Introduction
Ischemic cardiomyopathy arising from myocardial
ischemia is the leading cause of morbidity and mortality worldwide
(1). There are numerous
therapeutic options for ischemic cardiomyopathy, including the
potential use of mesenchymal stem cells (MSCs) for tissue repair,
as demonstrated in clinical trials for intractable diseases
(2,3). However, the therapeutic efficacy of
MSCs has been hindered by the low survival rate of transplanted
cells. The survival and retention of MSCs following transplantation
are adversely affected by the harmful ischemic microenvironment
(4), and myocardial oxidative
stress restricts the therapeutic effects of MSCs on cardiac repair
(5). It is therefore important to
develop novel strategies to promote donor cell survival to improve
the efficacy of stem-cell-based therapy for ischemic
cardiomyopathy.
Macrophage migration inhibitory factor (MIF) is a
widely expressed pleiotropic cytokine and is considered to be an
important therapeutic target for treating cardiovascular disease
(6). MIF regulates cellular
activities through transcriptional regulation of inflammatory gene
products, modulation of cell proliferation, cell cycle control and
metabolism, and by inhibition of apoptosis (7). MIF protects against myocardial
ischemia/reperfusion injury primarily through preventing redox
stress (8). MIF is a good
anti-apoptotic factor candidate, and in an ischemia/reperfusion
injury model, MIF was also demonstrated to protect against
oxidative stress-mediated cardiomyocyte apoptosis (9). In the current study, the protective
effects of exogenous MIF were determined in MSCs exposed to
hypoxia/serum deprivation (SD) to mimic the ischemic
environment.
Long non-coding RNAs (lncRNAs) are RNA transcripts
>200 bp in length with no apparent protein-coding ability
(10). Increasing evidence
suggests that lncRNAs affect numerous cellular functions, and
participate in diverse physiological and pathological processes
(11), including development,
differentiation, stem cell pluripotency and apoptosis (12). Long intergenic noncoding RNA-p21
(lincRNA-p21) is a p53-dependent transcriptional target gene
involved in proliferation, the cell cycle, metabolism and apoptosis
(13). Previous studies have
demonstrated that lincRNA-p21 is associated with
oxidative-stress-induced apoptosis (14,15), and participates in
senescence-induced cellular injury through the induction of
oxidative stress (16). MIF
inhibited the p21-dependent death signaling in keratinocytes
(17). We therefore hypothesized
that lincRNA-p21 may be a target gene inhibited by MIF,
subsequently preventing hypoxia/SD-induced injury.
Wnt/β-catenin signaling is known to serve essential
roles in cell growth, survival and apoptosis (18). As a target gene of lincRNA-p21,
β-catenin is associated with cellular apoptosis, proliferation and
oxidative stress (19).
lincRNA-p21 was reported to inhibit hepatic stellate cell
proliferation through inactivation of the Wnt/β-catenin signaling
pathway (20). Furthermore,
lincRNA-p21negatively regulates β-catenin translation at the
post-transcriptional level and contributes to glioma stem cell
apoptosis (15). In addition,
inhibition of Wnt/β-catenin signaling induced by lincRNA-p21
contributes to cellular senescence in MSCs by inhibiting cellular
oxidative stress (16). However,
the role of lincRNA-p21-associated inhibition of the Wnt/β-catenin
pathway in hypoxia/SD-induced MSC apoptosis remains unclear.
We proposed that exogenous MIF prevents
hypoxia/SD-induced apoptosis and inhibits oxidative stress. Thus,
the effect of MIF on hypoxia/SD-induced apoptosis of MSCs and its
associated signaling pathways were examined in the present
study.
Materials and methods
Reagents
Dulbecco’s modified Eagle’s medium (DMEM) and fetal
bovine serum (FBS) were purchased from GE Healthcare Life Sciences
(HyClone; Logan, UT, USA), TRIzol® reagent was obtained
from Thermo Fisher Scientific, Inc. (Invitrogen; Waltham, MA, USA),
and the Transcriptor First Strand cDNA Synthesis kit, FastStart
Universal SYBR®-Green Master (Rox) and X-tremeGENE HP
DNA transfection reagent were purchased from Roche Diagnostics
(Basel, Switzerland). The Annexin V-fluorescein isothiocyanate
(FITC) Apoptosis Detection kit was obtained from BD Biosciences (BD
Pharmingen; Franklin Lakes, NJ, USA). Rabbit monoclonal antibodies
against β-catenin (#8480; 1:1,000) and β-actin (#4970; 1:1,000)
were obtained from Cell Signaling Technology, Inc. (Danvers, MA,
USA) and horseradish peroxidase-conjugated anti-rabbit secondary
antibodies (#7074; 1:2,000) were from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). Small interfering RNAs (siRNAs) targeting
lincRNA-p21 and β-catenin transcripts were purchased from Thermo
Fisher Scientific, Inc. The ELISA kit for MIF was purchased from
Abcam (#ab7207; Cambridge, UK), and the Mitochondrial Membrane
Potential assay kit with JC-1 (#C2006) and Reactive Oxygen Species
(ROS) assay kit (#S0033) were purchased from Beyotime Institute of
Biotechnology (Jiangsu, China). The Superoxide Dismutase (SOD)
Activity Colorimetric assay (#ab211096), Lipid Peroxidation
(malondialdehyde; MDA) assay kits (#ab118970) and mouse recombinant
MIF were purchased from Abcam.
Cell culture and treatment
Bone marrow-derived mesenchymal stem cells (MSCs)
were isolated using a standard protocol, as described previously
(16). A total of 12 male mice
(mean age, 6 months; mean weight, 22.85±2.62 g) were purchased from
the Laboratory Animal Center of Wenzhou Medical University
(Wenzhou, China). Mice were kept to a 12 h light/12 h dark cycle at
21±2°C with 30-70% relative humidity. Food and water was freely
available throughout. All animal procedures were approved by the
Wenzhou Medical University Institutional Animal Care and Use
Committee (Wenzhou, China). Briefly, bone marrow was isolated from
mouse femurs and tibias by flushing with PBS. Adherent MSCs were
cultured at 37°C and 5% CO2 in high-glucose DMEM
supplemented with 10% FBS and 1% penicillin/streptomycin.
Third-passage MSCs were used for experiments.
Apoptosis was induced in vitro by hypoxia and
SD to mimic the in vivo conditions of the ischemic
myocardium, as previously reported (21). Apoptosis was induced by incubating
MSCs in serum-free DMEM in a glove box (model no. 855-AC; Plas
Labs, Inc., Lansing, MI, USA) with a regulated atmosphere
(anaerobic chamber) to scavenge free oxygen (hypoxia/SD group). For
MIF treatment, cells were cultured with DMEM containing 100 ng/ml
recombinant MIF and incubated at 37°C for various periods of time,
as reported previously (22).
Untreated cells were used as the control group throughout.
Flow cytometric analysis of cell
apoptosis
The effects of MIF on apoptosis were determined by
detecting phosphatidylserine exposure on cell plasma membranes
using an Annexin V-FITC Apoptosis Detection kit, according to the
manufacturer’s protocol. Briefly, cells were harvested (4°C, 5 min,
12,000 × g) and washed in ice-cold PBS, resuspended in 300
μl binding buffer, and incubated with 5 μl Annexin
V-FITC solution for 30 min at 4°C in the dark, followed by further
incubation in 5 μl propidium iodide for 5 min at 4°C. The
cells were then analyzed immediately using bivariate flow cytometry
with a BD FACSCanto II equipped with BD FACSDiva software (version
8.0.1; BD Biosciences).
Calculation of caspase 3/7/8
activities
Caspase 3/7/8 activities in MSCs were determined as
described previously (23).
Briefly, activities of caspases 3/7/8 in cell lysates of MSCs, MSCs
under hypoxia/SD conditions and MSCs treated with MIF under
hypoxia/SD conditions were measured using a Cell Meter Caspase
3/7/8 Activity Apoptosis assay kit (AAT Bioquest, Sunnyvale, CA,
USA) according to the manufacturer’s protocol. Results were read at
520 nm with a microplate reader and expressed as the fold change in
caspase 3/7/8 activity compared with the control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Expression levels of several genes were analyzed
using RT-qPCR. Briefly, total cellular RNA was isolated using
TRIzol reagent and reverse transcribed using a Transcriptor First
Strand cDNA Synthesis kit according to the manufacturer’s protocol.
qPCR was performed using the Fast Start Universal SYBR Master and
the Applied Biosystems Step One Plus Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling
conditions were as follows: 95°C for 10 min, followed by 40 cycles
of 15 sec at 95°C and 1 min at 60°C. The threshold number of cycles
(Cq) was set within the exponential phase of the PCR. The ΔCq value
for each target gene was calculated by subtracting the Cq value for
GAPDH (internal control) from that of the target gene. Relative
gene expression levels were calculated by comparing the ∆Cq values
between the control and experimental conditions for each target PCR
using the following equation: 2−(ΔCq sample−∆Cq control)
(24). The primer pairs used to
detect the mRNA levels of target genes are listed in Table I.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Genes | Sequences |
|---|
| lincRNA-p21 | |
| F | 5′-CCT GTC CAC TCG
CTT TC-3′ |
| R | 5′-GGA ACT GGA GAC
GGA ATG TC-3′ |
| β-catenin | |
| F | 5′-TAG TGT GAC AAG
CTG AGT ATG CGA-3′ |
| R | 5′-CTG GAG CGT CTG
ATG AG-3′ |
| GAPDH | |
| F | 5′-GGA GCC AAA AGG
GTC ATC AT-3′ |
| R | 5′-GTG ATG GCA TGG
ACT GTG GT-3′ |
|
siRNA-LincRNA-p21 | UGA AAA GAG CCG UGA
GCU A |
|
siRNA-β-catenin | CTC ACT TGC AAT AAT
TAC AAA |
| siRNA-NT | CTC UCC GAA CGU GUC
ACG UTT |
Western blot analysis
MSCs were lysed with ice-cold lysis buffer (Beyotime
Institute of Biotechnology) to obtain total protein, then β-catenin
and β-actin expression levels were evaluated using western
blotting. Cellular extracts were prepared according to the
manufacturer’s protocol. Protein samples were quantified and
separated by SDS-PAGE. Western blotting was performed as described
previously (25). Quantity One
software (version 4.5.2; Bio-Rad Laboratories, Inc., Hercules, CA,
USA) was used for densitometric analysis.
lincRNA-p21 and β-catenin siRNA
knockdown
MSCs were transfected using X-treme GENE HP DNA
Transfection reagent according to the manufacturer’s protocol.
Briefly, MSCs (1×105 cells/well) were cultured in 6-well
plates for 24 h and then treated with the transfection reagent
(siRNA weight ratio of 3:1) for 20 min. This was followed by the
addition of a mixture containing 100 nM siRNA and incubation in 2
ml DMEM for 48 h at 37°C. Scrambled non-targeting siRNA (siRNA-NT)
was used as a negative control. The knockdown efficiency was
determined by RT-qPCR, as aforementioned. The sequences are listed
in Table I.
Plasmid transfection
Adenoviral vectors expressing lincRNA-p21
(Ad-lincRNA-p21 group) and control scrambled sequence (Ad-ctrl
group) were designed and synthesized by Shanghai GeneChem Co., Ltd.
(Shanghai, China). MSCs were transfected using Lipofectamine™ 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) at a final
concentration of 100 nM.
Evolution of mitochondrial transmembrane
potential
Cells were cultured in complete DMEM in 96-well
microtiter plates at 37°C for 1 day to achieve 1×105
cells/well. The cells were then washed with PBS and incubated with
5 μg/ml JC-1 at 37°C for 15 min. Following two washes with
PBS, time-dependent JC-1 fluorescence was recorded using an ELISA
plate reader. The fluorescent probe was excited at 490 nm and the
emission was read alternately at 530 and 590 nm.
ROS measurement
Levels of intracellular ROS were determined using
2,7-dichlorodihydrofluorescein diacetate (Beyotime Institute of
Biotechnology), following the manufacturer’s protocol. The
fluorescence intensity of the cells was measured using a
fluorescence spectrophotometer, with excitation and emission
wavelengths of 488 and 525 nm, respectively.
SOD activity
SOD activity in MSCs was determined using a SOD
Activity Colorimetric assay according to the manufacturer’s
protocol. Briefly, protein was isolated from MSCs using lysis
buffer, and SOD activity was measured in 10 μg of total
protein extract. Absorbance was measured at 450 nm.
Lipid peroxidation assays
Lipid peroxidation was monitored using an assay kit
to measure the formation of MDA, according to the manufacturer’s
protocol. Briefly, MSCs (1×106 cells) were homogenized
on ice in 300 μl of MDA lysis buffer (with 3 μl of
100X butylated hydroxytoluene), and then centrifuged (4°C, 13,000 ×
g, 10 min) to remove insoluble material. The supernatant (200
μl) was added to 600 μl of thiobarbituric acid and
incubated at 95°C for 60 min. The samples were cooled to room
temperature in an ice bath for 10 min, and the absorbance at 532 nm
was measured spectrophotometrically.
Statistical analysis
Data are expressed as the mean ± standard deviation
following three repeats. Differences among groups were analyzed
using one-way analysis of variance followed by Tukey’s multiple
comparisons test, and comparisons between two groups were evaluated
by Student’s t-tests using SPSS software (version 19.0; IBM, Corp.,
Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
MIF ameliorates cell injury induced by
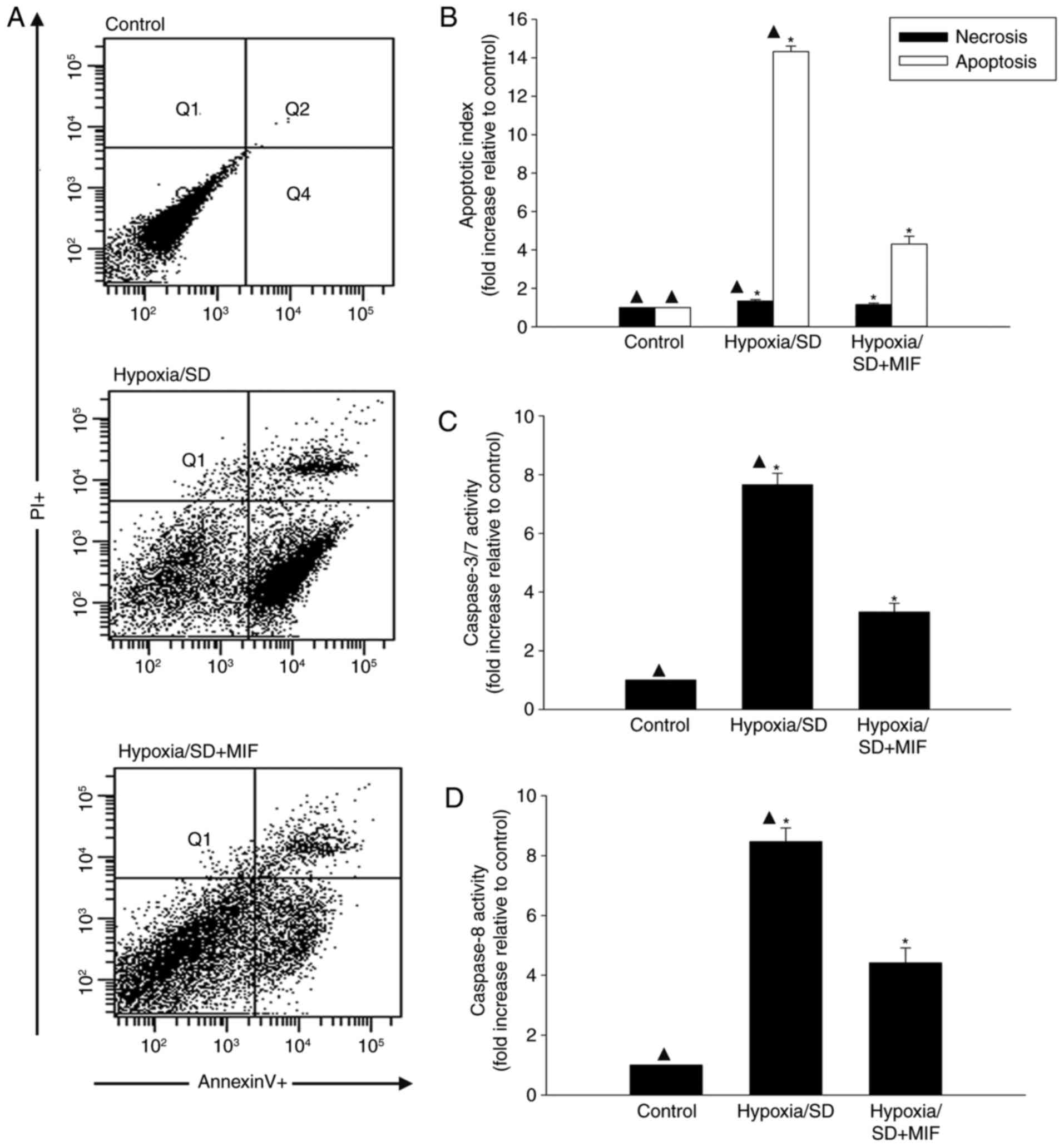
hypoxia/SD in MSCs
Hypoxia/SD induced MSC injury, with maximal injury
at 24 h. To determine if MIF protects MSCs from hypoxia/SD-induced
injury, MSCs were exposed to MIF (100 ng/ml) followed by hypoxia/SD
for 24 h and apoptosis rates were determined by flow cytometry. MIF
demonstrated a significant anti-apoptotic effect of the hypoxia/SD
model, as demonstrated by Annexin V-FITCFACS analysis (Fig. 1A and B).
The anti-apoptotic effects of MIF were further
examined by measuring the effects of MIF pretreatment on changes in
caspases 3/7 and caspase 8 following hypoxia/SD induction. MIF
pretreatment significantly reduced the stress-induced increases in
caspase activities (Fig. 1C and
D).
MIF protects MSCs from hypoxia/SD-induced
apoptosis by inhibiting the expression of lincRNA-p21
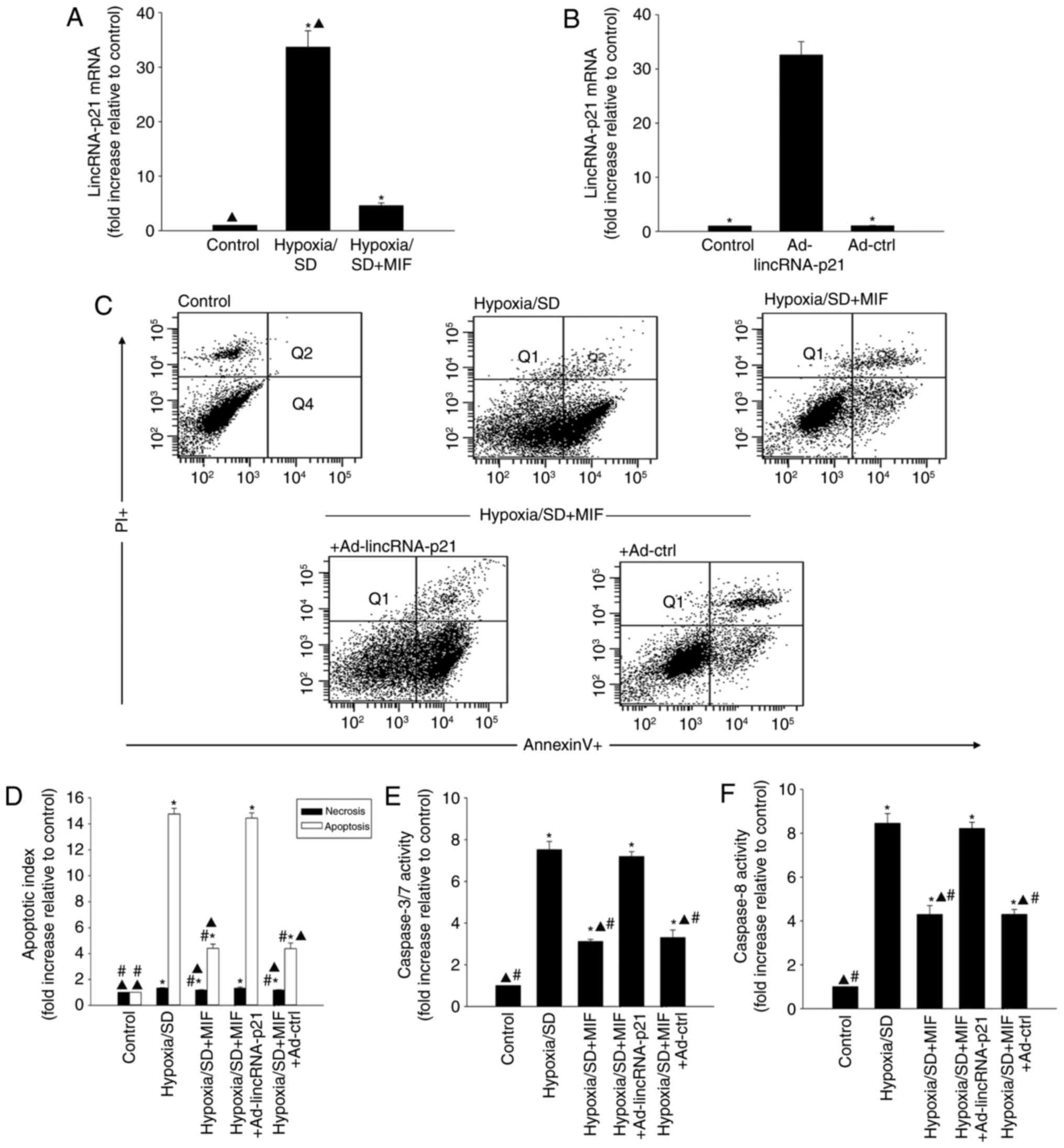
lincRNA-p21 has been reported to be associated with
cellular apoptosis (26). To
determine if lincRNA-p21 was involved in hypoxia/SD-induced
apoptosis, its expression was evaluated in MSCs exposed to
hypoxia/SD for 24 h. lincRNA-p21 was significantly increased in
MSCs following hypoxia/SD treatment, as demonstrated by RT-qPCR
analysis (Fig. 2A). Furthermore,
the hypoxia/SD-induced increase was significantly attenuated by
exogenous MIF treatment (Fig.
2A).
The role of MIF-induced inhibition of lincRNA-p21in
protecting MSCs from hypoxia/SD-induced apoptosis was further
examined. MIF was added to MSCs prior to hypoxia/SD treatment and
apoptosis was measured by flow cytometry. In a parallel experiment,
MSCs were transfected with Ad-lincRNA-p21 prior to treatment with
MIF (Fig. 2B) and cultured under
hypoxia/SD conditions. MIF treatment significantly decreased
cellular apoptosis (Fig. 2C and
D), and reduced caspases 3/7 and caspase 8 activities compared
with cells under hypoxia/SD treatment alone (Fig. 2E and F). In addition, these
effects were significantly abolished by lincRNA-p21 overexpression
(Fig. 2C-F).
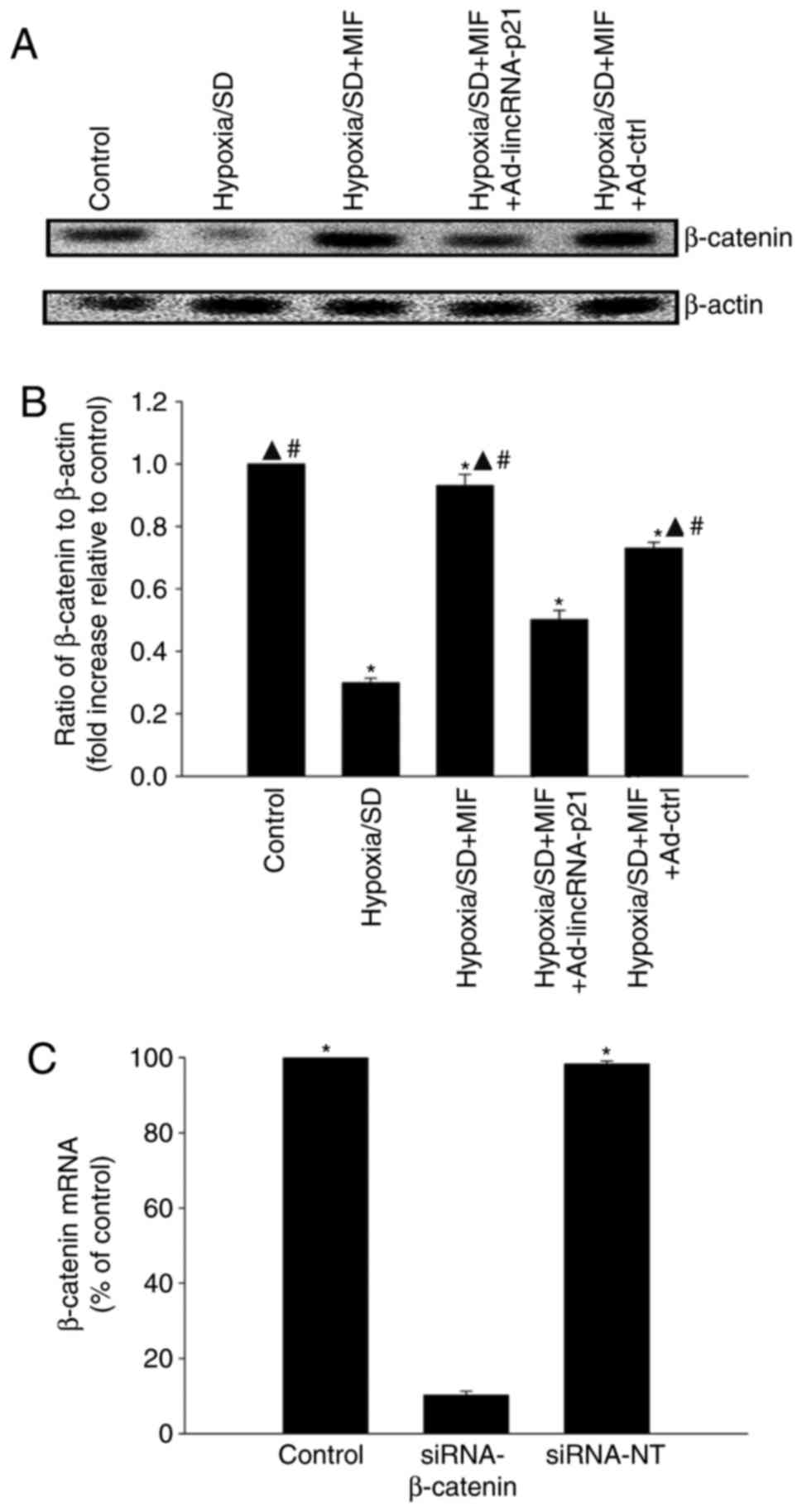
MIF restores the
lincRNA-p21-Wnt/β-catenin signaling pathway altered by hypoxia/SD
induction
The Wnt/β-catenin signaling pathway is a known
target of lincRNA-p21, and has been reported to be involved in
cellular injury in MSCs (16). In
the present study, β-catenin protein expression levels in MSCs were
significantly decreased by hypoxia/SD compared with control cells,
which was restored following pre-treatment with MIF. Overexpression
of lincRNA-p21by Ad-lincRNA-p21 transfection abolished the effect
of MIF, while transfection with the control vector had no
significant effect (Fig. 3A and
B). The mechanism underlying the modulation of
lincRNA-p21-Wnt/β-catenin signaling by MIF in hypoxia/SD-associated
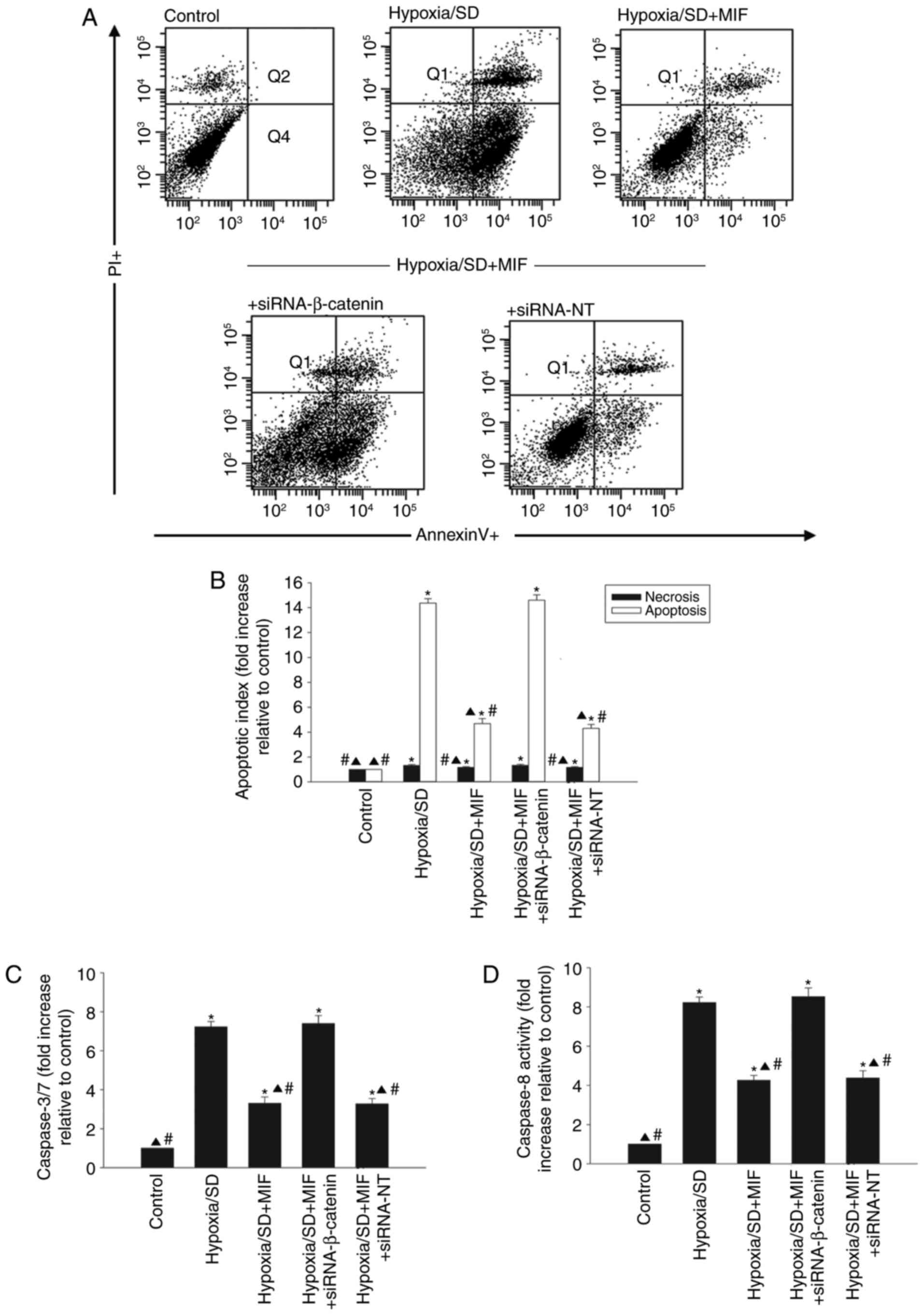
cellular apoptosis was examined by silencing β-catenin using siRNA.
β-catenin mRNA expression levels were significantly reduced in
cells transfected with siRNA-β-catenin compared with cells
transfected with siRNA-NT control (Fig. 3C). Pretreatment with MIF protected
MSCs from apoptosis induced by hypoxia/SD (Fig. 4A and B), and significantly
decreased the activities of caspases 3/7 and caspase 8 (Fig. 4C and D). However, these effects
were abolished by silencing β-catenin and not by transfection with
siRNA-NT (Fig. 4).
MIF enhances MSC survival via inhibition
of oxidative stress
Oxidative stress is associated with cellular
apoptosis (27), thus, the
feedback loop between oxidative stress and the
lincRNA-p21-Wnt/β-catenin signaling pathway modulated by MIF was
explored. Mitochondrial transmembrane potential, ROS generation,
SOD activation and lipid peroxidation were examined (Fig. 5). Hypoxia/SD significantly
decreased the mitochondrial transmembrane potential (Fig. 5A) and SOD activation (Fig. 5C), while increasing ROS generation
(Fig. 5B) and MDA activation
(Fig. 5D). Pretreatment with MIF
significantly increased the mitochondrial transmembrane potential
and SOD activation, and decreased ROS generation of ROS and MDA
activation. These anti-oxidant effects of MIF were abolished by
ectopic expression of lincRNA-p21 or by silencing β-catenin
(Fig. 5A-D).
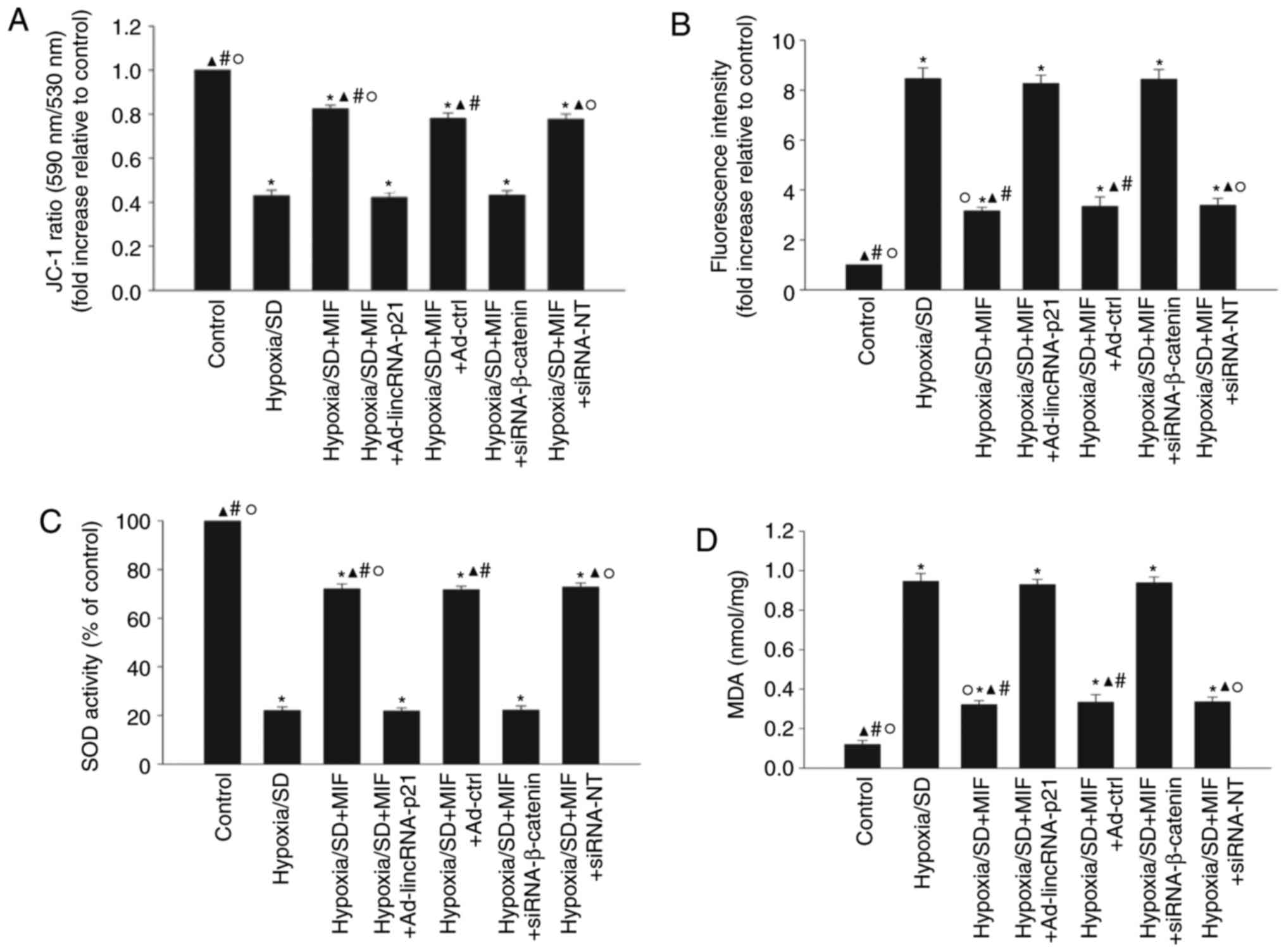 | Figure 5MIF enhanced MSC survival via
inhibition of oxidative stress. MSCs were incubated under
hypoxic/SD conditions for 24 h. In parallel experiments, cells were
transfected with Ad-lincRNA-p21, Ad-ctrl, siRNA-β-catenin, or
siRNA-NT before exposure to hypoxia/SD, and then treated with MIF.
MIF (100 ng/ml) was added at the beginning of exposure to
hypoxia/SD. Untreated MSCs were used as a control. (A)
Mitochondrial membrane potential was measured using JC-1 stain. (B)
Intracellular reactive oxygen species production was analyzed by
fluorescence spectrophotometry. (C) Superoxide dismutase activity
was evaluated by colorimetric assay. (D) Lipid peroxidation was
evaluated by malondialdehyde formation. Data represent mean ±
standard deviation from three independent experiments;
*P<0.05 vs. control, ▲P<0.05 vs.
hypoxia/SD; #P<0.05 vs.
hypoxia/SD+MIF+Ad-lincRNA-p21; ○P<0.05 vs.
hypoxia/SD+MIF+siRNA-β-catenin. MIF, macrophage migration
inhibitory factor; MSC, mesenchymal stem cells; SD, serum
deprivation; siRNA, small interfering RNA; linc, long intergenic
noncoding; NT, non-targeting; Ad-lincRNA-p21, adenoviral vectors
expressing lincRNA-p21; Ad-ctrl, adenoviral vectors expressing
control scrambled sequence. |
Discussion
MSCs are immune-protected and secrete a wide variety
of growth factors that protect the heart from ischemic injury
(28). The regenerative potency
of these cells has thus been investigated in numerous preclinical
and clinical studies, and MSC transplantation in the heart is
considered to be a safe therapeutic option in patients with
myocardial injury (29,30). However, numerous transplanted
cells undergo apoptosis, which is considered to be one of the
primary barriers limiting the effectiveness of cell therapy
(31). In the present study,
hypoxia/SD culture conditions were used to mimic the ischemic
environment in vitro, in order to determine whether the
survival and potency of transplanted MSCs increase following
treatment with MIF.
Plasma MIF levels are increased in patients with
myocardial infarction (32), and
ischemia also increases MIF expression in rat hearts (33). Excessive MIF reduces cardiac
contractility in isolated, perfused rat hearts (34), and has also been reported to
enhance the survival of cultured cardiomyocytes (35). The present study revealed that MIF
treatment significantly reduced apoptosis in hypoxia/SD-cultured
MSCs in vitro, and this reduction was accompanied by
inhibition of caspase 3/7 and caspase 8 activities, which are known
to be involved in the cellular apoptosis process.
Mammalian genomes encode >10,000 lncRNAs, which
are RNA molecules >200 nucleotides that do not appear to encode
proteins (36). lncRNAs have
emerged as important regulators in various cellular processes,
including proliferation, survival, autophagy and apoptosis
(37). A recent study
demonstrated that lncRNAs regulate cell proliferation, apoptosis
and autophagy in response to energy stress (38). lincRNA-p21 is a p53-dependent
transcriptional target gene with roles in cell cycle arrest and
apoptosis in response to DNA damage, and has been revealed to
activate damage signaling and cell cycle arrest (39). MIF inhibited p53-dependent death
signaling in keratinocytes and exerted a protective effect against
cellular apoptosis (17). The
results of this study demonstrated that hypoxia/SD induced
apoptosis in MSCs, accompanied by induction of lincRNA-p21, while
MIF decreased lincRNA-p21 expression and protected MSCs from injury
induced by hypoxia/SD. Overexpression of lincRNA-p21 abolished the
anti-apoptotic effect of MIF, confirming that this anti-apoptotic
effect was due to inhibition of lincRNA-p21 by MIF.
Wnt/β-catenin is a stress signaling kinase pathway
and key regulator of energy-generating and consuming pathways. It
serves as an essential sensor of cellular energy status and is
activated under energy stress conditions, to protect cells against
hypoxic injury and death (18).
The Wnt/β-catenin signaling pathway also regulates
organelle-compartmentalized protein folding, and is relevant in the
cross-talk between mitochondria and endoplasmic reticulum, thus
protecting against oxidative stress (40). β-Catenin is an important target
gene of lincRNA-p21, and lincRNA-p21was demonstrated to inhibit
β-catenin signaling, thereby attenuating the viability,
self-renewal and glycolysis of cancer stem cells in vitro
(41). Furthermore, MIF enhances
the proliferation of neural stem/progenitor cells and promotes
neuronal differentiation by activating the Wnt/β-catenin signal
pathway (42). The current
results suggest that the Wnt/β-catenin signaling pathway was
inhibited by hypoxia/SD and reactivated by exogenous MIF, and this
restoration was abolished by lincRNA-p21 overexpression. The
relevance of MIF regulation of β-catenin in hypoxia/SD-induced
apoptosis was confirmed by silencing β-catenin, which attenuated
the anti-apoptotic effect of MIF.
Excess oxidative stress causes cell death in the
heart (43), and excessive
oxidative stress below physiological levels may not only induce
myocardial injury in response to ischemia/reperfusion, but may also
cause apoptosis of transplanted stem cells (44). lncRNAs are involved in cellular
oxidative stress, and recent research revealed that the
lncRNAFOXD3-AS1was involved in hypoxia/ROS-induced injury (45). lincRNA-p21was also demonstrated to
inhibit HepG2 cell growth by activating endoplasmic reticulum
stress (14). A recent study
revealed that lincRNA-p21 participated in cellular senescence in
MSCs through inducing the oxidative process (16). In addition, MIF-knockout
exacerbated doxorubicin-induced mortality and cardiomyopathy,
accompanied by cellular apoptosis and ROS generation (46). The results of the present study
demonstrated that hypoxia/SD-induced oxidative stress was
accompanied by decreased mitochondrial transmembrane potential and
activation of SOD, and increased generation of ROS and MDA
activation, and these effects were reversed by MIF treatment. The
anti-oxidant effects of MIF were in turn abolished by
overexpression of lincRNA-p21 or silencing of β-catenin.
In conclusion, the results of this study suggest
that MIF acts as an anti-apoptotic factor to counteract
hypoxia/SD-induced apoptosis, via a mechanism involving rebalancing
the lincRNA-p21-Wnt/β-catenin signaling pathway and decreasing
oxidative stress.
Acknowledgments
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81600278 to WX and
81500261 to MH) and the Medical Science and Technology Project of
Zhejiang Province (grant no. 2018KY517 to MH).
Availability of data and materials
All data generated or analyzed during the present
study are included in the published article.
Authors’ contributions
WX and LZ made substantial contributions to the
acquisition of data, analysis and interpretation of data; and MH
was involved in conception and design, drafting the manuscript and
revising it critically for important intellectual content.
Ethics approval and consent to
participate
All animal procedures were approved by the
Institutional Animal Care and Use Committee of Wenzhou Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chareonthaitawee P, Gersh BJ, Araoz PA and
Gibbons RJ: Revascularization in severe left ventricular
dysfunction: The role of viability testing. J Am Coll Cardiol.
46:567–574. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Karantalis V, Suncion-Loescher VY, Bagno
L, Golpanian S, Wolf A, Sanina C, Premer C, Kanelidis AJ, McCall F,
Wang B, et al: Synergistic Effects of combined cell therapy for
chronic ischemic cardiomyopathy. J Am Coll Cardiol. 66:1990–1999.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Natsumeda M, Florea V, Rieger AC, Tompkins
BA, Banerjee MN, Golpanian S, Fritsch J, Landin AM, Kashikar ND,
Karantalis V, et al: A combination of allogeneic stem cells
promotes cardiac regeneration. J Am Coll Cardiol. 70:2504–2515.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abdelwahid E, Kalvelyte A, Stulpinas A, de
Carvalho KA, Guarita-Souza LC and Foldes G: Stem cell death and
survival in heart regeneration and repair. Apoptosis. 21:252–268.
2016. View Article : Google Scholar
|
|
5
|
Golpanian S, Wolf A, Hatzistergos KE and
Hare JM: Rebuilding the damaged heart: Mesenchymal stem cells,
cell-based therapy, and engineered heart tissue. Physiol Rev.
96:1127–1168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luedike P, Hendgen-Cotta UB, Sobierajski
J, Totzeck M, Reeh M, Dewor M, Lue H, Krisp C, Wolters D, Kelm M,
et al: Cardioprotection through S-nitros(yl)ation of macrophage
migration inhibitory factor. Circulation. 125:1880–1889. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Calandra T and Roger T: Macrophage
migration inhibitory factor: A regulator of innate immunity. Nat
Rev Immunol. 3:791–800. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qi D, Hu X, Wu X, Merk M, Leng L, Bucala R
and Young LH: Cardiac macrophage migration inhibitory factor
inhibits JNK pathway activation and injury during
ischemia/reperfusion. J Clin Invest. 119:3807–3816. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zernecke A, Bernhagen J and Weber C:
Macrophage migration inhibitory factor in cardiovascular disease.
Circulation. 117:1594–1602. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun Q, Hao Q and Prasanth KV: Nuclear long
noncoding RNAs: Key regulators of gene expression. Trends Genet.
S0168-9525(17)30207-X. 2017.PubMed/NCBI
|
|
11
|
Liu C, Yang Z, Wu J, Zhang L, Lee S, Shin
DJ, Tran M and Wang L: Long noncoding RNA H19 interacts with
polypyrimidine tract-binding protein 1 to reprogram hepatic lipid
homeostasis. Hepatology. 67:1768–1783. 2018. View Article : Google Scholar
|
|
12
|
Chew CL, Conos SA, Unal B and Tergaonkar
V: Noncoding RNAs: Master regulators of inflammatory signaling.
Trends Mol Med. 24:66–84. 2018. View Article : Google Scholar
|
|
13
|
Bao X, Wu H, Zhu X, Guo X, Hutchins AP,
Luo Z, Song H, Chen Y, Lai K, Yin M, et al: The p53-induced
lincRNA-p21 derails somatic cell reprogramming by sustaining
H3K9me3 and CpG methylation at pluripotency gene promoters. Cell
Res. 25:80–92. 2015. View Article : Google Scholar :
|
|
14
|
Yang N, Fu Y, Zhang H, Sima H, Zhu N and
Yang G: LincRNA-p21 activates endoplasmic reticulum stress and
inhibits hepatocellular carcinoma. Oncotarget. 6:28151–28163.
2015.PubMed/NCBI
|
|
15
|
Yang W, Yu H, Shen Y, Liu Y, Yang Z and
Sun T: MiR-146b-5p overexpression attenuates stemness and
radioresistance of glioma stem cells by targeting
HuR/lincRNA-p21/β-catenin pathway. Oncotarget. 7:41505–41526.
2016.PubMed/NCBI
|
|
16
|
Xia W, Zhuang L, Deng X and Hou M: Long
noncoding RNA-p21 modulates cellular senescence via the
Wnt/β-catenin signaling pathway in mesenchymal stem cells. Mol Med
Rep. 16:7039–7047. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoshihisa Y, Rehman MU, Kondo T and
Shimizu T: Role of macrophage migration inhibitory factor in
heat-induced apoptosis in keratinocytes. Faseb J. 30:3870–3877.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gammons M and Bienz M: Multiprotein
complexes governing Wnt signal transduction. Curr Opin Cell Biol.
51:42–49. 2018. View Article : Google Scholar
|
|
19
|
Yoon JH, Abdelmohsen K, Srikantan S, Yang
X, Martindale JL, De S, Huarte M, Zhan M, Becker KG and Gorospe M:
LincRNA-p21 suppresses target mRNA translation. Mol Cell.
47:648–655. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu F, Guo Y, Chen B, Shi L, Dong P, Zhou M
and Zheng J: LincRNA-p21 inhibits the Wnt/β-catenin pathway in
activated hepatic stellate cells via sponging MicroRNA-17-5p. Cell
Physiol Biochem. 41:1970–1980. 2017. View Article : Google Scholar
|
|
21
|
Zhu W, Chen J, Cong X, Hu S and Chen X:
Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem
cells. Stem Cells. 24:416–425. 2006. View Article : Google Scholar
|
|
22
|
Gore Y, Starlets D, Maharshak N,
Becker-Herman S, Kaneyuki U, Leng L, Bucala R and Shachar I:
Macrophage migration inhibitory factor induces B cell survival by
activation of a CD74-CD44 receptor complex. J Biol Chem.
283:2784–2792. 2008. View Article : Google Scholar
|
|
23
|
Liu Y, Xiong Y, Xing F, Gao H, Wang X, He
L, Ren C, Liu L, So KF and Xiao J: Precise regulation of miR-210 is
critical for the cellular homeostasis maintenance and
transplantation efficacy enhancement of mesenchymal stem cells in
acute liver failure therapy. Cell Transplant. 26:805–820. 2017.
View Article : Google Scholar :
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Xia W, Xie C, Jiang M and Hou M: Improved
survival of mesen-chymal stem cells by macrophage migration
inhibitory factor. Mol Cell Biochem. 404:11–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shen Y, Liu Y, Sun T and Yang W:
LincRNA-p21 knockdown enhances radiosensitivity of hypoxic tumor
cells by reducing autophagy through HIF-1/Akt/mTOR/P70S6K pathway.
Exp Cell Res. 358:188–198. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maj T, Wang W, Crespo J, Zhang H, Wang W,
Wei S, Zhao L, Vatan L, Shao I, Szeliga W, et al: Oxidative stress
controls regulatory T cell apoptosis and suppressor activity and
PD-L1-blockade resistance in tumor. Nat Immunol. 18:1332–1341.
2017. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nakamura Y, Wang X, Xu C, Asakura A,
Yoshiyama M, From AH and Zhang J: Xenotransplantation of
long-term-cultured swine bone marrow-derived mesenchymal stem
cells. Stem Cells. 25:612–620. 2007. View Article : Google Scholar
|
|
29
|
Amado LC, Schuleri KH, Saliaris AP, Boyle
AJ, Helm R, Oskouei B, Centola M, Eneboe V, Young R, Lima JA, et
al: Multimodality noninvasive imaging demonstrates in vivo cardiac
regeneration after mesenchymal stem cell therapy. J Am Coll
Cardiol. 48:2116–2124. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hare JM, Traverse JH, Henry TD, Dib N,
Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE,
Gammon RS, et al: A randomized, double-blind, placebo-controlled,
dose-escalation study of intravenous adult human mesenchymal stem
cells (prochymal) after acute myocardial infarction. J Am Coll
Cardiol. 54:2277–2286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ye L, Zhang P, Duval S, Su L, Xiong Q and
Zhang J: Thymosin β4 increases the potency of transplanted
mesenchymal stem cells for myocardial repair. Circulation. 128(11
Suppl 1): S32–S41. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takahashi M, Nishihira J, Katsuki T,
Kobayashi E, Ikeda U and Shimada K: Elevation of plasma levels of
macrophage migration inhibitory factor in patients with acute
myocardial infarction. Am J Cardiol. 89:248–249. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu CM, Lai KW, Chen YX, Huang XR and Lan
HY: Expression of macrophage migration inhibitory factor in acute
ischemic myocardial injury. J Histochem Cytochem. 51:625–631. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chagnon F, Metz CN, Bucala R and Lesur O:
Endotoxin-induced myocardial dysfunction: Effects of macrophage
migration inhibitory factor neutralization. Circ Res. 96:1095–1102.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang J, Tong C, Yan X, Yeung E, Gandavadi
S, Hare AA, Du X, Chen Y, Xiong H, Ma C, et al: Limiting cardiac
ischemic injury by pharmacological augmentation of macrophage
migration inhibitory factor-AMP-activated protein kinase signal
transduction. Circulation. 128:225–236. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ulitsky I and Bartel DP: lincRNAs:
Genomics, evolution, and mechanisms. Cell. 154:26–46. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schmitt AM, Garcia JT, Hung T, Flynn RA,
Shen Y, Qu K, Payumo AY, Peres-da-Silva A, Broz DK, Baum R, et al:
An inducible long noncoding RNA amplifies DNA damage signaling. Nat
Genet. 48:1370–1376. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu X, Xiao ZD, Zhang J, Lee SW, Wang W,
Lee H, Zhuang L, Chen J and Lin HK: LncRNA NBR2 engages a metabolic
checkpoint by regulating AMPK under energy stress. Nat Cell Biol.
18:431–442. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen S, Liang H, Yang H, Zhou K, Xu L, Liu
J, Lai B, Song L, Luo H, Peng J, et al: LincRNa-p21: Function and
mechanism in cancer. Med Oncol. 34:982017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lettini G, Sisinni L, Condelli V, Matassa
DS, Simeon V, Maddalena F, Gemei M, Lopes E, Vita G, Del Vecchio L,
et al: TRAP1 regulates stemness through Wnt/β-catenin pathway in
human colorectal carcinoma. Cell Death Differ. 23:1792–1803. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang J, Lei ZJ, Guo Y, Wang T, Qin ZY,
Xiao HL, Fan LL, Chen DF, Bian XW, Liu J and Wang B:
miRNA-regulated delivery of lincRNA-p21 suppresses β-catenin
signaling and tumorigenicity of colorectal cancer stem cells.
Oncotarget. 6:37852–37870. 2015.PubMed/NCBI
|
|
42
|
Zhang X, Chen L, Wang Y, Ding Y, Peng Z,
Duan L, Ju G, Ren Y and Wang X: Macrophage migration inhibitory
factor promotes proliferation and neuronal differentiation of
neural stem/precursor cells through Wnt/β-catenin signal pathway.
Int J Biol Sci. 9:1108–1120. 2013. View Article : Google Scholar :
|
|
43
|
Matsushima S, Kuroda J, Zhai P, Liu T,
Ikeda S, Nagarajan N, Oka S, Yokota T, Kinugawa S, Hsu CP, et al:
Tyrosine kinase FYN negatively regulates NOX4 in cardiac
remodeling. J Clin Invest. 126:3403–3416. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Han YS, Lee JH, Jung JS, Noh H, Baek MJ,
Ryu JM, Yoon YM, Han HJ and Lee SH: Fucoidan protects mesenchymal
stem cells against oxidative stress and enhances vascular
regeneration in a murine hindlimb ischemia model. Int J Cardiol.
198:187–195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang D, Lee H, Haspel JA and Jin Y: Long
noncoding RNA FOXD3-AS1 regulates oxidative stress-induced
apoptosis via sponging microRNA-150. FASEB J. 31:4472–4481. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xu X, Bucala R and Ren J: Macrophage
migration inhibitory factor deficiency augments doxorubicin-induced
cardiomyopathy. J Am Heart Assoc. 2:e0004392013. View Article : Google Scholar : PubMed/NCBI
|