Introduction
Brain-derived neurotrophic factor (BDNF), a major
type of neurotrophin, and its receptor BDNF/NT-3 growth factors
receptor (TrkB), are known to participate in the regulation of
different processes including neuronal development,
differentiation, survival and function (1-3).
In addition to regulating several nervous system-associated
processes, the BDNF/TrkB axis serves a cardioprotective role in
certain adult cardiac diseases, a phenomenon that has attracted
increasing attention in previous years (4-6).
The BDNF/TrkB axis may exert its cardioprotective effects, at least
partly, by promoting angiogenesis in the ischaemic myocardium
(6). A previous study has
demonstrated that BDNF-knockout mice exhibit increased
endotheliocyte apoptosis in the coronary arteries and capillary
tissue; however, BDNF overexpression in cardiac tissue increases
capillary density (6). The
exogenous delivery of BDNF to the ischaemic hearts of myocardial
infarction (MI) rats improved angiogenesis and cardiac function
(7,8). In addition, BDNF/TrkB signalling has
been identified to be essential for in vivo myocardial
performance (9). Loss of TrkB-T
in cardiomyocytes caused cardiomyopathy (10). Clinical data also indicated that
BDNF serves a beneficial role in cardiovascular homeostasis and/or
cardiovascular disease pathogenesis (11-13).
Exercise training has been indicated to induce
benefits, including angiogenesis, specifically in ischaemic hearts:
Clinical studies have demonstrated that treadmill training yields
significant improvements in collateral vessel growth in patients
with coronary artery disease (14), and animal experiments have
revealed increased microvessel density and improved left
ventricular function in post-MI rats following several weeks of
moderate exercise (15,16). However, the mechanisms by which
these changes occur remain to be elucidated. Previous data have
suggested that improvements in endothelial function and structure
in response to exercise correlate with shear stress (SS) (17). Exercise training-induced blood
flow-mediated elevations in laminar SS have been demonstrated to
stimulate growth of collateral vessels in heart tissue (18), and to serve an atheroprotective
role in the vasculature (19).
The hypothesis that the BDNF/TrkB axis mediates the
cardioprotective effects of exercise in ischaemic hearts derives
from studies in which physical training elicited increases in
plasma or serum BDNF concentrations in humans (20). In MI rats, exercise was identified
to increase cardiac BDNF expression (21). In addition, Prigent-Tessier et
al (22) suggested that
increases in physiological SS intensity, which are expected with
exercise training, have been used to mimic the effects of physical
training on the endothelium. In that study, cellular BDNF secretion
levels were identified to be proportional to shear stress intensity
(22). However, an additional
study regarding the effects of SS on the endothelium yielded
conflicting results (23). More
studies are required to establish the role of the BDNF/TrkB axis in
the exercise-induced increase in neoangiogenesis and cardiac
function, and additional data are required to establish the effect
of exercise on circulating BDNF changes in post-MI status and to
associate BDNF levels with exercise-induced improvements in
cardiovascular health.
Therefore, the current study aimed to address these
issues. For this purpose, prolonged BDNF/TrkB axis activation
following exercise training was first determined in post-MI rat
hearts. Increased circulating BDNF levels were also identified to
be correlated with an exercise-induced increase in neoangiogenesis
and cardiac function. Then, the effect of endothelial nitric oxide
synthase (eNOS)/nitric oxide (NO) on exercise-associated activation
of the BDNF/TrkB axis was assessed by using the post-MI rats and
human umbilical vein endothelial cells (HUVECs) subjected to 12
dyn/cm2 SS and exposed to NG-nitro-L-arginine methyl
ester (L-NAME), the NOS inhibitor. Finally, whether the inhibition
of the BDNF/TrkB pathway attenuated exercise-responsive
neoangiogenesis and improved cardiac function was investigated by
using post-MI rats exposed to K252a, the TrkB inhibitor.
Materials and methods
Animals
The present study was approved by the Animal Care
and Research Committee of Southeast University, and all experiments
were conducted in accordance with the European Convention for the
Protection of Vertebrates Used for Experiments and Other Scientific
Purposes (European Union Directive 86/609EEC, 1986). A total of 120
male Sprague-Dawley rats (12 weeks old, 220-250 g) were purchased
from Shanghai Laboratory Animal Centre (Shanghai, China) and housed
in the animal room of the Medical School of Southeast University
(Nanjing, China). All rats were randomly divided into the following
experimental groups: A sham infarction (Sham) group, a sedentary MI
(MIC) group, an MI + exercise (MIE) group, an MI + exercise +
L-NAME (MIE+L) group, an MI+exercise+K252a (MIEK) group and an MI +
exercise + vehicle (MIEV) group. The MIE+L group included rats
subjected to exercise and treatment with L-NAME (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). These rats received L-NAME (100
µg/ml) orally in their drinking water during an 8-week period.
Fluid consumption was monitored daily. The dose of L-NAME (25
mg/kg/day) used here was selected based on the results of a
previous study (24). The MIEK
group included rats subjected to exercise and daily intraperitoneal
injections of a TrkB inhibitor (K252a; Sigma-Aldrich; Merck KGaA),
which was administered at a dose of 100 µg/kg/day during the
seventh and eighth weeks of the experiment. K252a was prepared in
25% dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA) at a
working concentration of 10 µg/ml (25). The MIEV group included rats
subjected to exercise and daily intraperitoneal injections of an
equivalent volume of DMSO, which was administered during the
seventh and eighth weeks of the experiment. The Sham and MIC
animals were maintained in separate cages and were allowed ad
libitum access to chow and water. The room temperature was
20−25°C and the humidity was 40-70%.
Rat model of MI
The animals were anaesthetized with intra-peritoneal
injections of pentobarbital sodium (0.4 mg/10 g). The rats were
endotracheally intubated and then ventilated with a rodent
ventilator (Harvard Apparatus, Holliston, MA, USA) at 70 breaths
per min. Subsequent to exposure of the heart via left thoracotomy,
the left anterior descending (LAD) coronary artery was ligated
proximally with an 8-0 silk suture. For the rats in the Sham group,
the chest was opened but the LAD was left patent. Each animal was
allowed to recover for 48 h prior to initiation of exercise
training.
Exercise training protocol
The training schedule proposed by Leosco et
al (15) was used, with
specific modifications. Prior to the exercise protocol, all animals
underwent a preconditioning training regimen for 3 days comprising
30 min of daily running at a speed of 15 m/min on a rodent
treadmill (FT-200; Chengdu Taimeng Technology Co., Ltd., Chengdu,
China). A total of 1 week following cardiac surgery, the animals in
the MIE, MIE+L, MIEK and MIEV groups began training. In the first
week of the running regimen, the animals began with a running speed
of 0.3 km/h for 10 min/day. The animals' exercise speed was
gradually increased from 0.3 to 1.2 km/h. The animals participated
in the exercise regimen 5 times per week throughout the 8-week
period, with two days of relaxation allowed per week. At the end of
the exercise protocol, certain animals in each group were allowed 3
h of rest prior to undergoing tissue preparation, and the other
animals were allowed 48 h of rest prior to cardiac functional
evaluations.
Echocardiographic analysis and heart/body
weight measurement
The rats underwent cardiac functional evaluations,
prior to exercise training, at 1 week after cardiac surgery and
following the 8-week period of exercise training. Cardiac function
was evaluated using transthoracic echocardiography (Visual Sonics
Vevo 770; VisualSonics, Inc., Toronto, OT, Canada). When performing
echocardiography, isoflurane was used for the induction (4.5%) and
maintenance (1.5%) of inhaled anaesthesia. Left parasternal
short-axis two-dimensional M-mode images were recorded just below
the level of the papillary muscles using a 30-MHz linear
transducer. The following haemodynamic parameters were measured:
The left ventricular internal diameter at end-systole (LVIDs); the
left ventricular internal diameter at end-diastole (LVIDd); the
ejection fraction (EF); and left ventricular fractional shortening
(LVFS). Each ultrasound examination was performed by an experienced
examiner blinded to the study data. All the values are averages of
5 consecutive cardiac cycles.
Following the ultrasound examination, the animals
were anaesthetized with intraperitoneal injections of pentobarbital
sodium (0.4 mg/10 g) and the heart was perfused with PBS and
rapidly resected. The heart was desiccated with filter paper and
then weighed using an electronic analytical balance.
Tissue preparation
Prior to exercise training at 1 week after cardiac
surgery, venous blood samples were collected from the rats in the
Sham, MIC, MIE and MIE+L groups from the angular or caudal veins.
The animals were anaesthetized with pentobarbital sodium (0.4 mg/10
g) after 3 h of rest at the end of the exercise protocol. A total
of 0.5 ml of venous blood was collected by cardiac puncture, and
then the animals were sacrificed. The left ventricles were
dissected and then cut in half transversally at the level of the
ligation. One section was fixed in 10% formalin and embedded in
paraffin prior to use in immunohistochemical assessments at room
temperature for 24 h, and the other section was snap-frozen prior
to storage at −80°C for subsequent analyses.
Immunohistochemistry
BDNF, TrkB and hematopoietic progenitor cell antigen
CD34 (CD34) expression levels were detected in serial
paraffin-embedded sections of the left ventricle using standard
histological and immunostaining procedures. The heart sections were
deparaffinized and then rehydrated in a graded alcohol series
(xylene I for 20 min, xylene II for 20 min, 100% alcohol I for 5
min, 100% alcohol II for 5 min, 95% alcohol for 5 min, 80% alcohol
for 5 min and PBS for 3×3 min). Endogenous peroxidase activity was
quenched with 3% hydrogen peroxide. Subsequent to washing with PBS,
the sections were subjected to microwave antigen retrieval (800 W;
5 min) in sodium citrate buffer (0.01 mol/l; pH 6.0). Bovine serum
albumin (5%; Beyotime Institute of Biotechnology, Haimen, China)
was used as blocking agent and the blocking step was performed at
37°C for 30 min. The following antibodies were used for the
experiment: A sheep polyclonal antibody to BDNF (cat. no. ab75040;
Abcam, Cambridge, UK; 1:200), a rabbit polyclonal antibody to TrkB
(cat. no. ab33655; Abcam; 1:50), and a rabbit polyclonal antibody
to CD34 (cat. no. pab18289; Abnova, Taipei, Taiwan; 1:200). The
sections were incubated with the antibodies overnight at 4°C. The
sections were then washed prior to incubation with the appropriate
secondary antibodies, including horseradish peroxidase-conjugated
anti-goat secondary antibody (cat. no. 81-1620; Thermo Fisher
Scientific, Inc., Waltham, MA, USA; 1:800) and anti-rabbit
secondary antibody (cat. no. PV-6001; ZsBio, Beijing, China;
1:800), and then colour-developed by DAB (ZsBio) and counterstained
with 1 g/l haematoxylin (Sigma-Aldrich; Merck KGaA) at room
temperature for 3 min. Light microscopy was used to capture images
of the border zone, and 5 high-power fields (HPFs) in each sample
were selected at a magnification of x200. The cells were then
analysed and counted in a blinded manner.
Cell culture and shear stress
treatment
HUVECs were purchased from Wuxi Puhe
Biotechnological Co., Ltd. (Wuxi, China). The cells were placed in
a streamer laminar flow chamber and then exposed to static
conditions or high SS (12 dyn/cm2, HSS) for 1, 3, 6 or
12 h. Circulating and non-circulating flow patterns were used to
produce the SS. In certain experimental protocols, the cells were
treated with the NO synthase inhibitor L-NAME (10−4
mol/l), the NO donor S-nitroso-N-acetyl-D, L-penicillamine (SNAP;
10−6 mol/l; Sigma-Aldrich; Merck KGaA), or a TrkB-Fc
chimera (1 µg/ml; Sigma-Aldrich; Merck KGaA). The cells and medium
were stored at −80°C until BDNF, TrkB and phosphorylated TrkB
expression levels were measured.
Measurement of serum and media BDNF
levels
Serum and media BDNF concentrations were detected
using an ELISA kit (cat. no. DBNT00; R&D Systems, Inc.,
Minneapolis, MN, USA) according to the manufacturer's protocol. The
samples were analysed in duplicate, and then the mean BDNF
concentration for each sample was calculated. No significant
cross-reactivity between the BDNF antibodies and any other
cytokines was observed. The limit of sensitivity was fixed at
<20 pg/ml.
Western blot analysis
The heart tissue samples were homogenized, and the
HUVECs were isolated and washed twice with cold PBS. Total protein
was lysed by radioimmunoprecipitation assay lysis buffer (cat. no.
89900; Thermo Fisher Scientific, Inc.) as described previously
(4). The supernatants were
collected, and the protein concentrations were determined using a
DC Protein Assay kit (Beyotime Institute of Biotechnology). Equal
amounts of protein extracts (20 µg) were separated by SDS-PAGE (10%
separating gel and 4% stacking gel) and then transferred to
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA), which were blocked in TBS containing 5% (w/v) non-fat dry
milk at room temperature overnight. BDNF, TrkB and phosphorylated
TrkB (p-TrkB) expression levels were detected with the following
primary antibodies: An anti-BDNF antibody (cat. no. ab75040; Abcam;
1:200), an anti-TrkB antibody (cat. no. ab33655; Abcam; 1:200), and
an anti-TrkB (phospho Y515) antibody (cat. no. ab109684; Abcam;
1:200). The membranes were incubated with these primary antibodies
overnight at 4°C and then visualized using an anti-rabbit IgG
secondary antibody (cat. no. A27036; Thermo Fisher Scientific,
Inc.; 1:10,000) or anti-goat IgG secondary antibody (cat. no.
81-1620; Thermo Fisher Scientific, Inc.; 1:5,000) for 1 h at room
temperature and an EZ-ECL system (ChemiDoc XRS+ System, Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Equal protein loading was
confirmed by measuring total actin expression levels (cat. no.
ab8226; 1:2,000; Abcam), and the data pertaining to target protein
expression levels were analysed using ImageJ 4.0.1 software
(National Institutes of Health, Bethesda, MD, USA).
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from HUVECs and purified with
TRIzol® reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The isolated total RNA (3
µg) was converted into cDNA in a 20 µl reaction mixture using the
RevertAid First Strand cDNA Synthesis kit (Fermentas; Thermo Fisher
Scientific, Inc., Pittsburgh, PA, USA) according to the
manufacturer's protocol. qPCR was performed on a StepOnePlus PCR
System (Bio-Rad Laboratories, Inc.) using Fast SYBR Green PCR
Master Mix (Toyobo Life Sciences, Osaka, Japan) according to the
manufacturer's protocol. The 2−ΔΔCq method was used for
quantification (26).
Pre-denaturation process was at 95°C for 5 min, denaturation
process was at 95°C for 15 sec, annealing process was at 60°C for
20 sec, and stretching process was at 72°C for 40 sec. Melting
curve analysis of the PCR products was performed to ensure the
specificity of the amplification. The expression levels of the
target genes were normalized to those of GADPH, which served as an
endogenous control. Primers specific for BDNF, TrkB and GAPDH were
constructed by Genescript Co., Ltd. (Nanjing, China). The sequences
of the primers were as follows: BDNF forward, 5-AAC ATA AGG ACG CGA
CTT-3; BDNF reverse, 5-TGC AGT CTT TTT ATC TGC CG-3; TrkB forward,
5-GAT CTT CAC CTA CGG CAA GC-3; TrkB reverse, 5-TCG TCA AGT TCT GAA
GGA GT-3; GAPDH forward: 5-AAC TTT GGC ATT GTG GAA GG-3; and GAPDH
reverse: 5-TGT GAG GGA GAT GCT CAG TG-3.
Tube formation
HUVECs (1.5×104) exposed to SS or static
conditions for 6 h were suspended in Dulbecco's modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc.) with 5% FBS (Gibco;
Thermo Fisher Scientific, Inc.) and then seeded in a 96-well plate
precoated with Matrigel® (BD Biosciences, Franklin
Lakes, NJ, USA). The precoating step was performed at 0°C and the
plate was placed in the cell incubator at 37°C for 1 h. The cells
were incubated for 24 h at 37°C, and then the capillary-like tube
structures that had formed from the HUVECs were observed by a
phase-contrast microscope (×100). The number of tubes per low-power
field was calculated and compared using ImageJ 4.0.1 software.
Statistical analysis
SPSS 21.0 (IBM Corp., Armonk, NY, USA) was used for
statistical analysis. The data in the present study conformed to
Gaussian distribution. The data are expressed as the mean ±
standard error of the mean and are representative of at least 3
independent experiments. Differences among different groups were
assessed using one-way analysis of variance followed by a Least
Significant Difference post-hoc test. The associations between
serum BDNF levels and LV function and CD34+ cell counts
were analysed using Pearson's correlation coefficient analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
BDNF and TrkB protein expression
increases in the ischaemic heart zone (IHZ) following exercise
training
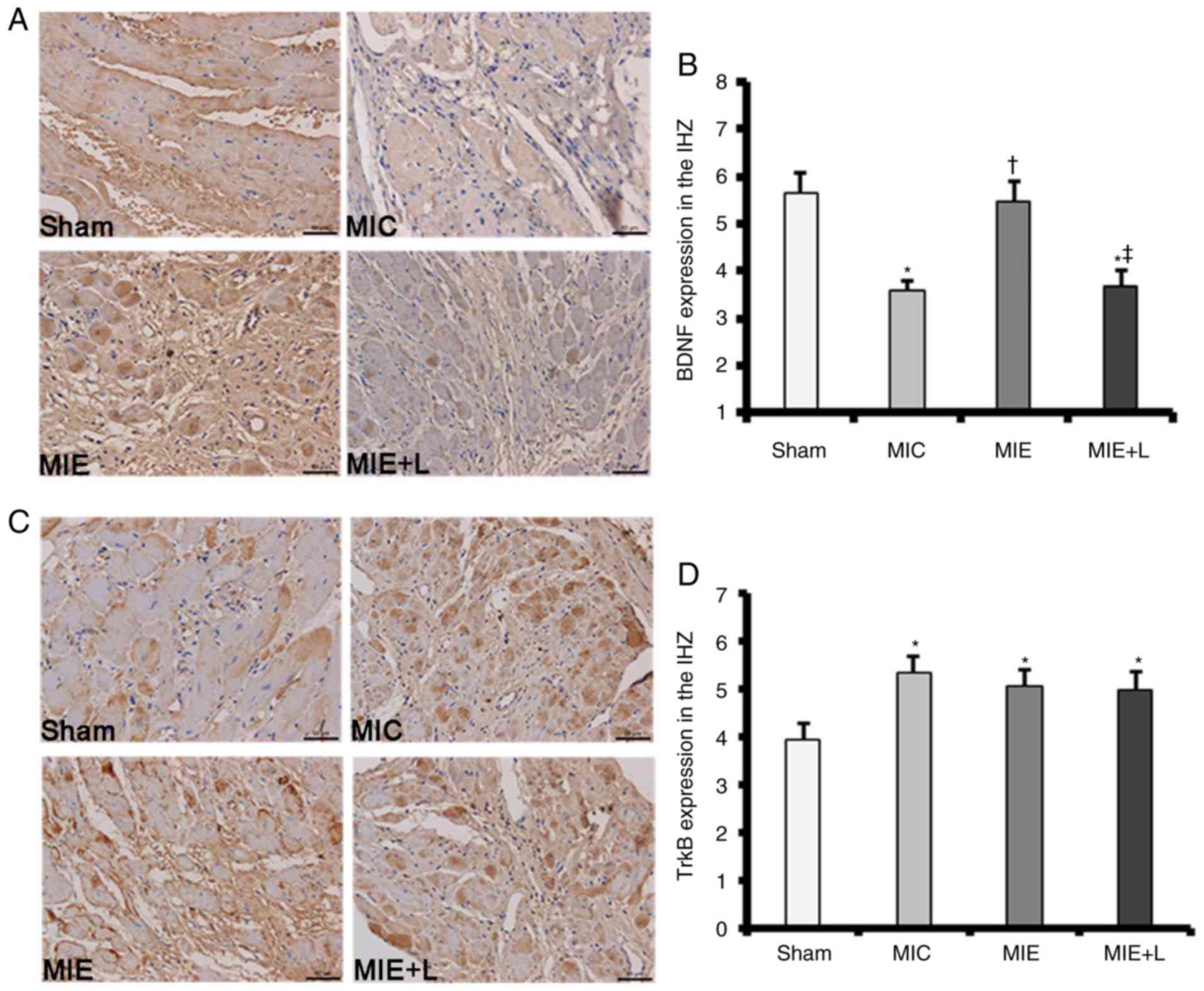
BDNF and TrkB expression levels in MI heart tissues
were measured by immunostaining. MI rats subjected to exercise
exhibited markedly increased BDNF protein expression in the IHZ
compared with sedentary MI rats. The exercise-induced increase in
BDNF protein expression was inhibited by L-NAME (Fig. 1A). Representative original images
indicating BDNF staining are presented in Fig. 1B. TrkB protein expression in the
ischaemic heart tissues was increased compared with that in Sham
heart tissues; however, no significant difference in TrkB protein
expression was identified among the MIC, MIE and MIE+L groups
(Fig. 1C). Representative
original images demonstrating TrkB staining are presented in
Fig. 1D.
BDNF/TrkB axis is activated in the IHZ
following exercise training
BDNF expression in the IHZ by was examined by
western blot analysis. As demonstrated in Fig. 2A, BDNF protein expression in the
ischaemic heart tissues was decreased compared with that in the
Sham heart tissues. Exercise training increased BDNF protein
expression, which was inhibited by L-NAME. The MIEK rats indicated
increased BDNF expression compared with the MIE and MIEV rats.
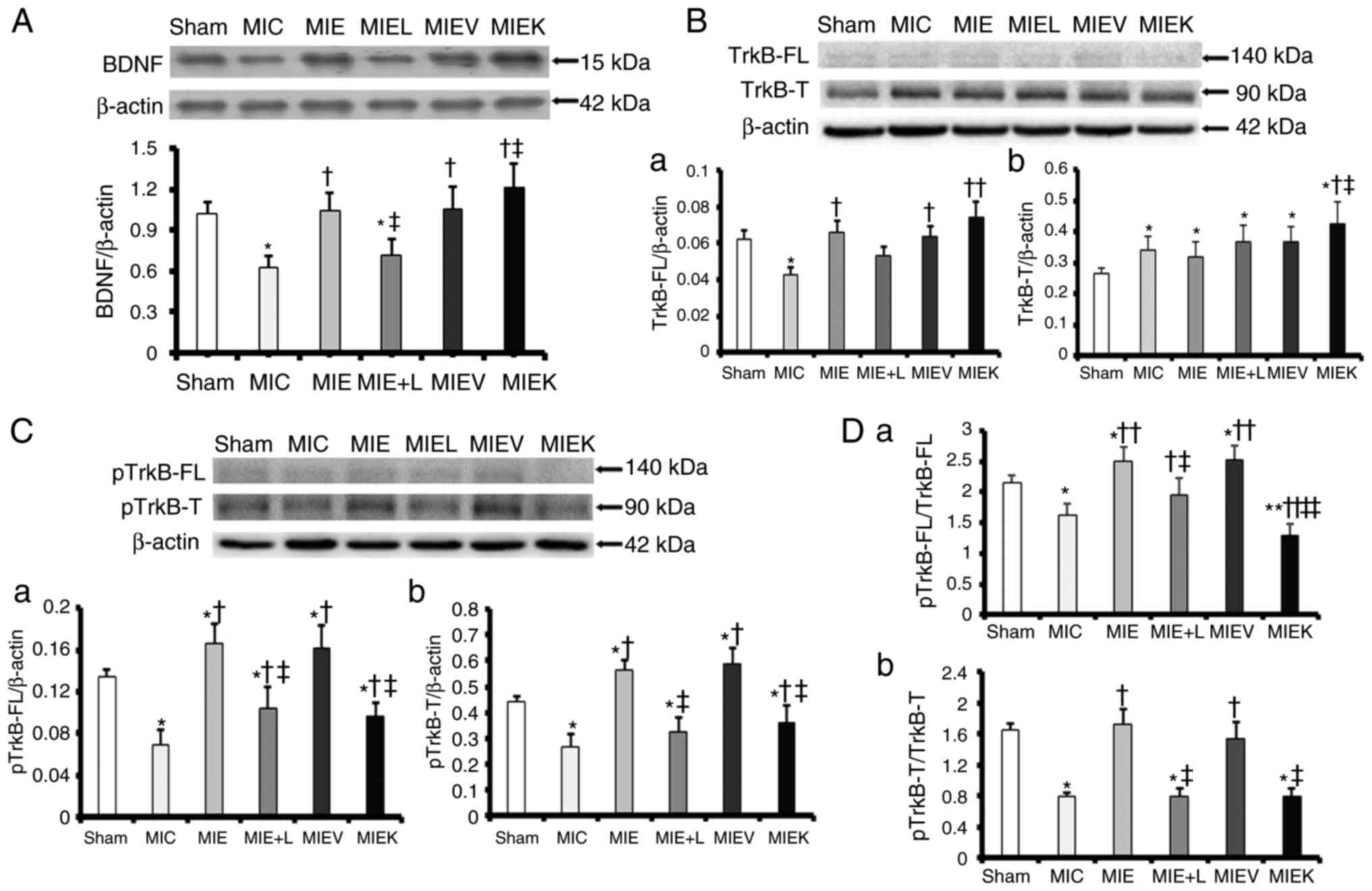 | Figure 2Exercise training activates the
BDNF/TrkB axis in the IHZ of the myocardium, which is attenuated by
L-NAME and the TrkB inhibitor. (A) Representative western blot
analysis images for BDNF and the protein level analysis.
*P<0.05 vs. Sham, †P<0.05 vs. MIC and
‡P<0.05 vs. MIE. (B) Representative western blot
analysis images for TrkB-FL and TrkB-T: (a) TrkB-FL protein level
analysis. *P<0.05 vs. Sham, †P<0.05 vs.
MIC and ‡P<0.01 MIEK vs. MIC; (b) TrkB-T protein
level analysis. *P<0.05 vs. Sham,
†P<0.05 vs. MIC and ‡P<0.05 vs. MIE.
(C) Representative western blot analysis images for phosphorylated
TrkB-FL and TrkB-T: (a) Phosphorylated TrkB-FL protein level
analysis. *P<0.01 vs. Sham, †P<0.01 vs.
MIC, ‡P<0.01 vs. MIE; (b) phosphorylated TrkB-T
protein level analysis. *P<0.01 vs. Sham,
†P<0.01 vs. MIC and ‡P<0.01 vs. MIE.
(D) The ratio of (a) pTrkB-FL to total TrkB-FL
(*P<0.05 MIC, MIE, MIEV vs. Sham,
**P<0.01 MIEK vs. Sham, †P<0.05 MIE+L
vs. MIC, ††P<0.01 MIE, MIEV, MIEK vs. MIC;
‡P<0.05 MIE+L vs. MIE, ‡‡P<0.01 MIEK
vs. MIE) and the ratio of (b) phosphorylated TrkB-T to total TrkB-T
(*P<0.01 vs. Sham, †P<0.01 vs. MIC and
‡P<0.01 vs. MIE). The values are presented as the
mean ± standard error of the mean (n=6). BDNF, brain-derived
neurotrophic factor; TrkB, BDNF/NT-3 growth factors receptor; p,
phosphorylated; MI, myocardial infarction; L-NAME,
NG-nitro-L-arginine methyl ester; MIC, sedentary MI group; MIE, MI
+ exercise group; MIE+L, MI + exercise + L-NAME group; MIEK,
MI+exercise+K252a group; MIEV, MI + exercise + vehicle group. |
The expression and phosphorylation levels of the two
types of TrkB [the full-length (FL) and truncated (T) isoforms]
were examined by western blot analysis. As demonstrated in Fig. 2B-a, the MIE, MIEV and MIEK rats
exhibited increased TrkB-FL expression compared with the MIC rats.
No signifi-cant differences among the Sham, MIE, MIE+L, MIEV and
MIEK groups were observed (Fig.
2B-a). As indicated in Fig.
2B-b, TrkB-T protein expression levels in MI hearts were
increased compared with those in Sham hearts; no significant
difference in TrkB-T expression was observed among the MIC, MIE,
MIE+L and MIEV groups. The MIEK rats exhibited an increased TrkB-T
protein content compared with the other groups (Fig. 2B-b).
It was also identified that TrkB-FL and TrkB-T
phosphorylation levels in the IHZs of the groups subjected to
exercise, with the exception of the MIEK group, were increased
compared with those in the IHZs of the other groups (Fig. 2C and D). The phosphorylation
levels of the two receptors in the MIE+L-NAME and MIEK groups were
decreased compared with those in the MIE and MIEV groups (Fig. 2C and D).
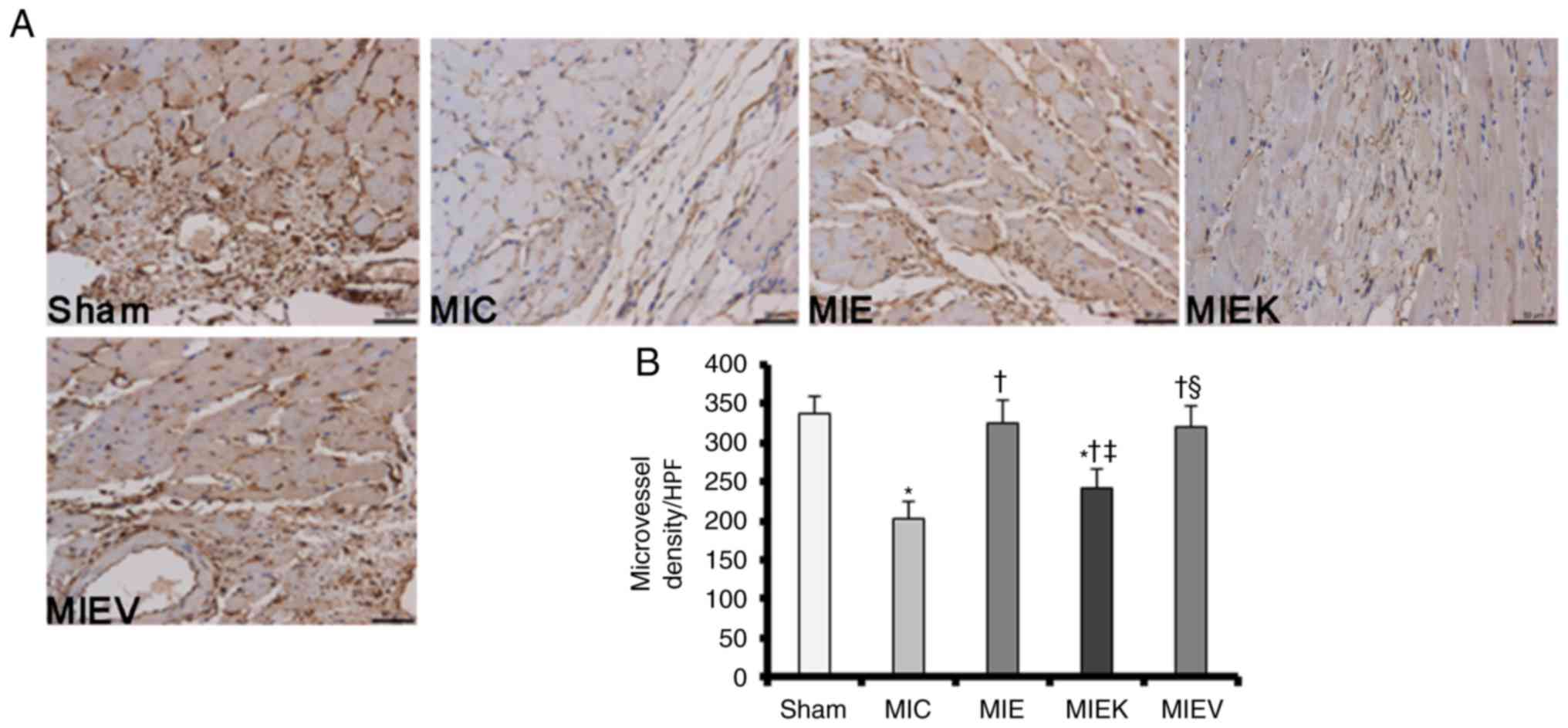
Angiogenic responses to exercise training
are attenuated by the TrkB inhibitor in post-MI rat hearts
Subsequent to the assessment of the effects of
exercise on BDNF/TrkB axis activation in post-MI rats, CD34
expression was quantified by immunohistochemistry. CD34 is an
antibody identified specifically in vascular endothelial cells, and
its expression may be used to evaluate angiogenesis in ischaemic
myocardial tissue (27). The
surviving myocardium was stained with standard immunohistochemical
reagents, and then the numbers of CD34+ microvascular
endothelial cells were counted. As demonstrated in Fig. 3B, the numbers of CD34+
microvascular cells were increased in the MI exercise groups
compared with those in the MIC group, indicating that exercise
promoted angiogenesis in the surviving myocardium. Notably, the
number of CD34+ microvascular cells was decreased in the
MIEK group compared with those in the MIE and MIEV groups; however,
the number of CD34+ microvascular cells in the MIEK
group was increased compared with that in the MIC group.
Representative original images demonstrating CD34+
staining are presented in Fig.
3A. These results suggest a possible explanation of why cardiac
function was decreased in the MIEK group and additionally
demonstrate the necessity of BDNF/TrkB axis activation in
exercise-induced angiogenesis following MI.
Effects of exercise training and K252a on
cardiac function and the echocardiographic data
As summarized in Table
I, training decreased body weight in the MI rats subjected to
exercise compared with the sedentary rats. In addition, heart
weight and the heart weight: Body weight ratio were increased in
the trained rats compared with those in the sedentary rats. Heart
weight and the heart weight: body weight ratio measurements
indicated modest but non-significant reductions in the MIEK group
compared with those in the MIE and MIEV groups. At 9 weeks after
surgery, the EF was evidently improved in the MIE and MIEV groups;
however, the EF remained sub-optimal in the MIEK group compared
with those in the other exercise groups. Training favourably
affected LV geometry, as the rats in the training groups exhibited
decreased LVIDd and LVIDs diameters compared with those in the MIC
group. Nevertheless, the MIEK group demonstrated larger LV
diastolic and systolic diameters compared with the MIE and MIEV
groups. LVFS was increased in the exercise groups compared with
that in the MIC group; however, the MIEK group indicated less LVFS
compared with the MIE and MIEV groups. These data indicate that the
effects of exercise on cardiac function were antagonized by K252a
and suggest that the BDNF/TrkB axis served a role in mediating the
cardioprotective effects of exercise (Table I).
 | Table IPhysiological, haemodynamic and
echocardiographic data for the Sham and MI rats prior and
subsequent to the exercise training protocol. |
Table I
Physiological, haemodynamic and
echocardiographic data for the Sham and MI rats prior and
subsequent to the exercise training protocol.
| Measurements | Groups
|
|---|
| Sham | MIC | MIE | MIEK | MIEV |
|---|
| Physiological
data | | | | | |
| Body wt, kg | 0.389±0.022 | 0.360±0.019a | 0.317±0.023b | 0.335±0.021b,c | 0.321±0.025b |
| Heart wt, g | 1.003±0.053 | 1.998±0.178a | 2.287±0.106b | 2.199±0.109b | 2.278±0.137b |
| Heart wt/body wt
ratio | 2.59±0.13 | 5.53±0.53a | 7.17±0.29b | 6.89±0.33b | 7.11±0.39b |
| Haemodynamic
and | | | | | |
| echocardiographic
data | | | | | |
| EF, % | | | | | |
| 1 week | 71.68±5.19 | 32.97±3.22a | 33.76±3.21a | 35.4±2.77a | 34.12±2.93a |
| 9 weeks | 75.45±5.63 | 36.96±8.05a | 56.89±9.21b | 45.4±8.54b,c | 55.72±8.90b |
| LVID (mm) | | | | | |
| Diastolic | | | | | |
| 1 week | 7.27±0.51 | 11.29±0.57a | 11.23±0.61a | 11.20±0.63a | 11.30±0.69a |
| 9 weeks | 7.41±0.22 | 11.69±0.63a | 9.06±0.31b | 11.09±0.55a,c | 9.34±0.34b |
| Systolic | | | | | |
| 1 week | 3.77±0.19 | 10.07±0.69a | 10.04±0.53a | 10.01±0.55a | 10.11±0.61a |
| 9 weeks | 3.82±0.20 | 9.95±0.60a | 6.54±0.40b | 8.88±0.40b,c | 6.98±0.30b |
| LVFS, % | | | | | |
| 1 week | 48.19±4.94 | 10.84±1.98a | 10.69±1.97a | 10.75±1.41a | 10.67±1.79a |
| 9 weeks | 48.45±4.40 | 14.88±2.37a | 27.81±2.92b | 19.93±2.13b,c | 25.27±3.17b |
Serum BDNF levels increase in response to
chronic exercise training and are correlated with angiogenesis and
cardiac function
No significant differences in serum BDNF
concentrations were observed among the Sham, MIC, MIE and MIE+L
groups at the beginning of the exercise programme (Fig. 4A-a). However, the BDNF
concentrations were increased in the MIE group (236.3±35.7 pg/ml)
compared with those in the MIC (186.5±19.1 pg/ml) and MIE+L groups
(180.4±20.9 pg/ml) at the end of exercise programme (Fig. 4A-b). Simultaneously, a significant
decrease in serum BDNF was observed in the rats of the MIC and
MIE+L groups compared with that in the Sham rats (223.9±30.1 pg/ml;
Fig. 4A-b).
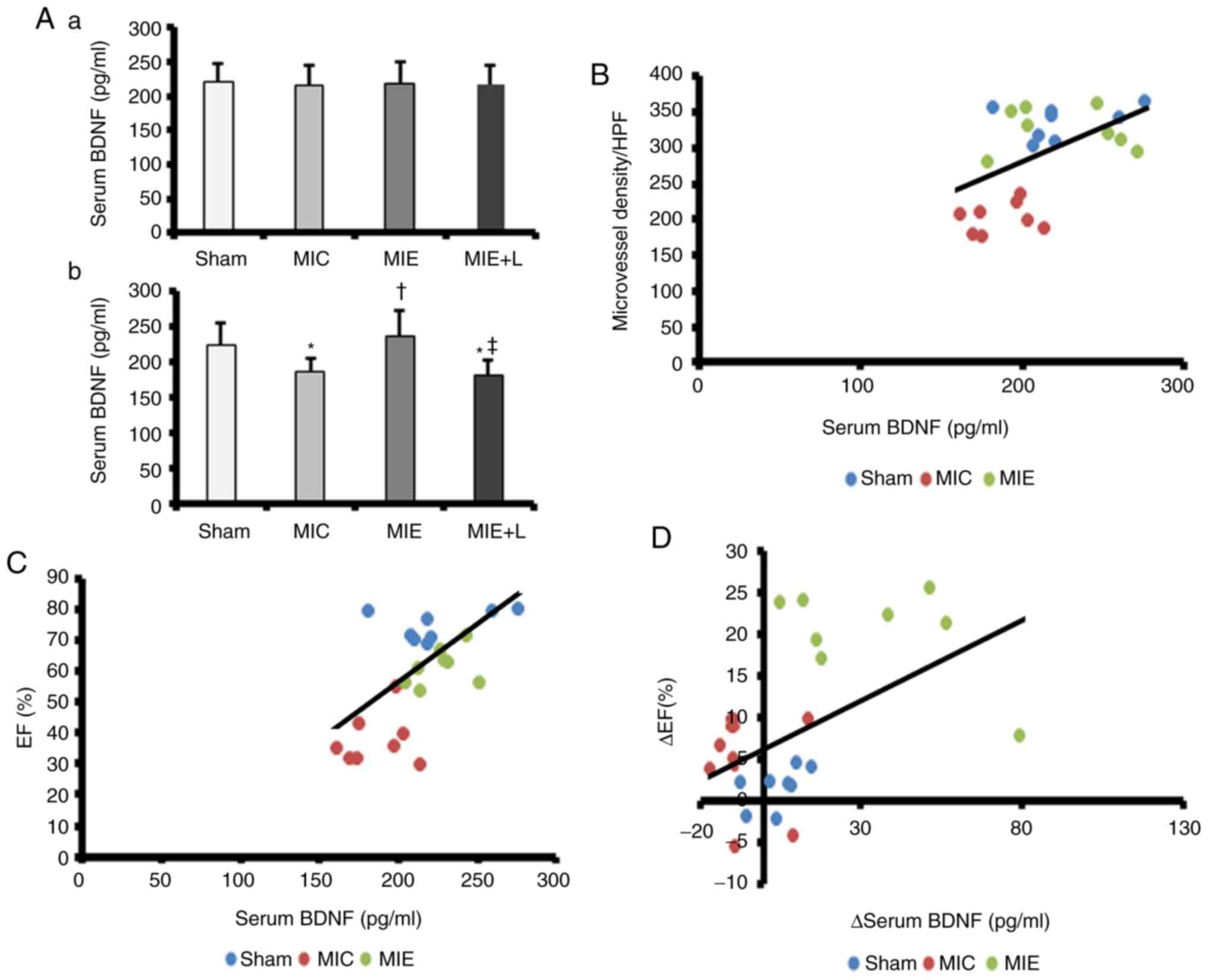 | Figure 4Serum BDNF level is associated with
myocardium angiogenesis and left ventricle function. (A) ELISA
measurements of the serum BDNF concentration at the (a) beginning
and the (b) end of the exercise programme. *P<0.05
vs. Sham and †P<0.05 vs. MIC. (B) The serum BDNF
concentration was positively correlated with myocardial microvessel
density (r=0.542, P=0.006). (C) The serum BDNF concentration was
positively correlated with the EF (r=0.631, P=0.001). (D) A
positive correlation was identified between improved serum BDNF
levels and the EF (r=0.502, P=0.013). The values are presented as
the mean ± standard error of the mean (n=8). MI, myocardial
infarction; L-NAME, NG-nitro-L-arginine methyl ester; MIC,
sedentary MI group; MIE, MI + exercise group; MIE+L, MI + exercise
+ L-NAME group; BDNF, brain-derived neurotrophic factor; EF,
ejection fraction; HPF, high-powered fields. |
The associations between serum BDNF concentrations
and LV function in vivo and the numbers of CD34+
endothelial cells in the IHZ were also examined; LV function was
determined by the EF value. A positive association was identified
between serum BDNF levels and the CD34+ cell counts
(r=0.542, P=0.006; Fig. 4B) and
the EF (r=0.502, P=0.013; Fig.
4C). Therefore, increased levels of serum BDNF were associated
with cardiac function and angiogenesis. In addition, the
association between the variation of BDNF serum levels and the EF
prior and subsequent to exercise is demonstrated in Fig. 4D. Improvement of the EF exhibited
a positive association with changes in serum BDNF.
SS of 12 dyn/cm2 increases
BDNF/TrkB-FL protein levels and sustains TrkB-FL activation in
HUVECs
To gain insight into the mechanisms by which
physical training induces angiogenesis and to explain the
upregulated BDNF/TrkB protein expression observed in the animal
experiments, the effects of SS on the expression of BDNF and the
two types of TrkB were examined. HUVECs were placed in a laminar
flow chamber and exposed to high SS at 12 dyn/cm2 for 1,
3, 6 or 12 h. The western blot analysis results, which are
demonstrated in Fig. 5A, revealed
that BDNF protein expression levels were persistently upregulated
by exposure to SS of 12 dyn/cm2. Specifically, BDNF
protein expression levels were increased markedly after 1 h of SS
exposure (147±17% of the static control), peaked after 3 h of SS
exposure (347±41%) prior to partially normalizing after 6 h of SS
exposure (265±33%), and remained elevated after 12 h of SS exposure
(163±21%). Consistent with the data indicating that SS elicited
sustained increases in cellular BDNF secretion, additional data
suggested that BDNF levels in the medium exposed to SS for 12 h
were 379±27% (407.65±27.31 pg/ml) increased compared with those in
the medium exposed to static conditions (Fig. 5B).
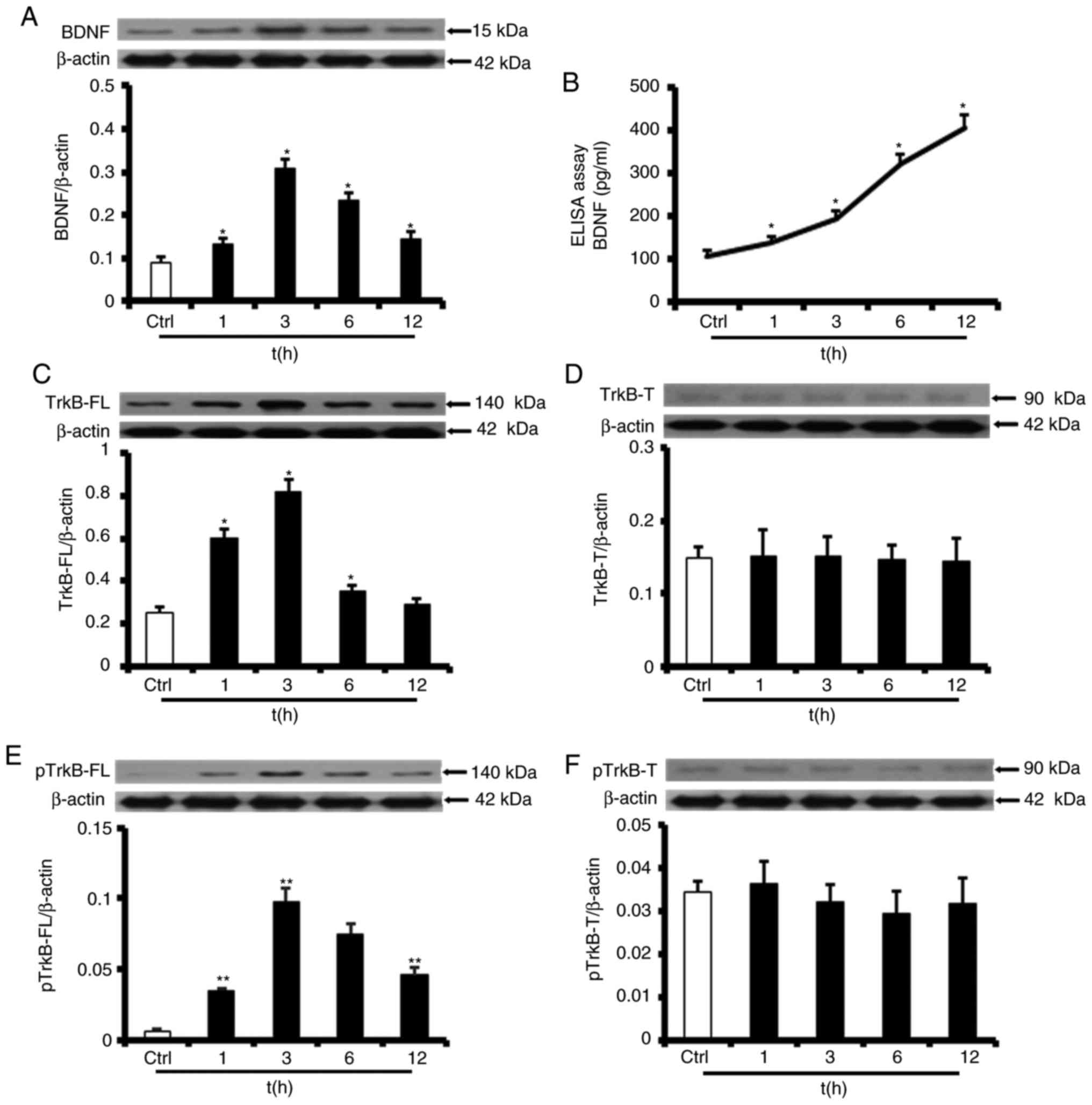 | Figure 5SS of 12 dyn/cm2 increased
BDNF/TrkB-FL protein levels and sustained TrkB-FL activation in
HUVECs. (A) Representative western blot analysis images for BDNF
and the protein level analysis in HUVECs exposed to SS
(*P<0.01 vs. Ctrl). (B) ELISA measurement of BDNF
levels in the culture medium following exposure of HUVECs for 1, 3,
6 and 12 h of SS (*P<0.01 vs. Ctrl). (C)
Representative western blot analysis images for TrkB-FL and the
protein level analysis in HUVECs exposed to SS
(*P<0.01 vs. Ctrl). (D) TrkB-T protein expression and
phosphorylation levels were not modified by SS at 12
dyn/cm2. (E) Representative western blot analysis images
for phosphorylated TrkB-FL and the protein level analysis in HUVECs
exposed to SS (**P<0.001 vs. Ctrl). (F) TrkB-T
phosphorylation levels were not modified by SS at 12
dyn/cm2. The values are presented as the mean ± standard
error of the mean (n=6). SS, shear stress; BDNF, brain-derived
neurotrophic factor; TrkB, BDNF/NT-3 growth factors receptor; FL,
full-length; T, truncated; p, phosphorylated; ctrl, control;
HUVECs, human umbilical vein endothelial cells; t, time. |
Western blot analysis also revealed that TrkB-FL
protein expression levels were upregulated by SS. Specifically, as
indicated in Fig. 5C, TrkB-FL
protein expression levels increased after 1 h of SS exposure
(211±32% of the static control), peaked after 3 h of SS exposure
(388±27%) prior to partial normalization after 6 h of SS exposure
(146±20%), and decreased to baseline after 12 h of SS exposure
(114±17%). As demonstrated in Fig.
5E, phosphorylated TrkB-FL protein expression levels increased
more markedly compared with TrkB-FL protein expression levels and
remained elevated after 12 h of SS exposure (558±65% after 1 h;
1,509±193% after 3 h; 1,216±107% after 6 h; and 739±79% after 12
h).
Notably, it was identified that TrkB-T protein
expression and phosphorylation levels were not modified by SS at 12
dyn cm−2 (Fig. 5D and
F).
eNOS/NO mediates SS-induced BDNF/TrkB
regulation
To assess whether eNOS/NO participates in the
regulation of BDNF/TrkB expression, HUVECs were treated with the
eNOS inhibitor L-NAME (10−4 mol/l). Fig. 6A and B indicate that SS-induced
increases in BDNF/TrkB-FL expression were completely abolished by
L-NAME. To additionally assess whether NO contributes to
upregulation of BDNF/TrkB expression, HUVECs were then exposed to
SNAP, an NO donor, under static conditions (10−6 mol/l).
SNAP treatment increased BDNF protein expression levels after 1 h
(227±26%) but did not have an effect on BDNF protein expression at
subsequent time points (Fig. 6C).
However, TrkB-FL protein levels were similar between unstimulated
HUVECs and HUVECs exposed to SNAP (Fig. 6D). Therefore, SS regulates
BDNF/TrkB expression in an NO-dependent manner in HUVECs, and NO
release is sufficient to increase BDNF expression.
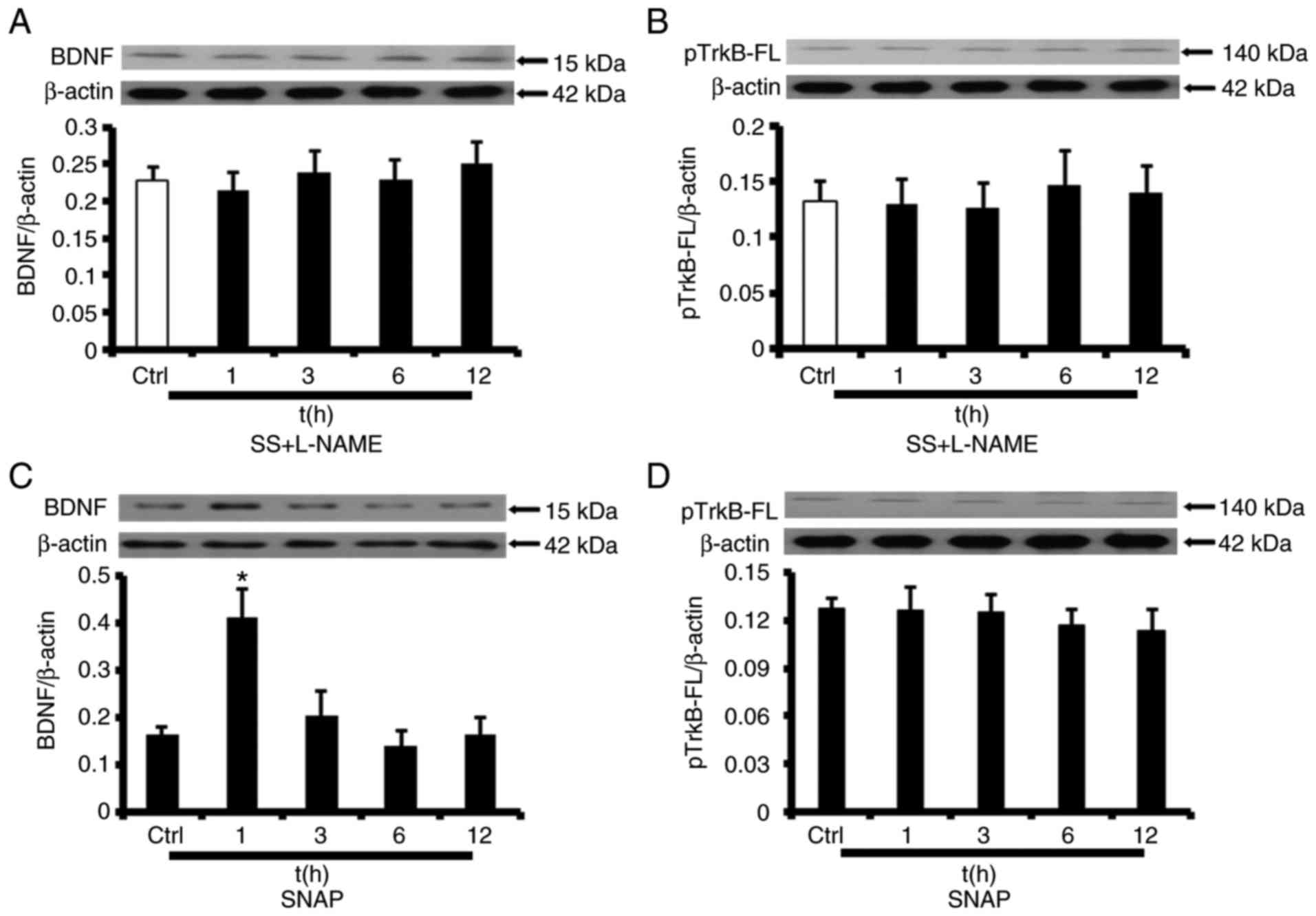 | Figure 6NO-dependent activation of the
BDNF/TrkB axis. Representative western blot analysis images and
protein level analysis for BDNF and phosphorylated TrkB-FL in
HUVECs. (A and B) Cultured HUVECs were incubated with L-NAME and
exposed to SS at 12 dyn/cm2 for 1, 3, 6 or 12 h. (A)
Representative western blot analysis images for BDNF and the
protein level analysis. (B) Representative western blot analysis
images for phosphorylated TrkB-FL and the protein level analysis.
(C and D) Static HUVECs incubated with the NO donor SNAP. (C)
Representative western blot analysis images for BDNF and the
protein level analysis (*P<0.01 vs. Ctrl). (D)
Western blot analysis images for phosphorylated TrkB-FL and the
protein level analysis. The values are presented as the mean ±
standard error of the mean (n=6). BDNF, brain-derived neurotrophic
factor; TrkB, BDNF/NT-3 growth factors receptor; FL, full-length;
p, phosphorylated; ctrl, control; NO, nitric oxide; HUVECs, human
umbilical vein endothelial cells; L-NAME, NG-nitro-L-arginine
methyl ester; SS, shear stress; t, time. |
SS-induced TrkB-FL upregulation is
dependent on increases in BDNF levels in media
To determine whether the aforementioned increases in
BDNF/TrkB-FL protein levels reflected a transcriptional regulatory
event, BDNF/TrkB-FL mRNA expression levels were assessed by RT-qPCR
in HUVECs exposed to high SS (12 dyn/cm2). Prolonged
increases in BDNF mRNA expression in HUVECs exposed to high SS for
1, 3, 6 or 12 h (190±15, 430±31, 525±53 and 641±52% of the static
control, respectively) were observed (Fig. 7A). Prolonged increases in TrkB-FL
mRNA expression in HUVECs exposed to high SS for 1, 3, 6 or 12 h
(258±17, 387±32, 498±43 and 697±55% of the static control,
respectively) were observed (Fig.
7B). To determine whether these SS-mediated increases in
TrkB-FL expression were dependent on changes in BDNF levels in the
media, HUVECs were treated with non-circulating fluid and
BDNF/TrkB-FL mRNA expression levels were examined after 1, 3, 6 and
12 h. As demonstrated in Fig. 7C,
BDNF mRNA expression levels increased continuously from 1-12 h
post-treatment (251±15, 348±17, 525±21 and 693±25% of the static
control, respectively), while TrkB-FL mRNA expression levels
decreased steadily from 1-12 h post-treatment (35.3±4, 16.2±2,
11.0±1 and 6.98±0.7% of static control, respectively) (Fig. 7D).
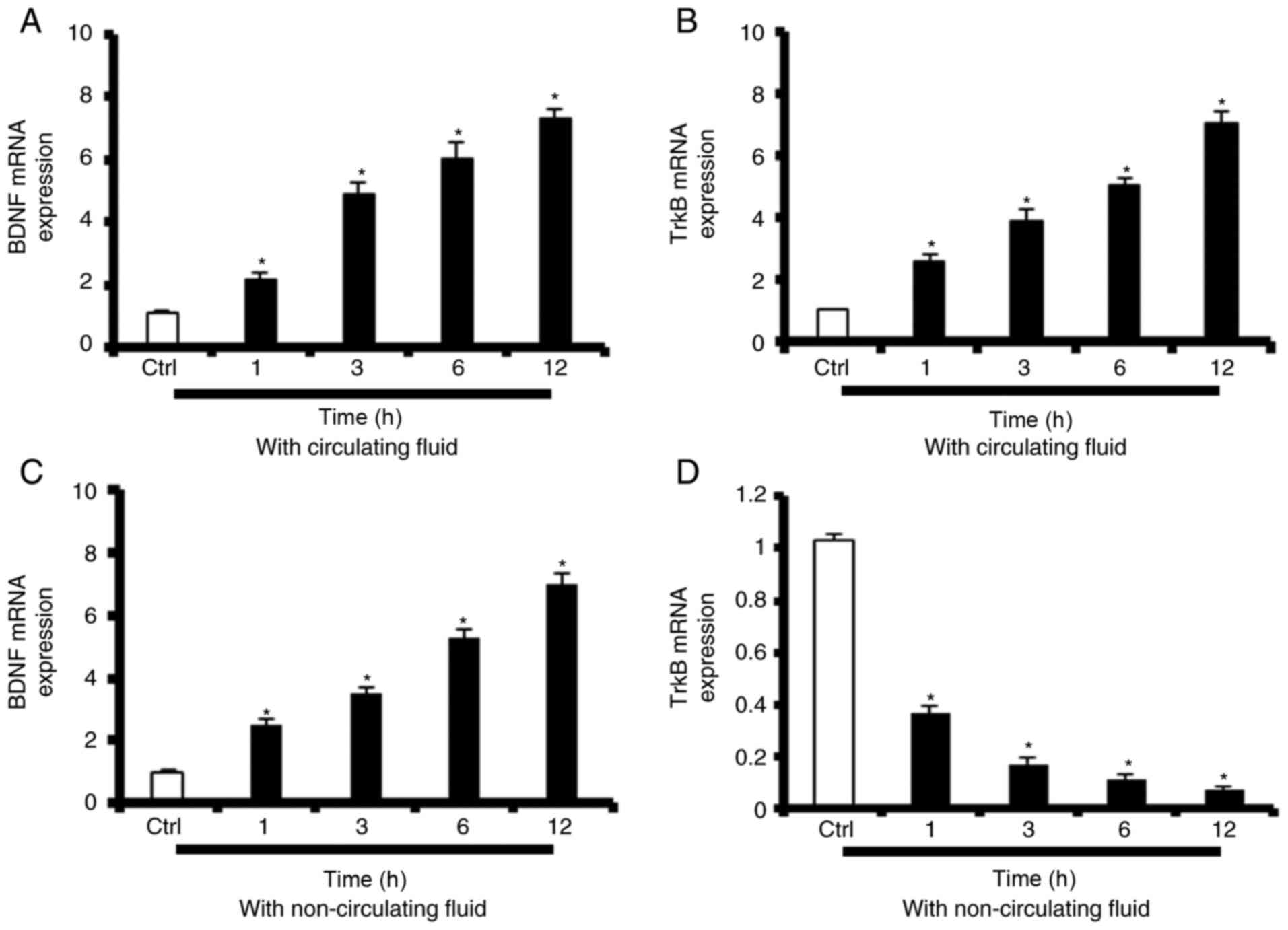 | Figure 7SS-induced TrkB-FL upregulation is
dependent on increases in BDNF levels in the media. (A and B) mRNA
expression levels of BDNF and TrkB-FL in HUVECs exposed to SS for
1, 3, 6 or 12 h. (A) BDNF mRNA levels. *P<0.001 vs.
Ctrl. (B) TrkB-FL mRNA levels. *P<0.001 vs. Ctrl. (C
and D) mRNA expression levels of BDNF and TrkB-FL in HUVECs exposed
to non-circulating fluid SS for 1, 3, 6 or 12 h. (C) BDNF mRNA
levels. *P<0.001 vs. Ctrl. (D) TrkB-FL mRNA levels.
*P<0.001 vs. Ctrl. The values are presented as the
mean ± standard error of the mean (n=6). BDNF, brain-derived
neurotrophic factor; TrkB, BDNF/NT-3 growth factors receptor; FL,
full-length; ctrl, control; HUVECs, human umbilical vein
endothelial cells; SS, shear stress; t, time. |
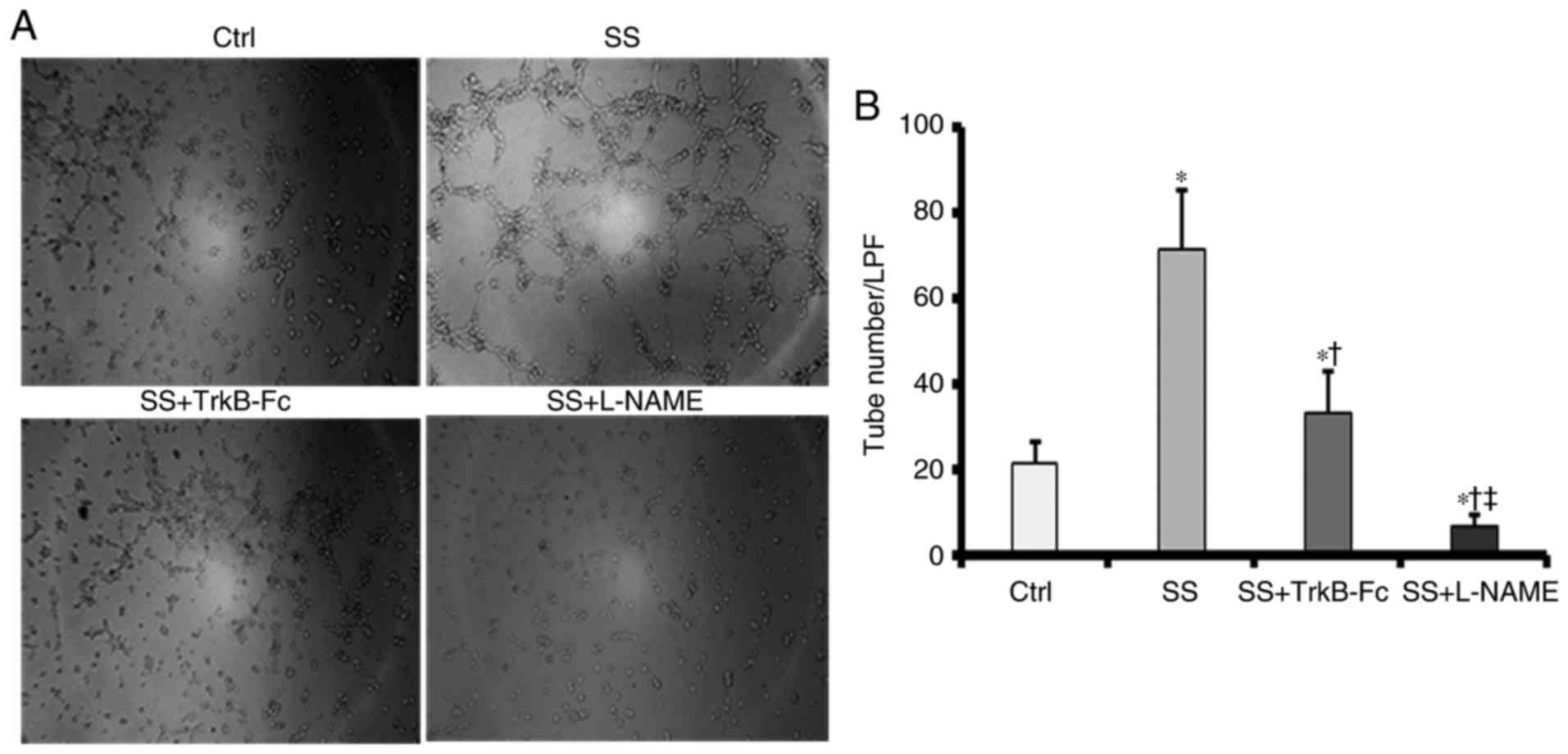
BDNF/TrkB activation is associated with
increases in tube-forming capacity
To investigate whether the BDNF/TrkB pathway
participated in high SS-induced capillary-like tube formation, a
tube formation assay was performed, the results of which were
examined microscopically. As indicated in Fig. 8A and B, HUVECs exposed to high SS
formed increased numbers of tubes compared with control HUVECs
cultured under static conditions at 12 h post-SS initiation.
SS-induced tube formation was completely inhibited by the eNOS
inhibitor L-NAME. To determine whether the BDNF/TrkB pathway
mediated high SS-induced improvements in tube-forming capacity in
HUVECs, a chimeric TrkB-Fc (1 µg/ml) was used to neutralize BDNF
secretion under fluid conditions. It was identified that tube
formation was impaired in the chimera-treated group compared with
that in the SS-treated group; however, the number of tubes that
formed in the chimera-treated group was increased compared with
that in the control group. These data suggested that SS confers a
proportion of its tube-forming effects through the activation of
the BDNF/TrkB axis.
Discussion
The present study provided evidence associating the
BDNF/TrkB axis with exercise-induced improvements in cardiovascular
health. The data indicated that circulating and cardiac BDNF
protein levels were increased within 8 weeks in rats subjected to
exercise training, and that increases in BDNF expression coincided
with improved vascularization and cardiac function. The decreases
in capillary density and cardiac function noted in the trained rats
treated with the known TrkB inhibitor K252a supported this
result.
One hypothesis explaining the association between
the BDNF/TrkB axis and improved cardiovascular function in response
to exercise implicates alterations in TrkB expression and
phosphorylation in the IHZ. There are 2 major isoforms of TrkB that
exist, including a full-length functional isoform (140 KDa) and a
truncated non-functional isoform (90 KDa). Increased TrkB-T
expression levels in the nervous system have been associated with
neurologic disorders, and TrkB-T production is induced in response
to brain injury (28). BDNF/TrkB
signalling was identified to modulate heart contraction force and
long-term heart tissue homeostasis (10). TrkB-FL was demonstrated to exist
in cardiomyocytes isolated from healthy and failing rodent hearts,
and TrkB-T expression levels were markedly increased in failing
hearts compared with those in healthy hearts (9). In the present study, it was also
identified that TrkB-T protein expression levels were increased in
MI rat hearts, indicating a response to ischaemic injury. Notably,
exercise training induced a modest but non-significant decrease in
TrkB-T protein expression. The marked decrease in TrkB-T protein
expression that occurred in response to training may be attributed
to improvements in myocardial damage due to exercise training.
However, TrkB-T phosphorylation levels increased markedly in the
MIE group. These novel data regarding exercise-induced increases in
TrkB-T phosphorylation also support the previous hypothesis that
exercise training has compensatory effects and contributes to
post-MI enhancement of cardiac performance via TrkB-T activation
(9). This hypothesis requires
additional investigation.
To the best of our knowledge, the western blot
analysis results of the present study represent the first evidence
demonstrating that exercise training upregulates TrkB-FL protein
expression and phosphorylation levels in the IHZs of MI rats.
Previous studies have identified that the TrkB-FL receptor was
expressed primarily in young cardiac microvascular endothelial
cells (CMECs) (7). BDNF may
induce young CMECs to migrate via BDNF-TrkB-FL-phosphoinositide
3-kinase/protein kinase B pathway activation (7). Therefore, we hypothesized that
exercise-induced TrkB-FL upregulation and phosphorylation may be
the primary factors driving angiogenesis following exercise
training. The rats in the exercise group that received K252a
exhibited decreased phosphorylation levels of TrkB and decreased
capillary density and cardiac function, which additionally
confirmed the role of the activated BDNF/TrkB axis in
exercise-induced cardioprotection.
The animal studies also exhibited elevated
peripheral BDNF levels following exercise, and the serum BDNF
concentration was positively correlated with myocardial
angiogenesis and cardiac function. However, the increased serum
BDNF observed subsequent to physical exercise in patients following
MI events, and the association between its variation and an
improved cardiac state, have not been fully investigated. The data
from the present study indicate that circulating BDNF may be a
potential biomarker for assessing the effect of exercise
rehabilitation in patients following MI events; however, additional
clinical studies are required for verification.
At present, how exercise training activates the
BDNF/TrkB axis remains unknown. The cardiovascular benefits of
physical training are known to depend heavily on exercise-induced
uniaxial laminar flow and shear stress, which is considered one of
the central signalling mechanisms underlying improvements in
vascular function (29-31). The average haemodynamic SS level
in human veins under resting conditions is ~6 dyn/cm2
(32,33). Laminar shear stress levels >10
dyn/cm2 have been demonstrated to trigger endothelial
cell sprouting (34,35). Exercise increases the heart rate,
resulting in increases in blood flow and vascular shear stress;
therefore, a circulatory system that produces SS (12
dyn/cm2) similar to the in vivo conditions
elicited by exercise was developed to mimic the effects of physical
training on the BDNF/TrkB axis in the vasculature. It was
identified that SS-induced increases in tube-formation capacity
were substantially inhibited by chimeric TrkB-Fc treatment. Similar
to the animal experimental results, this result additionally
indicated that the BDNF/TrkB axis is involved in exercise-induced
angiogenesis.
Dynamic observations of BDNF secretion under SS in
HUVECs were also conducted. Exposure of HUVECs to SS at 12
dyn/cm2 was associated with a significant and persistent
increase in BDNF protein expression. These data contradict the
results described by Nakahashi et al (23), who noted that BDNF levels were
decreased in the medium of HUVECs exposed to SS (24
dyn/cm2) for 24 h (23). However, Prigent-Tessier et
al (22) identified that BDNF
secretion levels were proportional to shear stress intensity in
cultured endothelial cells exposed to SS at 14 dyn/cm2
for 24 h. These contradictory results suggest that the responses of
BDNF expression to SS are likely to be cell- and
context-specific.
Notably, increased SS increased the expression and
phosphorylation of TrkB-FL but not TrkB-T in HUVECs. Concomitantly,
the variation trend of TrkB-FL phosphorylation was similar to that
for BDNF expression. These data also support the results of the
animal experiments, which demonstrated that TrkB-FL may be the
primary receptor mediating the angiogenic response to exercise,
while TrkB-T primarily mediates exercise-induced increases in
cardiac contraction force.
Exogenous BDNF (70 ng/ml) has been suggested to
induce only a transient increase in TrkB receptor levels in
cultured neurons and CMECs (7,36).
Therefore, we hypothesized that the sustained increases in TrkB
expression that occurred under flow conditions are
ligand-dependent. This hypothesis was verified using a laminar flow
chamber containing non-circulating fluid, in which only a small
amount of BDNF was present. Time-dependent increases in BDNF mRNA
expression and decreases in TrkB-FL mRNA expression, were observed,
confirming the previous hypothesis. It was also identified that SS
induced sustained TrkB-FL phosphorylation, whereas exogenous BDNF
application triggered only a transient increase in TrkB
phosphorylation (37). The
increased BDNF concentration that manifested under flow conditions
was decreased compared with the dose of exogenous BDNF
administered; however, the increase resulted in more significant
and longer TrkB receptor activation (12 h). The cellular mechanisms
responsible for this phenomenon are currently unknown. A previous
study identified that increases in Ca++ levels induced
by neuronal stimulation caused transient TrkB activation that
progressed to sustained activation (36). Substantial previous evidence also
indicated that vascular endothelial cells increased their
intracellular Ca++ levels in accordance with the flow
rate (38,39). Based on the present results and
those of previous studies, we hypothesized that sustained TrkB
activation resulted from SS-induced increases in Ca++
levels. The data from the present study suggested that
exercise-induced endogenous BDNF possessed greater bioactivity than
exogenous BDNF, and confirmed the amplified effect of BDNF within
endothelial cells exposed to increased shear force, indicating that
endogenous BDNF expression depended on endothelial function in
vivo; studies from Jin et al (40) and Prigent-Tessier et al
(22) validated these
conclusions. Based on these data and previous studies, we
hypothesized that exogenous BDNF administration combined with
exercise training may assist in alleviating circulatory
disturbances in diabetic or hypertensive patients with poor
vascular function. Therefore, the present study demonstrated the
potential of utilizing BDNF to improve vascular function in
clinical practice. However, additional animal and clinical
experiments are warranted.
To gain insight into the mechanisms by which
exercise activated the BDNF/TrkB axis, the effects of NO, an
important inducer of exercise-associated angiogenesis in ischaemic
myocardial tissue, were investigated (41). Several lines of evidence suggest
that NO modulates BDNF expression and relaxation in vivo and
in vitro (42,43). However, these studies were limited
to nerve tissues. In the present study, it was demonstrated that
exogenous L-NAME blocked exercise-induced BNDF/TrkB axis activation
in heart tissue. To the best of our knowledge, the present study is
the first to demonstrate that eNOS/NO participates in BDNF/TrkB
axis activation in organs other than the brain. A previous study
has confirmed that high SS-induced increases in eNOS expression may
be the basis of SS-mediated angiogenesis (44). The in vitro data from the
present study indicated that an NO donor upregulated BDNF
expression without TrkB-FL phosphorylation in HUVECs under static
conditions, which may contribute to weak and short TrkB-FL
activation in the absence of SS. Simultaneously, the eNOS inhibitor
L-NAME blocked SS-induced BDNF/TrkB axis activation in HUVECs.
These data, at least partially, explained the inhibitory effects of
L-NAME observed in the animal studies.
In summary, the data from the present study indicate
that exercise training activated the BDNF/TrkB (TrkB-FL and TrkB-T)
axis in post-MI rat hearts and that only BDNF/TrkB-FL pathway
activation is involved in exercise-induced angiogenesis. The
laminar SS response to exercise may contribute to BDNF/TrkB-FL axis
activation in an NO-dependent manner. Increased circulating BDNF
concentrations may be important for activating TrkB-FL and were
associated with improved cardiac function and angiogenesis in the
ischaemic myocardium in post-MI rats subjected to exercise. These
results provide novel insights not only into monitoring of cardiac
function but also into the development of effective cardiac
rehabilitation programmes for patients following MI events.
Funding
The present study was supported by the Science and
Technology Support, Social Development Research Fund, a division of
Health Bureau, Jiangsu Province Government (grant no. BE2011793)
awarded to Dr Nai-Feng Liu, and the Postgraduate Research
Innovation Plan of Jiangsu Province (grant no. KYCX17_0174) awarded
to Dr Xi-Qiong Han.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
The study was conceived and designed by NFL, HJ and
BLW. BLW conducted the experimental protocols with assistance from
XQH and YX. The paper was written by BLW, with contributions from
NFL and HJ.
Ethics approval and consent to
participate
The present study was approved by the Animal Care
and Research Committee of Southeast University, and all experiments
were conducted in accordance with the European Convention for the
Protection of Vertebrates Used for Experiments and Other Scientific
Purposes (European Union Directive 86/609EEC, 1986).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Dr Yu-Yu Yao
(Department and Institute of Cardiology, Zhongda Hospital, Medical
School of Southeast University) and Dr Yi-Ping Li (Department of
Pathophysiology, Medical School of Southeast University) for their
technical advice and support.
References
|
1
|
Barbacid M: Neurotrophic factors and their
receptors. Curr Opin Cell Biol. 7:148–155. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chao MV: Neurotrophins and their
receptors: A convergence point for many signalling pathways. Nat
Rev Neurosci. 4:299–309. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Monteggia LM, Barrot M, Powell CM, Berton
O, Galanis V, Gemelli T, Meuth S, Nagy A, Greene RW and Nestler EJ:
Essential role of brain-derived neurotrophic factor in adult
hippocampal function. Proc Natl Acad Sci USA. 101:10827–10832.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Okada S, Yokoyama M, Toko H, Tateno K,
Moriya J, Shimizu I, Nojima A, Ito T, Yoshida Y, Kobayashi Y, et
al: Brain-derived neurotrophic factor protects against cardiac
dysfunction after myocardial infarction via a central nervous
system-mediated pathway. Arterioscler Thromb Vasc Biol.
32:1902–1909. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kermani P, Rafii D, Jin DK, Whitlock P,
Schaffer W, Chiang A, Vincent L, Friedrich M, Shido K, Hackett NR,
et al: Neurotrophins promote revascularization by local recruitment
of TrkB+ endothelial cells and systemic mobilization of
hematopoietic progenitors. J Clin Invest. 115:653–663. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Donovan MJ, Lin MI, Wiegn P, Ringstedt T,
Kraemer R, Hahn R, Wang S, Ibañez CF, Rafii S and Hempstead BL:
Brain derived neurotrophic factor is an endothelial cell survival
factor required for intramyocardial vessel stabilization.
Development. 127:4531–4540. 2000.PubMed/NCBI
|
|
7
|
Cao L, Zhang L, Chen S, Yuan Z, Liu S,
Shen X, Zheng X, Qi X, Lee KK, Chan JY and Cai D: BDNF-mediated
migration of cardiac microvascular endothelial cells is impaired
during ageing. J Cell Mol Med. 16:3105–3115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y, Sun L, Huan Y, Zhao H and Deng J:
Application of bFGF and BDNF to improve angiogenesis and cardiac
function. J Surg Res. 136:85–91. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feng N, Huke S, Zhu G, Tocchetti CG, Shi
S, Aiba T, Kaludercic N, Hoover DB, Beck SE, Mankowski JL, et al:
Constitutive BDNF/TrkB signaling is required for normal cardiac
contraction and relaxation. Proc Natl Acad Sci USA. 112:1880–1885.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fulgenzi G, Tomassoni-Ardori F, Babini L,
Becker J, Barrick C, Puverel S and Tessarollo L: BDNF modulates
heart contraction force and long-term homeostasis through truncated
TrkB. T1 receptor activation J Cell Biol. 210:1003–1012. 2015.
|
|
11
|
Fukushima A, Kinugawa S, Homma T, Masaki
Y, Furihata T, Yokota T, Matsushima S, Abe T, Suga T, Takada S, et
al: Decreased serum brain-derived neurotrophic factor levels are
correlated with exercise intolerance in patients with heart
failure. Int J Cardiol. 168:e142–e144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Swardfager W, Herrmann N, Marzolini S,
Saleem M, Shammi P, Oh PI, Albert PR, Daigle M, Kiss A and Lanctôt
KL: Brain derived neurotrophic factor, cardiopulmonary fitness and
cognition in patients with coronary artery disease. Brain Behav
Immun. 25:1264–1271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Circulating brain-derived neurotrophic
factor concentrations and the risk of cardiovascular disease in the
community. J Am Heart Assoc. 5:e0020982016.PubMed/NCBI
|
|
14
|
Möbius-Winkler S, Uhlemann M, Adams V,
Sandri M, Erbs S, Lenk K, Mangner N, Mueller U, Adam J, Grunze M,
et al: Coronary collateral growth induced by physical exercise:
Results of the impact of intensive exercise training on coronary
collateral circulation in patients with stable coronary artery
disease (EXCITE) trial. Circulation. 133:1438–1448. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leosco D, Rengo G, Iaccarino G, Golino L,
Marchese M, Fortunato F, Zincarelli C, Sanzari E, Ciccarelli M,
Galasso G, et al: Exercise promotes angiogenesis and improves
beta-adrenergic receptor signalling in the post-ischaemic failing
rat heart. Cardiovasc Res. 78:385–394. 2008. View Article : Google Scholar
|
|
16
|
Tang XY, Hong HS, Chen LL, Lin XH, Lin JH
and Lin Z: Effects of exercise of different intensities on the
angiogenesis, infarct healing, and function of the left ventricle
in postmyocardial infarction rats. Coron Artery Dis. 22:497–506.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tinken TM, Thijssen DH, Hopkins N, Dawson
EA, Cable NT and Green DJ: Shear stress mediates endothelial
adaptations to exercise training in humans. Hypertension.
55:312–318. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Duncker DJ and Bache RJ: Regulation of
coronary blood flow during exercise. Physiol Rev. 88:1009–1086.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marsh SA and Coombes JS: Exercise and the
endothelial cell. Int J Cardiol. 99:165–169. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zoladz JA and Pilc A: The effect of
physical activity on the brain derived neurotrophic factor: From
animal to human studies. J Physiol Pharmacol. 61:533–541. 2010.
|
|
21
|
Lee HW, Ahmad M, Wang HW and Leenen FH:
Effects of exercise training on brain-derived neurotrophic factor
in skeletal muscle and heart of rats post myocardial infarction.
Exp Physiol. 102:314–328. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Prigent-Tessier A, Quirié A, Maguin-Gaté
K, Szostak J, Mossiat C, Nappey M, Devaux S, Marie C and Demougeot
C: Physical training and hypertension have opposite effects on
endo-thelial brain-derived neurotrophic factor expression.
Cardiovasc Res. 100:374–382. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakahashi T, Fujimura H, Altar CA, Li J,
Kambayashi J, Tandon NN and Sun B: Vascular endothelial cells
synthesize and secrete brain-derived neurotrophic factor. FEBS
Lett. 470:113–117. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hiraoka Y, Kishimoto C, Takada H, Nakamura
M, Kurokawa M, Ochiai H and Shiraki K: Nitric oxide and murine
coxsackievirus B3 myocarditis: Aggravation of myocarditis by
inhibition of nitric oxide synthase. J Am Coll Cardiol.
28:1610–1615. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiménez-Maldonado A, de Álvarez-Buylla ER,
Montero S, Melnikov V, Castro-Rodríguez E, Gamboa-Domínguez A,
Rodríguez-Hernández A, Lemus M and Murguía JM: Chronic exercise
increases plasma brain-derived neurotrophic factor levels,
pancreatic islet size, and insulin tolerance in a TrkB-dependent
manner. PLoS One. 9:e1151772014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Method. 25:402–408. 2001.
View Article : Google Scholar
|
|
27
|
Siemerink MJ, Klaassen I, Vogels IM,
Griffioen AW, Van Noorden CJ and Schlingemann RO: CD34 marks
angiogenic tip cells in human vascular endothelial cell cultures.
Angiogenesis. 15:151–163. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Frisén J, Verge VM, Fried K, Risling M,
Persson H, Trotter J, Hökfelt T and Lindholm D: Characterization of
glial trkB receptors: Differential response to injury in the
central and peripheral nervous systems. Proc Natl Acad Sci USA.
90:4971–4975. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Suh GY, Les AS, Tenforde AS, Shadden SC,
Spilker RL, Yeung JJ, Cheng CP, Herfkens RJ, Dalman RL and Taylor
CA: Hemodynamic changes quantified in abdominal aortic aneurysms
with increasing exercise intensity using mr exercise imaging and
image-based computational fluid dynamics. Ann Biomed Eng.
39:2186–2202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rodriguez I and Gonzalez M: Physiological
mechanisms of vascular response induced by shear stress and effect
of exercise in systemic and placental circulation. Front Pharmacol.
5:2092014.PubMed/NCBI
|
|
31
|
Wragg JW, Durant S, McGettrick HM, Sample
KM, Egginton S and Bicknell R: Shear stress regulated gene
expression and angiogenesis in vascular endothelium.
Microcirculation. 21:290–300. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Papaioannou TG and Stefanadis C: Vascular
wall shear stress: Basic principles and methods. Hellenic J
Cardiol. 46:9–15. 2005.PubMed/NCBI
|
|
33
|
Barnes JM, Nauseef JT and Henry MD:
Resistance to fluid shear stress is a conserved biophysical
property of malignant cells. PLoS One. 7:e509732012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Galie PA, Nguyen DH, Choi CK, Cohen DM,
Janmey PA and Chen CS: Fluid shear stress threshold regulates
angiogenic sprouting. Proc Natl Acad Sci USA. 111:7968–7973. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ueda A, Koga M, Ikeda M, Kudo S and
Tanishita K: Effect of shear stress on microvessel network
formation of endothelial cells with in vitro three-dimensional
model. Am J Physiol Heart Circ Physiol. 287:H994–H1002. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guo W, Ji Y, Wang S, Sun Y and Lu B:
Neuronal activity alters BDNF-TrkB signaling kinetics and
downstream functions. J Cell Sci. 127:2249–2260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ji Y, Lu Y, Yang F, Shen W, Tang TT, Feng
L, Duan S and Lu B: Acute and gradual increases in BDNF
concentration elicit distinct signaling and functions in neurons.
Nat Neurosci. 13:302–309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ando J, Ohtsuka A, Korenaga R and Kamiya
A: Effect of extracellular ATP level on flow-induced
Ca++ response in cultured vascular endothelial cells.
Biochem Biophys Res Commun. 179:1192–1199. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yamamoto K, Korenaga R, Kamiya A and Ando
J: Fluid shear stress activates Ca(2+) influx into human
endothelial cells via P2X4 purinoceptors. Circ Res. 87:385–391.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jin H, Chen Y, Wang B, Zhu Y, Chen L, Han
X, Ma G and Liu N: Association between brain-derived neurotrophic
factor and von Willebrand factor levels in patients with stable
coronary artery disease. BMC Cardiovasc Disord. 18:232018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Calvert JW: Cardioprotective effects of
nitrite during exercise. Cardiovasc Res. 89:499–506. 2011.
View Article : Google Scholar :
|
|
42
|
Cui X, Chopp M, Zacharek A, Ning R, Ding
X, Roberts C and Chen J: Endothelial nitric oxide synthase
regulates white matter changes via the BDNF/TrkB pathway after
stroke in mice. PLoS One. 8:e803582013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li ST, Pan J, Hua XM, Liu H, Shen S, Liu
JF, Li B, Tao BB, Ge XL, Wang XH, et al: Endothelial nitric oxide
synthase protects neurons against ischemic injury through
regulation of brain-derived neurotrophic factor expression. CNS
Neurosci Ther. 20:154–164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kolluru GK, Sinha S, Majumder S, Muley A,
Siamwala JH, Gupta R and Chatterjee S: Shear stress promotes nitric
oxide production in endothelial cells by sub-cellular
delocalization of eNOS: A basis for shear stress mediated
angiogenesis. Nitric Oxide. 22:304–315. 2010. View Article : Google Scholar : PubMed/NCBI
|