Introduction
Zinc finger and AT-hook domain containing (Zfat) is
a transcriptional regulator that contains 18 zinc-finger domains
and one AT-hook domain (1,2).
Zfat regulates the transcription of the Zfat target genes by
binding directly to the proximal region of transcription start
sites (1). From the evolutionary
point of view, Zfat is highly conserved from fish to humans, and is
considered an essential molecule in development and cellular
differentiation (3).
Zfat was originally identified as a candidate
susceptibility gene for autoimmune thyroid disease (4). Previous studies have reported that
genetic variants of Zfat are strongly associated with
interferon-β therapy responsiveness in multiple sclerosis (5), Hashimoto disease severity (6) and the susceptibility of pigs to
enterotoxigenic Escherichia coli infection (7). Genetic variants of Zfat have
also been reported to be associated with non-immune-associated
diseases, including cerebral aneurysms (8), hypertension (9) and cancer (10). Furthermore, genome-wide
association studies have revealed that genetic variants of
Zfat affect adult height in Japanese and Korean populations
(11,12), and horse body size (13,14). These findings indicated that Zfat
may have critical roles in particular human diseases and
development, as well as in immune-related cells.
In mice, Zfat is expressed during embryonic
development, and in adult tissues, such as the spleen and thymus
(3,15). Zfat-deficient
(Zfat−/−) mice exhibit embryonic lethality and
severe defects in the differentiation of hematopoietic progenitor
cells in yolk sac blood islands, thus indicating that Zfat serves a
critical role in primitive hematopoiesis (15). Our previous studies reported that
Zfat gene ablation in thymic T cells in
Zfatf/f-LckCre mice induces a marked
decrease in the number of cluster of differentiation
(CD)4+CD8+ double-positive (DP) cells,
alongside impaired positive selection and excessive apoptosis
(16,17). Furthermore, Zfat deficiency
in peripheral T cells in Zfatf/f-CD4Cre
mice results in a decrease in peripheral T cells, as well as
decreased expression of interleukin-7Rα (18) and forkhead box O1 (19), thus indicating that Zfat is an
essential molecule associated with thymic and peripheral T cells.
However, the detailed pattern of Zfat expression during embryonic
development and in adult tissues remains to be elucidated.
The present study established a knock-in reporter
mouse strain (ZfatZsG/+ mice), which expressed a
green fluorescent protein, ZsGreen, in the Zfat locus. Using
this reporter mouse, ZsGreen signals were examined during
development and in various adult tissues, leading to elucidation of
the pattern of Zfat expression. The present findings may have
implications for the novel functions of Zfat in thymic epithelial
cells (TECs) and definitive erythropoiesis in the fetal liver and
bone marrow.
Materials and methods
Generation of Zfat-ZsGreen reporter
mice
All animal experiments were approved by the Animal
Care and Use Committee of the National Center for Global Health and
Medicine (NCGM) Research Institute (NCGM#14032; Tokyo, Japan) and
the Institutional Animal Care and Use Committee of Fukuoka
University (Fukudai#157; Fukuoka, Japan). The present study was
approved by the ethics committee of Fukuoka University
(Fukudai#372). All mice were maintained in a temperature-controlled
(23°C) facility under a 12-h light/dark cycle with free access to
water and standard rodent chow. Between five and 10 mice were kept
in one cage (500 cm2). All mice (17–35 g) were
sacrificed by cervical dislocation under standard anesthetized
conditions using isoflurane or carbon dioxide, and tissues were
removed for further analysis.
To construct a ZsGreen-FRT-pGKneo-FRT cassette,
ZsGreen cDNA was amplified by polymerase chain reaction (PCR) from
the pIRES2-ZsGreen1 vector (Clontech Laboratories, Mountainview,
CA, USA) using KOD-Plus-Neo DNA polymerase (Toyobo Life Science,
Osaka, Japan) and the following primers: Forward primer, 5′-ATG GCC
CAG TCC AAG CAC GGC C-3′ and reverse primer, 5′-TCA GGG CAA GGC GGA
GCC G-3′. PCR products were inserted at cloning sites upstream of
the FRT-pGKneo-FRT cassette in the pPE7neoW-F2LR vector (provided
by Dr Kiyoshi Takeda, Laboratory of Immune Regulation, Graduate
School of Medicine, Osaka University, Osaka, Japan). The
ZsGreen-FRT-pGKneo-FRT cassette was inserted at the ATG
translational start site of the Zfat gene in-frame in the
bacterial artificial chromosome (BAC) clone (clone number,
RP23-57E24; DNAFORM, Yokohama, Japan) using the pRed/ET
recombination kit (Gene Bridges GmbH, Heidelberg, Germany), in
accordance with the manufacturer's protocol. To construct the
targeting vector, a 22.5-kb fragment, which consisted of the
ZsGreen-FRT-pGKneo-FRT cassette, 19 kb of a 5′ homology arm and 1
kb of a 3′ homology arm, was retrieved from the BAC clone and
inserted into a minimal vector carrying a ColE1 origin plus
ampicillin-resistant gene (Gene Bridges GmbH) using the pRed/ET
recombination kit.
The targeting vector was linearized by SalI
and electroporated into TT2 embryonic stem (ES) cells (RIKEN
BioResource Center, Tsukuba, Japan), as described previously
(20). To select mutant ES cells,
cells were cultured on embryonic fibroblast feeder cells (Cell
Biolabs, Inc., San Diego, CA, USA) in the presence of 350
μg/ml G418 (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). To identify mutant ES cell clones, genomic DNA was extracted
from ES cells and subjected to genotyping analysis by Southern
blotting and PCR. Mutant ES cells were microinjected into the
blastocysts of ICR mice (Japan SLC, Inc., Hamamatsu, Japan), as
described previously (20). One
8-week-old male chimera (ZfatZsG-neo/+ mouse) was
mated with two 8-week-old FLPe deleter mice [B6-Tg(CAG-FLPe)36;
RIKEN BioResource Centre] to eliminate the pGKneo cassette and to
generate ZfatZsG/+ mice. To identify the
ZfatZsG/+ mice, genomic DNA was extracted from
the mouse tail and subjected to genotyping analysis by Southern
blotting and PCR. ZfatZsG/+ mice were backcrossed
with three 8-week-old C57BL/6 mice (weight, 23–28 g; Charles River
Laboratories Japan, Inc., Yokohama, Japan) six times in order to
maintain the genetic background of C57BL/6 mice.
Genotyping by Southern blotting and
PCR
For Southern blotting, genomic DNA was extracted
using Wizard® Genomic DNA Purification kit (Promega
Japan, Tokyo, Japan). A total of 25 μg of DNA was digested
with BglII, separated by 0.8% agarose gel electrophoresis
and transferred to a Biodyne B nylon membrane (Thermo Fisher
Scientific, Inc.). Membranes were prehybridized for 1 h at 42°C in
hybridization buffer (50% formamide, 20% SDS, 1 M
Na2HPO4, 0.5 M EDTA and 3 M NaCl).
Subsequently, hybridization was conducted for 16 h at 42°C in the
hybridization buffer containing 32P-radiolabeled probes.
After hybridization, the membranes were washed twice in 2X
saline-sodium citrate (SSC) at room temperature for 5 min, in 1X
SSC/0.1% SDS at 42°C for 20 min, and then in 1X SSC/0.1% SDS at
45°C for 10 min. Hybridized membranes were exposed to BIOMAX MR
film (Carestream Health, Inc., Rochester, NY, USA) for 3–4 days at
−70°C with BIOMAX MS intensifying screen (Carestream Health,
Inc.).
DNA fragments used to prepare the external probe
were amplified by PCR from mouse genomic DNA using the following
primers: Forward primer, 5′-GGG TGC AAA GGT TTC TGC TTC-3′ and
reverse primer, 5′-AAA GCA AAT GCA TGG TGA CA-3′. DNA fragments
used to prepare the ZsGreen probe were amplified by PCR from the
pIRES2-ZsGreen1 plasmid DNA using the following primers: Forward
primer, 5′-ATG GCC CAG TCC AAG CAC GGC C-3′ and reverse primer,
5′-TCA GGG CAA GGC GGA GCC G-3′. PCR products were radiolabeled
with 32P using High Prime kit (Merck, Kenilworth, NJ,
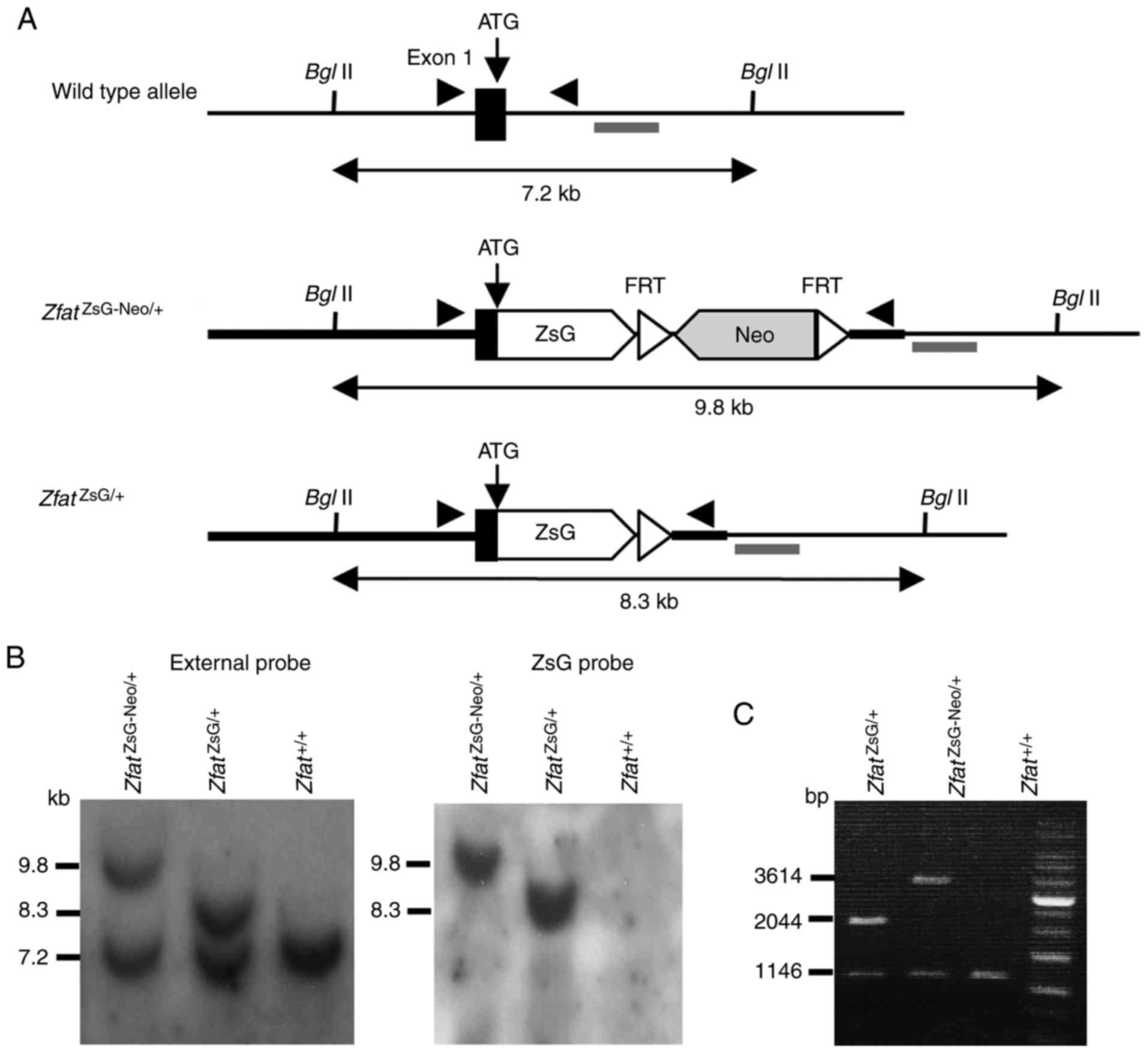
USA). BglII digestion of genomic DNA identified a 9.8-kb
ZsGreen-FRT-pGKneo-FRT recombinant allele, a 8.3-kb pGKneo
cassette-deleted recombinant allele and a 7.2-kb wild-type (WT)
allele, when hybridized with the external probe. The ZsGreen probe
was used to confirm homologous recombination and ensure that there
was only one integration site.
For PCR genotyping analysis, genomic DNA was
extracted from mouse tails by incubating samples in 50 mM NaOH at
95°C for 10 min; the samples were then mixed with 1 M Tris-HCl (pH
8.0) for neutralization. PCR was performed for 26 cycles at 98°C
for 10 sec and 68°C for 3 min using KOD FX Neo DNA polymerase
(Toyobo Life Science) and the following primers: Forward primer,
5′-GAT GTG CGA GGC ACT GTC ACT TCC-3′ and reverse primer, 5′-TGG
CCG CTC CCT CTG AAG GTC ACT AG-3′. PCR products were analyzed by
0.8% agarose gel electrophoresis.
Histological examination
Embryos with yolk sacs were obtained by sacrificing
pregnant ZfatZsG/+ mice at embryonic day (E)9.5
or 10.0, were fixed in 10% neutral-buffered formalin solution (Wako
Pure Chemical Industries, Osaka, Japan) for 24 h at 4°C and then
embedded in paraffin. Samples were cut into 3 μm sections
and were then stained with hematoxylin and eosin (H&E) using
the Cryosection preparation kit (1) (Section-lab Co., Ltd., Hiroshima,
Japan), in accordance with the manufacturer's protocol. To prepare
cryosections from brain and placenta samples of 4–8-week-old mice
and 8–12-week-old pregnant mice, respectively, tissues were fixed
in 10% neutral-buffered formalin solution for 24 h at 4°C, embedded
in super cryo-embedding medium (SCEM) and then frozen in accordance
with methods described by Kawamoto and Kawamoto (21). The SCEM-embedded frozen tissues
were cut into 6 μm sections, and adjacent sections were
processed for fluorescence microscopy or H&E staining. All
fluorescence and bright field images were obtained using a Biorevo
BZ-9000 inverted-phase microscope (Keyence Corporation, Osaka,
Japan).
Flow cytometry
To prepare single-cell suspensions from cerebrum,
cerebellum, testis, retina, lung and liver samples of 4–10-week-old
mice, tissues were minced in PBS and then incubated in Cell
Dissociation Buffer (Thermo Fisher Scientific, Inc.) for 10–30 min
at 37°C. To prepare single-cell suspensions from spleen and thymus
of 4–8-week-old mice, the lymphoid organs were gently ground by
pressing the tissues between frosted-glass slides. To prepare
single-cell suspensions from bone marrow, tibias and femurs were
dissected from 4–10-week-old mice, the ends of the bones were cut,
and marrow was flushed out with PBS using a needle and syringe.
Fetal livers were obtained by sacrificing pregnant mice at E12.5.
To prepare single-cell suspensions from fetal liver, the tissues
were gently ground by pressing between frosted-glass slides. Cell
suspensions were filtered through a 100-μm cell strainer.
Flow cytometric analysis was performed using FACSAria II (BD
Biosciences, Franklin Lakes, NJ, USA), as described previously
(17,22). ZsGreen was excited with the 488 nm
laser and detected using the 530/30 nm emission filter. The
fluorophore-conjugated antibodies used were as follows:
Allophycocyanin (APC)-conjugated CD4 (cat. no. 561091; BD
Biosciences), phycoerythrin (PE)-conjugated CD4 (cat. no. 100512;
BioLegend, Inc., San Diego, CA, USA], APC-conjugated CD8 (cat. no.
100711; BioLegend, Inc.) PE/Cy7-conjugated CD8 (cat. no. 100722;
BioLegend, Inc.), CD45R (B220) (cat. no. 25-0452-81; Thermo Fisher
Scientific, Inc.), epithelial cell adhesion molecule (EpCAM) (cat.
no. 17-5791-80; Thermo Fisher Scientific, Inc.), major
histocompatibility complex (MHC)-II (cat. no. 107627; BioLegend,
Inc.), c-kit (cat. no. 17-1171-81; Thermo Fisher Scientific, Inc.),
CD71 (cat. no. 561937; BD Biosciences) and Ter119 (cat. no. 116221;
BioLegend, Inc.). Data were analyzed using FlowJo™ 10.2 software
(FlowJo, LLC, Ashland, OR, USA).
Results
Generation of Zfat-ZsGreen knock-in
mice
To produce Zfat reporter
(ZfatZsG/+) mice, ZsGreen, a bright green
fluorescent protein derived from Zoanthus sp. reef coral,
was used to replace exon 1 of the Zfat gene through
homologous recombination (Fig.
1A-C). The pGKneo cassette was removed by crossing chimeric
mice with a deleter strain expressing FLPe recombinase. After FLPe
recombinase-mediated excision of the selection cassette, the
knock-in allele, which contains the ZsGreen gene inserted
in-frame with the Zfat ATG translation initiation site,
carried transcriptional regulatory elements identical to those in
the WT allele. As expected from the Zfat knock-out mice
reported previously (15),
intercrossing of ZfatZsG/+ mice never produces
ZfatZsG/ZsG mice, whereas heterozygous knock-in
mice were born and indistinguishable from WT mice.
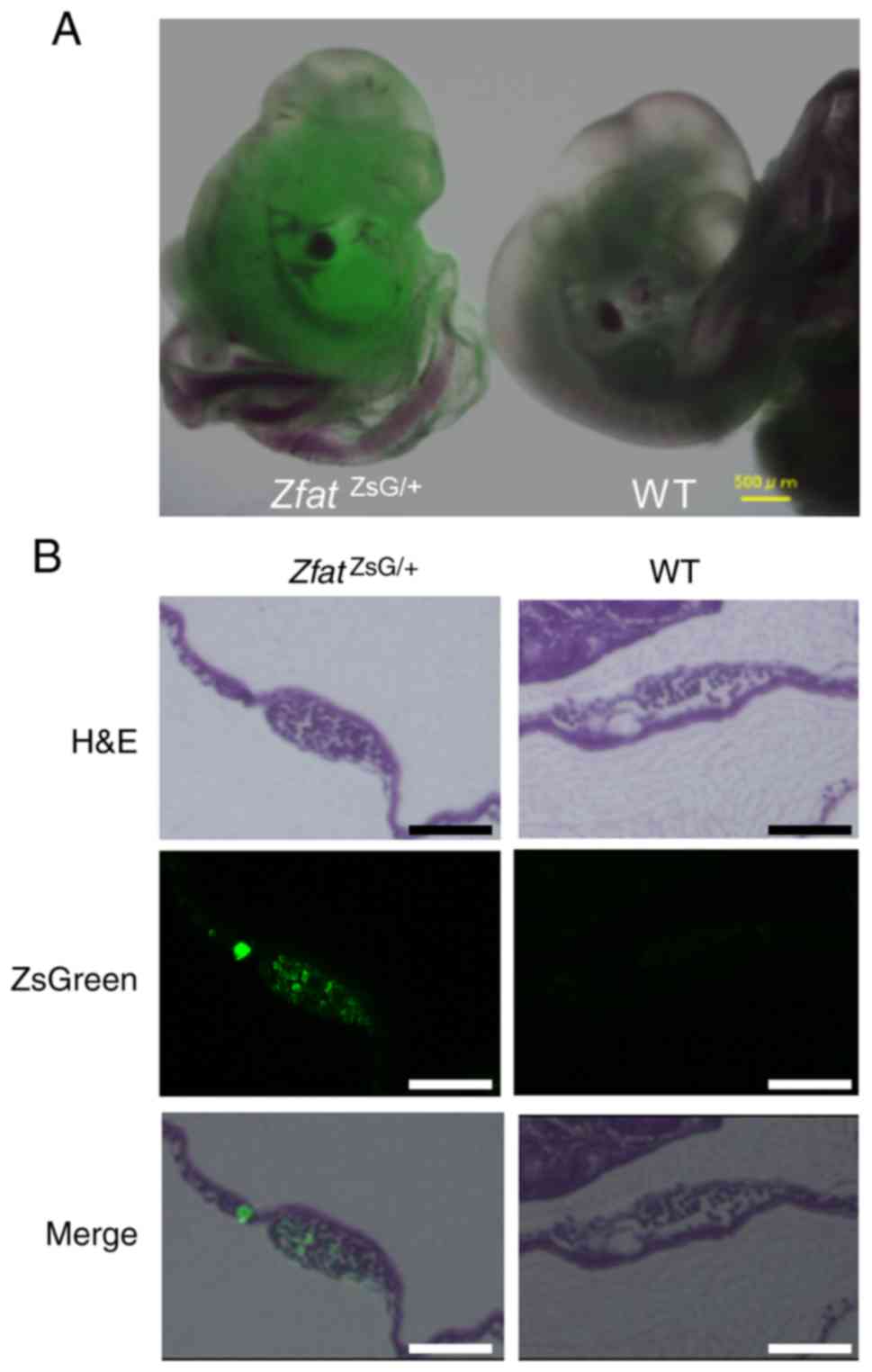
To confirm the validity of the reporter system,
ZsGreen signals were examined in E10 embryos using fluorescence
microscopy. No green fluorescence was detected in the WT embryos,
whereas ZsGreen signals were observed in the
ZfatZsG/+ embryos (Fig. 2A). Furthermore, histological
analysis indicated that ZsGreen signals were observed in
hematopoietic progenitor cells in the yolk sac blood islands from
ZfatZsG/+ embryos, but not in those from WT
embryos, at E9.5 (Fig. 2B). These
results are consistent with the results of our previous study,
which demonstrated that Zfat is expressed in the embryo and yolk
sac blood islands (15). These
results collectively indicated that endogenous Zfat expression was
precisely reflected by ZsGreen signals in
ZfatZsG/+ mice.
Zfat expression in TECs
Using flow cytometric analysis for ZsGreen, Zfat
expression was evaluated in immune cells from the spleen and thymus
of ZfatZsG/+ mice. The majority of splenocytes
from ZfatZsG/+ mice exhibited ZsGreen signals
that were higher than those from WT mice (Fig. 3A). ZsGreen+ cells were
observed in CD4+ T, CD8+ T and
B220+ cells from ZfatZsG/+ spleen
tissues (Fig. 3B). These results
are consistent with the results of our previous study, which
conducted immunoblotting analysis using the anti-Zfat antibody and
detected high Zfat expression in these cell populations (3). In addition, thymocytes from
ZfatZsG/+ mice exhibited high ZsGreen signals
(Fig. 3A). ZsGreen+
cells were observed not only in CD4+CD8+ DP,
CD4+CD8− single-positive and
CD4−CD8+ single-positive cells but also in
CD4−CD8− double-negative (DN) cells (Fig. 3C). To address whether ZsGreen
signals in the DN cell population are derived from T-cell
progenitor cells or non-T-lineage cells in the thymus, ZsGreen
signals were examined in TECs, which are essential for the
establishment of central immunological tolerance by presenting
self-antigens. Thymocytes were stained with EpCAM or MHC-II, both
of which are known to be markers of TECs (23,24). Notably, ZsGreen signals were
identified in EpCAM+ and MHC-II+ cells in the
DN cell population from ZfatZsG/+ thymus
(Fig. 3D). These results
suggested that Zfat serves particular roles in thymic T-cell
development through the regulation of TECs as well as T-lineage
cells.
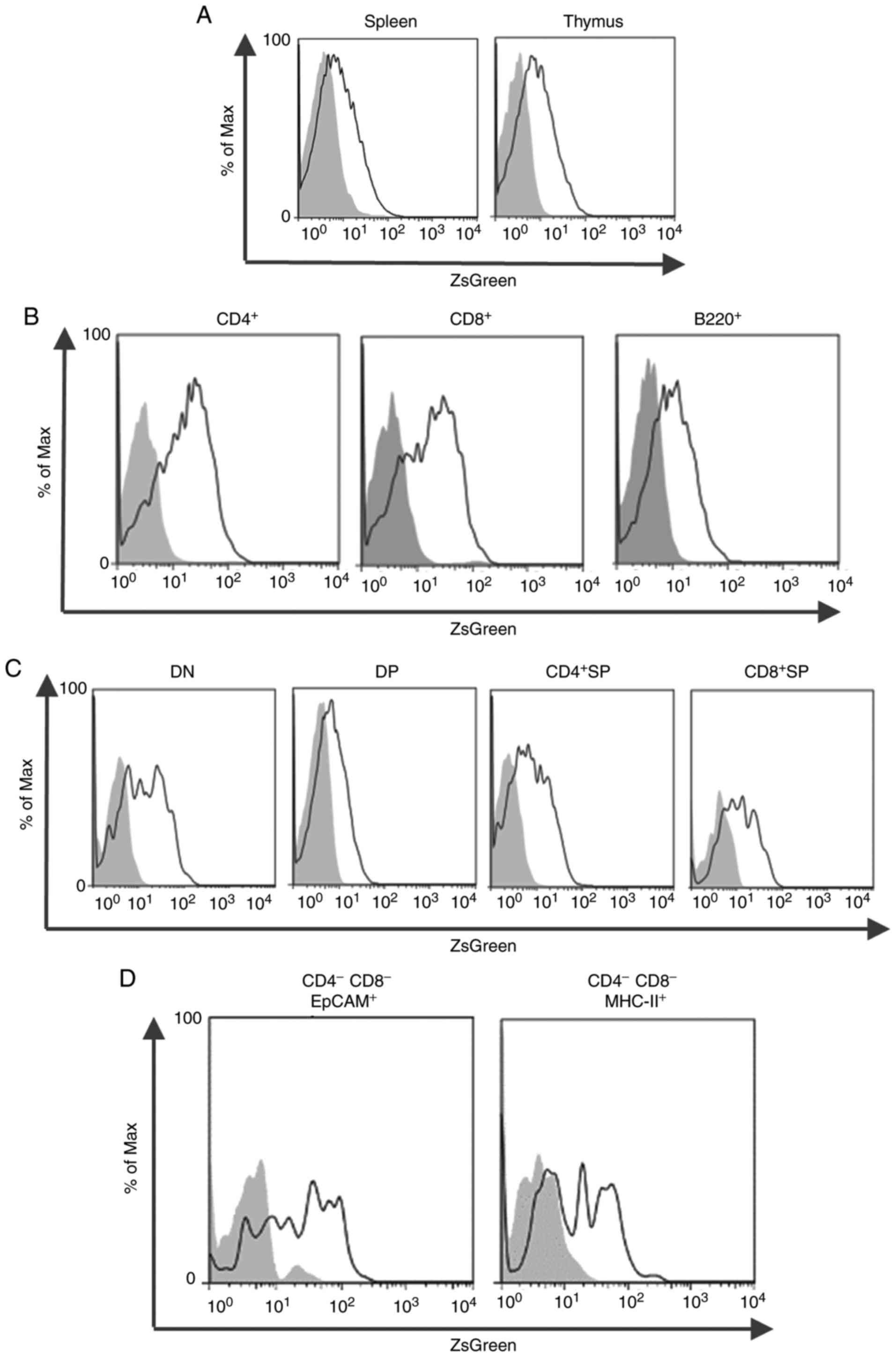 | Figure 3ZsGreen signals in splenocytes and
thymocytes. (A) Flow cytometric analysis of ZsGreen in splenocytes
or thymocytes isolated from 4–8-week-old
ZfatZsG/+ (black line) and WT (gray-filled) mice.
(B) ZsGreen signals in CD4+ T, CD8+ T or
B220+ cells isolated from 4–8-week-old
ZfatZsG/+ (black line) and WT (gray-filled)
spleen. (C) ZsGreen signals in CD4−CD8− DN,
CD4+CD8+ DP, CD4+CD8−
SP or CD4−CD8+ SP cells isolated from
4–8-week-old ZfatZsG/+ (black line) and WT
(gray-filled) thymus. (D) ZsGreen signals in
CD4−CD8−EpCAM+ or
CD4−CD8−MHC−II+ cells
isolated from 4–8-week-old ZfatZsG/+ (black line)
and WT (gray-filled) thymus. Data are representative of three
independent experiments. CD, cluster of differentiation; DN, double
negative; DP, double positive; EpCAM, epithelial cell adhesion
molecule; MHC, major histocompatibility complex; SP, single
positive; WT, wild-type; Zfat, zinc finger and AT-hook domain
containing. |
Zfat expression in definitive erythroid
progenitor cells
Our previous study reported that Zfat serves a key
role in primitive erythropoiesis (15); however, its involvement in the
regulation of definitive erythropoiesis remains to be determined.
Therefore, the present study examined ZsGreen signals in the fetal
liver from ZfatZsG/+ embryos at E12.5, where
definitive erythropoiesis takes place. As definitive erythroid
progenitor cells are known to express c-kit (25), the association between ZsGreen
signals and c-kit expression was analyzed using flow cytometry.
c-kit+ cells in the fetal liver from
ZfatZsG/+ embryos exhibited higher ZsGreen
signals than those from WT embryos (Fig. 4A). Conversely, ZsGreen+
cells were rarely detected in c-kit− cells in the
ZfatZsG/+ fetal liver (Fig. 4A). Subsequently, ZsGreen signals
were analyzed during the maturation of erythroid progenitor cells
in definitive erythropoiesis in the fetal liver. Fetal liver cells
in ZfatZsG/+ embryos were stained with CD71 and
Ter119, were analyzed by flow cytometry and were classified into P1
(CD71−Ter119low), P2
(CD71+Ter119low), P3
(CD71+Ter119mid) and P4
(CD71+Ter119high) over the course of the
maturation of erythroid progenitor cells (Fig. 4B). The proportion of
ZsGreen+ cells was highest in the P1 cell population,
and it was also high in the P2 cell population (Fig. 4C). ZsGreen+ cells were
hardly detected in P3 and P4 populations in the
ZfatZsG/+ fetal liver, indicating that the
expression levels of Zfat gradually decreased during the
progression of erythroid maturation. Furthermore, the majority of
c-kit+ cells in the P1 population from the
ZfatZsG/+ fetal liver expressed ZsGreen, and
there were also substantial numbers of ZsGreen+ cells
among the c-kit+ P2 cells (Fig. 4D), indicating that Zfat is
expressed in c-kit+ definitive erythroid progenitor
cells, particularly at the P1 and P2 stages.
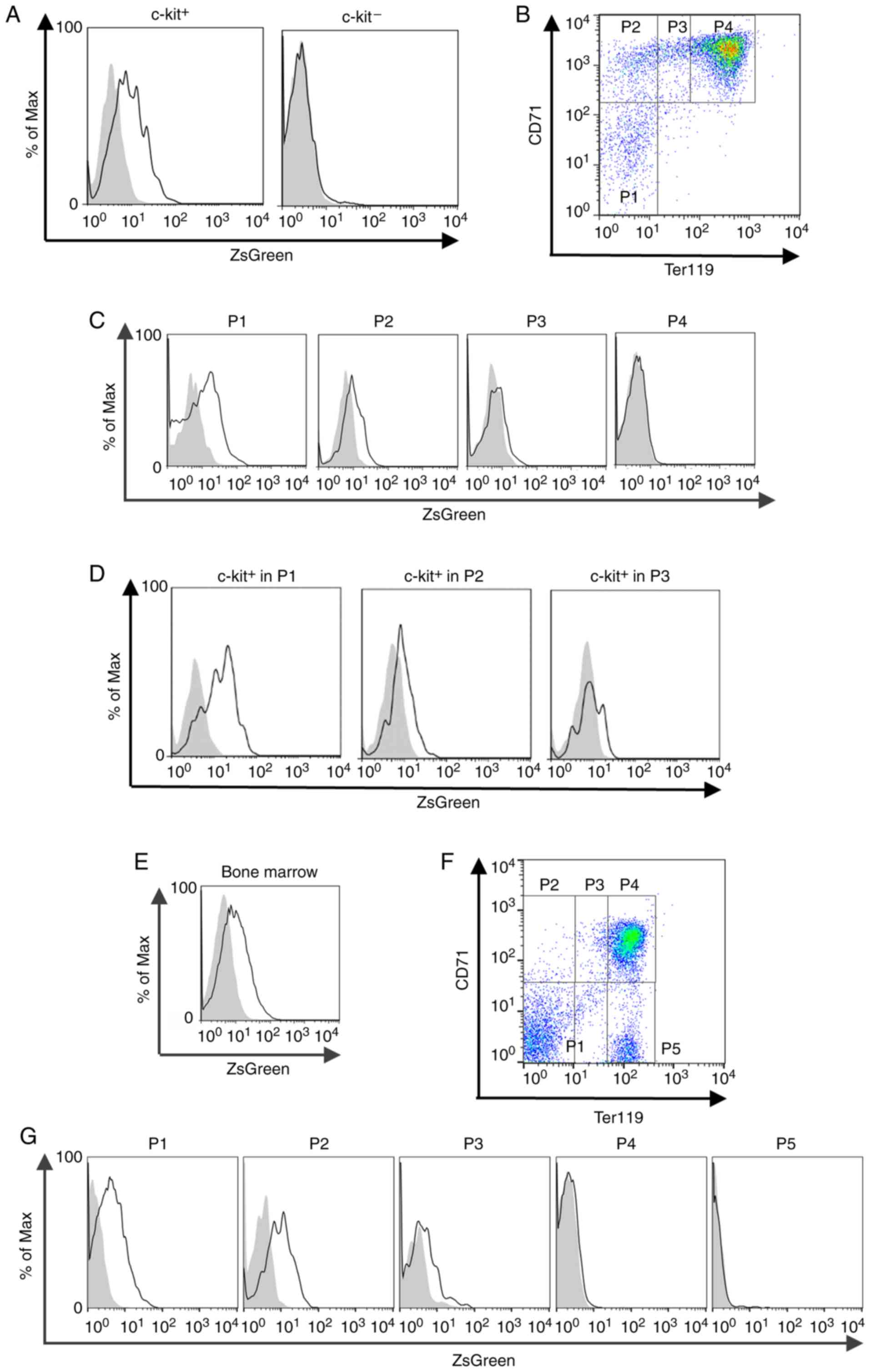 | Figure 4ZsGreen signals in erythroid
progenitor cells in the fetal liver and adult bone marrow. (A)
ZsGreen signals in c-kit+ or c-kit− cells
from the ZfatZsG/+ (black line) and WT
(gray-filled) fetal liver at E12.5. (B) Definition of stages during
erythroid maturation in the fetal liver at E12.5. Fetal liver cells
from ZfatZsG/+ embryos were stained with CD71 and
Ter119, and analyzed by flow cytometry. P1,
CD71-Ter119low; P2,
CD71+Ter119low; P3,
CD71+Ter119mid; and P4,
CD71+Ter119high. (C) ZsGreen signals in P1-P4
cells from the ZfatZsG/+ (black line) and WT
(gray-filled) fetal liver at E12.5. (D) ZsGreen signals in
c-kit+ cells in the P1-P3 population from the
ZfatZsG/+ (black line) and WT (gray-filled) fetal
liver at E12.5. (E) ZsGreen signals in the bone marrow cells
isolated from 4-week-old ZfatZsG/+ (black line)
and WT (gray-filled) mice. (F) Definition of stages during
erythroid maturation in the adult bone marrow. Bone marrow cells
from ZfatZsG/+ mice were stained with CD71 and
Ter119, and analyzed by flow cytometry. P1,
CD71−Ter119low; P2,
CD71+Ter119low; P3,
CD71+Ter119mid; P4,
CD71+Ter119high; and P5,
CD71−Ter119high. (G) ZsGreen signals in P1-P5
cells from the ZfatZsG/+ (black line) and WT
(gray-filled) bone marrow. Data are representative of three
independent experiments. CD, cluster of differentiation; E,
embryonic day; WT, wild-type; Zfat, zinc finger and AT-hook domain
containing. |
Notably, bone marrow in adult
ZfatZsG/+ mice contained substantial numbers of
ZsGreen+ cells (Fig.
4E). Similar to the cells in fetal liver, the cells in
ZfatZsG/+ bone marrow were stained with CD71 and
Ter119, and were classified according to the course of erythroid
progenitor cell maturation (Fig.
4F). The proportion of ZsGreen+ cells was the
highest in the P1 and P2 cell populations, and a few cells in the
P3 population exhibited modest ZsGreen signals (Fig. 4G). Conversely, ZsGreen+
cells were rarely detected in P4 and P5 populations in the
ZfatZsG/+ bone marrow (Fig. 4G). These results suggested that
Zfat may serve particular roles during the early stage of
definitive erythropoiesis in the fetal liver and adult bone
marrow.
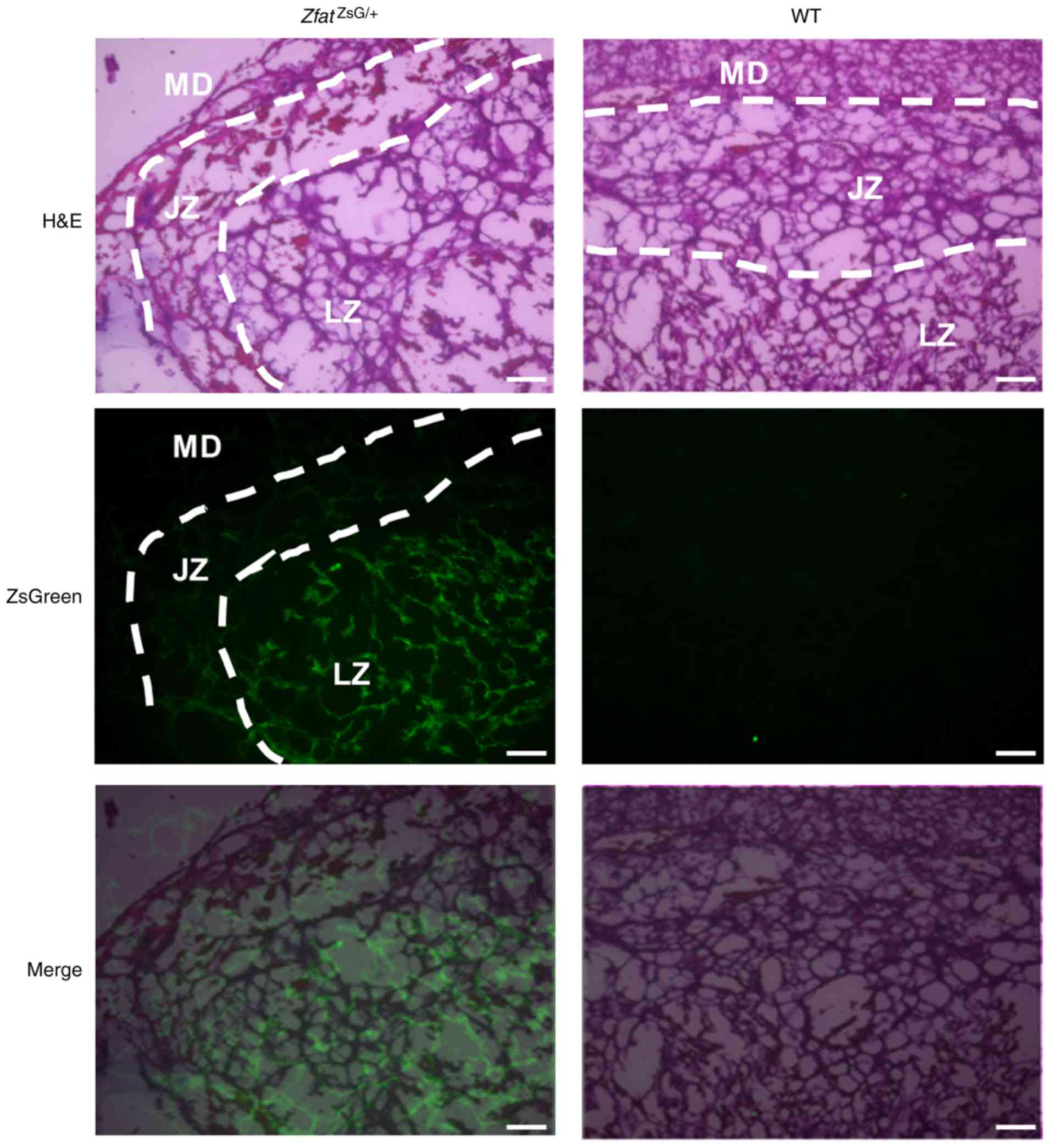
Zfat expression in the placenta and
nervous tissue
Our previous study reported a defect in the
development of spongiotrophoblast layers in the
Zfat−/− placenta (15). To address whether Zfat is
expressed in the placenta, histological analysis was performed on
E13.5 placenta, and ZsGreen signals were detected in the labyrinth
zone in the ZfatZsG/+ placenta (Fig. 5). Due to impaired development in
Zfat−/− placenta, these findings indicated that Zfat may
have a critical role in this organ.
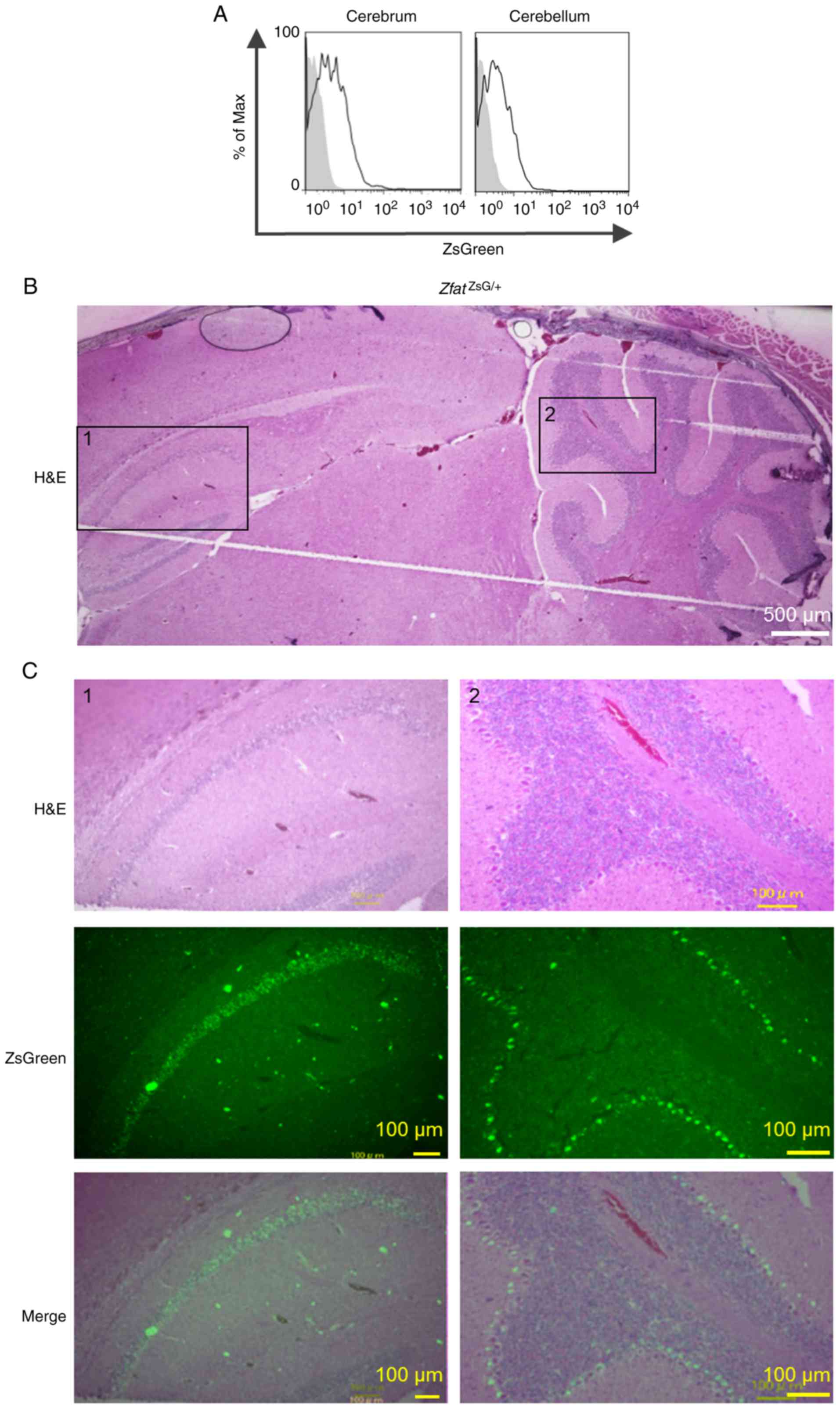
To the best of our knowledge, the expression and
functions of Zfat in nervous tissue are unknown. Flow cytometric
analysis of brain tissues from adult ZfatZsG/+
mice indicated that the cerebrum and cerebellum contained
ZsGreen+ cells (Fig.
6A). Histological analysis revealed that ZsGreen+
cells were specifically observed in the pyramidal cell layer in the
hippocampal CA1 region and the Purkinje cell layer in the
cerebellum of ZfatZsG/+ brain (Fig. 6B and C), thus suggesting novel
functions of Zfat in nervous tissue.
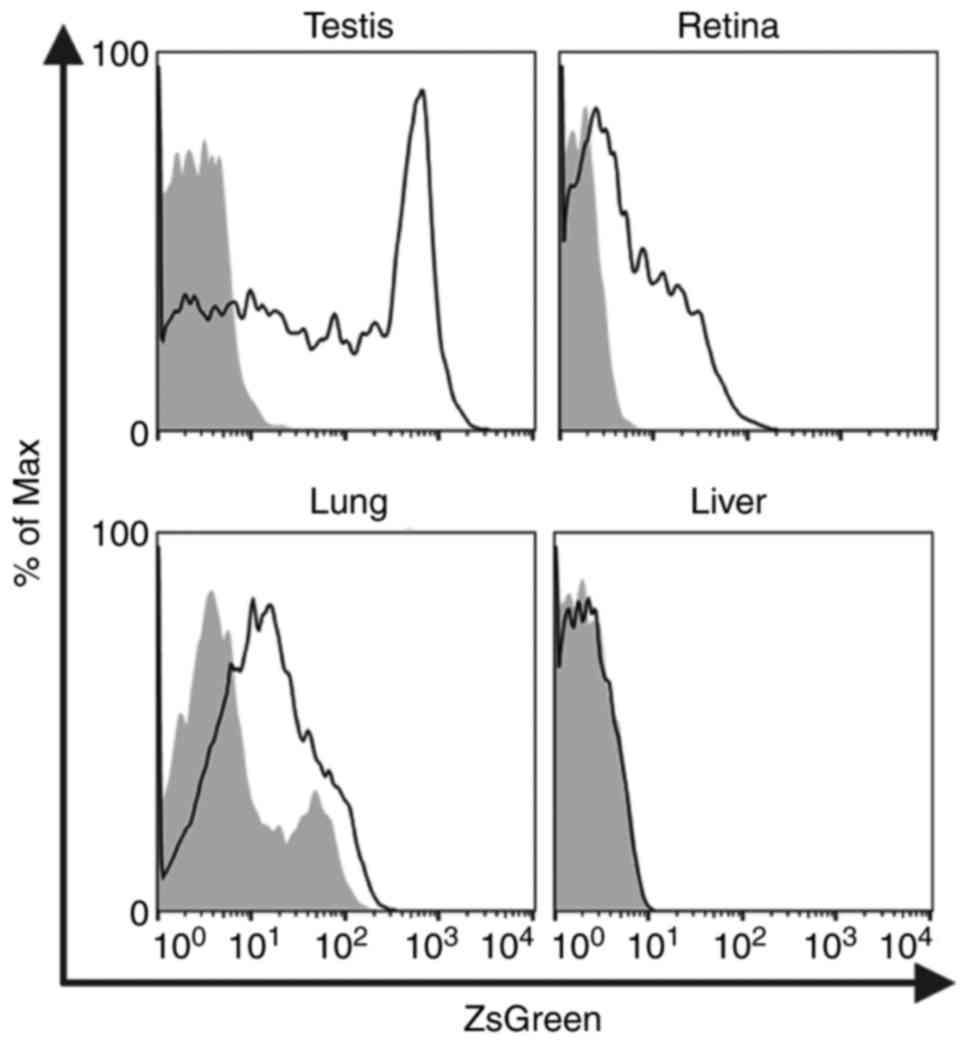
Finally, Zfat expression was evaluated in various
tissues of ZfatZsG/+ mice using flow cytometric
analysis of ZsGreen. Among the tissues examined in this study,
cells from the testes of ZfatZsG/+ mice exhibited
the highest intensity of ZsGreen signals (Fig. 7). ZsGreen signals were also
observed in the majority of cells from the retina of
ZfatZsG/+ mice (Fig. 7). Furthermore, cells from the
lungs of ZfatZsG/+ mice exhibited moderate
ZsGreen signals, whereas ZsGreen+ cells were rarely
detected in the ZfatZsG/+ liver (Fig. 7). These results suggested that
Zfat serves particular roles in the tissues where ZsGreen signals
were detected.
Discussion
The nuclear protein Zfat is a transcriptional
regulator that harbors DNA binding domains, including AT-hook and
zinc-finger domains (2,4). Our previous study demonstrated that
Zfat has critical roles in thymic T-cell development and peripheral
T-cell homeostasis (16,26,27). In addition, accumulating evidence
has suggested that Zfat has critical functions in non-immune cells
(27,28). However, the precise expression
pattern of Zfat remains to be determined.
Our previous study reported that Zfat gene
deletion in mice results in impaired differentiation of
hematopoietic progenitor cells in the blood islands, indicating
critical roles of Zfat in primitive erythropoiesis (15). The present study used
ZfatZsG/+ mice and detected Zfat-expressing cells
during definitive erythroid maturation in the fetal liver and adult
bone marrow. The majority of cells in the P1 cell population in
these tissues exhibited ZsGreen signals, whereas the proportion of
ZsGreen+ cells gradually decreased during the
progression of erythroid maturation. These results indicated that
Zfat expression may be restricted to the early stage of definitive
erythropoiesis, thus suggesting that Zfat is involved in the
differentiation of erythroid progenitors in both fetal and
postnatal definitive erythropoiesis. Because
Zfat−/− mice exhibit embryonic lethality by E8.5
(15), the roles of Zfat in
definitive erythropoiesis remain to be elucidated. Therefore,
conditional or inducible Zfat gene knock-out mice may be
useful in elucidating the roles of Zfat in definitive
erythropoiesis. Further studies are required to obtain a
comprehensive understanding of the roles of Zfat in definitive
erythropoiesis.
Zfat gene ablation in thymic T cells in
Zfatf/f-LckCre mice induces severe defects
in T-cell differentiation in the thymus, accompanied by impaired
positive selection and excessive apoptosis, thus indicating the
intrinsic roles of Zfat in thymic T-cell development (16,17). In the present study, in
ZfatZsG/+ mice, Zfat was expressed in TECs as
well as in thymic T-lineage cells. TECs generate the
microenvironment required for T-cell development within the thymus.
Zfat expression in TECs suggests that Zfat may have roles in thymic
T-cell development through the functional regulation of TECs,
particularly antigen presentation to T cells.
The present study further identified novel tissues
and cells that express Zfat using ZfatZsG/+ mice
through a combination of histological and flow cytometric analyses.
ZsGreen signals were detected in particular tissues, including the
labyrinth zone in the placenta, the pyramidal cell layer in the
hippocampal CA1 region and the Purkinje cell layer in the
cerebellum, suggesting specific roles of Zfat in these tissues.
In conclusion, the present study established
Zfat-ZsGreen knock-in mice to elucidate the expression and
functions of Zfat during embryonic development and in adult
tissues. Using this reporter mouse system, the results demonstrated
that Zfat was expressed in TECs, definitive erythroid progenitor
cells in the fetal liver and bone marrow, the labyrinth zone in the
placenta and in nervous tissues, thus suggesting its novel
functions in these cells and tissues. The
ZfatZsG/+ reporter mouse may be considered a
useful tool for elucidating the physiological roles and functions
of Zfat.
Funding
The present study was supported, in part, by grants
from the Ministry of Education, Culture, Sport, Science and
Technology of Japan.
Availability of data and materials
All data generated or analyzed during this study
are included in this published article.
Authors' contributions
TT, SI, HM, YT and TO generated the knock-in mice.
TT and MK performed the histological examinations. KD, HL and KN
performed the flow cytometry experiments. All experiments were
conducted by SS. The manuscript was written by TT, SI and SS. All
authors read and approved the manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Care and Use Committee of the National Center for Global Health and
Medicine (NCGM) Research Institute (NCGM#14032; Tokyo, Japan), and
the Institutional Animal Care and Use Committee of Fukuoka
University (Fukudai#157; Fukuoka, Japan). The study was approved by
the ethics committee of Fukuoka University (Fukudai#372).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Ms. Yumiko Hirose
(Fukuoka University, Fukuoka, Japan) and Ms. Takami Danno (Fukuoka
University) for their valuable technical assistance.
References
|
1
|
Ishikura S, Tsunoda T, Nakabayashi K, Doi
K, Koyanagi M, Hayashi K, Kawai T, Tanaka Y, Iwaihara Y, Luo H, et
al: Molecular mechanisms of transcriptional regulation by the
nuclear zinc-finger protein Zfat in T cells. Biochim Biophys Acta.
1859:1398–1410. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tochio N, Umehara T, Nakabayashi K,
Yoneyama M, Tsuda K, Shirouzu M, Koshiba S, Watanabe S, Kigawa T,
Sasazuki T, et al: Solution structures of the DNA-binding domains
of immune-related zinc-finger protein ZFAT. J Struct Funct Genom.
16:55–65. 2015. View Article : Google Scholar
|
|
3
|
Koyanagi M, Nakabayashi K, Fujimoto T, Gu
N, Baba I, Takashima Y, Doi K, Harada H, Kato N, Sasazuki T and
Shirasawa S: ZFAT expression in B and T lymphocytes and
identification of ZFAT-regulated genes. Genomics. 91:451–457. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shirasawa S, Harada H, Furugaki K, Akamizu
T, Ishikawa N, Ito K, Ito K, Tamai H, Kuma K, Kubota S, et al: SNPs
in the promoter of a B cell-specific antisense transcript,
SAS-ZFAT, determine susceptibility to autoimmune thyroid disease.
Hum Mol Genet. 13:2221–2231. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Comabella M, Craig DW, Morcillo-Suárez C,
Río J, Navarro A, Fernández M, Martin R and Montalban X:
Genome-wide scan of 500,000 single-nucleotide polymorphisms among
responders and nonresponders to interferon beta therapy in multiple
sclerosis. Arch Neurol. 66:972–978. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Inoue N, Watanabe M, Yamada H, Takemura K,
Hayashi F, Yamakawa N, Akahane M, Shimizuishi Y, Hidaka Y and
Iwatani Y: Associations between autoimmune thyroid disease
prognosis and functional polymorphisms of susceptibility genes,
CTLA4, PTPN22, CD40, FCRL3, and ZFAT, previously revealed in
genome-wide association studies. J Clin Immunol. 32:1243–1252.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ji HY, Yang B, Zhang ZY, Ouyang J, Yang M,
Zhang XF, Zhang WC, Su Y, Zhao KW, Xiao SJ, et al: A genome-wide
association analysis for susceptibility of pigs to enterotoxigenic
Escherichia coli F41. Animal. 10:1602–1608. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sabatino G, Rigante L, Minella D, Novelli
G, Della Pepa GM, Esposito G, Albanese A, Maira G and Marchese E:
Transcriptional profile characterization for the identification of
peripheral blood biomarkers in patients with cerebral aneurysms. J
Biol Regul Homeost Agents. 27:729–738. 2013.PubMed/NCBI
|
|
9
|
Slavin TP, Feng T, Schnell A, Zhu X and
Elston RC: Two-marker association tests yield new disease
associations for coronary artery disease and hypertension. Hum
Genet. 130:725–733. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ramakrishna M, Williams LH, Boyle SE,
Bearfoot JL, Sridhar A, Speed TP, Gorringe KL and Campbell IG:
Identification of candidate growth promoting genes in ovarian
cancer through integrated copy number and expression analysis. PLoS
One. 5:e99832010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takeuchi F, Nabika T, Isono M, Katsuya T,
Sugiyama T, Yamaguchi S, Kobayashi S, Yamori Y, Ogihara T and Kato
N: Evaluation of genetic loci influencing adult height in the
Japanese population. J Hum Genet. 54:749–752. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cho YS, Go MJ, Kim YJ, Heo JY, Oh JH, Ban
HJ, Yoon D, Lee MH, Kim DJ, Park M, et al: A large-scale
genome-wide association study of Asian populations uncovers genetic
factors influencing eight quantitative traits. Nat Genet.
41:527–534. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Signer-Hasler H, Flury C, Haase B, Burger
D, Simianer H, Leeb T and Rieder S: A genome-wide association study
reveals loci influencing height and other conformation traits in
horses. PLoS One. 7:e372822012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Makvandi-Nejad S, Hoffman GE, Allen JJ,
Chu E, Gu E, Chandler AM, Loredo AI, Bellone RR, Mezey JG, Brooks
SA and Sutter NB: Four loci explain 83% of size variation in the
horse. PLoS One. 7:e399292012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsunoda T, Takashima Y, Tanaka Y, Fujimoto
T, Doi K, Hirose Y, Koyanagi M, Yoshida Y, Okamura T, Kuroki M, et
al: Immune-related zinc finger gene ZFAT is an essential
transcriptional regulator for hematopoietic differentiation in
blood islands. Proc Natl Acad Sci USA. 107:14199–14204. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ishikura S, Ogawa M, Doi K, Matsuzaki H,
Iwaihara Y, Tanaka Y, Tsunoda T, Hideshima H, Okamura T and
Shirasawa S: Zfat-deficient CD4+ CD8+
double-positive thymocytes are susceptible to apoptosis with
deregulated activation of p38 and JNK. J Cell Biochem. 116:149–157.
2015. View Article : Google Scholar
|
|
17
|
Ogawa M, Okamura T, Ishikura S, Doi K,
Matsuzaki H, Tanaka Y, Ota T, Hayakawa K, Suzuki H, Tsunoda T, et
al: Zfat-deficiency results in a loss of CD3ζ phosphorylation with
dysregulation of ERK and Egr activities leading to impaired
positive selection. PLoS One. 8:e762542013. View Article : Google Scholar
|
|
18
|
Doi K, Fujimoto T, Okamura T, Ogawa M,
Tanaka Y, Mototani Y, Goto M, Ota T, Matsuzaki H, Kuroki M, et al:
ZFAT plays critical roles in peripheral T cell homeostasis and its
T cell receptor-mediated response. Biochem Biophys Res Commun.
425:107–112. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ishikura S, Iwaihara Y, Tanaka Y, Luo H,
Nishi K, Doi K, Koyanagi M, Okamura T, Tsunoda T and Shirasawa S:
The nuclear zinc finger protein Zfat maintains FoxO1 protein levels
in peripheral T cells by regulating the activities of autophagy and
the Akt signaling pathway. J Biol Chem. 291:15282–15291. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fujimoto T, Miyasaka K, Koyanagi M,
Tsunoda T, Baba I, Doi K, Ohta M, Kato N, Sasazuki T and Shirasawa
S: Altered energy homeostasis and resistance to diet-induced
obesity in KRAP-deficient mice. PLoS One. 4:e42402009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kawamoto T and Kawamoto K: Preparation of
thin frozen sections from nonfixed and undecalcified hard tissues
using Kawamot's film method 2012. Methods Mol Biol. 1130:149–164.
2014. View Article : Google Scholar
|
|
22
|
Nishi K, Iwaihara Y, Tsunoda T, Doi K,
Sakata T, Shirasawa S and Ishikura S: ROS-induced cleavage of
NHLRC2 by caspase-8 leads to apoptotic cell death in the HCT116
human colon cancer cell line. Cell Death Dis. 8:32182017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang SJ, Ahn S, Park CS, Holmes KL,
Westrup J, Chang CH and Kim MG: The quantitative assessment of MHC
II on thymic epithelium: Implications in cortical thymocyte
development. Int Immunol. 18:729–739. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gray DH, Chidgey AP and Boyd RL: Analysis
of thymic stromal cell populations using flow cytometry. J Immunol
Methods. 260:15–28. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fraser ST, Isern J and Baron MH:
Maturation and enucleation of primitive erythroblasts during mouse
embryogenesis is accompanied by changes in cell-surface antigen
expression. Blood. 109:343–352. 2007. View Article : Google Scholar
|
|
26
|
Iwaihara Y, Ishikura S, Doi K, Tsunoda T,
Fujimoto T, Okamura T and Shirasawa S: Marked reduction in FoxO1
protein by its enhanced proteasomal degradation in Zfat-deficient
peripheral T-cells. Anticancer Res. 35:4419–4423. 2015.PubMed/NCBI
|
|
27
|
Doi K, Ishikura S and Shirasawa S: The
roles of ZFAT in thymocyte differentiation and homeostasis of
peripheral naive T-cells. Anticancer Res. 34:4489–4495.
2014.PubMed/NCBI
|
|
28
|
Tsunoda T and Shirasawa S: Roles of ZFAT
in haematopoiesis, angiogenesis and cancer development. Anticancer
Res. 33:2833–2837. 2013.PubMed/NCBI
|