Introduction
Intracerebral hemorrhage (ICH) is the most severe
stroke subtype, and is a leading cause of morbidity and mortality
(1). ICH accounts for 10–15% of
all strokes in Europe, USA and Australia, and 20–30% of all strokes
in Asia; ~2 million cases of ICH occur annually worldwide (2). Intestinal injury, particularly upper
gastrointestinal (GI) bleeding, is a potential complication in
patients with ICH, which leads to malnutrition, impaired immunity,
longer periods of hospitalization, dependence, poor outcome and
even mortality (3–7). Furthermore, compared with other
common complications that occur following ICH, including pneumonia,
seizures and fever, intestinal injury has received much less
attention, and the associated pathophysiological alterations
require further discussion and evaluation.
It is generally accepted that the intestine is the
core organ of posttraumatic stress, and the initiating organ of
multiple organ dysfunction in the development of severe
complications under critically stressful events, including burns
(8), heat stroke (9), trauma (10), brain events (11,12) and transplantation (13). These stressful events may initiate
a cascade of intestinal events, including destruction of the gut
mucosa, barrier dysfunction, translocation of intestinal bacteria
and endotoxin, and upper GI bleeding, which may lead to systemic
inflammatory response syndrome (SIRS) and multiple organ
dysfunction syndrome (MODS) (14). ICH is a severe type of
pathological stress and a critical brain event, which may induce
numerous intestinal events, as aforementioned. Following ICH, not
only the general stress state, but also central nervous system
damage-induced neural, humoral and endocrine system disorders may
disrupt intestinal integrity and function. However, the underlying
molecular mechanism remains to be elucidated.
To the best of our knowledge, no previous study has
focused on intestinal aspects following ICH in vivo. Due to
the important role of intestinal function in the prognosis of
patients with ICH, the development of effective therapeutic
strategies for the treatment of intestinal complications is
required. However, the pathophysiological mechanisms of ICH-induced
intestinal complications are currently undefined. The present study
aimed to investigate the sequentially pathological alterations and
molecular mechanisms underlying ICH-induced intestinal injury.
Materials and methods
Animals and experimental groups
The present study was approved by the Laboratory
Animal Ethics Committee of Rui Jin Hospital (Shanghai, China).
Animal experiments were conducted in accordance with the US
National Institutes of Health Guidelines (15) and followed the guidelines of the
National Animal Protection of China. Male C57BL/6 mice (age, 6–8
weeks) were purchased from the Experimental Animal Center of the
Chinese Academy of Sciences (Shanghai, China). Mice were maintained
under specific pathogen-free conditions at a constant room
temperature (18–22°C) and humidity (40–70%), under a 12-h
light/dark cycle. Sterilized food and water were provided ad
libitum. To evaluate the intestinal damage following ICH, 42
mice were randomly divided into the following seven groups (n=6
mice/group): Control group and six ICH groups (2, 6, 12 and 24 h,
and 3 and 7 days). All efforts were made to minimize suffering and
to reduce the number of mice used.
ICH mouse model
Mice were anesthetized with a single intraperitoneal
dose of 100 mg/kg ketamine/10 mg/kg xylazine (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany). Mice were positioned in a stereotactic
frame (RWD Life Science Co., Ltd., Shenzhen, China) and ICH was
induced by autologous blood infusion. A 1 mm burr hole was drilled
2.5 mm lateral to the midline and 0.2 mm anterior to the bregma.
Autologous blood (25 µl) was collected from a cut in the
tail using a Hamilton syringe, which was flushed with heparin prior
to blood collection. A needle was advanced 3.0 mm into the right
striatum. A total of 25 µl autologous blood was injected via
a double injection technique using a microinfusion pump (World
Precision Instruments, Sarasota, FL, USA). An initial amount of 5
µl was delivered at a rate of 1.5 µl/min. Following a
10 min interval without injection, the remaining 20 µl was
delivered at the same rate (1.5 µl/min). The needle was left
in place for a further 10 min to minimize blood backflow. Following
withdrawal of the needle, the scalp was sutured. In the sham
control mice, only the needle was inserted. After the operation,
mice were returned back to their cages.
Small intestinal motility
Mice in each group were sacrificed at the
corresponding time point. A total of 30 min before sacrifice,
charcoal meal (0.1 ml; 5% activated charcoal suspended in 10%
aqueous gum arabic) was injected into the stomach through a gastric
tube. After laparotomy, the entire small intestine was harvested.
The distance traveled by the charcoal meal from the pylorus to the
furthest point of small intestine was recorded. The intestinal
impelling force was measured according to the following formula:
Impelling force (%) = distance traveled by charcoal meal/total
length of the small intestine × 100.
Histopathology
After fixation in 10% formalin for 24 h at room
temperature, ileal segments from each group were embedded in
paraffin. Subsequently, the samples were cut into 4 µm
sections and were mounted onto slides. For histopathological
examination, the sections were stained with hematoxylin for 10 min
and eosin for 5 min at room temperature. Intestinal morphological
alterations were detected by an observer blinded to the
experimental group using an inverted microscope (Leica Microsystems
GmbH, Wetzlar, Germany). As described by Chiu et al
(16) the histological scoring
system was used to quantify the degree of intestinal damage
following ICH.
Plasma endotoxin
All blood samples were carefully obtained from the
hearts of the mice prior to sacrifice and were centrifuged at 6,000
× g for 5 min at 4°C. Endotoxin content in the plasma samples was
assessed using the limulus amebocyte lysate kit (TAL, Xiamen,
China) with a kinetic modification according to the manufacturer’s
protocol. Endotoxin concentrations were expressed as EU/ml.
Immunohistological staining
Segments of the terminal ileum were fixed in 10%
neutral formalin for 24 h at room temperature, and 4 µm
sections were cut from each paraffin-embedded block. The sections
were dewaxed in xylene and rehydrated, after which antigen
retrieval was conducted. Briefly, the sections were immersed in
citrate buffer and heated using a microwave oven for 15 min at
92–98°C. Subsequently, the sections were incubated in 3%
H2O2 in PBS for 10 min, and blocked in PBS
containing 3% normal goat serum (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), 0.3% (w/v) Triton X-100 and 0.1% bovine serum
albumin (Sigma-Aldrich; Merck KGaA) at room temperature for 1 h.
The sections were then incubated with a primary antibody against
intercellular adhesion molecule 1 (ICAM-1; 1:100 dilution; cat. no.
ab171123; Abcam, Cambridge, UK) at 4°C overnight. Subsequently,
sections were developed with the ABC kit and detected with
diaminobenzidine (both Vector Laboratories, Inc., Burlingame, CA,
USA). The sections were then counterstained with hematoxylin,
dehydrated and mounted. Histopathological alterations were observed
using an inverted microscope (Leica Microsystems GmbH) following a
blinded procedure. Images were analyzed by Image-Pro Plus version
6.0 (Media Cybernetics, Inc., Rockville, MD, USA). Staining
intensity was evaluated as follows: ‘0’ indicates no detectable
positive staining; ‘1’ indicates very low density of positive
staining; ‘2’ indicates a moderate density of positive staining;
‘3’ indicates higher, but not maximal, density of positive
staining; ‘4’ indicates the highest density of positive
staining.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from intestinal samples
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer’s protocol. RNA quantity was
determined according to spectrophotometric analysis (optical
density260/280). RT was performed using a PrimeScript RT
reagent kit (Takara Bio, Inc., Otsu, Japan). The RT temperature
protocol parameters were as follows: 37°C for 15 min and 85°C for
15 sec. Forward and reverse oligonucleotide primers (Table I) were designed to amplify target
genes, and were synthesized by Sangon Biotech Co., Ltd. (Shanghai,
China). qPCR amplification was performed on an ABI 7500 PCR
instrument (Applied Biosystems; Thermo Fisher Scientific, Inc.)
using SYBR Green (Takara Bio, Inc.) according to the manufacturer’s
protocol. PCR thermocycling conditions were as follows: 1 cycle of
predenaturation at 95°C for 30 sec, followed by 40 cycles of
denaturation at 95°C for 5 sec, annealing at 60°C for 30 sec and
extension at 72°C for 30 sec, and a final step at 72°C for 10 min.
β-actin mRNA was quantified as an endogenous control to calculate
ΔCq using SDS software version 2.4.1 (Applied Biosystems; Thermo
Fisher Scientific, Inc.). Results were calculated by using the
2−ΔΔCq method (17).
 | Table IPolymerase chain reaction primer
sequences. |
Table I
Polymerase chain reaction primer
sequences.
| Target gene | Sense primer (5′ to
3′) | Antisense primer
(5′ to 3′) |
|---|
| IL-1β |
AACCTGCTGGTGTGTGACGTTC |
CAGCACGAGGCTTTTTTGTTGT |
| IL-6 |
ACAACCACGGCCTTCCCTACTT |
CACGATTTCCCAGAGAACATGTG |
| TNF-α |
CAGGCGGTGCCTATGTCTC |
CGATCACCCCGAAGTTCAGTAG |
| ICAM-1 |
CCTGATGGGCAGTCAACAGCTA |
ACAGCTGGCTCCCGTTTCA |
| MCP-1 |
CCACAGCATGGACGAATTCA |
AGCTTGCTTTGTGGCCTTCA |
| CCL-5 |
TCGTGCCCACGTCAAGGAGTATTT |
TCTTCTCTGGGTTGGCACACACTT |
| Nrf2 |
CAGTCTTCACCACCCCTGAT |
CTAATGGCAGCAGAGGAAGG |
| MnSOD |
CGTCATTCACTTCGAGCAGA |
CACCTTTGCCCAAGTCATCT |
| HO-1 |
ATGACACCAAGGACCAGAGC |
GTGTAAGGACCCATCGGAGA |
| β-actin |
GTGACGTTGACATCCGTAAAGA |
GCCGGACTCATCGTACTCC |
Western blot analysis
Total protein was extracted from intestinal samples
in each group using radioimmunoprecipitation assay lysis buffer
(EMD Millipore, Bedford, MA, USA) for 30 min. Following
centrifugation at 12,000 × g for 10 min at 4°C, the supernatant was
collected. Protein concentrations were quantified using an enhanced
bicinchoninic acid (BCA) protein assay kit (Thermo Fisher
Scientific, Inc.). All samples were diluted in loading buffer
(Nanjing Sunshine Biotechnology Co., Ltd., Nanjing, China) and
boiled at 95°C for 5 min. Total protein (20 µg) was
separated by 10% SDS-PAGE and the fractionated proteins were
electrotransferred to a polyvinylidene fluoride membrane. The
membrane was blocked with 5% skim milk for 1 h at room temperature
and probed with the following primary antibodies: Anti-nuclear
factor (erythroid-derived 2)-like 2 (Nrf2; 1:1,000 dilution; cat.
no. ab137550), anti-manganese super-oxide dismutase (MnSOD; 1:5,000
dilution; cat. no. ab13533), anti-heme oxygenase (HO)-1 (1:2,000
dilution; cat. no. ab189491) and anti-β-actin (1:10,000 dilution;
cat. no. ab3280) (all Abcam) at 4°C overnight. Subsequently, the
membrane was incubated with goat anti-rabbit (cat. no. HA1001-100)
or goat anti-mouse (cat. no. HA1006) horseradish
peroxidase-conjugated secondary antibodies (1:5,000; Hangzhou
Hua’an Medical & Health Instruments Co., Ltd., Hangzhou, China)
for 1 h at room temperature. Immunoreactive bands were detected
using an enhanced chemiluminescence reagent (Pierce; Thermo Fisher
Scientific, Inc.) and were visualized using Gel-Pro Analyzer gel
image analysis software version 6.3 (Media Cybernetics, Inc.).
Myeloperoxidase (MPO) activity
The intestinal tissue homogenate was extracted from
each group and the protein concentration of each supernatant was
assessed as aforementioned. Tissue protein concentrations from
different groups were measured using an enhanced BCA protein assay
kit (Thermo Fisher Scientific, Inc.). MPO activity in tissue
homogenates was analyzed using a detection kit according to the
manufacturer’s protocol (cat. no. A044; Nanjing Jiancheng
Bioengineering Institute, Nanjing, China). MPO activity was
expressed as U/mg tissue, and 1 unit of MPO activity represents the
amount of enzyme degrading 1 µmol
H2O2/min at 25°C.
ELISA analysis
Blood samples were collected from the hearts of mice
prior to sacrifice and were centrifuged at 6,000 × g for 5 min at
4°C. Serum levels of interleukin (IL)-1β were quantified using an
ELISA kit (cat. no. ab197742; Abcam) according to the
manufacturer’s protocol. Absorbance was measured at 450 nm, and
concentration of the target protein was determined according to the
standard curve. Protein levels were expressed as pg/mg protein.
Malondialdehyde (MDA) content and
superoxide dismutase (SOD) activity
Intestinal tissue levels of oxidative stress (OS)
were indirectly assessed using MDA content and SOD activity assays.
Intestine samples from each group were homogenized in cold saline,
with a weight-to-volume ratio of 1:9, and the homogenate was
centrifuged at 12,000 × g for 10 min at 4°C. The protein
concentration in the supernatant was determined using the enhanced
bicinchoninic acid protein assay kit (Thermo Fisher Scientific,
Inc.). Measurements of MDA content and SOD activity in tissue
homogenates were determined using detection kits (cat. nos. A003-1
and A001-1-1; Nanjing Jiancheng Biological Engineering Institute)
according to the manufacturer’s protocol.
Statistical analyses
Statistical analysis was performed using SPSS 16.0
(SPSS, Inc., Chicago, IL, USA). Data were analyzed by one-way
analysis of variance followed by post hoc Tukey’s test. All data
are representative of at least three independent experiments.
Quantitative data are presented as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Histopathological alterations in the
intestinal mucosa
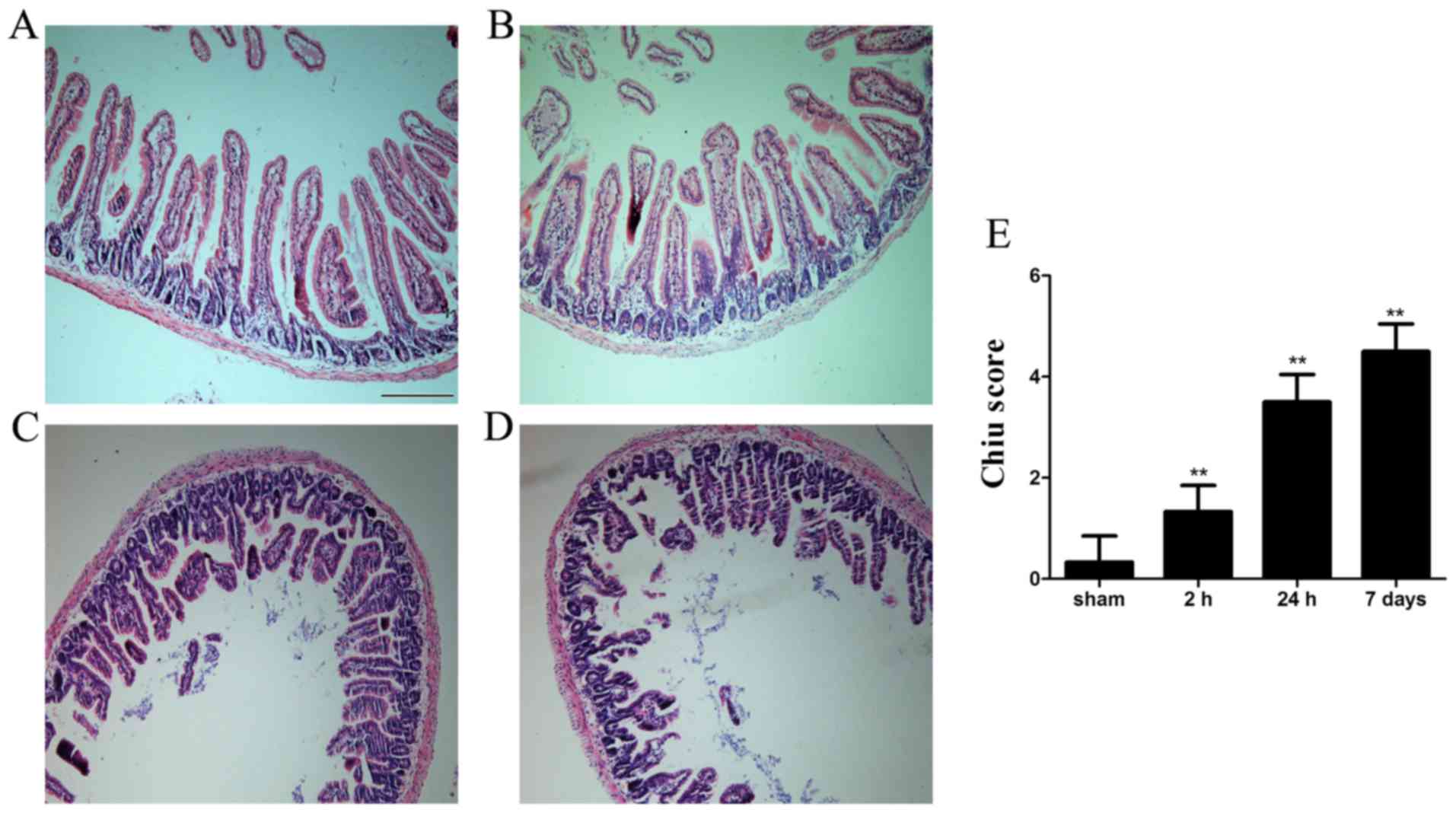
Histopa thological examination indicated that
morphology of the intestinal mucosa was intact in the sham group.
Conversely, mucosal damage was detected as early as 2 h after ICH;
shed ding of epithelial cells from the top of villi, intestinal
villi thickening and decurtation were detected. Furthermore, fusion
of adjacent villi, inflammatory cell infiltration, exposed lamina
propria and damaged villus tip were detected at 24 h and the
histopathological alterations persisted for 7 days (Fig. 1).
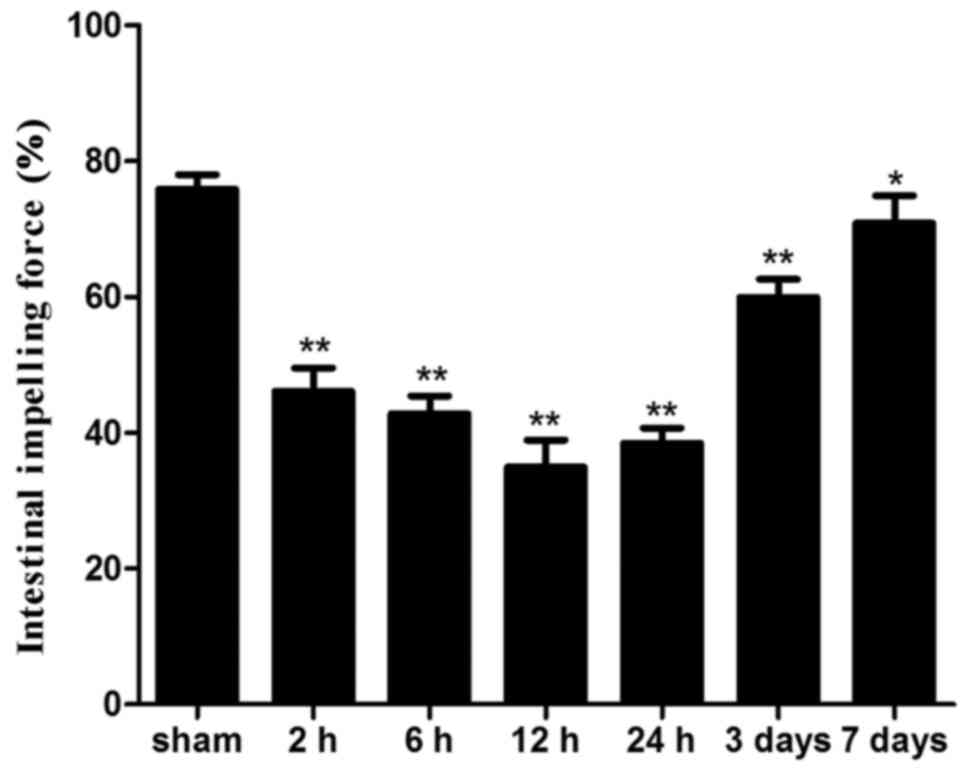
Small intestinal motility
Intestinal impelling force was significantly lower
in all ICH groups compared with in the sham group (P<0.05). The
lowest level was detected 12 h after ICH and the impelling force
remained significantly reduced until day 7 following ICH (Fig. 2).
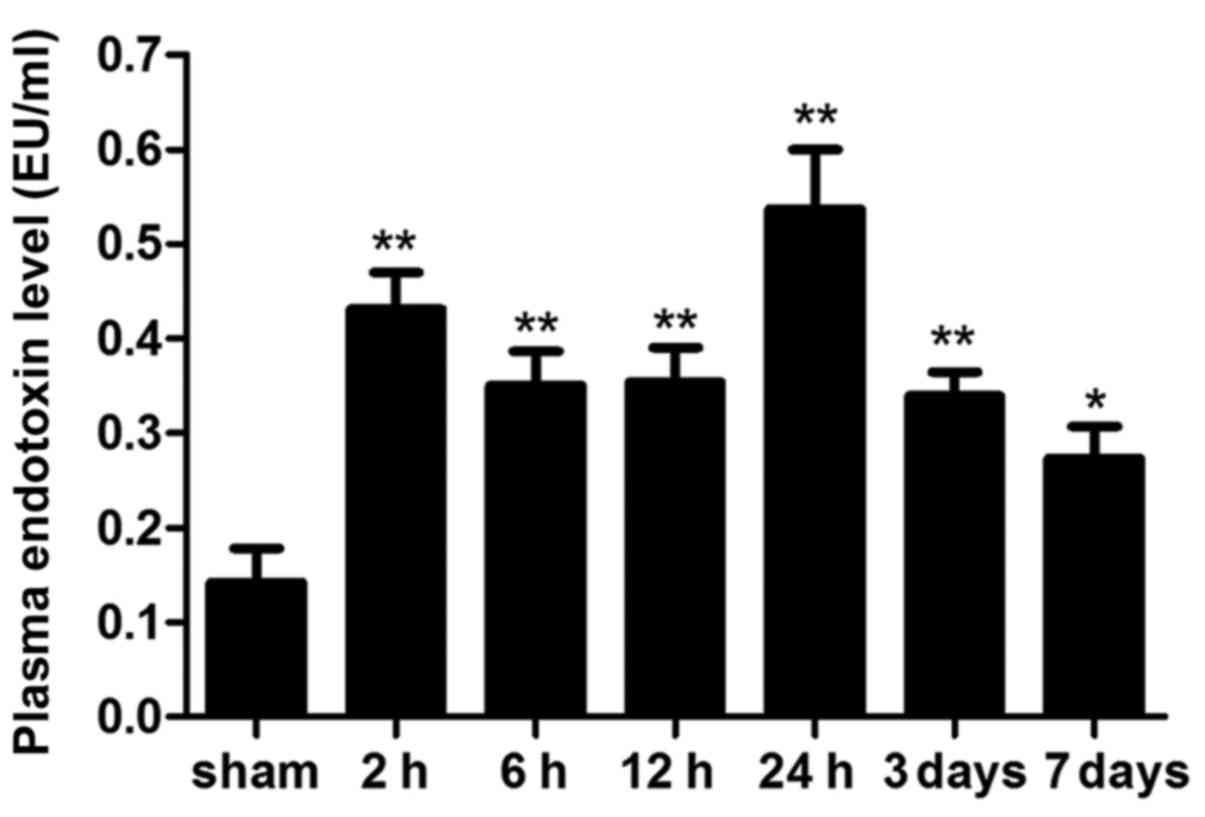
Intestinal barrier dysfunction
Plasma levels of endotoxin are considered the
hallmark of intestinal permeability and barrier function. Plasma
endotoxin levels were markedly increased 2 h following ICH, and the
levels peaked at 24 h. Notably, plasma endotoxin levels declined on
day 7; however, they were still significantly higher compared with
in the sham group (Fig. 3;
P<0.05).
mRNA expression levels of inflammatory
cytokines
The mRNA profile of intestinal inflammatory
cytokines was significantly altered post-ICH. IL-1β, IL-6 and tumor
necrosis factor (TNF)-α are important proinflammatory cytokines,
which are considered molecular markers of inflammatory responses
(18,19). The mRNA expression levels of IL-6
and TNF-α were significantly elevated after ICH at each time-point
(P<0.05). However, the mRNA expression levels of IL-1β were not
significantly increased 2 h and 7 days post-ICH (P>0.05). ICAM-1
is a member of the immunoglobulin superfamily, which may initiate
adhesion and infiltration of leukocytes into the lesion site,
leading to damage. In the present study, ICAM-1 expression was
elevated as early as 2 h post-ICH and was increased for 7 days
(P<0.05). Chemokines, including monocyte chemotactic protein
(MCP)-1 and chemokine (C-C motif) ligand (CCL)-5, participate in
the inflammatory process by promoting the infiltration and
chemotactic activation of inflammatory cells in lesions. In the
present study, MCP-1 and CCL-5 in the ICH groups were also
significantly elevated compared with in the sham group (P<0.05);
however, CCL-5 mRNA expression was not significantly increased on
day 7 post-ICH (Fig. 4). These
results indicated that ICH may induce rapid and persistent
upregulation of inflammatory cytokines in the intestine.
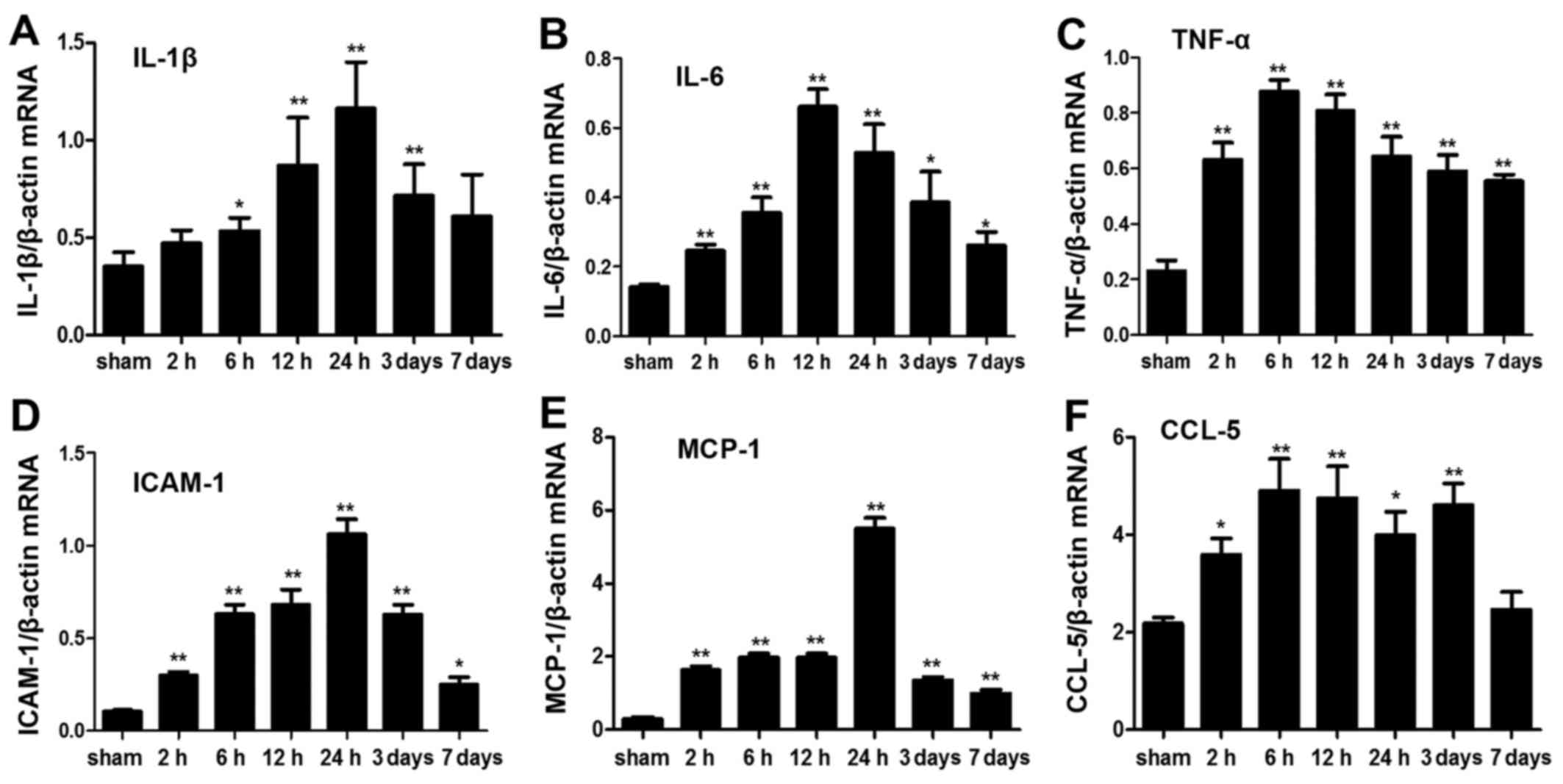 | Figure 4Analysis of inflammatory cytokine
expression in intestinal tissue following ICH. (A) IL-1β, (B) IL-6,
(C) TNF-α, (D) ICAM-1, (E) MCP-1 and (F) CCL-5 mRNA expression was
detected in the sham group, and in the ICH group at various
time-points, by quantitative polymerase chain reaction. β-actin
served as a loading control. Data are representative of three
independent experiments. *P<0.05,
**P<0.01 vs. the sham group. CCL-5, chemokine (C-C
motif) ligand-5; ICAM-1, intercellular adhesion molecule 1; ICH,
intracerebral hemorrhage; IL, interleukin; MCP-1, monocyte
chemotactic protein 1; TNF-α, tumor necrosis factor-α. |
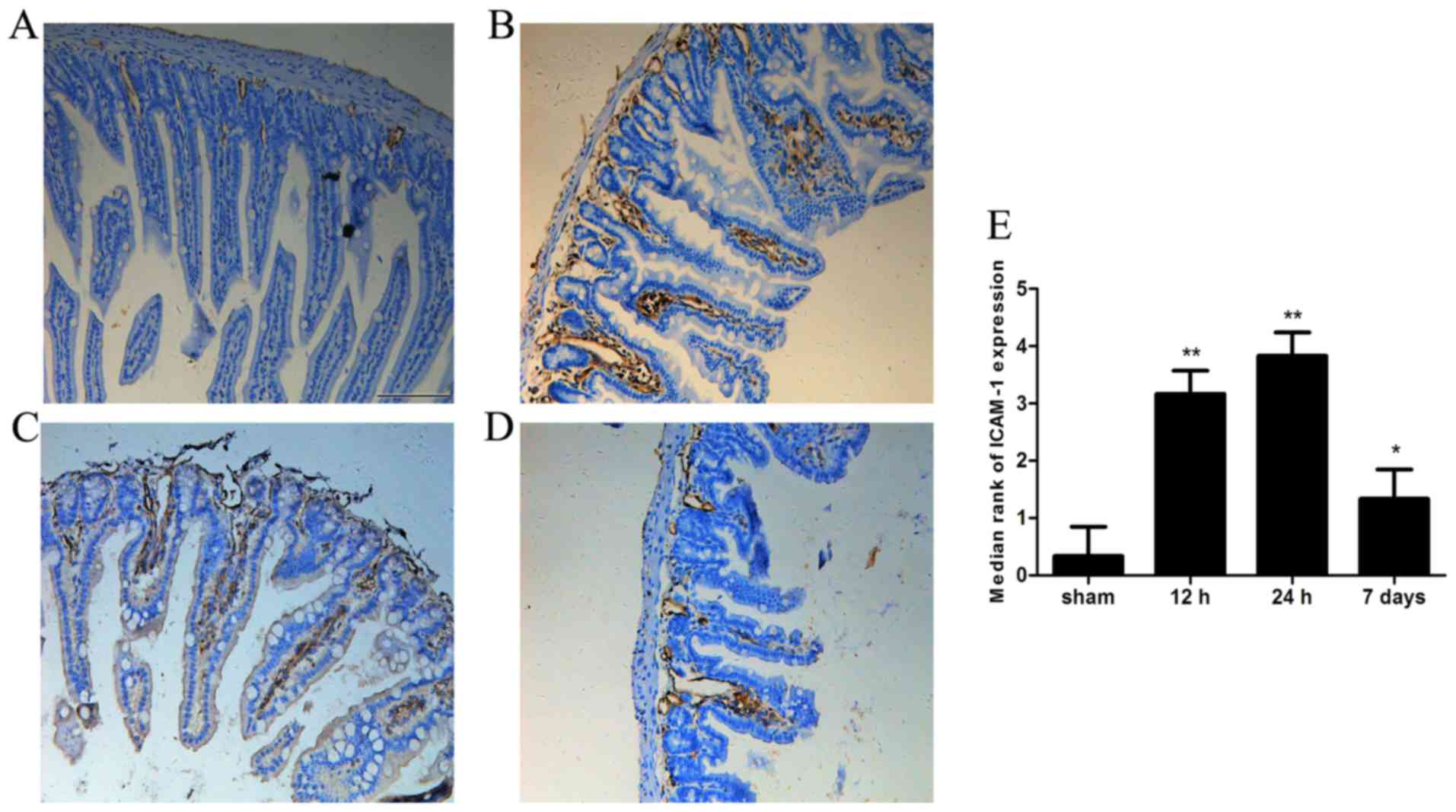
Expression of ICAM-1 in intestinal
tissue
ICAM-1 immunoreactivity was almost undetectable in
the sham group. However, strong ICAM-1 immunoreactivity was
observed in the intestine 12 or 24 h after ICH (P<0.05).
Furthermore, ICAM-1 immunoreactivity remained increased on day 7
post-ICH compared with in the sham group (Fig. 5).
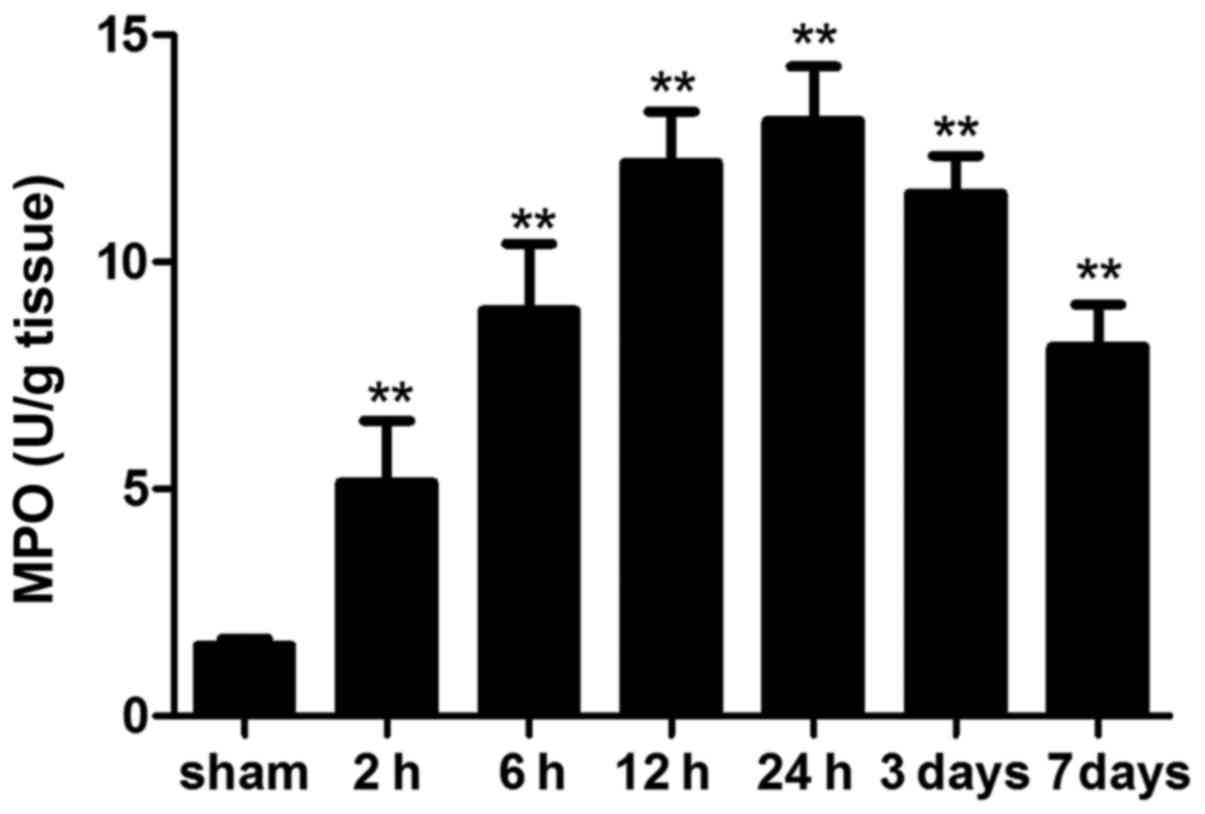
MPO activity
MPO is an enzyme that is abundantly stored in
azurophilic granules of neutrophils. MPO activity is a prognostic
hallmark of inflammatory response in various acute and chronic
inflammatory conditions (20,21). In the present study, MPO activity
was significantly increased in ICH mice compared with in the sham
mice at each time-point. In addition, MPO activity on day 7 was
still higher than that in the sham group (Fig. 6).
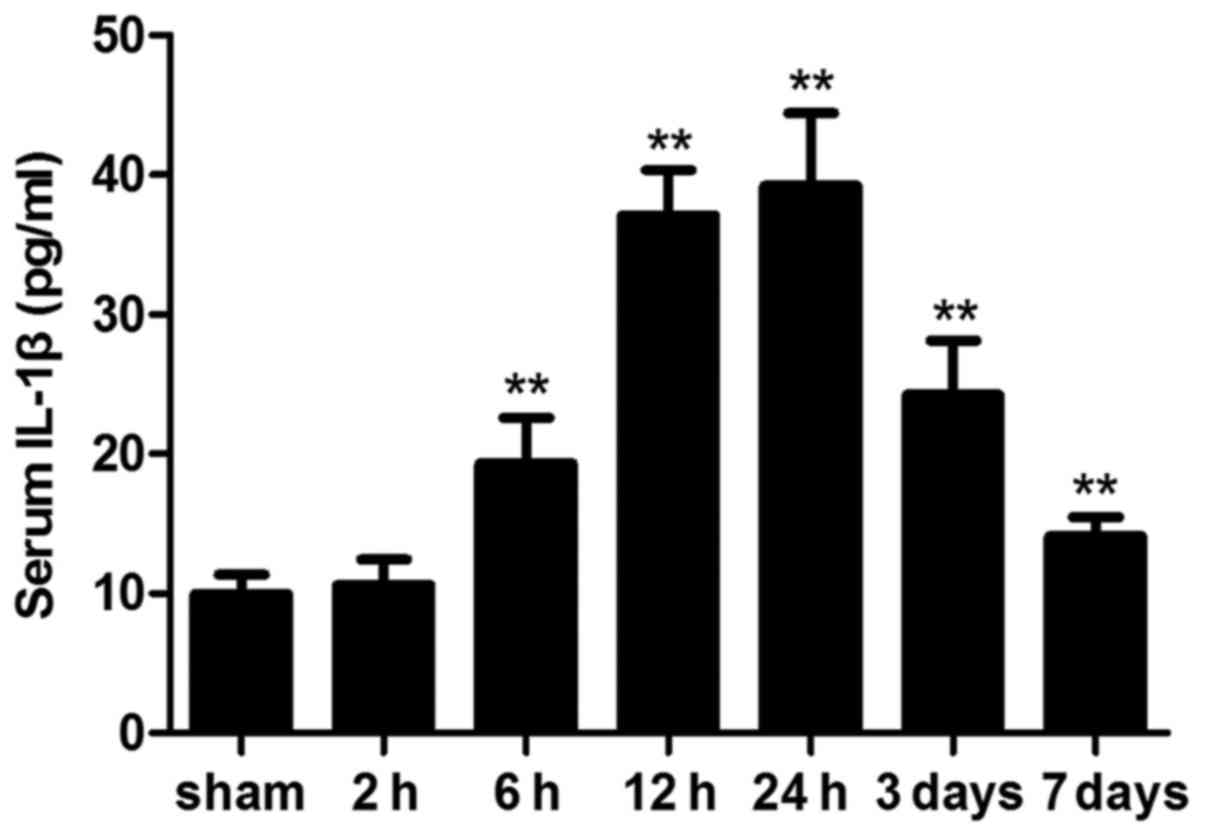
Circulating IL-1β levels
ICH significantly altered the circulating levels of
the cytokine, IL-1β. Following ICH, serum IL-1β levels were
significantly elevated compared with in the sham group; however,
this difference was not statistically significant at 2 h
(P>0.05). On day 7 post-ICH, serum IL-1β levels were still
significantly elevated compared with in the sham mice (Fig. 7).
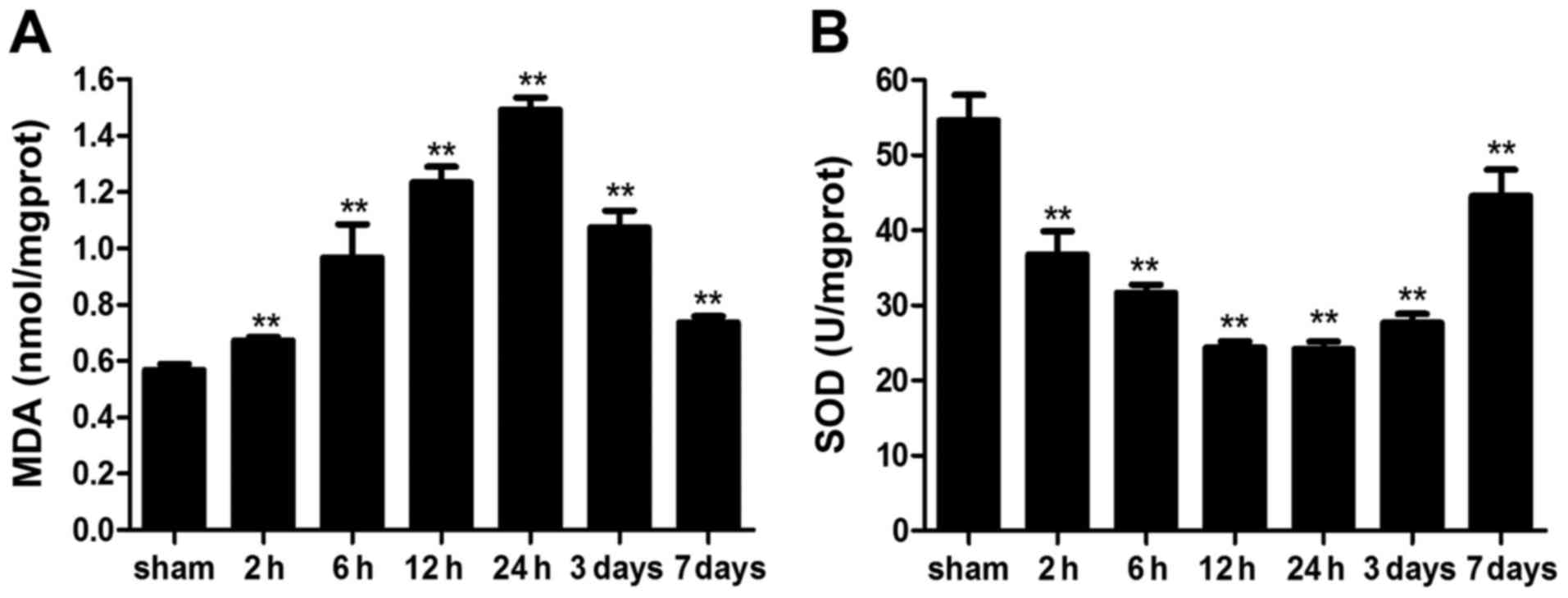
Intestinal reactive oxygen species (ROS)
levels
To analyze whether ICH induces intestinal OS in
mice, ROS levels were measured indirectly, by determining MDA
content and SOD activity. MDA levels were significantly elevated
following ICH; elevation was detected as early as 2 h post-ICH and
remained higher on day 7 compared with in the sham group.
Conversely, SOD activity was significantly reduced by ICH (Fig. 8). These results indicated that ICH
may markedly increase intestinal ROS levels in mice.
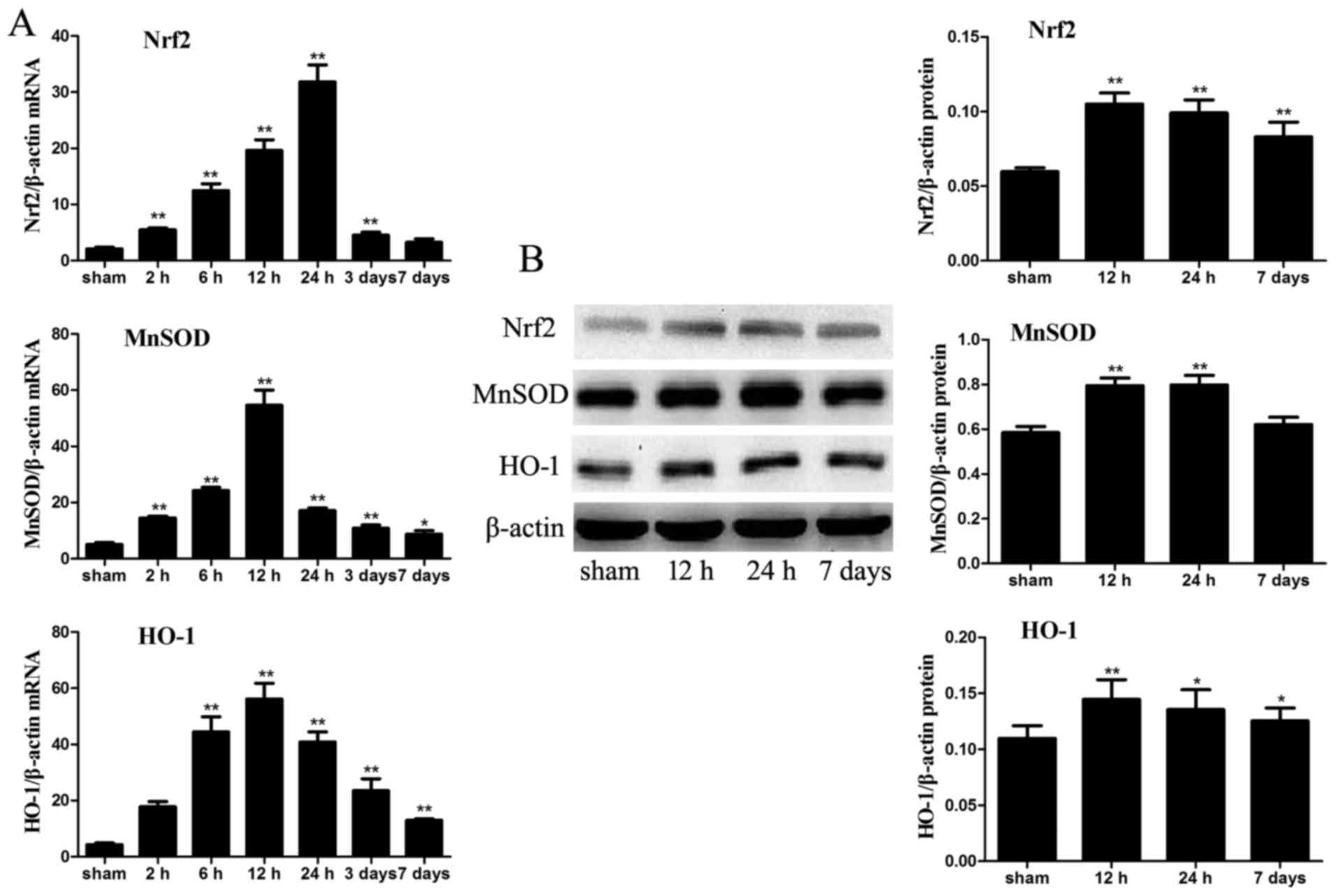
mRNA and protein expression levels of
antioxidants
The mRNA expression levels of the antioxidant
enzymes, Nrf2, MnSOD and HO-1, were significantly increased in the
intestinal samples from ICH mice compared with in the sham group,
particularly at 12 and 24 h post-ICH. To analyze the protein levels
of these antioxidant enzymes in the intestine, the protein
expression levels of Nrf2, MnSOD and HO-1 were detected in the
intestinal tissue of the various groups by western blot analysis.
The protein expression levels of Nrf2, MnSOD and HO-1 were also
markedly increased in the ICH mice compared with in the sham mice.
Nrf2 and HO-1, but not MnSOD, levels remained higher in the ICH
group at day 7 compared with in the sham group (P<0.05; Fig. 9).
Discussion
Compared with intestinal injury following ischemic
stroke or traumatic brain injury (TBI), few studies have focused on
intestinal injury post-ICH, particularly in experimental research.
Clinically, Misra et al (4) reported that the frequency of GI
bleeding post-ICH in India was 30%, and Yang et al (5) revealed that the prevalence of GI
bleeding after ICH was 26.7% in a retrospective review of 808 cases
in China. Furthermore, Yang et al (5) indicated that patients with GI
bleeding had significantly longer hospital stays and increased
in-hospital mortality compared with patients without GI bleeding.
However, the pathophysiological intestinal alterations following
ICH and the molecular mechanism involved in triggering the
inflammatory response and OS are unclear. On the basis of
experiments in mice, the present study systematically indicated
that ICH could induce significant intestinal damage, which occurred
as early as 2 h post-ICH and lasted for 7 days. In the present
study, ICH induced significant gut mucosal damage, barrier
dysfunction, delayed small intestinal motility, inflammatory
responses characterized by leukocyte infiltration into the
intestinal tissue, elevated levels of inflammatory cytokines in
intestinal tissue and serum, and OS characterized by indirect ROS
levels and antioxidant gene levels.
Stressful events can induce alterations in gut
mucosa, barrier function and small intestinal motility. To the best
of our knowledge, no previous studies regarding intestinal mucosa
structure and barrier function post-ICH have been conducted. The
present study demonstrated that damage to the intestinal mucosa
occurred rapidly, as early as 2 h post-ICH, and persisted for 7
days. The observed morphological alterations in the gut mucosa
included intestinal villi engorgement, thickening, decurtation,
fusion of adjacent villi, inflammatory cell infiltration, exposed
lamina propria and damaged villus tip. Recovery of small intestinal
motility is considered a vital hallmark of clinical outcome
following ICH. In a previous study, gastrointestinal motility
alterations were investigated in a model of middle cerebral artery
occlusion (MCAO)-induced intestinal injury; decreased small
intestinal motility was confirmed within 48 h of MCAO (12). In the present study, the
intestinal impelling ratio was significantly lower in the ICH
groups compared with in the sham group. The results indicated that
ICH may induce weakening in the tension of the lower esophageal
sphincter, descent of gastric contraction and emptying function,
and depression of gastrointestinal motility. In addition,
gastrointestinal motility, both 2 h and 7 days post-ICH, was
significantly lower than in the sham group, thus suggesting that
intestinal dyskinesis occurs in the early stage of ICH and persists
for a long period.
Impaired gut barrier function can increase
intestinal permeability and translocation of endotoxin. The present
study detected significantly increased plasma levels of endotoxin,
with peaks detected at 2 and 12 h post-ICH. Hang et al
(11) hypothesized the reason for
this phenomenon in a TBI-induced gastrointestinal dysfunction
study; it was suggested that the first peak may be due to excited
sympathetic nerve-induced acute gut mucosal damage. Subsequently,
the plasma levels of endotoxin may slightly decline due to recovery
of liver antitoxic function and lipopolysaccharide antibodies.
Finally, the second peak may be associated with severe mucosal
damage, which usually occurs at later stages following a brain
event. In addition, previous studies (22,23) have reported that endotoxin may not
only ultimately induce breakdown of the intestinal mucosal barrier,
potentially resulting in lethal SIRS and MODS, but may also lead to
inflammatory cell infiltration in the brain and breakdown of the
blood-brain barrier via inducing production and release of
proinflammatory cytokines by tissue macrophages and circulating
monocytes.
To understand the molecular mechanism underlying
ICH-induced intestinal injury, the present study focused on two
potential mechanisms. Firstly, the inflammatory response was
analyzed by detecting the expression levels of IL-1β, IL-6, TNF-α,
ICAM-1, MCP-1 and CCL-5, MPO activity in intestinal tissue, and
serum levels of IL-1β. The gut is a core organ where the
inflammatory response occurs with the release of various
inflammatory cytokines under stressful conditions, which has a
potent impact on the small intestine. Notably, the inflammatory
response may not only affect the gut itself, but may also influence
the function and integrity of remote organs and tissues, including
the brain, thus inducing SIRS and MODS. The gut has been labeled
the ‘trigger’ of inflammatory responses under severe stress, which
are mainly mediated by various inflammatory cytokines. Previous
studies (24,25) indicated that TBI could induce a
rapid and persistent inflammatory response, which was associated
with the upregulation of proinflammatory cyto-kines in the gut,
including nuclear factor-κB, TNF-α, IL-6 and ICAM-1. Consistent
with these findings, a significant increase in the mRNA and protein
expression levels of proinflammatory cytokines (IL-1β, IL-6, TNF-α,
ICAM-1, MCP-1 and CCL-5) was detected using RT-qPCR,
immunohistochemistry and ELISA at various time-points in the
intestine and serum of ICH mice in the present study. Furthermore,
detection of MPO activity confirmed that an inflammatory response
had been induced. The majority of these inflammatory cytokines
rapidly increased as early as 2 h post-ICH and persisted for 7
days. Notably, among these cytokines, IL-1β, IL-6 and TNF-α can
initiate the infiltration of inflammatory cells into the intestine
by activating ICAM-1 and other chemokines, including MCP-1 and
CCL-5, and vice versa (26).
Numerous studies have demonstrated that the release of inflammatory
cytokines induces a delay in small intestinal motility, and
intestinal obstruction (27–30). In addition, inflammatory cytokines
exert cytotoxic effects that induce damage to microvilli, resulting
in destruction of intercellular tight junctions and increased
intestinal barrier dysfunction (31,32). Taken together, the present study
suggested that ICH could significantly increase the inflammatory
response in the intestine through increased release of inflammatory
cytokines, MPO activity, and peripheral circulating concentrations
of IL-1β. Therefore, the intestinal inflammatory response may be
responsible for intestinal injury, and may serve an important role
in the pathogenesis of gut mucosal damage, small intestinal
motility and barrier dysfunction.
Secondly, the present study revealed that ICH
triggered a pathological intestinal imbalance between oxidant and
antioxidant systems. OS mediators, including ROS, which cause lipid
peroxidation, protein oxidation, inhibition of mitochondrial
activity, and increased membrane permeability of the cell and
lysosome, lead to cell swelling/rupture. As a product of lipid
peroxidation induced by ROS, MDA content can indirectly reflect the
degree of OS. Under normal conditions, ROS may be cleared by
antioxidants, including SOD. Redundant oxygen free radicals are
generated during periods of ischemia followed by reperfusion in the
ICH-induced intestine, leading to excessive MDA accumulation and
SOD consumption. In the present study, MDA levels were
significantly increased in the ICH group compared with in the sham
group. Conversely, SOD activity was markedly reduced. Nrf2, which
is a key endogenous regulator of cellular defense against OS, is a
positive regulator of the antioxidant response element that
modulates the expression of numerous genes and mobilizes the
internal antioxidant system, including antioxidant genes (MnSOD and
HO-1) (33,34). Consistent with the aforementioned
results regarding MDA and SOD, the mRNA and protein expression
levels of Nrf2, MnSOD and HO-1 were increased following ICH in the
intestine, further indicating that OS was activated and served a
critical role in ICH-induced intestinal damage. Notably, Jin et
al (35,36) reported that Nrf2-deficient mice
were more susceptible to TBI-induced acute intestinal mucosal
injury than wild-type Nrf2+/+ mice, as characterized by
decreased intestinal mRNA expression and antioxidant enzyme
activities. These findings indicated that an oxidant-antioxidant
imbalance participates in the pathogenesis of ICH-induced
intestinal injury.
It should be noted that OS is not a secondary event
that results from the inflammatory response. OS is now thought to
contribute to, rather than result from, the inflammatory response.
Previous studies have reported that antioxidant genes, particularly
the key upstream transcription factor Nrf2, serve a critical role
in counteracting inflammation during tissue injury in various
experimental models (35–38). These studies have indicated that
increased Nrf2 activity has an important protective role in
limiting tissue inflammatory response and injury. Notably, our
previous study demonstrated that Nrf2, and its downstream
antioxidant genes, were significantly increased in the ICH rat
brain, and activation of Nrf2 may represent a potential target for
treatment of brain injury following ICH (39). Therefore, it is hypothesized that
Nrf2 may be considered a novel therapeutic target for the
systematic treatment of not only ICH-induced brain damage but also
intestinal injury. Further studies using Nrf2 overexpression or
Nrf2 knockout mice are required to examine the exact role of Nrf2
in animal models of ICH-induced intestinal injury.
In conclusion, the results of the present study
systematically demonstrated that ICH may markedly induce gut
mucosal damage, barrier dysfunction, delayed small intestinal
motility, inflammatory response and OS in the intestine. To the
best of our knowledge, the present study is the first to elucidate
the pathophysiological intestinal alterations that occur following
ICH. Ultimately, based on a better understanding of the
physiological and pathological processes of ICH-induced intestinal
injury, strategies that attenuate the severe complication of
intestinal injury-induced SIRS and MODS following severe ICH will
be a vital therapeutic target for future ICH research.
Acknowledgments
The present study was supported by the Natural
Science Fund (grant nos. 14ZR1426000 and 16ZR14212000) from the
Science and Technology Commission of Shanghai Municipality.
Competing interests
The authors declare that they have no competing
interests to disclose.
References
|
1
|
Keep RF, Hua Y and Xi G: Intracerebral
haemorrhage: Mechanisms of injury and therapeutic targets. Lancet
Neurol. 11:720–731. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Adeoye O and Broderick JP: Advances in the
management of intracerebral hemorrhage. Nat Rev Neurol. 6:593–601.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu WY, Rhoney DH, Boling WB, Johnson JD
and Smith TC: A review of stress ulcer prophylaxis in the
neurosurgical intensive care unit. Neurosurgery. 41:416–425;
discussion 425–426. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Misra UK, Kalita J, Pandey S and Mandal
SK: Predictors of gastrointestinal bleeding in acute intracerebral
haemorrhage. J Neurol Sci. 208:25–29. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang TC, Li JG, Shi HM, Yu DM, Shan K, Li
LX, Dong XY and Ren TH: Gastrointestinal bleeding after
intracerebral hemorrhage: A retrospective review of 808 cases. Am J
Med Sci. 346:279–282. 2013. View Article : Google Scholar
|
|
6
|
Shinohara Y, Yanagihara T, Abe K,
Yoshimine T, Fujinaka T, Chuma T, Ochi F, Nagayama M, Ogawa A,
Suzuki N, et al: III. Intracerebral hemorrhage. J Stroke
Cerebrovasc Dis. 20(Suppl 4): S74–S99. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang WJ, Lu JJ, Wang YJ, Wang CX, Wang YL,
Hoff K, Yang ZH, Liu LP, Wang AX and Zhao XQ; China National Stroke
Registry (CNSR): Clinical characteristics, management, and
functional outcomes in Chinese patients within the first year after
intracerebral hemorrhage: Analysis from China National Stroke
Registry. CNS Neurosci Ther. 18:773–780. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li X, Hammer AM, Rendon JL and Choudhry
MA: Intestine immune homeostasis after alcohol and burn injury.
Shock. 43:540–548. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Phillips NA, Welc SS, Wallet SM, King MA
and Clanton TL: Protection of intestinal injury during heat stroke
in mice by interleukin-6 pretreatment. J Physiol. 593:739–753.
2015. View Article : Google Scholar :
|
|
10
|
Timmermans K, Sir Ö, Kox M, Vaneker M, de
Jong C, Gerretsen J, Edwards M, Scheffer GJ and Pickkers P:
Circulating iFABP Levels as a marker of intestinal damage in trauma
patients. Shock. 43:117–120. 2015. View Article : Google Scholar
|
|
11
|
Hang CH, Shi JX, Li JS, Wu W and Yin HX:
Alterations of intestinal mucosa structure and barrier function
following traumatic brain injury in rats. World J Gastroenterol.
9:2776–2781. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu X, Zhu Y and Chuai J: Changes in serum
ghrelin and small intestinal motility in rats with ischemic stroke.
Anat Rec (Hoboken). 295:307–312. 2012. View
Article : Google Scholar
|
|
13
|
Low G, Jaremko JL and Lomas DJ:
Extravascular complications following abdominal organ
transplantation. Clin Radiol. 70:898–908. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Doig CJ, Sutherland LR, Sandham JD, Fick
GH, Verhoef M and Meddings JB: Increased intestinal permeability is
associated with the development of multiple organ dysfunction
syndrome in critically ill ICU patients. Am J Respir Crit Care Med.
158:444–451. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
National Research Council: Guide for the
care and use of laboratory animals. 8th edition. National Academies
Press; Washington DC: pp. 1–246. 2011
|
|
16
|
Chiu CJ, McArdle AH, Brown R, Scott HJ and
Gurd FN: Intestinal mucosal lesion in low-flow states. I. A
morphological, hemodynamic, and metabolic reappraisal. Arch Surg.
101:478–483. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Molmenti EP, Ziambaras T and Perlmutter
DH: Evidence for an acute phase response in human intestinal
epithelial cells. J Biol Chem. 268:14116–14124. 1993.PubMed/NCBI
|
|
19
|
Liu XH, Yang YW, Dai HT, Cai SW, Chen RH
and Ye ZQ: Protective role of adiponectin in a rat model of
intestinal ischemia reperfusion injury. World J Gastroenterol.
21:13250–13258. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arndt H, Kubes P, Grisham MB, Gonzalez E
and Granger DN: Granulocyte turnover in the feline intestine.
Inflammation. 16:549–559. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nussbaum C, Klinke A, Adam M, Baldus S and
Sperandio M: Myeloperoxidase: A leukocyte-derived protagonist of
inflammation and cardiovascular disease. Antioxid Redox Signal.
18:692–713. 2013. View Article : Google Scholar
|
|
22
|
Xue B, Kasparek MS, Müller MH and Kreis
ME: Modulation of intestinal afferent nerve sensitivity to
inflammatory mediators following systemic endotoxin in mice.
Neurogastroenterol Motil. 27:550–558. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bohatschek M, Werner A and Raivich G:
Systemic LPS injection leads to granulocyte influx into normal and
injured brain: Effects of ICAM-1 deficiency. Exp Neurol.
172:137–152. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hang CH, Shi JX, Li JS, Li WQ and Wu W:
Expressions of intestinal NF-kappaB, TNF-α, and IL-6 following
traumatic brain injury in rats. J Surg Res. 123:188–193. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hang CH, Shi JX, Li JS, Li WQ and Yin HX:
Upregulation of intestinal nuclear factor kappa B and intercellular
adhesion molecule-1 following traumatic brain injury in rats. World
J Gastroenterol. 11:1149–1154. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hang CH, Shi JX, Tian J, Li JS, Wu W and
Yin HX: Effect of systemic LPS injection on cortical NF-kappaB
activity and inflammatory response following traumatic brain injury
in rats. Brain Res. 1026:23–32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kalff JC, Schraut WH, Billiar TR, Simmons
RL and Bauer AJ: Role of inducible nitric oxide synthase in
postoperative intestinal smooth muscle dysfunction in rodents.
Gastroenterology. 118:316–327. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wehner S, Vilz TO, Stoffels B and Kalff
JC: Immune mediators of postoperative ileus. Langenbecks Arch Surg.
397:591–601. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
The FO, de Jonge WJ, Bennink RJ, van den
Wijngaard RM and Boeckxstaens GE: The ICAM-1 antisense
oligonucleotide ISIS-3082 prevents the development of postoperative
ileus in mice. Br J Pharmacol. 146:252–258. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wehner S, Schwarz NT, Hundsdoerfer R,
Hierholzer C, Tweardy DJ, Billiar TR, Bauer AJ and Kalff JC:
Induction of IL-6 within the rodent intestinal muscularis after
intestinal surgical stress. Surgery. 137:436–446. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Marchiando AM, Shen L, Graham WV, Weber
CR, Schwarz BT, Austin JR II, Raleigh DR, Guan Y, Watson AJ,
Montrose MH, et al: Caveolin-1-dependent occludin endocytosis is
required for TNF-induced tight junction regulation in vivo. J Cell
Biol. 189:111–126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Turner JR: ‘Putting the squeeze’ on the
tight junction: Understanding cytoskeletal regulation. Semin Cell
Dev Biol. 11:301–308. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Giudice A, Arra C and Turco MC: Review of
molecular mechanisms involved in the activation of the Nrf2-ARE
signaling pathway by chemopreventive agents. Methods Mol Biol.
647:37–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nguyen T, Nioi P and Pickett CB: The
Nrf2-antioxidant response element signaling pathway and its
activation by oxidative stress. J Biol Chem. 284:13291–13295. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jin W, Wang H, Ji Y, Hu Q, Yan W, Chen G
and Yin H: Increased intestinal inflammatory response and gut
barrier dysfunction in Nrf2-deficient mice after traumatic brain
injury. Cytokine. 44:135–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jin W, Wang HD, Hu ZG, Yan W, Chen G and
Yin HX and Yin HX: Transcription factor Nrf2 plays a pivotal role
in protection against traumatic brain injury-induced acute
intestinal mucosal injury in mice. J Surg Res. 157:251–260. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rangasamy T, Guo J, Mitzner WA, Roman J,
Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, et
al: Disruption of Nrf2 enhances susceptibility to severe airway
inflammation and asthma in mice. J Exp Med. 202:47–59. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee JM and Johnson JA: An important role
of Nrf2-ARE pathway in the cellular defense mechanism. J Biochem
Mol Biol. 37:139–143. 2004.PubMed/NCBI
|
|
39
|
Shang H, Yang D, Zhang W, Li T, Ren X,
Wang X and Zhao W: Time course of Keap1-Nrf2 pathway expression
after experimental intracerebral haemorrhage: Correlation with
brain oedema and neurological deficit. Free Radic Res. 47:368–375.
2013. View Article : Google Scholar : PubMed/NCBI
|