Introduction
Fulminant hepatic failure (FHF), which is
characterized by extensive hepatocyte necrosis (1), is a clinical syndrome that results
from the severe impairment of liver function induced by drugs,
toxins or viral hepatitis (2).
Even in developed countries, the mortality rate of patients with
FHF is as high as 50-90% without liver transplantation (3). However, patients with FHF usually
suffer multiple organ failure, which prevents liver transplantation
(4). In addition, due to donor
liver shortages, high costs, complications, and the risk of organ
rejection, the application of liver transplantation is limited
(5). Biochemical and pathological
studies have suggested that hepatocyte apoptosis is important in
the development of FHF, resulting in substantial hepatocyte loss
when the rate and extent of hepatocellular apoptosis are not
adequately compensated by regenerative activity (5).
Under normal physiological conditions,
proto-oncogenes are involved in the maintenance of tissue
homeostasis (6). Proto-oncogene
activation has been used to repair cardiac ischemia injury
(7). The proto-oncogene
C-sis encodes the B chain of platelet-derived growth factor
(PDGF-B) (8). PDGF is a potent
mitogen, which is released from activated hepatocytes and hepatic
stellate cells (HSCs), which are involved in liver repair (9-11).
At the cellular level, PDGF is one of the most well characterized
fibrogenic and proliferative cytokines for HSCs. In addition,
hepatic injury is associated with the upregulation of autocrine
PDGF and PDGF receptor (10,12). Hao et al (13) demonstrated that the neutralization
of PDGF-B suppressed the proliferation and activation of HSCs in
the fibrotic mouse liver. PDGF-B may exist as a homodimer (PDGF-BB)
or as a heterodimer with chain A (PDGF-AB). PDGF-BB serum levels
are positively associated with survival rates among patients with
FHF (14), indicating its
potential role in the progression of FHF. PDGF-BB is the main
stimulus for the proliferation of mesenchymal cells and is secreted
by several cells residing in or passing through the liver (15). Hirota et al (16) reported that the overexpression of
PDGF-BB resulted in airway hyper-responsiveness, decreased lung
compliance, increased airway smooth muscle cell numbers, positive
proliferating cell nuclear antigen-stained airway smooth muscle
cells, and a reduction in genes encoding contractile proteins.
Additionally, PDGF-BB induces the proliferation of HSCs (12,17-23) and is also essential in the
progression of liver fibrosis (23,24). Therefore, it was hypothesized that
C-sis may be involved in the repair of liver injury in FHF
by regulating hepatocellular apoptosis.
To validate the above hypothesis in vitro and
in vivo, respectively, Buffalo rat liver (BRL) cells were
treated with hydrogen peroxide (H2O2) to
induce apoptosis or were transfected with a C-sis
overexpression vector. A rat model of FHF was established, and
C-sis was overexpressed. Cell viability and apoptosis were
assessed. The results showed that the overexpression of
C-sis inhibited the H2O2-induced
apoptosis of BRL cells in vitro, and alleviated liver injury
and decreased mortality rates in the FHF rats.
Materials and methods
Plasmid construction
The full-length cDNA encoding rat C-sis
(GenBank™ accession no. NM24628) was generated by reverse
transcription-polymerase chain reaction (RT-PCR) from the liver
tissues of Sprague-Dawley rats, using the following primers:
Forward, 5′-CGCGAATTCATGAATCGCTGCTGGGC-3′ (the EcoRI site is
underlined) and reverse, 5′-CCCTCTAGACTAGGCTCCAAGGATCTC-3′ (the
XbaI site is underlined). The PCR products were digested and
cloned into the mammalian expression vector pcDNA3.1 (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). The plasmids
were purified using the AxyPrep DNA Gel Recovery kit (Axygen
Biotechnology Co., Ltd., Hangzhou, China).
Cell culture, transfection and
H2O2 treatment
The BRL cells (Shanghai Cell Bank, Chinese Academy
of Sciences, Shanghai, China) were cultured in DMEM containing 10%
FBS (Gibco; Thermo Fisher Scientific, Inc.) in a humidified
incubator at 37°C under 5% CO2. Cells in the logarithmic
phase were digested with 0.25% trypsin and 0.02% EDTA, and then
centrifuged at 513 × g for 5 min at 37°C. The cells
(8×104 cells/well) were placed in an incubator under 5%
CO2 at 37°C. Following complete attachment to the walls,
2 µg of plasmid and 4 µl of Lipo3000 (Invitrogen;
Thermo Fisher Scientific, Inc.) were added. After 6-8 h, the medium
was discarded and replaced with DMEM with or without the addition
of 200 µM of H2O2 for 48 h. The groups
used were as follows: Control (no H2O2),
empty plasmid (no H2O2), C-sis plasmid
(no H2O2), H2O2 group
(no plasmid), empty plasmid+H2O2, and
C-sis plasmid+H2O2.
Animals
Female Sprague-Dawley rats (n=100, age, 8-10 weeks,
weight, 200±10 g) were purchased from Changzhou Cavens Laboratory
Animal Co., Ltd. (Changzhou, China). The rats were housed one per
cage in a room maintained at 24-25°C on a 12-h light/dark cycle
with free access to food and water. The present study was approved
by the Animal Ethics Committee of the Second Affiliated Hospital of
Nanchang University (Nanchang, China). All animal procedures were
performed in strict accordance with the guidelines for the Care and
Use of Laboratory Animals published by the US National Institutes
of Health (NIH publication no. 85-23, revised 1996). Transfection
of the target C-sis gene was performed using
hydrodynamics-based transfection in vivo, as described
previously (25,26).
FHF modeling and grouping
At 48 h post-transfection, 40 rats were randomized
into four groups (n=10 in each group): Group A, normal control
rats; group B, FHF and Ringer's solution (FHF+Ringer's solution
injection group); group C, FHF and empty vector pcDNA3.1 (FHF+empty
plasmid group); and group D, FHF and constructed plasmid
pcDNA3.1/C-sis (FHF+C-sis plasmid group). FHF was induced
through an intraperitoneal injection of 50 µg/kg of
lipopolysaccharide (LPS) and 300 mg/kg of D-galactosamine (D-GalN)
(27). At 8 h post-injection, the
liver tissues and blood samples of the normal control group (n=10),
the FHF+Ringer's solution injection group (n=9), the FHF+empty
plasmid group (n=9), and the FHF+C-sis plasmid group (n=10)
were collected. Another 40 rats were grouped as above (n=10 in each
group) to evaluate the 24-h mortality.
C-sis mRNA
Total RNA was extracted using the Rapid Extraction
kit for total RNA (Generay Biotech Co., Ltd., Shanghai, China).
First-strand cDNA was synthesized from 2 µg of total RNA by
using the TaqMan Reverse Transcription Reagents kit (Thermo Fisher
Scientific, Inc.) with oligo(dT)16 primer. The
RNA-primer mix was heated at 42°C for 5 min, and then incubated on
ice for at least 1 min The PCR primers were designed using Primer
Premier 5.0 software (Premier Biosoft International, Palo Alto, CA,
USA) based on previously reported sequences (GenBank™ accession no.
NM_24628 for C-sis, NM_81822 for β-actin). The primers were
as follows: C-sis, forward, 5′-ATGACCCGAGCACATTCTGG-3′ and
reverse, 5′-ACACCTCTGTACGCGTCTTG-3′; and β-actin, forward,
5′-CCCATCTATGAGGGTTACGC-3′ and reverse,
5′-TTTAATGTCACGCACGATTTC-3′. The PCR analysis was performed with
500 ng of cDNA, 1 µl of each primer, 10 µl of 2X
SuperReal premix plus, and ddH2O to achieve a final
volume of 20 µl. The conditions were as follows: i) 95.0°C
for 15 min; ii) 40 cycles at 95.0°C for 10 sec, 60.0°C for 20 sec,
and 72.0°C for 20 sec. The results were calculated and analyzed
using quantitative fluorescence PCR analysis software (BIO-RAD CFX
Manager version 3.0; Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Western blot analysis
Total proteins were extracted using a total protein
extraction kit. The protein concentrations were determined using
BCA protein assay kit (Beyotime Institute of Biotechnology,
Shanghai, China). The proteins (100 µg, 7
µg/µl) were separated by 10% denaturing
SDS-polyacrylamide gel electrophoresis and transferred onto a
polyvinylidene difluoride membrane. The membrane was blocked with
5% non-fat dry milk for 1 h at room temperature, washed, and
blotted with primary antibodies against C-sis (cat. no.
ab78409; 1:400), cleaved poly (ADP-ribose) polymerase 1 (PARP1;
cat. no. ab32064; 1:1,000), cleaved caspase-3 (cat. no. ab2302;
1:1,000), B-cell lymphoma 2 (Bcl-2; cat. no. ab196495; 1:1,000),
Bcl-2-associated X protein (Bax; cat. no. ab32503; 1:1,000) (all
from Abcam, Cambridge, MA, USA), or GAPDH (cat. no. AP0063; 1:400;
Bioworld Technology, Inc., Louis Park, MN, USA), and incubated
overnight at 4°C. This was followed by incubation with a
horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G
(cat. no. A50-106P; 1:1,000; Origene Technologies, Inc, Beijing,
China) at room temperature for 1 h. The bands were quantified using
Quantity One 4.62 software (Bio-Rad Laboratories, Inc.). GAPDH was
used as an internal control.
Histological examination
The liver tissues were fixed in 10% formaldehyde
solution for 30 min at room temperature, paraffin-embedded,
sectioned at 4-µm, and stained using hematoxylin and eosin
at room temperature for 10 min under an Olympus BH-2 light
microscope (Olympus Corporation, Tokyo, Japan).
Cell viability
Cell viability was assessed using the CCK8 assay.
The cells (100 µl; 5,000 cells/well) were added to a 96-well
plate. The plate was incubated for 48 h at 37°C under 5%
CO2. The medium was then discarded and replaced with
fresh medium containing 10 µl of CCK8 solution (Beyotime
Institute of Biotechnology, Shanghai, China). Blank control wells
received 0.9% saline. The plates were incubated at 37°C for 1 h in
the dark. The optical density was measured at 450 nm.
TUNEL assay
Cell apoptosis was detected using a commercial TUNEL
assay (cat. no. 11684817910; Roche Applied Science, Penzberg,
Germany), in strict accordance with the manufacturer's protocol.
The slides were counterstained using hematoxylin at room
temperature for 1 min, and sealed with neutral gum.
Cell apoptosis
A Cell Apoptosis kit (UNOCI Biological Technology
Co., Ltd., Hangzhou, China) was used to detect cell apoptosis, in
strict accordance with the manufacturer's protocol. A flow
cytometer (ACCURI C6; BD Biosciences, Franklin Lake, NJ, USA) was
used to detect apoptotic cells. The data was analyzed using FlowJo
software version 10.0.5 for Microsoft (FlowJo LLC, Ashland, OR,
USA).
Cell cycle analysis
A Cell Cycle Analysis kit (Beyotime Institute of
Biotechnology) was used to measure cell cycle data. Briefly, the
cells were collected by centrifugation at 513 × g for 15 min at
37°C, washed twice with 4°C PBS, and fixed with 75% cold ethanol at
4°C for 24 h. The cells were collected by centrifugation at 513 × g
for 5 min at 37°C, dried, and washed twice with PBS. Subsequently,
1 ml of Reagent A was added; the tube was then mixed well for 5-10
sec and incubated for 30 h. Flow cytometry (ACCURI C6; BD
Biosciences) was used to measure cell cycle data.
Immunohistochemistry
The liver tissues were fixed with 4%
paraformaldehyde solution at room temperature for 30 min,
paraffin-embedded, sectioned at 4-µm, and dewaxed.
Endogenous peroxidase was blocked and inactivated, and the sections
were blotted overnight at 4°C with a primary antibody against
caspase-3 (cat. no. ab2302; 1:100; Abcam), followed by incubation
with the biotinylated rabbit secondary antibody (cat. no. BA1100;
1:375; Vector Laboratories, Inc., Burlingame, CA, USA) at 37°C for
1 h. Finally, the sections were stained with DAB and then
counterstained with hematoxylin at room temperature for 1 min.
Three different fields were selected from each section under an
Olympus BH-2 light microscope (Olympus Corporation; magnification,
×200). The intensity of the expression and the positivity rate were
measured. The expression score was calculated as the staining
intensity multiplied by the percentage of positive cells, as
previously described (28).
Alanine transaminase (ALT) and aspartate
transaminase (AST) measurement
An ALT detection kit (Nanjing Jiancheng
Bioengineering Institute, cat. no. C009-2, batch no. 20141105) and
an AST detection kit (Nanjing Jiancheng Bioengineering Institute,
cat. no. C0010-2, batch no. 20141104) were used in strict
accordance with the manufacturer's protocol.
Statistical analysis
Data are presented as the mean ± standard deviation
and were analyzed using one-way analysis of variance followed by
the least significant difference test. Statistical analysis was
performed using SPSS 13.0 (SPSS, Inc., Chicago, IL, USA). Each
experiment was repeated three times. P<0.05 was considered to
indicate a statistically significant difference.
Results
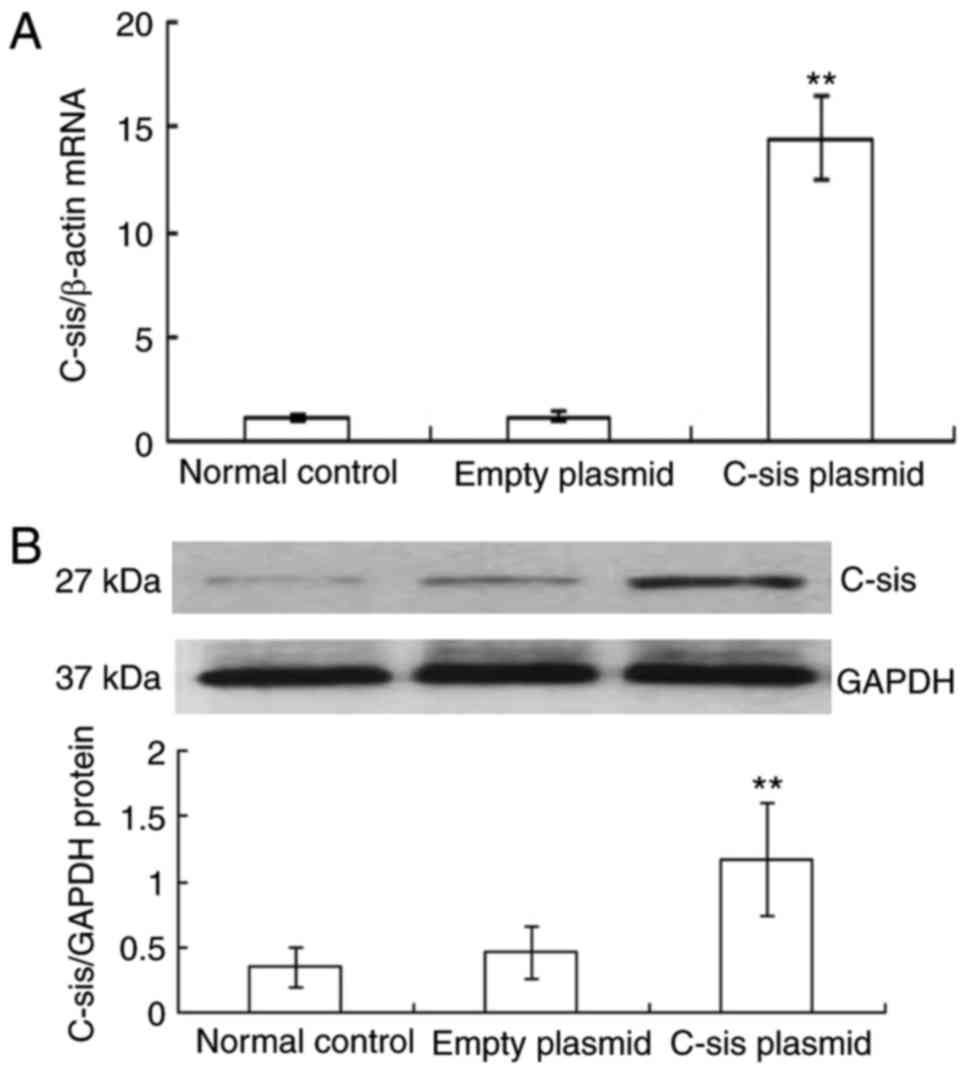
Overexpression of C-sis in BRL cells
Reactive oxygen species are crucial in FHF and acute
hepatic failure (29). The
present study used H2O2, which is known to
induce apoptosis in mouse primary cultured hepatocytes (30), to cause oxidative stress and
thereby induce apoptosis of BRL cells. Compared with the control
and empty plasmid groups, the mRNA expression of C-sis in
the C-sis plasmid group was significantly increased at 48 h
post-transfection (P<0.01; Fig.
1A). Similarly, the protein expression of C-sis in the
C-sis plasmid group at 48 h post-transfection was
significantly increased when compared with the control and the
empty plasmid groups (P<0.01; Fig.
1B). These results showed that C-sis was successfully
overexpressed in the BRL cells.
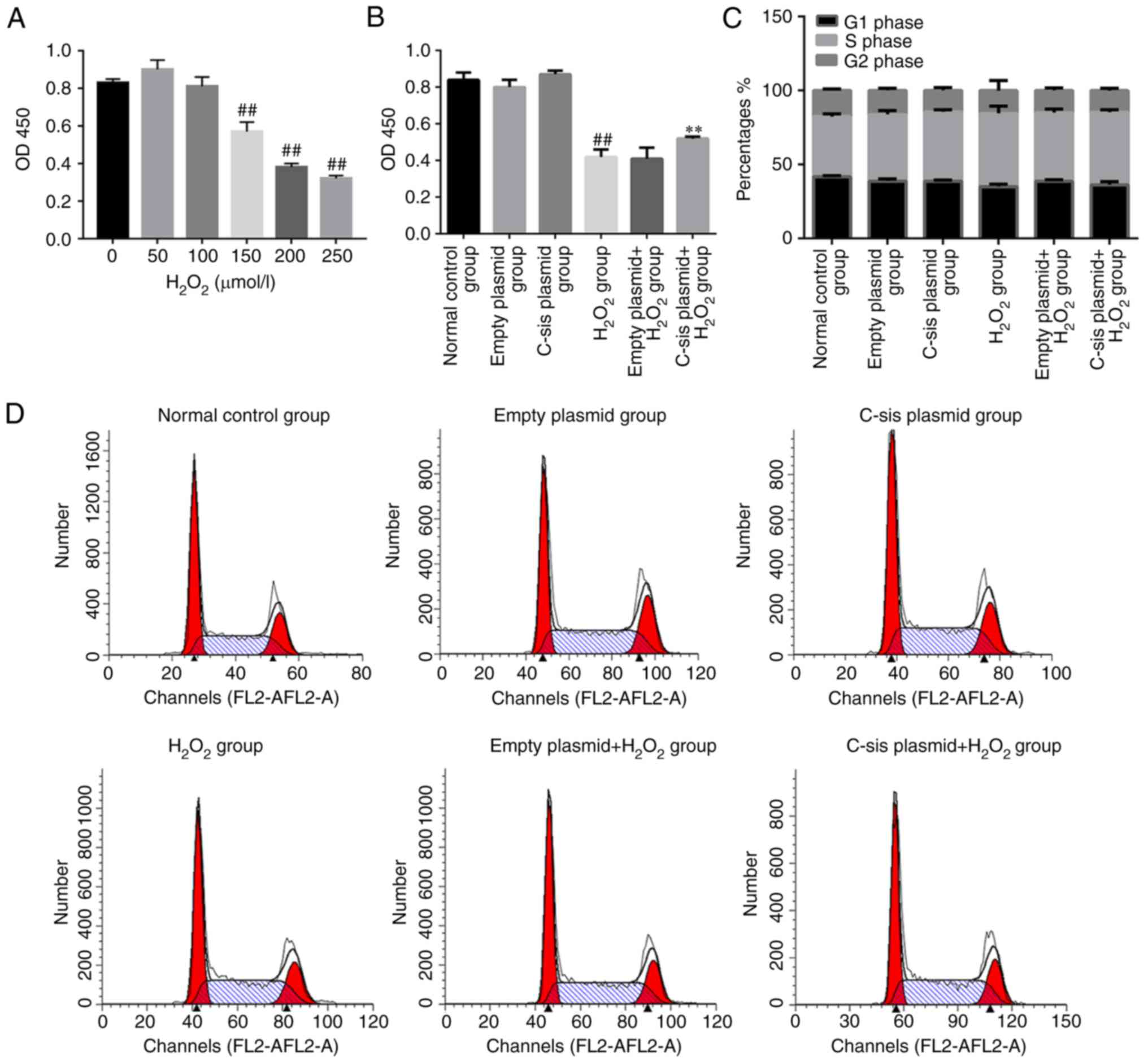
Cell viability and cell cycle analysis in
each group
Reactive oxygen species are crucial in FHF and acute
hepatic failure (29).
H2O2 treatment led to a significant decrease
in cell viability when the concentration of
H2O2 reached 150 µmol/l (Fig. 2A). As shown in Fig. 2B, the cell viability in the
H2O2 group was significantly decreased
compared with that in the control group (P<0.01). Compared with
the empty plasmid+H2O2 group, the cell
viability in the C-sis plasmid+H2O2
group was significantly increased (P<0.01). Cell cycle was
assessed by flow cytometry and the results demonstrated no
significant differences in cell cycle among the groups (Fig. 2C and D).
Cell apoptosis in each group
Four populations of cells were detected using flow
cytometry: Q1-UL (necrotic cells and debris), Q1-UR (late stage
apoptotic cells and necrotic cells), Q1-LL (normal cells), and
Q1-LR (apoptotic cells at the early stage, as detected by CCK8).
The sum of Q1-UR and Q1-LR was considered the apoptotic rate.
Compared with the normal control group, cell apoptosis in the
H2O2 group was significantly increased
(P<0.01). Compared with the empty
plasmid+H2O2 group, cell apoptosis of the
C-sis plasmid+H2O2 group was
significantly decreased (P<0.01; Fig. 3A and B). The results of the TUNEL
assay were consistent with the flow cytometry results (Fig. 3C and D).
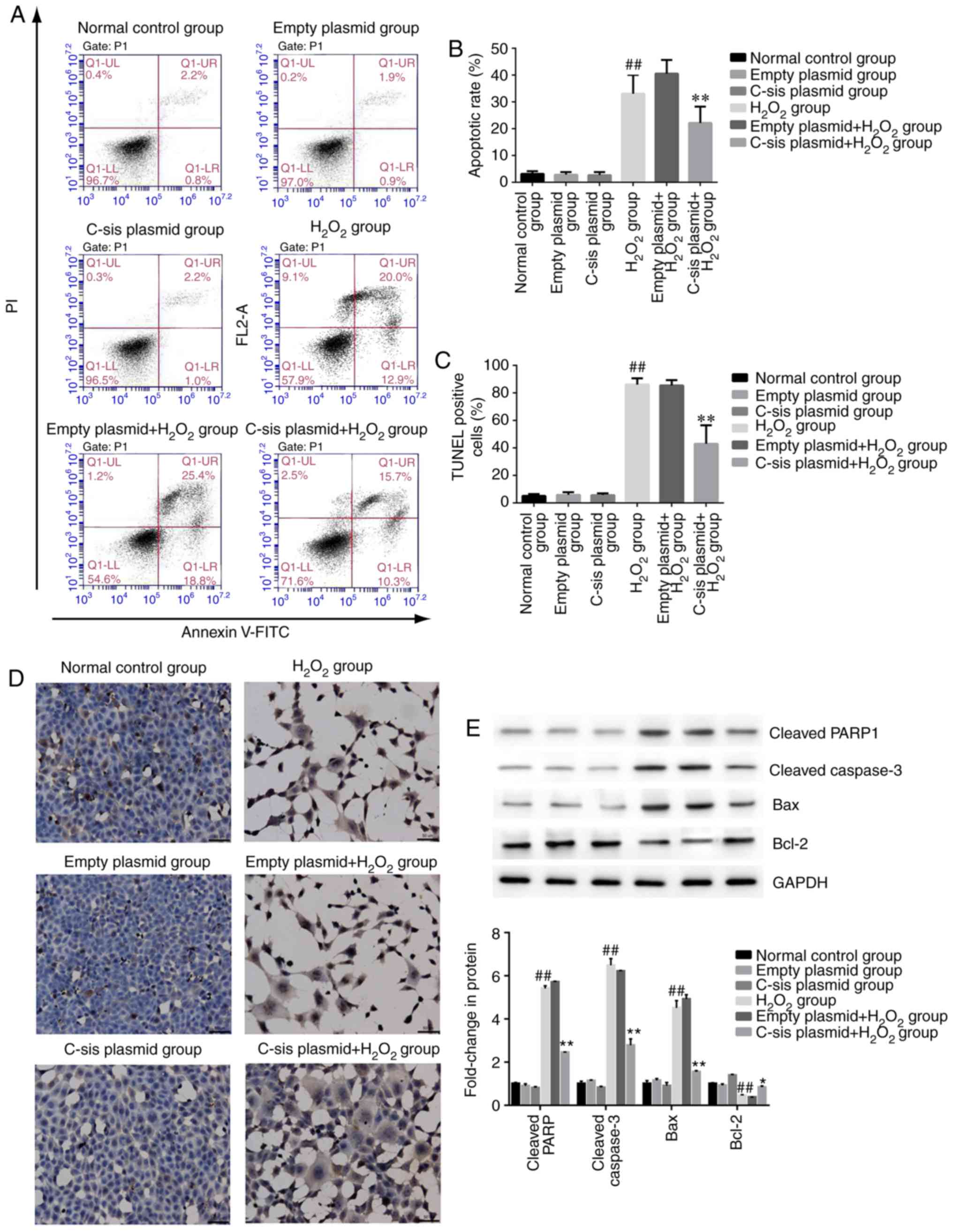 | Figure 3Cell apoptosis in each group. (A)
Representative results of BRL cell apoptosis, measured by flow
cytometry. (B) BRL cell apoptosis detected by flow cytometry. The
results are presented as the mean ± standard deviation calculated
from five replicates. ##P<0.01 vs. normal control
group; **P<0.01 vs. empty
plasmid+H2O2 group. (C) Detection of BRL cell
apoptosis using the TUNEL assay. The results are presented as the
mean ± standard deviation calculated from four replicates.
##P<0.01 vs. normal control group;
**P<0.01 vs. empty plasmid+H2O2
group. (D) Representative images of sections stained with TUNEL.
Magnificatiion, ×200. (E) Western blot analysis of cleaved PARP1,
cleaved caspase-3, Bax, and Bcl-2 in BRL cells. Values are
presented as the mean ± standard deviation calculated from five
replicates. ##P<0.01. vs. normal control group.
*P<0.05 vs. empty plasmid+H2O2
group. BRL, Buffalo rat liver; H2O2, hydrogen
peroxide; PARP1, poly (ADP-ribose) polymerase 1; Bcl-2, B-cell
lymphoma 2; Bax, Bcl-2-associated X protein. |
In addition, as shown in Fig. 3E, the protein expression levels of
the pro-apoptotic proteins, cleaved caspase-3, cleaved PARP1 and
Bax, were significantly increased in the H2O2
group, compared with those in the normal control group (P<0.01).
Compared with the empty plasmid+H2O2 group,
the expression levels of cleaved caspase-3, cleaved PARP1 and Bax
were significantly decreased in the C-sis
plasmid+H2O2 group (P<0.01). However, the
anti-apoptotic protein Bcl-2 showed the opposite protein expression
pattern compared with the three pro-apoptotic proteins.
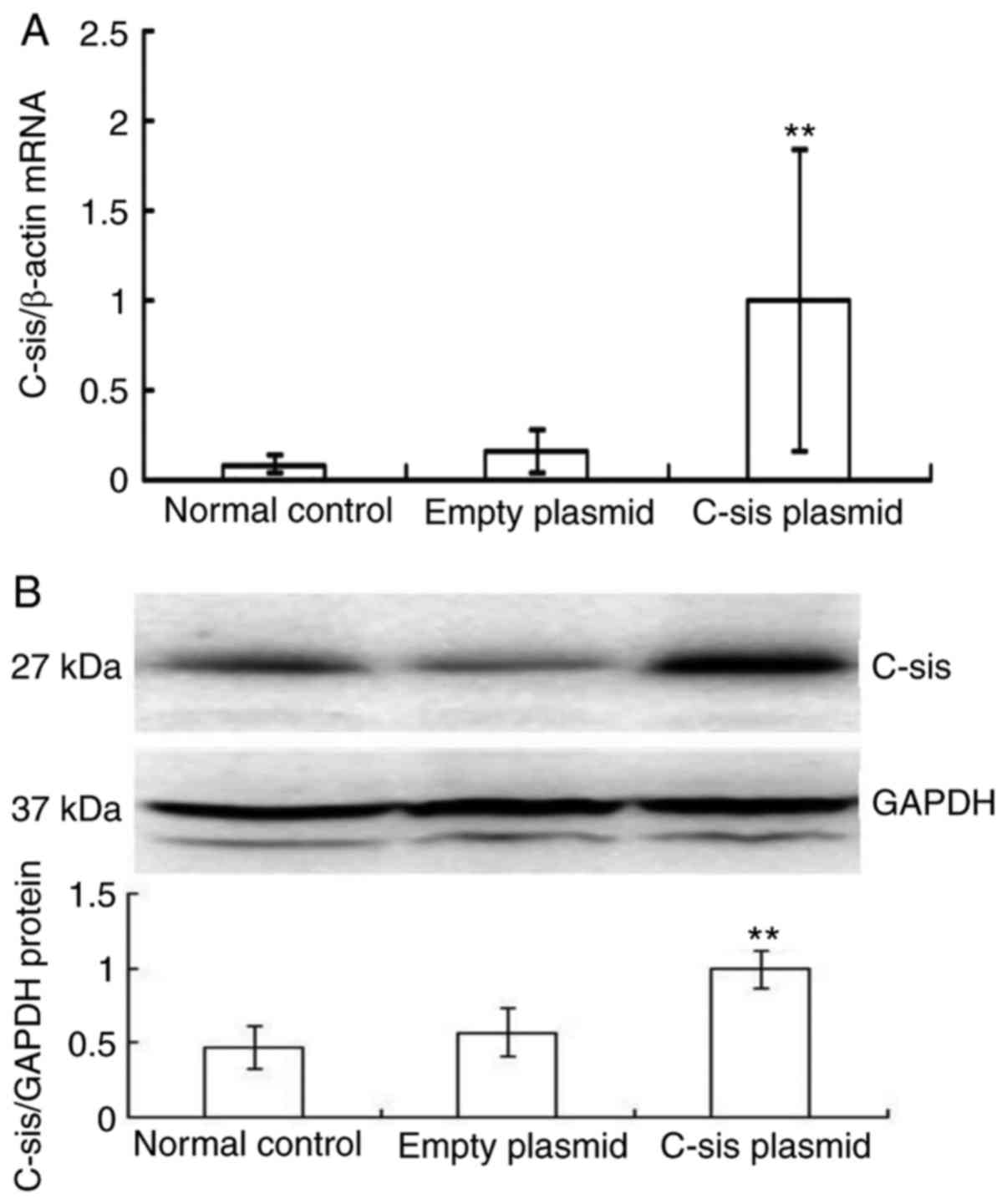
Overexpression of C-sis in the FHF rat
models
There was a significant increase in the mRNA
expression of C-sis in the C-sis plasmid group
compared with the normal control and empty plasmid groups
(P<0.01; Fig. 4A), and there
was a significant increase in the protein expression of
C-sis in the C-sis plasmid group compared with the
normal control and empty plasmid groups (P<0.01; Fig. 4B). These results showed that
C-sis was successfully overexpressed in the FHF rats.
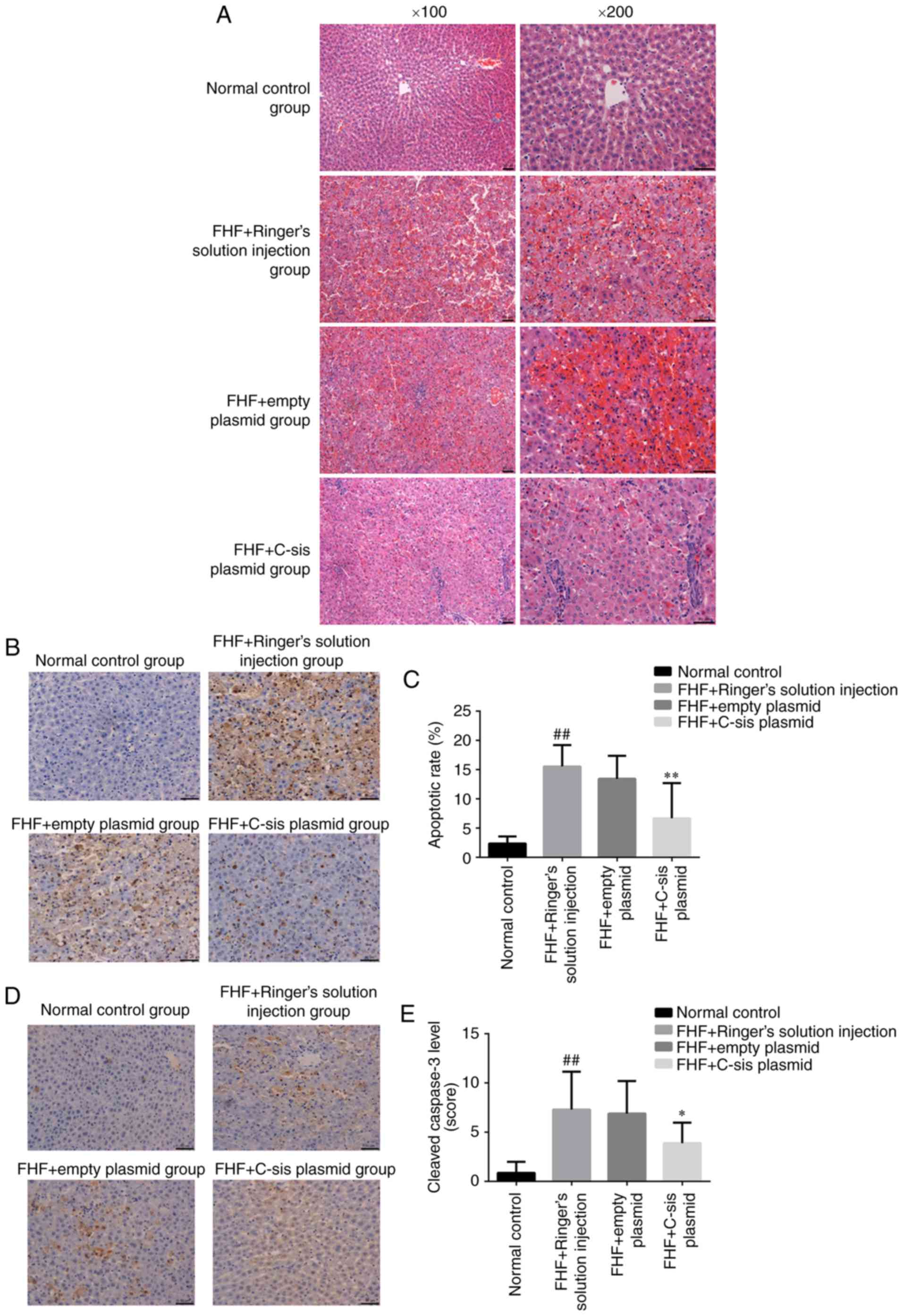
Histological examination in each
group
In the normal control group, the cellular structure
of the liver tissue was normal, and the liver cells were tightly
arranged. The nuclei were round, and the cells were without lesions
in the form of lipid droplets, inflammation or necrosis. The
lobular structure was normal, and hepatocytes and sinusoidal cells
were radially arranged around the central vein (Fig. 5A). In the FHF+Ringer's solution
injection group, the infiltration of inflammatory cells, mainly
neutrophils, was observed. Scattered cell apoptosis was observed
inside the liver of eight rats, and extensive necrosis was observed
in one rat. The organizational structure of the liver had
disappeared, and blood stasis was observed within the sinusoid
(Fig. 5A). In the FHF+empty
plasmid group, the infiltration of inflammatory cells, mainly
neutrophils, was observed. Scattered cell apoptosis was observed in
the liver of seven rats, whereas extensive necrosis was observed in
two rats. Marked blood stasis was found in the sinusoid (Fig. 5A). In the FHF+C-sis plasmid
group, small focal inflammation with neutrophils was observed in
the liver tissue. Cell apoptosis was observed in four rats, in
which the nuclei were condensed. Cell structure was destroyed and
extensive necrosis was present in one rat (Fig. 5A). Together, these results showed
that the overexpression of C-sis alleviated liver
injury.
Apoptosis in each group
Compared with the normal control group, there was a
significant increase in cell apoptosis in the FHF+Ringer's solution
injection group (P<0.01). Compared with the FHF+empty plasmid
group, there was a significant decrease in cell apoptosis in the
FHF+C-sis plasmid group (P<0.01; Fig. 5B and C). Compared with the normal
control group, there was a significant increase in cleaved
caspase-3 in the FHF+Ringer's solution injection group (P<0.01).
Compared with the FHF+empty plasmid group, there was a significant
decrease in cleaved caspase-3 in the FHF+C-sis plasmid group
(P<0.05; Fig. 5D and E).
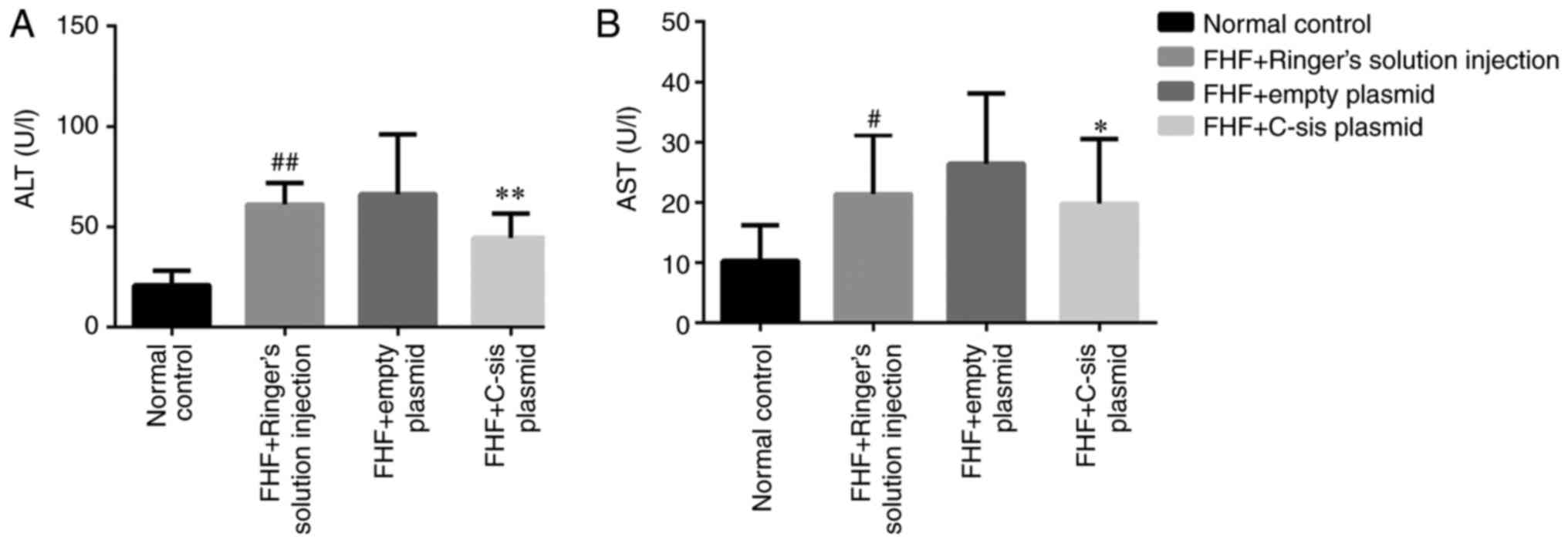
Serum levels of ALT and AST in each
group
Compared with the normal control group, the serum
levels of ALT (P<0.01) and AST (P<0.05) in the FHF+Ringer's
solution injection group were significantly increased. Compared
with the FHF+empty plasmid group, the serum levels of ALT
(P<0.01) and AST (P<0.05) in the FHF+C-sis plasmid
group were significantly decreased (Fig. 6A and B).
Animal mortality rates in each group
Within the 24-h observation period, all rats in the
normal control group survived, whereas 70.0 and 80.0% of the rats
died within 24 h in the FHF+Ringer's injection and FHF+empty
plasmid groups, respectively. Transfection of cells with the
C-sis gene effectively protected the animals from death
induced by LPS/D-GalN, and only two of these rats (20.0%) died
within the 24-h observation period (Table I).
 | Table IMortality rate of the rats in the
animal model of FHF. |
Table I
Mortality rate of the rats in the
animal model of FHF.
| Group | 0 h | 4 h | 8 h | 12 h | 24 h | Mortality (%) |
|---|
| Normal control | 0 | 0 | 0 | 0 | 0 | 0.00 |
| FHF+Ringer's
solution injection | 0 | 0 | 3 | 2 | 2 | 70.00a |
| FHF+empty
plasmid | 0 | 0 | 3 | 3 | 2 | 80.00 |
| FHF+C-sis
plasmid | 0 | 0 | 0 | 2 | 0 | 20.00b |
Discussion
The present study aimed to investigate the role of
the C-sis gene in the apoptosis of hepatocytes in
vitro and in liver function in a rat model of FHF. The results
showed that the overexpression of C-sis not only inhibited
the H2O2-induced apoptosis of hepatocytes
in vitro but also improved liver function and decreased
mortality in the rat models of FHF. Previous studies have shown
that FHF mainly involves hepatocyte apoptosis rather than necrosis
(14,31-33). Therefore, the inhibition of
hepatocyte apoptosis can assist in preventing hepatocyte
necrosis.
Previous studies have shown that PDGF-BB is involved
in the resistance against oxidative stress in vascular smooth
muscle cells (34), neurons
(35,36), the intestine (37), and liver (38). The results of the present study
showed that the overexpression of C-sis inhibited
H2O2-induced BRL cell apoptosis. However, no
significant difference in cell cycle was observed among the groups.
The possible reason for this may be that the C-sis gene can
function only through cell apoptosis and exerts no effect on the
cell cycle. The in vivo experiments showed that the
overexpression of C-sis alleviated histological damage,
improved liver function, and decreased mortality rate in the FHF
rats. To a certain extent, these results are consistent with a
previous study showing that low plasma levels of PDGF-BB were
associated with poor prognosis in patients with FHF (14). Taken together, the results of the
present study suggested that C-sis d cell viability and
inhibited apoptosis, and promoted tissue repair. Therefore, it was
hypothesized that C-sis may have a positive role in the
repair of damaged liver tissue and in the treatment of FHF.
The basal function of C-sis is to promote the
intracellular transduction of mitotic signals and promote cell
proliferation (39,40). C-sis encodes PDGF-B, which
is a potent mitogenic source that can stimulate the division and
proliferation of mesenchymal cells, and has a positive role in
promoting vascular regeneration and wound healing (41,42). PDGF-B can stimulate the healing of
ulcers in diabetic rats (43).
Novel technologies are being developed that focus on the slow
release of PDGF-B from a membrane to accelerate wound healing in
patients with diabetes (44). The
loss of function caused by PDGF-B mutation can lead to primary
familial cerebral calcification, resulting in neurodegenerative
disease (45,46).
The present study also considered the problem of
carcinogenesis that arises following the long-term presence of the
proto-oncogene C-sis. The majority of current findings
related to C-sis are focused on the fact that C-sis
can promote cell growth and tissue repair, however, certain studies
have focused on the occurrence and progression of tumors (20). This is the reason why C-sis
was selected for the treatment of FHF in the present study rather
than other proto-oncogenes. Second, the present study aimed to
observe the efficacy of C-sis on an acute life-threatening
disease, FHF. Proto-oncogenes exist in the normal human body but
are usually inactive. The occurrence of cancer is a process that
depends on numerous steps, and involves the activation of several
proto-oncogenes and the inactivation of tumor suppressor genes; the
activation of a single proto-oncogene may not cause cancer to
arise. In addition, the inactivation of tumor suppressor genes is
the main cause of cancer. Finally, previous studies performed
experiments in which the proto-oncogene Pim-3 was used for the
treatment of heart or liver failure; however, no tumor occurrence
was observed (25,47).
H2O2 is a potent oxidant, and
studies have shown that H2O2 can induce
hepatocyte apoptosis (48,49).
In the present study, LPS and D-GalN did not exert a significant
effect on the proliferation of the BRL liver cell line (data not
shown). Fas and ischemia-reperfusion can be separately used for the
induction of mouse models of liver failure, whereas
H2O2 is used to induce injury in cells
(50,51). Therefore, in the present study,
different methods had to be used to induce liver cell damage in the
animals and in the cell line, which is a limitation of the study.
Other limitations include the lack of comprehensive molecular
analyses to determine the exact pathways involved in the response
to the overexpression of C-sis, and the lack of
investigation of the cytokines involved. In addition, only the
short-term effects of the overexpression of C-sis were
examined, although it is known that PDGF-BB can be involved in
long-term liver fibrosis (52).
These issues are to be examined in future investigations.
Increasing evidence suggests the novel involvement
of the nucleolus in sensing cellular stress signals (53). Under stress conditions, the
structure of the nucleolus is perturbed and certain nucleolar
proteins, including ribosomal proteins, are released from the
nucleolus to the nucleoplasm where they associate with Mouse Double
Minute 2 (MDM2) to inhibit its activity and stabilize p53 (53,54). Reactive oxygen species are crucial
in FHF (55). The present study
used H2O2 to induce oxidative stress in order
to induce the apoptosis of BRL cells. Upon cell exposure to
H2O2, redox changes in the nucleolar
compartment are associated with activation of the ribosomal
protein/MDM2/p53 pathway leading to apoptosis. In addition,
previous data suggests that nuclear factor (NF)-κB is involved in
apoptosis as part of the cell response to the nucleolar stress
triggered by 5-fluorouracil (56). Inflammation is essential for the
pathogenesis of FHF (55), and
the NF-κB pathway has been shown to be key in the activation of the
pro-inflammatory mechanism in FHF (55,57). These findings may assist in
understanding the plausible molecular mechanisms underlying the
role of C-sis in apoptosis.
In conclusion, the results of the present study
showed that the overexpression of C-sis inhibited the
H2O2-induced apoptosis of BRL cells in
vitro, and alleviated liver injury, improved liver function and
decreased mortality rate in rat model of FHF. These findings may
assist in understanding the progression of FHF and may provide
potential therapeutic approaches.
Acknowledgments
Not applicable.
Funding
This study was funded by the National Natural
Science Foundation of China (grant no. 81300348).
Availability of data and materials
The datasets used and/or analysed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
HD and ZW conceived and designed the study. HD
performed the experiments and wrote the paper. ZW reviewed and
edited the manuscript. All authors read and approved the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Committee of the Second Affiliated Hospital of Nanchang University.
All animal procedures were performed in strict accordance with the
guidelines for the Care and Use of Laboratory Animals published by
the US National Institutes of Health (NIH publication no. 85-23,
revised 1996).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Moreau R: The pathogenesis of ACLF: The
inflammatory response and immune function. Semin Liver Dis.
36:133–140. 2016. View Article : Google Scholar
|
|
2
|
Tuñón M, San-Miguel B, Crespo I, Jorquera
F, Santamaría E, Alvarez M, Prieto J and González-Gallego J:
Melatonin attenuates apoptotic liver damage in fulminant hepatic
failure induced by the rabbit hemorrhagic disease virus. J Pineal
Res. 50:38–45. 2011. View Article : Google Scholar
|
|
3
|
Hoofnagle JH, Carithers RL Jr, Shapiro C
and Ascher N: Fulminant hepatic failure: Summary of a workshop.
Hepatology. 21:240–252. 1995.
|
|
4
|
Campbell DA Jr, Ham JM, Mccurry KR, Lucey
MR, Turcotte JG and Merion RM: Liver transplant for fulminant
hepatic failure. Am Surg. 57:546–549. 1991.
|
|
5
|
Andrew SdMD and Parsia AVMD: Expanding the
donor pool in liver transplantation: Extended criteria donors. Clin
Liver Dis. 2:156–159. 2013. View Article : Google Scholar
|
|
6
|
Dunn S and Cowling VH: Myc and mRNA
capping. Biochim Biophys Acta. 1849.501–505. 2015.
|
|
7
|
Landau E, Tirosh R, Pinson A, Banai S,
Even-Ram S, Maoz M, Katzav S and Bar-Shavit R: Protection of
thrombin receptor expression under hypoxia. J Biol Chem.
275:2281–2287. 2000. View Article : Google Scholar
|
|
8
|
Hellström M, Kalen M, Lindahl P, Abramsson
A and Betsholtz C: Role of PDGF-B and PDGFR-beta in recruitment of
vascular smooth muscle cells and pericytes during embryonic blood
vessel formation in the mouse. Development. 126:3047–3055.
1999.
|
|
9
|
Zhao S, Zhang Z, Qian L, Lin Q, Zhang C,
Shao J, Zhang F and Zheng S: Tetramethylpyrazine attenuates carbon
tetrachloride-caused liver injury and fibrogenesis and reduces
hepatic angiogenesis in rats. Biomed Pharmacother. 86:521–530.
2017. View Article : Google Scholar
|
|
10
|
Sun WY, Song Y, Hu SS, Wang QT, Wu HX,
Chen JY and Wei W: Depletion of β-arrestin2 in hepatic stellate
cells reduces cell proliferation via ERK pathway. J Cell Biochem.
114:1153–1162. 2013. View Article : Google Scholar
|
|
11
|
Wilhelm A, Aldridge V, Haldar D, Naylor
AJ, Weston CJ, Hedegaard D, Garg A, Fear J, Reynolds GM, Croft AP,
et al: CD248/endosialin critically regulates hepatic stellate cell
proliferation during chronic liver injury via a PDGF-regulated
mechanism. Gut. 65:1175–1185. 2016. View Article : Google Scholar
|
|
12
|
Kastanis GJ, Hernandez-Nazara Z, Nieto N,
Rincón-Sanchez AR, Popratiloff A, Dominguez-Rosales JA, Lechuga CG
and Rojkind M: The role of dystroglycan in PDGF-BB-dependent
migration of activated hepatic stellate cells/myofibroblasts. Am J
Physiol Gastrointest Liver Physiol. 301:G464–G474. 2011. View Article : Google Scholar
|
|
13
|
Hao ZM, Fan XB, Li S, Lv YF, Su HQ, Jiang
HP and Li HH: Vaccination with platelet-derived growth factor B
kinoids inhibits CCl(4)-induced hepatic fibrosis in mice. J
Pharmacol Exp Ther. 342:835–842. 2012. View Article : Google Scholar
|
|
14
|
Takayama H, Miyake Y, Nouso K, Ikeda F,
Shiraha H, Takaki A, Kobashi H and Yamamoto K: Serum levels of
platelet-derived growth factor-BB and vascular endothelial growth
factor as prognostic factors for patients with fulminant hepatic
failure. J Gastroenterol Hepatol. 26:116–121. 2011. View Article : Google Scholar
|
|
15
|
van Dijk F, Olinga P, Poelstra K and
Beljaars L: Targeted therapies in liver fibrosis: Combining the
best parts of platelet-derived growth factor BB and interferon
gamma. Front Med (Lausanne). 2:722015.
|
|
16
|
Hirota JA, Ask K, Farkas L, Smith JA,
Ellis R, Rodriguez-Lecompte JC, Kolb M and Inman MD: In vivo role
of platelet-derived growth factor-BB in airway smooth muscle
proliferation in mouse lung. Am J Respir Cell Mol Biol. 45:566–572.
2011. View Article : Google Scholar
|
|
17
|
Rovida E, Navari N, Caligiuri A, Dello
Sbarba P and Marra F: ERK5 differentially regulates PDGF-induced
proliferation and migration of hepatic stellate cells. J Hepatol.
48:107–115. 2008. View Article : Google Scholar
|
|
18
|
Gressner AM and Weiskirchen R: Modern
pathogenetic concepts of liver fibrosis suggest stellate cells and
TGF-beta as major players and therapeutic targets. J Cell Mol Med.
10:76–99. 2006. View Article : Google Scholar
|
|
19
|
Patsenker E, Popov Y, Wiesner M, Goodman
SL and Schuppan D: Pharmacological inhibition of the vitronectin
receptor abrogates PDGF-BB-induced hepatic stellate cell migration
and activation in vitro. J Hepatol. 46:878–887. 2007. View Article : Google Scholar
|
|
20
|
Lin X, Kong LN, Huang C, Ma TT, Meng XM,
He Y, Wang QQ and Li J: Hesperetin derivative-7 inhibits
PDGF-BB-induced hepatic stellate cell activation and proliferation
by targeting Wnt/β-catenin pathway. Int Immunopharmacol.
25:311–320. 2015. View Article : Google Scholar
|
|
21
|
Fang L, Zhan S, Huang C, Cheng X, Lv X, Si
H and Li J: TRPM7 channel regulates PDGF-BB-induced proliferation
of hepatic stellate cells via PI3K and ERK pathways. Toxicol Appl
Pharmacol. 272:713–725. 2013. View Article : Google Scholar
|
|
22
|
Shin HW, Park SY, Lee KB, Shin E, Nam SW,
Lee JY and Jang JJ: Transcriptional profiling and Wnt signaling
activation in proliferation of human hepatic stellate cells induced
by PDGF-BB. Korean J Hepatol. 15:486–495. 2009. View Article : Google Scholar
|
|
23
|
Chen SW, Chen YX, Zhang XR, Qian H, Chen
WZ and Xie WF: Targeted inhibition of platelet-derived growth
factor receptor-beta subunit in hepatic stellate cells ameliorates
hepatic fibrosis in rats. Gene Ther. 15:1424–1435. 2008. View Article : Google Scholar
|
|
24
|
Zeng Y, Liu H, Kang K, Wang Z, Hui G,
Zhang X, Zhong J, Peng W, Ramchandran R, Raj JU and Gou D: Hypoxia
inducible factor-1 mediates expression of miR-322: Potential role
in proliferation and migration of pulmonary arterial smooth muscle
cells. Sci Rep. 5:120982015. View Article : Google Scholar
|
|
25
|
Liu LM, Zhang JX, Wang XP, Guo HX, Deng H
and Luo J: Pim-3 protects against hepatic failure in
D-galactosamine (D-GalN)-sensitized rats. Eur J Clin Invest.
40:127–138. 2010. View Article : Google Scholar
|
|
26
|
Maruyama H, Higuchi N, Nishikawa Y, Kameda
S, Iino N, Kazama JJ, Takahashi N, Sugawa M, Hanawa H, Tada N, et
al: High-level expression of naked DNA delivered to rat liver via
tail vein injection. J Gene Med. 4:333–341. 2002. View Article : Google Scholar
|
|
27
|
Sayed RH, Khalil WK, Salem HA, Kenawy SA
and El-Sayeh BM: Sulforaphane increases the survival rate in rats
with fulminant hepatic failure induced by D-galactosamine and
lipopolysaccharide. Nutr Res. 34:982–989. 2014. View Article : Google Scholar
|
|
28
|
Kara S, Gencer B, Karaca T, Tufan HA,
Arikan S, Ersan I, Karaboga I and Hanci V: Protective effect of
hesperetin and naringenin against apoptosis in
ischemia/reperfusion-induced retinal injury in rats. Sci World J.
2014:7978242014. View Article : Google Scholar
|
|
29
|
Xiong Q, Hase K, Tezuka Y, Namba T and
Kadota S: Acteoside inhibits apoptosis in D-galactosamine and
lipopolysaccha-ride-induced liver injury. Life Sci. 65:421–430.
1999. View Article : Google Scholar
|
|
30
|
Kanno S, Ishikawa M, Takayanagi M,
Takayanagi Y and Sasaki K: Characterization of hydrogen
peroxide-induced apoptosis in mouse primary cultured hepatocytes.
Biol Pharm Bull. 23:37–42. 2000. View Article : Google Scholar
|
|
31
|
Ogasawara J, Watanabe-Fukunaga R, Adachi
M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T and Nagata S:
Lethal effect of the anti-Fas antibody in mice. Nature.
364:806–809. 1993. View Article : Google Scholar
|
|
32
|
Tanoi T, Tamura T, Sano N, Nakayama K,
Fukunaga K, Zheng YW, Akhter A, Sakurai Y, Hayashi Y, Harashima H
and Ohkohchi N: Protecting liver sinusoidal endothelial cells
suppresses apoptosis in acute liver damage. Hepatol Res.
46:697–706. 2016. View Article : Google Scholar
|
|
33
|
Gao G, Yu Z, Yan J, Li J, Shen S, Jia B,
Guan K, Gao X and Kan Q: Lowering blood ammonia prevents hepatocyte
injury and apoptosis. Int J Clin Exp Med. 8:12347–12355. 2015.
|
|
34
|
Salabei JK, Cummins TD, Singh M, Jones SP,
Bhatnagar A and Hill BG: PDGF-mediated autophagy regulates vascular
smooth muscle cell phenotype and resistance to oxidative stress.
Biochem J. 451:375–388. 2013. View Article : Google Scholar
|
|
35
|
Zheng L, Ishii Y, Tokunaga A, Hamashima T,
Shen J, Zhao QL, Ishizawa S, Fujimori T, Nabeshima Y, Mori H, et
al: Neuroprotective effects of PDGF against oxidative stress and
the signaling pathway involved. J Neurosci Res. 88:1273–1284.
2010.
|
|
36
|
Zheng LS, Ishii Y, Zhao QL, Kondo T and
Sasahara M: PDGF suppresses oxidative stress induced
Ca2+ overload and calpain activation in neurons. Oxid
Med Cell Longev. 2013:3672062013. View Article : Google Scholar
|
|
37
|
Krzystek-Korpacka M, Neubauer K and
Matusiewicz M: Platelet-derived growth factor-BB reflects clinical,
inflammatory and angiogenic disease activity and oxidative stress
in inflammatory bowel disease. Clin Biochem. 42:1602–1609. 2009.
View Article : Google Scholar
|
|
38
|
Urtasun R, Conde de la Rosa L and Nieto N:
Oxidative and nitrosative stress and fibrogenic response. Clin
Liver Dis. 12:769–790. 2008. View Article : Google Scholar
|
|
39
|
Saito Y, Hojo Y, Tanimoto T, Abe J and
Berk BC: Protein kinase C-alpha and protein kinase C-epsilon are
required for Grb2-associated binder-1 tyrosine phosphorylation in
response to platelet-derived growth factor. J Biol Chem.
277:23216–23222. 2002. View Article : Google Scholar
|
|
40
|
Lindqvist A, Nilsson BO, Ekblad E and
Hellstrand P: Platelet-derived growth factor receptors expressed in
response to injury of differentiated vascular smooth muscle in
vitro: Effects on Ca2+ and growth signals. Acta Physiol
Scand. 173:175–184. 2001. View Article : Google Scholar
|
|
41
|
Cheng M, Park H, Engelmayr GC, Moretti M
and Freed LE: Effects of regulatory factors on engineered cardiac
tissue in vitro. Tissue Eng. 13:2709–2719. 2007. View Article : Google Scholar
|
|
42
|
Zhang L, Ma J, Shen T, Wang S, Ma C, Liu
Y, Ran Y, Wang L, Liu L and Zhu D: Platelet-derived growth factor
(PDGF) induces pulmonary vascular remodeling through 15-LO/15-HETE
pathway under hypoxic condition. Cell Signal. 24:1931–1939. 2012.
View Article : Google Scholar
|
|
43
|
Geiger A, Walker A and Nissen E: Human
fibrocyte-derived exosomes accelerate wound healing in genetically
diabetic mice. Biochem Biophys Res Commun. 467:303–309. 2015.
View Article : Google Scholar
|
|
44
|
Chiang CH, Wu WW, Li HY, Chien Y, Sun CC,
Peng CH, Lin AT, Huang CS, Lai YH, Chiou SH, et al: Enhanced
antioxidant capacity of dental pulp-derived iPSC-differentiated
hepatocytes and liver regeneration by injectable HGF-releasing
hydrogel in fulminant hepatic failure. Cell Transplant. 24:541–559.
2015. View Article : Google Scholar
|
|
45
|
Vanlandewijck M, Lebouvier T, Andaloussi
Mäe M, Nahar K, Hornemann S, Kenkel D, Cunha SI, Lennartsson J,
Boss A, Heldin CH, et al: Functional characterization of germline
mutations in PDGFB and PDGFRB in primary familial brain
calcification. PloS One. 10:e01434072015. View Article : Google Scholar
|
|
46
|
Fjaer R, Brodtkorb E, Oye AM, Øye AM,
Sheng Y, Vigeland MD, Kvistad KA, Backe PH and Selmer KK:
Generalized epilepsy in a family with basal ganglia calcifications
and mutations in SLC20A2 and CHRNB2. Eur J Med Genet. 58:624–628.
2015. View Article : Google Scholar
|
|
47
|
Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y,
Shi S and Le AD: Mesenchymal stem cells derived from human gingiva
are capable of immunomodulatory functions and ameliorate
inflammation-related tissue destruction in experimental colitis. J
Immunol. 183:7787–7798. 2009. View Article : Google Scholar
|
|
48
|
Hwang GH, Jeon YJ, Han HJ, Park SH, Baek
KM, Chang W, Kim JS, Kim LK, Lee YM and Lee S: Protective effect of
butylated hydroxylanisole against hydrogen peroxide-induced
apoptosis in primary cultured mouse hepatocytes. J Vet Sci.
16:17–23. 2015. View Article : Google Scholar
|
|
49
|
Ma X, Han S, Zhang W, Fan YJ, Liu MN, Liu
AY and Liu BR: Protection of cultured human hepatocytes from
hydrogen peroxideinduced apoptosis by relaxin-3. Mol Med Rep.
11:1228–1234. 2015. View Article : Google Scholar
|
|
50
|
Moniaux N, Song H, Darnaud M, Garbin K,
Gigou M, Mitchell C, Samuel D, Jamot L, Amouyal P, Amouyal G, et
al: Human
hepatocarcinoma-intestine-pancreas/pancreatitis-associated protein
cures fas-induced acute liver failure in mice by attenuating
free-radical damage in injured livers. Hepatology. 53:618–627.
2011. View Article : Google Scholar
|
|
51
|
Ramsey HE, Da Silva CG, Longo CR,
Csizmadia E, Studer P, Patel VI, Damrauer SM, Siracuse JJ, Daniel S
and Ferran C: A20 protects mice from lethal liver
ischemia/reperfusion injury by increasing peroxisome
proliferator-activated receptor-alpha expression. Liver Transpl.
15:1613–1621. 2009. View Article : Google Scholar
|
|
52
|
Lou SM, Li YM, Wang KM, Cai WM and Weng
HL: Expression of platelet-derived growth factor-BB in liver
tissues of patients with chronic hepatitis B. World J
Gastroenterol. 10:385–388. 2004. View Article : Google Scholar
|
|
53
|
Boulon S, Westman BJ, Hutten S, Boisvert
FM and Lamond AI: The nucleolus under stress. Mol Cell. 40:216–227.
2010. View Article : Google Scholar
|
|
54
|
Russo A, Saide A, Smaldone S, Faraonio R
and Russo G: Role of uL3 in multidrug resistance in 53-mutated lung
cancer cells. Int J Mol Sci. 18:E5472017. View Article : Google Scholar
|
|
55
|
Lv H, Qi Z, Wang S, Feng H, Deng X and Ci
X: Asiatic acid exhibits anti-inflammatory and antioxidant
activities against lipopolysaccharide and d-Galactosamine-induced
fulminant hepatic failure. Front Immunol. 8:7852017. View Article : Google Scholar
|
|
56
|
Russo A, Saide A, Cagliani R, Cantile M,
Botti G and Russo G: rpL3 promotes the apoptosis of p53 mutated
lung cancer cells by down-regulating CBS and NFκB upon 5-FU
treatment. Sci Rep. 6:383692016. View Article : Google Scholar
|
|
57
|
Lawrence T: The nuclear factor NF-kappaB
pathway in inflammation. Cold Spring Harb Perspect Biol.
1:a0016512009. View Article : Google Scholar
|