Introduction
Hyperplasia and phenotypic remodelling of pulmonary
arterial smooth muscle cells (PASMCs) serve key roles in the
progressive narrowing and occlusion of the distal pulmonary
arterioles in pulmonary arterial hypertension (PAH) (1,2).
As in cancer cells, mitochondria from proliferating PASMCs may
undergo diverse molecular changes and respond by switching their
cellular metabolism towards cytoplasm-based glycolysis, a condition
associated with apoptosis resistance (3,4).
Previous studies indicated that platelet-derived growth factor
(PDGF) expression was increased in neointimal lesions of rats
treated with hypoxia or monocrotaline; the application of a
tyrosine receptor inhibitor, imatinib, exhibited a beneficial
effect in abrogating these pulmonary vasculature lesions (5,6).
Previous results from Xiao et al (7) suggested that PDGF promoted the
Warburg effect, which refers to enhanced glycolysis effects under
normoxic conditions, and induced excessive proliferation and
apoptosis resistance in PASMCs (8). The activation of the
phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)
signalling pathway accounted for this transition in metabolic
phenotype (glucose oxygenation to glycolysis), as pharmacological
ablation of PI3K with LY294002 facilitated the reversal of the
Warburg effect (7).
Dichloroacetate (DCA), a selective inhibitor of
pyruvate dehydrogenase kinase-1 (PDK-1), activates pyruvate
dehydrogenase (PDH), which catalyses the decarboxylation of
pyruvate into acetyl-CoA, promoting substrate entry into the Krebs
cycle. DCA-mediated inhibition of PDKs triggers a switch in
pyruvate metabolism towards glucose oxidation to CO2 in
mitochondria, along with a destabilisation of hypoxia-inducible
factor-1α (HIF-1α), which reverses the hyperpolarised mitochondrial
membrane potential (ΔΨm) and induces apoptosis of cancer cells
(3). DCA also has beneficial
effects on PAH due to its 'lung selective' characteristic and
ameliorates pulmonary vascular remodelling in several rodent models
of PAH (9–11). The reversal of HIF-1α activation
and the Warburg effect is considered to serve a pivotal role in
mitigating the vascular lesions in rats subjected to carotid
intimal balloon injury (12).
Furthermore, the hypoxia response element is ubiquitously present
in the promoter regions of genes encoding glycolytic enzymes,
including PDK-1, glucose transporter-1 and hexokinase (13). Hexokinases (HKs) catalyse the
first essential step in glucose metabolism by phosphorylation of
glucose to glucose-6-phosphate. There are 4 major isoforms (HK-1,
HK-2, HK-3, and HK-4) characterised in mammalian tissue (14). Among these, HK-2 has been
extensively investigated and identified to be associated with a
blocking of cytochrome c release through the induction of
the pro-apoptotic proteins B-cell lymphoma 2 (Bcl-2)-associated X
protein and BH3 interacting-domain death agonist, and protection of
cancer cells from apoptosis (15). The interaction between HIF-1α and
HK-2 in cancer cells is well-documented yet poorly exploited in
vascular cells (16,17). A study by Lambert et al
(12) indicated that the
hyper-proliferating smooth muscle cells (SMCs) from injured carotid
arteries exhibited activation of HIF-1α and HK-2 and increased
glycolysis. Functional suppression using small interfering RNA
(siRNA) targeting HIF-1α promoted SMC apoptosis through the
inhibition of HK-2 expression and translocation of HK-2 with
voltage dependent anion channel (VDAC) on the outer membrane of the
mitochondria (12). Additionally,
HK-2 was identified to be upregulated in neointimal lesion regions
in pre-capillary pulmonary arterioles in a rat model of severe PAH,
accompanied by a high expression of HIF-1α (18). In addition to the transcriptional
regulation of HK-2 by HIF-1α, HK-2 expression is precisely
controlled by multiple signalling pathways, particularly the
PI3K/Akt/glycogen synthase kinase-3β (GSK-3β) axis (19). A study by Bonnet et al
(20) indicated that
dehydroepiandrosterone, a steroid hormone, decreased proliferation
and induced apoptosis of vessel SMCs in viv and in
vitro, which was mediated by inhibition of Akt/GSK-3β,
interfering with the HK-2/VDAC interaction.
In the present study, whether PDK-1 and HK-2
inactivation was involved in DCA-induced growth retardation and
whether the pro-apoptotic effect of DCA in PASMCs exposure to PDGF
was potentiated by blocking the PI3K/Akt signalling pathway were
investigated. The results of the present study revealed that DCA
induced a pro-apoptotic effect on human PASMCs associated with the
downregulation of PDK-1 and HIF-1α and subsequent HK-2 inactivation
via blocking of the mitochondrial interaction. Furthermore, this
effect may be potentiated via the suppression of PDK-1 by blocking
the Akt/GSK-3β/HIF-1α signalling pathway.
Materials and methods
Ethics approval
The present study was approved by the Ethics
Committee of Nanjing University Drum Tower Hospital for
Institutional Animal Care and Use.
Cell culture
Human PASMCs were purchased from Wuxi BioHermes
Biomedical Technology Co., Ltd (Wuxi, China). They were trypsinised
and subcultured subsequent to reaching 90% confluence. A monoclonal
antibody against smooth muscle α-actin (1:1,000; cat. no.
M085129-2; Agilent Technologies, Inc., Santa Clara, CA, USA) was
used to assess the purity (>99%) of the SMC culture according to
the manufacturer's protocol. Unless otherwise indicated, primary
cultures of PASMCs were maintained in RPMI-1640 containing 10%
foetal bovine serum (FBS) and 1% antibiotics (penicillin and
streptomycin mixture), all purchased from Thermo Fisher Scientific
Inc. (Waltham, MA, USA).
All experiments examining HK-2, HIF-1α activation,
Akt, GSK-3β, PDH phosphorylation and PDH kinase-1 (PDK-1)
expression, and cell viability analysis, were performed on cells
that were cultured for 3 days at passages 3-5. Following this,
cells were trypsinised and seeded at a distinct cell density into
96-well plates (5,000 cells/well) or culture flasks
(5×105 cells/flask) and subcultured in RPMI-1640
containing 10% FBS and 1% antibiotics in a humidified 5%
CO2 atmosphere at 37°C overnight. Cells were then
exposed to PDGF-BB (Cytolab Ltd., Rehovot, Israel), SB216763
(S1075; Selleck Chemicals, Houston, TX, USA), DCA (5, 10, 20 and 50
mM) and LY294002 (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at
numerous concentrations (5, 10 and 20 µM) or a combination
of two drugs (10 mM DCA and 5 µM LY294002) as indicated. In
the control groups, an equal volume of PBS was substituted for the
reagents.
Determination of cell viability
[Cell-Counting-Kit 8 (CCK-8) colorimetric assay]
Cells were seeded at 5,000 cells/well into 96-well
plates and grown in RPMI-1640 supplemented with 10% FBS for 72 h.
After 48 h of serum starving with 0.5% FBS, the cells were then
incubated with 20 ng/ml PDGF, followed by increasing concentrations
of DCA (5, 10, 20 and 50 µM), LY294002 or a combination of
the two drugs for 72 h. Following drug exposure, cells were
incubated in a humidified 5% CO2 atmosphere at 37°C with
10 µl CCK-8 (Dojindo Molecular Technologies, Inc., Kumamoto,
Japan) for 1 h. The optical densities in the 96-well plates were
determined using a microplate reader at a wavelength of 450 nm. The
cell proliferation percentage was determined in the treated (AT),
PDGF (Ap), blank (Ab) groups using the following formula:
Proliferation Index = (AT-Ab/Ap-Ab) × 100; optical density was
measured at a 450 nm wavelength.
Cell apoptosis and
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazol-carbocyanine
iodide (JC-1) assays
Cells were seeded in 25 cm2 tissue
culture flasks at 5×105 cells/flask and incubated in
standard conditions. Cells were maintained in complete medium for
16 h, followed by serum starvation for 24 h prior to treatment with
20 ng/ml PDGF with 5 µM LY294002, DCA at 10 mM, a
combination of LY294002 and 10 mM DCA or alone for 48 h. Following
drug treatment, PASMCs were harvested and centrifuged at 4°C at 600
× g for 5 min; the pellets were washed twice with PBS and then
resuspended in 100 µl Annexin V binding buffer (0.14 M NaCl,
2.5 mM CaCl2, 0.01 M HEPES pH 7.4). Annexin V (1 µl;
Invitrogen; Thermo Fisher Scientific, Inc.) and 5 µl
propidium iodide (50 µg/ml) were added to the samples and
incubated at room temperature in the dark for 15 min. Samples were
kept on ice following incubation until fluorescence activated cell
sorting (FACS) analysis was performed. For the assays measuring
mitochondrial membrane potential (ΔΨm), the cells were treated as
indicated above, and then 1 µl JC-1 (Beyotime Institute of
Biotechnology, Haimen, China) was added in the medium at a final
concentration of 2 µM, and the cells were stained at room
temperature for 30 min in the dark. Subsequent to JC-1 staining,
cells were trypsinised, washed twice with PBS and resuspended in
100 µl PBS. JC-1 fluorescence intensity was examined using
FACS and data was analysed with FlowJo 7.61 software (Tree Star
Inc., Ashland, OR, USA).
Lactate measurement
The PASMCs were seeded into 25 cm2 tissue
culture flasks at a cell density of 5×105 cell/flask and
cultured with RPMI-1640 complete culture medium for 16 h followed
by serum starvation with 0.5% FBS for 24 h. The cells were then
incubated at 37°C with 20 ng/ml PDGF with 5 µM LY294002, DCA
at 10 or 20 mM, a combination of LY294002 and 10 mM DCA, or alone
for 48 h, subsequent to which the medium was removed from cells and
lactate levels in the extracellular medium were measured at room
temperature using the Lactate Colorimetric Assay kit (Abcam,
Cambridge, MA, USA) according to the manufacturer's protocol.
Lactate concentration was normalised to sample cell number.
Immunoblotting and densitometric
analysis
The PASMCs were seeded into T25 culture flasks at a
cell density of 1×106 and cultured with RPMI-1640
complete culture medium for 16 h followed by serum starvation with
0.5% FBS for 24 h. Thereafter, the cells were incubated under
different interventions for 24 h, as aforementioned, while PBS was
substituted for reagents in control cells cultured in medium with
0.5% FBS. Immunoblotting was performed as described previously
(18). Briefly, PASMCs were
harvested and resuspended in ice-cold cell lysis solution (Thermo
Fisher Scientific, Inc.) and the homogenate was centrifuged at 400
× g for 15 min at 4°C. Protein concentration was determined using a
Bicinchoninic Acid Protein Assay kit (cat. no. PICPI23223; Thermo
Fisher Scientific, Inc.). The supernatant was subsequently
transferred to a fresh tube and 75 µg protein from each
sample was then separated via 12% SDS-PAGE. Following this, the
proteins were then transferred to polyvinylidene difluoride
membranes. Membranes were then blocked using 5% fat-free milk for 1
h at room temperature and subsequently incubated at 4°C overnight
with the following primary antibodies specific to Akt,
phosphorylated (p)-Akt, GSK3β, p-GSK3β (Cell Signaling Technology,
Inc., Danvers, MA, USA; cat. nos., 4685, 4060, 9315 and 9313,
respectively), HK-2, PDK-1 (1:1,000; Cell Signaling Technology,
Inc.; cat. nos., 2867 and 3820), phospho(S293)-PDH E1α subunit
(1:1,000; Abcam, Cambridge, UK; cat. no., ab177461), HIF-1α (1:500;
Novus Biologicals, LLC, Littleton, CO, USA; cat. no., NB100-449)
and β-actin (1:2,000; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA; cat. no., sc-47778). Following this, membranes were incubated
for 2 h at room temperature with the following secondary
antibodies: Horseradish peroxidase-conjugated goat anti-rabbit IgG
(cat. no. ab6721; 1:5,000; Abcam) or HRP-conjugated anti-mouse IgG
(cat. no. 5450-0011; 1:5,000; KPL, Gaithersburg, MD, USA). β-actin
protein expression served as an internal control and was used to
normalise the protein band intensity. To determine the
phosphorylation levels of AKT and GSK-3β, membranes was washed with
stripping buffer (cat. no. P0025S; Beyotime Institute of
Biotechnology) at 50°C for 30 min, followed by blocking the
membrane with 5% bovine serum albumin (cat. no. 37525; Thermo
Fisher Scientific Inc.) in PBST at room temperature for 4 h. The
membrane was then re-probed at 4°C overnight with p-AKT (1:1,000)
and p-GSK-3β antibodies (1:1,000; Cell Signalling Technology, Inc.,
Danvers, MA, USA). Western blots were detected using the Western
chemiluminescent detection system (Pierce; Thermo Fisher
Scientific, Inc.) and exposure to X-ray film. Images were acquired
using a CanoScan 8600F flatbed scanner, quantified using ImageJ
software (version 1.4; National Institutes of Health, Bethesda, MD,
USA) and standardised to β-actin in each lane. Experiments were
repeated in triplicate.
Immunofluorescence confocal
microscopy
Human PASMCs were fixed with 1% paraformaldehyde and
permeabilised with 0.2% Triton X-100. The ΔΨm was determined using
MitoTracker® Red CMXROS (Invitrogen; Thermo Fisher
Scientific, Inc.). The PASMCs were then incubated at 37°C with
M7512- Mito tracker Red (50 nM) for 30 min and maintained in a dark
room at 4°C overnight for confocal detection. HK-2 detection was
performed using Alexa Fluor 488 (10 µg/ml; Invitrogen;
Thermo Fisher Scientific, Inc.) for green fluorescent protein
staining at 37°C for 1.5 h. Cell imaging was performed using an
FV1000 confocal microscope (magnification, ×100) equipped with a
live cell apparatus (Olympus Corporation, Tokyo, Japan).
Statistical analysis
Data are presented as the mean ± standard deviation.
Calculations were performed using the GraphPad Prism software
package (version 4.0.1; GraphPad Software, Inc., La Jolla, CA,
USA), and one-way analysis of variance with Tukey's post-hoc test
was applied. P<0.05 was considered to indicate a statistically
significant difference.
Results
DCA inhibits the proliferation of human
PASMCs stimulated with PDGF-BB in a dose-dependent manner, which
was improved by co-incubation with LY294002
As demonstrated in Fig. 1A, DCA suppressed human PASMC
proliferation in a dose-dependent manner (0–50 mM). DCA at 5 mM
exhibited minimal inhibitory effects on PASMC proliferation after
72 h incubation. The cell proliferation index was significantly
decreased in the 10, 20 and 50 mM groups compared with the 5 mM DCA
group (P<0.01). The inhibitory effect of DCA on the growth of
human PASMCs was mimicked by LY294002. LY294002 (10 µM)
caused a 27.7% decrease in cell proliferation rate, whereas only a
13.0% decrease was observed after 72 h of treatment with 5
µM LY294002 (P>0.05; Fig.
1B). Notably, the cell proliferation index was significantly
decreased to 57.2% after 72 h incubation with a combination of DCA
(10 mM) and LY294002 (5 µM) compared with the cells treated
by DCA alone (76.4%; P<0.05; Fig.
1C).
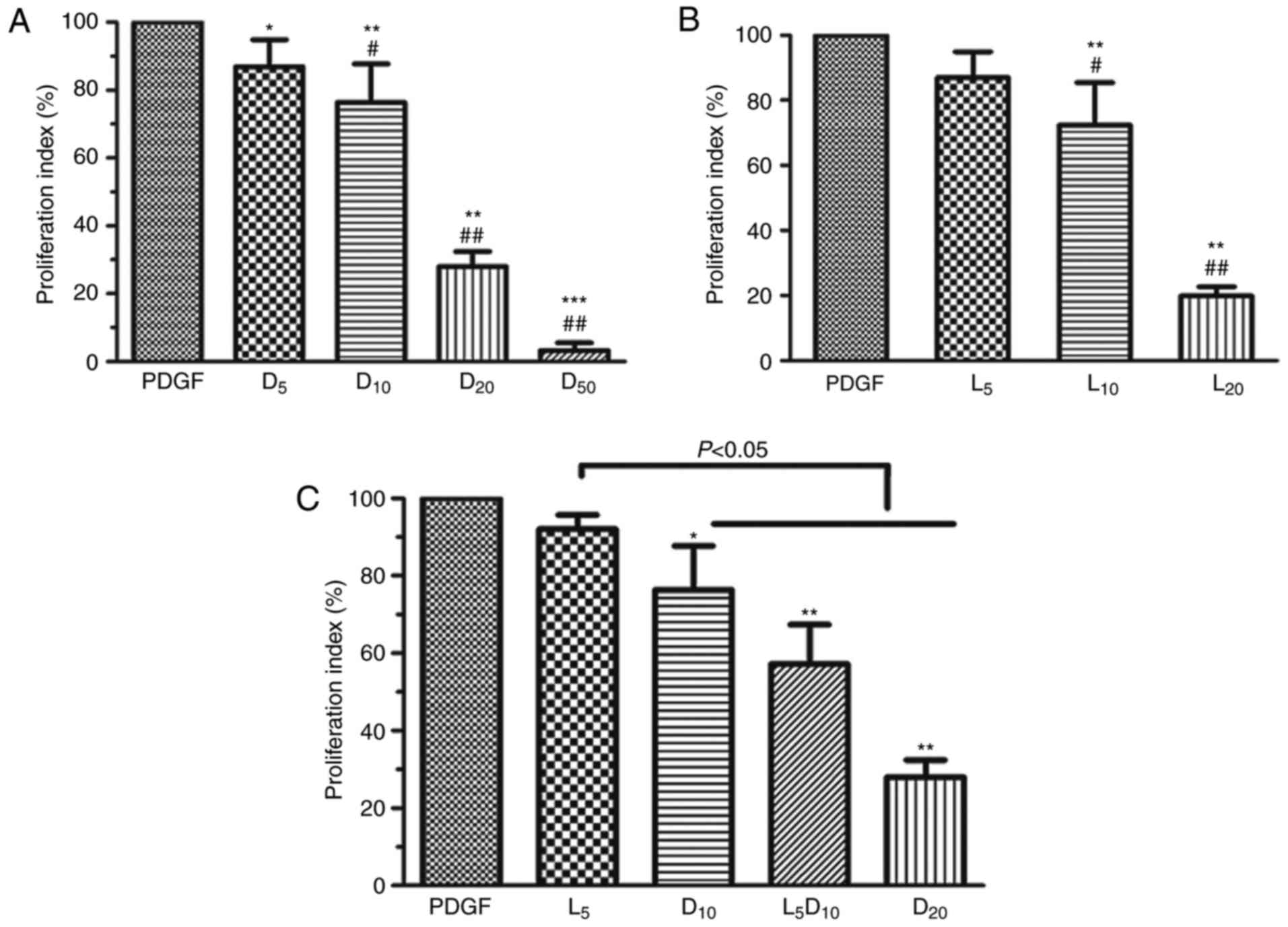 | Figure 1Effect of DCA, LY294002 or
combination of DCA and LY294002 on the growth of human PASMCs. The
human PASMCs were seeded in 96-well plates in RPMI-1640 medium
supplemented with 10% FBS followed by 48 h serum starving prior to
exposure to 20 ng/ml PDGF or with increased concentrations of (A)
DCA or (B) LY294002 in fresh culture medium with 0.5% foetal bovine
serum for 72 h. *P<0.05, **P<0.01 and
***P<0.001 vs. PDGF-treated cells; #P<0.05 and
##P<0.01 vs. cells treated with 5 mM DCA or 5
µM LY294002. (C) Cells were exposed to PDGF or 5 µM
LY294002, 10 or 20 mM DCA and combination of 5 µM LY294002
and DCA (10 mM) for 24 h. *P<0.05,
**P<0.01 as compared with PDGF cells. The data are
presented as the mean ± standard deviation of 6 duplicated wells in
three separate experiments. PASMCs, pulmonary arterial smooth
muscle cells; DCA, dichloroacetate; PDGF, platelet-derived growth
factor; D5, D10, D20 and D50, cells treated with dichloroacetate at
5, 10, 20, 50 mM, respectively, following PDGF exposure. L5, 10 and
20, cells treated with LY294002 at 5, 10, 20 µM,
respectively, following PDGF exposure. L5D10, cells treated in a
combination of 5 µM LY294002 and 10 mM dichloroacetate
following PDGF exposure. |
Growth inhibitory effect of DCA on PASMCs
potentiated by LY294002 may be attributed to increased
mitochondria-associated apoptosis
As indicated in Fig.
2A, the percentage of apoptotic cells was ~18.8% following
treatment with 10 mM DCA, which was significantly increased
compared with cells treated with 5 µM LY294002 alone (9.2%;
P<0.05). However, the combined treatment with LY294002 and 10 mM
DCA markedly increased the apoptotic cell percentage to 44.9%
following 48 h incubation. As demonstrated in Fig. 2C and D, although 5 µM
LY294002 only marginally affected the expression of cleaved
caspase-3, it significantly increased cleaved caspase-3 expression
levels when added in combination with 10 mM DCA. The human PASMCs
exhibited an increased ΔΨm, following PDGF incubation for 48 h
compared with the control cells (P<0.05), as expressed by the
ratio of red and blue fluorescence intensity following JC-1
staining in Fig. 2B. DCA at 10 mM
or LY294002 significantly decreased the ΔΨm in PASMCs. Furthermore,
the ΔΨm was markedly decreased after 48 h of treatment with a
combination of 10 mM DCA and LY294002 compared with DCA or LY294002
alone (P<0.05).
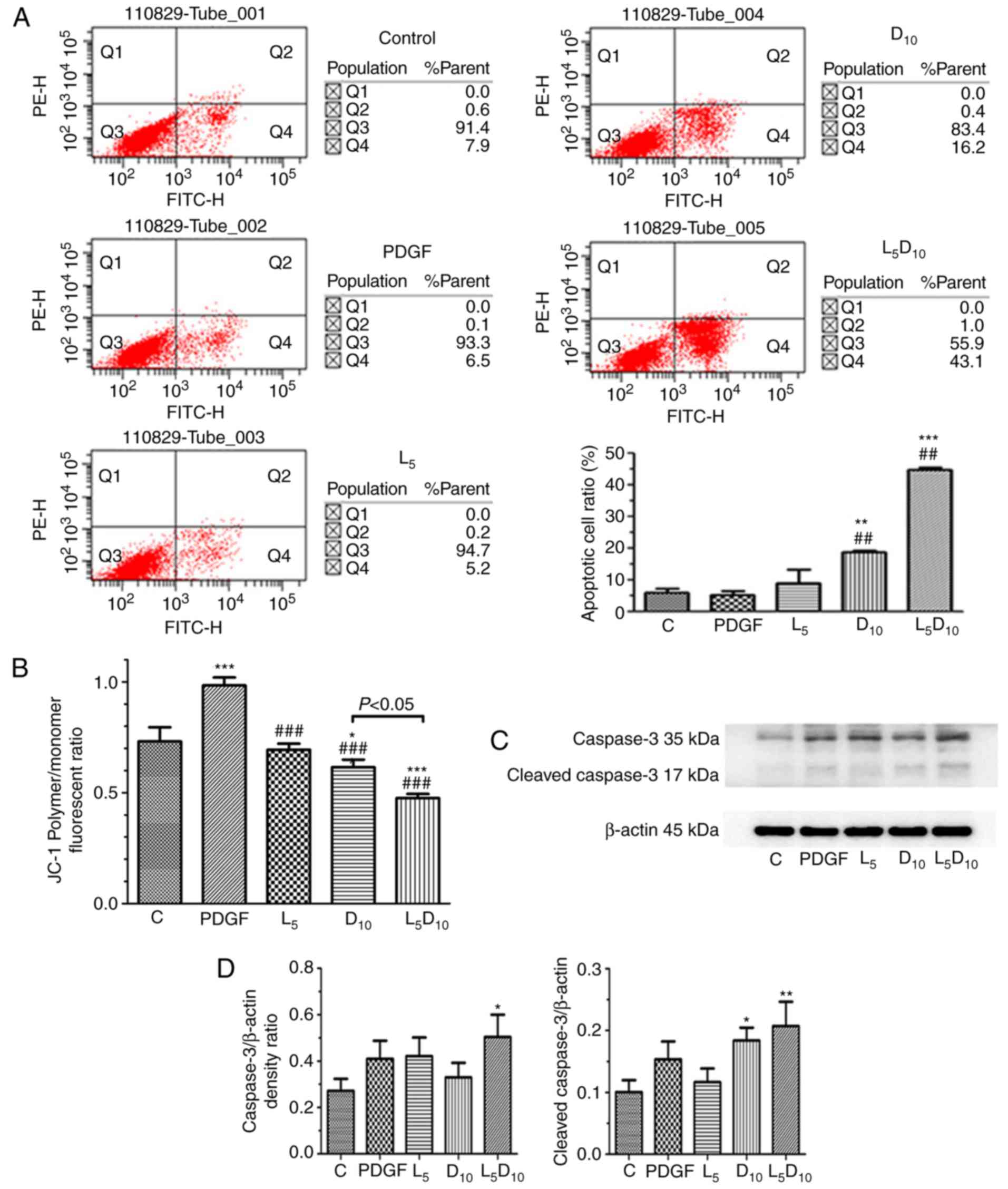 | Figure 2Effect of DCA, LY294002 or
combination of DCA and LY294002 on the apoptosis and mitochondria
membrane potential of human PASMCs. The PASMCs were seeded into 25
cm2 tissue culture flask at a density of
5×105 cell/flask and cultured in RPMI-1640 complete
culture medium for 16 h followed by serum starvation for 24 h. The
cells were then exposed to PDGF alone or 5 µM LY294002, DCA
at 10 mM or a combination of 5 µM LY294002 and 10 mM DCA for
48 h prior to (A) apoptosis or (B) JC-1 assay. (C) The expression
levels of caspase-3 and cleaved caspase-3 were analyzed with
western blot analysis. The representative change of one of the
three experiments is presented, as all assays exhibited identical
results. (D) Results were pooled from three separate experiments
and are presented as mean ± standard deviation.
*P<0.05, **P<0.01 and
***P<0.001 vs. control cells. ##P<0.01
and ###P<0.001 vs. cells treated with PDGF. C,
control; PASMCs, pulmonary arterial smooth muscle cells; DCA,
dichloroacetate; PDGF, platelet-derived growth factor; D5 and D10,
cells treated with DCA at 5 and 10 mM, respectively, following PDGF
exposure; L5, cells treated with LY294002 at 5 µM following
PDGF exposure; L5D10, cells treated with a combination of 5
µM LY294002 and 10 mM DCA following PDGF exposure. |
Co-treatment with LY294002 and DCA
decreases lactate concentration in extracellular culture medium,
PDK-1, p-PDH, HIF-1α and HK-2 expression and potentially represses
HK-2 activation, which are all associated with a reversal of the
Warburg effect
As suggested in Fig.
3A, DCA at 10 mM or 5 µM LY294002 significantly
decreased the lactate concentration in PASMCs stimulated with
PDGF-BB. Additionally, the increased concentration of DCA (20 mM)
markedly decreased PDGF-induced lactate production (3.55 vs. 6.28
mM, respectively; P<0.01). The lactate concentration markedly
decreased after 48 h of treatment with the combination of 10 mM DCA
and LY294002 compared to 10 mM DCA alone (3.97 vs. 5.18 mM;
P<0.05). Furthermore, the upregulation of PDK-1, p-PDH and
HIF-1α induced by PDGF was abrogated by treatment with 10 mM DCA in
PASMCs. The inhibitory effect on PDK-1, p-PDH and HIF-1α expression
in PASMCs was improved by a combination of 10 mM DCA with LY294002.
The protein expression of HK-2 increased significantly following
PDGF treatment compared with the control cells. Treatment with 5
µM LY294002 or 10 mM DCA significantly decreased HK-2
expression. The combination of LY294002 and DCA or 20 mM of DCA
elicited an additional decrease in HK-2 expression compared with
the cells treated with 10 mM DCA (Fig. 3B). In addition, the inhibitory
effect of DCA or LY294002 resulted in an uncoupling of HK-2 from
the mitochondria. The increased co-localisation of HK-2 with
mitochondria in the cytoplasm following PDGF-BB stimulation was
significantly attenuated by 10 and 20 mM DCA treatment. Notably,
LY294002 increased the dislocation of HK-2 from the mitochondria
induced by 10 mM DCA, which is demonstrated clearly in Fig. 3C as a visible decrease in the
yellow fluorescence intensity in the cytoplasm.
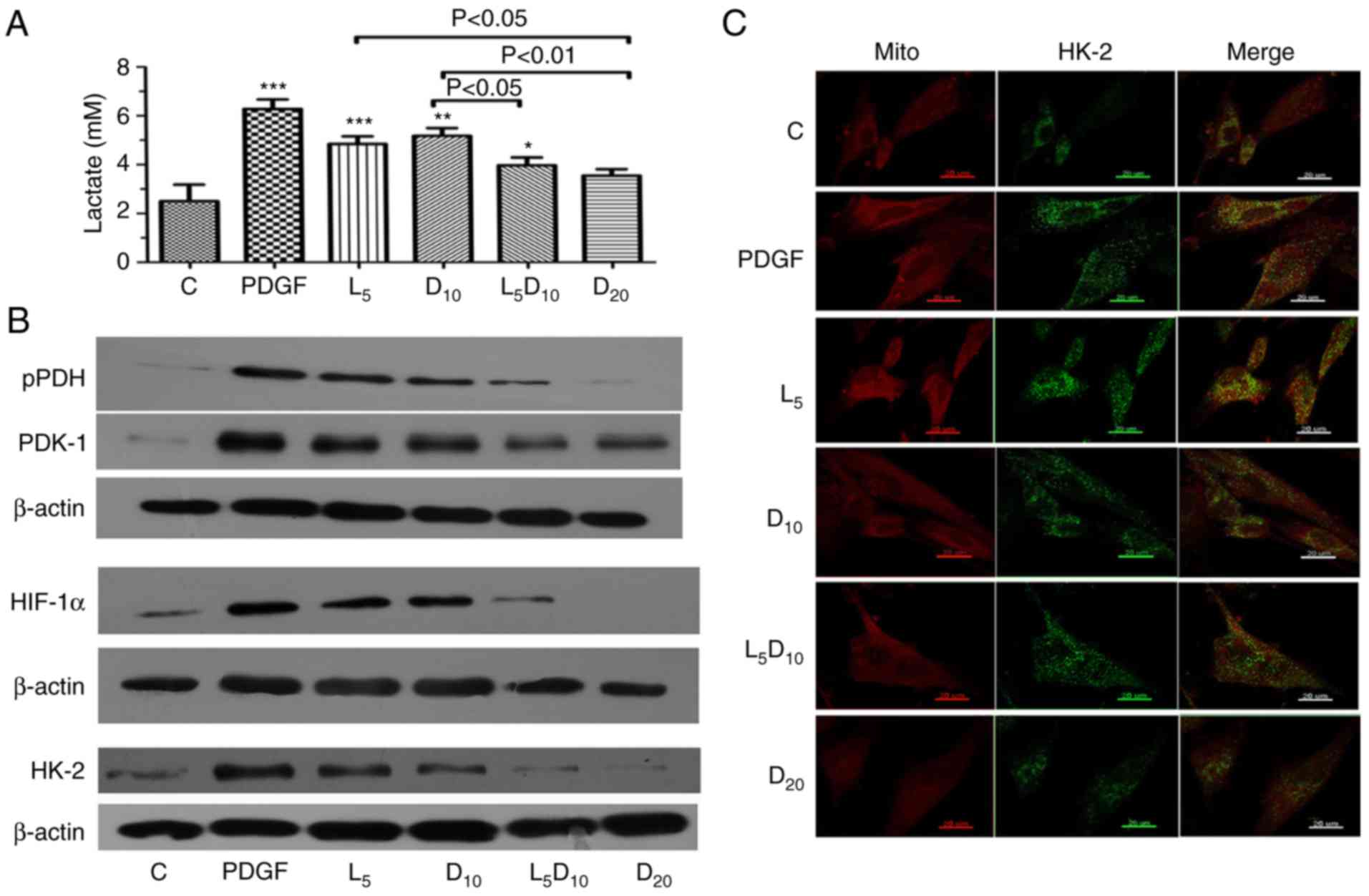 | Figure 3Effect of DCA, LY294002 or
combination of DCA and LY294002 on extracellular lactate
concentration, key glycolysis-associated enzymes expression and
HK-2 activation. The PASMCs were seeded into 25 cm2
tissue culture flask at a density of 5×105 cell/flask
and cultured with RPMI-1640 complete culture medium for 16 h
followed by serum starvation for 24 h. (A) The cells were then
exposed to PDGF or 5 µM LY294002, DCA at 10 or 20 mM, and a
combination of LY294002 and 10 mM DCA for 48 h prior to measurement
of lactate concentration. *P<0.05,
**P<0.01 and ***P<0.001 vs. control
cells. (B) Cell lysates were extracted for PDK-1, PDH, HIF-1α and
HK-2 expression analysis using western blot analysis. The
representative change of one of the three experiments is presented,
as all assays exhibited identical results. (C) HK-2 activation was
analyzed with immune-fluorescent confocal microscopy.
PDGF-stimulated PASMCs exhibited significant co-localization
between HK-2 (green) and the mitochondria (red), giving a yellow
pattern in the merged images. The cells treated with 20 mM DCA and
the combination of LY294002 and 10 mM DCA demonstrated diffuse
cytoplasmic staining of HK-2 (no colocalization of HK-2 to the
mitochondria). C, control; DCA, dichloroacetate; PDGF,
platelet-derived growth factor; L5, D10 and D20, cells treated with
DCA at 5, 10 and 20 mM, respectively, following PDGF exposure; L5,
cells treated with LY294002 at 5 µM following PDGF exposure;
L5D10, cells treated in combination of 5 µM LY294002 and 10
mM dichloroacetate following PDGF exposure; PASMCs, pulmonary
arterial smooth muscle cells; HK-2, hexokinase-2; PDK-1, pyruvate
dehydrogenase kinase-1; PDH, pyruvate dehydrogenase; HIF-1α,
hypoxia-inducible factor-1α; mito, mitochondria. |
Downregulation of PDK-1 through
Akt/GSK-3β signal blocking may be involved in the potentiation of
DCA-induced human PASMCs apoptosis by LY294002
As indicated in Fig.
4A–D, LY294002 inhibited Akt/GSK-3β axis activation in PASMCs
exposed to PDGF-BB in a concentration-dependent manner (1–20
µM). The PDK-1 upregulation induced by PDGF in PASMCs was
apparently inhibited by LY294002 in a concentration-dependent
manner (1–20 µM), as demonstrated in Fig. 4E and F (P<0.05). To investigate
the role of the Akt/GSK-3β axis in the downregulation of PDK-1 by
LY294002, PASMCs were treated with a GSK-3β inhibitor (5 µM
SB216763) for 30 min prior to PDGF-BB and 5 µM LY294002
exposure for 24 h. The inhibitory effect of LY294002 on PDK-1 was
completely abrogated by SB216763 (Fig. 4G–H).
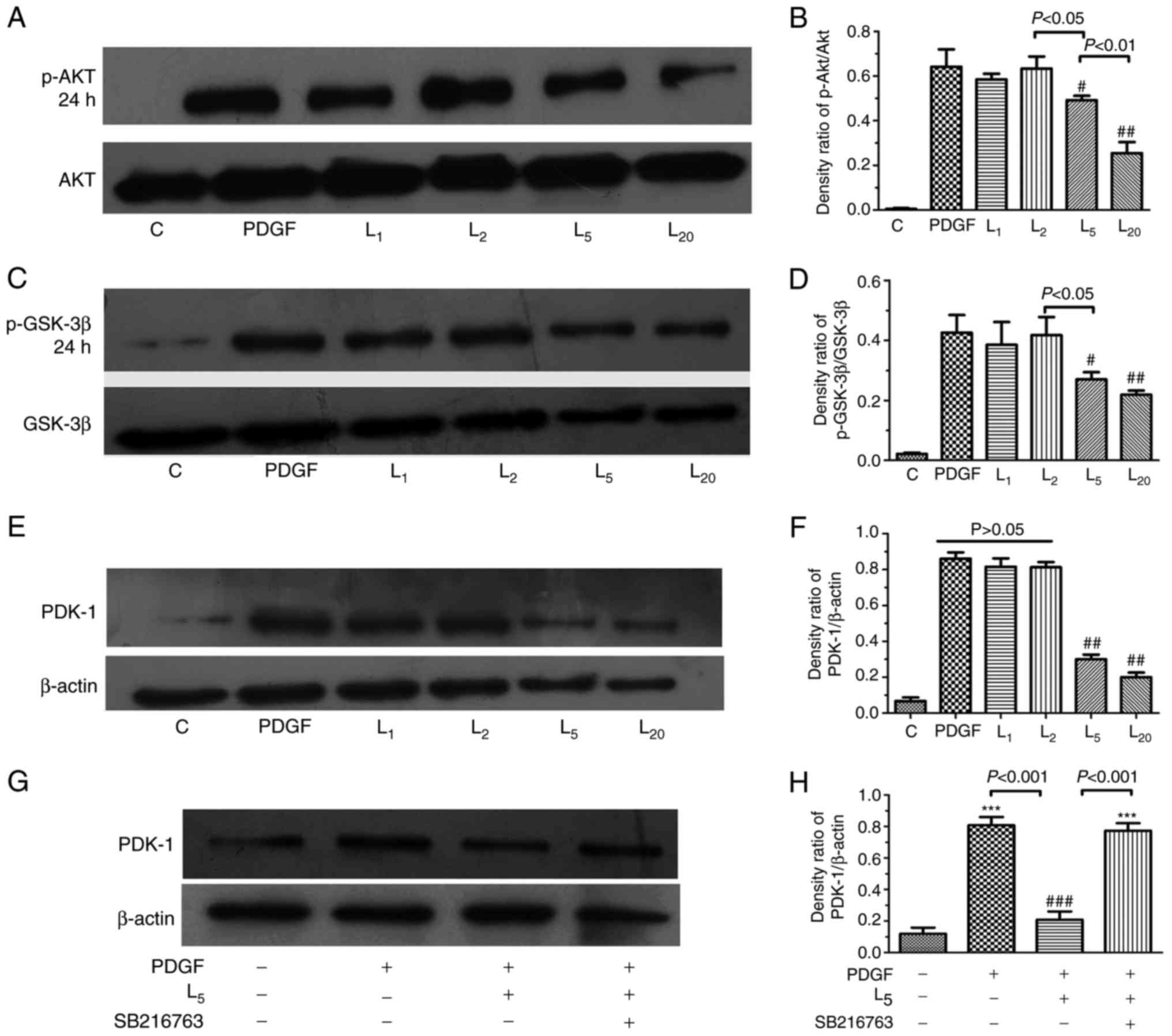 | Figure 4Effect of LY294002 on
Akt/GSK-3β/PDK-1 signaling and the inhibition of GSK-3β activity on
PDK-1 expression. The cells were exposed to PDGF, or with
increasing LY294002 concentrations of 1, 2, 5 and 20 µM for
24 h, prior to detection of the expression of (A and B) p-Akt, (C
and D) GSK-3β or (E and F) PDK-1. (G and H) The cells were treated
with PDGF, or with 5 µM LY294002 with/without 30 min
pre-exposure to 5 µM SB216763 for 24 h, prior to the
detection of PDK-1 expression. #P<0.05 and
##P<0.01 vs. cells treated with PDGF.
***P<0.001 vs. control cells. Results were pooled
from three separate experiments and are presented as mean ±
standard deviation. A, C and E are the representative change of
p-Akt or p-GSK-3β and PDK-1 expression, respectively. The
representative change of one of the three experiments is presented,
as all assays exhibited identical results. Semi-quantitative
analyses of p-Akt, p-GSK-3β and PDK-1 expression levels are
presented in B, D, F and H, respectively. C, control; PDGF,
platelet-derived growth factor; L1, L2, L5 and L10, cells treated
with LY294002 at 1, 2, 5 and 20 µM following PDGF exposure;
Akt, protein kinase B; GSK-3β, glycogen synthase kinase-3β; PDK-1,
pyruvate dehydrogenase kinase-1; p-, phosphorylated. |
Discussion
The hyperplasia of PASMCs within the vascular wall
of distal pulmonary arterioles, causing medial hypertrophy and
neointimal lesion formation, is a hallmark of pulmonary vascular
remodelling in PAH (2).
Abnormality in energy metabolism is one of the contributing factors
that results in aberrant growth of PASMCs (21,22). In accordance with previous studies
in cancer cell lines (23,24),
DCA in the present study inhibited human PASMC proliferation
stimulated by PDGF in a dose-dependent manner (0–50 mM). Reversal
of the Warburg effect by downregulating PDK-1 and HIF-1α and
inactivation of HK-2 may account for the repression of the aberrant
growth of human PASMCs treated by DCA. Notably, the pro-apoptotic
effect of DCA may also be potentiated by the inhibition of PDK-1,
PDH phosphorylation and HK-2 inactivity through blocking
Akt/GSK-3β/HIF-1α signalling with a PI3K inhibitor.
Accumulating evidence has suggested that PDGF was
highly expressed in the neointimal lesions of pulmonary arterioles
from rats subjected to monocrotaline or hypoxia insult (5,25).
Targeting of the PDGF receptor using imatinib exhibited beneficial
effects on PASMC proliferation and mitigated the neointimal
formation of the affected pulmonary arterioles. Repression of PDGF
receptor phosphorylation and subsequent mitogen activated protein
kinase activation underlies the alleviation of PAH by imatinib
(5), but the precise mechanism
remains unclear. A previous study from Xiao et al (7) provided novel insights into the
current understanding of PDGF-induced PASMC proliferation.
Consistent with the mitochondrial metabolic shifting paradigm in
cancer (26), PDGF induced
increased expression of a glycolysis-associated gate-keeper enzyme
PDK-1, glucose transporter-1 and HIF-1α, preferentially converting
pyruvate to lactate in PASMCs (7). DCA selectively inhibits PDK
activity, thereby indirectly inactivating PDH and shifting
metabolism to mitochondrial glucose oxygenation, thereby reversing
apoptotic resistance; this change has been demonstrated to be
important in attenuating PASMCs hyperplasia in several rodent
models of PAH (9,27). Consistent with these studies, the
results of the present study suggested that a high dose of DCA
facilitated the reversal of PASMCs glycolytic status, which was
confirmed by decreased lactate content in culture medium. Along
with rectifying this abnormal glycolysis metabolism phenotype, DCA
treatment promoted apoptotic sensitivity and inhibited
proliferation of PASMCs.
One notable result from the present study was that
the inhibitory effect of DCA on PASMCs growth was potentiated by
blocking the PI3K/Akt signalling pathway. Although 5 µM
LY294002 exhibited a negligible effect on cell apoptosis, it
increased caspase-3 levels and promoted apoptosis in PASMCs treated
with 10 mM DCA. Previous studies demonstrated that the median
lethal dose of DCA causing growth retardation in human cancer cell
lines was 5–20 mM (28–30). DCA has a relatively wide
therapeutic margin, and few adverse reactions have been described,
even following long-term ingestion, with the exception of a few
case reports of peripheral nerve lesions (31). However, a previous study suggested
that the incubation of rat sciatic nerves with 20 mM DCA caused a
15% decrease in the amplitude of the compound action potential,
while 10 mM DCA had no effect on the nerve transduction (32). Therefore, combined pharmaceutical
intervention is recommended to avoid a potential adverse reaction
due to overdose by single drug use, as multiple studies have
indicated that the anti-tumour effect of DCA may be improved by
sensitising the pro-apoptotic signalling pathway in cancer cell
lines (33,34).
The results of the present study indicated that the
reversal of the Warburg effect mediated through the downregulation
of HIF-1α and inactivation of HK-2 may account for the increased
PASMC apoptosis following DCA intervention. DCA is a
well-characterised inhibitor of the protein kinase PDH.
DCA-mediated inhibition of PDKs triggers a switch in pyruvate
metabolism towards glucose oxidation to CO2 in the
mitochondria; during the process of electron transport in the Krebs
cycle, the intermediate substrates (mitochondrial reactive oxygen
species or α-ketoglutarate) serve key roles in the destabilisation
of HIF-1α, which also reverses hyper-polarised ΔΨm and induces
apoptosis of cancer cells (3).
HIF-1α upregulation was also demonstrated by Lambert et al
(12), in a rodent model with
carotid arterial neointimal formation induced by balloon injury.
Targeting HIF-1α using siRNA effectively reversed the aerobic
glycolysis of SMCs and mitigated the neointimal lesions in diseased
carotid arteries. In Fawn-Hooded rats, DCA inhibited proliferation
of PASMCs by reversing the mitochondrial metabolic phenotype and
inactivation of HIF-1α and downstream voltage-gated potassium
channel subtype 1.5 (27). HK-2
has been recognized as one of the key molecules in cell
proliferation, differentiation and metastasis during
carcinogenesis: Inhibition of HK-2 using 2-DG or siRNA exhibited
beneficial effects on tumour growth in a xenograft model (35,36). However, the role of HK-2 in
DCA-induced PASMC apoptosis remains unclear. Previous studies
indicated that the HK-2 gene promoter comprising a 5′-RCGTG-3′ box
known as the hypoxia response element was under precise
transcriptional regulation by HIF-1α (37). HIF-1α activation was demonstrated
to serve a critical role in the subsequent upregulation of HK-2 in
diverse cancer cells, but has rarely been described in vascular
cells, particularly in PASMCs. The present study indicated that
with the downregulation of HIF-1α following DCA treatment, the
expression and activity of HK-2 decreased. Therefore, the present
results suggested that HIF-1α induced HK-2 activation in the
apoptotic-resistant PASMCs. Consistent with previous studies
(12,20), these data indicated that in
addition to HK-2 downregulation, the dislocation of HK-2 from VDAC
on the mitochondrial outer membrane was involved in cell apoptosis
following DCA treatment, which was associated with an efflux of
cytochrome c into the cytoplasm and caspase-3 activation.
However, although there was no significant difference in the
expression levels of cleaved caspase-3 in cells treated with 10 mM
DCA alone or a combination of LY294002 and DCA, the number
apoptotic cells was markedly increased in the combined treatment
group. We hypothesize that the activation of PI3K/Akt signalling is
associated with the cytosolic sequestration of the apoptotic
protein B-cell lymphoma 2 (Bcl-2)-associated death protein (BAD);
therefore, pharmaceutic ablation of PI3K facilitates BAD
translocation with B-cell lymphoma-extra large on the outer
membrane of the mitochondria, which activates caspase-9 and -3 and
causes the opening of the mitochondrial membrane transit pore
(MTP). In addition to the release of cytochrome c following
the MTP opening, the apoptotic inducing factor may also be released
into the cytoplasm. The latter may initiate the
non-caspase-3-dependent apoptosis pathway.
PDK-1 has been proposed as a key regulator of the
Warburg effect and inhibits acetyl-CoA entry into the Krebs cycle,
accompanied with decreased ketoglutarate synthesis, leading to
decreased HIF-1α proteasomal degradation (38). DCA is a pyruvate mimetic that
targets PDKs, inhibiting the phosphorylation of PDH (31). Therefore, we hypothesised that
inhibiting the activity of PDK-1 promoted the degradation of HIF-1α
and rectified the pseudo-hypoxic status in mitochondria of PASMCs
exposed to PDGF followed by DCA treatment. In addition, the results
of the present study suggested that DCA inhibited PDK-1 expression
in addition to downregulating PDK-1 activity. The mechanism of the
DCA-induced decrease in PDK-1 is poorly delineated, and we
postulate that PDK-1 activation induces a pseudo-hypoxic state in
mitochondria and HIF-1α activation, while HIF-1α inhibits the
tricarboxylic acid cycle by directly activating the gene encoding
PDK-1, which initiates a positive regulatory loop. The results of
the present study suggested that DCA inhibits PDK-1 activity and
disrupts the vicious cycle among aerobic glycolysis-associated
molecules, accounting for the decreased expression of PDK-1 in
PASMCs. A previous study indicated that the activation of PDGF
receptor increased PDK-1 activity, mitochondrial respiration and
cell proliferation (7); however,
the effect of PI3K suppression on PDK-1 and its target molecule
(PDH) in growth factor receptor tyrosine kinase-induced glycolytic
flux remains unclear (39).
Several previous data indicate that the PI3K/Akt/HIF-1α axis serves
an essential role in PDGF-induced vascular SMC proliferation
mediated through enhanced glycolytic flux (7,12).
Inhibition of PI3K decreases extracellular acidification rates
through HIF-1α downregulation in vascular SMCs stimulated with
growth factors (40). PDK-1
downregulation with LY294002 was abrogated by the GSK-3β inhibitor
in the present study; therefore, the downregulation of HIF-1α by
GSK-3β signal blockade is the key factor responsible for disrupting
the mutual positive feedback regulation between HIF-1α and PDK-1
when co-incubated with DCA. In contrast to the results of the
present study, PI3K blockade with LY294002 facilitated the
phosphorylation of PDH at the E1α-subunit (inactivation of its
function) and decreased the oxygen consumption rate in SQ20B cells
(41). Therefore, the role of
PI3K/Akt activation in regulation of downstream signals of
glycolysis-associated enzymes varies among diverse cells.
There are certain limitations in the present study
to be addressed: Firstly, with the exception of GSK-3β, other
downstream molecules of Akt, including mechanistic target of
rapamycin, may be affected following PI3K/Akt signal blocking
(42), which is closely
associated with cell survival and autophagy, and may have potential
effects on PASMCs apoptosis. Therefore, the possibility that other
signal molecules may be involved in the DCA-induced decrease in
cell growth cannot not be excluded. Secondly, LY-294002 may also
affect cell cycle proteins, including cyclin-dependent kinases, and
DCA was indicated to inhibit cell cycle progression, had no
association with cell apoptosis and inhibited cell hyperplasia in
certain cell lines (43).
Therefore, the synergistic effect of DCA and PI3K blockade on PASMC
growth may be mediated by cell cycle suppression, and additional
studies are warranted.
Collectively, the data in the present study
indicated that the pro-apoptotic effect of DCA on human PASMCs was
associated with PDK-1 and HIF-1α downregulation, which led to HK-2
inactivation by blocking the interaction with mitochondria.
Furthermore, this effect may be potentiated by the suppression of
PDK-1 via blockade of Akt/GSK-3β/HIF-1α signalling. These results
suggest that the inhibition of Akt/GSK-3β signalling may improve
the pro-apoptotic effect of DCA on human PASMCs, which suggests
that combined therapy may be used in the treatment of PAH and may
decrease the probability of peripheral neuropathic lesions or liver
damage following long-term ingestion of high doses of DCA.
Acknowledgments
The authors would like to thank Dr Yong Liu for his
technical support in fluorescence activated cell sorting detection
for cell apoptosis.
Abbreviations:
|
DCA
|
dichloroacetate
|
|
PASMCs
|
pulmonary arterial smooth muscle
cells
|
|
HK-2
|
hexokinase-2
|
|
GSK-3β
|
glycogen synthase kinase-3β
|
|
ΔΨm
|
mitochondria membrane potential
|
|
HIF-1α
|
hypoxia inducible factor-1α
|
|
PAH
|
pulmonary arterial hypertension
|
|
PDH
|
pyruvate dehydrogenase
|
|
PDK
|
pyruvate dehydrogenase kinase
|
|
VDAC
|
voltage dependent anion channel
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
FBS
|
foetal bovine serum
|
|
JC-1
|
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazol-carbocyanine
iodide
|
Funding
The present study was supported by National Natural
Science Foundation of China (project no., 81470242), the Six Talent
Summit Project of Jiangsu Province (project no., WSN-147) and the
Medical Science and Technique Development Foundation of Nanjing
Municipal Government (grant no., RX17013).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
BL and JY contributed to experimental design,
performed experiments, data analysis and wrote the final
manuscript. YZ, QS, LC, YT and CY performed experiments and
contributed to preparation of the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The approval of this study was obtained from the
ethic committee of Drum tower Hospital of Nanjing University
Medical School for Laboratory Animal Use and Care.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gurtu V and Michelakis ED: Emerging
therapies and future directions in pulmonary arterial hypertension.
Can J Cardiol. 31:489–501. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tuder RM: Pathology of pulmonary arterial
hypertension. Semin Respir Crit Care Med. 30:376–385. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Michelakis ED, Webster L and Mackey JR:
Dichloroacetate (DCA) as a potential metabolic-targeting therapy
for cancer. Br J Cancer. 99:989–994. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paulin R and Michelakis ED: The metabolic
theory of pulmonary arterial hypertension. Circ Res. 115:148–164.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schermuly RT, Dony E, Ghofrani HA,
Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N,
Seeger W, et al: Reversal of experimental pulmonary hypertension by
PDGF inhibition. J Clin Invest. 115:2811–2821. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Berghausen E, ten Freyhaus H and
Rosenkranz S: Targeting of platelet-derived growth factor signaling
in pulmonary arterial hypertension. Handb Exp Pharmacol.
218:381–408. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiao Y, Peng H, Hong C, Chen Z, Deng X,
Wang A, Yang F, Yang L, Chen C and Qin X: PDGF promotes the warburg
effect in pulmonary arterial smooth muscle cells via activation of
the PI3K/AKT/mTOR/HIF-1α signaling pathway. Cell Physiol Biochem.
42:1603–1613. 2017. View Article : Google Scholar
|
|
8
|
Rehman J and Archer SL: A proposed
mitochondrial-metabolic mechanism for initiation and maintenance of
pulmonary arterial hypertension in fawn-hooded rats: The Warburg
model of pulmonary arterial hypertension. Adv Exp Med Biol.
661:171–185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McMurtry MS, Bonnet S, Wu X, Dyck JR,
Haromy A, Hashimoto K and Michelakis ED: Dichloroacetate prevents
and reverses pulmonary hypertension by inducing pulmonary artery
smooth muscle cell apoptosis. Circ Res. 95:830–840. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guignabert C, Tu L, Izikki M, Dewachter L,
Zadigue P, Humbert M, Adnot S, Fadel E and Eddahibi S:
Dichloroacetate treatment partially regresses established pulmonary
hypertension in mice with SM22alpha-targeted overexpression of the
serotonin transporter. FASEB J. 23:4135–4147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Michelakis ED, McMurtry MS, Wu XC, Dyck
JR, Moudgil R, Hopkins TA, Lopaschuk GD, Puttagunta L, Waite R and
Archer SL: Dichloroacetate, a metabolic modulator, prevents and
reverses chronic hypoxic pulmonary hypertension in rats: Role of
increased expression and activity of voltage-gated potassium
channels. Circulation. 105:244–250. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lambert CM, Roy M, Robitaille GA, Richard
DE and Bonnet S: HIF-1 inhibition decreases systemic vascular
remodelling diseases by promoting apoptosis through a hexokinase
2-dependent mechanism. Cardiovasc Res. 88:196–204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Semenza GL: Hypoxia-inducible factor 1:
Oxygen homeostasis and disease pathophysiology. Trends Mol Med.
7:345–350. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wilson JE: Isozymes of mammalian
hexokinase. Structure, subcellular localization and metabolic
function. J Exp Biol. 206:2049–2057. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pastorino JG and Hoek JB: Hexokinase II:
The integration of energy metabolism and control of apoptosis. Curr
Med Chem. 10:1535–1551. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yasuda S, Arii S, Mori A, Isobe N, Yang W,
Oe H, Fujimoto A, Yonenaga Y, Sakashita H and Imamura M: Hexokinase
II and VEGF expression in liver tumors: Correlation with
hypoxia-inducible factor 1 alpha and its significance. J Hepatol.
40:117–123. 2004. View Article : Google Scholar
|
|
17
|
Riddle SR, Ahmad A, Ahmad S, Deeb SS,
Malkki M, Schneider BK, Allen CB and White CW: Hypoxia induces
hexokinase II gene expression in human lung cell line A549. Am J
Physiol Lung Cell Mol Physiol. 278:L407–L416. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yan J, Shen Y, Wang Y and Li BB: Increased
expression of hypoxia-inducible factor-1α in proliferating
neointimal lesions in a rat model of pulmonary arterial
hypertension. Am J Med Sci. 345:121–128. 2013. View Article : Google Scholar
|
|
19
|
Zhuo B, Li Y, Li Z, Qin H, Sun Q, Zhang F,
Shen Y, Shi Y and Wang R: PI3K/Akt signaling mediated Hexokinase-2
expression inhibits cell apoptosis and promotes tumor growth in
pediatric osteosarcoma. Biochem Biophys Res Commun. 464:401–406.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bonnet S, Paulin R, Sutendra G, Dromparis
P, Roy M, Watson KO, Nagendran J, Haromy A, Dyck JR and Michelakis
ED: Dehydroepiandrosterone reverses systemic vascular remodeling
through the inhibition of the Akt/GSK3-{beta}/NFAT axis.
Circulation. 120:1231–1240. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dromparis P, Sutendra G and Michelakis ED:
The role of mitochondria in pulmonary vascular remodeling. J Mol
Med. 88:1003–1010. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Assad TR and Hemnes AR: Metabolic
dysfunction in pulmonary arterial hypertension. Curr Hypertens Rep.
17:202015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Haugrud AB, Zhuang Y, Coppock JD and
Miskimins WK: Dichloroacetate enhances apoptotic cell death via
oxidative damage and attenuates lactate production in
metformin-treated breast cancer cells. Breast Cancer Res Treat.
147:539–550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Allen KT, Chin-Sinex H, DeLuca T,
Pomerening JR, Sherer J, Watkins JB III, Foley J, Jesseph JM and
Mendonca MS: Dichloroacetate alters Warburg metabolism, inhibits
cell growth, and increases the X-ray sensitivity of human A549 and
H1299 NSC lung cancer cells. Free Radic Biol Med. 89:263–273. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Antoniu SA: Targeting PDGF pathway in
pulmonary arterial hypertension. Expert Opin Ther Targets.
16:1055–1063. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peng H, Xiao Y, Deng X, Luo J, Hong C and
Qin X: The Warburg effect: A new story in pulmonary arterial
hypertension. Clin Chim Acta. 461:53–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bonnet S, Michelakis ED, Porter CJ,
Andrade-Navarro MA, Thébaud B, Bonnet S, Haromy A, Harry G, Moudgil
R, McMurtry MS, et al: An abnormal mitochondrial-hypoxia inducible
factor-1alpha-Kv channel pathway disrupts oxygen sensing and
triggers pulmonary arterial hypertension in fawn hooded rats:
Similarities to human pulmonary arterial hypertension. Circulation.
113:2630–2641. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wong JY, Huggins GS, Debidda M, Munshi NC
and De Vivo I: Dichloroacetate induces apoptosis in endometrial
cancer cells. Gynecol Oncol. 109:394–402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Madhok BM, Yeluri S, Perry SL, Hughes TA
and Jayne DG: Dichloroacetate induces apoptosis and cell-cycle
arrest in colorectal cancer cells. Br J Cancer. 102:1746–17521.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun RC, Board PG and Blackburn AC:
Targeting metabolism with arsenic trioxide and dichloroacetate in
breast cancer cells. Mol Cancer. 10:1422011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stacpoole PW, Nagaraja NV and Hutson AD:
Efficacy of dichloroacetate as a lactate-lowering drug. J Clin
Pharmacol. 43:683–691. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kagiava A and Theophilidis G: High
concentrations of dichloroacetate have minor effects on the
vitality of the mammalian nerve fibers: An ex-vivo
electrophysiological study. Anticancer Drugs. 22:273–276. 2011.
View Article : Google Scholar
|
|
33
|
Cao W, Yacoub S, Shiverick KT, Namiki K,
Sakai Y, Porvasnik S, Urbanek C and Rosser CJ: Dichloroacetate
(DCA) sensitizes both wild-type and over expressing Bcl-2 prostate
cancer cells in vitro to radiation. Prostate. 68:1223–1231. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tong J, Xie G, He J, Li J, Pan F and Liang
H: Synergistic antitumor effect of dichloroacetate in combination
with 5-fluorouracil in colorectal cancer. J Biomed Biotechnol.
2011:7405642011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang H, Wang L, Zhang Y, Wang J, Deng Y
and Lin D: Inhibition of glycolytic enzyme hexokinase II (HK2)
suppresses lung tumor growth. Cancer Cell Int. 16:92016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ahn KJ, Hwang HS, Park JH, Bang SH, Kang
WJ, Yun M and Lee JD: Evaluation of the role of hexokinase type II
in cellular proliferation and apoptosis using human hepatocellular
carcinoma cell lines. J Nucl Med. 50:1525–1532. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Masoud GN and Li W: HIF-1α pathway: Role,
regulation and intervention for cancer therapy. Acta Pharm Sin B.
5:378–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Semenza GL: HIF-1: Upstream and downstream
of cancer metabolism. Curr Opin Genet Dev. 20:51–56. 2010.
View Article : Google Scholar :
|
|
39
|
Velpula KK, Bhasin A, Asuthkar S and Tsung
AJ: Combined targeting of PDK1 and EGFR triggers regression of
glioblastoma by reversing the Warburg effect. Cancer Res.
73:7277–7289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Perez J, Hill BG, Benavides GA, Dranka BP
and Darley-Usmar VM: Role of cellular bioenergetics in smooth
muscle cell proliferation induced by platelet-derived growth
factor. Biochem J. 428:255–267. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cerniglia GJ, Dey S, Gallagher-Colombo SM,
Daurio NA, Tuttle S, Busch TM, Lin A, Sun R, Esipova TV, Vinogradov
SA, et al: The PI3K/Akt pathway regulates oxygen metabolism via
pyruvate dehydrogenase (PDH)-E1α phosphorylation. Mol Cancer Ther.
14:1928–1938. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang X, Cheng Y, Li P, Tao J, Deng X,
Zhang X, Gu M, Lu Q and Yin C: A lentiviral sponge for miRNA-21
diminishes aerobic glycolysis in bladder cancer T24 cells via the
PTEN/PI3K/AKT/mTOR axis. Tumour Biol. 36:383–391. 2015. View Article : Google Scholar
|
|
43
|
Delaney LM, Ho N, Morrison J, Farias NR,
Mosser DD and Coomber BL: Dichloroacetate affects proliferation but
not survival of human colorectal cancer cells. Apoptosis. 20:63–74.
2015. View Article : Google Scholar
|


















