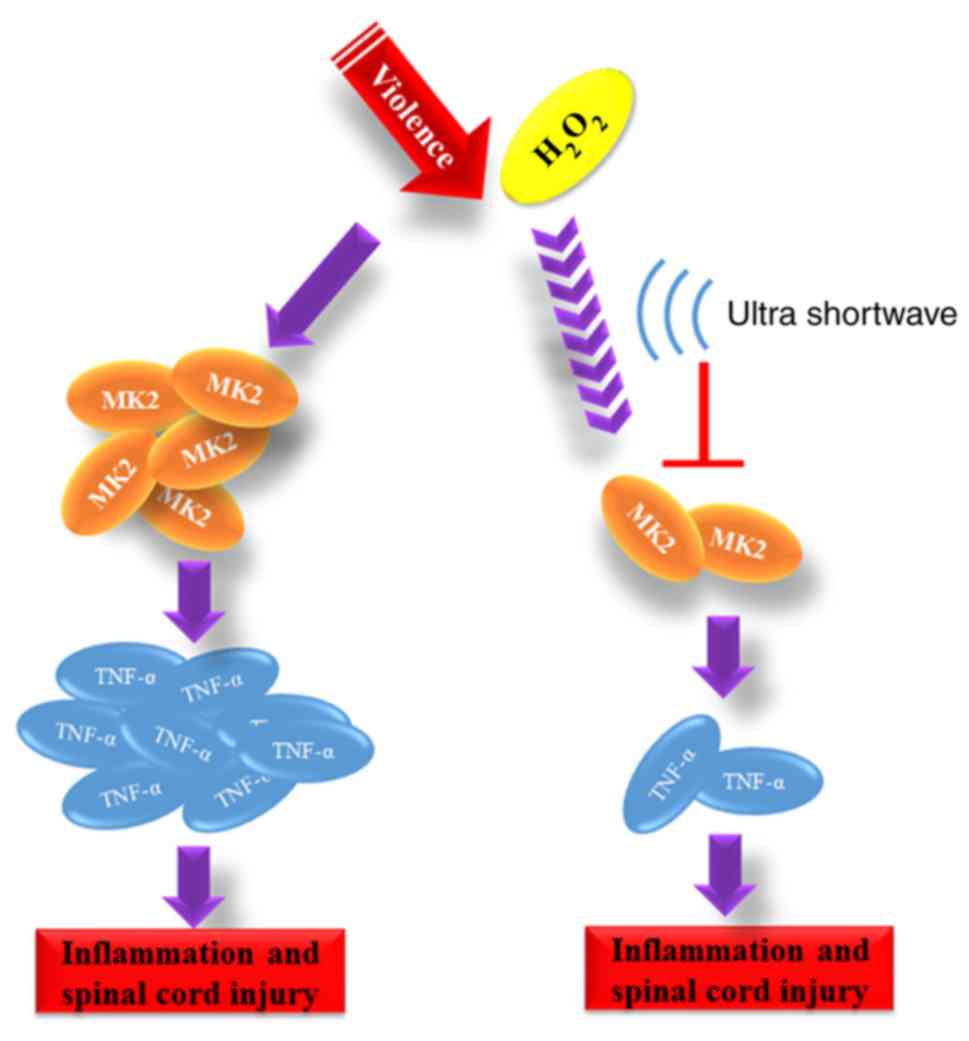
Introduction
Traumatic spinal cord injury (SCI) is a problematic
disease that results in a substantial burden on human society, and
has a marked effect on the mind and body of patients (1,2).
In general, the pathological process of SCI is divided into two
stages: The acute phase and subacute phase, in which secondary
damage to central nervous system (CNS) tissue occurs due to a
variety of factors, including ischemia, hemorrhage, excitotoxic
damage and inflammation (3). It
is widely accepted that innumerable cytokines and signaling
pathways mediating the inflammatory response contribute to the
secondary damage following SCI (4-7).
Mitogen-activated protein kinase (MAPK)-activated
protein kinase 2 (MK2) has been reported as a direct substrate of
p38α and p38β MAPKs, and is a novel molecular target for the
anti-inflammatory response (8).
Based on an animal study of Parkinson's disease, Gómez-Nicola found
that MK2-knockout mice exhibited reduced neurotoxicity and reduced
neuroinflammation (9). In
addition, Tietz et al revealed that MK2 is key in the
regulation and limitation of the immune response in the CNS
(10). To date, there have been
few studies on MK2 and SCI. Ghasemlou et al reported that a
lack of MK2 contributed to a reduction in tissue damage following
SCI and an improvement in locomotor recovery (11).
In our previous study, it was found that bone marrow
stromal cell (BMSC) transplantation combined with exposure to USW
(USW) radiation promoted tissue repair and functional recovery
following SCI. It was also demonstrated that the transplantation of
BMSCs combined with exposure to USW radiation inhibited ED1, a
marker of activated macrophages, and the expression of glial
fibrillary acidic protein, which indicated a weakening of the
inflammatory response (12). In
another previous study, it was found that USW therapy promotes
nerve axon regeneration and Schwann cell proliferation (13). The present study focused on the
effect of USW radiation on astrocyte inflammation. Using
H2O2-treated astrocyte SCI cell models and
construction of an SCI rat model, it was revealed that USW
radiation inhibited the MK2-mediated inflammatory response and
promoted the repair of SCI. The findings of the present study
provide an improved understanding of USW radiation in the treatment
of SCI.
Materials and methods
Patients and tissue samples
Serum specimens from 20 cases of patients with acute
SCI and paired serum specimens from 20 cases without SCI were
collected at Shengjing Hospital of China Medical University
(Shenyang, China) between January 2016 and December 2016. All cases
were diagnosed according to the American Spinal Injury Association
(ASIA; 2011) (14). Written
informed consent was obtained from the patients whose serum
specimens were used in the present study. The Institute Research
Medical Ethics Committee of Shengjing Hospital of China Medical
University granted approval for the study.
Astrocyte culture
C8-D1A murine astrocytes (cat. no. CRL-2541) were
purchased from America Type Culture Collection (Manassas, VA, USA.
The culture medium consisted of Dulbecco's Modified Eagle's Medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10%
(v/v) fetal bovine serum (Invitrogen; Thermo Fisher Scientific,
Inc.), 100 IU/ml penicillin and 100 mg/ml streptomycin (Baomanbio,
Shanghai, China). All C8-D1A cells were maintained at 37°C in a
humidified atmosphere containing 5% CO2. The culture
medium was replaced every third day. Cells were passaged with a
ratio of 1:4 when growing to 80% confluence.
H2O2 intervention
and USW radiation exposure
For the H2O2 intervention, 200
µM H2O2 was added to different groups
of C8-D1A cells (5×105) for 12 h, according to a
previous study (14), and these
were then harvested for subsequent detection. For the USW radiation
exposure, a USW device (Shanghai Electrical Device Company,
Shanghai, China) was used for USW radiation exposure at a frequency
of 40.68 MHz, with a maximum and actual export power of 11.32
W.
Plasmid construction and
transfection
The full length MK2 was amplified and cloned into
the KpnI and XhoI restriction sites (Promega
Corporation, Madison, WI, USA) of the pcDNA3.1 vector (Invitrogen;
Thermo Fisher Scientific, Inc.) to synthetize an MK2-overexpression
plasmid (pcDNA3.1-MK2). The pcDNA3.1-MK2 plasmids were transfected
into the cultured C8-D1A cells in the presence of
H2O2 to construct the MK2-overexpression SCI
cell model by using of Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). The pcDNA3.1 vector was transfected into
cells in the control SCI cell model. Total RNAs and total proteins
were harvested 24 h following transfection for further
detection.
Immunofluorescence analysis
The immunofluorescence procedure was performed as
previously reported (15).
Briefly, the C8-D1A cells (5×105) were passaged onto
glass coverslips (0.8×0.8 cm) and cultured to 50-60% confluence.
Following rinsing twice with PBS, the coverslips were fixed with 4%
paraformaldehyde for 15 min and blocked with 5% BSA (Cell Signaling
Technologies, Inc., Danvers, MA, USA) for 1 h at room temperature.
Subsequently, rabbit anti-MK2 antibody (Abcam, Cambridge, UK;
1:100; cat. no. ab131504) was added for incubation at 4°C
overnight. The following day, following incubation with fluorescent
secondary antibodies (1:200; cat. no. ab150081, Abcam) at room
temperature for 1 h, the coverslips were observed under a
fluorescent microscope (Leica Microsystems GmbH, Wetzlar, Germany).
Images were captured and analyzed using Image-Pro Plus 6.0 software
(Media Cybernetics, Inc., Rockville, MD, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
All the procedures were performed as described
previously (16). In brief, total
RNA was extracted using TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. cDNA
was synthesized using the PrimeScript RT Reagent kit (Takara
Biotechnology Co., Ltd., Dalian, China). The RT-qPCR analysis was
performed using SYBR-Green Premix Ex Taq (Takara Biotechnology Co.,
Ltd.) on Light Cycler 480 system (Roche Diagnostics, Basel,
Switzerland) according to the manufacturer's protocol. The
expression of tumor necrosis factor (TNF)-α, interleukin (IL)-1β
and MK2 were calculated using β-actin as an internal control by the
2−ΔΔCq method (17).
The primers used in the present study were as follows: TNF-α,
forward 5′-CCA CGT CGT AGC AGC AAA CCA CCA AG-3′ and reverse 5′-CAG
GTA CAT GGG CTC CTC ATA CC-3′; IL-1β, forward 5′-CCC AAC TGG TAC
ATC AGC ACC TC-3′ and reverse 5′-GAC ACG GAT TCC ATG GTG AAG TC-3′;
MK2, forward 5′-GAC ATG TGG TCC TTG GGT GTC ATC ATG-3′ and reverse
5′-GAG ATG GCA AGG CCG TGA TTG GAA TAG-3′; β-actin, forward 5′-AGT
GTG ACG TGG ACA TCC GCA AAG-3′ and reverse 5′-ATC CAC ATC TGC TGG
AAG GTG GAC-3′. The thermocycling conditions were: Pre-denaturation
at 95°C for 15 sec, followed by a denaturation at 95°C for 5 sec,
annealing and extension at 60°C for 30 sec for 45 cycles.
Western blot analysis
Total protein from the cells and tissue specimens
were extracted using RIPA lysis buffer (Santa Cruz Biotechnology,
Inc., Dallas, TX, USA). Protein concentration was quantified using
the BCA protein assay kit (Santa Cruz Biotechnology, Inc.) A total
of 40 µg proteins were subjected to 10% SDS-PAGE and
transferred onto a PVDF membrane, and then blocked by 5% BSA (Cell
Signaling Technologies, Inc.) for 1 h at room temperature. Each
membrane was incubated with primary antibodies at 4°C overnight,
followed by incubation with secondary antibodies [goat anti-rabbit
IgG horseradish peroxidase (HRP), 1:2,000; cat. no. ab205718;
Abcam] at room temperature for 1 h the following day. The following
antibodies were used: Rabbit anti-MK2 antibody (1:500); rabbit
anti-TNF-α antibody (1:500; ab6671; Abcam) and rabbit anti-GAPDH
antibody (1:10,000; ab128915; Abcam). After three washes with TBST,
an ECL Western Blotting Substrate Kit (cat. no. ab65623; Abcam) was
applied for chemiluminescence imaging by using of Image J2X
software (Rawak Software Inc., Stuttgart, Germany).
Terminal deoxynucleotidyl transferase
(TdT) dUTP nick-end labeling (TUNEL) assay
Cell apoptosis was determined using a TUNEL assay,
as previously described (18).
Briefly, the C8-D1A cells were seeded on coverslips and were then
fixed using 4% paraformaldehyde for 30 min, followed by
permeabilizing with 0.1% Triton X-100 for 2 min on ice. The cells
were then labeled using a TUNEL kit (Nanjing Keygen Biotech Co.,
Ltd., Nanjing, China) according to the manufacturer's protocol. The
apoptotic index was calculated using the following formula:
Apoptotic index = (total number of apoptotic cells/total number of
cells) × 100%.
Establishment of animal models and
Basso-Beattie-Bresnahan (BBB) evaluation
All the procedures were performed as previously
reported (12). A total of 48
Sprague-Dawley female rats (average weight: 180-220 g) with an
average age of 12-weeks-old, provided by the Experimental Animal
Center at Shengjing Hospital of China Medical University, were
randomly divided into three groups (n=16 each): Sham-operated
group, control group (SCI animals) and USW group (SCI animals
exposed to USW radiation). All animals were housed in a condition
of 20-25°C with 6-80% humidity and all animals were maintained
under a 12 h light/dark cycle with free access to food and water.
Spinal cord contusion was inflicted using Allen's method (Allen,
1911) when the animals were under intraperitoneal anesthesia
(12). A 2-cm midline incision
was made over the T9-T11 spinal region, and the soft tissue and
T9-T11 vertebrae were removed. Laminectomy was performed at the T10
level under a surgical microscope, avoiding dura matter laceration.
The spinal cord was injured using a modified Allen's impactor,
where a guided 9-g rod was dropped 10 cm onto the exposed dura
mater, representing moderate SCI. Following recovery from
anesthesia, the rats exhibited paralysis of both hind limbs, urine
and stool disorder, mental weakness, and slow and poor feeding, and
received manual bladder expression twice daily until recovery of
sphincter control. For the postoperative treatment of animals in
the USW group, a pair of disc electrodes 4 cm in diameter was
placed bilaterally on the T9-T11 vertebrae at a distance of 2 cm
from the skin. The USW device (Shanghai Electrical Device Company)
was operated at a frequency of 40.68 MHz, with maximum and actual
export powers of 40 and 11.32 W, respectively. USW treatment was
executed daily with a radiating exposure of 7 min/time until the
animals were sacrificed.
A 21-point BBB scale was used to assess
the hind limb locomotor function (19)
The tests were performed prior to surgery and at
1-week intervals following SCI for 4 weeks. The animals were placed
individually on an open field (radius, 90 cm) and allowed to move
freely for 4 min. Performance was evaluated by two experienced
observers blinded to the treatment groups.
Immunohistochemistry
All the procedures were performed as described
previously (20). In brief,
tissue slides (4-µm thick) were incubated with a TNF-α/MK2
primary antibody at 4°C overnight, and subsequently incubated with
biotinylated secondary antibodies (2 µg/ml; Abcam) at 37°C
for 30 min. This was followed by streptavidin-HRP complex
incubation and diaminobenzidine tetrahydrochloride (Abcam)
staining, and hematoxylin (Abcam) counterstaining. All slides were
assessed independently under a Leica DMILLED inverted microscope
(Olympus Corporation, Tokyo, Japan) by two experienced
pathologists, who were blinded to patient clinical pathology and
other information. The expression level of TNF-α/MK2 was evaluated
as described previously (21).
Statistical analysis
All data are presented as the mean ± standard
deviation and were analyzed using GraphPad Prism 5.0 software
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Two-way analysis of variance was used to compare groups
(between-subject) and time following surgery (within-subject and
repeated measures), and one-way analysis of variance was used to
examine differences in the between-subject factor. A post hoc
Bonferroni test was used when necessary to examine specific
differences between groups.
Results
MK2 is elevated in patients with SCI and
H2O2-treated C8-D1A cells
The expression of MK2 was detected in serum
specimens of 20 patients with SCI and in paired serum specimens of
20 patients without SCI. As shown in Fig. 1A and B, the expression of MK2 was
elevated in serum specimens of patients with SCI, compared with
that in serum specimens of patients without SCI (P<0.01).
Secondly, H2O2 was used to simulate an
astrocyte inflammation cell model. As shown in Fig. 1C and D, the expression levels of
TNF-α and IL-1β, two common markers of inflammation, were
significantly increased in the H2O2-treated
C8-D1A cells, compared with those in the control group (P<0.01).
Thirdly, the expression of MK2 was measured at the cellular level.
As shown in Fig. 1E–G, the
expression of MK2 was also elevated in the
H2O2-treated C8-D1A cells (P<0.01). All
the above findings indicated that an elevated level MK2 was present
in patients with SCI and in the H2O2-treated
astrocyte cell model.
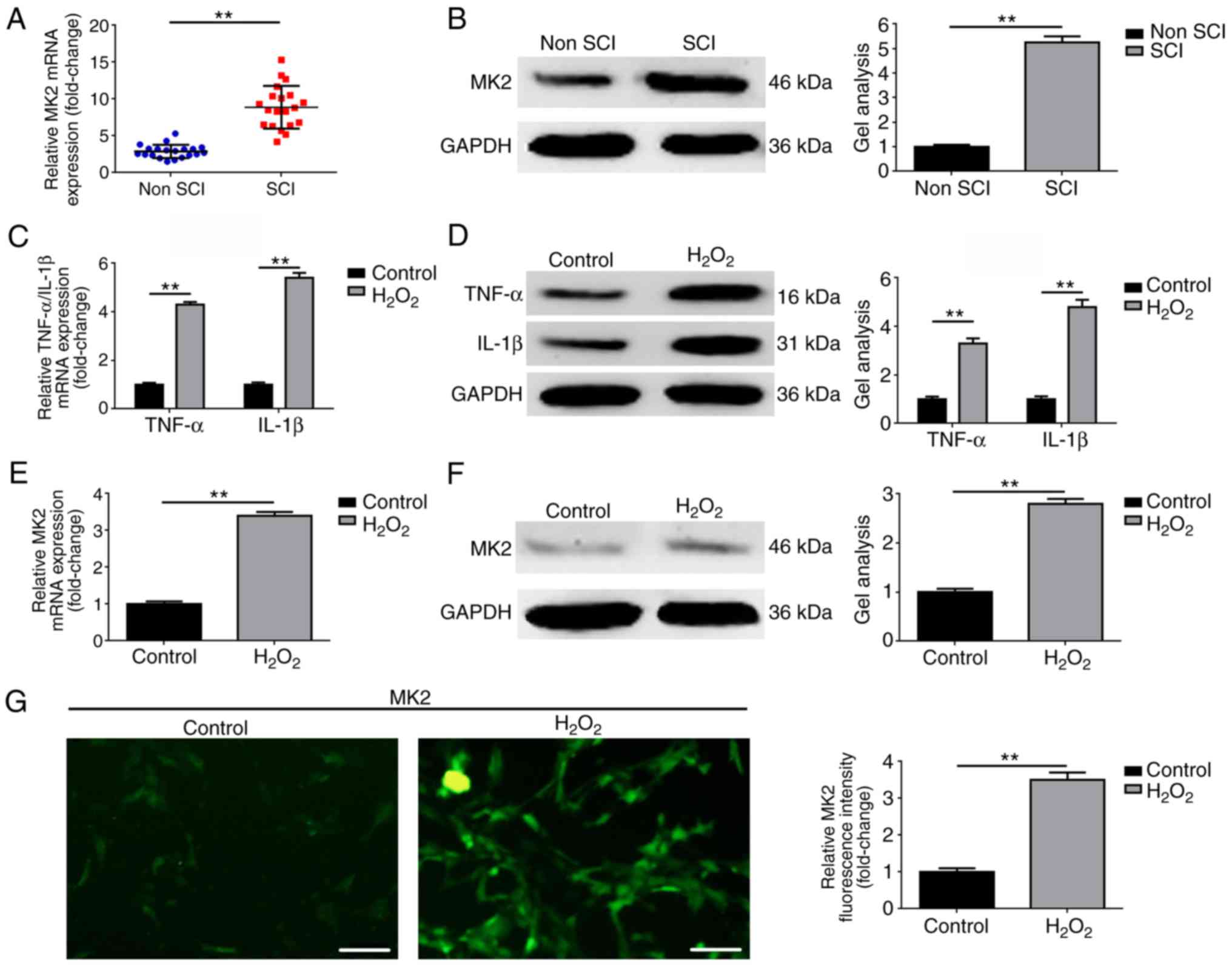 | Figure 1MK2 is elevated in patients with SCI
and H2O2-treated C8-D1A cells. MK2 was
elevated in patients with SCI, compared with non SCI group as
determined by (A) RT-qPCR and (B) western blot analyses.
**P<0.01, vs. non SCI group. Expression levels of
TNF-α and IL-1β were also elevated in the patients with SCI,
compared with the non SCI group, as determined by (C) RT-qPCR and
(D) western blot analyses. **P<0.01, vs. non SCI
group. MK2 was upregulated in H2O2-treated
C8-D1A cells, compared with the control group, as detected by (E)
RT-qPCR, (F) western blot and (G) immunofluorescence analyses
(magnification, ×200; scale bar=100 µm).
**P<0.01, vs. control group. SCI, spinal cord injury;
MK2, mitogen-activated protein kinase-activated protein kinase 2;
IL interleukin; TNF-α, tumor necrosis factor-α; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction. |
USW radiation inhibits the inflammatory
response and apoptosis in C8-D1A cells
In our previous study, it was found that USW therapy
combined with BMSC transplantation promoted the functional recovery
of SCI in rats (12). Therefore,
the present study examined whether USW radiation was able to
alleviate the astrocyte inflammatory response following SCI at the
cellular level. The expression levels of TNF-α and IL-1β were
detected in each group. As shown in Fig. 2A and B, compared with the control
group, the expression levels of TNF-α and IL-1β in the
H2O2 group and H2O2 +
USW group were elevated (P<0.01). Compared with the
H2O2 group, the expression levels of TNF-α
and IL-1β in the H2O2 + USW group were
significantly decreased (P<0.05). These results indicated that
USW radiation alleviated the inflammatory response of the
astrocytes following USW exposure. In addition, the expression of
MK2 was measured in the above-mentioned cell models. As shown in
Fig. 2C and D, USW radiation
inhibited the expression of MK2 in the
H2O2-treated C8-D1A cells (P<0.05).
Furthermore, the effect of USW treatment on the apoptosis of C8-D1A
cells was determined using a TUNEL assay. As shown in Fig. 2E, USW treatment inhibited cell
apoptosis in the H2O2-treated C8-D1A cells
(P<0.01). The above results supported the hypothesis that USW
radiation alleviates the inflammatory response and apoptosis
following H2O2 intervention, and it was
revealed that USW radiation had a protective effect in astrocytes
following H2O2 intervention.
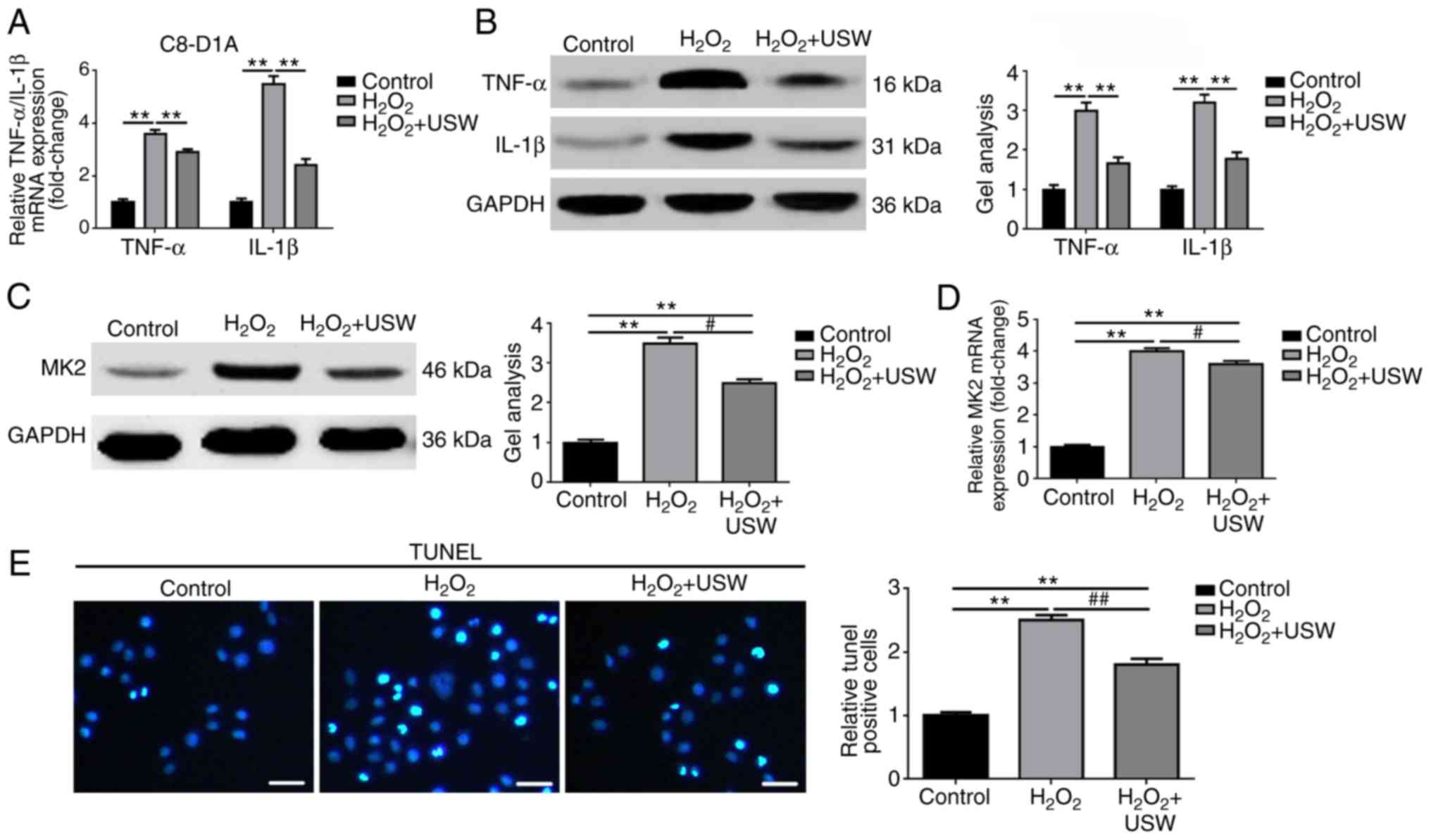 | Figure 2USW radiation inhibits the
inflammatory response and apoptosis in C8-D1A cells. USW radiation
inhibited the expression of TNF-α and IL-1β in
H2O2-treated C8-D1A cells, compared with the
control group, as detected by (A) RT-qPCR and (B) western blot
analyses. **P<0.01 vs. control group;
#P<0.05, vs. H2O2 group. USW
radiation inhibited the expression of MK2 in
H2O2-treated C8-D1A cells, compared with the
control group, as detected by (C) western blot and (D) RT-qPCR
analyses. **P<0.01, vs. control group;
#P<0.05, vs. H2O2 group. (E)
USW radiation inhibited apoptosis of
H2O2-treated C8-D1A cells, compared with the
control group, as determined using a TUNEL assay (magnification,
×400; scale bar=75 µm). **P<0.01, vs. control
group; ##P<0.05, vs. H2O2
group. USW, ultrashortwave; MK2, mitogen-activated protein
kinase-activated protein kinase 2; IL interleukin; TNF-α, tumor
necrosis factor-α; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; TUNEL, terminal deoxynucleotidyl
transferase (TdT) dUTP nick-end labeling. |
Upregulation of MK2 reverses the
protective effect USW radiation on C8-D1A cells
As described above, it was found that USW radiation
alleviated the inflammatory response and inhibited the expression
of MK2 in H2O2-treated C8-D1A cells.
Therefore, whether the protective effect of USW radiation on the
inflammatory response was achieved through the MK2 pathway was
investigated. An MK2-overexpression plasmid (pcDNA3.1-MK2) was
constructed, and the constructed pcDNA3.1-MK2 plasmid was then
transfected into the USW radiation-treated C8-D1A cells. As shown
in Fig. 3A–C, transfection of the
cells with pcDNA3.1-MK2 led to a significant elevation in the
expression of MK2 in the USW radiation-treated C8-D1A cells
(P<0.01). It was also confirmed that the elevation of MK2
promoted the expression of TNF-α and IL-1β with the presence of USW
radiation (Fig. 3D–G; P<0.01).
In addition, the results revealed that the overexpression of MK2
promoted C8-D1A cell apoptosis in the presence of USW radiation
(Fig. 3H; P<0.01). Therefore,
the findings revealed that the upregulation of MK2 reversed the
protective effect USW radiation on the C8-D1A cells.
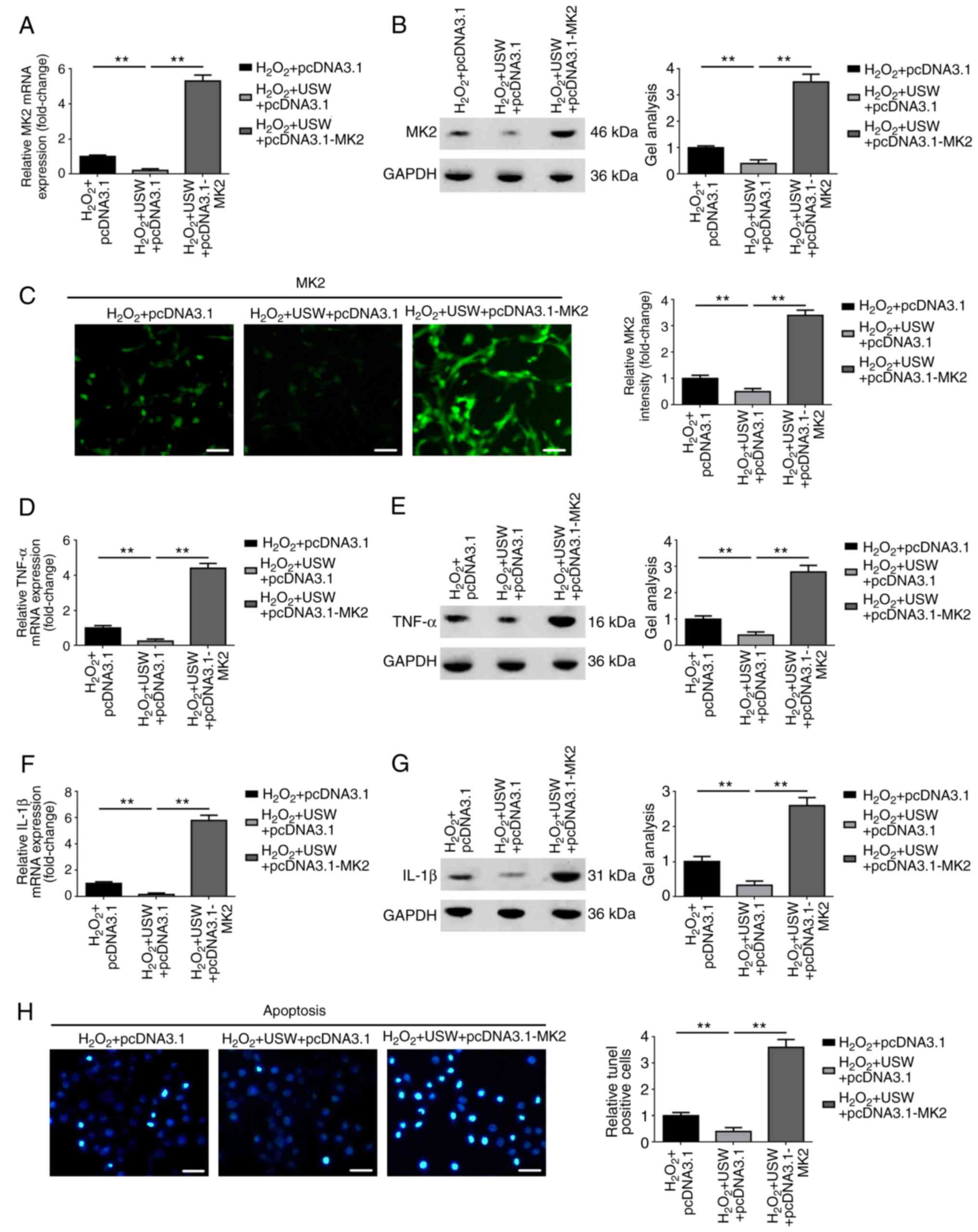 | Figure 3Upregulation of MK2 reverses the
protective effect USW radiation on C8-D1A cells. Expression of MK2
in USW radiation-treated C8-D1A cells was elevated by transfection
with pcDNA3.1-MK2, as detected by (A) RT-qPCR, (B) western blot and
(C) immunofluorescence analyses (Magnification, ×200; scale bar=100
µm). **P<0.01, vs. H2O2
+ USW + pcDNA3.1 group. Elevation of MK2 reversed the inhibitory
effect USW radiation on TNF-α, as determined by (D) RT-qPCR and (E)
western blot analyses, and on IL-1β, as determined by (F) RT-qPCR
and (G) western blot analyses. **P<0.01, vs.
H2O2 + USW + pcDNA3.1 group. (H)
Overexpression of MK2 attenuated the suppressive effect USW
radiation on apoptosis, as detected using a TUNEL assay
(magnification, ×400; scale bar=75 µm).
**P<0.01, vs. H2O2 + USW +
pcDNA3.1 group. USW, ultrashortwave; MK2, mitogen-activated protein
kinase-activated protein kinase 2; IL interleukin; TNF-α, tumor
necrosis factor-α; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; TUNEL, terminal deoxynucleotidyl
transferase (TdT) dUTP nick-end labeling. |
USW suppresses the expression of MK2 and
promotes functional recovery following SCI in vivo
To further confirm the protective effect of USW
radiation on SCI in vivo, an SCI rat model was constructed
as previously reported (12). USW
radiation was applied in treating the rats in the USW group
(Fig. 4A). As shown in Fig. 4B, compared with the sham surgery
group, the average BBB scores of the control group and USW group
were markedly decreased, indicating that the animal model was
successfully constructed. When comparing the differences between
the control group and USW group, it was found that the BBB scores
in the USW group were prominently higher than those in the control
group (Fig. 4B). These results
demonstrated that USW promoted the functional recovery of SCI. The
expression level of TNF-α was then determined in each group by IHC,
RT-qPCR and western blot analyses. As shown in Fig. 4C and D, compared with the control
group, USW radiation treatment markedly inhibited the expression of
TNF-α and IL-1β. The expression of MK2 was determined using the
same methods and, as shown in Fig.
4F, USW radiation markedly suppressed the expression of MK2
in vivo. Finally, apoptosis in the animal model was
determined. As shown in Fig. 4F,
USW radiation significantly inhibited apoptosis in the SCI
rats.
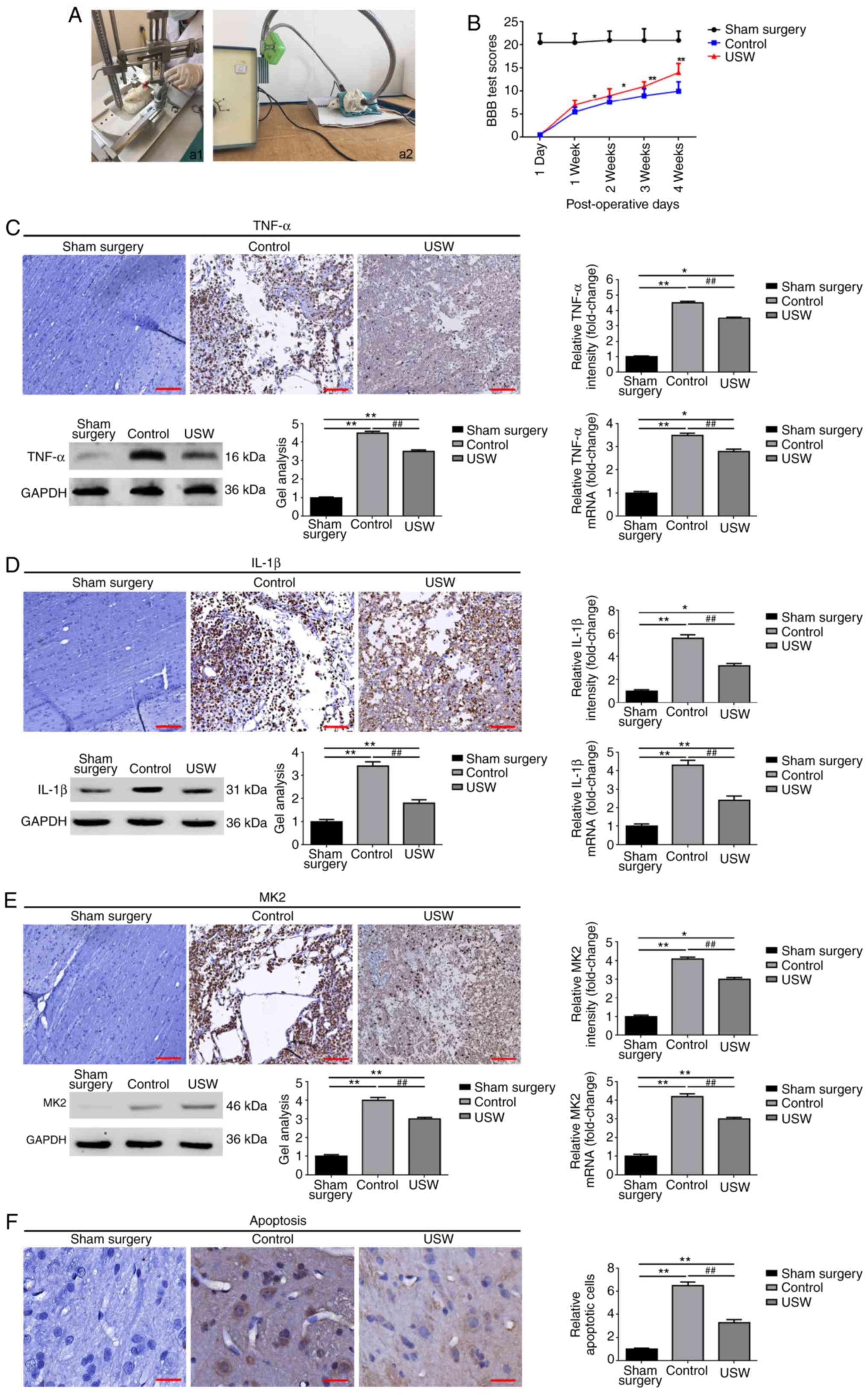 | Figure 4USW radiation suppresses the
expression of MK2 and promotes functional recovery following SCI in
rats. (A) Construction of SCI models using (a1) Allen's methods and
(a2) USW treatment. (B) BBB score system was applied to evaluate
the changes in locomotor function following USW radiation
intervention. *P<0.05 and **P<0.01, vs.
control group. USW radiation inhibited the expression of (C) TNF-α
and (D) IL-1β in vivo, as determined by IHC (magnification,
×100; scale bar=200 µm), western blot and a RT-qPCR
analyses. *P<0.05 and **P<0.01, vs.
sham surgery group; ##P<0.01, vs. control group. (E)
Expression of MK2 was decreased by USW radiation in vivo, as
determined by IHC (magnification, ×100; scale bar, 200 µm),
western blot and RT-qPCR analyses. *P<0.05 and
**P<0.01, vs. sham surgery group;
##P<0.01, vs. control group. (F) USW radiation
suppressed apoptosis following SCI, as measured using IHC
(magnification, ×400; scale bar=50 µm).
**P<0.01, vs. sham surgery group;
##P<0.01, vs. control group. USW, ultrashortwave;
MK2, mitogen-activated protein kinase-activated protein kinase 2;
IL interleukin; TNF-α, tumor necrosis factor-α; BBB,
Basso-Beattie-Bresnahan; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction. |
In brief, as the mechanism in Fig. 5 shows, the results described above
confirmed that USW radiation promoted SCI recovery by inhibiting
inflammation via suppression of the MK2/TNF-α pathway.
Discussion
Inflammation is an essential defense mechanism,
which is vital in protecting against various pathogenic attacks,
including SCI (3,22,23). As a downstream substrate of p38
MAPKs and a novel molecular target of the anti-inflammatory
response, MK2 is located at human chromosome 1q32.1 and functions
as a key regulator in the treatment of inflammatory diseases,
particularly in nervous system inflammation (8,24).
Ghasemlou et al reported that a lack of MK2 promoted
loco-motor recovery and reduced tissue damage following SCI
(11). Kroner et al
reported that MK2 facilitated TNF-induced M1 macrophage
polarization following SCI (25).
The present study focused on the expression and function of MK2
following SCI. It was found that the expression of MK2 was elevated
in patients with SCI and in an H2O2-treated
astrocyte cell model, which confirmed the role of MK2 as an
inflammatory factor in SCI, as previously reported. In addition,
through a constructed MK2-overexpression plasmid, the present study
demonstrated that the elevation of MK2 reversed the protective
effect of USW radiation on C8-D1A cells via promotion of the
expression of TNF-α and IL-1β.
As a downstream gene of MK2, TNF-α and IL-1β are
known markers of various inflammatory conditions (26,27). Tietz et al reported that
TNF-α and IL-6 signaling were mediated by the MK2 pathway, and that
the knockdown of MK2 inhibited the expression of TNF-α/IL-6 and
protected against cerulein-induced pancreatitis (28). In the present study, TNF-α and
IL-1β were selected as molecular markers in SCI-related
inflammation. The cellular and animal experiments confirmed that
TNF-α and IL-1β were elevated following the different
inflammation-related interventions. Of note, it was revealed again
that the up-regulation of MK2 led to elevated expression levels of
TNF-α and IL-1β, indicating that MK2 was upstream of these
genes.
As a type of high frequency electric field, USW
radiation exerts its function via a mechanism of heating and
non-heating effects. A low field intensity of electric field (40
mW/cm2) caused by a small dose of USW radiation may lead
to full extension or contraction on cells and may result in
biological effects via specific stimulation on cells or tissues.
Pelletier et al reported that the direct exposure to current
electric field stimulation resulted in morphological and molecular
changes of brain cells (29). In
the present study, it was confirmed that USW radiation alleviated
the inflammatory response in H2O2-treated
C8-D1A cells. In addition, it was confirmed that USW inhibited
H2O2-induced apoptosis in C8-D1A cells.
Furthermore, through in vivo experiments, the present study
also demonstrated the protective effect of USW radiation on the
constructed SCI rat model. In terms of the molecular mechanism, it
was shown that USW radiation suppressed the expression of MK2 in
the in vivo and in vitro experiments.
The inflammatory response of SCI and its treatment
are complex issues. The findings of the present study revealed that
USW promoted SCI recovery and alleviation of the inflammatory
response following SCI via the suppression of MK2; however, the
detailed mechanism of this effect on MK2 occurs remains to be fully
elucidated. The results of the present study may provide a novel
target in the molecular treatment of SCI.
Acknowledgments
Not applicable.
Funding
This study was supported by grants from National
Natural Science Foundation of China (grant no. 81101462), the
Liaoning Province Public Science Foundation (grant no. 2016 003001)
and the Liaoning Province Natural Science Foundation (grant no.
201602875).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
NW made substantial contributions to the analysis
and interpretation of data; ZF and NW contributed equally to
drafting the manuscript. WZ and ZZ made substantial contributions
to the conception of the present study and conducted the
statistical analysis. LZ critically revised the manuscript for
important intellectual content.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patients whose serum specimens were used in the present study. The
Institute Research Medical Ethics Committee of Shengjing Hospital
of China Medical University granted approval for the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stein DM and Sheth KN: Management of acute
spinal cord injury. Continuum. 21:159–187. 2015.PubMed/NCBI
|
|
2
|
Witiw CD and Fehlings MG: Acute spinal
cord injury. J Spinal Disord Tech. 28:202–210. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
David S, Zarruk JG and Ghasemlou N:
Inflammatory pathways in spinal cord injury. Int Rev Neurobiol.
106:127–152. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Allison DJ and Ditor DS: Immune
dysfunction and chronic inflammation following spinal cord injury.
Spinal Cord. 53:14–18. 2015. View Article : Google Scholar
|
|
5
|
Ambrozaitis KV, Kontautas E, Spakauskas B
and Vaitkaitis D: Pathophysiology of acute spinal cord injury.
Medicina. 42:255–261. 2006.In Lithuanian.
|
|
6
|
Bowes AL and Yip PK: Modulating
inflammatory cell responses to spinal cord injury: All in good
time. J Neurotrauma. 31:1753–1766. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang N, Yin Y, Xu SJ, Wu YP and Chen WS:
Inflammation & apoptosis in spinal cord injury. Indian J Med
Res. 135:287–296. 2012.PubMed/NCBI
|
|
8
|
Duraisamy S, Bajpai M, Bughani U, Dastidar
SG, Ray A and Chopra P: MK2: A novel molecular target for
anti-inflammatory therapy. Expert Opin Ther Targets. 12:921–936.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gómez-Nicola D, Valle-Argos B, Pita-Thomas
DW and Nieto-Sampedro M: Interleukin 15 expression in the CNS:
Blockade of its activity prevents glial activation after an
inflammatory injury. Glia. 56:494–505. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tietz SM, Hofmann R, Thomas T, Tackenberg
B, Gaestel M and Berghoff M: MK2 and Fas receptor contribute to the
severity of CNS demyelination. PLoS One. 9:e1003632014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ghasemlou N, Lopez-Vales R, Lachance C,
Thuraisingam T, Gaestel M, Radzioch D and David S:
Mitogen-activated protein kinase-activated protein kinase 2 (MK2)
contributes to secondary damage after spinal cord injury. J
Neurosci. 30:13750–13759. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yin YM, Lu Y, Zhang LX, Zhang GP and Zhang
ZQ: Bone marrow stromal cells transplantation combined with
ultrashort-wave therapy promotes functional recovery on spinal cord
injury in rats. Synapse. 69:139–147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang LX, Tong XJ, Sun XH, Tong L, Gao J,
Jia H and Li ZH: Experimental study of low dose ultrashortwave
promoting nerve regeneration after acellular nerve allografts
repairing the sciatic nerve gap of rats. Cell Mol Neurobiol.
28:501–509. 2008. View Article : Google Scholar
|
|
14
|
Kirshblum SC, Burns SP, Biering-Sorensen
F, Donovan W, Graves DE, Jha A, Johansen M, Jones L, Krassioukov A,
Mulcahey MJ, et al: International standards for neurological
classification of spinal cord injury (revised 2011). J Spinal Cord
Med. 34:535–546. 2011. View Article : Google Scholar
|
|
15
|
Wang Y, Yang T, Liu Y, Zhao W, Zhang Z, Lu
M and Zhang W: Decrease of miR-195 promotes chondrocytes
proliferation and maintenance of chondrogenic phenotype via
targeting FGF-18 pathway. Int J Mol Sci. 18:E9752017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Zhang Y, Yang T, Zhao W, Wang N,
Li P, Zeng X and Zhang W: Long non-coding RNA MALAT1 for promoting
metastasis and proliferation by acting as a ceRNA of miR-144-3p in
osteosarcoma cells. Oncotarget. 8:59417–59434. 2017.PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔC T method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Kyrylkova K, Kyryachenko S, Leid M and
Kioussi C: Detection of apoptosis by TUNEL assay. Methods Mol Biol.
887:41–47. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Basso DM, Beattie MS and Bresnahan JC: A
sensitive and reliable locomotor rating scale for open field
testing in rats. J Neurotrauma. 12:1–21. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Wang N, Zeng X, Sun J, Wang G, Xu
H and Zhao W: MicroRNA-335 and its target Rock1 synergistically
influence tumor progression and prognosis in osteosarcoma. Oncol
Lett. 13:3057–3065. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Sun J, Wei X, Luan L, Zeng X, Wang
C and Zhao W: Decrease of miR-622 expression suppresses migration
and invasion by targeting regulation of DYRK2 in colorectal cancer
cells. Onco Targets Ther. 10:1091–1100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
da Silva Alves E, de Aquino Lemos V, Ruiz
da Silva F, Lira FS, Dos Santos RV, Rosa JP, Caperuto E, Tufik S
and de Mello MT: Low-grade inflammation and spinal cord injury:
Exercise as therapy? Mediators Inflamm. 2013:9718412013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ren Y and Young W: Managing inflammation
after spinal cord injury through manipulation of macrophage
function. Neural Plast. 2013:9450342013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Corrêa SA and Eales KL: The role of p38
MAPK and its substrates in neuronal plasticity and
neurodegenerative disease. J Signal Transduct. 2012:6490792012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kroner A, Greenhalgh AD, Zarruk JG, Passos
Dos Santos R, Gaestel M and David S: TNF and increased
intracellular iron alter macrophage polarization to a detrimental
M1 phenotype in the injured spinal cord. Neuron. 83:1098–1116.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sedger LM and McDermott MF: TNF and
TNF-receptors: From mediators of cell death and inflammation to
therapeutic giants-past, present and future. Cytokine Growth Factor
Rev. 25:453–472. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zelová H and Hošek J: TNF-α signalling and
inflammation: Interactions between old acquaintances. Inflamm Res.
62:641–651. 2013. View Article : Google Scholar
|
|
28
|
Tietz AB, Malo A, Diebold J, Kotlyarov A,
Herbst A, Kolligs FT, Brandt-Nedelev B, Halangk W, Gaestel M, Göke
B, et al: Gene deletion of MK2 inhibits TNF-alpha and IL-6 and
protects against cerulein-induced pancreatitis. Am J Physiol
Gastrointest Liver Physiol. 290:G1298–G1306. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pelletier SJ, Lagacé M, St-Amour I,
Arsenault D, Cisbani G, Chabrat A, Fecteau S, Lévesque M and
Cicchetti F: The morphological and molecular changes of brain cells
exposed to direct current electric field stimulation. Int J
Neuropsychopharmacol. 18:pyu0902014. View Article : Google Scholar : PubMed/NCBI
|