Introduction
Chronic kidney disease (CKD) and its terminal stage,
end-stage renal disease, are important health concerns, as both are
associated with high morbidity and mortality (1,2).
The high global prevalence of CKD is increasing, requiring urgent
interventions (3). Despite its
importance, no established therapeutic approaches exist that can
restore the kidney to its healthy state. Therefore, elucidating the
pathophysiology of CKD is highly important, as is translating basic
research results to clinical practice.
Podocytes form the glomerular filtration barrier,
through interactions with endothelial cells and basement membrane
components. If podocyte injury occurs or if there is loss to their
intercalated structure, glomerular filtration capacity decreases,
which results in proteinuria (4).
Ongoing podocyte injury is a representative feature of CKD
(4), and is attributable to
several underlying problems, including primary glomerulonephritis,
diabetic nephropathy and other secondary forms (5-7).
Notably, podocyte injury is directly associated with patient
mortality (8). Therefore, aiming
to reduce podocyte injury is a research focus, as current
therapeutic options are not fully sufficient. Some of the available
options, such as modulators of the renin-angiotensin-aldosterone
system, do not target podocyte pathophysiology, while steroids have
several side effects following prolonged use (9).
Krüppel-like factor (KLF) is a member of the
zinc-finger family of transcription factors, which preferentially
binds to GC-rich sequences (for example, CACCC) (10). To date, 17 subclasses have been
identified in the human genome, and each regulates diverse and
distinct cellular processes. Their expression levels also differ
according to the type of tissue. KLF15 is abundant in podocytes
(11), hepatocytes, and
cardiomyocytes (12,13). Of note, KLF15 regulates the
transcription of podocyte-specific genes, such as nephrin and
podocin (11). This property may
represent a potential novel target that can be manipulated for
therapeutic purposes in cases of podocyte injury. A follow-up study
supported the hypothesis that KLF15 may serve as a therapeutic
target, reporting that dexamethasone, which is used for treating
podocyte inflammation, induced KLF15 expression and reduced
podocyte injury in a model of focal segmental glomerulosclerosis
(14). In a model of hypertensive
nephropathy, Sirtuin-3 was demonstrated to be responsible for KLF15
deacetylation, which prevented renal injury (15).
The present study hypothesized that
podocyte-specific expression of KLF15 may be altered in CKD. Using
both in vitro (primary human podocytes) and in vivo
(the 5/6 nephrectomy mouse model) systems, the results revealed
that low expression of KLF15 was associated with severe renal
injury. The present results also demonstrated that cyclosporine,
which is known to reduce podocyte injury (16), increased KLF15 expression.
Finally, KLF15 expression was examined in biopsied kidney samples
from patients, which may serve as a useful biomarker in conditions
such as membranous nephropathy and diabetic nephropathy.
Materials and methods
Mice
All animal experiments were performed under the
approval of the Institutional Animal Care and Use Committee of
Seoul National University Hospital (Seoul, Korea; approval no.
12-0132), according to the Guide for the Care and Use of Laboratory
Animals of the National Research Council. Wild type and C-C
chemokine receptor (CCR) type 5-deficient (CCR5−/−) mice were
obtained from the Orient Company (Seoul, Korea) and the Jackson
Laboratory (Bar Harbor, ME, USA) on a C57BL/6 background. Animals
were maintained at 22±2°C with a relative humidity of 40–60% and a
12 h light/dark cycle. Animals had ad libitum access to a
commercial rodent diet and tap water. A total of 72 adult male mice
(7-8 weeks old) were used for the 5/6 nephrectomy model. To produce
visible renal ischemia in one-third of the kidney, the lower branch
of the right renal artery was suture ligated, and the upper branch
of the right kidney was amputated using electrocoagulation. The
upper branch of the left renal artery was then ligated, and the
lower pole was placed back in the renal fossa by cauterization.
Left nephrectomy was performed 1 week later, which indicated the
onset of moderate-to-severe kidney injury (day 0). Parameters such
as blood pressure, serum blood urea nitrogen, creatinine, and
proteinuria were subsequently monitored.
Isolation and culture of primary human
podocytes
The study protocol complied with the Declaration of
Helsinki and received full approval from the Institutional Review
Board at Seoul National University Hospital (approval no.
1607-133-777). Primary human podocytes were obtained in January
2017 from the unaffected pole of a nephrectomy specimen from a
patient with renal cell carcinoma (45 years old, male) from the
Seoul National University Hospital (Seoul, Korea). Kidney cortices
were mechanically dissected. Glomerular cells were sieved using
optimized media (17), and
isolated by flow cytometry for a podocalyxin-positive (cat. no.
FAB1658P; R&D Systems, Inc., Minneapolis, MN, USA) and
CD90-negative (cat. no. 11-0909-41; eBioscience; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) population. The purities of
sorted cells were >98%. Subsequent gating analyses were
conducted with the FlowJo software (version 10; FlowJo LLC,
Ashland, OR, USA). The overall procedure was performed as
previously described (18).
For in vitro stimulation, cells were
maintained at a concentration of 1×105 cells per well in
6-well plates, and starved for 24 h. They were then treated with 1
μM retinoic acid (cat. no. R2625; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) or 2 ng/ml recombinant transforming growth
factor-β (TGF-β) for 48 h (R&D Systems, Inc.). In other
experiments, cyclosporine (Calbiochem; Merck KGaA) was used as a
renoprotective agent. Small interfering RNA (siRNA) was transfected
into cells in order to knockdown the KLF15 expression as suggested
in instructor's protocol (cat. no. sc-45567; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). For KLF15 silencing, 1
μmol human KLF15 siRNA or 1 μmol scrambled
non-targeting siRNA (cat. no. sc-36869) was added.
Briefly, 24 h prior to transfection, cells were
washed, detached with trypsin, diluted to 1:5 with fresh medium
without antibiotics (2×105 cells/ml), transferred to
6-well plates (1 ml/well) and grown to 60–80% confluency. A mixture
of KLF15 siRNA with transfection reagent (cat. no. sc-29528; Santa
Cruz Biotechnology) into siRNA transfection medium (cat. no.
sc-36868; Santa Cruz Biotechnology) was prepared and incubated for
30 min at room temperature. Complete medium was subsequently
replaced with 1 ml of the above transfection mixture. Approximately
6 h later, this was replaced with 1 ml normal complete medium.
After 24 h, cells were treated with recombinant TGF-β (2 ng/ml) and
cyclosporine (2 μmol) in fresh complete medium for 48 h.
Specific silencing of KLF15 was confirmed using western blot
analysis in three independent experiments.
Microscopy imaging analysis
Harvested mouse or biopsied human kidney tissue
samples were fixed in 4% paraformaldehyde at 4°C overnight.
Paraffin-embedded tissue sections (4 μm) were deparaffinized
and rehydrated with xylene three times (5 min each) and a
descending ethanol series (100, 100, 95, 90, 80 and 70%; 5 min
each), respectively. Endogenous streptavidin activity was blocked
in 0.3% hydrogen peroxide. Antigens were retrieved by heating
paraffin-embedded sections to ~95°C in 10% citrate buffer in a
microwave oven, five times for 5 min each. Blocking reagent (cat.
no. X0909; Agilent; Santa Clara, CA, USA) was incubated with
sections at room temperature for 1 h to block non-specific
background staining. The sections were subsequently stained with
periodic acid-Schiff and Sirius red (cat. no. ab150681; Abcam,
Cambridge, UK), and antibodies against KLF15 (1:100; cat. no.
AV32587; Sigma-Aldrich; Merck KGaA), and counterstained with
hematoxylin at 4°C overnight. Morphometric parameters were captured
using a microscope coupled to a computerized morphometry system
(Leica Microsystems, Rijswijk, Netherlands). By the use of isotype
controls for the anti-KLF15 antibody (1:100; cat. no. A0545;
Sigma-Aldrich; Merck KGaA), non-specific staining could be
excluded.
Confocal imaging was also performed following
staining with DAPI (1:1,000; cat. no. 62248; Thermo Fisher
Scientific, Inc.). Blocking reagent (cat. no. X0909; Agilent) was
added for 1 h at room temperature. PBS-diluted antibodies against
synaptopodin (1:100; cat. no. 61094; Progen Biotechnik, Heidelberg,
Germany), nephrin (1:100; cat. no. PA5-25932; Thermo Fisher
Scientific, Inc.), CD31 (1:50; cat. no. ab56299; Abcam), Wilms
tumor-1 (1:100; cat. no. ab8990-1; WT-1; Abcam), and KLF15 (1:100;
cat. no. AV32587; Sigma-Aldrich; Merck KGaA) were subsequently
incubated with the sections at 4°C overnight. Alexa Fluor 488-
(1:400; cat. no. A-10631; Thermo Fisher Scientific, Inc.), Alexa
Fluor 555-(1:400; cat. no. A-21433; Thermo Fisher Scientific,
Inc.), and Alexa Fluor 647-antibodies (1:400; cat. no. A-21445;
Thermo Fisher Scientific, Inc.) were then added at room temperature
for 30 min. Confocal microscopy was performed with a Leica TCS SP8
STED CW (Leica Microsystems).
Western blot analysis
Primary human podocytes were lysed with
radioimmunoprecipitation assay lysis buffer (cat. no. 89900; Thermo
Fisher Scientific, Inc.) supplemented with complete protease
inhibitor (cat. no. 78420; Thermo Fisher Scientific, Inc.). The
kidney homogenate was centrifugated at 12,000 × g and 4°C for 30
min. Protein concentration of the supernatant was determined with
the Bradford method. Equal amounts (80 μg) of extracted
protein were separated by 10% SDS-PAGE and transferred onto
Immobilon-FL 0.4 μM poly-vinylidene difluoride membranes
(Millipore, Bedford, MA, USA). Blocking was conducted at room
temperature for 1 h with blocking buffer (5% skin milk and 2%
bovine serum albumin (cat. no. 10735086001; Sigma-Aldrich; Merck
KGaA) in PBS with 0.2% Tween 20). The membranes were then incubated
with antibodies against fibronectin (1:300; cat. no. sc-6952; Santa
Cruz Biotechnology, Inc.), zonula occludens-1 (ZO-1; 1:300; cat.
no. 339100; Invitrogen; Thermo Fisher Scientific, Inc.), WT-1
(1:400; cat. no. sc-192; Santa Cruz Biotechnology, Inc.), β-actin
(1:10,000; cat. no. a5316; Sigma-Aldrich; Merck KGaA), and KLF15
(1:200; cat. no. ab167192; Abcam) at 4°C overnight. Horseradish
peroxidase-conjugated secondary anti-rabbit (1:4,000; cat. no.
7074S; Cell Signaling Technology, Inc. Danvers, MA, USA), anti-goat
(1:4,000; cat. no. sc-2005; Santa Cruz Biotechnology, Inc.), and
anti-mouse (1:4,000; cat. no. 7076S; Cell Signaling Technology,
Inc.) immunoglobulin G antibodies were added for 1 h at room
temperature. The blots were developed and enhanced using a Super
Signal West Pico Chemiluminescence kit. Band intensity was assessed
using ImageJ software (version 1.5; National Institutes of Health,
Bethesda, MD, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from primary human cells and
mouse renal tissue with an RNeasy Micro kit (Qiagen, GmbH, Hilden,
Germany). Subsequently, RNA was converted to cDNA using a kit
according to the manufacturer's instructions (cat. no. A3500;
Promega Corporation, Madison, WI, USA). The TaqMan gene expression
assay was used for human fibronectin and GAPDH (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The SYBR Green expression assay
was used for mouse fibronectin, interleukin (IL)-6, monocyte
chemotactic protein (MCP)-1, IL-10, CCR5, KLF15, and GAPDH (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Primer sequences were
as follows: Mouse fibronectin, forward 5′-ATGCAACGATCAGGACACAA-3′
and reverse 5′-TGTGCCTCTCACACTTCCAC-3′; mouse IL-6, forward
5′-CCTCTGGTCTTCTGGAGTACC-3′ and reverse 5′-CGACCTCAGTGTCTTCCTCA-3′;
mouse MCP-1, forward 5′-GCTCAGCCAGATGCAGTTAA-3′ and reverse
5′-TCAAAAACAGTGGTTCGAGTTCT-3′; mouse IL-10, forward
5′-ATAACTGCACCCACTTCCCA-3′ and reverse 5′-TGGACCATCTTCACTACGGG-3′;
mouse CCR5, forward 5′-TTGTCTACTTTCTCTTCTGG-3′ and reverse
5′-ATCGGGTATAGACTGAGC-3′; mouse KLF15, forward
5′-CACCAAGAGCAGCCACCTCA-3′ and reverse 5′-CGGGACACTGGTACGGCTTC-3′;
mouse GAPDH, forward 5′-CACCAAGAGCAGCCACCTCA-3′ and reverse
5′-CGGGACACTGGTACGGCTTC-3′. Thermocycling conditions were as
follows: 95°C for 10 min followed by 40 cycles of dissociation
(95°C for 10 sec), annealing (55°C for 30 sec), and elongation
(72°C for 30 sec). Data were quantified using the comparative Cq
(2−∆∆Cq) method (19).
Patient sample analysis
Biopsy samples from human kidney tissue were
obtained from patients with primary membranous nephropathy (n=26;
27-76 years old; male, 69.2%) or diabetic nephropathy (n=21; age,
24-67 years old; male, 61.9%) from the Seoul National University
Hospital (Seoul, Korea) between April 2013 and April 2015.
Glomerular KLF15 expression was quantified microscopically, and its
association with clinical outcomes was analyzed. The clinical
outcomes that were analyzed for membranous nephropathy and diabetic
nephropathy were spontaneous remission and end-stage renal disease,
respectively.
Statistical analysis
All analyses and calculations were performed using
SPSS (version 23.0; IBM Corp., Armonk, NY, USA) and GraphPad Prism
(version 5.0; GraphPad Software, Inc., La Jolla, CA, USA). The
results are expressed as mean ± standard error of mean. Differences
between groups in the western blot, RT-qPCR, and
immunohistochemistry analyses were evaluated using one-way analysis
of variance, followed by the least significant difference post hoc
test. Survival curves were drawn using the Kaplan-Meier method. To
compare survival curves between groups, the log-rank test was
applied. P<0.05 was considered to indicate a statistically
significant difference.
Results
Podocyte KLF15 expression in the chronic
kidney injury model
The 5/6 nephrectomy model was used to induce chronic
podocyte injury. Additionally, CCR5-/- mice were used to induce
podocyte injury with increased severity through alteration of
macrophage composition (20).
Twenty weeks post-injury, kidney markers such as blood urea
nitrogen, creatinine, and the urine protein-to-creatinine ratio
were increased in nephrectomized mice compared with sham-operated
mice (Fig. 1A). Other surrogate
markers, such as systolic blood pressure and body weight exhibited
significant differences between nephrectomized and sham-operated
mice (Fig. 1A). When these
markers were compared between nephrectomized wild type and CCR5−/−
mice, aggravated injury was observed in the CCR5−/− mice. In
specific, the measurements were: Body weight (g), 26.1±0.44 vs.
24.5±0.33; blood urea nitrogen (mg/dl), 51.8±3.10 vs. 70.0±4.21;
creatinine (mg/dl), 0.58±0.03 vs. 0.77±0.04; and urine
protein-to-creatinine ratio (mg/mg), 13.9±0.97 vs. 17.6±1.00 in
wild type and CCR5−/− mice, respectively (Fig. 1A). Fibronectin, a fibrosis marker,
was expressed in higher levels in nephrectomized mice, but podocyte
KLF15 expression was decreased and correlated with the degree of
injury (Fig. 1B–1D). Production
of inflammatory cytokines, IL-6 and MCP-1, was significantly
increased (Fig. 1D), while IL-10
production was decreased depending on the degree of podocyte injury
(wide type vs. CCR5−/− mice; Fig.
1D). These results suggest that chronic podocyte injury is
associated with low KLF15 expression.
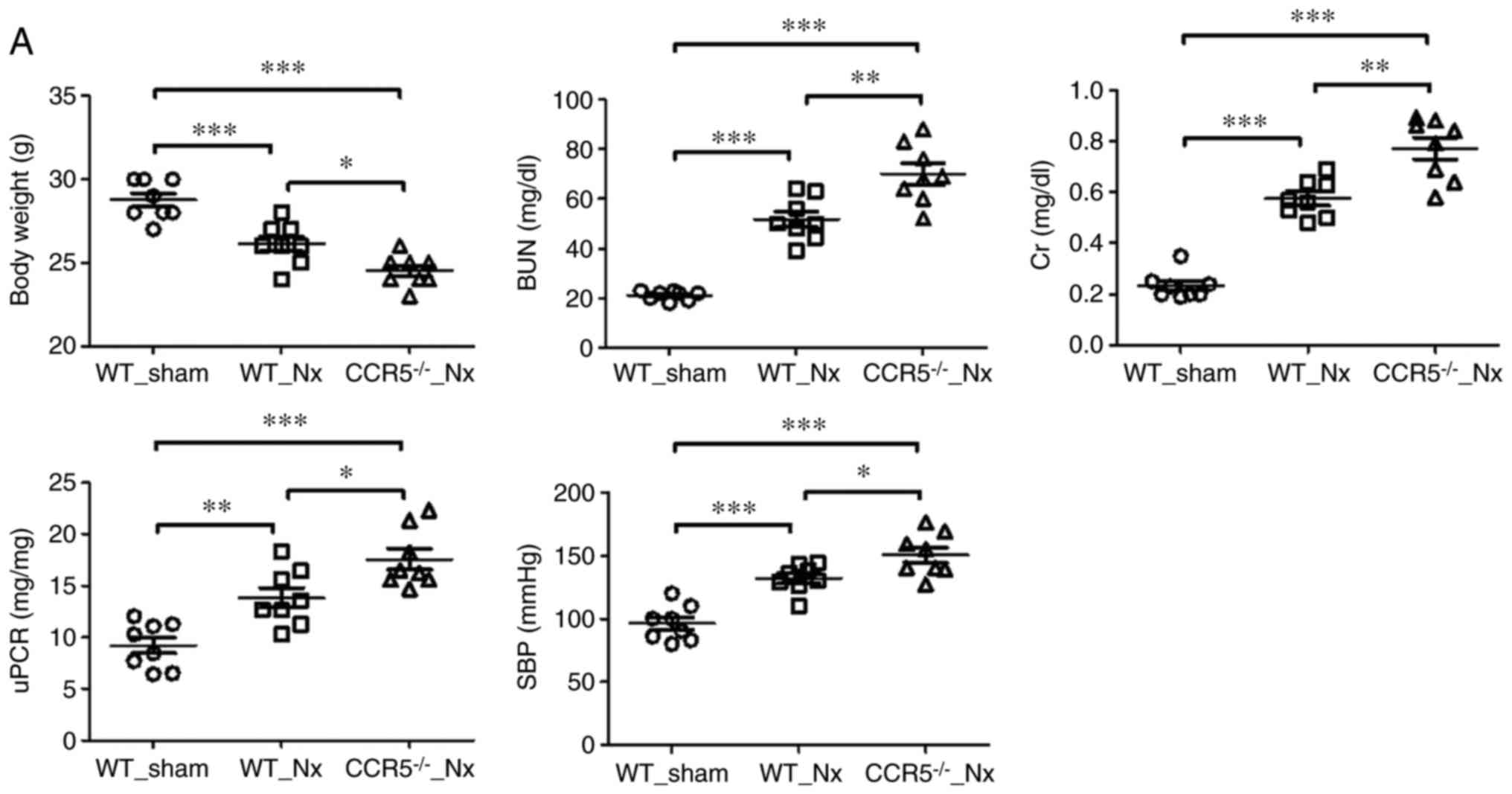 | Figure 1KLF15 expression in an in vivo
model of chronic podocyte injury. (A) Renal injury and systemic
markers at 20 weeks following 5/6 nephrectomy. Each parameter was
evaluated in eight mice per group. (B) Periodic acid-Schiff
staining and immunohistochemistry for fibrosis (Sirius red) and
KLF15 (magnification, ×400). The experiments were performed with
eight mice per group. *P<0.05, **P<0.01
and ***P<0.001, with comparisons indicated by
brackets. KLF15, Krüppel-like factor 15; IL, interleukin; MCP-1,
monocyte chemotactic protein-1; CCR5, C-C chemokine receptor type
5; WT, wild-type; Nx, 5/6 nephrectomy; BUN, blood urea nitrogen;
Cr, creatinine; uPCR, urine protein to creatinine ratio; SBP,
systolic blood pressure; PAS, periodic acid-Schiff. KLF15
expression in an in vivo model of chronic podocyte injury.
(C) Representative western blots and quantitative band intensity
analysis for KLF15 expression levels. (D) Quantitative polymerase
chain reaction analysis for the mRNA expression levels of
fibronectin, IL-6, MCP-1, IL-10, CCR5 and KLF15 from total kidney
extracts. The experiments were performed with eight mice per group.
*P<0.05, **P<0.01 and
***P<0.001, with comparisons indicated by brackets.
KLF15, Krüppel-like factor 15; IL, interleukin; MCP-1, monocyte
chemotactic protein-1; CCR5, C-C chemokine receptor type 5; WT,
wild-type; Nx, 5/6 nephrectomy; BUN, blood urea nitrogen; Cr,
creatinine; uPCR, urine protein to creatinine ratio; SBP, systolic
blood pressure; PAS, periodic acid-Schiff |
KLF15 expression is altered by a
renoprotective agent in primary human podocytes
Next, we aimed to confirm that low KLF15 expression
is associated with chronic podocyte injury using an in vitro
model of primary human cells. Primary human podocytes were isolated
and demonstrated to express synaptopodin and nephrin as podocyte
markers, while they were negative for the endothelial cell marker,
CD31 (data not shown). TGF-β is a potent inducer of fibrosis
(21), and therefore it was used
to induce podocyte fibrosis in vitro. Results from western
blot analysis confirmed that fibronectin levels as a fibrosis
marker increased when cells were treated with TGF-β for 48 h
(Fig. 2).
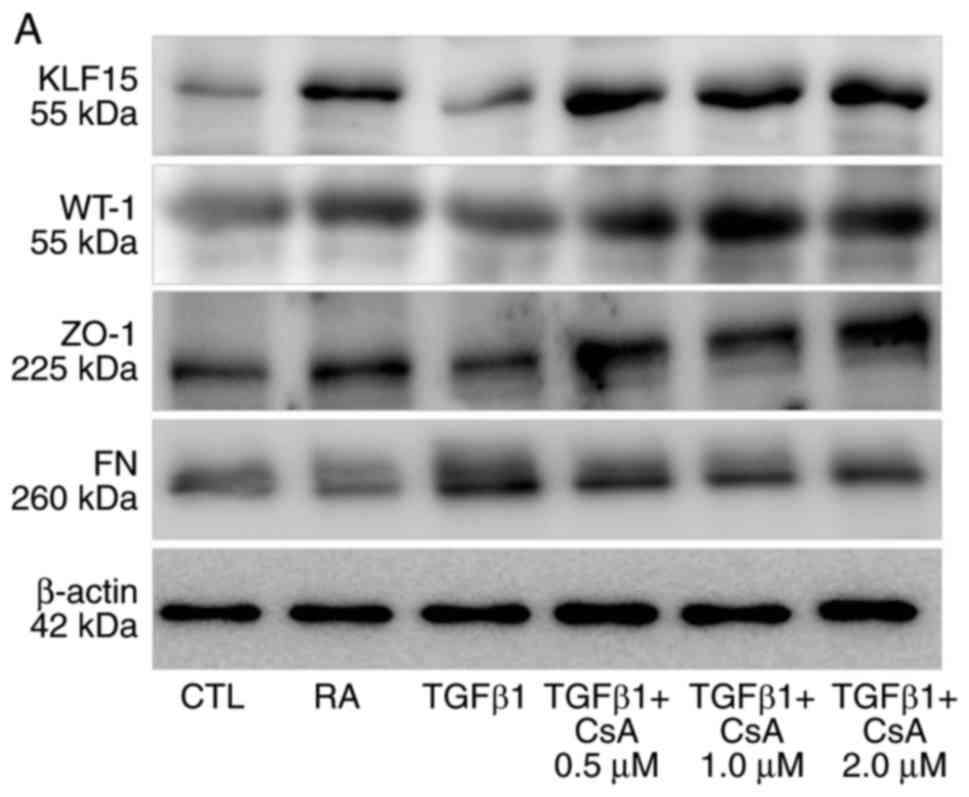 | Figure 2Renoprotective agent-induced changes
in podocyte KLF15 expression. (A) Representative western blots for
KLF15, WT-1, ZO-1, and fibrosis markers. (B) Quantitative analysis
of band intensities from the western blot analysis. The experiments
were independently repeated three times. *P<0.05,
**P<0.01 and ***P<0.001, with
comparisons indicated by brackets. KLF15, Krüppel-like factor 15;
WT-1, Wilms tumor-1; ZO-1, zonula occludens-1; si, small
interfering; TGF-β, transforming growth factor-β; FN, fibronectin;
CTL, control; RA, retinoic acid; CsA, cyclosporine. Renoprotective
agent-induced changes in podocyte KLF15 expression. (C)
Quantitative polymerase chain reaction analysis for the mRNA
expression levels of KLF15 and fibronectin. (D) Representative
western blots and quantitative band intensity analysis for KLF15
protein expression levels in scramble- or KLF15 siRNA-treated
podocytes. (E and F) Representative western blots and quantitative
band intensity analysis for the protein expression levels of ZO-1
and fibronectin in cells treated with retinoic acid, TGF-β,
cyclosporine (2 μM), scramble and/or KLF15 siRNA. The
experiments were independently repeated three times.
*P<0.05, **P<0.01 and
***P<0.001, with comparisons indicated by brackets.
KLF15, Krüppel-like factor 15; WT-1, Wilms tumor-1; ZO-1, zonula
occludens-1; si, small interfering; TGF-β, transforming growth
factor-β; FN, fibronectin; CTL, control; RA, retinoic acid; CsA,
cyclosporine. |
Retinoic acid and glucocorticosteroids are known to
induce the expression of KLF15 in podocytes (11,14). These observations suggest other
renoprotective agents may regulate KLF15 expression as part of
their protective function. Cyclosporine, a calcineurin inhibitor,
was evaluated in the present study, because it is routinely
administered to patients with podocytopathy in clinical practice
(22). Compared with
vehicle-treated cells, cells treated with cyclosporine displayed
increased levels of KLF15 and decreased levels of fibronectin that
were comparable with the positive control group treated with
retinoic acid (Fig. 2A–C). siRNA
was then used to knockdown expression of KLF15 in the cells.
Following siRNA transfection, the protein expression levels of
KLF15 were successfully decreased (Fig. 2D). The siRNA-treated cells
exhibited lower ZO-1 and higher fibronectin expressions compared
with scramble-treated cells, following TGF-β treatment (Fig. 2E and F). The renoprotective effect
of cyclosporine was not evident in the KLF15-knockdown cells
(Fig. 2E and F). Cyclosporine
could maintain podocytes in a healthier state throughout enrichment
of KLF15, in addition to the previous knowledge regarding
cytoskeleton stabilization (16).
KLF15 levels and their relationship with
clinical outcomes
Previous studies have demonstrated that the
decreased KLF15 expression in biopsied kidney tissue from patients
with minimal change disease and focal segmental glomerulonephritis
was associated with low response to glucocorticosteroids (14). In the present study,
immunohistochemistry results demonstrated that decreased KLF15
expression was predominant in segmental sclerotic glomeruli
(Fig. 3A). Next, we aimed to
explore the relationship between KLF15 expression and renal
outcomes in other podocytopathic diseases, such as membranous
nephropathy and diabetic nephropathy. Regarding membranous
nephropathy, cases with spontaneous remission (i.e. no use of
immunosuppressive drugs) exhibited higher KLF15 expression compared
with cases without spontaneous remission (Fig. 3B and C). However, KLF15 expression
did not differ between the remission and non-remission cases
following administration of immunosuppressive drugs (Fig. 3C). Regarding diabetic nephropathy,
the patients were divided into three groups according to KLF15
expression levels (i.e. tertiles), and their renal function was
followed for 30 months. The third tertile group (highest
expression) did not exhibit doubling of serum creatinine (P=0.020)
or end-stage renal disease (P=0.039; Fig. 3D and E). These results indicate
that KLF15 expression is associated with renal outcomes commonly
observed in several podocytopathic diseases.
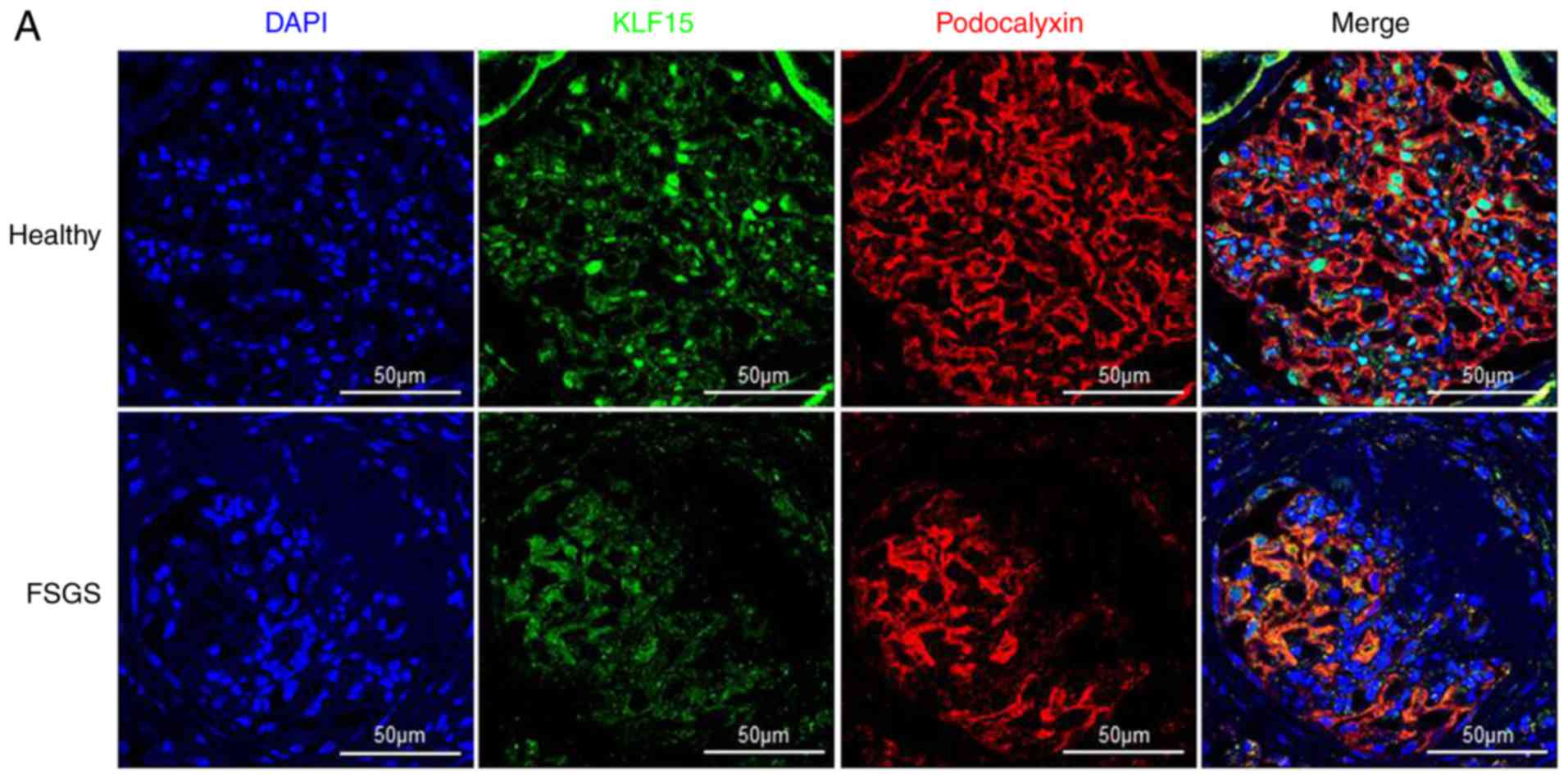 | Figure 3KLF15 expression in biopsied human
kidney tissue samples. (A) Representative confocal images of KLF15
and podocalyxin staining in sections of a glomerulus from a healthy
control and a patient with focal segmental glomerulosclerosis
(magnification, ×600). (B) Immunofluorescent images of KLF15 and
WT-1 staining of samples from a healthy control and a patient with
primary membranous nephropathy (magnification, ×400). (C)
Association of KLF15 expression levels with spontaneous remission
of membranous nephropathy. *P<0.05,
**P<0.01 and ***P<0.001, with
comparisons indicated by brackets. KLF15, Krüppel-like factor 15;
WT-1, Wilms tumor-1; FSGS, focal segmental glomerulosclerosis; DN,
diabetic nephropathy; MN, membranous nephropathy; ESRD end-stage
renal disease. KLF15 expression in biopsied human kidney tissue
samples. (D) Representative images from immunohistochemical
staining for KLF15 of samples from healthy control and patients
with diabetic nephropathy (magnification, ×400). (E) Association of
KLF15 expression levels with doubling of serum creatinine and the
risk of end-stage renal disease following kidney biopsy.
*P<0.05, **P<0.01 and
***P<0.001, with comparisons indicated by brackets.
KLF15, Krüppel-like factor 15; WT-1, Wilms tumor-1; FSGS, focal
segmental glomerulosclerosis; DN, diabetic nephropathy; MN,
membranous nephropathy; ESRD end-stage renal disease. |
Discussion
Current therapeutic regimens cannot reverse chronic
podocyte injury, and the prevalence and socioeconomic impact of CKD
are increasing significantly (23,24). This may be partially because the
underlying pathophysiology of CKD is not fully understood. The
present study focused on podocyte KLF15 expression in models of
CKD. Injured podocytes in 5/6 nephrectomized mice exhibited
decreased levels of KLF15. This effect was aggravated in a severe
form of podocyte injury (CCR5−/− mice), while it was reverted to
high KLF15 expression and low fibrotic phenotype in response to
cyclosporine treatment. The present findings suggest that KLF15 may
serve as a biomarker related to renal outcomes in patients with
podocytopathy.
The KLF family of transcription factors has
C-terminal zinc fingers. Since the initial discovery (25), 16 additional proteins have been
added to the family. Among them, KLF15 is highly expressed in
podocytes and binds the promoter of podocyte-specific genes, such
as nephrin and podocin (11,26). Both nephrin and podocin are key
components of the filtration slits of podocytes. Mutations in these
genes are a representative disorder of congenital nephrotic
syndrome (27,28). If these proteins are not
successfully produced, the glomerular filtration barrier cannot be
maintained and the resultant sign is proteinuria. Accordingly, low
or deficient KLF15 expression of podocytes might reduce nephrin and
podocin production and subsequently lead to broken glomerular
filtration barrier, detachment of podocytes from the glomerular
basement membrane, and progressive glomerular injuries. These
processes are collectively known as podocytopathies (29). Therefore, KLF15 may have
podocyte-specific functions, such as in differentiation and
treatment response (11,14). Previous studies have used several
murine models, such as lipopolysaccharide- or adriamycin-induced
proteinuric models. In the present study, the 5/6 nephrectomy mouse
was used as model of chronic podocyte injury. Furthermore, primary
human podocytes were used in vitro to confirm the results.
In specific, silencing of KLF5 by siRNA resulted in an increase of
podocyte injury markers. This might be because the structural
contexts including nephrin and podocin were not appropriately
maintained and cells were transformed to fibrotic phenotype by
depletion or low production of KLF15. These results support the
hypothesis that KLF15 is associated with podocyte function in
several disease models, despite differences in the mechanism of
podocyte injury.
Current therapeutic regimens for chronic kidney
injury, especially those that include management of comorbidities,
cannot halt the progression of proteinuria. This is in part because
the underlying mechanisms of podocyte injury are incompletely
understood. Nonetheless, agents such as glucocorticosteroids and
calcineurin inhibitors have been recommended for clinical use. A
previous study demonstrated the effect of glucocorticosteroids on
the expression of KLF15 in patients with focal segmental
glomerulosclerosis, and corresponding murine models (14). The present study also demonstrated
the potential role of cyclosporine (a representative calcineurin
inhibitor) in the podocyte injury model, by increasing KLF15
expression. The beneficial effect of cyclosporine was diminished in
KLF15-knockdown cells, which indicates that KLF may be one of
mediators between cyclosporine and podocyte homeostasis. This is a
novel function of cyclosporine, although the direct mechanism could
not be fully explored in the present study. Recently, resveratrol
was also demonstrated to alter KLF15 expression, and to improve
outcomes in an ischemic heart model (30). It can be speculated that several
other agents that induce KLF15 expression will be identified, and
may be used therapeutically for maintaining podocyte
homeostasis.
The mechanisms of podocyte injury may differ between
different renal diseases. However, based on the observation that
KLF15 is highly expressed in podocytes, the loss of KLF15 may be a
common late event following initial injury. The present study aimed
to verify this hypothesis in patients with membranous nephropathy
and diabetic nephropathy (the most common podocyte diseases in
proteinuric adult patients). KLF15 expression was related to
clinical outcomes, such as disease remission and progression. These
results suggest that KLF15 may serve as a useful biomarker in
tissue biopsies.
The present results further validate the association
between low KLF15 expression and chronic podocyte injury, which is
a typical feature of CKD. Furthermore, altered KLF15 expression may
be predictive of outcomes of several podocytopathic diseases.
Therapeutic agents that target podocytes are needed in the clinic,
ideally ones applicable for several different diseases. In this
aspect, future therapies that target KLF15 may be valuable in the
clinic.
Acknowledgments
Not applicable.
Funding
This work was supported by a grant from the Korea
Healthcare Technology R&D Project, Ministry of Health and
Welfare, Republic of Korea (grant no. A111336). Biospecimens were
provided by the Seoul National University Hospital Human Biobank, a
member of the National Biobank of Korea, which is supported by the
Ministry of Health and Welfare, Republic of Korea.
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
SSH designed the study, collected, analyzed and
interpreted the data, performed the experiments and drafted the
manuscript. MYY and KDY performed the experiments. JPL and DKK
collected the data. YSK designed the study. SHY designed the study,
performed the experiments, interpreted the data and reviewed the
final manuscript. All authors read and approved the manuscript.
Ethics approval and consent to
participate
All experiments involving animals were performed
under the approval of the Institutional Animal Care and Use
Committee of Seoul National University Hospital (Seoul, Korea;
approval no. 12-0132). The experiments involving human tissues
complied with the Declaration of Helsinki and received full
approval from the Institutional Review Board at Seoul National
University Hospital (approval no. 1607-133-777).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schiffrin EL, Lipman ML and Mann JF:
Chronic kidney disease: Effects on the cardiovascular system.
Circulation. 116:85–97. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tonelli M, Wiebe N, Culleton B, House A,
Rabbat C, Fok M, McAlister F and Garg AX: Chronic kidney disease
and mortality risk: A systematic review. J Am Soc Nephrol.
17:2034–2047. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meguid El Nahas A and Bello AK: Chronic
kidney disease: The global challenge. Lancet. 365:331–340. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reiser J and Sever S: Podocyte biology and
pathogenesis of kidney disease. Ann Rev Med. 64:357–366. 2013.
View Article : Google Scholar
|
|
5
|
Floege J and Amann K: Primary
glomerulonephritides. Lancet. 387:2036–2048. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dalla Vestra M, Masiero A, Roiter AM,
Saller A, Crepaldi G and Fioretto P: Is podocyte injury relevant in
diabetic nephropathy? Studies in patients with type 2 diabetes.
Diabetes. 52:1031–1035. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Trivedi S, Zeier M and Reiser J: Role of
podocytes in lupus nephritis. Nephrol Dial Transplant.
24:3607–3612. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chronic Kidney Disease Prognosis
Consortium; Matsushita K, van der Velde M, Astor BC, Woodward M,
Levey AS, de Jong PE, Coresh J and Gansevoort RT: Association of
estimated glomerular filtration rate and albuminuria with all-cause
and cardiovascular mortality in general population cohorts: A
collaborative meta-analysis. Lancet. 375:2073–2081. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lv J, Xu D, Perkovic V, Ma X, Johnson DW,
Woodward M, Levin A, Zhang and Wang H: Corticosteroid therapy in
IgA nephropathy. J Am Soc Nephrol. 23:1108–1116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Swamynathan SK: Krüppel-like factors:
Three fingers in control. Hum Genomics. 4:263–270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mallipattu SK, Liu R, Zheng F, Narla G,
Ma'ayan A, Dikman S, Jain MK, Saleem M, D'Agati V, Klotman P, et
al: Kruppel-like factor 15 (KLF15) is a key regulator of podocyte
differentiation. J Biol Chem. 287:19122–19135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gray S, Feinberg MW, Hull S, Kuo CT,
Watanabe M, Sen-Banerjee S, DePina A, Haspel R and Jain MK: The
Krüppel-like factor KLF15 regulates the insulin-sensitive glucose
transporter GLUT4. J Biol Chem. 277:34322–34328. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fisch S, Gray S, Heymans S, Haldar SM,
Wang B, Pfister O, Cui L, Kumar A, Lin Z, Sen-Banerjee S, et al:
Kruppel-like factor 15 is a regulator of cardiomyocyte hypertrophy.
Proc Natl Acad Sci USA. 104:7074–7079. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mallipattu SK, Guo Y, Revelo MP, Roa-Peña
L, Miller T, Ling J, Shankland SJ, Bialkowska AB, Ly V, Estrada C,
et al: Krüppel-like factor 15 mediates glucocorticoid-induced
restoration of podocyte differentiation markers. J Am Soc Nephrol.
28:166–184. 2017. View Article : Google Scholar
|
|
15
|
Li N, Zhang J, Yan X, Zhang C, Liu H, Shan
X, Li J, Yang Y, Huang C, Zhang P, et al: SIRT3-KLF15 signaling
ameliorates kidney injury induced by hypertension. Oncotarget.
8:39592–39604. 2017.PubMed/NCBI
|
|
16
|
Faul C, Donnelly M, Merscher-Gomez S,
Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim
K, et al: The actin cytoskeleton of kidney podocytes is a direct
target of the antiproteinuric effect of cyclosporine A. Nat Med.
14:931–938. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mundel P, Reiser J and Kriz W: Induction
of differentiation in cultured rat and human podocytes. J Am Soc
Nephrol. 8:697–705. 1997.PubMed/NCBI
|
|
18
|
Yang SH, Choi JW, Huh D, Jo HA, Kim S, Lim
CS, Lee JC, Kim HC, Kwon HM, Jeong CW, et al: Roles of fluid shear
stress and retinoic acid in the differentiation of primary cultured
human podocytes. Exp Cell Res. 354:48–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Turner JE, Paust HJ, Bennstein SB, Bramke
P, Krebs C, Steinmetz OM, Velden J, Haag F, Stahl RA and Panzer U:
Protective role for CCR5 in murine lupus nephritis. Am J Physiol
Renal Physiol. 302:F1503–F1515. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meng XM, Nikolic-Paterson DJ and Lan HY:
TGF-beta: The master regulator of fibrosis. Nat Rev Nephrol.
12:325–338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cattran DC, Feehally J, Cook HT, Liu ZH,
Fervenza FC, Mezzano SA, Floege J, Nachman PH, Gipson DS, Praga M,
et al: Kidney disease: Improving global outcomes (KDIGO)
glomeru-lonephritis work group. KDIGO clinical practice guideline
for glomerulonephritis. Kidney Int Suppl. 2:139–274. 2012.
|
|
23
|
Coresh J, Selvin E, Stevens LA, Manzi J,
Kusek JW, Eggers P, Van Lente F and Levey AS: Prevalence of chronic
kidney disease in the United States. JAMA. 298:2038–2047. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bommer J: Prevalence and socio-economic
aspects of chronic kidney disease. Nephrol Dial Transplant.
17(Suppl 11): S8–S12. 2002. View Article : Google Scholar
|
|
25
|
Turner J and Crossley M: Mammalian
Kruppel-like transcription factors: More than just a pretty finger.
Trends Biochem Sci. 24:236–240. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cohen CD, Klingenhoff A, Boucherot A,
Nitsche A, Henger A, Brunner B, Schmid H, Merkle M, Saleem MA,
Koller KP, et al: Comparative promoter analysis allows de novo
identification of specialized cell junction-associated proteins.
Proc Natl Acad Sci USA. 103:5682–5687. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Patrakka J, Kestilä M, Wartiovaara J,
Ruotsalainen V, Tissari P, Lenkkeri U, Männikkö M, Visapää I,
Holmberg C, Rapola J, et al: Congenital nephrotic syndrome (NPHS1):
Features resulting from different mutations in Finnish patients.
Kidney Int. 58:972–980. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mollet G, Ratelade J, Boyer O, Muda AO,
Morisset L, Lavin TA, Kitzis D, Dallman MJ, Bugeon L, Hubner N, et
al: Podocin inactivation in mature kidneys causes focal segmental
glomerulosclerosis and nephrotic syndrome. J Am Soc Nephrol.
20:2181–2189. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barisoni L and Mundel P: Podocyte biology
and the emerging understanding of podocyte diseases. Am J Nephrol.
23:353–360. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rogers RG and Otis JS:
Resveratrol-mediated expression of KLF15 in the ischemic myocardium
is associated with an improved cardiac phenotype. Cardiovasc Drugs
Ther. 31:29–38. 2017. View Article : Google Scholar : PubMed/NCBI
|

















