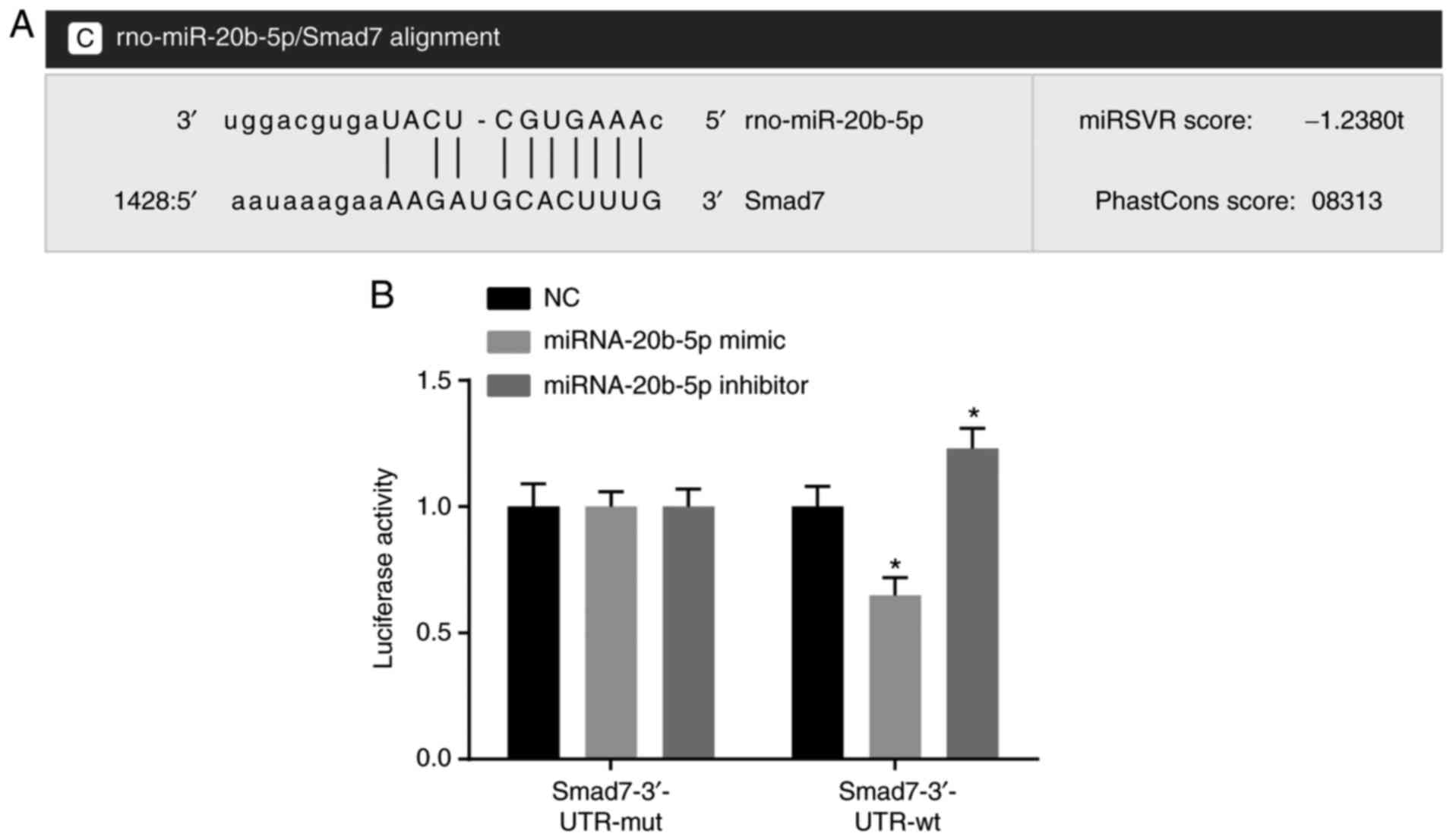
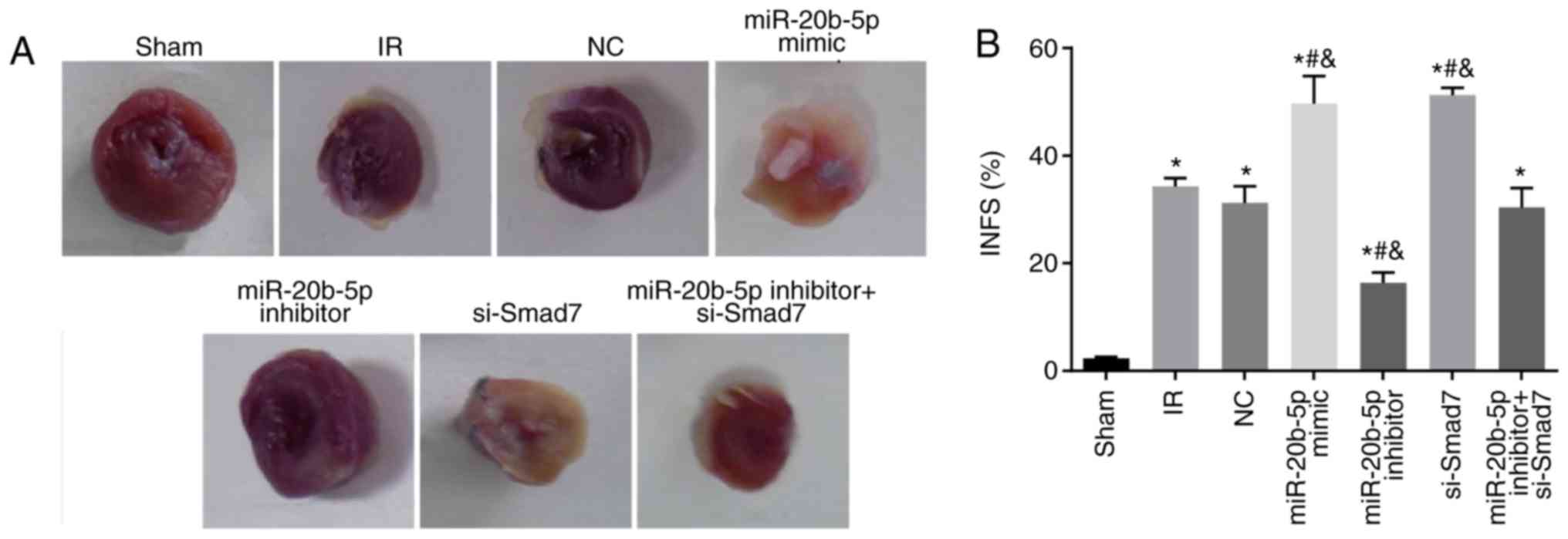
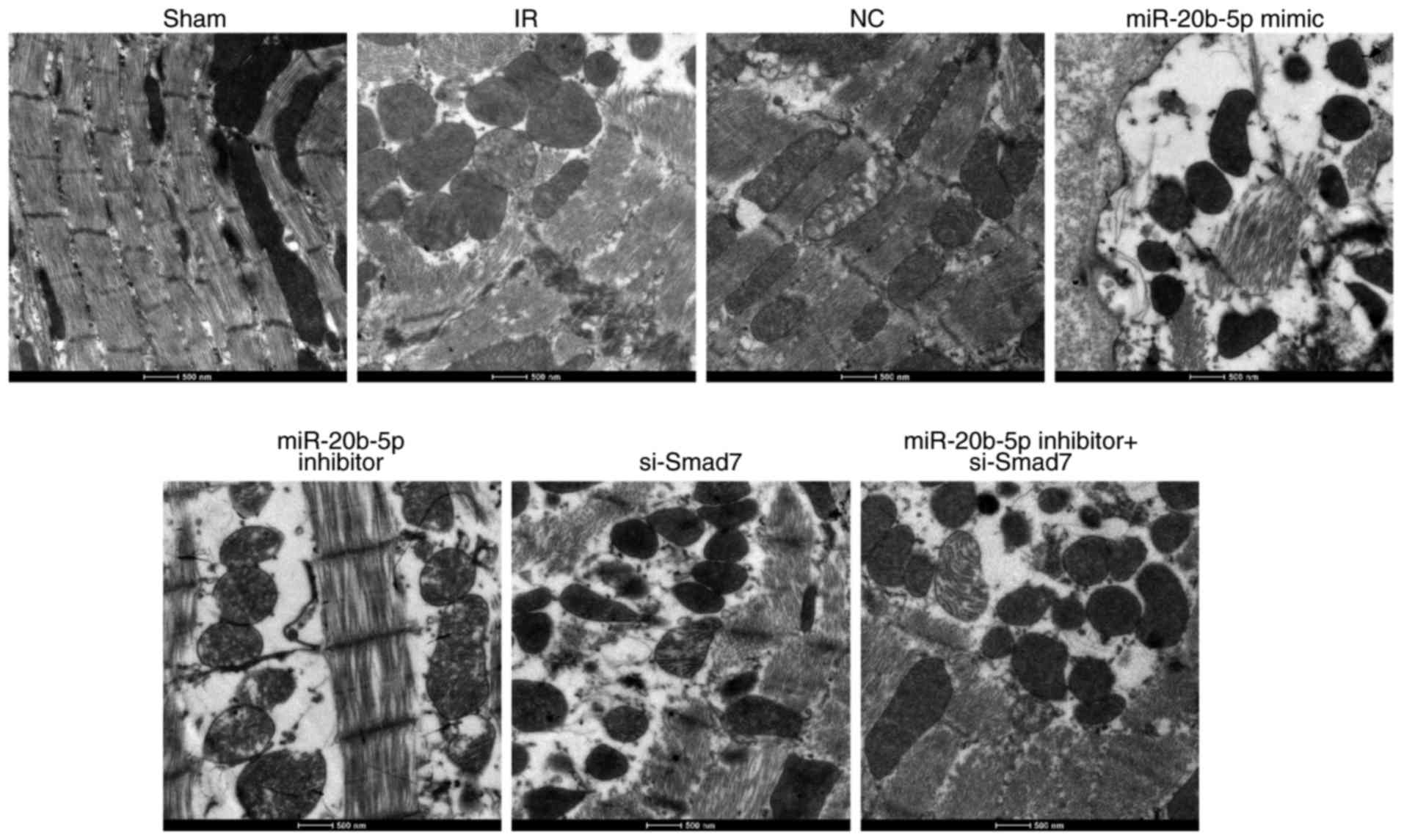
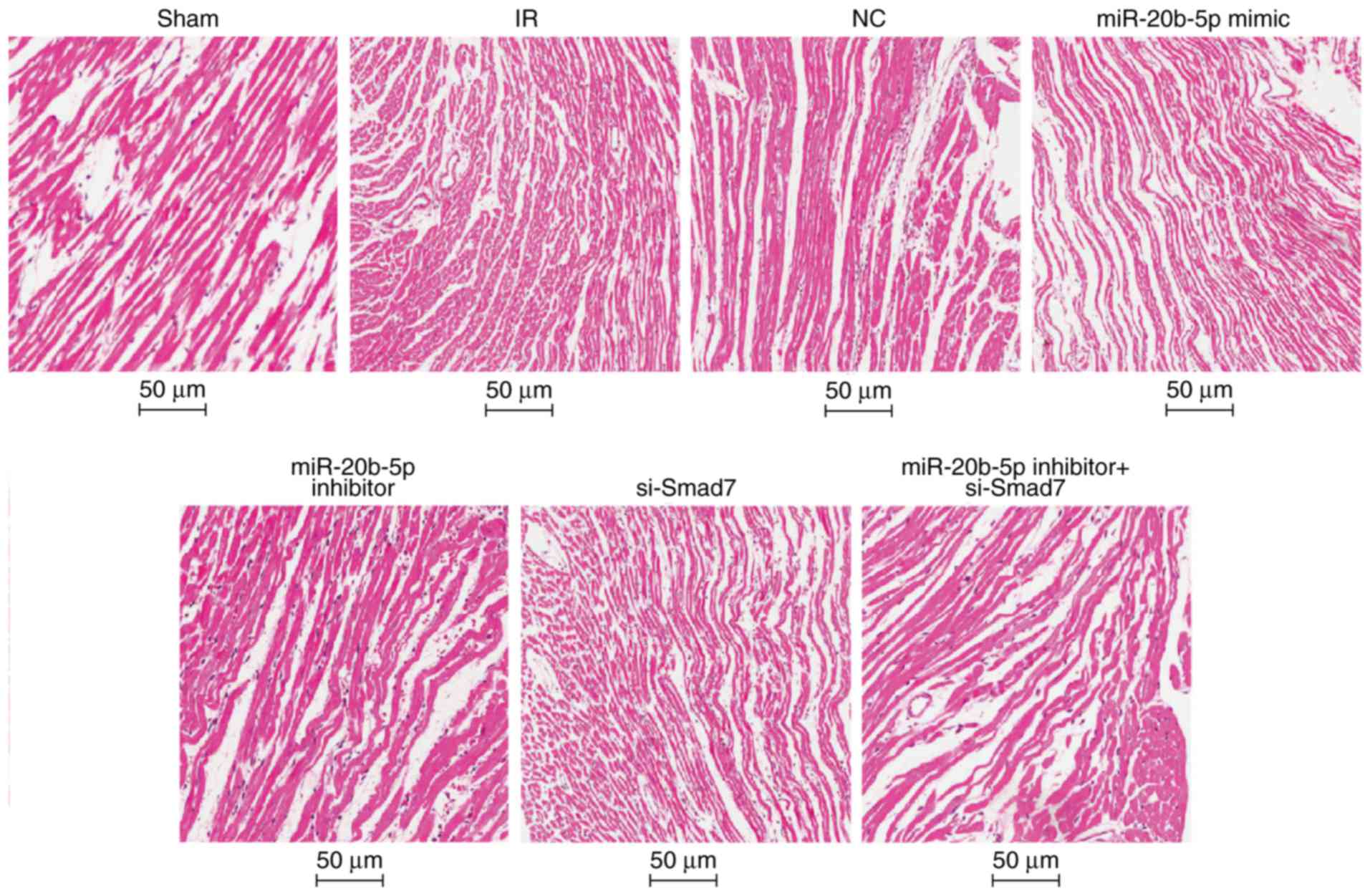
|
1
|
De Roeck L, Vandamme S, Everaert BR,
Hoymans V, Haine S, Vandendriessche T, Bosmans J, Ronsyn MW,
Miljoen H, Van Berendoncks A, et al: Adiponectin and
ischemia-reperfusion injury in ST segment elevation myocardial
infarction. Eur Heart J Acute Cardiovasc Care. 5:71–76. 2016.
View Article : Google Scholar
|
|
2
|
Selmer R, Halvorsen S, Myhre KI, Wisløff
TF and Kristiansen IS: Cost-effectiveness of primary percutaneous
coronary intervention versus thrombolytic therapy for acute
myocardial infarction. Scand Cardiovasc J. 39:276–285. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ong SB, Samangouei P, Kalkhoran SB and
Hausenloy DJ: The mitochondrial permeability transition pore and
its role in myocardial ischemia reperfusion injury. J Mol Cell
Cardiol. 78:23–34. 2015. View Article : Google Scholar
|
|
4
|
Fröhlich GM, Meier P, White SK, Yellon DM
and Hausenloy DJ: Myocardial reperfusion injury: Looking beyond
primary PCI. Eur Heart J. 34:1714–1722. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wick G, Grundtman C, Mayerl C,
Wimpissinger TF, Feichtinger J, Zelger B, Sgonc R and Wolfram D:
The immunology of fibrosis. Annu Rev Immunol. 31:107–135. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Neri M, Fineschi V, Di Paolo M, Pomara C,
Riezzo I, Turillazzi E and Cerretani D: Cardiac oxidative stress
and inflammatory cytokines response after myocardial infarction.
Curr Vasc Pharmacol. 13:26–36. 2015. View Article : Google Scholar
|
|
7
|
Zhang M, Xu YJ, Saini HK, Turan B, Liu PP
and Dhalla NS: TNF-alpha as a potential mediator of cardiac
dysfunction due to intracellular Ca2+-overload. Biochem
Biophys Res Commun. 327:57–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hausenloy DJ and Yellon DM: The
mitochondrial permeability transition pore: Its fundamental role in
mediating cell death during ischaemia and reperfusion. J Mol Cell
Cardiol. 35:339–341. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
hao ZQ, Nakamura M, Wang NP, Velez DA,
Hewan-Lowe KO, Guyton RA and Vinten-Johansen J: Dynamic progression
of contractile and endothelial dysfunction and infarct extension in
the late phase of reperfusion. J Surg Res. 94:133–144. 2000.
View Article : Google Scholar
|
|
10
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: A neglected therapeutic target. J Clin
Invest. 123:92–100. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fan ZX and Yang J: The role of microRNAs
in regulating myocardial ischemia reperfusion injury. Saudi Med J.
36:787–793. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Suzuki HI and Miyazono K: Emerging
complexity of microRNA generation cascades. J Biochem. 149:15–25.
2011. View Article : Google Scholar
|
|
13
|
Huntzinger E and Izaurralde E: Gene
silencing by microRNAs: Contributions of translational repression
and mRNA decay. Nat Rev Genet. 12:99–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He B, Xiao J, Ren AJ, Zhang YF, Zhang H,
Chen M, Xie B, Gao XG and Wang YW: Role of miR-1 and miR-133a in
myocardial ischemic postconditioning. J Biomed Sci. 18:222011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mukhopadhyay P, Das S, Ahsan MK, Otani H
and Das DK: Modulation of microRNA 20b with resveratrol and
longevinex is linked with their potent anti-angiogenic action in
the ischaemic myocardium and synergestic effects of resveratrol and
γ-tocotrienol. J Cell Mol Med. 16:2504–2517. 2012. View Article : Google Scholar
|
|
16
|
Dickinson BA, Semus HM, Montgomery RL,
Stack C, Latimer PA, Lewton SM, Lynch JM, Hullinger TG, Seto AG and
van Rooij E: Plasma microRNAs serve as biomarkers of therapeutic
efficacy and disease progression in hypertension-induced heart
failure. Eur J Heart Fail. 15:650–659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Y, Chen D, Jin L, Liu J, Su Z, Li Y,
Gui Y and Lai Y: MicroRNA-20b-5p functions as a tumor suppressor in
renal cell carcinoma by regulating cellular proliferation,
migration and apoptosis. Mol Med Rep. 13:1895–1901. 2016.
View Article : Google Scholar
|
|
18
|
Blahna MT and Hata A: Smad-mediated
regulation of microRNA biosynthesis. FEBS Lett. 586:1906–1912.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hanyu A, Ishidou Y, Ebisawa T, Shimanuki
T, Imamura T and Miyazono K: The N domain of Smad7 is essential for
specific inhibition of transforming growth factor-beta signaling. J
Cell Biol. 155:1017–1027. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zorzi F, Angelucci E, Sedda S, Pallone F
and Monteleone G: Smad7 antisense oligonucleotide-based therapy for
inflammatory bowel diseases. Dig Liver Dis. 45:552–555. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cozzolino D, Sasso FC, Salvatore T,
Torella M, Gentile S, Torella R and Giugliano D: Acute effects of
beta-endorphin on cardiovascular function in patients with mild to
moderate chronic heart failure. Am Heart J. 148:E132004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takagawa J, Zhang Y, Wong ML, Sievers RE,
Kapasi NK, Wang Y, Yeghiazarians Y, Lee RJ, Grossman W and Springer
ML: Myocardial infarct size measurement in the mouse chronic
infarction model: Comparison of area- and length-based approaches.
J Appl Physiol. 102:2104–2111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Timmers L, Lim SK, Arslan F, Armstrong JS,
Hoefer IE, Doevendans PA, Piek JJ, El Oakley RM, Choo A, Lee CN, et
al: Reduction of myocardial infarct size by human mesenchymal stem
cell conditioned medium. Stem Cell Res. 1:129–137. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li XW, Wang XM, Li S and Yang JR: Effects
of chrysin (5,7-dihydroxyf lavone) on vascular remodeling in
hypoxia-induced pulmonary hypertension in rats. Chin Med. 10:42015.
View Article : Google Scholar
|
|
25
|
Brown RS and Wahl RL: Overexpression of
Glut-1 glucose transporter in human breast cancer. An
immunohistochemical study. Cancer. 72:2979–2985. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li R, Yan G, Li Q, Sun H, Hu Y, Sun J and
Xu B: MicroRNA-P145 protects cardiomyocytes against hydrogen
peroxide (H2O2)-induced apoptosis through
targeting the mitochondria apoptotic pathway. PLoS One.
7:e449072012. View Article : Google Scholar
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-delta delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
28
|
Liu H, Shang J, Chu F, Li A, Wu B, Xie X,
Liu W, Yang H and Tong T: Protective effects of Shen-Yuan-Dan, a
traditional Chinese medicine, against myocardial
ischemia/reperfusion injury in vivo and in vitro. Evid Based
Complement Alternat Med. 2013:9563972013. View Article : Google Scholar
|
|
29
|
Cohn JN, Ferrari R and Sharpe N: Cardiac
remodeling-concepts and clinical implications: A consensus paper
from an international forum on cardiac remodeling. Behalf of an
international forum on cardiac remodeling. J Am Coll Cardiol.
35:569–582. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ito Y, Ito K, Shiroto T, Tsuburaya R, Yi
GJ, Takeda M, Fukumoto Y, Yasuda S and Shimokawa H: Cardiac shock
wave therapy ameliorates left ventricular remodeling after
myocardial ischemia-reperfusion injury in pigs in vivo. Coron
Artery Dis. 21:304–311. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hagiwara S, Iwasaka H, Shingu C, Matumoto
S, Hasegawa A and Noguchi T: Heat shock protein 47 (HSP47)
antisense oligonucleotides reduce cardiac remodeling and improve
cardiac function in a rat model of myocardial infarction. Thorac
Cardiovasc Surg. 59:386–392. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Apstein CS: Increased glycolytic substrate
protection improves ischemic cardiac dysfunction and reduces
injury. Am Heart J. 139:S107–S114. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Galang N, Sasaki H and Maulik N: Apoptotic
cell death during ischemia/reperfusion and its attenuation by
antioxidant therapy. Toxicology. 148:111–118. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fedak PW, Verma S, Weisel RD, Skrtic M and
Li RK: Cardiac remodeling and failure: From molecules to man (Part
III). Cardiovasc Pathol. 14:109–119. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Donekal S, Venkatesh BA, Liu YC, Liu CY,
Yoneyama K, Wu CO, Nacif M, Gomes AS, Hundley WG, Bluemke DA and
Lima JA: Interstitial fibrosis, left ventricular remodeling, and
myocardial mechanical behavior in a population-based multiethnic
cohort: The multi-ethnic study of Atherosclerosis (MESA) study.
Circ Cardiovasc Imaging. 7:292–302. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu M, Chen J, Huang Y, Ke J, Li L, Huang
D and Wu W: Triptolide alleviates isoprenaline-induced cardiac
remodeling in rats via TGF-β1/Smad3 and p38 MAPK signaling pathway.
Pharmazie. 70:244–250. 2015.PubMed/NCBI
|
|
37
|
Kasama S, Toyama T, Hatori T, Sumino H,
Kumakura H, Takayama Y, Ichikawa S, Suzuki T and Kurabayashi M:
Evaluation of cardiac sympathetic nerve activity and left
ventricular remodelling in patients with dilated cardiomyopathy on
the treatment containing carvedilol. Eur Heart J. 28:989–995. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fazi F and Nervi C: MicroRNA: Basic
mechanisms and transcriptional regulatory networks for cell fate
determination. Cardiovasc Res. 79:553–561. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Landgraf P, Rusu M, Sheridan R, Sewer A,
Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M,
et al: A mammalian microRNA expression atlas based on small RNA
library sequencing. Cell. 129:1401–1414. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao Y, Samal E and Srivastava D: Serum
response factor regulates a muscle-specific microRNA that targets
Hand2 during cardiogenesis. Nature. 436:214–220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kretzschmar M and Massague J: SMADs:
Mediators and regulators of TGF-beta signaling. Curr Opin Genet
Dev. 8:103–111. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen Q, Chen H, Zheng D, Kuang C, Fang H,
Zou B, Zhu W, Bu G, Jin T, Wang Z, et al: Smad7 is required for the
development and function of the heart. J Biol Chem. 284:292–300.
2009. View Article : Google Scholar :
|
|
44
|
Wei LH, Huang XR, Zhang Y, Li YQ, Chen HY,
Yan BP, Yu CM and Lan HY: Smad7 inhibits angiotensin II-induced
hypertensive cardiac remodelling. Cardiovasc Res. 99:665–673. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nagarajan RP, Zhang J, Li W and Chen Y:
Regulation of Smad7 promoter by direct association with Smad3 and
Smad4. J Biol Chem. 274:33412–33418. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhu X, Topouzis S, Liang LF and Stotish
RL: Myostatin signaling through Smad2, Smad3 and Smad4 is regulated
by the inhibitory Smad7 by a negative feedback mechanism. Cytokine.
26:262–272. 2004. View Article : Google Scholar : PubMed/NCBI
|