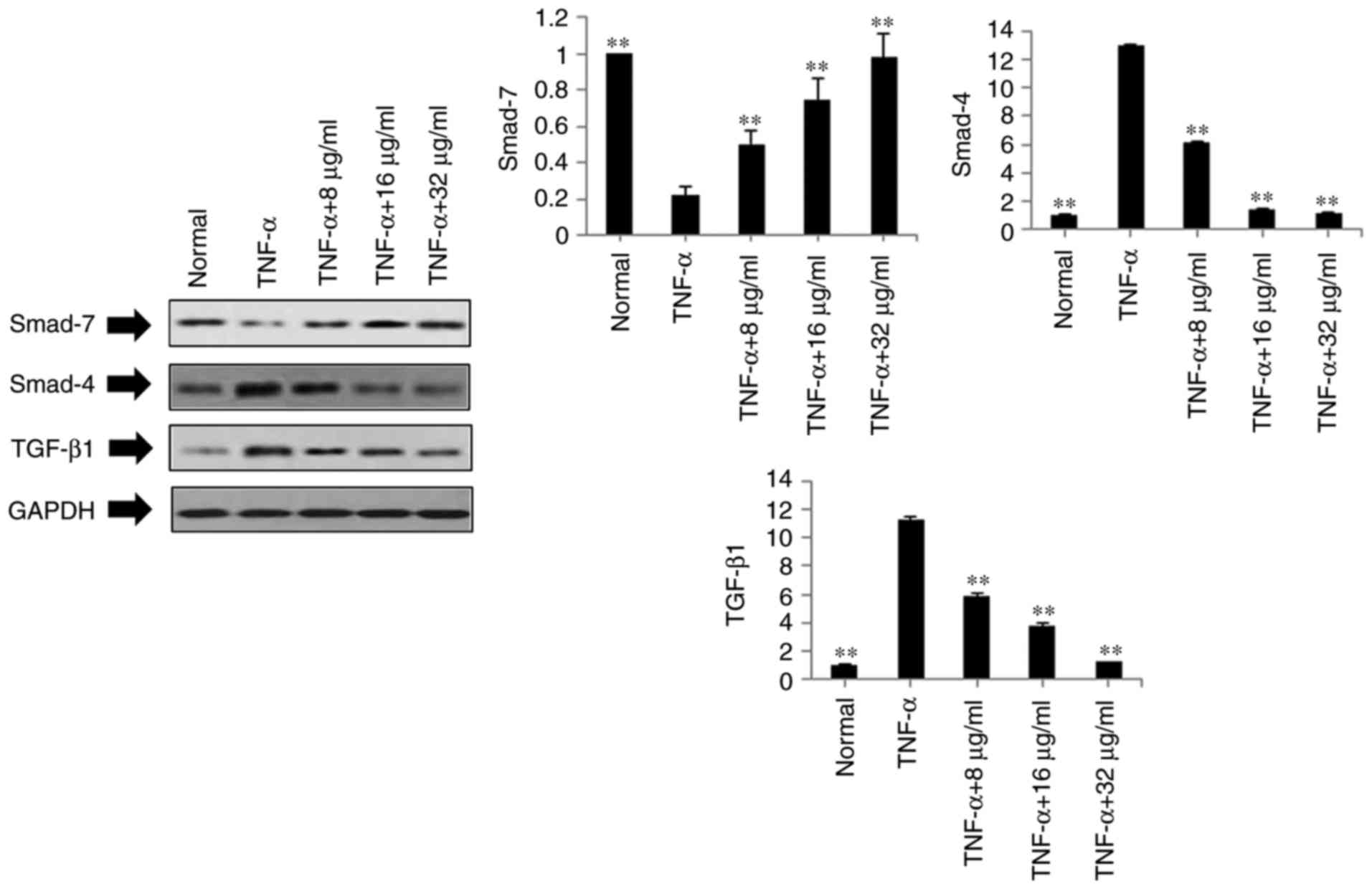
Effects of POGD on expression levels of
TGF-β1, Smad4 and Smad7 in TNF-α-stimulated MH7A cells
Based on the above results, POGD not only inhibited
the expression levels of pro-inflammatory cytokines IL-1β, IL-6,
IL-8, IL-12 and IL-17A in the TNF-α-stimulated MH7A cells, but also
decreased the mRNA expression levels of MMP-2, MMP-3, MMP-9, VEGF
and COX-2. To further investigate the possible mechanisms
underlying these changes, the protein expression levels of TGF-β1,
Smad4 and Smad7 were measured in the TNF-α-stimulated MH7A cells
using western blot analysis. As shown in Fig. 5, compared with the TNF-α group,
the protein expression levels of TGF-β1 and Smad4 were markedly
down-regulated in the POGD groups, whereas the protein expression
of Smad7 was significantly upregulated in the TNF-α-induced MH7A
cells.
Effects of POGD on expression levels of
ERK1/2, P-ERK1/2, JNK, P-JNK, P-38, P-P38 in TNF-α-stimulated MH7A
cells
The present study also examined the protein
expression levels of ERK1/2, P-ERK1/2, JNK, P-JNK, P-38 and P-P38
in the TNF-α-stimulated and POGD-treated MH7A cells using western
blot analysis. As shown in Fig.
6, POGD significantly inhibited the protein expression levels
of P-JNK, JNK, P-P38, P38, P-ERK1/2 and ERK1/2 in the TNF-α-induced
MH7A cells (P<0.01).
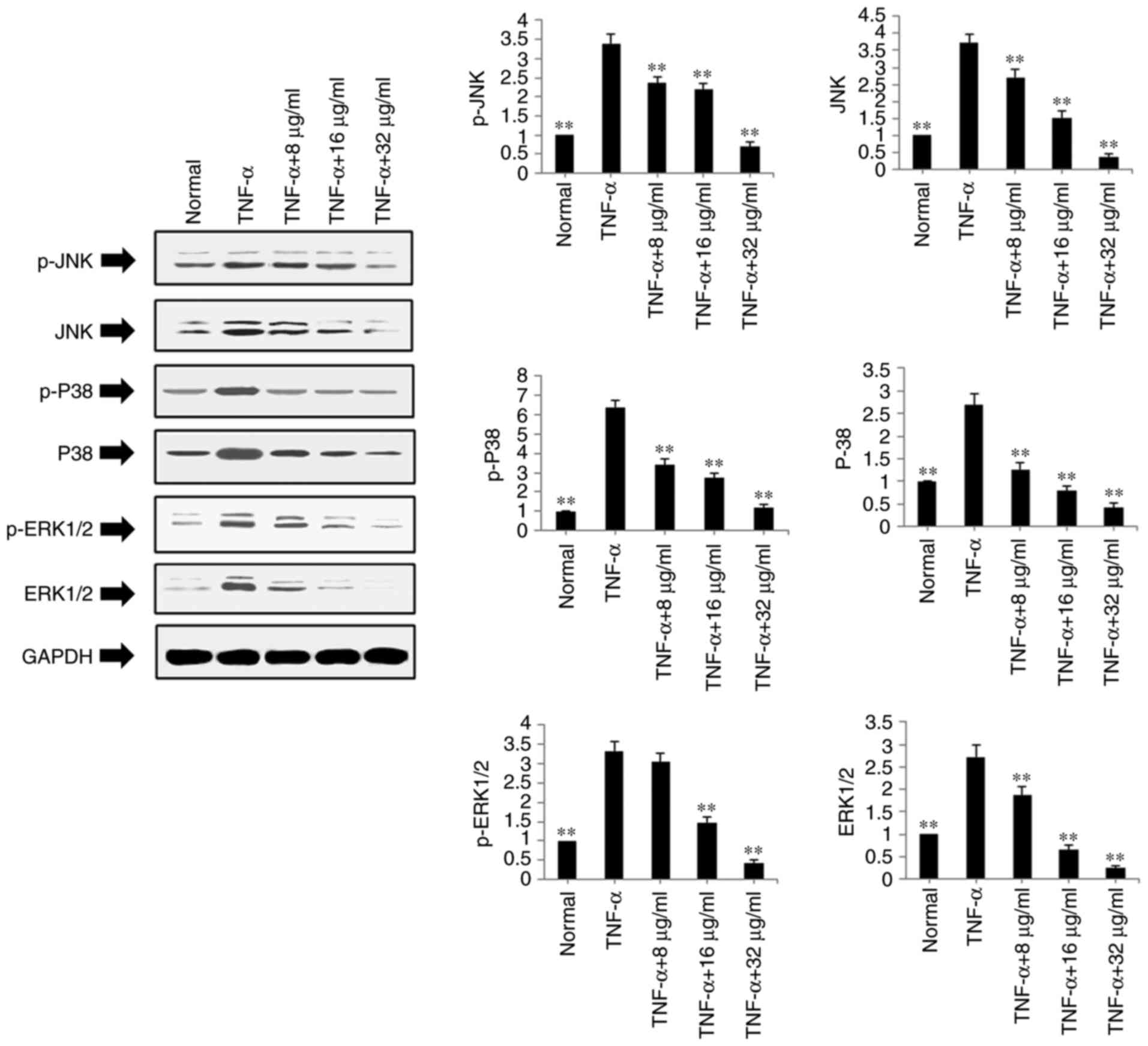 | Figure 6Regulatory effects of POGD on the
expression of p-JNK, JNK, p-P38, P38, p-ERK1/2 and ERK1/2 in
TNF-α-stimulated MH7A cells. Cells were pretreated with TNF-α (10
ng/ml) for 12 h, following which the cells were exposed to POGD (8,
16 and 32 μg/ml) for another 24 h; western blot assays were
performed to determine the expression levels of p-JNK, JNK, p-P38,
P38, p-ERK1/2 and ERK1/2 (n=4). **P<0.01, vs. control
(TNF-α group). POGD, physcion 8-O-β-glucopyranoside; TNF-α, tumor
necrosis factor-α; JNK, c-Jun N-terminal kinase; ERK, extracellular
signal-regulated kinase; p-, phosphorylated. |
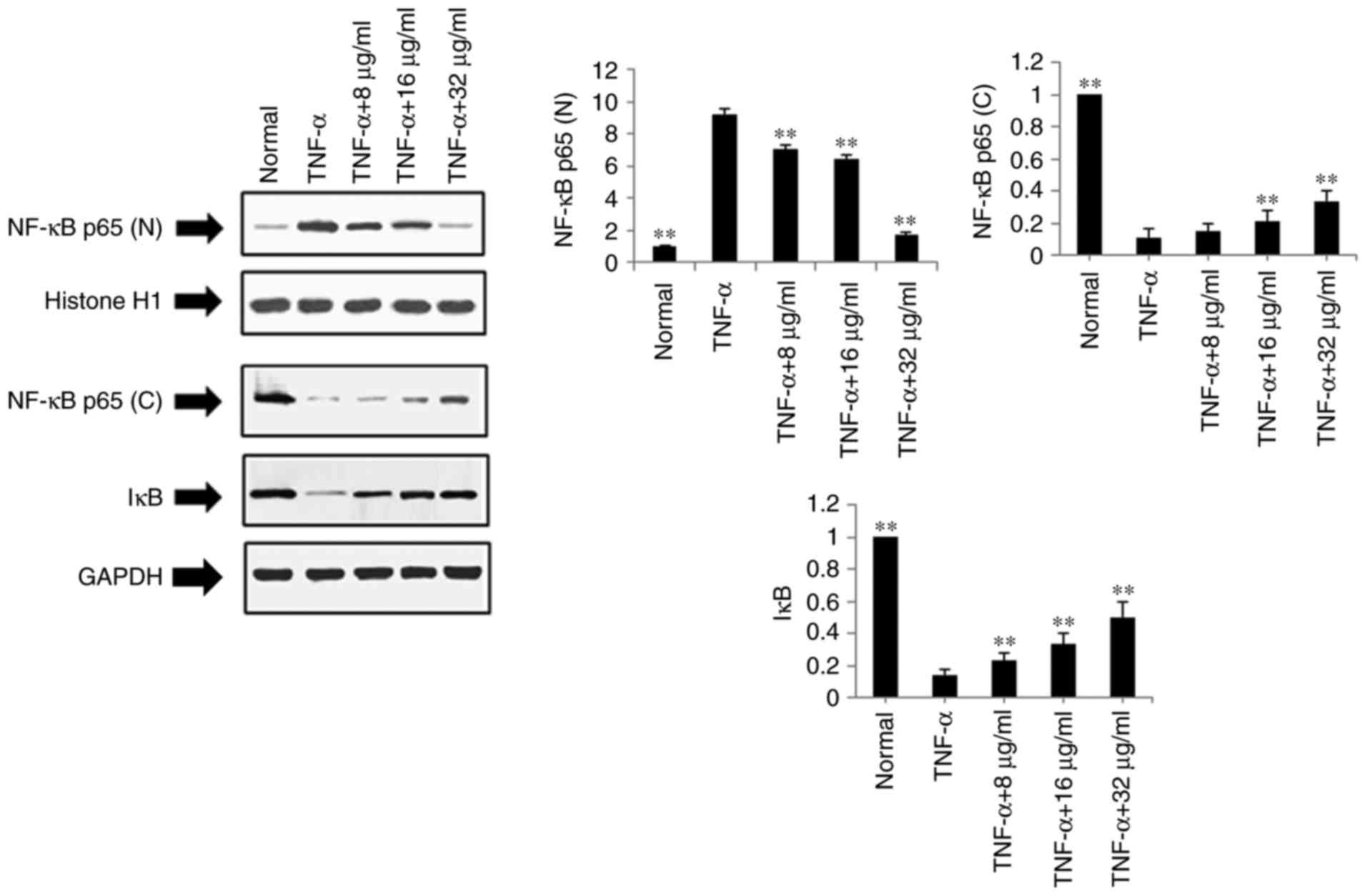
Effects of POGD on expression levels of
NF-κB p65 (N), NF-κB p65 (C) and IκB in TNF-α-stimulated MH7A
cells
The present study also examined the effects of POGD
on the expression levels of NF-κB p65 (N), NF-κB p65 (C) and IκB
using western blot analysis in TNF-α-stimulated MH7A cells.
Following treatment with POGD for 24 h, the protein expression
level of NF-κB p65 (N) was significantly reduced, whereas the
protein expression levels of NF-κB p65 (C) and IκB were
significantly increased (P<0.01), as shown in Fig. 7.
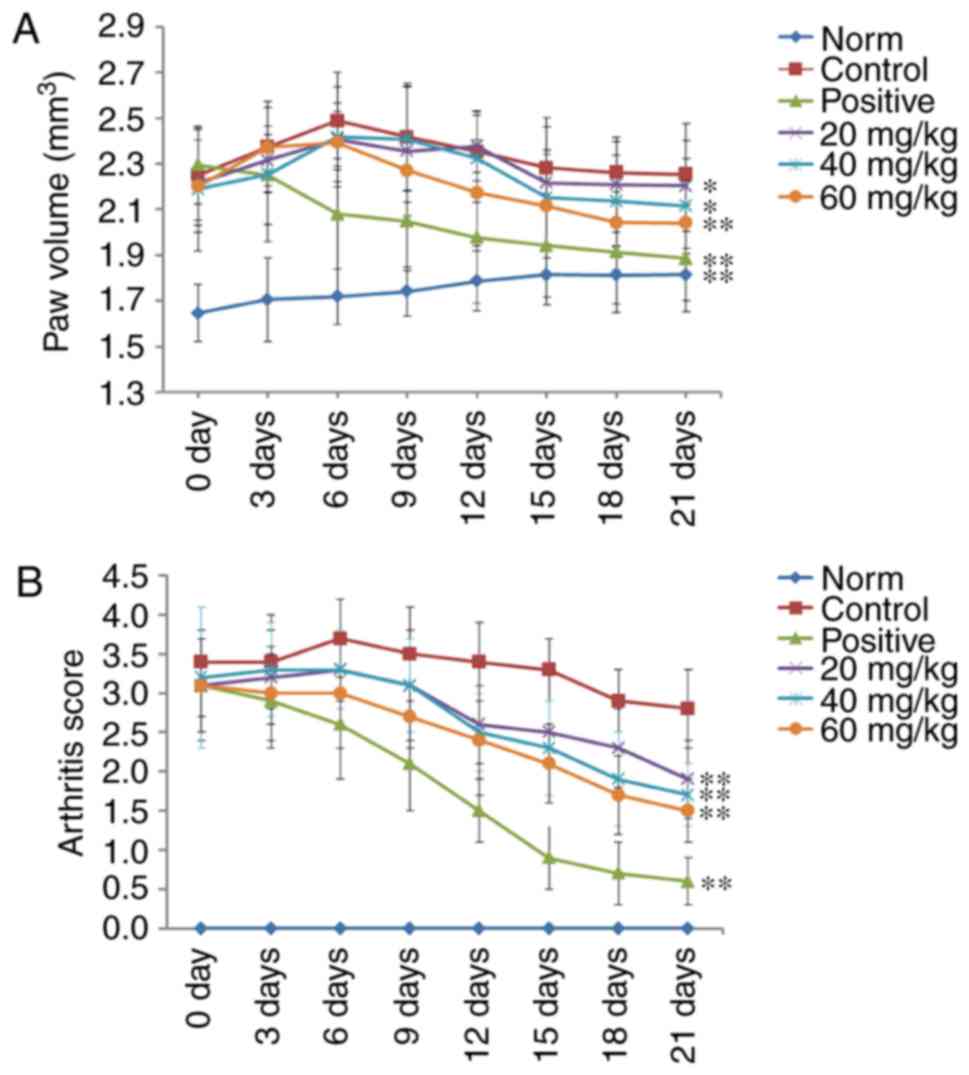
POGD decreases paw swelling and arthritis
indices in CIA rats
A CIA rat model was established to evaluate the
therapeutic effects of POGD in rats with RA. As shown in Fig. 8, RA symptoms were observed when
the experimental rats were immunized twice with CII, including paw
swelling and higher arthritis indices (P<0.01), compared with
the normal rats. These symptoms were significantly improved
following treatment with dexamethasone. The results indicated that,
from the 6th day following initial dexamethasone administration,
paw swelling was decreased in the CIA rats, compared with that in
the control group (P<0.01; Fig.
8A). Additionally, the arthritis indices in the dexamethasone
(0.05 mg/kg)-treated CIA rats were significantly decreased,
compared with those in the control group (P<0.01; Fig. 8B). Similar to the results from
dexamethasone treatment, POGD significantly decreased paw swelling
when administered at a dose of 60 mg/kg (P<0.01), and moderately
decreased paw swelling at doses of 20 and 40 mg/kg. In addition, at
POGD doses of 20, 40 and 60 mg/kg, the arthritis indices were
significantly reduced.
POGD decreases the release of TNF-α,
IL-1β, IL-6, IL-8 and IL-17A into the serum of CIA rats
As shown in Fig.
9, there was an increase in the levels of TNF-α, IL-1β, IL-6,
IL-8 and IL-17A in the CIA rats (P<0.01), compared with those in
the normal rats. Of note, daily treatment of the CIA rats with POGD
at a dose of 20, 40 or 60 mg/kg significantly decreased the release
of these regulatory cytokines into the serum (P<0.01), compared
with the release observed in the control CIA rats. Similar results
were observed in the dexamethasone treatment group.
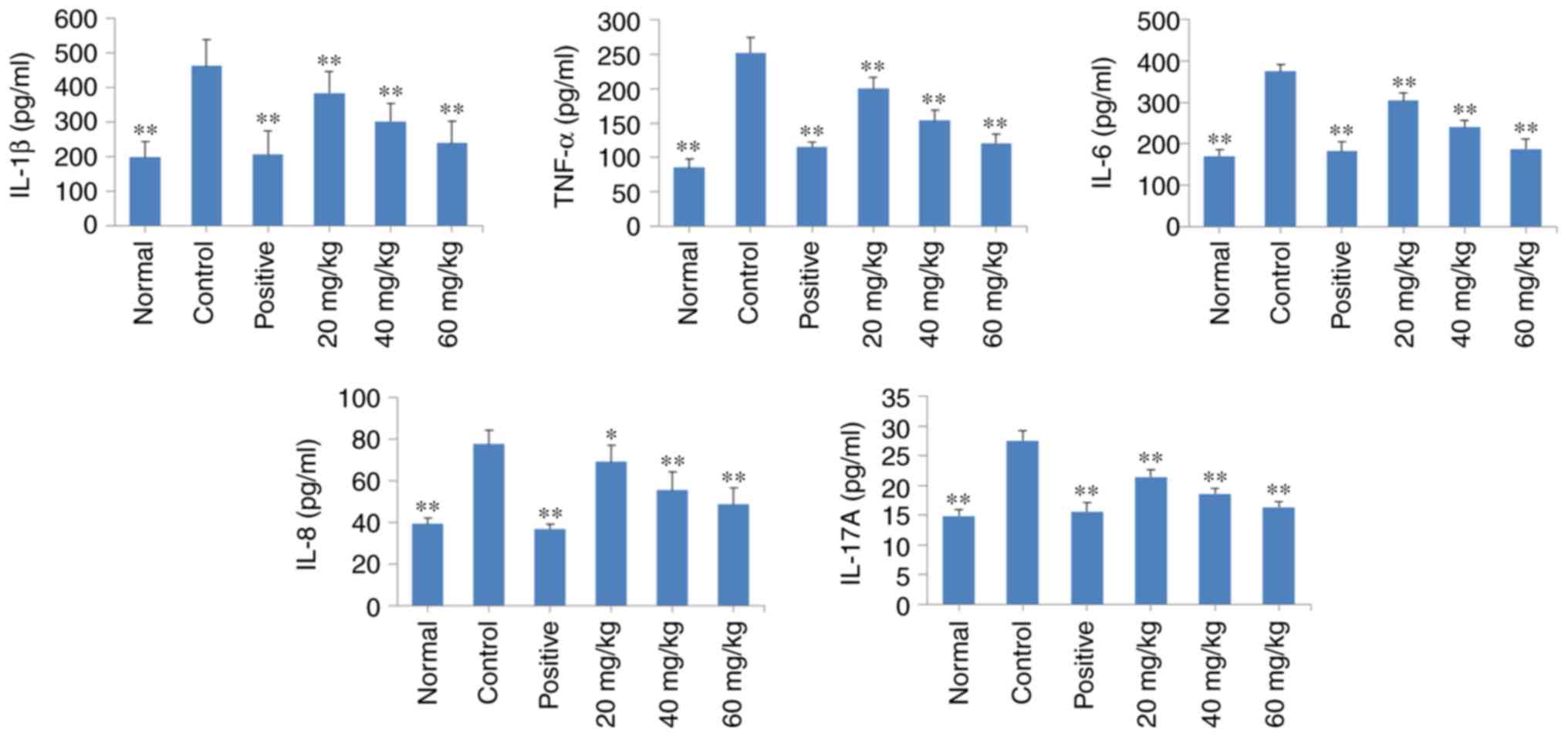 | Figure 9Effects of POGD on the release of
TNF-α, IL-1β, IL-6, IL-8 and IL-17A into the serum of CIA rats.
Following establishment of the CIA rat model, rats were orally
administered with saline, dexamethasone or different doses of POGD.
Following 30 days of treatment, blood samples were collected, and
the serum levels of TNF-α, IL-1β, IL-6, IL-8 and IL-17A were
determined using enzyme-linked immunosorbent assays.
*P<0.05 and **P<0.01, compared with the
control group. POGD, physcion 8-O-β-glucopyranoside; TNF-α, tumor
necrosis factor-α; IL, interleukin; CIA, type II collagen-induced
arthritis; CIA, type II collagen-induced arthritis. |
Discussion
In the present study, the anti-proliferative and
anti-inflammatory activities of POGD against MH7A] RA-derived
fibroblast-like synoviocytes and CIA rats were analyzed. POGD
significantly inhibited MH7A cell growth, and decreased the levels
of inflammatory cytokines IL-1β, IL-6, IL-8, IL-12 and IL-17A
following TNF-α stimulation. In addition, the mRNA expression
levels of MMP-2, MMP-3, MMP-9, VEGF and COX-2 were markedly
decreased, compared with those in the TNF-α group. POGD also
significantly decreased the protein expression levels of TGF-β1,
Smad4, P-JNK, JNK, P-P38, P38, P-ERK1/2, ERK1/2 and NF-κB p65 (N)
in the TNF-α-induced MH7A cells, but increased the protein
expression levels of Smad7, NF-κB p65 (C) and IκB. Furthermore,
POGD inhibited paw swelling, arthritis indices and the release of
pro-inflammatory cytokines in CIA rats.
The expansion of synovial cells is one of the main
pathological findings in the inflamed synovium of patients with RA
(17). The invasion of synovial
hyperplasia into cartilage and bones causes direct joint damage and
chronic inflammation in situ (18). In the hyperplastic synovium,
RA-FLS is the main cell type and tumor-like expansion is the
characteristic feature. It has been reported that RA-FLS always
causes aggressive invasion into the cartilage (7,19).
Therefore, the anti-proliferative effect on MH7A cells is
considered to be an efficient therapeutic method in the treatment
of RA. The inhibition of RA-FLS also reduced the production of
proinflammatory cytokines. In the present study, POGD exhibited
potent anti-proliferative and anti-inflammatory activities against
MH7A cells in vitro. These effects are important for
attenuating synovial hyperplasia, inflammation and bone
degeneration in joints.
It is known that the CIA model is the most commonly
used autoimmune model of RA, which induces immunological and
pathological features similar to those in the RA disease observed
in humans (20). In the present
study, a CIA rat model was successfully established, which was used
to evaluate the anti-arthritic activity of POGD. The resulting data
demonstrated that treatment with POGD decreased paw swelling and
arthritis indices in the CIA rats, indicating that POGD may have
potential therapeutic effects in vivo.
TNF-α is a proinflammatory cytokine and has been
reported to be important in contributing to the development and
progression of human RA (21). It
has been reported that TNF-α can induce lymphocytes, macrophages
and synovial fibroblasts to release proinflammatory cytokines
(22). In the present study, it
was found that the levels of inflammatory cytokines IL-1β, IL-6,
IL-8, IL-10, IL-12 and IL-17 were increased in TNF-α-stimulated
MH7A cells, which indicated that the inflammatory cell model of
human RA had been successfully established. The data obtained in
the present study demonstrated that POGD significantly inhibited
the expression levels of these cytokines (IL-1β, IL-6, IL-8, IL-12
and IL-17), suggesting that POGD exerted anti-inflammatory
activities against the TNF-α-induced MH7A cells. In addition, the
inhibition ratio of POGD (32 μg/ml) against MH7A cells was
<30% at 36 h and <20% at 24 h (Fig. 2B). Therefore, it was hypothesized
that the POGD-induced inhibition of inflammatory factor release was
not caused by the anti-proliferative activity of the MH7A
cells.
Previous studies have reported that cytokines,
including TNF-α and IL-1β, induce the expression of MMPs and COX-2
(23,24). The production of MMPs is largely
responsible for joint destruction, involving cartilage, bone and
tendons (25). COX-2, a
proinflammatory enzyme, is also important in the inflammatory
reaction, and is upregulated in inflammatory sites and tumor
tissues (26). COX-2 catalyzes
the conversion of arachidonic acid into prostaglandins, in
particular prostaglandin E2 (PGE2) (27). PGE2 is an important
pro-inflammatory mediator involved in inflammatory diseases. In the
present study, POGD significantly decreased the mRNA expression
levels of MMP-2, MMP-3, MMP-9 and COX-2 in the MH7A cells treated
with TNF-α, which indicated that POGD inhibited the targeted joint
destruction mediated by MMPs and decreased the release of
proinflammatory cytokines by reducing the expression of COX-2 in
RA.
Angiogenesis is considered to be an essential
process in the development of arthritis (28). Expression of VEGF has been
demonstrated in the synovium and synovial fluids, where it can
cause vascular permeability and angiogenesis in arthritic joints
(29). Cytokines, particularly
TNF-α, IL-1β and TGF-β1, can stimulate the release of VEGF, which
may contribute significantly to angiogenesis in the synovium
(30,31). Additionally, in RA, cartilage and
bone matrix are degraded, and extracellular matrix (ECM) proteins,
which act as cellular activators, are liberated (32). TGF-β may be important in cartilage
repair, as it can induce collagen synthesis to result in synovial
hyperplasia. In the present study, POGD inhibited the expression of
VEGF, TGF-β1 and Smad4, and increased the expression of Smad7,
which indicated that the inhibitory effect of POGD on the
expression of VEGF was mediated by the TGF-β1 pathway.
Mitogen-activated protein kinases (MAPKs) have been
reported to be involved in cell proliferation and the inflammatory
response (33,34). Regulating MAPK kinases offer
potential benefits for treating tumor and inflammatory diseases. As
ERK and JNK can inhibit the expression of c-Jun and activator
protein-1, which regulate the gene transcription of
pro-inflammatory cytokines, and inhibiting ERK and JNK is
beneficial in slowing the progression of RA (35). In the present study, POGD
treatment downregulated the expression of p-JNK, JNK, p-ERK1/2 and
ERK1/2 in the MH7A cells, which led to the inhibition of
proinflammatory cytokine secretion. In addition, the decreased
cytokine levels contributed to the anti-proliferative activity
against MH7A cells. The modulation of p38 is considered to be key
in the inhibitory properties of POGD against MH7A cells, with the
upregulation of p38 being essential for the anti-proliferative
activity.
The NF-κB signaling pathway is also pivotal in
inflammatory and immune responses (36). Transcription factors of NF-κB are
key coordinators of innate immunity and inflammation (37). p65, an NF-κB subunit, is generally
localized to the cytoplasm by its inhibitor IκB (38). As IκB dissociates from NF-κB,
NF-κB p65 can be translocated to the nucleus and can exert its
transcriptional activity, including regulating the expression of
inflammatory enzyme COX-2, and cytokines IL-1β, IL-6, IL-8, IL-12
and IL-17A (39). In the present
study, the protein expression of nuclear p65 was significantly
decreased by POGD, whereas the protein levels of IκB and cytosolic
p65 were significantly increased, suggesting that the
anti-inflammatory effect of POGD may be associated with a reduction
in NF-κB protein by increasing the expression of its inhibitor
IκB.
In conclusion, the present study demonstrated that
POGD exhibited significant anti-proliferative and anti-inflammatory
activities in vitro and in vivo. Furthermore, the
possible mechanisms may involve reducing the release of
pro-inflammatory cytokines, including IL-1β, IL-6, IL-8, IL-12 and
IL-17A, and decreasing the expression of MMP-2, MMP-3, MMP-9, VEGF
and COX-2 by inhibiting the TGF-β, NF-κB and MAPK signaling
pathways.
Funding
No funding was received.
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QG and SW contributed to the design of the study.
QG, QW, SW, HQ, QZ, XIA LIU and XS contributed to the acquisition,
collection and assembly of the data. QG and QW contributed to the
statistical analysis. QG and SW wrote the main manuscript text. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The experimental protocols were approved by the
Animal Care and Use Committee of Dezhou People's Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Okada Y, Wu D, Trynka G, Raj T, Terao C,
Ikari K, Kochi Y, Ohmura K, Suzuki A, Yoshida S, et al: Genetics of
rheumatoid arthritis contributes to biology and drug discovery.
Nature. 506:376–381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fry J, Moulds A, Strube G and Gambrill E:
Rheumatic diseases. In: The family good health guide. Springer.
157–164. 1982.
|
|
3
|
Collison J: Experimental arthritis: Do you
want to treat arthritis? IDO2. Nat Rev Rheumatol. 13:196–197.
2017.PubMed/NCBI
|
|
4
|
Firestein GS and McInnes IB:
Immunopathogenesis of rheumatoid arthritis. Immunity. 46:183–196.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Calabrese LH, Calabrese C and Kirchner E:
The 2015 American college of rheumatology guideline for the
treatment of rheumatoid arthritis should include new standards for
hepatitis B screening: Comment on the article by Singh et al.
Arthritis Care Res. 68:723–724. 2016. View Article : Google Scholar
|
|
6
|
Singh JA, Saag KG, Bridges SL Jr, Akl EA,
Bannuru RR, Sullivan MC, Vaysbrot E, McNaughton C, Osani M,
Shmerling RH, et al: 2015 American college of rheumatology
guideline for the treatment of rheumatoid arthritis. Arthritis
Rheumatol. 68:1–26. 2016. View Article : Google Scholar
|
|
7
|
Mor A, Abramson SB and Pillinger MH: The
fibroblast-like synovial cell in rheumatoid arthritis: A key player
in inflammation and joint destruction. Clin Immunol. 115:118–128.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peng W, Qin R, Li X and Zhou H: Botany,
phytochemistry, pharmacology, and potential application of
Polygonum cuspidatum Sieb.et Zucc.: A review. J Ethnopharmacol.
148:729–745. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xie QC and Yang YP: Anti-proliferative of
physcion 8-O-β-glucopyranoside isolated from Rumex japonicus Houtt.
on A549 cell lines via inducing apoptosis and cell cycle arrest.
BMC Complement Altern Med. 14:3772014. View Article : Google Scholar
|
|
10
|
Zhao KS, Jin C, Huang X, Liu J, Yan WS,
Huang Q and Kan W: The mechanism of Polydatin in shock treatment.
Clin Hemorheol Microcirc. 29:211–217. 2003.
|
|
11
|
Bralley EE, Greenspan P, Hargrove JL,
Wicker L and Hartle DK: Topical anti-inflammatory activity of
Polygonum cuspidatum extract in the TPA model of mouse ear
inflammation. J Inflamm. 5:12008. View Article : Google Scholar
|
|
12
|
Hsu CY, Chan YP and Chang J: Antioxidant
activity of extract from Polygonum cuspidatum. Biol Res. 40:13–21.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kimura Y and Okuda H: Resveratrol isolated
from Polygonum cuspidatum root prevents tumor growth and metastasis
to lung and tumor-induced neovascularization in Lewis lung
carcinoma-bearing mice. J Nutri. 131:1844–1849. 2001. View Article : Google Scholar
|
|
14
|
Zhang H, Yu CH, Jiang YP, Peng C, He K,
Tang JY and Xin HL: Protective effects of polydatin from Polygonum
cuspidatum against carbon tetrachloride-induced liver injury in
mice. PLoS One. 7:e465742012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔC T method. Methods. 25:402–408.
2001. View Article : Google Scholar
|
|
16
|
Pu J, Fang FF, Li XQ, Shu ZH, Jiang YP,
Han T, Peng W and Zheng CJ: Matrine exerts a strong anti-arthritic
effect on type II collagen-induced arthritis in rats by inhibiting
inflammatory responses. Int J Mol Sci. 17:E14102016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tran CN, Lundy SK and Fox DA: Synovial
biology and T cells in rheumatoid arthritis. Pathophysiology.
12:183–189. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ospelt C and Gay S: The role of resident
synovial cells in destructive arthritis. Best Prac Res Clin
Rheumatol. 22:239–252. 2008. View Article : Google Scholar
|
|
19
|
Zuo J, Xia Y, Li X, Ou-Yang Z and Chen JW:
Selective modulation of MAPKs contribute to the anti-proliferative
and anti-inflammatory activities of
17-dihydroxy-3,4-dimethoxyxanthone in rheumatoid arthritis-derived
fibroblast-like synoviocyte MH7A cells. J Ethnopharmacol.
168:248–254. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Myers LK, Rosloniec EF, Cremer MA and Kang
AH: Collagen-induced arthritis, an animal model of autoimmunity.
Life Sci. 61:1861–1878. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Giles JT, Bartlett SJ, Gelber AC, Nanda S,
Fontaine K, Ruffing V and Bathon JM: Tumor necrosis factor
inhibitor therapy and risk of serious postoperative orthopedic
infection in rheumatoid arthritis. Arthritis Rheum. 55:333–337.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Umar S, Hedaya O, Singh AK and Ahmed S:
Thymoquinone inhibits TNF-α-induced inflammation and cell adhesion
in rheumatoid arthritis synovial fibroblasts by ASK1 regulation.
Toxicol Appl Pharmacol. 287:299–305. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lianxu C, Hongti J and Changlong Y:
NF-kappaBp65-specific siRNA inhibits expression of genes of COX-2,
NOS-2 and MMP-9 in rat IL-1beta-induced and TNF-alpha-induced
chon-drocytes. Osteoarthritis Cartilage. 14:367–376. 2006.
View Article : Google Scholar
|
|
24
|
Tian J, Chen JW, Gao JS, Li L and Xie X:
Resveratrol inhibits TNF-α-induced IL-1β, MMP-3 production in human
rheumatoid arthritis fibroblast-like synoviocytes via modulation of
PI3k inase/Akt pathway. Rheumatol Int. 33:1829–1835. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Burrage PS, Mix KS and Brinckerhoff CE:
Matrix metalloproteinases: Role in arthritis. Front Biosci.
11:529–543. 2006. View
Article : Google Scholar
|
|
26
|
Aggarwal BB, Shishodia S, Sandur SK,
Pandey MK and Sethi G: Inflammation and cancer: How hot is the
link. Biochem Pharmacol. 72:1605–1621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hawkey CJ: COX-2 inhibitors. Lancet.
353:307–314. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Koch A: Angiogenesis as a target in
rheumatoid arthritis. Ann Rheum Dis. 62(Suppl 2): ii60–ii67. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nagashima M, Yoshino S, Ishiwata T and
Asano G: Role of vascular endothelial growth factor in angiogenesis
of rheumatoid arthritis. J Rheumatol. 22:1624–1630. 1995.PubMed/NCBI
|
|
30
|
Paleolog EM, Young S, Stark AC, McCloskey
RV, Feldmann M and Maini RN: Modulation of angiogenic vascular
endothelial growth factor by tumor necrosis factor α and
interleukin-1 in rheumatoid arthritis. Arthritis Rheum.
41:1258–1265. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheon H, YU SJ, Yoo DH, Chae IJ, Song GG
and Sohn J: Increased expression of pro-inflammatory cytokines and
metalloproteinase-1 by TGF-beta1 in synovial fibroblasts from
rheumatoid arthritis and normal individuals. Clin Exp Immunol.
127:547–552. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Goldring S: Pathogenesis of bone and
cartilage destruction in rheumatoid arthritis. Rheumatology.
42(Suppl 2): ii11–ii16. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Keyse SM: Dual-specificity MAP kinase
phosphatases (MKPs) and cancer. Cancer Metastasis Rev. 27:253–261.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kaminska B: MAPK signalling pathways as
molecular targets for anti-inflammatory therapy-from molecular
mechanisms to therapeutic benefits. Biochim Biophys Acta.
1754:253–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Baker RG, Hayden MS and Ghosh S: NF-κB,
inflammation, and metabolic disease. Cell Metab. 13:11–22. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vallabhapurapu S and Karin M: Regulation
and function of NF-kappaB transcription factors in the immune
system. Ann Rev Immunol. 27:693–733. 2009. View Article : Google Scholar
|
|
38
|
Beg AA and Baldwin AS Jr: The I kappa B
proteins: Multifunctional regulators of Rel/NF-kappa B
transcription factors. Genes Dev. 7:2064–2070. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lyss G, Knorre A, Schmidt TJ, Pahl HL and
Merfort I: The anti-inflammatory sesquiterpene lactone helenalin
inhibits the transcription factor NF-kappaB by directly targeting
p65. J Biol Chem. 273:33508–33516. 1998. View Article : Google Scholar : PubMed/NCBI
|