Introduction
Dimethylglycine sodium (DMG-Na) has the ability to
improve cellular antioxidant capacity; thus, treatment with DMG-Na
may prevent intracellular oxidative damage by scavenging excess
free radicals in cells (1,2).
Indeed, previous studies have indicated that DMG can reduce
oxidative damage and improve animal growth performance (3). Oxidative damage induced by the
imbalance of the antioxidant and free radical generation systems
can increase the level of reactive oxygen species (ROS), leading to
tissue damage (4). Previous
studies have indicated that the generation of free radicals is
likely one mechanism that leads to the development of diseases,
including cancer and neuronal disorders (5,6).
ROS, specifi-cally, are generated along with adenosine triphosphate
in the mitochondria (7). The
production of excess ROS results in an imbalance in cellular redox
states, which leads to a decrease in the mitochondrial membrane
potential (MMP) and a disorder in mitochondrial functioning
(8). There is a complex system of
natural enzymatic and non-enzymatic antioxidants in the human body
that defend against oxidative damage caused by free radicals and
oxidative materials. Recent studies have suggested that dietary
supplementation with antioxidants may be beneficial in preventing
diseases and improving quality of life (2). Antioxidant enzymes or natural
products can reduce oxidative damage via their antioxidation
capabilities.
The small intestine is the most important digestive
organ, and is the first organ damaged when oxidative stress occurs
(9). Previous studies have
indicated that the generation of free radicals is one of the main
mechanisms that lead to many diseases, including gastrointestinal
diseases (4). Lipid peroxi-dation
is one of the major causes of oxidative damage, which can
contribute to the development of oxygen radical-related damages via
increasing level of ROS. Oxidative stress often leads to serious
damage to the gastrointestinal tract; therefore, it was proposed
that oxidative stress is associated with the clinical risk of
gastrointestinal damage failure (10,11). It has been suggested that dietary
supplementation with non-enzymatic antioxidants may be beneficial
in preventing diseases and improving quality of life. Few studies
have focused on the effects of DMG-Na on the small intestine due to
the complexity of designing and developing proper human trials. The
use of cell cultures is a valid method to overcome this challenge.
Particularly, IPEC-J2 cells, which exhibit the properties of normal
intestinal epithelium, have been widely used as a suitable model to
simulate the gastrointestinal system (12-14). The present study was designed to
evaluate the protective effects of DMG-Na against oxidative damage
in IPEC-J2 cells, which would provide a potential solution for the
prevention and treatment of intestinal damage via enhancing the
antioxidant capacity, increasing the antioxidant-associated gene
expression, relieving mitochondrial dysfunction and suppressing
apoptosis.
Materials and methods
Radical scavenging assay
1,1-diphenyl-2-pierylhydrazy (DPPH) radical
scavenging activity was calculated according to the method
described by Moon and Shibamoto (15). The absorbance was determined with
a spectrophotometer at 517 nm and the formula is as follows: DPPH
radical scavenging activity (%) =
(Acontrol-Asample)/Acontrol x100;
where Acontrol was the absorbance of control and
Asample was the absorbance of water-soluble pigments
(DMG-Na, betalain, capsanthin, and cyanidin-3-rutinoside) or
Trolox.
2,2'-Azinobis-(3-ethylbenzthiazoline-6-sulphonate)
(ABTS+) assay
The ABTS+ radical scavenging activity was
measured according to the method of Siddhuraju and Manian (16). The absorbance was recorded with a
spectrophotometer at 534 nm and the formula is as follows:
ABTS+ radical scavenging activity (%) =
(Acontrol-Asample)/Acontrol x100.
Where Acontrol was the absorbance of control and
Asample was the absorbance of water-soluble pigments or
Trolox.
Superoxide radical
(O2-) assay
O2- scavenging activity was
determined using the method of Chen and Yen (17). The absorbance was determined with
a spectrophotometer at 560 nm and the formula is as follows:
O2- scavenging activity (%) =
(Acontrol-Asample)/Acontrol x100.
Where Acontrol was the absorbance of control and
Asample was the absorbance of water-soluble pigments or
Trolox.
Hydrogen peroxide
(H2O2) assay
The H2O2 scavenging activity
was performed following the procedure of Zhang et al
(18). The absorbance was
recorded with a spectrophotometer at 230 nm and the formula is as
follows: H2O2 scavenging activity (%) =
(Acontrol-Asample)/Acontrol x100.
Where Acontrol was the absorbance of control and
Asample was the absorbance of water-soluble pigments or
Trolox.
Ferric-reducing antioxidant power (FRAP)
assay
The ferric reducing antioxidant power of
water-soluble pigments or Trolox was measured according to the
method described by Benzie and Strain (19). The absorbance at 593 nm was
calculated with a spectrophotometer. The reducing power was
expressed as µmol Fe(II)/g fresh weight. A standard curve of
FeSO4·7H2O was used for calibration.
Assay for hemolysis of porcine
erythrocytes
Freshly collected swine blood, obtained from a
single genetic line [swine from Yangzhou Fangling Agricultural and
Pastoral Co., Ltd. (Yangzhou, China); all procedures were approved
by the Institutional Animal Care and Use Committee of Nanjing
Agricultural University (Nanjing, China)], was mixed with heparin
and then centrifuged at 1,500 x g for 10 min at 4°C. Following
removal of plasma, erythrocytes were washed three times with cool
PBS buffer (pH 7.4) and finally resuspended in this PBS buffer. In
order to induce the free radical chain oxidation in erythrocytes,
the aqueous peroxyl radicals were generated by thermal
decomposition of 2,2'-azobis(2-amidino-propane) dihydrochloride
(AAPH; an azo compound) in oxygen. Briefly, a 2% suspension of
porcine erythrocytes in PBS buffer were pre-incubated for 30 min at
37°C with DMG-Na (32 µM), followed by incubation with or
without AAPH (75 mM) for 5 h with gentle shaking. Hemolysis of
erythrocytes was determined spectrophotometrically as described by
Magalhães et al (20). The
hemolysis percentage (%) was calculated using the formula:
Absorbance of supernatant/complete hemolysis x100.
Measurement of erythrocyte antioxidant
activity and ROS level
Following repeated sonication (three times),
superoxide dismutase (SOD; cat. no. A001-2-1), glutathione
peroxidase (GSH-Px; cat. no. A005), glutathione (GSH; cat. no.
A006-1) activity and malondialdehyde (MDA; cat. no. A003-4) level
in erythrocytes were measured using the corresponding kits (Nanjing
Jiancheng Institute of Bioengineering, Nanjing, China) (21,22). The ROS level was detected using a
ROS assay kit (cat. no. E004; Nanjing Jiancheng Institute of
Bioengineering) (23).
Experimental design of IPEC-J2 cells
DMG-Na was obtained from Qilu Sheng Hua
Pharmaceutical Co., Ltd. (Jinan, China). All other chemicals were
commercially available and of reagent grade. The non-transformed
intestinal cell line IPEC-J2 (Leibniz-Institut DSMZ-Deutsche
Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig,
Germany) was derived from the jejunum, and cultured as described
previously (12). Namely, the
IPEC-J2 cells were cultured in Dulbecco's modified Eagle medium,
Ham's F-12 mixture, 1.5 mM HEPES, 5% (v/v) fetal bovine serum, 1%
(v/v) insulin-transferrin-selenium mixture, 1% (v/v)
penicillin-streptomycin mixture and 2.5 µg/ml fungizone
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
(37°C, 5% CO2). Cells were harvested by scraping them
into 200 ml sodium chloride-tris-ethylenediaminetetraacetic acid
(EDTA) buffer (250 mM sucrose, 3.59 mM Trizma®-base,
16.4 mM tris-HCl, 2 mM EDTA and 40 mM KCl). Subsequently, cells
were lysed by sonication at 40% amplitude for 10 sec (three times)
and centrifuged at 600 x g for 10 min at 4°C to remove cell
debris.
Oxidative damage was induced by exposing IPEC-J2
cells to 20 µM oleic acid hydroperoxides (OAHPx) in
lipopolysaccharide, supplemented with 1% L-glutamine and 1%
non-essential amino acids on the 4th day of culturing when
confluency had been reached (24,25). IPEC-J2 cells were randomly
assigned to four groups (n=6 per group): i) Cells treated with PBS
(T); ii) cells treated with 32 µM DMG-Na (TD); iii) cells
treated with 20 µM OAHPx (TO); iv) cells treated with 32
µM DMG-Na followed by 20 µM OAHPx (DTO).
Culture medium was removed at 24 h, and the cells
were harvested via brief trypsinization following washing with PBS.
Cell suspensions were then centrifuged at 200 x g for 5 min and
washed with PBS, the cell supernatant was discarded, and the cell
pellets were re-suspended in PBS on ice. Cells were lysed using a
mini-bead beater for 10 sec at 2,800 x g, and the cell lysates were
obtained via centrifugation at 14,000 x g for 10 min at 4°C.
Assay for cell viability of IPEC-J2
cells
The cells were pre-treated with or without DMG-Na
(8-56 µM) overnight for 18 h. Neutral red dye (Janssen
Pharmaceutica NV, Beerse, Belgium) was prepared as a 0.1% (w/v)
stock solution and diluted 1/10 with washing buffer. After removing
the culture medium, 100 µl washing buffer plus 50 µl
0.01% (w/v) neutral red dye was added to each well, and incubated
for 2 h (37°C, 5% CO2). Afterwards, the cells were
washed twice with PBS. The neutral red dye is re- tained in
lysosomes of living cells (26).
This dye was then extracted from the cells using 100 µl 50%
(v/v) ethanol solution (in 0.05 M NaH2PO4).
The absorbance was measured at 550 nm using a spectrophotometer
(Table II).
 | Table IICell viability of IPEC-J2 cells. |
Table II
Cell viability of IPEC-J2 cells.
| DMG-Na
(µM) | Cell viability
(%) |
|---|
| 0 | 100.00±2.17 |
| 8 | 98.35±2.54 |
| 16 | 98.32±3.28 |
| 24 | 98.44±2.65 |
| 32 | 99.07±3.02 |
| 40 | 97.24±3.87 |
| 48 | 97.57±3.51 |
| 56 | 97.33±2.79 |
Antioxidant capacity assay
The culture supernatants and cell lysate of IPEC-J2
cells at 24 h were obtained to measure the activity SOD (cat. no.
A001-2-1), GSH-Px (cat. no. A005), GSH (cat. no. A006-1) and
glutathione reductase (GR; cat. no. A062) using their corresponding
assay kits (Nanjing Jiancheng Institute of Bioengineering, Nanjing,
China) (21,22).
Assay for mitochondrial isolation
The mitochondria were prepared according to the
method described previously by Tang et al (27). Mitochondria were lysed and protein
concentration was measured using an BCA assay kit (cat. no. A045-3;
Nanjing Jiancheng Institute of Bioengineering) (27).
Mitochondrial antioxidant capacity
assay
Manganese superoxide dismutase (MnSOD; cat. no.
A001-2-1), GSH-Px (cat. no. A005), GSH (cat. no. A006-1), GR (cat.
no. A062), γ-glutamylcysteine ligase (γ-GCL; cat. no. A120)
activity, and protein concentration (BCA assay kit; cat. no.
A045-4) of mitochondria were calculated using their corresponding
assay kits (Nanjing Jiancheng Institute of Bioengineering)
(21,28,29).
Assay for lipid peroxidation, protein
oxidation and 8-hydroxy-2-deoxyguanosine assay
Lipid peroxidation, expressed as MDA concentration,
was determined using an MDA assay kit (cat. no. A003-4; Nanjing
Jiancheng Bioengineering Institute) according to the instructions
of the manufacturer. The results were expressed in nmol/100 mg
protein. Protein oxidation of mitochondria was expressed as the
concentration of protein carbonyls (PC), which was calculated
according to the method described previously (30), and the results were presented in
nmol/mg protein. The content of 8-hydroxy-2-deoxyguanosine (8-OhdG)
in mitochondria was measured using an ELISA assay kit (cat. no.
H165; Nanjing Jiancheng Institute of Bioengineering) and the
results were presented as ng/mg protein.
Assay for ROS, MMP and cell
apoptosis
The ROS level was detected using a ROS assay kit
(cat. no. E004; Nanjing Jiancheng Institute of Bioengineering)
(23). Briefly, the mitochondria
were incubated with dichlorodihydrofluorescein diacetate (DCFH-DA;
10 µM) and DNA stain Hoechst 33342 (10 mmol/l) at 37°C for
30 min. Then the DCFH fluorescence of the mitochondria was measured
at an emission wavelength of 530 nm and an excitation wavelength of
485 nm with a fluorescence reader (FACS Aria III; BD Biosciences,
Franklin Lakes, NJ, USA). The results were expressed as the mean
DCFH-DA fluorescence intensity over that of the control.
The MMP level was calculated according to a method
described previously (31). In
brief, the mitochondria were loaded with 1 X JC-1 dye at 37°C for
20 min, and then analyzed, after washing, by flow cytometry (FACS
Aria III). The MMP was calculated as the increase in ratio of green
and red fluorescence. When the MMP levels are low, JC-1 exists
mainly as a monomer, which emits green fluorescence (excitation
wavelength of 490 nm and emission wavelength of 540 nm). However,
when the MMP levels are high, JC-1 exists mainly as a polymer,
which emits red fluorescence (excitation wavelength of 525 nm and
emission wavelength of 590 nm). The results were calculated in
triplicate as the ratio of the fluorescence of aggregates (red) to
that of the monomers (green).
Phosphatidylserine exposure on the outer leaflet of
the cell membrane was measured using an Alexa Fluor® 488
Annexin V/Dead Cell Apoptosis kit (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions (16). Briefly, following the
preincubation of AAPH and DMG-Na, the cells were washed twice with
cool PBS buffer (pH=7.4, containing 150 mM NaCl, 1.9 mM
Na2HPO4 and 8.1 mM
NaH2PO4) and resuspended (2% suspension) in 1
X Annexin-binding buffer. Then, the cell density was determined and
diluted in 1 X Annexin-binding buffer to ~1x106
cells/ml. A sufficient volume of the above cell suspension was
stained with Annexin V-fluorescein isothiocyanate and propidium
iodide (1:9 dilution) staining solution in dark for 15 min at room
temperature. After incubation, the forward scatter of cells was
determined, and Annexin V fluorescence intensity was measured in
FL-1 with 488 nm excitation wavelength and 530 nm emission
wavelength on a FACS caliber (BD Biosciences).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
Total RNA was obtained from the cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and then
reverse-transcribed using a commercial kit (Perfect Real Time,
SYBR® PrimeScript™; Thermo Fisher Scientific, Inc.)
following the instructions of the manufacturer. The gene expression
levels were quantified via qPCR, using SYBR1 Premix Ex Taq II (Tli
RNaseH Plus; Thermo Fisher Scientific, Inc.) and an ABI 7300 Fast
Real-Time PCR detection system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The SYBR-Green PCR reaction mixture consisted of
10 µl SYBR1 Premix Ex Taq (2 X), 0.4 µl forward and
reverse primers, 0.4 µl ROX reference dye (50 X), 6.8
µl ddH2O and 2 µl cDNA template. The
cycling conditions were as follows: Pre-run at 95°C for 30 sec, 40
cycles of denaturation at 95°C for 5 sec, followed by a 60°C
annealing step for 30 sec. Each sample was run in triplicate. The
fold-expression of each gene (Table
I) was calculated according to the 2-ΔΔCq method
(32), in which β-actin was used
as an internal standard.
 | Table IPrimer sequences used for reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Sequence
(5'-3') | GenBank accession
number |
|---|
| β-actin | Forward:
CTGTCCCTGTATGCCTCTG | NM_007393.3 |
| Reverse:
ATGTCACGCACGATTTCC | |
| Nrf2 | Forward:
GATGTCACCCTGCCCTTAG | NM_205117.1 |
| Reverse:
CTGCCACCATGTTATTCC | |
| HO1 | Forward:
GGTCCCGAATGAATGCCCTTG | HM237181.1 |
| Reverse:
ACCGTTCTCCTGGCTCTTGG | |
|
Cu/ZnSOD | Forward:
CCGGCTTGTCTGATGGAGAT | NM_205064.1 |
| Reverse:
TGCATCTTTTGGTCCACCGT | |
| GSH-Px | Forward:
GACCAACCCGCAGTACATCA | NM_001277853.1 |
| Reverse:
GAGGTGCGGGCTTTCCTTTA | |
| MnSOD | Forward:
AGGAGGGGAGCCTAAAGGAGA | NM_204211.1 |
| Reverse:
CCAGCAATGGAATGAGACCTG | |
| Sirt1 | Forward:
TGCAGACGTGGTAATGTCCAAAC | NM_019812.2 |
| Reverse:
ACATCTTGGCAGTATTTGTGGTGAA | |
| γ-GCLc | Forward:
TGCGGTTCTGCACAAAATGG | XM_419910.3 |
| Reverse:
TGCTGTGCGATGAATTCCCT | |
| γ-GCLm | Forward:
CCAGAACGTCAAAGCACACG | NM_001007953.1 |
| Reverse:
TCCTCCCATCCCCCAGAAAT | |
| Trx2 | Forward:
AGTACGAGGTGTCAGCAGTG | NM_001031410.1 |
| Reverse:
CACACGTTGTGAGCAGGAAG | |
| Trx-R2 | Forward:
CCGGGTCCCTGACATCAAA | NM_001122691.1 |
| Reverse:
TAGCTTCGCTGGCATCAACA | |
| Prx3 | Forward:
ACCTCGTGCTCTTCTTCTACC | XM_004942320.1 |
| Reverse:
ACCACCTCGCAGTTCACATC | |
| OCLN | Forward:
CCGTAACCCCGAGTTGGAT | NM_205128.1 |
| Reverse:
ATTGAGGCGGTCGTTGATG | |
| CLDN2 | Forward:
CCTGCTCACCCTCATTGGAG | NM_001277622.1 |
| Reverse:
GCTGAACTCACTCTTGGGCT | |
| CLDN3 | Forward:
CCCGTCCCGTTGTTGTTTTG | NM_204202.1 |
| Reverse:
CCCCTTCAACCTTCCCGAAA | |
| ZO1 | Forward:
TGTAGCCACAGCAAGAGGTG | XM_413773.4 |
| Reverse:
CTGGAATGGCTCCTTGTGGT | |
Western blotting
Antibodies against nuclear factor erythroid 2 like 2
(Nrf2), heme oxygenase 1 (HO1) and sirtuin 1 (Sirt1) were purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA). Cells were
lysed using Laemmli sample buffer [2% SDS, 62 mM Tris, 10%
glycerol, 5% β-mercaptoethanol, 0.005% bromphenol blue (pH 6.8)]
The cellular protein content was measured using a bicinchoninic
protein assay kit (Beyotime Institute of Biotechnology, Haimen,
China). For western blotting analysis, 50 µg protein from
each sample was subjected to SDS-PAGE (10%). Following
electrophoresis, the separated proteins were transferred to
polyvinylidene difluoride membranes, which were blocked with
blocking buffer (5% nonfat dry milk) for 12 h at 4°C. The membranes
were then probed with an appropriate primary antibody for 10 h at
4°C [Nrf2 (cat. no. 12721; 1:1,000), HO1 (cat. no. 82206; 1:1,000),
Sirt1 (cat. no. 9475; 1:1,000), and β-actin (cat. no.
4970;1:1,000), respectively] and a secondary antibody for 2 h at
4°C [goat-anti-rabbit secondary antibody (1:1,000)] purchased from
Cell Signaling Technology, Inc. The blots were detected using
enhanced chemiluminescence reagents (ECL-Kit; Beyotime Institute of
Biotechnology, Haimen, China) followed by autoradiography. Images
of the membranes were taken using a Luminescent Image Analyzer-4000
system (Fujifilm, Tokyo, Japan) and quantified with ImageJ 1.42 q
software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All the data were statistically analyzed by one-way
analysis of variance procedure of Statistical Analysis System (SAS
2003, v. 9.1; SAS Institute, Inc., Cary, NC, USA). This was
followed by the Tukey's test, when significant differences were
found. Experiments were repeated three times, and the sample size
was six. The data are expressed as the mean ± standard error.
P<0.05 was considered to indicate a statistically significant
different.
Results
Free radicals scavenging capacity of
DMG-Na
The free radical scavenging capacity of the
water-soluble pigments (DMG-Na, betalain, capsanthin and
cyanidin-3-rutinoside) in comparison to that of the synthetic
antioxidant Trolox is presented in Fig. 1. The free radical scavenging
capacity of DMG-Na was measured relative to Trolox, which was set
as 100% at all concentrations measured (from 0.08-0.64 M). DMG-Na
exhibited the highest free radical scavenging capacity (DPPH,
ABTS+, O2-,
H2O2 and FRAP) at a concentration of 0.32 M,
from the concentrations of 0.08-0.64 M that were tested.
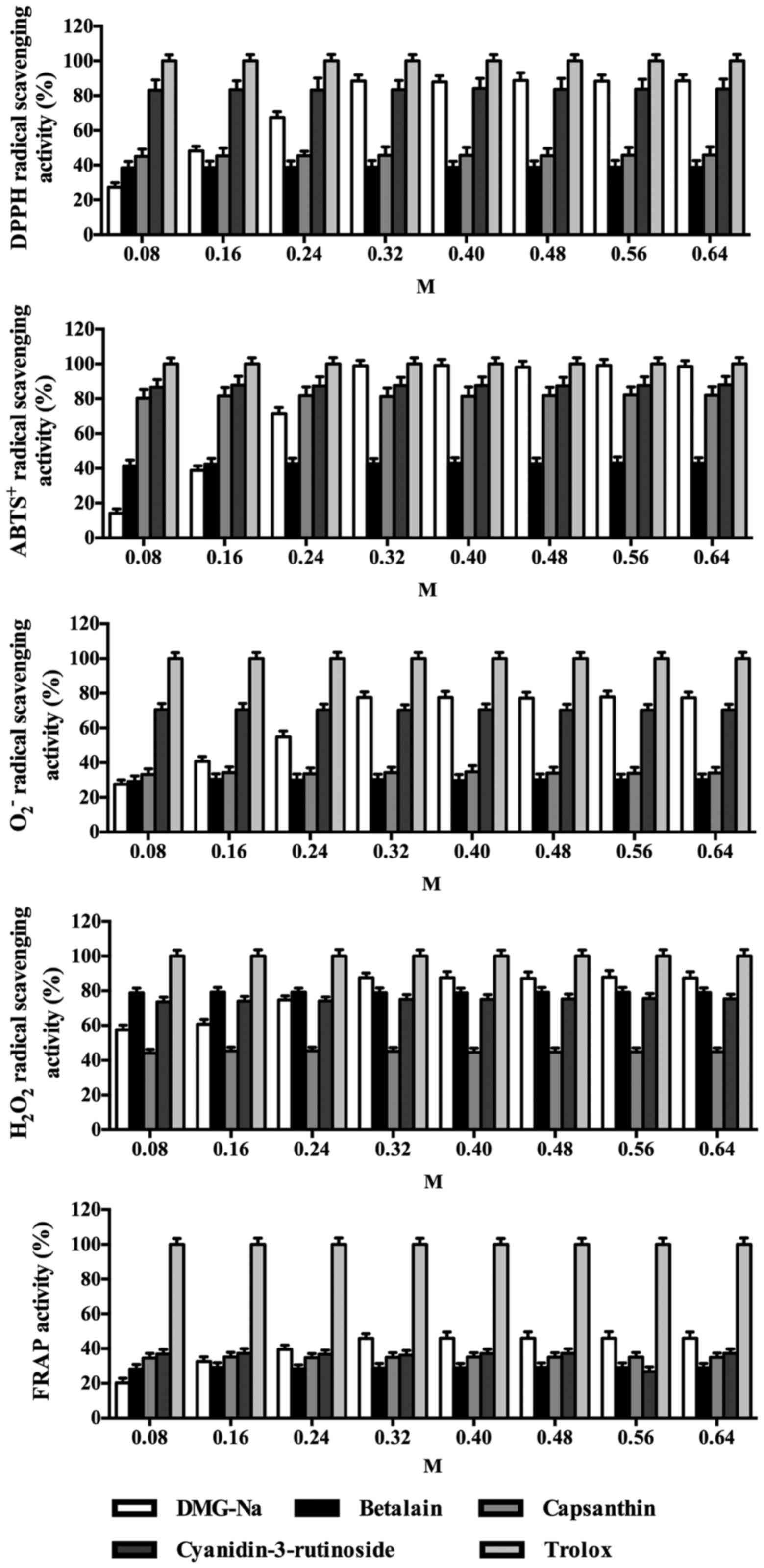 | Figure 1Free radical scavenging capacity of
DMG-Na. DPPH, ABTS+, O2- and
H2O2 radical scavenging activity, and FRAP
activity were measured. The values of Trolox are set to 100%. The
experiment was repeated three times, and six repetitions per group.
Values are presented as the mean ± standard error. DPPH,
1,1-diphenyl-2-pierylhydrazy; ABTS+,
2,2'-azinobis-(3-ethylbenzthiazoline-6-sulphonate);
O2-, superoxide radical;
H2O2, hydrogen peroxide; FRAP,
ferric-reducing antioxidant power; DMG-Na, dimethylglycine sodium
salt. |
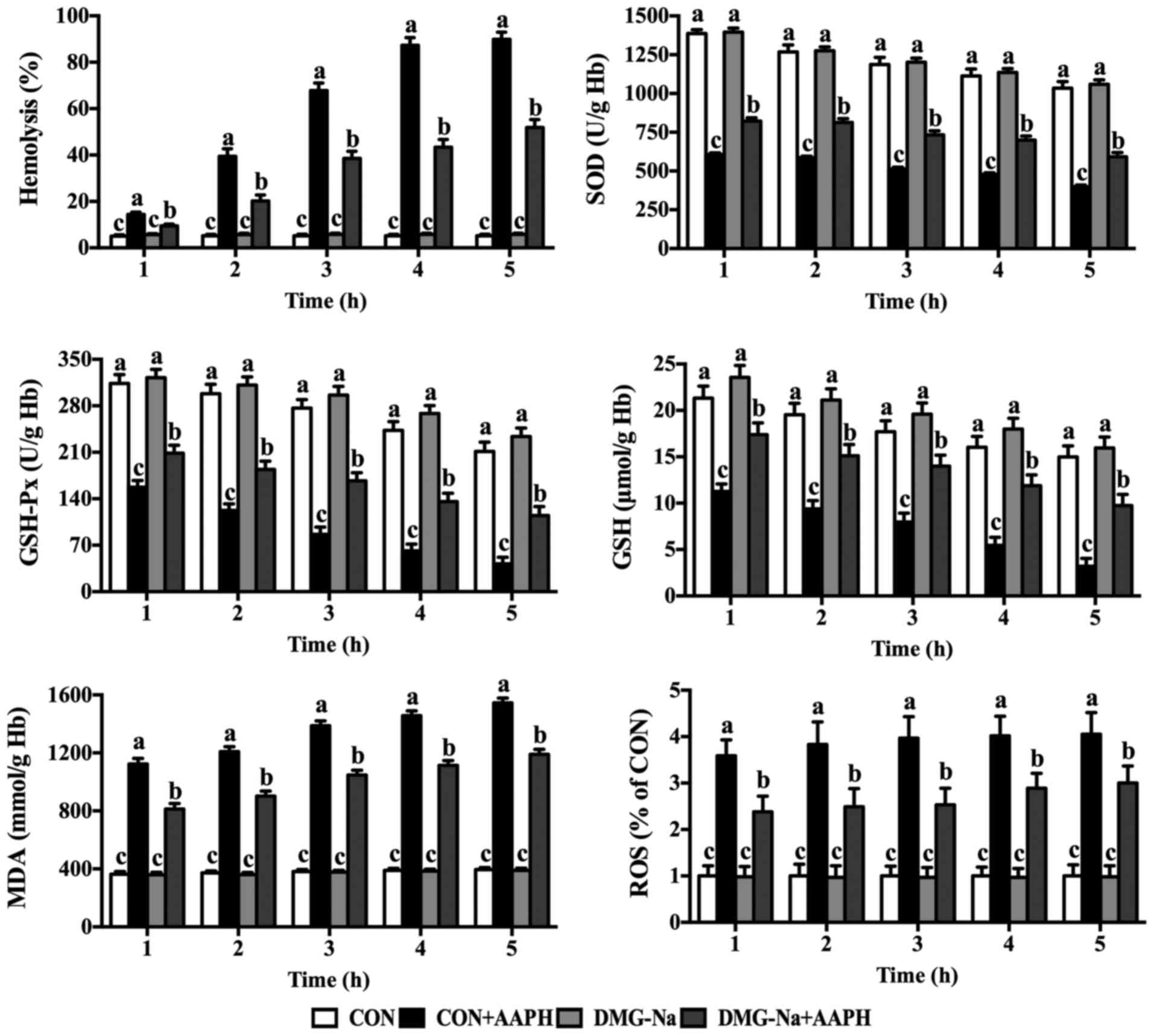
Hemolysis and antioxidant system of
porcine erythrocytes
Fig. 2
demonstrates the effects of DMG-Na on porcine erythrocytes exposed
to the water-soluble radical initiator AAPH. When AAPH was added to
the suspension, hemolysis induction increased in a time-dependent
manner. DMG-Na prevented hemolysis in AAPH-challenged erythrocytes
(P<0.05). As demonstrated in Fig.
2, DMG-Na protected the porcine erythrocytes from AAPH damage
by increasing the cellular antioxidant enzyme activity (P<0.05)
while inhibiting intracellular MDA and ROS formation
(P<0.05).
Antioxidant system of IPEC-J2 cells
The effects of DMG-Na on the antioxidant capacity
(SOD, GSH-Px, GSH, GR and MDA) of IPEC-J2 cells are presented in
Figs. 3 and 4. The culture supernatant and cell
lysate in the DTO group had greater antioxidant enzyme levels
(P<0.05) and lower MDA concentrations in comparison to those in
the TO group (P<0.05). Compared with the TO group, the DTO group
also exhibited increased enzyme activity of MnSOD, GPx, GSH, GR and
γ-GCL (P<0.05; Fig. 5).
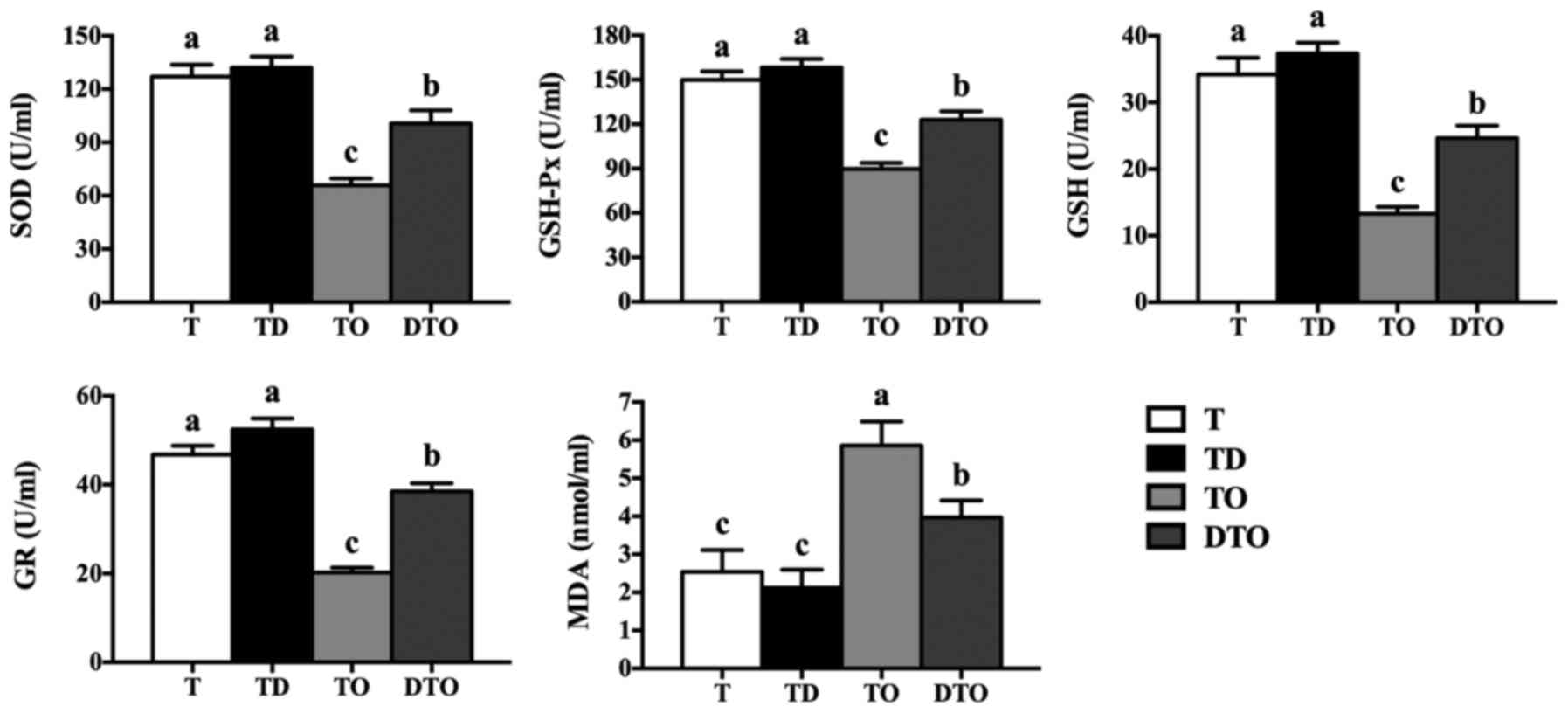 | Figure 3Effects of DMG-Na on the antioxidant
capacity of OAHPx-damaged IPEC-J2 cells culture supernatants. The
experiment was repeated three times, and six repetitions per group.
Values are presented as the mean ± standard error. Groups with
different letters were significantly different to each other
(P<0.05). OAHPx, oleic acid hydroperoxides; T, PBS group; TD, 32
µM DMG-Na; TO, 20 µM OAHPx; DTO, 32 µM DMG-Na
followed by 20 µM OAHPx; SOD, superoxide dismutase; GSH-Px,
glutathione peroxidase; GSH, glutathione; GR, glutathione
reductase; MDA, malondialdehyde. |
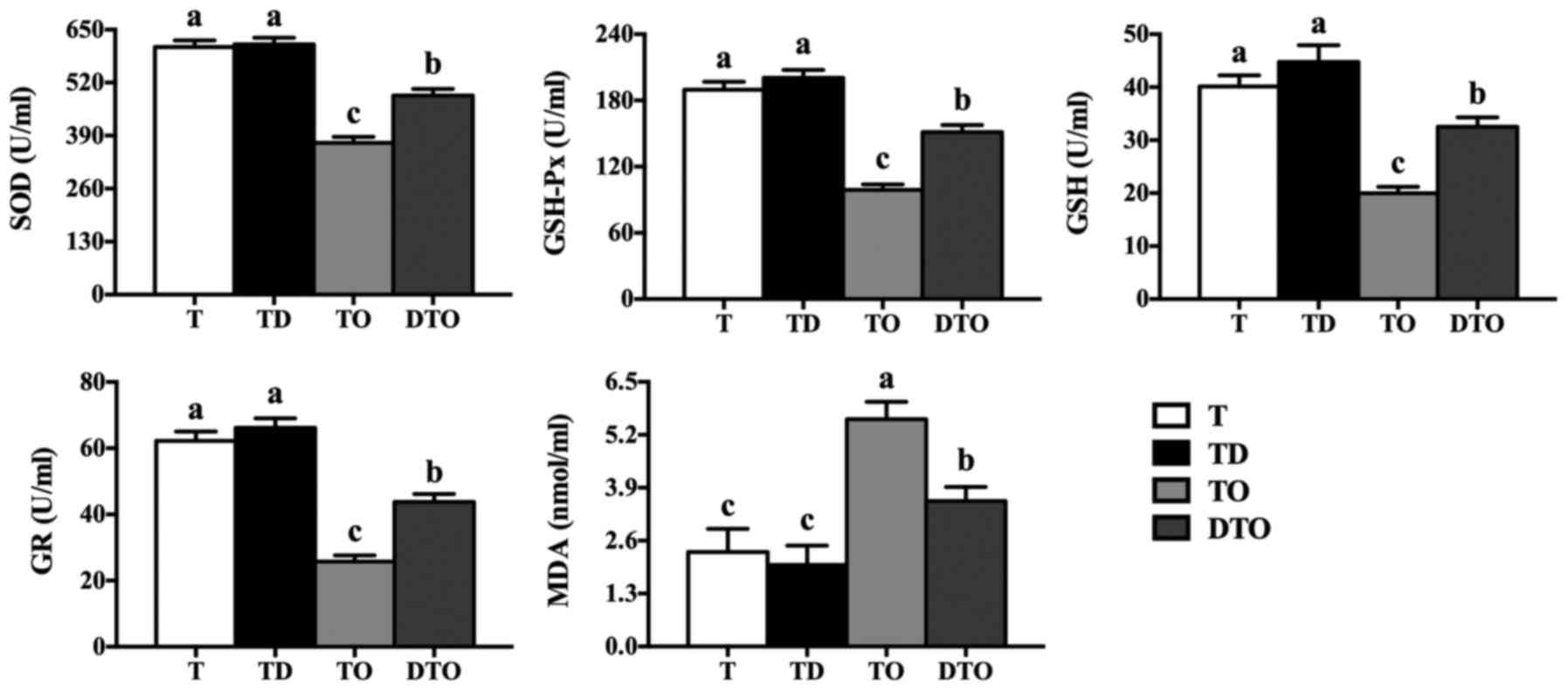 | Figure 4Effects of DMG-Na on the antioxidant
capacity of OAHPx-damaged IPEC-J2 cell lysates. The experiment was
repeated three times, and six repetitions per group. Values are
presented as the mean ± standard error. Groups with different
letters were significantly different to each other (P<0.05).
OAHPx, oleic acid hydroperoxides; T, PBS group; TD, 32 µM
DMG-Na; TO, 20 µM OAHPx; DTO, 32 µM DMG-Na followed
by 20 µM OAHPx; SOD, superoxide dismutase; GSH-Px,
glutathione peroxidase; GSH, glutathione; GR, glutathione
reductase; MDA, malondialdehyde. |
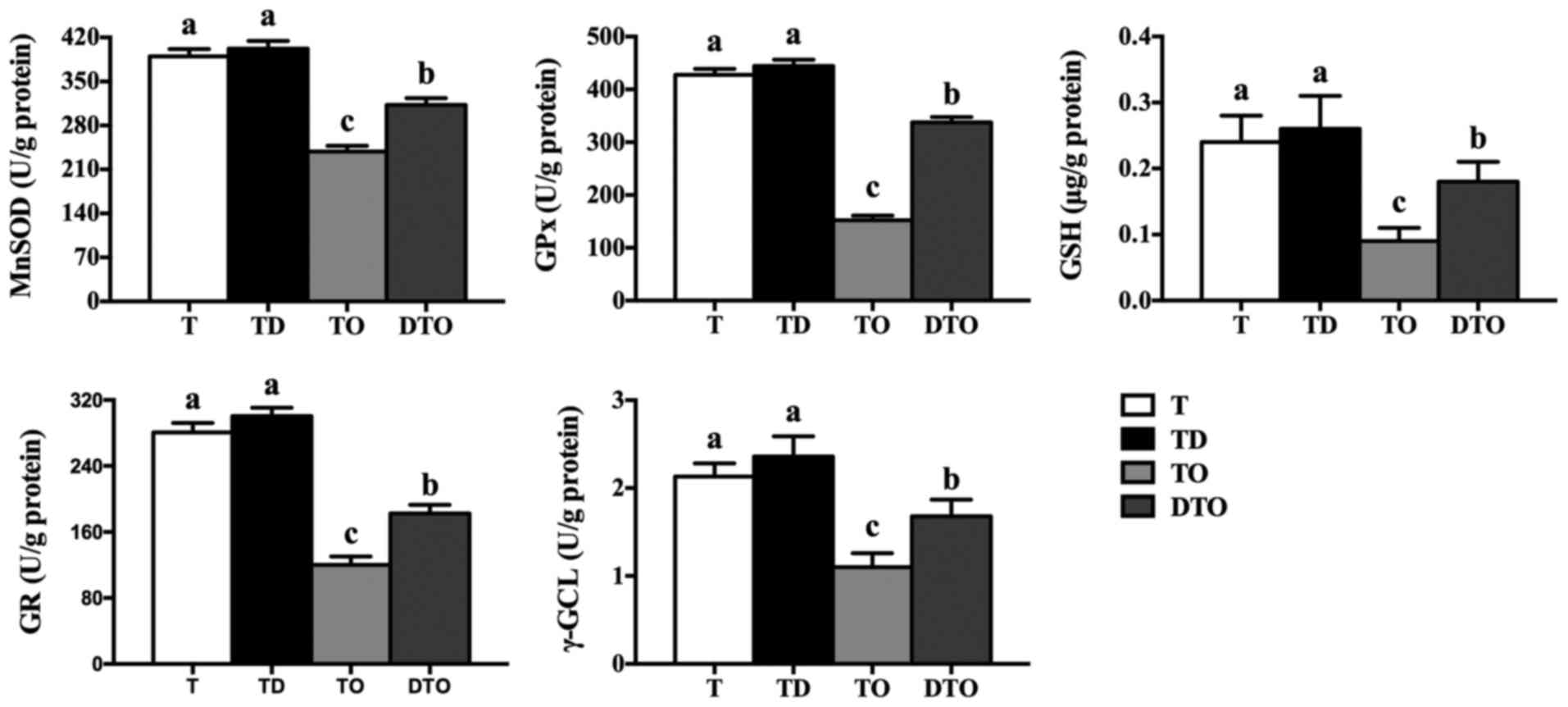 | Figure 5Effects of DMG-Na on the
mitochondrial antioxidant capacity of OAHPx-damaged IPEC-J2 cells.
The experiment was repeated three times, and six repetitions per
group. Values are presented as the mean ± standard error. Groups
with different letters were significantly different to each other
(P<0.05). OAHPx, oleic acid hydroperoxides; T, PBS group; TD, 32
µM DMG-Na; TO, 20 µM OAHPx; DTO, 32 µM DMG-Na
followed by 20 µM OAHPx; MnSOD, manganese superoxide
dismutase; GPx, glutathione peroxidase; GSH, glutathione; GR,
glutathione reductase; γ-GCL, γ-glutamylcysteine ligase. |
The effect of DMG-Na on the oxidative damage (ROS,
PC, 8-OHdG, MMP, apoptosis and necrosis level) of IPEC-J2 cells is
presented Fig. 6. Compared with
the TO group, the DTO group exhibited lower ROS, PC, 8-OHdG,
apoptosis and necrosis levels (P<0.05), along with higher MMP
levels (P<0.05).
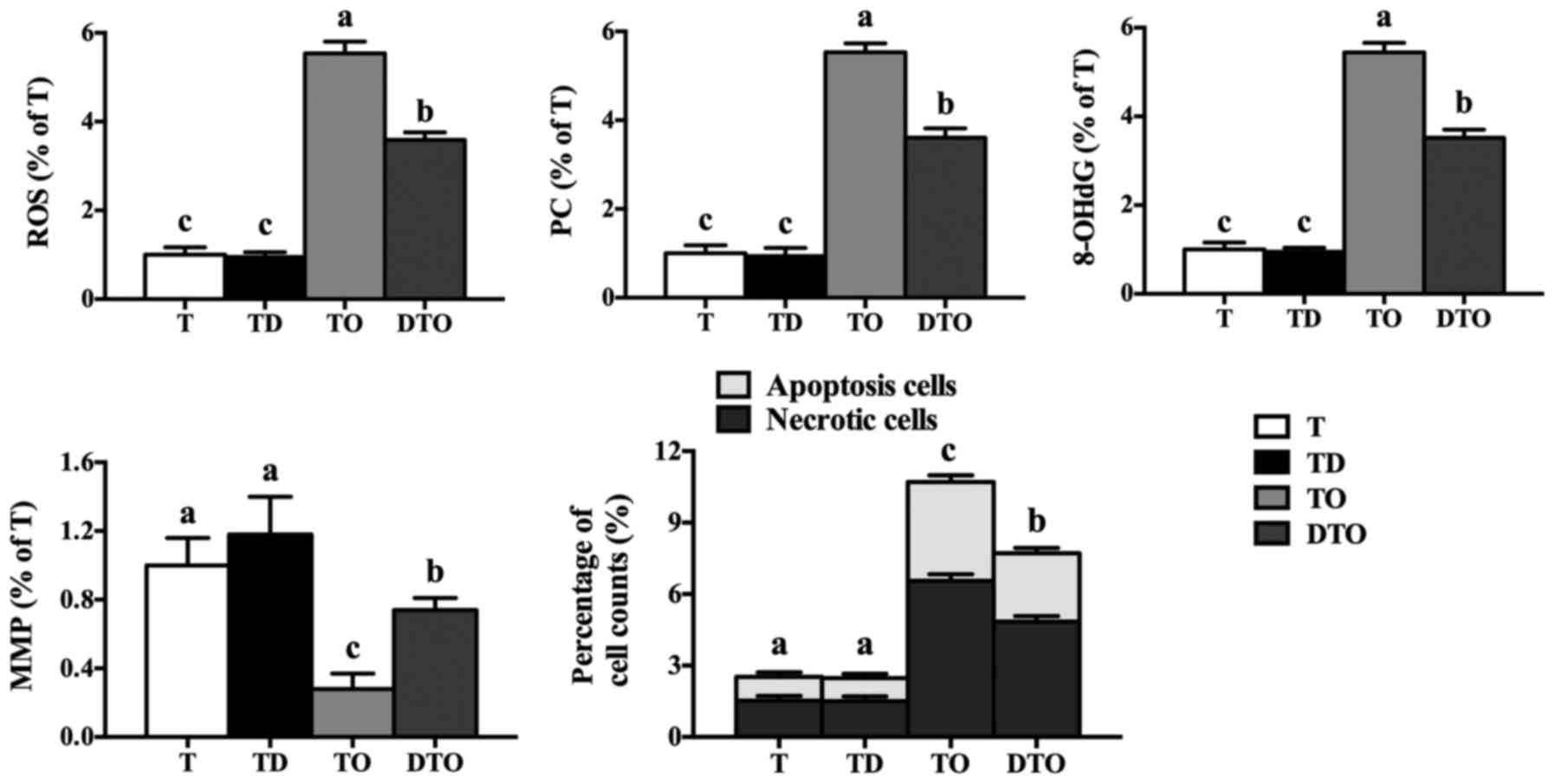 | Figure 6Effects of DMG-Na on the oxidative
damage of OAHPx-damaged IPEC-J2 cells. The experiment was repeated
three times, and six repetitions per group. Values are presented as
the mean ± standard error. Groups with different letters were
significantly different to each other (P<0.05). OAHPx, oleic
acid hydroperoxides; T, PBS group; TD, 32 µM DMG-Na; TO, 20
µM OAHPx; DTO, 32 µM DMG-Na followed by 20 µM
OAHPx; ROS, reactive oxygen species; PC, protein carbonyls; 8-OHdG,
8-hydroxy-2-deoxyguanosine; MMP, mitochondrial membrane
potential. |
Anti-oxidant gene and protein expression
in IPEC-J2 cells
The effects of DMG-Na on gene expression levels in
IPEC-J2 cells are demonstrated in Fig. 7. Compared with the TO group, the
DTO group exhibited greater levels of the genes copper and zinc
superoxide dismutase, GSH-Px, MnSOD, Sirt1, γ-GCLc, γ-GCLm,
thioredoxin 2 (Trx2), thioredoxin reductase 2 (Trx-R2),
peroxiredoxin 3 (Prx3), occludin (OCLN) and zonula occludens-1
(ZO1; P<0.05), and lower levels of the genes Nrf2, HO-1, claudin
2 and claudin 3 (P<0.05). The DTO group also exhibited a higher
protein level of Sirt1 (P<0.05), and lower (P<0.05) levels of
the proteins Nrf2 and HO-1 compared with the TO group (Fig. 8).
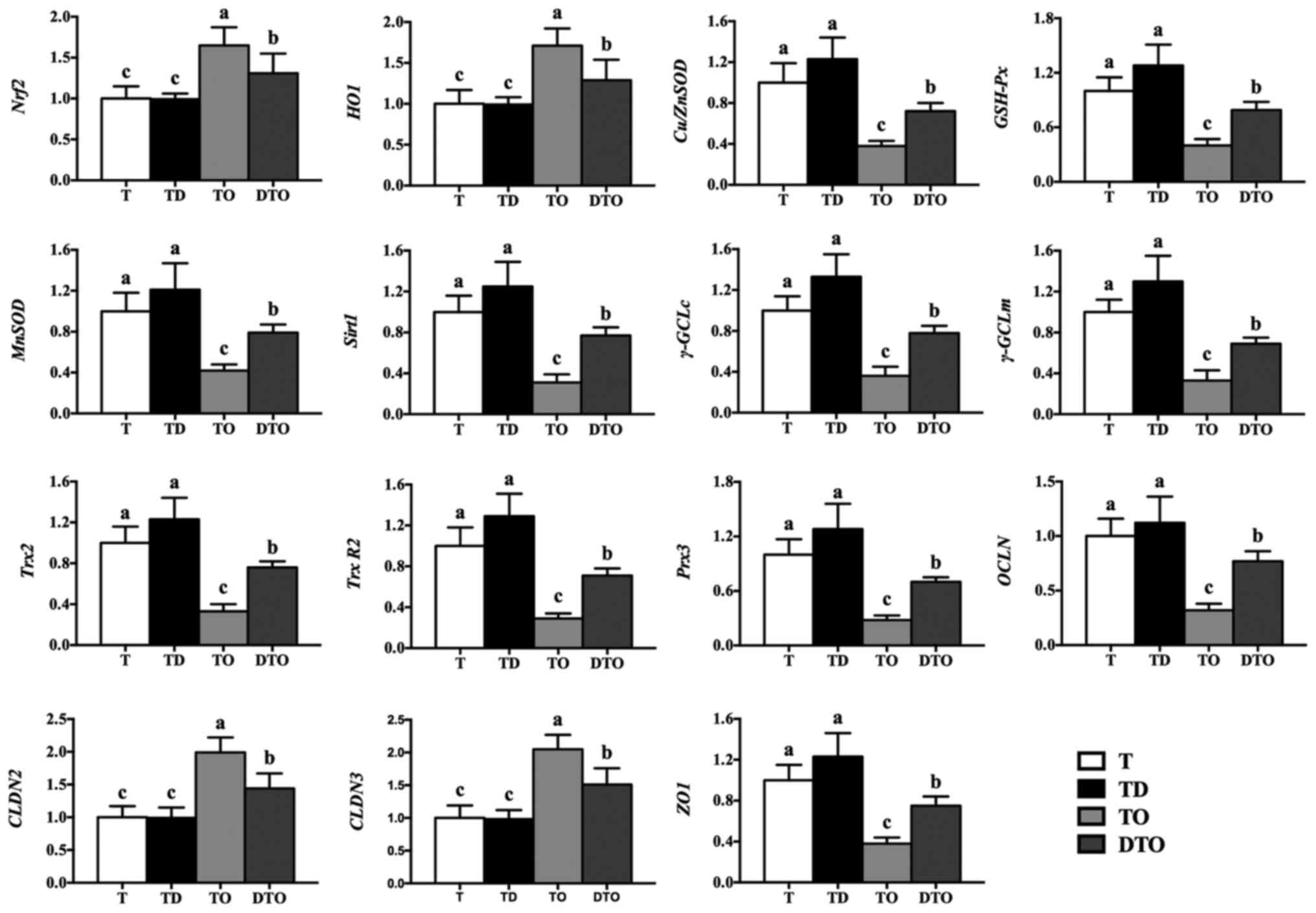 | Figure 7Effects of DMG-Na on gene expression
in OAHPx-damaged IPEC-J2 cells. The experiment was repeated three
times, and six repetitions per group. Values are presented as the
mean ± standard error. Groups with different letters were
significantly different to each other (P<0.05). OAHPx, oleic
acid hydroperoxides; T, PBS group; TD, 32 µM DMG-Na; TO, 20
µM OAHPx; DTO, 32 µM DMG-Na followed by 20 µM
OAHPx; Nrf2, nuclear factor ery-throid 2-related factor 2;
HO1, heme oxygenase 1; Cu/ZnSOD, copper and zinc
superoxide dismutase; GSH-Px, glutathione peroxidase;
MnSOD, manganese superoxide dismutase; Sirt1, sirtuin
1; γ-GCLc, γ-glutamylcysteine ligase c; γ-GCLm,
γ-glutamylcysteine ligase m; Trx2, thioredoxin 2;
Trx-R2, thioredoxin reductase 2; Prx3, peroxiredoxin
3; OCLN, occludin; CLDN2, claudin 2; CLDN3,
claudin 3; ZO1, zonula occludens-1. |
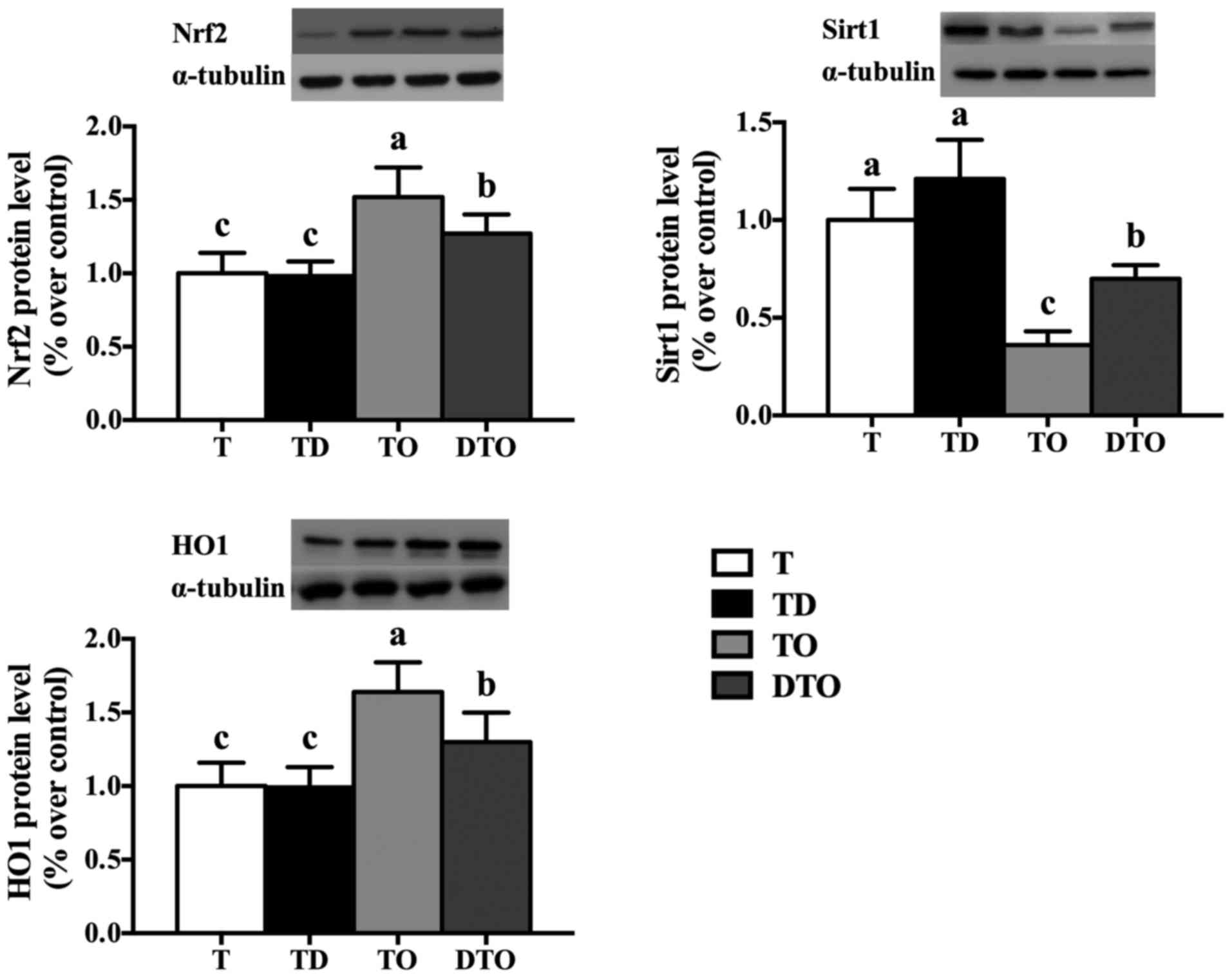 | Figure 8Effects of DMG-Na on Nrf2, HO1, and
Sirt1 protein level of OAHPx-damaged IPEC-J2 cells. The experiment
was repeated three times, and six repetitions per group. Values are
presented as the mean ± standard error. Groups with different
letters were significantly different to each other (P<0.05).
OAHPx, oleic acid hydroperoxides; T, PBS group; TD, 32 µM
DMG-Na; TO, 20 µM OAHPx; DTO, 32 µM DMG-Na followed
by 20 µM OAHPx; Nrf2, nuclear factor erythroid 2-related
factor 2; Sirt1, sirtuin 1; HO1, heme oxygenase 1. |
Discussion
DPPH and ABTS+ radicals have excellent
reproducibility and stability, and for these reasons, are the two
chromogenic compounds most commonly used to determine the capacity
of a compound to inhibit free radicals. Reaction with the
antioxidant DPPH neutralizes excess free radicals by transferring
either an electron or a hydrogen atom (33). The ABTS+
decol-orization assay is used for the rapid measurement of the
total antioxidant activity of individual chemical compounds
(34). In the case of cellular
oxidation reactions, O2- and
H2O2 are two of the most common free radicals
produced, that then produce other cell-damaging free radicals and
oxidizing agents in vivo (35,36). Of all the antioxidant capacity
detection assays, the FRAP assay is the only one that directly
measures antioxidants in samples and is useful in the measurement
of oxidative damage (37). In the
present study, Trolox was used as a standard substance that exerts
strong free radical scavenging capacity in vitro. DMG-Na
exhibited the greatest free radical scavenging activity at a
concentration of 0.32 M, indicating the potential of DMG-Na as a
free radical scavenger. Similar to our results, Hariganesh and
Prathiba (2) reported the free
radical scavenging potential of orally-administered DMG-Na in rats
subjected to oxidative stress. As DMG-Na exerts a strong free
radical scavenging capacity in vitro, whether DMG-Na could
prevent oxidative damage in a cell model was assessed in the
current study.
Based on the results of free radical scavenging
assays, DMG-Na was selected for further evaluation of its
protective effect against hemolysis in AAPH-challenged porcine
erythrocytes. Erythrocytes possess a high membrane concentration of
polyunsaturated fatty acids, have a specific role as oxygen
carriers and are considered to be the major targets for free
radical attacks (38). In the
present study, DMG-Na prevented AAPH-induced hemolysis of
erythrocyte membranes. It has been reported that DMG, a raw
material used in the synthesis of glutathione, is an important
antioxidant that can protect the body from oxidative damage
(1). Thus, the powerful
protection of DMG-Na against AAPH-induced hemolysis in the porcine
erythrocyte model may be explained by its strong free radical
scavenging capacity. Erythrocyte membrane lipids can lose a
hydrogen atom from an unsaturated fatty acyl chain when they are
subjected to oxidative damage (39).
This step initiates lipid peroxidation, which then
propagates as a chain reaction. Oxidative damage can destroy the
lipid bilayer membrane structure, alter membrane permeability,
disrupt ion channels and ultimately lead to dysfunction of whole
erythrocytes (40). In
erythrocytes, oxidative damage induced by free radicals can be
prevented by the SOD enzyme, which protects cells by dismutating
the highly reactive O2- species to
H2O2, which is then neutralized by GSH-Px and
GSH (41,42). These results are consistent with
those reported by Deng et al (43) and Tedesco et al (44) that reported the protective roles
of antioxidant substances against oxidative damage in human
erythrocytes. The results of the present study suggest that DMG-Na
exerted strong protective effects against lipid peroxidation and
indicates that it could reduce the MDA and ROS levels in
AAPH-challenged porcine erythrocytes. The present results provide
strong evidence for DMG-Na application in oxidative
damage-associated diseases; however, further study regarding the
specific mechanisms involved is still required.
The small intestine is the main organ that digests
and absorbs nutrients, and is the first line of defense against
foreign pathogenic microorganisms and toxins. The ability of
foreign substances to cross the cell membrane into intestinal cells
depends on either diffusion or active transport (45). Oxidative damage is caused by
enhanced levels of ROS, which can destroy the mitochondrial
structure and decrease antioxidant capacity. Previous studies have
indicated that DMG is an important antioxidant that can prevent
oxidative damage in the body by scavenging excess free radicals
(1). In cells, oxidative damage
induced by free radicals can be prevented by the enzyme, SOD, which
catalyzes the conversion of endogenous superoxide anions to
hydrogen peroxide through disproportionation; hydrogen peroxide is
then neutralized by the intracellular GSH-Px enzyme (41). The mitochondrial antioxidant
system is composed of enzymatic and non-enzymatic antioxidants that
protect the body from oxidative damage (46). It has been reported that the MnSOD
enzyme and GSH-associated metabolic enzymes are important members
of the mitochondrial antioxidant system and are crucial in
suppressing oxidative damage by neutralizing excess ROS and acting
as a substrate for various other antioxidant enzymes (27). γ-GCL is an important rate-limiting
enzyme that is involved in the synthesis of γ-glutamylcysteine and
is crucial for relieving oxidative damage (28). A previous study reported that
DMG-Na acts as an important component in relieving oxida-tive
damage by enhancing antioxidant capacity (1,47).
Results from the present study suggest that DMG-Na may improve the
total antioxidant capacity by scavenging excess ROS, so as to
maintain the intracellular redox balance.
When oxidative damage occurs, the blockade of
oxidative phosphorylation reactions can increase the levels of ROS
(48). The excess ROS harms
biological macromolecular substances, and exacerbates the oxidative
damage through lipid peroxidation and protein oxidation reactions
(49). Mitochondria are also
prone to becoming damaged by the excess ROS (50). This information is supported by
the findings of the present study, where the damaged IPEC-J2 cells
exhibited higher concentrations of MDA, PC, 8-OHdG and ROS compared
with those in the T group. It has been reported that DMG, a natural
antioxidant, can effectively neutralize free radicals to alleviate
oxidative damage in the body (2).
It was hypothesized that pre-treatment with DMG-Na could minimize
lipid peroxidation and protein oxidation reactions by scavenging
excess ROS in cells. MMP expression is negatively associated with
ROS concentration, and acts as an indicator for the beginning of
mitochondria-dependent apoptosis. When MMP levels are reduced,
there is uncoupling of oxidative phosphorylation in the
mitochondria, which results in an increase in ROS levels and
inevitably leads to apoptosis (46). The present study indicates that
the administration of DMG-Na may lead to relief of oxidative
damage. This is supported by previous studies demonstrating that
natural antioxidants could inhibit apoptosis by protecting cells
from oxidative damage (1,51). We hypothesize that there is a
correlation between the anti-apoptotic effect of DMG-Na and the
protective effects of DMG-Na on mitochondrial function in damaged
IPEC-J2 cells; however, further study is still required.
The activation of the genes Nrf2, HO-1
and SIRT1 is important in relieving oxidative damage, and is
also crucial in regulating the gene expressions of certain
important antioxidant enzymes (52-55). Mitochondria are rich in Trx2,
Trx-R2 and Prx3, which together compose a unique antioxidant system
that prevents oxidative stress by scavenging free radicals and
regulating mitochondrial-dependent apoptotic pathways (56). The gene ZO1 is correlated
with paracellular permeability, and together with the genes
OCLN and CLDN from the same gene family, it is a key
regulator of intestinal permeability (57). A previous study suggested that a
natural antioxidant was beneficial in the regulation of
antioxidant-associated gene expression (47,51). The current study indicated that
the regulation of antioxidant-associated genes may be one mechanism
used by IPEC-J2 cells to prevent oxidative stress and maintain
mitochondrial function.
The present study indicates that DMG-Na is a strong
free radical quencher. Furthermore, DMG-Na reduced lipid
peroxidation, increased antioxidant enzyme activity and attenuated
hemolysis in AAPH-challenged porcine erythrocytes. The present
study also demonstrated that DMG-Na effectively prevented the
oxidative damage of IPEC-J2 cells by scavenging excess ROS,
preventing mitochondrial dysfunction and inhibiting cell apoptosis.
Based on these results, it is speculated that DMG-Na may directly
neutralize excess free radicals, and act indirectly on the
improvement of antioxidant enzyme activity and inhibit the abnormal
expression of stress-associated factors.
Acknowledgements
Not applicable
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KB and LJ analyzed and interpreted the IPEC- J2 cell
data. KB, LigenZ, YZ, YL, JZ, JC, LiliZ and TW performed the whole
experiment, and LJ was a major contributor in writing the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures were approved by the Institutional
Animal Care and Use Committee of Nanjing Agricultural University
(Nanjing, China)
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
DMG-Na
|
dimethylglycine sodium salt
|
|
OAHPx
|
oleic acid hydroperoxides
|
|
ROS
|
reactive oxygen species
|
|
DPPH
|
1,1-diphenyl-2-pierylhydrazy
|
|
ABTS+
|
2,2'-azinobis-
(3-ethylbenzthiazoline-6-sulphonate)
|
|
O2-
|
superoxide radical
|
|
H2O2
|
hydrogen peroxide
|
|
FRAP
|
ferric-reducing antioxidant power
|
|
SOD
|
superoxide dismutase
|
|
GSH-Px
|
glutathione peroxidase
|
|
GSH
|
glutathione
|
|
GR
|
glutathione reductase
|
|
MDA
|
malondialdehyde
|
|
MnSOD
|
manganese superoxide dismutase
|
|
GPx
|
glutathione peroxidase
|
|
γ-GCL
|
γ-glutamylcysteine ligase
|
|
PC
|
protein carbonyls
|
|
8-OHdG
|
8-hydroxy-2-deoxyguanosine
|
|
MMP
|
mitochondrial membrane potential
|
References
|
1
|
Friesen RW, Novak EM, Hasman D and Inniset
SM: Relationship of dimethylglycine, choline, and betaine with
oxoproline in plasma of pregnant women and their newborn infants. J
Nutr. 137:2641–2646. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hariganesh K and Prathiba J: Effect of
dimethylglycine on gastric ulcers in rats. J Pharm Pharmacol.
52:1519–1522. 2000. View Article : Google Scholar
|
|
3
|
Clapes P and Infante MR: Amino acid-based
surfactants: Enzymatic synthesis, properties and potential
applications. J Biocatalys Biotransformation. 20:215–233. 2002.
View Article : Google Scholar
|
|
4
|
Giacco F and Brownlee M: Oxidative stress
and diabetic complications. Circ Res. 107:1058–1070. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grune T: Oxidative stress, aging and the
proteasomal system. Biogerontology. 1:31–40. 2000. View Article : Google Scholar
|
|
6
|
Ma K, Zhang Y, Zhu D and Lou Y: Protective
effects of asiatic acid against
D-galactosamine/lipopolysaccharide-induced hepa-totoxicity in
hepatocytes and kupffer cells co-cultured system via
redox-regulated leukotriene C4 synthase expression pathway. Eur J
Pharmacol. 603:98–107. 2009. View Article : Google Scholar
|
|
7
|
Balaban RS, Nemoto S and Finkel T:
Mitochondria, oxidants, and aging. Cell. 120:4832005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Andreyev AY, Kushnareva YE and Starkov AA:
Mitochondrial metabolism of reactive oxygen species. Biochemistry
(Mosc). 70:200–214. 2005. View Article : Google Scholar
|
|
9
|
Dong L, Zhong X, He J, Zhang L, Bai K, Xu
W, Wang T and Huang X: Supplementation of tributyrin improves the
growth and intestinal digestive and barrier functions in
intrauterine growth-restricted piglets. Clin Nutr. 35:399–407.
2016. View Article : Google Scholar
|
|
10
|
Inoue J, Miki I, Tanahashi T, Kawauchi S,
Azuma T and Mizuno S: Mo2016 effect of ghrelin on
indomethacin-induced small intestinal injury in mice.
Gastroenterology. 144:S-7192013. View Article : Google Scholar
|
|
11
|
Konaka A, Kato S, Tanaka A, Kunikata T,
Korolkiewicz R and Takeuchi K: Roles of enterobacteria, nitric
oxide and neutrophil in pathogenesis of indomethacin-induced small
intestinal lesions in rats. Pharmacol Res. 40:517–524. 1999.
View Article : Google Scholar
|
|
12
|
Liu F, Li G, Wen K, Bui T, Cao D, Zhang Y
and Yuan L: Porcine small intestinal epithelial cell line (IPEC-J2)
of rotavirus infection as a new model for the study of innate
immune responses to rota-viruses and probiotics. Viral Immunol.
23:135–149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brosnahan AJ and Brown DR: Porcine IPEC-J2
intestinal epithelial cells in microbiological investigations. Vet
Microbiol. 156:229–237. 2012. View Article : Google Scholar :
|
|
14
|
Arce C, Ramírez-Boo M, Lucena C and
Garrido JJ: Innate immune activation of swine intestinal epithelial
cell lines (IPEC-J2 and IPI-2I) in response to LPS from salmonella
typhimurium. Comp Immunol Microbiol Infect Dis. 33:161–174. 2010.
View Article : Google Scholar
|
|
15
|
Moon JK and Shibamoto T: Antioxidant
assays for plant and food components. J Agric Food Chem.
57:1655–1666. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Siddhuraju P and Manian S: The antioxidant
activity and free radical-scavenging capacity of dietary phenolic
extracts from horse gram (Macrotyloma uniflorum (Lam) Verdc) seeds.
Food Chem. 105:950–958. 2007. View Article : Google Scholar
|
|
17
|
Chen HY and Yen GC: Antioxidant activity
and free radical-scavenging capacity of extracts from guava
(Psidium guajava L.) leaves. Food Chem. 101:686–694. 2007.
View Article : Google Scholar
|
|
18
|
Zhang J, Hou X, Ahmad H, Zhang H, Zhang L
and Wang T: Assessment of free radicals scavenging activity of
seven natural pigments and protective effects in AAPH-challenged
chicken erythrocytes. Food Chem. 145:57–65. 2014. View Article : Google Scholar
|
|
19
|
Benzie IF and Strain JJ: The ferric
reducing ability of plasma (FRAP) as a measure of ‘antioxidant
power’: The frap assay. Anal Biochem. 239:70–76. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Magalhães AS, Silva BM, Pereira JA,
Andrade PB, Valentão P and Carvalho M: Protective effect of quince
(cydonia oblonga miller) fruit against oxidative hemolysis of human
erythrocytes. Food Chem Toxicol. 47:1372–1377. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lawrence RA and Burk RF: Glutathione
peroxidase activity in selenium-deficient rat liver. Biochem
Biophys Res Commun. 71:952–958. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Panchenko LF, Brusov OS, Gerasimov AM and
Loktaeva TD: Intramitochondrial localization and release of rat
liver superoxide dismutase. FEBS Lett. 55:84–87. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sang H, Zhang L and Li J:
Anti-benzopyrene-7,8-diol-9,10-epoxide induces apoptosis via
mitochondrial pathway in human bronchiolar epithelium cells
independent of the mitochondria permeability transition pore. Food
Chem Toxicol. 50:2417–2423. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
AOAC: Official Methods of Analysis. 14th
edition. Association of Official Analytical Chemists; Washington,
DC: 1985
|
|
25
|
Aw TY, Williams MW and Gray L: Absorption
and lymphatic transport of peroxidized lipids by rat small
intestine in vivo: Role of mucosal GSH. Am J Physiol. 262:G99–G106.
1992.PubMed/NCBI
|
|
26
|
Repetto G, Peso AD and Zurita JL: Neutral
red uptake assay for the estimation of cell viability/cytotoxicity.
Nat Protoc. 3:1125–1131. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang X, Gao J, Wang Y, Fan YM, Xu LZ, Zhao
XN, Xu Q and Qian ZM: Effective protection of terminalia catappa l.
Leaves from damage induced by carbon tetrachloride in liver
mitochondria. J Nutr Biochem. 17:177–182. 2006. View Article : Google Scholar
|
|
28
|
Langston JW, Li W, Harrison L and Aw TY:
Activation of promoter activity of the catalytic subunit of
γ-glutamylcysteine ligase (GCL) in brain endothelial cells by
insulin requires antioxidant response element 4 and altered
glycemic status: Implication for GCL expression and GSH synthesis.
Free Radic Biol Med. 51:1749–1757. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Van Remmen H, Ikeno Y, Hamilton M,
Pahlavani M, Wolf N, Thorpe SR, Alderson NL, Baynes JW, Epstein CJ,
Huang TT, et al: Life-long reduction in MnSOD activity results in
increased DNA damage and higher incidence of cancer but does not
accelerate aging. Physiol Genomics. 16:29–37. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wei QY, Chen WF, Zhou B, Yang L and Liu
ZL: Inhibition of lipid peroxidation and protein oxidation in rat
liver mitochondria by curcumin and its analogues. Biochim Biophys
Acta. 1760:70–77. 2006. View Article : Google Scholar
|
|
31
|
Zhang Q, Zou P, Zhan H, Zhang M, Zhang L,
Ge RS and Huang Y: Dihydrolipoamide dehydrogenase and cAMP are
associated with cadmium-mediated leydig cell damage. Toxicol Lett.
205:183–189. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative pcr and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
33
|
Naik GH, Priyadarsini KI, Satav JG,
Banavalikar MM, Sohoni DP, Biyani MK and Mohan H: Comparative
antioxidant activity of individual herbal components used in
ayurvedic medicine. Phytochemistry. 63:97–104. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
JiSang K and YoungSoon L: Antioxidant
activity of Maillard reaction products derived from aqueous
glucose/glycine, diglycine, and triglycine model systems as a
function of heating time. Food Chem. 116:227–232. 2009. View Article : Google Scholar
|
|
35
|
Rollet-Labelle E, Grange MJ, Elbim C,
Marquetty C, Gougerot-Pocidalo MA and Pasquier C: Hydroxyl radical
as a potential intracellular mediator of polymorphonuclear
neutrophil apoptosis. Free Radic Biol Med. 24:563–572. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang J, Yuan X, Jin Z, Tian Y and Song H:
Free radical and reactive oxygen species scavenging activities of
peanut skins extract. Food Chem. 104:242–250. 2007. View Article : Google Scholar
|
|
37
|
Bernaert N, De Paepe D, Bouten C, De
Clercq H, Stewart D, Van Bockstaele E, De Loose M and Van
Droogenbroeck B: Antioxidant capacity, total phenolic and ascorbate
content as a function of the genetic diversity of leek (Allium
ampeloprasum var. porrum) Food Chem. 134:669–677. 2012. View Article : Google Scholar
|
|
38
|
Ajila CM and Prasada Rao UJ: Protection
against hydrogen peroxide induced oxidative damage in rat
erythrocytes by mangifera indica L. peel extract. Food Chem
Toxicol. 46:303–309. 2008. View Article : Google Scholar
|
|
39
|
Mendes L, de Freitas V, Baptista P and
Carvalho M: Comparative antihemolytic and radical scavenging
activities of strawberry tree (Arbutus unedo L.) leaf and fruit.
Food Chem Toxicol. 49:2285–2291. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lang F, Lang KS, Lang PA, Huber SM and
Wieder T: Mechanisms and significance of eryptosis. Antioxid Redox
Signal. 8:1183–1192. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bayrak O, Uz E, Bayrak R, Turgut F, Atmaca
AF, Sahin S, Yildirim ME, Kaya A, Cimentepe E and Akcayet A:
Curcumin protects against ischemia/reperfusion injury in rat
kidneys. World J Urol. 26:285–291. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Forman HJ, Zhang H and Rinna A:
Glutathione: Overview of its protective roles, measurement, and
biosynthesis. Mol Aspects Med. 30:1–12. 2009. View Article : Google Scholar :
|
|
43
|
Deng SL, Chen WF, Zhou B, Yang L and Liu
ZL: Protective effects of curcumin and its analogues against free
radical-induced oxidative haemolysis of human red blood cells. Food
Chem. 98:112–119. 2006. View Article : Google Scholar
|
|
44
|
Tedesco I, Luigi Russo G, Nazzaro F, Russo
M and Palumbo R: Antioxidant effect of red wine anthocyanins in
normal and catalase-inactive human erythrocytes. J Nutr Biochem.
12:505–511. 2001. View Article : Google Scholar
|
|
45
|
Nusrat A, Parkos CA, Verkade P, Foley CS,
Liang TW, Innis-Whitehouse W, Eastburn KK and Madara JL: Tight
junctions are membrane microdomains. J Cell Sci. 113:1771–1781.
2000.PubMed/NCBI
|
|
46
|
Kowaltowski AJ and Vercesi AE:
Mitochondrial damage induced by conditions of oxidative stress.
Free Radic Biol Med. 26:463–471. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bai K, Xu W, Zhang J, Kou T, Niu Y, Wan X,
Zhang L, Wang C and Wang T: Assessment of free radical scavenging
activity of dimethylglycine sodium salt and its role in providing
protection against lipopolysaccharide-induced oxidative stress in
mice. PLoS One. 11:e01553932016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lee HJ, Oh YK, Rhee M, Lim JY, Hwang JY,
Park YS, Kwon Y, Choi KH, Jo I, Park SI, et al: The role of
STAT1/IRF-1 on synergistic ROS production and loss of mitochondrial
transmembrane potential during hepatic cell death induced by
LPS/d-GalN. J Mol Biol. 369:967–984. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wilhelm EA, Jesse CR, Roman SS, Nogueira
CW and Savegnago L: Hepatoprotective effect of 3-alkynyl
selenophene on acute liver injury induced by D-galactosamine and
lipopoly-saccharide. Exp Mol Pathol. 87:20–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen JJ and Yu BP: Alterations in
mitochondrial membrane fluidity by lipid peroxidation products.
Free Radic Biol Med. 17:411–418. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang J, Xu L, Zhang L, Ying Z, Su W and
Wang T: Curcumin attenuates
D-galactosamine/lipopolysaccharide-induced liver injury and
mitochondrial dysfunction in mice. J Nutr. 144:1211–1218. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chiu H, Brittingham JA and Laskin DL:
Differential induction of heme oxygenase-1 in macrophages and
hepatocytes during acetaminophen-induced hepatotoxicity in the rat:
Effects of hemin and biliverdin. Toxicol Appl Pharmacol.
181:106–115. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chung S, Yao H, Caito S, Hwang JW,
Arunachalam G and Rahman I: Regulation of SIRT1 in cellular
functions: Role of polyphenols. Arch Biochem Biophys. 501:79–90.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hwang JW, Yao H, Caito S, Sundar IK and
Rahman I: Redox regulation of SIRT1 in inflammation and cellular
senescence. Free Radic Biol Med. 61:95–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lee JM and Johnson JA: An important role
of Nrf2-are pathway in the cellular defense mechanism. J Biochem
Mol Biol. 37:139–143. 2004.PubMed/NCBI
|
|
56
|
Michelet L, Zaffagnini M, Massot V, Keryer
E, Vanacker H, Miginiac-Maslow M, Issakidis-Bourguet E and Lemai
SD: Thioredoxins, glutaredoxins, and glutathionylation: New
crosstalks to explore. Photosynth Res. 89:225–245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Gu L, Li N, Gong J, Li Q, Zhu W and Li J:
Berberine ameliorates intestinal epithelial tight-junction damage
and down-regulates myosin light chain kinase pathways in a mouse
model of endotoxinemia. J Infect Dis. 203:1602–1612. 2011.
View Article : Google Scholar : PubMed/NCBI
|