Introduction
In light of social continuous improvement, rapid
economic development and aggravation of population aging, the
number of patients with cardiovascular disease has increased
annually and cardiovascular disease has become an important factor
that threatens human health (1).
The tolerance of myocardial cells to hypoxia injury is poor, and
hypoxia-ischemia can lead to abnormal cardiac electric activity,
necrosis of myocardial cells and cell apoptosis, which may induce
various cardiovascular diseases (2-4).
At present, there is no known radical cure. The normal
physiological function of the heart can be maintained only by
pharmacological remission and stent implantation. Therefore, it is
urgent to develop novel treatment methods to improve and repair the
damaged myocardial cells, eventually achieving a radical cure.
Ghrelin is a specific growth hormone (GH)
secretagogue (GHS) containing 28 amino acid residues, which was
identified in 1999 (5). It is an
endogenous ligand for the GHS receptor (GHSR). GHSR abundantly
exists in the cardiovascular system. The expression of GHSR mRNA is
also detected in human atrial and ventricular myocytes. Ghrelin
combines with a G protein coupled receptor to regulate GH (6-8).
Ghrelin and its receptors are widely distributed in all tissues and
organs, and have numerous biological effects, including increasing
appetite, promoting fat accumulation and protecting the heart
(8,9). In particular, as a vasoactive
peptide, it has a number of protective and repair effects on the
cardiovascular system (8,9). A number of studies have indicated
that in addition to GHSR, ghrelin may have other unknown receptors
in the cardiovascular system and may work independently of GH
(10-13). The present study investigated the
effect of ghrelin on myocardial repair and function through
investigating the expression of ghrelin in the myocardial cells to
provide solid support for the treatment of hypoxic myocardial
injury by ghrelin.
Ghrelin has been known to exert positive effects in
cardiovascular protection and repair. Although based on a
cardiopulmonary bypass (CPB) study, Cao et al (14) reported that cardioprotective
effects elicited by ghrelin may contribute toward the inhibition of
inflammatory response through the protein kinase B (Akt) activated
pathway, this is only one of a number of theories regarding the
mechanism of ghrelin and there remains a great possibility of other
mechanisms of action. Insulin-like growth factor-1 (IGF-1) is a
type of active polypeptide that is necessary for GH to produce a
physiological effect. IGF-1 has functions of regulating
physiological and pathological states of the heart, relaxing blood
vessels, decreasing vascular resistance and increasing cardiac
blood flow (12). Therefore, the
expression of IGF-1 is an important indicator of the normal
function of myocardial cells and tissues. Akt is a kind of Ser/Thr
protein kinase and serves an important role in cell survival and
apoptosis (15). Akt is a
significant component of the phosphatidylinositol 3-kinase
(PI3K)/Akt signaling pathway that is a classic signaling pathway
serving an important role in numerous physiological and
pathological processes, including cell survival, differentiation,
growth and apoptosis via regulating gene expression (16). Akt is generally overexpressed in
damaged or apoptotic cells, thereby promoting the PI3K/Akt
signaling pathway to aggravate cell apoptosis. By contrast, Akt
will be phosphorylated in normal cells to inhibit its activity,
such that the level of phosphorylated Akt (p-Akt) will be
upregulated and the cells or tissues are improved and repaired
(17,18).
Therefore, the present study further revealed the
molecular mechanism of ghrelin expression improving and repairing
cardiac myocytes and myocardium by investigating GH, GHSR, IGF-1,
Akt and p-Akt targets associated with cardiomyocyte metabolism and
apoptosis. This would also provide promising insights into the
treatment of hypoxic myocardial injury by ghrelin.
Materials and methods
Materials and animals
BSA blocking buffer (5%), typsin-EDTA (0.25%) and
type II collagenase were obtained from Beijing Solarbio Science
& Technology Co., Ltd. (Beijing, China). 3,3′-Diaminobenzidine
(DAB) developing kit (cat. no. CW0125), TRIzol reagent (cat. no.
CW0580S), Ultrapure RNA extraction kit (cat. no. CW0581M),
HiFiScript cDNA synthesis kit (cat. no. CW2569M), UltraSYBR mixture
(cat. no. CW0957M) and 2X Taq MasterMix were obtained from CWbio
Co., Ltd. (Beijing, China). DMEM/F12 (1:1) and Lipofectamine 3000
reagent were purchased from Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). Fetal bovine serum (FBS; cat. no. SKU
04-007-1A) was purchased from Biological Industries (Kibbutz Beit
Haemek, Israel). Hoechst apoptosis kit was purchased from Beyotime
Institute of Biotechnology (Haimen, China). Endo-free plasmid mini
kit II (cat. no. D6950-01) was obtained from Omega Bio-Tek, Inc.
(Norcross, GA, USA). TIANgel midi purification kit (cat. no. DP209)
was purchased from Tiangen Biotech Co., Ltd. (Beijing, China).
pLVX-Puro vector (VECT231322) was purchased from Huayueyang Bio
(Beijing, China). pUC57 plasmid was purchased from BioVector NTCC,
Inc. (Beijing, China). Ghrelin-pUC57 plasmid was routinely cloned
in our laboratory. Mouse anti-GH antibody (cat. no. ab9821;
dilution, 1:1,200), rabbit anti-IGF-1 antibody (cat. no. ab182408;
dilution, 1:1,000), rabbit anti-Akt antibody (cat. no. ab81283;
dilution, 1:1,000) and rabbit anti-p-Akt antibody (cat. no.
ab38449; dilution, 1:1,000) were purchased from Abcam (Cambridge,
MA, USA). Mouse anti-β-actin antibody (cat. no. TA-09; dilution,
1:2,000), horseradish peroxidase (HRP)-conjugated goat anti-mouse
IgG (H+L; cat. no. ZB-2305; dilution, 1:2,000) and HRP-conjugated
goat anti-rabbit IgG (H+L; cat. no. ZB-2301; dilution, 1:2,000)
were obtained from OriGene Technologies, Inc. (Beijing, China).
Rabbit anti-GH antibody (cat. no. bs-0467R), rabbit anti-GHSR
antibody (cat. no. bs-11529R; dilution, 1:1,200), rabbit anti-IGF-1
receptor antibody (cat. no. bs-0227R), rabbit anti-Akt antibody
(cat. no. bs-0115R), rabbit anti-α-sarcomeric actinin antibody
(cat. no. bs-10367R) and ghrelin (cat. no. bs-0467P) were from
BIOSS (Beijing, China). HRP-conjugated polymer anti-rabbit IgG
(cat. no. SV0002) was obtained from Boster Biological Technology
(Pleasanton, CA, USA).
A total of 28 specific pathogen-free (SPF) male
Sprague Dawley (SD) rats (aged 8 weeks; weight, 300±30 g) and 8 SPF
neonatal SD rats (4 male and 4 female; aged 1-3 days) were obtained
from Hunan Slac JD Laboratory Animal Co., Ltd. [License
SCXK(XIANG)2016-0002; Hunan, China]. The rats were housed with
ad libitum access to water and food in an environment of
20-26°C, 40-70% relative humidity and a 12/12 h light/dark cycle.
The study protocol was reviewed and approved by the Ethics
Committee of Children’s Hospital of Suzhou University (approval no.
2016LW009).
Construction of ghrelin expression
vector
Ghrelin-pUC57 plasmid was digested by incubating
with BamHI and EcoRI at 37°C for 2 h. The enzyme
digestion system (50.0 µl) contained 10X Tango buffer 5.0 µl,
ghrelin-pUC57 (1 µg) 4.0 µl, BamHI (10 U) 1.0 µl,
EcoRI (10 U) 1.0 µl and ddH2O 39.0 µl. Next, the
product was separated by 1% agarose gel electrophoresis. The gel
was visualized on an electrophoresis imaging system (Tanon1600;
Tanon Science and Technology Co., Ltd., Shanghai, China). Target
fragment (363 bp) was harvested quickly under the imaging system to
prevent the destruction of the gene fragments by ultraviolet
radiation. DNA was recovered from the harvested gel with a gel
extraction kit according to the manufacturer’s protocol.
pLVX-Puro vector was digested with BamHI and
EcoRI at 37°C for 2 h. The enzyme digestion system (10.0 µl)
was composed of 10X Tango buffer 1.0 µl, pLVX-Puro vector (0.5 µg)
2.0 µl, BamHI (10 U) 1.0 µl, EcoRI (10 U) 1.0 µl and
ddH2O 5.0 µl. The product was also separated by 1%
agarose gel electrophoresis. The gel was visualized on an
electrophoresis imaging system (Tanon1600; Tanon Science and
Technology Co., Ltd.). Target fragment (8,102 bp) was harvested and
recovered using a gel purification kit according to the
manufacturer’s protocols.
The ghrelin gene was ligated to pLVX-Puro vector in
a 20 µl system (linear vector 4.0 µl, target gene 1.0 µl, 10X
ligation buffer 2.0 µl, T4 DNA ligase (5 U/µl) 0.3 µl and
ddH2O 12.7 µl) at 22°C for 3 h. Next, the ligated
product was transformed into DH5α. The transformation product was
coated on an LB plate containing 100 µg/ml ampicillin and incubated
at 37°C for 22 h.
Two single colonies were selected from the
ghrelin-pLVX- Puro plate following overnight incubation to perform
colony polymerase chain reaction (PCR) verification. The PCR
amplification system (20.0 µl) was comprised of 2X Taq MasterMix
10.0 µl, template 1.0 µl, forward primer (10 µm) 1.0 µl, reverse
primer (10 µm) 1.0 µl and ddH2O 7.0 µl. Sequences of the
forward and reverse primers were: 5′-CAC GCT GTT TTG ACC TCC AT-3′
and 5′-GGA TGT GGA ATG TGT GCG AG-3′. PCR parameters were as
follows: Predenaturation at 94°C for 2 min, denaturation at 94°C
for 30 sec, annealing at 55°C for 30 sec, elongation at 72°C for 45
sec (30 cycles of denaturation to elongation), sufficient
elongation at 72°C for 10 min. The PCR product was separated by 1%
agarose gel electrophoresis. The gel was visualized on an
electrophoresis imaging system (Tanon1600; Tanon Science and
Technology Co., Ltd.).
The ghrelin-pLVX-Puro colonies were incubated in LB
broth containing 100 µg/ml ampicillin overnight. The
ghrelin-pLVX-Puro vectors were extracted using the plasmid
extraction kit according to the manufacturer’s protocols for the
subsequent experiments.
Isolation and identification of primary
neonatal rat cardiac myocytes
A total of 8 neonatal SD rats (aged 1-3 days) were
anaesthetized by inhaling isoflurane (2%) and were sacrificed by
cervical dislocation. Following the heart being isolated, the apex
cordis was obtained using scissors and immersed in precooled PBS.
The apex cordis was washed three times with PBS to remove residual
blood and uniformly cut into ~1 mm3 fragments in 0.1%
trypsin. The apex cordis fragments were trypsinized in 0.1% trypsin
at 37°C for 6 min. Following continuous agitating and natural
sedimentation, the supernatant was removed. Next, the tissues were
trypsinized in a mixture of 0.08% trypsin and 0.05% type II
collagenase at 37°C for 5 min. During this 5 min, agitating was
conducted for 3 min. The supernatant was collected into another
pre-cooled centrifuge tube and an equal volume of DMEM/F12
containing 10% FBS was added to terminate the trypsinization. The
aforementioned trypsinization steps were repeated 3-4 times. All
the trypsinized mixture was filtered into centrifuge tubes through
a 200-mesh sieve. The filtrate was centrifuged at 800 x g at 4°C
for 8 min and the pellets were resuspended in 20% DMEM/F12.
Following transfer to culture plates, cells were cultured at 37°C
in 5% CO2.
Cells were washed with PBS three times for 3 min
each time and fixed in 4% paraformaldehyde at room temperature for
15 min. Following washing with PBS again, cells were incubated in
0.5% Triton X-100 at room temperature for 20 min. Following washing
with PBS, cells were incubated in 5% BSA buffer at 37°C for 30 min.
The BSA buffer was removed and diluted rabbit anti-α-sarcomeric
actinin antibody (1:300) was added at 4°C overnight. Cells were
washed with PBS and incubated in secondary antibody buffer (1:200)
at 37°C for 30 min. Following washing, cells were stained in
4′,6-diamidino-2-phenylindole (DAPI) solution for 5 min in the
dark. The remaining DAPI was removed by PBS washing. Finally, the
culture dish was mounted with 20% glycerin and visualized under a
fluorescence microscope (magnification, ×200).
Cell transfection and
hypoxia/reoxygenation (H/R) treatment
Cells were divided into four groups: Control, H/R,
empty (empty pLVX-Puro plasmid + H/R) and ghrelin
(ghrelin-pLVX-Puro plasmid + H/R). The plasmid was mixed with
Lipofectamine 3000 reagent according to the manufacturer’s
protocol. When cell confluence reached 80%, cells were incubated
with a mixture of plasmid and Lipofectamine 3000 at 37°C and 5%
CO2 for 4 days. Subsequently, hypoxia (4 h) and
reoxygenation (1 h) treatments were performed. Cells in the control
group did not undergo any treatments, including transfection or
H/R. H/R treatment is a common method used to establish the model
of myocardial injury (19).
Cell viability
At 24, 48 and 72 h after the aforementioned
treatments, Cell Counting Kit-8 (CCK-8) reagent (10 µl) was added
to each well and then cells were cultured at 37°C in 5%
CO2 for 4 h. The absorbance was determined at 550 nm on
a microplate reader. The cell viability was calculated
accordingly.
Cell apoptosis
Cells were seeded into the 6-well plate where
coverslips had been laid. Once the confluence reached ~80%, the
treatments, including transfection and/or H/R were conducted. The
culture medium was discarded later and the cells were incubated in
4% paraformaldehyde at room temperature for 10 min. Subsequently,
the cells were washed twice with PBS for 3 min each time. Hoechst
33258 (0.5 ml) was added to each well for 5 min. The cells were
washed twice with PBS again. A drop of antifading mounting medium
(S2100; Beijing Solarbio Science & Technology Co., Ltd.) was
added to the slide and the slide was then covered with the
coverslip carrying the cells. Eventually, the slide was visualized
under a fluorescence microscope (magnification, ×100; IX51; Olympus
Corporation, Tokyo, Japan) with excitation at 350 nm and emission
at 460 nm.
Rat cardiac perfusion tests ex vivo
K-H buffer (600 ml) was added to the reservoir of a
Langendorff cardiac perfusion system (Beijing, Zhishuduobao
Biological Technology, Beijing, China) and the temperature was
adjusted to 37°C. Next, a gas mixture of 95% O2-5%
CO2 was aerated for 30 min. A total of 28 SD rats (aged
8 weeks) were anesthetized by intra-peritoneal injection of 1%
pentobarbital sodium at a dosage of 45 mg/kg. Immediately, the
heart was placed in the reservoir containing oxygenic K-H buffer at
37°C. Other tissues around the heart were removed and the remaining
blood in the atria and ventricles was extruded by gently squeezing
the heart with cotton swabs. Retrograde perfusion was performed
from the aortic cannula. The heart was fixed with 4-0 sutures.
Coronary ischemia and reperfusion were controlled by the switch of
perfusion pathway. The flow rate of the perfusate for balancing was
~15 ml/min.
Tests were divided into four groups: Control, sham,
H/R and ghrelin (ghrelin + H/R) (n=7). The untreated hearts served
as the control. In the sham group, the balancing perfusion was
conducted for 20 min. In the H/R group, following balancing for 20
min, improved Thomas II cardioplegic solution was perfused for 3
min to induce cardiac arrest and then the perfusion was stopped for
30 min. Subsequently, oxygenic K-H buffer was perfused again for 2
h to induce cardioversion. In the ghrelin group, following
balancing for 20 min, ghrelin (5 mg/l) was perfused for 15 min and
then the normal aerobic perfusion was restored for 15 min.
Subsequently, the H/R treatment was performed as demonstrated in
the H/R group.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from multiple primary
cardiac myocytes and ex vivo myocardial tissues following
various treatments using TRIzol reagent according to the
manufacturer’s protocol. The concentration and purity of the RNA
were determined by measuring the absorbance at 260 and 280 nm.
Next, the RNA was reverse transcribed to cDNA using a HiFiScript
cDNA synthesis kit according to the manufacturer’s protocol. The
reverse transcription system (20 µl) was comprised of dNTP Mix (4
µl), primer Mix (2 µl), RNA template (7 µl), 5X RT Buffer (4 µl),
dithiothreitol (DTT, 2 µl) and HiFiScript (1 µl). Sequences of the
primers, which were synthesized by General Biosystems (Anhui,
China), are presented in Table I.
The PCR system (25 µl) comprised RNase free dH2O (9.5
µl), cDNA/DNA (1 µl), forward primer (1 µl), reverse primer (1 µl)
and 2X UltraSYBR Mixture (12.5 µl). Reaction parameters were as
follows: Predenaturation at 95°C for 10 min, denaturation at 95°C
for 10 sec, annealing at 58.5°C for 30 sec and elongation for 30
sec at 72°C, for 40 cycles. Dissociation curve was analyzed as
follows: 15 sec at 95°C, 1 min at 58.5°C, 15 sec at 95°C, 15 sec at
58.5°C and 15 sec at 58.5°C, and measured stepwise from 95°C, every
0.5°C. It was finally evaluated on a RT-PCR detection system (CFX
Connect™; Bio-Rad, Laboratories, Inc., Hercules, CA, USA). β-actin
served as an internal control and the expression level relative to
β-actin was calculated using 2−ΔΔCq method (20).
 | Table ISequences of the primers in reverse
transcription-polymerase chain reaction. |
Table I
Sequences of the primers in reverse
transcription-polymerase chain reaction.
| Primer | Sequence
(5′-3′) | Length (bp) |
|---|
| GH | | |
| Forward |
CTGTTTGCCAATGCTGTGC | 19 |
| Reverse |
GCTGTCCCTCGGGAATGTA | 19 |
| GHSR | | |
| Forward |
CTTCTGCCTCACTGTGCTCTA | 21 |
| Reverse |
GCATCTTCACTGTCTGCTTGT | 21 |
| IGF-1 | | |
| Forward |
GCACTCTGCTTGCTCACCT | 19 |
| Reverse |
CATCCACAATGCCCGTCT | 18 |
| Akt | | |
| Forward |
GGCATCTTCTCCTTCCAGC | 19 |
| Reverse |
AGAGTTCCTCCACCACCGT | 19 |
| β-actin | | |
| Forward |
AGGGAAATCGTGCGTGAC | 18 |
| Reverse |
ATACCCAGGAAGGAAGGCT | 19 |
Western blot analysis
Following various treatments, primary cardiac
myocytes were incubated in radioimmunoprecipitation assay (RIPA)
lysis buffer in an ice bath for 15 min and sonicated in an ice bath
for another 15 min. Following various treatments, ex vivo
myocardial tissues were ground repeatedly in RIPA lysis buffer on
ice and sonicated for 15 min. The two types of lysates were
centrifuged at 10,000 x g and 4°C for 10 min. The supernatant was
collected and mixed with PBS. The mixture was boiled for 5 min and
then centrifuged at 10,000 x g for 5 min (cells) or 10 min
(tissues). The supernatant was collected to prepare total protein.
The concentration was determined using a bicinchoninic acid assay
kit (Beyotime Institute of Biotechnology, Haimen, China). Next,
protein (20 µg per lane) was loaded to perform SDS-PAGE on 10%
gels. The protein was condensed at 60 V and separated at 80 V.
Polyvinylidene difluoride membranes were activated by absolute
methanol at room temperature for 15 sec. Membrane transfer was
conducted for β-actin and GHSR at 300 mA for 1.5 h, for IGF-1 at
300 mA for 2 h, for GH at 200 mA for 50 min, and for Akt at 200 mA
for 1 h. Next, it was blocked in 5% BSA buffer at room temperature
overnight. The membrane was subsequently incubated in primary
antibody buffer at 4°C for 3 h. It was rinsed three times for 10
min each time and incubated in secondary antibody buffer at room
temperature for 2 h. It was rinsed three times for 10 min each
time. Following chemiluminescent substrate being added, the
membrane was exposed on an imaging system (ChemiDoc XRS+, Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Blots were
semi-quantitatively analyzed with a Quantity one soft-ware (v4.62;
Bio-Rad Laboratories, Inc.). β-actin served as the internal
control.
Immunohistochemical analysis
Following various treatments, ex vivo
myocardial tissues were collected, fixed in 4% paraformaldehyde at
room temperature for 30 min, embedded with paraffin and cut into
slices (thickness, 4 µm). Following heating at 65°C for 2 h, the
slices were incubated in xylene for 10 min and in fresh xylene for
another 10 min. Subsequently, the slices were immersed successively
in 100% ethanol, 100 ethanol, 95 ethanol, 80% ethanol and water
each for 5 min. The slices were later incubated in citrate buffer
in a box and heated to automatic air release in a pressure cooker.
After 2 min, the slices were removed and naturally cooled.
Following the citrate buffer being removed and the slices being
eluted with PBS, the slices were incubated in 3% fresh hydrogen
peroxide in a wet box for 10 min at room temperature. Subsequently,
the slices were washed in PBS three times for 5 min each time and
5% BSA was added dropwise onto the slices at 37°C for 30 min. The
excess blocking buffer around the tissue was absorbed with
absorbent papers. Diluted primary antibodies (all 1:500) were
dropwise added onto each slice. Following incubation at 4°C
overnight in a wet box, the slices were removed for 45 min at room
temperature and then washed in PBS three times for 5 min each time.
Diluted secondary antibody (1:200) was added dropwise onto the
slices, which were subsequently incubated at room temperature for
30 min. The slices were rinsed and developed in DAB for 5-10 min
followed by rinsing with PBS for 1 min. The slices were then
counterstained with hematoxylin at room temperature for 3 min,
differentiated in 1% hydrochloric alcohol, blued, rinsed,
dehydrated, transparentized, mounted and examined under a
fluorescence microscope (magnification, ×200; CKX31; Olympus
Corporation).
Statistical analysis
Each experiment was repeated three times. Data are
expressed as the mean ± standard deviation (SD; n=7). Following
confirmation of normal distribution by the Kolmogorov-Smirnov test,
statistical differences among different groups were analyzed by
analysis of variance followed by least significant difference post
hoc test using SPSS 19.0 software (IBM Corp., Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Construction of ghrelin expression
vector
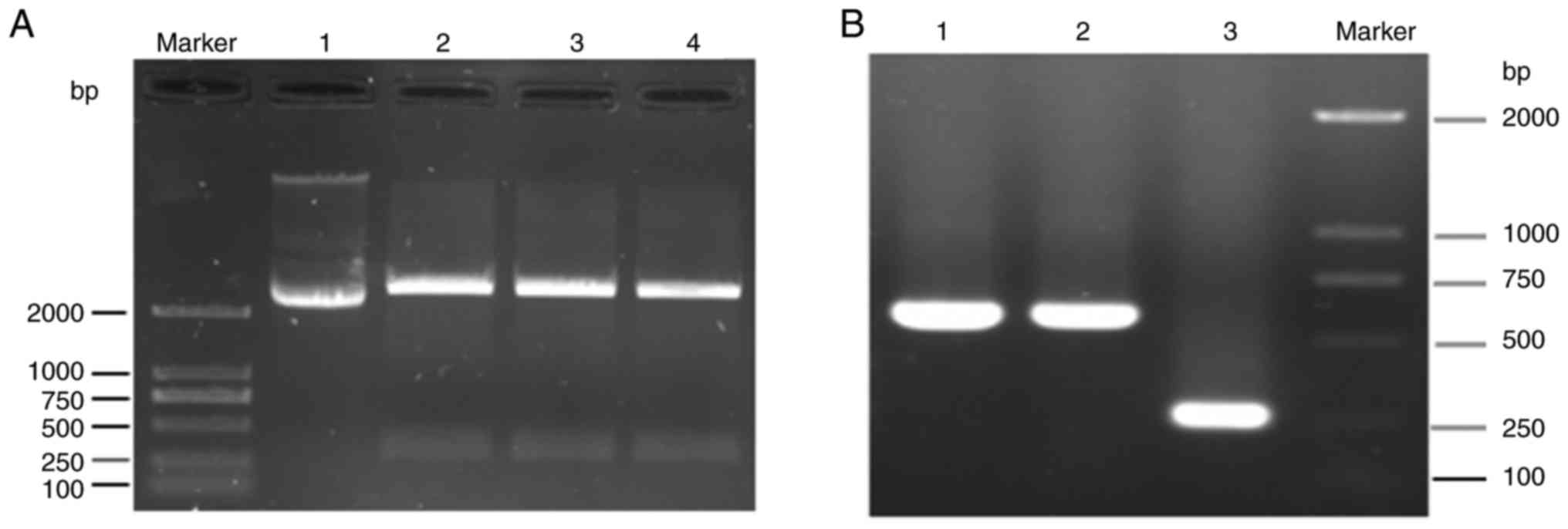
The electrophoretogram of the enzyme digestion
product from ghrelin-pUC57 plasmid is shown in Fig. 1A. There was no band between 250
and 500 bp in the lane of the ghrelin-pUC57 plasmid without enzyme
digestion. However, the target bands of ghrelin (363 bp) were
observed in the lanes of the ghrelin-pUC57 plasmid following
incubation with BamHI and EcoRI.
The isolated ghrelin from the ghrelin-pUC57 plasmid
was ligated to a pLVX-Puro vector, and two single colonies
containing the ghrelin-pLVX-Puro vector were verified by PCR. In
the electrophoretogram of colony PCR verification, the target genes
were identified, which demonstrated that the two colonies were
positive colonies (Fig. 1B). This
confirmed the successful construction of the ghrelin-pLVX-Puro
vector.
Isolation and identification of primary
neonatal rat cardiac myocytes
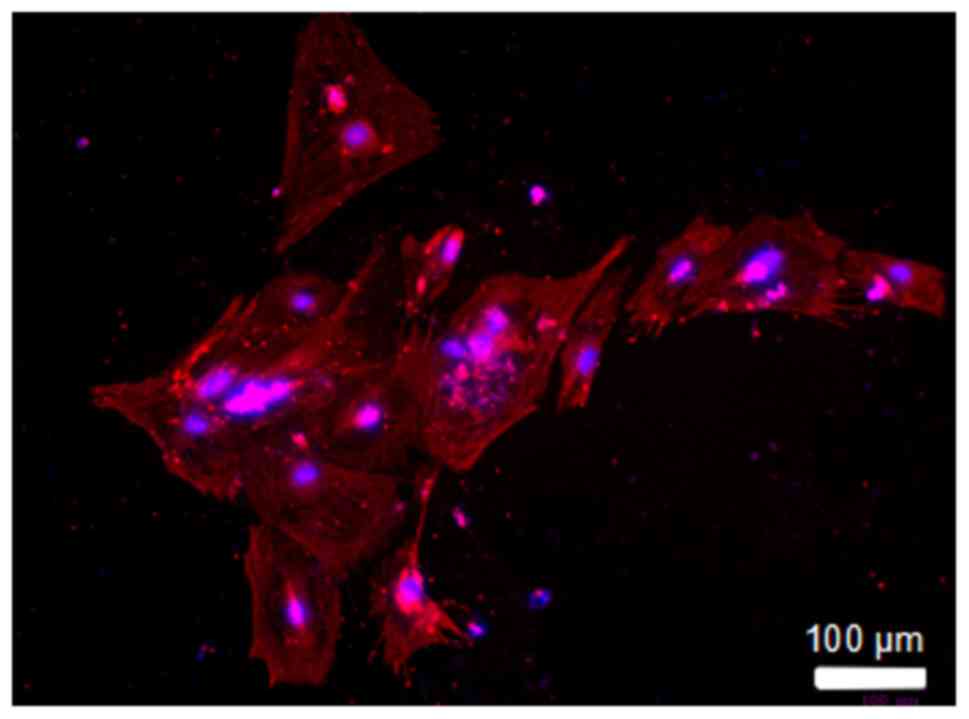
The immunofluorescent staining of primary neonatal
rat cardiac myocytes was shown in Fig. 2. α-sarcomeric actinin was a
specific protein of cardiac myocytes. Red and blue fluorescence
represented the α-sarcomeric actinin and the cell nuclei,
respectively. It was demonstrated that all the isolated cells had
red α-sarcomeric actinin. It was indicated that the primary
neonatal rat cardiac myocytes were successfully isolated and
cultured.
Cell viability
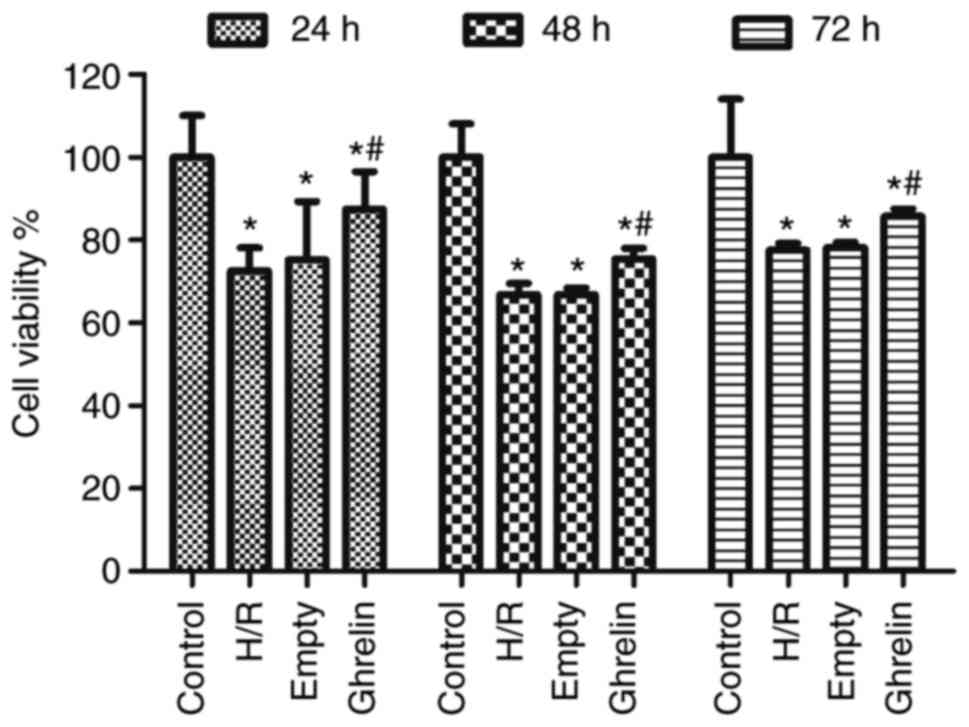
Fig. 3
demonstrates the viability of primary neonatal rat cardiac myocytes
in various groups (control, H/R, empty and ghrelin) at 24, 48 and
72 h after treatment (if any), which was examined by CCK-8 assay.
Compared with the control group, the viabilities in other three
groups were significantly reduced (P<0.05), suggesting the
inhibition of cell growth by H/R treatment. There was no
significant difference in the cell viability between the H/R and
empty groups. The empty pLVX-Puro vector did not promote cell
proliferation. However, the cell viability in the ghrelin group was
significantly higher than that in the empty group (P<0.05),
indicating that ghrelin was capable of improving the viability of
primary neonatal rat cardiac myocytes.
Cell apoptosis
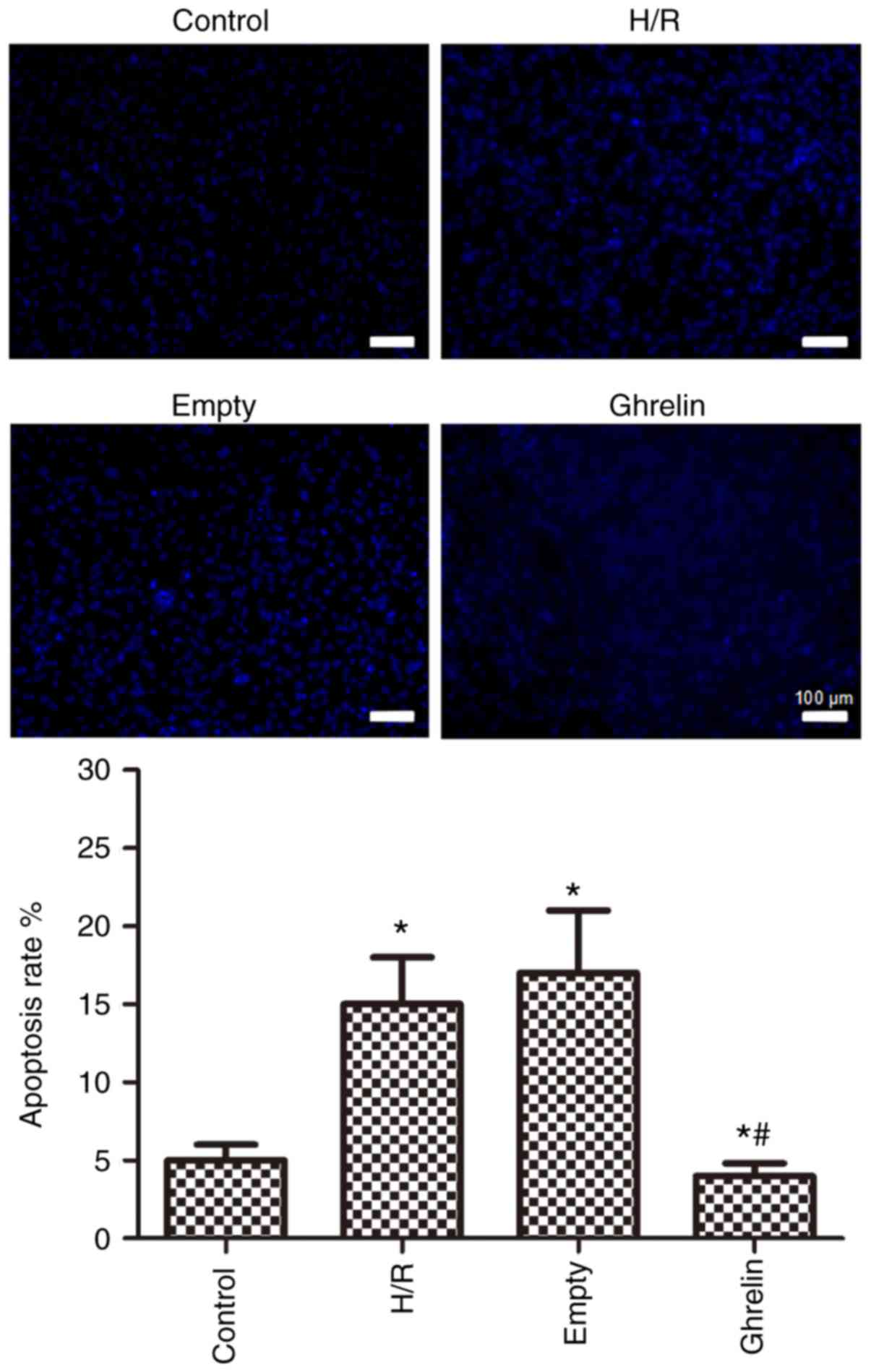
Fig. 4
demonstrates the apoptosis of primary neonatal rat cardiac myocytes
in various groups (control, H/R, empty and ghrelin), which was
evaluated by Hoechst staining. Compared with the control group, the
apoptosis rates in the other three groups were significantly
increased (P<0.05), suggesting the promotion of cell apoptosis
by H/R treatment. The H/R and empty groups exhibited similar
apoptosis rates, demonstrating that the empty pLVX-Puro vector had
no effect on cell apoptosis. However, the apoptosis rate in the
ghrelin group was significantly lower than that in the empty group
(P<0.05), indicating that ghrelin was able to suppress the
apoptosis of primary neonatal rat cardiac myocytes and repair the
hypoxic cardiac myocytes.
Levels of GH, GHSR, IGF-1, Akt and p-Akt
in primary cardiac myocytes following various treatments
The mRNA levels of GH, GHSR, IGF-1 and Akt in
primary cardiac myocytes in various groups (control, H/R, empty and
ghrelin), which were determined by RT-PCR, are presented in
Fig. 5A. The protein expression
levels of GH, GHSR, IGF-1, Akt and p-Akt in primary cardiac
myocytes in various groups (control, H/R, empty and ghrelin), which
were evaluated by western blot analysis, are presented in Fig. 5B. Compared with the control group,
the mRNA and protein levels of GH, GHSR and IGF-1 in the other
three groups were significantly decreased (P<0.05), suggesting
the downregulation of GH, GHSR and IGF-1 in primary cardiac
myocytes by H/R treatment. Similar mRNA and protein levels of GH,
GHSR and IGF-1 were discovered between the H/R and empty groups,
demonstrating that the empty pLVX-Puro vector did not affect the
expression of GH, GHSR and IGF-1 in primary cardiac myocytes.
Notably, the mRNA and protein levels of GH, GHSR and IGF-1 in the
ghrelin group were significantly higher than those in the empty
group (P<0.05), indicating that ghrelin could upregulate the
expression of GH, GHSR and IGF-1 in primary cardiac myocytes. It
was demonstrated that the mRNA and protein expression levels of Akt
were similar among the four groups. It was implied that ghrelin
transfection and H/R treatment did not influence the expression of
Akt in primary cardiac myocytes. However, compared with the control
group, the ratios of p-Akt to Akt protein expression (p-Akt/Akt) in
the other three groups were significantly decreased (P<0.05).
The ratio of p-Akt/Akt was similar between the H/R and empty
groups. Compared with the empty group, the ghrelin transfection in
the ghrelin group significantly increased the ratio of p-Akt/Akt
(P<0.05).
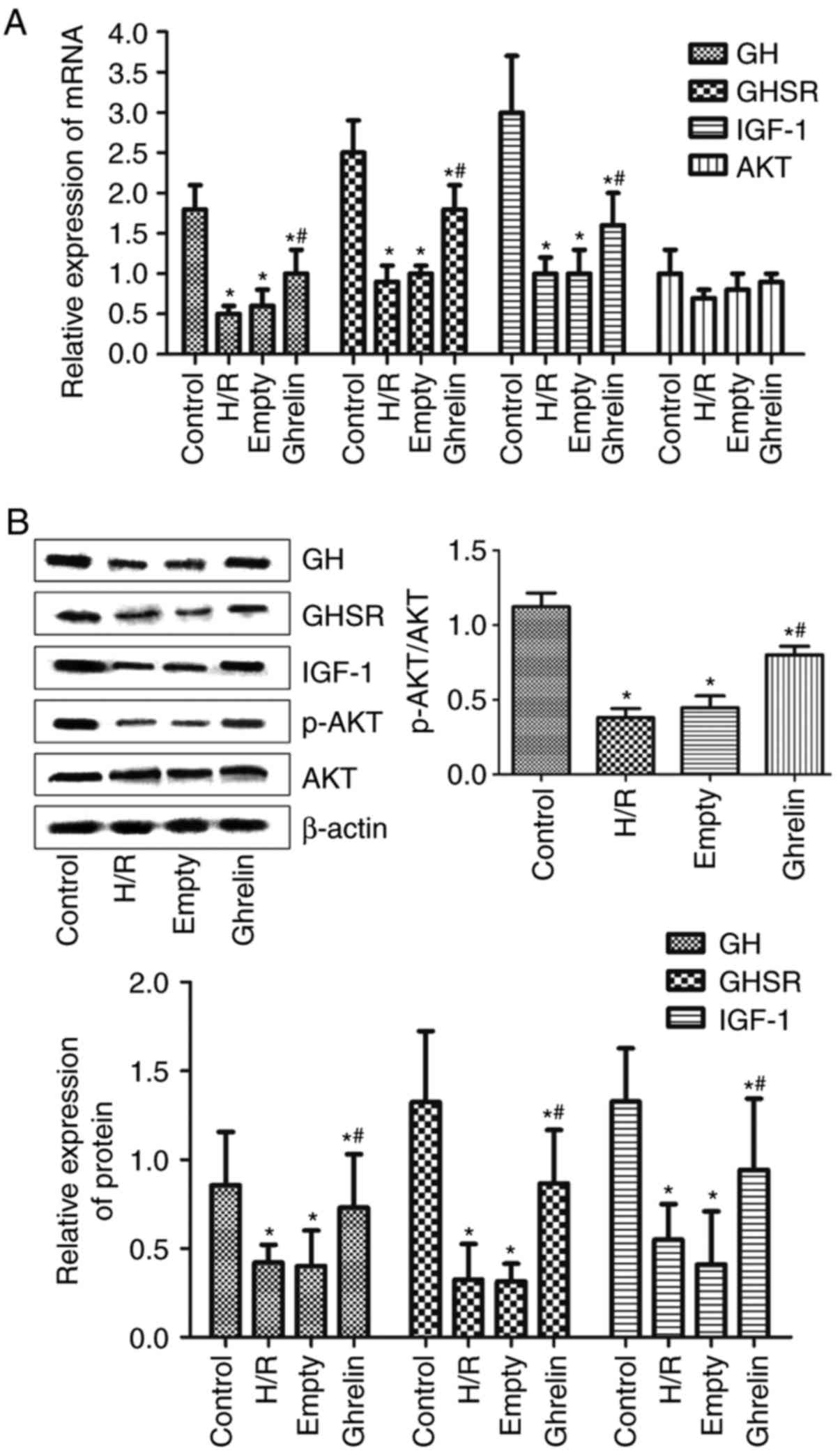 | Figure 5The expression levels of GH, GHSR,
IGF-1, Akt and p-Akt in primary cardiac myocytes in various groups.
(A) The mRNA expression levels of GH, GHSR, IGF-1 and Akt in
primary cardiac myocytes in various groups [control, H/R, empty
(empty pLVX-Puro plasmid + H/R) and ghrelin (ghrelin-pLVX-Puro
plasmid + H/R)], which was determined by reverse
transcription-polymerase chain reaction. (B) The protein expression
levels of GH, GHSR, IGF-1, Akt and p-Akt in primary cardiac
myocytes in various groups, which was evaluated by western blot
analysis. GH, growth hormone; GHSR, growth hormone secretagogue
receptor; IGF-1, insulin-like growth factor-1; Akt, protein kinase
B; p-Akt, phosphorylated Akt. *P<0.05 vs. the control
group; #P<0.05 vs. the empty group. H/R,
hypoxia/reoxygenation. |
Levels of GH, GHSR, IGF-1, Akt and p-Akt
in myocardial tissues following various treatments
The mRNA expression levels of GH, GHSR, IGF-1 and
Akt in myocardial tissues in various groups (control, sham, H/R and
ghrelin) determined by RT-PCR are shown in Fig. 6A. The protein expression levels of
GH, GHSR, IGF-1, Akt and p-Akt in myocardial tissues in various
groups (control, sham, H/R and ghrelin) evaluated by western blot
analysis were demonstrated in Fig.
6B. Compared with the control group, the mRNA and protein
expression levels of GH, GHSR and IGF-1 in the H/R and ghrelin
groups were significantly decreased (P<0.05), suggesting the
down-regulation of GH, GHSR and IGF-1 in myocardial tissues by H/R
treatment. Similar mRNA and protein expression levels of GH, GHSR
and IGF-1 were observed between the control and sham groups.
Notably, the mRNA and protein levels of GH, GHSR and IGF-1 in the
ghrelin group were significantly higher than those in the H/R group
(P<0.05), indicating that ghrelin could upregulate the
expression of GH, GHSR and IGF-1 in myocardial tissues. The mRNA
and protein expression levels of Akt were revealed to be similar
among the four groups. It was implied that ghrelin and H/R
treatment did not influence the expression of Akt in myocardial
tissues. However, compared with the control group, the ratios of
p-Akt/Akt in the H/R and ghrelin groups were significantly
decreased (P<0.05). The ratio of p-Akt/Akt was similar between
the control and sham groups. Compared to the H/R group, the ghrelin
group exhibited a notably larger ratio of p-Akt/Akt
(P<0.05).
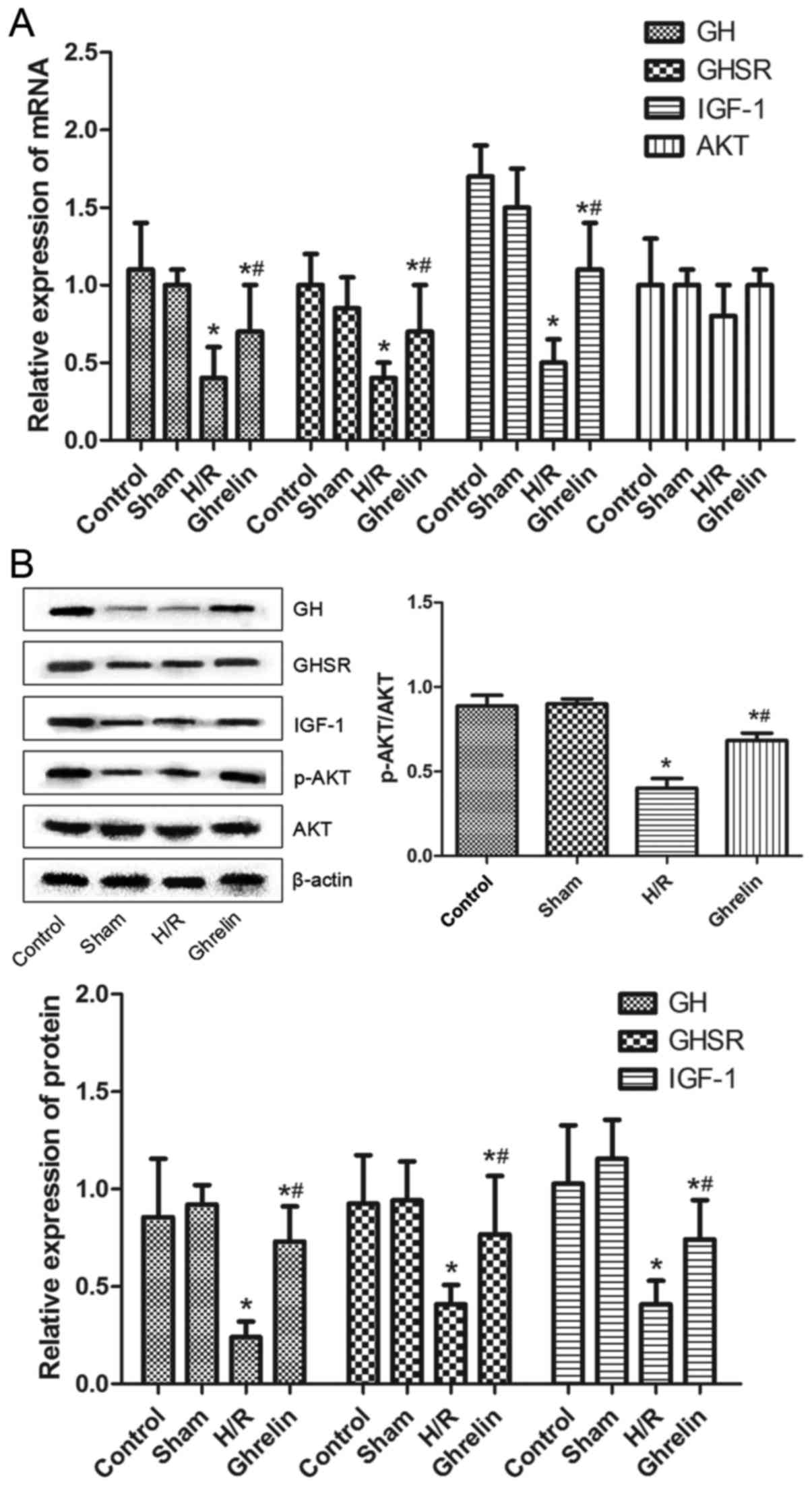 | Figure 6(A) The mRNA expression levels of GH,
GHSR, IGF-1 and Akt in myocardial tissues in various groups
[control, sham, H/R and ghrelin (ghrelin + H/R)], which was
determined by reverse transcription-polymerase chain reaction. (B)
The protein expression levels of GH, GHSR, IGF-1, Akt and p-Akt in
myocardial tissues in various groups, which was evaluated by
western blot analysis. GH, growth hormone; GHSR, growth hormone
secretagogue receptor; IGF-1, insulin-like growth factor-1; Akt,
protein kinase B; p-Akt, phosphorylated Akt; H/R,
hypoxia/reoxygenation. *P<0.05 vs. the control group;
#P<0.05 vs. the H/R group. |
Immunohistochemical analysis of GH, GHSR,
IGF-1 and Akt in myocardial tissues following various
treatments
Fig. 7 depicts the
immunohistochemical staining images of GH, GHSR, IGF-1 and Akt in
myocardial tissues in various groups (control, sham, H/R and
ghrelin). The corresponding protein and the nuclei are stained
brown and bluish violet, respectively. The expression levels of GH,
GHSR, IGF-1 and Akt in the H/R and ghrelin groups were intuitively
lower than those in the control group. This demonstrated that the
myocardial tissues following H/R treatment were damaged. However,
the expression level of brown protein in the ghrelin group was
notably higher than that in the H/R group, particularly for GH and
GHSR. Furthermore, there were varying degrees of rupture, shrinkage
and irregular appearance of the myocardial tissues in the H/R
group, and this phenomenon was particularly evident in the IGF-1
protein group. Although there remained certain injuries of the
myocardial tissues in the ghrelin group, this was markedly improved
compared with the H/R group. Ghrelin enhanced the integrity of
cardiac myocytes, and reduced shrinkage and apoptosis.
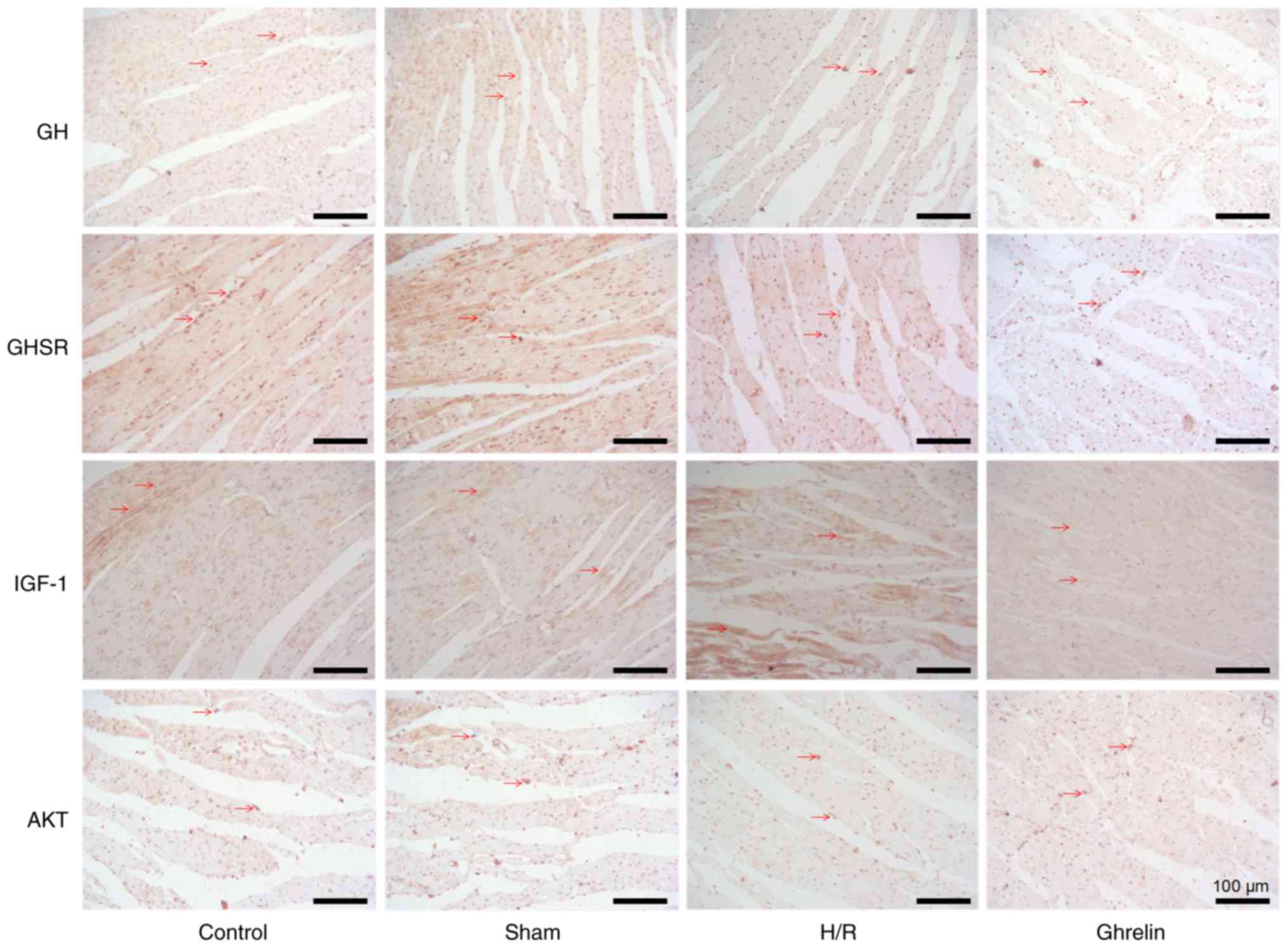 | Figure 7The immunohistochemical staining
images of GH, GHSR, IGF-1 and Akt in myocardial tissues in various
groups [control, sham, H/R and ghrelin (ghrelin + H/R)]. The
corresponding protein and the nuclei are shown by brown (arrows)
and bluish violet, respectively. GH, growth hormone; GHSR, growth
hormone secretagogue receptor; IGF-1, insulin-like growth factor-1;
Akt, protein kinase B; H/R, hypoxia/reoxygenation. Magnification,
×200. |
Discussion
The reduction of coronary blood flow due to various
reasons results in insufficient supply of myocardial oxygen and the
reduced elimination of metabolic products; therefore, this clinical
condition is known as myocardial hypoxia (21). The majority of cardiac diseases
can cause myocardial ischemia and hypoxia, but no radical cure is
currently available in clinic. Therefore, to the best of our
knowledge, the present study was the first to reveal the improving
effect of ghrelin on hypoxic myocardium and the involved molecular
mechanisms through constructing primary neonatal rat cardiac
myocytes transfected with ghrelin lentiviral expression vector, and
evaluating the subsequent cell viability and apoptosis, as well as
the expression of associated genes at the cell and tissue
levels.
Primary neonatal rat cardiac myocytes were isolated
and the immunofluorescent staining of α-sarcomeric actinin proved
that the isolated cells were the target cells. Improved cellular
activity could be obtained through the cell characterization using
the isolated primary cells, and this was consistent with the
characterization of subsequent ex vivo myocardial tissues.
This could more accurately reflect the repair effect of ghrelin on
the myocardium at the cell and tissue levels.
CCK-8 and Hoechst assays demonstrated that ghrelin
could inhibit the apoptosis of hypoxic cardiac myocytes, and that
it had a protective and repair effect on hypoxic cardiac myocytes,
which was in agreement with the reported heart protection function
of ghrelin (22,23).
Apoptosis is regulated by intracellular apoptosis
regulating proteins, which are divided into two categories:
Apoptotic protein and anti-apoptotic protein (24-26). The relative balance between
apoptotic protein and anti-apoptotic protein following a series of
stimuli or injuries determines whether the cell is alive or
apoptotic (27). RT-PCR and
western blot analysis were conducted to evaluate the expression of
five genes, including GH, GHSR, IGF-1, Akt and p-Akt in primary
cardiac myocytes following H/R treatment. The present study
demonstrated that ghrelin transfection upregulated the expression
of GH, GHSR and IGF-1 at the mRNA and protein levels. Furthermore,
ghrelin transfection could elevate the ratio of p-Akt/Akt. It was
suggested that ghrelin promoted the phosphorylation of Akt,
inhibited the activity of Akt (28), and upregulated the expression of
GH, GHSR and IGF-1, consequently enhancing the viability and
suppressing the apoptosis of cardiac myocytes. Additionally, the
PI3K/Akt signaling pathway may be inhibited following the
phosphorylation of Akt, such that the cardiac myocytes may be
repaired (17,18). Ghrelin inhibited the expression of
apoptotic proteins and promoted the expression of anti-apoptotic
proteins in neonatal rat cardiac myocytes, thereby inhibiting the
apoptosis of cardiac myocytes following heart failure and resisting
ventricular remodeling; therefore, this may be a mechanism of the
anti-apoptotic effect of ghrelin (28,29).
Furthermore, a rat cardiac perfusion model was
established ex vivo and the expression of GH, GHSR, IGF-1,
Akt and p-Akt in the myocardial tissues following H/R treatment was
investigated by RT-PCR, western blot analysis and
immunohistochemical analysis. Immunohistochemical results
demonstrated that ghrelin enhanced the integrity of cardiac
myocytes and reduced shrinkage and apoptosis. These results
suggested that ghrelin may protect and repair the myocardium
through upregulating the expression of GH, GHSR and IGF-1, and
ghrelin promoted the phosphorylation of Akt and inhibited the
activity of Akt in myocardial tissues, consequently alleviating the
injury of myocardial tissues. This was the same as the results at
the cell level. Immunohistochemical analysis of myocardial tissues
was performed, but cardiac function was not analyzed. A negative
control was not included in the immunohistochemical analysis. These
were limitations to the present study.
Ghrelin can improve the cardiac output, left
ventricular ejection fraction and change rate of left ventricular
maximum pressure, and inhibit left ventricular hypertrophy in rats
with chronic heart failure (30).
Ghrelin is a natural endogenous ligand for GHSR and is also
considered to be a powerful stimulant for the release of GH.
Certain studies have demonstrated that the main role of ghrelin in
cardiac myocytes is to promote the expression of non-functional
GHSR (31). Another report also
indicated that, in addition to GHSR, ghrelin may have other unknown
receptors in the cardiac repair system (32). According to the results of RT-PCR
and western blot analysis, ghrelin promoted the expression of GHSR
and GH, and repaired hypoxic cardiac myocytes. Therefore, the
possible mechanism was that ghrelin upregulated the expression of
GHSR through binding with GHSR, and subsequently GHSR stimulated
the overexpression of GH. GH is one of the hormones synthesized and
secreted in the adeno-hypophysis, which has a positive function in
the treatment of cardiovascular disease (33). It has been demonstrated that it
can stimulate the central nervous system and local organs to
produce IGF-1 through autocrine and paracrine mechanisms, which can
directly or indirectly influence cardiac tissues, enhance cardiac
contractility, reduce cardiac load, improve cardiac function and
postpone cardiomyocyte apoptosis (34,35).
Cao et al (14) reported that the cardioprotective
effects elicited by ghrelin may contribute toward the inhibition of
inflammatory response through the Akt activated pathway. The basic
difference between this previous paper and the present study was in
the different animal models. This previous paper used a CPB model,
while the present study used a model of myocardial injury caused by
H/R (14). This was the first
time to investigate the therapeutic effect of ghrelin on the model
of myocardial injury induced by H/R. Furthermore, in the present
study, the cells used in the in vitro cell experiments were
isolated from the neonatal rats in the same batch of rats used in
the animal experiments ex vivo. Homologous cells and animal
materials were selected for the cell and animal experiments to
conduct similar H/R treatments, which made the results of the
present study more accurate and reliable.
In conclusion, to the best of our knowledge, the
present study was the first to reveal that ghrelin protected the
primary cardiac myocytes and the myocardium ex vivo with H/R
treatment through upregulating the expression of GH, GHSR and
IGF-1, promoting the phosphorylation of Akt, and inhibiting the
activity of Akt. These results may provide novel insight into the
understanding of the mechanisms of ghrelin in cardiovascular
protection and repair, and provide promising guidance for the
clinical application of ghrelin. One limitation of the present
study was that inflammation was not investigated. Additionally, a
ghrelin blocker group will be added in future studies.
Funding
The present study was supported by the Natural
Science Foundation of Jiangxi Province of China (grant no. 20171BA
B205042) and Jinlei Growth Science Research Foundation for
Pediatric Endocrinologist with Young and Middle-Age (grant no.
PEGRF201607011).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
YL, ZC and GG designed study and wrote the
manuscript. YangL, YanlingL and GL collected and analyzed the data.
All authors performed the study.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of Children’s Hospital of Suzhou University (Suzhou,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Heather LC, Cole MA, Tan JJ, Ambrose LJ,
Pope S, Abd-Jamil AH, Carter EE, Dodd MS, Yeoh KK, Schofield CJ, et
al: Metabolic adaptation to chronic hypoxia in cardiac
mitochondria. Basic Res Cardiol. 107:2682012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Suematsu M, Katsuki A, Sumida Y, Gabazza
EC, Murashima S, Matsumoto K, Kitagawa N, Akatsuka H, Hori Y,
Nakatani K, et al: Decreased circulating levels of active ghrelin
are associated with increased oxidative stress in obese subjects.
Eur J Endocrinol. 153:403–407. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shimizu Y, Nagaya N, Teranishi Y, Imazu M,
Yamamoto H, Shokawa T, Kangawa K, Kohno N and Yoshizumi M: Ghrelin
improves endothelial dysfunction through growth hormone-independent
mechanisms in rats. Biochem Biophys Res Commun. 310:830–835. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lim JA, Baek HJ, Jang MS, Choi EK, Lee YM,
Lee SJ, Lim SC, Kim JY, Kim TH, Kim HS, et al: Loss of β2-spectrin
prevents cardiomyocyte differentiation and heart development.
Cardiovasc Res. 101:39–47. 2014. View Article : Google Scholar
|
|
5
|
Li CJ, Madhu V, Balian G, Dighe AS and Cui
Q: Cross-talk between VEGF and BMP-6 pathways accelerates
osteogenic differentiation of human adipose-derived stem cells. J
Cell Physiol. 230:2671–2682. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsuura K, Honda A, Nagai T, Fukushima N,
Iwanaga K, Tokunaga M, Shimizu T, Okano T, Kasanuki H, Hagiwara N
and Komuro I: Transplantation of cardiac progenitor cells
ameliorates cardiac dysfunction after myocardial infarction in
mice. J Clin Invest. 119:2204–2217. 2009.PubMed/NCBI
|
|
7
|
Reich H, Tseliou E, de Couto G, Angert D,
Valle J, Kubota Y, Luthringer D, Mirocha J, Sun B, Smith RR, et al:
Repeated transplantation of allogeneic cardiosphere derived cells
boosts therapeutic benefits without immune sensitization in a rat
model of myocardial infarction. J Heart Lung Transplant.
35:1348–1357. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mishra PK, Chavali V, Metreveli N and
Tyagi SC: Ablation of MMP9 induces survival and differentiation of
cardiac stem cells into cardiomyocytes in the heart of diabetics:a
role of extra-cellular matrix. Can J Physiol Pharmacol. 90:353–360.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nam YJ, Song K and Olson EN: Heart repair
by cardiac reprogramming. Nat Med. 19:413–415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mouquet F, Pfister O, Jain M,
Oikonomopoulos A, Ngoy S, Summer R, Fine A and Liao R: Restoration
of cardiac progenitor cells after myocardial infarction by
self-proliferation and selective homing of bone marrow-derived stem
cells. Circ Res. 97:1090–1092. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shirai M, Joe N, Tsuchimochi H, Sonobe T
and Schwenke DO: Ghrelin supresses sympathetic hyper-excititation
in acute heart failure in male rats-assessing centrally and
peripherally mediated pathways. Endocrinology. 156:3309–3316. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang B, He K, Zheng F, Wan L, Yu X, Wang
X, Zhao D, Bai Y, Chu W, Sun Y, et al: Overexpression of
hypoxia-inducible factor-1 alpha in vitro protects the cardiac
fibroblasts from hypoxia-induced apoptosis. J Cardiovasc Med.
15:579–586. 2014. View Article : Google Scholar
|
|
13
|
Ahluwalia A and Tarnawski AS: Critical
role of hypoxia sensor - HIF-1α in VEGF gene activation.
Implications for angiogenesis and tissue injury healing. Curr Med
Chem. 19:90–97. 2012. View Article : Google Scholar
|
|
14
|
Cao Y, Tang J, Yang T, Ma H, Yi D, Gu C
and Yu S: Cardioprotective effect of ghrelin in cardiopulmonary
bypass involves a reduction in inflammatory response. PLoS One.
8:e550212013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhong W and Xie W: New strategy for the
treatment of hepatic fibrosis. J Clin Hepatol. 27:233–235. 2011.In
Chinesee.
|
|
16
|
Yuan TL and Cantley LC: PI3K pathway
alterations in cancer: Variations on a theme. Oncogen e.
27:5497–5510. 2008. View Article : Google Scholar
|
|
17
|
Zheng H, Fu G, Dai T and Huang H:
Migration of endothelial progenitor cells mediated by stromal
cell-derived factor-1alpha/CXCR4 via PI3K/Akt/eNOS signal
transduction pathway. J Cardiovasc Pharmacol. 50:274–280. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chu K, Jung KH, Lee ST, Park HK, Sinn DI,
Kim JM, Kim DH, Kim JH, Kim SJ, Song EC, et al: Circulating
endothelial progenitor cells as a new marker of endothelial
dysfunction or repair in acute stroke. Stroke. 39:1441–1447. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu S, Wang H, Yu T and Liu X: Role of
Nrf2-ARE signaling pathway in protective effect of hypoxia or
pinacidil postconditioning against hypoxia-reoxygenation injury in
adult rat cardiomyocytes. Chin J Pathophysiol. 29:1696–1703.
2013.
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔC T method. Methods. 25:402–408.
2001. View Article : Google Scholar
|
|
21
|
Kamegai J, Tamura H, Shimizu T, Ishii S,
Tatsuguchi A, Sugihara H, Oikawa S and Kineman RD: The role of
pituitary ghrelin in growth hormone (GH) secretion: GH-releasing
hormone-dependent regulation of pituitary ghrelin gene expression
and peptide content. Endocrinology. 145:3731–3738. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cokkinos DV and Pantos C: Myocardial
protection in man-from research concept to clinical practice. Heart
Fail Rev. 12:345–362. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Q, Li Z, Xu XY, Guo YL and Du F:
Neuroprotective properties of picroside II in a rat model of focal
cerebral ischemia. Int J Mol Sci. 11:4580–4590. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liao YH, Xia N, Zhou SF, Tang TT, Yan XX,
Lv BJ, Nie SF, Wang J, Iwakura Y, Xiao H, et al: Interleukin-17A
contributes to myocardial ischemia/reperfusion injury by regulating
cardiomyocyte apoptosis and neutrophil infiltration. J Am Coll
Cardiol. 59:420–429. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fu Y, Yang W, Wei S, Zhang W, Dai G, Gao
H, Zheng P and Dang H: Inhibitory effect of matrine on
isoproterenol-induced cardiac hypertrophy via regulation of
p-Akt/Akt protein in rat. Chin Hosp Pharm J. 34:505–509. 2014.In
Chinese.
|
|
26
|
Park M, Youn B, Zheng XL, Wu D, Xu A and
Sweeney G: Globular adiponectin, acting via AdipoR1/APPL1, protects
H9c2 cells from hypoxia/reoxygenation-induced apoptosis. PLoS One.
6:e191432011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xia B, Liu H, Xie J, Wu R and Li Y: Akt
enhances nerve growth factor-induced axon growth via activating the
Nrf2/ARE pathway. Int J Mol Med. 36:1426–1432. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hwa JS, Jin YC, Lee YS, Ko YS, Kim YM, Shi
LY, Kim HJ, Lee JH, Ngoc TM, Bae KH, et al: 2-Methoxycinnamaldehyde
from Cinnamomum cassia reduces rat myocardial ischemia and
reperfusion injury in vivo due to HO-1 induction. J Ethnopharmacol.
139:605–615. 2012. View Article : Google Scholar
|
|
29
|
Silambarasan T, Manivannan J, Priya MK,
Suganya N, Chatterjee S and Raja B: Sinapic acid protects heart
against ischemia/reperfusion injury and H9c2 cardiomyoblast cells
against oxidative stress. Biochem Biophys Res Commun. 456:853–859.
2015. View Article : Google Scholar
|
|
30
|
Wu W, Zhou X, Liu P, Fei W, Li L and Yun
H: Isoflurane reduces hypoxia/reoxygenation-induced apoptosis and
mitochondrial permeability transition in rat primary cultured
cardiocytes. BMC Anesthesiol. 14:172014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Catak Z, Aydin S, Sahin I, Kuloglu T,
Aksoy A and Dagli AF: Regulatory neuropeptides (ghrelin, obestatin
and nesfatin-1) levels in serum and reproductive tissues of female
and male rats with fructose-induced metabolic syndrome.
Neuropeptides. 48:167–177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Soliman AT, Madkour A, Galil MA, El
Zalabany M, Aziz SM and Ansari BM: Growth parameters and endocrine
function in relation to echocardiographic parameters in children
with ventricular septal defect without heart failure. J Trop
Pediatr. 47:146–152. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hudson B, Hidalgo C, Saripalli C and
Granzier H: Hyper-phosphorylation of mouse cardiac titin
contributes to transverse aortic constriction-induced diastolic
dysfunction. Circ Res. 109:858–866. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu JP, Wang HX, Wang W, Zhang LK and Tang
CS: Ghrelin improves disturbed myocardial energy metabolism in rats
with heart failure induced by isoproterenol. J Pept Sci.
16:392–402. 2010.PubMed/NCBI
|
|
35
|
Tsukamoto Y, Mano T, Sakata Y, Ohtani T,
Takeda Y, Tamaki S, Omori Y, Ikeya Y, Saito Y, Ishii R, et al: A
novel heart failure mice model of hypertensive heart disease by
angiotensin II infusion, nephrectomy, and salt loading. Am J
Physiol Heart Circ Physiol. 305:H1658–H1667. 2013. View Article : Google Scholar : PubMed/NCBI
|