Introduction
Congenital diaphragmatic hernia (CDH) is a common
structural fetal malformation, with an incidence of ~1/3,000
(1,2). According to its anatomical
classification, CDH can be divided into posterolateral (Bochdalek
hernia), central diaphragmatic, and anterior diaphragmatic hernias.
Anterior diaphragmatic hernias include Morgnani (poststernal and
parasternal hernias) and other rare hernias. Posterolateral hernias
account for 80–90% of all CDHs, and include left diaphragmatic
hernias (~85%), right diaphragmatic hernias (~10%), and bilateral
diaphragmatic hernias (~5%) (3–5).
Infants with CDH have a high mortality rate, with respiratory
dysfunction, mainly due to concomitant pulmonary hypoplasia and
persistent pulmonary hypertension, being the main cause of
mortality in CDH neonates. Long-term follow-up of patients with CDH
has shown that pulmonary dysplasia can lead to pulmonary diseases
in surviving children, including obstructive airway disease and
restrictive lung function, and children with CDH are often prone to
develop asthma-related difficulties, with a propensity for
pulmonary infections and chronic pulmonary hypertension (6–8).
The present study found that the main pathological
lung tissue changes in CDH were abnormal pulmonary branch
development, pulmonary hypoplasia and pulmonary hypertension caused
by pulmonary vascular dysplasia (9–11).
It has been suggested that fetal lung hypoplasia in patients with
CDH is mainly caused by the abdominal visceral hernia protruding
into the chest, resulting in compression of the developing lung.
However, fetal lung dysplasia occurs prior to the occurrence of
diaphragmatic hernia in a CDH rat model (12).
Ephrin (Eph) proteins belong to the superfamily of
trans-membrane tyrosine kinase receptors and were originally
identified in human tumors (13).
Eph receptors exhibit the prototypical receptor tyrosine kinase
topology, with a multidomain extracellular region that includes the
ephrin ligand-binding domain, a single transmembrane segment, and a
cytoplasmic region containing the kinase domain. Eph receptors have
been divided into class A and B receptors, termed EphA and EphB,
based on sequence similarity and their preference for binding a
particular subclass of ephrins (14). Ephrin type-B receptor 4 (EPHB4) is
the receptor for ephrin-B2 (EFNB2) and Eph family members interact
with each other through ligand-receptor interactions, and exert
their biological effects by altering intracellular signaling
pathways (15). The biological
functions of Eph proteins include neural development, axon
guidance, synaptogenesis, blood and lymphatic vessel development,
skeletal patterning, adult stem cell regeneration, and bone
homeostasis (16,17). Among these, Eph family molecules
have demonstrated dynamic expression during pulmonary vascular
development. The expression of EFNB2 and EPHB4 at the
arterial-venous interface may restrict the intermingling of
arterial and venous endothelial cells, thereby stimulating the
formation of new capillary sprouts. In addition, Eph family
receptor-ligand interactions on different cell surfaces can promote
vascular assembly and are critical in the differentiation of
mesenchymal cells into perivascular supporting cells, which is
essential for the maintenance of stable and mature vessels
(18). The roles of ephrins and
their receptors in vascular development, cell migration indicate
potential involvement in lung branching morphogenesis
Based on these facts, it is hypothesized that EFNB2
and EPHB4 may have regulatory roles in CDH fetal lung development.
The aim of the present study was to investigate the pathological
changes in the CDH fetal lung, changes in the expression of EFNB2
and EPHB4, and the mechanism involved in affecting lung development
in a nitrofen-induced CDH rat model.
Materials and methods
Animals
All procedures/protocols were approved by the Animal
Research Committee of China Medical University (Shenyang, Liaoning,
China). Experimental Sprague-Dawley, specific pathogen-free (SPF)
rats were provided by Liaoning Changsheng Biotechnology Co., Ltd.
(Benxi, Liaoning, China), and were fed in the SPF-grade laboratory
of the Animal Center at Shengjing Hospital Affiliated to China
Medical University. The rats were fed a normal diet and were
maintained under a 12/12 h day/night cycle at a temperature of
20–25°C. Female rats (weight 240–260 g) were divided randomly into
two groups, and housed in cages with male rats (260–280 g) at a
ratio of 3:1 for mating. The female rats were then fed alone
following confirmation of sperm in the vaginal smear on the
following day, with 12 o'clock denoted as embryonic day (E)0.5. On
E8.5, the experimental group was administered with 100 mg nitrofen
(cat. no. N141413; Aladdin, Shanghai, China) by oral gavage (100 mg
nitrofen dissolved in 1 ml edible oil), whereas the control group
was provided with the same quantity of edible oil. Fetuses were
then removed by cesarean section on E13.5, E15.5, E17.5, E19.5, and
E21.5, following anesthesia with 1% pentobarbital intraperitoneal
injection. The incidence of CDH in the fetal rats was 64%. Fetal
lung tissue was collected under an anatomical microscope and stored
at −80°C. Certain lung tissues were fixed in 4% paraformaldehyde
for 24–48 h.
Hematoxylin and eosin (H&E)
staining
The anterior halves of the paraffin-embedded lungs
(4-μm sections) were deparaffinized in dimethylbenzene and
hydrated by ethanol. Three E21.5 lung tissue slices from each group
were selected randomly for H&E staining; H&E staining was
performed according to the manufacturer's protocol (cat. no. G1120,
Solarbio Science & Technology Co., Ltd., Beijing, China). Five
fields (upper, middle, lower, left, and right) in each section were
also selected randomly (avoiding large vessels and bronchi),
observed under Olympus BX61 light microscope (Olympus Corporation,
Tokyo, Japan) at ×100 magnification, and images were captured using
a digital camera. Cross lines were drawn at the center of each
field of view, and the number of alveolar spaces (Ns) intersecting
the cross line and the number of alveoli in each visual field (Na)
were calculated. The total length of the line (L) and the area of
each visual field (S) were also measured. The mean linear intercept
(MLI) and mean alveolar number (MAN) in the lung tissues were
calculated according to the following formulae: MLI = L/Ns (which
reflects the mean alveolar diameter) and MAN = Na/S (which reflects
alveolar density). Image analysis was performed using Image
Pro-Plus 6.0 (Media Cybernetics, Inc., Washington, DC, USA).
Fetal lung explant cultures
The pregnant rats received an intraperitoneal
injection of 1% pentobarbital on E13.5, and the fetuses were
removed by cesarean section. The fetal lung tissue was removed
under aseptic conditions, transferred to Costar-Transwell® cells
(Corning Incorporated, Corning, NY, USA), and cultured at the
air-liquid interface in serum-free DMEM/F12 medium (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
100 U/ml penicillin and 100 μg/ml streptomycin. The three
experimental groups (control, CDH and CDH + EFNB2 groups) comprised
eight lungs/group. The explants were placed in humidified
incubators at 37°C in an atmosphere of air plus 5% CO2
and cultured for 96 h. In the CDH + EFNB2 group, following 1 h of
incubation, recombinant EFNB2 (cat. no. 496-EB-200; R&D
Systems, Inc., Minneapolis, MN, USA) was added to the lung explants
at a final concentration of 0.01 μg/ml. The recombinant
EFNB2 was added daily. An equal volume of phosphate-buffered saline
(PBS) was added to the control group. The medium was replaced at 48
h. Images of the lung explants were captured daily using an
inverted phase contrast microscope, and lung buds were counted in
the digitized images using Image Pro-Plus software 6.0 (Media
Cybernetics, Inc.). At the end of the incubation period, the
explants were washed in PBS and stored at −80°C until use.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was extracted from the fetal pulmonary
tissues using RNAiso Plus extraction reagent (cat. no. 9108; Takara
Biotechnology Co., Ltd., Beijing, China) according to the
manufacturer's protocol. A PrimeScript™ RT reagent kit with gDNA
Eraser (Perfect Real Time; cat. no. RR047A, Takara Biotechnology
Co., Ltd.) was used for reverse transcription. Total RNA (1
μg) was reverse transcribed into cDNA for qPCR, which was
performed in accordance with the manufacturer's protocol using
SYBR® Premix Ex Taq™ II (Tli RNaseH Plus; cat. no. RR820A; Takara
Biotechnology Co., Ltd.) on an Applied Biosystems® 7500 Fast
Real-Time PCR system. The qPCR system included the following: TB
Green Premix Ex Taq II 10 μl; PCR Forward Primer (10
μM) 0.8 μl; PCR Reverse Primer (10 μM) 0.8
μl; ROX Reference Dye II (50×) 0.4 μl; cDNA 2
μl; ddH2O 6 μl; Total: 20 μl.
Primer synthesis was performed by Sangon Biotech Co., Ltd.
(Shanghai, China), as listed in Table
I. The specific conditions were as follows: Stage i) 95°C for
30 sec; stage ii) 95° for 3 sec, and then 60° for 30 sec, repeating
40 times; stage iii) melt curve establishment. The relative mRNA
levels were calculated using the 2−ΔΔCq method (19) following normalization with the
housekeeping gene β-actin.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Target gene | ID | Sequence
(5′-3′) | Temp (°C) | Size (bp) |
|---|
| Rat β-actin | NM_031144.3 | Forward:
GGAGATTACTGCCCTGGCTCCTA | 63.7 | 127 |
| | Reverse:
GACTCATCGTACTCCTGCTTGCTG | 63.7 | |
| Rat EFNB2 | NM_001107328.2 | Forward:
GCCTTATTCGCAGGGATTG | 57.4 | 98 |
| | Reverse:
CGTGTGCTGTGGAGAGTGTT | 54.1 | |
| Rat EPHB4 | XM_003751157.4 | Forward:
TGAGGTGTGCGATATGAAGC | 55.2 | 108 |
| | Reverse:
CAGGGACAGACATTCCATCA | 57.3 | |
Western blot analysis
The fresh frozen lungs were thawed and sonicated,
and proteins were isolated using the Mem-PER™ Plus Membrane Protein
Extraction kit (cat. no. 89842; Thermo Fisher Scientific, Inc.).
Protein concentrations were measured using the Pierce™ BCA Protein
Assay kit (cat. no. 23225; Thermo Fisher Scientific, Inc.) and
diluted with gel-loading buffer (cat. no. P0015; Beyotime Institute
of Biotechnology, Shanghai, China) prior to gel loading. Protein
(quantity, 50 μg) separation was achieved by gel
electrophoresis using 10% SDS-polyacrylamide gels (cat. no. P0012A;
Beyotime Institute of Biotechnology) in SDS running buffer. The
proteins were transferred onto polyvinylidene difluoride membranes
(EMD Millipore, Billerica, MA, USA) by western blotting. Following
western blotting, the membranes were blocked in 5% non-fat milk for
60 min prior to antibody detection. Primary antibodies against
EPHB4 [rabbit polyclonal; cat. no. 20883-1-AP, 1:500 dilution in
TRIS-buffered saline with 0.1% Tween-20 (TBST), ProteinTech Group,
Inc., Wuhan, China], EFNB2 (rabbit monoclonal; cat. no. ab150411;
1:500 dilution in TBST; Abcam, Cambridge, MA, USA), β-actin (mouse
monoclonal; cat. no. 60008-1-Ig; 1:4,000 dilution in TBST;
ProteinTech Group, Inc.), non-phosphorylated and phosphorylated
forms of p38, p44/42 [extracellular signal-regulated kinase
(ERK)1/2], c-Jun NH2-terminal kinase (JNK) (cat. nos. 9926 and
9910; 1:1,000 dilution in TBST; Cell Signaling Technology, Inc.,
Beverly, MA, USA) and non-phos-phorylated and phosphorylated forms
of signal transducer and activator of transcription (STAT)3 (cat.
nos. 12640 and 9131; 1:1,000 dilution in TBST; Cell Signaling
Technology, Inc.), were incubated overnight at 4°C. Following
extensive washing with TBST, the membranes were incubated with the
following horseradish peroxidase (HRP)-conjugated secondary
antibodies for 1.5 h at room temperature: Anti-rabbit IgG,
HRP-linked antibody (cat. no. 7074; Cell Signaling Technology,
Inc.) and anti-mouse IgG, HRP-linked antibody (cat. no. 7076; Cell
Signaling Technology, Inc.), followed by further extensive washing.
Detection was performed using an enhanced chemiluminescence kit
SuperSignal™ West Pico PLUS chemiluminescent substrate (cat. no.
34580; Thermo Fisher Scientific, Inc.). β-actin was used to control
for equal loading and transfer of the samples. The optical density
(OD) of the protein bands was analyzed using Quantity One software
4.6.7 (Bio-Rad Laboratories, Inc.). The expression of a target
protein was calculated as a percentage of the corresponding β-actin
OD. Experiments were performed at least three times for each
antibody.
Immunohistochemistry
The paraffin-embedded lungs (4-μm sections)
were deparaffinized in dimethylbenzene and hydrated in ethanol. The
tissue preparations were boiled for 7 min in a microwave oven and
then cooled to room temperature. Immunohistochemistry was performed
using the streptav-idin-peroxidase method (cat. no. SP-9001; SPlink
detection kit; OriGene Technologies, Inc., Beijing, China)
according to the kit protocol. Rabbit polyclonal anti-EPHB4
antibody was added at 4°C (1:250, overnight). Rabbit monoclonal
anti-EFNB2 was added at 4°C (1:200, overnight). The sections were
counterstained with hematoxylin (cat. no. G1080; Solarbio Science
& Technology Co., Ltd.) for 90 sec. Negative controls were
performed without primary antibody. Images of 60 slides were
captured and semi-quantitative analysis was performed using Image
Pro-Plus 6.0 (Media Cybernetics, Inc.).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Statistical analyses were performed using GraphPad Prism
5 (GraphPad Software, Inc., San Diego, CA, USA). Statistical
comparison of experimental groups was achieved using unpaired
Student's t-test or a one-way analysis of variance. The
Student-Newman-Keuls test was used for post hoc analysis. P<0.05
was considered to indicate a statistically significant
difference.
Results
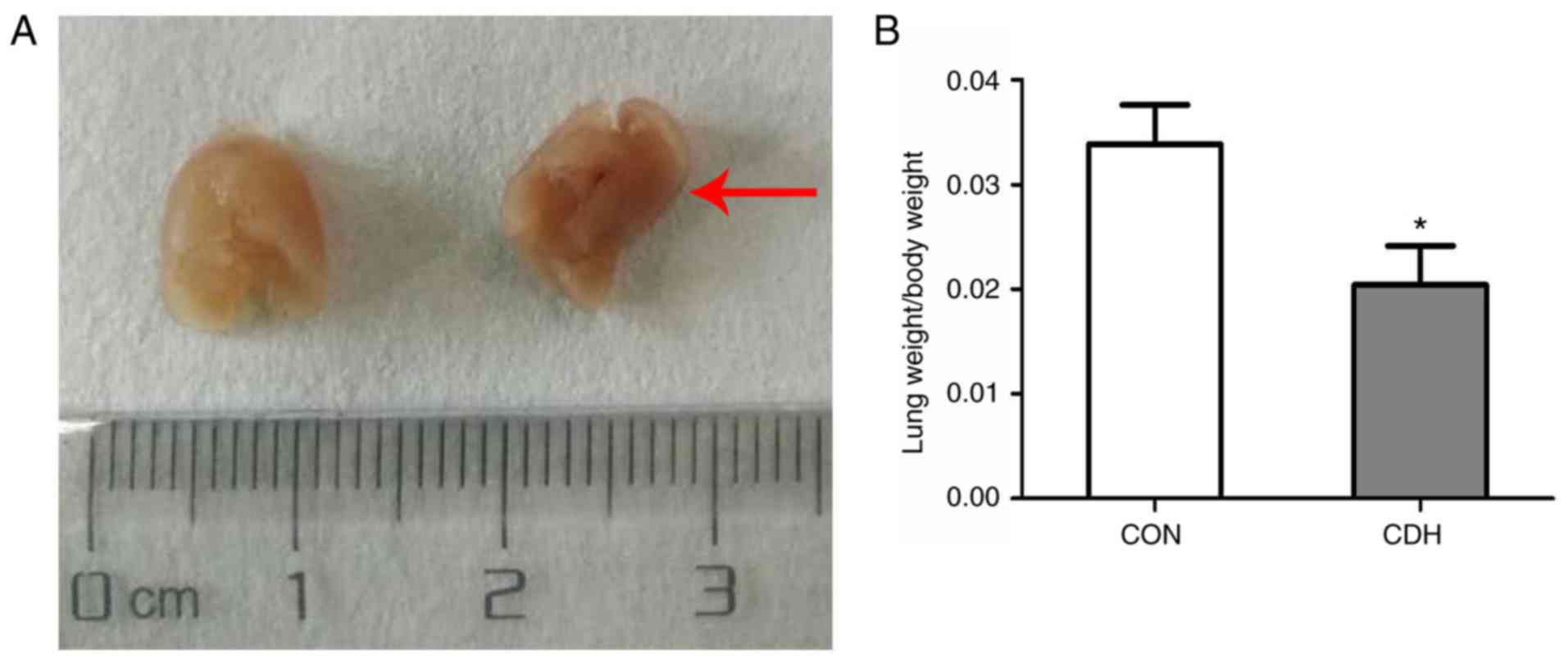
Lung development in CDH fetal rats
Herniation of the liver and stomach from the
abdominal cavity into the left thoracic cavity was observed on
E21.5 in the CDH fetal rats, and the volume of lung tissue on the
hernia side was significantly smaller in the CDH fetuses compared
with the control fetuses. The lung index (lung wet weight/body
weight) was also significantly lower in the CDH group compared with
that in the control group (Fig. 1A
and B).
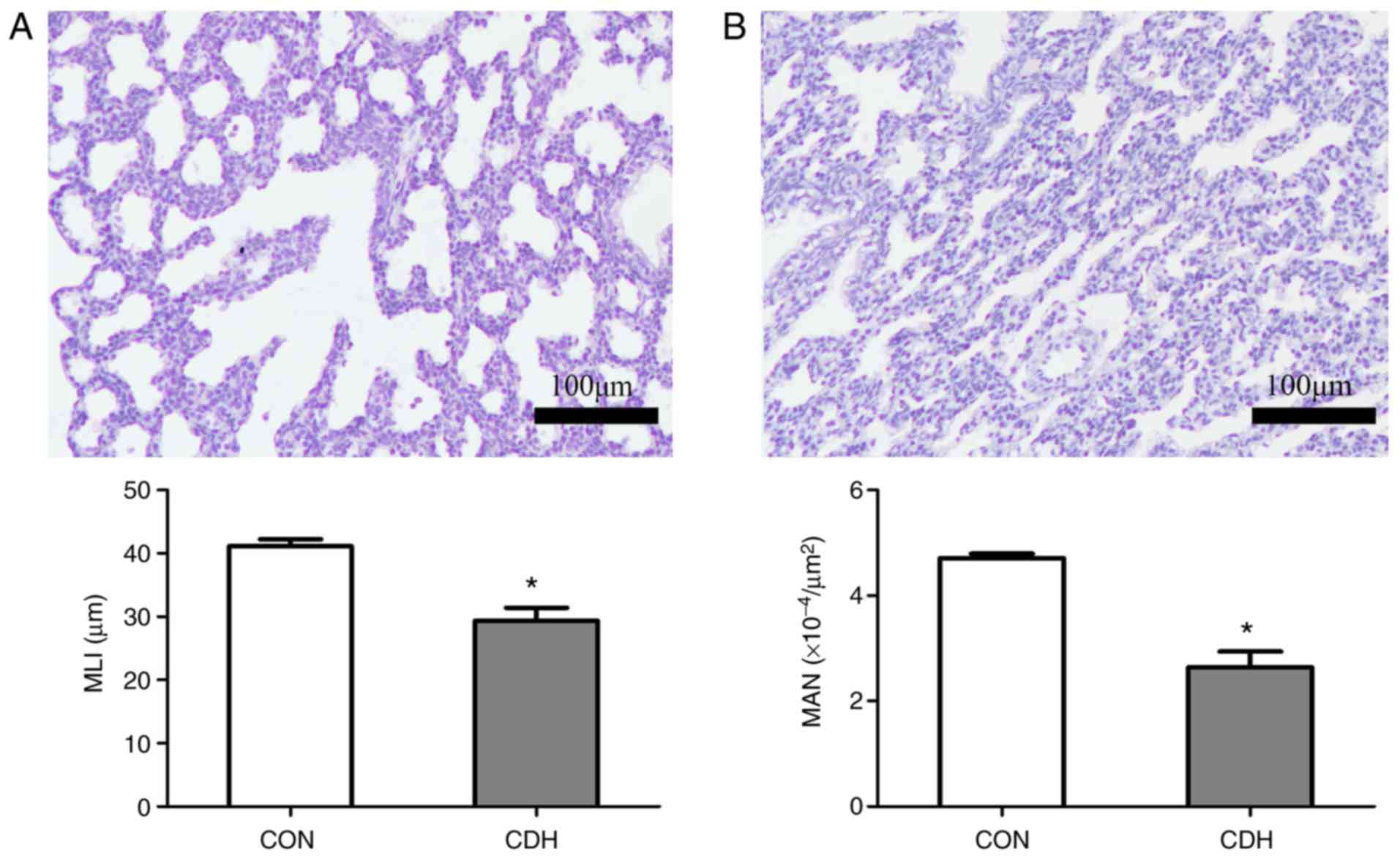
Pathological changes of CDH fetal
pulmonary development
Paraffin sections of lung tissue from E21.5 fetuses
were subjected to H&E staining to reveal morphological changes.
The MAN was significantly lower in the CDH group compared with that
in the control group. The MLI in the CDH group was also
significantly reduced compared with that in the control group.
These results suggested the existence of pulmonary dysplasia in the
CDH group, with widened alveolar septa and decreased numbers of
alveoli (Fig. 2A and B).
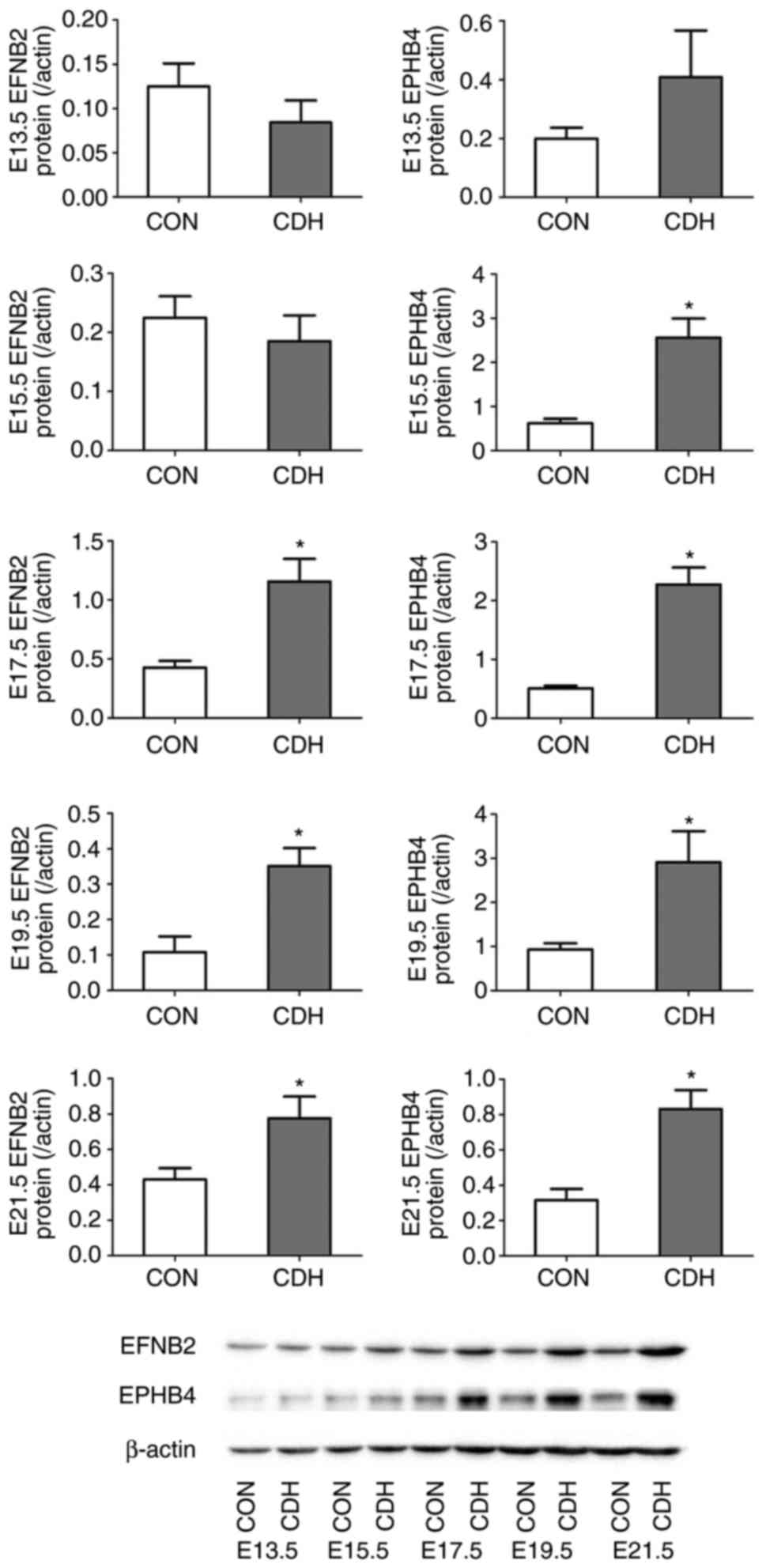
Expression of EPHB4 and EFNB2 in lung
tissues
The gene expression levels of EFNB2 and EPHB4 were
detected in the CDH and control fetal lung tissues by RT-qPCR
analysis at E13.5, E15.5, E17.5, E19.5, and E21.5. No significant
differences in expression levels were observed at E13.5 (embryonic
stage) or E15.5 (pseudoglandular stage) in the CDH group compared
with the control group, however, EFNB2 and EPHB4 were significantly
upregulated at E17.5 (canalicular stage) and E19.5 and E21.5
(saccular/alveolar stages) (Fig.
3). Western blot analysis also showed that the difference in
protein expression levels between the two groups were more marked
with increasing embryonic age (Fig.
4).
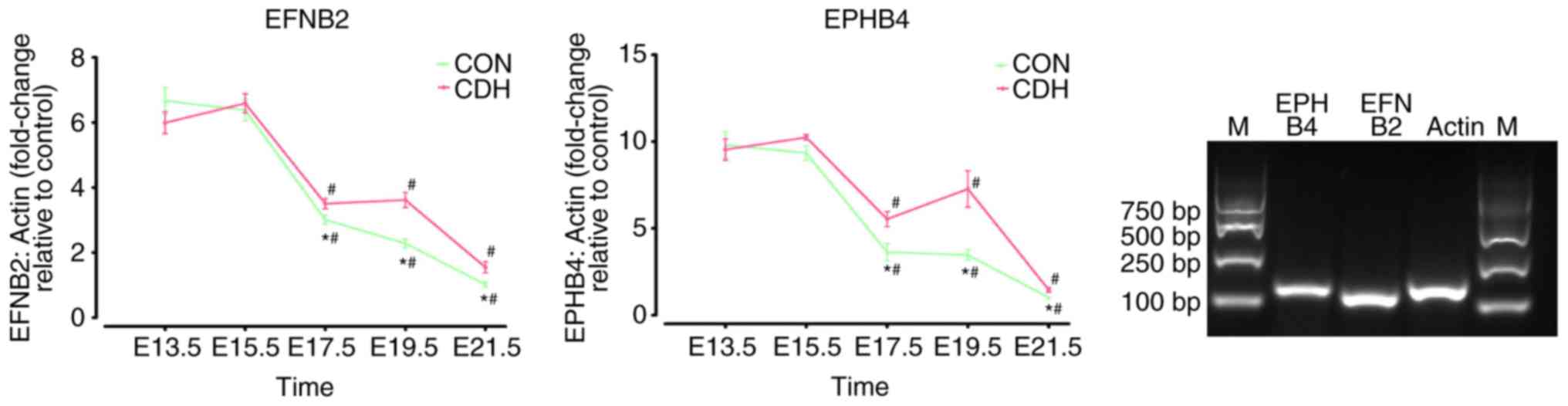 | Figure 3Tempo ral expression of EFNB2 and
EPHB4 in the lungs of the CDH and CON groups. mRNA levels of EFNB2
and EPHB4 in develo ping lungs were determined by reverse
transcription-quantitative polymerase chain reaction analysis and
results are expressed rela tive to the cont rol at E13.5, E15.5,
E17.5, E19.5 and E21.5. Specificity of the products were confirmed
by visualization on 2% agarose gels. Results are presented as the
mean ± standard error of the mean. *P<0.05, vs. CON
at the same time point. #P<0.05, vs. E13.5,
inner-group. CDH, congenital diaphragmatic hernia; CON, control; E,
embryonic day; EFNB2, ephrin-B2; EPHB4, ephrin type-B receptor 4;
M, marker. |
The results of immunohistochemistry showed that the
expression of EFNB2 and EPHB4 at different time periods of
pregnancy had similarities. EFNB2 was consistently expressed in
epithelial cells; however, at E13.5 and E15.5, EFNB2 was also
expressed in cells surrounding the epithelium. At E17.5 and E19.5,
the expression levels of EFNB2 in the alveolar epithelium and
terminal and respiratory bronchioles were higher in the CDH group
than in the control group. At E21.5, EFNB2 was mainly expressed in
terminal and respiratory bronchioles, with expression in the CDH
group higher than that in the control group, according to the
result of semi-quantitative analysis. EFNB2 was not detected in
vascular smooth muscle cells, whereas weak expression was observed
in the endothelial cells (Fig.
5A).
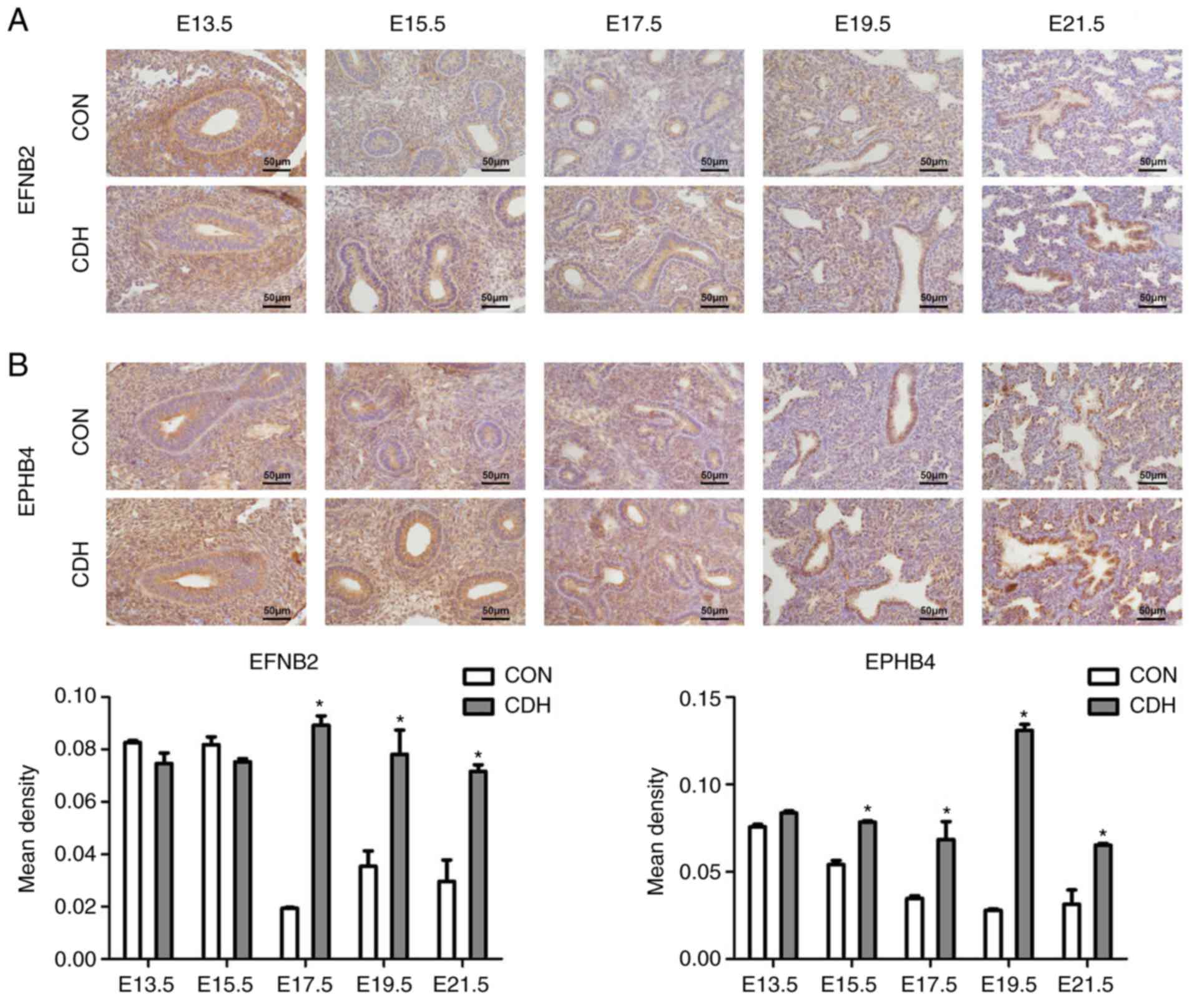 | Figure 5Expression of EFNB2 and EPHB4.
Representative photomicrographs of IHC staining for (A) EFNB2 and
(B) EPHB4 in the lung sections from CON and CDH groups at five
gestational stages: E13.5, E15.5, E17.5, E19.5 and E21.5 days.
EFNB2 and receptor EPHB4 exhibited marked epithelial expression. In
the semi-quantitative analysis, at E17.5, E19.5 and E21.5, the
expression of EFNB2 and EPHB4 was higher in the CDH group than in
the CON group (*P<0.05 vs. CON at the same time
point). Original magnification, ×400, scale bar=50 μm. CDH,
congenital diaphragmatic hernia; CON, control; E, embryonic day;
EFNB2, ephrin-B2; EPHB4, ephrin type-B receptor 4. |
In a similar manner, EPHB4 was expressed in less
differentiated cells surrounding the epithelium at E13.5 and E15.5.
Unlike EFNB2, EPHB4 was expressed in the alveolar epithelium during
late lung development, although no expression was detected in the
vascular smooth muscle cells (Fig.
5B).
Fetal lung culture
The lung tissues were extracted from the CDH and
control fetal rats on E13.5 and cultured in vitro. The
number of terminal lung buds, and the lung tissue explant perimeter
and surface were measured at 0, 24, 48, 72 and 96 h in the two
groups, and these were significantly lower in the CDH group
compared with those in the control group (Fig. 6A–C). Significant differences in
all three parameters remained following 96 h of culture (Fig. 6D and E).
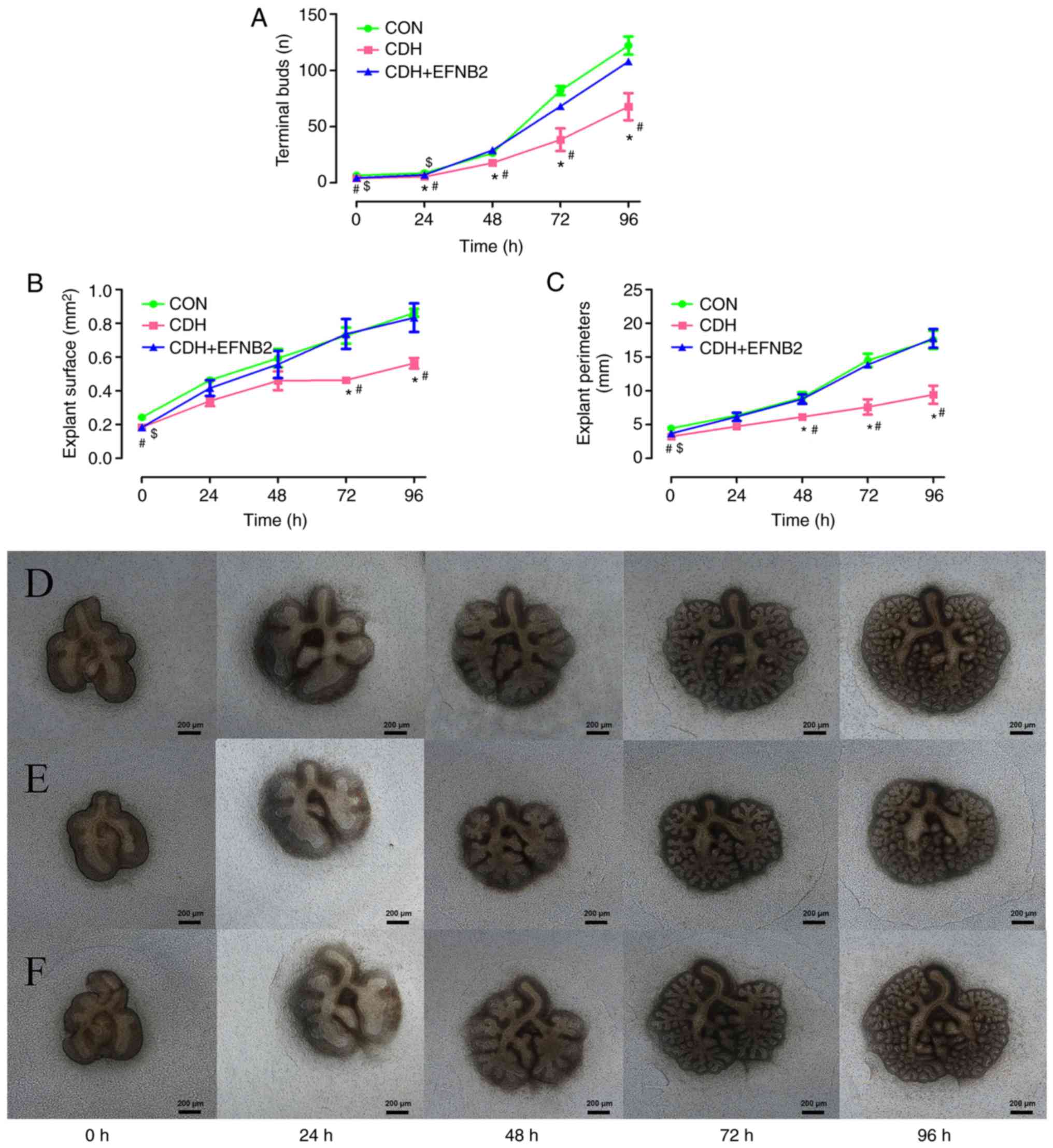 | Figure 6Fetal lung explant cultures in
vitro. (A) Terminal bud count, (B) explant surface, and (C)
explant perimeter were significantly smaller in the E13.5 CDH group
than in the CON group (#P<0.05, vs. CON at the same
time point). Lung tissue development in the CDH group lagged behind
that in the CON group by 96 h of culture. Lung explants of the (D)
CON group (E) CDH group, and (F) CDH + EFNB2 group at 0, 24, 48, 72
and 96 h. EFNB2 supplementation promoted branching of rat fetal
lung explants. E13.5 lungs were treated with EFNB2 recombinant
protein (0.01 μg/ml) daily. Terminal buds counts, explant
surface, and perimeter were significantly increased in the
EFNB2-treated group, compared with the CDH group. Compared with the
untreated lung, EFNB2 treatment markedly increased airway branching
morphogenesis, termi nal bud count, explant surface, and explant
perimeter at 96 h (*P<0.05, CDH, vs. CDH + EFNB2 at
the same time point). After 96 h in vitro, no significant
differences in termi nal bud count, explant surface, or explant
perimeter were observed between the CDH + EFNB2 group and CON group
($P<0.05, CDH+EFNB2, vs. CON at the same time point).
Results are presented as the mean ± standard error of the mean.
Original magnification, ×40, scale bar=200 μm (all images at
same magnification). CDH, congenital diaphragmatic hernia; CON,
control; E13.5, embryonic day 13.5; EFNB2, ephrin-B2. |
Effect of EFNB2 on fetal lung
morphogenesis in rats
To clarify the role of EFNB2 in the development of
congenital diaphragmatic hernia in fetal lungs, functional
experiments were performed using cultured lung tissue in
vitro. Recombinant EFNB2 (final concentration 0.01
μg/ml) was added to the medium daily and images of the lung
tissues were captured every 24 h (Fig. 6F). It was found that, following
the addition of EFNB2 to fetal lung tissue, the development of lung
branches was improved, compared with that in the control group at
24, 48, 72, and 96 h (Fig. 6A).
At 72 and 96 h of culture, there was a significant difference in
the lung tissue area of the two groups (Fig. 6B), and following 48, 72, and 96 h
of incubation, there was a significant difference in lung tissue
circumference between the two groups (Fig. 6C).
EFNB2 influences the phosphorylated forms
of P38, ERK, JNK and STAT3
At present, the mechanism of lung development
remains to be fully elucidated. To clarify the mechanism involving
the effects of EFNB2 on the development of lung branch tissue
during congenital diaphragmatic hernia development, western blot
analysis was performed on the cultured lung tissues.
Samples from the CDH and CDH + EFNB2 groups were
mixed (n=10/each group), and changes in the phosphorylation levels
of P38, ERK, JNK, and STAT3 were determined. The results showed
that EFNB2 treatment inactivated the P38, ERK, JNK, and STAT
signaling pathways in the fetal lung explants (Fig. 7A and B).
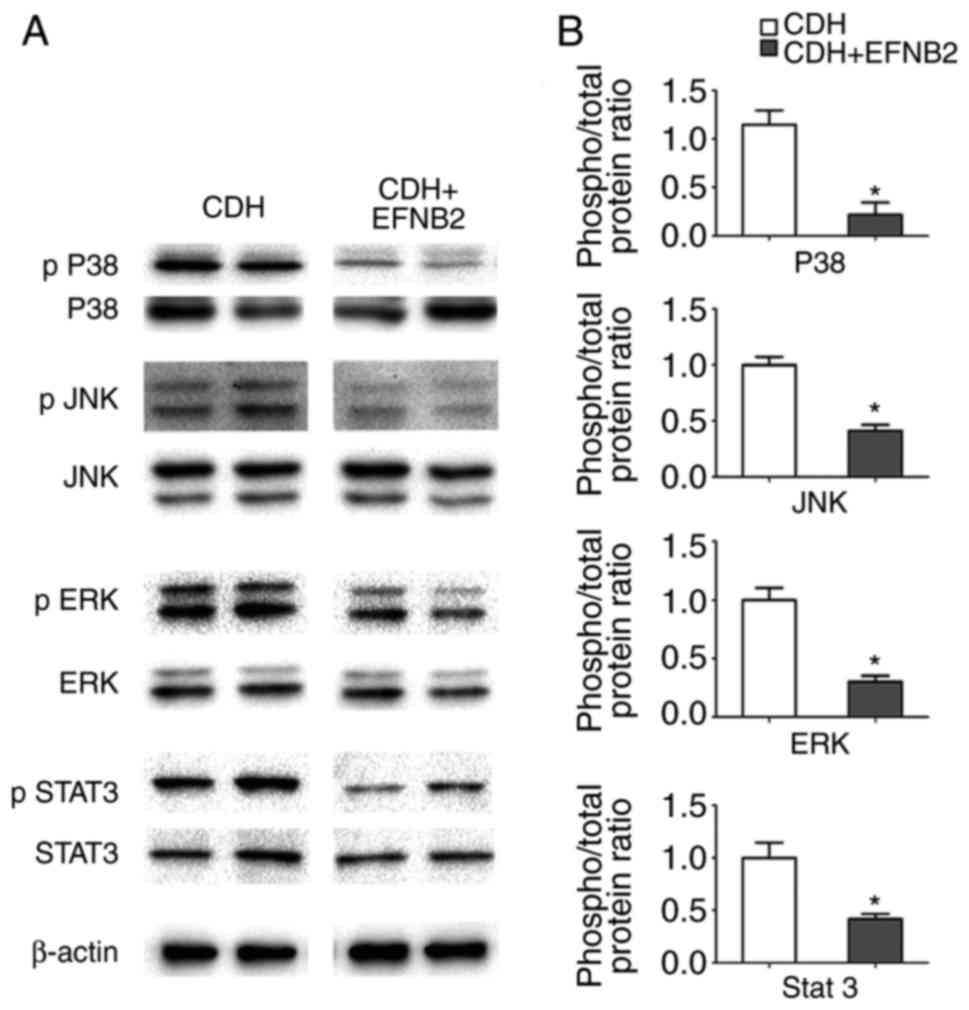 | Figure 7Analysis of the intracellular
signaling pathways that the mediate effects of EFNB2 in lung
morphogenesis. (A) Representative immunoblots and densitometric
analysis of p38, JNK, ERK and STAT3, and p-p38, p JNK, p ERK and p
STAT3 in CDH- and EFNB2-treated lung explants (fetal lung explant
cultures in vitro for 96 h). Results were normalized
relative to the expression of β-actin. (B) Semi-quantitative
analysis of phosphorylated forms of intracellular signaling
pathways that mediate lung growth. EFNB2 caused a significant
decrease in p38, JNK, ERK and STAT3 signaling activity. The
activity of intracellular signaling pathways was measured by the
ratio between phosphorylated protein and total protein. All data
were normalized against the CDH group. *P<0.05, vs.
CDH. EFNB2, ephrin-B2; EPHB4, ephrin type-B receptor 4; CDH,
congenital diaphragmatic hernia; CON, control; JNK, c-Jun
NH2-terminal kinase; ERK, extracellular signal-regulated kinase;
STAT3, signal transducer and activator of transcription 3; p,
phosphorylated. |
Discussion
CDH is a common congenital anomaly in which an
abdominal visceral hernia is caused by diaphragmatic or muscular
insufficiency, potentially leading to pulmonary hypoplasia and
pulmonary hypertension. Including the effects of induced labor, the
mortality rate of CDH is at least 50% (20). The majority of CDHs are
left-Bochdaleck type diaphragmatic hernias. Although rapid
developments in maternal fetal medicine and surgical techniques
have enabled resolution of the problem of lung tissue oppression by
surgery following birth, pulmonary hypoplasia and pulmonary
hypertension caused by abnormal intrauterine lung development
remain the main causes of mortality in children with CDH. The
quality of life of those surviving CDH has attracted increasing
attention, with children who survive CDH being particularly prone
to lung diseases, including chronic pulmonary infection and
obstructive pulmonary disease (3,6–8).
Lung development is influenced by several factors,
including genetic and environmental factors, involving complex
regulatory mechanisms. Rat lung development is divided into five
distinct stages: Embryonic stage (E9.5-E14), pseudoglandular stage
(E14-E16.5), canalicular stage (E16.5-E17.5), and saccular and
alveolar stages (>E17.5) (21). Various growth factors (sonic
hedgehog and fibroblast growth factors, particularly fibroblast
growth factor 10), transcription factors (hepatocyte nuclear
factor-3, and Hox and Gli genes), and signaling pathways, [vascular
endothelial growth factor (VEGF) and Ephrin/Eph pathways], have
important roles in lung development (22–26).
EFNB2 and EPHB4 are important regulators of
pulmonary branch and vascular development. EFNB2 has been shown to
control VEGF-induced angiogenesis and lymphangiogenesis (27). A previous study showed that
alveolar development was inhibited when the expression of EFNB2 was
inhibited by small interfering RNA in newborn rats in vivo.
In a rat model of high-pressure oxygen-induced lung injury, EFNB2
preserved alveolar epithelial cell viability in oxygen, decreased
oxygen-induced alveolar epithelial cell apoptosis, and accelerated
alveolar epithelial cell wound healing, which had a protective
effect in alveolar development. An in vitro study showed
that the intranasal administration of EFNB2 inhibited apoptosis in
rat alveolar epithelial cells (28). Furthermore, intracellular pathways
downstream of EFNB2 were inactivated in EFNB2 mutants, thus
mediating distal lung dysplasia and alveolar crest formation. In
addition, EFNB2 reverse signaling reduced distal lung compliance by
increasing the deposition of α5β1 integrin-mediated fibronectin
(29).
In the present study, the structure of fetal lung
tissue in a nitrofen-induced rat model of CDH was examined.
Abnormal lung structure was observed in full-term CDH fetal rats
(E21.5), with a reduced alveolar MLI, and reduced numbers of
alveoli. The number of lung tissue terminal buds, and the lung
tissue explant surface and perimeter were also reduced in the CDH
fetuses compared with the control rat fetuses at E13.5, and lung
tissue development continued to lag behind the control group at 96
h of culture. Previous studies have suggested that compression of
the developing lung by the abdominal organs during pregnancy is the
main cause of lung dysplasia, however, the development of CDH lung
tissue was delayed even in vitro in the present study. This
suggests that molecular defects in the lung tissue itself, in
addition to compression, contribute to the development of lung
dysplasia.
The present study examined the temporal and spatial
changes in the expression of EFNB2 and EPHB4 during lung
development. No significant difference was observed in the
expression levels of either of these factors between the CDH and
control fetuses during the embryonic (E13.5) and pseudoglandular
(E15.5) stages, however, EFNB2 and EPHB4 were significantly
upregulated during the canalicular stage (E17.5) and the
saccular/alveolar stages (E19.5, E21.5) in the CDH group, with
consistent results for mRNA and protein levels. In the present
study, lung development in the CDH fetuses lagged behind that in
the controls at E13.5, even following 96 h of in vitro
culture. However, no signifi-cant differences were observed in the
expression levels of EFNB2 and EPHB4 between the two groups at the
embryonic (E13.5) or pseudoglandular (E15.5) stages, possibly due
the effect of the hernia-induced compression into the chest cavity
on the developing lung being not particularly severe during these
stages. However, as the pregnancy progresses, the lung volume
increases and its compression becomes more marked, resulting in
reconstruction of the lung structure and changes in the expression
levels of a series of signaling molecules regulating lung
development. It was hypothesized that the expression levels EFNB2
and EPHB4 are increased at the canalicular, saccular, and alveolar
stages, to provide compensatory promotion of lung branch
development.
The EFNB2 molecule was used to elucidate its role in
the development of CDH lungs. Compared with ephrin forward
signaling, less is known about ephrin reverse signaling, in which
the signal initiation factor is EFNB2, or EFNB2 as a key molecule
capable of affecting other molecules involved in lung development
(15). Therefore, EFNB2 was
selected in the present study as a target gene in the functional
experiments. When recombinant EFNB2 was added to fetal lung explant
cultures in vitro, it not only promoted the development of
CDH lung tissue, but also increased the area and perimeter of this
tissue. These results demonstrated that the promotion of EFNB2
during lung development in CDH fetal rats significantly improved
hypoplastic lung conditions.
To clarify the mechanism of how EFNB2 affects lung
development, the phosphorylation levels of P38, ERK, JNK, and STAT3
were examined. EFNB2 stimulation induced a decrease in the
phosphorylated levels of P38, ERK, JNK, and STAT3, indicating a
decrease in these intracellular signaling pathways. The regulation
of pulmonary branch development is complex, involving interactions
among multiple signaling pathways (30–34). The ERK signaling pathway is a
classic signaling pathway, and previous studies have suggested that
the ERK/mitogen-activated protein kinase (MAPK) pathway is
important in tracheal progenitor cell maintenance and
differentiation. The ERK/MAPK pathway is also required for the
integration of mesenchymal and epithelial signals required for the
development of the entire respiratory tract (35,36). DA-Raf-dependent inhibition of the
Ras-ERK signaling pathway in type 2 alveolar epithelial cells is
required for alveolar formation (37). The JNK pathway is another
distinct family included in the MAPK signal transduction pathway,
which may also be important in lung development. In a previous
study of hyperoxia-induced cell death and impaired alveolarization
in the developing lung, of a transforming growth factor-β1
transgenic mouse model, inhibition of the JNK signaling pathway
significantly improved spontaneously impaired alveolarization in
room air and decreased mortality on exposure to hyperoxia (38). For
the development of lung branches, rat fetal lung explants at E13.5
cultured in the presence of piceatannol, a known STAT3 signaling
inhibitor, have been shown to increase in growth in a
dose-dependent manner (39). The possible role of the P38, ERK, JNK
and STAT3 signaling pathways in lung growth remains an indirect or
even a balancing effect, involving the activation of other
pathways, or it may simply be contextual. Further investigations
are required to determine whether inactivation of this pathway is
crucial for the function of EFNB2 in lung development.
In conclusion, the results of the present study
suggested that abnormal intrauterine lung branch development may
contribute to pulmonary dysplasia in CDH fetal rats. The increased
expression of the angiogenesis factors, EFNB2 and EPHB4, in CDH
fetal lung tissues promoted fetal lung development, however, this
was insufficient to completely reverse the CDH-related defects. At
present, the effects of fetoscopic endotracheal occlusion on the
treatment of severe congenital diaphragmatic hernia remain to be
fully elucidated, therefore, EFNB2 may be used as a novel target
for intrauterine treatment of severe diaphragmatic hernia. Further
investigations are required to determine the exact molecular
mechanisms involved in CDH fetal lung branch development, and the
ways in which the Eph family factors can compensate for the
abnormal regulation of this process.
Funding
The present study was supported by The Establishment
and Optimization of Common High-risk Fetal Diagnosis and Treatment
Technology Standards and Specifications from the National Health
and Family Planning Commission, China (grant no. 201402006).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL contributed to the study design, data acquisition
and analysis and wrote the manuscript; XL and WY were involved in
data acquisition. CL was involved in study design and assisted in
writing the manuscript. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
All procedures performed in experiments involving
animals were in accordance with the Animal Research Committee of
China Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Torfs CP, Curry CJ, Bateson TF and Honoré
LH: A population-based study of congenital diaphragmatic hernia.
Teratology. 46:555–565. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Skari H, Bjornland K, Haugen G, Egeland T
and Emblem R: Congenital diaphragmatic hernia: A meta-analysis of
mortality factors. J Pediatr Surg. 35:1187–1197. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pober BR, Russell MK and Ackerman KG:
Congenital Diaphragmatic Hernia Overview.
|
|
4
|
van Loenhout RB, Tibboel D, Post M and
Keijzer R: Congenital diaphragmatic hernia: Comparison of animal
models and relevance to the human situation. Neonatology.
96:137–149. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pober BR: Overview of epidemiology,
genetics, birth defects, and chromosome abnormalities associated
with CDH. Am J Med Genet C Semin Med Genet. 145C:158–171. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peetsold MG, Heij HA, Kneepkens CM,
Nagelkerke AF, Huisman J and Gemke RJ: The long-term follow-up of
patients with a congenital diaphragmatic hernia: A broad spectrum
of morbidity. Pediatr Surg Int. 25:1–17. 2009. View Article : Google Scholar
|
|
7
|
Muratore CS, Kharasch V, Lund DP, Sheils
C, Friedman S, Brown C, Utter S, Jaksic T and Wilson JM: Pulmonary
morbidity in 100 survivors of congenital diaphragmatic hernia
monitored in a multidisciplinary clinic. J Pediatr Surg.
36:133–140. 2001. View Article : Google Scholar
|
|
8
|
Badillo A and Gingalewski C: Congenital
diaphragmatic hernia: Treatment and outcomes. Semin Perinatol.
38:92–96. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bargy F, Beaudoin S and Barbet P: Fetal
lung growth in congenital diaphragmatic hernia. Fetal Diagn Ther.
21:39–44. 2006. View Article : Google Scholar
|
|
10
|
Acker SN, Mandell EW, Sims-Lucas S, Gien
J, Abman SH and Galambos C: Histologic identification of prominent
intrapul-monary anastomotic vessels in severe congenital
diaphragmatic hernia. J Pediatr. 166:178–183. 2015. View Article : Google Scholar
|
|
11
|
Sluiter I, Reiss I, Kraemer U, Krijger Rd,
Tibboel D and Rottier RJ: Vascular abnormalities in human newborns
with pulmonary hypertension. Expert Rev Respir Med. 5:245–256.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guilbert TW, Gebb SA and Shannon JM: Lung
hypoplasia in the nitrofen model of congenital diaphragmatic hernia
occurs early in development. Am J Physiol Lung Cell Mol Physiol.
279:L1159–L1171. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hirai H, Maru Y, Hagiwara K, Nishida J and
Takaku F: A novel putative tyrosine kinase receptor encoded by the
eph gene. Science. 238:1717–1720. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pasquale EB: Eph-ephrin bidirectional
signaling in physiology and disease. Cell. 133:38–52. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Klein R: Eph/ephrin signalling during
development. Development. 139:4105–4109. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kania A and Klein R: Mechanisms of
ephrin-eph signalling in development, physiology and disease. Nat
Rev Mol Cell Biol. 17:240–256. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lisabeth EM, Falivelli G and Pasquale EB:
Eph receptor signaling and ephrins. Cold Spring Harb Perspect Biol.
5:a0091592013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng N, Brantley DM and Chen J: The
ephrins and eph receptors in angiogenesis. Cytokine Growth Factor
Rev. 13:75–85. 2002. View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods.
25:402–408. 2001. View Article : Google Scholar
|
|
20
|
Downard CD, Jaksic T, Garza JJ, Dzakovic
A, Nemes L, Jennings RW and Wilson JM: Analysis of an improved
survival rate for congenital diaphragmatic hernia. J Pediatr Surg.
38:729–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kinane TB: Lung development and
implications for hypoplasia found in congenital diaphragmatic
hernia. Am J Med Genet C Semin Med Genet. 145C:117–124. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Warburton D, Schwarz M, Tefft D,
Flores-Delgado G, Anderson KD and Cardoso WV: The molecular basis
of lung morphogenesis. Mech Dev. 92:55–81. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cardoso WV: Molecular regulation of lung
development. Annu Rev Physiol. 63:471–494. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Groenman F, Unger S and Post M: The
molecular basis for abnormal Human lung development. Biol Neonate.
87:164–177. 2005. View Article : Google Scholar
|
|
25
|
Cardoso WV and Lü J: Regulation of early
lung morphogenesis: Questions, facts and controversies.
Development. 133:1611–1124. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ware LB and Matthay MA: Keratinocyte and
hepatocyte growth factors in the lung: Roles in lung development
inflammation and repair. Am J Physiol Lung Cell Mol Physiol.
282:L924–L940. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Nakayama M, Pitulescu ME, Schmidt
TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Lüthi U,
et al: Ephrin-B2 controls VEGF-induced angiogenesis and
lymphangiogenesis. Nature. 465:483–486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vadivel A, van Haaften T, Alphonse RS,
Rey-Parra GJ, Ionescu L, Haromy A, Eaton F, Michelakis E and
Thébaud B: Critical role of the axonal guidance cue EphrinB2 in
lung growth, angiogenesis, and repair. Am J Respir Crit Care Med.
185:564–574. 2012. View Article : Google Scholar
|
|
29
|
Bennett KM, Afanador MD, Lal CV, Xu H,
Persad E, Legan SK, Chenaux G, Dellinger M, Savani RC, Dravis C, et
al: Ephrin-B2 reverse signaling increases α5β1 integrin mediated
fibronectin deposition and reduces distal lung compliance. Am J
Respir Cell Mol Biol. 49:680–687. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sikkema AH, den Dunnen WF, Hulleman E, van
Vuurden DG, Garcia-Manero G, Yang H, Scherpen FJ, Kampen KR, Hoving
EW, Kamps EW, et al: EphB2 activity plays a pivotal role in
pediatric medulloblastoma cell adhesion and invasion. Neuro Oncol.
14:1125–1135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nogueira-Silva C, Piairo P, Carvalho-Dias
E, Veiga C, Moura RS and Correia-Pinto J: The role of glycoprotein
130 family of cytokines in fetal rat lung development. PLoS One.
8:e676072013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nogueira-Silva C, Piairo P, Carvalho-Dias
E, Peixoto FO, Moura RS and Correia-Pinto J: Leukemia inhibitory
factor in rat fetal lung development: Expression and functional
studies. PLoS One. 7:e305172012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kling DE, Lorenzo HK, Trbovich AM, Kinane
TB, Donahoe PK and Schnitzer JJ: MEK-1/2 inhibition reduces
branching morphogenesis and causes mesenchymal cell apoptosis in
fetal rat lungs. Am J Physiol Lung Cell Mol Physiol. 282:L370–L378.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Piairo P, Moura RS, Nogueira-Silva C and
Correia-Pinto J: The apelinergic system in the developing lung:
Expression and signaling. Peptides. 32:2474–2483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Boucherat O, Nadeau V, Bérubé-Simard FA,
Charron J and Jeannotte L: Crucial requirement of ERK/MAPK
signaling in respiratory tract development. Development.
21:3197–3211. 2014. View Article : Google Scholar
|
|
36
|
Boucherat O, Landry-Truchon K, Aoidi R,
Houde N, Nadeau V, Charron J and Jeannotte L: Lung development
requires an active ERK/MAPK pathway in the lung mesenchyme. Dev
Dyn. 246:72–82. 2017. View Article : Google Scholar
|