Introduction
There are several mechanisms associated with the
pathogenesis of non-alcoholic fatty liver disease (NAFLD) (1), including a potential role for the
intestinal microbiota (IM) (2–6)
and the hepatic immune system (7–9).
Elevated hepatic immune cell markers such as cluster of
differentiation (CD)45, CD163, CD20 and CD3 have been detected in
NAFLD, mostly in pediatric studies (7–9).
CD45 is a marker of hematopoietic cells (10), and an increase of CD45 indicates
activation of one or more inflammatory cell types (9). CD163 is another immune cell marker,
which is expressed on Kupffer cells (KCs). When activated, KCs
produce cytokines and chemokines, contributing to hepatic injury
(11). In addition, markers of B
cells such as CD20 (8,12) and CD3, a marker for T-cell
activation (10), have been
reported to be elevated in the livers of patients with NAFLD;
however the results are conflicting (7–9).
Significant differences in IM have also been
reported in NAFLD compared with controls (2–6).
IM may contribute to NAFLD by increasing intestinal permeability
which can lead to the translocation of viable bacteria and/or
bacterial products across the gut mucosa (13), causing the production of
pro-inflammatory cytokines and activation of hepatic inflammatory
cells (14). This is supported by
studies reporting elevated serum endotoxin levels in NAFLD vs.
healthy controls (2). One such
study investigated endotoxin and plasminogen activator inhibitor 1
(PAI-1) concentrations in NAFLD vs. healthy controls (HC) and
demonstrated that these concentrations were higher in those with
NAFLD (15). PAI-1 stimulates
inflammation through upregulation of inflammatory factors,
promoting inflammatory cell migration (15). Similar findings were also
demonstrated in a previous pediatric study (16). In addition, translocation of
bacteria can trigger an inflammatory response through the
activation of Toll-like-receptor-4 (17), which in turn activates
proinflammatory cytokines (18).
These results suggest a role for IM in hepatic immune cell
activation. To the best of our knowledge, no previous studies have
assessed both IM and hepatic immune cell markers in the same
patients to determine if there are associations between specific IM
and immune cell markers.
The aim of the present study was to investigate
whether there are differences in specific hepatic inflammatory cell
markers between patients with NAFLD and HC, using the antigens
CD45, CD163, CD20 and CD3, and to explore whether these markers are
associated with specific IM.
Materials and methods
Methodology
The present prospective, cross-sectional study was
conducted at the University Health Network (UHN; Toronto, Canada)
between January 2008 and September 2012. The study protocol was
approved by the UHN and University of Toronto (Toronto, Canada)
Research Ethics Boards and conformed to the ethical guidelines of
the 1975 Declaration of Helsinki. All participants provided
informed written consent.
Subjects
A total of 42 participants were included in the
present study: 12 with simple steatosis (SS), 22 with non-alcoholic
steatohepatitis (NASH) and 8 with HC. Adults with persistently
elevated alanine aminotransferase (ALT) levels were assessed by a
hepatologist in order to rule out other causes of liver disease.
Patients with biopsy-proven NAFLD were eligible for recruitment.
Healthy adults undergoing assessment for live liver donation at the
Living Liver Donor Transplant Program at UHN were eligible for
recruitment as HC. One stool sample, fasting blood work and
anthropometric measurements were collected from participants prior
to liver biopsy.
Participants were excluded if they had: Liver
disease other than NAFLD; end-stage liver disease; anticipated need
for a liver transplant within 12 months; chronic gastrointestinal
diseases; previous surgery of the gastrointestinal tract that
modifies the anatomy; use of medications associated with
steatosis/steatohepatitis (e.g. corticosteroids); use of pre- or
probiotics within the past 6 months; use of vitamin E or fish oil
supplements; consumption of >20 g of alcohol per day; and
pregnancy or lactating state.
Biochemical and clinical data
Participants’ anthropometrics, smoking status,
alcohol consumption habits, medication usage and samples were
collected prior to the liver biopsy. The UHN Laboratory Medicine
Program analyzed fasting blood samples for hemoglobin A1c, insulin,
glucose, liver enzymes, lipid profile and platelets. Hemoglobin A1C
in plasma was measured by ion exchange high performance liquid
chromatograph (Variant II analyzer; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Serum fasting insulin was determined by
semi-automated immunoassay (Abbott IMX Architect i2000 system;
Abbott Laboratories, Abbott Park, IL, USA). Fasting blood glucose
was measured by the enzymatic hexokinase method on an Architect
c8000 system (Abbott Laboratories). ALT, aspartate aminotransferase
(AST) and alkaline phosphatase (ALP) in plasma were measured using
an automated method on the Architect c8000 system (Abbott
Laboratories). Triglycerides, total cholesterol and high-density
lipoprotein (HDL) were measured using an enzymatic reaction on the
Architect C16000 system (Abbott Laboratories). Low-density
lipoprotein (LDL) was calculated by subtracting HDL from total
cholesterol. Platelet aggregation was measured using a manual
platelet aggregometer. The homeostatic model assessment-insulin
resistance (HOMA-IR) was calculated using fasting serum glucose and
insulin (19).
Histology
Liver histology assessment
Liver samples for patients with NAFLD were harvested
using percutaneous needle biopsy, and an intraoperative wedge
biopsy was obtained for HC. Within 15 min of collection, biopsies
were preserved in 10% buffered formalin for 24 h at room
temperature, embedded in paraffin and cut into 4-5-µm thick
sections. Stains including hematoxylin-eosin, Masson trichrome and
Perl’s Prussian blue were performed at room temperature for 15 sec
and were used for the diagnosis of NAFLD, morphologic evaluation
and to rule out iron loading. A single pathologist assessed the
liver histology using a light microscope (020-525.024; Leica
Microsystems GmbH, Wetzlar, Germany) for the presence of steatosis,
inflammation and fibrosis using the validated and reproducible
Brunt system (20). Additionally,
the NAFLD Activity Score (NAS) was used to evaluate disease
severity (21). The NAS is scored
out of 8 points and includes steatosis, hepatocyte ballooning and
lobular inflammation (21).
Scores corresponded to level of steatosis as follows: 0, <5%; 1,
5–33%; 2, <33–66%; and 3, >66%. Scores corresponded to level
of hepatocyte ballooning as follows: 0, no ballooning; 1, few
ballooning; and 2 prominent ballooning. Scores corresponded to
level of lobular inflammation as follows; 0, no foci; 1, <2
foci; 2, 2–4 foci; and 3, >4 foci/200x field (21).
Hepatic immune cells
The formalin-fixed paraffin-embedded liver tissue
underwent immunostaining using primary monoclonal mouse antibodies
raised against cell markers CD45, CD163, CD20 and CD3, as detailed
in Table I. The ImmPress
anti-Rabbit kit (cat. no. MP-7401; Vector Laboratories, Inc.,
Burlingame, CA, USA) blocking was performed at room temperature for
20 min, and the Mach 4 (cat. no. BC-MU534G; Inter-Medico, Markham,
ON, Canada) blocking was performed at room temperature for 5 min.
The serum used for all blocking was provided by the ImmPress
anti-Rabbit kit (Vector Laboratories, Inc.). Staining was performed
at the UHN Laboratory Medicine Program. For this, 4 µm
formalin-fixed paraffin-embedded sections were dewaxed at room
temperature for 15 min in 5 changes of xylene and brought down to
water through graded alcohols. Antigen retrieval or unmasking
procedures were applied. Endogenous peroxidase was blocked with 3%
hydrogen peroxide at room temperature for 15 min. The detection
systems used were dependent on the marker. Two detections kits were
used: ImmPress anti-Rabbit kit (Vector Laboratories, Inc.) and Mach
4 (Inter-Medico). After following the kit instructions, color
development was performed with freshly prepared
3,3′-diaminobenzidine (Dako; Agilent Technologies, Inc., Santa
Clara, CA, USA). Finally, sections were counterstained lightly at
room temperature for 15 sec with Mayer’s Hematoxylin, dehydrated in
alcohol, cleared in xylene and mounted with Permount mounting
medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA). A
pathologist blinded to the study groups determined the density of
the cells that stained positive for the aforementioned monoclonal
antibodies in both the portal tract and liver lobule. This was
determined by counting the number of positive cells in 10 random
portal tracts and in 10 areas of the lobular region at a
magnification of x200 under light microscopy.
 | Table IMaterials and methods for
immunohistochemical staining. |
Table I
Materials and methods for
immunohistochemical staining.
| Antibody | Source | Cat. no. | Clonality | Pretreatment | Antigen retrieval
temperature | Dilution,
incubation, temerature | Blocking serum | Detection kit used
(polymer system) |
|---|
| CD3 | Dako | A0452 | Polyclonal
antibody | Tris-EDTA pH
9.0 | 120°C | 1:300, 1 h, RT | 2.5% normal horse
serum | Impress Rabbit |
| CD20 | Dako | M0755 | L26 | Citrate pH 6.0 | 120°C | 1:500, 1 h, RT | 2.5% normal horse
serum | MACH 4 kit |
| CD45 | Dako | M0701 | PD7+2B11 | Citrate pH 6.0 | 120°C | 1:1,000, 1 h,
RT | 2.5% normal horse
serum | MACH 4 kit |
| CD 163 | Leica | NCL-L-CD163 | 10D6 | Citrate pH 6.0 | 120°C | 1:200, 1 h, RT | 2.5% normal horse
serum | MACH 4 kit |
Stool sample collection and
measurements
A subset of 39 patients, 12 with SS, 22 with NASH
and 5 HC provided stool samples for IM. A total of 3 patients with
HC were unable to produce stool samples and thus were excluded from
this analysis. Participants provided a stool sample according to
previously published methods (22). Stools samples were stored at −80°C
prior to analyses. Total DNA from fecal samples was extracted as
previously described (6).
Quantitative polymerase chain reaction (qPCR) was then performed in
a 7900HT thermocycler (Applied Biosystems; Thermo Fisher
Scientific, Inc.), using 50 ng DNA, 16S rRNA-based qPCR primers and
TaqMan or Power SYBR Green 2X Master Mixes (Thermo Fisher
Scientific, Inc.) to quantify total bacteria and selected
groups/genera of gut microorganisms as previously described
(6). These assays were adapted
from the literature to target total bacteria, Bacteroidetes,
Prevotella, Alistipes, Coprococcus, Ruminococcus, Clostridium
leptum, C. coccoides, Lactobacillus, Faecalibacterium
prausnitzii, Bifidobacterium and Escherichia coli
(23–25). Most of these bacteria were chosen
based on previous literature suggesting an association with NAFLD
(2–6,25).
The specific primers used are provided in a previously published
study from the present authors (25). The number of microbial cells in
the fecal samples was calculated by interpolation of standard
curves and expressed as colony forming unit per gram (CFU/g) of wet
feces and normalized to total counts (relative abundance) as
previously described (6).
Statistical analysis
Measured parameters were compared between groups
using the Kruskal-Wallis test followed by Wilcoxon ranked sum, or
χ2 and Fisher’s exact test as necessary, with Bonferroni
correction for multiple comparisons between diagnostic groups.
P<0.05 was considered to indicate a statistically significant
difference. Spearman correlation coefficients and partial Spearman
correlation coefficients were used to evaluate the association
between the bacterial relative abundances and immunohistochemistry.
Analysis was performed using SAS (version 9.4; SAS Institute, Inc.,
Cary, NC, USA) and R (version 3.2.5; https://cran.r-project.org/bin/windows/base/old/3.2.5/).
Results
Demographic and laboratory results
Table II
summarizes demographic and laboratory results. Patients with SS and
NASH were older and had higher AST and ALT levels compared with HC.
Additionally, those with NASH had higher BMI, fasting insulin,
HOMA-IR score and triglycerides compared with those with HC.
 | Table IIDemographic and laboratory
results. |
Table II
Demographic and laboratory
results.
| Variables | HC (n=8) | SS (n=12) | NASH (n=22) |
|---|
| Sex, % male | 62.5 | 58.3 | 45.5 |
| Age (years) | 35.50 (23.00,
48.00) | 50.50 (33.00,
68.00)a | 45.50 (29.00,
61.00)a |
| BMI
(kg/m2) | 26.75 (23.76,
35.27) | 26.60 (23.54,
37.15) | 31.80 (24.17,
49.53)a |
| Waist-to-hip
ratio | 0.90 (0.77,
1.03) | 0.93 (0.85,
1.04) | 0.98 (0.81,
1.05) |
| AST (U/l) | 17.50 (14.00,
20.00) | 26.00 (19.00,
112.00)a | 44.00 (18.00,
114.00)a,b |
| ALT (U/l) | 15.00 (11.00,
21.00) | 45.50 (21.00,
168.00)a | 68.00 (22.00,
141.00)a |
| ALP (U/l) | 85.00 (64.00,
105.00) | 64.50 (40.00,
105.00) | 72.50 (37.00,
107.00) |
| Glucose
(mmol/l) | 5.00 (4.40,
6.00) | 5.80 (4.70,
11.40) | 5.60 (4.20,
7.60) |
| Insulin
(pmol/l) | 49.00 (20.00,
85.00) | 33.00 (15.00,
437.00) | 110.00 (36.00,
720.00)a |
| HOMA-IR | 1.73 (0.68,
3.78) | 2.99 (0.54,
21.04) | 4.89 (1.21,
40.00)a |
| HbA1c | 0.05 (0.05,
0.06) | 0.06 (0.05,
0.09)a | 0.06 (0.05,
0.07) |
| Triglycerides
(mmol/l) | 1.01 (0.57,
1.38) | 1.02 (0.62,
3.97) | 1.52 (0.28,
5.90)a |
| Total cholesterol
(mmol/l) | 5.13 (4.85,
6.35) | 4.80 (3.88,
6.88) | 4.64 (2.63,
9.83) |
| HDL (mmol/l) | 1.33 (0.83,
2.07) | 1.28 (0.95,
1.72) | 1.12 (0.34,
1.60) |
| LDL (mmol/l) | 3.44 (3.07,
3.88) | 2.73 (2.18,
5.06) | 2.80 (0.73,
4.94) |
Immunohistochemistry and liver
histology
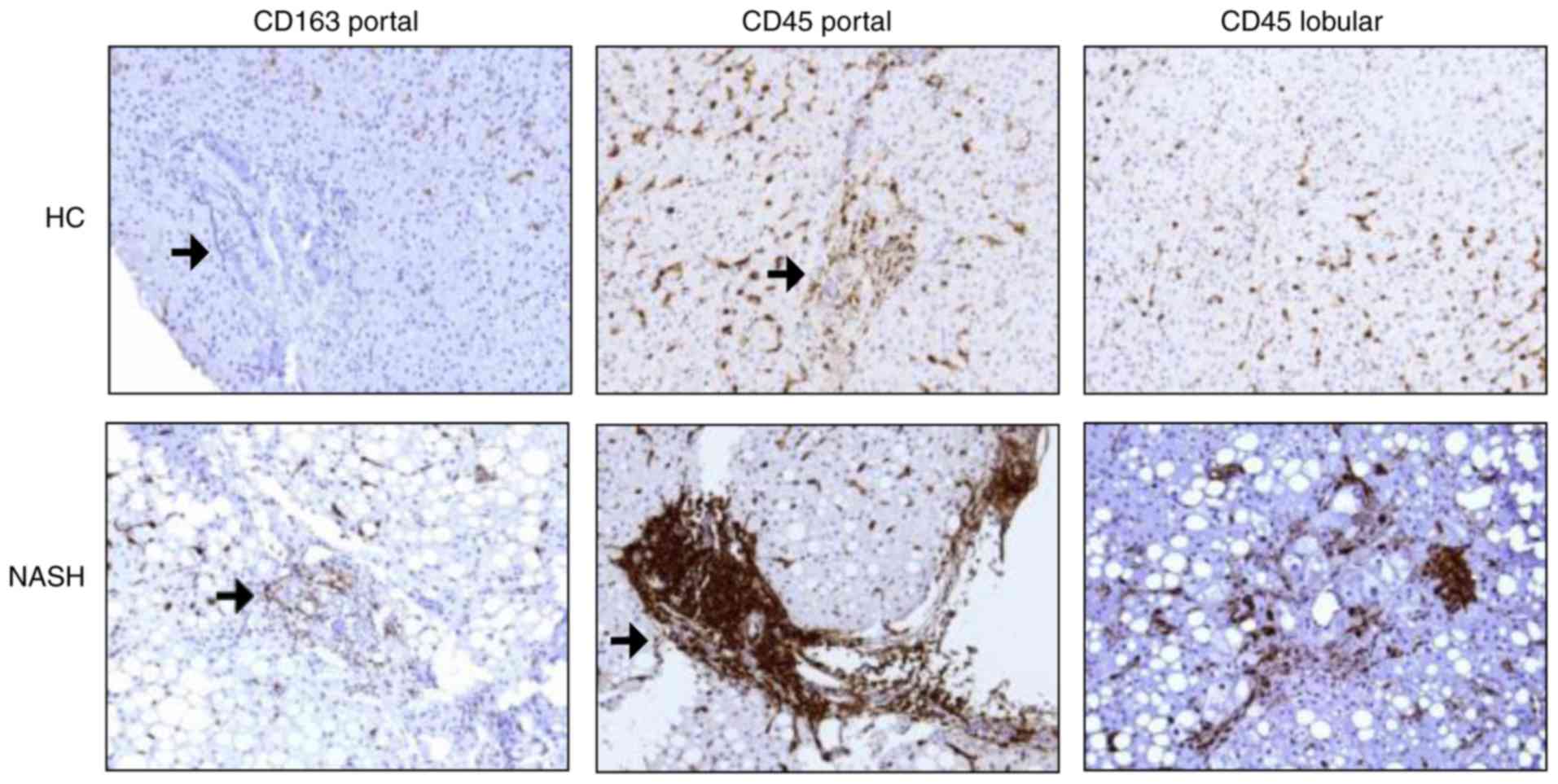
As presented in Table III and Fig. 1, patients with SS and NASH
exhibited a significantly higher number of CD45+ cells
in liver lobule and more CD163+ cells in the portal
tract compared with HC. Additionally, those with NASH had a
significantly higher CD45+ cells in the portal tract
compared with HC.
 | Table IIIImmunohistochemistry by diagnosis of
liver disease. |
Table III
Immunohistochemistry by diagnosis of
liver disease.
| Parameters | HC (n=8) | SS (n=12) | NASH (n=22) |
|---|
| CD45P | 21.1 (6.6,
42.7) | 53.8 (10.8,
94.9) | 45.0 (12.1,
189.0)a |
| CD45L | 122.1 (45.3,
155.0) | 148.7 (78.3,
196.4)a | 139.7 (102.9,
221.8)a |
| CD3P | 25.8 (7.9,
43.5) | 38.3 (7.4,
95.8) | 32.6 (8.8,
144.0) |
| CD3L | 53.2 (30.0,
86.6) | 67.8 (30.3,
112.3) | 62.2 (21.7,
119.0) |
| CD20P | 1.5 (0.3, 7.9) | 5.3 (0.2,
19.2) | 5.1 (0.1,
63.9) |
| CD20L | 5.2 (1.7, 8.2) | 4.5 (1.7,
10.7) | 6.1 (2.3,
23.0) |
| CD163P | 1.4 (0.0, 4.0) | 5.8 (0.0,
28.7)a | 5.7 (1.3,
24.4)a |
| CD163L | 149.6 (37.0,
226.3) | 132.2 (43.3,
226.3) | 137.9 (65.5,
264.1) |
Patients were also assessed according to NAS
(Table IV). Those with an NAS ≥5
had a significantly higher number of CD45+ and
CD163+ cells in the portal tract compared with those
with an NAS of 0. Additionally, those with an NAS ≥5 had
significantly higher CD20+ cells in the liver lobule
compared with those with an NAS of 0 or 1–4. Those with an NAS of
1–4 also had significantly higher CD163+ cells in the
portal tract compared with those with an NAS of 0.
 | Table IVImmunohistochemistry by NAS. |
Table IV
Immunohistochemistry by NAS.
| Parameters | NAS=0 (n=8) | NAS 1–4 (n=22) | NAS ≥ 5 (n=11) |
|---|
| CD45P | 21.1 (6.6,
42.7) | 46.5 (10.8,
102.8) | 47.3 (30.7,
189.0)a |
| CD45L | 122.1 (45.3,
155.0) | 144.4 (78.3,
196.4) | 133.4 (111.5,
221.8) |
| CD3P | 25.8 (7.9,
43.5) | 27.4 (7.4,
106.4) | 37.8 (25.2,
144.0) |
| CD3L | 53.2 (30.0,
86.6) | 65.6 (24.75,
119.0) | 68.9 (21.7,
112.3) |
| CD20P | 1.5 (0.3, 7.9) | 4.2 (0.1,
30.3) | 5.5 (2.4,
63.9) |
| CD20L | 5.2 (1.7, 8.2) | 4.9 (1.7,
11.2)a | 7.40 (3.8,
23.0)a,b |
| CD163P | 1.4 (0.0, 4.0) | 5.2 (0.0,
28.7)a | 6.3 (2.6,
19.5)a |
| CD163L | 149.6 (37.0,
226.3) | 159.5 (43.3,
264.1) | 121.4 (65.5,
157.7) |
Immunohistochemistry, intestinal
microbiota and liver histology
A subset of 39 patients, 12 with SS, 22 with NASH
and 5 HC provided stool samples for IM. Correlations between CD
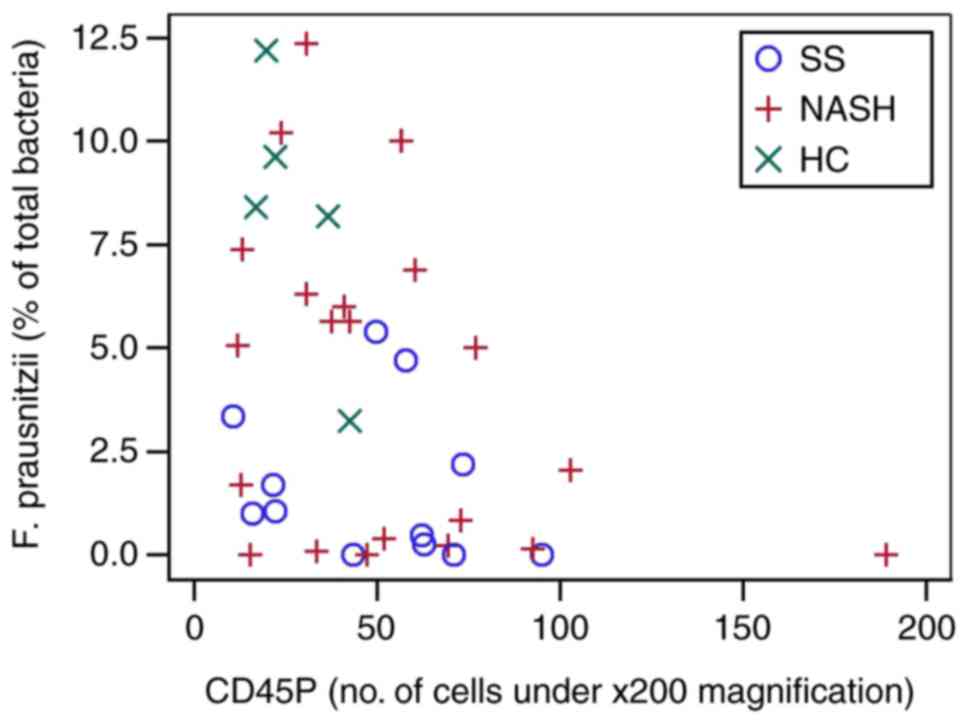
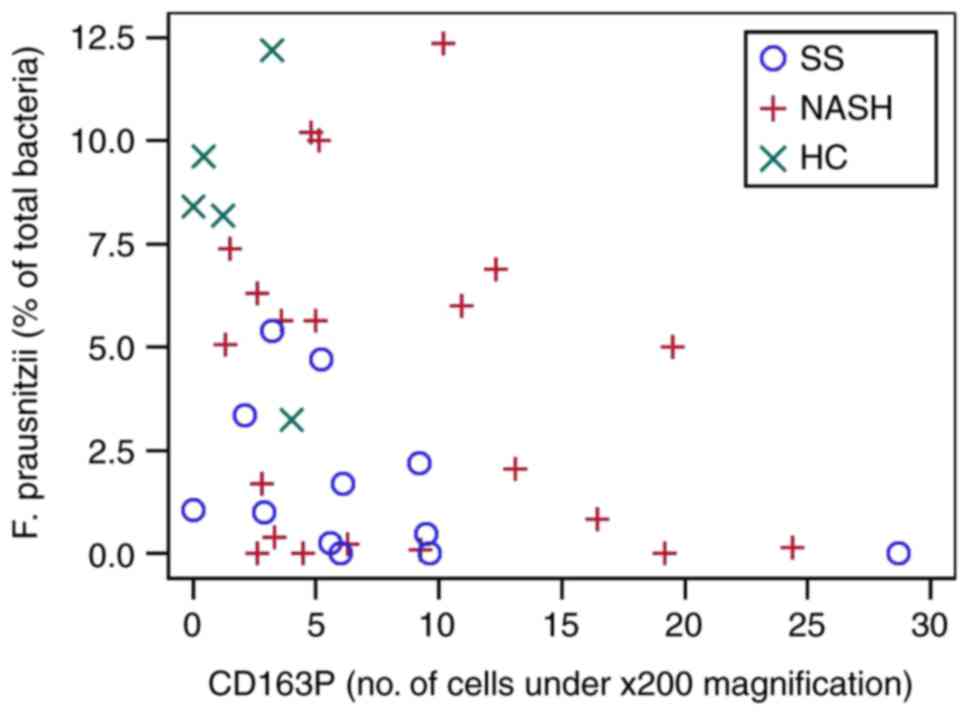
cell counts and specific bacteria taxa are presented in Table V. In the entire cohort, F.
prausnitzii was significantly negatively correlated with
CD45+ (Fig. 2) and
CD163+ (Fig. 3) cells
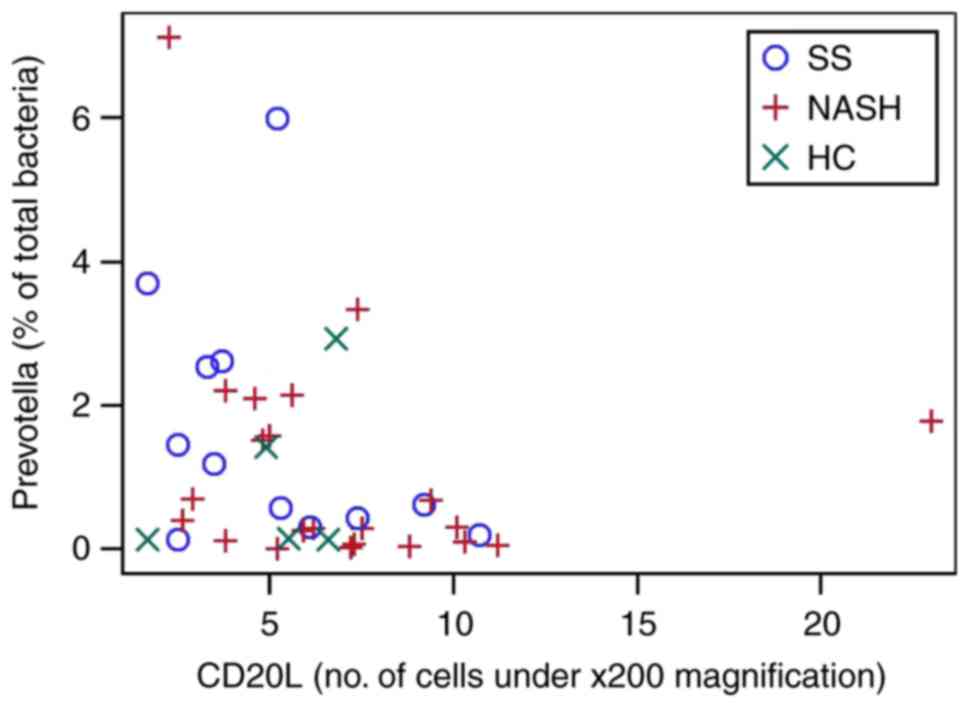
in the portal tract. Prevotella was significantly negatively
correlated (Fig. 4) with the
CD20+ cell count in the liver lobule. Other taxa did not
correlate.
 | Table VCorrelation between
immunohistochemistry and relative abundances of microbial taxa. |
Table V
Correlation between
immunohistochemistry and relative abundances of microbial taxa.
| Parameters |
Prevotella |
Alistipes |
Copro-coccus |
Rumino-coccus | Clostridium
leptum | C.
coccoides |
Lacto-bacillus | F.
prausnitzii |
Bifido-bacterium | E. coli |
|---|
| CD45P | −0.041 (0.804) | 0.042 (0.801) | −0.194 (0.244) | 0.138 (0.403) | −0.291 (0.072) | 0.220 (0.178) | 0.037 (0.825) | −0.394 (0.015) | −0.173 (0.300) | 0.064 (0.698) |
| CD45L | −0.086 (0.602) | −0.162 (0.331) | −0.158 (0.343) | −0.122 (0.459) | −0.126 (0.443) | −0.181 (0.269) | −0.123 (0.455) | −0.192 (0.248) | −0.074 (0.659) | 0.077 (0.639) |
| CD3P | −0.069 (0.675) | 0.051 (0.762) | −0.044 (0.793) | 0.186 (0.256) | −0.196 (0.233) | 0.220 (0.178) | −0.027 (0.870) | −0.233 (0.160) | −0.194 (0.244) | 0.023 (0.889) |
| CD3L | −0.155 (0.348) | −0.083 (0.620) | 0.102 (0.544) | 0.151 (0.360) | 0.076 (0.647) | −0.150 (0.361) | −0.121 (0.463) | −0.195 (0.240) | 0.239 (0.083) | 0128 (0.436) |
| CD20P | −0.132 (0.424) | −0.019 (0.910) | −0.053 (0.750) | 0.133 (0.421) | −0197 (0.230) | 0.180 (0.274) | −0.025 (0.880) | −0.259 (0.116) | −0.133 (0.426) | −0.085 (0.608) |
| CD20L | −0.353 (0.028) | −0.079 (0.637) | 0.216 (0.192) | 0.063 (0.705) | −0.048 (0.774) | −0.067 (0.682) | −0.053 (0.748) | 0.055 (0.745) | 0.022 (0.897) | −0.092 (0.576) |
| CD163P | −0.008 (0.962) | −0.081 (0.628) | −0.202 (0.225) | −0.057 (0.732) | −0.181 (0.269) | 0.140 (0.394) | 0.054 (0.742) | −0.371 (0.022) | −0.208 (0.211) | 0.014 (0.934) |
| CD163L | 0.070 (0.674) | −0.101 (0.547) | 0.005 (0.974) | −0.165 (0.315) | 0.016 (0.921) | −0.126 (0.445) | −0.197 (0.229) | −0.043 (0.797) | 0.022 (0.894) | 0.069 (0.675) |
Subsequently, the correlation of IM and
immunohistochemistry was investigated based on histological
diagnosis (Table VI). It was
demonstrated that F. prausnitzii was negatively correlated
with CD163P in patients with SS, however this was not significant
in patients with NASH or HC. Finally, it was demonstrated that
Bifidobacterium was negatively correlated with
CD20+ and CD163+ cell counts in the portal
tract in patients with SS.
 | Table VICorrelation between
immunohistochemistry and relative abundances of microbial taxa
based on non-alcoholic fatty liver disease diagnosis. |
Table VI
Correlation between
immunohistochemistry and relative abundances of microbial taxa
based on non-alcoholic fatty liver disease diagnosis.
| Taxa | CD cell | Diagnosis | Correlation
coefficient | P-value |
|---|
| F.
prausnitzii | CD45P | HC | −0.700 | 0.188 |
| SS | −0.399 | 0.199 |
| NASH | −0.309 | 0.174 |
| CD163P | HC | −0.300 | 0.624 |
| SS | −0.594 | 0.042 |
| NASH | −0.144 | 0.535 |
|
Bifidobacterium | CD20P | HC | 0.700 | 0.188 |
| SS | −0.615 | 0.033 |
| NASH | 0.001 | 0.996 |
| CD163P | HC | 0.500 | 0.391 |
| SS | −0.608 | 0.036 |
| NASH | −0.127 | 0.582 |
|
Prevotella | CD20L | HC | 0.300 | 0.624 |
| SS | −0.431 | 0.162 |
| NASH | −0.367 | 0.093 |
Discussion
The present results demonstrate that patients with
NAFLD have elevated CD cell markers that are associated with
disease severity. In addition, some of these CD cell markers are
associated with the relative abundance of specific bacterial taxa
in feces, particularly F. prausnitzii, Prevotella and
Bifidobacterium. To the best of our knowledge, this finding
has not been reported before and requires further investigation.
Very few previous studies, mostly pediatric, (7–9)
demonstrated elevation of specific CD markers in NAFLD as well as
associations with disease severity. Other studies demonstrated
differences in IM between NAFLD and HC (2,3,5,13),
and associations with disease severity (26). However, none have assessed both
parameters in the same patients to determine if there are potential
associations between hepatic immune cells and specific IM.
Previous evidence supports a role for the immune
system in the progression of NAFLD (17). In accordance with previous
findings, (7,9) the present study demonstrated that
patients with NAFLD, specifically NASH, had significantly higher
numbers of total immune cell population (CD45+) in both
the lobule and the portal tract when compared with controls.
However, the number of KCs (CD163+) was higher only in
the portal tract, and there was no significant difference in the
numbers of T cells or B cells in either portal or lobular areas
between HC and SS/NASH. The present data also demonstrated that
patients with higher NAS had altered numbers of total immune cell
population (CD45+) and KCs (CD163+), but only
in the portal tract. Although NAFLD is histologically assessed
based on lobular features of inflammation, portal inflammatory
infiltrate was previously studied by Gadd et al (8) because of its role in the development
of portal fibrosis. In that study, the presence of portal
inflammation was strongly correlated with fibrosis stage (8). Additionally, using immunostaining
for broad leukocyte subset markers (CD68, CD3, CD8, CD4, CD20 and
neutrophil elastase) and selected inflammatory markers, it was
observed that cells expressing all markers examined were identified
throughout the liver lobules and in portal tracts. Portal tracts
were more densely populated by these cells, and the population was
dominated by CD68 macrophages and CD8 lymphocytes at all stages of
disease (8). As in that previous
study, the present findings also demonstrated that the number of B
cells (CD20+) was higher in the liver lobules of those
with higher NAS scores, but there was no significant difference in
T cell (CD3+) counts in either the lobular or the portal
area. CD20+ and CD3+ cells levels were also
not significantly different between NAFLD and HC. In contrast, one
previous pediatric study revealed lower CD3+ in children
with NASH (9). Notably, two other
human studies identified that the intrahepatic ratio of
CD3+/CD56+ cells was increased in patients
with NAFLD with more severe liver disease (27,28). One previous study, using flow
cytometric analysis, demonstrated that as NAS increased so did the
amount of intrahepatic CD3+/CD56+ cells
(28), whereas another study
indicated that the percentage of intrahepatic
CD3+/CD56+ cells was significantly higher in
patients with moderate to severe steatosis when compared with those
with mild and no steatosis (27).
Therefore, one of the reasons that a significant difference was not
observed in in CD3+ cells is because the differences are
seen in a cell subgroup.
In regard to CD45+ and CD163+,
the present results were also comparable to others. Previous
pediatric studies (7,9) have identified increased numbers of
CD45+ and CD163+ cells in children with
greater disease severity, and another study in adults revealed that
activated KCs (CD163+) contributed to the development of
NASH (29). However, a recent
study of 45 individuals with NAFLD undergoing bariatric surgery
identified no difference in the intrahepatic numbers of
CD163+ between those with and without NASH (30), thereby suggesting that perhaps
patients with morbid obesity are different. In the present study,
higher CD163+ cell counts were observed in NAFLD vs. HC,
and an association was identified with disease severity as
previously reported in pediatric patients (9).
Taken together, these results suggest that in NAFLD
there is an infiltration of inflammatory cells that is associated
with disease severity, suggesting a possible role in the
pathogenesis of the disease. Previous research suggests that
altered IM, which has been demonstrated in NAFLD (2,3,5,6,13,26), may serve a role in pathogenesis
through bacterial translocation leading to systemic chronic
inflammation (31), which has the
potential to activate hepatic inflammatory cells through the
production of cytokines. Using qPCR to evaluate several bacterial
taxa, it was demonstrated that F. prausnitzii,
Prevotella and Bifidobacterium were negatively
correlated with specific CD cell markers. F. prausnitzii was
the most abundant bacterium in the healthy IM, and as hypothesized,
was revealed to be negatively correlated with hepatic immune cells
(32). In animal models, F.
prausnitzii has been demonstrated to have anti-inflammatory
effects (33,34) and is also reported to be low in
other human inflammatory conditions such as inflammatory bowel
disease (34). Therefore, lower
F. prausnitzii may have a role in the higher hepatic
inflammatory cell infiltrate. Prevotella, which produces
high levels of short-chain fatty acids (SCFAs), has been identified
in lower abundance in individuals with NASH (13,26) and SCFAs have been reported to have
an anti-inflammatory effect (35), supporting a role for
Prevotella. Bifidobacterium is another SCFA producer
that has been reported to be reduced in NASH (2). SCFAs have been demonstrated to
protect against gut inflammation (36).
The strengths of the present study are the defined
characterization of the study subjects with liver histology and the
quantification of specific bacterial taxa reported to be associated
with NAFLD. Another is that liver immunostaining allowed for the
assessment of associations between IM and CD markers. The
limitations include the cross-sectional design, which does not
indicate causality and the small sample size due to the
invasiveness of the liver biopsy. However, similar studies
assessing immunohistochemistry in NAFLD had comparable samples
sizes (8,9) but did not include IM assessment. The
association reported between immune cell infiltrate and IM is based
on associations and does not prove causality. Additional studies
are required to determine the role of specific bacterial taxa in
the immunopathogenesis of NAFLD.
In conclusion, the present findings indicated that B
cells and Kupffer cells, but not T cells, were associated with
NAFLD and disease severity, and specific immune cells in portal or
lobular areas were correlated with specific gut microbial taxa,
particularly Faecalibacterium prausnitzii. Future research
should investigate the specific immune cell subgroups, inflammatory
mediators and IM during intervention studies to further explore
their roles in NAFLD pathogenesis.
Acknowledgments
EMC holds the Lawson Family Chair in Microbiome
Nutrition Research at the University of Toronto. This abstract was
presented at the Canadian Digestive Diseases Week, on February 9th
2018 at the Fairmount Royal York (Toronto, Canada), and was
published as Abstract 1.
Funding
The present study was funded through operating
grants from the Canadian Institutes of Health Research (grant nos.
NMD-86922 and MOP-89705) and the 2012 American College of
Gastroenterology Clinical Research Award. Some of the equipment
used in the present study was supported by the 3D (Diet, Digestive
Tract and Disease) Centre, funded by the Canadian Foundation for
Innovation and Ontario Research Fund (grant nos. 19442 and
30961).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
KJPS helped analyze and interpret statistical data
and was a major contributor to writing of manuscript. LC performed
study conception and design, drafting manuscript and critical
revision. AC performed acquisition of immune cell data. HEDS
performed acquisition of patient clinical and biochemical data. ATe
performed analysis and interpretation of statistical data. EMC
performed study conception and design, drafting of manuscript and
critical revision. Ata performed acquisition of microbiota data and
drafting of manuscript. BMA performed study conception and design,
acquisition of clinical and biochemical data. SF performed study
conception and design, analysis and interpretation of histological
data, drafting of manuscript and critical revision. JPA performed
study conception and design, analysis and interpretation of
statistical data, drafting of manuscript and critical revision and
supervised entire project. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study protocol was approved by the
University Health Network (Toronto, Canada) and University of
Toronto (Toronto, Canada) Research Ethics Boards and conformed to
the ethical guidelines of the 1975 Declaration of Helsinki. All
participants gave their informed written consent.
Patient consent for publication
All participants gave their informed written
consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Review Team; LaBrecque DR, Abbas Z, Anania
F, Ferenci P, Khan AG, Goh KL, Hamid SS, Isakov V, Lizarzabal M, et
al: World Gastroenterology Organisation global guidelines:
Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis.
J Clin Gastroenterol. 48:467–473. 2014.PubMed/NCBI
|
|
2
|
Zhu L, Baker SS, Gill C, Liu W, Alkhouri
R, Baker RD and Gill SR: Characterization of gut microbiomes in
nonalcoholic steatohepatitis (NASH) patients: A connection between
endogenous alcohol and NASH. Hepatology. 57:601–609. 2013.
View Article : Google Scholar
|
|
3
|
Wong VW, Tse CH, Lam TT, Wong GL, Chim AM,
Chu WC, Yeung DK, Law PT, Kwan HS, Yu J, et al: Molecular
characterization of the fecal microbiota in patients with
nonalcoholic steatohepatitis-a longitudinal study. PLoS One.
8:e628852013. View Article : Google Scholar
|
|
4
|
Spencer MD, Hamp TJ, Reid RW, Fischer LM,
Zeisel SH and Fodor AA: Association between composition of the
human gastrointestinal microbiome and development of fatty liver
with choline deficiency. Gastroenterology. 140:976–986. 2011.
View Article : Google Scholar :
|
|
5
|
Raman M, Ahmed I, Gillevet PM, Probert CS,
Ratcliffe NM, Smith S, Greenwood R, Sikaroodi M, Lam V, Crotty P,
et al: Fecal microbiome and volatile organic compound metabolome in
obese humans with nonalcoholic fatty liver disease. Clin
Gastroenterol Hepatol. 11:868–875. e1–e3. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mouzaki M, Comelli EM, Arendt BM, Bonengel
J, Fung SK, Fischer SE, McGilvray ID and Allard JP: Intestinal
microbiota in patients with nonalcoholic fatty liver disease.
Hepatology. 58:120–127. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nobili V, Cutrera R, Liccardo D, Pavone M,
Devito R, Giorgio V, Verrillo E, Baviera G and Musso G: Obstructive
sleep apnea syndrome affects liver histology and inflammatory cell
activation in pediatric nonalcoholic fatty liver disease,
regardless of obesity/insulin resistance. Am J Respir Crit Care
Med. 189:66–76. 2014.
|
|
8
|
Gadd VL, Skoien R, Powell EE, Fagan KJ,
Winterford C, Horsfall L, Irvine K and Clouston AD: The portal
inflammatory infiltrate and ductular reaction in human nonalcoholic
fatty liver disease. Hepatology. 59:1393–1405. 2014. View Article : Google Scholar
|
|
9
|
De Vito R, Alisi A, Masotti A, Ceccarelli
S, Panera N, Citti A, Salata M, Valenti L, Feldstein AE and Nobili
V: Markers of activated inflammatory cells correlate with severity
of liver damage in children with nonalcoholic fatty liver disease.
Int J Mol Med. 30:49–56. 2012.PubMed/NCBI
|
|
10
|
Torlakovic EE, Naresh K, Kremer M, van der
Walt J, Hyjek E and Porwit A: Call for a European programme in
external quality assurance for bone marrow immunohistochemistry;
report of a European Bone Marrow Working Group pilot study. J Clin
Pathol. 62:547–551. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arrese M, Cabrera D, Kalergis AM and
Feldstein AE: Innate immunity and inflammation in NAFLD/NASH. Dig
Dis Sci. 61:1294–1303. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Caldeira PC, Oliveira e Silva KR, Vidigal
PV, Grossmann Sde M and do Carmo MA: Inflammatory cells in minor
salivary glands of patients with chronic hepatitis C:
Immunophenotype, pattern of distribution, and comparison with liver
samples. Hum Immunol. 75:422–427. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang W, Wu N, Wang X, Chi Y, Zhang Y, Qiu
X, Hu Y, Li J and Liu Y: Dysbiosis gut microbiota associated with
inflammation and impaired mucosal immune function in intestine of
humans with non-alcoholic fatty liver disease. Sci Rep. 5:80962015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kudo H, Takahara T, Yata Y, Kawai K, Zhang
W and Sugiyama T: Lipopolysaccharide triggered TNF-alpha-induced
hepatocyte apoptosis in a murine non-alcoholic steatohepatitis
model. J Hepatol. 51:168–175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thuy S, Ladurner R, Volynets V, Wagner S,
Strahl S, Königsrainer A, Maier KP, Bischoff SC and Bergheim I:
Nonalcoholic fatty liver disease in humans is associated with
increased plasma endotoxin and plasminogen activator inhibitor 1
concentrations and with fructose intake. The J Nutr. 138:1452–1455.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alisi A, Manco M, Devito R, Piemonte F and
Nobili V: Endotoxin and plasminogen activator inhibitor-1 serum
levels associated with nonalcoholic steatohepatitis in children. J
Pediatr Gastroenterol Nutr. 50:645–649. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ganz M and Szabo G: Immune and
inflammatory pathways in NASH. Hepatol Int. 7(Suppl 2): S771–S781.
2013. View Article : Google Scholar
|
|
18
|
Aron-Wisnewsky J, Gaborit B, Dutour A and
Clement K: Gut microbiota and non-alcoholic fatty liver disease:
New insights. Clin Microbiol Infect. 19:338–348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matthews DR, Hosker JP, Rudenski AS,
Naylor BA, Treacher DF and Turner RC: Homeostasis model assessment:
Insulin resistance and beta-cell function from fasting plasma
glucose and insulin concentrations in man. Diabetologia.
28:412–419. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brunt EM, Janney CG, Di Bisceglie AM,
Neuschwander-Tetri BA and Bacon BR: Nonalcoholic steatohepatitis: A
proposal for grading and staging the histological lesions. Am J
Gastroenterol. 94:2467–2474. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brunt EM, Kleiner DE, Wilson LA and Belt
P: Nonalcoholic fatty liver disease (NAFLD) activity score and the
histopathologic diagnosis in NAFLD: Distinct clinico-pathologic
meanings. Hepatology. 53:810–820. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qin J, Li R, Raes J, Arumugam M, Burgdorf
KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al: A
human gut microbial gene catalogue established by metagenomic
sequencing. Nature. 464:59–65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Furet JP, Firmesse O, Gourmelon M,
Bridonneau C, Tap J, Mondot S, Doré J and Corthier G: Comparative
assessment of human and farm animal faecal microbiota using
real-time quantitative PCR. FEMS Microbiol Ecol. 68:351–362. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mariat D, Firmesse O, Levenez F, Guimarăes
V, Sokol H, Doré J, Corthier G and Furet JP: The
Firmicutes/Bacteroidetes ratio of the human microbiota changes with
age. BMC Microbiol. 9:1232009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Da Silva HE, Teterina A, Comelli EM, Taibi
A, Arendt BM, Fischer SE, Lou W and Allard JP: Nonalcoholic fatty
liver disease is associated with dysbiosis independent of body mass
index and insulin resistance. Sci Rep. 8:14662018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boursier J, Mueller O, Barret M, Machado
M, Fizanne L, Araujo-Perez F, Guy CD, Seed PC, Rawls JF, David LA,
et al: The severity of nonalcoholic fatty liver disease is
associated with gut dysbiosis and shift in the metabolic function
of the gut microbiota. Hepatology. 63:764–775. 2016. View Article : Google Scholar :
|
|
27
|
Adler M, Taylor S, Okebugwu K, Yee H,
Fielding C, Fielding G and Poles M: Intrahepatic natural killer T
cell populations are increased in human hepatic steatosis. World J
Gastroenterol. 17:1725–1731. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tajiri K, Shimizu Y, Tsuneyama K and
Sugiyama T: Role of liver-infiltrating
CD3+CD56+ natural killer T cells in the
pathogenesis of nonalcoholic fatty liver disease. Eur J
Gastroenterol Hepatol. 21:673–680. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lotowska JM, Sobaniec-Lotowska ME and
Lebensztejn DM: The role of Kupffer cells in the morphogenesis of
nonalcoholic steatohepatitis-ultrastructural findings. The first
report in pediatric patients. Scand J Gastroenterol. 48:352–357.
2013. View Article : Google Scholar
|
|
30
|
Kazankov K, Tordjman J, Møller HJ,
Vilstrup H, Poitou C, Bedossa P, Bouillot JL, Clement K and
Grønbaek H: Macrophage activation marker soluble CD163 and
non-alcoholic fatty liver disease in morbidly obese patients
undergoing bariatric surgery. J Gastroenterol Hepatol.
30:1293–1300. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cani PD and Delzenne NM: The role of the
gut microbiota in energy metabolism and metabolic disease. Curr
Pharm Des. 15:1546–1558. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Miquel S, Martín R, Rossi O,
Bermúdez-Humarán LG, Chatel JM, Sokol H, Thomas M, Wells JM and
Langella P: Faecalibacterium prausnitzii and human intestinal
health. Curr Opin Microbiol. 16:255–261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang M, Qiu X, Zhang H, Yang X, Hong N,
Yang Y, Chen H and Yu C: Faecalibacterium prausnitzii inhibits
interleukin-17 to ameliorate colorectal colitis in rats. PLoS One.
9:e1091462014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sokol H, Pigneur B, Watterlot L, Lakhdari
O, Bermúdez-Humarán LG, Gratadoux JJ, Blugeon S, Bridonneau C,
Furet JP, Corthier G, et al: Faecalibacterium prausnitzii is an
anti-inflammatory commensal bacterium identified by gut microbiota
analysis of Crohn disease patients. Proc Natl Acad Sci USA.
105:16731–16736. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mattace Raso G, Simeoli R, Russo R, Iacono
A, Santoro A, Paciello O, Ferrante MC, Canani RB, Calignano A and
Meli R: Effects of sodium butyrate and its synthetic amide
derivative on liver inflammation and glucose tolerance in an animal
model of steatosis induced by high fat diet. PLoS One.
8:e686262013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kles KA and Chang EB: Short-chain fatty
acids impact on intestinal adaptation, inflammation, carcinoma, and
failure. Gastroenterology. 130(Suppl 1): S100–S105. 2006.
View Article : Google Scholar : PubMed/NCBI
|