Introduction
Spirulina polysaccharide (PSP), a type of
water-soluble, physiologically active polysaccharide extracted from
spirulina, has a large and complex molecular structure, which is
mainly composed of glycosidic bonds (1). PSP is reported to have an effect on
inhibiting tumor cell growth through inhibiting the synthesis of
nucleic acid and proteins in cancer cells, but not directly killing
cancer cells. In addition, the inhibitory effect of PSP on cancer
cells has been reported to be time-dependent (2,3).
It is well known that free radicals can oxidize biomolecules and
are important in several degenerative and pathological processes
(4,5). As an antioxidant, PSP can maintain
cellular health and inhibit senescence in the body by removing
excess free radicals and preventing the oxidation of cellular
oxidative substrates (6,7). PSP can enhance the non-specific
cellular immune function in the body, and improve the ability to
resist the invasion of viruses (8). Therefore, it has potential
application and development value.
Nanoemulsions (NEs) (9) are formed spontaneously by mixing
together an aqueous phase, an oil phase, surfactant, and
co-surfactant; can be a thermodynamically stable, isotropic,
transparent, or translucent homogeneous dispersion; they have a
particle size of 1–100 nm. NEs are stabilized and not layered by
autoclaving and high-speed centrifugation (10). NEs have been used since 1930,
particularly in floor polishing products, including liquid waxes,
fuels and dry lotions (11). At
present, NE technology is employed in the production of commonly
used chemicals, specialized chemicals, and in the petroleum and
materials science (12,13), biotechnology and pharmaceutical
industries (14). Furthermore,
NEs are novel drug carriers and offer numerous beneficial
properties (15). In particular,
they exhibit thermodynamic stability and low viscosity, and are
thus easy to prepare and store. They also increase the solubility
of fat-soluble drugs.
The pseudo-ternary phase diagram method is always
applied to reflect the mutual change of a three-component system on
a plane triangle under isothermal isobaric conditions. In NEs, the
three vertices in the pseudo-ternary phase diagram respectively
represent the aqueous phase, the oil phase, and the surfactant. The
ratio between two components is determined by the distance from
this point to the endpoints on both sides, and the proportional
relationship between the three components is within the
pseudo-ternary phase diagram. This method turns the association
among the three components in the NE formation into a flat graph,
which is intuitive, concise and enables easy determination of the
proportion of each component.
Studies have indicated that a PSP-loaded NE offers a
wide application in clinical use, including increasing stability
and sustained drug release. Furthermore, a PSP-loaded NE has been
shown to improve clinical medication safety by reducing its
irritant or toxic side effects, enhancing its availability and
bioavailability by avoiding destruction when passing through the
digestive tract, and increasing specificity to drug targeting by
extending the release time of water-soluble drugs. In addition to
achieving the above effects, a PSP-loaded NE can mask strong odors
to improve patient compliance (16,17). Therefore, the fish-flavored PSP
was encapsulated into NE to prepare PSP-NE, which aimed to improve
the oral bioavailability and stability of PSP, as well as the
antitumor and antioxidative effects.
Materials and methods
Materials
The PSP (polysaccharide content of 71.65%) produced
by the Department of Traditional Chinese Medicine Department of
Jinan University (Guangzhou, China) was a spirulina lye extract
product, and the monosaccharide composition was as follows: Glucose
(21.3±1.4%), rhamnose (43.6±2.7%), xylose (2.4±0.6%), galactose
(1.3±0.2%) and arabinose (1.1±0.1%). Other reagents included
indocyanine green (MedChem Express, Monmouth Junction, NJ, USA),
1,9-dimethylmethylene blue (DMB Sigma; EMD Millipore, Billerica,
MA, USA), Span 80 (Aladdin Reagent Co., Ltd., Shanghai, China),
Tween-80 (Aladdin Reagent Co., Ltd.), injection-grade soybean oil
(Emerging Tieling Pharmaceutical Co., Ltd., Tieling, China),
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
(MTT; Sigma; EMD Millipore), RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific. Inc.) Sudan Red III, methylene
blue, dimethyl sulfoxide, ethanol, iron sulfate, isopropanol,
n-butanol, n-octanol and polyethylene glycol 400 (Tianjin Daming
Chemical Reagent Factory, Tianjin, China).
Experimental animals and tumor cell
line
A total of 9 BALB/c-nu mice (age: 8 weeks; weight:
~22 g) with equal numbers of males and females were provided by
Beijing Huafu Kang Biotechnology Co., Ltd. (Beijing, China), and
were maintained in our facility with free access to water and food,
under a 12-h light/dark cycle, with 40% humidity. The HepG2 human
hepatoblastoma cell line and MCF-7 human breast adenocarcinoma cell
line were obtained from Jiangsu KeyGen Biotech Co., Ltd., (Jiangsu,
China).
Surfactant preparation
The NEs were prepared with a high-speed homogenizer.
For formulation screening, a surfactant or mixed surfactant was
selected according to the NE type declared. A low
hydrophile-lipophile balance (HLB) value (<8) was used for the
preparation of a water-oil (W/O) NE and a high HLB value (>10)
was used for an oil-water (O/W) NE. As PSP was readily soluble, the
PSP was mixed with the water-in-oil NE.
Screening of PSP-NE components
Combination of surfactants
The effects of seven complexes of mixed surfactants
(Span 80 and glycerolmonooleate at ratios of 3:2 and 3:4;
glycerolmonooleate and Tween-80 at ratios of 2:1, 3:1 and 4:1, and
Span 80/Tween-80 at ratios of 3:1 and 4:1) were investigated on the
NE-forming area of a pseudo-ternary phase diagram in the
surfactant/anhydrous ethanol/soybean oil/water system [surfactant
and co-surfactant ratio (Km) of 2:1] at
room temperature (18).
Screening of co-surfactants
At room temperature, the effects of five
co-surfactants (ethanol, isopropanol, polyethylene glycol 400,
n-butanol and n-octanol) on the NE-forming area of the
pseudo-ternary phase diagram were investigated in a Span
80/Tween-80/co-surfactant/soybean oil/water system
(Km 2:1).
Effect of Km on the
formation of NEs
The effects of Km values at 2:1,
3:1 and 4:1 on the NE-forming area of the pseudo-ternary phase
diagram were investigated in a Span 80/Tween-80/anhydrous
ethanol/soybean oil/water system at room temperature.
Screening of oil phase
Liquid paraffin, medium-chain fatty acid
triglyceride, soybean oil and sesame oil were used to determine
whether they have individual effects on the NE-forming regions.
This step was performed on a Span 80/Tween-80/anhydrous ethanol/oil
phase/water (Km 2:1) system at room
temperature.
Screening of the aqueous phase
The individual effects of 1, 2, 5, 10 and 20% PSP
aqueous solutions on the NE-forming areas were investigated in a
Span 80/Tween-80/anhydrous ethanol/soybean oil/PSP aqueous solution
system (Km 2:1) at room temperature.
Titration to construct the
pseudo-ternary phase diagram
The pseudo-ternary phase diagram was drawn by
titration. For the determination of the optimal prescription as the
main evaluation index for the NE formation, the mixed (Smix), oil
and aqueous phases were used as the three vertices of the
pseudo-ternary phase diagram on the basis of the size of the
NE-forming area (19,20).
Evaluation of NE quality
Appearance and type
The appearance of the prepared NE with respect to
clarity, transparency and homogeneity was examined macroscopically.
The type of NE was determined by using different modes of the
spreading speed of methylene blue (water-soluble dye) and Sudan red
(oil-soluble dye) (21).
Shape and particle size
The shape was observed using transmission electron
microscopy (TEM; Tecnai 10; Philips Healthcare, DA Best, The
Netherlands) following diluting of the NE with soybean oil. The
particle size and polydispersity index (PDI) of the prepared NE
were measured with a laser particle size analyzer (Nano ZS 90;
Malvern Instruments, Inc., Westborough, MA, USA) (22).
Viscosity and pH
The viscosities and pH values of the empty NE and
PSP-NE were measured with a viscometer (SV-10 type; A&D Co.,
Ltd., Tokyo, Japan) and pH meter (pH 10/100 type, Shanghai Di Yim
Instrument Co., Ltd., Shanghai, China), respectively.
Stability
The stability of the NEs were determined through a
hot and cold cycle test (4 and 60°C), light experiment (4,500±500
lx) and accelerated experiment (40±2°C, 75±5% RH).
PSP content in the PSP-NE and
entrapment rate
Following extraction from the prepared PSP-NE (1 ml)
with a mixed solvent of chloroform and ethanol (1:1), an
appropriate volume of acetone was added, and the PSP-NE was then
subjected to ultrasonication until breakage. The PSP content in the
prepared PSP-NE (C1) was measured by high-performance liquid
chromatography (HPLC; Agilent 1260, Agilent Technologies, Inc.,
Santa, Clara, CA, USA) for refractive index (RI) detection.
The PSP-NE (1 ml) was placed in a 10 ml volumetric
flask. An equal volume of acetone was then added. The PSP-NE was
subjected to ultrasonication until it breakage and maintained
constant at 10 ml. The total quantity of PSP (C2) was also measured
by HPLC and RI detection. The entrapment rate was calculated
according to the following formula: Entrapment rate (%)=C1/C2
×100%.
Distribution of PSP-NE following oral
administration
Indocyanine green was dissolved in the PSP aqueous
solution (ICG-PSP) and then added to the NE to form an ICG-PSP-NE
complex (The final concentration of ICG in both solutions is 0.05
mg/ml). A total of 9 BALB/C nude mice were assigned to each of
three experimental groups according to treatment type (0.1 ml/100 g
of ICG-PSP, ICG-PSP-NE, and physiological saline, ig). The mice
were subjected to fasting 4 h following their respective treatment
and then placed in a live imaging device (Lumina XR Series III;
Perkin-Elmer, Inc., Waltham, MA, USA) to observe fluorescence
distribution in vivo at 0.5, 1, 2, 4, 8, 24 and 36 h.
Antitumor effects of PSP-NE and PSP
aqueous solution
The cytotoxicities of PSP and PSP-NE were determined
by measuring the inhibition of cell growth using an MTT assay
(23). The cells were maintained
in RPMI 1640 medium with 10% FBS and 0.1 mg/ml penicillin G and 100
U/ml streptomycin in a humidified atmosphere of 5% CO2
at 37°C. The HepG2 cells and MCF-7 cells were seeded separately on
a 96-well plate at a cellular density of 5,000 cells/well when at a
confluence of ~80%. Following treatment of the cells with the
various concentrations of PSP and PSP-NE over 48 h, 20 µl
MTT solution was added to the cells. The cell viability was
determined at 570 nm in a microplate reader (Synergy HT; BioTek
Instruments, Inc., Winooski, VT, USA). Cell toxicity was calculated
according to the following equation: Cell viability
(%)=Abssample/Abscontrol ×100%, where
Abssample is the absorbance of cells in the presence of
different formulations, Abscontrol is the absorbance of
cells in the absence of drug.
Antioxidant effects of PSPNE and PSP
aqueous solution 1 1,1-diphenyl-2-picrylhydrazyl radical (DPPH•)
scavenging assay
The DPPH• is a stable free radical, which is often
used as a tool to estimate the antioxidant capacity. The DPPH free
radical scavenging activities of PSP and PSP-NE were measured using
DPPH according to a published method with modifications (7,24).
Vitamin C (Vc) was used as a positive control. The sample solution
(2 ml), including Vc, PSP or PSP-NE samples at different
concentrations (1, 2, 3, 4 and 5 mg/ml) was added to a tube
containing 2 ml DPPH solution (0.04 mg/ml in ethanol). Following
incubation in the dark for 20 min at 37°C, the absorbance was
measured at 517 nm. The DPPH radical scavenging activity (%) was
calculated using the following equation (24): Scavenging activity
(%)=1−(A1−A2)/A0 ×100%, where
A0 is the absorbance of DPPH solution without samples.
A1 is the absorbance of DPPH with the samples, and
A2 is the absorbance of background solution (distilled
water instead of DPPH solution).
Hydroxyl radical (OH•) scavenging
assay
The OH• scavenging activities of PSP and PSP-NE were
measured following the modified method of Wu et al (24). Vc was also used as a reference
material for a positive control. The sample solution (1 ml) with
different concentrations (1, 2, 3, 4 and 5 mg/ml), 1 ml
FeSO4 solution (9 mmol/l) and 1 ml salicylic acid
ethanol solution (9 mmol/l) were fully mixed, and the reaction was
started with 1 ml H2O2 solution (8 mmol/l).
The absorbance of the mixture solution was measured at 510 nm
following incubation at 37°C for 40 min. The OH• scavenging
activity was calculated as follows: Scavenging activity (%)=1−
(A1−A2)/A0 ×100%, where
A0 is the absorbance of the negative control (without
samples), A1 is the absorbance of the sample mixture,
and A2 is the absorbance of background solution
(distilled water instead of H2O2).
Statistical analysis
Quantitative data are expressed as the mean ±
standard deviation. Statistical analysis (one-way analysis of
variance) was performed with GraphPad Prism. V6.0 software
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Optimization of the PSP-NE
formulation
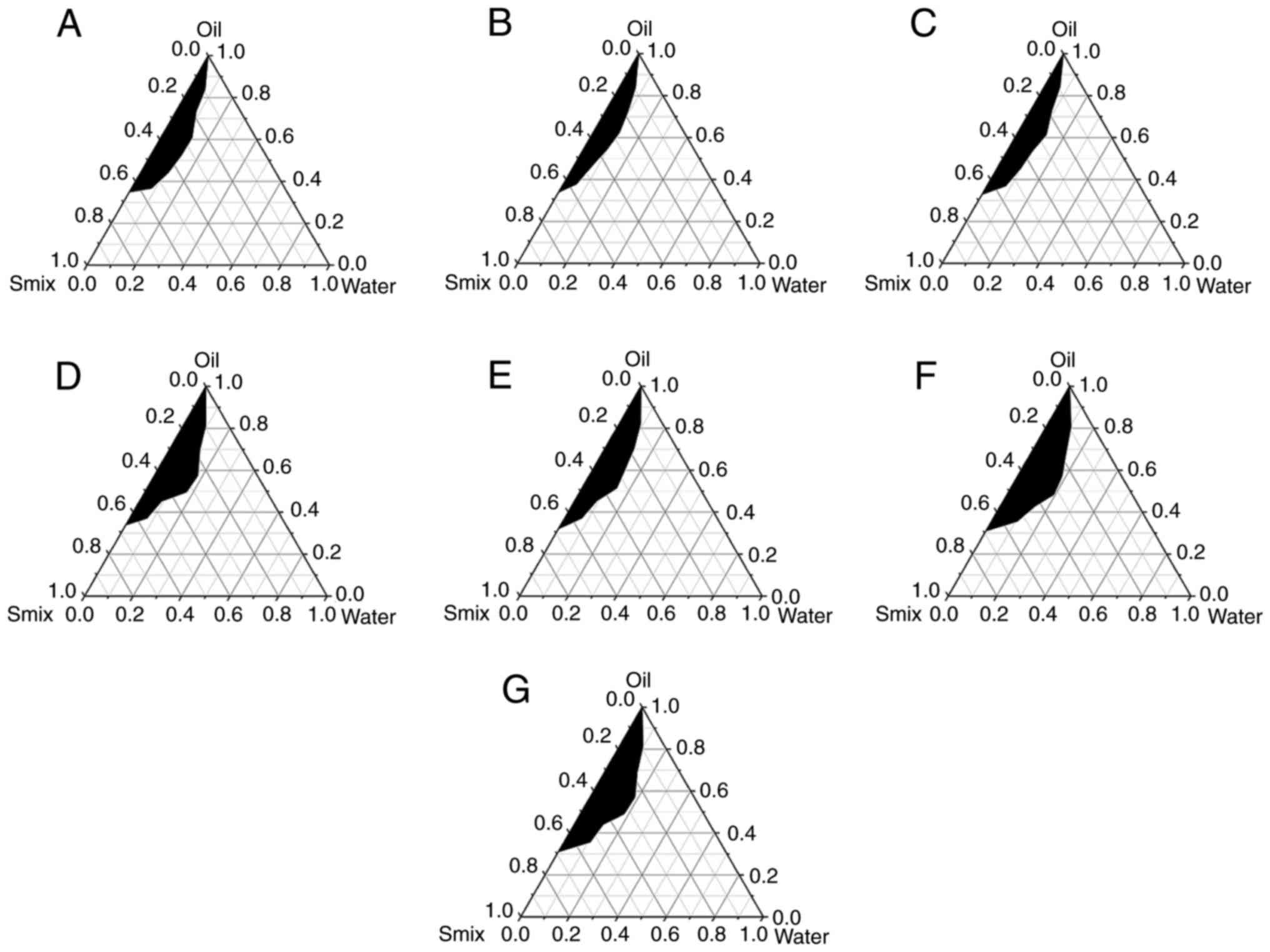
Surfactant complexes
The seven ratios of surfactant complexes all had
good emulsifying ability to form NEs. The results of the
pseudo-ternary phase diagram are shown in Fig. 1. The largest NE-forming area was
observed at Span 80/Tween-80=3:1, and this ratio was selected as
the optimal surfactant complex for subsequent experiments.
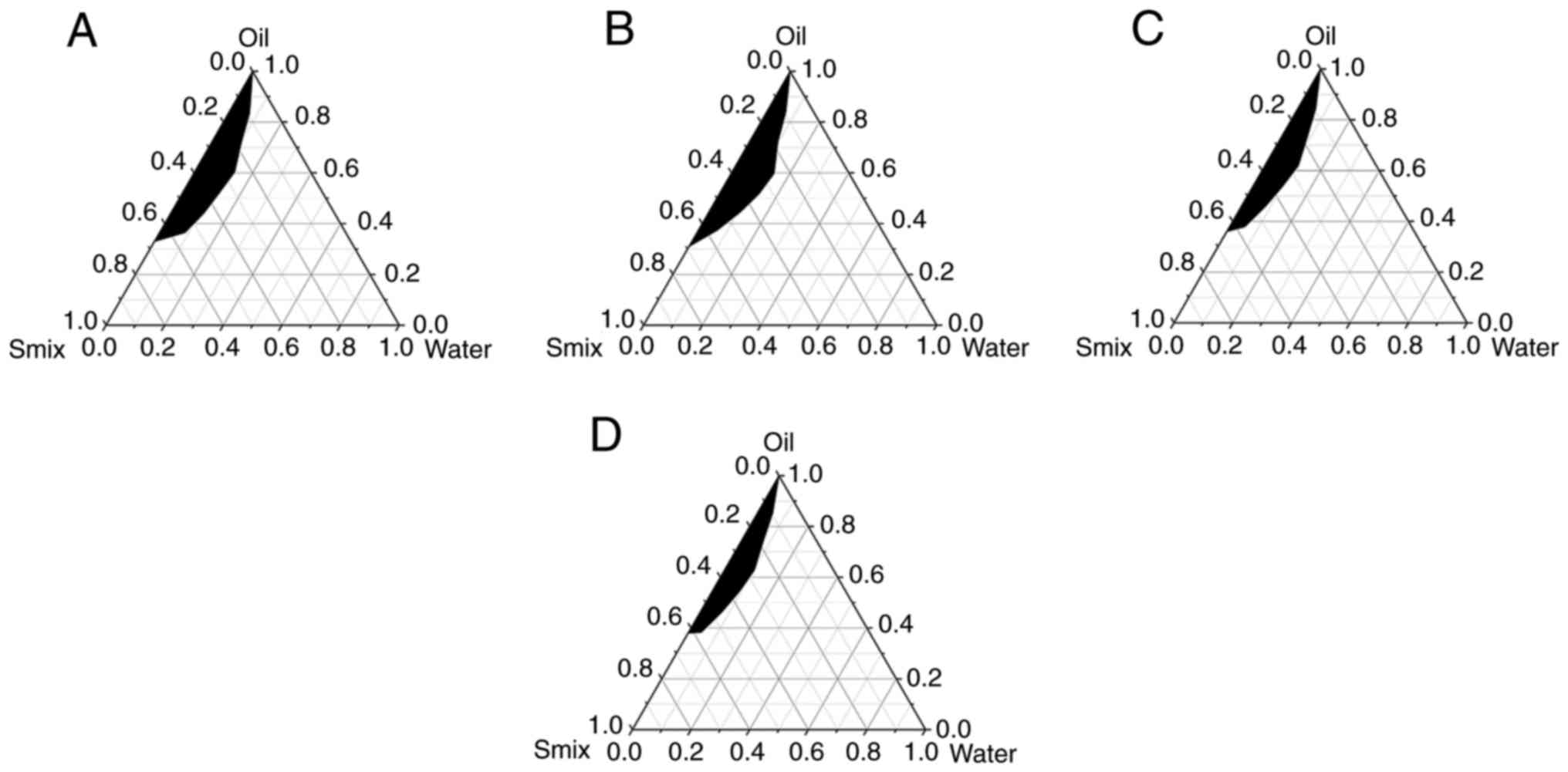
Screening of co-surfactant and
Km
Among the several commonly used co-surfactants, only
anhydrous ethanol formed NE when the Km values
were 4:1, 3:1, and 2:1. In addition, it did not significantly
change in appearance regardless of storage in either hot or cold
temperatures. Therefore, anhydrous ethanol was selected as a
co-surfactant. The pseudo-ternary phase diagram drawn by different
Km values is shown in Fig. 2. The NE-forming area at c
(Km=2:1) was the largest, and was selected for
subsequent experiments.
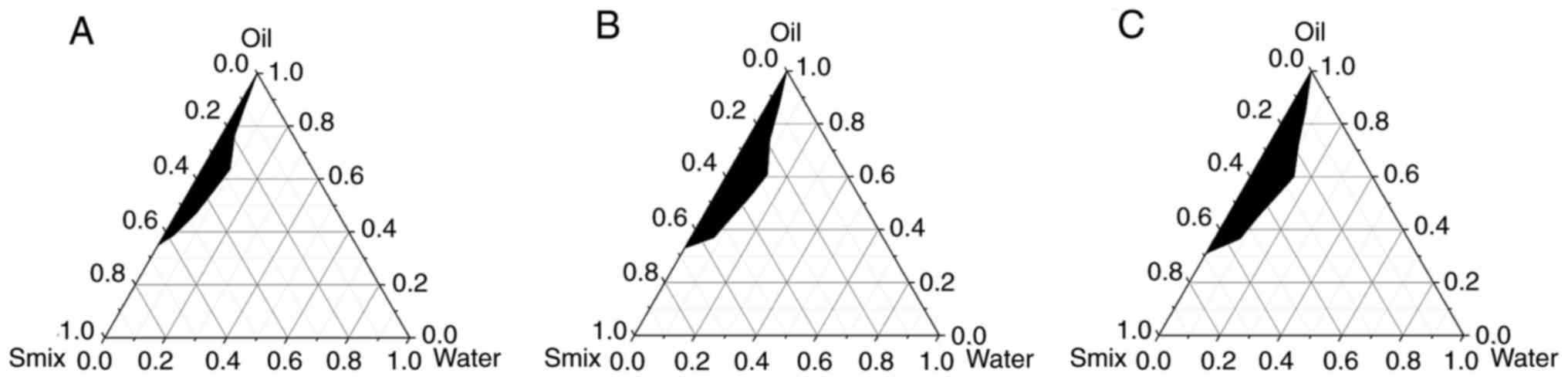
Oil phase screening
Four oil phases were assessed for their ability to
form NE and the results are shown in Fig. 3. The NE-forming areas were as
follows: Soybean oil >liquid paraffin >medium-chain fatty
acid triglycerides >sesame oil. Therefore, soybean oil was
selected as the preferred oil phase for subsequent experiments.
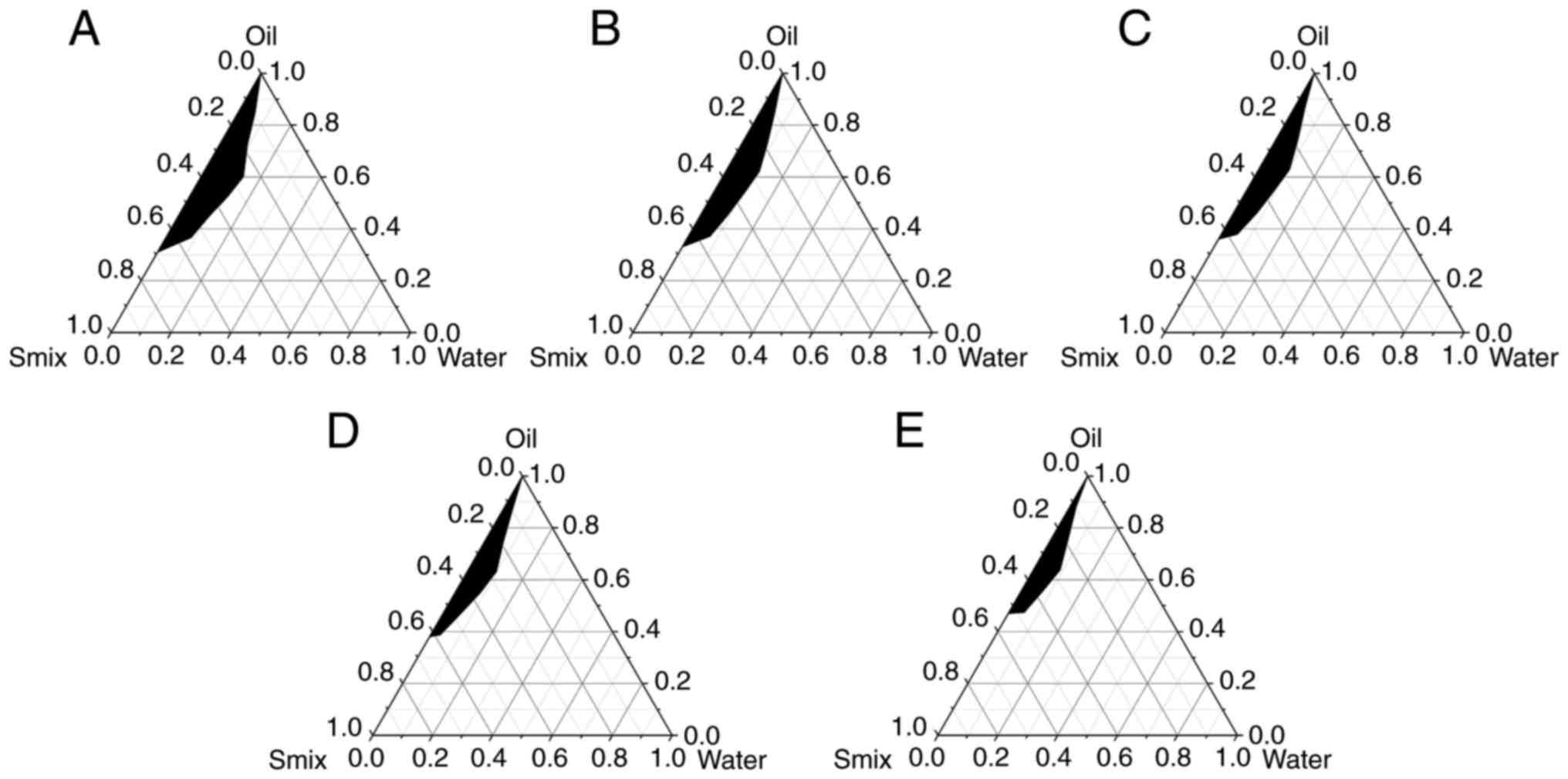
Aqueous phase screening
PSP solution at <20% had no significant effect on
NE formation. At higher concentrations, the NE became turbid. As
shown in the pseudo-ternary phase diagram (Fig. 4), the NE-forming area was as
follows: 1 >2 >5 >10 >20% PSP. There were no
significant differences among the areas at 1, 2 and 5% PSP,
however, the NE formation was considerably reduced when PSP
concentration exceeded 10%. PSP at 10% was the final water phase
used in consideration of the drug loading.
Therefore, the final NE formulation consisted of
11.9% Span 80, 6.0% Tween-80, 9.0% absolute ethanol, 62.8% soybean
oil, and 10.3% aqueous PSP.
Characteristics of the PSP-NE
formulation
Appearance and type
identification
The PSP-NE formed according to the above formulation
was light yellow, transparent and uniform. Following high-speed
centrifugation, the NE remained in a single phase, and the
diffusion rate of the Sudan red exceeded that of the methylene blue
(Fig. 5). These observations
demonstrated that the PSP-NE was a W/O NE.
Morphological observation and particle
size determination
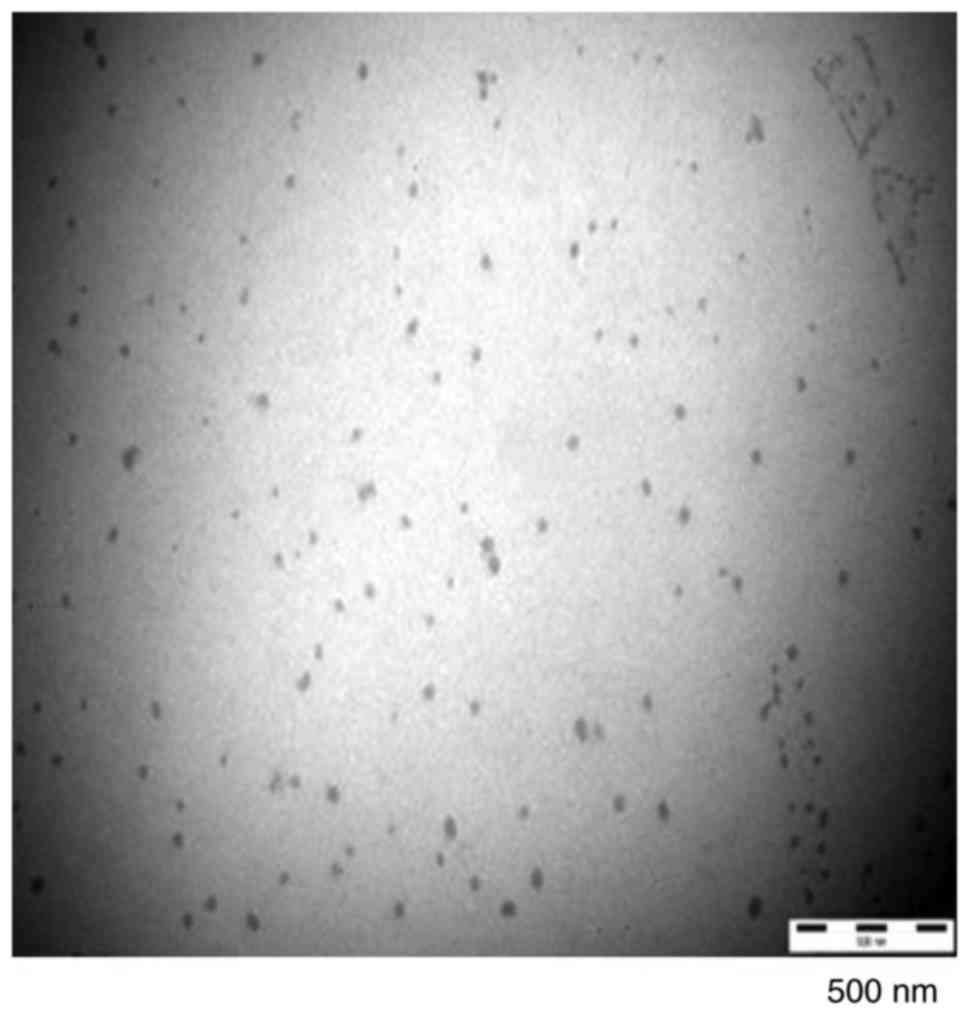
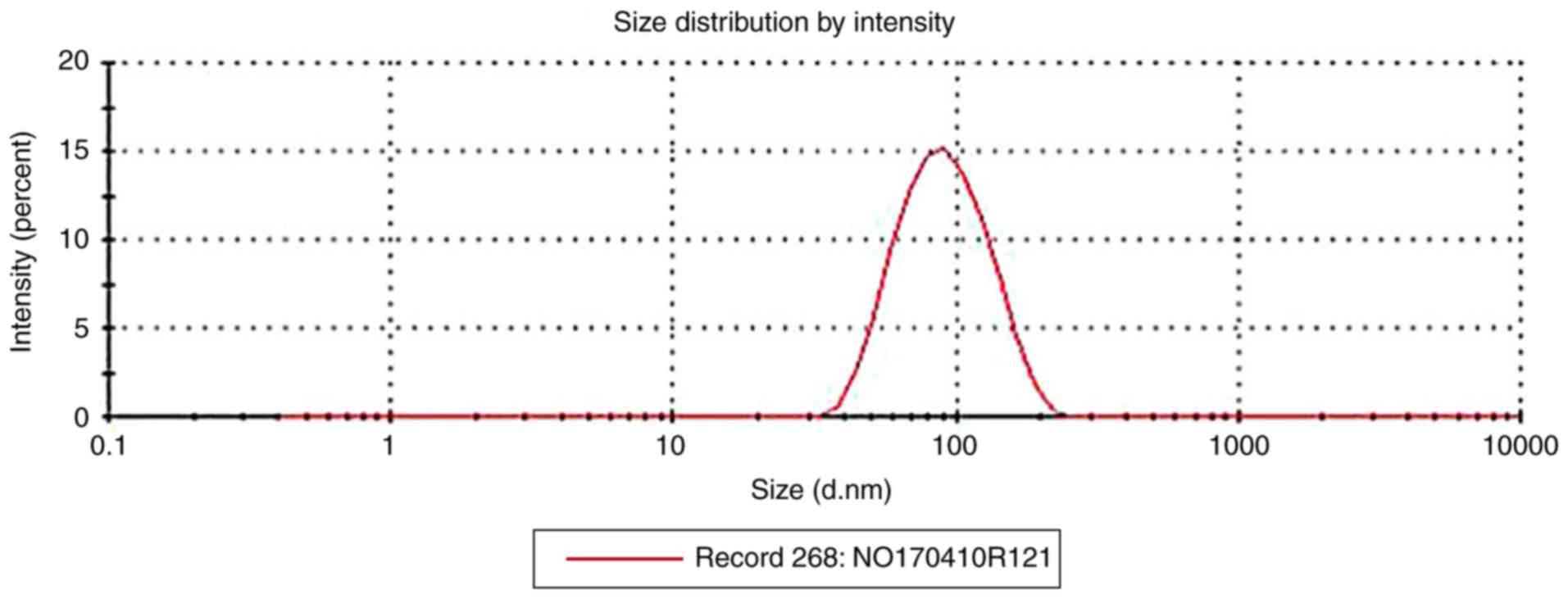
The PSP-NE droplets were homogeneous, spherical and
non-adhesive. As shown in Fig. 6,
the average particle sizes were 79.93±19 nm, and the PDI values
were 0.185±0.04 (n=3).
Viscosity and pH
The viscosity and pH values obtained in the
determination of empty NE and PSP-NE are shown in Table I.
 | Table IpH and viscosity of empty NE and
PSP-NE. |
Table I
pH and viscosity of empty NE and
PSP-NE.
| Sample | pH | Viscosity
(relative to water; °E) |
|---|
| NE only | 7.37±0.03 | 11.68±0.03 |
| PSP-NE | 7.24±0.02 | 12.37±0.06 |
Stability
The PSP-NE did not exhibit a muddy consistency
following stratification or sedimentation, and remained clear and
transparent. As indicated in Table
II, the results of the light experiment showed that PSP-NE was
stable for up to 10 days. As indicated in Table III, the PSP-NE was stable for up
to 2 months during the accelerated experiment. Together, these
observations demonstrated that PSP-NE was stable.
 | Table IILight properties of PSP-NE (n=3). |
Table II
Light properties of PSP-NE (n=3).
| Time (days) | Appearance | Particle size | PSP content
(mg/ml) | pH | Viscosity
(relative to water; °E) |
|---|
| 0 | Clear and
transparent | 79.16±0.76 | 5.17±0.03 | 7.23±0.03 | 11.52±0.04 |
| 5 | Clear and
transparent | 79.42±0.65 | 5.16±0.01 | 7.21±0.00 | 11.52±0.05 |
| 10 | Clear and
transparent | 80.02±1.06 | 5.16±0.03 | 7.18±0.01 | 11.55±0.05 |
 | Table IIIAccelerated test results of PSP-NE
(n=3). |
Table III
Accelerated test results of PSP-NE
(n=3).
Time
(mins) | Appearance | Particle size | PSP
content
(mg/ml) | pH | Viscosity
(relative to water; °E) |
|---|
| 0 | Clear and
transparent | 78.63±0.50 | 5.16±0.02 | 7.24±0.01 | 11.50±0.01 |
| 1 | Clear and
transparent | 79.06±0.41 | 5.15±0.01 | 7.22±0.02 | 11.49±0.03 |
| 2 | Clear and
transparent | 79.66±0.46 | 5.12±0.01 | 7.20±0.02 | 11.51±0.07 |
| 3 | Clear, transparent,
slight darker color | 80.43±1.02 | 5.10±0.02 | 7.16±0.02 | 11.53±0.02 |
| 6 | Clear, transparent,
darker color | 80.75±1.30 | 5.11±0.02 | 7.14±0.03 | 11.54±0.03 |
PSP content in the PSP-NE and
entrapment rate
The content of PSP measured was 5.14±0.06 mg/ml. The
entrapment rate measured was 62%.
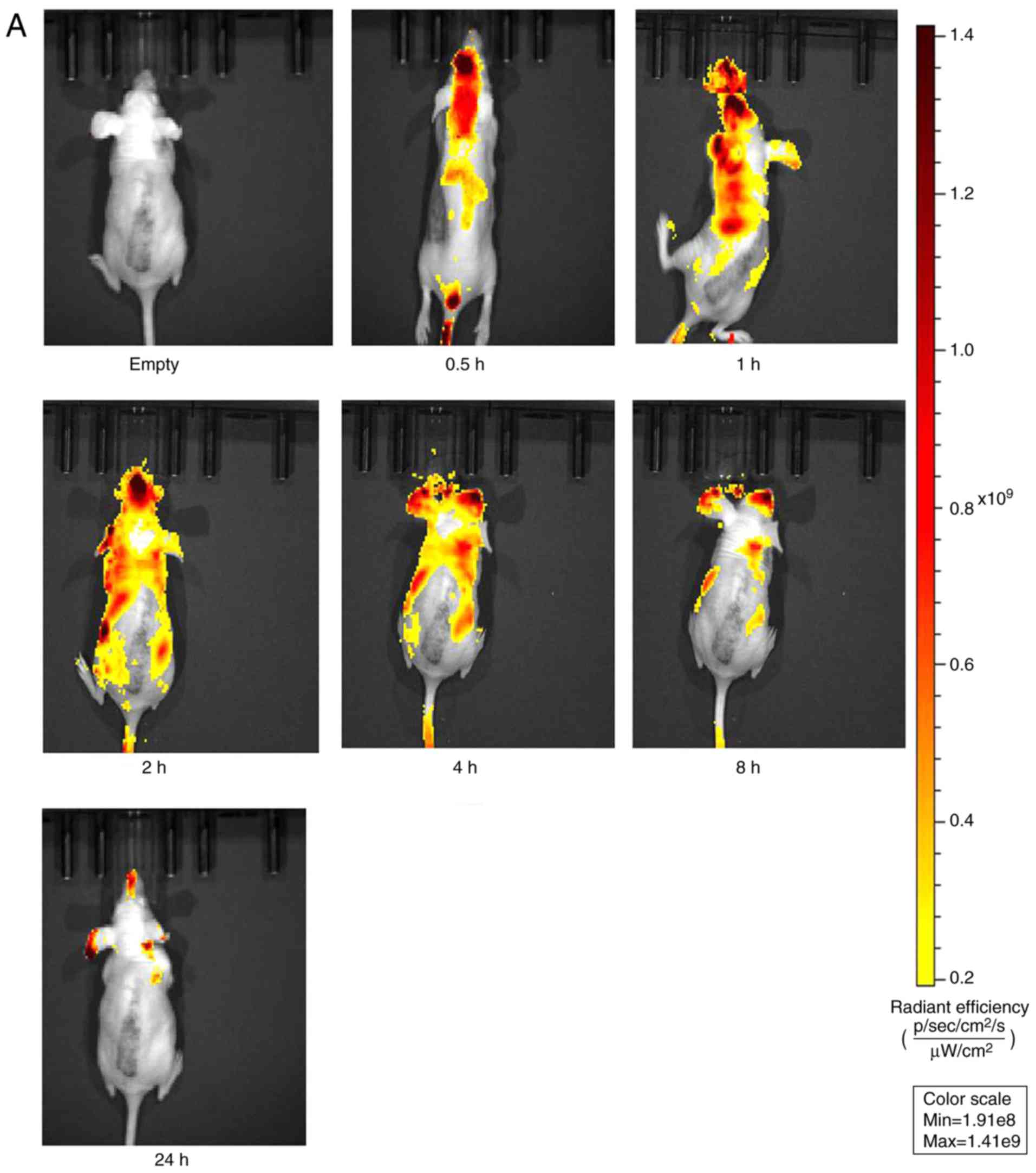
Biodistribution following oral
administration in nude mice
The fluorescence distribution at different time
points are shown in Fig. 7A (PSP)
and Fig. 7B (PSP-NE). In the PSP
group, fluorescence was distributed throughout the body by ~2 h,
mostly eliminated by 8 h, and eliminated at 24 h. By contrast, the
fluorescence of the PSP-NE group was distributed throughout the
body in ~4 h, and the fluorescence intensity was significantly
higher than that of the PSP group. In addition, significant
fluorescence accumulated in the liver and the kidney, and remained
detectable at ~24 h, and almost eliminated at 36 h. These findings
indicated that the PSP-NE exhibited a sustained-release and tissue
effect.
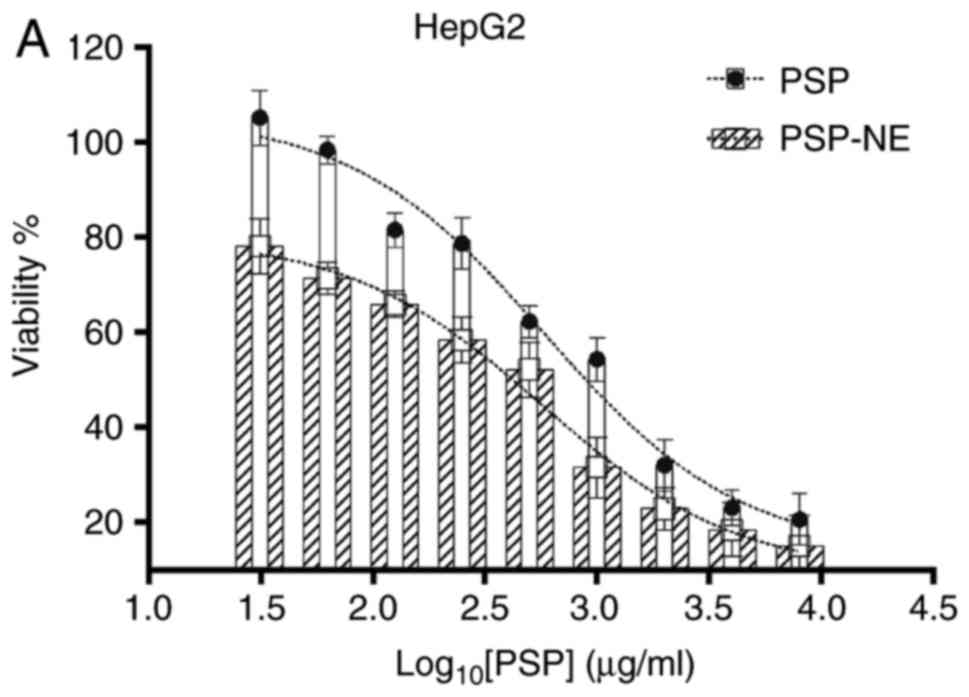
Antitumor assay
As shown in Fig. 8A
and B, PSP-NE exhibited a superior inhibitory effect on MCF-7
cells (IC50 at 500 µg/ml) and HepG2 cells
(IC50 at 500 µg/ml), compared with PSP (MCF-7
cell IC50 at 1,000 µg/ml; HepG2 cell
IC50 at 2,000 µg/ml).
Antioxidant assay
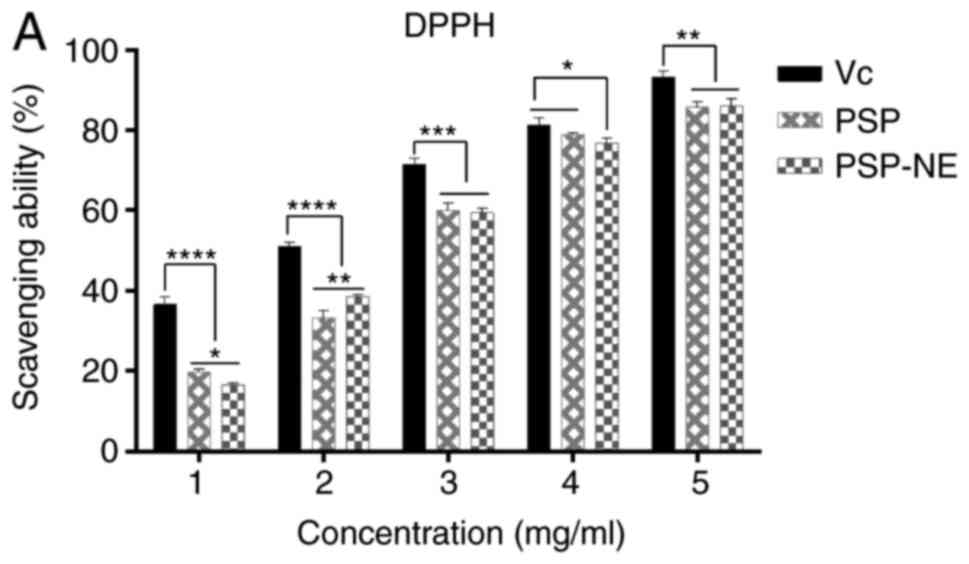
In the present study, the potent free radical
scavenging ability of PSP and PSP-NE on DPPH• and OH• was
determined at concentrations between 1 and 5 mg/ml, which showed
concentration-dependent effects. The scavenging abilities increased
with increasing concentration. As shown in Fig. 9A and B, Vc exerted higher
antioxidant scavenging of DPPH•, but a lower ability to scavenge
OH•, compared with PSP or PSP-NE. However, no significant
differences were observed in the DPPH• and OH• scavenging activity
between PSP-NE and PSP at any concentrations.
Discussion
The surfactant screening process revealed that a
single surfactant was not able to form an NE with superior
properties. Therefore, using combinations of surfactants is
suitable for the adjustment of HLB values and enhancement of
emulsion stability. The HLB of Span 80 was 4.3 and that of Tween-80
was 15.0, and a 3:1 mixture ratio produced the desired HLB for
soybean oil emulsification.
When the co-surfactant was screened, ethanol was
selected, which presented with the largest NE formation area at a
Km 2:1. In the process of NE formation, liquid
crystal and gel phenomenon (soybean phospholipids as a surfactant)
may occur, and alcohol is conducive to the transition of a liquid
crystal phase to an NE phase.
When screening the oil phase, the smaller the
molecular volume, the higher the ability to dissolve the drug, and
the faster the NE formation. Peanut and corn oils formed NE, but
the resulting NE appeared turbid at high- or low-temperature
storage (data not shown). Other oil phases not only formed NEs with
good qualities but also remained unchanged at low and high
temperatures. Therefore, liquid paraffin, sesame oil,
injection-grade soybean oil, and medium-chain fatty acid
triglycerides were selected as alternative oil phases. The screened
mixed surfactants and co-surfactants were weighed according to the
selected Km value and mixed with the above oil
phase, and the resulting formulation was screened, finally
confirming that soybean oil was the optimal oil phase.
When the water phase was selected, the effects of
PSP concentration on NE formation were observed, and the optimum
PSP concentration was determined. The results showed that the PSP
concentration within 20% did not markedly affect NE formation, and
the NE became turbid at higher PSP concentrations. As
polysaccharides are macromolecule materials, the area for NE
formation gradually decreased at increased PSP concentrations. On
considering the drug loading problem, 10% PSP aqueous solution was
finally selected.
The majority of polysaccharides neither absorb light
nor chromophores, making them difficult to quantify. However, the
present study observed the distribution of the dosage form in the
body by using living imaging aids. In this experiment, ICG was
embedded in PSP-NE, and the ICG-PSP and ICG-PSP-NE solutions were
administered in nude mice in parallel. The body distribution of
fluorescence was observed over time. The distribution and
elimination rates of the PSP-treated group were faster than those
of the PSP-NE group. These results indicated that the prepared NEs
exhibited slow release.
The in vitro antitumor experiments on HepG2
and MCF-7 cells demonstrated that PSP-NE had a superior inhibitory
effect compared with PSP, which may be attributed to the fact that
PSP-NE increased the uptake of cells and increased the effective
concentration of drugs.
It has been reported that PSP can affect the
metabolism in the body (25).
Ravi et al (26)
demonstrated that PSP reduced the ratio of low-density lipoprotein
to high-density lipoprotein (HDL), decreased the levels of plasma
lipid, and modified the total cholesterol and HDL cholesterol
levels. PSP has also been found to decrease the levels of
postprandial blood glucose and glycosylated hemoglobin (HbA1c), and
exhibit long-term glycemic regulation (27). Therefore, further investigation of
the glucose and lipid metabolism of PSP-NE is required.
In conclusion, PSP-NE was prepared through a phase
transition method, and the surfactant, co-surfactant, oil phase and
water phase were screened through the use of a pseudo-ternary phase
diagram. The prepared PSP-NE was transparent and uniform. Under
TEM, the average particle size was 79.93±19 nm; the PDI was
0.185±0.04, PSP content was 5.14±0.06 mg/ml, and the entrapment
rate was 62%. The PSP-NE did not exhibit demulsification in the
stability assessment, indicating its stability. The live imaging
distributions of PSP and PSP-NE in nude mice were observed with a
small-animal live imaging instrument and fluorescein ICG. The
distribution and elimination rates of the PSP-treated group were
faster than those of the PSP-NE-treated group. Furthermore, the
PSP-NE treated group exhibited marked liver and kidney
accumulation, indicating that the release of NEs was sustained and
specifically targeted to the tissue. The free radical scavenging
activity and antitumor ability of PSP-NE were also analyzed. The
antioxidant effect of PSP-NE was not affected but it exhibited
enhanced antitumor ability. The in vitro experiments showed
that PSP-NE had good antioxidant and antitumor abilities.
Acknowledgments
Not applicable.
Funding
This study was financially supported by the Ministry
of Education in the New Century Excellent Talents (grant no.
NECT-12-0677), the Science and Technology Program of Guangzhou
(grant nos. 2014J4500005 and 201704030141), the Science Program of
the Department of Education of Guangdong (grant no. 2015KGJHZ012)
and the Special Project of International Scientific and
Technological Cooperation in Guangzhou Development District (grant
no. 2017GH16).
Availability of data and materials
All relevant materials and datasets are within the
manuscript and are available.
Authors’ contributions
BW performed the screening of PSP-NE formulation
components, and was a major contributor in writing the manuscript;
TC was responsible for the evaluation of NE quality; QL undertook
the live imaging of distribution of PSP-NE in animals following
oral administration; MD and QM performed the anti-tumor and
anti-oxidant effects of PSP-NE; JCCW, RZ and LY made substantial
contributions to acquisition, analysis and interpretation of data.
JCCW, SPCC and YC were involved in drafting the manuscript and
revising it critically for intellectual content. SPCC and YC made
contributions to conception and design, and assisted in the
analysis of all experiments. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The protocol for the study was approved by the
College of Pharmacy, Jinan University. The Laboratory Animal Ethics
Committee of Jinan University approved all protocols (date of
approval, 13/09/2016; certification no. 20160913003).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu Q, Huang Y, Zhang R, Cai T and Cai Y:
Medical application of spirulina platensis derived c-phycocyanin.
Evid Based Complement Alternat Med. 2016:78038462016.PubMed/NCBI
|
|
2
|
Yang F, Tang Q, Zhong X, Bai Y, Chen T,
Zhang Y, Li Y and Zheng W: Surface decoration by Spirulina
polysaccharide enhances the cellular uptake and anticancer efficacy
of selenium nanoparticles. Int J Nanomedicine. 7:835–844.
2012.PubMed/NCBI
|
|
3
|
Kurd F and Samavati V: Water soluble
polysaccharides from Spirulina platensis: Extraction and in vitro
anti-cancer activity. Int J Biol Macromol. 74:498–506. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Capek P, Machová E and Turjan J:
Scavenging and antioxidant activities of immunomodulating
polysaccharides isolated from Salvia officinalis L. Int J Biol
Macromol. 44:75–80. 2009. View Article : Google Scholar
|
|
5
|
Klaus A, Kozarski M, Niksic M, Jakovljevic
D, Todorovic N and Van Griensven LJLD: Antioxidative activities and
chemical characterization of polysaccharides extracted from the
basidiomycete Schizophyllum commune. LWT-Food Sci Technol.
44:2005–2011. 2011. View Article : Google Scholar
|
|
6
|
Lai F, Wen Q, Li L, Wu H and Li X:
Antioxidant activities of water-soluble polysaccharide extracted
from mung bean (Vigna radiata L.) hull with ultrasonic assisted
treatment. Carbohydr Polym. 81:323–329. 2010. View Article : Google Scholar
|
|
7
|
Chaiklahan R, Chirasuwan N, Triratana P,
Loha V, Tia S and Bunnag B: Polysaccharide extraction from
Spirulina sp. and its antioxidant capacity. Int J Biol Macromol.
58:73–78. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Balachandran P, Pugh ND, Ma G and Pasco
DS: Toll-like receptor 2-dependent activation of monocytes by
Spirulina polysaccharide and its immune enhancing action in mice.
Int Immunopharmacol. 6:1808–1814. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang H, Cui Y, Zhu S, Feng F and Zheng X:
Characterization and antimicrobial activity of a pharmaceutical
microemulsion. Int J Pharm. 395:154–160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
López-Quintela MA and Rivas J: Chemical
reactions in micro-emulsions: A powerful method to obtain ultrafine
particles. J Colloid Interface Sci. 158:446–451. 1993. View Article : Google Scholar
|
|
11
|
Hoar TP and Schulman JH: Transparent
water-in-oil dispersions: The oleopathic hydro-micelle. Nature.
152:201–203. 1943. View
Article : Google Scholar
|
|
12
|
López-Quintela MA: Synthesis of
nanomaterials in microemulsions: Formation mechanisms and growth
control. Curr Opinion Colloid Interface Sci. 8:137–144. 2003.
View Article : Google Scholar
|
|
13
|
Malik MA, Wani MY and Hashim MA:
Microemulsion method: A novel route to synthesize organic and
inorganic nanomaterials: 1st Nano Update. Arabian J Chem.
5:397–417. 2012. View Article : Google Scholar
|
|
14
|
Shafiq-un-Nabi S, Shakeel F, Talegaonkar
S, Ali J, Baboota S, Ahuja A, Khar RK and Ali M: Formulation
development and optimization using nanoemulsion technique: A
technical note. AAPS PharmSciTech. 8:Article 282007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kong M and Park HJ: Stability
investigation of hyaluronic acid based nanoemulsion and its
potential as transdermal carrier. Carbohydr Polym. 83:1303–1310.
2011. View Article : Google Scholar
|
|
16
|
Schmalfußa U, Neubert R and Wohlrab W:
Modification of drug penetration into human skin using
microemulsions. J Control Release. 46:279–285. 1997. View Article : Google Scholar
|
|
17
|
Sintov AC, Levy HV and Botner S: Systemic
delivery of insulin via the nasal route using a new microemulsion
system: In vitro and in vivo studies. J Control Release.
148:168–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xavier-Junior FH, Huang N, Vachon JJ,
Rehder VL, do Egito ES and Vauthier C: Match of solubility
parameters between oil and surfactants as a rational approach for
the formulation of microemulsion with a high dispersed volume of
copaiba oil and low surfactant content. Pharm Res. 33:3031–3043.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moghimipour E, Salimi A and Leis F:
Preparation and evaluation of tretinoin microemulsion based on
pseudo-ternary phase diagram. Adv Pharm Bull. 2:141–147.
2012.PubMed/NCBI
|
|
20
|
Pons R, Carrera I, Caelles J, Rouch J and
Panizza P: Formation and properties of mini-emulsions formed by
microemulsions dilution. Adv Colloid Interface Sci. 106:129–146.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roohinejad S, Oey I, Wen J, Lee SJ,
Everett DW and Burritt DJ: Formulation of oil-in-water β-carotene
microemulsions: Effect of oil type and fatty acid chain length.
Food Chem. 174:270–278. 2015. View Article : Google Scholar
|
|
22
|
Li Q, Tiange C, Yinghong H, Ronghua Z,
Susan C and Yu C: The preparation and evaluation of
cepharanthine-nanostructured lipid carriers in vitro and in vivo. J
Bio Tissue Eng. 7:848–857. 2017. View Article : Google Scholar
|
|
23
|
Yoo HS, Lee KH, Oh JE and Park TG: In
vitro and in vivo anti-tumor activities of nanoparticles based on
doxorubicin-PLGA conjugates. J Control Release. 68:419–431. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu X, Li R, Zhao Y and Liu Y: Separation
of polysaccharides from Spirulina platensis by HSCCC with
ethanol-ammonium sulfate ATPS and their antioxidant activities.
Carbohydr Polym. 173:465–472. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
González-Torres L, Vázquez-Velasco M,
Olivero-David R, Bastida S, Benedí J, González RR, González-Muñoz
MJ and Sánchez-Muniz FJ: Glucomannan and glucomannan plus spirulina
added to pork significantly block dietary cholesterol effects on
lipoproteinemia, arylesterase activity, and CYP7A1 expression in
Zucker fa/fa rats. J Physiol Biochem. 71:773–784. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ravi M, De SL, Azharuddin S and Paul SFD:
The benefcial effects of spirulina focusing on its immunomodulatory
and antioxidant properties. Nut Diet Suppl. 2:73–83. 2010.
|
|
27
|
Parikh P, Mani U and Iyer U: Role of
spirulina in the control of glycemia and lipidemia in type 2
diabetes mellitus. J Med Food. 4:193–199. 2001. View Article : Google Scholar
|