Introduction
Metastasis is the main reason of poor prognosis in
gastric cancer patients (1). The
roles of various key factors in cancer metastasis continue to be
evaluated, and targeted drug discovery and selection are important
for preventing and treating metastatic cancer (2). The results of a recent study have
suggested that the epithelial-mesenchymal transition (EMT) is a key
step in tumor metastasis (3). EMT
represents the loss of cell polarity and cell-cell adhesion by
epithelial cells, which results in transformation to a mesenchymal
phenotype, as well as migration and invasion, providing a mechanism
for cancer development (4).
Peritoneal metastasis of cancer cells occurs due to decreased
adhesion between cells, resulting in primary cancer cell lesions
breaking free from the serosa in the abdominal cavity.
The tissue oxygen balance is an important condition
for the regulation of cell proliferation and survival (5), while hypoxia is one of the most
important initiators of EMT during metastasis. Hypoxia-inducible
factor 1α (HIF-1α) is a specific transcription factor that serves
an active role in hypoxia by connecting upstream and downstream
genes, activating a wide range of factors in mammals (6). Hypoxia increases the expression
levels of transforming growth factor β (TGF-β), lysyl oxidase
(LOX), matrix metalloproteinase (MMP), vascular endothelial growth
factor (VEGF) and the mesenchymal cell marker vimentin, while it
decreases the expression level of the endothelial marker
E-cadherin. This leads to decreased tumor cell adhesion and
increased mobility, facilitating metastasis and invasion of tumor
cells (7).
TGF-β signaling serves an important role in cancer
cell EMT, metastasis and invasion (8). High TGF-β expression promotes
extracellular matrix (ECM) instability, consequently affecting the
activity of the ECM protein, LOX (9). The TGF-β/Smad4 pathway is closely
associated with cancer tissue differentiation. However, Smad4 and
TGF-β expression levels exhibit opposite effects on the survival
prognosis of gastric cancer patients, with TGF-β expression
positively correlated and Smad4 expression negatively correlated
with survival (10). Through the
promotion of the transfer process, serum TGF-β expression is
significantly higher in patients with peritoneal metastasis as
compared with that in patients without peritoneal metastasis,
although no independent factor has been identified to date
(11). It has also been reported
that TGF-β and LOX are closely linked with the process of
metastasis (12).
LOX is a copper-dependent amine oxidase that is
considered to participate in the ECM. It serves a pivotal role in
morphological and structural integrity, while LOX overexpression
leads to ECM remodeling and sclerosis, which provides the basis for
tumor migration and invasion (13). Furthermore, LOX is considered a
hypoxic response gene that is closely associated with the migration
and invasion of tumor cells (14). LOX overexpression upregulates
Snail expression and down-regulates E-cadherin expression under
hypoxic conditions, thereby promoting EMT (15). It has been suggested that HIF
regulates LOX expression during the metastasis of breast, head and
neck cancer, and inhibition of HIF decreases LOX expression, since
these pathways are closely associated (16). Another study reported that TGF-β
signaling may upregulate LOX expression and promote cancer
metastasis under hypoxic conditions (17). In addition, LOX interference
significantly reduces TGF-β expression by upregulating VEGF
expression and P38 phosphorylation, inhibiting cancer cell
migration and invasion (18).
Thus, LOX has a potential clinical value in the diagnosis of
gastric cancer lymph node and peritoneal metastases (19).
Overall, HIF-1α, TGF-β and LOX are closely
correlated and serve key roles in cancer metastasis. Based on these
findings, the use of dextran sulfate (DS) as an intervention drug
was evaluated in the present study. Our previous study showed that
DS reduced the peritoneal metastasis of BGC-823 gastric cancer
cells in nude mice and decreased the number of nodules and tumor
cell volume with downregulated expression of HIF-1α and ITGβ1
expression in gastric cancer cells (20). However, the mechanism underlying
the effect of DS on the peritoneal metastasis of gastric cancer
cells remains unclear. The present study aimed to evaluate the
influence of DS on human gastric cancer cell migration and invasion
under hypoxic conditions. It is speculated that DS may inhibit the
mechanism of action of gastric cancer peritoneal metastasis.
Materials and methods
Gastric cancer tissues
A total of 40 specimens from gastric cancer patients
who had not received radiotherapy or chemotherapy were obtained
from the Department of Pathology at the Ningxia People's Hospital
(Yinchuan, China) between March 2015 and March 2016. Informed
consent was obtained from all individuals participating in the
present study. Gastric cancer was diagnosed by two pathologists
according to the ESMO-ESSO-ESTRO clinical practice guidelines
(21). Gastric cancer and normal
gastric peritumoral tissues were collected for immunohistochemical
experiments.
Cell culture
The primary human gastric cancer BGC-823 cell line
(Beijing Jin ZiJing, Beijing, China) was routinely cultured in RPMI
1640 medium (HyClone; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin
and 100 mg/l streptomycin (HyClone; GE Healthcare Life Sciences).
The cells were incubated in a humidified atmosphere at 37°C and 5%
CO2.
Drugs and cell groups
DS (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
was dissolved in phosphate-buffered saline (PBS) for cell culture
and then sterilized using a 22-μm filter, to obtain a final
concentration of 0.3%. β-aminopropionitrile (BAPN; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) at a final concentration of 300
μM/ml was used. The appropriate concenratiions of DS and
BAPN for invasion and migration were obtained in the CCK-8 and flow
cytometry (FCM) apoptosis assay under normoxic conditions. Then the
cells were treated with PBS, DS, BAPN and DS combined with BAPN
under hypoxia, respectively.
Cell Counting Kit-8 (CCK-8) assay
The effect of DS and BAPN on cell proliferation was
determined using the CCK-8 assay (Dojindo Molecular Technologies,
Inc., Kumamoto, Japan). Briefly, cells in the logarithmic growth
phase were seeded at a density of 1,000 cells/well into 96-well
plates and cultured under normoxic conditions at 37°C for 24 h.
Subsequently, the cells were treated with different concentrations
of DS (0.1, 0.3, 0.6 and 1.0%) and BAPN (200, 300, 500, 700 and
1,000 μM), and cultured for 24 h. Next, 10 μl CCK-8
reagent was added to each well, and the cells were incubated at
37°C for an additional 3 h. The absorbance of each well,
representing the cell viability, was measured at 450 nm using a
Multiskan FC microplate reader (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA).
Flow cytometry (FCM) apoptosis assay
BGC-823 cells were seeded at a density of
5×105 cells/well in a 6-well plate, treated with
different DS concentrations (0.1, 0.3, 0.6 and 1.0%) and incubated
for 24 h. The re-suspended cells were treated with 400 μl
Annexin V-fluorescein isothiocyanate (FITC) binding buffer
(BestBio, Shanghai, China) and digested in 0.25% trypsin without
ethylenediaminetetraacetic acid. The cells were centrifuged at
1,000 × g at room temperature for 5 min and washed three times with
cold PBS. Annexin V-FITC (5 μl) was applied for 20 min,
followed by the addition of propidium iodide (10 μl) and
incubation for 5 min at 4°C. The stained cells were immediately
analyzed by FCM (BD Biosciences, San Jose, CA, USA).
In vitro invasion and migration
assays
BGC-823 cells were subjected to serum deprivation
for 24 h and seeded at a density of 2.5×104 cells/well
on the top of Matrigel-coated filters. Cells were then transferred
to chambers containing 600 μl of 10% FBS as a
chemo-attractant and incubated with DS (0.3%) and BAPN (300
μM) under oxygen-deprived conditions for 24 h (22). The non-migrating or non-invading
cells in the upper chamber were removed with a cotton swab. The
invading cells were fixed with 4% paraformaldehyde (Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China) for 15
min at room temperature, stained with 0.1% crystal violet and
manually counted under a phase-contrast microscope at ×400
magnification. The mean of each assay for six randomly selected
fields was considered as the sample value. Experiments were
repeated three times. The in vitro cellular migration assay
was based on the described membrane invasion culture system, but
with absence of Matrigel coating in the filters used.
Immunocytochemical analyses
Cells were seeded on the culture dish at a density
of 30×104/dish and treated with DS for 24 h under
hypoxia as aforementioned. Then the cells were fixed in 4%
paraformaldehyde (PFA) for 15 min and incubated in hydrogen
peroxide for 15 min. The cells were then blocked with goat serum at
37°C for 30 min. Following incubation with anti-HIF-1α (1:100;
20960-1-AP; ProteinTech Group, Inc., Wuhan, China), anti-TGF-β
(1:150; ab27969; Abcam, Cambridge, MA, USA) and anti-LOX (1:150;
ab174316, Abcam) and β-actin (1:1,000; E-AB-30422; Elabscience
Biotechnology Co., Ltd., Wuhan, China).
Primary antibodies at 4°C overnight, the samples
were incubated with goat anti-rabbit secondary antibodies
(Histostain™- SP kits, SPN-9001; OriGene Technologies, Inc.,
Rockville, MD, USA) at room temperature for 1 h. Signals were
detected using 3,3′-diaminobenzidine tetrahydrochloride (1:20;
OriGene Technologies, Inc.), and the cells were counter-stained
with hematoxylin. The staining intensity score criteria were as
follows: 0, no staining; 1, light yellow staining; 2, yellow
staining; and 3, brown staining. The following scores were assigned
for different percentages tumor-positive cells: 0, negative; 1,
1-25% positive cells; 2, 25-50%; and 3, >50%. The staining
intensity, percentage of positive samples and tumor grades were
scored between 0 and 9 (with 0 indicating a lack of brown
immunoreactivity and 9 reflecting intense dark brown staining)
(23), and divided into the
following categories: 0-1, negative; 2, +; 3-4, ++; and ≥5, +++,
corresponding to low, moderate and high expression,
respectively.
Immunofluorescence analysis
Cells were seeded on the culture dish at a density
of 30×104/dish and treated with DS for 24 h under
hypoxia as aforementioned. Then, the cells were fixed in 4%
paraformaldehyde at 37°C for 15 min and permeabilized with 0.5%
Triton X-100 for 30 min. The cells were then blocked with goat
serum at 37°C for 30 min. Following incubation with TGF-β (1:150)
and LOX (1:150) with primary antibodies overnight at 4°C, followed
by incubation with FITC-conjugated and
tetramethylrhodamine-conjugated secondary antibodies, all samples
were counterstained with 4′,6-diamidino-2-phenylindole (BIOSS,
Beijing, China) and examined under an fluorescence microscope
(IX73P1F, Olympus Corporation, Tokyo, Japan). Images were analyzed
using Image-Pro Plus 6.0 software (Media Cybernetics, Inc.,
Rockville, MD, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-PCR) analysis
Total RNA of treated cells was extracted from cells
after treatment. Next, RNA was reverse transcribed into cDNA using
a total mRNA extraction kit (Total RNA kit I, R6834-01; OMEGA,
Guangzhou, China) and an RevertAid RT kit (K1691; Sangon Biotech
Co., Ltd., Shanghai, China), according to the manufacturer's
protocols. qPCR was subsequently performed using PCR mixture/kit
(2XTaq MasterMix, CW0682; CWBio, Beijing, China). with the cDNA
primer sequences provided in Table
I. qPCR was performed under the following conditions: 94°C for
2 min, 94°C 30 sec, 58°C for 30 sec, 72°C for 30 sec under 30
cycles, 7°C for 2 min and hold at 4°C. For qPCR, the mRNA
expression levels of TGF-β, Smad4 and LOX were analyzed using
target/internal relative grayscale values (ImageJ software 1.48u;
National Institutes of Health, Bethesda, MD, USA) based on the
expression level of 18S rRNA QuantumRNA™ Technology in Multiplex
Quantitative RT-PCR using 18S rRNA as an Internal Control.
(QuantumRNA™ Classic 18S Internal Standard, AM1716; Thermo Fisher
Scientific, Inc.).
 | Table IOligonucleotide primers for
quantitative polymerase chain reaction. |
Table I
Oligonucleotide primers for
quantitative polymerase chain reaction.
| Gene | cDNA primer
sequence (5′-3′) | Annealing
temperature (°C) | Product size
(bp) |
|---|
| TGF-β | Forward:
GACACCAACTATTGCTTCAG | | |
| Reverse:
CAGGCTCCAAATGTAGGG | 59 | 156 |
| Smad4 | Forward:
CCCCATCTGAGTCTAATGCTAC | | |
| Reverse:
GCAGTCCTACTTCCAGTCCAG | 60 | 217 |
| LOX | Forward:
TTGAGTCCTGGCTGTTATGATACC | | |
| Reverse:
TGATGTCCTGTGTAGCGAATGTC | 60 | 179 |
| β-actin | Forward:
TGACGTGGACATCCGCAAAG | | |
| Reverse:
CTGGAAGGTGGACAGCGAGG | 60 | 284 |
Western blot analysis
Total proteins were isolated by Total Extraction Kit
(KGP2100; Nanjing KeyGen Biotech Co., Ltd., Nanjing, China)
following the manufacturer's protocols. Protein concentration was
determined by the BCA reagent kit (KGPBCA; Nanjing KeyGen Biotech
Co., Ltd.). Equal amounts of protein (60 μg) were separated
by 10% SDS-PAGE and transferred to polyvinylidene difluoride
membranes, which were blocked in 5% non-fat milk, and then
incubated with the primary antibodies against anti-HIF-1α (1:1,000;
ProteinTech Group, Inc.), anti-TGF-β (1:500; Abcam, USA),
anti-Smad4 (1:1,000; ProteinTech Group, Inc.), anti-LOX (1:2,000;
Abcam, USA), anti-E-cadherin (1:200; Cell Signaling Technology,
Danvers, MA, USA) and anti-β-actin (1:1,000; Elabscience
Biotechnology Co., Ltd.). The samples were incubated with the
antibodies overnight at 4°C. Subsequently, the membrane was
incubated with horseradish peroxidase-conjugated secondary
antibodies (1:6,000; OriGene, Beijing, China) at room temperature
for 1 h. An enhanced chemiluminescence reagent (ECL kit, KGP1121;
Nanjing KeyGen Biotech Co., Ltd.) was applied as a chromogenic
substrate for 1 min, and then visualized with an Amersham Imager
600 instrument (GE Healthcare Life Sciences). Grayscale analysis
was performed with ImageJ software.
Statistical analysis
All experiments were assayed in triplicate (n=3).
Data are expressed as the mean ± standard error of the mean. All
statistical analyses were performed using SPSS version 17.0
statistical software (SPSS, Inc., Chicago, IL, USA). Two-sample
comparison was performed using to t-test, Multiple samples were
compared using one-way analysis of variance. The correlation
between LOX and TGF-β was analyzed using four samples and three
replications by Pearson's correlation analysis. Differences with a
P<0.05 were considered to exhibit a statistically significant
difference.
Results
HIF-1α, TGF-β and LOX expression levels
in poorly differentiated gastric carcinoma and peritumoral
tissues
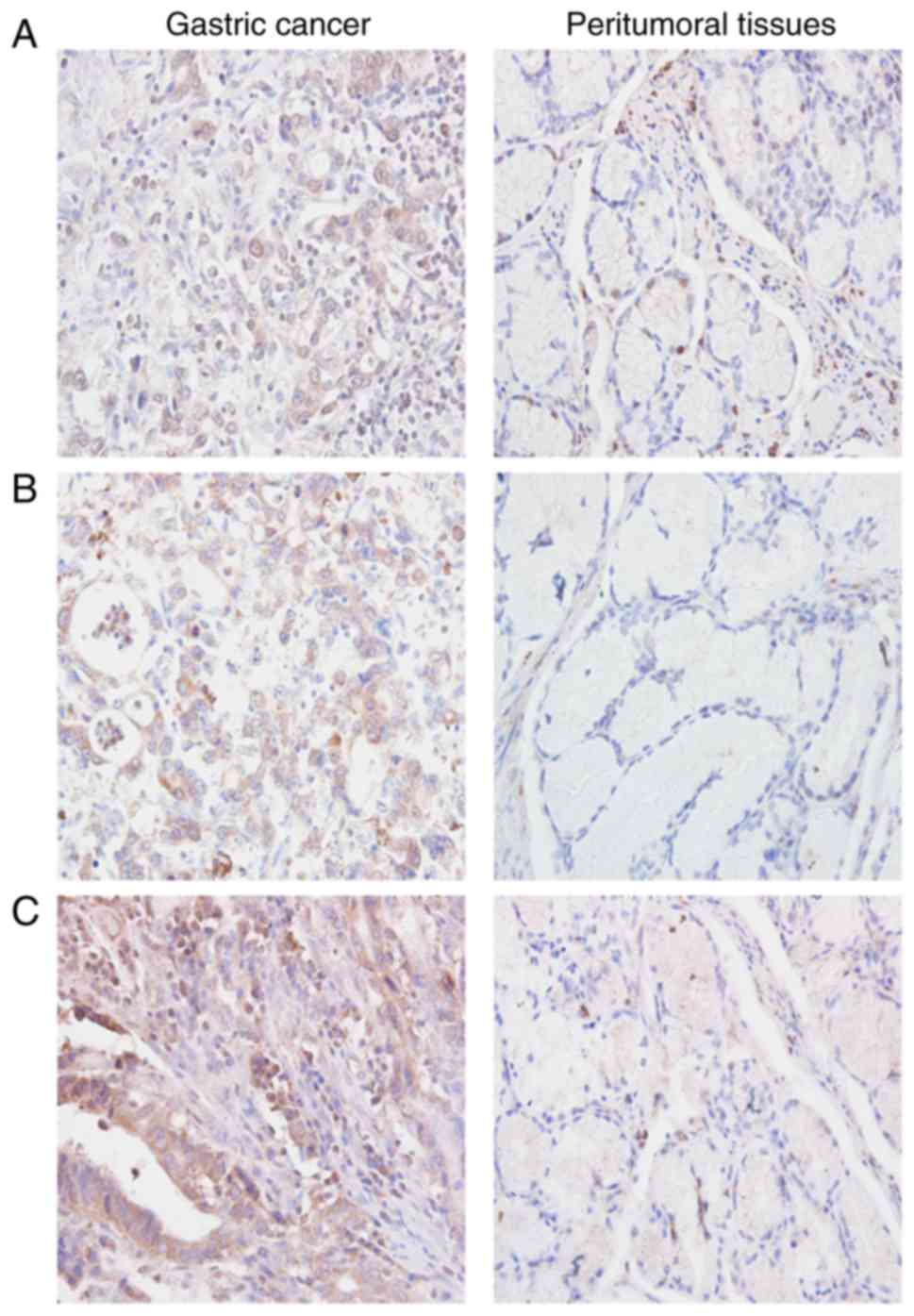
Immunohistochemical staining revealed that HIF-1α
was mainly located in the nucleus of cells in gastric carcinoma
tissues (Fig. 1A), whereas TGF-β
and LOX were mainly located in the cytoplasm (Fig. 1B and C) as indicated by positively
stained brown particles or clusters. The levels of HIF-1α, TGF-β
and LOX expression were markedly higher in gastric cancer as
compared with those in peritumoral tissues (Table II).
 | Table IIHIF-1α, TGF-β and LOX expression
levels in poorly differentiated gastric cancer and peritumoral
tissues. |
Table II
HIF-1α, TGF-β and LOX expression
levels in poorly differentiated gastric cancer and peritumoral
tissues.
| Tissues | Number | HIF-1α+
(%) | TGF-β+
(%) | LOX+
(%) |
|---|
| Gastric cancer | 40 | 26 (65)a | 31 (77.5)b | 29 (72.5)b |
| Peritumoral | 40 | 16 (40) | 13 (32.5) | 14 (35) |
Effects of DS on BGC-823 cell
proliferation and apoptosis
To determine the toxic effects of DS and BAPN on
BGC-823 cells, the same number of BGC-823 cells was incubated with
different concentrations of DS and BAPN, and cell survival was
assessed using the CCK-8 assay. The relative cell survival rates
were 89.9, 77.2, 70.0 and 57.8% at DS concentrations of 0.1, 0.3,
0.6 and 1.0%, respectively, while the survival rates were 94.6,
72.7, 81.8, 76.6 and 73.7% at BAPN concentrations of 200, 300, 500,
700 and 1,000 μM, respectively (Fig. 2A and B). In addition, the combined
use of DS (0.3%) and different concentrations of BAPN (200, 300 and
500 μM) resulted in relative survival rates of 86.6, 66.9
and 64.4%, respectively (Fig.
2C). Importantly, 0.3% DS combined with 300 μM BAPN
exerted an effective inhibition effect on BGC-823 cell than 300
μM BAPN. FCM was also used to detect the effect of different
DS concentrations on apoptosis under the same conditions. When the
DS concentrations were 0.1, 0.3, 0.6 and 1.0%, the relative
apoptosis rates were 10.5, 14.1, 13.7 and 16.1%, respectively
(Fig. 2D). The FCM and CCK-8
assay results demonstrated the dose dependence of the DS effect.
Furthermore, these data indicate that BGC-823 apoptosis and
survival are influenced by DS and BAPN in a dose-dependent manner.
For subsequent analysis, 0.3% DS was selected as the optimal
concentration.
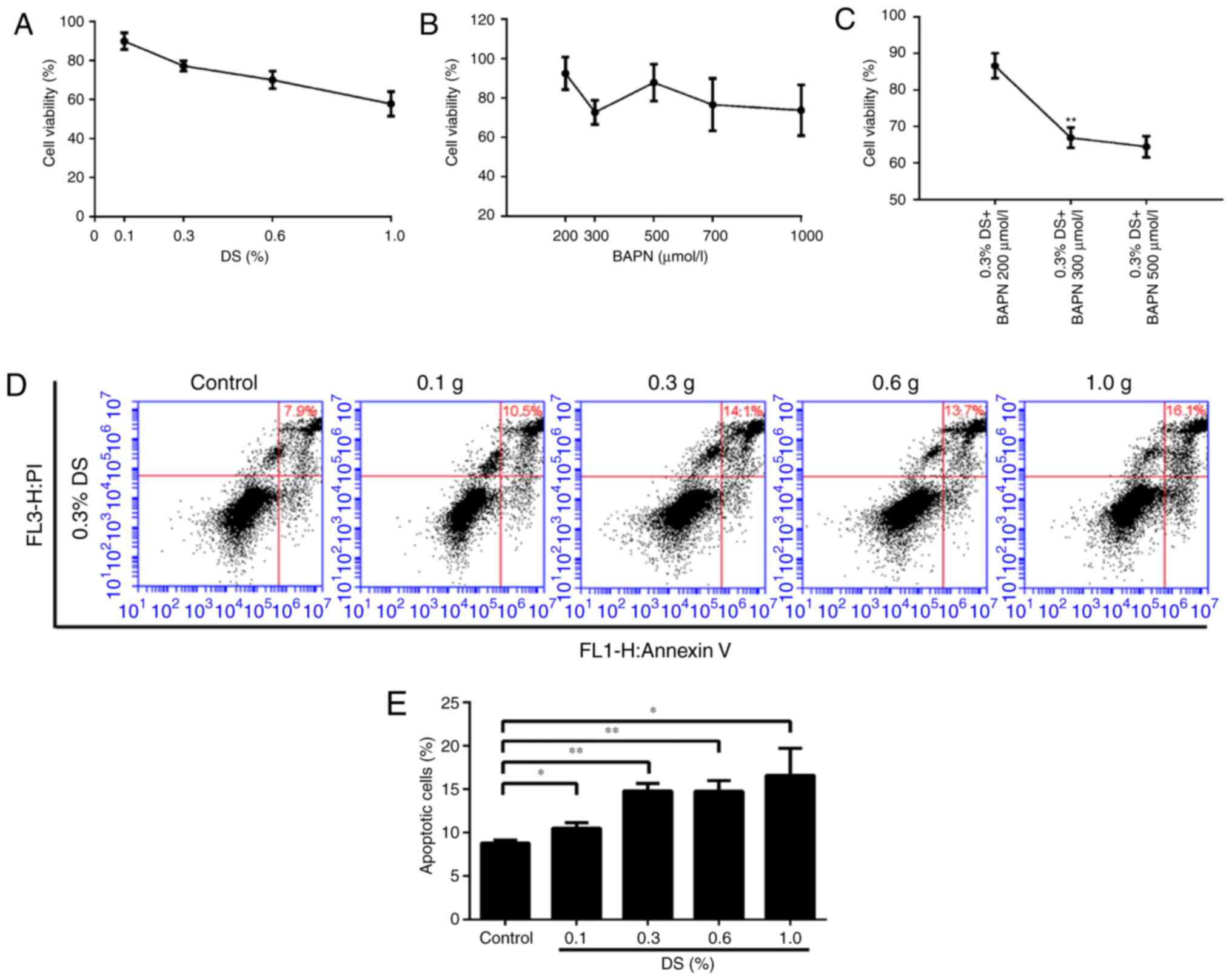 | Figure 2Effects of different DS
concentrations on the proliferation and apoptosis of gastric cancer
cells. Under normoxic conditions, Cell Counting Kit-8 was used to
detect the BGC-823 cell proliferation ability. Relative survival
rate of cells exposed to different concentrations of (A) DS (0.1,
0.3, 0.6 and 1.0%) and (B) BAPN (200, 300, 500, 700 and 1,000
μM). (C) Effect of the combination of 0.3% DS and BAPN (200,
300, and 500 μM). **P<0.01 vs. 300 μM
BAPN. (D) Flow cytometry analysis and (E) percentage of apoptotic
cells. The induction of apoptosis in human gastric cancer cells was
determined by flow cytometry following treatment with DS (0.1, 0.3,
0.6 and 1.0%) for 24 h. *P<0.05 and
**P<0.01, vs. control group. DS, dextran sulfate;
BAPN, β-aminopropionitrile. |
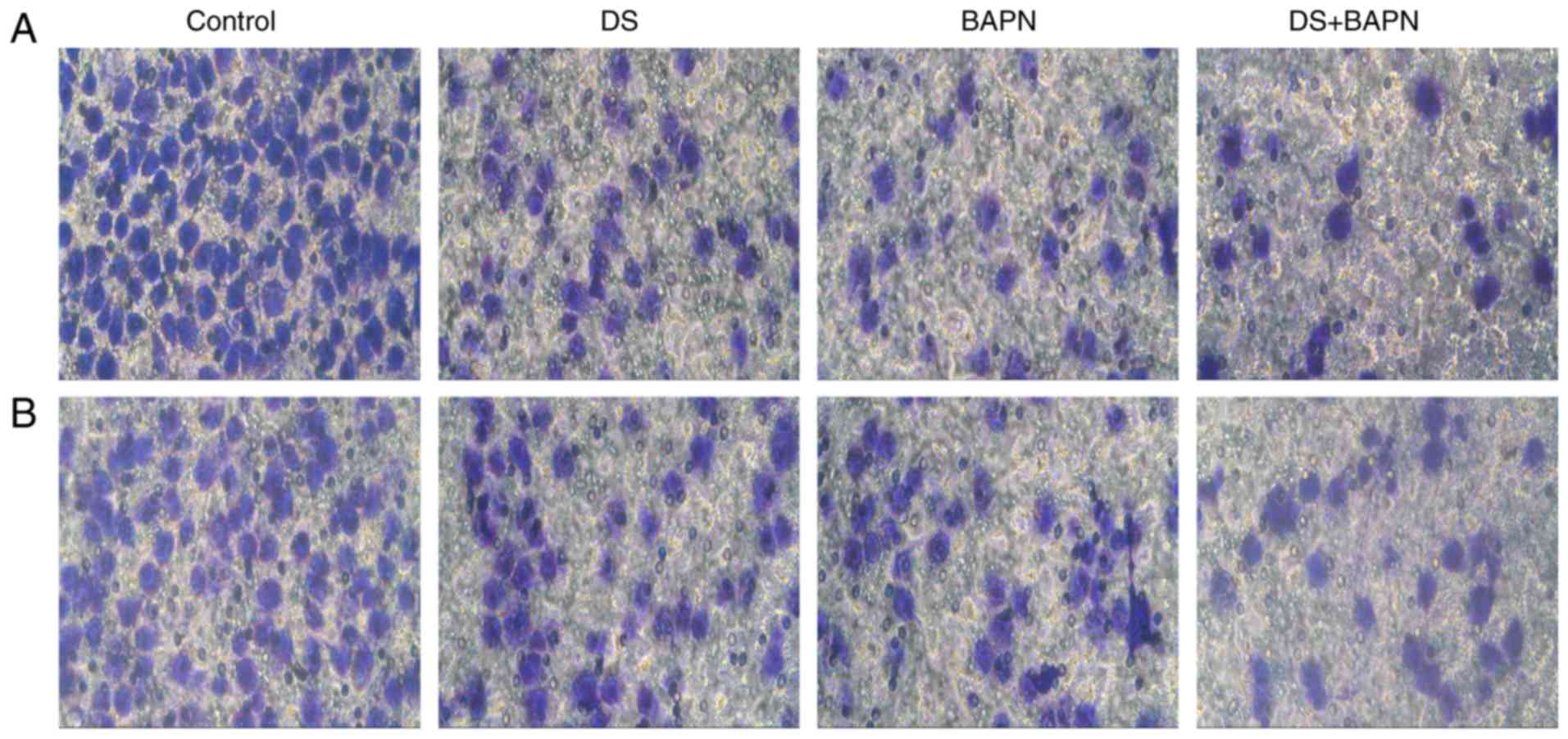
Effects of DS on BGC-823 cell migration
and invasion
First, cells were treated with 0.3% DS and/or 300
μM BAPN for 24 h under hypoxia, and incubation with these
continued throughout the experiment. Subsequently, invading and
migrating cells were fixed, stained with crystal violet and
counted. Both DS and BAPN were observed to significantly reduce the
invasion and migration of BGC-823 cells in comparison with the
control group. Compared with DS or BAPN alone, the combination of
DS and BAPN resulted in significantly reduced cell invasion and
migration (Fig. 3).
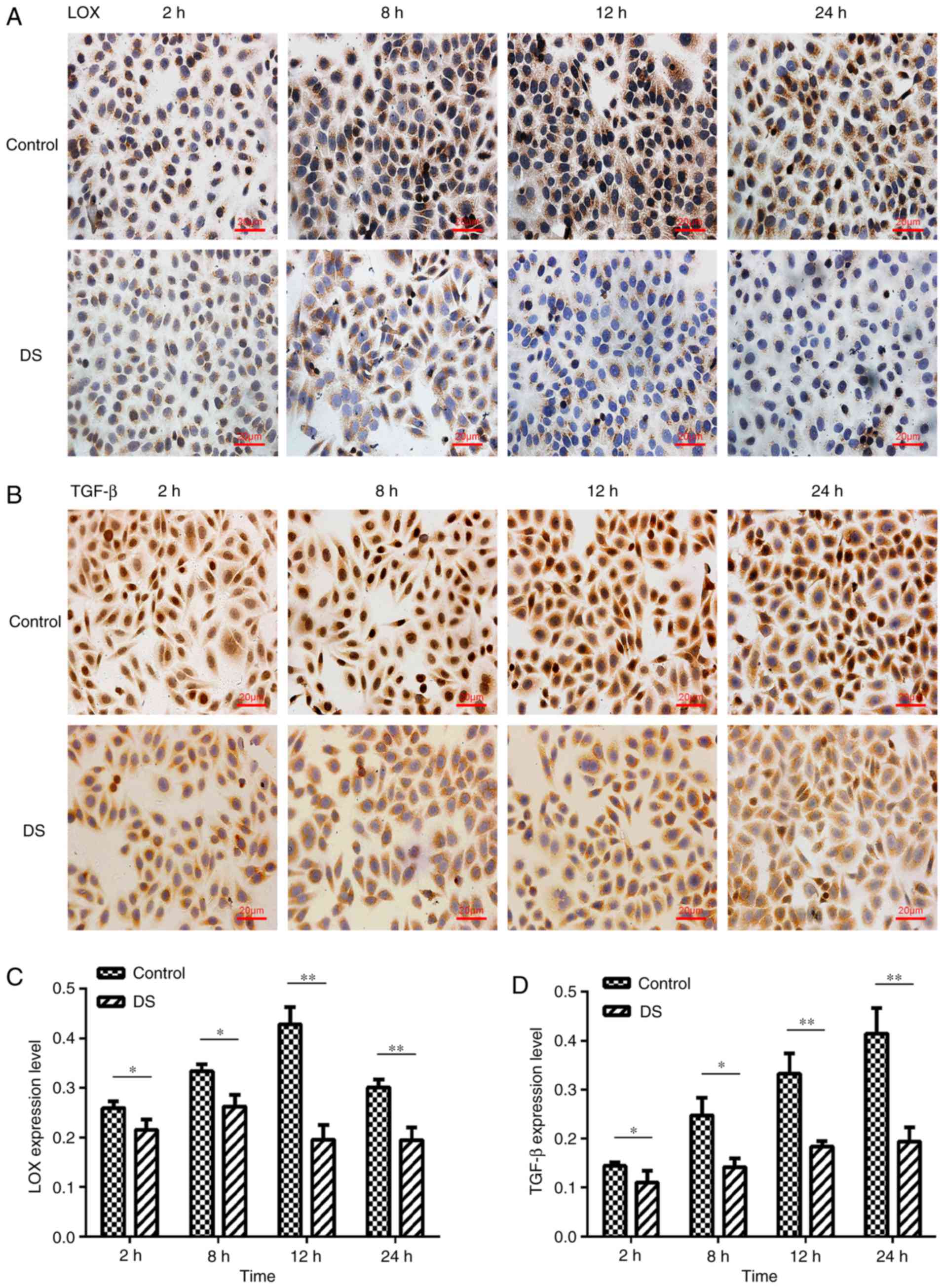
Immunocytochemical staining
LOX was observed to be mainly expressed in the
cytoplasm of BGC-823 cells and in a few nuclei, with brown staining
indicating positive LOX expression (Fig. 4A). Positive TGF-β expression was
detected in the nucleus and cytoplasm by brown staining with high
expression in the nucleus. (Fig.
4B). The LOX and TGF-β expression levels were significantly
decreased at 2 h (P<0.05), 8 h (P<0.05), 12 h (P<0.01) and
24 h (P<0.01) of incubation with DS, as compared with the
corresponding control group (Fig. 4C
and D). Thus, these results indicate that DS inhibited LOX and
TGF-β expression levels in BGC-823 cells.
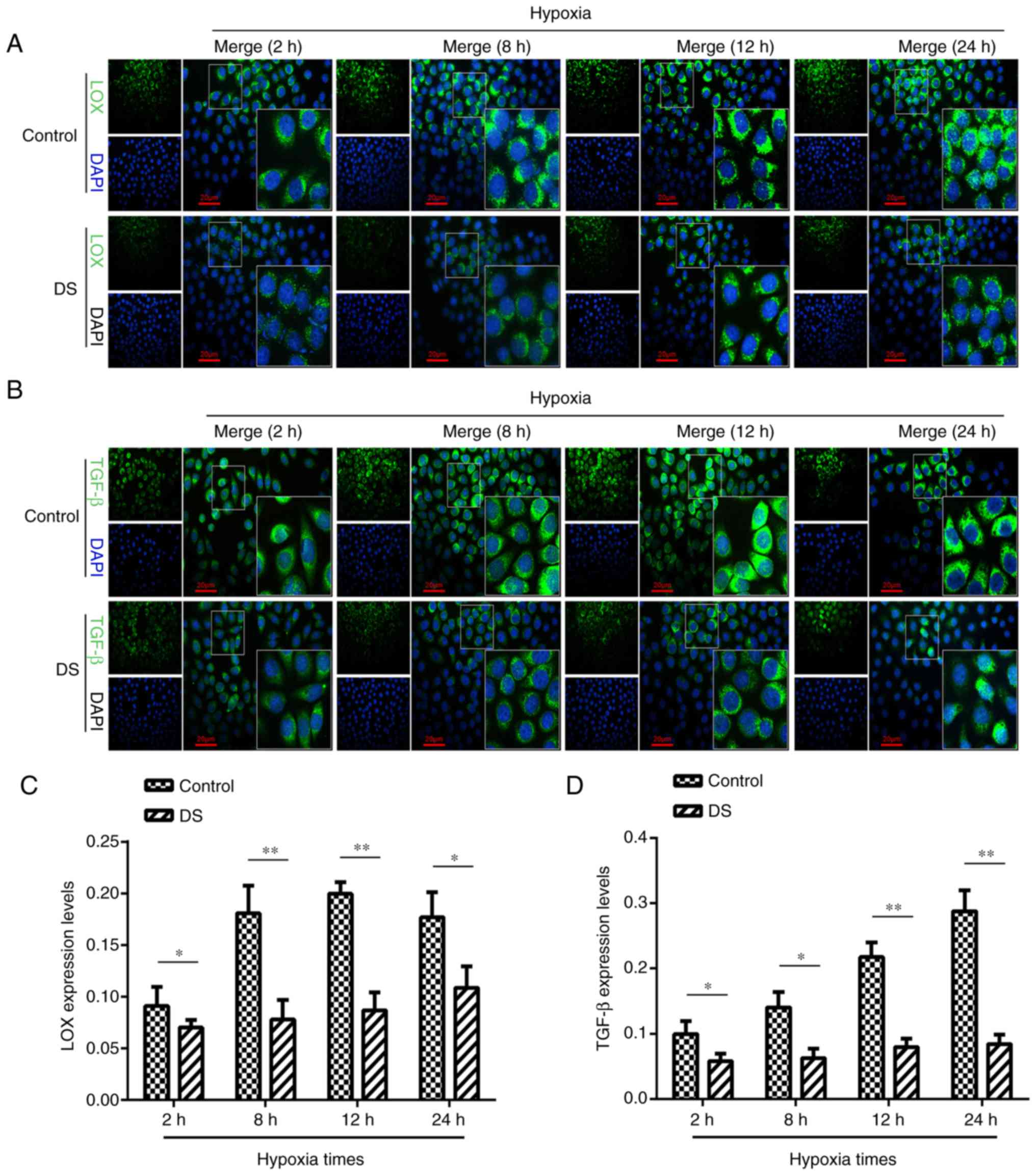
Immunocytofluorescence analysis of LOX
and TGF-β expression levels
The current study attempted to further elucidate the
association between LOX and TGF-β expression levels in gastric
cancer cells under hypoxic conditions by immunocytofluorescence
analysis (Fig. 5A and B). LOX
expression in the DS group was significantly reduced at 2 h
(P<0.05), 8 h (P<0.01), 12 h (P<0.01) and 24 h (P<0.05)
when compared with that in the control group (Fig. 5C). Similarly, the level of TGF-β
expression was significantly lower at 2 h (P<0.05), 8 h
(P<0.05), 12 h (P<0.01), and 24 h (P<0.01) in the DS group
in comparison with that in the control group (Fig. 5D). This suggested that, under
hypoxia, DS may inhibit the expression TGF-β; however LOX
expression levels may be inhibited in a time dependent manner.
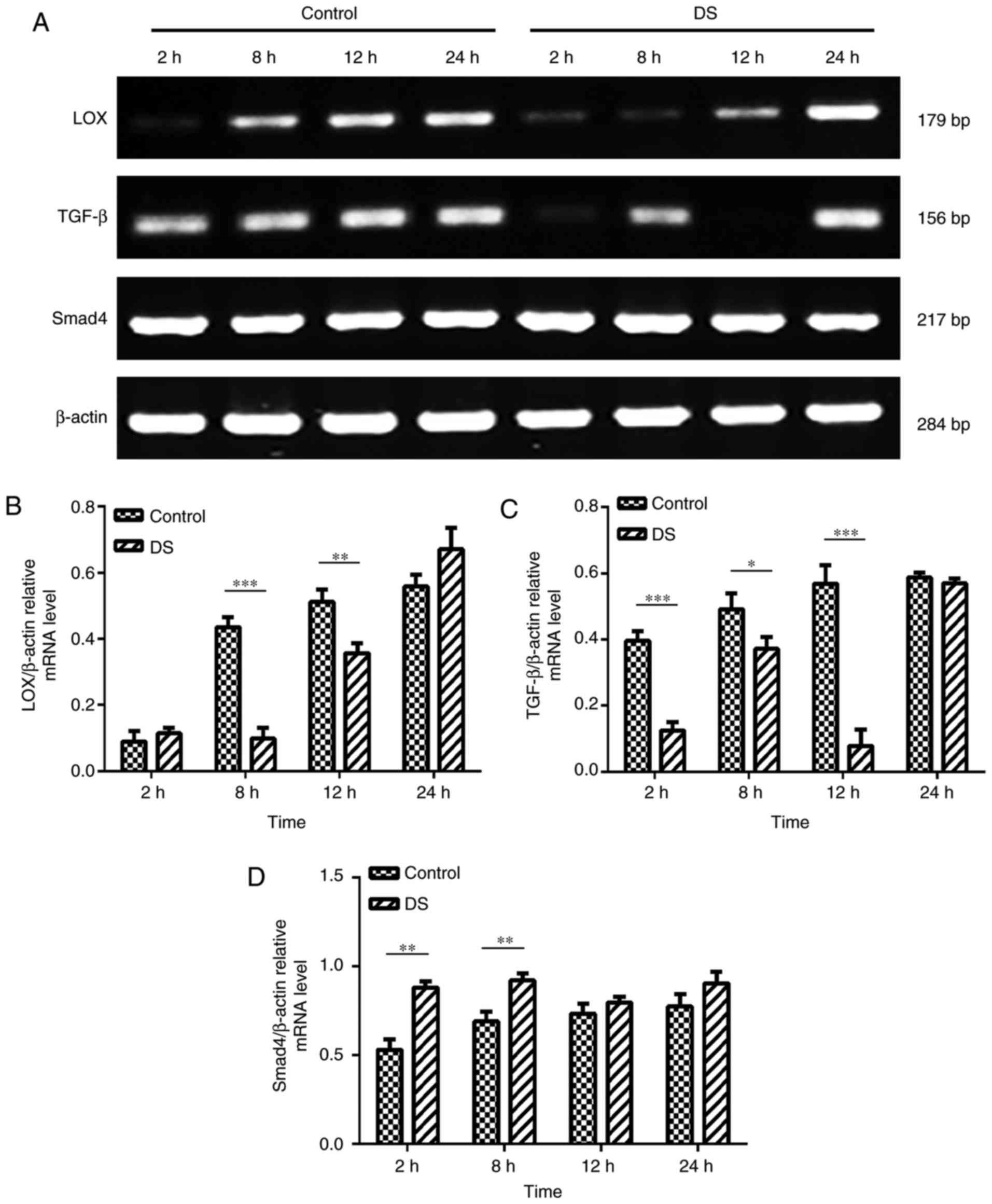
RT-PCR analysis of LOX, TGF-β and Smad4
mRNA expression levels in BGC-823 cells
As LOX, TGF-β and Smad4 serve a significant role in
the process of invasion and migration and is a prognostic indicator
of gastric cancer (10,19), the present study investigated
their expression profiles. The RT-PCR results revealed that the
mRNA expression levels of LOX (8 and 12 h, P<0.01) and TGF-β (2,
8 and 12 h, P<0.01) were significantly decreased, while the
expression of Smad4 (2 and 8 h, P<0.01) was significantly
increased in BGC-823 cells under hypoxic conditions at certain time
points in the DS group, when compared with the levels in the
control group (Fig. 6). According
these results, DS may regulate the expression of LOX, TGF-β and
Smad4 thereby inhibiting the invasion and migration of gastric
cancer cells.
Effect of DS on metastasis-associated
protein expression levels in BGC-823 cells
Western blot analysis was conducted to determine the
levels of various metastasis-associated proteins (Fig. 7A). Following treatment with 0.3%
DS, LOX, HIF-1α and TGF-β protein expression levels were
significantly decreased at 8,12 and 24 h compared with in the
control group (Fig. 7B-D). In
contrast, the expression levels of Smad4 were significantly
increased at 2 and 8 h in response to DS treatment compared with in
the control, but no alterations were observed at 12 and 24 h
following treatment of DS (Fig.
7E). In addition, the expression levels of E-cadherin at 2, 8,
12 and 24 h were signficantly upregulated under hypoxia and DS
compared with control; (Fig.
7F).
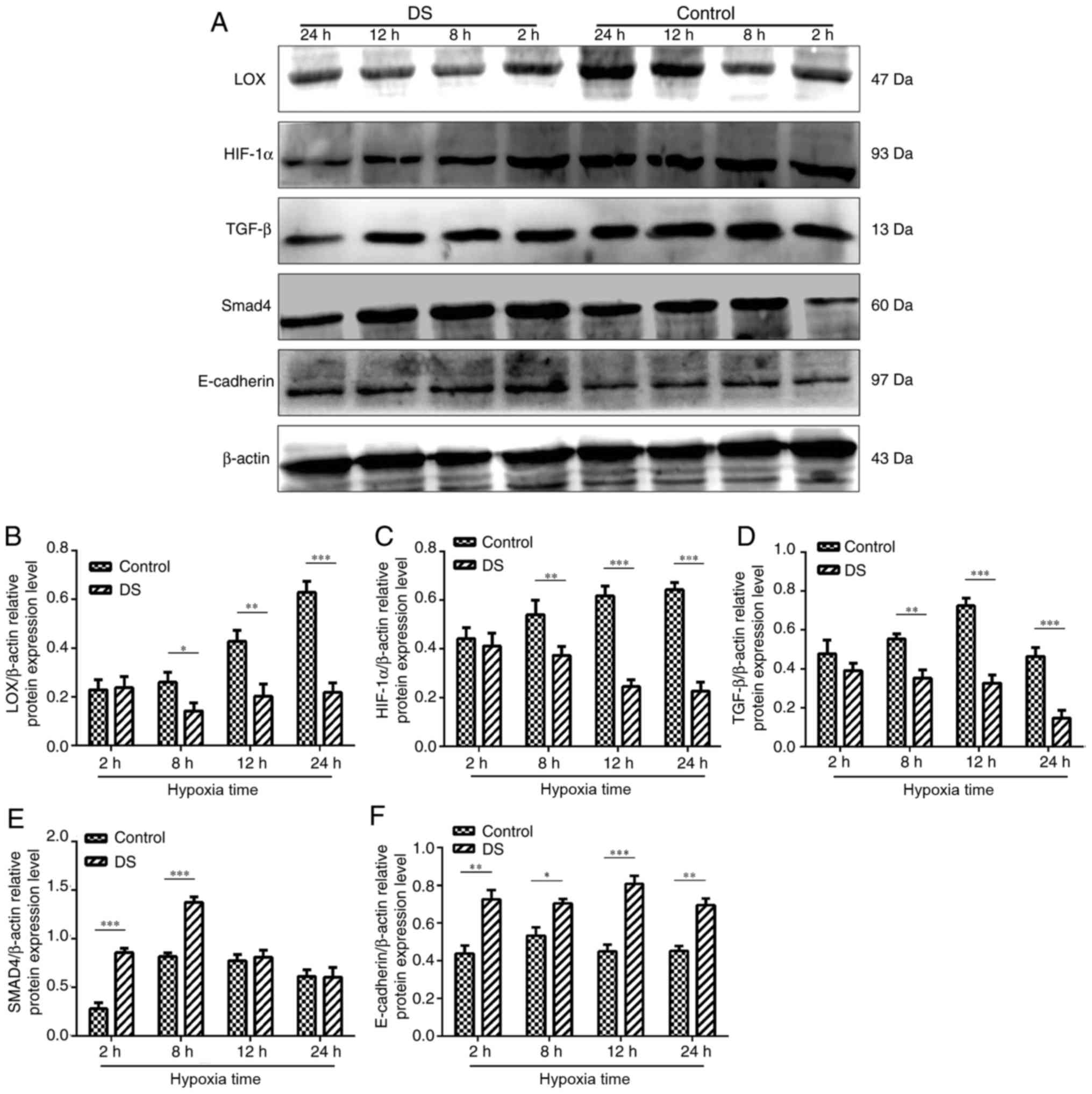 | Figure 7(A) Western blot analysis of HIF-1α,
TGF-β, LOX, Smad4 and E-cadherin protein expression levels in
BGC-823 experimental and control cell groups. (B) LOX, (C) HIF-1α
and (D) TGF-β protein expression levels were significantly reduced
in the DS group. By contrast, DS significantly increased (E) Smad4
and (F) E-cadherin protein expression compared with the control
group. The western blot analysis results were analyzed using ImageJ
software and are shown as bar graphs. Similar results were obtained
in at least three independent experiments. Data are presented as
the mean ± standard error. *P<0.05,
**P<0.01 and ***P<0.001. DS, dextran
sulfate; LOX, lysyl oxidase; HIF-1α, hypoxia-inducible factor 1α;
TGF-β, transforming growth factor β. |
Furthermore, the inhibitory effects of 0.3% DS and
300 μM BAPN on LOX, HIF-1α and TGF-β were enhanced when the
cells were treated by DS in combination with BAPN, compared with DS
or BAPN, respectively. This suggested that there may a synergic
inhibition of BGC-823 cells between DS and BAPN (Fig. 8). Additionally, the correlation
between LOX and TGF-β expression levels were investigated using
Person's correlation analysis. This indicated that the expression
of LOX was positively associated with TGF-β (Table III).
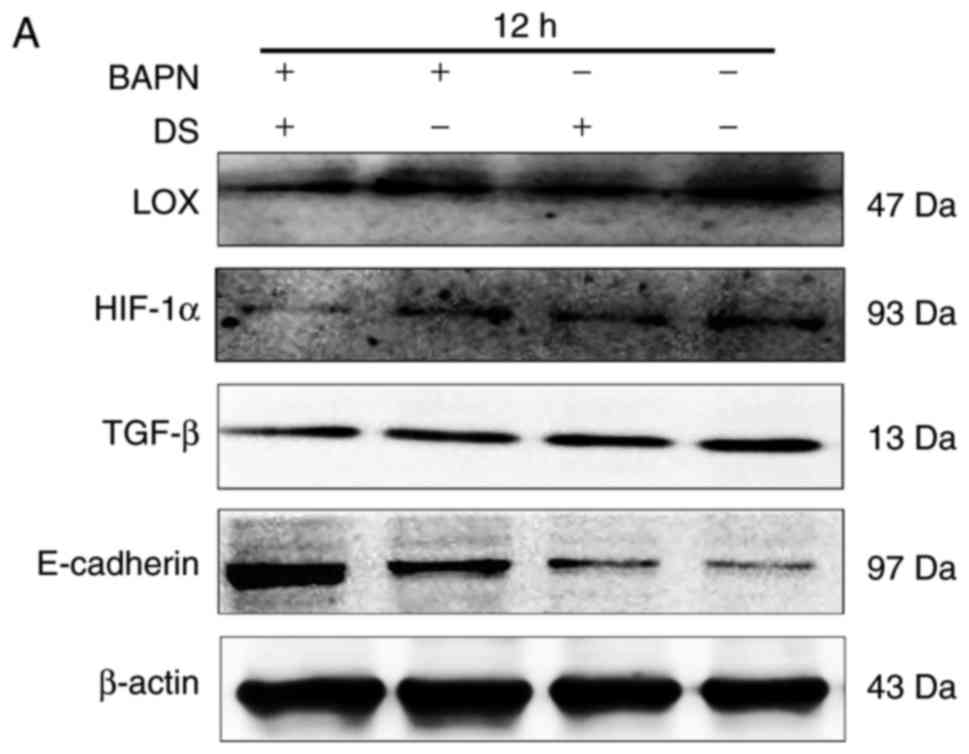 | Figure 8Combination of DS and BAPN inhibited
LOX, HIF-1α and TGF-β expression levels, and promoted E-cadherin
expression. (A) Western blot analysis, and quantified results of
the expression levels of (B) LOX, (C) HIF-1α, (D) TGF-β and (E)
E-cadherin at 12 h. β-actin was used as a loading control. The
western blotting results were analyzed using ImageJ software and
are shown as bar graphs. Data are presented as the mean ± standard
error. *P<0.05. DS, dextran sulfate; BAPN,
β-aminopropionitrile; LOX, lysyl oxidase; HIF-1α, hypoxia-inducible
factor 1α; TGF-β, transforming growth factor β. |
 | Table IIICorrelation between LOX and TGF-β
protein levels in vitro. |
Table III
Correlation between LOX and TGF-β
protein levels in vitro.
| Protein | Statistical
value | LOX | TGF-β |
|---|
| LOX | Pearson's
correlation | 1 | 0.898a |
| P-value
(two-sided) | - | <0.001 |
| n | 4 | 4 |
| TGF-β | Pearson's
correlation | 0.898a | 1 |
| P-value
(two-sided) | <0.001 | - |
| m | 4 | 4 |
Discussion
Metastasis and recurrence are major causes of
mortality in gastric cancer patients, and tumor molecular markers
are important for determining the prognosis and predicting the
recurrence of tumors (24).
Despite improvements in surgical approaches and chemotherapy
regimens for the treatment of gastric cancer, patients with
metastasis and recurrence still have a poor prognosis. Therefore,
limiting tumor metastasis is the key to effectively increasing
patient survival rates.
Several signaling factors are expressed at higher
levels in cancerous tissue as compared with their expression in
tissues adjacent to the tumor. Therefore, these signaling factors
have an important clinical value in the study of cancer metastasis.
It has been suggested that high HIF-1α expression under hypoxic
conditions can promote tumor cell angiogenesis, EMT, invasion and
migration (25). A previous study
demonstrated that DS can prevent B16 melanoma cells from implanting
on greater omentum milky spots and the peritoneum, thus serving an
important role in the inhibition of abdominal cavity metastasis
(26). When DS was introduced
into the murine visceral endoderm through endocytosis, the endoderm
cells maintained normal functions and their unique membrane
dynamics (27). The present study
demonstrated that DS inhibited hypoxia-induced BGC-823 cell
migration and invasion under hypoxic conditions, while
significantly reducing the high level of HIF-1α expression.
Under anoxic conditions, LOX is closely connected
with key signaling pathways that are important in the cancer
metastasis process (28). In
eukaryotic cells, HIF-1α mainly adjusts the oxygen balance and, in
turn, is regulated by oxygen. A hypoxic environment exists in the
majority of tumors due to tumor growth-induced blood vessel
anfractuosity, which alters the tumor microenvironment. Despite the
rich oxygenation state of growth in surrounding tissues, a growth
state with relatively low oxygenation exists in the central
organization of tumor tissues (29). HIF-1α has been demonstrated to
increase LOX, Twist and Snail expression levels, and to reduce
E-cadherin expression under hypoxic conditions, thereby
accelerating cancer cell migration and invasion in vitro
(30). In addition to HIF-1α,
TGF-β is another key factor that promotes LOX expression (12) and is the key regulatory factor in
cell proliferation, apoptosis, differentiation and migration
processes, as well as in ECM synthesis and precipitation (31). In the present study, it was
observed that TGF-β was mainly expressed in the nucleus and
cytoplasm under hypoxia and that TGF-β was able to translocate from
the cytoplasm to nucleus. DS reduced TGF-β expression at different
time points, with significantly reduced nuclear expression,
suggesting that DS can inhibit the nuclear translocation of TGF-β.
Under hypoxic conditions, TGF-β protein expression increased
gradually, whereas this expression was decreased after 24 h. It has
previously been reported that the development of cancer TGF-β
signaling has a dual role in tumor suppression and promotion
(32); therefore, the present
study speculated that TGF-β may affect BGC-823 cell autocrine
secretion through other unknown pathways.
Studies on cancer metastasis have reported that
TGF-β is closely linked to LOX expression. TGF-β signaling
increases LOX expression to promote breast cancer cell metastasis.
However, the capacity of cancer cell metastasis was found to be
reduced by inhibition of LOX activity (12). Another study reported that the
application of a LOX inhibitor reduced the number of cells with a
polygon or long spindle shape under hypoxic conditions (9). Although the experiments of the
current study demonstrated that 0.3% DS reduced LOX expression, the
BGC-823 cell morphology was not significantly altered (data not
shown). One possible cause for this discrepancy may be due to the
poor differentiation of BGC-823 cells (20).
Smad4 is a key factor in the TGF-β signaling pathway
(33). It was initially
identified as a tumor suppressor gene and as the central regulation
factor of TGF-β signal transduction, however, with the continuous
progression of the tumor, the expression of Smad4 decreased
(10). Hu et al (34) reported that a significantly higher
TGF-β expression in gastric cancer tissues compared with that in
para-carcinoma tissues, and continuous alterations in the tumor
microenvironment accelerated the invasion and migration of gastric
cancer cells in clinical pathological specimens. In addition, Leng
et al (10) reported that
Smad4 expression was higher in gastric cancer tissues, poorly
differentiated carcinomas and patients without lymphatic metastasis
in comparison with that in para-carcinoma tissues, highly
differentiated gastric cancer and lymphatic metastases.
The present study found that DS significantly
reduced TGF-β and increased Smad4 expression under hypoxic
conditions. Therefore, it is speculated that DS can affect gastric
cancer cell metastasis through the TGF-β/Smad signaling
pathway.
In cancer research, inhibition of LOX expression can
effectively reduce EMT and the migration of cancer cells. Under
hypoxic conditions, the autocrine activity of LOX increased Snail
expression and enhanced invasive ability; indeed, cancer cell
migration, invasion and EMT capacity were reduced following
application of the LOX activity inhibitor BAPN (35). It has been demonstrated that BAPN
inactivates LOX and decreases its upstream factors HIF-1α and
TGF-β, while it increases the expression of E-cadherin, which
affects tumor cell metastasis (22). Furthermore, BAPN was reported to
exert the most effective inhibitory effects on invasion and
metastasis at a concentration of 500 μM against low
oxygen-induced ovarian cancer cell invasion and metastasis
(36). In a breast cancer study,
higher LOX expression occurred with a change in the tumor
microenvironment, which promoted the early dissolution of bone
lesions and cancer cell metastases (37). Furthermore, in a hepatocellular
carcinoma study, TGF-β caused increased cancer cell expression of
LOX and VEGF protein in a dose-dependent manner, and LOX gene
silencing was able to decrease the phosphorylation of TGF-β
expression by p38, indicating that LOX may influence the expression
of TGF-β via the mitogen-activated protein kinase pathway (18). The involvement of LOX in the
process of cancer metastasis has gradually emerged from a number of
studies (9,38). In the current study, experiments
focused on investigating the influence of DS on certain key factors
during hypoxia-induced cancer cell migration. Under hypoxic
conditions, LOX expression increased over time in the control
group, but was significantly decreased by DS in a time-dependent
manner, concomitant with an increase in E-cadherin expression.
Preliminary experiments also revealed that DS may reduce cancer
cell TGF-β and LOX expression and increase E-cadherin
expression.
Notably, combined application of the LOX inhibitor
BAPN (300 μM) and 0.3% DS decreased HIF-1α, TGF-β and LOX
expression levels at 8 and 12 h, while it significantly increased
Smad4 and E-cadherin expression levels. These results indicate that
combined application of BAPN and DS is better than the single
application of BAPN or DS. The current study also speculated that
DS may inhibit the expression of LOX, thereby affecting TGF-β
signaling in the invasion and migration of human gastric cancer
cells. The DS-mediated inhibition of key signaling pathways should
be further explored in the future to improve the prevention and
treatment options for cancer metastasis. Although an inhibitory
effect of DS on gastric cancer cell migration and invasion was
demonstrated in the present study, further research is needed to
determine whether this effect is generalizable to other cancer
types.
In conclusion, the data presented in the current
study revealed a positive correlation between LOX and TGF-β protein
expression, and that DS may inhibit the invasion and migration of
gastric cancer cells by inhibiting LOX expression under hypoxic
conditions. Furthermore, the combined application of DS and BAPN
had a more prominent effect. Elucidating the mechanism through
which DS inhibits intra-abdominal gastric cancer cell migration and
its clinical application value require further analysis.
Funding
The present study was supported by the Ningxia
Science and Technology Support Projects (grant nos. 2002310201 and
2015BY076). West China First Class Discipline Construction Project
in Basic Medicine funded by Ningxia Medical University.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YX conceived the design of the present study. XW
wrote the manuscript with support from all the other authors. XW
and XJ conducted cell culture, immunohistochemistry and
immunofluorescence experiments. YH guided and performed CCK-8 and
cell flow cytometry experiments. YM, XJ, and HW conducted western
blotting and reverse transcription-quantitative polymerase chain
reaction. JW performed immunofluorescence experiments and data
analysis. All authors read and approved the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Ningxia Medical University (Yinchuan, China). Informed
consent was obtained from all individual participants included in
the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of
interest.
Acknowledgments
Not applicable.
References
|
1
|
Ajani JA: Evolving chemotherapy for
advanced gastric cancer. Oncologist. 3(Suppl 10): 49–58. 2005.
View Article : Google Scholar
|
|
2
|
Gou WF, Shen DF, Yang XF, Zhao S, Liu YP,
Sun HZ, Su RJ, Luo JS and Zheng HC: ING5 suppresses proliferation,
apoptosis, migration and invasion, and induces autophagy and
differentiation of gastric cancer cells: A good marker for
carcinogenesis and subsequent progression. Oncotarget.
6:19552–19579. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fioroni I, Dell'aquila E, Pantano F,
Intagliata S, Caricato M, Vincenzi B, Coppola R, Santini D and
Tonini G: Role of c-mesenchymal-epithelial transition pathway in
gastric cancer. Expert Opin Pharmacother. 16:1195–1207. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: EMT: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peng Z, Wang CX, Fang EH, Wang GB and Tong
Q: Role of epithelial-mesenchymal transition in gastric cancer
initiation and progression. World J Gastroenterol. 20:5403–5410.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang YJ, Na HJ, Suh MJ, Ban MJ, Byeon HK,
Kim WS, Kim JW, Choi EC, Kwon HJ, Chang JW and Koh YW: Hypoxia
induces epithelial-mesenchymal transition in follicular thyroid
cancer: Involvement of regulation of twist by hypoxia inducible
factor-1α. Yonsei Med J. 56:1503–1514. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo J, Wang B, Fu Z, Wei J and Lu W:
Hypoxic microenvironment induces EMT and upgrades stem-like
properties of gastric cancer cells. Technol Cancer Res Treat.
15:60–68. 2016. View Article : Google Scholar
|
|
8
|
Tian M, Neil JR and Schiemann WP:
Transforming growth factor-β and the hallmarks of cancer. Cell
Signal. 23:951–962. 2011. View Article : Google Scholar
|
|
9
|
Kasashima H, Yashiro M, Kinoshita H,
Fukuoka T, Morisaki T, Masuda G, Sakurai K, Kubo N, Ohira M and
Hirakawa K: Lysyl oxidase is associated with the
epithelial-mesenchymal transition of gastric cancer cells in
hypoxia. Gastric Cancer. 19:431–442. 2016. View Article : Google Scholar
|
|
10
|
Leng A, Liu T, He Y, Li Q and Zhang G:
Smad4/Smad7 balance: A role of tumorigenesis in gastric cancer. Exp
Mol Pathol. 87:48–53. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Noda S, Yashiro M, Nshii T and Hirakawa K:
Hypoxia upregulates adhesion ability to peritoneum through a
transforming growth factor-beta-dependent mechanism in diffuse-type
gastric cancer cells. Eur J Cancer. 46:995–1005. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Taylor MA, Amin JD, Kirschmann DA and
Schiemann WP: Lysyl oxidase contributes to
mechanotransduction-mediated regulation of transforming growth
factor-β signaling in breast cancer cells. Neoplasia. 13:406–418.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oskarsson T: Extracellular matrix
components in breast cancer progression and metastasis. Breast.
2(Suppl 22): S66–S72. 2013. View Article : Google Scholar
|
|
14
|
Barry-Hamilton V, Spangler R, Marshall D,
McCauley S, Rodriguez HM, Oyasu M, Mikels A, Vaysberg M, Ghermazien
H, Wai C, et al: Allosteric inhibition of lysyl oxidase-like-2
impedes the development of a pathologic microenvironment. Nat Med.
16:1009–1017. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cuevas EP, Moreno-Bueno G, Canesin G,
Santos V, Portillo F and Cano A: LOXL2 catalytically inactive
mutants mediate epithelial-to-mesenchymal transition. Biol Open.
3:129–137. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Erler JT, Bennewith KL, Nicolau M,
Dornhöfer N, Kong C, Le QT, Chi JT, Jeffrey SS and Giaccia AJ:
Lysyl oxidase is essential for hypoxia-induced metastasis. Nature.
440:1222–1226. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matsuoka J, Yashiro M, Doi Y, Fuyuhiro Y,
Kato Y, Shinto O, Noda S, Kashiwagi S, Aomatsu N, Hirakawa T, et
al: Hypoxia stimulates the EMT of gastric cancer cells through
autocrine TGFβ signaling. PLoS One. 8:e623102013. View Article : Google Scholar
|
|
18
|
Zhu J, Huang S, Wu G, Huang C, Li X, Chen
Z, Zhao L and Zhao Y: Lysyl oxidase is predictive of unfavorable
outcomes and essential for regulation of vascular endothelial
growth factor in hepatocellular carcinoma. Dig Dis Sci.
60:3019–3031. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lai H, Jin Q, Lin Y, Mo X, Li B, He K and
Chen J: Combined use of lysyl oxidase, carcino-embryonic antigen,
and carbohydrate antigens improves the sensitivity of biomarkers in
predicting lymph node metastasis and peritoneal metastasis in
gastric cancer. Tumour Biol. 35:10547–10554. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu Y, Jin X, Huang Y, Dong J, Wang H, Wang
X and Cao X: Inhibition of peritoneal metastasis of human gastric
cancer cells by dextran sulphate through the reduction in HIF-1α
and ITGβ1 expression. Oncol Rep. 35:2624–2634. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Waddell T, Verheij M, Allum W, Cunningham
D, Cervantes A and Arnold D: Gastric cancer: ESMO-esso-estro
clinical practice guidelines for diagnosis, treatment and
follow-up. Radiother Oncol. 110:189–194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang X, Li S, Li W, Chen J, Xiao X, Wang
Y, Yan G and Chen L: Inactivation of lysyl oxidase by
β-aminopropionitrile inhibits hypoxia-induced invasion and
migration of cervical cancer cells. Oncol Rep. 29:541–548. 2013.
View Article : Google Scholar
|
|
23
|
Ding W, Zhang W, Hui FM, Zhang YH, Zhang
FF, Li XM and Shi FX: Cell-specific expression and
immunolocalization of nitric oxide synthase isoforms and soluble
guanylyl cyclase α1 and β1 subunits in the ovary of fetal, neonatal
and immature pigs. Anim Reprod Sci. 131:172–180. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Riquelme I, Saavedra K, Espinoza J A,
Weber H, García P, Nervi B, Garrido M, Corvalán AH, Roa JC and
Bizama C: Molecular classification of gastric cancer: Towards a
pathway-driven targeted therapy. Oncotarget. 6:24750–24779. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gordan JD and Simon MC: Hypoxia-inducible
factors: Central regulators of the tumor phenotype. Curr opin Genet
Dev. 17:71–77. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hagiwara A, Sakakura C, Shirasu M, Togawa
T, Sonoyama Y, Fujiyama J, Ebihara Y, Itoh T and Yamagishi H:
Intraperitoneal injection of dextran sulfate as an anti-adherent
drug for the prevention of peritoneal metastasis of cancer shows
low toxicity in animals. Anticancer Drugs. 11:393–399. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kawamura N, Sun-Wada GH, Aoyama M, Harada
A, Takasuga S, Sasaki T and Wada Y: Delivery of endosomes to
lysosomes via microautophagy in the visceral endoderm of mouse
embryos. Nat Commun. 3:10712012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Erez N: Cancer: Opening lox to metastasis.
Nature. 522:41–42. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vaupel P and Harrison L: Tumor hypoxia:
Causative factors, compensatory mechanisms, and cellular response.
Oncologist. 5(Suppl 9): S4–S9. 2004. View Article : Google Scholar
|
|
30
|
Wang Y, Ma J, Shen H, Wang C, Sun Y,
Howell SB and Lin X: Reactive oxygen species promote ovarian cancer
progression via the HIF-1α/LOX/E-cadherin pathway. Oncol Rep.
32:2150–2158. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lim S, Bae E, Kim HS, Kim TA, Byun K, Kim
B, Hong S, Im JP, Yun C, Lee B, et al: TRAF6 mediates
IL-1β/LPS-induced suppression of TGF-β signaling through its
interaction with the type III TGF-β receptor. PLoS One.
7:e327052012. View Article : Google Scholar
|
|
32
|
Massague J: TGFβ signalling in context.
Nat Rev Mol Cell Biol. 13:616–630. 2012. View Article : Google Scholar
|
|
33
|
Vincent T, Neve EP, Johnson JR, Kukalev A,
Rojo F, Albanell J, Pietras K, Virtanen I, Philipson L, Leopold PL,
et al: A SNAIL1SMAD3/4 transcriptional repressor complex promotes
TGF-beta mediated epithelial-mesenchymal transition. Nat Cell Biol.
11:943–950. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu WQ, Wang LW, Yuan JP, Yan SG, Li JD,
Zhao HL, Peng CW, Yang GF and Li Y: High expression of transform
growth factor beta 1 in gastric cancer confers worse outcome:
Results of a cohort study on 184 patients. Hepatogastroenterology.
61:245–250. 2014.PubMed/NCBI
|
|
35
|
Moon HJ, Finney J, Xu L, Moore D, Welch DR
and Mure M: MCF-7 cells expressing nuclear associated lysyl
oxidase-like 2 (LOXL2) exhibit an epithelial-to-mesenchymal
transition (EMT) phenotype and are highly invasive in vitro. J Biol
Chem. 288:30000–30008. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Osawa T, Ohga N, Akiyama K, Hida Y,
Kitayama K, Kawamoto T, Yamamoto K, Maishi N, Kondoh M, Onodera Y,
et al: Lysyl oxidase secreted by tumour endothelial cells promotes
angio-genesis and metastasis. Br J Cancer. 109:2237–2247. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cox TR, Rumney RMH, Schoof EM, Perryman L,
Høye AM, Agrawal A, Bird D, Latif NA, Forrest H, Evans HR, et al:
The hypoxic cancer secretome induces pre-metastatic bone lesions
through lysyl oxidase. Nature. 522:106–110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hua YJ, Wang HY, Tang LQ, Chen QY, Shao JY
and Mai HQ: LOX expression in primary nasopharyngeal carcinoma:
Correlation with prognostic parameters and outcome. Oncotarget.
7:8200–8207. 2016. View Article : Google Scholar : PubMed/NCBI
|