Introduction
Preeclampsia (PE) is a disorder that is
characterized by pregnancy-induced hypertension. The incidence of
PE among primiparas is ~8%, and approximately 50,000 mortality
cases in pregnant women worldwide are reported annually due to PE
and its complications (1,2). PE usually occurs after 20 weeks of
gestation, and the main clinical manifestations include
hypertension, proteinuria, and placental and renal tissue injury.
Severe PE can advance to eclampsia, leading to complications, such
as HELLP syndrome (involving hemolysis, increased liver enzyme
levels and thrombocytopenia), coagulation dysfunction,
cardiopulmonary syndrome and cardiorenal syndrome, which directly
threaten the safety of pregnant women (3,4).
PE not only produces adverse effects on the maternal body, but also
leads to fetal hypoxic-ischemic injury, resulting in intrauterine
growth retardation and even fetal death (5-7).
Although numerous efforts have been made in order to fully
understand PE, the pathogenesis of PE remains unclear. This
contributes to the current lack of an effective screening method
and treatment of PE; therefore, PE currently remains one of the
most refractory diseases in clinical practice. Therefore, it is
necessary to explore the pathogenesis of PE, identify novel
biological markers for early diagnosis and develop new strategies
for treating this disorder.
Annexin A1 (ANXA1), also known as Annexin I, is a
member of the calcium-dependent phospholipid-binding protein
superfamily of Annexins, which can bind to negatively charged
phospholipids (8). Annexin A is
usually used to detect cell apoptosis as it can bind to
phosphatidylserine, a marker of apoptosis on the outer leaflet of
the membrane (9). Annexin A1 is
considered to be able to mitigate inflammation by interacting with
Annexin A1 receptors (10). ANXA1
is located in chromosome 9q24, which contains 13 exons and 12
introns, and is an anti-inflammatory protein (11,12). It can participate in various cell
activities, including the anti-inflammatory response,
differentiation and proliferation, cell signal regulation, as well
as phagocytosis of apoptotic cells (13,14). Nevertheless, the specific
mechanism of ANXA1 in PE is not fully clear.
The Janus kinase 2 (JAK2)/signal transducer and
activator of transcription 3 (STAT3) signaling pathway is thought
to act as a signal transduction pathway that is activated by
cytokines. JAK2/STAT3 pathway is associated with the occurrence and
development of numerous diseases, such as tumors, PE and bone
diseases among others, and it mainly participates in inflammatory
response, growth, differentiation and apoptosis of cells (15-18). Extracellular signals or cytokines
are able to change the receptor structure by binding to the
receptor on the cell membrane, enabling JAK2 to move onto the
membrane receptor and activate the tyrosine phosphorylation of JAK2
(19). Next, activated JAK2
phosphorylates STAT3 and these activated STATs formed dimers, then
the dimers enter into cell nucleus and regulate the downstream gene
expression (20).
In the present study, a model of PE was constructed
in rats, and the expression of ANXA1 in the model was detected.
Placental trophoblasts were obtained from PE rats, and the roles
and molecular mechanism of ANXA1 in these cells were explored. The
data revealed that the knockdown of ANXA1 decreased the apoptosis
and inflammatory response of PE trophoblasts.
Materials and methods
Animal model construction and
grouping
A total of 20 male and 20 female Sprague-Dawley rats
(weight, 250-300 g; age, 7-9 weeks; Guangdong Medical Laboratory
Animal Center, Guangzhou, China) were kept in cages at a
temperature set between 23 and 26°C (50-70% humidity and 12-h
light/dark cycle). The rats had free access to food and water, and
the ratio of male to female was 1:1. After mating of the rats, in
the following morning, small wet cotton swabs with saline were used
to collect vaginal secretions of female rats, and this was recorded
as the day 0 of pregnancy (vaginal plugs were used to determine if
the rats were pregnant). A hypertensive model group was established
by gavaging 6 pregnant rats using 80 mg/kg/day Nω-nitro-L-arginine
methyl ester (L-NAME; Sigma-Aldrich; Merck KGaA, Darmstadt Germany)
for 8 days starting on day 12 of the pregnancy, and this was termed
the L-NAME group. In the control group, 6 pregnant rats were
gavaged using 80 mg/kg/day normal saline for 8 days. All animal
tests conducted in the present study were approved by the Ethics
Committee of Hebei General Hospital (Shijiazhuang, China).
Detection of blood pressure and urine
protein
The systolic blood pressure (SBP), diastolic blood
pressure (DBB) and mean arterial pressure (MAP) of rats were
detected on days 12, 16 and 20 of pregnancy, using a non-invasive
rat tail arterial blood pressure monitor (Tail Cuff Blood Pressure
Systems; IITC Life Science, Woodland Hills, CA, USA). The method
was as follows: The pregnant rats were placed in a fixator, which
was adjusted in terms of the size of the pregnant rat. An air bag
was sleeved in the middle of the tail, and the measuring instrument
was well-connected. The blood pressure of the rat tail artery was
detected when the rat was calm and the pulse wave of the blood
pressure measuring instrument was stable. The mean value of each
rat was obtained based on five measured values.
In addition, the rats were kept in standard
metabolic cages on days 12, 16 and 20 of their pregnancy, and their
urine volume was collected for 24 h. The 24-h urinary protein (UP)
level was tested with an automatic biochemical analyzer (AU5800;
Beckman Coulter, Inc., Brea, CA, USA).
Specimen collection
Prior to placental tissue collection, urine was
obtained from the rats on day 20 of pregnancy. Under anesthesia by
an intraperitoneal injection of chloral hydrate (350 mg/kg), the
animals were sacrificed by rapid cervical dislocation (21). The uterus was exposed and cut
open, and the placenta was removed. The placenta was fixed using 4%
polymethylene for 24 h. Next, the placenta was embedded in paraffin
and prepared for hematoxylin and eosin (H&E) and
immunohistochemical staining.
Following the collection of placental tissue, the
abdominal organs and the abdominal viscera were pushed to one side
in order to expose the inferior vena cava and the abdominal aorta.
An index finger was used to touch the blood vessel, and the
abdominal aorta was more evident. The blood of the rats was
collected from the bifurcation of the abdominal aorta and
centrifuged at 1,500 × g for 10 min at 4°C. The serum was then used
for enzyme-linked immunosorbent assay (ELISA).
H&E staining
Paraffin-embedded placental tissue was sliced, and
the thickness of each slice was ~4 µm. The slices were
routinely dewaxed with dimethylbenzene for 15 min and 95% ethanol
for 3 min at room temperature, and then washed three times with
distilled water. Next, the slices were stained by hematoxylin
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) for 15 min at room temperature. The slices were then washed
three times with distilled water, followed by treatment with 1%
hydrochloric acid alcohol. The slices were then washed three times
with distilled water and stained with eosin staining solution
(Beijing Solarbio Science & Technology Co., Ltd.).
Subsequently, the slices were dehydrated using gradient alcohol
(80% ethanol for 2 sec, 95% ethanol for 3 min and absolute alcohol
for 6 min), and neutral gum (Beijing Solarbio Science &
Technology Co., Ltd.) was used for mounting. Finally, the slices
were observed under an inverted fluorescence microscope (MF53;
Micro-shot Technology Co., Ltd., Guangzhou, China).
Immunohistochemical assay
The tissue slices were routinely dewaxed, washed
with distilled water, soaked in 0.01 M citrate buffer (pH=6.0) and
heated to 95°C using a microwave. Subsequent to cooling, the slices
were washed using PBS (pH=7.4), following which 3% hydrogen
peroxide was added to the slices and maintained for 20 min. The
slices were then incubated with anti-ANXA1 antibody (cat. no.
ab214486; 1:1,000; Abcam, Cambridge, MA, USA), at 4°C for 24 h. On
the following day, goat anti-mouse IgG secondary antibodies (cat.
no. ab6708; 1:8,000; Abcam) were added to the slices at 37°C and
maintained for 30 min. The slices were then stained with
3,3′-diaminobenzidine solution (Leica Microsystems, Shanghai,
China) for 10 min. After washing three times with distilled water,
the slices were dyed using hematoxylin for 30 sec at room
temperature. The slices were finally observed under an inverted
fluorescence microscope.
ELISA
The levels of the inflammatory factors-tumor
necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6 and IL-8 were
measured using TNF-α ELISA kit (cat. no. RTA00; R&D Systems,
Inc., Minneapolis, MN, USA), IL-1β ELISA kit (cat. no. RLB00;
R&D Systems, Inc.), IL-6 ELISA kit (cat. no. ESK6029; Sangon
Biotech Co., Ltd., Shanghai, China) and respectively. All the
regents used for the ELISA assay were provided by the kits and the
determination was performed according to the manufacture’s
protocols. The optical density value at 450 nm was measured using
SMR16.1 multimode reader (USCN Business Co., Ltd., Wuhan,
China).
Cell culture and transfection
The placental trophoblasts were obtained from the
placenta of pregnant rats with hypertension. In brief, the villi
tissues were cut into −1 mm3 pieces and digested with
0.25% trypsin (Beijing Solarbio Science & Technology Co., Ltd.)
and 0.1% collagenase (Beijing Solarbio Science & Technology
Co., Ltd.) at 37°C for 25 h. The reaction was stopped by addition
of the serum. The solution was centrifuged at 1,200 × g for 30 min
at 4°C. The collected trophoblasts were cultured in Dulbecco’s
modified Eagle’s medium (DMEM; Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) with 10% fetal bovine serum
(FBS; Invitrogen; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 µg/ml streptomycin at 37°C in an
incubator that contained 5% CO2. The cells were
identified under an inverted microscope.
For ANXA1 knockdown, ANXA1 small interfering RNA
(siRNA) and empty vectors (30 nM) were purchased from the
Genomeditech (Shanghai, China). The vectors were transfected into
trophoblasts by Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.).
Immunofluorescence staining
The trophoblasts were isolated from placental
tissues and then the expression of vimentin was examined by
immunofluorescence staining to identify trophoblasts. Briefly, the
villi tissues were homogenized and soaked in Hank’s balanced salt
solution supplemented with HEPES (25 mmol), DNase1 and collagenase
(15 U/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C
with agitation for 30 min. The dispersed cells were separated by
filtering through a screen mesh (40-mm diameter per pore). The
cells in Ficoll suspension were centrifuged at 1,200 × g for 30 min
at 4°C, and the obtained cells were maintained in DMEM with 10%
FBS. Subsequent to culturing, the trophoblasts were washed using
PBS and fixed with 4% paraformaldehyde at 4°C for 20 min. Goat
serum (Beyotime Institute of Biotechnology, Shanghai, China) was
then added to block the cells (at room temperature for 30 min),
followed by incubation with the anti-vimentin primary antibody
(cat. no. ab8978; 1:1,000; Abcam) 4°C for 24 h. PBS was used as the
negative control for the anti-vimentin primary antibody. Next, the
cells were incubated using a fluorescence-conjugated secondary
antibody (Alexa-Fluor® 594 goat anti-rabbit antibody
IgG; Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 1.5 h at
room temperature. Nuclei were counterstained by Hoechst 33258
(Beyotime, Shanghai, China), and images were obtained using an
inverted fluorescence microscope.
Cell apoptosis
Cell apoptosis was analyzed using an Annexin
V-FITC/propidium iodide (PI) apoptosis detection kit (Beijing
Solarbio Science & Technology Co., Ltd.). The trophoblasts were
first seeded in a 6-well plate (5×104 cells/well) for 24
h, and then treated with PBS, siRNA-ANXA1 or empty vector using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) and
incubated at 37°C for further 24 h. The cells were digested by
trypsin (Beijing Solarbio Science & Technology Co., Ltd.) and
re-suspended with a 1X binding buffer. Next, the cells were
double-stained with Annexin V-FITC/PI for 20 min, and the cell
apoptosis was determined using flow cytometry (BD Biosciences,
Franklin Lakes, NJ, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
The total RNA of cells was isolated using a TRIzol
reagent (Promega Corporation, Beijing, China). The concentration of
RNA was measured by ultra-micro protein nucleic acid analyzer
(NanoDrop; Thermo Fisher Scientific, Inc., Wilmington, DE, USA). In
total, 1 µg RNA was used for cDNA synthesis by an ABScript
II cDNA First Strand synthesis kit (ABclonal Biotech Co., Ltd.,
Wuhan, China) following the manufacturer’s protocols. A SYBR Premix
Taq™ II kit (Dalian Meilun Biology Technology Co., Ltd, Dalian,
China) was then used to amplify cDNA, according to the instructions
of the kit. The primers used were purchased from BersinBio Co.,
Ltd. (Guangzhou, China), and are listed in Table I. The data were analyzed with the
2−ΔΔCq calculation (22), and the relative mRNA expression
levels were normalized to that of GAPDH.
 | Table ISequences of primers used in
polymerase chain reaction. |
Table I
Sequences of primers used in
polymerase chain reaction.
| Primer name | Sequence
(5′-3′) | Product size
(bp) |
|---|
| ANXA1 forward |
CTGGAGGAGGTTGTTTTGGC | 238 |
| ANXA1 reverse |
GAGCAAGCAAGGCATTACGA | |
| Bax forward |
GAGACACCTGAGCTGACCTT | 187 |
| Bax reverse |
CGTCTGCAAACATGTCAGCT | |
| Bcl-2 forward |
GCCTTCTTTGAGTTCGGTGG | 221 |
| Bcl-2 reverse |
CTGAGCAGCGTCTTCAGAG | |
| GAPDH forward |
AGTCTACTGGCGTCTTCACC | 225 |
| GAPDH reverse |
CCACGATGCCAAAGTTGTCA | |
Western blot analysis
The cells were lysed by radioimmunoprecipitation
assay buffer (Thermo Fisher Scientific, Inc.). The protein
concentrations of samples were measured using a BCA protein assay
kit (Qiyi Biological Technology Co., Ltd., Shanghai, China). Next,
the proteins (25 µg) were separated by 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and then transferred
onto a polyvinylidene fluoride membrane (EMD Millipore, Billerica,
MA, USA). Tris-buffered saline/Tween 20 buffer containing 5%
skimmed milk was added to block the membranes at room temperature
for 2 h. The corresponding primary antibodies were subsequently
used to incubate the membranes overnight at 4°C, including
anti-ANXA1 (cat. no. ab214486; 1:1,000; Abcam), anti-pro-caspase-3
(cat. no. ab32499; 1:1,000; Abcam), anti-cleaved-caspase-3 (cat.
no. ab2302; 1:700; Abcam), anti-B-cell lymphoma-2 (Bcl-2; cat. no.
ab692; 1:800; Abcam), anti-Bcl-2-associated X protein (Bax; cat.
no. 2774; 1:1,000; Cell Signaling Technology, Inc., Danvers, MA,
USA), anti-JAK2 (cat. no. ab108596; 1:600; Abcam),
anti-phosphorylated (p)-JAK2 (cat. no. ab195055; 1:800; Abcam;
anti-STAT3 (cat. no. ab119352; 1:1,000; Abcam), anti-p-STAT3 (cat.
no. ab32143; 1:800; Abcam) and anti-GAPDH (cat. no. ab8245; 1:600;
Abcam). On the following day, the membranes were incubated with the
following secondary antibodies at room temperature for 1 h: Rabbit
anti-mouse IgG (cat. no. ab6709; 1:6,000; Abcam), mouse anti-rabbit
IgG (cat. no. 93702; 1:8,000, Cell Signaling Technology, Inc.), and
goat anti-rabbit (cat. no. ab205718; 1:6,000; Abcam). The blots
were subsequently developed using a chemiluminescence substrate kit
(BeyoECL Star; Beyotime Institute of Biotechnology, Haimen, China).
The results were analyzed by the ECL system (Amersham; GE
Healthcare, Chicago, IL, USA).
Statistical analysis
Statistical analysis was conducted using SPSS
version 20.0 software (IBM Corporation, Armonk, NY, USA). Data are
expressed as the mean ± standard deviation. Student’s t-test was
used to compare the differences between two groups, while one-way
analysis of variance and the post-hoc Dunnett’s test were performed
to compare the differences among more than two groups. P<0.05
was considered to denote a statistically significant
difference.
Results
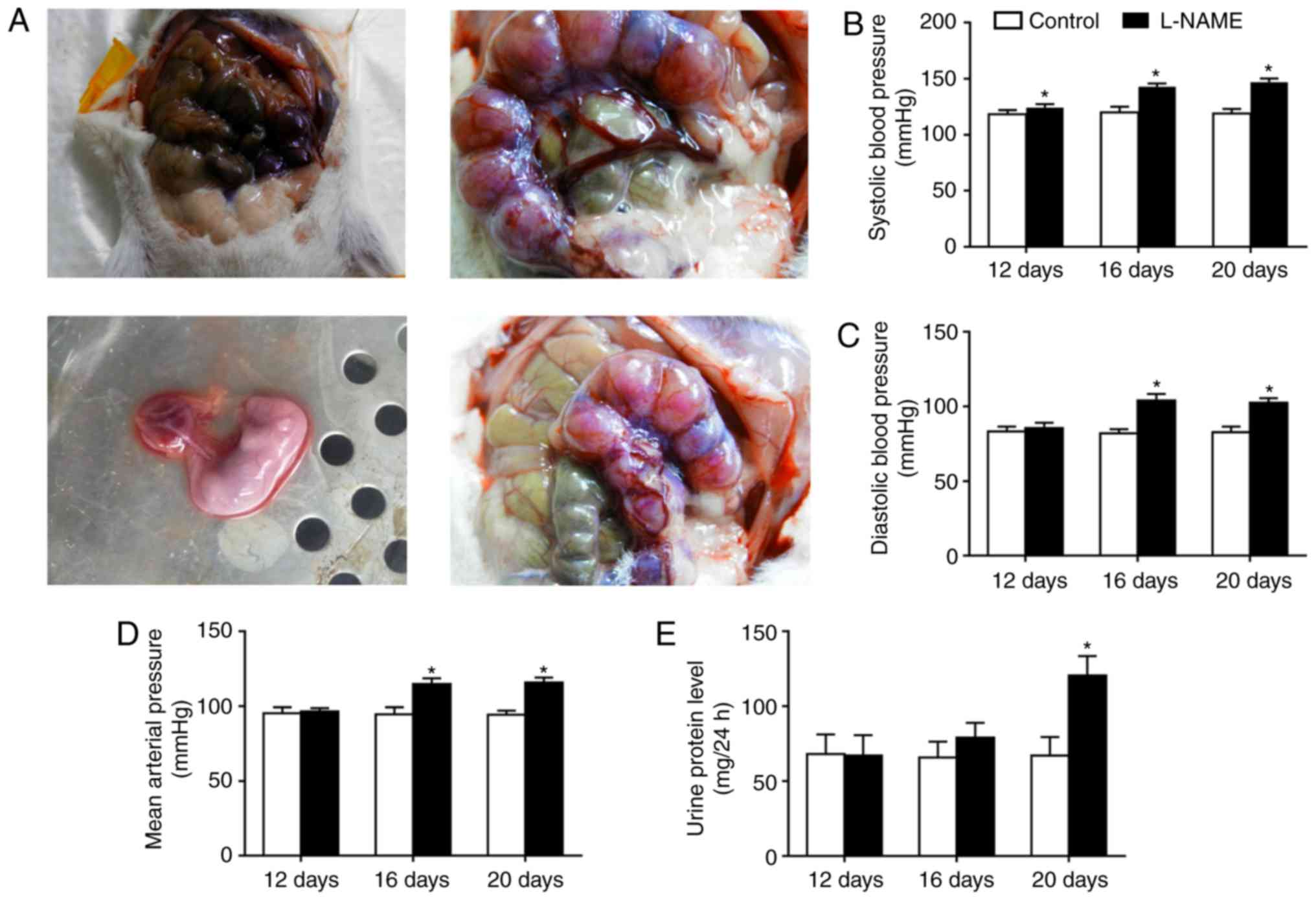
Blood pressure and UP levels are
increased in the L-NAME group
The uterus and placenta were examined, and serum was
obtained (Fig. 1A). The results
revealed that the SBP, DBP, MAP and UP levels remained stable on
day 12 of the rat pregnancy in the L-NAME group, as compared with
those in the control group. However, the SBP, DBP, MAP and UP
levels in the L-NAME group were significantly increased in
comparison with those in the control group on day 20 of the
pregnancy (P<0.05; Fig. 1B-E).
These results demonstrated that the PE model was successfully
established in the L-NAME-treated rats.
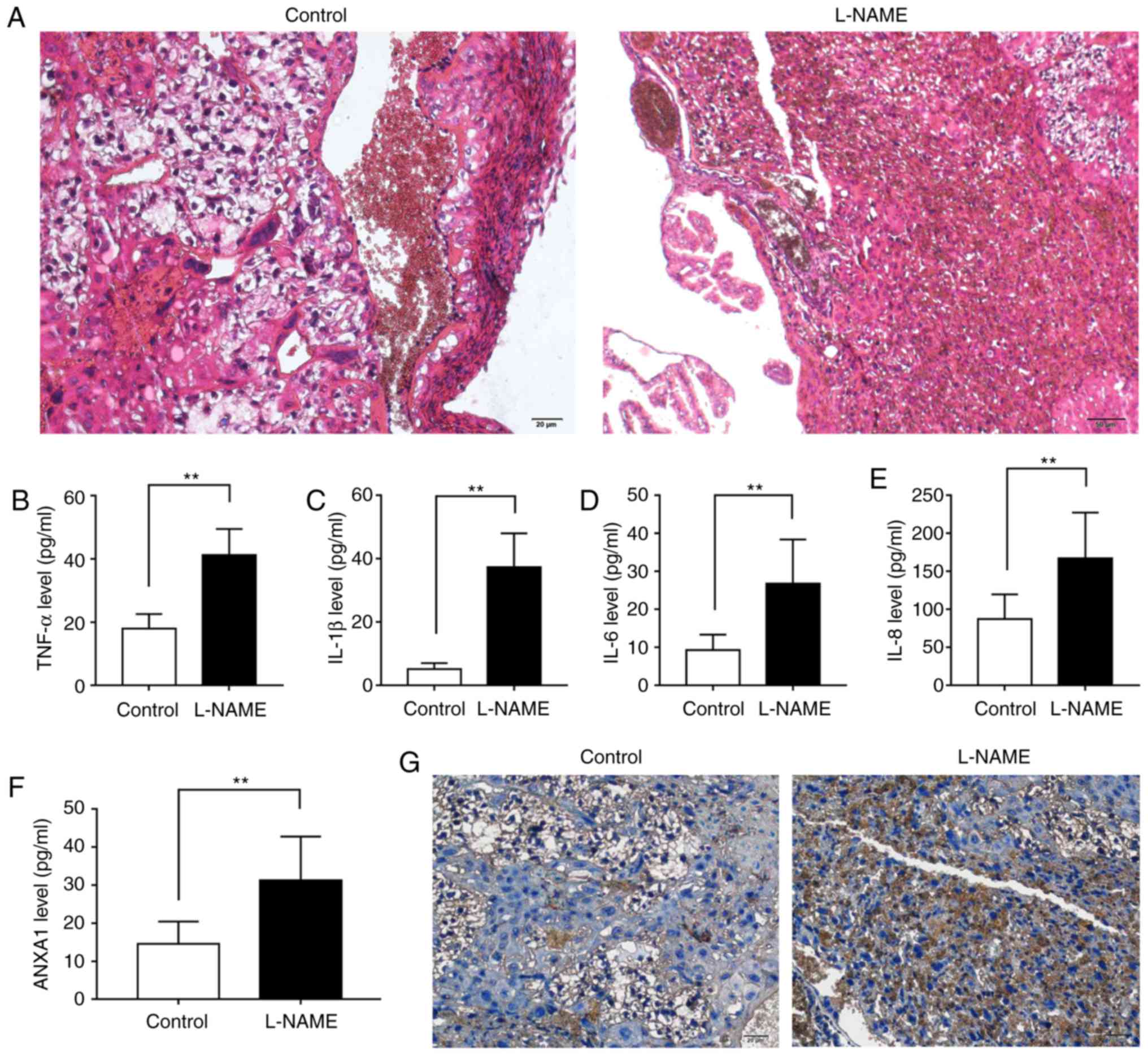
Inflammatory response and upregulated
ANXA1 expression are observed in the L-NAME group
H&E staining, ELISA and immunohistochemical
assay were performed to investigate the histopathological changes,
inflammatory reaction and ANXA1 expression in placental tissues.
The H&E staining images displayed that, in comparison with the
control group, the trophoblast proliferation and inflammatory cell
infiltration were evidently increased in the L-NAME group (Fig. 2A). The ELISA data revealed that
the levels of TNF-α, IL-1β, IL-6 and IL-8 in L-NAME group were
higher as compared with those in the control group (Fig. 2B-E; P<0.01). The
immunohistochemical assay results also revealed that there was a
considerable amount of brown particles in the L-NAME group, which
represented positive expression of ANXA1 (Fig. 2F and G). These results indicated
that the expression of inflammatory factors and ANXA1 was
upregulated during PE.
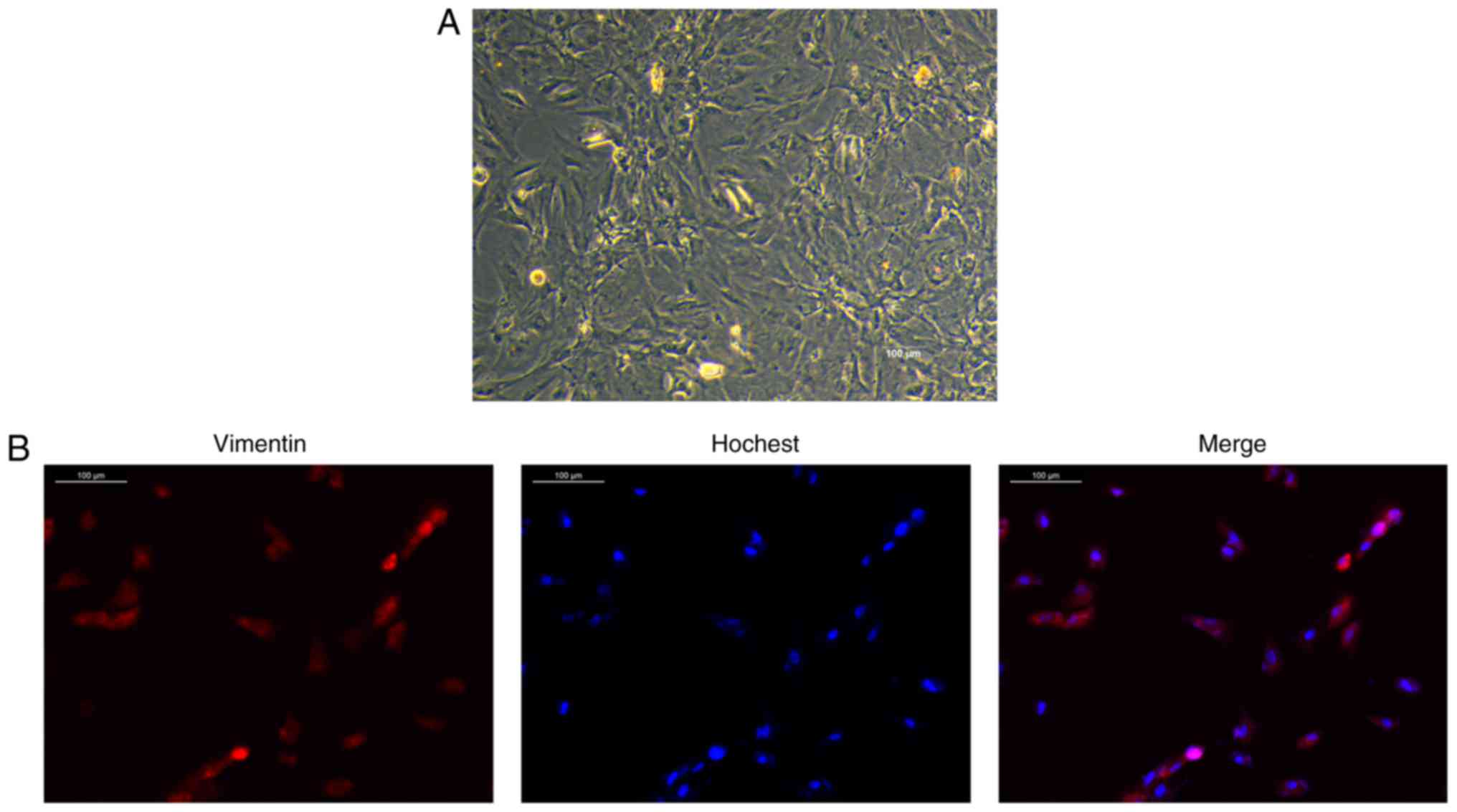
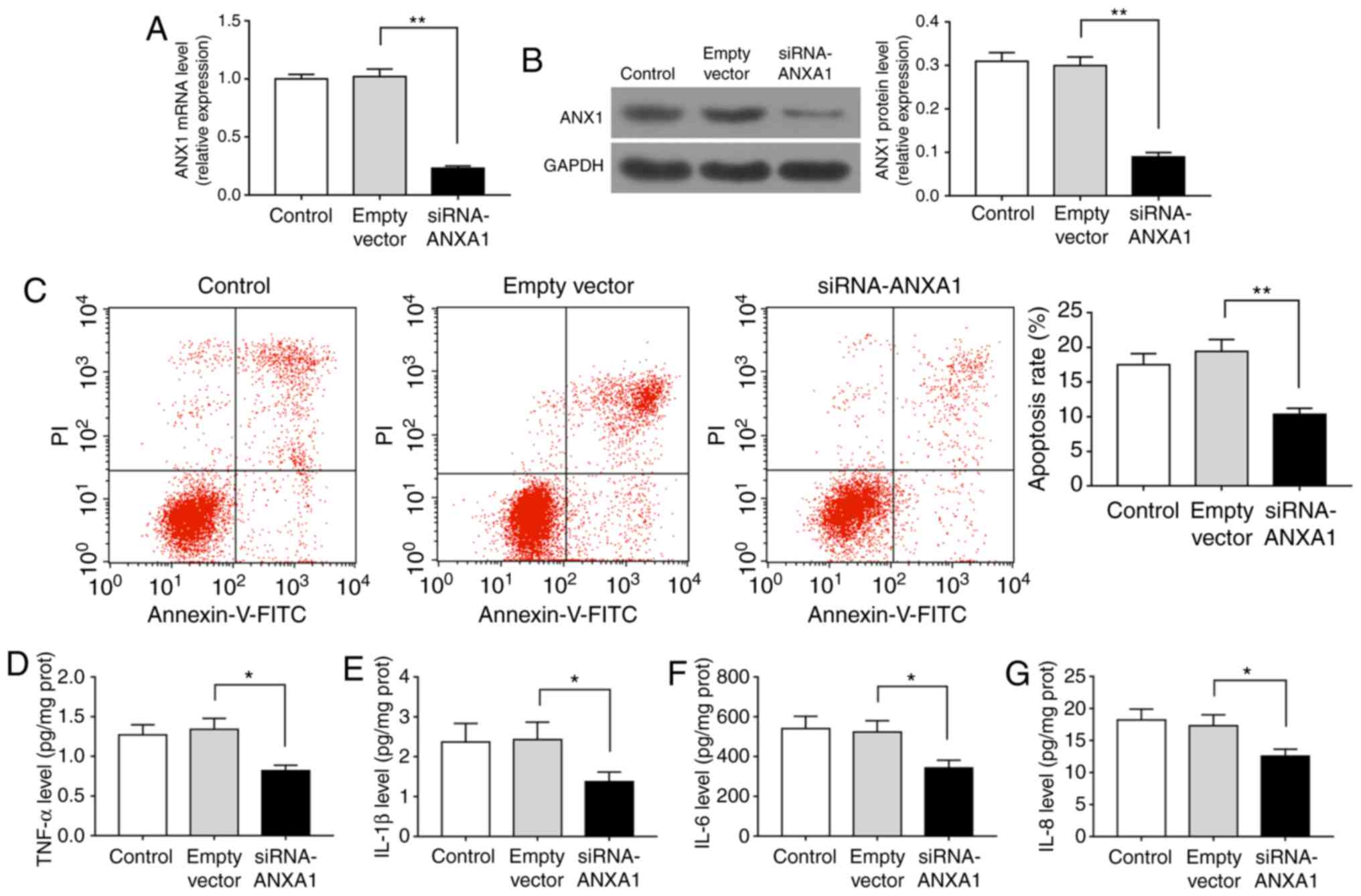
Knockdown of ANXA1 inhibits apoptosis and
inflammatory response in trophoblasts
The trophoblasts were identified by staining with
vimentin antibody (Fig. 3). The
RT-qPCR and western blotting data demonstrated that the mRNA and
protein levels of ANXA1 were reduced in cells transfected with
siRNA-ANXA1 as compared with those transfected with empty vector
(Fig. 4A and B; P<0.01). The
flow cytometry results also observed that the apoptosis rate was
significantly decreased when cells were transfected with
siRNA-ANXA1 (Fig. 4C; P<0.01).
As displayed by the ELISA data, siRNA-ANXA1 markedly suppressed the
levels of TNF-α, IL-1β, IL-6 and IL-8 (Fig. 4D-G; P<0.05). Therefore,
downregulation of ANXA1 decreased the apoptosis during PE.
Knockdown of A N X A1 regulates the
levels of apoptosis-associated factors in trophoblasts
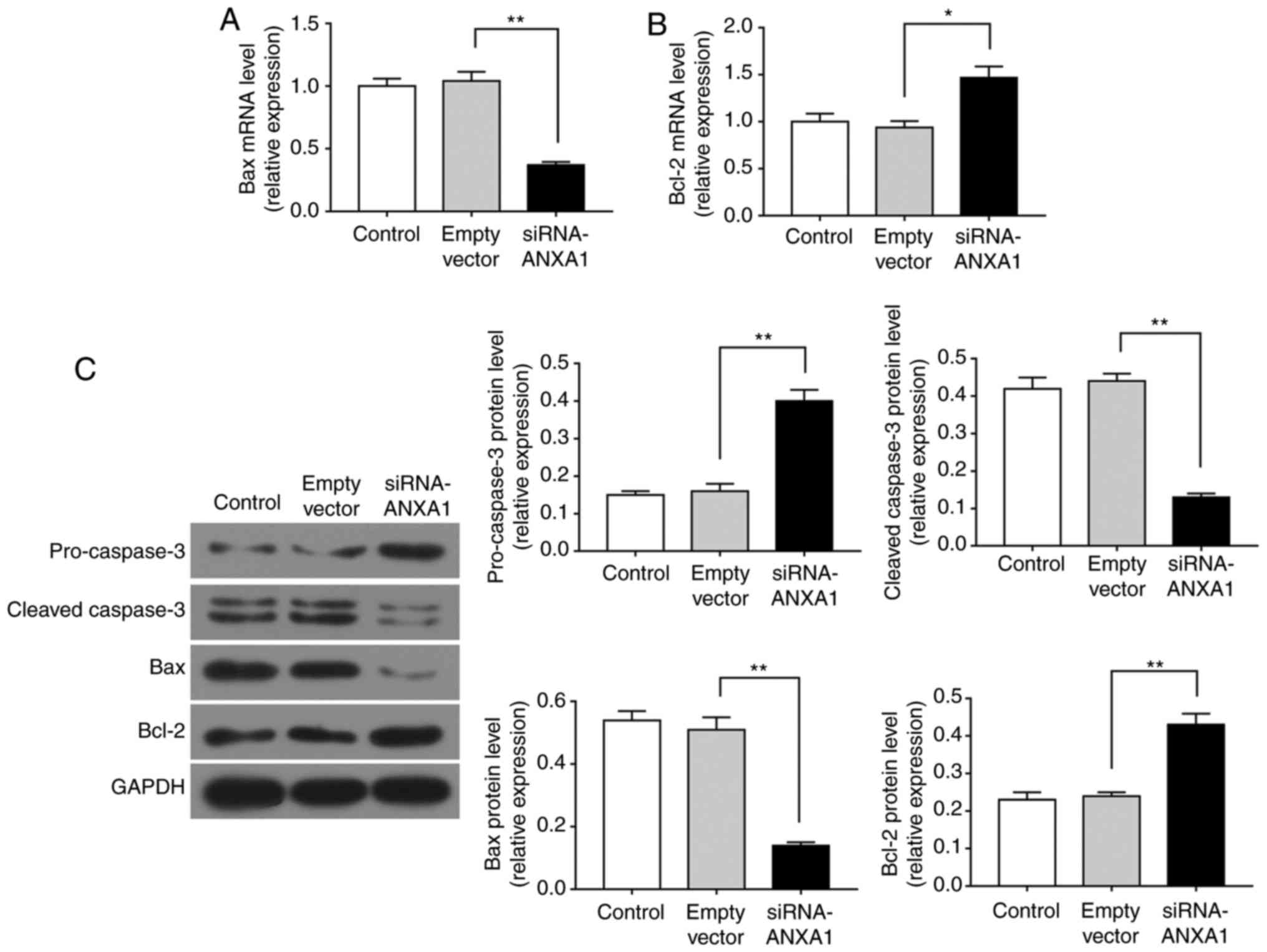
Bcl-2 and Bax are known to regulate the cell
apoptosis (23), while caspase-3
is activated by multiple apoptotic signals by cleaving
pro-caspase-3 into cleaved-caspase-3 (24). Therefore, RT-qPCR and western blot
analysis were performed to explore the effect of ANXA1 on these
apoptosis-associated factors in trophoblasts. As demonstrated by
the RT-qPCR data, siRNA-ANXA1 transfection significantly decreased
Bax mRNA expression and increased Bcl-2 mRNA expression (Fig. 5A and B; P<0.05). Furthermore,
the western blot analysis data revealed that siRNA-ANXA1 promoted
the expression levels of pro-caspase-3 and Bcl-2 protein, whereas
it reduced the expression levels of cleaved-caspase-3 and Bax
protein (Fig. 5C; P<0.01).
Therefore, downregulation of ANXA1 inhibited the apoptosis during
PE through regulating the expression of apoptosis-related
genes.
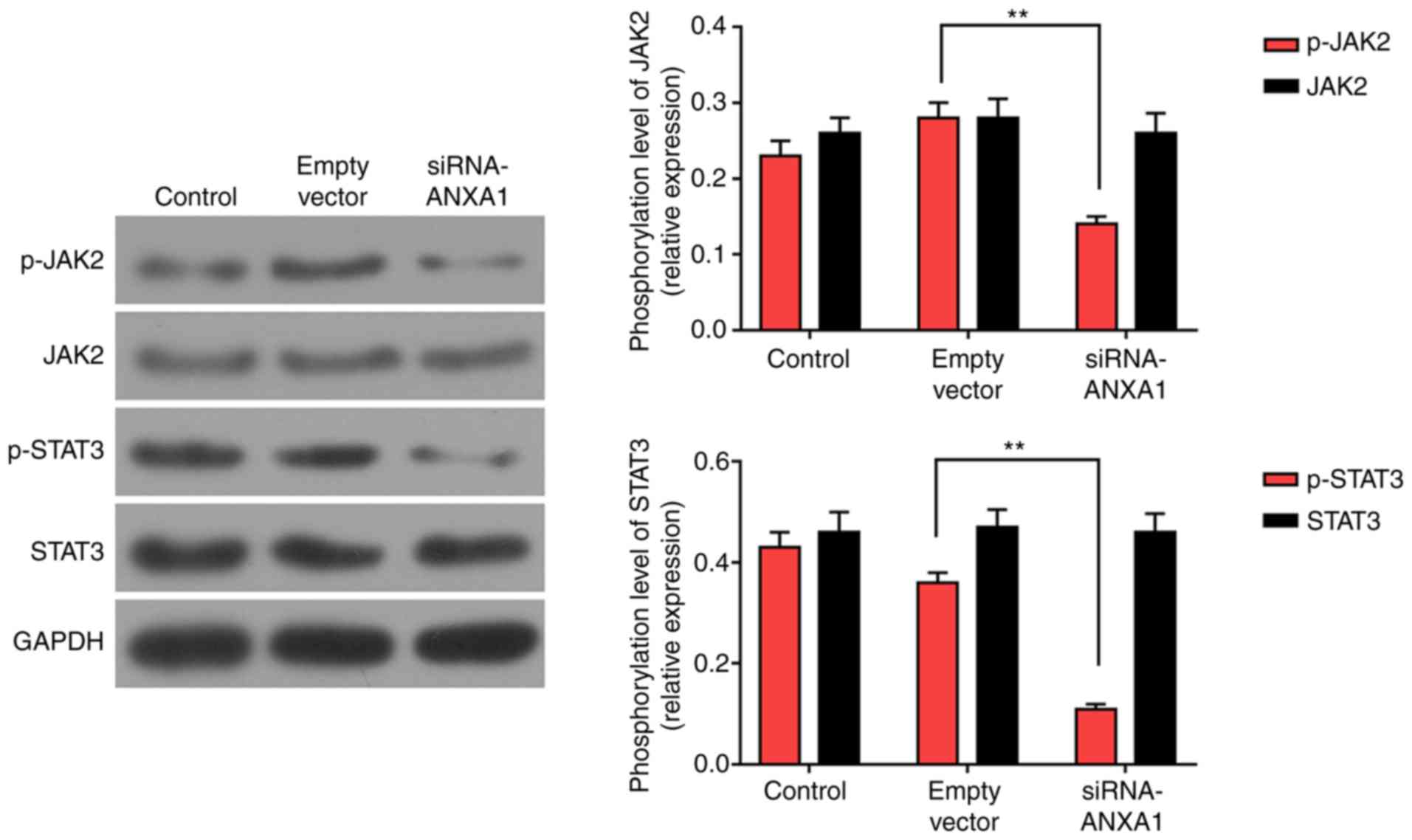
Knockdown of ANXA1 suppresses the
JAK2/STAT3 signaling pathway in trophoblasts
JAK2/STAT3 pathway was examined using western blot
analysis to help investigate the pathway of ANXA1 in trophoblasts.
The data revealed that the phosphorylation of JAK2 and STAT3 was
downregulated in siRNA-ANXA1-treated cells as compared with the
empty vector group. The levels of JAK2 and STAT3 remained stable in
all groups (Fig. 6; P<0.01).
Therefore, the inhibition of JAK2/STAT3 signaling pathway may be
related to the effect produced by si-ANXA1.
Discussion
L-NAME is an inhibitor of nitric oxide (NO) synthase
(25,26). NO is produced by vascular
endothelial cells, and its functions include the dilation of blood
vessels, and regulation of blood vessel tension and of the
cardiovascular system during preg-nancy (27,28). Increasing evidence suggested that
L-NAME can be used as an inducer of PE symptoms in pregnant rats
(29-31). In the present study, it was
observed that SBP, DBP, MAP and UP level in the L-NAME group were
higher compared with those in the control group. Therefore, this
suggested that the PE model was successfully established in
pregnant rats using L-NAME.
The placenta is a unique temporary organ during
pregnancy that serves an indispensable role in maintaining a normal
and stable pregnancy, and preventing pregnancy-associated diseases
(32-34). Placental tissue has been widely
used as a research object for studying PE (35,36). Therefore, in the current study,
normal and PE placental tissues were obtained from rats.
Furthermore, previous studies have reported that excessive
inflammatory response is an important factor leading to the onset
of PE (37-39). Several inflammatory factors,
including TNF-α, IL-1β, IL-6 and IL-8, are suggested to contribute
to the development of PE. Hence, the levels of these factors in the
placental tissues were detected in the present study. The results
demonstrated that TNF-α, IL-1β, IL-6 and IL-8 levels in the L-NAME
group were higher compared with those in the control group. These
observations indicated that there was a strong inflammatory
response in PE placental tissue.
ANXA1 was demonstrated to exhibit various
anti-inflammatory properties (40). Researchers have reported that
ANXA1Ac2-26 peptide reduced the inflammatory response in
ARPE-19 cells and peritonitis rats (41,42). However, ANXA1 is also modulated by
pro-inflammatory proteins, suggesting that it may act as a ‘brake’
in controlling the inflammatory response (43). According to previous studies,
ANXA1 expression was enhanced in PE patients (44,45). The increased concentration of
ANXA1 may be a compensatory mechanism underlying systemic
inflammation (45). Similarly,
the data of the present study displayed that ANXA1 level in the
L-NAME group was higher compared with that of the control group. In
order to explore the effect of ANXA1 on PE, the trophoblasts from
PE placental tissue were transfected with siRNA-ANXA1. It was
observed that the ANXA1 expression in the siRNA-ANXA1 group was
lower in comparison with that in the group transfected with empty
vector. These results indicated that the transfection efficiency of
siRNA-ANXA1 in the trophoblasts was high. It was also observed that
siRNA-ANXA1 inhibited the inflammatory response of trophoblasts
through decreasing the levels of TNF-α, IL-1β, IL-6 and IL-8.
Consistently, a previous study demonstrated that transfection with
ANXA1 short hairpin RNA inhibited IL-1β expression in
ischemia/reperfusion-induced retinal ganglion cells (46).
The inflammatory response during PE alters the
inflammation microenvironment, and such a change leads to the
trophoblasts being more susceptible to apoptosis (47). In addition, PE is associated with
abnormal lipid metabolism (48),
and apoptosis of trophoblasts may be induced by oxidative stress
during PE (49). PE is usually
considered to be associated with insufficient perfusion of the
uterus and placenta from the mother, which contributes to the
ischemic and hypoxic microenvironment of placental trophoblasts
(49). According to a previous
study (49), the apoptosis rate
of a trophoblast cell line reached nearly 40% when the cells were
cultured in hypoxic conditions. It has also been reported that
ANXA1 enhanced the apoptosis of retinal ganglion cells (46). Furthermore, Huang et al
(50) proved that the knockdown
of ANXA1 decreased ischemia/reperfusion-induced apoptosis in
nasopharyngeal carcinoma cells. In the current study, ANXA1
silencing noticeably suppressed the apoptosis of the trophoblasts.
Subsequently, the apoptosis-associated factors were examined by
performing RT-qPCR and western blot analysis. The data revealed
that siRNA-ANXA1 markedly enhanced the expression levels of
pro-caspase-3 and Bcl-2, while it reduced the expression levels of
cleaved-caspase-3 and Bax, and these results are consistent with
those reported in previous studies (51,52). Thus, the data suggested that
deletion of ANXA1 suppressed the apoptosis of the trophoblasts by
upregulating pro-caspase-3 and Bcl-2, and downregulating
cleaved-caspase-3 and Bax.
JAK2/STAT3 pathway contributes to development of
complications in pregnancy. A previous study reported that
5-hydroxytryptamine 2A receptor activated JAK2/STAT3 pathway in
human choriocarcinoma cells (17). IL-1 was observed to be able to
regulate the invasion of trophoblasts via controlling the
phosphorylation of JAK2 and STAT3 (53). Hence, it can be hypothesized that
ANXA1 regulates the JAK2/STAT3 pathway in trophoblasts. As
expected, the present study identified that siRNA-ANXA1
significantly downregulated the phosphorylation of JAK2 and STAT3,
but did not affect the levels of JAK2 and STAT3. This verified that
silencing of ANXA1 inhibited the regulation of JAK2/STAT3 pathway
in trophoblasts.
In conclusion, in the current study, an
L-NAME-induced model of PE was constructed in rats. The
inflammatory response and the increase of ANXA1 expression were
observed in the PE model. Silencing of ANXA1 decreased the
apoptosis and inflammatory response of trophoblasts obtained from
PE placental tissue and downregulated the JAK2/STAK3 pathway. The
aforementioned results revealed that ANXA1 may contribute to the
pathological mechanism of PE and provided a solid foundation for
further study on the specific mechanism of ANXA1 on PE in
vivo.
Funding
Not applicable.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors’ contributions
JF wrote the main manuscript. JF, XW, HL and LW
performed the experiments. QQ, JF and ZT designed the study. XW, HL
and LW performed data analysis. JF, XW, HL, LW and ZT contributed
to manuscript revisions. All authors reviewed, read and approved
the final manuscript.
Ethics approval and consent to
participate
All animal tests conducted in the present study were
approved by the Ethics Committee of Hebei General Hospital
(Shijiazhuang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Roberts JM and Cooper DW: Pathogenesis and
genetics of pre-eclampsia. Lancet. 357:53–56. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singh SK and Bhatia K: Ultrasonographic
optic nerve sheath diameter as a surrogate measure of raised
intracranial pressure in severe pregnancy-induced hypertension
patients. Anesth Essays Res. 12:42–46. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Contini C, Jansen M, König B,
Markfeld-Erol F, Kunze M, Zschiedrich S, Massing U, Merfort I,
Prömpeler H, Pecks U, et al: Lipoprotein turnover and possible
remnant accumulation in preeclampsia: Insights from the Freiburg
Preeclampsia H.E.L.P.-apheresis study. Lipids Health Dis.
17:492018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kaitu’u-Lino TJ, Brownfoot FC, Beard S,
Cannon P, Hastie R, Nguyen TV, Binder NK, Tong S and Hannan NJ:
Combining metformin and esomeprazole is additive in reducing sFlt-1
secretion and decreasing endothelial dysfunction-implications for
treating preeclampsia. PLoS One. 13:e01888452018. View Article : Google Scholar
|
|
5
|
Asgharnia M, Mirblouk F, Kazemi S,
Pourmarzi D, Mahdipour Keivani M and Dalil Heirati SF: Maternal
serum uric acid level and maternal and neonatal complications in
preeclamptic women: A cross-sectional study. Int J Reprod Biomed
(Yazd). 15:583–588. 2017. View Article : Google Scholar
|
|
6
|
Maruotti GM, Giudicepietro A, Saccone G,
Castaldo G, Sarno L, Zullo F, Berghella V and Martinelli P: Risk of
preeclampsia in of women who underwent chorionic villus sampling. J
Matern Fetal Neonatal Med. 1–4. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang F, Fan F, Wang L, Ye W, Zhang Q and
Xie S: Maternal cadmium levels during pregnancy and the
relationship with preeclampsia and fetal biometric parameters. Biol
Trace Elem Res. Apr 12–2018.Epub ahead of print. View Article : Google Scholar
|
|
8
|
Gerke V and Moss SE: Annexins: From
structure to function. Physiol Rev. 82:331–371. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Van ME, Nieland LJ, Ramaekers FC, Schutte
B and Reutelingsperger CP: Annexin V-affinity assay: A review on an
apoptosis detection system based on phosphatidylserine exposure.
Cytometry. 31:1–9. 1998. View Article : Google Scholar
|
|
10
|
Hannon R, Croxtall JD, Getting SJ,
Roviezzo F, Yona S, Paul-Clark MJ, Gavins FN, Perretti M, Morris
JF, Buckingham JC and Flower RJ: Aberrant inflammation and
resistance to glucocorticoids in annexin 1−/− mouse. FASEB J.
17:253–255. 2003. View Article : Google Scholar
|
|
11
|
Ansari J, Kaur G and Gavins FNE:
Therapeutic potential of annexin A1 in ischemia reperfusion injury.
Int J Mol Sci. 19:E12112018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sheikh MH and Solito E: Annexin A1:
Uncovering the many talents of an old protein. Int J Mol Sci.
19:E10452018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alli-Shaik A, Wee S, Lim LHK and Gunaratne
J: Phospho-proteomics reveals network rewiring to a pro-adhesion
state in annexin-1-deficient mammary epithelial cells. Breast
Cancer Res. 19:1322017. View Article : Google Scholar
|
|
14
|
Lee SH, Lee PH, Kim BG, Seo HJ, Baek AR,
Park JS, Lee JH, Park SW, Kim DJ, Park CS and Jang AS: Annexin A1
in plasma from patients with bronchial asthma: Its association with
lung function. BMC Pulm Med. 18:12018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bi J, Sun K, Wu H, Chen X, Tang H and Mao
J: PPARgamma alleviated hepatocyte steatosis through reducing SOCS3
by inhibiting JAK2/STAT3 pathway. Biochem Biophys Res Commun.
498:1037–1044. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li CH, Xu LL, Jian LL, Yu RH, Zhao JX, Sun
L, Du GH and Liu XY: Stattic inhibits RANKL-mediated
osteoclastogenesis by suppressing activation of STAT3 and NF-kappaB
pathways. Int Immunopharmacol. 58:136–144. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oufkir T, Arseneault M, Sanderson JT and
Vaillancourt C: The 5-HT 2A serotonin receptor enhances cell
viability, affects cell cycle progression and activates MEK-ERK1/2
and JAK2 STAT3 signalling pathways in human choriocarcinoma cell
lines. Placenta. 31:439–447. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun YX, Zhang HY, Wei YM, Zhu F, Wang M
and Liao YH: The mechanism of signal transduction during vascular
smooth muscle cell proliferation induced by autoantibodies against
angiotensin AT1 receptor from hypertension. Chin Med J (Engl).
121:43–48. 2008.
|
|
19
|
Parganas E, Wang D, Stravopodis D, Topham
DJ, Marine JC, Teglund S, Vanin EF, Bodner S, Colamonici OR, van
Deursen JM, et al: Jak2 is essential for signaling through a
variety of cytokine receptors. Cell. 93:385–395. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schindler C, Levy DE and Decker T:
JAK-STAT signaling: From interferons to cytokines. J Biol Chem.
282:20059–20063. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nagy A, Gertsenstein M, Vintersten K and
Behringer R: Quick and humane sacrifice of a mouse by cervical
dislocation. CSH Protoc. 2006.2006.
|
|
22
|
Kenneth J and Livak TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)). Method Method. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Knudson CM and Korsmeyer SJ: Bcl-2 and Bax
function independently to regulate cell death. Nat Genet.
16:358–363. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Salvesen GS: Caspases: Opening the boxes
and interpreting the arrows. Cell Death Differ. 9:3–5. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Paredes MD, Romecin P, Atucha NM, O’Valle
F, Castillo J, Ortiz MC and García-Estañ J: Beneficial effects of
different flavonoids on vascular and renal function in L-NAME
hyper-tensive rats. Nutrients. 10:E4842018. View Article : Google Scholar
|
|
26
|
Vimalraj S, Bhuvaneswari S, Lakshmikirupa
S, Jyothsna G and Chatterjee S: Nitric oxide signaling regulates
tumor-induced intussusceptive-like angiogenesis. Microvasc Res.
119:47–59. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Osol G, Ko NL and Mandala M: Altered
endothelial nitric oxide signaling as a paradigm for maternal
vascular maladaptation in preeclampsia. Curr Hypertens Rep.
19:822017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Palei AC, Spradley FT and Granger JP: Role
of nitric oxide synthase on blood pressure regulation and vascular
function in pregnant rats on a high-fat diet. Am J Hypertens.
30:240–248. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Acurio J, Herlitz K, Troncoso F, Aguayo C,
Bertoglia P and Escudero C: Adenosine A2A receptor regulates
expression of vascular endothelial growth factor in feto-placental
endothelium from normal and late-onset pre-eclamptic pregnancies.
Purinergic Signal. 13:51–60. 2017. View Article : Google Scholar :
|
|
30
|
Amaral TAS, Ognibene DT, Carvalho LCRM,
Rocha APM, Costa CA, Moura RS and Resende AC: Differential
responses of mesenteric arterial bed to vasoactive substances in
L-NAME-induced preeclampsia: Role of oxidative stress and
endothelial dysfunction. Clin Exp Hypertens. 40:126–135. 2018.
View Article : Google Scholar
|
|
31
|
Baijnath S, Murugesan S, Mackraj I,
Gathiram P and Moodley J: The effects of sildenafil citrate on
urinary podocin and nephrin mRNA expression in an L-NAME model of
pre-eclampsia. Mol Cell Biochem. 427:59–67. 2017. View Article : Google Scholar
|
|
32
|
Deng Q, Liu X, Yang Z and Xie L:
Expression of N-Acetylglucosaminyltransferase III promotes
trophoblast invasion and migration in early human placenta. Reprod
Sci. Jan 1–2018.Epub ahead of print. View Article : Google Scholar
|
|
33
|
Sahay AS, Jadhav AT, Sundrani DP, Wagh GN,
Mehendale SS and Joshi SR: Matrix metalloproteinases-2 (MMP-2) and
matrix metalloproteinases-9 (MMP-9) are differentially expressed in
different regions of normal and preeclampsia placentae. J Cell
Biochem. 119:6657–6664. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang A, Xiao XH, Wang ZL, Wang ZY and Wang
KY: T2-weighted balanced steady-state free procession MRI evaluated
for diagnosing placental adhesion disorder in late pregnancy. Eur
Radiol. 28:3770–3778. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Luo S, Pei J, Li X and Gu W: Decreased
expression of JHDMID in placenta is associated with preeclampsia
through HLA-G. J Hum Hypertens. 32:448–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zardoya-Laguardia P, Blaschitz A,
Hirschmugl B, Lang I, Herzog SA, Nikitina L, Gauster M, Häusler M,
Cervar-Zivkovic M, Karpf E, et al: Endothelial indoleamine
2,3-dioxygenase-1 regulates the placental vascular tone and is
deficient in intra-uterine growth restriction and pre-eclampsia.
Sci Rep. 8:54882018. View Article : Google Scholar
|
|
37
|
Redman CW, Sacks GP and Sargent IL:
Preeclampsia: An excessive maternal inflammatory response to
pregnancy. Am J Obstet Gynecol. 180:499–506. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mihu D, Razvan C, Malutan A and Mihaela C:
Evaluation of maternal systemic inflammatory response in
preeclampsia. Taiwan J Obstet Gynecol. 54:160–166. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mazouni C, Capo C, Ledu R, Honstettre A,
Agostini A, Capelle M, Mege JL and Bretelle F: Preeclampsia:
Impaired inflammatory response mediated by toll-like receptors. J
Reprod Immunol. 78:80–83. 2008. View Article : Google Scholar
|
|
40
|
Parente L and Solito E: Annexin 1: More
than an anti-phospholipase protein. Inflamm Res. 53:125–132. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cardin LT, Sonehara NM and Mimura KK:
Ramos Dinarte Dos Santos AANXA1Ac2-26 peptide, a possible
therapeutic approach in inflammatory ocular diseases. Gene.
614:26–36. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stuqui B, de Paula-Silva M, Carlos CP,
Ullah A, Arni RK, Gil CD and Oliani SM: Ac2-26 mimetic peptide of
annexin A1 inhibits local and systemic inflammatory processes
induced by bothrops moojeni venom and the Lys-49 phospholipase A2
in a rat model. PLoS One. 10:e01308032015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Solito E, de Coupade C, Parente L, Flower
RJ and Russo-Marie F: IL-6 stimulates annexin 1 expression and
translocation and suggests a new biological role as class II acute
phase protein. Cytokine. 10:514–521. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Perucci LO, Carneiro FS, Ferreira CN,
Sugimoto MA, Soriani FM, Martins GG, Lima KM, Guimarães FL,
Teixeira AL and Dusse LM: Annexin A1 is increased in the plasma of
preeclamptic women. PLoS One. 10:e01384752015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Perucci LO, Vieira ELM, Teixeira AL, Gomes
KB, Dusse LM and Sousa LP: Decreased plasma concentrations of
brain-derived neurotrophic factor in preeclampsia. Clin Chim Acta.
464:142–147. 2017. View Article : Google Scholar
|
|
46
|
Zhao Y, Li X, Gong J, Li L, Chen L, Zheng
L, Chen Z, Shi J and Zhang H: Annexin A1 nuclear translocation
induces retinal ganglion cell apoptosis after ischemia-reperfusion
injury through the p65/IL-1beta pathway. Biochim Biophys Acta.
1863.1350–1358. 2017.
|
|
47
|
Mor G and Abrahams VM: Potential role of
macrophages as immunoregulators of pregnancy. Reprod Biol
Endocrinol. 1:1192003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Belo L, Caslake M, Gaffney D, Santos-Silva
A, Pereira-Leite L, Quintanilha A and Rebelo I: Changes in LDL size
and HDL concentration in normal and preeclamptic pregnancies.
Atherosclerosis. 162:425–432. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zou Y, Zuo Q, Huang S, Yu X, Jiang Z, Zou
S, Fan M and Sun L: Resveratrol inhibits trophoblast apoptosis
through oxidative stress in preeclampsia-model rats. Molecules.
19:20570–20579. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Huang L, Liao L, Wan Y, Cheng A, Li M,
Chen S, Li M, Tan X and Zeng G: Downregulation of Annexin A1 is
correlated with radioresistance in nasopharyngeal carcinoma. Oncol
Lett. 12:5229–5234. 2016. View Article : Google Scholar
|
|
51
|
D’Acunto CW, Fontanella B, Rodriquez M,
Taddei M, Parente L and Petrella A: Histone deacetylase inhibitor
FR235222 sensitizes human prostate adenocarcinoma cells to
apoptosis through up-regulation of Annexin A1. Cancer Lett.
295:85–91. 2010. View Article : Google Scholar
|
|
52
|
Vago JP, Nogueira CR, Tavares LP, Soriani
FM, Lopes F, Russo RC, Pinho V, Teixeira MM and Sousa LP: Annexin
A1 modulates natural and glucocorticoid-induced resolution of
inflammation by enhancing neutrophil apoptosis. J Leukoc Biol.
92:249–258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bao L, Devi YS, Bowen-Shauver J,
Ferguson-Gottschall S, Robb L and Gibori G: The role of
interleukin-11 in pregnancy involves up-regulation of
alpha2-macroglobulin gene through janus kinase 2-signal transducer
and activator of transcription 3 pathway in the decidua. Mol
Endocrinol. 20:3240–3250. 2006. View Article : Google Scholar : PubMed/NCBI
|