Introduction
The liver is known as the primary organ important
for drug and chemical substance metabolism (1). Liver injury may be induced by drug
abuse, viral infection and heavy alcohol consumption, and is
considered to be a common clinical disease (2,3).
CCl4 is a well-known hepatotoxin, which may induce liver
injury through various mechanisms, including oxidative stress,
inflammatory response and apoptosis (4,5).
The CCl4-induced animal model of acute liver injury is
well established, leading to fibrosis, inflammation and apoptotic
response in mice (6). Previous
studies have assessed the possible molecular mechanism of liver
toxicity induced by CCl4 (7,8).
Certain hepatoprotective agents have been investigated and applied
in clinical practice, but a large proportion of them have potential
adverse effects (9,10). Therefore, application of natural
products isolated from plants as an effective and safe therapeutic
strategy for liver disease has been in the focus of recent research
(11,12).
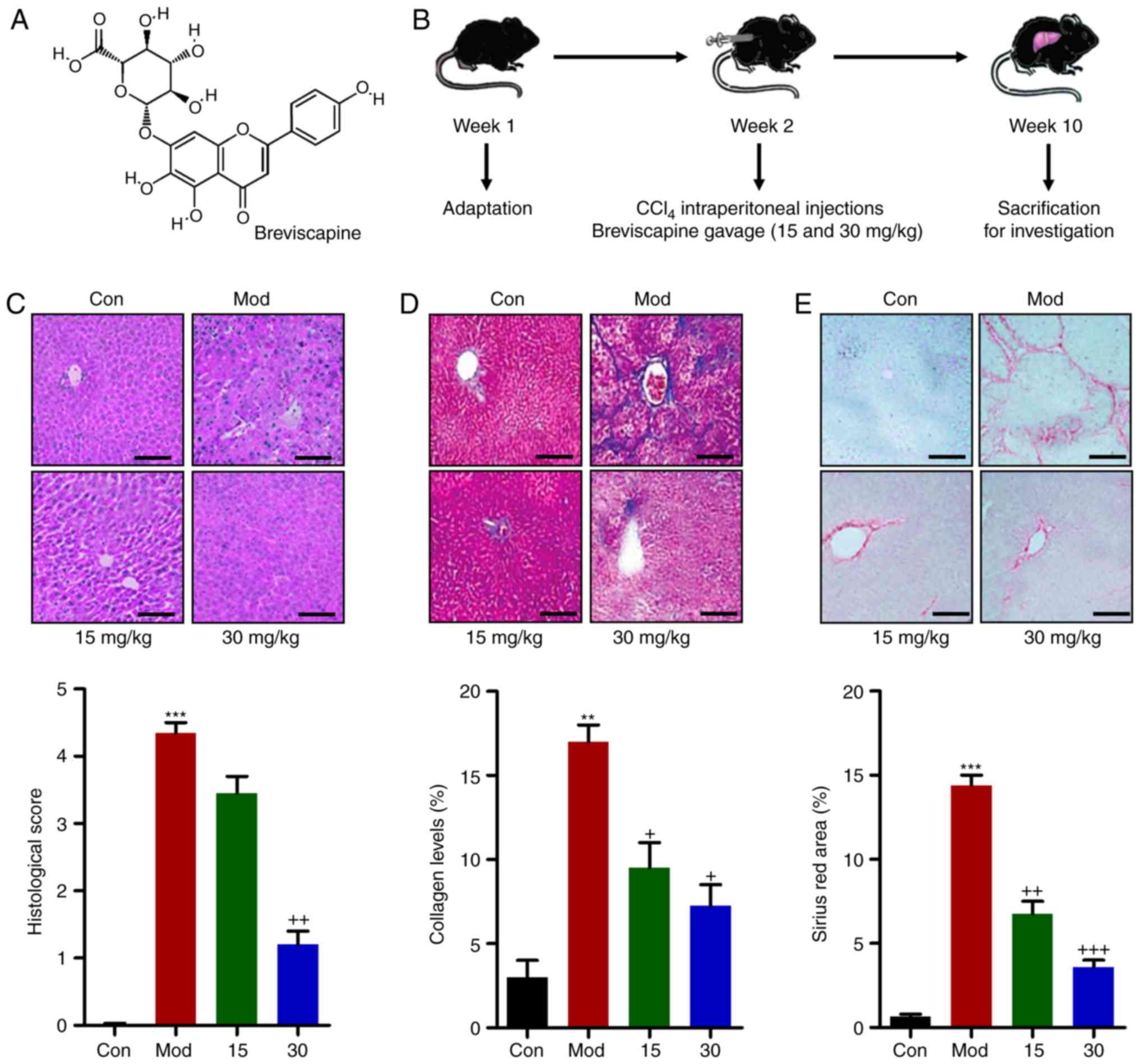
Breviscapine (Fig.
1A) is a mixture of flavonoid glycosides isolated from Chinese
herbs, including Erigerin breviscapus (Vant.) (13). Breviscapine has been reported to
have various biological activities, including anti-oxidant,
anticancer, anti-degenerative and anti-angiogenesis effects
(14-16). It has been indicated that
breviscapine administration is safe and has low toxicity to various
normal cell types (17,18). In addition, the anti-inflammatory
effect of breviscapine has been reported (16). Accordingly, breviscapine is
extensively used for the treatment of cerebrovascular diseases
caused by cerebral infarction, hypertension and chronic
arachnoiditis along with their sequelae, and is suggested to
inhibit tumor proliferation and angiogenesis, thereby limiting
tumor development (19). However,
the effects of breviscapine on liver injury and the underlying
molecular mechanisms still remain to be fully elucidated.
The present study attempted to investigate whether
breviscapine may be effectively used as a therapeutic drug, and to
test this, it was administered to mice with CCl4-induced
liver injury and L02 cells challenged with lipopolysaccharide
(LPS). It was indicated that inflammatory cytokines were
down-regulated by breviscapine through inactivating the Toll-like
receptor (TLR)4/nuclear factor (NF)-κB signaling pathways.
Breviscapine co-administered with CCl4 reduced apoptosis
by inactivating the caspase-3 signaling pathway. In addition,
CCl4-induced oxidative stress was blocked by
breviscapine through improving anti-oxidants and impeding
mitogen-activated protein kinase (MAPK) signaling. The present
study indicated that breviscapine exerted protective effects
against acute liver injury by suppressing inflammation, apoptosis
and oxidative stress, which may be used as a therapeutic strategy
for patients with liver injury.
Materials and methods
Reagents
CCl4 was obtained from Tianjin Baishi
Chemical (Tianjin, China). A Cell Counting Kit-8 (CCK-8) was
purchased from Beyotime Institute of Biotechnology (Shanghai,
China). Breviscapine (purity, ≥98%) and Picrosirius red were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Antibodies to B-cell lymphoma-2 (Bcl-2)-associated X protein (Bax;
cat. no. 2772; 1:1,000 dilution), Bcl-2 (cat. no. 3498; 1:1,000
dilution), myeloid differentiation primary response gene 88 (MyD88;
cat. no. 4283; 1:1,000 dilution), phospho (p)-NF-κB (cat. no. 3033;
1:1,000 dilution), NF-κB (cat. no. 8242; 1:1,000 dilution),
phospho-inhibitor of NF-κB (p-IκBα; cat. no. 2859; 1:1,000
dilution), phospho-IκB kinase α (p-IKKα; cat. no. 2078; 1:1,000
dilution), extracellular signal-regulated kinase (ERK)1/2 (cat. no.
4377; 1:1,000 dilution) p-ERK1/2 (cat. no. 9101; 1:1,000 dilution),
p38 (cat. no. 8690; 1:1,000 dilution), p-p38 (cat. no. 9215;
1:1,000 dilution), superoxide dismutase (SOD1; cat. no. 13141;
1:1,000 dilution) and GAPDH (cat. no. 5174; 1:1,000 dilution), all
raised in rabbit, were obtained from Cell Signaling Technology Inc.
(Danvers, MA, USA). Antibodies against TLR4 (cat. no. ab13556;
1:1,000 dilution), c-Jun N-terminal kinase (JNK; cat. no. ab4821;
1:1,000 dilution), p-JNK (cat. no. ab47337; 1:1,000 dilution),
nuclear factor erythroid 2-related factor 2 (Nrf2; cat. no.
ab62352; 1:1,000 dilution), heme oxygenase (HO)-1 (cat. no.
ab13248; 1:1,000 dilution), NAD(P) H quinone dehydrogenase-1
(NQO-1; cat. no. ab34173, 1:1000 dilution) activated caspase-3
(cat. no. ab52293; 1:1,000 dilution), pro-caspase-3 (cat. no.
ab90437; 1:1,000 dilution), cleaved poly(ADP ribose) polymerase
(PARP; cat. no. ab13907; 1:1,000 dilution), PARP (cat. no.
ab218132; 1:1,000 dilution) and apoptotic protease activating
factor-1 (Apaf-1; cat. no. ab2000; 1:1,000 dilution), all raised in
rabbit, were purchased from Abcam (Cambridge, MA, USA). A
HRP-labelled secondary antibody (cat. no. KIT-5902; 1:200 dilution;
Max Vision HRP-polymer anti-rabbit IHC kit) was purchased from
Maxim BioTechnology Co., Ltd. (Fuzhou, China).
Animals and treatments
A total of 40 healthy male C57BL/6 mice (age, 6-8
weeks, weight, 20-22 g), were purchased from Shanghai Experimental
Animal Center (Shanghai, China) and kept under standard conditions
of 25±2°C and 50±10% humidity with a 12-h light/dark cycle with
food and water provided ad libitum in cages. The
experimental protocols, which were in accordance with the Guide for
the Care and Use of Experimental Animals of the National Institutes
of Health (NIH) from 1996 (20)
and were approved by the Institutional Animal Care and Use
Committee of the Beijing Chao-Yang Hospital (Beijing, China). Prior
to experimental treatment, all mice were kept under the standard
conditions for 1 week for adaptation. Next, the mice were divided
into four groups (n=10 each): i) Control group (Con); ii)
CCl4-treated model group (Mod); iii) CCl4 and
breviscapine co-treatment (15 mg/kg) and iv) CCl4 and
breviscapine co-treatment (30 mg/kg). A total of 30 mice were
treated twice a week with 8 consecutive intraperitoneal (i.p.)
injections of 1 ml/kg CCl4 (diluted at 1:10 in olive
oil) to induce liver fibrosis. As for the breviscapine-treated
groups, 15 or 30 mg/kg breviscapine was administered to the mice
each day by oral gavage (Fig. 1B)
for 8 weeks. At the end of the experiment, blood samples and liver
tissues were collected from the mice for further assays.
Cell culture and treatment
The L02 human normal liver cell line was purchased
from KeyGEN BioTECH (Nanjing, China). The BRL-3A rat normal liver
line and the AML-12 mouse normal liver cell line were all purchased
from the cell bank of the Chinese Academy of Sciences (Shanghai,
China). All cells were cultured and grown in Dulbecco's modified
Eagle's medium supplemented with 10% heat-inactivated fetal bovine
serum (both from Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA), 100 U/ml penicillin and 100 mg/ml streptomycin at 37°C in
a humidified atmosphere containing 5% CO2. The cells
were then exposed to LPS (100 ng/ml) for 24 h in the absence or
presence of breviscapine at different concentrations (20 and 40
µM) (21-24).
Cell viability assessment
L02 cells were seeded in a 96-well plate at
1×103 cells/well overnight, prior to the addition of
breviscapine. The cells were treated with various concentrations of
breviscapine (0, 5, 10, 20 and 40 µM) for different
durations ranging from 0-72 h (0, 6, 12, 24, 36, 48 and 72 h) in
the absence of LPS. Subsequently, 10 µl CCK-8 solution
(Dojindo Laboratories, Kumamoto, Japan) was added to each well and
the plate was incubated at 37°C for 1 h. Finally, the absorbance at
450 nm was measured to determine the cell viability. The protocol
was performed according to the manufacturer's protocols.
Analysis of biochemical indicators
To evaluate liver injury, serum alanine
aminotransferase (ALT) and aspartate aminotransferase (AST) levels
were determined using ALT and AST reagent kits (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China) according to
manufacturer's protocols. Albumin levels in the serum were measured
by using an albumin reagent kit (NanJing JianCheng Bioengineering
Institute). As for the hepatic hydroxyproline content, 200 mg
Snap-frozen liver specimens were weighed and hydrolyzed in NaOH (2
M). The hydroxyproline content was quantified following the
manufacturer's instructions of the hydroxyproline measurement kit
(Nanjing Jiancheng Bioengineering Institute). SOD, glutathione
(GSH), malondialdehyde (MDA), H2O2 and
myeloperoxidase (MPO) levels were measured using the standard
diagnostic kits purchased from NanJing JianCheng Bioengineering
Institute.
ELISA
The levels of tumor necrosis factor (TNF)-α,
interleukin (IL)-1β, IL-6 and monocyte chemoattractant protein
(MCP)-1 in serum and liver tissue samples were determined by using
the ELISA kits purchased from R&D systems (Minneapolis, MN,
USA) following the manufacturer's protocols.
Terminal deoxynucleotidyl transferase
deoxyuridinetriphosphate nick end labelling (TUNEL) analysis
TUNEL analysis was used to indicate apoptosis in the
liver tissue samples. The assay detects the 3′ hydroxyl ends of DNA
fragments. The staining was performed with the In Situ Cell
Death Detection kit (TUNEL assay; KeyGen Biotech; Nanjing, China)
following the manufacturer's protocols. Recombinant DNase I (Takara
Biotechnology Co., Ltd., Dalian, China) was included in the
positive control. The immunostained liver cells of were quantified
using a light microscope and results are presented as a percentage
of the control.
Assessment of reactive oxygen species
(ROS) generation
A 5-µM intracellular probe of non-fluorescent
2′,7′-dichlorofluorescein-diacetate (DCFH-DA; KeyGen Biotech) was
used to detect the cellular ROS formation. The L02 cells
(5×105 cells/well) were treated with LPS (100 ng/ml)
with or without breviscapine for 24 h. DCFH-DA was then added and
the cells were cultured continuously in the dark at 37°C for 20
min. Subsequently, the fluorescent cells were visualized using a
fluorescence microscope.
Immunofluorescent staining
For in vivo analysis, the frozen liver
sections were blocked using a solution containing 4% normal goat
serum (OriGene Technologies, Inc., Rockville, MD, USA), 1% Triton
X-100 and 1% bovine serum albumin (Xi'an Guanyu Bio-Tech Co., Ltd.,
Xi'an, China) for 1 h at the room temperature. Next, the samples
were incubated with anti-TLR4 primary antibody (1:100 dilution) at
4°C overnight. Subsequently, they were washed three times with PBS
(0.1 M) containing 0.5% Triton X-100 for 5 min each time, and the
sections were then incubated with Alexa Fluor 594-labeled
anti-rabbit secondary antibody (cat. no. A-11072, 1:500 dilution;
Invitrogen; Thermo Fisher Scientific, Inc.) at room temperature for
1 h prior to analysis with a fluorescent microscope equipped with a
digital camera. Finally, the fluorescent cells were quantified.
In vitro, the L02 cells were washed three
times with chilled PBS and fixed with 3.7% (v/v) formaldehyde in
PBS for 15 min. The specimens were then permeabilized for 5 min
using 0.1% Triton X-100. For Apaf-1 fluorescence staining, 50
µg/ml mouse anti-Apaf-1 antibody was applied and incubated
at 4°C overnight, followed by staining with anti-mouse secondary
antibody labelled with Alexa Fluor 488 (cat. no. A-21441; 1:500
dilution; Invitrogen; Thermo Fisher Scientific, Inc.) for 30 min.
Subsequent to washing in PBS for 3 times, the immunofluorescence
was visualized and quantified with a Zeiss LSM 710 confocal laser
system (Carl Zeiss AG, Oberkochen, Germany).
Immunohistological analysis
The liver tissue samples obtained from mice were
fixed in 4% paraformaldehyde, embedded in paraffin, sectioned at 4
mm and then stained with haematoxylin and eosin (H&E) for 10
min or Masson's trichrome blue for 5 min at room temperature to
analyze the liver injury and collagen deposition, respectively. The
score of injured level was described in a previous study (25). The sections stained with Masson's
trichrome were scanned and analyzed using a digital image analyzer
(Image-Pro Analyzer 7 software; Media Cybernetics, Inc., Rockville,
MD, USA). Sirius red staining was also used to calculate the
fibrotic area. In brief, the liver sections were incubated with
picrosirius red for 2 h at room temperature and then washed with
acetic acid and water. The percentage of the fibrotic area was
calculated in 5 randomly selected fields per slide. Furthermore,
liver tissue samples were also subjected to immunohistochemical
staining for the assessment of Bax and Apaf-1 expression. The
sections were stained with rabbit anti-Bax (1:200 dilution) or
rabbit anti-Apaf-1 (1:200 dilution) at 4°C overnight. Slides were
incubated in a humidified chamber for 1 h and incubated for 10 min
with a HRP-labelled secondary antibody (Max Vision HRP-polymer
anti-rabbit IHC kit, 1:200 dilution) at room temperature.
Immunoreactive proteins were visualized using a DAB substrate (cat.
no. SK-4100; Vector Laboratories, Inc., Burlingame, CA, USA),
followed by counterstaining with hematoxylin at room temperature
for 50 min. Immunohistochemical quantification was carried out
using image analysis software (ImageJ; 1.46a; National Institutes
of Health, Bethesda, MD, US). The histological protocol was in line
with standard procedures (26,27).
Western blot analysis
The liver tissues and L02 cells were homogenized
with 10% (wt/vol) hypotonic buffer (1 mM EDTA, 1 mM Pefabloc SC
(Roche Applied Science, Penzberg, Germany), 5 µg/ml
leupeptin, 25 mM Tris-HCl, 5 µg/ml soybean trypsin inhibitor
(Sigma-Aldrich; Merck KGaA), 4 mM benzamidine and 50 µg/ml
aprotinin; pH 8.0). The final supernatants were obtained by
centrifugation at 12,000 × g rpm for 20 min. The protein
concentration was determined using a bicinchoninic acid protein
assay kit (Thermo Fisher Scientific, Inc.) with bovine serum
albumin as a standard. Equal amounts (20-40 µg) of total
protein were subjected to 10 or 12% SDS-PAGE and
electrophoretically transferred to the polyvinylidene difluoride
membranes (Merck KGaA). The membranes were then blocked with 5%
skim milk Tris buffered saline with 0.1% Tween-20 (TBST), washed,
and then incubated with primary antibodies (Bax, Bcl-2, MyD88,
p-NF-κB, NF-κB, p-IκBα, p-IKKα, ERK1/2, p-ERK1/2, p38, p-p38, SOD1,
GAPDH, TLR4, JNK, p-JNK, Nrf2, HO-1, NQO-1, activated caspase-3,
pro-caspase-3, PARP, Apaf-1) overnight at 4°C. Then, the membrane
was washed with TBST three times, followed by incubation with a
horseradish peroxidase (HRP)-conjugated secondary antibody (cat.
no. ab191866; 1:2,500 dilution; Abcam) at room temperature for 2 h.
Western blot bands were observed using a GE Healthcare ECL Western
Blotting Analysis System (GE Healthcare, Little Chalfont, UK) and
exposed to X-ray film (Eastman Kodak, Rochester NY, USA). For
enhanced chemiluminescence, detection reagents A and B was mixed at
a 1:1 ratio, which was immediately added to the blotting membrane.
In total, 0.125 ml working solution was used per cm2 for
each membrane. The blot was incubated for 1 min at room temperature
and excess reagents were drained. The blot was then exposed to
X-ray film. The protein expression levels were defined as the grey
value determined with ImageJ software version 1.4.2b (NIH,
Bethesda, MD, USA) and standardized by presenting them as a fold of
the housekeeping gene GAPDH as the control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues and cells with
the TRI Reagent (Sigma-Aldrich; Merck KGaA) following the
manufacturer's instructions and treated with deoxyribonuclease I
(Roche Applied Science). The mRNA was then reverse transcribed to
complementary DNA using the miScript II RT kit (Qiagen, Hilden,
Germany), which was then amplified and quantified by real-time qPCR
using All-in-One qPCR Mix (GeneCopoeia, Inc., Rockville, MD, USA)
in a 20 ml reaction volume containing 10 ml of 2X All-in-One qPCR
Mix (GeneCopoeia, Inc.), 1 µl of 2 µM forward primer,
1 µl of 2 µM reverse primer, 1 µl of cDNA, and
6 µl of nuclease-free water. The thermocycling conditions
were for 35 cycles of 95°C for 20 sec, 54°C for 30 sec and 72°C for
30 sec according to a previous study (28). Fold changes in mRNA levels of
target genes relative to the endogenous control GAPDH were
calculated. In brief, the Cq method was used for quantification
cycle values of each target gene were subtracted from the Ct values
of the housekeeping gene GAPDH (ΔCt). Target gene ΔΔCt was
calculated as ΔCt of the target gene minus the ΔCt of the control.
The fold change in mRNA expression was calculated as
2−ΔΔCq (29). The
primer sequences (5′-3′) were as follows: TNF-α forward,
CAAGAGAGTAGGGAAGTGG and reverse, AGCAGAACGCCGACTAGCTAAC; IL-1β
forward, AGAAAGATAGCAGGTACGC and reverse, CGTTCTAGAACTATTGGAGC;
IL-6 forward, GAGAGCCGACGGTGAGAC and reverse, CGAGAATGAAGAGGCACACT;
IL-18 forward, TGACTAGAGAGCGCAGTCAAC and reverse,
TGCTCAAGGCAAGTGTAATTCC; Bcl-2 forward, GATGGATCATGACACAGGACA and
reverse, TCAACAGCCTAGAGATTATGTG; Bax forward,
AACAGAGACATAAGGCGGCTAC and reverse, CATCACCTAGGAGGTGGCTTAT; Apaf-1
forward, TCGGTAAGAGCTCAAGAGCC and reverse, TCTGCAACTTCAAGGCGTGCAA;
GAPDH forward, AGGAACGCAGTTCGCATCGCAA and reverse,
TCACACCTACAGCCAACACGA.
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. Statistical analyses were performed using GraphPad Prism
version 6.0 (Graph Pad Software, Inc., La Jolla, CA, USA). Analysis
of variance followed by Dunnett's least-significant differences
post-hoc test was performed for comparison between groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Breviscapine improves
CCl4-induced histological changes and collagen
deposition in mice
CCl4 is well-known to cause hepatic
injury, apoptosis and necrosis (4,5).
In order to investigate the effects of breviscapine on hepatic
damage, male mice were subjected to a 8-week treatment with
CCl4 with optional co-treatment with 15 or 30 mg/kg
breviscapine. H&E staining indicated that CCl4
treatment produced liver injury, resulting in higher histological
score compared with those in the control group. Of note,
breviscapine administration ameliorated liver injury induced by
CCl4, resulting in lower histological score compared
with those in the Mod group (Fig.
1C). In addition, CCl4 treatment generated higher
collagen accumulation, as indicated by Masson trichrome staining,
which was reduced by breviscapine administration (Fig. 1D). Finally, Sirius Red staining
indicated that marked fibrosis occurred in the CCl4
treated-group. However, breviscapine reduced the fibrotic area in
the liver tissue of CCl4-induced mice (Fig. 1E). These results indicated that
breviscapine administration significantly attenuated
CCl4-induced liver damage.
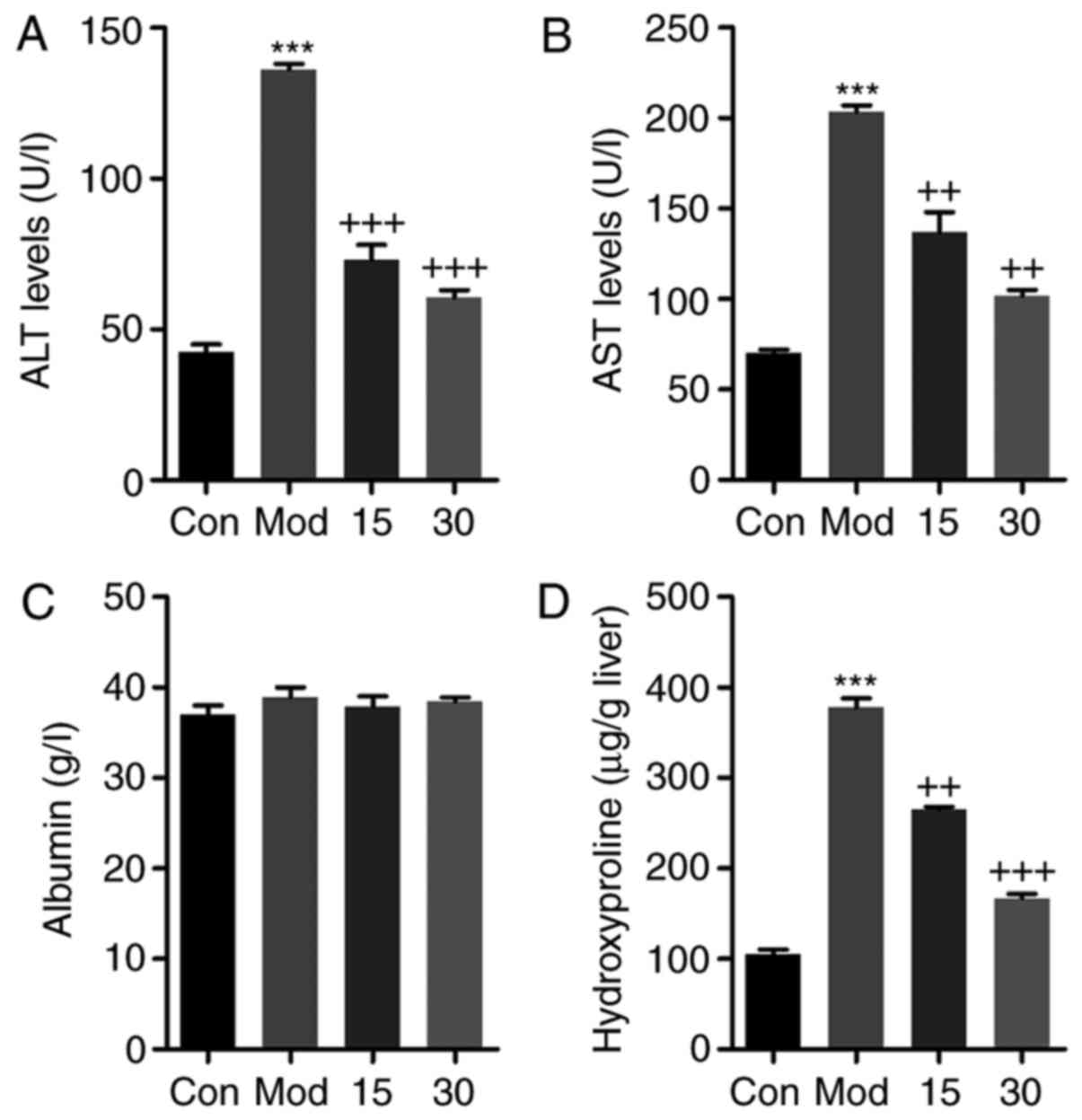
Breviscapine attenuates liver injury in
mice induced by CCl4
To further clarify the therapeutic effect of
breviscapine on liver injury induced by CCl4, serum ALT,
AST, albumin and liver hydroxyproline were calculated. As presented
in Fig. 2A and B, respectively,
ALT and AST levels in the serum were markedly elevated in the
CCl4-group compared with those in the control group,
while breviscapine significantly decreased the ALT and AST levels
in a dose-dependent manner. As for albumin, no significant
difference was observed among the four groups (Fig. 2C). Furthermore, hydroxyproline
levels in the in liver were markedly increased after
CCl4 treatment. However, breviscapine administration
reduced the hydroxyproline levels in comparison to those in the
CCl4-treated group (Fig.
2D). These results indicated that breviscapine had preventive
effects against liver injury caused by CCl4
treatment.
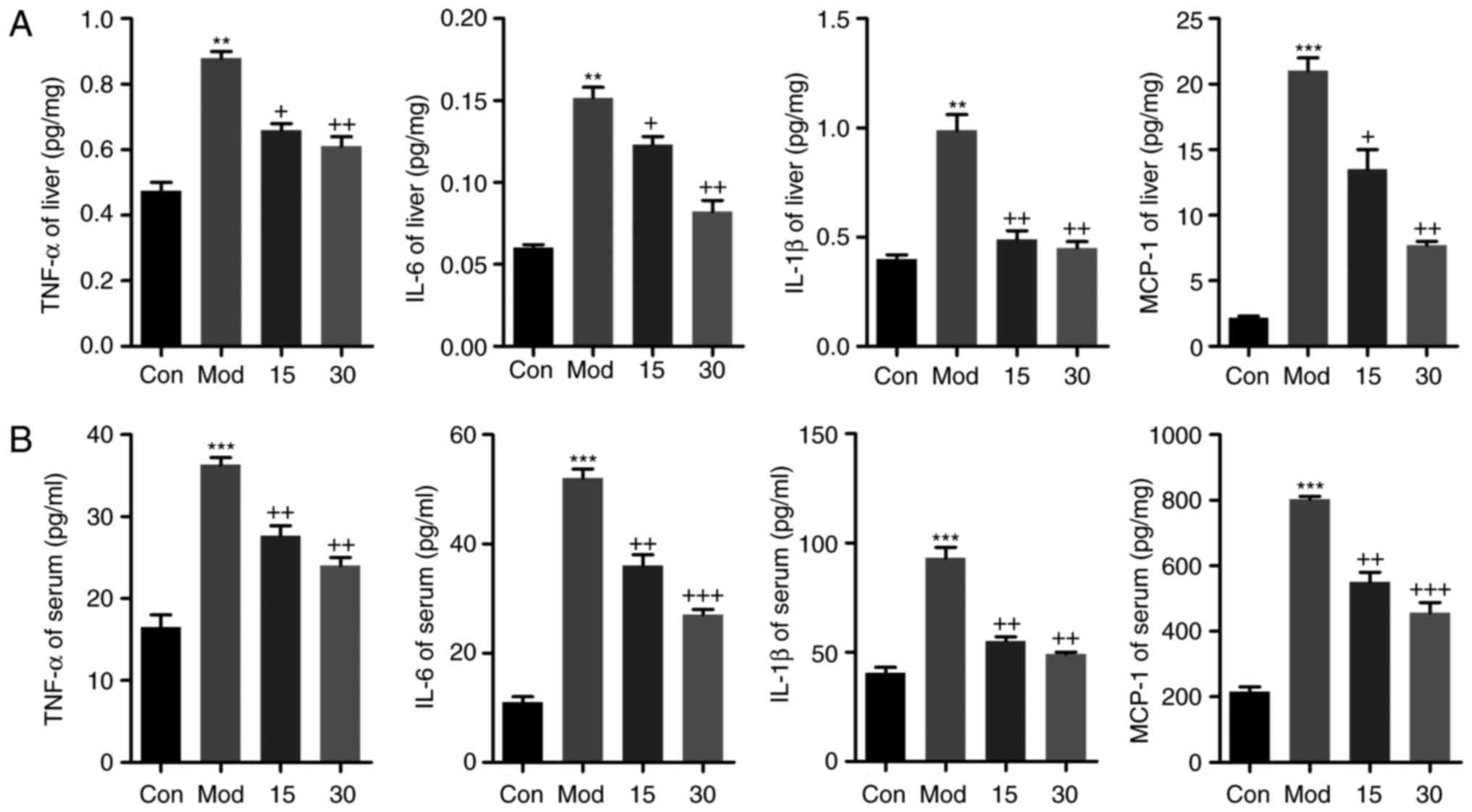
Breviscapine ameliorates
CCl4-induced liver injury by reducing pro-inflammatory
cytokine secretion
The levels of TNF-α, IL-6, IL-1β and MCP-1 in the
liver tissues were also elevated after CCl4 treatment,
which was inhibited by breviscapine administration, as determined
by ELISA (Fig. 3A). In addition,
the circulating pro-inflammatory cytokines TNF-α, IL-6 and IL-1β,
as well as the chemokine MCP-1, were assessed by ELISA (Fig. 3B). Compared with those in the
control group, the serum levels of TNF-α, IL-6, IL-1β and MCP-1
were markedly elevated in the CCl4-treated group.
However, mice treated with breviscapine exhibited a significant
downregulation of TNF-α, IL-6, IL-1β and MCP-1 levels in the serum.
Taken together, the results indicated that breviscapine suppressed
the secretion of pro-inflammatory cytokines and chemokines in serum
and their expression in liver tissues.
The attenuation of
CCl4-induced liver injury by breviscapine proceeds via
suppression of TLR4/NF-κB signaling
According to previous studies, TLR4 is involved in
liver injury induced by CCl4, initiating the
inflammatory response by stimulating NF-κB activity (30). To determine whether breviscapine
exerted its protective effects against CCl4-induced
liver injury by suppressing inflammation via TLR4/NF-κB signaling,
immunofluorescence and western blot analyses were used to determine
the expression of TLR4 and NF-κB pathway components.
Immunofluorescence staining indicated that TLR4 was significantly
upregulated in the liver tissue samples of mice induced with
CCl4, when compared with those in the control group
(Fig. 4A). The number
TLR4-positive cells was markedly decreased in breviscapine-treated
mice, when compared with that in the CCl4 group. To
further explore the mechanistic involvement of the TLR4 signaling
pathway, TLR4 and its downstream signaling molecule MyD88 were
assessed by western blot analysis. As presented in Fig. 4B, a significant increase in TLR4
and MyD88 expression levels was observed in the CCl4 vs.
the control group. However, breviscapine-treated mice exhibited
reduced TLR4 and MyD88 protein levels compared with those in the
CCl4 group. Next, the NF-κB signaling pathway was
examined. As indicated in Fig.
4C, mice subjected to CCl4 treatment had higher
levels of phosphorylated IKKα and IκBα levels in comparison with
those in the control group, accompanied with significantly higher
level of phosphorylated NF-κB. Of note, IKKα, IκBα and NF-κB
activation were downregulated in breviscapine-treated mice
subjected to CCl4 treatment, compared with those in mice
only treated with CCl4. In conclusion, the results
demonstrated that breviscapine administration de-activated the
TLR4/NF-κB signaling pathway in CCl4-treated mice.
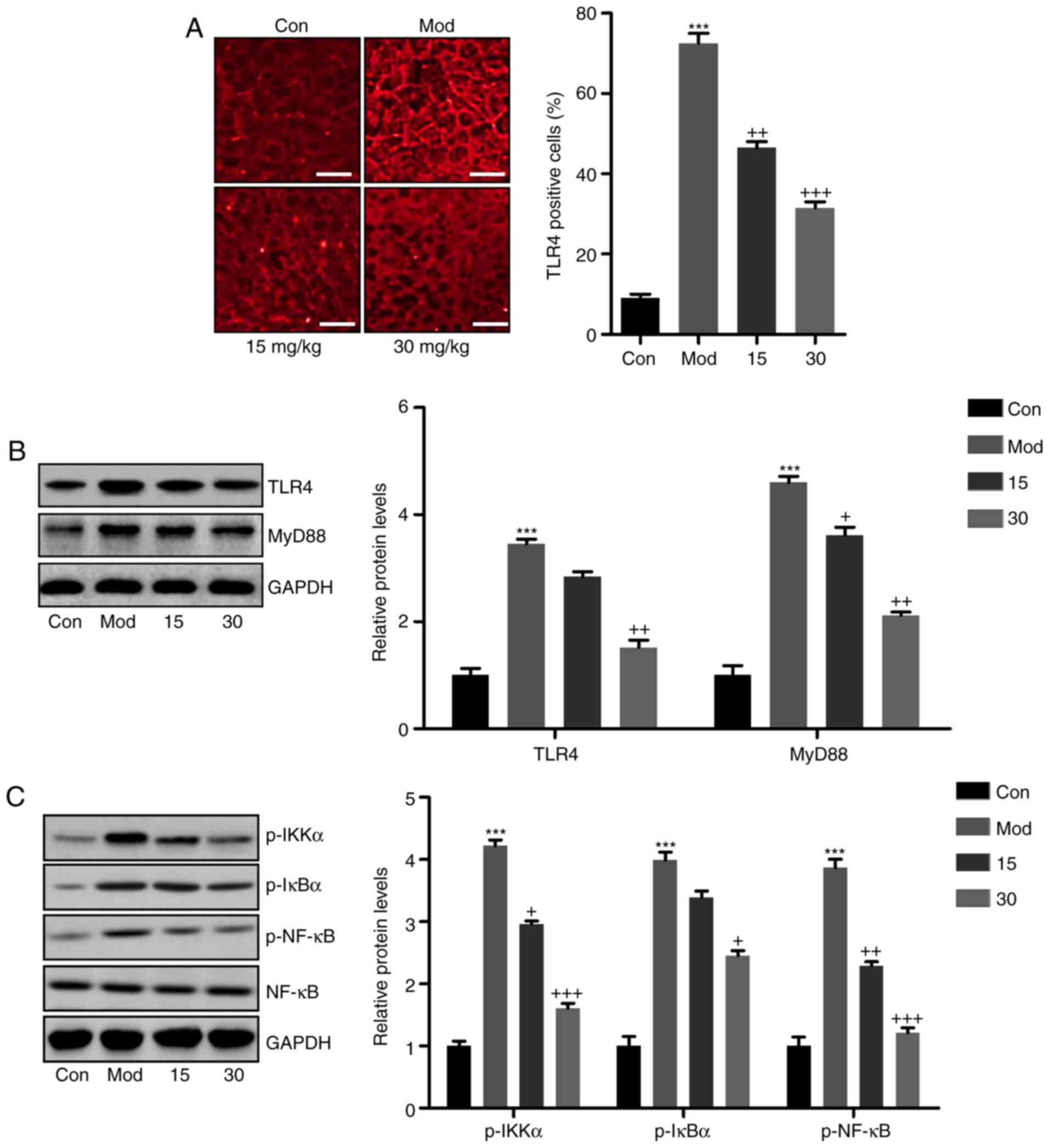 | Figure 4Breviscapine ameliorates
CCl4-induced liver injury by via suppression of
TLR4/NF-κB signaling. (A) Immunofluorescence microscopy was
performed to explore the TLR4 intensity in the liver sections of
mice after CCl4 induction. Scale bar, 100 µm. (B)
TLR4 and MyD88 protein levels were calculated by western blot
analysis, displayed with the representative images and quantified
levels. (C) Phosphorylated IKKα, IκBα and NF-κB levels were
determined by immunoblot analysis. The quantified levels were also
displayed. Values are expressed as the mean ± standard error of the
mean (n=10). ***P<0.001 vs. Con;
+P<0.05, ++P<0.01 and
+++P<0.001 vs. CCl4-induced mice. Mod,
CCl4-treated group; Con, control group; TLR, Toll-like
receptor; p-NF-κB, phosphorylated nuclear factor κB; IκBα,
inhibitor of NF-κB; IKKα, IκB kinase α; MyD88, myeloid
differentiation primary response gene 88. |
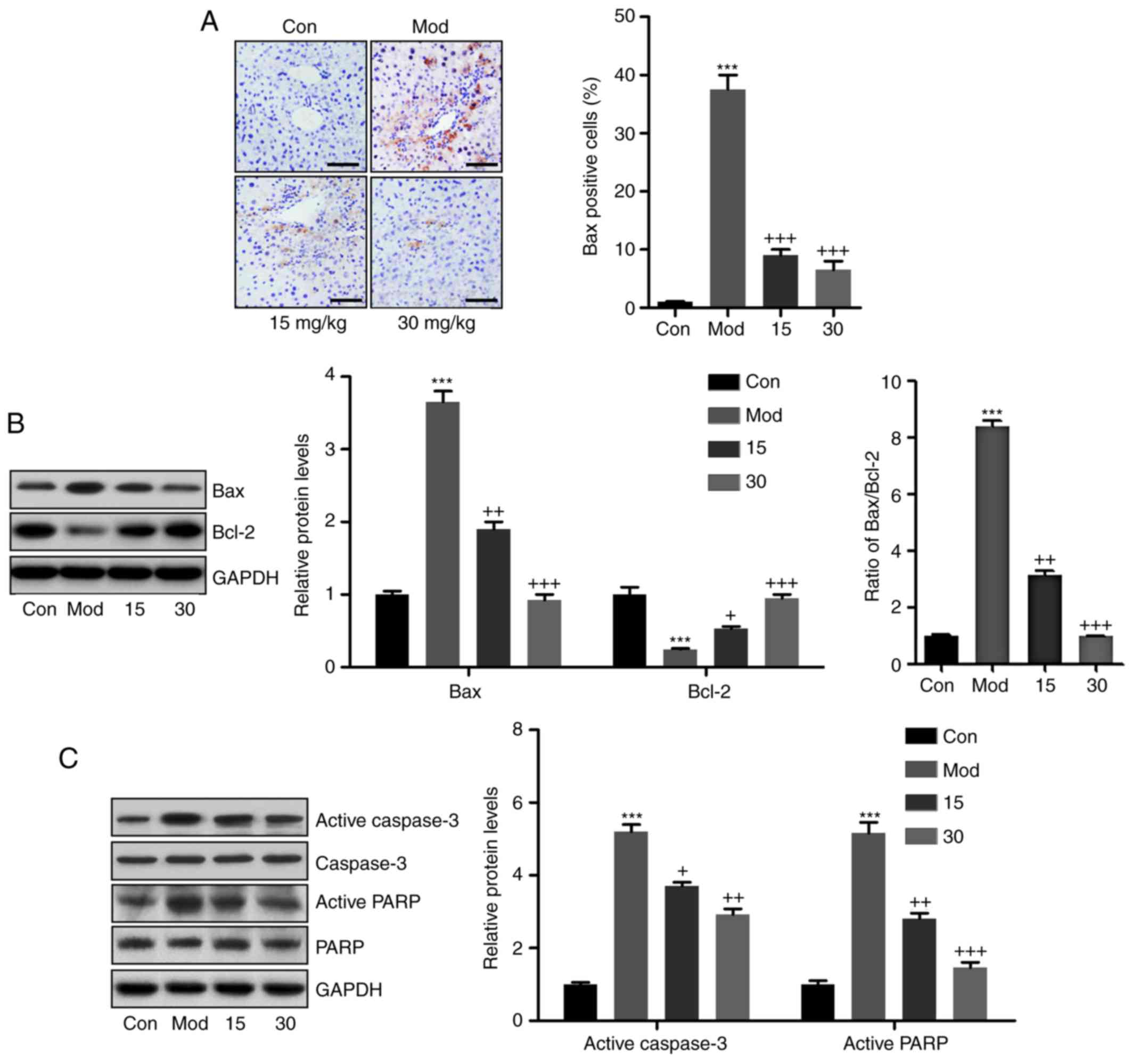
Breviscapine attenuates liver injury by
inhibiting apoptosis in CCl4-induced mice
To determine whether CCl4 caused
apoptosis, the expression levels of apoptosis-associated molecules
were determined. CCl4 treatment markedly increased the
mRNA levels of the pro-apoptotic mitochondrial protein Bax, which
was decreased when the mice were co-treated with breviscapine
(Fig. 5A). In addition, western
blot analysis indicated that the expression levels of the
anti-apoptotic molecule Bcl-2 exhibited an opposite trend to that
of the protein expression levels of Bax in the
CCl4-treated mice, indicating that CCl4
induced apoptosis in the liver tissue samples. Of note,
breviscapine treatment caused a significant upregulation of Bcl-2
expression to prevent apoptosis (Fig.
5B). Finally, casapse-3 and PARP were determined by western
blot to further clarify the role of breviscapine in
CCl4-induced apoptosis. As presented in Fig. 5C, active caspase-3 and PARP were
increased in the CCl4-treated group in comparison with
the control group, while breviscapine had a suppressive effect on
caspase-3 and PARP cleavage.
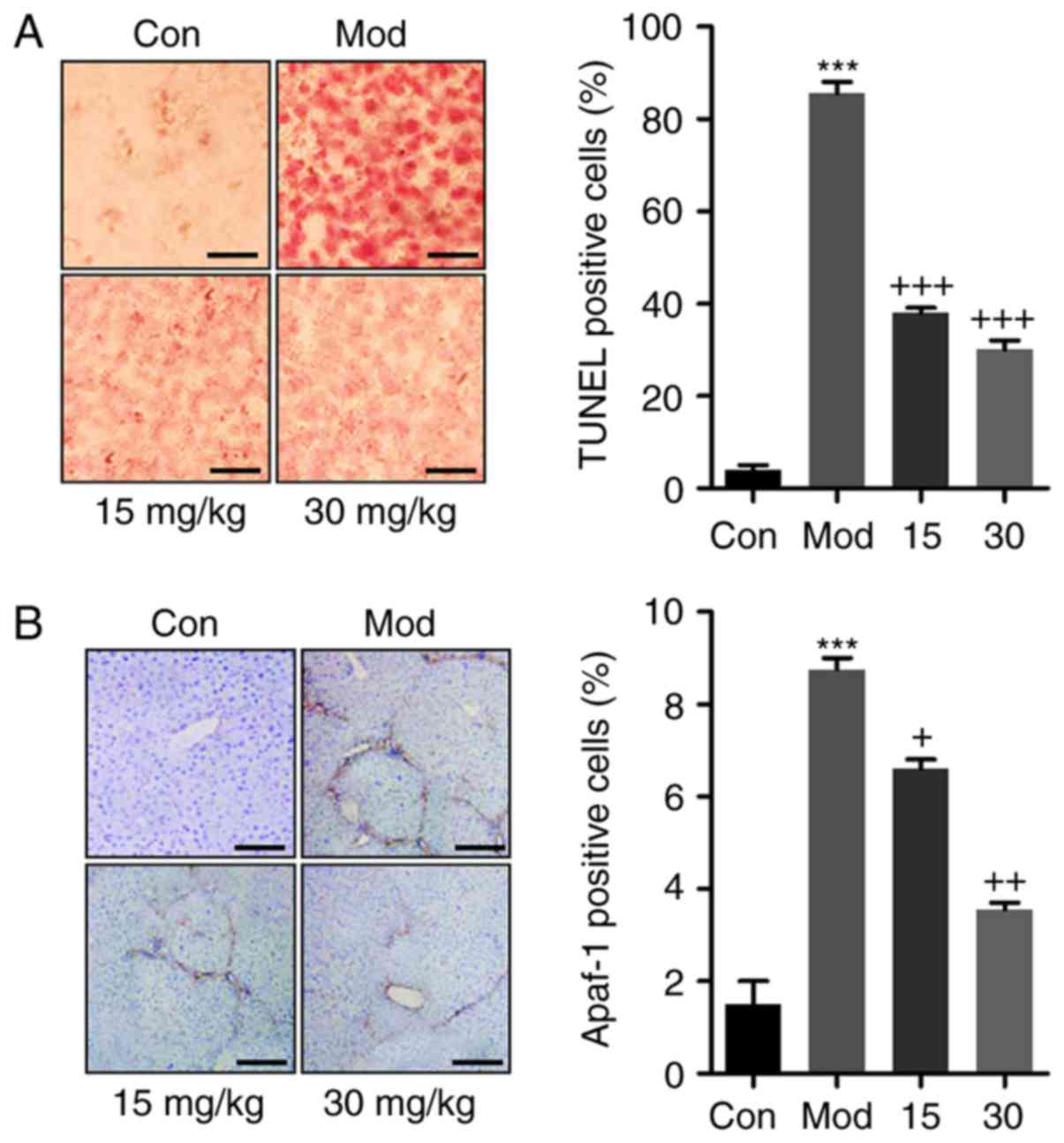
In addition, TUNEL analysis was performed to further
evaluate cellular apoptosis. The representative images and the
histogram in Fig. 6A confirmed
that CCl4 induced massive apoptosis, while breviscapine
administration significantly reduced the amount of TUNEL-positive
cells. Apaf-1 is important during caspase-dependent mitochondrial
apoptosis (31). As indicated in
Fig. 6B, Apaf-1 was significantly
upregulated following CCl4 treatment, while breviscapine
administration reduced Apaf-1 protein levels to inhibit apoptosis.
These results indicated that breviscapine attenuated
CCl4-induced liver injury by suppressing the apoptotic
response.
Breviscapine reduces oxidative stress in
the livers of mice treated with CCl4
Oxidative stress is another essential factor
contributing to acute liver injury (32,33). Thus, the present study further
explored whether breviscapine modulates oxidative stress to
attenuate liver injury induced by CCl4 in vivo.
As presented in Fig. 7A, liver
tissue samples of mice from the CCl4 group exhibited
reduced levels of the anti-oxidants SOD and GSH, which were
comparable to those in the Con group. Breviscapine significantly
upregulated SOD activity and GSH levels in liver tissues. By
contrast, MDA and H2O2 levels were enhanced
by CCl4 treatment, but were downregulated after
breviscapine administration. Furthermore, western blot analysis
indicated that the CCl4-induced decrease of
anti-oxidants, including SOD1, HO-1, NQO-1 and Nrf2, was inhibited
by treatment with breviscapine (Fig.
7B). MAPK signaling is closely associated with the progression
of oxidative stress (34,35). The ratios of phosphorylated p38,
ERK1/2 and JNK vs. total proteins are shown. As presented in
Fig. 7C, p38, ERK1/2 and JNK were
phosphorylated by CCl4 stimulation, and breviscapine
inhibited the activation of these MAPKs. Therefore, the above
results indicated that breviscapine reduced oxidative stress to
alleviate liver injury induced by CCl4.
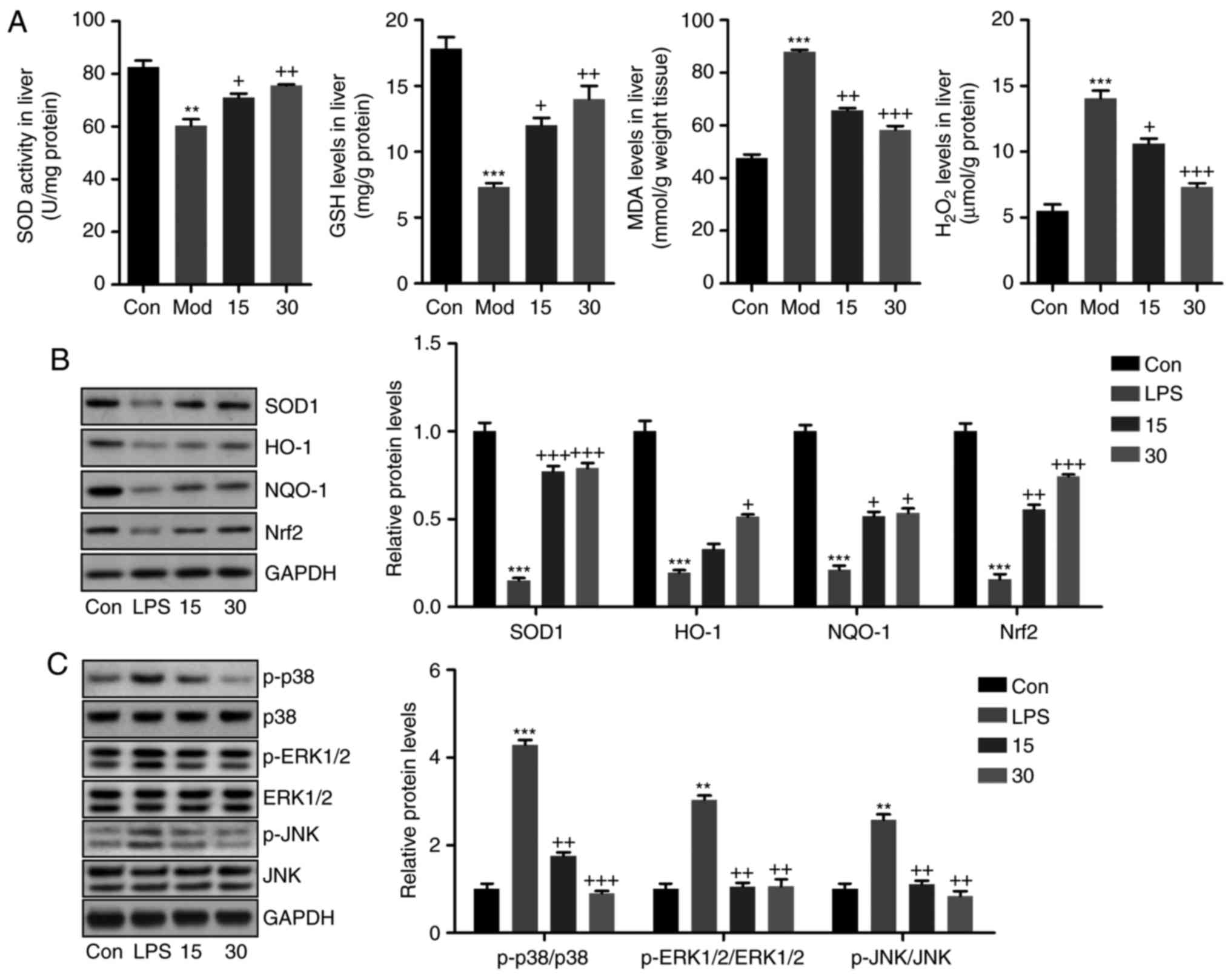 | Figure 7Breviscapine reduces oxidative stress
in the livers of mice induced with CCl4. (A) Liver SOD
activity, GSH levels, MDA levels and H2O2
levels were measured. (B) Western blot analysis was used to
determine SOD1, NQO-1, HO-1 and Nrf2 protein expression levels in
the liver tissue samples. (C) p-p38, p-ERK1/2 and p-JNK protein
levels in liver samples were calculated using western blot assays.
Values are expressed as the mean ± standard error of the mean
(n=8). **P<0.01 and ***P<0.001 vs. Con;
+P<0.05, ++P<0.01 and
+++P<0.001 vs. CCl4-induced mice. Mod,
CCl4-treated group; Con, control group. SOD, superoxide
dismutase; GSH, glutathione synthase; MDA, malondialdehyde; HO-1,
heme oxygenase 1; NQO-1, NAD(P)H quinone dehydrogenase 1; p-ERK,
phosphorylated extracellular signal-regulated kinase; JNK, c-Jun
N-terminal kinase; Nrf2, nuclear factor erythroid 2-related factor
2. |
Breviscapine downregulates the
LPS-induced inflammatory response in L02 cells in vitro
In order to further confirm the effects of
breviscapine on liver injury, an in vitro experiment was
performed. First, the viability of liver cell lines was examined by
CCK-8 analysis. As presented in Fig.
8A, the normal liver cell lines L02, BRL-3A and AML-12 were
treated with various concentrations of breviscapine (0, 5, 10, 20
and 40 µM) for different durations ranging from 0-72 h. The
cell viability assay indicated that the above treatment had almost
no significant effect on normal liver cell lines, except for the
BRL-3A cells treated with breviscapine at 40 µM for 72 h.
Therefore, it was suggested that breviscapine is safe for
application, exerting only little cytotoxicity to normal liver cell
lines isolated from a human, rat and mouse. In the next experiment,
L02 cells were treated with 100 ng/ml LPS for 24 h, in the presence
or absence of breviscapine at 20 or 40 µM. The
pro-inflammatory cytokines TNF-α, IL-6, IL-1β and IL-18, and the
chemokine MCP-1 were highly induced by LPS at the mRNA level, as
indicated by RT-qPCR analysis. Breviscapine co-treatment reduced
the mRNA levels of these factors, indicating that breviscapine
exerted anti-inflammatory effects on liver cells (Fig. 8B). LPS markedly increased the
protein levels of TLR4 and MyD88, which was markedly inhibited by
breviscapine (Fig. 8C). In
addition, LPS-induced increases in the levels of phosphorylated
IKKα, IκBα and NF-κB (p65) protein were suppressed by breviscapine,
as indicated by western blot analysis (Fig. 8D). The above evidence indicated
that breviscapine ameliorated the inflammatory response through
attenuating the activation of TLR4/NF-κB signaling.
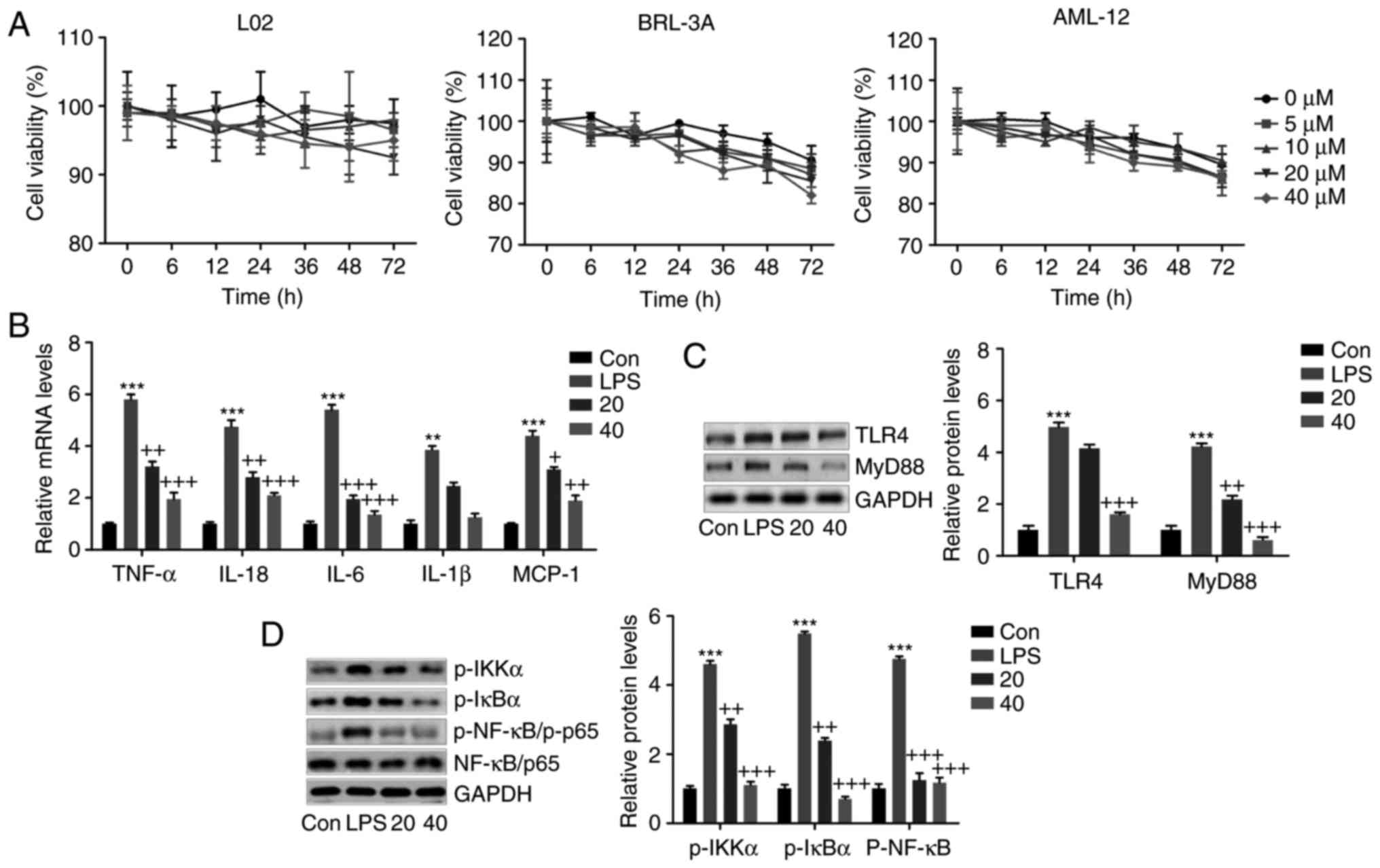 | Figure 8Breviscapine suppresses the
inflammatory response in L02 cells stimulated with LPS in
vitro. (A) The normal liver cell lines of L02, BRL-3A and
AML-12 were treated with breviscapine at different concentrations
(0, 5, 10, 20 and 40 µM) for various durations ranging from
0-72 h. The cell viability was measured by a cell counting kit-8
assay to evaluate the cytotoxicity of breviscapine to liver cells.
In addition, L02 cells were exposed to 100 ng/ml LPS for 24 h in
the absence or presence of breviscapine (20 or 40 µM). (B)
The pro-inflammatory cytokines TNF-α, IL-6, IL-1β and IL-18, and
the chemokine MCP-1 were assessed by reverse
transcription-quantitative polymerase chain reaction analysis. (C)
Western blot analysis was used to evaluate TLR4 and MyD88 levels in
L02 cells after various treatments. (D) IKKα, IκBα and NF-κB (p65)
phosphorylation were examined by immunoblotting analysis.
Representative western blot images and quantified protein levels
are provided. Values are expressed as the mean ± standard error of
the mean (n=8). *P<0.05, **P<0.01 and
***P<0.001 vs. Con; +P<0.05,
++P<0.01 and +++P<0.001 vs. LPS group.
LPS, lipopolysaccharide; Con, control group; p-NF-κB,
phosphorylated nuclear factor-κB; IκBα, inhibitor of NF-κB; IKKα,
IκB kinase α; TLR, Toll-like receptor; MyD88, myeloid
differentiation primary response gene 88; TNF, tumor necrosis
factor; IL, interleukin; MCP, monocyte chemoattractant protein. |
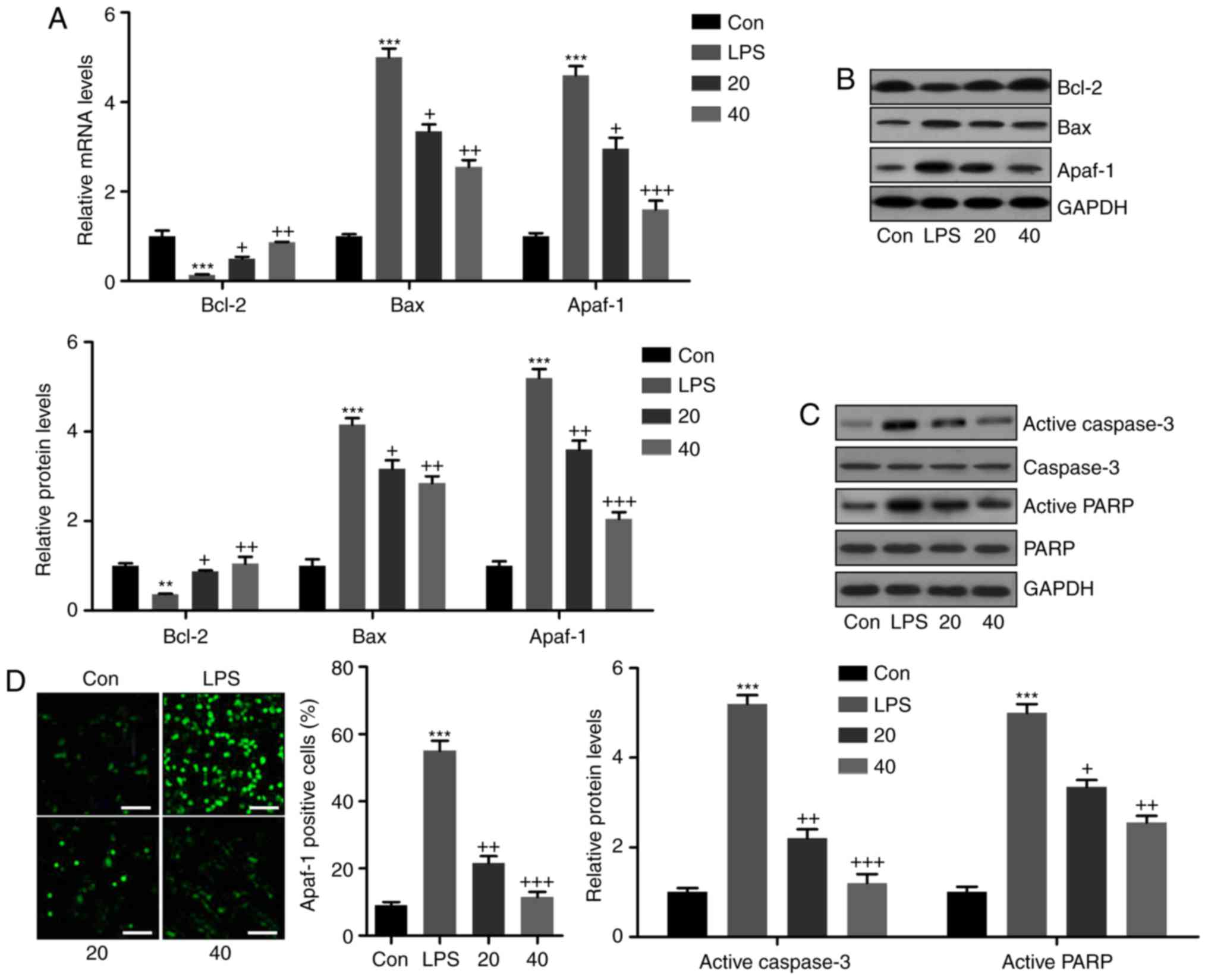
Breviscapine reduces LPS-induced
apoptosis of L02 cells
In L02 cells subjected to various treatments,
apoptotic markers were assessed. RT-qPCR and western blot analysis
indicated that LPS induced decreases in the mRNA and protein levels
of the anti-apoptotic molecule Bcl-2, which was inhibited by
breviscapine in a dose-dependent manner (Fig. 9A and B). By contrast, the mRNA and
protein expression levels of Bax and Apaf-1 were increased in the
LPS-treated group, which was inhibited by co-treatment with
breviscapine (Fig. 9A and B). In
addition, western blot analysis indicated that the cleavage of
caspase-3 and PARP was significantly induced by LPS, when compared
with that in the control group. Of note, breviscapine co-treatment
reduced caspase-3 and PARP activity in a dose-dependent manner,
which was in line with the in vivo results (Fig. 9C). Finally, immunofluorescence
analysis indicated that LPS-induced Apaf-1 expression in L02 cells
was attenuated by breviscapine treatment (Fig. 9D). The above results demonstrated
that breviscapine ameliorated apoptosis in LPS-induced L02 cells to
attenuate liver injury.
Breviscapine reduces LPS-induced
oxidative stress in L02 cells
In vivo, breviscapine exerted anti-oxidant
effects to attenuate CCl4-induced liver injury. A
further in vitro experiment was performed to confirm this
result. As presented in Fig.
10A, the DCFH-DA assay revealed that LPS treatment induced
massive ROS generation, which was inhibited by breviscapine. In
addition, breviscapine restored the levels of SOD1, NQO-1, HO-1 and
Nrf2, which were decreased in LPS-treated cells (Fig. 10B). However, phosphorylation of
p38, ERK1/2 and JNK stimulated by LPS was markedly reduced by
breviscapine in a dose-dependent manner (Fig. 10C). These results further
confirmed that breviscapine exerted protective effects against
liver injury through inhibiting ROS production.
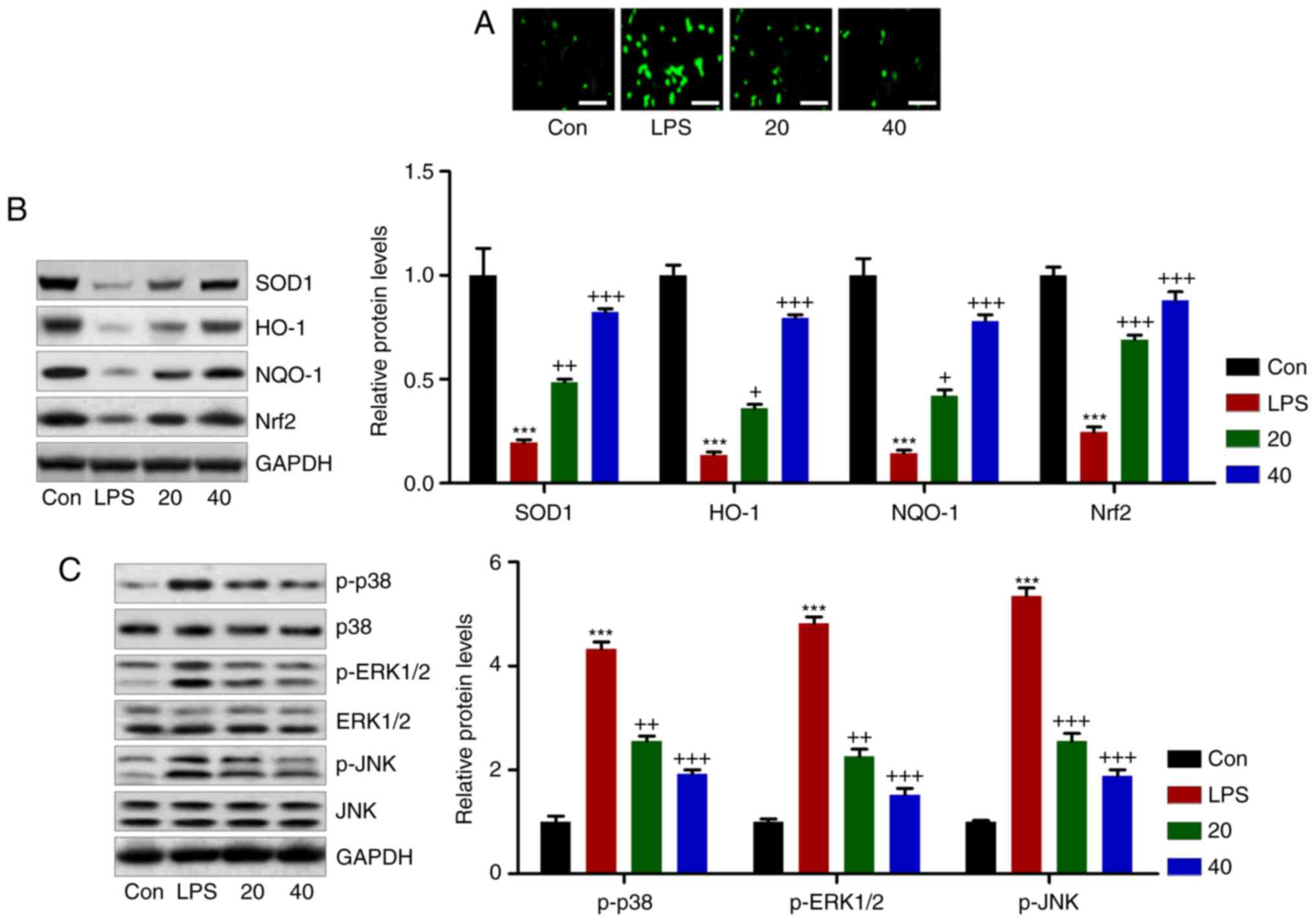 | Figure 10Breviscapine downregulates
LPS-induced oxidative stress in L02 cells. (A) A
2′,7′-dichlorofluoresceindiacetate assay was performed to measure
the generation of reactive oxygen species in L02 cells treated with
LPS with or without breviscapine treatment (20 or 40 µM).
Scale bar, 50 µm. (B) SOD1, NQO-1, HO-1 and Nrf2, as well as
(C) p-p38, p-ERK1/2 and p-JNK protein levels were measured using
western blot analysis. The quantified results are provided in the
bar graphs. Values are expressed as the mean ± standard error of
the mean (n=10). ***P<0.001 vs. Con.
+P<0.05, ++P<0.01 and
+++P<0.001 vs. LPS group. Con, control; LPS,
lipopolysaccharide; SOD, superoxide dismutase; HO-1, heme oxygenase
1; NQO-1, NAD(P)H quinone dehydrogenase 1; p-ERK, phosphorylated
extracellular signal-regulated kinase; JNK, c-Jun N-terminal
kinase; Nrf2, nuclear factor erythroid 2-related factor 2. |
Discussion
The liver is an essential organ, which is involved
in a variety of activities, including the generation of blood
clotting factors, bile acid secretion, destruction of bacteria in
the blood and detoxification. Liver injury may be triggered by
various factors, including microbes, drugs and xenobiotics, as well
as metabolites in the liver (1-3,36).
Previous studies have indicated that breviscapine has beneficial
properties, such as anti-oxidant, anticancer and anti-degenerative
effects (14-16). While breviscapine has been
investigated in various diseases, the current knowledge regarding
its protective effect against liver injury induced by
CCl4 is limited, and further study is required to
elucidate the underlying mechanism. In the present study,
histological analysis indicated that breviscapine attenuated
CCl4-induced liver cell injury. Masson staining further
demonstrated that the collagen deposition caused by CCl4
was significantly decreased by breviscapine. In addition, the
fibrotic area caused by CCl4 was reduced by breviscapine
administration. Supplementation with breviscapine in
CCl4-induced mice attenuated liver injury, reduced
fibrosis and improved hepatic function by reducing the
CCl4 -associated increases in ALT and AST levels, which
was in line with previous studies (37,38).
To identify the possible molecular mechanisms,
including the signaling pathways involved, the inflammatory
response was investigated. The inflammatory response has been
reported as a pivotal process leading to organ injury under various
stresses (39,40). As previously described,
CCl4 treatment induced acute liver injury, which was
closely associated with inflammation by elevating pro-inflammatory
cytokine secretion (41). In line
with previous studies, the present study confirmed that the
pro-inflammatory cytokines IL-1β, TNF-α, IL-18 and IL-6 were highly
expressed in CCl4-treated mice in vivo, as well
as in LPS-induced L02 cells in vitro, which represents a
major mechanism of liver injury. Of note, breviscapine
administration significantly reduced the release of these
cytokines, suggesting its role in ameliorating
CCl4-induced liver injury. The TLR4 signaling pathway
has a vital role in various physiological and pathological
processes, including CCl4-induced liver injury. It has
been demonstrated that TLR4 recruits specific adaptor molecules,
including MyD88, to initiate down-streaming signaling events
towards the phosphorylation of NF-κB, thereby inducing the release
of pro-inflammatory cytokines (42,43). In addition, NF-κB has a central
role in the inflammatory response and triggers the expression of
crucial inflammatory genes. Furthermore, NF-κB is one of the key
transcription factors in LPS-stimulated inflammation, which
regulates various inflammatory mediators, including TNF-α, IL-18,
IL-6 and IL-1β (44). The IKK
complex is activated by LPS through the TLR4 signaling pathway and
phosphorylates IκBα in the cytoplasm. Consequently, it undergoes
proteasomal degradation, leading to NF-κB release from the IKK
complex and its translocation into the nucleus to subsequently
enhance the expression of targeting genes involved in the
inflammatory response (45,46). Thus, downregulation of the NF-κB
signaling pathway regulated by TLR4/MyD88 is one of the major
targets to attenuate the inflammatory response and associated
diseases. Similarly, the present study indicated that the
TLR4/MyD88 signaling pathway was activated in vitro and
in vivo by LPS or CCl4. Accordingly, the
phosphorylation of IKKα, IκBα and NF-κB was stimulated,
contributing to the secretion of pro-inflammatory cytokines.
Breviscapine obviously exerted a suppressive effect on the
TLR4/NF-κB signaling pathway, which appears to be a major mechanism
of its anti-inflammatory action.
Apoptosis is a process that is tightly regulated by
specific genes, including several pro- and anti-apoptotic genes
expressing homologous proteins of the Bcl-2 family, including Bcl-2
and Bax, which are known to have an important role in determining
whether a cell undergoes apoptosis (47-50). Apoptosis may be induced via the
mitochondrial pathway and the Bcl-2/Bax equilibrium regulates the
mitochondrial apoptotic pathway (51). Bax is a typical pro-apoptotic
protein in the cytosol, which may translocate to the mitochondria
to induce apoptosis, while Bcl-2 is an anti-apoptotic protein that
suppresses Bax-induced apoptosis (52). Bax activation stimulates caspase-3
and PARP cleavage through Apaf-1 stimulation. Consequently, the
apoptotic response is induced, eventually leading to cell death
(53,54). Western blot analysis indicated
that Bax expression in the CCl4-treated mice was
upregulated compared with that in the control group, whereas Bcl-2
expression in the CCl4-treated group was downregulated.
Of note, co-treatment with breviscapine inhibited the increase of
Bax expression and the decrease of Bcl-2 expression induced by
CCl4. Consequently, the higher caspase-3 and PARP
cleavage caused by CCl4 was suppressed by breviscapine
administration. Therefore, these results indicated that
breviscapine ameliorated CCl4-induced liver cell
apoptosis by modulating the expression of the apoptosis-associated
molecules Bax and Bcl-2 to inhibit the caspase-3-dependent
apoptotic signaling pathway. To the best of our knowledge, the
present study was the first to indicate that breviscapine
alleviates liver injury through suppression of apoptosis and
oxidative stress. According to previous studies, an interaction
between apoptosis and inflammation is involved in regulating the
progression and development of various types of tumor (55,56). NF-κB is generally considered to be
a survival factor that activates the expression of various
anti-apoptotic genes, including Bcl-2, myeloid leukemia-1, Bcl
extra-large protein and cellular (FADD-like IL-1β-converting
enzyme)-inhibitory protein. Inhibition of NF-κB leads to
downregulation of the NF-κB-regulated anti-apoptotic proteins,
thereby promoting apoptotic cell death (57,58). As activation of NF-κB is a
frequent event in cancer cells, it may be an attractive potential
therapeutic target. However, NF-κB inhibition alone is not
sufficient to induce apoptosis (59).
The elevation of cellular ROS is thought to cause
various diseases and conditions, including diabetes, cardiovascular
diseases, cancer and aging (60-62). CCl4 causes severe liver
cell damage via elevation of ROS, leading to necrosis and apoptosis
to result in acute liver injury. It is evident that direct
reduction of ROS levels and inhibition of the
CCl4-induced oxidative chain reaction are critical for
the prevention and treatment of CCl4-induced acute liver
damage (63). Therefore,
supplementation with anti-oxidants is beneficial for human health.
According to a previous study, breviscapine exerted anti-oxidant
effects in the livers of rats (64). The present study indicated that
breviscapine reduced oxidative stress via enhancing anti-oxidants,
including SOD1, NQO-1, HO-1 and Nrf2. ROS may affect the activity
of MAPKs (p38, ERK1/2 and JNK), which are involved in important
signaling pathways regulating cell proliferation, differentiation
and death in response to a variety of stimuli. MAPKs also sense the
cellular redox status and are common targets for ROS (34,35,65,66). In the present study, breviscapine
de-activated MAPK signaling induced by CCl4 and LPS
in vivo and in vitro, respectively. Therefore, the
breviscapine-mediated attenuation of liver injury was also linked
to its suppression of oxidative stress, which was in line with the
results of a previous study (67).
In conclusion, the present study indicated the
potential protective effects of breviscapine against
CCl4-induced liver damage. The hepatoprotective effects
of breviscapine depend on its ability to reduce the generation of
ROS, as well as pro-inflammatory signalling and apoptosis through
de-activation of TLR4/NF-κB, caspase-3/PARP and MAPK signaling.
Overall, the present study provides evidence for the protective
effects of breviscapine against CCl4-induced liver
injury and suggests breviscapine as a potential hepatoprotective
agent to prevent oxidative liver damage.
Funding
This work was supported by the Institutional Animal
Care and Use Committee of the Beijing Chao-Yang Hospital (Beijing,
China) (grant no. Yt20115713c).
Availability of data and materials
The datasets generated and analyzed in the present
study are included in this published article.
Authors' contributions
YL, PW, XZ and YD contributed to the design of this
study and performed the experiments. QH drafted the manuscript. All
the authors read and approved the final manuscript.
Ethics and consent to participate
This work was approved by the Institutional Animal
Care and Use Committee of the Beijing Chao-Yang Hospital (Beijing,
China). The present study was conducted following the Guide for the
Care and Use of Experimental Animals of the National Institutes of
Health (NIH) from 1996 (20).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Rowland A, Miners JO and Mackenzie PI: The
UDP-glucuronosyltransferases: Their role in drug metabolism and
detoxification. Int J Biochem Cell Biol. 45:1121–1132. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mao S, Gao D, Liu W, Wei H and Lin JM:
Imitation of drug metabolism in human liver and cytotoxicity assay
using a micro-fluidic device coupled to mass spectrometric
detection. Lab Chip. 12:219–226. 2012. View Article : Google Scholar
|
|
3
|
Dawson S, Stahl S, Paul N, Barber J and
Kenna JG: In vitro inhibition of the bile salt export pump
correlates with risk of cholestatic drug-induced liver injury in
humans. Drug Metab Dispos. 40:130–138. 2012. View Article : Google Scholar
|
|
4
|
Huang HL, Wang YJ, Zhang QY, Liu B, Wang
FY, Li JJ and Zhu RZ: Hepatoprotective effects of baicalein against
CCl4-induced acute liver injury in mice. World J
Gastroenterol. 18:6605–6613. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen X, Meng Q, Wang C, Liu Q, Sun H, Huo
X, Sun P, Yang X, Peng J and Liu K: Protective effects of calycosin
against CCl4-induced liver injury with activation of FXR
and STAT3 in mice. Pharm Res. 32:538–548. 2015. View Article : Google Scholar
|
|
6
|
Chen X, Gong X, Jiang R, Wang B, Kuang G,
Li K and Wan J: Resolvin D1 attenuates CCl4-induced
acute liver injury involving up-regulation of HO-1 in mice.
Immunopharmacol Immunotoxicol. 38:61–67. 2016. View Article : Google Scholar
|
|
7
|
Perugorria MJ, Sharif O, Oakley F,
Esparza-Baquer A, Korosec A, Mann J, Labiano I, Tiniakos D, Gawish
R, Jiménez-Agüero R, et al: TREM-2 protects the liver from
immune-mediated hepatocellular damage. J Hepatol. 64:S133–S158.
2016. View Article : Google Scholar
|
|
8
|
Karkampouna S, Goumans MJ, Ten Dijke P,
Dooley S and Kruithof-de Julio M: Inhibition of TGFβ type I
receptor activity facilitates liver regeneration upon acute
CCl4 intoxication in mice. Arch Toxicol. 90:347–357.
2016. View Article : Google Scholar
|
|
9
|
Liu J, Wang X, Liu R, Liu Y, Zhang T, Fu H
and Hai C: Oleanolic acid co-administration alleviates
ethanol-induced hepatic injury via Nrf-2 and ethanol-metabolizing
modulating in rats. Chem Biol Interact. 221:88–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bansal R, Nagórniewicz B, Storm G and
Prakash J: PS-072-Relaxin-coated superparamagnetic iron-oxide
nanoparticles as a novel theranostic approach for the diagnosis and
treatment of liver fibrosis. J Hepatol. 66:S33–S62. 2017.
View Article : Google Scholar
|
|
11
|
Navarro VJ, Barnhart H, Bonkovsky HL,
Davern T, Fontana RJ, Grant L, Reddy KR, Seeff LB, Serrano J,
Sherker AH, et al: Liver injury from herbals and dietary
supplements in the US drug-induced liver injury network.
Hepatology. 60:1399–1408. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Salomone F, Godos J and Zelber-Sagi S:
Natural antioxidants for non-alcoholic fatty liver disease:
Molecular targets and clinical perspectives. Liver Int. 36:5–20.
2016. View Article : Google Scholar
|
|
13
|
Wang X, Xia H, Liu Y, Qiu F and Di X:
Simultaneous determination of three glucuronide conjugates of
scutellarein in rat plasma by LC-MS/MS for pharmacokinetic study of
breviscapine. J Chromatogr B Analyt Technol Biomed Life Sci.
965:79–84. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao Y, Ma X, Wang J, He X, Zhang Y, Wang
Y, Liu H, Shen H and Xiao X: A system review of anti-fibrogenesis
effects of compounds derived from chinese herbal medicine. Mini Rev
Med Chem. 16:163–175. 2016. View Article : Google Scholar
|
|
15
|
Qian LH, Li NG, Tang YP, Zhang L, Tang H,
Wang ZJ, Liu L, Song SL, Guo JM and Ding AW: Synthesis and
bio-activity evaluation of scutellarein as a potent agent for the
therapy of ischemic cerebrovascular disease. Int J Mol Sci.
12:8208–8216. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zeng J and Cai S: Breviscapine suppresses
the growth of non-small cell lung cancer by enhancing microRNA-7
expression. J Biosci. 42:121–129. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu Y, Fan Q, Lu N, Tao L, Gao Y, Qi Q and
Guo Q: Breviscapine-induced apoptosis of human hepatocellular
carcinoma cell line HepG2 was involved in its antitumor activity.
Phytother Res. 24:1188–1194. 2010.PubMed/NCBI
|
|
18
|
Jiang L, Xia QJ, Dong XJ, Hu Y, Chen ZW,
Chen K, Wang KH, Liu J and Wang TH: Neuroprotective effect of
breviscapine on traumatic brain injury in rats associated with the
inhibition of GSK3β signaling pathway. Brain Res. 1660:1–9. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He X, Long W, Dong H, Wang C, Chu X, Zheng
Q and Fan S: Evaluation of the protective effects of 13 traditional
Chinese medicine compounds on ionizing radiation injury: Bupleurum,
shenmai, and breviscapine as candidate radioprotectors. RSC Adv.
7:22640–22648. 2017. View Article : Google Scholar
|
|
20
|
Clark JD, Gebhart GF, Gonder JC, Keeling
ME and Kohn DF: Special Report: The 1996 guide for the care and use
of laboratory animals. ILAR J. 38:41–48. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Andrade-Cetto A and Wiedenfeld H:
Anti-hyperglycemic effect of opuntia streptacantha lem. J
Ethnopharmacol. 133:940–943. 2011. View Article : Google Scholar
|
|
22
|
Wang Y: Attenuation of berberine on
lipopolysaccharide- induced inflammatory and apoptosis responses in
β-cells via TLR4-independent JNK/NF-κB pathway. Pharm Biol.
2013.
|
|
23
|
Liang CJ, Lee CW, Sung HC, Chen YH, Wang
SH, Wu PJ, Chiang YC, Tsai JS, Wu CC, Li CY and Chen YL: Magnolol
reduced TNF-α-induced vascular cell adhesion molecule-1 expression
in endothelial cells via JNK/p38 and NF-κB signaling pathways. Am J
Chin Med. 42:619–637. 2014. View Article : Google Scholar
|
|
24
|
Xu MX, Wang M and Yang WW: Gold-quercetin
nanoparticles prevent metabolic endotoxemia-induced kidney injury
by regulating TLR4/NF-κB signaling and Nrf2 pathway in high fat
diet fed mice. Int J Nanomedicine. 12:327–345. 2017. View Article : Google Scholar :
|
|
25
|
Uesugi T, Froh M, Arteel GE, Bradford BU
and Thurman RG: Toll-like receptor 4 is involved in the mechanism
of early alcohol-induced liver injury in mice. Hepatology.
34:101–108. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li JM, Ge CX, Xu MX, Wang W, Yu R, Fan CY
and Kong LD: Betaine recovers hypothalamic neural injury by
inhibiting astrogliosis and inflammation in fructose-fed rats. Mol
Nutr Food Res. 59:189–202. 2015. View Article : Google Scholar
|
|
27
|
Kluk MJ, Chapuy B, Sinha P, Roy A, Dal Cin
P, Neuberg DS, Monti S, Pinkus GS, Shipp MA and Rodig SJ:
Immunohistochemical detection of MYC-driven diffuse large B-cell
lymphomas. PloS One. 7:e338132012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu MX, Zhu YF, Chang HF and Liang Y:
Nanoceria restrains PM2.5-induced metabolic disorder and
hypothalamus inflammation by inhibition of astrocytes activation
related NF-κB pathway in Nrf2 deficient mice. Free Radic Biol Med.
99:259–272. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
30
|
Tewari R, Choudhury SR, Ghosh S, Mehta VS
and Sen E: Involvement of TNFα-induced TLR4-NF-κB and TLR4-HIF-1α
feed-forward loops in the regulation of inflammatory responses in
glioma. J Mol Med (Berl). 90:67–80. 2012. View Article : Google Scholar
|
|
31
|
Brentnall M, Rodriguez-Menocal L, De
Guevara RL, Cepero E and Boise LH: Caspase-9, caspase-3 and
caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell
Biol. 14:322013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cichoż-Lach H and Michalak A: Oxidative
stress as a crucial factor in liver diseases. World J
Gastroenterol. 20:8082–8091. 2014. View Article : Google Scholar
|
|
33
|
Sutti S, Jindal A, Locatelli I, Vacchiano
M, Gigliotti L, Bozzola C and Albano E: Adaptive immune responses
triggered by oxidative stress contribute to hepatic inflammation in
NASH. Hepatology. 59:886–897. 2014. View Article : Google Scholar
|
|
34
|
Cordero-Herrera I, Martín MA, Goya L and
Ramos S: Cocoa flavonoids protect hepatic cells against
high-glucose-induced oxidative stress: Relevance of MAPKs. Mol Nutr
Food Res. 59:597–609. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ma JQ, Ding J, Zhang L and Liu CM: Ursolic
acid protects mouse liver against CCl 4-induced oxidative stress
and inflammation by the MAPK/NF-κB pathway. Environ Toxicol
Pharmacol. 37:975–983. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Luyendyk JP, Kassel KM, Allen K, Guo GL,
Li G, Cantor GH and Copple BL: Fibrinogen deficiency increases
liver injury and early growth response-1 (Egr-1) expression in a
model of chronic xenobiotic-induced cholestasis. Am J Pathol.
178:1117–1125. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Esmaeili MA and Alilou M: Naringenin
attenuates CCl4-induced hepatic inflammation by the
activation of an Nrf2-mediated pathway in rats. Clin Exp Pharmacol
Physiol. 41:416–422. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Y, Lian F, Li J, Fan W, Xu H, Yang X,
Liang L, Chen W and Yang J: Adipose derived mesenchymal stem cells
transplantation via portal vein improves microcirculation and
ameliorates liver fibrosis induced by CCl4 in rats. J
Transl Med. 10:1332012. View Article : Google Scholar
|
|
39
|
Pöling J, Gajawada P, Richter M, Lörchner
H, Polyakova V, Kostin S, Shin J, Boettger T, Walther T, Rees W, et
al: Therapeutic targeting of the oncostatin M receptor-β prevents
inflammatory heart failure. Basic Res Cardiol. 109:3962014.
View Article : Google Scholar
|
|
40
|
Szpechcinski A, Chorostowska-Wynimko J,
Struniawski R, Kupis W, Rudzinski P, Langfort R, Puscinska E,
Bielen P, Sliwinski P and Orlowski T: Cell-free DNA levels in
plasma of patients with non-small-cell lung cancer and inflammatory
lung disease. Br J Cancer. 113:476–483. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shi H, Dong L, Jiang J, Zhao J, Zhao G,
Dang X, Lu X and Jia M: Chlorogenic acid reduces liver inflammation
and fibrosis through inhibition of toll-like receptor 4 signaling
pathway. Toxicology. 303:107–114. 2013. View Article : Google Scholar
|
|
42
|
Zhu HT, Bian C, Yuan JC, Chu WH, Xiang X,
Chen F, Wang CS, Feng H and Lin JK: Curcumin attenuates acute
inflammatory injury by inhibiting the TLR4/MyD88/NF-κB signaling
pathway in experimental traumatic brain injury. J
Neuroinflammation. 11:592014. View Article : Google Scholar
|
|
43
|
Ma Y, He M and Qiang L: Exercise therapy
downregulates the overexpression of TLR4, TLR2, MyD88 and NF-κB
after cerebral ischemia in rats. Int J Mol Sci. 14:3718–3733. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang WY, Tan MS, Yu JT and Tan L: Role of
pro-inflammatory cytokines released from microglia in Alzheimer's
disease. Ann Transl Med. 3:1362015.PubMed/NCBI
|
|
45
|
Wei HY and Ma X: Tamoxifen reduces
infiltration of inflammatory cells, apoptosis and inhibits
IKK/NF-kB pathway after spinal cord injury in rats. Neurol Sci.
35:1763–1768. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Baldwin AS: Regulation of cell death and
autophagy by IKK and NF-κB: Critical mechanisms in immune function
and cancer. Immunol Rev. 246:327–345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View Article : Google Scholar
|
|
48
|
Shirali S, Aghaei M, Shabani M, Fathi M,
Sohrabi M and Moeinifard M: Adenosine induces cell cycle arrest and
apoptosis via cyclinD1/Cdk4 and Bcl-2/Bax pathways in human ovarian
cancer cell line OVCAR-3. Tumor Biol. 34:1085–1095. 2013.
View Article : Google Scholar
|
|
49
|
Sun Y, Lin Y, Li H, Liu J, Sheng X and
Zhang W: 2,5-Hexanedione induces human ovarian granulosa cell
apoptosis through BCL-2, BAX, and CASPASE-3 signaling pathways.
Arch Toxicol. 86:205–215. 2012. View Article : Google Scholar
|
|
50
|
Lee JS, Jung WK, Jeong MH, Yoon TR and Kim
HK: Sanguinarine induces apoptosis of HT-29 human colon cancer
cells via the regulation of Bax/Bcl-2 ratio and caspase-9-dependent
pathway. Int J Toxicol. 31:70–77. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hoshyar R, Bathaie SZ and Sadeghizadeh M:
Crocin triggers the apoptosis through increasing the Bax/Bcl-2
ratio and caspase activation in human gastric adenocarcinoma, AGS,
cells. DNA Cell Biol. 32:50–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Spampanato C, De Maria S, Sarnataro M,
Giordano E, Zanfardino M, Baiano S, Cartenì M and Morelli F:
Simvastatin inhibits cancer cell growth by inducing apoptosis
correlated to activation of Bax and down-regulation of BCL-2 gene
expression. Int J Oncol. 40:935–941. 2012. View Article : Google Scholar
|
|
53
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Roos WP and Kaina B: DNA damage-induced
cell death: From specific DNA lesions to the DNA damage response
and apoptosis. Cancer Lett. 332:237–248. 2013. View Article : Google Scholar
|
|
55
|
Jost PJ and Ruland J: Aberrant NF-kappaB
signaling in lymphoma: Mechanisms, consequences, and therapeutic
implications. Blood. 109:2700–2707. 2007.
|
|
56
|
Dutta J, Fan Y, Gupta N, Fan G and Gélinas
C: Current insights into the regulation of programmed cell death by
NF-kappaB. Oncogene. 25:6800–6816. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Fujioka S, Sclabas GM, Schmidt C, Niu J,
Frederick WA, Dong QG, Abbruzzese JL, Evans DB, Baker C and Chiao
PJ: Inhibition of constitutive NF-kappa B activity by I kappa B
alpha M suppresses tumorigenesis. Oncogene. 22:1365–1370. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Rogers R, Ouellet G, Brown C, Moyer B,
Rasoulpour T and Hixon M: Cross-talk between the akt and nf-κb
signaling pathways inhibits mehp-induced germ cell apoptosis.
Toxicol Sci. 106:497–508. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Gasparian AV, Yao YJ, Kowalczyk D, Lyakh
LA, Karseladze A, Slaga TJ and Budunova IV: The role of IKK in
constitutive activation of NF-κB transcription factor in prostate
carcinoma cells. J Cell Sci. 115:141–151. 2002.PubMed/NCBI
|
|
60
|
Harrison DG, Cai H, Landmesser U and
Griendling KK: Interactions of angiotensin II with NAD(P)H oxidase,
oxidant stress and cardiovascular disease. J Renin Angiotensin
Aldosterone Syst. 4:51–61. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Inoguchi T, Sonta T, Tsubouchi H, Etoh T,
Kakimoto M, Sonoda N, Sato N, Sekiguchi N, Kobayashi K, Sumimoto H,
et al: Protein kinase C-dependent increase in reactive oxygen
species (ROS) production in vascular tissues of diabetes: Role of
vascular NAD(P)H oxidase. J Am Soc Nephrol. 14(Suppl 3): S227–S232.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Singh KK: Mitochondrial dysfunction is a
common phenotype in aging and cancer. Ann NY Acad Sci.
1019:260–264. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ulicná O, Greksák M, Vancová O, Zlatos L,
Galbavý S, Bozek P and Nakano M: Hepatoprotective effect of rooibos
tea (Aspalathus linearis) on CCl 4-induced liver damage in rats.
Physiol Res. 52:461–466. 2003.
|
|
64
|
Wu YG, Xia LL, Lin H, Zhou D, Qian H and
Lin ST: Prevention of early liver injury by breviscapine in
streptozotocin-induced diabetic rats. Planta Med. 73:433–438. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Son Y, Cheong YK, Kim NH, Chung HT, Kang
DG and Pae HO: Mitogen-activated protein kinases and reactive
oxygen species: How can ROS activate MAPK pathways. J Signal
Transduct. 2011:7926392011. View Article : Google Scholar
|
|
66
|
Sauer H, Wartenberg M and Hescheler J:
Reactive oxygen species as intracellular messengers during cell
growth and differentiation. Cell Physiol Biochem. 11:173–186. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lin YZ, Lu ZY, Liang XH, Li K, Peng B and
Gong J: Effect of breviscapine against hepatic ischemia reperfusion
injury. J Surg Res. 203:268–274. 2016. View Article : Google Scholar : PubMed/NCBI
|