Introduction
With the continuous improvement of living standards
and the accelerated pace of life, the morbidity and mortality rates
of coronary atherosclerotic heart disease are increasing (1). The World Health Organization has
indicated that coronary heart disease has become the leading cause
of mortality in the world, and that acute myocardial infarction
(AMI) is the leading cause of coronary heart disease-related
mortality (2). With the
continuous development of treatment, the morbidity rate of heart
failure resulting from ventricular remodeling and of arrhythmia due
to sympathetic remodeling is gradually increasing, while the
acute-phase mortality rate of AMI is gradually decreasing (3,4).
Even though patients may receive optimized medical treatment, the
overall mortality rate remains high.
Myocardial apoptosis is an autonomous process of
programmed cell death in a series of gene regulation, of which the
essence is physiological cell death (5). Myocardial apoptosis exists in the
cardiovascular system, particularly during the physiological and
pathological changes of AMI, and is an important cellular basis for
a variety of cardiovascular diseases (6). The inhibition or reduction of
myocardial apoptosis can reflect ventricular remodeling and cardiac
function recovery following AMI, so the study of myocardial
apoptosis is essential to evaluate the post-myocardial infarction
heart function and associated drug efficacy (7).
AMI leads to an abnormal cellular environment due to
an insufficient energy supply. The increase in cardiac compensatory
contraction results in an elevated reactive oxygen species (ROS)
level caused by membrane nicotinamide adenine dinucleotide
phosphate (8). More seriously,
the elevated ROS level triggers mitochondria to generate a large
amount of oxidative stress (9).
Oxidative stress can not only attack the cell membrane and
organelles, but also cause the inflammatory response through mutual
reinforcement with inflammatory cytokines, to further aggravate
myocardial injury caused by myocardial infarction (10).
Heart inflammation can be summarized as a simple
natural immune response and/or the combination of a natural immune
response and an acquired immune response (11). The most typical characteristic of
a natural immune response is the induction of inflammatory cytokine
generation. In myocardial ischemia and heart failure, a natural
immune response and an inflammatory response usually occur
(12).
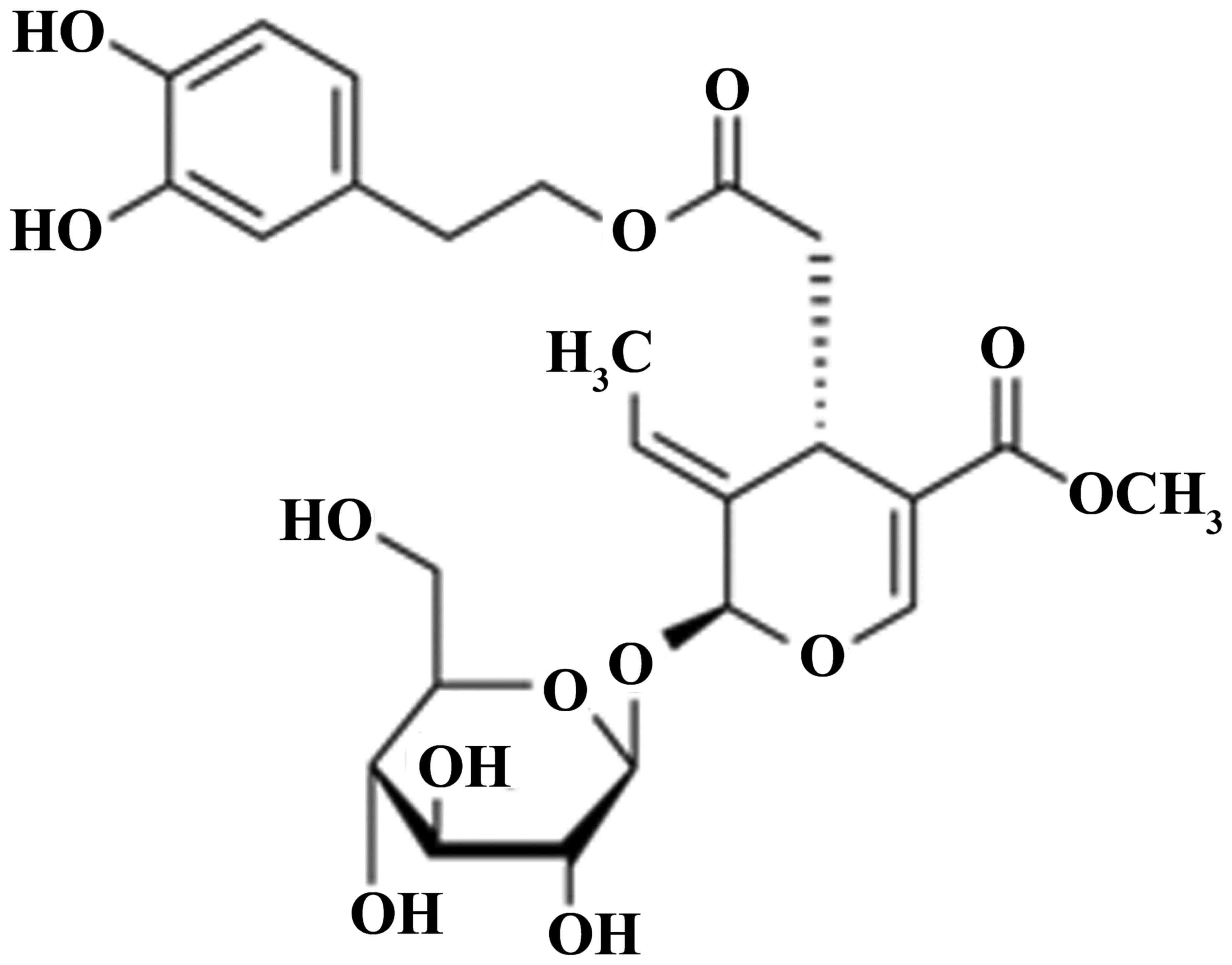
Oleuropein (Fig.
1) is a non-toxic split iridoid glycoside compound. Oleuropein
increases the coronary blood flow of the rabbit isolated heart by
50%, indicating antiarrhythmic and antispasmodic effects (13). Oleuropein obtained by the
hydrolysis of olive leaf extract has antihypertensive effect
(14). In addition, oleuropein is
a strong angiotensin-converting enzyme inhibitor, for which the
inhibitory effect is a result of the inherent 2,3-dihydroxy
glutaraldehyde structures and their high reactivity (15). The corresponding aglycone produced
by the enzymatic hydrolysis shows a similar effect to oleuropein,
which has long-lasting hypotensive effect on rats, cats and dogs
(16). Therefore, the present
study aimed to investigate the protective effect and mechanisms of
oleuropein in myocardial ischemia/reperfusion (I/R), and the
possible role of extracellular signal-regulated protein kinase
(ERK) signaling in the protective effects of oleuropein in
myocardial I/R injury.
Materials and methods
Animals
All procedures were performed in accordance with the
National Research Council's Guide of HARRISON International Peace
Hospital, Hebei Medical University (Hengshu, Hebei, China) for
Humane Care and Use of Laboratory Animals. All animal experiments
were approved by the Medical Ethics Committee of HARRISON
International Peace Hospital. Adult, male, Sprague-Dawley rats
weighing 250–300 g were purchased from Hebei Medical University and
maintained in controlled conditions of 22±2°C and 60–70% humidity
under a 12 h light-dark cycle (7:00 a.m. to 7:00 p.m.). Food and
water were available ad libitum.
In vivo myocardial I/R model and
experimental groups
Firstly, a total of 26 rats (age, 5–6 weeks; weight,
200–250 g) were randomized into 3 groups: i) Control group (n=6):
rats subjected to the surgical procedures without coronary
occlusion; ii) myocardial I/R model group (n=10): 30 min of
coronary occlusion followed by 3 h of reperfusion; and iii) Ole
(20) group (n=10): 20 mg/kg of
oleuropein for 2 consecutive days, then 30 min of coronary
occlusion followed by 3 h of reperfusion.
Next, a total of 36 rats (age, 5–6 weeks; weight,
200–250 g) were randomized into 4 groups: i) Control group (n=6):
rats subjected to the surgical procedures without coronary
occlusion; ii) myocardial I/R model group (n=10): 30 min of
coronary occlusion followed by 3 h of reperfusion; iii) Ole
(20) group (n=10): 20 mg/kg of
oleuropein for 2 consecutive days, then 30 min of coronary
occlusion followed by 3 h of reperfusion; and iv) PD0325901 group:
3 mg/kg of PD0325901 and 20 mg/kg of oleuropein for 2 consecutive
days, the 30 min of coronary occlusion followed by 3 h of
reperfusion.
Rats were anesthetized by an intraperitoneal
injection of 100 mg/kg ketamine (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). A left thoracotomy was performed to expose the
hearts and the left anterior descending (LAD) artery was ligated at
2–3 mm at the pulmonary artery conus. Next, the left atrium was
sutured using 6-0 silk Prolene. For reversible coronary occlusion,
a small vinyl tube was placed on top of the vessel to form a snare.
Regional ischemia was sustained in the heart for 30 min and
reperfusion was then performed with release of the slipknot. Rats
were sacrificed using decollation under anesthesia and the heart
was peeled and frozen at 80°C.
Measurement of myocardial infarction
size
Following reperfusion for 3 h, the LAD artery was
removed and then 2% Evans blue (1.8–2 ml) was injected
intravenously to denote the area at risk. The heart was peeled and
frozen at −80°C for 24 h. The heart was then vertically cut into
1.5-mm sections from the long axis to the area of ligation.
Sections were incubated in 1% TTC solution for 30 min at 37°C and
then incubated with 10% neutral buffered formalin overnight at room
temperature.
Measurements of lactate dehydrogenase
(LDH), creatinine kinase-MB (CK-MB), tumor necrosis factor-α
(TNF-α), interleukin-1β (IL-1β), IL-6, superoxide dismutase (SOD),
glutathione (GSH), malondialdehyde (MDA) and catalase levels
Subsequent to reperfusion, blood samples were
collected from the right ventricle of every rat. These were
centrifuged at 3,000 × g for 10 min at 4°C to separate the serum.
LDH and CK-MB levels were evaluated using commercially available
assay kits (Sigma-Aldrich; Merck KGaA). TNF-α, IL-1β, IL-6, SOD,
GSH, MDA and catalase levels were evaluated using commercial
enzyme-linked immunosorbent assay (ELISA) assay kits (Wuhan Boster
Biological Technology, Inc., Wuhan, China).
Determination of apoptosis
Cytoplasmic proteins were prepared from heart
tissues following reperfusion for 3 h and were lysed in ice-cold
extraction buffer containing protease inhibitor cocktail (both
Beyotime Institute of Technology, Shanghai, China) for 30 min.
Miscible liquids were centrifuged at 12,000 × g for 30 min and
levels determined using a modified Bradford assay (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Protein (10 µg) was
used to measure the activity levels of caspase-3 using caspase-3
activity kit (Beyotime Institute of Technology).
Western blot analysis
Cytoplasmic proteins were prepared from heart
tissues following reperfusion for 3 h and were lysed in ice-cold
extraction buffer containing protease inhibitor cocktail (both
Beyotime Institute of Technology) for 30 min. Miscible liquids were
centrifuged at 12,000 × g for 30 min and levels determined using a
modified Bradford assay (Bio-Rad Laboratories, Inc.). Protein
(50–60 µg) was separated by electrophoresis on 10% sodium
dodecyl sulfate-polyacrylamide gels and transferred to
nitrocellulose membranes (Bio-Rad Laboratories, Inc.). Membranes
were washed with 5% bovine serum albumin with Tris-buffered saline
(TBS; 0.01 M, pH 7.4) for 1 h at 37°C and incubated in a humidified
chamber at 4°C overnight with primary antibodies against p53
(catalog no. 2527, 1:2,000 dilution), p-mitogen-activated protein
kinase kinase (p-MEK; catalog no. 9127; 1:2,000 dilution), p-ERK
(catalog no. 4370; 1:1,000 dilution), p-IκBα (catalog no. 2859,
1:1,000 dilution; all from Cell Signaling Technology, Inc.,
Danvers, MA, USA), p-signal transducer and activator of
transcription 3 (p-STAT3; sc-8001-R; 1:1,000 dilution) and
glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:2,000; both from
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Membranes was
washed with TBS with Tween 20 for 1 h at 37°C and then incubated
with peroxidase-conjugated secondary antibodies (sc-2004; 1:5,000
dilution, Santa Cruz Biotechnology, Inc.) for 1 h at 37°C. The
signals were detected with the enhanced chemiluminescence system
(GE Healthcare, Chicago, IL, USA) and assayed by Image_Lab_3.0
(Bio-Rad Laboratories, Inc.).
Statistical analysis
Data are presented as the mean ± standard error.
Data were analyzed using StatSoft Statistica version 13.0 (StatSoft
Inc., Tulsa, OK, USA). Statistical analyses were performed using
one-way analysis of variance with repeated measures, followed by
Bonferroni's post-hoc test. Statistical significance was defined as
P<0.05.
Results
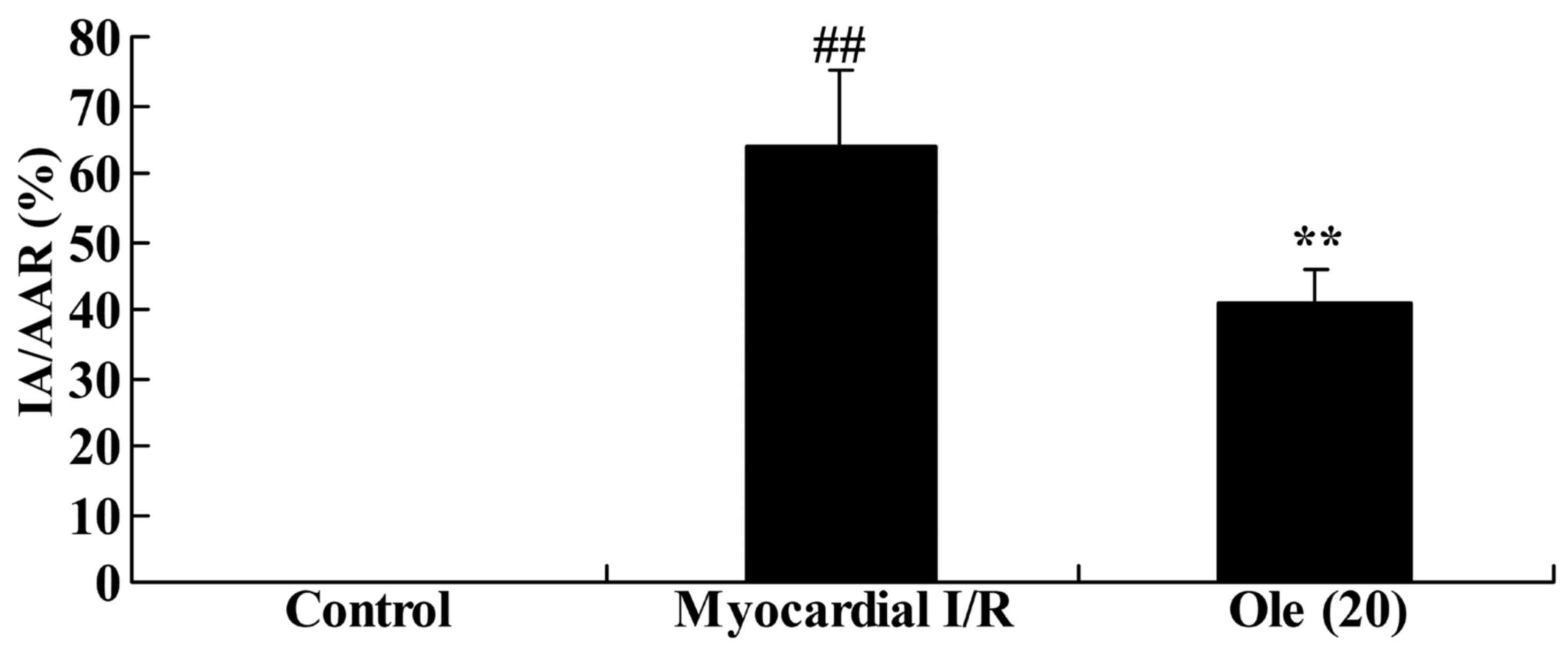
Protective effect of oleuropein against
myocardial infarction size in the myocardial I/R rats
Compared with rats in the normal control group, rats
in the myocardial I/R group exhibited a significant increase in
myocardial infarction size (Fig.
2). Administration of oleuropein significantly inhibited the
induction of myocardial infarction size by myocardial I/R injury
compared with the myocardial I/R model (Fig. 2).
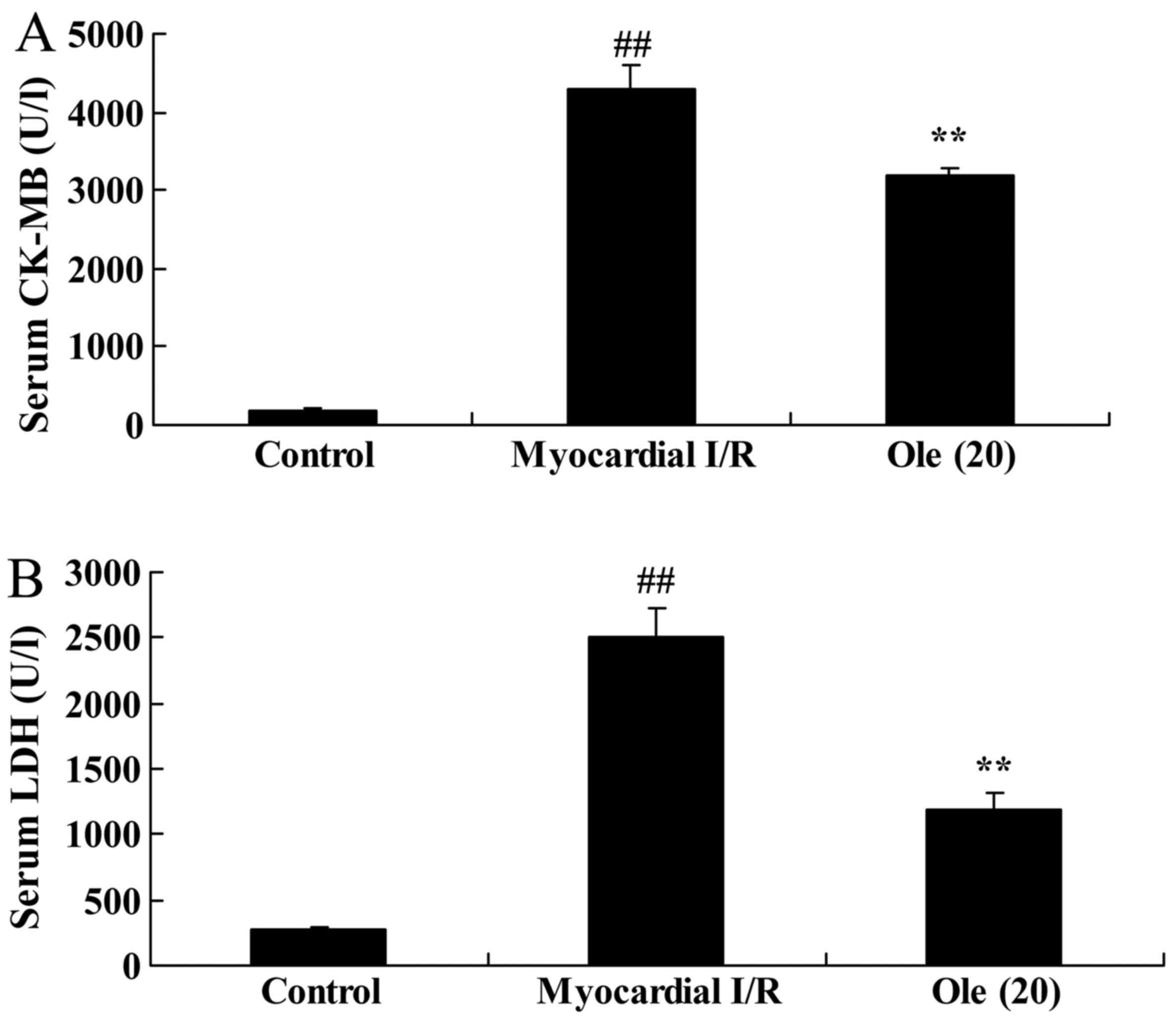
Protective effect of oleuropein against
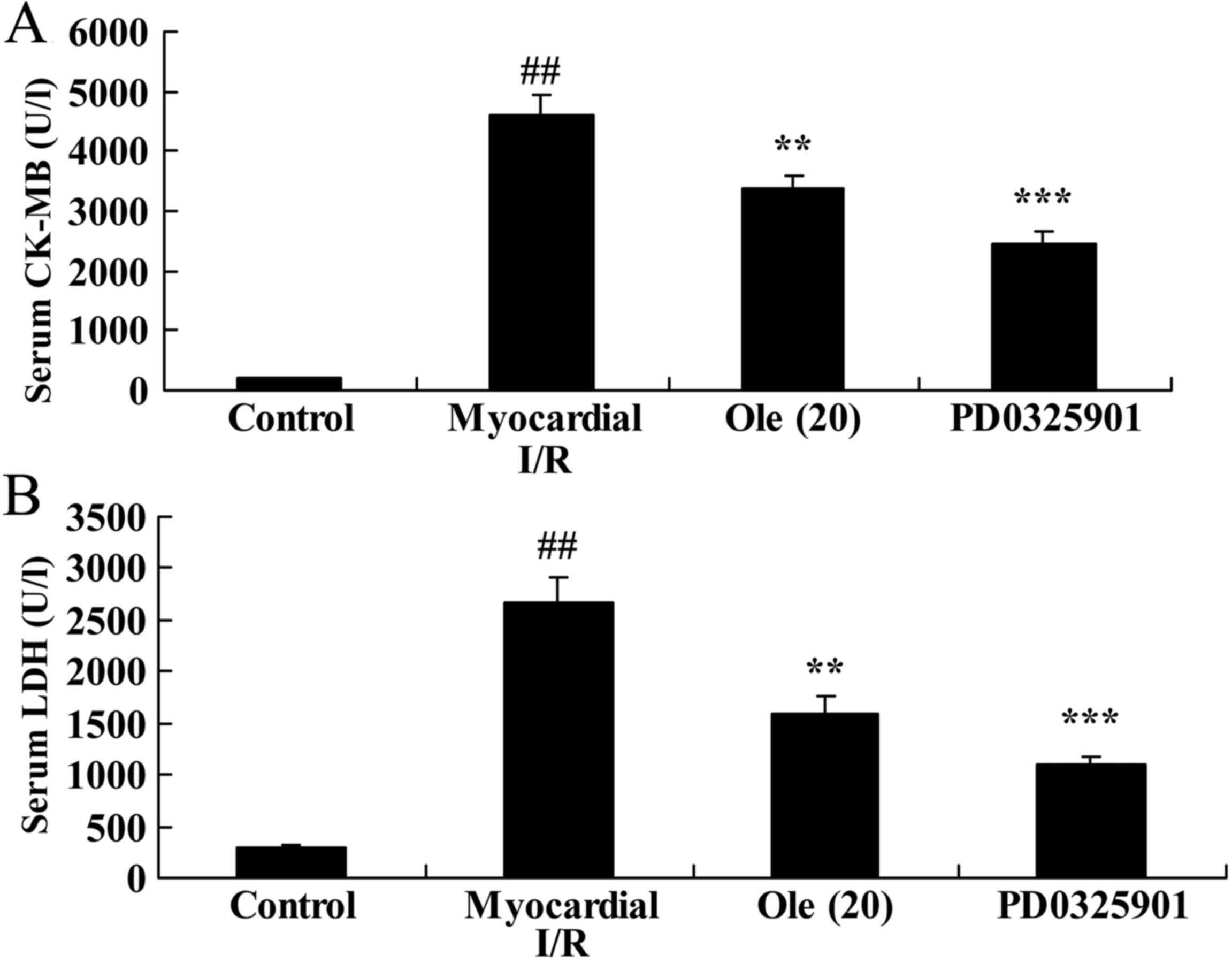
CK-MB and LDH serum levels in myocardial I/R rats
CK-MB and LDH levels in the serum were also examined
as indicators for myocardial injury evaluation in the present
study. Compared with levels in the normal control group, there was
a significant increase in CK-MB and LDH serum levels in the
myocardial I/R rat group (Fig.
3). Consistently, rats with oleuropein treatment exhibited
reduced levels of CK-MB and LDH compared with rats in the
myocardial I/R group (Fig.
3).
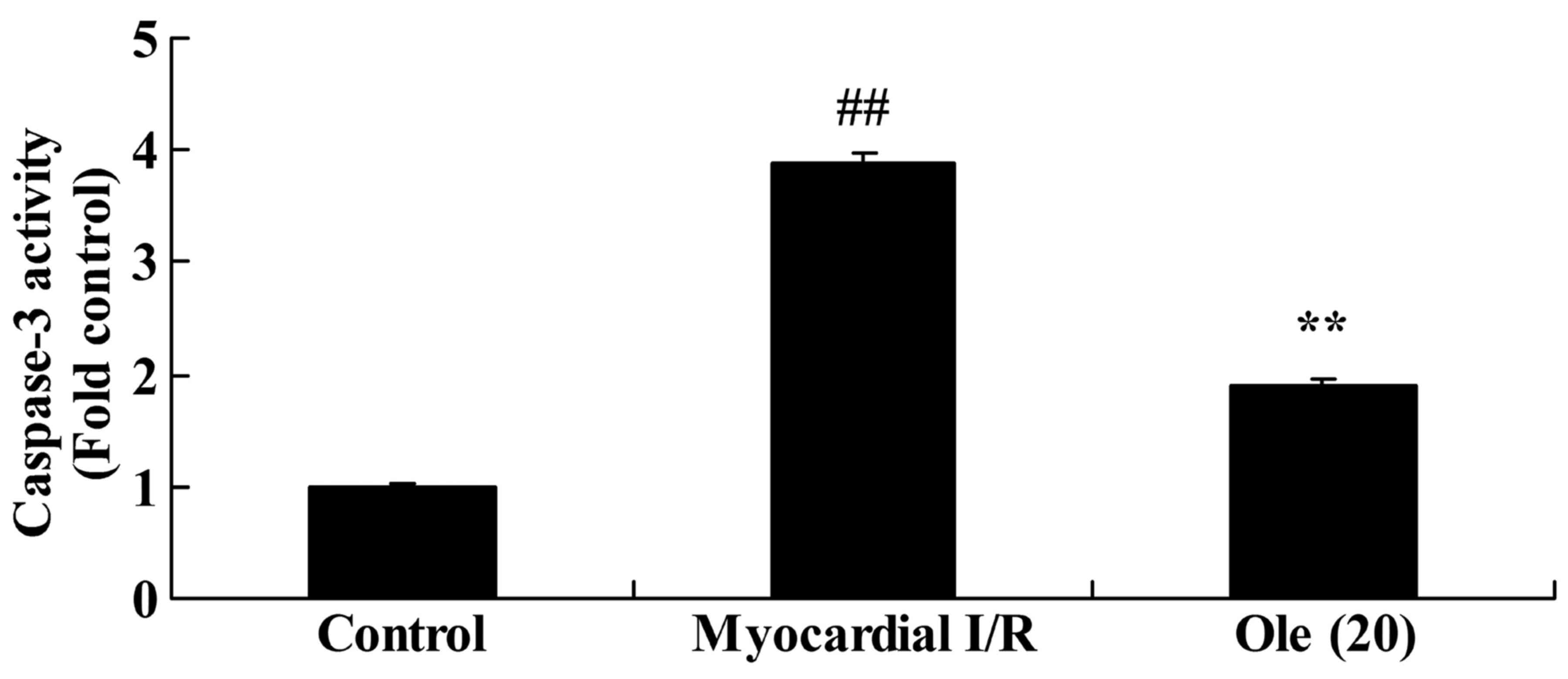
Protective effect of oleuropein against
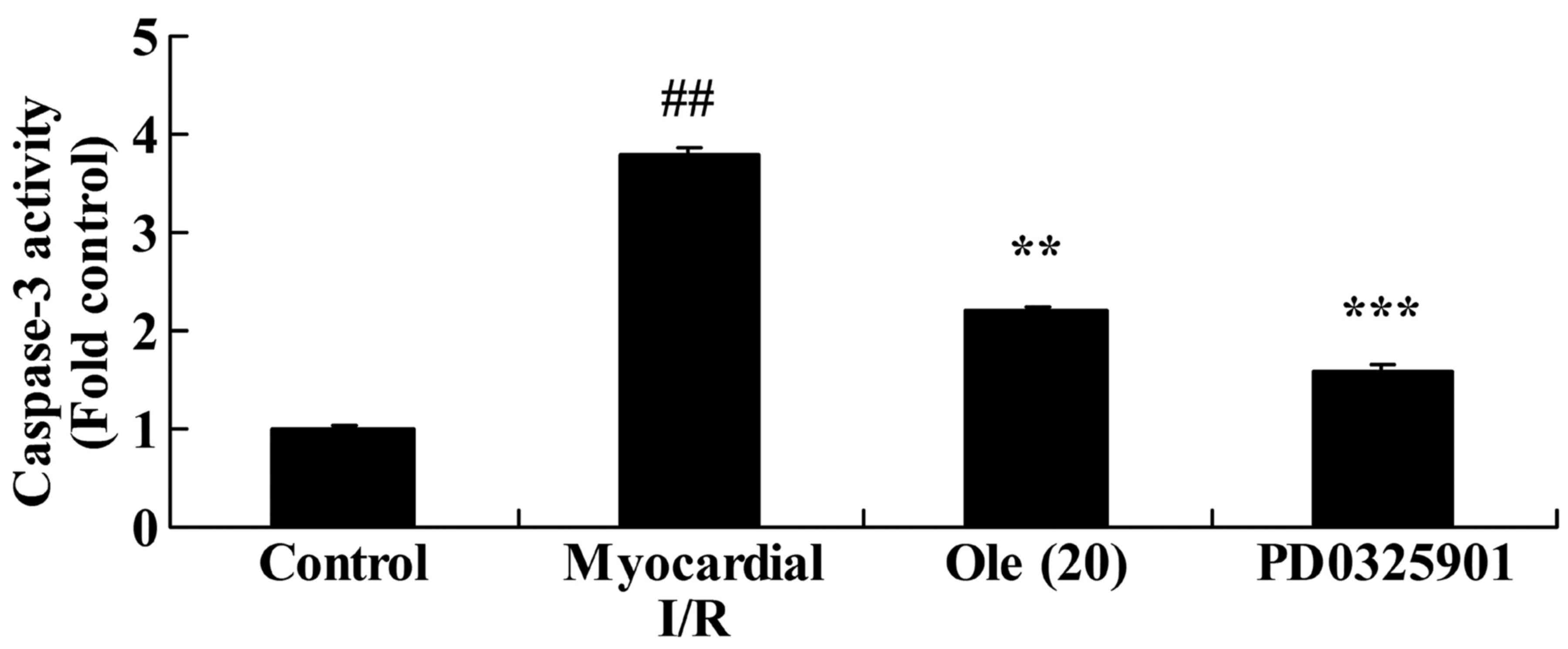
caspase-3 activity expression in myocardial I/R rats
To research the protective effect of oleuropein
against apoptosis, caspase-3 activity expression in the myocardial
I/R rat was researched. In comparison with that in the normal
control group, a significant induction in caspase-3 activity was
found in the myocardial I/R rat group (Fig. 4). However, rats with oleuropein
administration exhibited significantly inhibited caspase-3 activity
compared with rats in the myocardial I/R group (Fig. 4).
Protective effect of oleuropein against
p53, MEK and ERK protein expression in myocardial I/R rats
To further research the mechanism behind the
protective effect of oleuropein against apoptosis, p53, MEK and ERK
protein expression in myocardial I/R rats was measured using
western blot analysis. The induction of p53, p-MEK and p-ERK
protein expression was markedly observed in the myocardial I/R
model group in comparison with that in the normal control group
(Fig. 5). The oleuropein
treatment group showed significantly suppressed induction of p53,
p-MEK and p-ERK protein expression compared with the myocardial I/R
group.
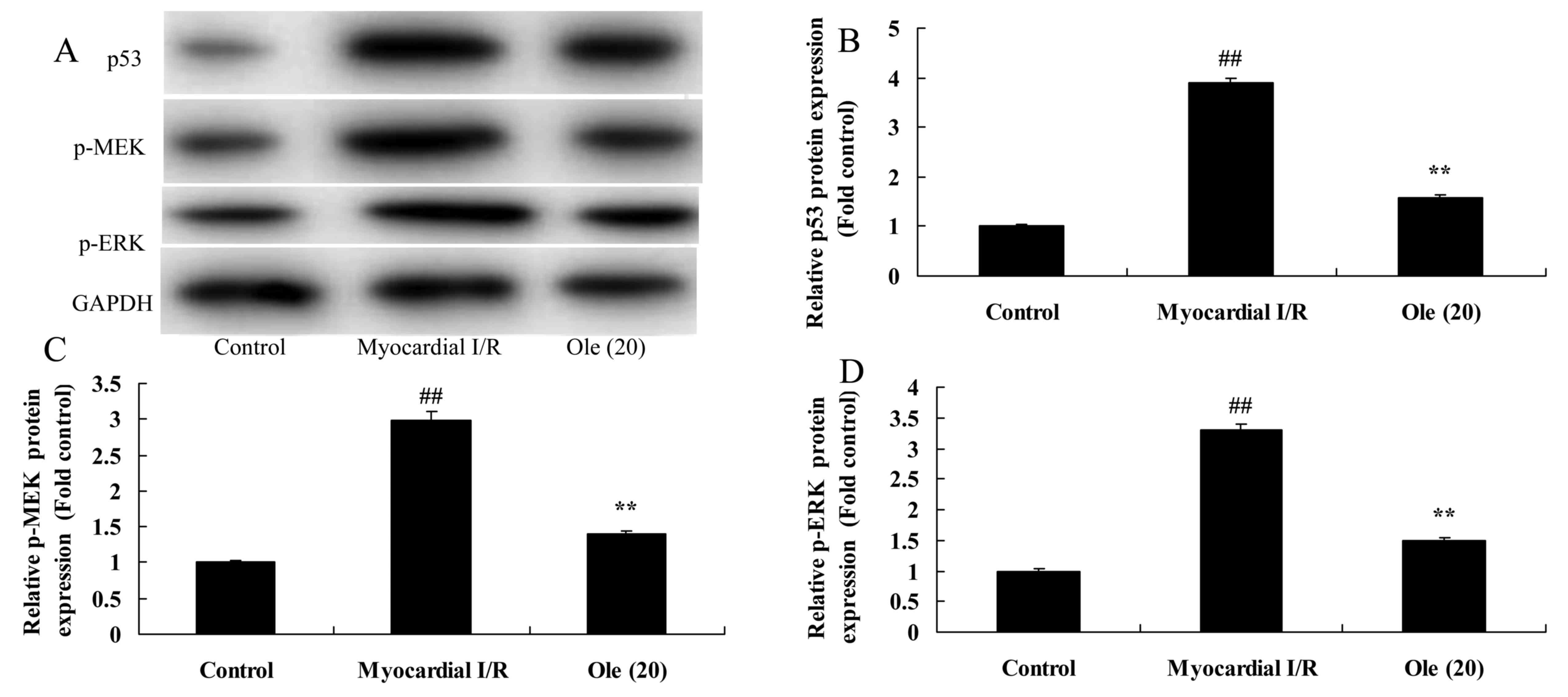 | Figure 5Protective effect of oleuropein
against p53, MEK and ERK protein expression in myocardial I/R rats,
as determined using (A) western blotting and statistical analysis
of (B) p53, (C) p-MEK and (D) p-ERK protein expression. Control,
control group; myocardial I/R, myocardial I/R model group; Ole
(20), 20 mg/kg/day oleuropein
treatment group. ##P<0.01 vs. control group;
**P<0.01 vs. myocardial I/R group. Ole, oleuropein;
I/R, ischemia/reperfusion; ER, extracellular signal-regulated
protein kinase; MEK, mitogen-activated protein kinase kinase;
GAPDH, glyceraldehyde 3-phosphate dehydrogenase; p-,
phosphorylated. |
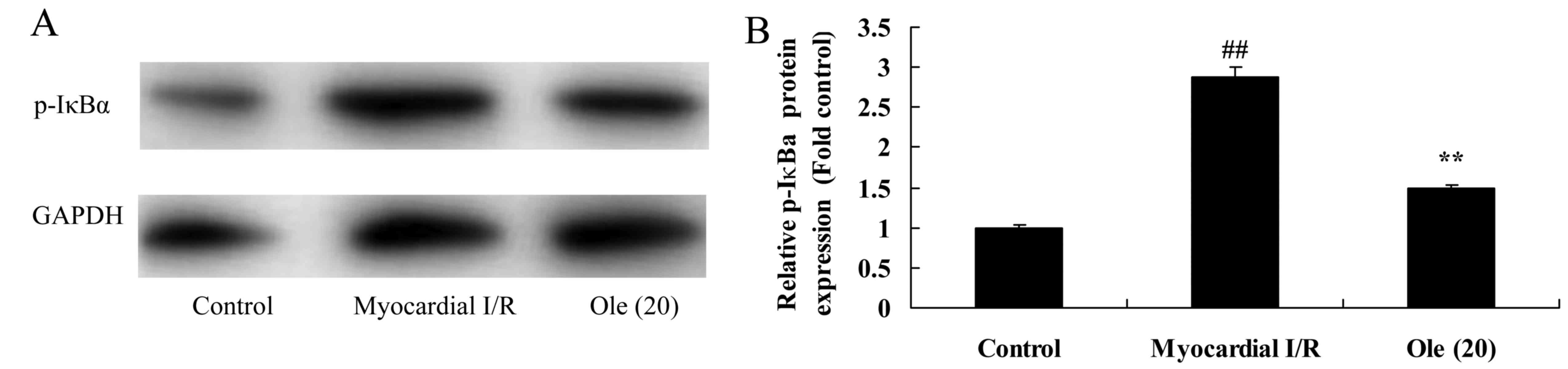
Protective effect of oleuropein against
p-IκBα protein expression in myocardial I/R rats
To investigate the mechanism behind the protective
effect of oleuropein against inflammation factors, p-IκBα protein
expression in myocardial I/R rats was measured using western blot
analysis. Myocardial I/R significantly induced the p-IκBα protein
expression in the myocardial I/R rat group compared with that in
the normal control group (Fig.
6). The oleuropein-treated group showed significant suppression
of p-IκBα protein expression compared with the myocardial I/R rat
group (Fig. 6).
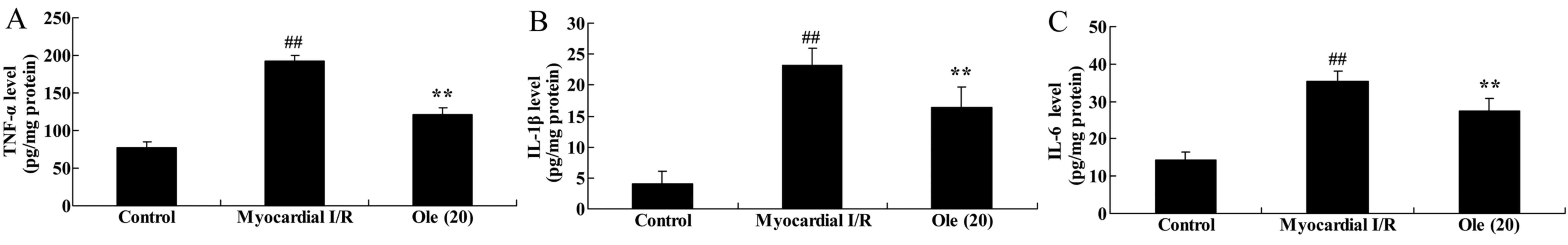
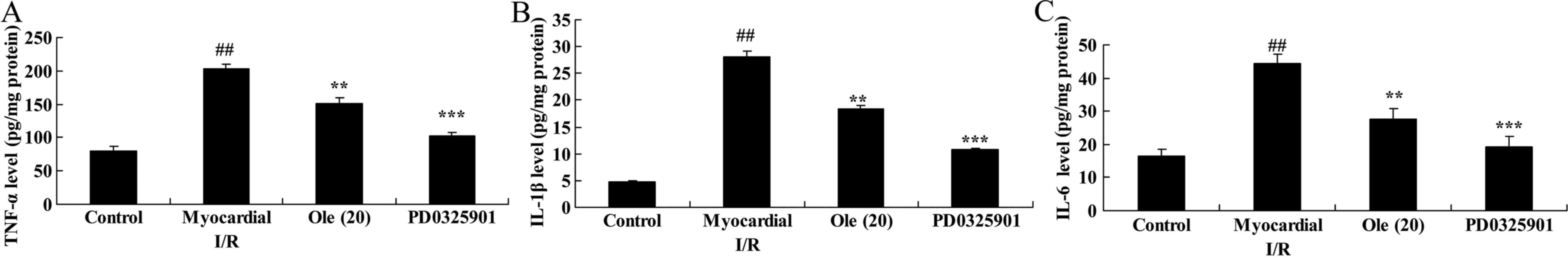
Protective effect of oleuropein against
TNF-α, IL-1β and IL-6 levels in myocardial I/R rats
To investigate the anti-inflammatory effect of
oleuropein against myocardial I/R, TNF-α, IL-1β and IL-6 levels
were measured using ELISA assay kits. The results demonstrated that
the TNF-α, IL-1β and IL-6 levels were significantly induced in the
myocardial I/R model group compared with that in the normal control
group (Fig. 7). The
oleuropein-treated group exhibited significantly reduced induction
of TNF-α, IL-1β and IL-6 levels by myocardial I/R compared with the
myocardial I/R group (Fig.
7).
Protective effect of oleuropein against
SOD, GSH, MDA levels in myocardial I/R rats
Furthermore, the protective effect of oleuropein
against oxidative stress in myocardial I/R rats was probed by
detecting SOD, GSH, MDA levels using ELISA assay kits. The
inhibition of SOD and GSH, and the increase in MDA levels was
significantly different in the myocardial I/R rat compared with
that in the normal control group (Fig. 8). The oleuropein-treated rats
exhibited significantly increased levels of SOD and GSH, and a
significantly decreased MDA level compared with rats in the
myocardial I/R model group (Fig.
8).
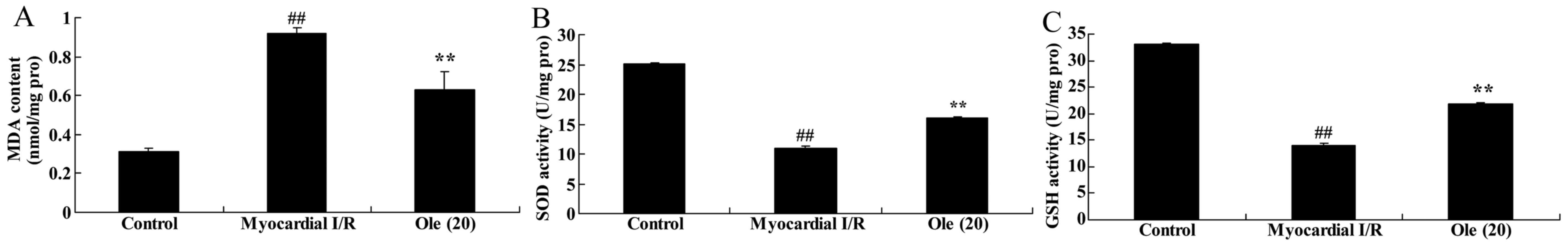 | Figure 8Protective effect of oleuropein
against (A) SOD, (B) GSH and (C) MDA levels in myocardial I/R rats.
Control, control group; myocardial I/R, myocardial I/R model group;
Ole (20), 20 mg/kg/day
oleuropein treatment group. ##P<0.01 vs. control
group; **P<0.01 vs. myocardial I/R group. Ole,
oleuropein; I/R, ischemia/reperfusion; SOD, superoxide dismutase;
GSH, glutathione; MDA, malondialdehyde; pro, protein. |
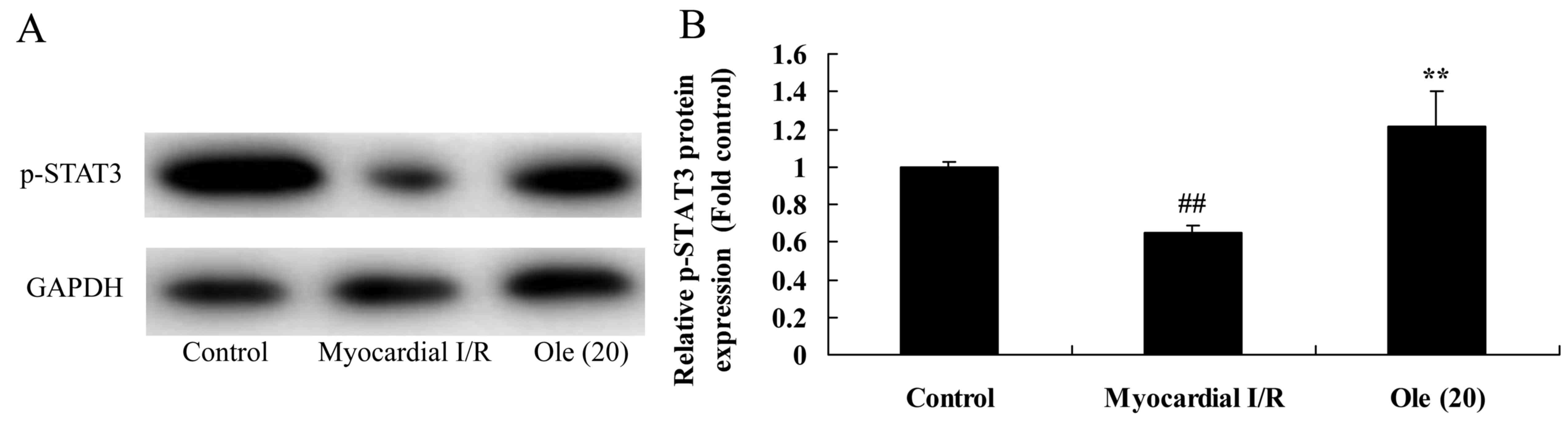
Protective effect of oleuropein on
p-STAT3 protein expression in myocardial I/R rats
Additionally, the protective effect of oleuropein
against p-STAT3 (p-STAT3) protein expression was investigated in
the myocardial I/R rat. As shown in Fig. 9, the suppression of p-STAT3
protein expression was markedly increased in the myocardial I/R rat
group compared with that in the normal control group. Rats treated
with oleuropein exhibited significantly induced p-STAT3 protein
expression compared with rats in the myocardial I/R group (Fig. 9).
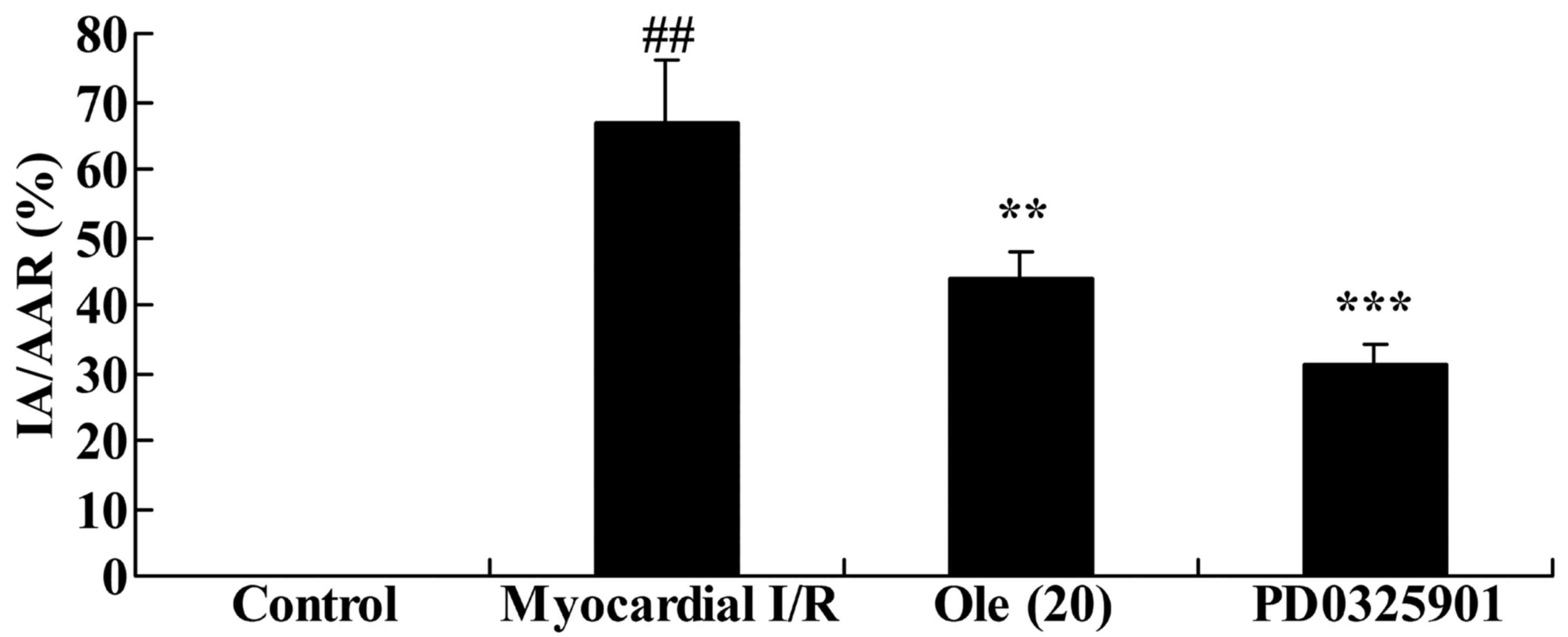
Inhibition of MEK increases the
protective effect of oleuropein against myocardial infarction size
in myocardial I/R rats
To provide evidence linking MEK to the effect of
oleuropein, PD0325901, a MEK inhibitor, was introduced to the
myocardial I/R rats to analyze how the inhibition of MEK affected
the protective effect of oleuropein against myocardial infarction
size. The group treated with PD0325901 and oleuropein exhibited a
significantly inhibited myocardial infarction size compared with
the group treated with oleuropein only (Fig. 10).
Inhibition of MEK increases the
protective effect of oleuropein against CK-MB and LDH serum levels
in myocardial I/R rats
To investigate the mechanism of oleuropein against
myocardial I/R, CK-MB and LDH serum levels were examined in
myocardial I/R rats treated with oleuropein following inhibition of
MEK. Rats with MEK inhibition exhibited significantly decreased
CK-MB and LDH serum levels compared rats treated with oleuropein
only (Fig. 11).
Inhibition of MEK increases the
protective effect of oleuropein against caspase-3 activity
expression in myocardial I/R rats
To determine whether the inhibition of MEK affects
apoptosis in oleuropein-treated myocardial I/R rats, caspase-3
activity expression was examined after the addition of PD0325901.
Rats with MEK inhibition showed significantly decreased caspase-3
activity compared with rats treated with oleuropein only (Fig. 12).
Inhibition of MEK increases the
protective effect of oleuropein against p53, MEK and ERK protein
expression in myocardial I/R rats
To further determine whether the inhibition of MEK
regulates p53, MEK and ERK protein expression in myocardial I/R
rats treated with oleuropein, protein levels were analyzed.
Following MEK inhibition, the protein expression of p53, p-MEK and
p-ERK was significantly suppressed compared with that in the group
treated with oleuropein only (Fig.
13A–D).
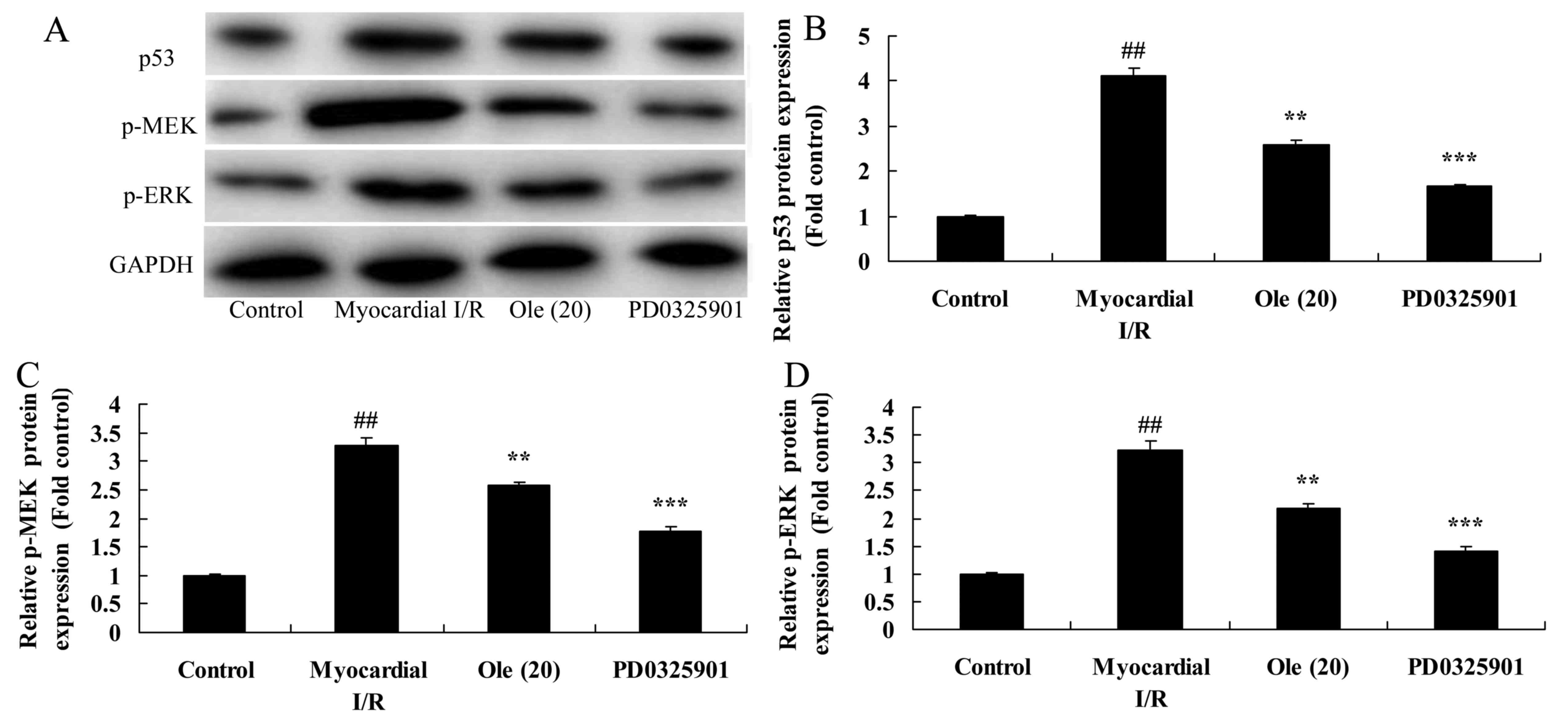 | Figure 13Inhibition of MEK increases the
protective effect of oleuropein against p53, MEK, ERK and p-IκBα
protein expression, as determined using western blotting (A) and
statistical analysis of (B) p53, (C) p-MEK, (D) p-ERK and (E)
p-IκBα protein expression in myocardial I/R rats. Control, control
group; myocardial I/R, myocardial I/R model group; Ole (20), 20 mg/kg/day of oleuropein treated
group; PD0325901, 3 mg/kg of PD0325901 + 20 mg/kg/day of oleuropein
treated group. ##p<0.01 vs. the control group;
**p<0.01 vs. the myocardial I/R group;
***p<0.01 vs. Ole (20) group. Ole, oleuropein; I/R,
ischemia/reperfusion; p-, phosphorylated; MEK, mitogen-activated
protein kinase kinase; ERK, extracellular signal-regulated protein
kinase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |
Inhibition of MEK increases the
protective effect of oleuropein against p-IκBα protein expression
in myocardial I/R rats
To determine whether the inhibition of MEK increased
the anti-inflammatory effect of oleuropein in myocardial I/R rats,
p-IκBα protein expression was detected using western blot analysis.
Following MEK inhibition, the protein expression of p-IκBα was also
significantly suppressed compared with that in the group treated
with oleuropein only (Fig. 13A and
E).
Inhibition of MEK increases the
protective effect of oleuropein against TNF-α, IL-1β and IL-6
levels in myocardial I/R rats
To further determine whether the inhibition of MEK
affects the anti-inflammatory effect of oleuropein in myocardial
I/R rats, TNF-α, IL-1β and IL-6 levels were analyzed. It was found
that the inhibition of MEK significantly inhibited TNF-α, IL-1β and
IL-6 levels in the rats treated with PD0325901 and oleuropein
compared with that in rats treated with oleuropein only (Fig. 14).
Inhibition of MEK increases the
protective effect of oleuropein against SOD, GSH and MDA levels in
myocardial I/R rats
Next, the effect of the inhibition of MEK on the
anti-oxidative action of oleuropein was examined in the myocardial
I/R rats. With the inhibition of MEK, a significant increase in SOD
and GSH levels, and a significant decrease in MDA level was found
in the PD0325901 group compared with that in the group treated with
oleuropein only (Fig. 15).
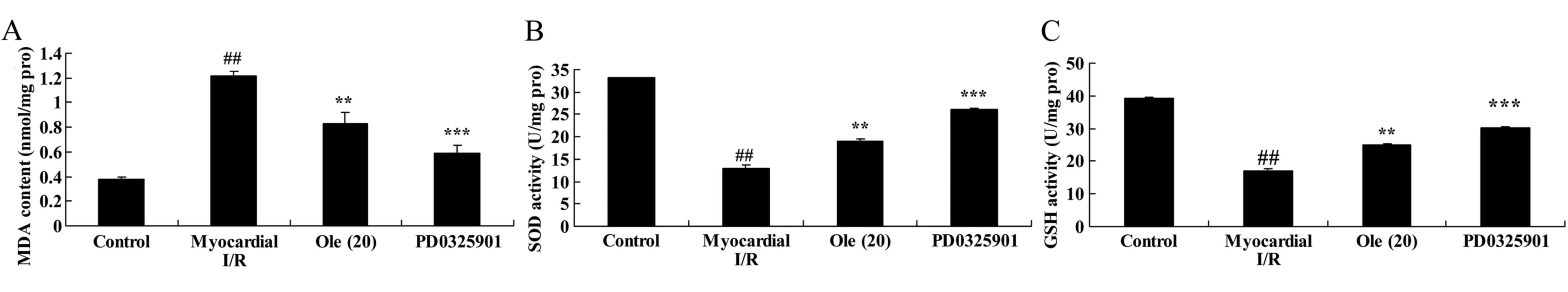 | Figure 15Inhibition of mitogen-activated
protein kinase kinase increases the protective effect of oleuropein
against (A) SOD, (B) GSH and (C) MDA levels in myocardial I/R rats.
Control, control group; myocardial I/R, myocardial I/R model group;
Ole (20), 20 mg/kg/day
oleuropein treatment group; PD0325901, 3 mg/kg PD0325901 + 20
mg/kg/day oleuropein treatment group. ##P<0.01 vs.
control group; **P<0.01 vs. myocardial I/R group;
***P<0.01 vs. Ole (20) group. Ole, oleuropein; I/R,
ischemia/reperfusion; SOD, superoxide dismutase; GSH, glutathione;
MDA, malondialdehyde; pro, protein. |
Inhibition of MEK increases the
protective effect of oleuropein on p-STAT3 in myocardial I/R
rats
Lastly, whether the inhibition of MEK increased the
protective effect of oleuropein against p-STAT3 was examined. The
protein expression of p-STAT3 was significantly promoted in the
PD0325901 group compared with that in the group treated with
oleuropein only (Fig. 16).
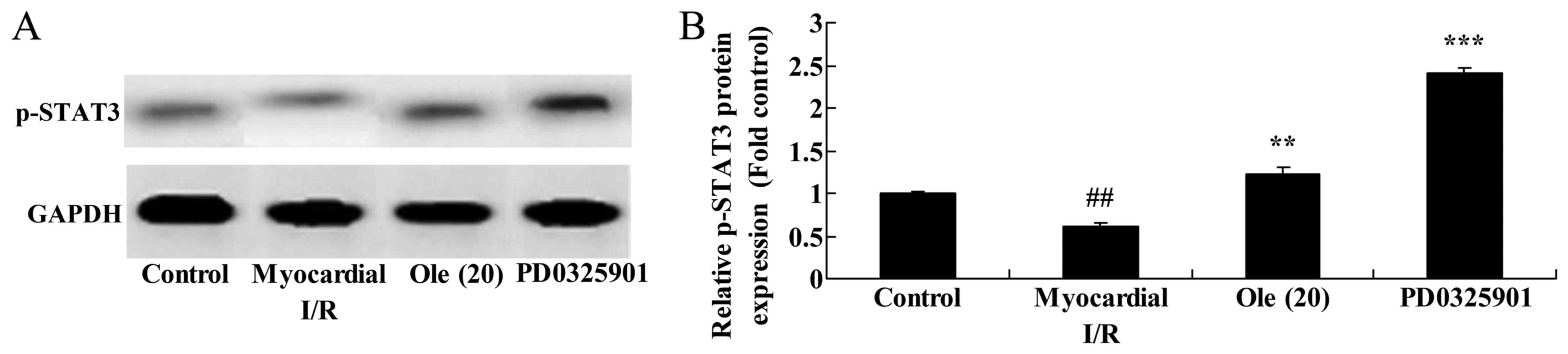 | Figure 16Inhibition of mitogen-activated
protein kinase kinase increases the protective effect of oleuropein
against p-STAT3 protein expression, as determined using (A) western
blotting and (B) statistical analysis of p-STAT3 protein expression
in myocardial I/R rats. Control, control group; myocardial I/R,
myocardial I/R model group; Ole (20), 20 mg/kg/day oleuropein treatment
group; PD0325901, 3 mg/kg PD0325901 + 20 mg/kg/day oleuropein
treatment group. ##P<0.01 vs. control group;
**P<0.01 vs. myocardial I/R group;
***P<0.01 vs. Ole (20) group. Ole, oleuropein; I/R,
ischemia/reperfusion; p-, phosphorylated; STAT3, signal transducer
and activator of transcription 3; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase. |
Discussion
Myocardial ischemia refers to the reduction of heart
blood perfusion, resulting in cardiac oxygen reduction and abnormal
myocardial energy metabolism, which is a pathological state that
cannot support heart function (1). Coronary plaques or occlusion and
instability of coronary atherosclerosis caused by coronary artery
stenosis are major causes of myocardial ischemia (17). Patients with recurrent myocardial
ischemia may suffer from angina, myocardial stunning, myocardial
hibernation, ischemic preconditioning, acute coronary syndrome, AMI
or even cardiac rupture (18). In
the present study, oleuropein significantly inhibited the
myocardial infarction size and reduced the levels of CK-MB and LDH
in the myocardial I/R rat. Nekooeian et al (15) reported that oleuropein offered
cardioprotection via its antioxidant properties.
ERK1/2 is a subfamily of the mitogen-activated
protein kinase (MAPK) signaling pathway, which exists widely in
various stages of the cell cycle and serves an important role in
gene transcription and cell cycle processes (19). At present, ERK1/2 is regarded to
promote both cell survival and proliferation, and apoptosis, for
which the mechanism depends on the sub-localization of ERK1/2 in
cells and the activated signaling molecules downstream (19). According to the traditional view,
ERK1/2 enters into the nucleus to activate cell survival and
proliferation; however, if the phosphorylated ERK1/2 stays in the
cytoplasm for the long term, it may interact with a series of
pro-apoptotic proteins to initiate the apoptosis of cells (20). The present study found that
oleuropein significantly suppressed the induction of p53, p-MEK and
p-ERK protein expression in myocardial I/R rats. Potočnjak et
al (21) suggest that
oleuropein attenuated cisplatin-induced acute renal injury through
inhibition of p53 and ERK signaling in mice.
Cardiac cells are a class of mature cells with
terminal differentiation and without the proliferation ability
(22). Myocardial cell apoptosis
is a type of programmed cell death that is performed in a series of
gene regulation by activating cell 'suicide' program through
certain signaling pathways under certain physiological and
pathological conditions (22). It
has been indicated that the apoptosis in myocardial cells serves an
important role in the physiological and pathological developmental
processes and maintenance of normal heart morphology, and is
considered to be the 'cellular basis' for the change from
compensatory changes to pathological changes (23). Meanwhile, the present study found
that oleuropein administration significantly inhibited caspase-3
activity in the myocardial I/R rat. Impellizzeri et al
(13) showed that oleuropein may
be useful in the treatment of various inflammatory diseases via
suppression of caspase-3 in mice with spinal cord injury.
In AMI, long-term hypoxia and ischemia of myocardial
cells leads to aerobic metabolic disorder due to coronary
occlusion, and then causes hyperemia and edema of myocardial
interstitial cells, and myocardial cell degeneration and necrosis,
accompanied by a large amount of inflammatory cell infiltration
(10). A large number of free
radicals are generated in the tissues and the peroxide destruction
of oxygen free radicals mainly damages the structure and function
of myocardial cell membranes, damages the mitochondria, cuts off
the cells energy supply and destroys lysosomes to cause cell
autolysis (24). The myocardium
of accelerated ischemia develops from reversible damage to
irreversible degeneration and necrosis. Malignant arrhythmia
appears, thereby causing ventricular remodeling and cardiac
dysfunction (24). Recanalization
and reperfusion therapy is the most effective treatment now, but
I/R can further damage the myocardium, for which the important
mechanism is oxidative stress; the greater the duration of
myocardial ischemia and hypoxia the greater the oxidative stress
and myocardial damage, and the more severe the disease (25). The present study showed that
oleuropein significantly increased the inhibition of SOD and GSH,
and inhibited the activation of MDA level in myocardial I/R rats.
Nekooeian et al (15)
indicated that oleuropein offered cardioprotection through its
antioxidant properties in rats with simultaneous type 2
diabetes.
AMI is the myocardial necrosis caused by acute and
persistent myocardial ischemia and hypoxia. Following AMI, changes
such as myocardial ischemia, hypoxia and increases in wall tension
among, other others, may induce the formation of the inflammatory
response (26). In the
inflammatory process subsequent to AMI, IL-6 and TNF-α serve
greater roles. IL-6 promotes the increased expression of
intercellular adhesion molecule-1 by myocardial cells via the
regulation of the synthesis of liver C-reactive protein (CRP) and a
series of biochemical processes of the liver in the acute phase
reaction, thereby enhancing the adhesion of neutrophils and the
release of oxygen free radicals (27). TNF-α directly damages endothelial
cells to induce the CRP synthesis pathway to produce an effect by
enhancing leukocyte chemotaxis (27). The aforementioned inflammatory
processes will inevitably result in myocardial cell injury or
myocardial fibrosis. Thus far, studies have shown that inflammation
serves an important role in the development of myocardial fibrosis
(28). In the present study, it
was found that oleuropein significantly reduced the induction of
TNF-α, IL-1β and IL-6 levels by myocardial I/R. Giner et al
(16) also showed oleuropein to
be a protective agent against colitis-associated colorectal cancer
via reduction of intestinal IL-6, IFN-γ, TNF-α and IL-17A
concentration in c57bl/6 mice.
In the resting condition of cells, nuclear factor-κB
(NF-κB) is bound to IκB to form a complex present in the cytoplasm.
When subjected to external stimuli, including cytokines, oxidants,
protein kinase C activator, viruses, ultraviolet and
lipopolysaccharides, IκBs are degraded, thus releasing the free
NF-κB dimers. At this time, the NF-κB is transported from the
cytoplasm to the nucleus, which influences the transcription of
various adhesion cytokines, immune receptors, acute-phase proteins
and stress-response protein genes (29). Studies have shown that NF-κB is a
central regulator for stress and the inflammatory response, and
that it not only serves a role in immune regulation, but that its
signaling pathway has also been extensively involved in cell
survival, differentiation, proliferation and apoptosis, playing an
important role in the occurrence, development and outcome of
numerous diseases (30,31). The present study showed that
p-IκBα protein expression was significantly suppressed in
myocardial I/R rats with oleuropein treatment compared with that in
rats without treatment. Campolo et al (32) demonstrated that oleuropein
inhibits secondary events of intestinal ischemia/reperfusion injury
through NF-κB and IκBα.
ERK is the most important and classical route that
is best studied for the MAPK path, the path is the nodes and common
pathway of multiple pro-proliferation signal transduction pathways
(33). Previous results have
shown that angiotensin II (AngII) may lead to pathological
myocardial hypertrophy and myocardial fibrosis by AngII type I
receptor (AT1R)-mediated protein kinase C
(PKC)-ERK1/ERK2 pathway signaling (34). The binding of AngII and
AT1R can activate a number of signaling molecules
downstream, including phospholipase C, phospholipase D and PKC. PKC
activates ERK1/2, thus achieving the signal transduction within the
cell and the activation of nuclear gene transcription (35). Notably, in the present study,
inhibition of MEK expression could inhibit myocardial infarction
size and CK-MB and LDH serum levels, suppress caspase-3 activity
and p53, p-MEK p-ERK and p-IκBα protein expression, and inhibit
TNF-α, IL-1β, IL-6 and MDA levels and increase SOD, GSH and
catalase levels in myocardial I/R rats treated with oleuropein.
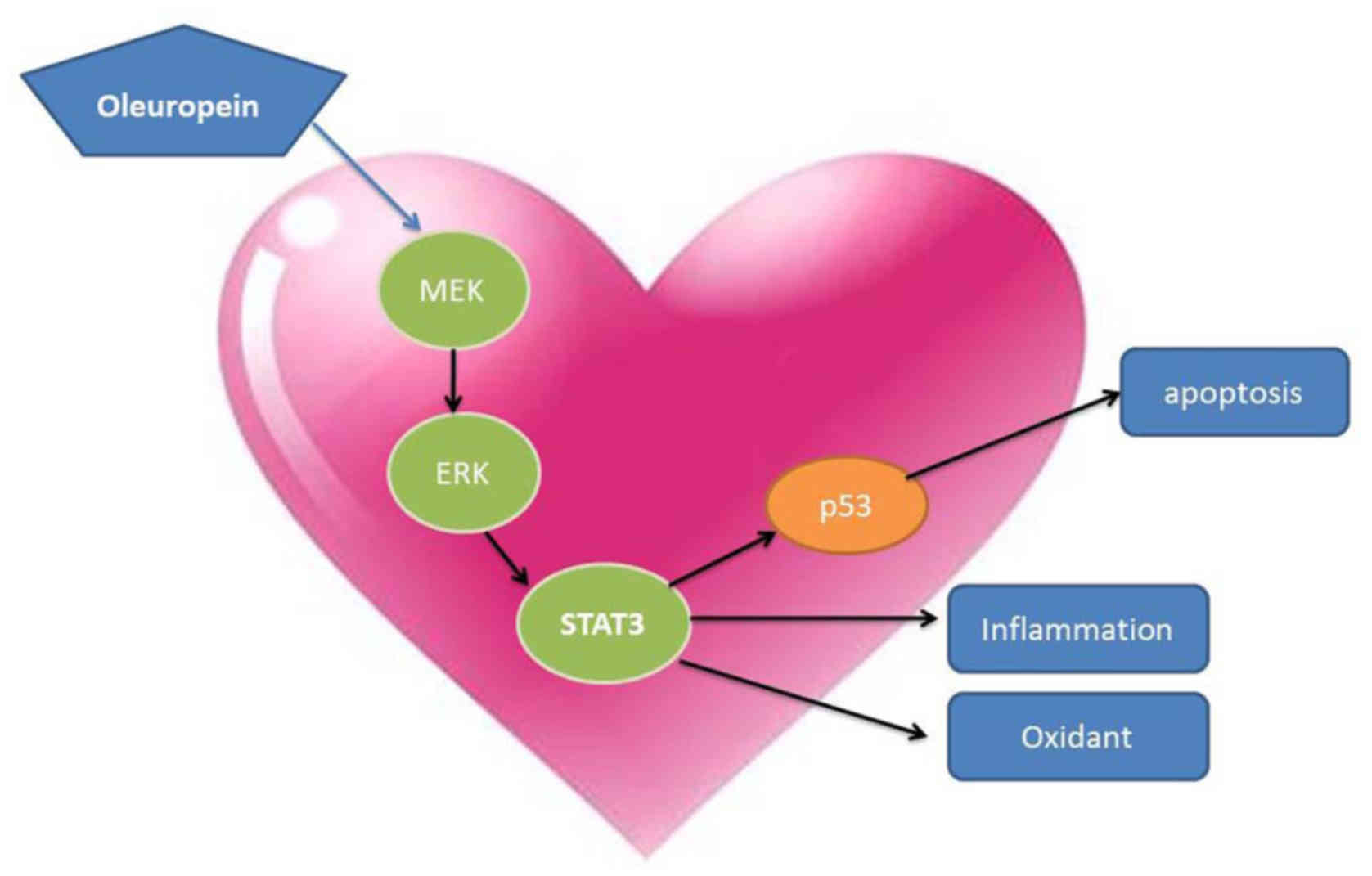
In conclusion, the results of the current study
showed that oleuropein inhibited myocardial infarction size, and
CK-MB and LDH serum levels in myocardial I/R rats through
anti-inflammation, anti-oxidant, anti-apoptosis and inhibition of
MEK/ERK/STAT3 signaling. Moreover, inhibition of the MEK/ERK/STAT3
signaling pathway may serve a key role in the protective effects of
oleuropein against myocardial I/R in rats (Fig. 17).
Acknowledgments
Not applocable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
HXJ designed the experiment. YHZ, RNG and SNZ
performed the experiment. HXJ analyzed the data and wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Medical
Ethics Committee of Harrisson International Peace Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nicholls SJ, Cavender MA, Kastelein JJ,
Schwartz G, Waters DD, Rosenson RS, Bash D and Hislop C: Inhibition
of secretory phospholipase A(2) in patients with acute coronary
syndromes: Rationale and design of the vascular inflammation
suppression to treat acute coronary syndrome for 16 weeks
(VISTA-16) trial. Cardiovasc Drugs Ther. 26:71–75. 2012. View Article : Google Scholar
|
|
2
|
Holme I, Szarek M, Cater NB, Faergeman O,
Kastelein JJ, Olsson AG, Tikkanen MJ, Larsen ML, Lindahl C and
Pedersen TR: Incremental Decrease in End Points Through Aggressive
Lipid Lowering Study Group: Adherence-adjusted efficacy with
intensive versus standard statin therapy in patients with acute
myocardial infarction in the IDEAL study. Eur J Cardiovasc Prev
Rehabil. 16:315–320. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vemulapalli S, Zhou Y, Gutberlet M, Kumar
AS, Mills JS, Blaxill J, Smalling R, Ohman EM and Patel MR:
Importance of total ischemic time and preprocedural infarct-related
artery blood flow in predicting infarct size in patients with
anterior wall myocardial infarction (from the CRISP-AMI Trial). Am
J Cardiol. 112:911–917. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Winter JL, Lindefjeld DS, Veas N, Guarda
E, Valdebenito M, Méndez M, Pérez O, Zuanic K, Mestas M and
Martínez A: Angiographic and electrocardiographic parameters of
myocardial reperfusion in angioplasty of patients with ST elevation
acute myocardial infarction loaded with ticagrelor or clopidogrel
(MICAMI-TICLO trial). Cardiovasc Revasc Med. 15:284–288. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wei C, Wang Y, Li M, Li H, Lu X, Shao H
and Xu C: Spermine inhibits endoplasmic reticulum stress-induced
apoptosis: A new strategy to prevent cardiomyocyte apoptosis. Cell
Physiol Biochem. 38:531–544. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang H, Wang Z, Feng SJ, Xu L, Shi HX,
Chen LL, Yuan GD, Yan W, Zhuang W, Zhang YQ, et al: PEDF improves
cardiac function in rats with acute myocardial infarction via
inhibiting vascular permeability and cardiomyocyte apoptosis. Int J
Mol Sci. 16:5618–5634. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ale A, Siebenhaar F, Kosanke K, Aichler M,
Radrich K, Heydrich S, Schiemann M, Bielicki I, Noel PB, Braren R,
et al: Cardioprotective C-kit+ bone marrow cells
attenuate apoptosis after acute myocardial infarction in mice -
in-vivo assessment with fluorescence molecular imaging.
Theranostics. 3:903–913. 2013. View Article : Google Scholar :
|
|
8
|
Li L, Guo Y, Zhai H, Yin Y, Zhang J, Chen
H, Wang L, Li N, Liu R and Xia Y: Aging increases the susceptivity
of MSCs to reactive oxygen species and impairs their therapeutic
potency for myocardial infarction. PLoS One. 9:e1118502014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Becatti M, Fiorillo C, Gori AM, Marcucci
R, Paniccia R, Giusti B, Violi F, Pignatelli P, Gensini GF and
Abbate R: Platelet and leukocyte ROS production and
lipoperoxidation are associated with high platelet reactivity in
Non-ST elevation myocardial infarction (NSTEMI) patients on dual
antiplatelet treatment. Atherosclerosis. 231:392–400. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Correa F, Martínez-Abundis E,
Hernández-Reséndiz S, García N, Buelna-Chontal M, Arreguín F and
Zazueta C: Pharmacological strategies to contend against myocardial
reperfusion damage: Diverse chemicals for multiple targets. Curr
Med Chem. 17:2261–2273. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun SJ, Wu XP, Song HL and Li GQ: Baicalin
ameliorates isoproterenol-induced acute myocardial infarction
through iNOS, inflammation, oxidative stress and P38MAPK pathway in
rat. Int J Clin Exp Med. 8:22063–22072. 2015.
|
|
12
|
Protti A, Dong X, Andia ME, Yu B, Dokukina
K, Chaubey S, Phinikaridou A, Vizcay-Barrena G, Taupitz M, Botnar
RM, et al: Assessment of inflammation with a very small iron-oxide
particle in a murine model of reperfused myocardial infarction. J
Magn Reson Imaging. 39:598–608. 2014. View Article : Google Scholar
|
|
13
|
Impellizzeri D, Esposito E, Mazzon E,
Paterniti I, Di Paola R, Bramanti P, Morittu VM, Procopio A, Perri
E, Britti D, et al: The effects of a polyphenol present in olive
oil, oleuropein aglycone, in an experimental model of spinal cord
injury in mice. Biochem Pharmacol. 83:1413–1426. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lapi D, Di Maro M, Mastantuono T,
Battiloro L, Sabatino L, Muscariello E and Colantuoni A: Effects of
oleuropein and pinoresinol on microvascular damage induced by
hypoperfusion and reperfusion in rat pial circulation.
Microcirculation. 22:79–90. 2015. View Article : Google Scholar
|
|
15
|
Nekooeian AA, Khalili A and Khosravi MB:
Oleuropein offers cardioprotection in rats with simultaneous type 2
diabetes and renal hypertension. Indian J Pharmacol. 46:398–403.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Giner E, Recio MC, Ríos JL, Cerdá-Nicolás
JM and Giner RM: Chemopreventive effect of oleuropein in
colitis-associated colorectal cancer in c57bl/6 mice. Mol Nutr Food
Res. 60:242–255. 2016. View Article : Google Scholar
|
|
17
|
Elbarouni B, Cantor WJ, Ducas J,
Borgundvaag B, Džavík V, Heffernan M, Buller CE, Langer A, Goodman
SG and Yan AT; TRANSFER-AMI Trial Investigators: Efficacy of an
early invasive strategy after fibrinolysis in ST-elevation
myocardial infarction relative to the extent of coronary artery
disease. Can J Cardiol. 30:1555–1561. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boghdady A and Elbadry MI: Comparison of
successful myocardial reperfusion and adverse events in patients
with ST-elevation myocardial infarction who underwent rescue
percutaneous coronary intervention after failed fibrinolytic
therapy with versus without manual coronary thrombus aspiration. Am
J Cardiol. 116:1185–1192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
She T, Wang X, Gan Y, Kuang D, Yue J, Ni
J, Zhao X and Wang G: Hyperglycemia suppresses cardiac stem cell
homing to peri-infarcted myocardium via regulation of ERK1/2 and
p38 MAPK activities. Int J Mol Med. 30:1313–1320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Przybyt E, Krenning G, Brinker MG and
Harmsen MC: Adipose stromal cells primed with hypoxia and
inflammation enhance cardiomyocyte proliferation rate in vitro
through STAT3 and Erk1/2. J Transl Med. 11:392013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Potočnjak I, Škoda M, Pernjak-Pugel E,
Peršić MP and Domitrović R: Oral administration of oleuropein
attenuates cisplatin-induced acute renal injury in mice through
inhibition of ERK signaling. Mol Nutr Food Res. 60:530–541. 2016.
View Article : Google Scholar
|
|
22
|
Yang X, Qin Y, Shao S, Yu Y, Zhang C, Dong
H, Lv G and Dong S: MicroRNA-214 Inhibits Left Ventricular
Remodeling in an Acute Myocardial Infarction Rat Model by
Suppressing Cellular Apoptosis via the Phosphatase and Tensin
Homolog (PTEN). Int Heart J. 57:247–250. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang RL, Guo Z, Wang LL and Wu J:
Degeneration of capsaicin sensitive sensory nerves enhances
myocardial injury in acute myocardial infarction in rats. Int J
Cardiol. 160:41–47. 2012. View Article : Google Scholar
|
|
24
|
Sygitowicz G, Maciejak A,
Piniewska-Juraszek J, Pawlak M, Góra M, Burzyńska B, Dłużniewski M,
Opolski G and Sitkiewicz D: Interindividual variability of
atorvastatin treatment influence on the MPO gene expression in
patients after acute myocardial infarction. Acta Biochim Pol.
63:10142016. View Article : Google Scholar
|
|
25
|
Mladenka P, Filipský T, Ríha M, Vávrová J,
Holecková M, Palicka V and Hrdina R: The relationship of oxidative
stress markers and parameters of myocardial function in a rat model
of cardiotoxicity. Free Radic Biol Med. 75(Suppl 1): S422014.
View Article : Google Scholar
|
|
26
|
Carvalheiro T, Velada I, Valado A, Mendes
F, Martinho A, António N, Gonçalves L, Providência L, Pais ML and
Paiva A: Phenotypic and functional alterations on inflammatory
peripheral blood cells after acute myocardial infarction. J
Cardiovasc Transl Res. 5:309–320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shrivastava AK, Singh HV, Raizada A and
Singh SK: Serial measurement of lipid profile and inflammatory
markers in patients with acute myocardial infarction. EXCLI J.
14:517–526. 2015.PubMed/NCBI
|
|
28
|
Prato FS, Butler J, Sykes J, Keenliside L,
Blackwood KJ, Thompson RT, White JA, Mikami Y, Thiessen JD and
Wisenberg G: Can the inflammatory response be evaluated using
18F-FDG within zones of microvascular obstruction after myocardial
infarction? J Nucl Med. 56:299–304. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xia KP, Ca HM and Shao CZ: Protective
effect of notoginsenoside R1 in a rat model of myocardial ischemia
reperfusion injury by regulation of vitamin D3 upregulated protein
1/NF-κB pathway. Pharmazie. 70:740–744. 2015.
|
|
30
|
Xu H, Wang D, Peng C, Huang X, Ou M, Wang
N, Wang P, Zhou L and Ye X: Rabbit sera containing compound danshen
dripping pill attenuate leukocytes adhesion to TNF-alpha-activated
human umbilical vein endothelial cells by suppressing endothelial
ICAM-1 and VCAM-1 expression through NF-kappaB signaling pathway. J
Cardiovasc Pharmacol. 63:323–332. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Arslan S, Korkmaz Ö, Özbilüm N and Berkan
Ö: Association between NF-κBI and NF-κBIA polymorphisms and
coronary artery disease. Biomed Rep. 3:736–740. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Campolo M, Di Paola R, Impellizzeri D,
Crupi R, Morittu VM, Procopio A, Perri E, Britti D, Peli A,
Esposito E, et al: Effects of a polyphenol present in olive oil,
oleuropein aglycone, in a murine model of intestinal
ischemia/reperfusion injury. J Leukoc Biol. 93:277–287. 2013.
View Article : Google Scholar
|
|
33
|
Grossi V, Lucarelli G, Forte G, Peserico
A, Matrone A, Germani A, Rutigliano M, Stella A, Bagnulo R, Loconte
D, et al: Loss of STK11 expression is an early event in prostate
carcinogenesis and predicts therapeutic response to targeted
therapy against MAPK/p38. Autophagy. 11:2102–2113. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Armstrong SC: Protein kinase activation
and myocardial ischemia/reperfusion injury. Cardiovasc Res.
61:427–436. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ruisong M, Xiaorong H, Gangying H,
Chunfeng Y, Changjiang Z, Xuefei L, Yuanhong L and Hong J: The
protective role of interleukin-33 in myocardial ischemia and
reperfusion is associated with decreased HMGB1 expression and
upregulation of the p38 MAPK signaling pathway. PLoS One.
10:e01430642015. View Article : Google Scholar
|