Introduction
Rheumatoid arthritis (RA) is a common chronic
autoimmune disease, which is characterized by the persistent
recurrence of synovitis, and intra-articular cartilage and bone
destruction. Worldwide, 0.5-1% of the population suffers from RA.
The prevalence rate of RA in China is 0.32-0.34% of the population,
among which the disability rate after 1 year can reach ≤20%,
seriously affecting the quality of life of patients (1). RA is generally associated with
numerous factors, including genetics, infection, smoking and immune
dysfunction (2). Although the
pathology and etiology are not yet fully distinct, numerous studies
have confirmed that T-cell dysfunction, particularly imbalances in
T helper (Th)1/Th2 and Th17/regulatory T cells, results in an
increase in proinflammatory cytokines, including tumor necrosis
factor (TNF)-α, interleukin (IL)-1β and IL-6, which have an
essential role in RA occurrence and progression (3,4).
Nuclear factor (NF)-κB is a transcription factor, which is
considered the core of inflammation and immune responses. NF-κB can
be activated by IL-1β, IL-6, transforming growth factor-β, TNF-α
and other cytokines, and further promotes the transcription of
various cytokines, which constitutes the feedback mechanism of
NF-κB and cytokines, thus leading to persistent inflammation and
joint damage in RA (5).
At present, RA remains one of the most incurable
diseases. Biological agents, including TNF-α or IL-1 inhibitors,
have garnered attention and are recommended for RA treatment as
they are able to halt disease progression (6). However, various unfavorable elements
of these drugs, such as severe adverse reactions, potential
toxicity and high costs, limit their application (7). Traditional Chinese medicine (TCM)
has been reported to regulate the systemic immune response
(6). Long-term clinical practice
and experience has indicated that TCM may have potential for the
development of novel therapeutic agents that not only prevent joint
damage, but also have less adverse effects and lower costs. Wang-Bi
Tablet (WB) is a product of Liaoning Herbapex Pharmaceutical
(Group) Co., Ltd. (Benxi, China); the SFDA approval number is
Z20044066. It has been used to treat arthritis for several years,
and has exhibited a good efficacy and few side effects. WB consists
of 16 herbal medicines, including Radix Rehmanniae, Radix
Rehmanniae Preparata, Radix aconiti lateralis preparata, Rhizoma
Drynariae, Cassia Twig, Radix Dipsaci, Epimedium brevicornu Maxim,
Rhizoma Cibotii Preparata, Carthami Flos, Radix Clematidis, Spina
Gleditsiae, Radix Angelicae Pubescentis, Radix Saposhnikoviae,
Radix Paeoniae Alba, Rhizoma Anemarrhenae and goat bone. It has
previously been reported that WB can relieve joint swelling and
pain, and improve joint motion (8); however, the pharmacological
mechanism of action of WB is largely unclear. The present study
aimed to evaluate the anti-inflammatory effects of WB on rat
adjuvant-induced arthritis (AIA) and to explore the underlying
molecular mechanism.
Materials and methods
Chemicals and reagents
Acetonitrile [high performance liquid chromatography
(HPLC) grade] was purchased from Caledon Laboratories, Ltd.
(Georgetown, ON, Canada). Timosaponin BII (batch no.
111839-201505), mangiferin (batch no. 111607-200402), naringin
(batch no. 110722-201312), hydroxysafflor yellow A (batch no.
111637-201308), paeoniflorin (batch no. 110736-201438),
prim-O-glucosylcimifugin (batch no. 111522-201511),
5-O-methylvisammioside (batch no. 111523-201509), icariin (batch
no. 110737-200415), protocatechuate (batch no. 110809-200604) were
purchased from the National Institutes for Food and Drug Control
(Beijing, China). Columbianadin (batch no. MUST-12072906), epimedin
A (batch no. MUST-14060312), epimedin B (batch no. MUST-14062312),
epimedin C (batch no. MUST-14022312), benzoylmesaconine (batch no.
MUST-15012216), benzoylaconitine (batch no. MUST-14052315) and
benzoylhypacoitine (batch no. MUST-14032209) were purchased from
Chengdu Must Bio-Technology Co., Ltd. (Chengdu, China). Alibiflorin
(batch no. 130824) was purchased from Shanghai Winherb Medical
Technology Co., Ltd. (Shanghai, China). Lipopolysaccharide (LPS)
was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Total glucosides of paeony (TGP) tablets were produced by Ningbo
Lihua Pharmaceuticals Co., Ltd. (Ningbo, China). For animal
experiments, drugs were suspended in sterilized 0.5%
carboxymethylcellulose sodium (CMC-Na). Heat-killed
Mycobacterium tuberculosis (MT) H37Ra was purchased from
Difco (BD Biosciences, Franklin Lakes, NJ, USA). Antibodies against
phosphorylated (p)-p65 (ab194926), p(IKK) α/β (ab194528), NF-κB
inhibitor α (IκBα) (ab32518), Toll-like receptor 4 (TLR4) (ab13867)
and p-signal transducer and activator of transcription 3 (STAT3)
(ab76315) were from Abcam (Cambridge, UK). β-actin (A5441) primary
antibody was purchased from Sigma-Aldrich (Merck KGaA). Cell
Counting Kit-8 (CCK-8) kit was from Beyotime Institute of
Biotechnology (Shanghai, China).
Preparation of herbs
WB was prepared by Liaoning Herbapex Pharmaceutical
(Group) Co., Ltd. It contains 16 herbal medicines and one animal
medicine, including Radix Paeoniae Alba and Rhizoma Anemarrhenae,
which were crushed into a fine powder; and Radix Rehmanniae, Radix
Rehmanniae Preparata, Rhizoma Drynariae, Rhizoma Cibotii and goat
bone, which were decocted with water two times, after which the
decoction was mixed with the alcohol precipitate from the decoction
of the remaining 10 herbs and was concentrated to acquire a thick
paste. A total of 1 g paste is equivalent to 8.92 g crude medicinal
herbs.
HPLC analysis
WB (1.0 g) was weighed and subjected to ultrasonic
extraction with 25 ml alcohol solution (529 ml ethanol diluted in 1
l water) for 60 min at 25°C. The extract solution was then filtered
through a 0.45-µm filter membrane prior to HPLC analysis.
The separation was carried out at 30°C, with a flow rate of 1.0
ml/min, on an Agilent ZORBAX SB-C18 (880975-902) 1100 HPLC system
(4.6×250 mm, 5 µm) (Agilent Technologies, Inc., Santa Clara,
CA, USA). The mobile phase consisted of solvent A (100%
acetonitrile) and solvent B (water and phosphoric acid, 100:0.1,
v/v), and 10 ml sample was injected onto the column. The elution
program was optimized and conducted as follows: 0-40 min, linear
gradient was increased from 5 to 18% solvent A; 40-60 min, linear
gradient was increased from 18 to 25% solvent A; 60-80 min, linear
gradient was increased from 25 to 40% solvent A; 80-90 min, linear
gradient was increased from 40 to 80% solvent A; and 90-95 min,
linear gradient was maintained at 80% solvent A. Monitoring was
performed at 235 nm using a VWD G1314A detector (Agilent
Technologies, Inc.).
The compounds were analyzed by comparing sample
retention times with those of authentic standards, including
paeoniflorin, benzoylaconine, epimedin C and icariin. Data analysis
of chromatographic fingerprints was performed using a similarity
evaluation system. OpenLAB CDS ChemStation software (Edition
Revision C. 01. 07; Agilent Technologies, Inc.) was used to
evaluate the similarities between different chromatograms and to
calculate the correlation coefficient of different patterns.
Preparation of medicated sera
Sprague-Dawley male rats (n=30; age, 6-7; weight,
~200 g) were purchased from Shanghai SLAC Laboratory Animal Co.,
Ltd. (Shanghai, China) and were housed in a specific pathogen-free
animal room under the following conditions: 50±5% humidity and
23±1°C ambient temperature. Rats were maintained under a 12-h
light/dark cycle, and food and water were provided ad
libitum during all experiments. The rats were divided into the
following groups: Normal group, WB group and TGP group. WB or TGP
were suspended in 0.5% CMC-Na solution and administered at a dose
of 2.8 g and 0.93 g/kg/day, respectively for 7 days. The dosage was
five times the equivalent clinical dose. The rats in the normal
group were administered the same volume of 0.5% CMC-Na solution. On
day 8, whole blood was obtained from the abdominal aorta after an
overnight fast (9). The medicated
serum samples were collected by centrifugation of whole blood at
2,000 × g for 20 min at room temperature. Dulbeccos's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) was added to the corresponding serum, and the
final concentration of medicated serum in the medium was 10%.
Animals and cells
Sprague-Dawley female rats (n=30; age, 6-7 weeks;
weight, 200 g) were purchased from Shanghai SLAC Laboratory Animal
Co., Ltd. The rats were housed in a controlled environment:
Temperature, 25±1°C; humidity, 50±5% under a 12-h light/dark cycle
with free access to sterilized food and water. All animal
procedures were performed following the Guide for the Care and Use
of Laboratory Animals published by the National Institutes of
Health (Bethesda, MD, USA) (10),
and the present study was approved by the ethics committee of
Experimental Research, Shanghai Medical College, Fudan University
(Shanghai, China). Raw264.7 cells, purchased from the cell
repository of Chinese Academy of Sciences (Shanghai, China), were
cultured in DMEM containing 10% heat-inactivated FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin at 37°C in a
humidified atmosphere containing 5% CO2. The cells were
treated with medium containing 10% normal serum, or TGP-(1:4
dilution with normal rat serum), WBL-(1:4 dilution with normal rat
serum), WBM-(4:6 dilution with normal rat serum) or WBH- (6:4
dilution with normal rat serum) medicated sera for 48 h, followed
by CCK-8 assay or western blotting. Cell viability was determined
using the CCK-8 assay based on water-soluble tetrazolium salt-8 as
described in our previous study (11).
Induction of AIA and WB treatment
Heat-killed MT H37Ra was ground in a roughened
mortar until its color changed to white, and mineral oil was added
gradually to make a paste. Each female rat was subcutaneously
injected at the base of the tail with 0.1 ml MT suspension
containing 62.5 µg MT (12). From day 0 after adjuvant
injection, the rats were treated by gavage once a day with 0.5%
CMC-Na solvent in the model group (n=8), WB (0.56 g/kg/day,
clinical equivalent dose) in the WB group (n=7), and TGP (0.186
g/kg/day) in the positive control TGP group (n=7). In addition,
rats (n=8) in the normal control group (without MT injection) were
treated with an equal volume of 0.5% CMC-Na. After 30 days of
treatment, all rats in each group were sacrificed following sodium
pentobarbital anesthesia.
Assessment of arthritis severity
Arthritis score was evaluated every 3 days from the
onset of arthritis according to the previously described method
(13). The highest score for each
rat was 16. Hind-limb volume was measured using a volumetric meter.
Radiological and histological evaluation was processed as described
in our previous study (13).
Hematoxylin and eosin staining
The left hind paw from each rat was fixed in 4%
phosphate-buffered formaldehyde for 3 days at room temperature,
then decalcified in 5% nitric acid solution for 48 h at room
temperature. The metatarsophalangeal joint from the third toe was
cut and embedded in paraffin. Longitudinal sections (6 µm)
were prepared for routine hematoxylin and eosin staining. After
routine deparaffinization, the sections were stained with
hematoxylin for 15 min at room temperature, followed by
counterstaining with eosin for 1 min. Images were captured using a
light microscope (Zeiss GmbH, Jena, Germany).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and western blotting
Total RNA was isolated from the paws of rats in each
group using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), and cDNA was synthesized using a Takara reverse
transcriptase kit (Takara Biotechnology Co., Ltd., Dalian, China),
according to the manufacturer's protocol. Finally, fluorescent qPCR
experiments (procedure: 95°C for 2 min, 39 cycles at 95°C for 15
sec, 60°C for 30 sec and 95°C for 15 sec; melt curve, 60 to 95°C,
increment 0.5°C) were conducted using a Bio-Rad iQ5 Real Time PCR
system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) using SYBR
Green (Vazyme Biotech Co., Ltd., Nanjing, China) according to the
manufacturer's protocol and results were quantified using the
2−ΔΔCq method (14).
The primer sequences used were as follows: TNF-α, forward (F)
5′-ATGGGCTCCCTCTCATCAGT-3′, reverse (R) 5′-GCTTGGTGGTTTGCTACGAC-3′;
IL-6, F 5′-ATGAACAGCGATGATGCACT-3′, R 5′-ACAAACTCCAGGTAGAAACGG-3′;
IL-1β, F 5′-GAGCTTCAGGAAGGCAGTGT-3′, R 5′-TCACACACTAGCAGGTCGTC-3′;
and β-actin, F 5′-ATCTATGAGGGTTACGCGCTCC-3′ and R
5′-CAGCTGTGGTGGTGAAGCTG-3′.
For western blotting, rat paw lysates were prepared
using Radioimmunoprecipitation Assay Lysis Buffer (Beyotime
Institute of Biotechnology), and protein concentrations were
quantified using the bicinchoninic acid method. Equal amounts of
protein (20 µg) were separated in different concentrations
of polyacrylamide gel (8-10%), according to the molecular weight of
the proteins. Proteins were transferred to polyvinylidene
difluoride membranes, which were blocked in 5% milk for 30 min at
room temperature. Subsequently, membranes were incubated with
various primary antibodies using the dilutions recommended by the
manufacturer's protocol, for 24 h at 4°C. The membranes were then
incubated with horseradish peroxidase-conjugated anti-rabbit or
anti-mouse immunoglobulin G secondary antibodies (1:5,000; cat.
nos. W4011 and W4021; Promega Corporation, Madison, USA) at room
temperature for 1 h and were detected using GeneGnomeXRQ (Syngene,
Frederick, MD, USA). The intensity of each lane was semi-quantified
using ImageJ 1.48 (National Institutes of Health).
Statistical analysis
Data are presented as the means ± standard error of
values from three experiments. Student's t-test was used to compare
two groups, and one-way analysis of variance followed by Bonferroni
post hoc test was used for multiple comparisons. All statistical
analyses were performed using SPSS 13.0 statistical analysis
software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
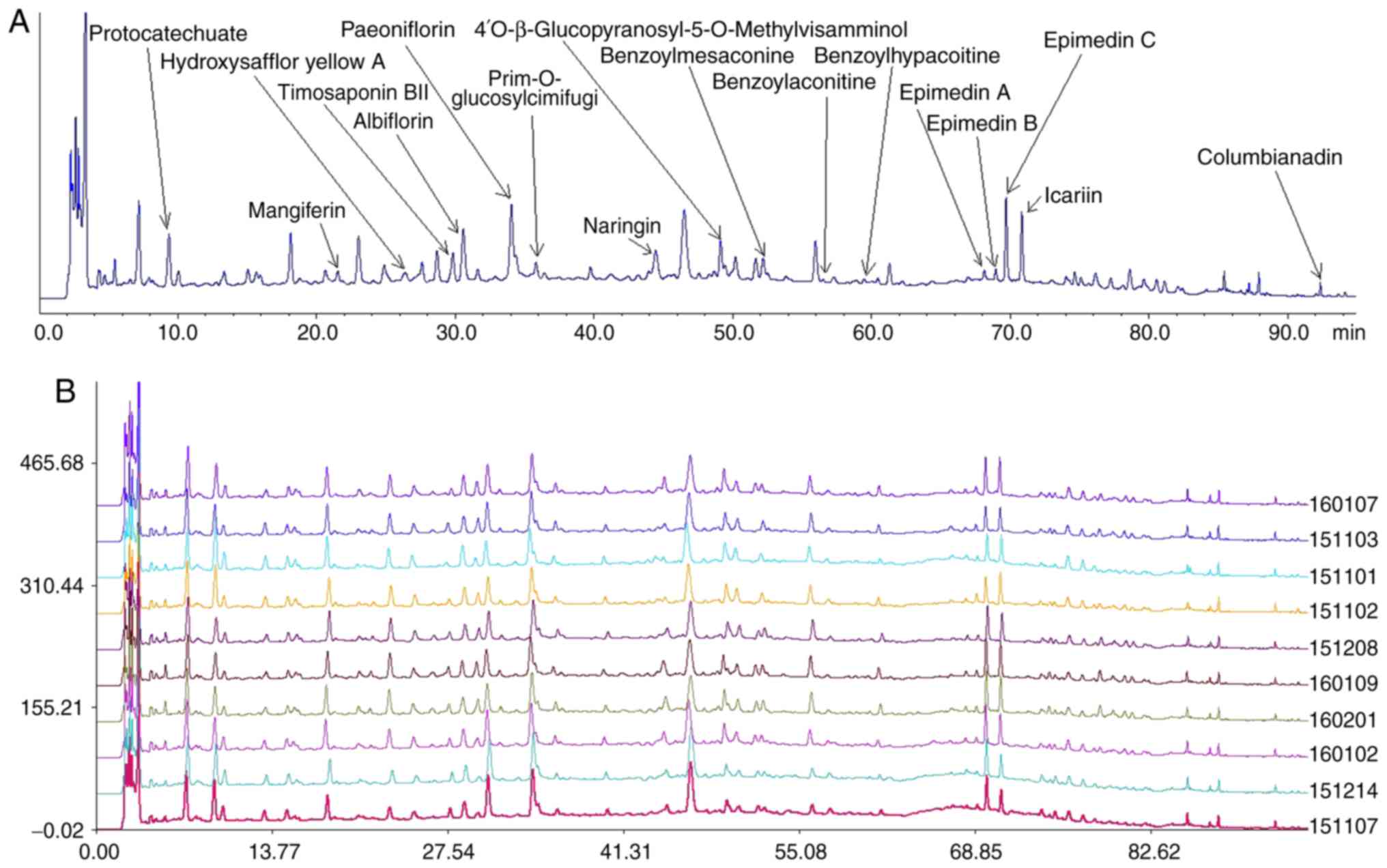
Quality control of WB
The HPLC profile of WB contained numerous visible
peaks. The compounds were identified by comparing the retention
times of the samples with authentic standards. When compared with
the standard references, 17 peaks, corresponding to relative
retention time and relative peak area of protocatechuate,
mangiferin, hydroxysafflor yellow A, timosaponin BII, albiflorin,
paeoniflorin, prim-O-glucosylcimifugi, naringin,
4′-O-β-glucopyranosyl-5O-methylvisamminol, benzoylmesaconine,
benzoylaconine, benzoylhypacoitine, epimedin A, epimedin B,
epimedin C, icariin and columbianadin were identified (Fig. 1A). The similarity indices of 10
batches of WB samples were calculated using a similarity evaluation
system (Fig. 1B). The results
demonstrated that the samples had good correlation and shared a
similar chromatographic pattern with similarity indices at
>0.900.
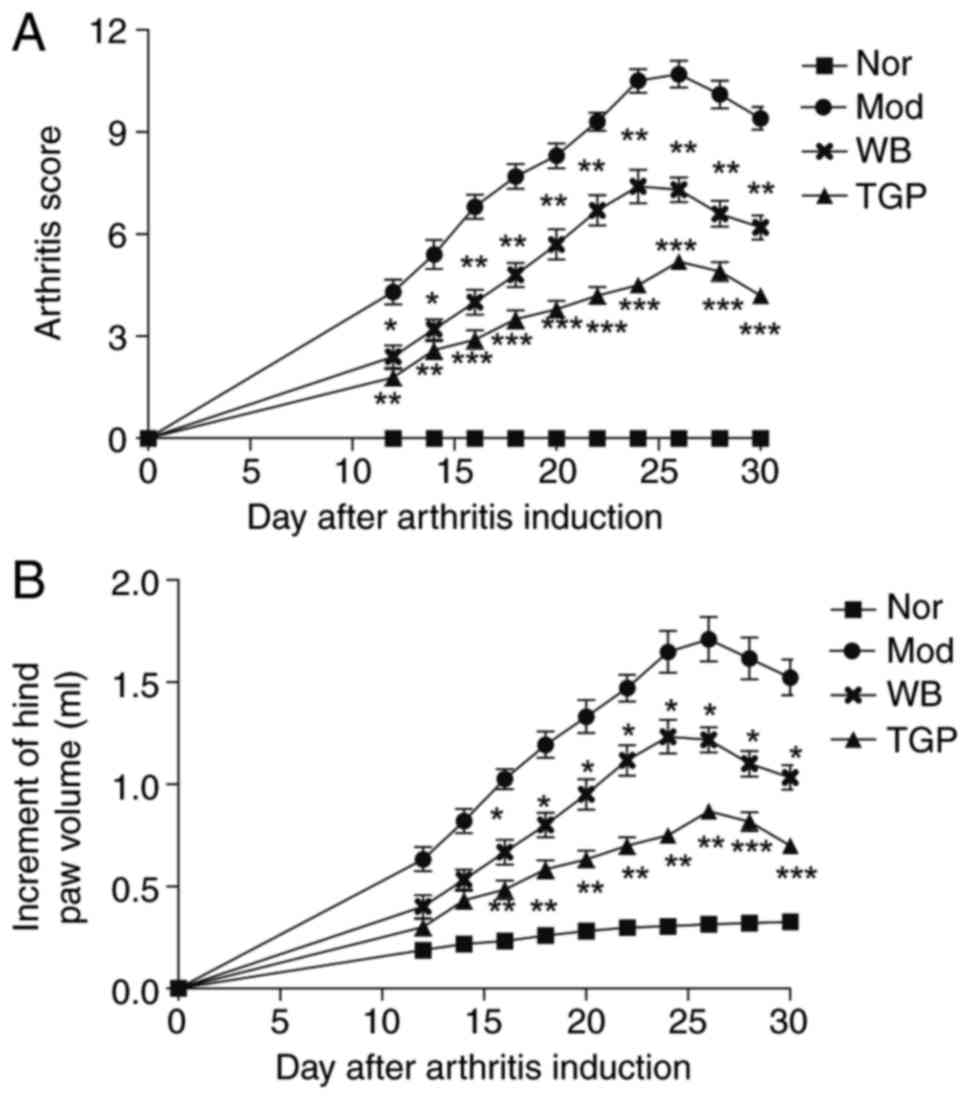
Inhibitory effects of WB on the symptoms
of AIA in rats
The onset of AIA occurred ~12 days following
immunization. Symptoms, such as swollen red paws and functional
joint impairment, were observed and the incidence rate was 100%.
The arthritis score and hind-paw volume were typically increased
and reached a peak 2 weeks following the onset of arthritis.
Treatment with WB or TGP decreased arthritis score and hind-paw
volume (Fig. 2A and B). The
arthritis score of WB and TGP groups began to decrease at day 10
following drug administration compared with the model group. In
addition, between days 10 and 30, arthritis scores were
continuously decreased and inflammatory symptoms were obviously
improved (Fig. 2A). Hind-paw
volumes were measured to evaluate the severity of swelling.
Following WB treatment for 16 days, hind-paw volume was
significantly decreased compared with the model group (Fig. 2B). A significant inhibition of
hind-paw volume was also observed in the TGP-treated group between
days 16 and day 30 compared with the model group (Fig. 2B). The inhibitory effects of TGP
on arthritis score and hind-paw volume were stronger than WB.
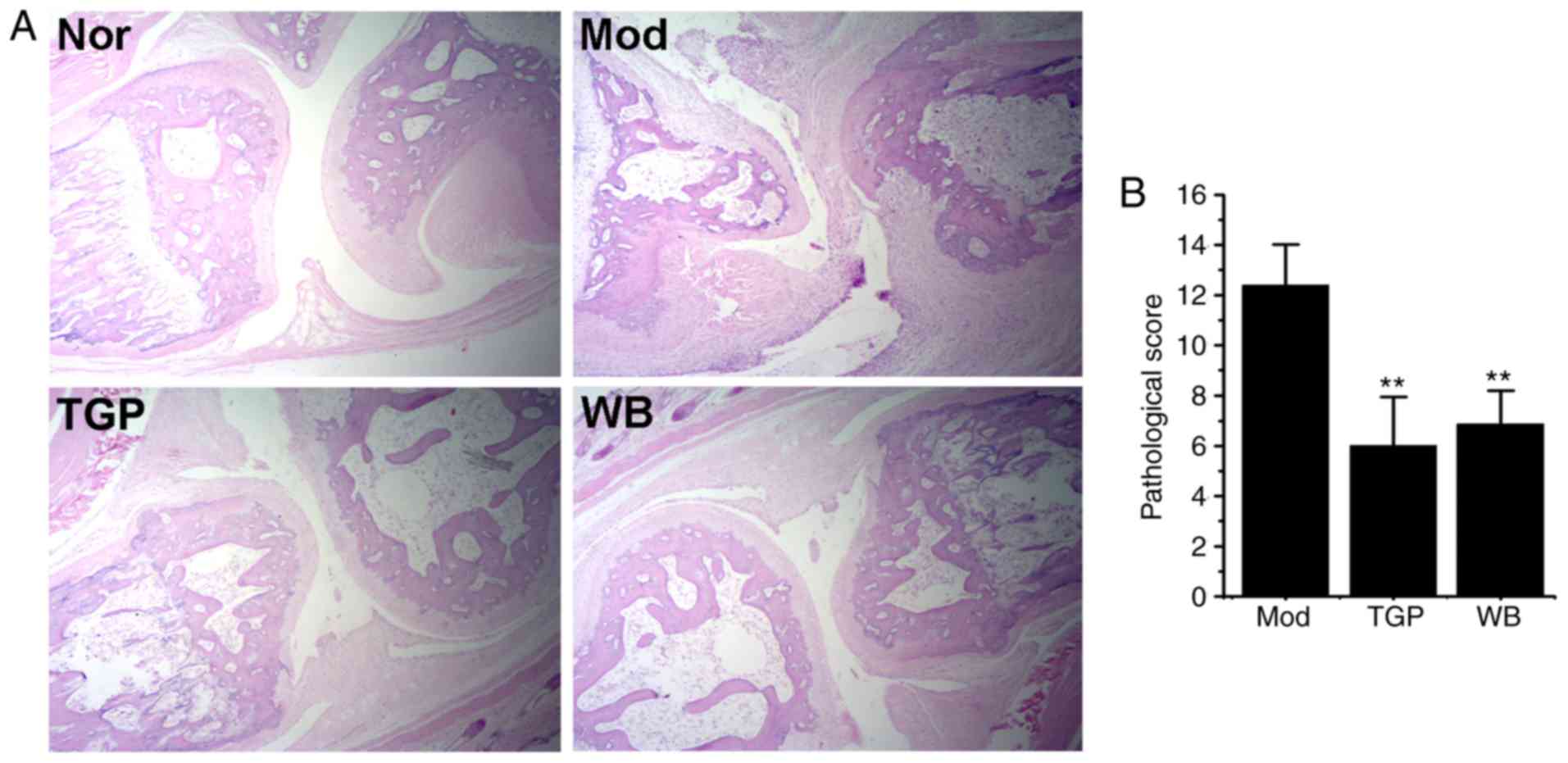
WB suppresses pathological alterations to
joints
Histological alterations in the metatarsophalangeal
joint of the third toe were evaluated. As shown in Fig. 3A, in the model group, inflammatory
cells extensively infiltrated into the joints, and pannus
formation, synovial tissue proliferation, cartilage destruction and
subchondral bone erosion were detected. Compared with in the model
group, the histological lesions of the TGP and WB groups were
markedly reduced; in addition, synovial hyperplasia, inflammatory
cell infiltration and joint destruction were moderate, and the
pathological score was significantly reduced (Fig. 3B, P<0.05). These findings
indicated that WB may improve the pathological alterations of
AIA.
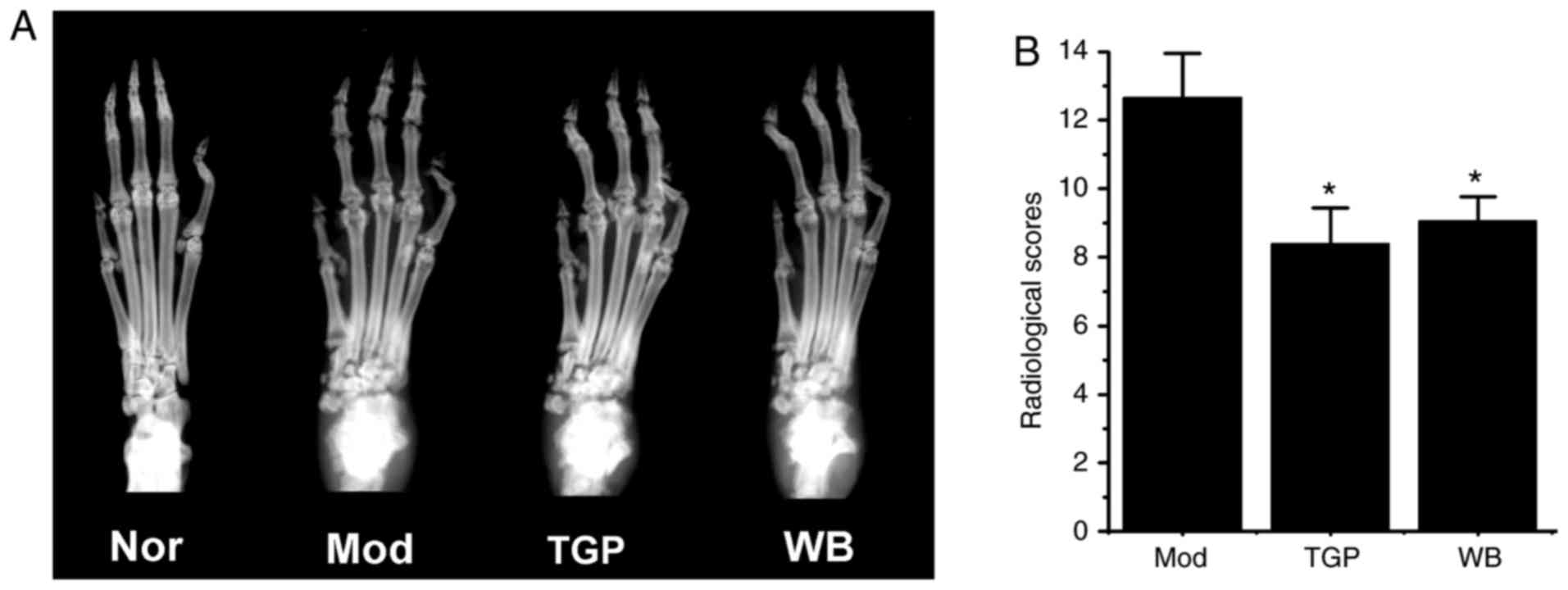
Protective effects of WB on joint
destruction
A total of 30 days from the onset of arthritis, the
left hind paws of rats were subjected to radiological examination.
As shown in Fig. 4A, the paws of
the model group exhibited severe bone resorption, enlarged joint
spaces and joint destruction. However, paws from rats in the WB- or
TGP-treated groups exhibited reduced joint destruction and
increased bone density. The radiological scores of the WB and TGP
groups were markedly decreased compared with in the model group
(Fig. 4B, P<0.05).
WB suppresses proinflammatory cytokine
expression
After 30 days of treatment, the rats were sacrificed
and total RNA was extracted from the right hind paws in each group.
The mRNA expression levels of proinflammatory cytokines, TNF-α,
IL-1β and IL-6, were examined by RT-qPCR. WB treatment
significantly decreased the mRNA expression levels of TNF-α, IL-1β
and IL-6 in paw tissues compared with in the model group. TGP
exhibited a stronger inhibitory effect than WB (Fig. 5).
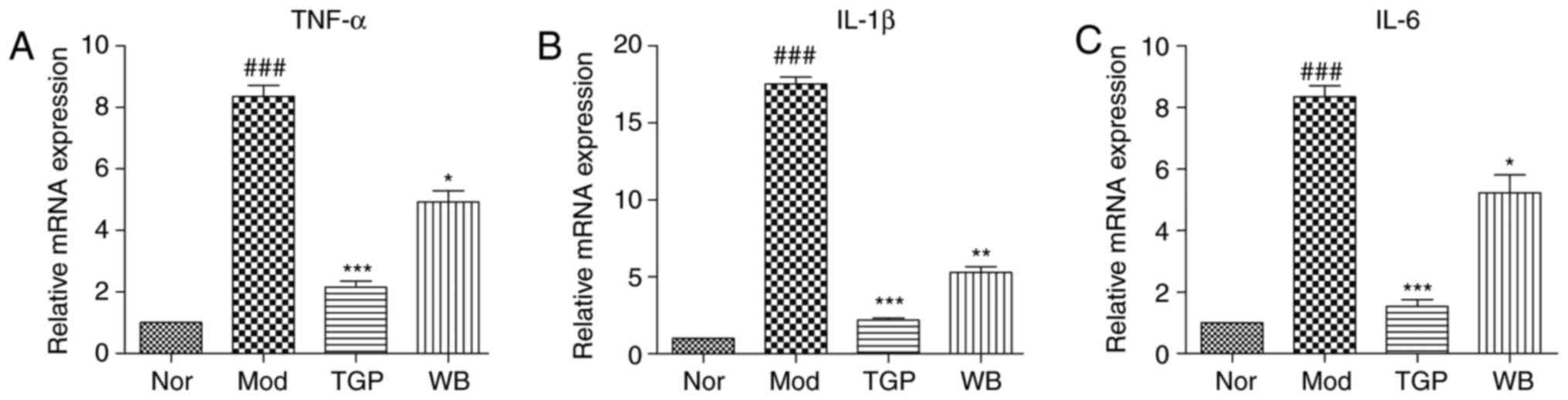 | Figure 5Effects of WB on the mRNA expression
levels of proinflammatory cytokines in the paws of adjuvant-induced
arthritis rats. The stomachs of rats were infused daily with WB or
TGP, from the day of adjuvant injection for 30 days. The mRNA
expression levels of (A) TNF-α, (B) IL-1β and (C) IL-6 were
detected. Data are expressed as the means ± standard error of the
mean. ###P<0.001 vs. the Nor group;
*P<0.05, **P<0.01,
***P<0.001 vs. the Mod group. IL, interleukin; Mod,
model (n=8); Nor, normal (n=8); TGP, total glycosides of paeony
(n=7); TNF-α, tumor necrosis factor-α; WB, Wang-Bi Tablet
(n=7). |
Inhibitory effects of WB on activation of
the STAT3 and NF-κB signaling pathways in AIA rats
It has previously been reported that excessive
generation of proinflammatory cytokines is closely associated with
activation of the NF-κB signaling pathway (3). In order to further study the
molecular pharmacological mechanism underlying the anti-AIA effects
of WB, total proteins were extracted from rat paws in each group,
and the expression levels of proteins crucial for the NF-κB
signaling pathway were detected by western blotting. As shown in
Fig. 6, treatment with WB
significantly decreased the expression levels of p-STAT3, p-IKK and
p-p65 compared with in the model group. In addition, TLR4 was
reduced and IκBα was increased by WB or TGP treatment (Fig. 6A). Densitometric
semi-quantification of western blotting confirmed the effects of WB
on activation of the STAT3 and NF-κB signaling pathways (Fig. 6B).
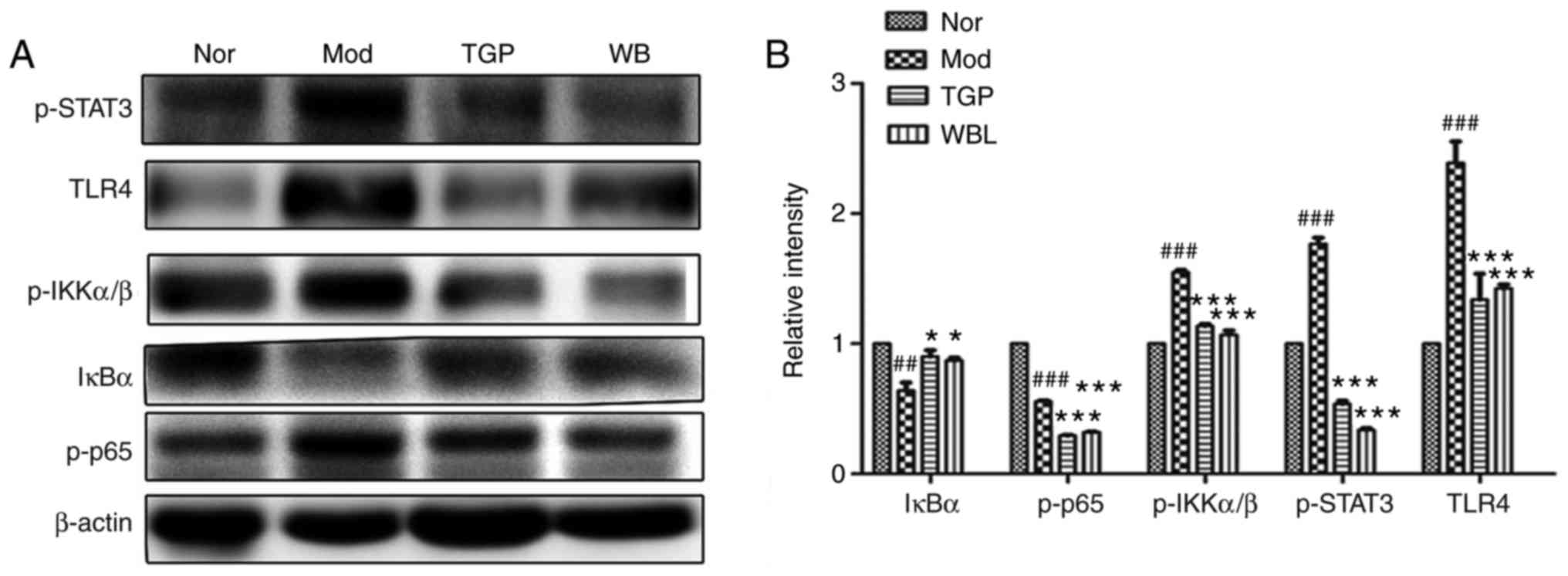 | Figure 6Effects of WB on activation of the
STAT3 and NF-κB signaling pathways in the paws of adjuvant-induced
arthritis rats. The stomachs of rats were infused daily with WB or
TGP, from the day of adjuvant injection for 30 days. (A)
Post-treatment, equal amounts of protein from the hind paws in each
group were merged into one sample, and immunoblotting was conducted
using the indicated antibodies. (B) Densitometric
semi-quantification of western blots. Data are expressed as the
means ± standard error of the mean (n=7-8 rats).
##P<0.05, ###P<0.01 vs. the Nor group;
*P<0.05, **P<0.01,
***P<0.001 vs. the Mod group. IκBα, NF-κB inhibitor
α; IKK, IκB kinase; Mod, model (n=8); NF-κB, nuclear factor-κB;
Nor, normal (n=8); p-, phosphorylated; STAT3, signal transducer and
activator of transcription 3; TGP, total glycosides of paeony
(n=7); TLR4, Toll-like receptor 4; TNF-α, tumor necrosis factor-α;
WB, Wang-Bi Tablet (n=7). |
Suppression of LPS-induced STAT3 and
NF-κB activation in Raw264.7 cells following treatment with
WB-medicated sera
In order to confirm the inhibitory effects of WB on
the STAT3 and NF-κB pathways at the cellular level, the present
study detected the toxicity of WB-medicated sera on Raw264.7 cells.
The results demonstrated that WB-medicated sera exerted no toxic
effect on Raw264.7 cells in the tested concentration range
(Fig. 7A). WB-medicated sera
hindered degradation of IκB and reduced the expression levels of
p-STAT3, p-IKK and p-p65 (Fig.
7B). Densitometric semi-quantification of western blotting
confirmed the effects of WB-medicated sera on STAT3 and NF-κB
activation (Fig. 7C).
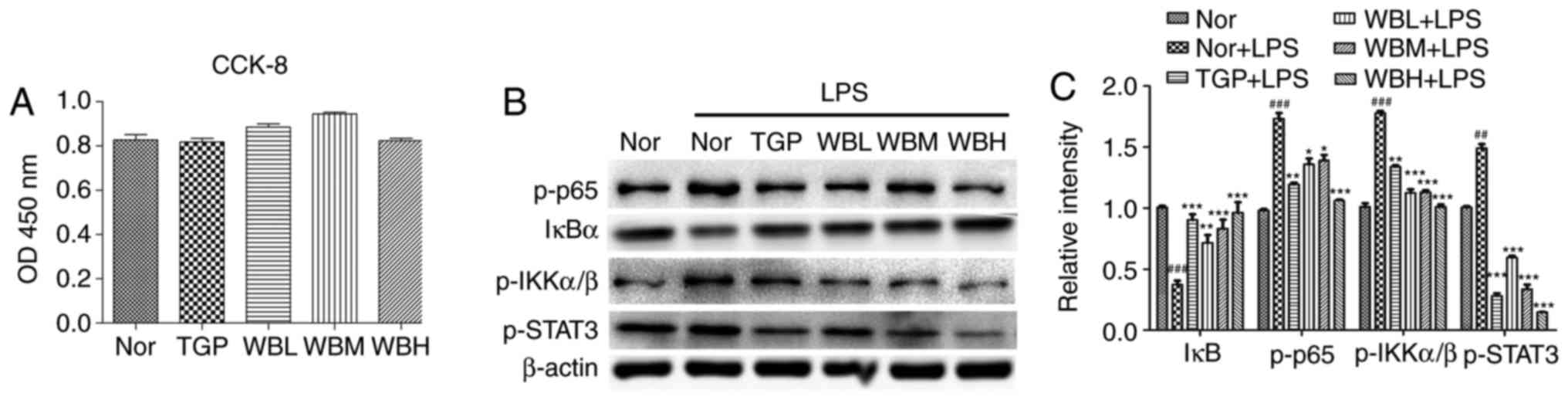 | Figure 7Suppression of LPS-induced STAT3 and
NF-κB activation in Raw264.7 macrophages following treatment with
WB medicated sera. (A) Raw264.7 cells were treated with medium
containing normal serum, or TGP-, WBL-, WBM- or WBH-medicated sera
for 48 h, followed by CCK-8 assay. (B) Raw264.7 cells were
pretreated, as described in (A), and were then treated with 100
ng/ml LPS for 15 min. Cell lysates were immunoblotted with the
indicated antibodies. (C) Densitometric semi-quantification of
western blots. Data are expressed as the means ± standard error of
the mean. ##P<0.01, ###P<0.001 vs. the
Nor group; *P<0.05, **P<0.01,
***P<0.001 vs. the Nor + LPS group. CCK-8, Cell
Counting Kit-8; IκBα, NF-κB inhibitor α; IKK, IκB kinase; LPS,
lipopolysaccharide; NF-κB, nuclear factor-κB; Nor, normal; OD,
optical density; p-, phosphorylated; STAT3, signal transducer and
activator of transcription 3; TGP, total glycosides of paeony TLR4,
Toll-like receptor 4; WB, Wang-Bi Tablet; WBH, high-dose WB; WBL,
low-dose WB; WBM, medium-dose WB. |
Discussion
WB has been applied in the clinical treatment of RA
for several years in China, due to its efficacy and few side
effects. The HPLC profile of WB confirmed that it contained several
main compounds that possess anti-inflammatory effects.
Protocatechuate has been reported to possess anti-inflammatory and
antioxidant activities via the inhibition of inflammatory
cytokines, including TNF-α, IL-1β and IL-6, through the NF-κB
pathway (15). Mangiferin has
been demonstrated to act as an effective inhibitor of the NF-κB
signaling pathway; its ability to regulate various transcription
factors, such as NF-κB and nuclear factor (erythroid-derived
2)-like 2, and modulate the expression of numerous proinflammatory
signaling intermediates, such as TNF-α, cyclooxygenase 2 (COX-2),
contributes to its anti-inflammatory potential (16). Hydroxysafflor-yellow A has been
reported to exert inhibitory effects on NF-κB, ultimately
suppressing abnormal proliferation of vascular endothelial cells
(17). Timosaponin B-II has also
been revealed to inhibit proinflammatory cytokine production by
attenuating increases in TNF-α, IL-1β and IL-6 (18). In addition, icariin may exert
therapeutic effects against osteoporosis; it was reported to be
able to synergistically increase osteoblast proliferation, alkaline
phosphatase activity and mineralized nodule formation, and to
promote bone matrix formation by upregulating bone morphogenetic
protein and the Wnt/β-catenin signaling pathway in osteoblasts
(19). Icariin may also stimulate
osteogenic differentiation of rat bone marrow stromal stem cells by
increasing tafazzin expression (20). Albiflorin and paeoniflorin are
often applied in RA treatment due to their anti-inflammatory
effects. It has been reported that they may inhibit LPS-induced
inducible nitric oxide synthase and COX-2 gene expression, as well
as the subsequent production of nitric oxide, prostaglandin E2
(PGE2) and COX-2. Furthermore, they both reduce the production of
cytokines (TNF-α and IL-6) induced by LPS in Raw264.7 macrophages
(21). Naringin has been revealed
to stimulate angiogenesis in the process of bone healing by
regulating the vascular endothelial growth factor (VEGF)/VEGF
receptor 2 signaling pathway in osteoporosis (22). Prim-O-glucosylcimifugin and
4′-O-β-Glucopyranosyl-5-O-Methylvisamminol both exert inhibitory
effects on the proliferation of smooth muscle cells stimulated by
TNF-α (23). Benzoylmesaconine is
known for its analgesic effects (24). Furthermore, benzoylhypacoitine,
benzoylaconitine and benzoylmesaconine have all been reported to
possess anti-inflammatory and immunosuppressive effects (25). Epimedin A, epimedin B and epimedin
C have been demonstrated to possess potential activity against
osteoporosis (26). In addition,
columbianadin is an active constituent of Radix Angelicae
Pubescentis, which exerts anti-inflammatory and analgesic effects
(27). Therefore, the numerous
herbal components of WB may exert effects that vary from one
another, and even a single component may initiate various actions.
Therefore, the overall pharmacology of WB may be achieved through
numerous pathways, factors and targets.
NF-κB is an important drug target for the treatment
of RA, which regulates the transcription of various inflammatory
cytokines, including TNF-α, IL-1β and IL-6 (28). Furthermore, the accumulation of
these inflammatory cytokines contributes to further activation of
the NF-κB signaling pathway (29). In the resting state, IκBα is
contained within the IκBα/p50/p65 inactive complex in the
cytoplasm; however, once cells are stimulated by external stimuli,
IκBα is rapidly phosphorylated by activation of p-IKKα/β, and
p-IκBα is then rapidly degraded. The p50/p65 complex subsequently
translocates into the nucleus, rapidly initiating the transcription
of NF-κB-associated genes (30-33). Clinical studies have revealed that
NF-κB signaling molecules are overactive in the synovial tissues of
patients with RA. Therefore, inhibition of the NF-κB signaling
pathway is a key point in RA therapy. In order to clarify the
pharmacological mechanism underlying the effects of WB on RA, the
therapeutic effects of WB were evaluated on AIA rats. The results
demonstrated that WB may significantly reduce the progression of
AIA in rats. As determined by western blotting, the expression
levels of NF-κB signal molecules were detected in rat paws and
Raw264.7 cells; the results confirmed that WB may inhibit
activation of the NF-κB signaling pathway, thereby inhibiting the
progression of inflammation.
Under normal conditions, proinflammatory and
anti-inflammatory cytokines are maintained in a dynamic balance. In
RA, proinflammatory cytokines are overly transcribed and expressed,
thus resulting in destruction of the normal cytokine balance
(34-36). Excessive IL-1β and TNF-α can
stimulate the production of vascular endothelial cells and induce
overexpression of cell adhesion factors. In addition, they
stimulate synovial cells and cartilage cells to release
collagenase, PGE2 and other inflammatory mediators, which have a
synergistic effect in arthritis. IL-6 amplifies the biological
effects of cytokines during the inflammatory response. It can
induce the production and release of other inflammatory cytokines,
including TNF-α and IL-1β, further enhancing inflammatory effects.
Therefore, reducing the production of proinflammatory cytokines,
such as TNF-α, IL-1β and IL-6, has a pivotal role in RA treatment
(37). In the present study, the
mRNA expression levels of TNF-α, IL-1β IL-6 were detected in AIA;
the results confirmed that WB significantly decreased the mRNA
expression levels of TNF-α, IL-1β and IL-6, and therefore reduced
RA-associated inflammation.
Various inflammatory cytokines and growth factors
may activate the Janus kinase (JAK)-STAT3 signaling pathway.
Firstly, inflammatory cytokines bind to their corresponding
receptors to induce receptor dimerization, after which,
phosphorylation of the upstream JAKs, as a result of tyrosine
phosphorylation, catalyzes phosphorylation of downstream STAT3.
Subsequently, p-STAT3 dissociates from the receptor and forms a
dimer that combines with the DNA target sequence in the nucleus,
thus participating in specific transcription of downstream genes
and completing the whole process of cytokine transduction (38-41). Finally, the concentrations of
various inflammatory cytokines are elevated in RA, which are
transduced via the JAK-STAT3 signaling pathway. Therefore, blocking
the JAK-STAT3 signaling pathway may inhibit the transduction of
inflammatory cytokines. The present data indicated that p-STAT3 is
decreased in a rat model of AIA in response to WB treatment, thus
suggesting that WB may reduce the inflammatory reaction of AIA
through suppressing activation of STAT3.
RA is a chronic systemic autoimmune disease, which
is associated with chronic inflammation and destructive events,
including joint pain and swelling, synovial hyperplasia, pannus
formation, joint deformity, and articular cartilage and bone
destruction. Articular cartilage and bone destruction poses a
particular problem. The present results demonstrated that WB not
only had an inhibitory effect on AIA-associated inflammation, but
also significantly improved joint pathology and bone destruction.
This may be associated with inhibition of the production of
proinflammatory cytokines, including TNF-α, IL-1β, and IL-6.
Although the molecular mechanisms underlying the pathological
effects of RA on joints are not completely understood, TNF-α, IL-1β
and IL-6 are known to have key roles in the pathological process of
RA. It has previously been suggested that an increased response of
T cells to pathogenic antigens may be an underlying reason for the
formation of RA via cell contact and the production of a large
number of proinflammatory cytokines (4). Stimulated T cells can activate
monocytes, macrophages and synovial cells to further produce
substantial proinflammatory cytokines, mainly TNF-α, IL-1β and
IL-6. Once bound to their receptors, these soluble molecules
trigger signal transduction, resulting in matrix metalloproteinase
production, periarticular connective tissue injury and joint
damage. In addition, TNF-α and IL-1β also induce the production of
receptor activator of NF-κB (RANK) in osteoblasts, which mediates
osteoclast differentiation and directly leads to joint bone
resorption and destruction (39).
In conclusion, the present study examined the
effects and mechanism of WB on AIA rats and in a Raw264.7 cell
model. The results demonstrated that WB may not only inhibit
inflammation, but may also prevent joint damage. The inhibitory
effects of WB on AIA might be achieved by inhibiting activation of
the NF-κB and STAT3 signaling pathways.
Acknowledgments
Not applicable.
Abbreviations:
|
RA
|
rheumatoid arthritis
|
|
WB
|
Wang-Bi Tablet
|
|
AIA
|
adjuvant-induced arthritis
|
|
TCM
|
traditional Chinese medicine
|
|
TGP
|
total glucosides of paeony
|
Funding
The present study was supported by the Science and
Technology Commission of Shanghai Municipality (grant nos.
15DZ1900103 and 15DZ1900100).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XS and GY conceived and designed the experiments.
Y-YG, L-XL, W-LB and H-DL performed the experiments. Y-YG, YZ and
DX analyzed the data. Y-YG, YZ, GY and XS wrote the paper.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Experimental Research, Shanghai Medical College, Fudan
University (Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dhawan SS and Quyyumi AA: Rheumatoid
arthritis and cardiovascular disease. Curr Atheroscler Rep.
10:128–133. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scott DL, Wolfe F and Huizinga TW:
Rheumatoid arthritis. Lancet. 376:1094–1108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feldmann M, Brennan FM and Maini RN: Role
of cytokines in rheumatoid arthritis. Annu Rev Immunol. 14:397–440.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cooles FA, Isaacs JD and Anderson AE: Treg
cells in rheumatoid arthritis: An update. Curr Rheumatol Rep.
15:3522013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaltschmidt B and Kaltschmidt C: NF-kappaB
in the nervous system. Cold Spring Harb Perspect Biol.
1:a0012712009. View Article : Google Scholar
|
|
6
|
O'Dell JR: Therapeutic strategies for
rheumatoid arthritis. N Engl J Med. 350:2591–2602. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Smolen JS and Steiner G: Therapeutic
strategies for rheumatoid arthritis. Nat Rev Drug Discov.
2:473–488. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu JW and Shen T: Clinic study of Wang-Bi
Tablet on rheumatoid arthritis. Liaon J Trad Chinese Med.
38:2392–232393. 2011.
|
|
9
|
Sergelius P, Lee JH, Fruchart O, Salem MS,
Allende S, Escobar RA, Gooth J, Zierold R, Toussaint JC, Schneider
S, et al: Intra-wire coupling in segmented Ni/Cu nanowires
deposited by electrodeposition. Nanotechnology. 28:0657092017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press; Washington, DC: 2011
|
|
11
|
Lin W, Wang Y, Lin S, Li C, Zhou C, Wang
S, Huang H, Liu P, Ye G and Shen X: Induction of cell cycle arrest
by the carbazole alkaloid Clauszoline-I from Clausena vestita D. D.
Tao via inhibition of the PKCδ phosphorylation. Eur J Med Chem.
47:214–220. 2012. View Article : Google Scholar
|
|
12
|
Cai X, Wong YF, Zhou H, Liu ZQ, Xie Y,
Jiang ZH, Bian ZX, Xu HX and Liu L: Manipulation of the induction
of adjuvant arthritis in Sprague-Dawley rats. Inflamm Res.
55:368–377. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Wang S, Wang Y, Zhou C, Chen G, Shen
W, Li C, Lin W, Lin S, Huang H, et al: Inhibitory effect of the
antimalarial agent artesunate on collagen-induced arthritis in rats
through nuclear factor kappa B and mitogen-activated protein kinase
signaling pathway. Transl Res. 161:89–98. 2013. View Article : Google Scholar
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−delta delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
15
|
Khan AK, Rashid R, Fatima N, Mahmood S,
Mir S, Khan S, Jabeen N and Murtaza G: Pharmacological activities
of protocatechuic acid. Acta Pol Pharm. 72:643–650. 2015.PubMed/NCBI
|
|
16
|
Saha S, Sadhukhan P and Sil PC:
Mangiferin: A xanthonoid with multipotent anti-inflammatory
potential. Biofactors. 42:459–474. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J, Wang J, Wang X, Liu L, Hu J, Yu X,
Xu Y, Niu X, Lin Z, Zhang Y, et al: Molecular mechanism of
inhibition of the abnormal proliferation of human umbilical vein
endothelial cells by hydroxysafflor-yellow A. Pharm Biol.
54:1800–1807. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu WQ, Qiu Y, Li TJ, Tao X, Sun LN and
Chen WS: Timosaponin B-II inhibits pro-inflammatory cytokine
induction by lipopolysaccharide in BV2 cells. Arch Pharm Res.
32:1301–1308. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li M, Zhang ND, Wang Y, Han T, Jiang YP,
Rahman K, Qin LP, Xin HL and Zhang QY: Coordinate regulatory
osteogenesis effects of icariin, timosaponin B II and ferulic acid
from traditional Chinese medicine formulas on UMR-106 osteoblastic
cells and osteoblasts in neonatal rat calvaria cultures. J
Ethnopharmacol. 185:120–131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wei Q, He M, Chen M, Chen Z, Yang F, Wang
H, Zhang J and He W: Icariin stimulates osteogenic differentiation
of rat bone marrow stromal stem cells by increasing TAZ expression.
Biomed Pharmacother. 91:581–589. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang QS, Gao T, Cui YL, Gao LN and Jiang
HL: Comparative studies of paeoniflorin and albiflorin from Paeonia
lactiflora on anti-inflammatory activities. Pharm Biol.
52:1189–1195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song N, Zhao Z, Ma X, Sun X, Ma J, Li F,
Sun L and Lv J: Naringin promotes fracture healing through
stimulation of angiogenesis by regulating the VEGF/VEGFR-2
signaling pathway in osteoporotic rats. Chem Biol Interact.
261:11–17. 2017. View Article : Google Scholar
|
|
23
|
Wang L, Liang RX, Cao Y and Ye JX: Effect
of prim-o-glucosylcimifugin and
4′-O-beta-D-glucosyl-5-O-methylvisamminol con on proliferation of
smooth muscle cell stimulated by TNF-alpha. Zhongguo Zhong Yao Za
Zhi. 33:2157–2160. 2008.In Chinese. PubMed/NCBI
|
|
24
|
Suzuki Y, Hayakawa Y, Oyama T, Isono T,
Ohmiya Y, Ikeda Y, Asami A and Noguchi M: Analgesic effect of
benzoylmesaconine. Nihon Yakurigaku Zasshi. 102:399–404. 1993.In
Japanese. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang F, Liang SY, Chen FL, Tang QF and Tan
XM: Study on material basis of Mahuang Fuzi Xixin decoction for
anti-inflammation and immune suppression based on combined method
of serum pharmacochemistry and serum pharmacology. Zhongguo Zhong
Yao Za Zhi. 40:1971–1976. 2015.In Chinese. PubMed/NCBI
|
|
26
|
Meng FH, Li YB, Xiong ZL, Jiang ZM and Li
FM: Osteoblastic proliferative activity of Epimedium brevicornum
Maxim. Phytomedicine. 12:189–193. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen YF, Tsai HY and Wu TS:
Anti-inflammatory and analgesic activities from roots of Angelica
pubescens. Planta Med. 61:2–8. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Granet C, Maslinski W and Miossec P:
Increased AP-1 and NF-kappaB activation and recruitment with the
combination of the proinflammatory cytokines IL-1beta, tumor
necrosis factor alpha and IL-17 in rheumatoid synoviocytes.
Arthritis Res Ther. 6:R190–R198. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Boyce BF and Xing L: Biology of RANK,
RANKL, and osteoprotegerin. Arthritis Res Ther. 9(Suppl 1): S12007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han Z, Boyle DL, Manning AM and Firestein
GS: AP-1 and NF-kappaB regulation in rheumatoid arthritis and
murine collagen-induced arthritis. Autoimmunity. 28:197–208. 1998.
View Article : Google Scholar
|
|
31
|
Lee HS, Ka SO, Lee SM, Lee SI, Park JW and
Park BH: Overexpression of sirtuin 6 suppresses inflammatory
responses and bone destruction in mice with collagen-induced
arthritis. Arthritis Rheum. 65:1776–1785. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee YR, Hwang JK, Lee HS, Cheon YJ, Ryu
JH, Lee SI, Kwak HB, Lee SM, Kim JS, Park JW, et al: SPA0355, a
thiourea analogue, inhibits inflammatory responses and joint
destruction in fibroblast-like synoviocytes and mice with
collagen-induced arthritis. Br J Pharmacol. 164:794–806. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamamoto Y and Gaynor RB: IkappaB kinases:
Key regulators of the NF-kappaB pathway. Trends Biochem Sci.
29:72–79. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ha MK, Song YH, Jeong SJ, Lee HJ, Jung JH,
Kim B, Song HS, Huh JE and Kim SH: Emodin inhibits proinflammatory
responses and inactivates histone deacetylase 1 in hypoxic
rheumatoid synoviocytes. Biol Pharm Bull. 34:1432–1437. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sivalingam SP, Thumboo J, Vasoo S, Thio
ST, Tse C and Fong KY: In vivo pro- and anti-inflammatory cytokines
in normal and patients with rheumatoid arthritis. Ann Acad Med
Singapore. 36:96–99. 2007.PubMed/NCBI
|
|
36
|
Isomaki P and Punnonen J: Pro- and
anti-inflammatory cytokines in rheumatoid arthritis. Ann Med.
29:499–507. 1997. View Article : Google Scholar
|
|
37
|
Aupperle KR, Bennett BL, Boyle DL, Tak PP,
Manning AM and Firestein GS: NF-kappa B regulation by I kappa B
kinase in primary fibroblast-like synoviocytes. J Immunol.
163:427–433. 1999.PubMed/NCBI
|
|
38
|
Coombs JH, Bloom BJ, Breedveld FC,
Fletcher MP, Gruben D, Kremer JM, Burgos-Vargas R, Wilkinson B,
Zerbini CA and Zwillich SH: Improved pain, physical functioning and
health status in patients with rheumatoid arthritis treated with
CP-690,550, an orally active Janus kinase (JAK) inhibitor: Results
from a randomised, double-blind, placebo-controlled trial. Ann
Rheum Dis. 69:413–416. 2010. View Article : Google Scholar
|
|
39
|
Mori T, Miyamoto T, Yoshida H, Asakawa M,
Kawasumi M, Kobayashi T, Morioka H, Chiba K, Toyama Y and Yoshimura
A: IL-1β and TNFα-initiated IL-6-STAT3 pathway is critical in
mediating inflammatory cytokines and RANKL expression in
inflammatory arthritis. Int Immunol. 23:701–712. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Williams NK, Bamert RS, Patel O, Wang C,
Walden PM, Wilks AF, Fantino E, Rossjohn J and Lucet IS: Dissecting
specificity in the Janus kinases: The structures of JAK-specific
inhibitors complexed to the JAK1 and JAK2 protein tyrosine kinase
domains. J Mol Biol. 387:219–232. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yoshimura A: Signal transduction of
inflammatory cytokines and tumor development. Cancer Sci.
97:439–447. 2006. View Article : Google Scholar : PubMed/NCBI
|