Introduction
Smallpox is a devastating disease in humans caused
by variola virus (VARV), which was eradicated worldwide in the late
1970s (1,2). However, poxvirus infection,
particularly Orthopoxvirus infection, remains a significant
public health concern due to the bioterror threat concerning the
release of VARV and the emergence of zoonotic poxvirus infections
caused by monkeypox virus (MPXV) (3–6).
Since VARV is a strict human pathogen, much of our current
understanding of the pathogenesis of smallpox and the host response
to infection is the result of studies using vaccinia virus (VACV)
infection and other types of Orthopoxvirus, such as
ectromelia virus (ECTV), MPXV and cowpox virus (6–8).
Due to genetic similarity and the common features of the resulting
disease with VARV, ECTV has been used as a model for the study of
Orthopoxvirus infection, including pathogenesis of viral
infection, viral immunology, genetic resistance to disease, and
antiviral vaccine discovery (9–11).
ECTV has a restricted host range, in that it only infects rodents
and causes mousepox (10). Viral
infection begins through abrasions in the skin, and then
disseminates via the afferent lymphatic system. Viraemia occurs
when the virus is released into the bloodstream, which permits
infection of the spleen, liver and other organs (7).
The complex cell-virus interactions involved in
poxvirus morphogenesis remain incompletely understood. Although
poxvirus has a greater reliance on viral proteins compared with
other double-stranded DNA viruses, host factors still serve a major
role during replication. Heat shock protein 70 (Hsp70) family
proteins are molecular chaperones that function to fold nascent
proteins, refold misfolded proteins and transport proteins between
cellular compartments (12–14). Under stress conditions, including
heat, oxidative stress and infection, Hsp70 proteins are activated
and expressed at high levels (14–16). During viral infection, Hsp70
proteins are frequently recruited to the viral replication sites
and are involved in various steps of the life cycle of numerous DNA
and RNA viruses (14–19). For example, viruses can recruit
Hsp70 proteins to promote folding of viral proteins involved in the
cell-to-cell movement of the virus, and interaction with viral
proteins or virions (20–23). In addition, Hsp70 proteins have
been reported to be involved in assembly of the replicase complex
to enhance viral genome replication (15,24). Conversely, Hsp70 proteins
interfere with the polymerase activity of influenza virus and
negatively regulate viral RNA replication, thus highlighting the
complexity of the virus-chaperone interaction (25,26). Furthermore, Hsp70 proteins have
the ability to enhance protective antiviral immunity by inducing
the production of interferon-β (27,28).
The present study demonstrated that the expression
levels of several isoforms of Hsp70 were highly elevated
during ECTV infection in vivo and in vitro, as
determined by micro-array and reverse transcription-quantitative
polymerase chain reaction (RT-qPCR). Subsequently, the present
study aimed to investigate the effects of Hspa1b on ECTV
replication, via overexpression and RNA interference (RNAi) of
Hsp70 member 1B (Hspa1b), and treatment with inhibitors of
cytosolic Hspa1b. The results demonstrated that Hspa1b may have an
important role in promoting the replication of ECTV.
Materials and methods
Animals, cell culture and viral
infection
Specific-pathogen-free male BALB/c mice (age, 9
weeks; weight, 25.74±0.25 g) were purchased from the Experimental
Animal Center of Lanzhou University (Lanzhou, China). Upon arrival,
mice were housed in a biosafety level 3 room under the following
conditions: Controlled temperature, 23±5°C; humidity 45–55%; 12-h
light/dark cycle. The mice were given free access to commercial
mouse chow and water. After a 1-week acclimation period, mice were
randomly assigned into three groups (n=5 mice/group). All mice were
handled in accordance with the Good Animal Practice Requirements of
the Animal Ethics Procedures and Guidelines of the People's
Republic of China (29). The
present study was reviewed and approved by the Animal Ethics
Committee of Lanzhou Veterinary Research Institute, Chinese Academy
of Agricultural Science (Lanzhou, China; permit no.
LVRIAEC2016-005).
The African green monkey kidney cell line Vero, the
murine fibroblast cell line NIH3T3, and the BALB/c mouse-derived
embryonic fibroblast cell line BALB/3T3 clone A31 were obtained
from China Center for Type Culture Collection (Wuhan, China). All
cell lines were cultured in Dulbecco's Modified Eagle's Medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), 100 IU/ml penicillin and 100 µg/ml
streptomycin at 37°C in the presence of 5% CO2. The
wild-type strain of ECTV was originally isolated from a naturally
infected laboratory mouse and propagated in Vero cells (30). The virus was confirmed by PCR
using specific primers (forward, 5′-ATGGACGGAACTCTTTTC-3′; reverse,
5′-AACTTCATCGTTGCGTTTAC-3′). The PCR was performed in a total
volume of 25 µl containing 2 µl viral genomic DNA, 2
µl of each forward and reverse primers (10 µM), 4
µl PrimeSTAR Buffer (5X, including Mg2+; Takara
Biotechnology Co., Ltd., Dalian, China), 1.5 µl dNTP
mixture, 0.2 µl PrimeSTAR HS DNA Polymerase (Takara
Biotechnology Co., Ltd.) and 8.3 µl diethylpyrocarbonate
water. PCR conditions were as follows: Denaturation at 95°C for 4
min, 35 cycles at 98°C for 10 sec, 60°C for 15 sec and 72°C for 2
min, with final extension at 72°C for 10 min. The PCR products were
analyzed by 1.0% agarose gel electrophoresis with 10 µg/ml
ethidium bromide (Sangon Biotech Co., Ltd., Shanghai, China), and
the positive products underwent Sanger sequencing by Genescript
Co., Ltd. (Nanjing, China). Experimental infection of susceptible
BALB/c mice exhibited less virulence than the ECTV-Moscow strain
(30). Plaque-purified ECTV was
serially passaged and viral titer was measured using a 50% tissue
culture infective dose (TCID50) assay.
Viral infection and microarray
analysis
To examine the host transcriptome profiles of cells
following ECTV infection, 5×105 BALB/3T3 cells were
seeded in each well of a 6-well plate and were infected in
triplicate with ECTV at a multiplicity of infection (MOI) of 1.
Control cells were mock infected with virus-free DMEM. The infected
and control cells were incubated in complete DMEM at 37°C in an
atmosphere containing 5% CO2 and were harvested for
total RNA extraction at 6, 12, 24, 48 and 96 h post-infection
(hpi). For cell harvesting, cells were washed three times with cold
PBS and lysed with 1 ml TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.).
In view of the high transfection efficiency in
NIH3T3 cells, NIH3T3 cells were chosen to carry out in vitro
tests. Briefly, 7×105 NIH3T3 cells were cultured in a
6-well plate and were infected in triplicate with ECTV (MOI, 1) for
2 h. Subsequently, the media were replaced with fresh media
containing 2% FBS and cells were harvested for total RNA extraction
at 2, 4, 8, 12, 24 and 48 hpi to detect the transcription levels of
Hspa1b by qPCR.
With regards to the infection of BALB/c mice, 10
mice were individually anesthetized and infected subcutaneously in
the abdomen with a single dose of 104
TCID50/ml ECTV (in 100 µl PBS). Furthermore, 5
uninfected mice served as a control group, which were euthanized by
cervical dislocation, after which spleen tissues were isolated. On
days 3 and 10 post-infection, 5 mice in 3 and 10 dpi group were
euthanized by cervical dislocation and whole spleen tissues were
harvested, respectively. All of the spleen tissues from each group
were pooled in a cell culture plate and cut into sections (0.2 cm)
using surgical scissors. A total of 2.0 g pooled spleen tissues
used for RNA extraction with TRIzol were separately stored in four
tubes and maintained at -70°C following freezing in liquid
nitrogen.
Spleen tissue samples and BALB/3T3 cells were sent
to Beijing CapitalBio Technology Co., Ltd. (Beijing, China) for
microarray analysis. Briefly, total RNA was extracted from the
samples, and the quality and quantity of RNA were assessed by
formaldehyde agarose gel electrophoresis and spectrophotometry.
Gene expression analysis was performed using the Affymetrix
GeneChip Mouse Genome Arrays (GeneChip® Mouse Genome 430
2.0; Affymetrix; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Microarray data were analyzed using Bio
Molecule Annotation System 3.0 software (Beijing CapitalBio
Technology Co., Ltd.). Using the cutoff criterion of a fold-change
≥2 or ≤0.5, differentially expressed genes were screened and
clustered. All data were deposited into the Gene Expression Omnibus
database (https://www.ncbi.nlm.nih.gov/gds/) under the accession
nos. GSE100644 (30) and
GSE102850. The differential expression of 10 isoforms of the HSP70
family contained in the genechip were filtered out for hierarchical
clustering using Cluster 3.0 (http://bonsai.hgc.jp/~mdehoon/software/cluster/software.htm),
and displayed by using Java TreeView (http://jtreeview.sourceforge.net/).
Plasmids and transfection
The murine Hspa1b gene (National Center for
Biotechnology Information accession no. NM_010478) was synthesized
from C57BL/6 mouse cDNA stored in our laboratory at −80°C (29), and was cloned into the pCMV-Tag2b
vector (Stratagene; Agilent Technologies, Inc., Santa Clara, CA,
USA) with a FLAG tag at the N-terminus. Subsequently, the plasmids
were transfected into Escherichia coli (Takara Biotechnology
Co., Ltd.). The nucleotide sequences of the plasmids were confirmed
using DNA sequencing, and the plasmid was extracted from the
confirmed clone of E. coli using Endo-Free Plasmid Maxi kit
(Omega Bio-Tek, Inc., Norcross, GA, USA). For transfection of cells
with the plasmid, FuGENE HD transfection reagent (Promega
Corporation, Madison, WI, USA) was used according to the
manufacturer's protocol. A total of 3.0 µg pCMV-HSPa1b or
pCMV-Tag2b plasmid mixed with 8.5 µl FuGENE HD reagent was
transfected into 7×105 NIH3T3 cells cultured in a 6-well
plate; the control cells were incubated with 8.5 µl FuGENE
HD reagent only. After 24 h transfection at 37°C, the cells were
collected for western blot analysis or were infected with ECTV
(MOI, 1 or 5) for 24 h. Furthermore, NIH3T3 cells were transfected
with various amounts (1.0, 2.0 and 3.0 µg) of pCMV-Hspa1b
plasmid or empty vector followed by ECTV infection (MOI, 1) for 24
h to explore the effects of Hspa1b on ECTV replication.
RNAi assay
Gene silencing of Hspa1b by small interfering
(si)RNA was conducted in NIH3T3 cells. siRNA sequences (Shanghai
GenePharma Co., Ltd., Shanghai, China) targeting Hspa1b were as
follows: S1, 5′-GAUUACUGUCAAGGUUAUUTT-3′; S2,
5′-AUCUGCUUGUCCAUGUUAATT-3′; and negative control (NC) siRNA,
5′-UUCUUCGAACGUGUCACGUTT-3′. A total of 7×104 cells were
seeded into 12-well plates and were transfected with a final
concentration of 100 nM Hspa1b siRNA or NC siRNA using the FuGENE
HD transfection reagent. Subsequently, the cells were incubated at
37°C for 36 h in an atmosphere containing 5% CO2 prior
to determination of knockdown efficiency by qPCR or infection with
ECTV (MOI, 1 or 5) for 24 h.
RT-qPCR assays
Total RNA was extracted from cellular or tissue
samples using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and first-strand cDNA was synthesized using
PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd.)
according to the manufacturer's protocol. The mRNA expression
levels of Hspa1b were detected using specific primers
(sense, 5′-TGG TGC AGT CCG ACA TGA AG-3′ and antisense,
5′-AGGTCGGTGTGAACGGATTTG-3′). GAPDH (sense,
5′-TCTTCCAGCCCTCCTTCCT-3′ and antisense,
5′-TGTAGACCATGTAGTTGAGGTCA-3′) was used as the internal
amplification control. qPCR was performed in a final volume of 20
µl containing 10 µl 2X SYBR-Green qPCR Master Mix
(Takara Biotechnology Co., Ltd.), 0.8 µl each primer (10
µM), 7.2 µl nuclease-free water and 2 µl cDNA.
PCR amplification was performed as described previously (30). Relative gene expression levels
were calculated using the 2−ΔΔCq method (31).
Virus titration
To determine viral titers at various time-points
post-infection, cells were lysed by three cycles of freeze-thawing
and the supernatant was clarified by centrifugation at 1,000 × g
for 10 min. Viral titers were determined in Vero cells according to
the TCID50 assay (32). Briefly, 2×104 Vero
cells in 100 µl DMEM supplemented with 10% FBS were seeded
in each well of a 96-well plate and incubated at 37°C in an
atmosphere containing 5% CO2. After 24 h of incubation,
25 µl serial 10-fold dilutions of viral samples were added
to each well, with eight replicates per dilution. The plates were
incubated for 7 days at 37°C and checked daily for characteristic
cytopathic effects. TCID50 end point titers were
calculated according to the Reed and Muench method (32).
Viral genome DNA copy number
measurements
Genomic DNA was extracted from the NIH3T3 cell
culture supernatants and whole-cell lysates using a viral RNA/DNA
extraction kit (Takara Biotechnology Co., Ltd.) according to the
manufacturer's protocol. ECTV genomic DNA copy numbers were
determined by qPCR using ECTV P4b gene specific primers: Forward,
5′-GTAGAACGACGCCAGAATAAGATA-3′ and reverse,
5′-AGAAGATATCAGACGATCCACAATC-3′. PCR amplification was performed as
described previously (33). A
standard curve was established from a cloned DNA fragment of the
ECTV P4b gene (33).
Quantification cycle values obtained by qPCR were plotted on the
standard curve to calculate the viral DNA copy number.
Western blot analysis
Protein expression levels of Hspa1b were determined
through the anti-FLAG tag by western blot analysis of total cell
proteins. Cells were lysed using radioimmunoprecipitation assay
buffer (Beyotime Institute of Biotechnology, Beijing, China) and
the protein concentration of cell lysates was determined using the
bicinchoninic acid (BCA) assay (QuantiPRO BCA Assay kit;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) according to the
manufacturer's protocol. Equal amounts of total protein (20
µg) were resolved by electrophoresis on 10% bis-Tris
polyacrylamide gels and transferred to polyvinylidene fluoride
membranes. Membranes were blocked for 1 h in 5% non-fat dry milk in
Tris-buffered saline with 0.1% Tween-20 (TBST) and were then probed
with the following primary antibodies: Anti-FLAG (F3165; 1:10,000;
Sigma-Aldrich; Merck KGaA) and anti-β-actin (60008-1; 1:2,000;
ProteinTech Group, Inc., Chicago, IL, USA). Membranes were
incubated with the primary antibodies diluted in 5% (wt/vol) bovine
serum albumin (Sangon Biotech Co., Ltd.) and 1X TSBT at 4°C
overnight. Antibody signals were detected by enhanced
chemiluminescence detection kit (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) following incubation with secondary antibodies
conjugated to horseradish peroxidase (A9044; 1:8,000;
Sigma-Aldrich; Merck KGaA) at room temperature for 30 min.
Quercetin and VER1550 08 treatment
Quercetin (Sigma-Aldrich; Merck KGaA) and VER155008
(VER; Sigma-Aldrich; Merck KGaA) were dissolved in dimethyl
sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA) to produce a 10 mM
stock solution, which was further diluted in DMEM supplemented with
2% FBS to the appropriate working concentrations. NIH3T3 or Vero
cells were seeded in 12-well plates and incubated at 37°C until the
cells reached 60–80% confluence. Subsequently, cells were infected
with ECTV at an MOI of 1 for 2 h, after which the media were
replaced with fresh media containing the appropriate concentrations
(10, 50 and 100 µM in NIH3T3 cells; 10, 20 and 50 µM
in Vero cells) of quercetin and VER. After 24 h incubation, cell
culture supernatants and whole-cell lysates were harvested for
virus titration. To investigate the effects of the two Hspa1b
inhibitors on viral growth kinetics, NIH3T3 cells were infected
with ECTV at an MOI of 1 for 2 h, after which the media were
replaced with fresh media containing 50 µM quercetin, VER,
DMSO or DMEM media (Mock). Subsequently, cell culture supernatants
and whole-cell lysates were harvested at the indicated time
points.
Cell viability assay
Cell viability was determined using the MTT Cell
Proliferation and Cytotoxicity Assay kit (Beyotime Institute of
Biotechnology). Briefly, NIH3T3 cells were incubated in a 96-well
plate and the medium was replaced with fresh medium containing
various concentrations of quercetin, VER or DMSO. After 24 h, cell
culture supernatants were replaced with 50 µl MTT (2
µg/ml) and incubated for 4 h at 37°C. Subsequently, 100
µl formazan-dissolving solution (Beyotime Institute of
Biotechnology) was added to each well and incubated at 37°C until
the crystals dissolved. Finally, the absorbance of the cell
solution was measured using a universal microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) at 570 nm wavelength.
Statistical analysis
Data are presented as the means ± standard deviation
from at least three independent experiments and were analyzed using
Student's t-test, or one-way analysis of variance followed by
Duncan's multiple range test. Statistical analyses were conducted
using SPSS software (SPSS 18.0 for Windows; SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
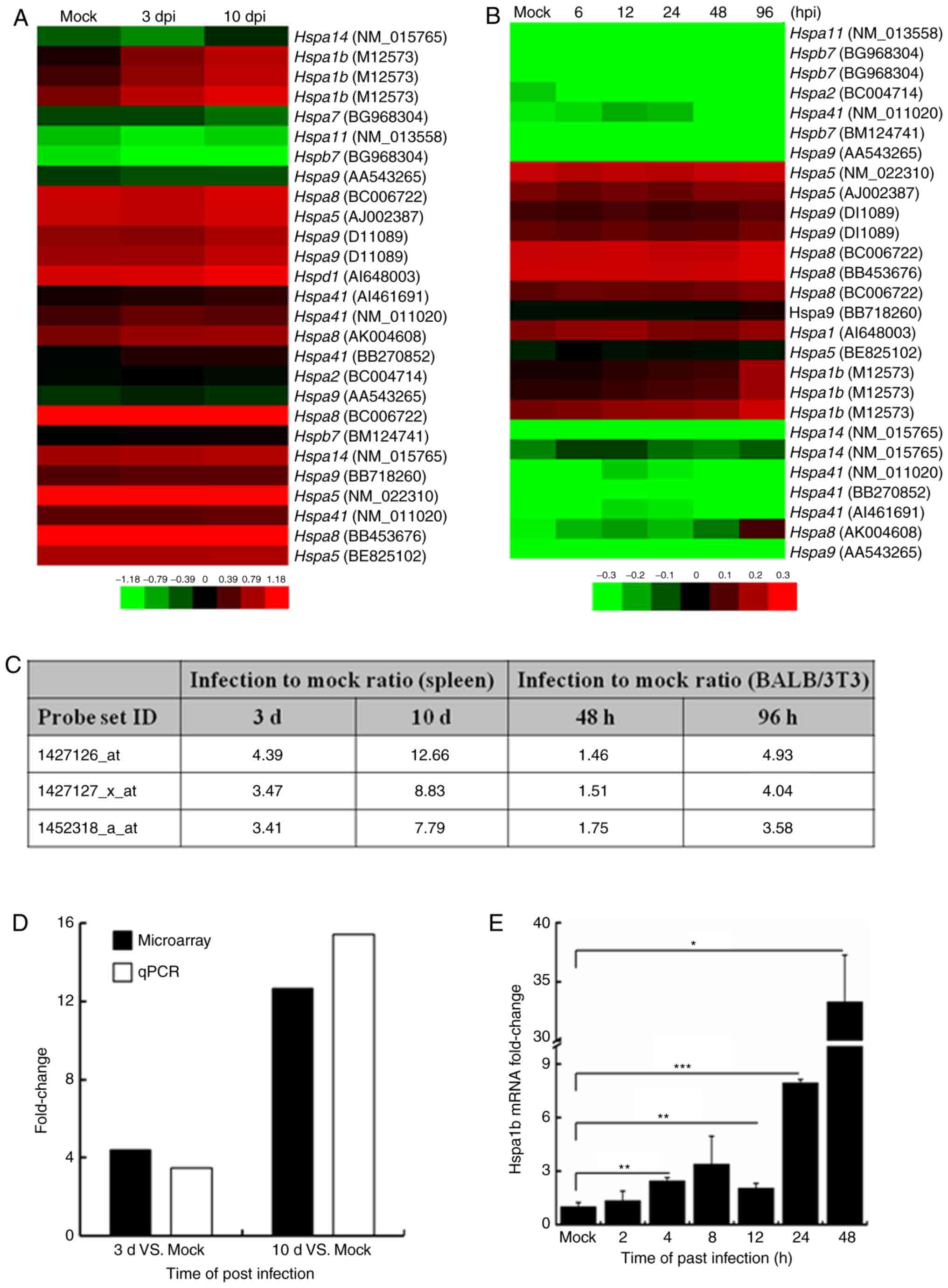
Elevated Hsp70 expression during ECTV
infection in vitro and in vivo
ECTV has a restricted host range and infection of
BALB/c mice with ECTV is associated with a high mortality rate. The
natural route of infection is believed to be through abrasions in
the skin, after which the virus uncontrollably replicates in the
spleen and the liver (10). In
the present study, infected mice began to exhibit disease symptoms
starting at 7 days post-infection (dpi) and one mouse succumbed to
the disease at 10 dpi (data not shown). To elucidate alterations in
the host transcriptome profile caused by ECTV infection, microarray
analysis was used to examine the spleen cells from ECTV-infected
BALB/c mice and BALB/3T3 cells. Following normalization, the
results demonstrated that Hspa1b was one of the most
upregulated genes in spleen tissues of infected mice and BALB/3T3
cells (30). To examine whether
other isoforms of the Hsp70 family were upregulated during ECTV
infection, the differential expression of 10 isoforms of the HSP70
family contained in the genechip were analyzed. Compared with in
the mock control groups, the heat maps demonstrated that the
expression levels of numerous Hsp70 isoforms were induced in
vivo (Fig. 1A) and in
vitro (Fig. 1B), including
Hspa1b, Hspa5, Hspa8 and Hspa9. To
examine the level of induction, the ratio of Hsp70 isoform mRNA
expression levels in the infected samples compared with the mock
control samples was determined; the results indicated that
Hspa1b was the most highly induced isoform in vivo
and in vitro (Fig. 1C). To
validate the microarray data, the transcription levels of
Hspa1b were determined in the spleen tissues by RT-qPCR
analysis. As shown in Fig. 1D,
the results of RT-qPCR were consistent with the microarray data.
Furthermore, the transcription levels of Hspa1b were
detected in NIH3T3 cells at various time-points. As demonstrated in
Fig. 1E, the expression levels of
Hspa1b were upregulated post-infection; the highest
expression was detected at 48 hpi.
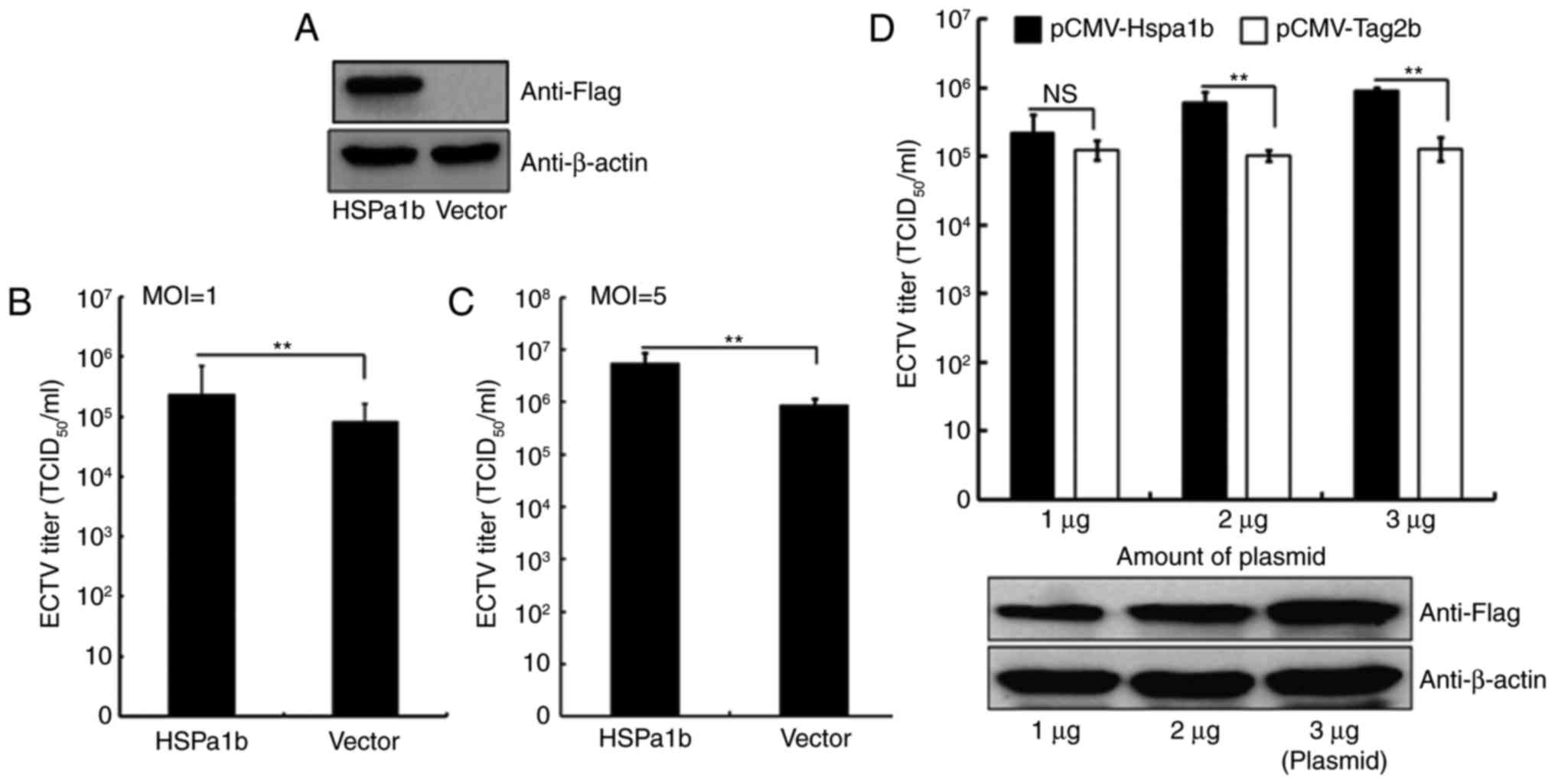
Overexpression of Hspa1b enhances ECTV
replication
To investigate the effects of Hspa1b on ECTV
replication, Hspa1b was overexpressed in NIH3T3 cells using the
pCMV-Hspa1b plasmid; the empty pCMV-Tag2b vector was used as a
negative control. A total of 24 h post-transfection, cells were
infected with ECTV (MOI, 1 or 5), and the protein expression levels
of FLAG (Fig. 2A) and viral
titers were determined. As shown in Fig. 2B, the viral yields from
Hspa1b-transfected cells were significantly higher compared with
the control cells. Cells overexpressing Hspa1b that were infected
with ECTV at an MOI of 5 also exhibited a significantly higher
viral yield (Fig. 2C). To further
confirm the positive effects of Hspa1b on ECTV replication, NIH3T3
cells were transfected with various amounts of pCMV-Hspa1b plasmid,
followed by ECTV infection (MOI, 1). As shown in Fig. 2D (lower panel), the protein
expression levels of FLAG were increased with the amount of
transfected pCMV-Hspa1b plasmid. The results demonstrated that
Hspa1b enhanced ECTV replication in a dose-dependent manner
(Fig. 2D, upper panel).
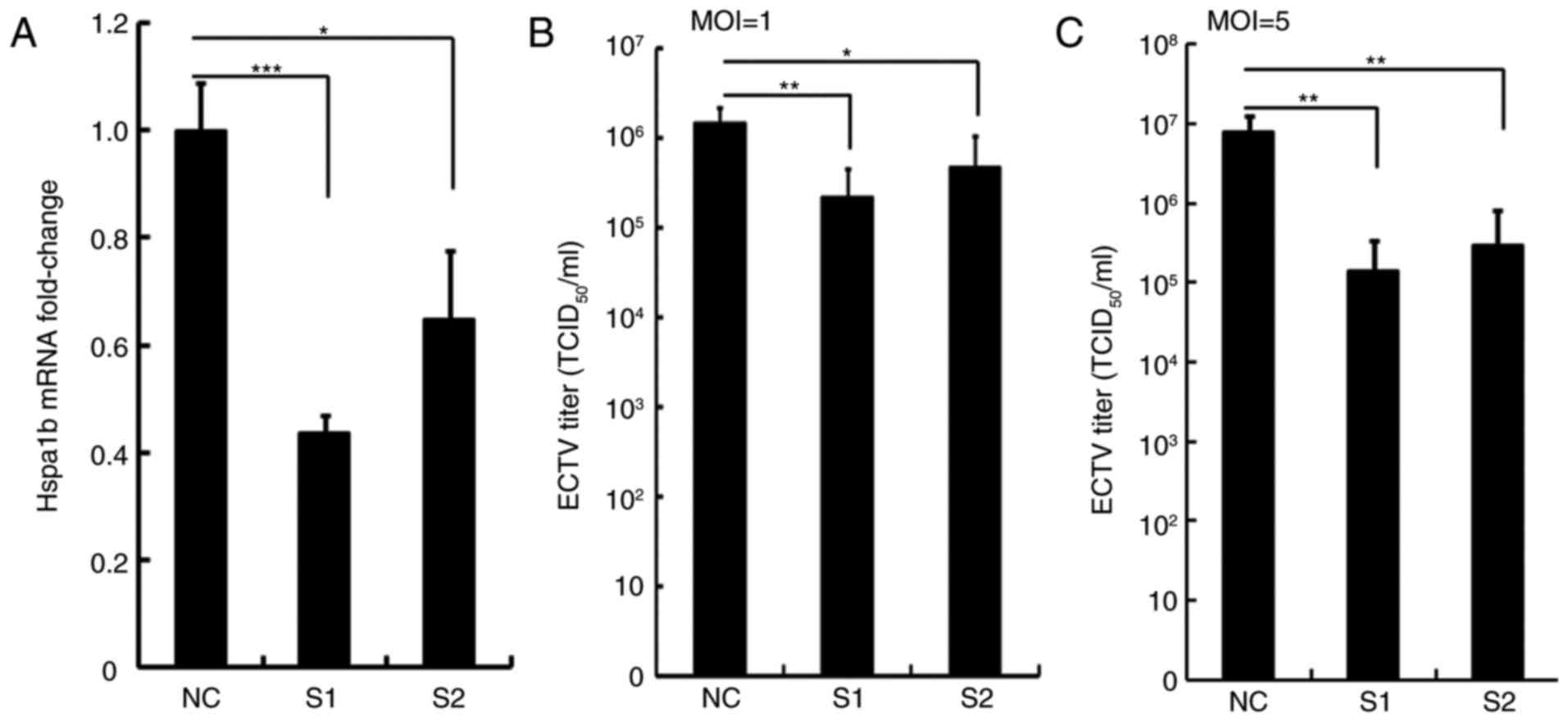
RNAi-mediated depletion of Hspa1b reduces
ECTV viral yields
To further investigate the effects of Hspa1b on ECTV
replication, Hspa1b expression in NIH3T3 cells was
suppressed following transfection with siRNAs for 36 h; the effects
of Hspa1b knockdown on viral yield were subsequently determined.
NIH3T3 cells were transfected with siRNAs, which either targeted
the Hspa1b sequence (S1 and S2) or a nonspecific sequence (NC), and
knockdown efficiency was confirmed by RT-qPCR analysis. siRNAs that
targeted Hspa1b significantly reduced Hspa1b mRNA expression
compared with in cells transfected with NC siRNA; knockdown with S1
was more pronounced compared with in cells transfected with S2 (57%
for S1 and 36% for S2; Fig. 3A).
Silencing Hspa1b with two siRNAs significantly impaired ECTV
replication at 24 hpi. A marked decrease in viral yield was
detected in Hspa1b siRNA-transfected cells following infection with
two doses of ECTV compared with in the control group (Fig. 3B and C).
Inhibitors of Hspa1b suppress ECTV
replication
To further examine the importance of Hspa1b in ECTV
replication, the effects of two Hspa1b inhibitors, quercetin and
VER, were determined on ECTV replication. Quercetin is one of the
most ubiquitous flavonoids, and as a Hsp inhibitor, its function is
not limited to Hsp70 (34,35).
VER is a small-molecule inhibitor of Hsp70, which can specifically
bind to the ATPase domain of Hsp70 consequently inhibiting its
function (36). In order to
assess the effects of quercetin and VER on ECTV replication, the
present study examined their cytotoxic effects on NIH3T3 and Vero
cells by MTT assay. NIH3T3 or Vero cells were incubated with
various concentrations of quercetin and VER. As shown in Fig. 4A, the viability of NIH3T3 cells
was not significantly affected by treatment with ≤100 µM
quercetin and VER. In addition, no cytotoxicity was detected
following treatment of Vero cells with ≤50 µM quercetin and
VER, whereas higher concentrations of quercetin and VER exhibited
cytotoxic effects in Vero cells (Fig.
4B). Therefore, the present study determined the ability of VER
and quercetin at 10, 20 and 50 µM to interfere with viral
replication in Vero, and VER and quercetin at 10, 50 and 100
µM in NIH3T3 cells. Treatment of ECTV-infected NIH3T3 and
Vero cells with various doses of quercetin or VER for 22 h resulted
in a reduction of viral replication in a dose-dependent manner
(Fig. 4C and D). In addition,
treatment with 100 µM quercetin in NIH3T3 cells led to a
significant reduction (~70-fold) in viral replication compared with
in the DMSO-treated control group. Under the same conditions, VER
(100 µM) only inhibited viral replication by ~15-fold. A
similar inhibition was observed in Vero cells (Fig. 4D).
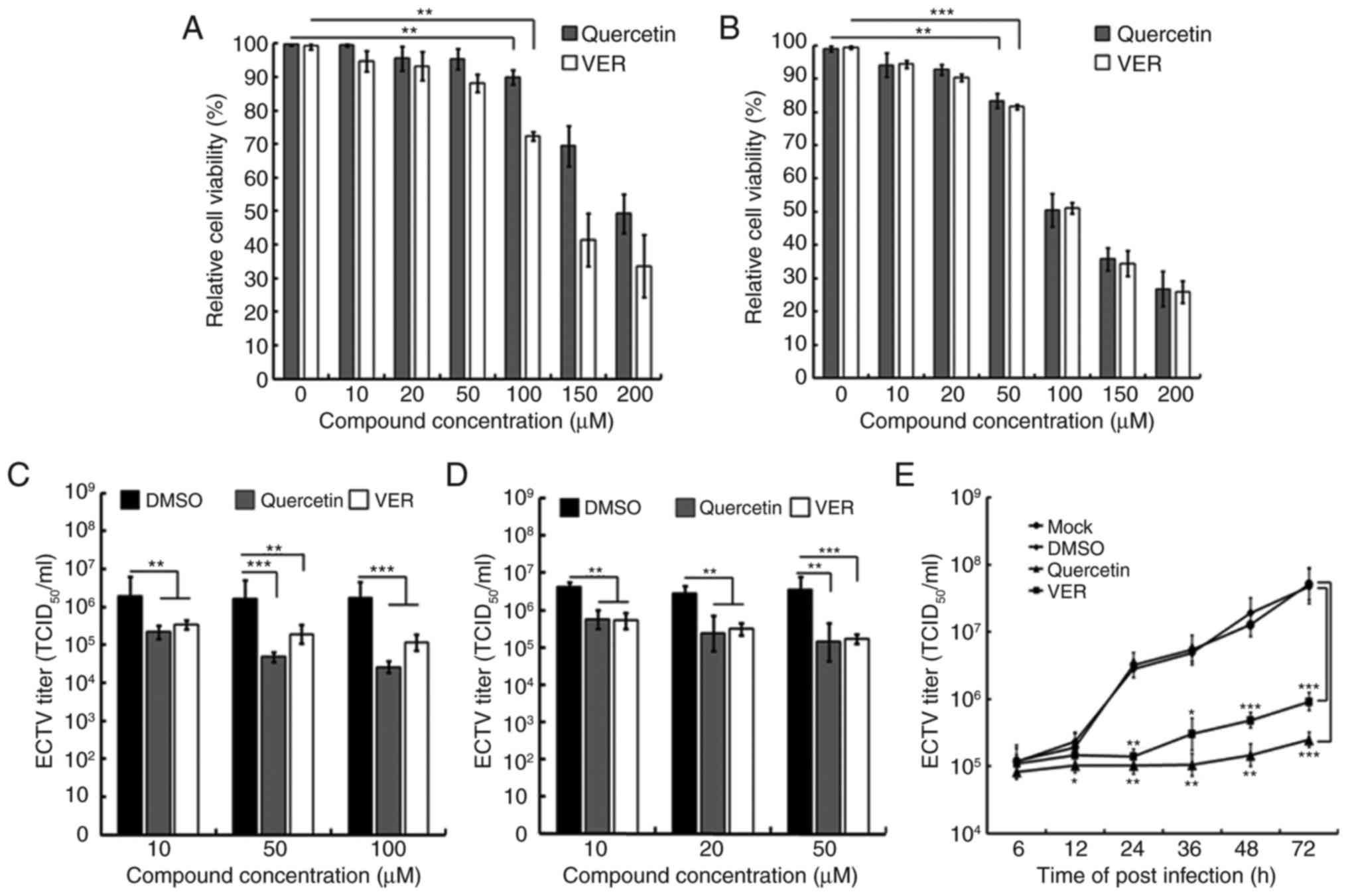 | Figure 4Effects of Hsp70 inhibitors VER and
quercetin on ECTV replication. Cytotoxicity of quercetin and VER in
(A) NIH3T3 or (B) Vero cells, as assessed by MTT assay following
treatment with various concentrations of quercetin and VER for 24 h
in the absence of ECTV. Effects of quercetin and VER treatment on
ECTV replication in (C) NIH3T3 or (D) Vero cells were determined by
TCID50, which revealed a reduction in ECTV titers
following treatment with subcytotoxic concentrations of quercetin
and VER. (E) NIH3T3 cells incubated with ECTV (multiplicity of
infection, 1) for 2 h were treated with 50 µM quercetin, 50
µM VER, DMSO control or mock treatment. Samples were
collected at several time-points (6, 12, 24, 36, 48 and 72 hpi) for
virus titration. *P<0.05; **P<0.01;
***P<0.01. DMSO, dimethyl sulfoxide; ECTV, ectromelia
virus; TCID50, 50% tissue culture infective dose; VER,
VER155008. |
To further assess the effects of the two Hspa1b
inhibitors on viral growth kinetics, NIH3T3 cells were infected
with ECTV at an MOI of 1 for 2 h, after which the media were
replaced with fresh media containing 50 µM quercetin or VER.
Infected cells and supernatants were harvested for virus titration
at 6, 12, 24, 36, 48 and 72 hpi. Compared with in the DMSO-treated
cells, the viral titer of quercetin- or VER-treated cells began to
reduce at 12 hpi; quercetin and VER treatment reduced viral titers
by ~2.3- and 1.7-fold, respectively. At 72 hpi, the effects were
enlarged to ~200-fold in quercetin-treated cells and ~52-fold in
VER-treated cells (Fig. 4E).
These results further confirmed the importance of Hspa1b in ECTV
replication and indicated that quercetin is a much more potent
inhibitor than VER in NIH3T3 cells.
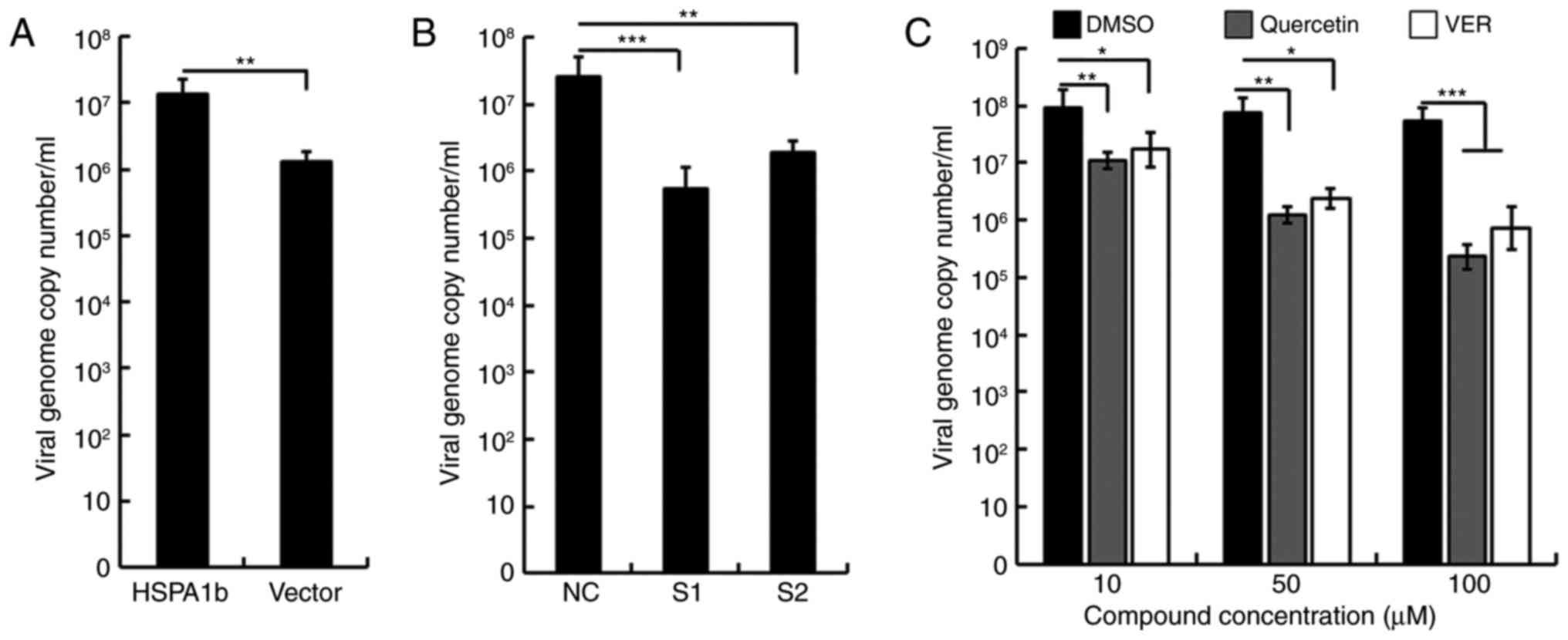
Cellular Hspa1b promotes viral DNA
replication
It has previously been reported that Hsp70 is
required in the genome replication of numerous viruses (18,37–39). To investigate whether Hspa1b
affects the genome replication of ECTV, the present study examined
the viral genome copy number of ECTV following overexpression or
knockdown of Hspa1b. The viral genome copy number was significantly
increased when Hspa1b was overexpressed in NIH3T3 cells (Fig. 5A). Cells transfected with two
Hspa1b siRNAs exhibited a marked decrease in viral genome copy
number compared with in the NC control group (Fig. 5B). Consistent with the viral
yields presented in Fig. 3B, the
viral genome was more impaired in S1-transfected cells compared
with in cells transfected with S2. Furthermore, the present study
determined the effects of quercetin and VER on viral genome
replication. A significant decrease in viral genome copy number was
observed in a dose-dependent manner, with ~30 and 25% reductions in
viral genome following treatment with 100 µM quercetin and
VER, respectively (Fig. 5C).
Taken together, these results suggested that cellular Hspa1b may
promote ECTV DNA replication. However, due to underdeveloped
technological skills and unavailable ECTV antibodies, the exact
mechanisms underlying how Hspa1b affects ECTV replication require
further study.
Discussion
ECTV infection in mice is a valuable model to
investigate the interplay between Orthopoxvirus species and
their hosts, such as the host immune response to poxvirus
infection, the host genetic control of antiviral resistance and the
development of small-molecule antivirals (40,41). The present study used microarrays
to examine the host gene expression profile in response to ECTV
infection in vivo and in vitro. The results of the
genetic analysis demonstrated that Hspa1b was one of the
most significantly upregulated genes at various time-points. To
validate the microarray data, RT-qPCR was performed to quantify the
mRNA expression levels of Hspa1b using the same samples as
those used for microarray analysis. In in vivo and in
vitro samples, RT-qPCR analysis confirmed the results of the
microarray analysis, in that Hspa1b was significantly upregulated
during ECTV replication. Therefore, these observations suggested
that Hspa1b may have an important role in ECTV replication.
Previous studies regarding the transcriptome of host cells during
VACV infection also indicated that Hspa1b was upregulated,
and data from RNAi screening suggested that Hspa1b may have a
necessary role in Orthopoxvirus infection, although the
candidate genes were not further validated (42–44).
The Hsps form an evolutionarily well-conserved
family of regulatory proteins, which are classified according to
their molecular weight; e.g. Hsp40, Hsp60, Hsp70, Hsp90, Hsp100,
Hsp110 and small Hsps (<34 kDa) (12). The Hsp70 family contains four
major isoforms: The constitutively expressed heat shock cognate 70
(Hsc70), the stress-inducible Hsp70, the endoplasmic reticulum
resident glucose-regulated protein (Grp)78 and the mitochondrial
form Grp75 (45). These chaperone
proteins are involved not only in cellular protein quality control,
but also in response to stress conditions. Growing evidence has
indicated that members of the Hsp70 family functionally interact
with viral proteins to regulate viral replication (15,21–23,36). The majority of previous studies
have focused on Hsp70/Hsc70, which positively regulates viral
replication through the transcription and replication of viral
RNA/DNA. Viruses use Hsp70 isoforms to aid in several stages of
viral replication, including viral entry, transcription, envelope
protein maturation, morphogenesis or DNA replication (46). Furthermore, the antiviral ability
of Hsp70, via the innate immune system, has also been detected in a
mouse model of measles virus brain infection and a model of
vesicular stomatitis virus infection (28,47). To investigate the functional
effects of Hspa1b on ECTV replication, the present study employed
plasmid-mediated overexpression and siRNA-mediated gene silencing
of Hspa1b in NIH3T3 cells. The results confirmed that Hspa1b
expression may benefit ECTV replication in a dose-dependent manner.
Viral genome copy number was altered by overexpression or
suppression of Hspa1b in NIH3T3 cells, thus suggesting a role for
Hspa1b in viral DNA replication. However, the role of this
chaperone in other aspects of the viral life cycle and the exact
mechanisms by which HSPa1b affects replication of ECTV require
further exploration. Data from VACV infection models have provided
evidence to suggest that Hspa1b is upregulated and heat shock
factor 1, which is a regulator of Hsp70, is critical for
Orthopoxvirus replication (44). Furthermore, a previous report
established that Hsp90 was required for VACV to grow in cultured
cells (48). Hsp90 interacts with
VACV core protein 4a (A10L) and colocalizes with the viral factory
during specific stages of the viral lifecycle (48). Hsp70 family proteins favor viral
replication through interaction with viral proteins at several
stages of viral replication. For example, Hsp72/Hsp70 interacts
with NS5 proteins of members of the Flaviviridae family,
such as classical swine fever virus, Japanese encephalitis virus
and dengue virus (22–23,49). The N protein of Crimean-Congo
hemorrhagic fever virus and PB2/PB1 protein of influenza A viruses
can also interact with Hsp70 to enhance viral replication,
respectively (15,26,36). It remains to be identified which
ECTV proteins may interact with Hsp70 or other chaperones to
facilitate viral replication.
To further confirm the effects of Hspa1b on ECTV
replication, the present study analyzed the effects of two
Hsps/Hsp70 inhibitors, quercetin and VER, on ECTV replication.
Treatment with these inhibitors suppressed viral replication in a
dose-dependent manner, and the effects of quercetin at the same
concentration were more noticeable. One of the possible
explanations is that besides Hsp70, quercetin may also inhibit the
function of other Hsp members, which may be involved in ECTV
infection, whereas VER only targets the Hsp70 family.
In conclusion, the present study identified a host
cell protein, Hspa1b, which may promote ECTV replication. Notably,
the Hsp chaperone involved in ECTV replication is a potential
target for the development of antiviral therapies, since two Hsp70
inhibitors, quercetin and VER, were able to significantly suppress
ECTV replication in cell culture.
Abbreviations:
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
ECTV
|
ectromelia virus
|
|
FBS
|
fetal bovine serum
|
|
hpi
|
hours post-infection
|
|
Hsc70
|
heat shock cognate 70
|
|
Hsp
|
heat shock protein
|
|
MOI
|
multiplicity of infection
|
|
MPXV
|
monkeypox virus
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
RNAi
|
RNA interference
|
|
VARV
|
variola virus
|
|
VACV
|
vaccinia virus
|
|
TCID50
|
50% tissue culture infective dose
|
Acknowledgments
The authors of the present study would like to thank
Miss Yang Yang (Lanzhou Veterinary Research Institute, Chinese
Academy of Agricultural Sciences, Lanzhou, China) for help with
data analysis and Professor Dingxiang Liu (School of Biological
Sciences, Nanyang Technological University, Singapore, Singapore)
for technical assistance.
Funding
The present study was supported by grants from the
Fundamental Research Funds for the Lanzhou Veterinary Research
Institute (grant no. 1610312016019) and the Agricultural Science
and Technology Innovation Program (grant no.
CAAS-ASTIP-2016-LVRI-06).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
WC, ZJ, JC and XW designed the study and critically
revised the manuscript. WC, HJ, XH and QJ performed the
experiments, analyzed the data and drafted the manuscript.
Ethics approval and consent to
participate
All mice were handled in accordance with the Good
Animal Practice Requirements of the Animal Ethics Procedures and
Guidelines of the People's Republic of China. The present study was
reviewed and approved by the Animal Ethics Committee of Lanzhou
Veterinary Research Institute, Chinese Academy of Agricultural
Science (Lanzhou, China; permit no. LVRIAEC2016-005).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Puissant B and Combadière B: Keeping the
memory of smallpox virus. Cell Mol Life Sci. 63:2249–2259. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Doellinger J, Schaade L and Nitsche A:
Comparison of the Cowpox virus and Vaccinia virus mature virion
proteome: Analysis of the species- and strain-specific proteome.
PLoS One. 10:e01415272015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Panchanathan V, Chaudhri G and Karupiah G:
Correlates of protective immunity in poxvirus infection: Where does
antibody stand? Immunol Cell Biol. 86:80–86. 2008. View Article : Google Scholar
|
|
4
|
Damon IK, Damaso CR and McFadden G: Are we
there yet? The smallpox research agenda using variola virus. PLoS
Pathog. 10:e10041082014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reardon S: 'Forgotten' NIH smallpox virus
languishes on death row. Nature. 514:5442014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Quiner CA, Moses C, Monroe BP, Nakazawa Y,
Doty JB, Hughes CM, McCollum AM, Ibata S, Malekani J, Okitolonda E,
et al: Presumptive risk factors for monkeypox in rural communities
in the Democratic Republic of the Congo. PLoS One. 12:e01686642017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Esteban DJ and Buller RM: Ectromelia
virus: The causative agent of mousepox. J Gen Virol. 86:2645–2659.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chapman JL, Nichols DK, Martinez MJ and
Raymond JW: Animal models of orthopoxvirus infection. Vet Pathol.
47:852–870. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Esteban D, Parker S, Schriewer J, Hartzler
H and Buller RM: Mousepox, a small animal model of smallpox.
Methods Mol Biol. 890:177–198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sigal LJ: The pathogenesis and
immunobiology of mousepox. Adv Immunol. 129:251–276. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garver J, Weber L, Vela EM, Anderson M,
Warren R, Merchlinsky M, Houchens C and Rogers JV: Ectromelia virus
disease characterization in the BALB/c mouse: A surrogate model for
assessment of smallpox medical countermeasures. Viruses.
8:E2032016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Temajo NO and Howard N: The virus-induced
HSPs regulate the apoptosis of operatus APCs that results in
autoimmunity, not in homeostasis. Autoimmun Rev. 13:1013–1019.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Makhoba XH, Burger A, Coertzen D, Zininga
T, Birkholtz LM and Shonhai A: Use of a chimeric Hsp70 to enhance
the quality of recombinant Plasmodium falciparum
s-adenosylmethionine decarboxylase protein produced in Escherichia
coli. PLoS One. 11:e01526262016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Burch AD and Weller SK: Nuclear
sequestration of cellular chaperone and proteasomal machinery
during herpes simplex virus type 1 infection. J Virol.
78:7175–7185. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Manzoor R, Kuroda K, Yoshida R, Tsuda Y,
Fujikura D, Miyamoto H, Kajihara M, Kida H and Takada A: Heat shock
protein 70 modulates influenza A virus polymerase activity. J Biol
Chem. 289:7599–7614. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Howe MK, Speer BL, Hughes PF, Loiselle DR,
Vasudevan S and Haystead TA: An inducible heat shock protein 70
small molecule inhibitor demonstrates anti-dengue virus activity,
validating Hsp70 as a host antiviral target. Antiviral Res.
130:81–92. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brown G, Rixon HW, Steel J, McDonald TP,
Pitt AR, Graham S and Sugrue RJ: Evidence for an association
between heat shock protein 70 and the respiratory syncytial virus
polymerase complex within lipid-raft membranes during virus
infection. Virology. 338:69–80. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mayer MP: Recruitment of Hsp70 chaperones:
A crucial part of viral survival strategies. Rev Physiol Biochem
Pharmacol. 153:1–46. 2005. View Article : Google Scholar
|
|
19
|
Kaufer S, Coffey CM and Parker JS: The
cellular chaperone hsc70 is specifically recruited to reovirus
viral factories independently of its chaperone function. J Virol.
86:1079–1089. 2012. View Article : Google Scholar :
|
|
20
|
Cobbold C, Windsor M and Wileman T: A
virally encoded chaperone specialized for folding of the major
capsid protein of African swine fever virus. J Virol. 75:7221–7229.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alam SB and Rochon D: Evidence that Hsc70
is associated with Cucumber necrosis virus particles and plays a
role in particle disassembly. J Virol. 91:e01555–16. 2017.
View Article : Google Scholar :
|
|
22
|
Taguwa S, Maringer K, Li X, Bernal-Rubio
D, Rauch JN, Gestwicki JE, Andino R, Fernandez-Sesma A and Frydman
J: Defining Hsp70 subnetworks in Dengue virus replication reveals
key vulnerability in Flavivirus infection. Cell. 163:1108–1123.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang C, Kang K, Ning P, Peng Y, Lin Z,
Cui H, Cao Z, Wang J and Zhang Y: Heat shock protein 70 is
associated with CSFV NS5A protein and enhances viral RNA
replication. Virology. 482:9–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ye J, Chen Z, Zhang B, Miao H, Zohaib A,
Xu Q, Chen H and Cao S: Heat shock protein 70 is associated with
replicase complex of Japanese encephalitis virus and positively
regulates viral genome replication. PLoS One. 8:e751882013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hirayama E, Atagi H, Hiraki A and Kim J:
Heat shock protein 70 is related to thermal inhibition of nuclear
export of the influenza virus ribonucleoprotein complex. J Virol.
78:1263–1270. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li G, Zhang J, Tong X, Liu W and Ye X:
Heat shock protein 70 inhibits the activity of Influenza A virus
ribonucleoprotein and blocks the replication of virus in vitro and
in vivo. PLoS One. 6:e165462011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim MY and Oglesbee M: Virus-heat shock
protein interaction and a novel axis for innate antiviral immunity.
Cells. 1:646–666. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim MY, Shu Y, Carsillo T, Zhang J, Yu L,
Peterson C, Longhi S, Girod S, Niewiesk S and Oglesbee M: Hsp70 and
a novel axis of type I interferon-dependent antiviral immunity in
the measles virus-infected brain. J Virol. 87:998–1009. 2013.
View Article : Google Scholar :
|
|
29
|
Li T, He X, Jia H, Chen G, Zeng S, Fang Y,
Jin Q and Jing Z: Molecular cloning and functional characterization
of murine toll-like receptor 8. Mol Med Rep. 13:1119–1126. 2016.
View Article : Google Scholar :
|
|
30
|
Cheng WY, Jia HJ, He XB, Chen GH, Feng Y,
Wang CY, Wang XX and Jing ZZ: Comparison of host gene expression
profiles in spleen tissues of genetically susceptible and resistant
mice during ECTV infection. Biomed Res Int. 2017:64561802017.
View Article : Google Scholar
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
32
|
Reed LJ and Muench H: A simple method of
estimating 50 percent endpoints. Am J Hyg. 27:493–497. 1938.
|
|
33
|
Cheng W, He X, Jia H, Chen G, Wang C,
Zhang J and Jing Z: Development of a SYBR Green I real-time PCR for
detection and quantitation of orthopoxvirus by using Ectromelia
virus. Mol Cell Probes. 38:45–50. 2018. View Article : Google Scholar
|
|
34
|
Gonzalez O, Fontanes V, Raychaudhuri S,
Loo R, Loo J, Arumugaswami V, Sun R, Dasgupta A and French SW: The
heat shock protein inhibitor quercetin attenuates hepatitis C virus
production. Hepatology. 50:1756–1764. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu J, Bai J, Zhang L, Jiang Z, Wang X, Li
Y and Jiang P: Hsp70 positively regulates porcine circovirus type 2
replication in vitro. Virology. 447:52–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Surtees R, Dowall SD, Shaw A, Armstrong S,
Hewson R, Carroll MW, Mankouri J, Edwards TA, Hiscox JA and Barr
JN: Heat shock protein 70 family members interact with
Crimean-Congo Hemorrhagic Fever virus and Hazara virus nucleocapsid
proteins and perform a functional role in the nairo-virus
replication cycle. J Virol. 90:9305–9316. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tanguy Le Gac N and Boehmer PE: Activation
of the herpes simplex virus type-1 origin-binding protein (UL9) by
heat shock proteins. J Biol Chem. 277:5660–5666. 2002. View Article : Google Scholar
|
|
38
|
Pogany J, Stork J, Li Z and Nagy PD: In
vitro assembly of the Tomato bushy stunt virus replicase requires
the host Heat shock protein 70. Proc Natl Acad Sci USA.
105:19956–19961. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Baquero-Pérez B and Whitehouse A: Hsp70
isoforms are essential for the formation of Kaposi's
sarcoma-associated Herpesvirus replication and transcription
compartments. PLoS Pathog. 11:e10052742015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Smee DF: Orthopoxvirus inhibitors that are
active in animal models: An update from 2008 to 2012. Future Virol.
8:891–901. 2013. View Article : Google Scholar
|
|
41
|
Parker S, Crump R, Foster S, Hartzler H,
Hembrador E, Lanier ER, Painter G, Schriewer J, Trost LC and Buller
RM: Co-administration of the broad-spectrum antiviral,
brincidofovir (CMX001), with smallpox vaccine does not compromise
vaccine protection in mice challenged with ectromelia virus.
Antiviral Res. 111:42–52. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mercer J, Snijder B, Sacher R, Burkard C,
Bleck CK, Stahlberg H, Pelkmans L and Helenius A: RNAi screening
reveals protea-some- and Cullin3-dependent stages in vaccinia virus
infection. Cell Rep. 2:1036–1047. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bourquain D, Dabrowski PW and Nitsche A:
Comparison of host cell gene expression in cowpox, monkeypox or
vaccinia virus-infected cells reveals virus-specific regulation of
immune response genes. Virol J. 10:612013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Filone CM, Caballero IS, Dower K, Mendillo
ML, Cowley GS, Santagata S, Rozelle DK, Yen J, Rubins KH, Hacohen
N, et al: The master regulator of the cellular stress response
(HSF1) is critical for orthopoxvirus infection. PLoS Pathog.
10:e10039042014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Frydman J: Folding of newly translated
proteins in vivo: The role of molecular chaperones. Annu Rev
Biochem. 70:603–647. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yu A, Li P, Tang T, Wang J, Chen Y and Liu
L: Roles of Hsp70s in stress responses of microorganisms, plants,
and animals. Biomed Res Int. 2015:5103192015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kim MY, Ma Y, Zhang Y, Li J, Shu Y and
Oglesbee M: hsp70-dependent antiviral immunity against cytopathic
neuronal infection by vesicular stomatitis virus. J Viro.
187:10668–10678. 2013. View Article : Google Scholar
|
|
48
|
Hung JJ, Chung CS and Chang W: Molecular
chaperone Hsp90 is important for vaccinia virus growth in cells. J
Virol. 76:1379–1390. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Khachatoorian R, Ganapathy E, Ahmadieh Y,
Wheatley N, Sundberg C, Jung CL, Arumugaswami V, Raychaudhuri S,
Dasgupta A and French SW: The NS5A-binding heat shock proteins
HSC70 and HSP70 play distinct roles in the hepatitis C viral life
cycle. Virology. 454–455:118–127. 2014. View Article : Google Scholar
|