Introduction
Cerebral thrombosis is an ischemic cerebrovascular
disease (1). It is an important
component type of atherosclerosis, accounting for 70–80% of all
patients with stroke. In cerebral thrombosis, ~90% of cases develop
on the basis of cerebral atherosclerosis (2). Therefore, it is frequently referred
to as atherosclerotic cerebral thrombosis. Atherosclerosis is a
chronic progressive disease. It develops during childhood but
clinical symptoms present in middle- and old-aged individuals. The
pathological changes of atherosclerosis mainly involve the aorta
and medium-sized elastic arteries (3). Among these, the coronary artery and
cerebral arteries are the most dominant (3). Furthermore, the lesion predominantly
involves multiple organs at the same time (2). With the increase in detailed
investigations on atherosclerosis over the last century, several
representative theories have formed successively. These include
lipid infiltration, thrombosis, smooth muscle cell clones, injury
response and chronic inflammation (4).
MicroRNAs (miRNAs) are a class of small, endogenous,
non-coding single-strand RNAs. They are one of the most important
gene expression regulatory factors (5). miRNAs interact with the specific
sequence of a target gene and inhibits target gene activity or
promotes its degradation at the post-transcriptional level
(5). In addition, miRNAs regulate
target gene expression, and are involved in biological processes
that include cell proliferation, differentiation, apoptosis and
metabolism (6). The expression of
brain-specific miRNAs in the central nervous system is rich,
whereas no or minimal expression is observed in the majority of
other organs (6). miRNAs are
important in nervous system development and function. It is
reported that miRNA may be passively leaked out from damaged tissue
cells or infiltrated cells, similar to other materials (7). Alternatively, it may be actively
secreted from the damaged tissue cells into the blood circulation.
The latter is considered to be the major mechanism (6).
The phosphoinositide 3-kinase (PI3K)/Akt signal
transduction pathway is an important pathway for the intracellular
transduction of membrane receptor signals (8). It is key in maintaining cell
survival and inhibiting cell apoptosis. In addition, it can affect
the activation process of effector molecules, including downstream
apoptosis-related proteins and cell cycle regulating proteins.
Therefore, it is crucial in inhibiting apoptosis and promoting
proliferation in cells (9). The
aim of the present study was to examine the effects of plasma
miRNA-634 on adjusting nerve inflammation and apoptosis in cerebral
infarction, and investigate its possible mechanism.
Materials and methods
Middle cerebral artery occlusion (MCAO)
model
Male Sprague-Dawley (SD) rats (170–210 g) were
purchased for animal experiments from Beijing Vital River
Laboratory Animal Technology Co., Ltd. (Beijing, China). All rats
were housed at 22–23°C with a 55–60% humidity, 12/12 h light/dark
cycle and free access to food and water. Cerebral
ischemia-reperfusion was induced by MCAO. The SD rats were
anesthetized with 35 mg/kg of pentobarbital (i.p) and randomly
assigned into two groups: Control and MCAO model group. A midline
neck incision was made and the left common and external carotid
arteries was isolated, and was ligated with a microvascular clip
(cat. no. FE691, Aesculap, Tuttlingen, Germany). An 8-0 nylon
monofilament with silicon resin (180–190 µm) was introduced
through a small incision into the common carotid artery and
advanced 9 mm distal to the carotid bifurcation to induce MCAO. In
the control group, the rats were anesthetized with 35 mg/kg of
pentobarbital only without MCAO. After 1 h, reperfusion was
initiated by withdrawal of the monofilament. After 1 day, the SD
rats were anesthetized with 35 mg/kg of pentobarbital and
sacrificed for subsequent investigations.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Brain tissue samples were subsequently obtained and
hippocampal tissues were isolated. Total RNA from the hippocampal
tissues samples was isolated using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). The cDNA was
reverse transcribed using the M-MLV Reverse Transcription system
(Takara Biotechnology Co., Ltd., Dalian, China). The qPCR analysis
was performed using 100 ng cDNA on an Applied Biosystems 7500 Fast
Real-Time PCR system with the SYBR-Green PCR kit (TransGen,
Beijing, China). The following primers were used for qPCR: miR-634
forward, 5′-CAGTCTCAAACCAGCACC-3′ and reverse,
5′-TATGGTTGTTCACGACTCCTTCAC-3′; and U6 forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′.
The qPCR analysis was performed by denaturation at 95°C for 10 min,
followed by 40 cycles of 95°C for 30 sec, 60°C for 30 sec and 72°C
for 30 sec. Relative expression levels of mRNA were calculated by
the 2−ΔΔCq method (10).
Gene expression microarrays
RNA (500 ng) from hippocampal tissues was amplified
using fluorescent complementary RNA and a RNA Labeling kit
(Arraystar Inc., Rockville, MD, USA) in accordance with the
manufacturer’s protocol. Array hybridization was performed
according to the Agilent One-Color Microarray-Based Gene Expression
Analysis protocol (Agilent Technologies, Inc., Santa Clara, CA,
USA) and subsequent washing was performed using a mRNA-ONLY™
Eukaryotic mRNA Isolation kit (Epicentre; Illumina, Inc., San
Diego, CA, USA). cDNA samples were labeled with Cy3 using the
SureTag DNA Labeling kit (Agilent Technologies, Inc.). Scanning of
the microarrays was performed using Feature Extraction software
(v.10.7.3.1; Agilent Technologies, Inc.).
Transient transfection of cells
BV2 cells were purchased from the Shanghai Cell Bank
of Chinese Academy of Sciences (Shanghai, China) and cultured in
Dulbecco’s modified Eagle’s medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), glutamine (2 mmol/l, Invitrogen; Thermo Fisher
Scientific, Inc.), penicillin (200 U/ml; Hyclone; GE Healthcare
Life Sciences, Logan, UT, USA) and streptomycin (100 µg/ml;
Hyclone; GE Health Care Life Sciences) at 37°C with 5%
CO2. The miRNA (miR)-634 inhibitor mimics (100 nM;
Sangon Biotech Co., Ltd., Shanghai, China) and negative mimics (100
nM; Sangon Biotech Co., Ltd.) were transfected into BV2 cells using
Lipofectamine 2000 according to the manufacturer’s protocol
(Invitrogen; Thermo Fisher Scientific, Inc.). A total of 4 h
post-transfection, BV2 cells were incubated in DMEM medium without
glucose or serum in hypoxic conditions (5% CO2 and 95%
N2) for 4 h at 37°C followed by incubation under
normoxic conditions for 14 h at 37°C.
Cell proliferation and cell apoptosis
assays
The cells (1×103 per well) were grown in
96-well plates and analyzed with an MTT assay for 4 h at 37°C. DMSO
was added to dissolve crystals for 20 min at 37°C. The luminescence
was then measured using a microplate reader (BioTek Instruments,
Inc., Vinooski, VT, USA) at 492 nm.
For the analysis of apoptosis, the cells were
proliferated in 6-well plates for 48 h following transfection,
following which the cells were washed once in PBS and resuspended
with binding buffer. The cells were stained with 5 µl
fluoro-chrome-conjugated Annexin V and 5 µl PI (BD
Biosciences, Franklin Lakes, NJ, USA). Apoptosis was detected using
flow cytometry (FACSCanto; BD Biosciences).
Enzyme-linked immunosorbent assay
(ELISA)
The cell super-natants were collected and protein
content was measured using a BCA assay. The levels of capsase-3,
capsase-8 and capsase-9 were quantified using commercially
available ELISA kits.
Western blot analysis
Total protein extracts were obtained from the
cultured cells using RIPA assay and centrifuged at 10,000 g for 5
min at 4°C. Protein content was measured using a BCA assay. The
protein samples (50 µg) were separated with 8–12% SDS-PAGE
and transferred onto nitrocellulose membranes. The membranes were
then blocked with 5% BSA in TBST and incubated overnight at 4°C
with the following primary antibodies: B-cell lymphoma 2
(Bcl-2)-associated X protein (Bax; cat. no. sc-6236; 1:1,000; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA), PI3K (cat. no.
sc-1331; 1:1,000; Santa Cruz Biotechnology, Inc.), phosphorylated
(p-)Akt (cat. no. sc-7985-R; 1:1,000; Santa Cruz Biotechnology,
Inc.), MDM2 proto-oncogene (MDM2; cat. no. sc-812; 1:1,000; Santa
Cruz Biotechnology, Inc.), p53 (cat. no. sc-47698; 1:1,000; Santa
Cruz Biotechnology, Inc.) and GAPDH (cat. no. sc-293335; 1:2,000;
Santa Cruz Biotechnology, Inc.). The membranes were then washed
with TBST and incubated for 1 h at 37°C with HRP-conjugated goat
anti-rabbit IgG (cat. no. sc-2004; 1:5,000; Santa Cruz
Biotechnology, Inc.) or HRP-conjugated goat anti-mouse IgG
secondary antibodies (cat. no. sc-2005; 1:5,000; Santa Cruz
Biotechnology, Inc.). The protein bands were visualized using ECL
western blotting detection reagents (Thermo Fisher Scientific,
Inc.).
Statistical analysis
All data are expressed as the mean ± standard error
of the mean using SPSS software (version 21.0; IBM Corp., Armonk,
NY, USA). All statistical significance was determined using one-way
analysis of variance with Student’s t-test or one-way analysis of
variance and Tukey’s post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of miRNA-634 in the cerebral
infarction rat model
To determine the expression level of miRNA-634 in
the cerebral infarction rat model, RT-qPCR and gene chip analyses
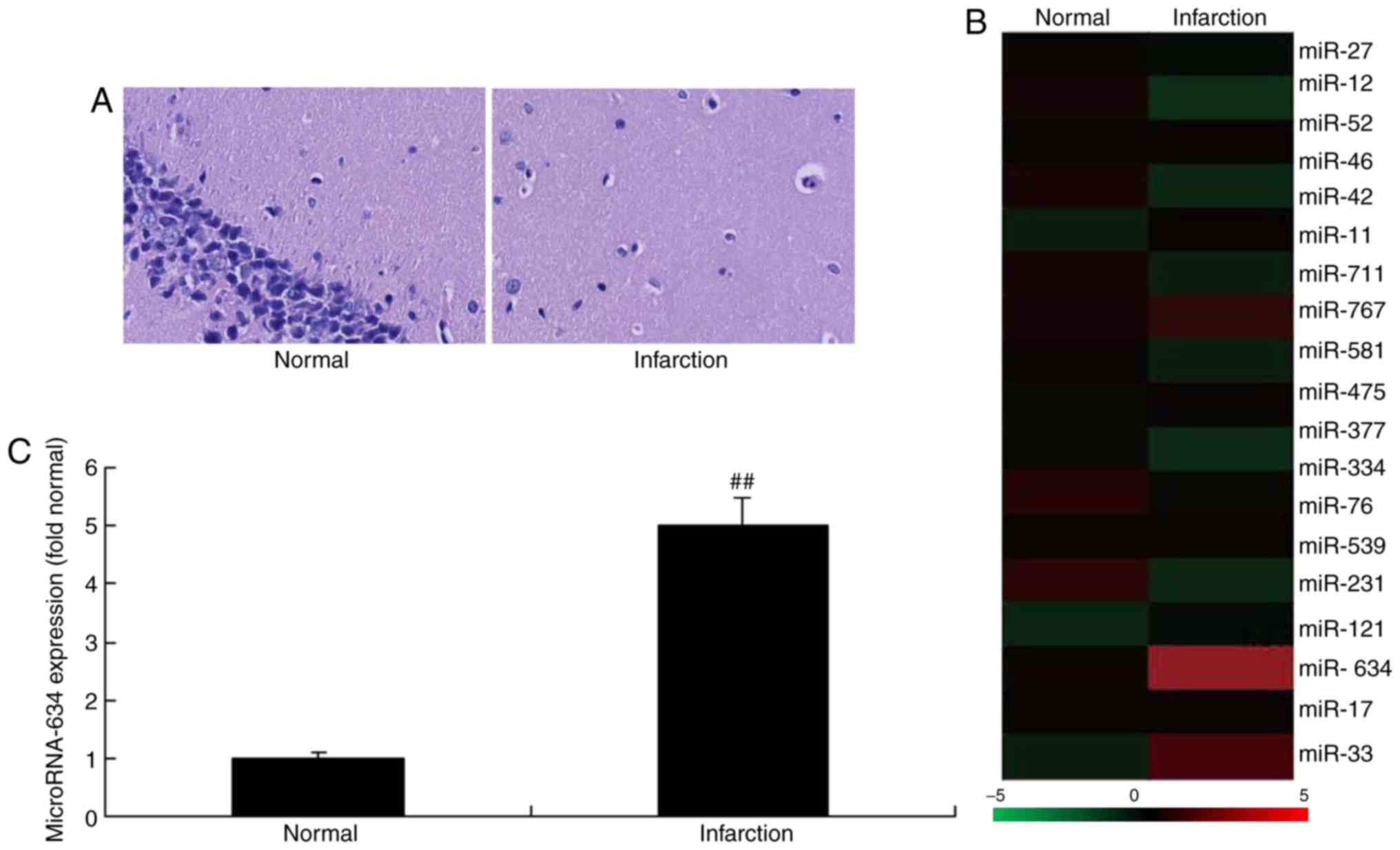
were performed to analyze the expression level of miRNA-634. As
shown in Fig. 1A, neurocyte death
was apparent in the cerebral infarction rat model, compared with
the normal group. The expression level of miRNA-634 in the cerebral
infarction rat group was increased compared with that in the normal
control group (Fig. 1B and
C).
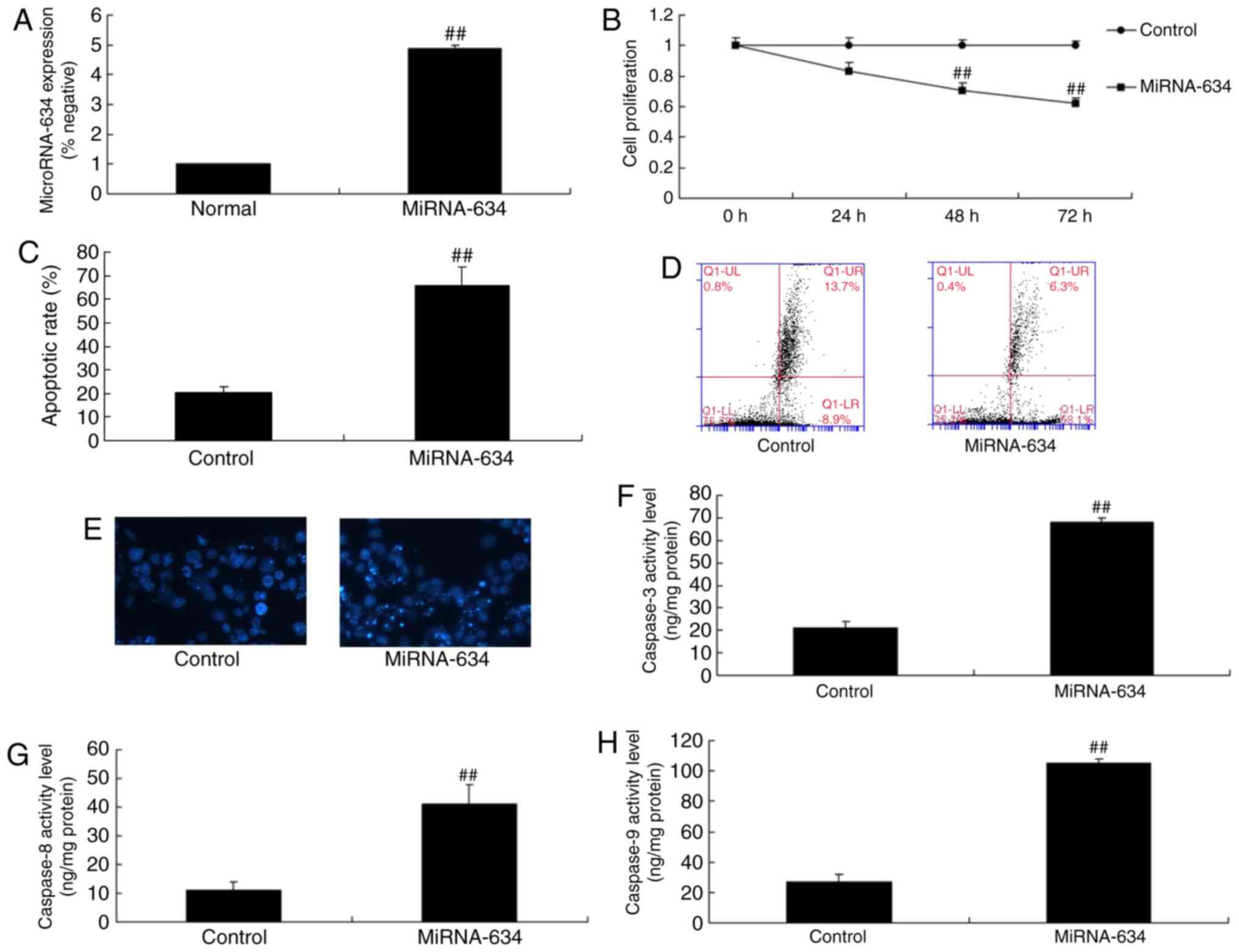
Upregulation of the expression of
miRNA-634 increases cell apoptosis in vitro
To investigate the effects of mature miRNA-634 on
nerve inflammation and apoptosis in cerebral infarction, miRNA-634
mimics were used to increase the expression of miRNA-634. As shown
in Fig. 2A-E, the miRNA-634
mimics significantly increased the expression of miRNA-634,
inhibited cell proliferation and induced apoptosis in the in
vitro model of cerebral infarction, compared with the control
negative group. There were also significant increases in the levels
of capsase-3, capsase-8 and capsase-9 in the in vitro model
of cerebral infarction with upregulated miRNA-634, compared with
levels in the negative control group (Fig. 2F-H).
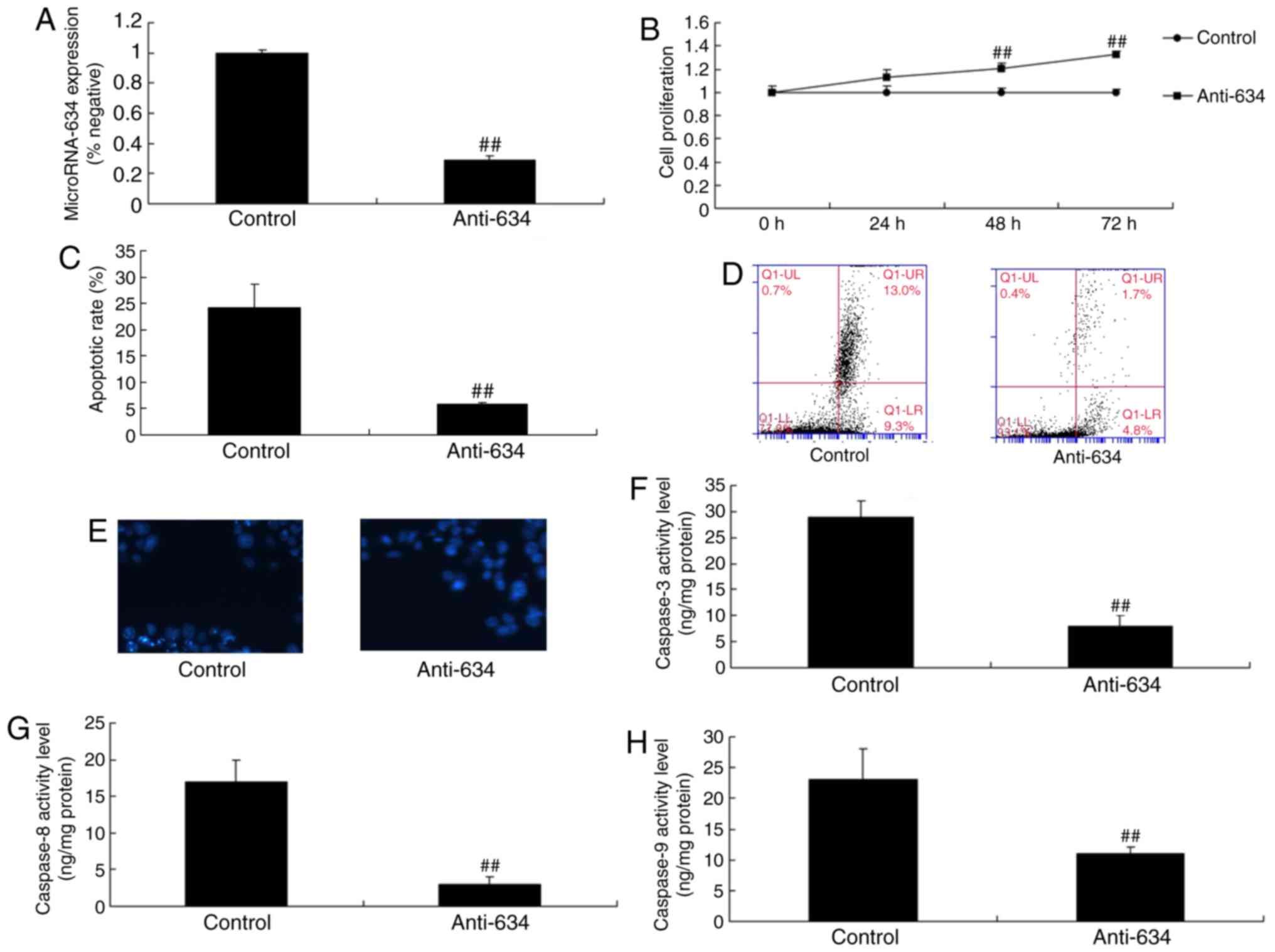
Downregulation of the expression of
miRNA-634 suppresses cell apoptosis in vitro
The present study also determined the function of
the downregulation of miRNA-634 on cell apoptosis in the in
vitro model of cerebral infarction. The downregulation of
miRNA-634 promoted cell proliferation and inhibited apoptosis in
the in vitro model of cerebral infarction, compared with the
control negative group (Fig.
3A-E). The downregulation of miRNA-634 decreased the levels of
capsase-3, capsase-8 and capsase-9 in the in vitro model of
cerebral infarction with downregulated miRNA-634, compared with
levels in the negative control group (Fig. 3F-H).
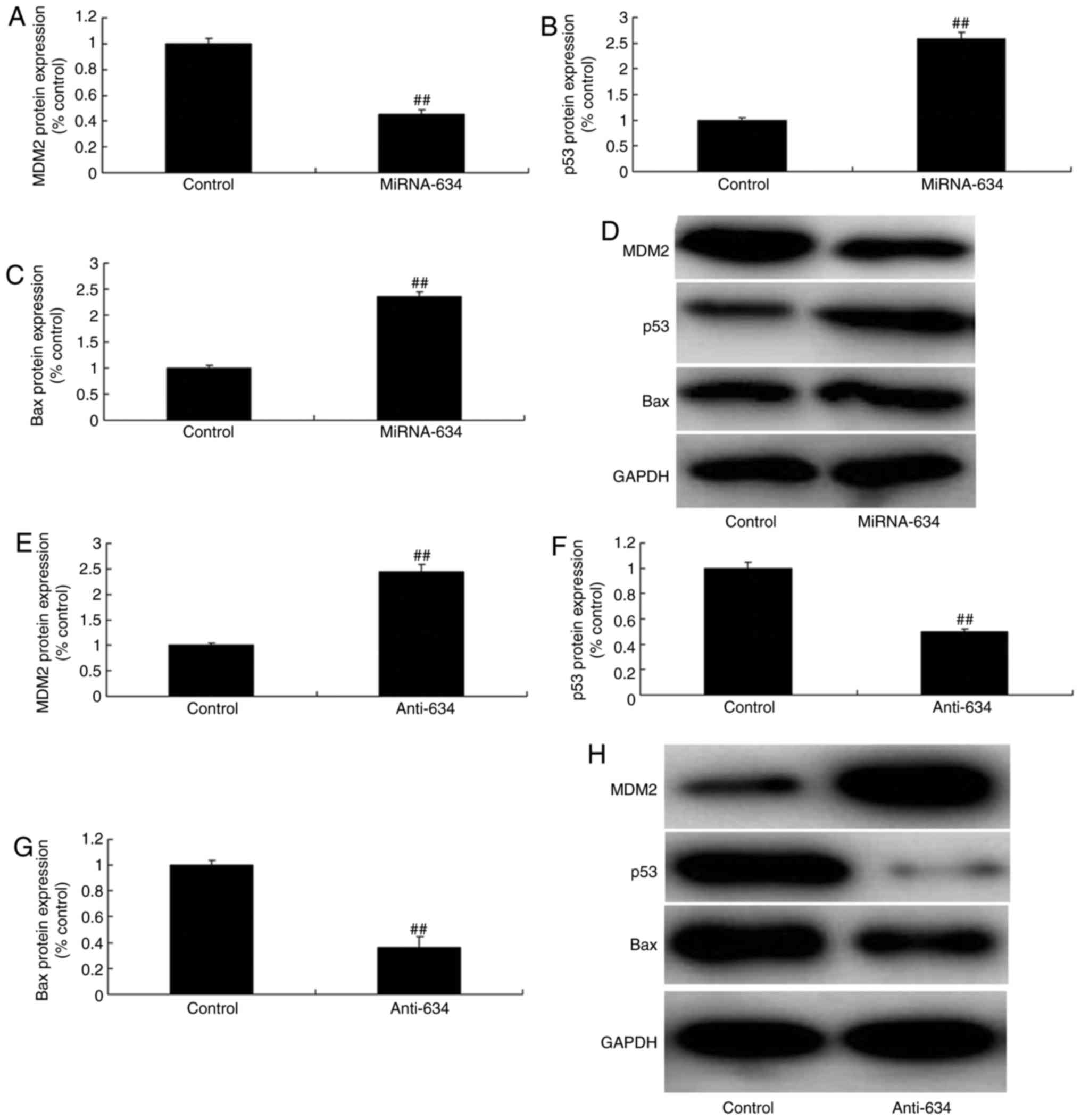
miRNA-634 regulates the MDM2/p53/Bax
pathway in vitro
The present study also aimed to validate the effect
on the 53/Bax pathway in the in vitro model of cerebral
infarction following miRNA-634 exposure. As shown in Fig. 4A-D, the upregulation of miRNA-634
suppressed the protein expression of MDM2, and induced the protein
expression of p53 and Bax in the in vitro model of cerebral
infarction, compared with expression in the negative control group.
The downregulation of miRNA-634 induced the protein expression of
MDM2, and suppressed the protein expression of p53 and Baxin the
in vitro model of cerebral infarction, compared with the
expression in the negative control group (Fig. 4E–H).
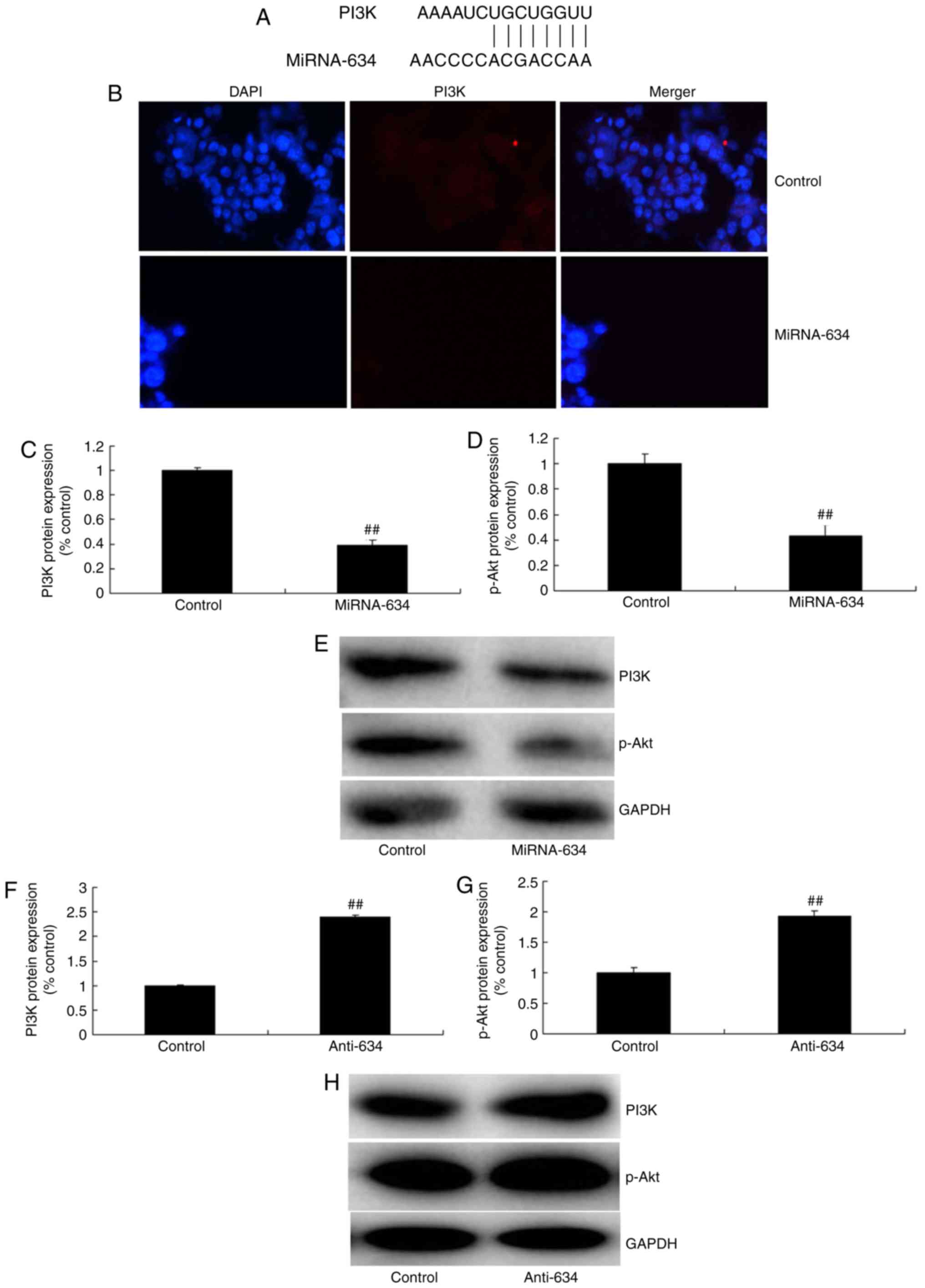
miRNA-634 regulates the PI3K/Akt pathway
in vitro
To determine the mechanism underlying the effect of
miRNA-634 on nerve apoptosis of cerebral infarction, the protein
expression levels of PI3K and p-Akt were measured using western
blot analysis and immunofluorescence. As the schematic in Fig. 5A shows, the 3′ untanslated region
of PI3K contains the miRNA-634 seed sites. The results of the
immunofluorescence showed that miRNA-634 suppressed the protein
expression of PI3K in the in vitro model of cerebral
infarction, compared with that in the negative control group
(Fig. 5B). As shown in Fig. 5C-E, the upregulated expression of
miRNA-634 suppressed the protein expression of PI3K and p-Akt in
the in vitro model of cerebral infarction, compared with the
expression in the negative control group. The down-regulation of
miRNA-634 induced the protein expression of PI3K and p-Akt in the
in vitro model of cerebral infarction, compared with the
expression in the negative control group (Fig. 5F–H).
Promotion of PI3K reduces the effects of
miRNA-634 in vitro
Additionally, the present study assessed the role of
PI3K in the effects of microRNA-634 in the in vitro model.
As shown in Fig. 6, the PI3K
agonist (10 ng/ml of 1,3-dicaffeoylquinic acid) induced the
expression of PI3K. The protein expression of p-Akt in the in
vitro model of cerebral infarction was also induced by
miRNA-634 and the PI3K agonist, compared with that in the miRNA-634
group (Fig. 6A-C). The PI3K
agonist induced the protein expression of MDM2, and suppressed the
protein expression of p53 and Bax in the in vitro model of
cerebral infarction by anti-miRNA-634, compared with the
anti-miRNA-634 group (Fig. 6D-G).
The PI3K agonist was shown to reduce the effects of miRNA-634 on
cell proliferation, apoptosis and caspase-3/8/9 activity levels in
the in vitro model of cerebral infarction by miRNA-634,
compared with levels in the miRNA-634 group (Fig. 7A-G).
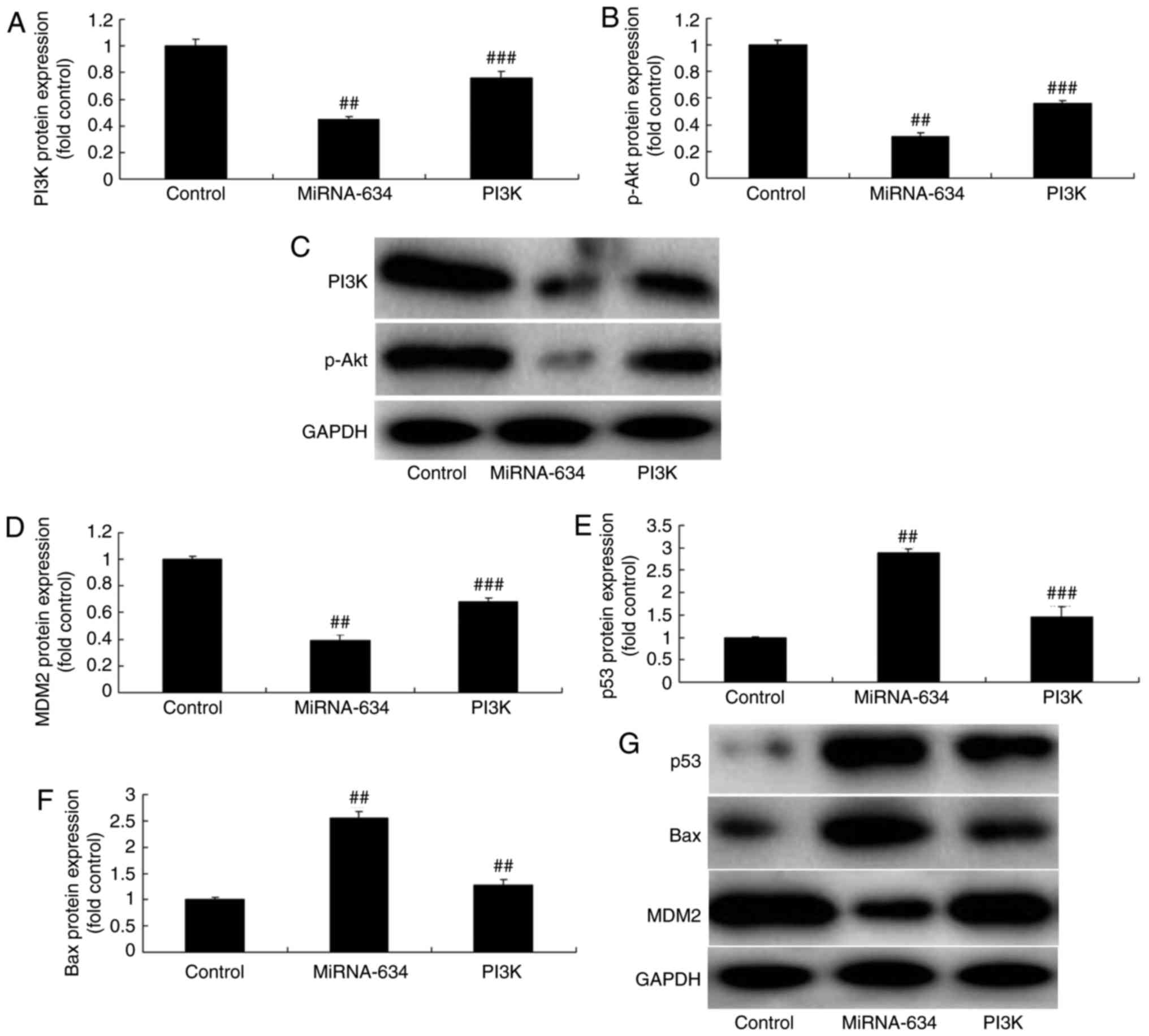 | Figure 6Promotion of PI3K reduces the effects
of miRNA-634 on the PI3K/Akt pathway in vitro. Statistical
analysis of protein expression levels of (A) PI3K and (B) from (C)
western blot analysis. Statistical analysis of protein expression
levels of (D) MDM2, (E) p53 and (F) Bax from (G) western blot
analysis in the in vitro model. ##P<0.01, vs.
control; ###P<0.01, vs. overexpression of miRNA-634
group. Control, negative control group; miRNA-634, overexpression
of miRNA-634 group; PI3K, overexpression of miRNA-634 and
1,3-dicaffeoylquinic acid group. miRNA, microRNA; PI3K,
phosphoinositide 3-kinase; p-, phosphorylated; MDM2, MDM2
proto-oncogene; Bax, B-cell lymphoma 2-associated X protein. |
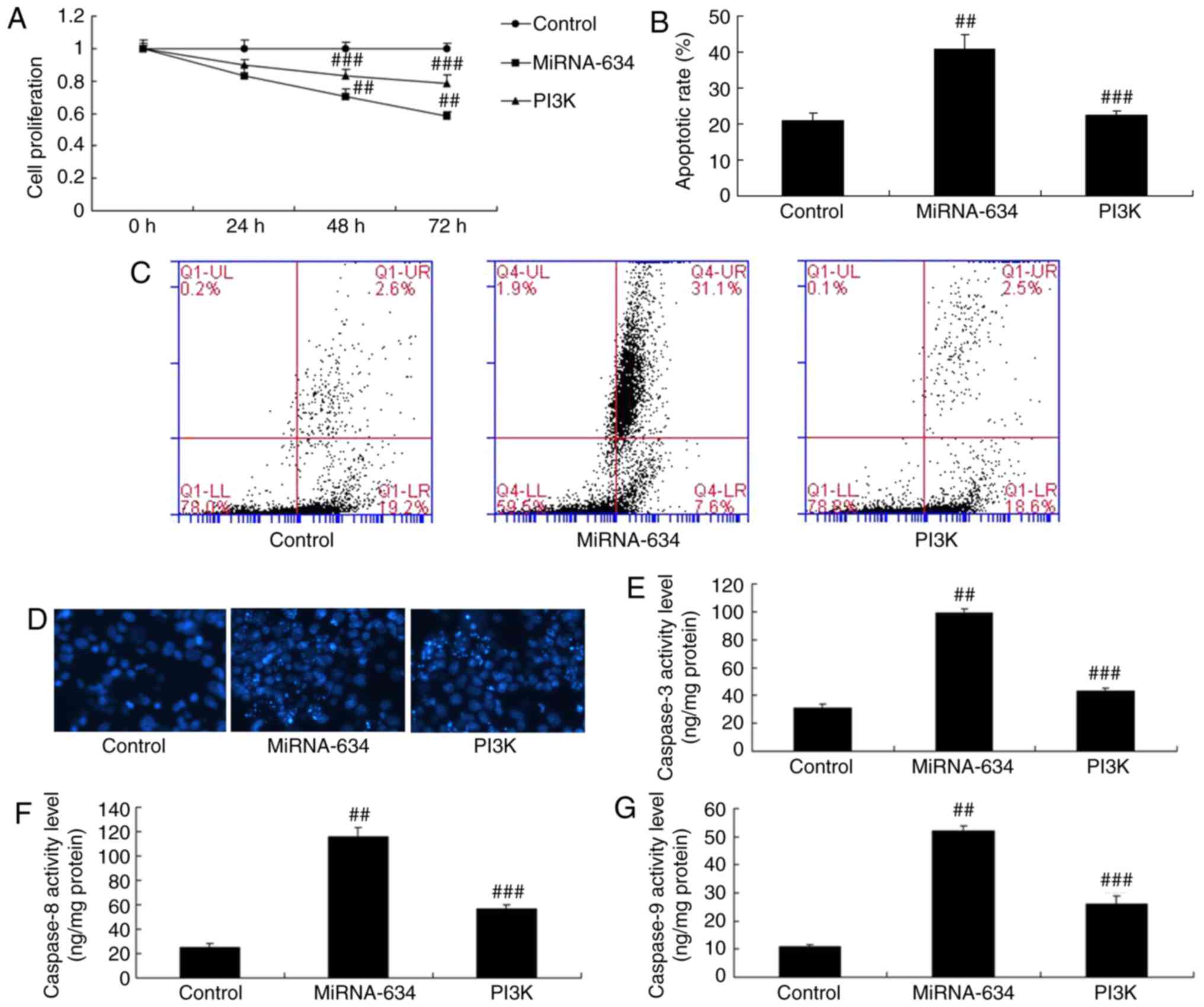 | Figure 7Promotion of PI3K reduces the effects
of miRNA-634 on cell apoptosis in vitro. (A) Cell
proliferation; (B) quantification of cell apoptosis from (C)
results of flow cytometry; (D) DAPI staining (magnification, ×100);
activity levels of (E) caspase-3, (F) caspase-8 and (G) caspase-9
in the in vitro model. ##P<0.01, vs. control;
###P<0.01, vs. miRNA-634 group. Control, negative
control group; miRNA-634, overexpression of miRNA-634 group; PI3K,
overexpression of miRNA-634 and 1,3-dicaffeoylquinic acid group;
miRNA, microRNA; PI3K, phosphoinositide 3-kinase. |
Inhibition of PI3K reduces the effects of
anti-miRNA-634 in vitro
To assess the mechanism underlying the effect of
miRNA-634 on apoptosis in the in vitro model of cerebral
infarction, the PI3K inhibitor (20 nM of NVP-BAG956), was used to
inhibit the protein expression of PI3K. The results of western blot
analysis showed that the PI3K inhibitor suppressed the protein
expression of PI3K and p-Akt in the in vitro model of
cerebral infarction by anti-miRNA-634, compared with the
anti-miRNA-634 group (Fig. 8A-C).
The PI3K inhibitor suppressed the protein expression of MDM2, and
induced the protein expression of p53 and Bax in the in
vitro model of cerebral infarction by anti-miRNA-634, compared
with the anti-miRNA-634 group (Fig.
8D-G). The PI3K inhibitor also reduced the effects of
anti-miRNA-634 on cell proliferation, apoptosis and caspase-3/8/9
activity levels in the in vitro model of cerebral infarction
by anti-miRNA-634, compared with the anti-miRNA-634 group (Fig. 9A–G).
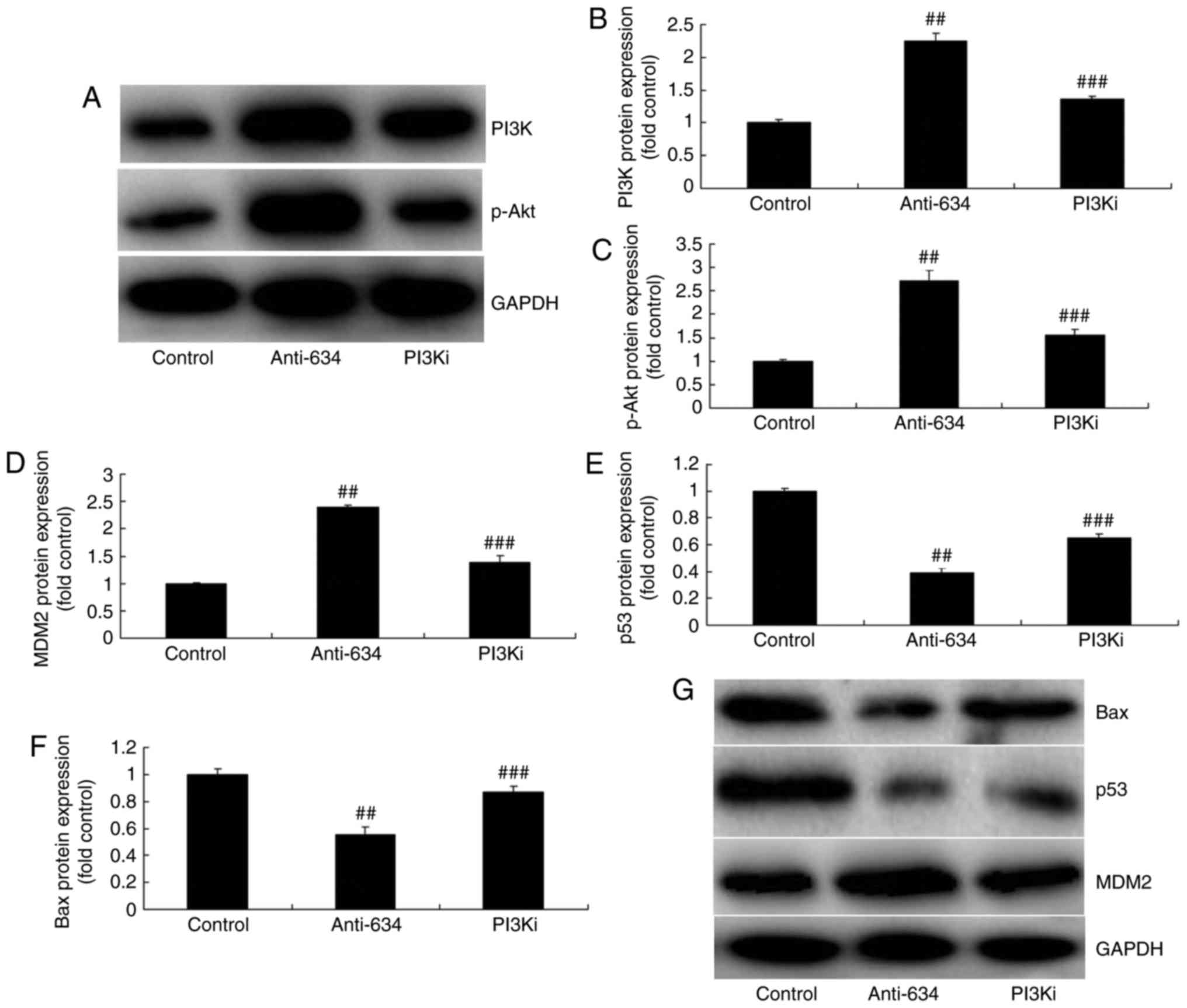 | Figure 8Inhibition of PI3K reduces the effects
of anti-miRNA-634 on the PI3K/Akt pathway in vitro. (A)
Western blot analysis of the protein expression of (B) PI3K and (C)
p-Akt. Statistical analysis of the protein expression of (D) MDM2,
(E) p53 and (F) Bax from (G) western blot analysis in the in
vitro model. ##P<0.01, vs. control;
###P<0.01, vs. downregulation of miRNA-634 group.
Control, negative control group; anti-634, downregulation of
miRNA-634 group; PI3Ki, downregulation of miRNA-634 and NVP-BAG956
group; miRNA, microRNA; PI3K, phosphoinositide 3-kinase; MDM2, MDM2
proto-oncogene; Bax, B-cell lymphoma 2-associated X protein; p-,
phosphorylated. |
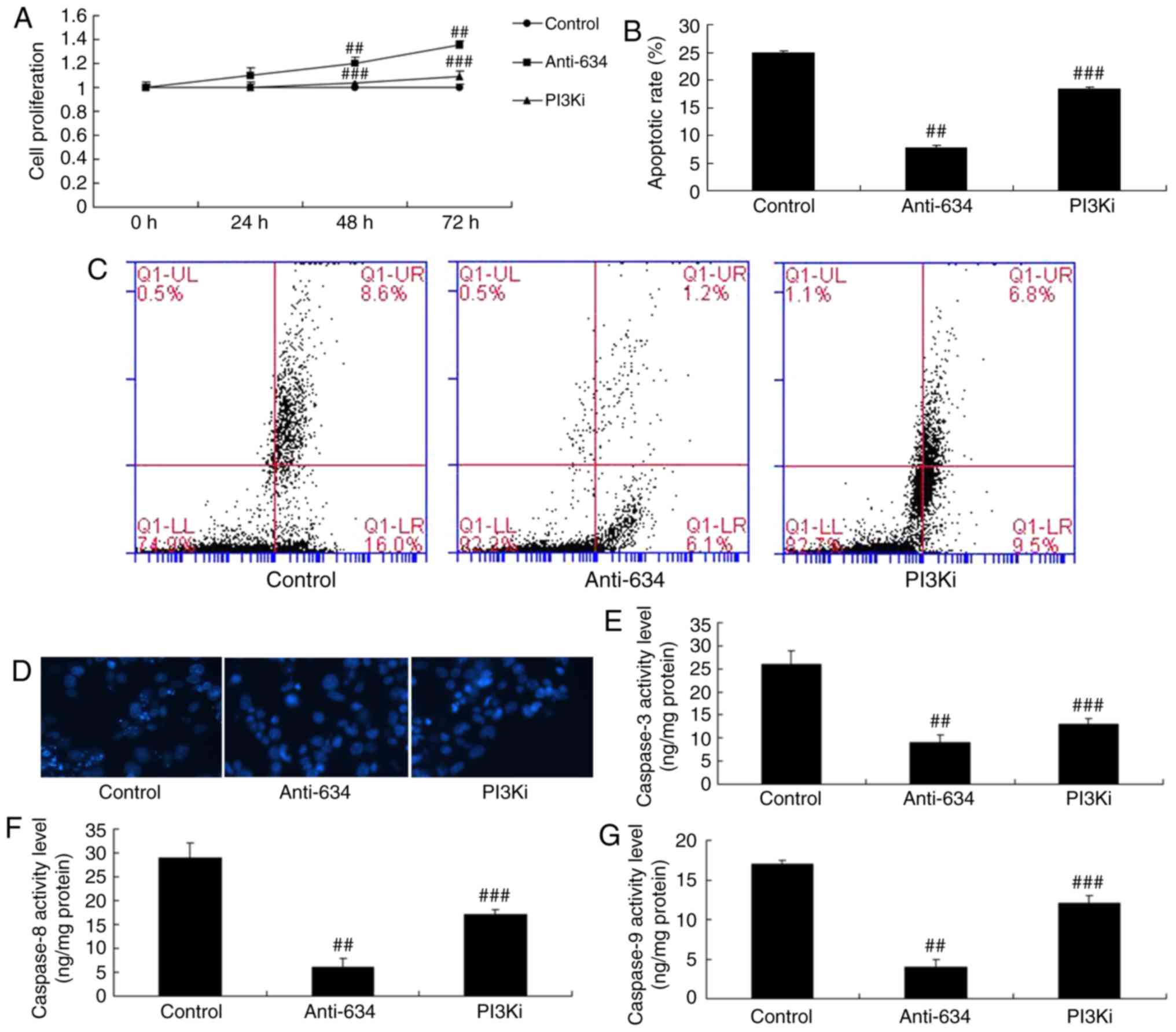 | Figure 9Inhibition of PI3K reduces the effects
of anti-miRNA-634 on cell apoptosis in vitro. (A) Cell
proliferation; (B) quantification of cell apoptosis from (C) flow
cytometry; (D) DAPI staining (magnification, ×100), activity levels
of (E) caspase-3, (F) caspase-8 and (G) caspase-9 in the in
vitro model. ##P<0.01, vs. control;
###P<0.01, vs. downregulation of miRNA-634 group.
Control, negative control group; anti-634, downregulation of
miRNA-634 group; PI3Ki, downregulation of miRNA-634 and NVP-BAG956
group; miRNA, microRNA; PI3K, phosphoinositide 3-kinase. |
Discussion
Acute cerebrovascular disease is a common and
frequently occurring disease in middle-aged and elderly individuals
(11). It is associated with high
rates of disability and mortality. The morbidity rate of
atherosclerosis has shown an increasing and younger trend year on
year with the population aging, and changes to living and social
environments. Therefore, it is a serious threat to human health and
life (3). In the present study,
it was showed that the expression level of miRNA-634 in a cerebral
infarction rat model was increased, compared with that in the
normal control group. Jeansonne et al (12) showed that the expression of
miRNA-634 was upregulated in glioblastoma.
The detection of miRNAs in human tissues is either
difficult to achieve or repeatedly used in clinic. It has been
found in previous studies that there are stable miRNA molecules in
the peripheral blood (13).
Additionally, different diseases are associated with different
miRNA expression profiles. This finding has led to novel thinking
in adopting peripheral blood miRNA for the non-invasive diagnosis
of disease (7). It is found that
peripheral blood miRNA with specific expression changes can be
observed in multiple diseases, including tumors, diabetes,
myocardial infarction, Parkinson’s disease and Alzheimer’s disease
(14). In addition, the present
study found that miRNA-634 mimics significantly inhibited cell
proliferation and induced apoptosis in an in vitro model of
cerebral infarction. Cong et al (15) showed that miRNA-634 also decreases
cell proliferation and induces apoptosis in cervical cancer
cells.
The PI3K/Akt signal transduction pathway is a
pro-survival signal. Its activation is important in
ischemic-hypoxic neuronal injury, and it has attracted increasing
attention (16). The PI3K/Akt
signal transduction pathway is an important pathway for the
intracellular transduction of membrane receptor signals. It is key
in maintaining cell survival and inhibiting cell apoptosis
(17). It can affect the
activation of effector molecules, including downstream
apoptosis-related protein and cell cycle regulatory protein
(17). Consequently, it is
important in inhibiting apoptosis and promoting proliferation in
cells (9). PI3K/Akt has a
definite anti-apoptotic protective effect on the brain in cerebral
ischemia. Inhibiting such a pathway can aggravate cerebral
ischemia-induced nerve cell apoptosis (9). The findings of the present study
suggested that downregulation of the expression of miRNA-634
significantly suppressed the protein expression of PI3K and p-Akt
in the in vitro model of cerebral infarction. Cui et
al (18) suggested that the
overexpression of miRNA-634 suppresses survival and matrix
synthesis by targeting PIK3 regulatory subunit 1 in human
osteoarthritic chondrocytes.
Caspase-3 is the most important apoptotic protease
during apoptosis. Its activation is dependent on the release of
cytochrome c (19). The
Bcl-2 and Bax genes of the Bcl-2 family are the most important
regulatory genes involved in cell apoptosis known at present. They
can mediate the release of substances, including cytochrome
c, through the mitochondrial pathway (20). A number of previous studies have
found that Bcl-2 and Bax can serve as the upstream regulatory
mechanism of caspase-3 and are involved in regulating the activity
of caspase-3 (20). In addition,
they can be treated as the direct substrate of caspase-3 to act on
the downstream caspase-3. The two correlate with and restrain each
other during cell apoptosis transduction (21). In the present study, it was shown
that the inhibition of PI3K increased the effect of the
downregulation of miRNA-634 on cell apoptosis and capsase-3/Bax
protein expression in the in vitro model of cerebral
infarction.
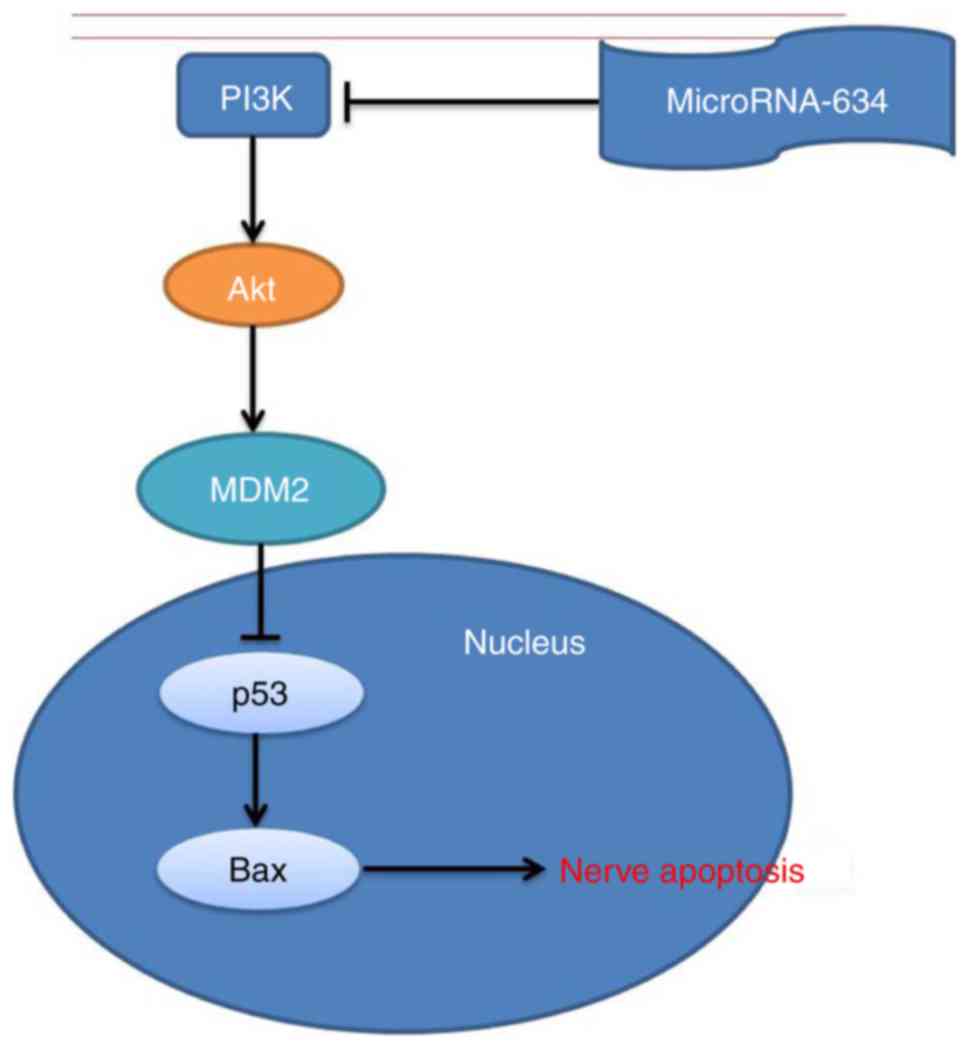
In conclusion, the findings of the present study
suggested that the plasma miRNA-634 adjusted nerve apoptosis in
cerebral infarction through the MDM2/p53/Bax pathway by PI3K/Akt
(Fig. 10). miRNA-634 may be used
as a potential therapeutic target in the treatment of cerebral
infarction for clinical use in the future.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analysed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors’ contributions
QS designed the study; YC, WH, SL, ZY, QW and XL
performed the experiments; QS and YC analyzed the data; and QS
wrote the manuscript.
Ethics approval and consent to
participate
The study was approved by the Animal Experimental
Ethics Committee of Tangshan, Worker Hospital (Tangshan,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Afshari D, Moradian N, Nasiri F, Razazian
N, Bostani A and Sariaslani P: The efficacy and safety of
low-molecular-weight heparin and unfractionated heparin in the
treatment of cerebral venous sinus thrombosis. Neurosciences
(Riyadh). 20:357–361. 2015. View Article : Google Scholar
|
|
2
|
Berge J, Blanco P, Rooryck C, Boursier R,
Marnat G, Gariel F, Wavasseur T, Desal H and Dousset V:
Understanding flow patterns and inflammatory status in intracranial
aneurysms: Towards a personalized medicine. J Neuroradiol.
43:141–147. 2016. View Article : Google Scholar
|
|
3
|
Zhou Y, Yang PF, Fang YB, Xu Y, Hong B,
Zhao WY, Li Q, Zhao R, Huang QH and Liu JM: Parent artery
reconstruction for large or giant cerebral aneurysms using a
Tubridge flow diverter (PARAT): Study protocol for a multicenter,
randomized, controlled clinical trial. BMC Neurol. 14:972014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cebral J, Ollikainen E, Chung BJ, Mut F,
Sippola V, Jahromi BJ, Tulamo R, Hernesniemi J, Niemelä M,
Robertson A and Frösen J: Flow conditions in the intracranial
aneurysm lumen are associated with inflammation and degenerative
changes of the aneurysm wall. AJNR Am J Neuroradiol. 38:119–126.
2017. View Article : Google Scholar
|
|
5
|
Yuan M, Zhan Q, Duan X, Song B, Zeng S,
Chen X, Yang Q and Xia J: A functional polymorphism at miR-491-5p
binding site in the 3′-UTR of MMP-9 gene confers increased risk for
atherosclerotic cerebral infarction in a Chinese population.
Atherosclerosis. 226:447–452. 2013. View Article : Google Scholar
|
|
6
|
Wang C, Pan Y, Cheng B, Chen J and Bai B:
Identification of conserved and novel microRNAs in cerebral
ischemia-reperfusion injury of rat using deep sequencing. J Mol
Neurosci. 54:671–683. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tian C, Li Z, Yang Z, Huang Q, Liu J and
Hong B: Plasma microRNA-16 is a biomarker for diagnosis,
stratification, and prognosis of hyperacute cerebral infarction.
PLoS One. 11:e01666882016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peng B, Guo QL, He ZJ, Ye Z, Yuan YJ, Wang
N and Zhou J: Remote ischemic postconditioning protects the brain
from global cerebral ischemia/reperfusion injury by up-regulating
endothelial nitric oxide synthase through the PI3K/Akt pathway.
Brain Res. 1445:92–102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu XY, Zhou XY, Hou JC, Zhu H, Wang Z,
Liu JX and Zheng YQ: Ginsenoside Rd promotes neurogenesis in rat
brain after transient focal cerebral ischemia via activation of
PI3K/Akt pathway. Acta Pharmacol Sin. 36:421–428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
11
|
Iveson T, Donehower RC, Davidenko I,
Tjulandin S, Deptala A, Harrison M, Nirni S, Lakshmaiah K, Thomas
A, Jiang Y, et al: Rilotumumab in combination with epirubicin,
cisplatin, and capecitabine as first-line treatment for gastric or
oesophagogastric junction adenocarcinoma: An open-label, dose
de-escalation phase 1b study and a double-blind, randomised phase 2
study. Lancet Oncol. 15:1007–1018. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jeansonne D, Pacifici M, Lassak A, Reiss
K, Russo G, Zabaleta J and Peruzzi1 F: Differential effects of
microRNAs on glioblas-toma growth and migration. Genes (Basel).
4:46–64. 2013. View Article : Google Scholar
|
|
13
|
Liu XS, Chopp M, Zhang RL and Zhang ZG:
MicroRNAs in cerebral ischemia-induced neurogenesis. J Neuropathol
Exp Neurol. 72:718–722. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kortvelyessy P, Huchtemann T, Heinze HJ
and Bittner DM: Progranulin and its related microRNAs after status
epilepticus: Possible mechanisms of neuroprotection. Int J Mol Sci.
18:2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cong J, Liu R, Wang X, Jiang H and Zhang
Y: miR-634 decreases cell proliferation and induces apoptosis by
targeting mTOR signaling pathway in cervical cancer cells. Artif
Cells Nanomed Biotechnol. 44:1694–1701. 2016. View Article : Google Scholar
|
|
16
|
Ji K, Xue L, Cheng J and Bai Y:
Preconditioning of H2S inhalation protects against cerebral
ischemia/reperfusion injury by induction of HSP70 through
PI3K/Akt/Nrf2 pathway. Brain Res Bull. 121:68–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liang K, Ye Y, Wang Y, Zhang J and Li C:
Formononetin mediates neuroprotection against cerebral
ischemia/reperfusion in rats via downregulation of the Bax/Bcl-2
ratio and upregulation PI3K/Akt signaling pathway. J Neurol Sci.
344:100–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cui X, Wang S, Cai H, Lin Y, Zheng X,
Zhang B and Xia C: Overexpression of microRNA-634 suppresses
survival and matrix synthesis of human osteoarthritis chondrocytes
by targeting PIK3R1. Sci Rep. 6:231172016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang X, Luo Y, Sun H, Feng J, Ma S, Liu J
and Huang B: Dynamic expression changes of Bcl-2, Caspase-3 and
Hsp70 in middle cerebral artery occlusion rats. Brain Inj.
29:93–97. 2015. View Article : Google Scholar
|
|
20
|
Zhang HR, Peng JH, Zhu GY and Xu RX:
Neuroprotective effects of Bcl-2 overexpression on nerve cells of
rats with acute cerebral infarction. Genet Mol Res. 14:7696–7703.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheng CY, Tang NY, Kao ST and Hsieh CL:
Ferulic acid administered at various time points protects against
cerebral infarction by activating p38 MAPK/p90RSK/CREB/Bcl-2
anti-apoptotic signaling in the subacute phase of cerebral
ischemia-reperfusion injury in rats. PLoS One. 11:e01557482016.
View Article : Google Scholar : PubMed/NCBI
|