Introduction
Recombinant proteins are the main biopharmaceutical
agents (1–4), and the reduction of primary cost of
recombinant proteins and improvement of their quality by selecting
novel efficient expression systems has received increasing
attention (1–4). The production of recombinant
proteins through the use of bacterial hosts, mammalian cells,
yeast, viruses, transgenic plants or other expression systems has
numerous disadvantages, including poor biological activity, high
cost and poor protein post-translation modification among other
(1–4). Recombinant proteins expressed in the
mammary gland of transgenic animals have become a research focus as
bioreactors due to their high expression, low cost, easy
purification process and similar protein modification to that in
the human body (4–11). Therefore, the animal mammary gland
is currently the most ideal expression system for the production of
recombinant proteins. Various recombinant proteins, such as ATryn,
hAAT, recombinant human protein C, superoxide dismutase (SOD) and
hormones, have been generated in the milk of transgenic animals
(8–12). However, although the first
production of recombinant proteins through the milk of transgenic
animal for therapeutic use was reported 30 years ago, the
recombinant proteins expression regulatory mechanism in the milk of
these animals remains unclear and only a few recombinant proteins
have been successfully produced through the transgenic animal
mammary gland (4,13).
The expression of recombinant proteins in the
mammary gland of transgenic animals is a complex process affected
by various factors, including the genetic structure, gene copy
number and their regulatory elements, gene integration site,
promoter and methylation (3,4).
At present, the expression level and stability of recombinant
proteins in transgenic animals are not sufficient to allow for
larger-scale production of the desired proteins (3,4).
Thrombotic diseases are serious threat to human
health, and thrombolytic agents are the main drugs used in the
treatment of these diseases (14–19). The human tissue-type plasminogen
activator (tPA) is a key kinase of fibrinolysis that serves an
important role in dissolving fibrin clots to promote thrombolysis
(16,17). To increase the half-life and
thrombolytic activity of tPA, a mutant of tPA containing only the
essential K2 fibrin-binding and P activating plasminogen domains of
the wild-type tPA transgenic rabbit has been generated (19). Recombinant human plasminogen
activator (rhPA) is a novel drug for the treatment of thrombolytic
disease (19). Compared with the
natural human tPA, rhPA has higher thrombolytic activity and some
other advantages, such as a high affinity to fibrin, low incidence
of side effects of systemic bleeding, long half-life in the blood
and low total dose (18–23). Therefore, as a representative of
the third-generation thrombolytic agents, rhPA has been
increasingly accepted by the majorities of patients (23).
Currently, there are no studies reporting the
difference in rhPA expression in the milk of homozygous and
hemizygous rhPA transgenic rabbits. Compared with other animals,
homozygous transgenic rabbits present numerous advantages,
including genetic stability, no need for PCR integrate testing on
offspring and reduced detection cost. In addition, all animals can
have the same genotype and an excellent reproductive rate (24–27).
The objective of the present study was to improve
the expression and stability of mammary gland-specific rhPA in
homozygote transgenic rabbits. Enzyme-linked immunosor-bent assay
(ELISA) and fibrin agarose plate assay (FAPA) were used to analyze
the rhPA expression level and stability in the milk of the
homozygous transgenic rabbits, as previously described (28–31). The results indicated that rhPA
expression in homozygous transgenic rabbit was markedly higher in
comparison with that in hemizygous transgenic rabbits. Thus,
transgenic rabbits with high rhPA expression and stability were
obtained. A new strain (K29) of rhPA transgenic rabbits was
established in the current study, and two homozygous transgenic
rabbits (K29 F2–05 and K29 F2-09) were obtained. With those
efforts, the current study aimed to increase the novel rhPA
expression level and stability.
Materials and methods
Animals and reagents
The present study was approved by the Institutional
Animal Care and Use Committee of Yangzhou University (Yangzhou,
China) and was performed according to the Guide for the Care and
Use of Laboratory Animals (32).
A total of 30 female New Zealand rabbits (2.5–3.0 kg, 210 days old,
Better Biotechnology Co., Ltd., Nanjing, China) used in the current
study were raised at room temperature (25±2°C) under
specific-pathogen-free conditions, and allowed free access to food
and water. All chemicals were purchased from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany) unless otherwise specified. The rhPA
transgenic rabbits were generated in our lab, as previously
described (5,6).
Construction of mammary gland-specific
rhPA gene expression vector
The rhPA mammary gland-specific expression vector in
rabbits was constructed in our lab. It included the effective
mammary-specific expression vector PCL25/rhPA (F,E,K1 domains
deletion mutant of tPA) and goat β-casein gene as regulatory
elements. Subsequently, rhPA transgenic rabbits were generated
through microinjection into rabbit’s fertilized eggs with vector
concentration at 5 ng/µl (5).
Generation of homozygote transgenic
rabbits
Male rhPA transgenic rabbits K29 were selected as
the F0 rabbits, and they were mated with normal nontransgenic
rabbits in order to obtain the F1 transgenic rabbits, and F1 male
and female rabbits were then mated to obtain the F2 transgenic
rabbits. Next, the F2 transgenic rabbits were mated with normal
nontransgenic rabbits to obtain F3 rabbits as a test cross.
Homozygous transgenic rabbits were then screened out from the F2
generation through F2 rabbit test cross and polymerase chain
reaction (PCR) according to the methods described in a previous
study (5). All the rhPA-positive
rabbits were identified through the PCR method. After the F2
generation rabbit mating with normal nontransgenic rabbits, F2
rabbits were consider as homozygous (>99.9%) if their
descendants were >5 and if all rabbits were positive for rhPA
(24,25).
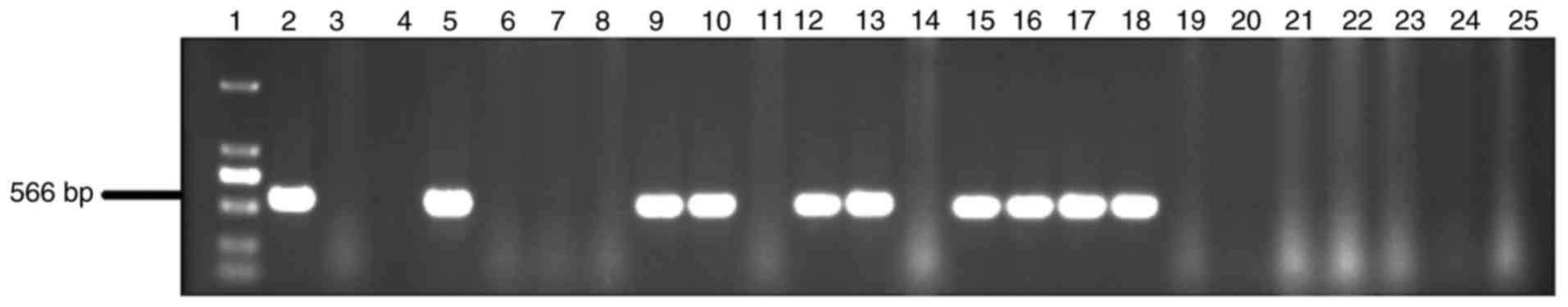
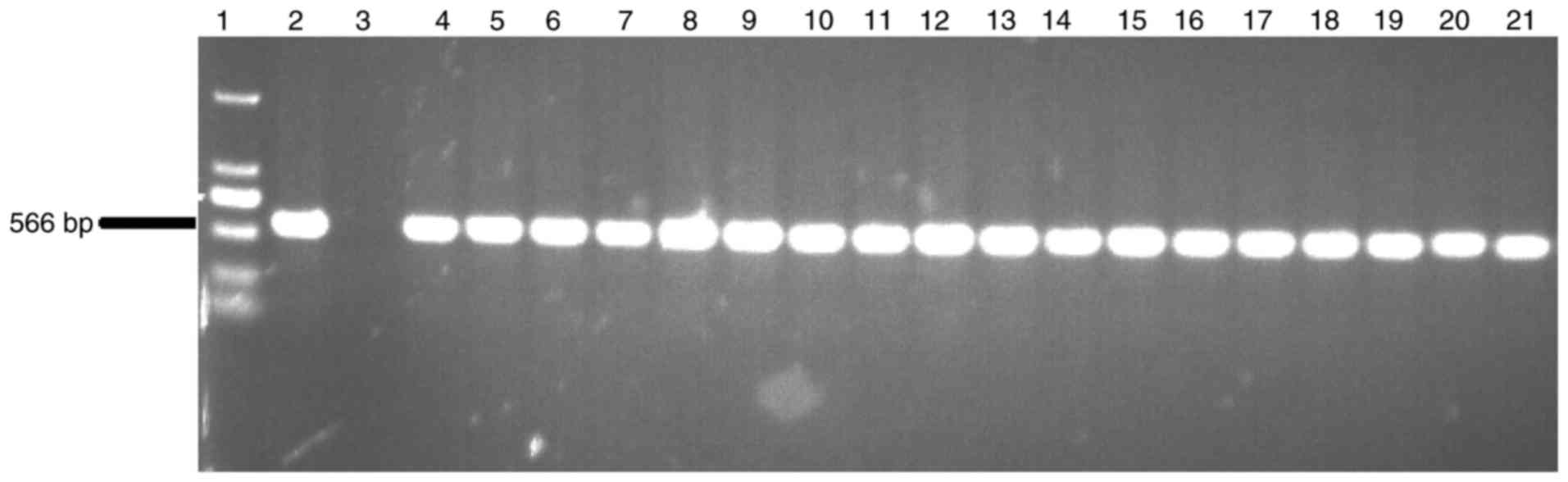
PCR test
Genomic DNA was extracted from rabbits with the
phenol-chloroform method. Transgenic rabbits were identified with
PCR analysis using the extracted genomic DNA. The upstream primer
was located on the promoter region, while the downstream primer was
located on the rhPA coding region. The amplification production
size was 566 bp at the CMV and rhPA joint area. Primers were
designed with the Primer Premier software (version 5.0; Premier
Biosoft, Palo Alto, CA, USA), and the primer sequences were
(5,30): Forward, 5′-CGTGGATAGCGGTTTGA-3′,
and reverse, 5′-GAGCCCTCCTTTGATGC-3′. PCR reaction conditions were:
95°C for 5 min, followed by 30 cycles of 94°C for 45 sec, 55°C for
45 sec, and 72°C for 1 min for a total of 30 cycles, and then 72°C
for 10 min. PCR Master Mix (Thermo Fisher Scientific) was used to
prepare PCR reaction system, and PCR product was confirmed by
agarose gel electrophoresis (1.1% gel) and gel images were analyzed
using a Digital Gel Imaging Analysis system (GIS-1000, Shanghai
Tianneng Technology Co., Ltd., Shanghai, China).
Pretreatment of transgenic rabbit
milk
Rabbit milk was collected everyday during the
lactation period (4 weeks after kindling), followed by
centrifugation at 4,000 × g, 4°C for 30 min, precipitation was
removed and the fat in the top layer was also removed. The rabbit
whey was collected and the pH value was adjusted to 2.5 with 50%
phosphoric acid and 0.5 mol/l HCl. Next, the milk was centrifuged
at 6,000 × g at 4°C for 30 min, the supernatant was collected and
the pH value was adjusted to 3.5 with 1 mol/l sodium hydroxide
(50%). A total of 5 centrifugation cycles were performed at 6,000
×g at 4°C for 10 min, the supernatant was collected after each
centrifugation, and 1 mol/l sodium hydroxide was added to adjust
the pH value to 4.5, 5.5, 6.5, 7.5 and 8.5 after collections of
supernatant, respectively, at each successive cycle (6,33,34). Finally, the supernatant was
filtered with a 0.22-µm aperture membrane filter to remove
any impurities.
Determination of rhPA in transgenic
rabbit milk by ELISA
Indirect ELISA was used to determine the rhPA level
in the milk of transgenic rabbits (29,35,36). ELISA kits (cat. no. QY-H11759)
were purchased from Westang Biotechnology Co., Ltd (Shanghai,
China) and the analysis was conducted according to the
manufacturer’s protocol. Briefly, 100 ul of rhPA transgenic rabbit
milk whey was immobilized to each well of ELISA plate (96-well) by
physical adsorption as the detecting antigen. The well containing
thrombolytic drug alteplase (Sigma-Aldrich; Merck KGaA) was used as
the positive control, while the normal nontransgenic rabbit milk
whey was used as the control antigen to exclude the false positive
results. PBS (containing 137 mM NaCl, 10 mM
Na2HPO4, 3 mM KCl and 2 mM
KH2PO4, pH 7.4) was used as the blank
control. Following thorough washing using washing solution in the
ELISA kit, the sample was mixed with the chromogenic substrate
tetramethyl benzidine. The intensity of the staining color and the
rhPA level in the samples were positively correlated. Absorbance
(optical density) was examined at 450 nm, and the concentrations of
rhPA in the samples were calculated according to the instructions
of the ELISA kit. Briefly, following incubation in blocking
solution at 37°C for 1.5 hours, washing was performed with washing
solution 3 times, for 5 min each time. The primary rhPA Monoclonal
antibodies (1:4,000; cat. no. sc-59721; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) was added and incubated at 37°C for 1.5 h,
then washed with washing solution 3 times, 5 min each time. Then
secondary horseradish peroxidase-conjugated goat anti-mouse
Immunoglobulin G (1:4,000; cat. no. sc-2005; Santa Cruz
Biotechnology, Inc.) was added and incubated at 37°C for 1.5 h.
Analysis was performed using a microplate reader with the internal
software (RT-6000; Rayto Life and Analytical Sciences Co., Ltd.,
Shenzen, China).
Detection of thrombolytic activity of
transgenic rabbit milk by FAPA
The thrombolytic bioactivity of rhPA transgenic
rabbit milk was evaluated in vitro through FAPA using a
fibrin-thrombin-agarose gel containing 1.0 % (w/v) agarose, 10
mg/ml fibrinogen containing a small amount of plasminogen, and 1
U/ml thrombin in PBS (31,35–37).
The rhPA transgenic rabbit whey samples were diluted for analysis
using PBS. In order to obtain the fibrin-thrombin-agarose gel, PBS
was used as a solvent and mixed with 1.0% agarose gel, 10 mg/ml
fibrinogen and 1 U/ml thrombin. Next, 15 ml agarose gel was boiled.
When the temperature dropped to 55–60°C, 1 ml fibrinogen 37°C was
preheated, and thrombin was heated to 42°C, then agarose gel,
fibrinogen and thrombin were immediately mixed and transferred to a
glass dish. When the solution cooled to room temperature,
fibrin-thrombin-agarose solidified to a gel state. Sample wells
were drilled in each gel, and each sample well filled with 20
µl sample solution, followed by incubation at 37°C for 24 h.
The thrombolytic activity of each sample was determined based on
the diameter size of the thrombin-dissolving ring.
Evaluation of rhPA expression in
transgenic rabbit milk by western blot analyses
Protein concentration was measured using a
bicinchoninic acid assay. Western blot analysis was used to detect
the expression of rhPA in the transgenic rabbit milk, as previously
described (6,29,35–37). Briefly, whey samples were
denatured in boiling water (100°C) for 5 min. The protein bands
were then separated under denaturing conditions by 12% SDS-PAGE
using Mini-Protean II Electrophoresis Cell (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Next, the unstained proteins were
electrotransferred, to a polyvinylidene difluoride membrane.
Following transfer, the membrane was blocked with 5% (v/v) skimmed
milk containing 0.1% Tween 20 for 90 min at 42°C, and the membrane
was then probed overnight at 4°C in the same buffer containing a
mouse anti-human rhPA primary antibody (1:2,000 dilution; sc-59721;
Santa Cruz Biotechnology, Inc.). Subsequently, the membrane was
incubated with the secondary antibody conjugated with horseradish
peroxidase (cat. no. A01854-200; 1:1,000; GenScript, Piscataway,
NJ, USA) for 2 h at 37°C. Finally, the membrane was washed three
times with the wash buffer, and the reaction was developed with DAB
colorimetric solution reagent.
Results
Generation of homozygous rhPA transgenic
rabbits
Transgenic rabbits K29 were selected to mate with
normal female New Zealand rabbits. A total of 25 F1 offspring
rabbits were obtained, and 16 of these were found to be transgenic
rabbits using the PCR method. F1 male transgenic rabbits were then
mated with female F1 transgenic rabbits to obtain F2 transgenic
rabbits. A total of 10 F2 offspring rabbits were obtained, and it
was observed that 5 F2 rabbits (4 female and 1 male) were
transgenic. Finally, F3 rabbits were obtained by mating F2
transgenic rabbits with normal rabbits. PCR was used to analyze the
genomic DNA of the F3 rabbits, and homozygous rhPA transgenic
rabbits were screened out from the F2 generation of transgenic
rabbits through a test cross. F3 generation rabbits were subjected
to DNA extraction, followed by PCR reaction and gel
electrophoresis. All the 16 rabbits from the F3 generation were
identified to be transgenic and the results demonstrated that 2 F2
transgenic rabbits (K29 F2-05 and K29 F2-09) were homozygous
(Figs. 1 and 2).
Evaluation of rhPA expression level in
the milk of homozygous transgenic rabbits by ELISA
ELISA was used to analyze the rhPA expression level
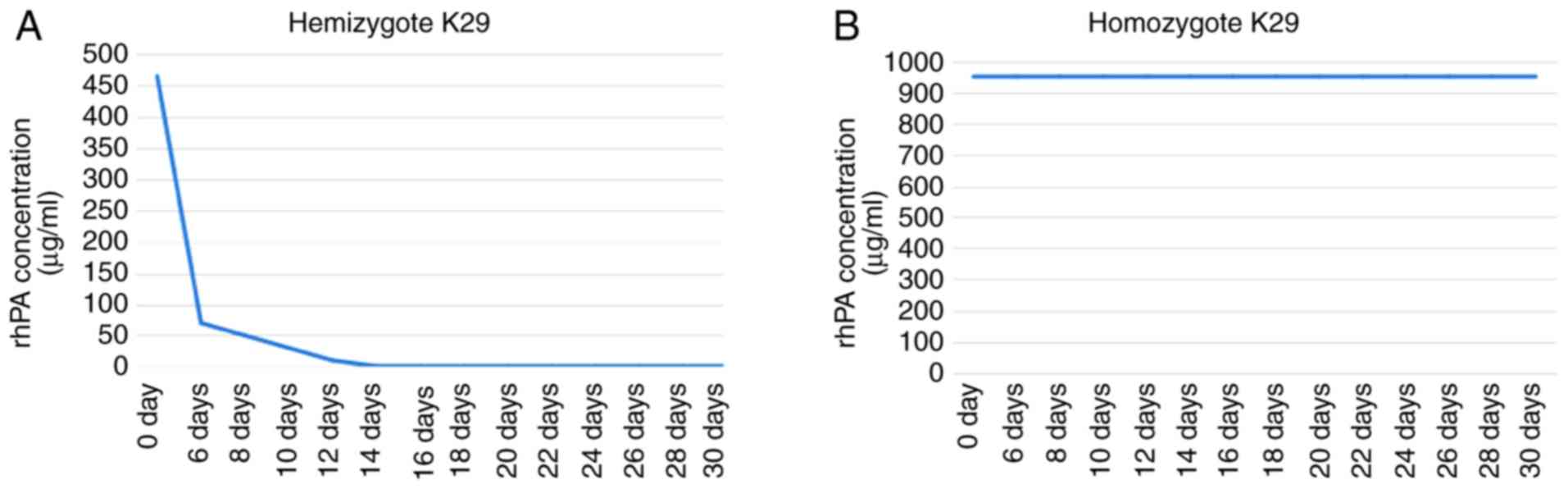
in the milk of transgenic rabbits. As shown in Figs. 3 and 4, ELISA results revealed that rhPA
expression in the milk of homozygous K29 strain transgenic rabbits
was approximately two times higher in comparison with that in the
hemizygote K29 strain transgenic rabbits. Notably, the rhPA
expression level in the milk of homozygous rabbits was stable
(~0.95 mg/ml) throughout the entire lactation period. However, the
rhPA expression level in hemizygous rabbit milk was stabilized at
only 0.465 mg/ml in the first 5 days of the lactation period
(Fig. 4).
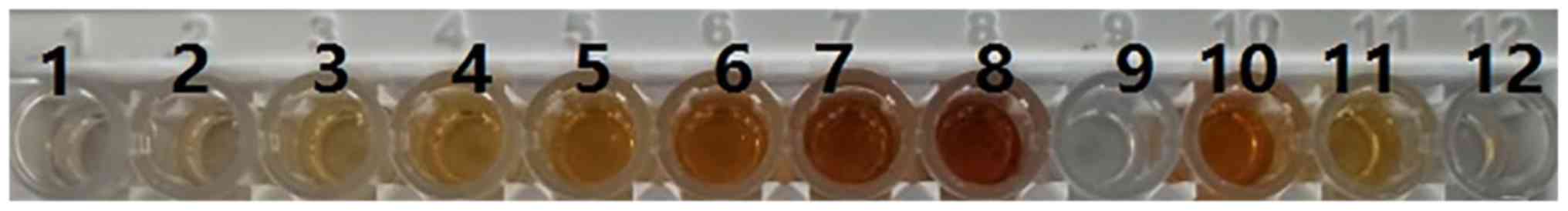 | Figure 3Enzyme-linked immunosorbent assay of
partial transgenic rabbit whey. Sample 1, PBS; samples 2–8,
alteplase serving as the injection standard at concentrations of 5,
10, 50, 500, 1,000, 2,000 and 4,000 ng/ml, respectively; sample 9,
whey from normal nontransgenic rabbits; sample 10, whey from
homo-zygote K29 transgenic rabbits (diluted to 1,000-fold); sample
11, whey obtained on days 1–5 from hemizygote K29 transgenic
rabbits (diluted to 1,000-fold); sample 12, whey obtained on days
6–30 from hemizygote K29 transgenic rabbits (diluted to
1,000-fold). |
Activity assay of rhPA in the milk of
transgenic rabbits
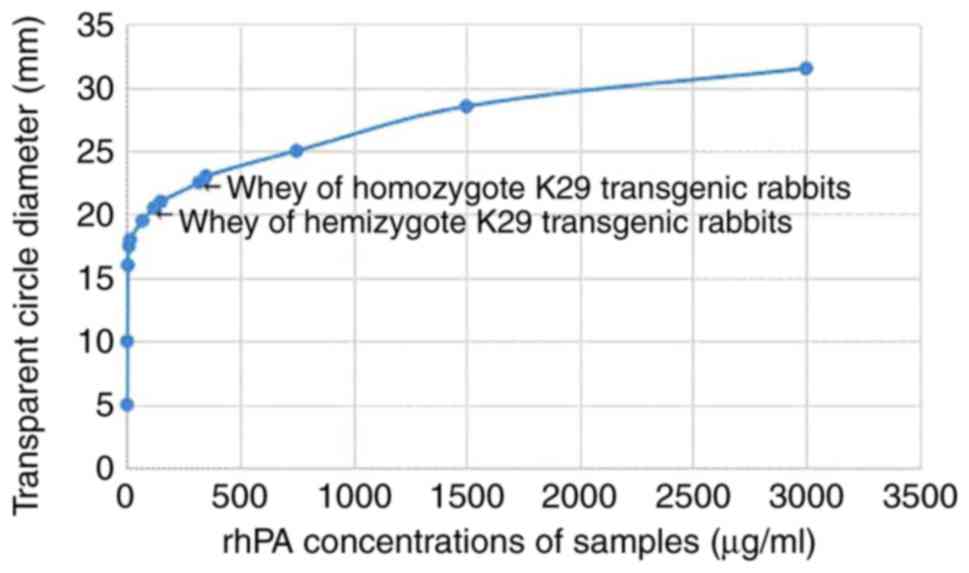
FAPA was conducted to determine the thrombolytic
activity of rhPA, which was measured according to the size of the
thrombolytic transparent circle. Activity analysis demonstrated
that the rhPA products expressed by the K29 strain of transgenic
rabbits exhibited a fibrinolytic activity in vitro (Fig. 5). The rhPA activity in samples was
calculated using regression equations of the standard curves (data
not shown) based on their transparent diameter size of the
thrombin-dissolving ring. Based on the standard curve, the
calculated activity of the expressed rhPA in the milk of transgenic
rabbits was approximately equivalent to the effects of alteplase
(Tables I and II). FAPA also revealed that the
proteins from the rhPA homozygote transgenic rabbits had a
thrombolytic activity in vitro, and that their specific
activity (biological activity/expression level) was 0.12×1,000
mg/ml, which was 2.7-fold higher compared with that of hemizygote
rabbits (0.36×1,000 mg/ml) (Fig.
6). These results demonstrated that the in vitro
activity of rhPA in the milk of transgenic rabbits was 110-360-fold
higher in comparison with that of the alteplase positive control
group.
 | Figure 5Fibrin agarose plate assay of partial
transgenic rabbit whey. Sample 1, PBS; samples 2–12, alteplase
serving as an injection standard, at concentrations of 3,000,
1,500, 750, 350, 150, 70, 15, 10, 5, 2 and 1 µg/ml,
respectively; sample 13, whey from homozygote K29 transgenic
rabbits (diluted to 1,000-fold); sample 14, whey from hemizygote
K29 transgenic rabbits (diluted to 1,000-fold); sample 15, whey
from normal nontransgenic rabbits. |
 | Table IEnzyme-linked immunosorbent assay of
rhPA level in the milk samples. |
Table I
Enzyme-linked immunosorbent assay of
rhPA level in the milk samples.
| Sample | rhPA
concentration
(ng/ml) | Optical density at
450 nm |
|---|
| 1 (non-transgenic
rabbit) | 0 | 0 |
| 2 | 5 | 0.125 |
| 3 | 10 | 0.208 |
| 4 | 50 | 0.267 |
| 5 | 500 | 0.891 |
| 6 | 1,000 | 1.112 |
| 7 | 2,000 | 1.245 |
| 8 | 4,000 | 1.360 |
| Homozygote K29 | 950 | 1.09 |
| Hemizygote K29 | 465 | 0.86 |
| Hemizygote K29 | 7 | 0.15 |
 | Table IISpecific activity of rhPA in the milk
samples. |
Table II
Specific activity of rhPA in the milk
samples.
| Sample | rhPA
concentration
(µg/ml) | Transparent circle
diameter (mm) |
|---|
| 1 (non-transgenic
rabbit) | 0 | 0 |
| 2 | 3,000 | 31.5 |
| 3 | 1,500 | 28.5 |
| 4 | 750 | 25.0 |
| 5 | 350 | 23.0 |
| 6 | 150 | 21 |
| 7 | 70 | 19.5 |
| 8 | 15 | 18.0 |
| 9 | 10 | 17.5 |
| 10 | 5 | 16.0 |
| 11 | 2 | 10.0 |
| 12 | 1 | 5.0 |
| Homozygote | 320 | 22.5 |
| Hemizygote | 120 | 20.5 |
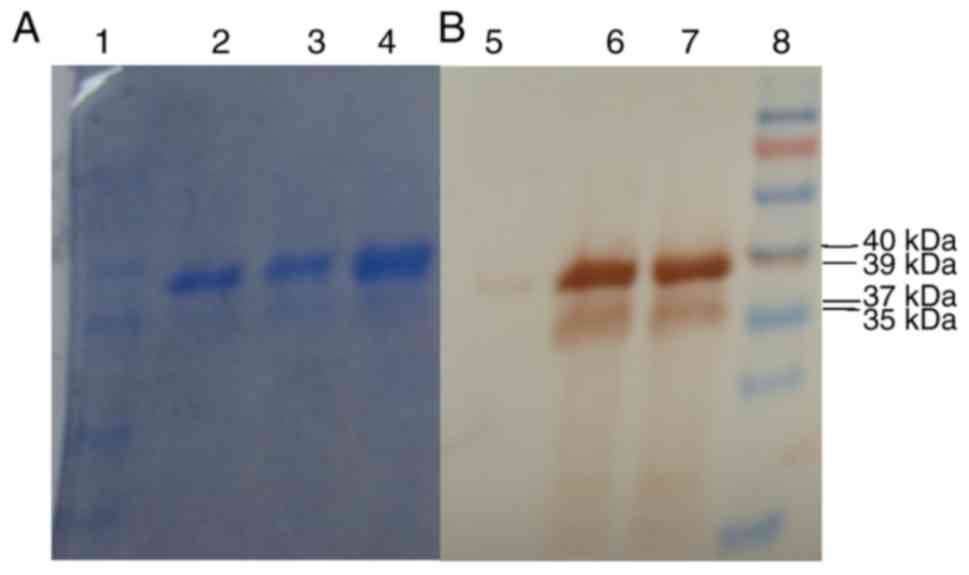
Analysis of rhPA expression in the milk
of transgenic rabbits by western blot analysis
Western blot analysis was also performed to
determine the rhPA levels in the different groups. The rhPA protein
is shown as two bands with molecular weight of 39 and 37 kDa in
SDS-PAGE gels (Fig. 7), which is
in accordance with the observations of a previous study (38). Lane 8 represents the protein
Marker (SN123, Nanjing Shengxing Biology Company, Nanjing, China),
Lanes 2 and 5 are reteplase National Institute for Biological
Standards and Control, 20 and 1 µg, respectively. Lanes 3
and 6 are hemizygous rabbit milk (40 and 80 µl purified
rabbit milk, respectively). Lanes 4 and 7 are homozygous rabbit
milk (40 and 80 µl purified rabbit milk, respectively).
These results demonstrated that rhPA homozygote transgenic rabbits
were successfully generated and efficiently expressed rhPA.
Discussion
To the best of our knowledge, the present study is
the first to report the expression level and stability of rhPA in
the milk produced by homozygote transgenic rabbits (4). A novel PCL25/rhPA transgenic rabbit
strain (K29) was established, and high expression levels of rhPA
was achieved in the homozygous transgenic rabbit (5,6).
Homozygous animals exhibit increased advantages, such as higher
genetic stability and no need for transgenic detection of offspring
rabbits. Thus, the cost may be markedly reduced, and higher levels
of rhPA can be obtained in the milk (24,25).
In the present study, individual K29 rabbits with
relatively high rhPA expression level were screened out, and
homozygous K29 F2 rabbits were generated. ELISA results revealed
that rhPA expression in homozygous K29 F2 transgenic rabbits
exhibited increased levels and stability compared with those in
hemizygous K29 strain rabbits. FAPA results also demonstrated that
the rhPA expression levels in homozygous K29 F2 transgenic rabbits
were 2.7-fold higher in comparison with that in hemizygous K29
strain rabbits. This result was not consistent with the ELISA
results, possibly due to the presence of certain substances in the
milk that influenced the FAPA results.
Transgenic rabbits were generated in the current
study by microinjection, and the exogenous gene was randomly
integrated into the animal genome. However, controlling the
integration loci and copy number is difficult at present. Due to
different integration sites, this may create various transgenic
exogenous gene loci, and influence the rhPA expression level and
stability (1–4,39,40). Based on the findings in a previous
study, the rhPA levels that individual transgenic rabbits expressed
were evidently different (5).
There are three reasons for this finding: Firstly, the expression
of foreign genes and the copy number are correlated. The
integration gene copy number of homozygous transgenic rabbits was
more than double compared with that of hemizygous transgenic
rabbits, which resulted in increased and stable expression.
Secondly, a PCL25/rhPA expression vector was built containing
enhancer regulation components, which can lead to raised
expression. Finally, in the present study, only one gene
integration loci site was obtained from the homozygous transgenic
rabbit milk sample, and the sample size was small; therefore, more
gene integration loci in transgenic rabbits should be investigated
in order to examine rhPA expression in the milk of homozygous
transgenic rabbits.
It has been reported that other strains of
homozygous transgenic rabbits are unable to express rhPA or other
recombinant proteins efficiently or stably, while certain studies
have also indicated that use of the same recombinant protein and
expression vector in different animals resulted in different
expression levels (1–4). This may be due to the vector
construction or locus, or the different recombinant proteins
influencing the expression level or stability (1–4).
Therefore, future studies should examine how to control the locus
and construct an efficient expression vector in transgenic animals,
and how to select expression vectors for specific recombinant
proteins, eventually identifying an ideal expression vector and
animal species to express different recombinant proteins. The yield
must also be further improved by optimization of the expression
vector construction or other methods (1–4,39–43).
rhPA expression in the milk of homozygous transgenic
rabbits has not been previously reported. The main reason for the
use of transgenic rabbits is that surgery in rabbits is simple, the
mating period is short, their lactation amount is large, and
finally rhPA in the milk is easily purified due to the simple
ingredients contained in their milk (26,27). Thus, the present study used
transgenic animals as a mammary gland bioreactor to produce
recombinant proteins. The present study may provide reference for
the large-scale production of recombinant proteins. High rhPA
expression through microinjection to generate rhPA transgenic
rabbits was conducted in the present study. The results revealed
that the exogenous genes in the genome of rabbits can be stably
expressed, while also proving that homozygous transgenic animals
can feasibly improve foreign gene expression levels and stability
in the animal mammalian gland. The present study generated a
homozygous rhPA rabbit strain and provided a reliable basis for
large-scale production of thrombolytic drugs in the future
(6).
In conclusion, the present study constructed
homozygous transgenic rabbits with a high expression of rhPA during
the entire lactation period. The results of ELISA indicated that
the rhPA expression level in homozygous K29 rabbits was markedly
higher as compared with that in hemizygous transgenic rabbits.
Furthermore, it was verified that improving the expression level of
foreign genes is feasible by cultivating homozygous transgenic
animals. The current study provided an experimental basis and
guidance for establishing a new strain of rhPA homozygous
transgenic rabbits, and may find applications in the large-scale
preparation of thrombolytic agents.
Acknowledgments
Not applicable
Funding
Financial support was provided by the National Key
Research and Development Program of China (grant no.
2016YFE0126000) and A Project Funded by the Priority Academic
Program Development of Jiangsu Higher Education Institutions.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors’ contributions
ZH and YC designed the experiments, ZH, LJ, TZ and
MZ performed the experiments. DW, TY and YY analyzed data. YC wrote
the manuscript. All authors read the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee of Yangzhou University (Yangzhou,
China) and was performed according to the Guide for the Care and
Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
References
|
1
|
Wang Y, Zhao S, Bai L, Fan J and Liu E:
Expression systems and species used for transgenic animal
bioreactors. Biomed Res Int. 2013:5804632013.PubMed/NCBI
|
|
2
|
Echelard Y, Williams JL, Destrempes MM,
Koster JA, Overton SA, Pollock DP, Rapiejko KT, Behboodi E,
Masiello NC, Gavin WG, et al: Production of recombinant albumin by
a herd of cloned transgenic cattle. Transgenic Res. 18:361–376.
2009. View Article : Google Scholar
|
|
3
|
Dyck MK, Lacroix D, Pothier F and Sirard
MA: Making recombinant proteins in animals-different systems,
different applications. Trends Biotechnol. 21:394–399. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khodarovicha YM, Goldman IL, Sadchikova ER
and Georgiev PG: Expression of eukaryotic recombinant proteins and
deriving them from the milk of transgenic animals. ISSN:
00036838Appl Biochem Microbiol. 49:711–722. 2013. View Article : Google Scholar
|
|
5
|
Song S, Ge X and Cheng Y, Lu R, Zhang T,
Yu B, Ji X, Qi Z, Rong Y, Yuan Y and Cheng Y: High-level expression
of a novel recombinant human plasminogen activator (rhPA) in the
milk of transgenic rabbits and its thrombolytic bioactivity in
vitro. Mol Biol Rep. 43:775–783. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song S, He Z, Mei J, Qi Z, Chen S, Xu S,
Yuan Y and Cheng Y: Affinity purification of recombinant human
plasminogen activator from transgenic rabbit milk using a novel
polyol responsive monoclonal antibody. Trop J Pharm Res.
15:905–911. 2016. View Article : Google Scholar
|
|
7
|
Meunier JM, Wenker E, Lindsell CJ and Shaw
GJ: Individual lytic efficacy of recombinant tissue plasminogen
activator in an in vitro human clot model: Rate of ‘nonresponse’.
Acad Emerg Med. 20:449–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bosze Z, Baranyi M and Whitelaw CB:
Producing recombinant human milk proteins in the milk of livestock
species. Adv Exp Med Biol. 606:357–393. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chevreux G, Faid V, Scohyers JM and
Bihoreau N: N-/O-glycosylation analysis of human FVIIa produced in
the milk of transgenic rabbits. Glycobiology. 23:1531–1546. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gil GC, Velander WH and Van Cott KE:
Analysis of the Nglycans of recombinant human factor IX purified
from transgenic pig milk. Glycobiology. 7:526–539. 2008. View Article : Google Scholar
|
|
11
|
Koles K, van Berkel PH, Pieper FR, Nuijens
JH, Mannesse ML, Vliegenthart JF and Kamerling JP: N- and O-glycans
of recombinant human C1 inhibit or expressed in the milk of
transgenic rabbits. Glycobiology. 14:51–64. 2004. View Article : Google Scholar
|
|
12
|
Zhang YL, Wan YJ, Wang ZY, Xu D, Pang XS,
Meng L, Wang LH, Zhong BS and Wang F: Production of dairy goat
embryos, by nuclear transfer, transgenic for human acid
Bglucosidase. Theriogenology. 73:681–690. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gordon K and Groet S: Transgenic animals
secreting desired proteins into milk. King George Holdings
Luxembourg Iia S.A.R.L, 2011. US Patent US7939317. Filed February
20, 1992; issued May 10, 2011.
|
|
14
|
Lian LF, Xu F, Tang ZP, Xue Z, Liang QM,
Hu Q, Zhu WH, Kang HC, Liu XY, Wang FR and Zhu SQ: Intra clot
recombinant tissuetype plasminogen activator reduces perihematomal
edema and mortality in patients with spontaneous intracerebral
hemorrhage. J Huazhong Univ Sci Technolog Med Sci. 34:165–171.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dotan A, Kaiserman I, Kremer I, Ehrlich R
and Bahar I: Intracameral recombinant tissue plasminogen activator
(r-tPA)for refractory toxic anterior segment syndrome. Br J
Ophthalmol. 98:252–255. 2014. View Article : Google Scholar
|
|
16
|
Wardlaw JM, Murray V, Berge E, del Zoppo
G, Sandercock P, Lindley RL and Cohen G: Recombinant tissue
plasminogen activator for acute ischaemic stroke: an updated
systematic review and meta-analysis. Lancet. 379:2364–2372. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fonarow GC, Zhao X, Smith EE, Saver JL,
Reeves MJ, Bhatt DL, Xian Y, Hernandez AF, Peterson ED and Schwamm
LH: Door-to-needle times for tissue plasminogen activator
administration and clinical outcomes in acute ischemic stroke
before and after a quality improvement t initiative. JAMA.
311:1632–1640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meyer MW, Witt AR, Krishnan LK, Yokota M,
Roszkowski MJ, Rudney JD and Herzberg MC: Therapeutic advantage of
recombinant human plasminogen activator in endocarditis: evidence
from experiments in rabbits. Thromb Haemost. 73:680–682.
1995.PubMed/NCBI
|
|
19
|
Smalling RW, Bode C, Kalbfleisch J, Sen S,
Limbourg P, Forycki F, Habib G, Feldman R, Hohnloser S and Seals A:
More rapid, complete, and stable coronary thrombolysis with bolus
administration of reteplase compared with alteplase infusion in
acute myocardial infarction. Circulation. 91:2725–2732. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aghaabdollahian S, Rabbani M, Ghaedi K and
Sadeghi HM: Molecular cloning of reteplase and its expression in E.
coli using tac promoter. Adv Biomed Res. 3:1902014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hong-Ying S, Si-Guo L, Jian-Quan C, Ai-Min
Z and Guo-Xiang C: Expression of a variant of human tissue-type
plasminogen activator in transgenic mouse milk. J Exp Anim Sci.
43:211–218. 2006. View Article : Google Scholar
|
|
22
|
Lijnen HR and Collen D: Strategies for the
improvement of thrombolytic agents. Thromb Haemost. 66:88–110.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Davami F, Sardari S, Majidzadeh-A K,
Hemayatkar M, Barkhrdari F, Omidi M, Azami M, Adeli A, Davoudi N
and Mahboudi F: Expression of a novel chimeric truncated t-PA in
CHO cells based on in silico experiments. J Biomed Biotechnol.
2010:1081592010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takezawa J, Yamada K, Miyachi M, Morita A,
Aiba N, Sasaki S and Watanabe S; Saku Control Obesity Program
(SCOP) Study Group: Preproghrelin gene polymorphisms in obese
Japanese women. Minor homozygotes are light eaters, do not prefer
protein or fat, and apparently have a poor appetite. Appetite.
63:105–111. 2013. View Article : Google Scholar
|
|
25
|
Knox-Macaulay HH, Rehman JU, Al Zadjali S,
Fawaz NA and Al Kindi S: Idiopathic thrombocytopenic purpura and
hypo-kalaemic dRTA with compensated haemolysis and striking
acanthocytosis in a band 3 (SLC4A1/AE1) A858D homozygote. Ann
Hematol. 92:553–554. 2013. View Article : Google Scholar
|
|
26
|
Xue F, Ma Y, Chen YE, Zhang J, Lin TA,
Chen CH, Lin WW, Roach M, Ju JC, Yang L, Du F and Xu J: Recombinant
rabbit leukemia inhibitory factor and rabbit embryonic fibroblasts
support the derivation and maintenance of rabbit embryonic stemb
cells. Cell Reprogram. 14:364–376. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fan J and Watanabe T: Transgenic rabbits
as therapeutic protein bioreactors and human disease models.
Pharmacol Ther. 99:261–282. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goldman IL, Georgieva SG, Gurskiy YG,
Krasnov CA, Deykin CA, Popov AN, Ermolkevich TG, Budzevich AI,
Chernousov AD and Sadchikova ER: Production of human lactoferrin in
animal milk. Biochem Cell Biol. 90:513–519. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pollock DP, Kutzko JP, Birck-Wilson E,
Williams JL, Echelard Y and Meade HM: Transgenic milk as a method
for the production of recombinant antibodies. J Immunol Methods.
231:147–157. 1999. View Article : Google Scholar
|
|
30
|
Zhou Q, Kyazike J, Echelard Y, Meade HM,
Higgins E, Cole ES and Edmunds T: Effect of genetic background on
glycosylation heterogeneity in human antithrombin produced in the
mammary gland of transgenic goats. J Biotechnol. 117:57–72. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Granelli-Piperno A and Reich E: A study of
proteases and protease-inhibitor complexes in biological fluids. J
Exp Med. 148:223–234. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bayne K: Developing guidelines on the care
and use of animals. Ann N Y Acad Sci. 862:105–110. 1998. View Article : Google Scholar
|
|
33
|
Denman J, Hayes M, O’Day C, Edmunds T,
Bartlett C, Hirani S, Ebert KM, Gordon K and McPherson JM:
Transgenic expression of a variant of human tissue-type plasminogen
activator in goat milk: Purification and characterization of the
recombinant enzyme. Biotechnology. 9:839–843. 1991.PubMed/NCBI
|
|
34
|
Parker MH, Birck-Wilson E, Allard G,
Masiello N, Day M, Murphy KP, Paragas V, Silver S and Moody MD:
Purification and characterization of a recombinant vers ion of
human A-feto protein expressed in the milk of transgenic goats.
Protein Expr Purif. 38:177–183. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim HJ: Glycosylation variant analysis of
recombinant human tissue plasminogen activator produced in
urea-cycle-enzyme-expressing Chinese hamster ovary (CHO) cell line.
J Biosci Bioeng. 102:447–451. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Farid SS: Process economics of industrial
monoclonal antibody manufacture. J Chromatogr B Analyt Technol
Biomed Life Sci. 848:8–18. 2007. View Article : Google Scholar
|
|
37
|
Barrett AJ, Rawlings N and Woessner J:
E-STREAMS: Electronic Reviews of Science and Technology. Handbook
of proteolytic enzymes. 2nd edition. Elsevier; Amsterdam: pp.
2946–2952. 2004
|
|
38
|
Kwon JY, Yang YS, Cheon SH, Nam HJ, Jin GH
and Kim DI: Bioreactor engineering using disposable technology for
enhanced production of hCTLA4Ig intransgenic rice cell cultures.
Biotechnol Bioeng. 110:2412–2424. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rader RA: Expression systems for process
and product improvement. BioProcess Int. 66(Suppl 4): pp. S4–S9.
2008, http://www.bioprocessintl.com/wp-content/uploads/2014/05/BPI_A_080606SUPAR01__78704a.pdf.
|
|
40
|
Ryll T: Antibody production using
mammalian cell culture-how high can we push productivity. SIM
annual meeting program & abstract San Diego: pp. S1462008
|
|
41
|
Luo Y, Wang Y, Liu J, Lan H, Shao M, Yu Y,
Quan F and Zhang Y: Production of transgenic cattle highly
expressing human serum albumin in milk by phiC31 integrase-mediated
gene delivery. Transgenic Res. 24:875–883. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou Y, Lin Y, Wu X, Xiong F, Lv Y, Zheng
T, Huang P and Chen H: The high-level expression of human tissue
plasminogen activator in the milk of transgenic mice with hybrid
gene locus strategy. Mol Biotechnol. 50:137–144. 2012. View Article : Google Scholar
|
|
43
|
Fujiwara Y, Miwa M, Takahashi R,
Hirabayashi M, Suzuki T and Ueda M: Position-independent and
high-level expression of human alpha-lactalbumin in the milk of
transgenic rats carrying a 210-kb YAC DNA. Mol Reprod Dev.
47:157–163. 1997. View Article : Google Scholar : PubMed/NCBI
|