Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune
disease with an increasing global prevalence (1), characterized by chronic synovial
inflammation, cartilage and joint erosion, pannus formation, joint
abnormalities, and ankylosis. Although RA is commonly considered to
arise due to exogenous factors (2,3),
the exact cause of RA remains to be fully elucidated and as such,
there is no known cure (4).
Therefore, patients with RA currently require novel therapeutic
treatments that can inhibit the inflammation and erosion of joints
without any unwanted side effects.
Although the pathogenesis of RA remains to be fully
elucidated, pro-inflammatory mediators, including autoantibodies
and cytokines, are considered to function within the mechanisms of
its expression (5).
Autoantibodies secreted by activated B cells and plasma cells
target various of the cartilage components affected by RA, which
results in the increased autoreactivity of B and T cells and the
progression of RA (6). An
overproduction of interleukin (IL)-6, tumor necrosis factor (TNF)α,
IL-1 and IL-17 is found in the synovial fluid of patients with RA
(7). Pro-inflammatory cytokines
produced by infiltrated immune cells influence osteoclast formation
by upregulating the expression of receptor activator of nuclear
factor-κB ligand (RANKL). Increased osteoclast formation may be a
factor in the acceleration of bone resorption and thus of RA
symptoms (8).
IL-6, a type of pro-inflammatory cytokine, has
pleio-tropic biological activities and is produced by various types
of cells (9). IL-6 stimulates
osteoclastogenesis by inducing the expression of RANKL in synovial
fibroblasts and osteoblasts (10). Osteoclasts are important in
maintaining bone homeostasis through the regulation of bone
resorption using proteolytic enzymes and acids. However, an
accumulation of IL-6 can lead to the hyperactivation of
osteoclasts, which leads to bone and cartilage erosion and
ankyloses (11).
Melicope ptelefolia (M. ptelefolia) is
a dioecious plant that is widespread across Asia, particularly in
Malaysia, Vietnam, and southern China. It is a source of
3,5-di-C-β-d-glucopyranosyl
phloroacetophenone (βGP). Phytochemical studies have established
that acetophenone, benzopyran, bis-isoquinoline alkaloids, and
benzopyrans dimers are the main components of M. ptelefolia
(12,13). M. ptelefolia leaves are
commonly used not only in the treatment of remittent fever, colds,
stomach ache and wounds, but are also used as a natural remedy for
rheumatism in certain parts of Asia (14-17). However, several of these reported
uses are not substantiated by any published documents, and there
are currently few reports on biologically active components of
M. ptelefolia showing these beneficial effects.
The present study investigated the effects of βGP, a
main component of M. ptelefolia, on RA and its underlying
mechanisms using a mouse model of collagen-induced arthritis
(CIA).
Materials and methods
Plant material
The leaves of M. ptelefolia were purchased
from a market in My Duc herbal distract of Hanoi, Vietnam in March
2014. The sample was botanically identified by Dr Tran The Bach at
the Institute of Ecology and Biological Resources (Hanoi, Vietnam).
A sample of the voucher (KRIBB 010471) has been deposited in the
herbarium of the Korea Research Institute of Bioscience and
Biotechnology (Daejeon, Korea).
General experimental procedures for
extraction and isolation
1D and 2D nuclear magnetic resonance (NMR)
spectroscopy were performed using the Bruker AVANCE 800 (Bruker
Corporation, Billerica, MA, USA) NMR spectrometer with TMS as the
internal standard. Thin layer chromatography was performed with
silica gel 60 F254 and RP-18 F254 plates.
High-performance liquid chromatography (HPLC) was performed using
the Gilson HPLC system with a 321 pump and a UV/VIS-155 detector.
An RS Tech Optima Pak C18 column (10×250 mm, 5-µm
particle size; RStech Corporation, Daejeon, Korea) was used as the
HPLC column. ESI-MS data were obtained using Agilent Technologies
6130 Quadrupole liquid chromatography/ionization mass spectrometry
(LC/MS). IR spectra (KBr) were obtained using Nicolet 6700 FT-IR
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). All solvents
used for extraction and isolation were of an analytical grade.
Extraction and isolation of βGP from M.
ptelefolia
The air-dried M. ptelefolia leaves (1.0 kg)
were sonicated with water three times at 2-h intervals. The crude
extract (174.2 g) was suspended in water and used for
Diaion® HP-20 column chromatography, eluted with water,
40% ethanol and acetone to obtain three fractions, respectively.
The 40% ethanol fraction (64.1 g) was pre-isolated on MPLC using
RP-C18 (Watcher® Flash Cartridge, 3×15 cm;
40–60-µm particle size), eluting with a stepwise gradient of
methanol/H2O (1:9-1:0) to obtain eight subfractions.
βGP-rich fractions 2 and 3 were chromatographed using an RP-C18
(4×40 cm; 75-µm particle size) open column, eluting with 40%
methanol. Eluted fraction 3 (8.79 g) was directly purified using
the Gilson HPLC system (Optima Pak C18 Column; 10×250 mm,
5-µm particle size, RS Tech Corporation) with a mobile phase
of methanol in H2O containing 0.1% HCO2H
(0-50 min: 15-30% methanol, 51-60 min: 100% methanol); a flow rate
of 2 ml/min; and UV detection at 205 and 254 nm to yield βGP
(tR=30 min, 1.90 g).
HPLC analysis of βGP
The βGP was quantified using reversed-phase HPLC
with an Agilent series 1260 liquid chromatography, a G1322A vacuum
degasser, G1312C binary pump, G1329B autosampler, and G1315D DAD
detector, and processed using Agilent ChemStation software (Edition
Rev.C.01.05 36; Agilent Technologies GmbH, Waldbronn, Germany). The
system used MeCN/H2O [INNO C18 Column; 4.6×250 mm,
5-µm particle size (RS Tech Corporation); mobile phase
acetonitrile in H2O containing 0.1% HCO2H
(3-15 min: 3-20% MeCN, 15-38 min: 20-100% MeCN); flow rate 0.6
ml/min; UV detection at 254 and 300 nm]. A stock solution of
purified βGP was prepared by dissolving 1.00 mg of βGP in 50% DMSO.
Five additional calibration levels were prepared by diluting this
stock solution with water. The calibration curves were constructed
by plotting the peak area ratios via OpenLAB CDS analysis
(ChemStation; Agilent Technologies GmbH). A good linearity
(correlation coefficient values
R2>0.999) was achieved with a
relatively wide concentration ranging between 0.31 and 2.5
µg/ml. The lowest concentration of the working solution was
mixed with water to yield a series of diluted concentrations. The
limits of detection and quantification under the chromatographic
conditions were separately determined at a signal-to-noise ratio of
~2 and 12, respectively.
Animal studies
Eight-week-old male DBA/1 (Orient Bio, Inc.,
Seongnam, Korea) and ICR (Koatech, Pyeongtaek, Korea) mice (20-25
g) were acclimatized to a 12-h light/dark cycle at 22±2°C for 2
weeks with unlimited access to food and water in a specific
pathogen-free facility. The DAB/1 mice were randomly divided into
four groups: i) Normal group (n=4); ii) vehicle group (n=16),
treated with 0.5% carboxymethylcellulose; iii) βGP 25 group (n=5),
treated with 25 mg/kg βGP; iv) βGP 50 group (n=15), treated with 50
mg/kg βGP. From 3 days prior to the second immunization, βGP was
administered daily by oral gavage for 18 days. The concentration of
βGP was determined based on the results of preliminary animal
experiments. Body weight was measured each day. The ICR mice were
randomly divided into six groups (0, 2, 4, 8, 24, and 48 h; n=4-5
for each group) and were administered with a single dose of 50
mg/kg βGP by oral gavage. The plasma and livers were obtained from
the ICR mice following the specified time periods (0, 2, 4, 8, 24,
or 48 h), and βGP concentration was determined by LC-MS. All mice
were sacrificed by CO2 asphyxiation. All animal
experiments were approved by the Institutional Animal Care and Use
Committee of the Korea Research Institute of Bioscience and
Biotechnology (KRIBB-AEC-18001) and performed in accordance with
the Guide for the Care and Use of Laboratory Animals published by
the US National Institutes of Health (Bethesda, MD, USA).
Cell culture
RAW 264.7 cells, a murine macrophage cell line, and
NIH/3T3 fibroblasts were purchased from the American Type Cell
Culture (Manassas, VA, USA). The cells were cultured in Dulbecco's
modified Eagle's medium (DMEM; Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) containing 10% fetal bovine serum (FBS;
Gibco, Thermo Fisher Scientific, Inc.), 100 U/ml penicillin, and
100 µg/ml streptomycin, in a humidified environment (5%
CO2/95% air) at 37°C. Mouse embryo fibroblasts (MEFs)
were isolated from day 13.5 C57BL/6 mouse embryos. Briefly, the
embryos were harvested from the uterus of female 13-14 days after
the appearance of the copulation plug. The isolated embryos were
transferred to a dish with phosphate buffered saline (PBS), and
blood was removed by swirling. The head, limbs, and internal organs
of the embryo were removed. The rest of the embryos were placed in
the covered dish with 0.25% trypsin buffer, and dissected with
scissors. Then, the tissues were minced into pieces of 1-2 mm with
a scalpel blade, and placed in the 37°C tissue culture incubator
for 10 min. The cell suspensions were transferred to a 50 ml tube,
and DMEM containing 10% FBS was added to inactivate the trypsin.
The cell suspension sat for 5 min to allow larger embryo fragments
to sink to the bottom the tube. The supernatant consisting of
single cells and cell clusters was transferred to a T75 flask, and
cultured in DMEM containing 10% FBS, 100 U/ml penicillin, and 100
µg/ml streptomycin, in a humidified environment (5%
CO2/95% air) at 37°C. The RAW 264.7 cells and MEFs at a
density of 500,000 cells in a 6 well plate were pre-treated with
different concentrations of βGP for 1 h. The RAW 264.7 cells were
then stimulated with lipopolysaccharide (LPS; 100 ng/ml; Sigma, EMD
Millipore, Billerica, MA, USA) or PBS for 6 h at 37°C. The MEF
cells were stimulated with TNFα (40 ng/ml; Gibco; Thermo Fisher
Scientific, Inc.) or PBS for 6 h at 37°C. NIH/3T3 fibroblasts at a
density of 100,000 cells in a 24 well plate were incubated with
different concentrations of βGP for 24 h at 37°C. Cell viability
and proliferation were assessed using a Cell Counting Kit-8
(Dojindo Molecular Technologies, Inc., Rockville, MD, USA) with
2-(2-methoxy-4-nitrophenyl)-3-
(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium monosodium
salt. The concentration of βGP used in the experiments was
determined based on the results of preliminary in vitro
experiments.
Induction and clinical assessment of
CIA
For the induction of arthritis, bovine type II
collagen (Chondrex, Redmond, WA, USA) was dissolved at 2 mg/ml in
PBS containing 0.1 M acetic acid and emulsified in an equal volume
of 2 mg/ml complete Freund's adjuvant (Chondrex). The mice in the
vehicle, βGP 25, and βGP 50 groups were immunized intradermally at
the base of the tail with 100 µl of emulsion containing 100
µg bovine type II collagen. The animals were boosted with an
intradermal injection 21 days later, using the same procedure. At 7
days following the second immunization, the animals were boosted
with an intraperitoneal injection of 40 µg LPS. The mice
were assessed for swelling of the paws and a clinical score was
assigned. Paw swelling was assessed by measuring the mean thickness
of all paws with micrometer calipers. The clinical score was
assessed using the following system: 0, normal paw; 1, one toe
inflamed and swollen; 2, >1 toe, but not the entire paw inflamed
and swollen, or mild swelling of the entire paw; 3, entire paw
inflamed and swollen; 4, markedly inflamed and swollen or ankylosed
paw (18). Each limb was graded,
with a maximum possible score of 16 per animal.
Histopathological analysis
The rear paws of each mouse were collected 35 days
following the first immunization. The paws were fixed, decalcified,
paraffin-embedded, sectioned (5 µm), and stained with
hematoxylin and eosin (H&E), safranin O, or toluidine blue.
Images were captured using a light microscope (BX51; Olympus
Corporation, Tokyo, Japan). The H&E-stained images were
analyzed microscopically for the degree of inflammation and for
cartilage and bone erosion, using the following scale: 0, normal
synovium; 1, synovial membrane hypertrophy and cell infiltrates; 2,
pannus and cartilage erosion; 3, major erosion of cartilage and
subchondral bone; 4, loss of joint integrity and ankyloses
(19).
Micro-computed tomography (µCT)
Images of the rear paw ankle joints of the arthritic
vehicle- and βGP-treated mice were captured on day 35 using a
Quantum FX imaging system (PerkinElmer, Inc., Waltham, MA, USA).
The samples were immersed in 10% formalin.
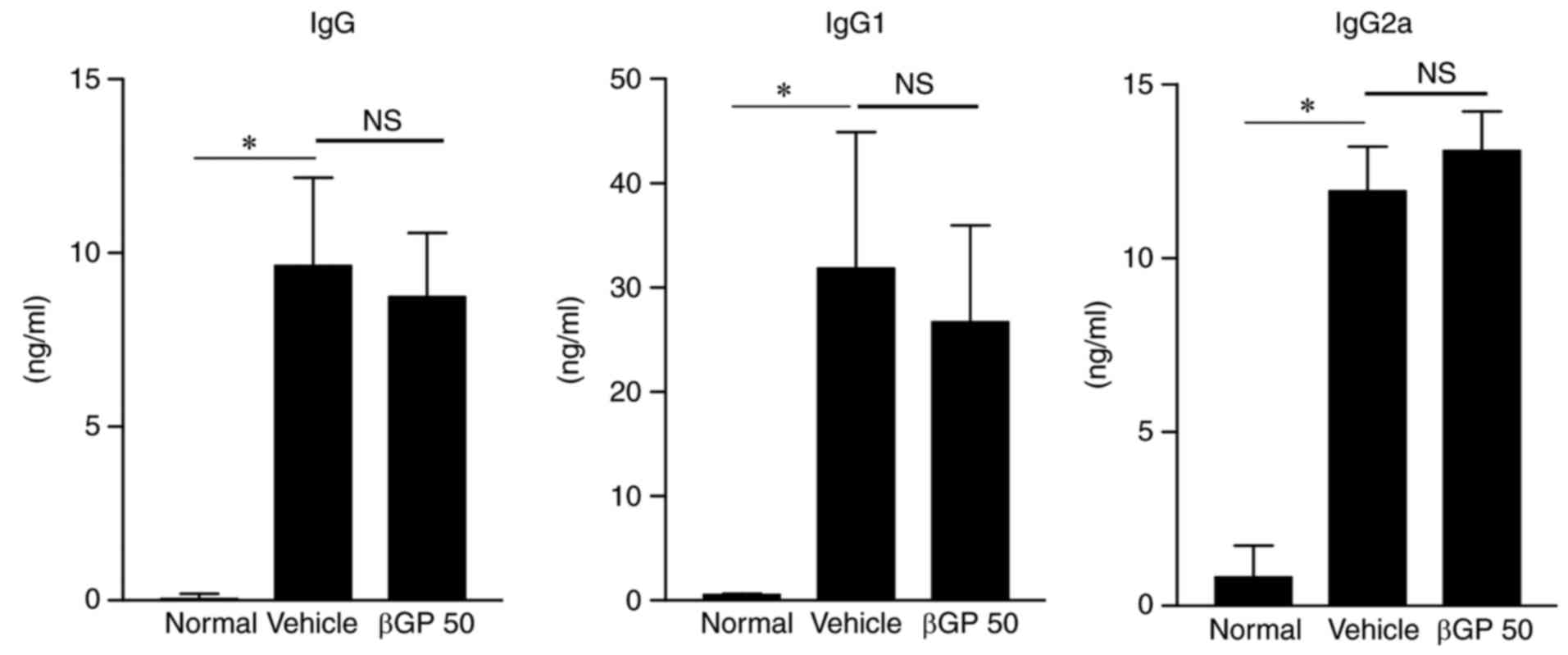
Measurement of serum anti-type II
collagen antibodies immunoglobulin (Ig)G, IgG1, and IgG2a by
ELISA
Serum samples were collected at the end of the
experiment (day 35) for the determination of IgG, IgG1, and IgG2a
antibody levels with three commercially available test kits
(Chondrex), according to the manufacturer's protocol. The antibody
levels were quantified using seven standard serum samples (0.16-10
ng/ml).
Measurement of serum cytokine levels by
ELISA
The serum levels of IL-6, IL-10, and TNF were
determined using the BD OptEIA™ set (BD Biosciences, San Diego, CA,
USA), according to the manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The total RNA was isolated from the paw tissues, RAW
264.7 cells or MEFs using TRIzol reagent (Invitrogen, Thermo Fisher
Scientific, Inc.), and reverse transcribed using the iScript™ cDNA
Synthesis kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
cycling conditions were as follows: Priming at 25°C for 5 min and
reverse transcription at 46°C for 20 min and RT inactivation at
95°C for 1 min. The resulting cDNA was subjected to RT-qPCR using
the StepOnePlus™ Real-Time PCR system (Applied Biosystems, Thermo
Fisher Scientific, Inc.) with AccuPower® 2X Greenstar
qPCR Master mix (Bioneer Corporation, Daejeon, Korea) according to
the manufacturers' protocols. A 10 µl reaction mixture was
used for one well, and the composition was as follows: 1 µl
cDNA (1 ng/µl), 1 µl primer mixtures (5 ρmol/l) for
target gene, 5 µl AccuPower® 2X Greenstar qPCR
Master mix, and 3 µl distilled water. The cycling conditions
were as follows: Pre-denaturation at 95°C for 10 min, followed by
denaturation at 95°C for 10 sec, and annealing and extension at
60°C for 30 sec, for 45 cycles of amplification. Relative gene
expression levels were analyzed using the 2−ΔΔCq method
(20), and normalized against the
expression of 18S rRNA. The primer sequences used in the
experiments are listed in Table
I.
 | Table ISequences of polymerase chain
reaction primers used in the present study. |
Table I
Sequences of polymerase chain
reaction primers used in the present study.
| Gene | GenBank accession
no. | Primer
sequence |
|---|
| Tnfα | NM_013693.3 | Forward
5′-TGGCCTCCCTCTCATCAGTT-3′ |
| Reverse
5′-CCTCCACTTGGTGGTTTGCT-3′ |
| Il-6 | NM_031168.2 | Forward
5′-TTCCATCCAGTTGCCTTCTTG-3′ |
| Reverse
5′-GGGAGTGGTATCCTCTGTGAAGTC-3′ |
| Il-10 | NM_010548.2 | Forward
5′-GGGTTGCCAAGCCTTATCG-3′ |
| Reverse
5′-TCTCACCCAGGGAATTCAAATG-3′ |
| Lif | NM_008501.2 | Forward
5′-GGTGGAGCTGTATCGGATGG-3′ |
| Reverse
5′-TACTTGTTGCACAGACGGCA-3′ |
| Cd68 | NM_001291058.1 | Forward
5′-TCACAGTTCACACCAGCTCC-3′ |
| Reverse
5′-CTTGGACCTTGGACTAGGCG-3′ |
| Cd45 | NM_001111316.2 | Forward
5′-GACAACCTTCGTGCCCAAAC-3′ |
| Reverse
5′-TGACGAGTTTACACCGCGA-3′ |
| Trap | NM_001102405.1 | Forward
5′-GGAACTTCCCCAGCCCTTAC-3′ |
| Reverse
5′-AGGTCTCGAGGCATTTTGGG-3′ |
| Oscar | NM_001290377.1 | Forward
5′-GTAACGGATCAGCTCCCCAG-3′ |
| Reverse
5′-TGCAAAACTCATGCCCGGTA-3′ |
| CtsK | NM_007802.4 | Forward
5′-TACCCATATGTGGGCCAGGA-3′ |
| Reverse
5′-TTCAGGGCTTTCTCGTTCCC-3′ |
| Calcr | NM_007588.2 | Forward
5′-TAGTTAGTGCTCCTCGGGCT-3′ |
| Reverse
5′-AGTACTCTCCTCGCCTTCGT-3′ |
| Rankl | NM_011613.3 | Forward
5′-AGGCTGGGCCAAGATCTCTA-3′ |
| Reverse
5′-GTCTGTAGGTACGCTTCCCG-3′ |
| 18s
rRNA | NR_003278.3 | Forward
5′-GACACGGACAGGATTGACAGATTGATAG-3′ |
| Reverse
5′-GTTAGCATGCCAGAGTCTCGTTCGTT-3′ |
Western blot analysis
The paws from the DBA/1 mice were collected at the
end of the experiment (day 35). The paw tissues and MEF cells were
prepared by homogenization in lysis buffer with a protease
inhibitor and phosphatase inhibitor. The protein concentration in
the supernatant was measured using the Bradford method. Protein
samples (25 µg) were separated by 10% sodium dodecyl sulfate
gel electrophoresis and transferred onto a PVDF membrane (EMD
Millipore). The membranes were stained with primary antibodies
specific to p-STAT3 (S727) (catalogue no. 9134; 1:1,000; Cell
Signaling Technology, Inc., Danvers, MA, USA) or STAT3 (catalogue
no. 9139; 1:1,000; Cell Signaling Technology, Inc.) overnight at
4°C. Horseradish peroxidase-conjugated goat anti-rabbit secondary
antibodies (catalogue no. 111-035-144; 1:1,000; Jackson
ImmunoResearch, West Grove, PA, USA) were then added and incubated
for 1 h at room temperature. Following washing with Tris-buffered
saline and Tween-20, the bands were detected using EzWestLumi plus
(Atto Corporation, Tokyo, Japan).
Statistical analysis
Numerical data are presented as the mean ± standard
error of the mean. Comparisons between two groups were performed
using a two-tailed Student's t-test or Wilcoxon's test.
Comparisons of multiple groups were performed using the
Tukey-Kramer HSD test following one-way analysis of variance. JMP
5.1 software was used for analysis (SAS Institute, Inc., Cary, NC,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Isolation and structure determination of
compound βGP from M. ptelefolia
βGP was isolated from M. ptelefolia using the
bioactive-guided method. As shown in Fig. 1A, the chemical structure of
purified βGP was determined by 1D and 2D NMR spectroscopy and by
comparison with its physical-chemical properties of a previously
published report (14,15).
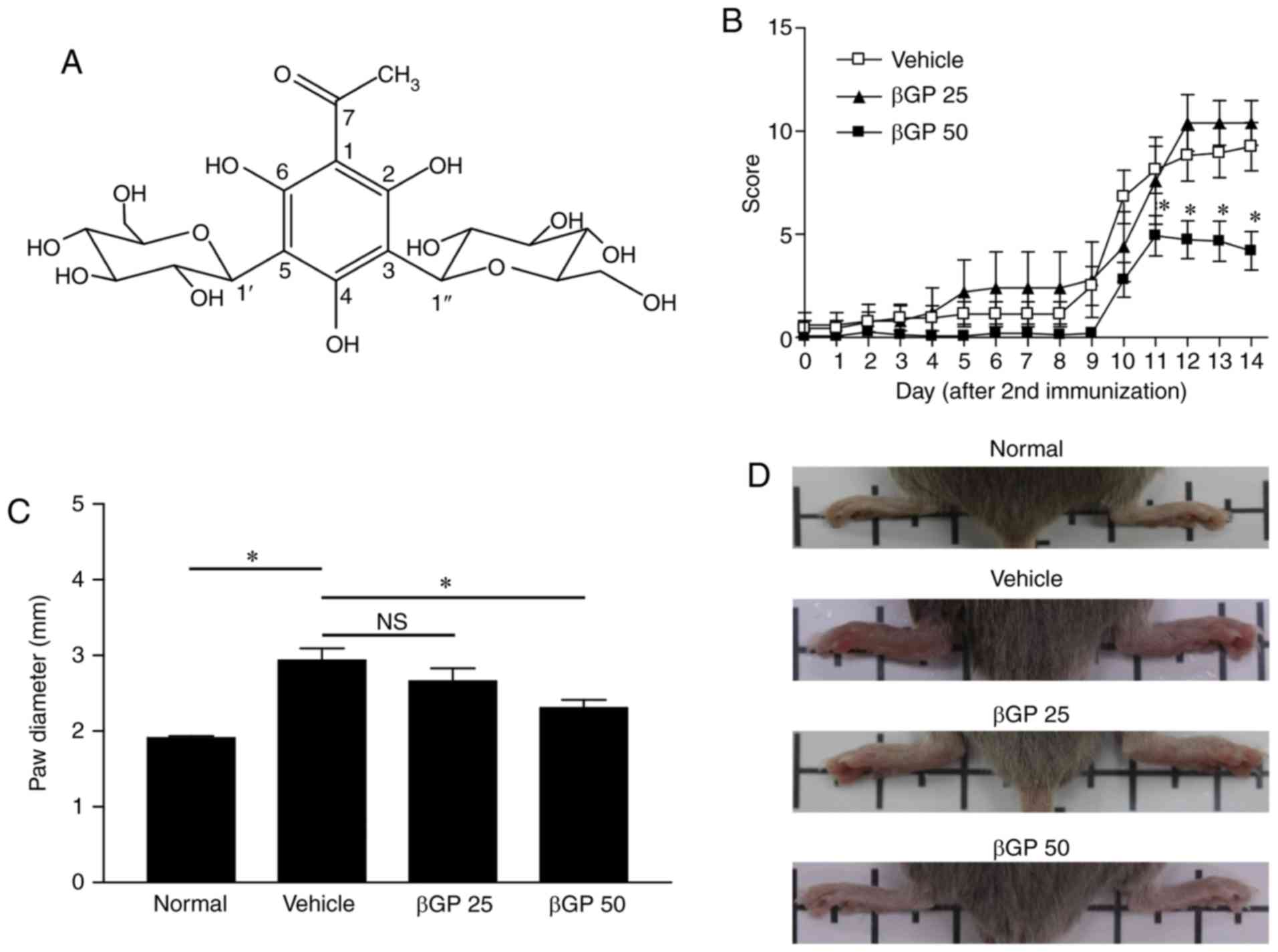 | Figure 1Alleviation of the progression of CIA
by administration of βGP. (A) Chemical structure of βGP, isolated
from Melicope ptelefolia. (B) Mice were intradermally
immunized with 100 µg bovine type II collagen and
administered with either vehicle, βGP 25, or 50 mg/kg βGP (βGP 50)
for 18 days. The clinical arthritis scores were evaluated from 1
day prior to second immunization. (C) Paw diameter in normal and
CIA mice at the end of experiment, measured using calipers. (D)
Images of the rear paws of normal mice, vehicle-treated mice, and
βGP-treated mice. Grouped quantitative data are presented as the
mean ± standard error of the mean (normal group, n=4; vehicle
group, n=16; βGP 25 group, n=5; βGP 50 group, n=15). Significance
was measured using the Tukey-Kramer HSD test following one-way
analysis of variance; *P<0.05. CIA, collagen-induced
arthritis; βGP, 3,5-di-C-β-d-glucopyranosyl
phloroacetophe-none; NS, no significant difference; βGP 25, 25
mg/kg GP; βGP 50, 50 mg/kg βGP. |
βGP: brownish gum; [α]D20+ 56.4 (c 0.3, methanol); UV
λmax (methanol) (log ε) (nm) 234 (3.48), 286
(3.49); IR (KBr) νmax 3,354, 1,621, 1,447, 1,277,
1,048 cm−1; ESIMS m/z 475.1 [M-H2O +
H]+, 493.2 [M + H]+, 491.1 [M-H]-;
1H NMR (800 MHz, DMSO-d6): 9.14 (1H,
s, 6-OH), 4.70 (2H, d, J=10.0 Hz, H-1', 1″), 3.60 (4H, m, H-6′,
6″), 3.47 (2H, t, J=9.0 Hz, H-2′, 2″), 3.33 (2H, t, J = 9.0 Hz,
H-4′, 4″), 3.25 (4H, m, H-3′, 3″, 5′, 5″), 2.60 (3H, s, H-8);
13C NMR (200 MHz, DMSO-d6): 104.8
(C-1), 161.4 (C-2, 6), 104.0 (C-3, 5), 161.3 (C-4), 203.4 (C-7),
33.0 (C-8), 74.6 (C-1′, 1″), 72.1 (C-2′, 2″), 77.8 (C-3′, 3″), 69.2
(C-4′, 4″), 81.1 (C-5′, 5″), 60.0 (C-6′, 6″).
Quantitative analysis of the major
compound βGP from M. ptelefolia
Quantitative analysis was performed with >98%
purity by HPLC and by 1D and 2D NMR spectroscopy following
purification of βGP. HPLC was used to quantitatively analyze βGP in
the water extract of M. ptelefolia and in the 40% ethanol
elute of the HP-20 column using the regression equation
(y=203.8991× − 3.862, R2=0.999). The UV spectrum of the
βGP was set to 300 nm to monitor the phenolic compound. The βGP
peak was set by spiking the sample with a reference standard and a
comparison of its UV, mass spectrum and retention time. The
concentration of βGP in the water extract was found to be 13.3%.
Following elution with 40% ethanol using Diaion HP-20 column
chromatography, the concentration of βGP was increased to
35.5%.
βGP treatment improves collagen-induced
arthritis
The gross score of paw arthritis was significantly
reduced from day 32 in the βGP 50 group compared to that of the
vehicle group (Fig. 1B). Paw size
was also significantly decreased in the βGP 50 group (vehicle
group, vs. βGP 50 group=2.94±0.16, vs. 2.30±0.11). However, the
severity of arthritis was comparable between the vehicle and βGP 25
groups (Fig. 1B and C). In line
with paw diameter, the development of swelling or redness of paws
was reduced in the rear paws of the βGP 50 group at day 35
(Fig. 1D). These results
suggested that βGP had ameliorative effects on CIA. To identify the
presence of βGP in the mice, a single dose of 50 mg/kg βGP was
administered by oral gavage to male ICR mice. βGP was identified in
the plasma and liver at various time points for 48 h following
administration. βGP concentration was determined by LC-MS. βGP was
retained in its original structure until ~4 h in the plasma and
until ~8 h in the liver (data not shown). The levels of blood
biochemical markers were comparable among all groups (Table II) and changes in body weight
were similar between groups (data not shown), suggesting that βGP
did not evoke significant toxicity.
 | Table IIEffects of βGP on plasma biomarkers
in collagen-induced arthritis mice. |
Table II
Effects of βGP on plasma biomarkers
in collagen-induced arthritis mice.
| Group | ALT (IU/l) | AST (IU/l) | BUN (mg/dl) | CHO (mg/dl) | CK (IU/l) | CREA (mg/dl) | TG (mg/dl) |
|---|
| Vehicle | 87.27±13.15 | 148.00±7.19 | 15.40±0.42 | 138.00±2.96 | 482.42±63.84 | 0.26±0.01 | 98.27±8.58 |
| βGP 25 |
43.54±12.15a | 123.69±18.98 | 13.38±0.71b | 131.00±4.10 |
219.17±29.85b | 0.24±0.01 | 85.31±5.07 |
| βGP 50 | 82.00±17.91 | 145.46±17.83 | 15.00±0.77 | 133.54±3.28 |
276.70±61.06b | 0.22±0.02 | 96.31±7.02 |
βGP reduces articular inflammation and
injury in CIA mice
As the βGP 25 group did not show any differences
compared with the vehicle group, further analysis were performed in
the vehicle and βGP 50 groups only. Articular inflammation,
cartilage damage, and bone erosion were the typical symptoms
(21). In H&E staining, the
vehicle group showed synovial membrane hypertrophy with marked
infiltration of immune cells and intra-articular pannus formation.
These observations were lower in the βGP 50 group (Fig. 2A). In line with the H&E
staining results, the histological scores were significantly
reduced in the βGP-treated mice compared with that in
vehicle-treated mice (vehicle, vs. βGP 50 group=1.69±0.25, vs.
0.82±0.24; Fig. 2B). To confirm
the extent of the cartilage damage, the paw tissues were stained
with safranin O and toluidine blue, which identify cartilage
proteoglycans and glycosaminoglycans (22). As shown in Fig. 2C and D, no positive regions for
safranin O (red staining) or toluidine blue (purple staining) were
observed in the vehicle group, but this was markedly recovered by
βGP treatment, indicating the protective effects of βGP on CIA. A
three-dimensional reconstruction of the tarsal bone using
µCT showed decreased bone erosion in the βGP 50 group
compared to that in the vehicle group (Fig. 2E). These results suggested that
βGP improved CIA via the regulation of inflammation, cartilage
injury, and bone erosion in the joints.
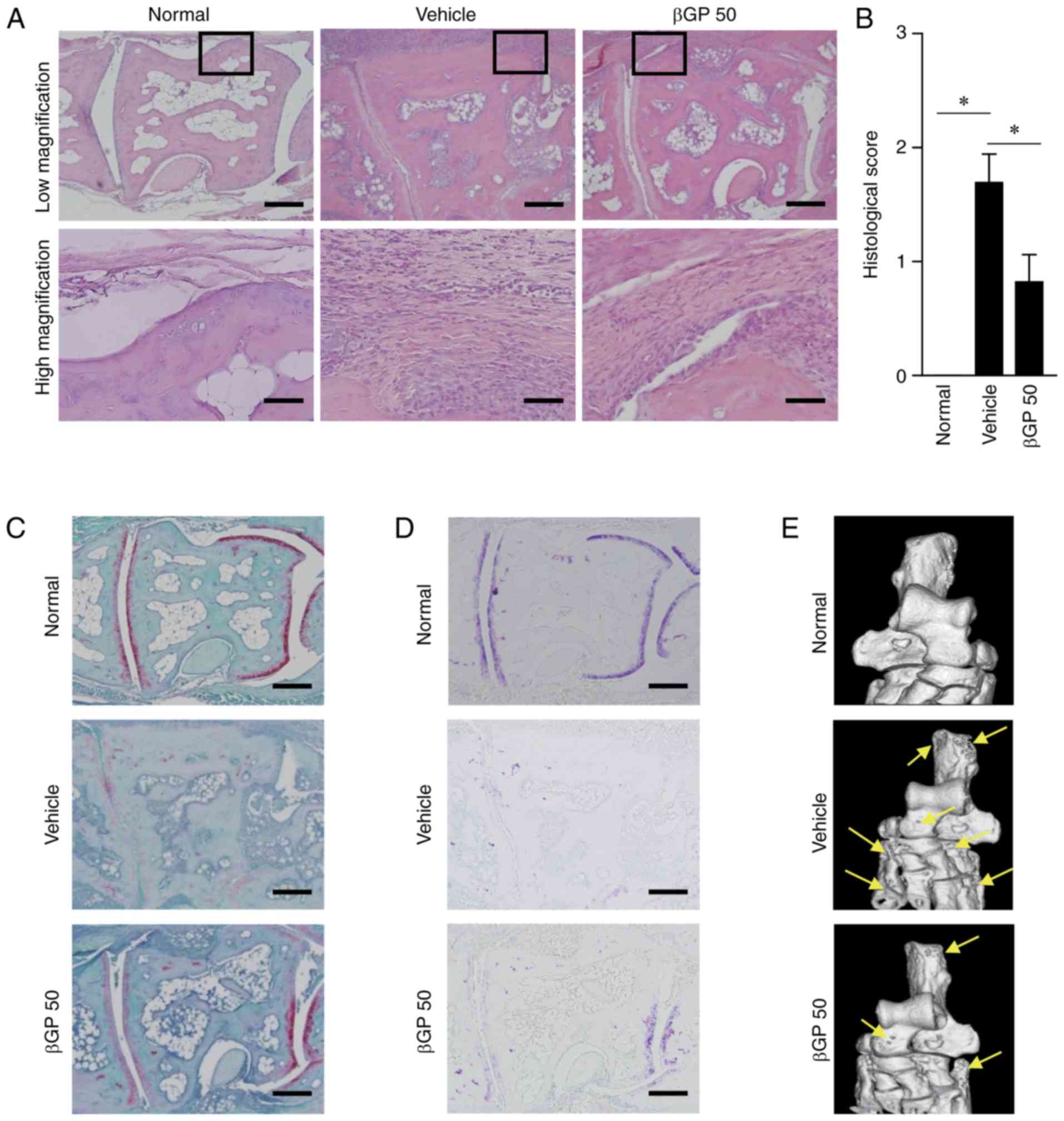 | Figure 2Histological analysis of the
anti-arthritic effects of βGP. At 35 days following the first
immunization, paws were obtained from all CIA mice treated with
either vehicle or βGP. (A) Representative images of mouse paws
stained with H&E (scale bar=200 µm). The region outlined
with a box in each group is shown at a higher magnification beneath
(scale bar=50 µm). (B) Histological scores of the
H&E-stained tissues. Representative images of paws stained with
(C) safranin O and (D) toluidine blue (scale bar, 200 µm).
(E) Representative images of three-dimensional reconstructions of
the tarsal bone at 35 days in each group. The arrows point to
regions of bone erosion. Grouped quantitative data are presented as
the mean ± standard error of the mean (normal group, n=4; vehicle
group, n=13; βGP 50 group, n=14). A Wilcoxon test was used to
compare the vehicle group with the βGP groups;
*P<0.05. CIA, collagen-induced arthritis; βGP,
3,5-di-C-β-d-glucopyranosyl
phloroacetophenone; βGP 50, 50 mg/kg βGP; H&E, hematoxylin and
eosin. |
βGP does not modulate autoantibody
production in CIA mice
Subsequently, how βGP affects inflammatory arthritis
in CIA mice was investigated. The increased production of
anti-collagen antibodies by the humoral immune system is a crucial
factor for inducing RA (23).
Therefore, to confirm whether βGP can regulate humoral immunity,
the present study analyzed the levels of anti-type II collagen
antibody in the plasma of CIA mice using ELISA. The levels of total
IgG and its subclasses, IgG1 and IgG2a, did not differ
significantly between the vehicle and the βGP 50 groups (Fig. 3). These results suggested that the
therapeutic effects of βGP on CIA were not associated to the
regulation of autoantibody production.
βGP decreases articular macrophage
infiltration and inflammatory cytokine expression in CIA mice
The degree of synovial macrophage infiltration is
correlated with the severity of joint erosion in RA (24). Therefore, the present study
investigated whether βGP influences articular macrophage
infiltration in CIA mice. Gene expression levels of the macrophage
marker Cd68 and leukocyte common antigen Cd45 were
significantly reduced in the paws of the βGP 50 group compared with
those of the vehicle group (Fig.
4A). Additionally, the gene expression levels of IL-6,
leukemia inhibitory factor (LIF) and IL-10 were
significantly decreased by βGP treatment. The gene expression of
TNF-α was also found to be reduced in the paws of the βGP 50
group (Fig. 4B). These results
suggested that βGP downregulated macrophage infiltration and the
levels of inflammatory cytokines in the paws of CIA mice.
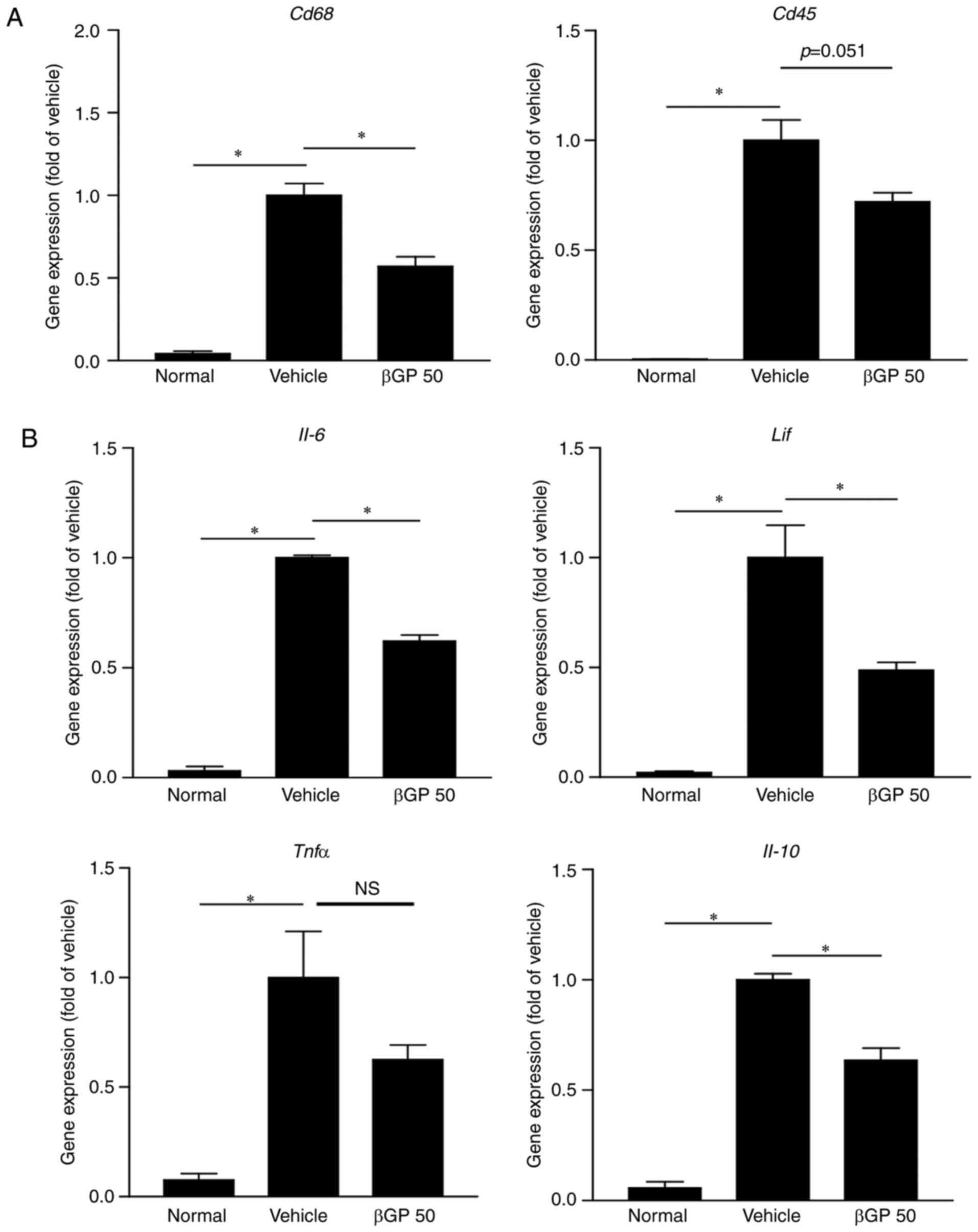 | Figure 4βGP influences gene expression of
pro-inflammatory cytokines in CIA mice. At 35 days following the
first immunization, paws were obtained from all CIA mice treated
with either vehicle or βGP, and gene expression levels were
analyzed by reverse transcription-quantitative polymerase chain
reaction analysis. (A) Gene expression levels of Cd68, a
macrophage marker, and Cd45, a common leukocyte antigen. (B)
Gene expression levels of IL-6, LIF, TNFα, and IL-10.
The vehicle group was set to a value of 1. Its average fold-change
is shown. Grouped quantitative data are presented as the mean ±
standard error of the mean (normal group, n=4; vehicle group, n=5;
βGP 50 group, n=5). Significance was measured using the
Tukey-Kramer HSD test following one-way analysis of variance;
*P<0.05. CIA, collagen-induced arthritis; βGP,
3,5-di-C-β-d-glucopyranosyl
phloroacetophenone; βGP 50, 50 mg/kg βGP; IL, interleukin;
LIF, leukocyte inhibitory factor; TNFα, tumor
necrosis factor-α; NS, no significant difference. |
βGP does not affect inflammatory cytokine
expression in macrophages
Although macrophages alone do not cause RA, they are
important in inflammation and in bone and cartilage erosion
(25). The activation and
infiltration of macrophages in the joints and in the synovial
membrane is crucial for the progression of RA (26). Previous studies and the results of
the present study prompted the investigation of the direct effect
of βGP on macrophages. To confirm whether βGP can regulate
inflammation by controlling macrophages, the LPS-stimulated mouse
macrophage cell line, RAW264.7, was treated with βGP. Neither the
LPS-induced elevation of gene expression nor protein secretion of
cytokines in RAW264.7 cells, except for TNF-α gene expression, was
affected by βGP treatment (Fig. 5A
and B). This result suggested that βGP does not directly
regulate the production of inflammatory cytokines in
macrophages.
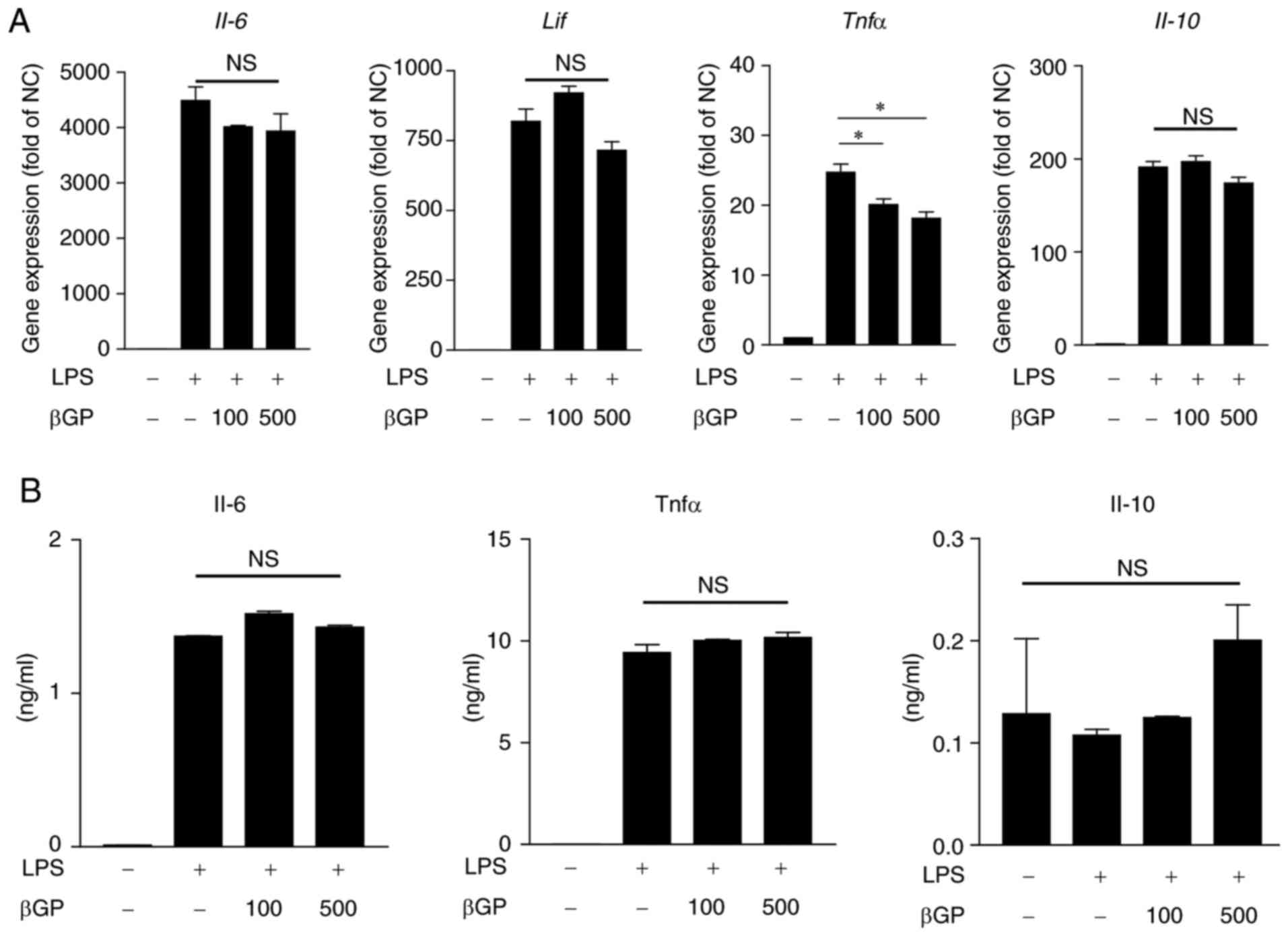 | Figure 5Effects of βGP on pro-inflammatory
cytokine changes in LPS-stimulated RAW 264.7 cells. RAW 264.7 cells
were pre-treated with different concentrations of βGP (0-500
µg/ml) for 1 h, and stimulated with either 100 ng/ml LPS or
vehicle for 6 h. (A) Gene expression levels of Il-6,
Lif, Tnfα, and Il-10 were analyzed by reverse
transcription-quantitative polymerase chain reaction analysis. The
vehicle group was set to a value of 1. Its average fold-change is
shown. (B) Protein levels of IL-6, TNFα, and IL-10 in the cell
culture supernatant were measured by ELISA. Grouped quantitative
data are presented as the mean ± standard error of the mean
(triplicate of RAW264.7 cell lysate cDNA samples and duplicate of
RAW264.7 cell culture supernatant). Significance was measured using
the Tukey-Kramer HSD test following one-way analysis of variance;
*P<0.05. βGP, 3,5-di-C-β-d-glucopyranosyl
phloroacetophenone; IL, interleukin; LIF, leukocyte inhibitory
factor; TNFα, tumor necrosis factor-α; LPS, lipopolysaccharide; NC,
negative control; NS, no significant difference. |
βGP regulates the expression of RANKL
following IL-6 and LIF modulation in fibroblast cells
It has been reported that synovial fibroblasts are a
source of inflammatory cytokines and are critical in RA through the
regulation of RANKL-mediated osteoclast activation (27). Among cytokines, IL-6 and its
family, LIF, regulate the expression of RANKL in synovial
fibroblast cells through autocrine or paracrine modes (27). In addition, IL-6 and LIF-dependent
activation of STAT3 is essential for the expression of RANKL in
fibroblast cells (27,28). As shown in Fig. 6A and B, the TNFα-stimulated
increases in IL-6 and LIF were significantly reduced
by βGP co-treatment in MEFs. In addition, βGP markedly reduced the
phosphorylation of STAT3 and significantly decreased the gene
expression of RANKL in the TNFα-treated MEFs (Fig. 6C and D). It has been reported
that, in the progression of RA, synovial fibroblasts proliferate,
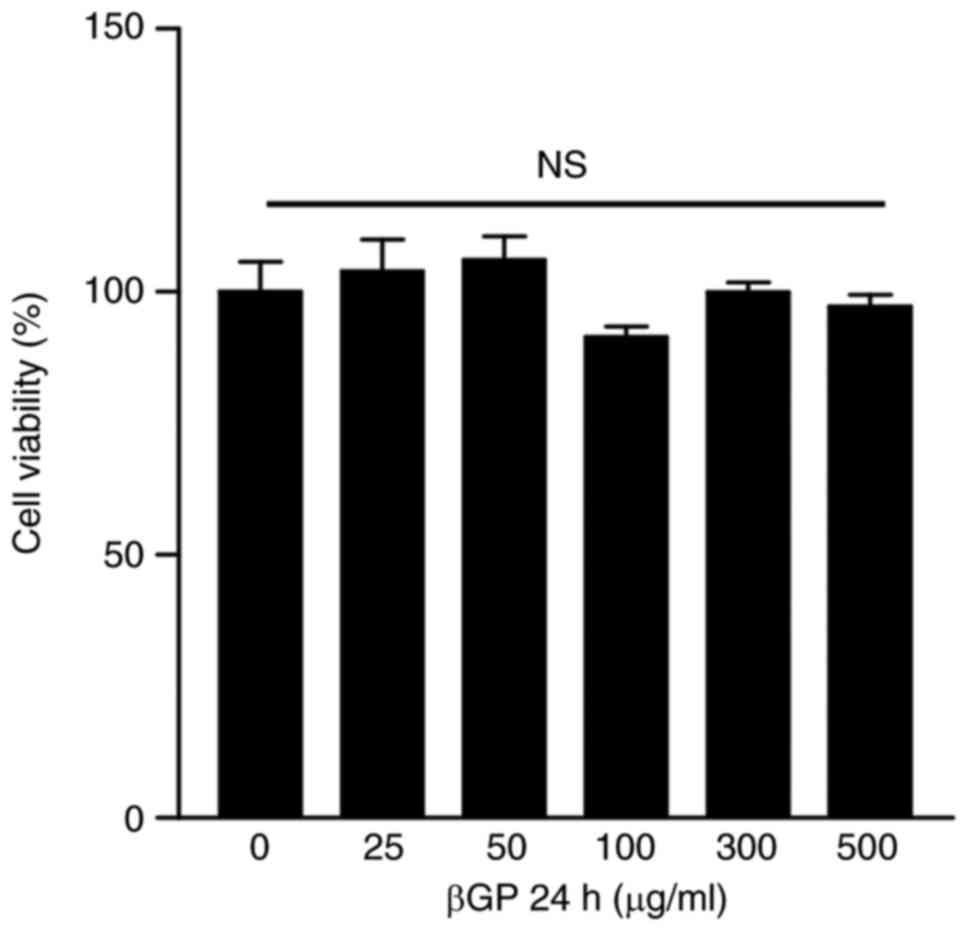
become activated, and invade and destroy adjacent cartilages
(29). Therefore, the present
study examined the cytotoxicity and effect on cell proliferation of
βGP in NIH/3T3 fibroblasts. However, βGP treatment did not induce
any significant changes compared with the control group (Fig. 7). These results suggested that βGP
may regulate the expression of RANKL through inhibiting the
expression of IL-6 and LIF in synovial fibroblast
cells.
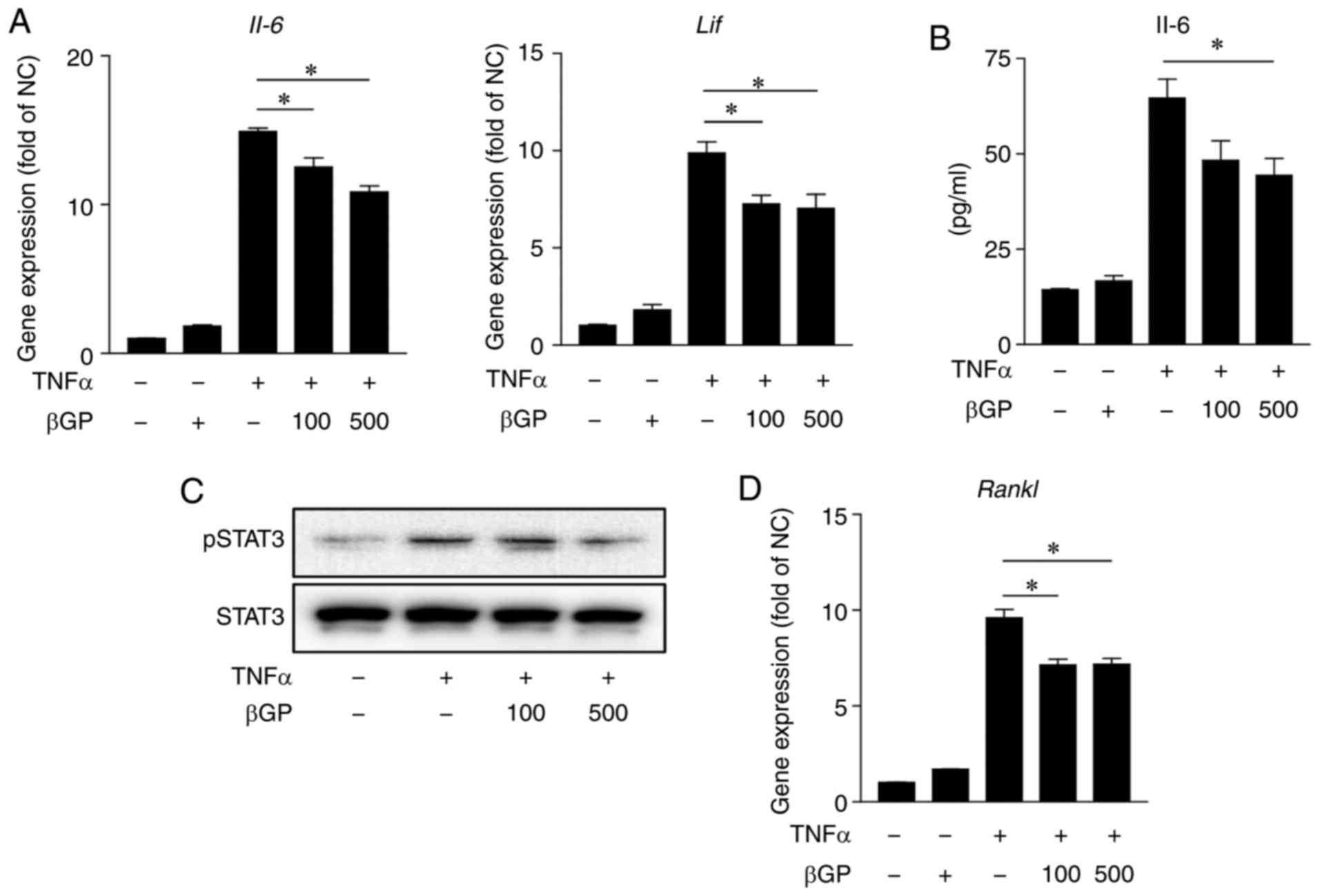 | Figure 6βGP downregulates TNFα-induced
inflammation and expression of RANKL in MEFs. MEFs were pre-treated
with different concentrations of βGP (0-500 µg/ml) for 1 h
and then stimulated with either 40 ng/ml TNFα or vehicle for 6 h.
(A) Gene expression levels of IL-6 and LIF were
analyzed by RT-qPCR analysis. The vehicle group was set to a value
of 1. Its average fold-change is shown. (B) Protein levels of IL-6
in the cell culture supernatant were measured by ELISA. (C) Protein
levels of pSTAT3 and STAT3 in the MEF cell lysate were analyzed by
western blot analysis. (D) Gene expression levels of Rankl
were analyzed by RT-qPCR analysis. The vehicle group was set to a
value of 1. Its average fold-change is shown. Grouped quantitative
data are presented as the mean ± standard error of the mean
(triplicate of MEFs lysate cDNA samples and triplicate of MEF cell
culture supernatant). Significance was measured using the
Tukey-Kramer HSD test following one-way analysis of variance;
*P<0.05. βGP, 3,5-di-C-β-d-glucopyranosyl
phloroacetophenone; IL, interleukin; LIF, leukocyte inhibitory
factor; TNFα, tumor necrosis factor-α; RANKL, receptor activator of
nuclear factor-κB ligand; STAT3, signal transducer and activator of
transcription 3; pSTAT3, phosphorylated STAT3; MEFs, mouse
embryonic fibroblasts; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction. |
βGP improves CIA through the regulation
of RANKL- dependent osteoclast activation in mice
Periarticular bone erosion and generalized bone loss
are hallmarks of RA. It has been reported that osteoclasts, cells
specialized in bone resorption, are important for the progression
of joint erosion in RA (30). In
addition, it is well known that RANKL, an osteoclast activator, is
promoted by phosphorylated STAT3 (31). In accordance with the results in
the MEFs (Fig. 6), treatment with
βGP significantly reduced the phosphorylation of STAT3 and gene
expression of Rankl in the paws of CIA mice (Fig. 8A and B). Accordingly, osteoclast
markers, including tartrate-resistant acid phosphatase, osteoclast
associated receptor, cathepsin K, and calcitonin receptor, were
also significantly reduced in the βGP 50 group compared to those in
the vehicle group (Fig. 8C).
These results suggested that βGP can improve CIA through
RANKL-dependent osteoclast regulation.
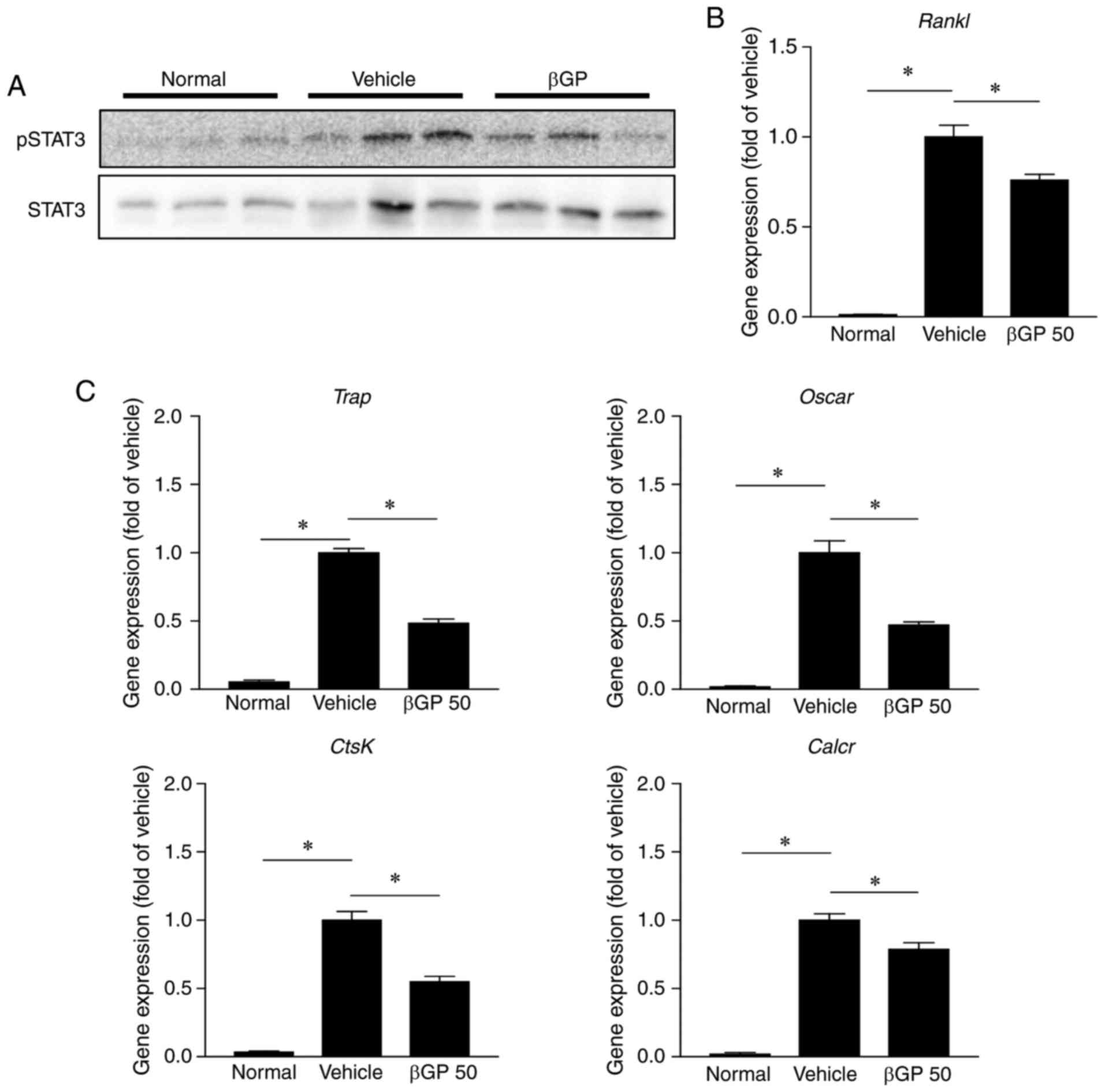 | Figure 8βGP influences osteoclast activity in
CIA mice. At 35 days following the first immunization, paws were
obtained from each CIA mouse treated with either vehicle or βGP.
(A) Protein levels of pSTAT3 and STAT3 in the paw were analyzed by
western blot analysis. (B) Gene expression levels of Rankl
in the paw were analyzed by RT-qPCR analysis. The vehicle group was
set to a value of 1. Its average fold-change is shown. (C) Gene
expression levels of osteoclast activity markers in the paw were
analyzed by RT-qPCR analysis. The vehicle group was set to a value
of 1. Its average fold-change is shown. Grouped quantitative data
are presented as the mean ± standard error of the mean (normal
group, n=4; vehicle group, n=5; βGP 50 group, n=5). Significance
was measured using the Tukey-Kramer HSD test following one-way
analysis of variance; *P<0.05. βGP,
3,5-di-C-β-d-glucopyranosyl
phloroacetophenone; STAT3, signal transducer and activator of
transcription 3; pSTAT3, phosphorylated STAT3; Rankl,
receptor activator of nuclear factor-κB ligand; Trap,
tartrate-resistant acid phosphatase; Oscar, osteoclast
associated receptor; CtsK, cathepsin K; Calcr,
calcitonin receptor. |
Discussion
The present study demonstrated the anti-rheumatic
potential of βGP as an active compound in the water extract of
M. ptelefolia. When βGP was administered to CIA mice, their
arthritis was significantly relieved, demonstrated by a reduced
gross and histologic arthritic score, decreased inflammatory
cytokines and expression of RANKL, and decreased expression of
osteoclast markers in the paws of mice.
RA is a systemic autoimmune disease characterized by
the presence of autoantibodies, and this is used as a diagnostic
marker for RA. Autoantibodies not only react with synovial
components but also activate T and B cells (6). Cytokines derived from T helper (Th)
cells enhance B cell differentiation into Ig-secreting plasma cells
and accelerate the production of autoantibodies in patients with RA
(32). Among the cytokines, Th2
cytokines regulate the switch from IgM/D to IgG1 and IgE, and Th1
cytokines induce IgG2a secretion in activated B cells (33). In addition, further insight into
the importance of B cells in the pathogenesis of RA is highlighted
by the efficacy of B cell depletion (34). However, in the present study, the
plasma levels of anti-type II collagen autoantibodies, including
total IgG, IgG1 and IgG2a, were not altered by the βGP
administration in CIA mice. These results demonstrate that the
therapeutic effects of βGP were not associated with the modulation
of B cell-derived autoantibody regulation in the context of
CIA.
Cytokines are directly implicated in several of the
immune processes that are associated with the pathogenesis of RA.
Numerous cytokines are expressed and are functionally active in the
synovial tissues of patients with RA (35). Accordingly, cytokine modulation
alters the outcome of several RA rodent models (36). Of note, TNF is targeted in the
standard treatment of patients with RA, and other cytokines,
including the IL-6 family (IL-6 and LIF), are also being
investigated as targets in the clinic, with promising results
(37,38). In the present study, βGP decreased
the expression levels of TNFα, IL-6 and LIF in
the paws of CIA mice. Of note, the expression of IL-10 was
also reduced by βGP administration, however, it was interpreted
that the expression of IL-10 may have decreased subsequent
to the βGP-mediated amelioration of articular inflammation. These
results suggest that βGP can improve RA through the modulation of
inflammatory cytokine levels in the joints.
It has been reported that cytokines derived from
macrophages and synovial fibroblasts, including TNFα and IL-6,
respectively, are important in RA pathogenesis (39). In the present study, to identify a
specific target cell of βGP, the effects of βGP on an LPS-treated
macrophage cell line and TNFα-stimulated MEFs were examined. βGP
did not regulate inflammatory cytokine levels in macrophages
following LPS treatment. However, IL-6 and LIF were
significantly decreased by βGP treatment in the TNFα-stimulated
MEFs. These results suggested that the inhibitory effects of βGP on
cytokine production in synovial fibroblasts may partially result in
decreased levels of cytokines in the paws of CIA mice. Although βGP
did not regulate the production of inflammatory cytokines in
macrophages in vitro, macrophage markers were markedly
reduced by βGP treatment in the paws of CIA mice. These results
suggested that the reduction of cytokines in the paws of
βGP-treated mice was not solely dependent on the direct modulation
of synovial fibroblasts and may result from a decrease of
infiltrated macrophages in the joints of CIA mice. Further
investigations are required to elucidate the effects of βGP on
macrophage migration into the joints of CIA mice and its mechanism
of action.
The compound βGP has a similar structure to aspirin,
which was derived from willow (Salix) bark and is one of the
most ideal drugs. Unlike aspirin, which has an acetyl group at
position 2, βGP has two sugars at positions 3 and 5, which gives
βGP a higher polarity than aspirin. In addition, the presence of
the two sugars in βGP is considered to be non-absorbable through
the brain barrier as a larger molecule. Therefore, the effects of
βGP may be driven not by central nervous system regulation, but by
peripheral nervous system modulation, considering the targeted
mechanism of βGP through the local mode of action of aspirin. The
principle mechanism considered to be responsible for the
anti-inflammatory effects of aspirin is the inhibition of
cyclooxygenase (COX)-dependent prostaglandin (PG) synthesis
(40). It has been reported that
aspirin inhibits the secretion of IL-6 through COX-dependent PG
regulation in adipose tissue (41). In addition, TNFα accelerates the
transactivation and COX-2 increases PG release in human gingival
fibroblasts (42). Taken
together, it is possible that aspirin regulates TNFα-stimulated
IL-6 induction via the downregulation of COX-dependent PG synthesis
in nonimmune cells. In the present study, the gene expression of
Tnfα was markedly increased in the paws of CIA mice,
compared with that in normal mice. Although the elevated level of
TNFα was similar between the vehicle and βGP groups, the level of
Il-6 was significantly reduced by βGP administration in the
paw. In addition, βGP treatment significantly decreased the
expression of IL-6 in the TNFα-stimulated MEFs and in the paws of
CIA mice. Although the exact mechanism of βGP in CIA was not
confirmed, the aspirin-like skeleton characteristics in compound
βGP suggested the possibility that βGP may target and inhibit the
COX-induced upregulation of PG and the subsequent downregulation of
IL-6.
Osteoclasts are terminally differentiated cells of
the monocyte/macrophage lineage that resorb bone matrix. Bone
destruction in RA is mainly attributable to the abnormal activation
of osteoclasts (43). Osteoclast
formation, activation and survival are mainly regulated by RANKL
(44). Among the cytokines, IL-6
and LIF upregulate the expression of RANKL in RA synovial
fibroblasts through the Janus kinase 2/STAT3 signaling pathway
(45,46). Therefore, osteoclast regulation
via inhibiting STAT3-dependent expression of RANKL is an efficient
way to treat RA (47,48). In the present study, βGP
significantly reduced the expression levels of IL-6 and LIF in
TNFα-stimulated MEFs and in the paws of CIA mice. Furthermore,
phosphorylated STAT3 and the expression of RANKL were significantly
reduced by βGP treatment, followed by decreased expression levels
of osteoclast markers. These results suggest that βGP modulates
osteoclast activity via regulation of the STAT3-dependent
expression of RANKL in the paws of CIA mice.
In terms of βGP metabolism in vivo, the
present study found that βGP remained until ~4 h and 8 h in the
plasma and the liver. Although detailed analysis of βGP metabolism
was not performed, the results suggested the possible rapid
metabolism of this compound. Future investigations aim to further
elucidate the metabolism of βGP.
In conclusion, the present study is the first
study, to the best of our knowledge, to demonstrate that the
compound βGP had therapeutic effects against RA in mice. βGP
suppressed osteoclasts by reducing the STAT3-mediated induction of
RANKL following inhibition of the expression of IL-6 and LIF in
synovial fibroblasts. The results of the present study provide
novel insights into the possibility of βGP and M. ptelefolia
active fraction applications as therapeutics to prevent RA.
Funding
This study was supported by grants from the Korea
Bioactive Natural Material Bank (grant no. NRF-2017M3A9B8069409)
and the Bio & Medical Technology Development Program (grant no.
NRF-2016K1A1A8A01939075) of the National Research Foundation, which
were funded by the Korean government (Ministry of Science and ICT)
and from the KRIBB Research Initiative Program of the Republic of
Korea.
Availability of data and materials
The datasets generated in the present study are not
currently available to the public but will be available from the
corresponding author upon reasonable request.
Authors' contributions
HJK, CHL, WKO, and YHK designed the experiments and
the study. HJK, JHC, DHC, SWK, JYL, JGP, and YHK collected the data
and performed experiments for the study. HJK, JRN, SJM, HYK, SWK,
JYL, JGP, and YHK analyzed the data. JHH, KSK, SC, SME, TTB, JR,
SRO, and CHL provided critical revisions to the text. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the
Institutional Animal Care and Use Committee of the Korea Research
Institute of Bioscience and Biotechnology (KRIBB-AEC-18001) and
were performed in accordance with the Guide for the Care and Use of
Laboratory Animals published by the US National Institutes of
Health.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Mr. I.B. Lee, Mr.
Y.K. Choi, Mrs. Y.J. Seo and Mrs. J.H. Choi (Laboratory Animal
Resource Centre, Korea Research Institute of Bioscience and
Biotechnology, Daejeon, Korea) for their technical assistance.
Abbreviations:
|
βGP
|
3,5-di-C-β-d-glucopyranosyl phloroace
tophenone
|
|
CIA
|
collagen-induced arthritis
|
|
H&E
|
hematoxylin and eosin
|
|
M. ptelefolia
|
Melicope ptelefolia
|
|
MPPE
|
Melicope ptelefolia ethanol
extract
|
|
µCT
|
micro-computed tomography
|
|
MEFs
|
mouse embryonic fibroblasts
|
|
RANKL
|
receptor activator of nuclear
factor-κB ligand
|
|
RA
|
rheumatoid arthritis
|
|
Th
|
T helper
|
References
|
1
|
Myasoedova E, Crowson CS, Kremers HM,
Therneau TM and Gabriel SE: Is the incidence of rheumatoid
arthritis rising? Results from olmsted county, minnesota,
1955–2007. Arthritis Rheum. 62:1576–1582. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang SH, Ping LF, Sun FY, Wang XL and Sun
ZJ: Protective effect of taraxasterol against rheumatoid arthritis
by the modulation of inflammatory responses in mice. Exp Ther Med.
12:4035–4040. 2016. View Article : Google Scholar
|
|
3
|
Xu H, Wang J, Wang C, Chang G, Lin Y,
Zhang H, Zhang H, Li Q and Pang T: Therapeutic effects of
micheliolide on a murine model of rheumatoid arthritis. Mol Med
Rep. 11:489–493. 2015. View Article : Google Scholar
|
|
4
|
Choy E: Understanding the dynamics:
Pathways involved in the pathogenesis of rheumatoid arthritis.
Rheumatology (Oxford). 5(Suppl 51): pp. v3–v11. 2012, View Article : Google Scholar
|
|
5
|
Shin JS, Yun CH, Chung KS, Bang MH, Baek
NI, Chung HG, Cho YW and Lee KT: Standardized ethyl acetate
fraction from the roots of brassica rapa attenuates the
experimental arthritis by down regulating inflammatory responses
and inhibiting NF-κB activation. Food Chem Toxicol. 66:96–106.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song YW and Kang EH: Autoantibodies in
rheumatoid arthritis: Rheumatoid factors and anticitrullinated
protein antibodies. QJM. 103:139–146. 2010. View Article : Google Scholar :
|
|
7
|
Hashizume M, Hayakawa N and Mihara M: IL-6
trans-signalling directly induces RANKL on fibroblast-like synovial
cells and is involved in RANKL induction by TNF-alpha and IL-17.
Rheumatology (Oxford). 47. pp. 1635–1640. 2008, View Article : Google Scholar
|
|
8
|
Weitzmann MN: The role of inflammatory
cytokines, the RANKL/OPG axis, and the immunoskeletal interface in
physiological bone turnover and osteoporosis. Scientifica (Cairo).
2013:1257052013.
|
|
9
|
Srirangan S and Choy EH: The role of
interleukin 6 in the pathophysiology of rheumatoid arthritis. Ther
Adv Musculoskelet Dis. 2:247–256. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kwan Tat S, Padrines M, Théoleyre S,
Heymann D and Fortun Y: IL-6, RANKL, TNF-alpha/IL-1: Interrelations
in bone resorption pathophysiology. Cytokine Growth Factor Rev.
15:49–60. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Charles JF and Aliprantis AO: Osteoclasts:
More than 'bone eaters'. Trends Mol Med. 20:449–459. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kamperdick C, Van NH, Sung TV and Adam G:
Benzopyrans from Melicope ptelefolia leaves. Phytochemistry.
45:1049–1056. 1997. View Article : Google Scholar
|
|
13
|
Hong Van N, Kamperdick C, Van Sung T and
Adam G: Benzopyran dimers from Melicope ptelefolia. Phytochemistry.
48:1055–1057. 1998. View Article : Google Scholar
|
|
14
|
Nguyen NH, Ha TK, Choi S, Eum S, Lee CH,
Bach TT, Chinh VT and Oh WK: Chemical constituents from Melicope
pteleifolia leaves. Phytochemistry. 130:291–300. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sulaiman MR, Mohd Padzil A, Shaari K,
Khalid S, Shaik Mossadeq WM, Mohamad AS, Ahmad S, Akira A, Israf D
and Lajis N: Antinociceptive activity of Melicope ptelefolia
ethanolic extract in experimental animals. J Biomed Biotechnol.
2010:9376422010. View Article : Google Scholar
|
|
16
|
Loi DT: Nhung cay Thuoc va vi thuoc Viet
Nam (Glossary of Vietnamese Medicinal Plants). Science and Technics
Publication; Hanoi, Vietnam: 1977
|
|
17
|
Perry LM and Metzger J: Medicinal Plants
of East and Southeast Asia: Attributed Properties and Uses. MIT
Press; Cambridge, UK: 1980
|
|
18
|
Lee CH, Bae SJ and Kim M:
Mucosa-associated lymphoid tissue lymphoma translocation 1 as a
novel therapeutic target for rheumatoid arthritis. Sci Rep.
7:118892017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun J, Jia Y, Li R, Guo J, Sun X, Liu Y,
Li Y, Yao H, Liu X, Zhao J and Li Z: Altered influenza virus
haemagglutinin (HA)-derived peptide is potent therapy for CIA by
inducing Th1 to Th2 shift. Cell Mol Immunol. 8:348–358. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Schett G and Gravallese E: Bone erosion in
rheumatoid arthritis: Mechanisms, diagnosis and treatment. Nat Rev
Rheumatol. 8:656–664. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schmitz N, Laverty S, Kraus VB and Aigner
T: Basic methods in histopathology of joint tissues. Osteoarthritis
Cartilage. 3(Suppl 18): S113–S116. 2010. View Article : Google Scholar
|
|
23
|
van Boekel MA, Vossenaar ER, van den
Hoogen FH and van Venrooij WJ: Autoantibody systems in rheumatoid
arthritis: Specificity, sensitivity and diagnostic value. Arthritis
Res. 4:87–93. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yanni G, Whelan A, Feighery C and
Bresnihan B: Synovial tissue macrophages and joint erosion in
rheumatoid arthritis. Ann Rheum Dis. 53:39–44. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kinne RW, Bräuer R, Stuhlmüller B,
Palombo-Kinne E and Burmester GR: Macrophages in rheumatoid
arthritis. Arthritis Res. 2:189–202. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Davignon JL, Hayder M, Baron M, Boyer JF,
Constantin A, Apparailly F, Poupot R and Cantagrel A: Targeting
monocytes/macrophages in the treatment of rheumatoid arthritis.
Rheumatology (Oxford). 52. pp. 590–598. 2013, View Article : Google Scholar
|
|
27
|
Baumann H and Kushner I: Production of
interleukin-6 by synovial fibroblasts in rheumatoid arthritis. Am J
Pathol. 152:641–644. 1998.PubMed/NCBI
|
|
28
|
Palmqvist P, Persson E, Conaway HH and
Lerner UH: IL-6, leukemia inhibitory factor, and oncostatin M
stimulate bone resorption and regulate the expression of receptor
activator of NF-kappa B ligand, osteoprotegerin, and receptor
activator of NF-kappa B in mouse calvariae. J Immunol.
169:3353–3362. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ospelt C: Synovial fibroblasts in 2017.
RMD Open. 3:e0004712017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Durand M, Boire G, Komarova SV, Dixon SJ,
Sims SM, Harrison RE, Nabavi N, Maria O, Manolson MF, Mizianty M,
et al: The increased in vitro osteoclastogenesis in patients with
rheumatoid arthritis is due to increased percentage of precursors
and decreased apoptosis-the In vitro osteoclast differentiation in
arthritis (IODA) study. Bone. 48:588–596. 2011. View Article : Google Scholar
|
|
31
|
Hikata T, Takaishi H, Takito J, Hakozaki
A, Furukawa M, Uchikawa S, Kimura T, Okada Y, Matsumoto M,
Yoshimura A, et al: PIAS3 negatively regulates RANKL-mediated
osteoclastogenesis directly in osteoclast precursors and indirectly
via osteoblasts. Blood. 113:2202–2212. 2009. View Article : Google Scholar
|
|
32
|
Cope AP, Schulze-Koops H and Aringer M:
The central role of T cells in rheumatoid arthritis. Clin Exp
Rheumatol. 25(Suppl 46): S4–S11. 2007.PubMed/NCBI
|
|
33
|
Kaplan C, Valdez JC, Chandrasekaran R,
Eibel H, Mikecz K, Glant TT and Finnegan A: Th1 and Th2 cytokines
regulate proteoglycan-specific autoantibody isotypes and arthritis.
Arthritis Res. 4:54–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Edwards JC, Szczepanski L, Szechinski J,
Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM and Shaw T:
Efficacy of B-Cell-targeted therapy with rituximab in patients with
rheumatoid arthritis. N Engl J Med. 350:2572–2281. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Brennan F and Foey A: Cytokine regulation
in RA synovial tissue: Role of T cell/macrophage contact-dependent
interactions. Arthritis Res. 3(Suppl 4): S177–S182. 2002.
View Article : Google Scholar
|
|
36
|
Alves CH, Farrell E, Vis M, Colin EM and
Lubberts E: Animal models of bone loss in inflammatory arthritis:
From cytokines in the bench to novel treatments for bone loss in
the bedside-a comprehensive review. Clin Rev Allergy Immunol.
51:27–47. 2016. View Article : Google Scholar
|
|
37
|
Siebert S, Tsoukas A, Robertson J and
McInnes I: Cytokines as therapeutic targets in rheumatoid arthritis
and other inflammatory diseases. Pharmacol Rev. 67:280–309. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
St C: Interleukin 10 treatment for
rheumatoid arthritis. Ann Rheum Dis. 58(Suppl 1): I99–I102. 1999.
View Article : Google Scholar
|
|
39
|
Brzustewicz E and Bryl E: The role of
cytokines in the pathogenesis of rheumatoid arthritis-practical and
potential application of cytokines as biomarkers and targets of
personalized therapy. Cytokine. 76:527–536. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vane JR: Inhibition of prostaglandin
synthesis as a mechanism of action for aspirin-like drugs. Nat New
Biol. 231:232–235. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ogston NC, Karastergiou K,
Hosseinzadeh-Attar MJ, Bhome R, Madani R, Stables M, Gilroy D,
Flachs P, Hensler M, Kopecky J and Mohamed-Ali V: Low-dose
acetylsalicylic acid inhibits the secretion of interleukin-6 from
white adipose tissue. Int J Obes (Lond). 32:1807–1815. 2008.
View Article : Google Scholar
|
|
42
|
Nakao S, Ogtata Y, Shimizu E, Yamazaki M,
Furuyama S and Sugiya H: Tumor necrosis factor alpha
(TNF-alpha)-induced prostaglandin E2 release is mediated by the
activation of cyclooxygenase-2 (COX-2) transcription via NFkappaB
in human gingival fibroblasts. Mol Cell Biochem. 238:11–18. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jones DH, Kong YY and Penninger JM: Role
of RANKL and RANK in bone loss and arthritis. Ann Rheum Dis.
2(Suppl 61): ii32–ii39. 2002. View Article : Google Scholar
|
|
44
|
Feng X: RANKing intracellular signaling in
osteoclasts. IUBMB Life. 57:389–395. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Boyce BF and Xing L: Functions of
RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem
Biophys. 473:139–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tawara K; Oxford JT; Jorcyk CL: Clinical
significance of interleukin (IL)-6 in cancer metastasis to bone:
Potential of anti-IL-6 therapies. Cancer Manag Res. 3:177–189.
2011.PubMed/NCBI
|
|
47
|
Miyamoto T, Mori T, Yoshimura A and Toyama
T: STAT3 is critical to promote inflammatory cytokines and RANKL
expression in inflammatory arthritis. Arthritis Res Ther. 14(Suppl
1): P432012. View
Article : Google Scholar :
|
|
48
|
O'Brien CA, Gubrij I, Lin SC, Saylorsi RL
and Manolagas SC: STAT3 activation in stromal/osteoblastic cells is
required for induction of the receptor activator of NF-κB ligand
and stimulation of osteoclastogenesis by gp130-utilizing cytokines
or interleukin-1 but not 1,25-dihydroxyvitamin d3 or parathyroid
hormone. J Biol Chem. 274:19301–19308. 1999. View Article : Google Scholar : PubMed/NCBI
|