Introduction
Surgery has developed rapidly in recent years, which
has led to a gradual increase in the proportion of patients
receiving general anesthesia (1).
General anesthesia has been applied for almost two centuries.
However, the precise mechanism of action remains to be fully
elucidated (2). Sevoflurane has
the advantages of rapid induction and resuscitation. In addition,
it has a small influence on liver and renal function, and
hemodynamics (2). Therefore, it
is applied extensively clinically. It is considered that
intravenous and inhaled drugs can induce the apoptosis of central
nervous system cells (2). In
particular, anesthetic drugs affect the construction of the central
nervous network at a critical stage of brain tissue development
(2). In addition, they can impair
learning and memory function in the future (1). Preclinical experiments have
indicated that the neurotoxicity of sevoflurane in general
anesthesia on the developing brain is associated with neuronal
apoptosis and subsequent cognitive impairment (3). Consequently, treatment against
neuronal apoptosis may alleviate sevoflurane-induced neurocognitive
impairment (4).
Cell apoptosis, the most commonly observed
sevoflurane-induced injury, is associated with complicated and
diverse mechanisms (5). The
phosphoinositide 3-kinase (PI3K)/Akt signal transduction pathway is
the most important among numerous signal transduction pathways in
the cell apoptotic mechanism (5).
Its mechanism of inhibiting cell apoptosis may be realized through
regulating apoptin and apoptotic genes (6). Alternatively, it can be achieved
through affecting the corresponding changes of mitochondrion and
endoplasmic reticulum. Neurotrophin can activate the PI3K/Akt
signal transduction pathway (7).
The nuclear transcription factor forkhead box O3a (FOXO3a) is a
downstream substrate of the PI3K/Akt signaling pathway (8). It is closely associated with cell
proliferation, aging, apoptosis, differentiation and tumor genesis
(8).
MicroRNAs (miRNAs) are highly conserved, non-coding,
single-stranded RNAs, which are only 21-24 nucleotides in length
(9). They can inhibit target gene
expression at the transcriptional level (10). In addition, they are involved in
regulating almost all physiopathological processes, including
growth, development, inflammation, swelling and neuro-degenerative
disease (10). The expression of
miRNAs is time- and space-specific. The expression level of a
specific miRNA may be different under various developmental stages.
Alternatively, it may be different under different histological or
pathological conditions (9). Wang
et al showed that miR-132 affects glioma cell growth
(11). Leinders et al also
reported that the expression of miR-132-3p is associated with
chronic neuropathic pain (12).
Yao et al suggested that the hypoxia-induced upregulation of
miR-132 promoted Schwann cell migration and facilitated peripheral
nerve regeneration (13).
Therefore, miR-32 has become a focus of investigations in the
medical community. In the present study, the mechanisms underlying
the protective effects of miRNA-132 on sevoflurane-induced neuronal
apoptosis were investigated.
Materials and methods
Animals and anesthesia treatment
The study protocol was approved by the Animal Use
and Care Committee for Research and Education of Shandong
University (Shandong, China). Sprague-Dawley rats (male, 220-250 g,
8-10 weeks) were purchased from Shandong Animal Laboratory
(Shandong, China) and housed at 22±1°C under a 12-h light/dark
cycle (7:00 am-7:00 pm). A total of 12 rats were randomly assigned
into two groups: Normal control group (n=6) and sevoflurane-induced
model group (n=6). In the sevoflurane-induced model group, the rats
were placed into a chamber and exposed to 2% sevoflurane for 6 h.
After 24 h, the hippocampus of rats in all groups was analyzed
following sacrifice of the rats.
Hematoxylin and eosin staining
The histology of the hippocampus and the apoptotic
status were examined using hematoxylin staining. The brains were
fixed in 4% paraformaldehyde for 24 h, paraffin-embedded and cut
into 5-µm-thick sections. The tissue samples were then
stained with hematoxylin and eosin, and the sections were examined
under an optical microscope using fluorescence (BX53; Olympus,
Tokyo, Japan).
Cell culture, transfection and
treatment
The human H4 neuroglioma cell line was obtained from
the Cell Bank of the Chinese Academy of Sciences (Shanghai, China)
and cultured in RPMI-1640 (HyClone; GE Healthcare Life Sciences,
Logan, UT, USA) supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) in a humidified
atmosphere of 5% CO2 at 37°C. The FOXO3 plasmid, miR-132
mimic, anti-miR-132 mimic and control mimic were synthesized by
GenePharma Co., Ltd. (Shanghai, China). The cells were plated at
70-80% confluence in a 6-well plate and were transfected with 100
ng mimics using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis and gene microarray
hybridization
Total RNA was extracted from the tissue and cells
using an RNA simple total RNA kit (Tiangen Biotech Co., Ltd.,
Beijing, China), and 200 nM total RNA was reverse transcribed into
cDNA using Super M-MLV reverse transcriptase (BioTeke Corporation,
Beijing, China) at 37°C for 1 h and 84°C for 5 min. RT-qPCR
analysis was performed in an ABI Prism 7500 Real-time PCR system
using SYBR-Green master mix (Solarbio Science and Technology Co.,
Ltd., Beijing, China) with the following cycling parameters: 10 min
at 95°C, followed by 40 cycles of 30 sec at 95°C and 30 sec at
60°C. The following primers were used in the present study:
miR-132: 5'-CCG CGT CTC CAG GGC AAC-3' and 5'-CCT CCG GTT CCC ACA
GTA ACA A-3'; and U6: 5'-TGG TAT TCG TGG AAG GAC TCA TGA C-3' and
5'-ATG CCA GTG AGC TTC CCG TTC AGC-3'. Analysis of relative gene
expression data was quantified using the 2−ΔΔCt method
(14).
Total RNA was labeled using Cyanine-5-CTP and
hybridized to the SurePrint G3 Mouse Whole Genome GE Microarray
G4852A platform with an equimolar concentration of
Cyanine-3-CTP-labelled universal rat reference (Stratagene; Agilent
Technologies, Inc., Santa Clara, CA, USA). Images were quantified
and feature-extracted using Agilent Feature Extraction software
(version A.10.7.3.1; Agilent Technologies, Inc.).
Cell viability assays
Cell viability (1×103 cell/well) was
measured using an MTT assay (20 µl; 5 mg/ml) at 96-well
plate and incubated for 4 h at 37°C. After 4 h, the medium was
removed, and 150 µl DMSO was added to the cells and
incubated for 20 min for 37°C. The absorbance was measured using
the ELX-800 absorbance microplate reader (Bio-Tek Instruments,
Inc., Winooski, VT, USA) at 450 nm.
Cell viability was measured according to lactate
dehydrogenase (LDH) activity (Beyotime Institute of Biotechnology,
Haimen, China) and the absorbance was measured using the ELX-800
absorbance microplate reader (Bio-Tek Instruments, Inc.) at 450
nm.
Analysis of apoptosis by flow cytometry
and caspase-3/9 activity
The cells (1×106 cell/well) were washed
with PBS and resuspended in 500 µl binding buffer (BD
Biosciences, Franklin Lakes, NJ, USA). The cells were stained with
anti-Annexin V-FITC antibody (5 µl) and PI (5 µl) for
15 min at room temperature in the dark. The apoptotic rates were
measured using flow cytometric analysis on a FACSCalibur flow
cytometer (BD Biosciences).
The cells were used to extract total proteins using
RIPA lysis buffer (Beyotime Institute of Biotechnology) and protein
concentrations were determined using BCA protein assays (Beyotime
Institute of Biotechnology). The proteins (10 µg) were used
to measure the activity of caspase-3/9 using caspase-3 or caspase-9
activity kits (Beyotime Institute of Biotechnology). The absorbance
was measured using the ELX-800 absorbance microplate reader
(Bio-Tek Instruments, Inc.) at 405 nm.
Immunofluorescence staining
The cells were fixed using 4% paraformaldehyde for
15 min at room temperature and permeabilized with 0.1% Triton X-100
(Beyotime Institute of Biotechnology) for 15 min at room
temperature. The cells (1×104 cell/well) were then
incubated with 1:100 diluted anti-nuclear factor (NF)-κB p65
antibody (1:100; cat. no. sc-8008; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), overnight at 4°C and incubated with FITC-labeled
goat anti-rabbit secondary antibody (1:200; cat. no. A0562;
Beyotime Institute of Biotechnology) for 1 h at room temperature.
The cells were then stained with DAPI for 30 min at room
temperature in the dark. The cells were observed under a
fluorescence microscope (BX53; Olympus).
Western blot analysis
The cells were used to extract total proteins using
RIPA lysis buffer (Beyotime Institute of Biotechnology) and protein
concentration was determined using BCA protein assays (Beyotime
Institute of Biotechnology). The proteins (40 µg) from each
sample were separated by 10% SDS-PAGE and transferred onto a PVDF
membrane (EMD Millipore, Bedford, MA, USA). The membrane was
blocked with 5% non-fat milk for 1 h at 37°C and incubated with the
following specific primary antibodies: B-cell lymphoma 2
(Bcl-2)-associated X protein (Bax, cat. no. sc-6236; 1:500; Santa
Cruz Biotechnology, Inc.), PI3K (cat. no. sc-7174; 1:500; Santa
Cruz Biotechnology, Inc.), phosphorylated (p-)AKT (sc-7985-R;
1:300; Santa Cruz Biotechnology, Inc.), FOXO3a (cat. no. 12829;
1:2,000; Cell Signaling Technology, Inc., Danvers, MA, USA) and
GAPDH (cat. no. sc-25778; 1:2,000; Santa Cruz Biotechnology, Inc.)
at 4°C overnight. Subsequently, the membrane was incubated with
corresponding horseradish peroxidase-conjugated secondary
antibodies (cat. no. sc-2004; 1:5,000; Santa Cruz Biotechnology,
Inc.) at 37°C for 1 h. The blots of the proteins were visualized
using Enhanced Chemiluminescence reagents (Beyotime Institute of
Biotechnology) and quantified using Image Lab 3.0 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Values are expressed as the mean ± standard
deviation using SPSS software (version 17.0; SPSS, Inc., Chicago,
IL, USA). All data were analyzed using Student's t-test or one-way
analysis of variance with Bonferroni's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of miR-132 in
sevoflurane-induced rats
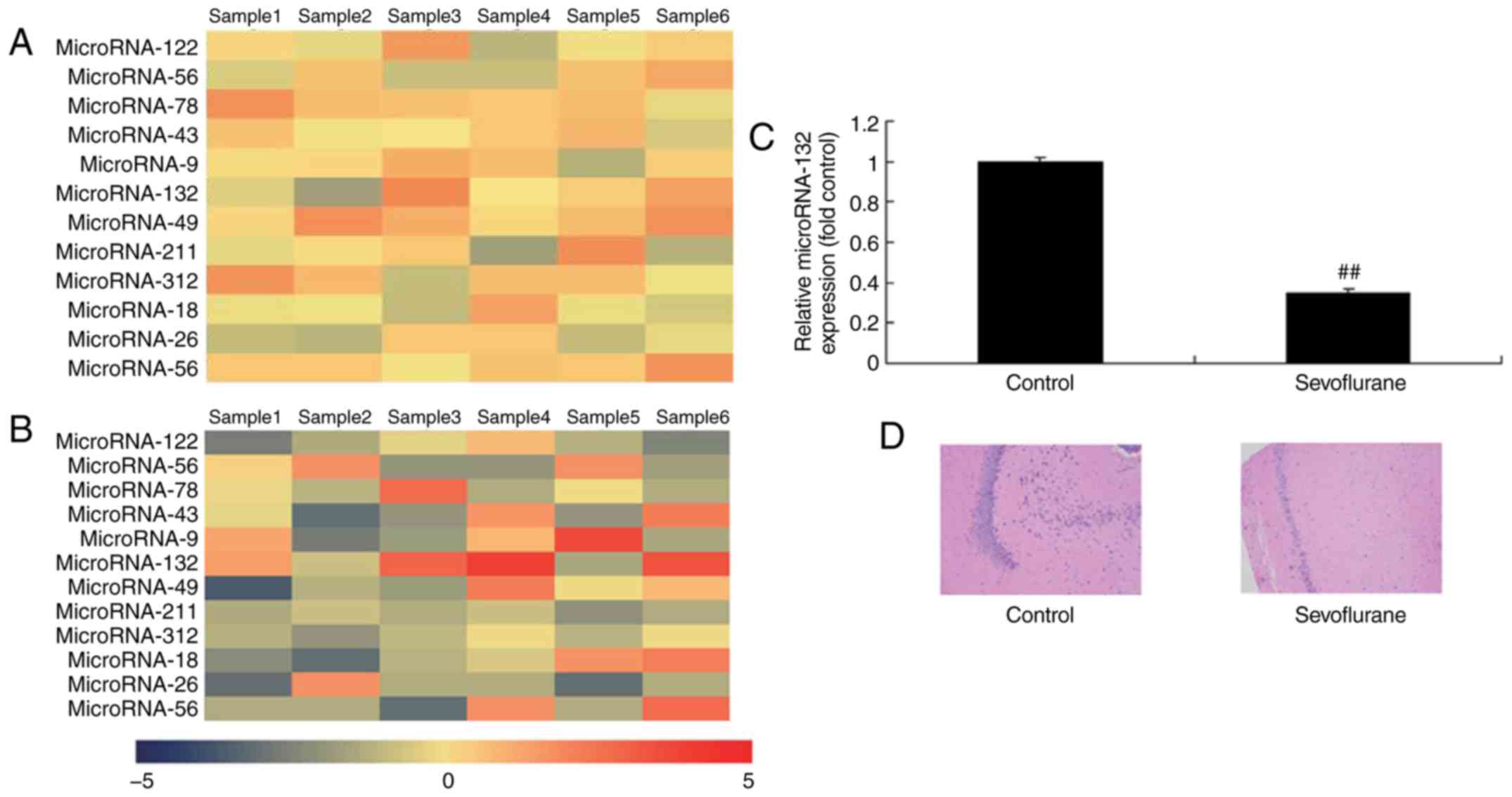
Gene chip and RT-qPCR analyses were used to measure
the expression of miRNA-132 in sevoflurane-induced rats, and it was
found that the expression of miRNA-132 was downregulated in
sevoflurane-induced rats, compared with that in the normal group
(Fig. 1A and B). A high level of
neuronal apoptosis was observed in the sevoflurane-induced rats,
compared with the normal group (Fig.
1C). These data suggested that the downregulation of miR-132
may be associated with sevoflurane-induced neuronal apoptosis.
miR-132 affects neuronal cell growth in a
sevoflurane-induced in vitro model
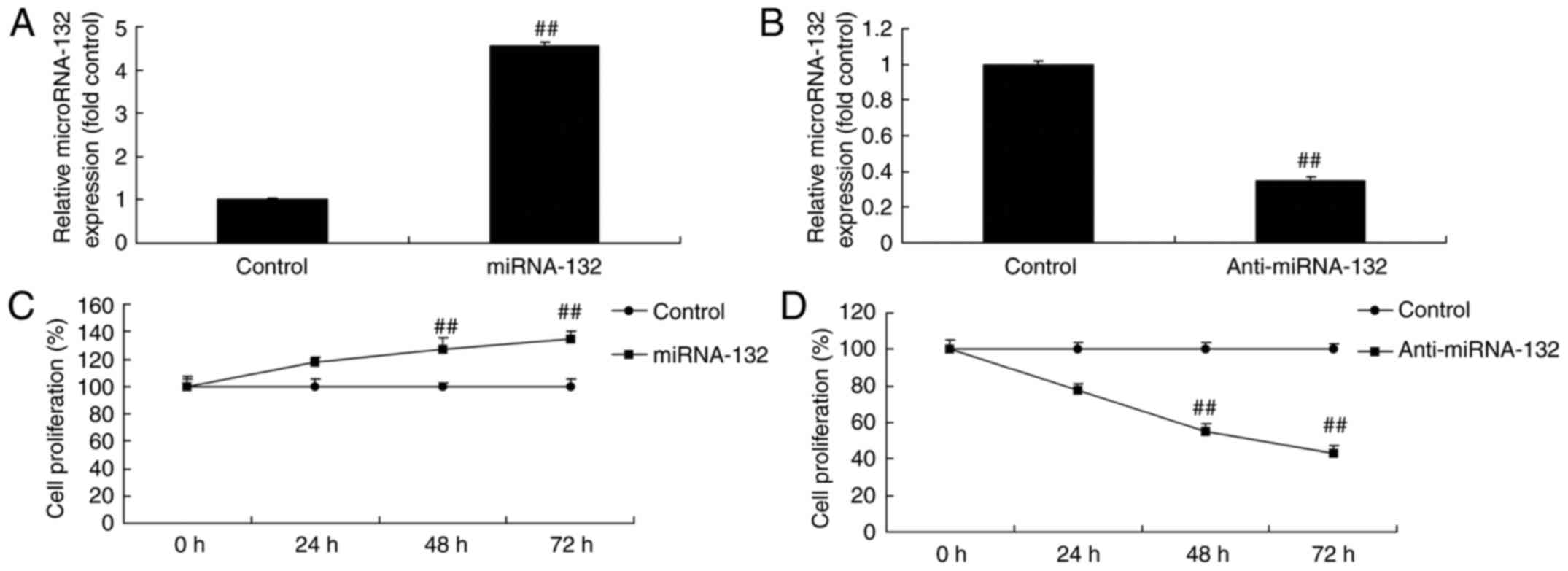
The expression levels of miR-132 in the H4 cells
transfected with miR-132, anti-miR-132 mimic or mimic control were
determined by RT-qPCR analysis. As shown in Fig. 2A and B, miR-132 and anti-miR-132
mimic increased and inhibited the expression level of miR-132 in
the sevoflurane-induced cells, respectively, compared with the
mimic control group. The overexpression of miR-132 and
downregulation of miR-132 promoted neuronal cell growth and
inhibited neuronal cell growth in the sevoflurane-induced in
vitro model, respectively, compared with the mimic control
group (Fig. 2C and D).
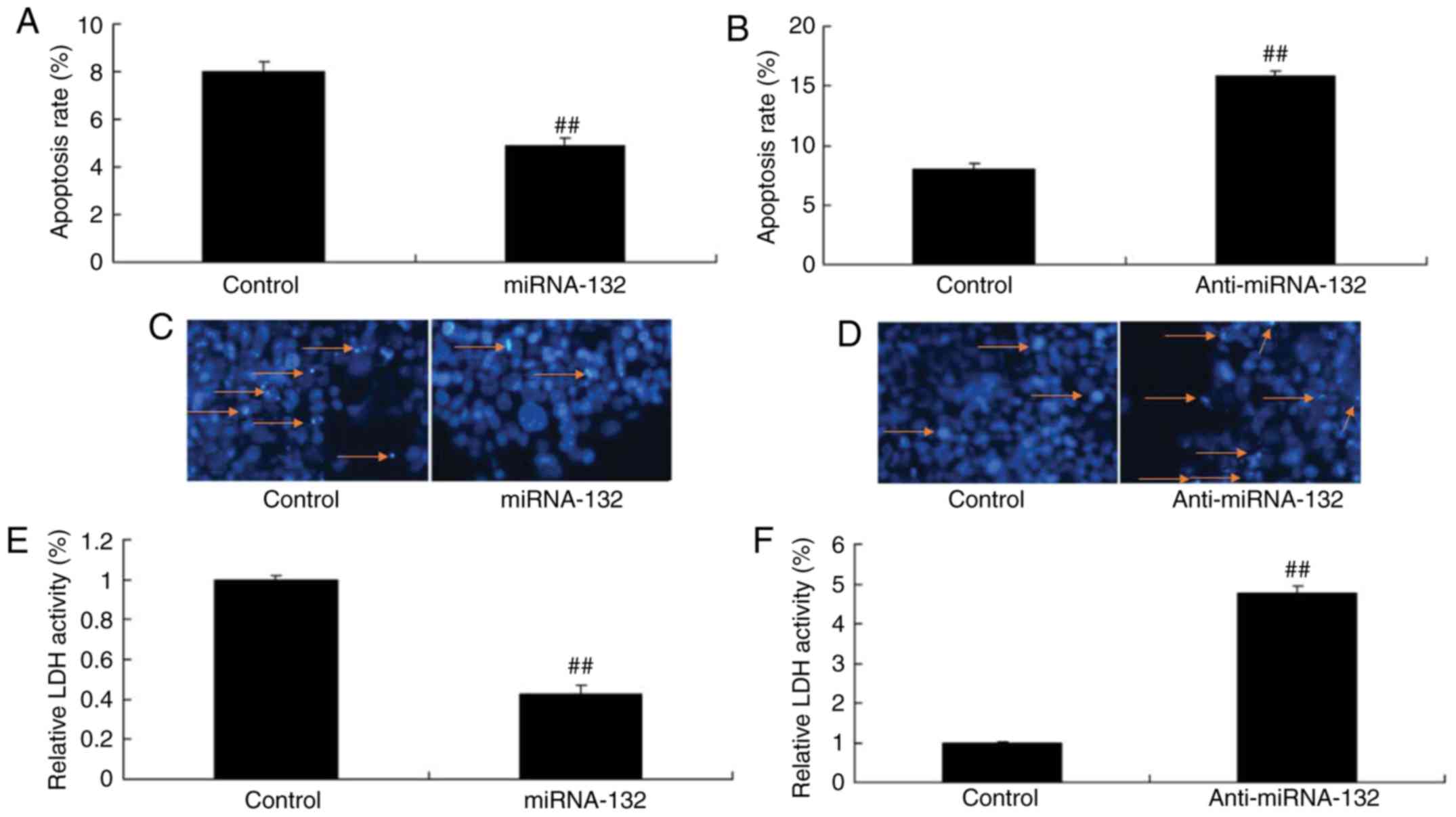
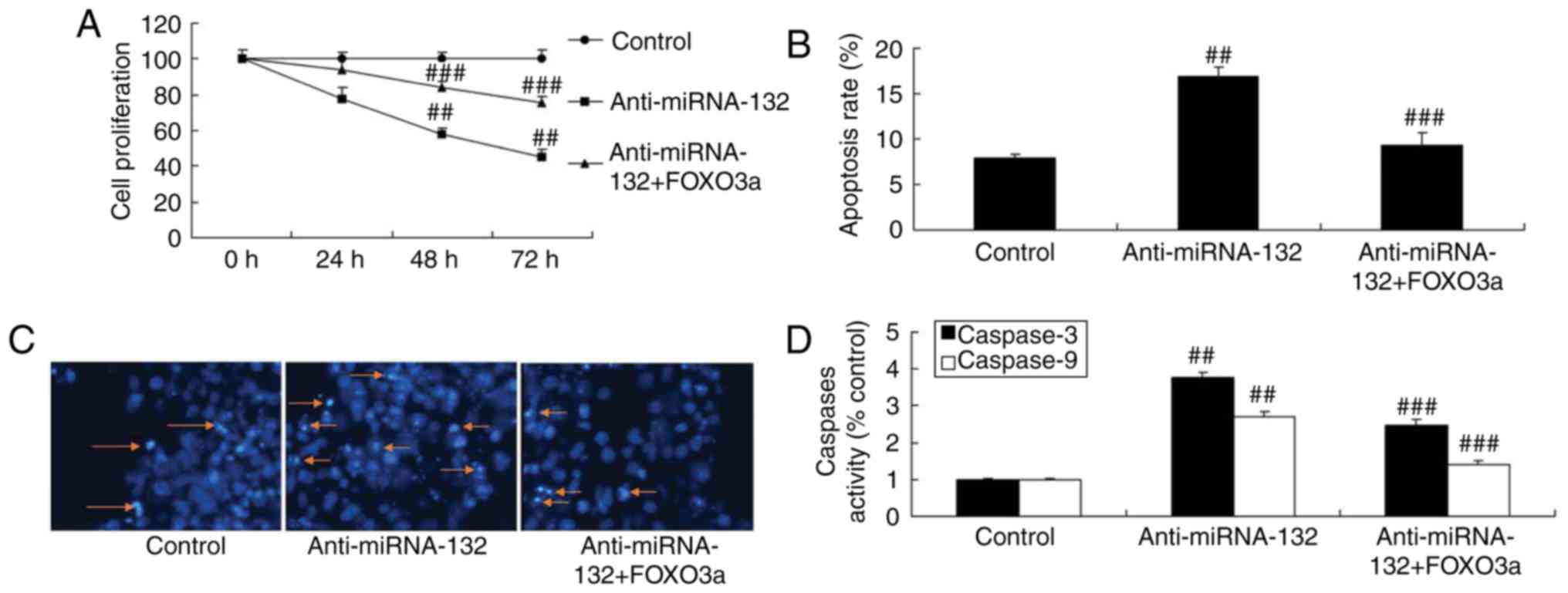
miRNA-132 affects neuronal cell apoptosis
in a sevoflurane-induced in vitro model
The overexpression of miRNA-132 and downregulation
of miRNA-132 reduced and increased the apoptotic rate in the
sevoflurane-induced in vitro model, respectively, compared
with the mimic control group (Fig. 3A
and B). DAPI was used to stain cells, and it was also found
that the overexpression of miRNA-132 and downregulation of
miRNA-132 inhibited and increased cell apoptosis in the
sevoflurane-induced in vitro model, respectively, compared
with the mimic control group (Fig. 3C
and D). The activity of LDH in the cells transfected with
miR-132 was reduced and that transfected with anti-miR-132 mimic
was increased, compared with that in the mimic control group
(Fig. 3E and F).
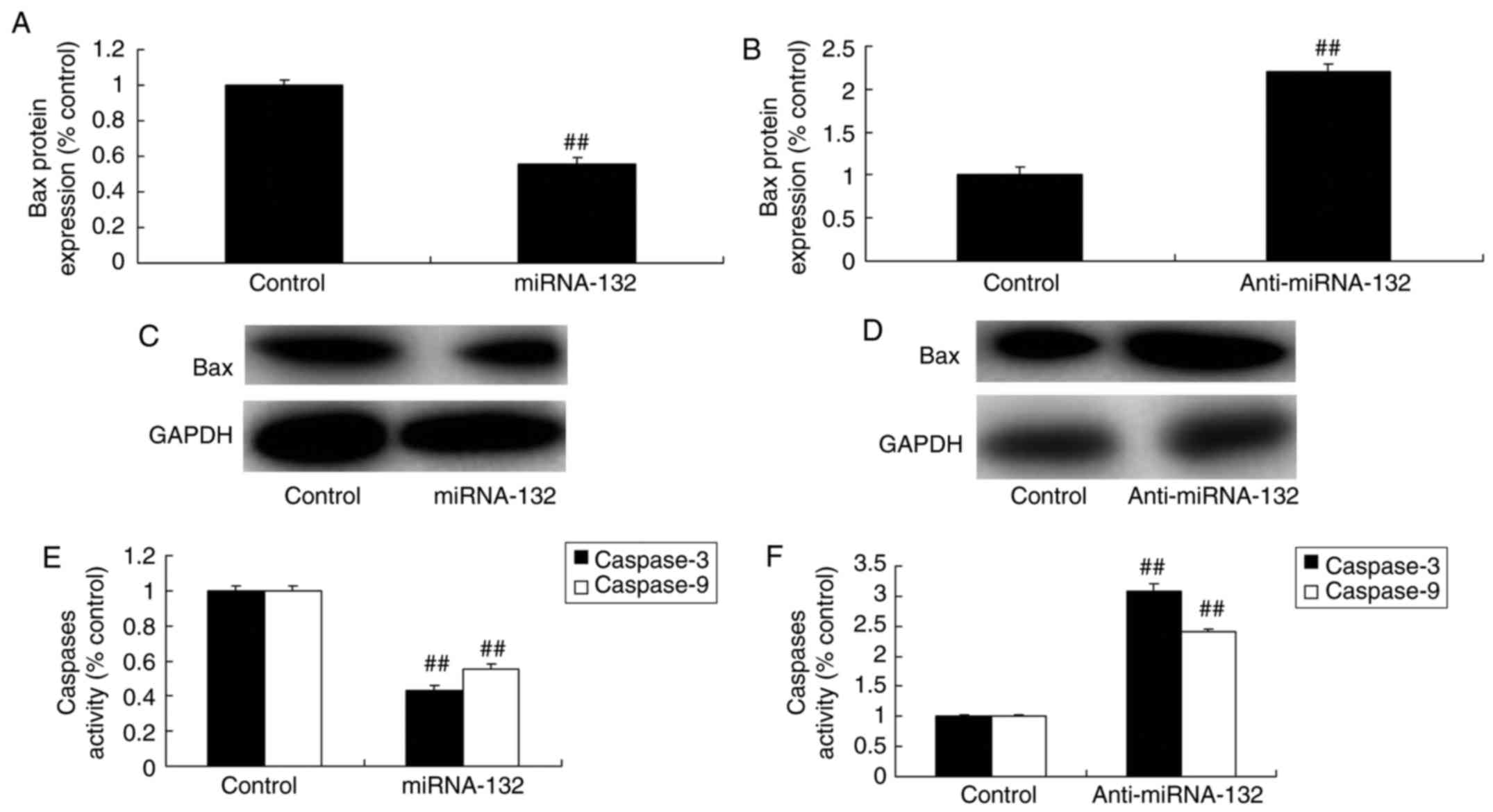
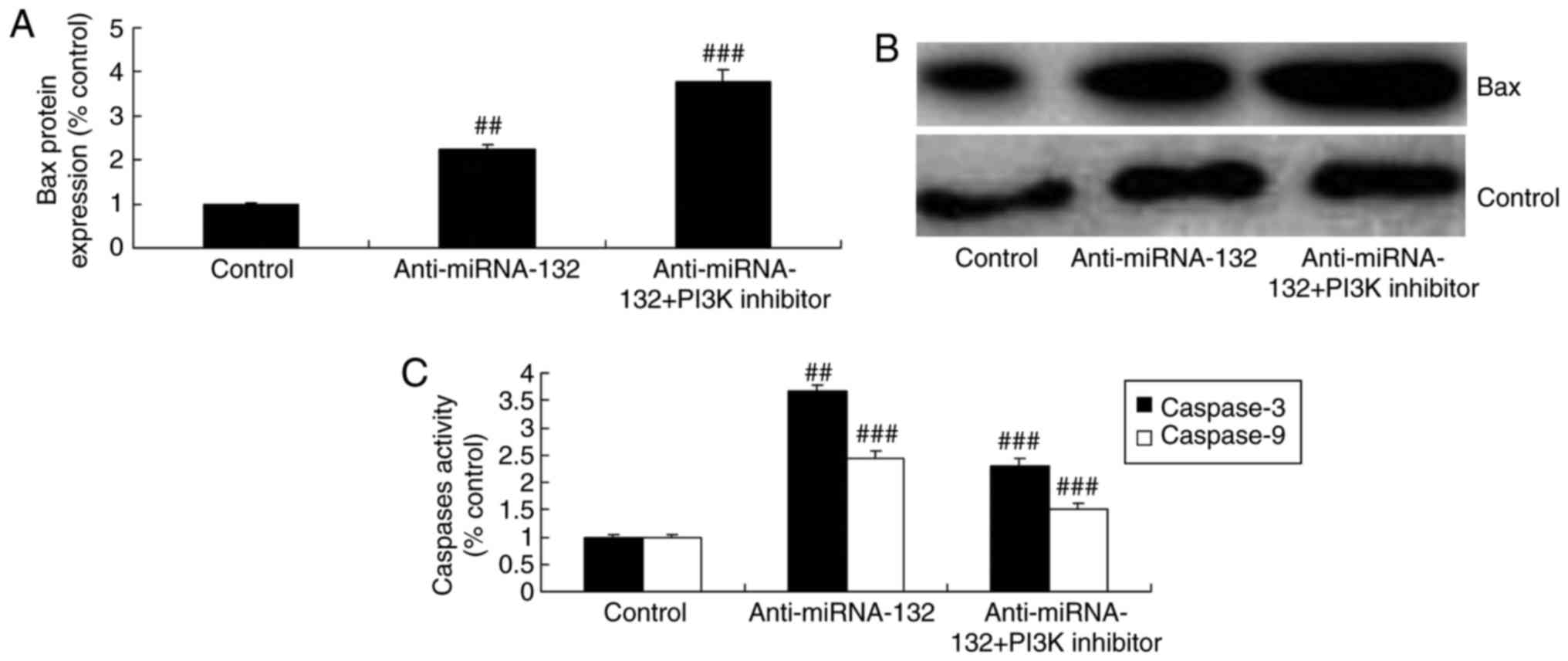
miRNA-132 affects the expression of Bax
and caspase-3/9 in the sevoflurane-induced in vitro model
The overexpression of miRNA-132 suppressed the
expression levels of Bax and caspase-3/9, and the downregulation of
miRNA-132 induced the expression of Bax and caspase-3/9 in the
sevoflurane-induced in vitro model, respectively, compared
with that in the mimic control group (Fig. 4A-F).
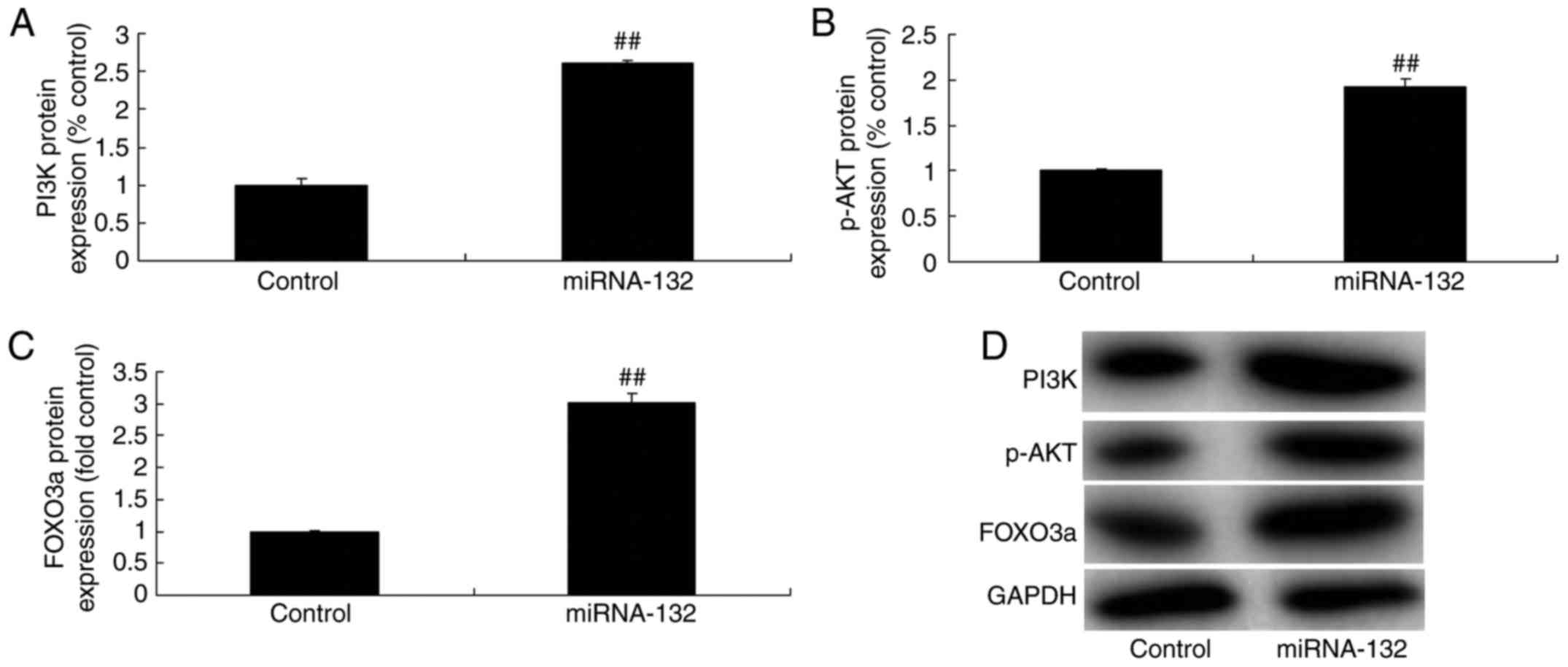
miRNA-132 affects the PI3K/AKT/FOXO3a
pathway in the sevoflurane-induced in vitro model
To investigate whether miRNA-132 exerts its
cytoprotective effect via the PI3K/AKT/FOXO3a pathway, the protein
expression levels of PI3K, p-AKT and FOXO3a were measured in the
sevoflurane-induced in vitro model. The overexpression of
miRNA-132 induced the PI3K/AKT/FOXO3a pathway in the
sevoflurane-induced in vitro model (Fig. 5A-D). The down-regulation of
miRNA-132 inhibited the PI3K/AKT/FOXO3a pathway in the
sevoflurane-induced in vitro model (Fig. 5E-H), as shown in the
immunofluorescence staining (Fig. 5I
and J).
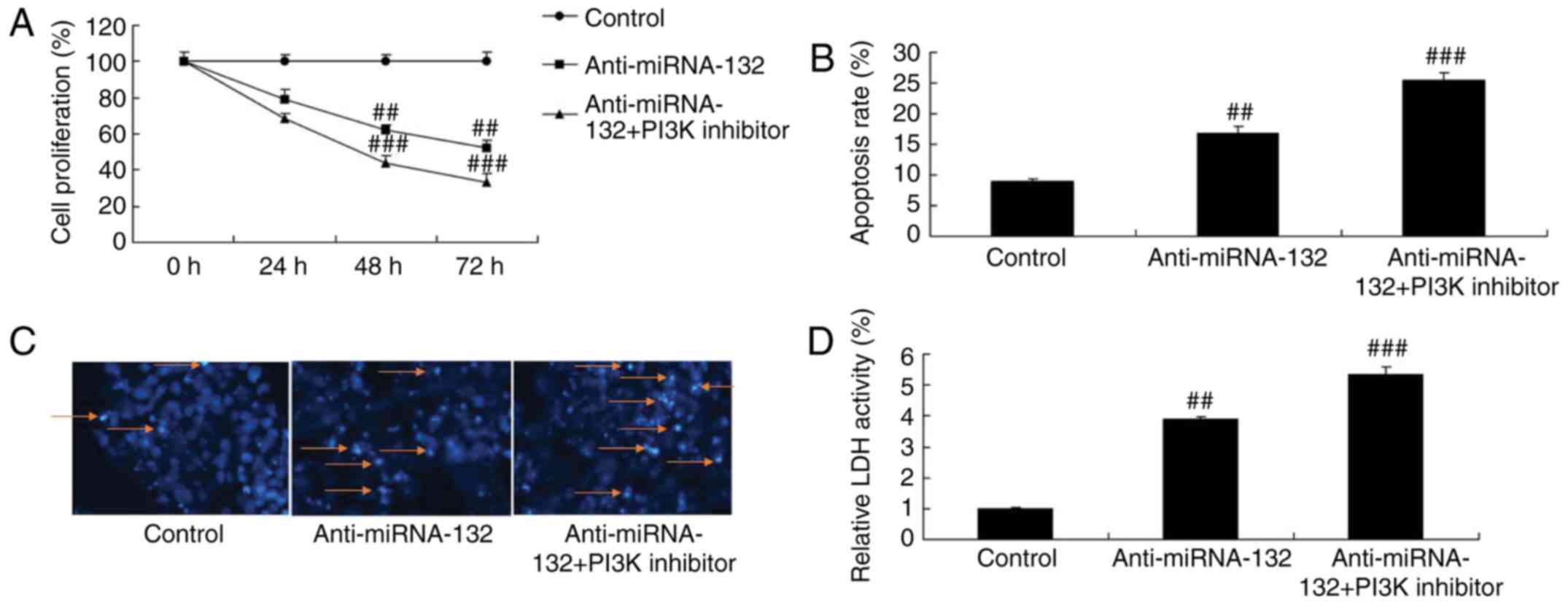
PI3K inhibitor increases the effects of
anti-miRNA-132 on the AKT/FOXO3a pathway in a sevoflurane-induced
in vitro model
The present study also examined whether PI3K
inhibitor increased the effects of anti-miRNA-132 on the
PI3K/AKT/FOXO3a pathway by using the PI3K inhibitor, LY294002 (100
nM), to reduce the PI3K/AKT/FOXO3a pathway in the
sevoflurane-induced in vitro model following anti-miRNA-132.
As shown in Fig. 6A-D, the PI3K
inhibitor suppressed the PI3K/AKT/FOXO3a pathway in the
sevoflurane-induced in vitro model following anti-miRNA-132
treatment, compared with the anti-miRNA-132 only-treated group.
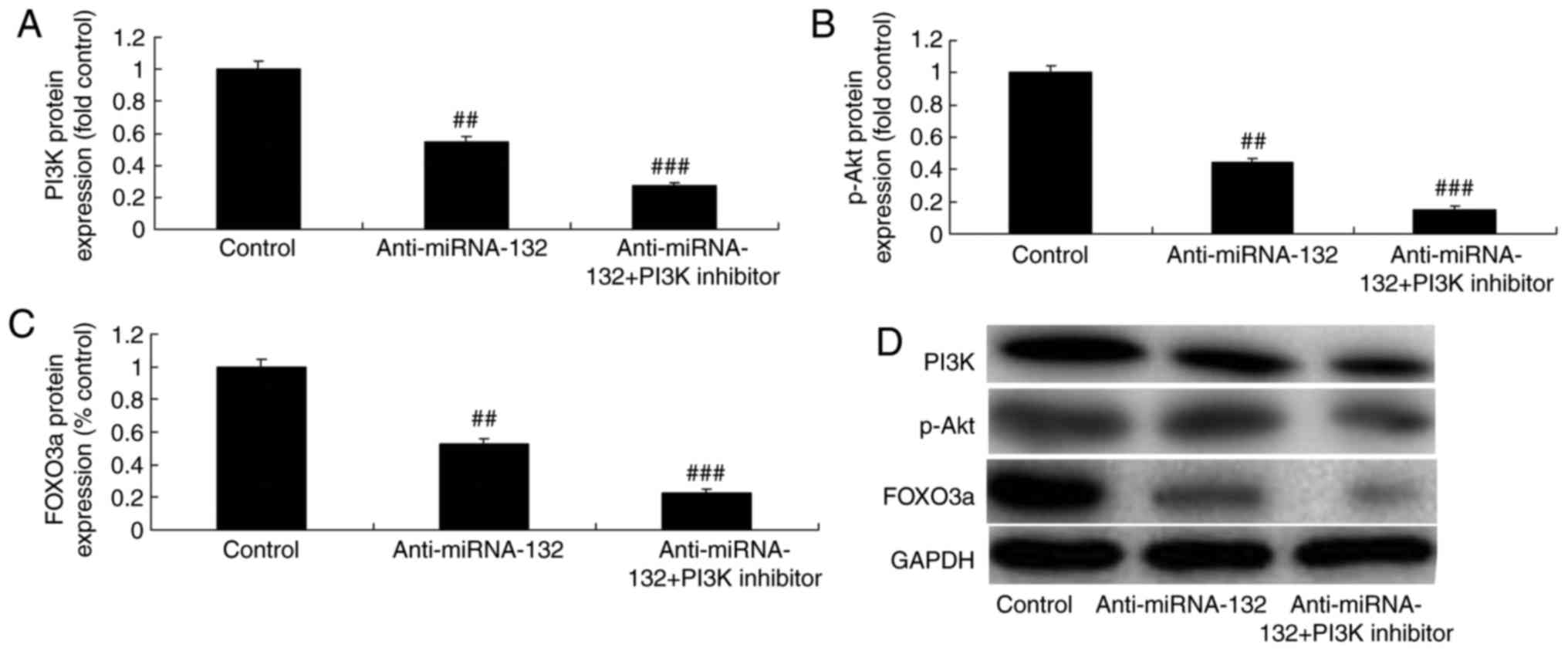 | Figure 6PI3K inhibitor increases the effects
of anti-miRNA-132 through the AKT/FOXO3a pathway in a
sevoflurane-induced in vitro model. Protein expression of
(A) PI3K, (B) p-Akt and (C) FOXO3a, determined via statistical
analysis of (D) western blot assays. Values are expressed as the
mean ± standard deviation (n=3). ##P<0.01, compared
with the control group; ###P<0.01, compared with the
anti-miRNA-132 group. miRNA, microRNA; control, negative control;
anti-miRNA-132, downregulated expression of miRNA-132; PI3K
inhibitor, LY294002; PI3K, phosphoinositide 3-kinase; p-Akt,
phosphorylated Akt; FOXO3a, forkhead box O3A. |
PI3K inhibitor increases the effects of
anti-miRNA-132 on neuronal apoptosis in the sevoflurane-induced in
vitro model
The PI3K inhibitor increased the effects of anti-
miRNA-132 on the inhibition of neuronal cell growth, activation of
cell apoptosis, activity of LDH, and induction of the expression of
Bax and caspase-3/9 in the sevoflurane-induced in vitro
model, compared with the effects in the anti-miRNA-132 group
(Figs. 7A-D and 8A-C).
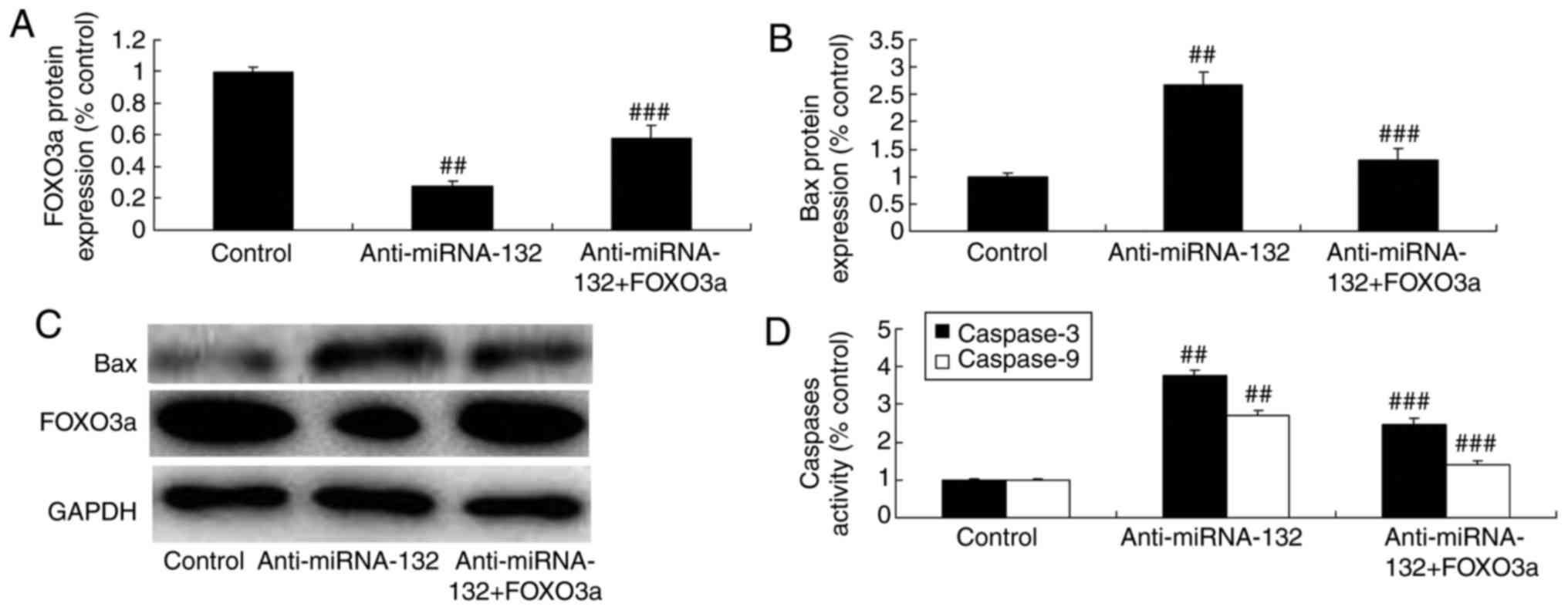
Promotion of FOXO3a inhibits the effects
of anti-miRNA-132 on neuronal apoptosis through the FOXO3a pathway
in a sevoflurane-induced in vitro model
To confirm the activation of the FOXO3a signaling
pathway following anti-miRNA-132 treatment in in
sevoflurane-induced in vitro model, western blot analysis
was performed. The FOXO3 plasmid induced the protein expression of
FOXO3a and suppressed the expression of Bax and caspase-3/9 in the
sevoflurane-induced in vitro model induced by
anti-miRNA-132, compared with the expression levels in the
anti-miRNA-132 group (Fig.
9A-D).
Promotion of FOXO3a inhibits the effects
of anti-miRNA-132 on neuronal apoptosis through the AKT/FOXO3a
pathway in a sevoflurane-induced in vitro model
Finally, the promotion of FOXO3a inhibited the
effects of anti-miRNA-132 on the inhibition of neuronal cell
growth, activation of cell apoptosis and activity of caspase-3/9 in
the sevoflurane-induced in vitro model, compared with the
effects in the anti-miRNA-132 group (Fig. 10A-D).
Discussion
Sevoflurane is a common inhaled anesthetic gas
(15). At present, the problem of
whether sevoflurane anesthesia produces neuroprotective or
neurotoxic effects is controversial (15). Numerous existing preclinical
studies indicate that general inhaled anesthetics, including
sevoflurane, induce extensive neuronal apoptosis (3). In addition, they are accompanied
with definite long-term cognitive and memory impairment (4). Neuronal apoptosis is considered a
potential mechanism of cell injury. The activated caspase-3 can
damage the corresponding cytoplasm and nuclear substrate during
early apoptosis, thus inducing cell apoptosis. The results of the
present study showed that the expression of miRNA-132 was
downregulated in sevoflurane-induced rats, compared with that in
the normal group. The downregulated expression of miRNA-132 induced
neuronal cell apoptosis in the sevoflurane-induced in vitro
model.
There are numerous downstream effector molecules of
PI3K. Of these, Akt is the central link in the pathway (16). The activation of Akt can act on
multiple targets, including the downstream NF-κB family, Bcl-2
family and caspase protein family. It loses activity through
phosphorylating the apoptotic protein, enhancing the transcription
and expression of apoptotic genes (17). The decreased phosphorylation of
Akt downregulates the expression of Bcl-2 and upregulates the
expression of Bax in the cytoplasm. This was observed in Akt
gene-knockout mice and further aggravated the degree of brain
damage (17). The results of the
present study indicated that the downregulation of miRNA-132
induced the expression of Bax and caspase-3/9, and promoted the
PI3K/AKT/FOXO3a pathway in the sevoflurane-induced in vitro
model. Lian et al demonstrated that the overexpression of
miR-132 enhanced cell proliferation and tumor growth in laryngeal
squamous cell carcinoma through the PI3K/AKT/FOXO1 pathway
(18). Zhang et al showed
that miRNA-132 attenuated the neurobehavioral and neuropathological
changes in mice with intracerebral hemorrhage (19). These results indicate that
miRNA-132 attenuates sevoflurane-induced neuronal apoptosis, and
its mechanism requires further investigation.
The PI3K/AKT signaling pathway is one of the
important intracellular signal transduction pathways (7). It is mainly involved in regulating
processes, including cell growth, differentiation, metabolism and
apoptosis (20). It can also
affect the activity of numerous downstream target genes and target
proteins. Therefore, it can enhance cell survival (6), inhibit cell apoptosis and exert
brain protective effects. Its role in mental health disease has
attracted extensive attention (6). Notably, the present study found that
the PI3K inhibitor increased the effects of anti-miRNA-132 on
neuronal apoptosis through the PI3K/AKT/FOXO3a pathway in the
sevoflurane-induced in vitro model. Qi and Zhang also showed
that miRNA-132 regulates fluid shear stress-induced differentiation
in periodontal ligament cells through the PI3K/AKT/mammalian target
of rapamycin signaling pathway (21).
FoxO3a is an important downstream target of the
PI3K/Akt signaling pathway. It is important in the anti-apoptotic
mechanism of insulin-like growth factor-1 (22). The dephosphorylation of FoxO3a can
affect the Bcl-2 family genes, pre-apoptotic gene Bcl-2-interacting
mediator of cell death and growth arrest. In addition, it can
affect the DNA damage-inducible gene Gadd45α and non-specific
cyclin-dependent kinase inhibitor P27kip1 (22). Therefore, it can induce cell
apoptosis. Activation of the PI3K/Akt pathway can phosphorylate
FoxO3a, which loses its transcription activity (20). Finally, it can reduce neuronal
apoptosis. FoxO3a is a transcription factor, which is located in
the cell nucleus. It can bind with the response elements on DNA and
activate target genes (23). It
is also involved in regulating cell proliferation, differentiation,
metabolism and apoptosis (23).
It has been suggested that FoxO3a can upregulate the expression of
cell death-related genes, including P53, which indicates that
FoxO3a is regulated by the PI3K/Akt pathway (24). The present study showed that the
promotion of FOXO3a inhibited the effects of anti-miRNA-132 on
neuronal apoptosis through the AKT/FOXO3a pathway in a
sevoflurane-induced in vitro model (25). miRNA-132 may have a
neuroprotective effect against sevoflurane-induced neuronal
apoptosis through the PI3K/AKT/FOXO3a pathway.
In conclusion, the present study provided evidence
that the expression of miRNA-132 was downregulated in
sevoflurane-induced rats, compared with that in normal rats. The
inhibition of miRNA-132 caused sevoflurane-induced neuronal
apoptosis via the PI3K/AKT/FOXO3a pathway. The
miRNA-132/PI3K/AKT/FOXO3a signaling pathway responsible for
sevoflurane-induced neuronal apoptosis requires elucidation in
vivo in future experiments.
Funding
This study was supported by the Major Project of
Science and Technology of Shandong Province (grant no.
2016GSF201070).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
BY designed the experiments. PD, XD, JZ, DL and LL
performed the experiments. BY and PD analyzed the data. BY wrote
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Animal Use
and Care Committee for Research and Education of Shandong
University (Shandong, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Tian Y, Guo S, Wu X, Ma L and Zhao X:
Minocycline alleviates sevoflurane-induced cognitive impairment in
aged rats. Cell Mol Neurobiol. 35:585–594. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang ZJ, Wang YW, Li CL, Ma LQ and Zhao X:
Pre-treatment with a Xingnaojing preparation ameliorates
sevoflurane-induced neuroapoptosis in the infant rat striatum. Mol
Med Rep. 11:1615–1622. 2015. View Article : Google Scholar
|
|
3
|
Yufune S, Satoh Y, Akai R, Yoshinaga Y,
Kobayashi Y, Endo S and Kazama T: Suppression of ERK
phosphorylation through oxidative stress is involved in the
mechanism underlying sevoflurane-induced toxicity in the developing
brain. Sci Rep. 6:218592016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tian Y, Wu X, Guo S, Ma L, Huang W and
Zhao X: Minocycline attenuates sevoflurane-induced cell injury via
activation of Nrf2. Int J Mol Med. 39:869–878. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xiang J, Pan J, Chen F, Zheng L, Chen Y,
Zhang S and Feng W: L-3-n-butylphthalide improves cognitive
impairment of APP/PS1 mice by BDNF/TrkB/PI3K/AKT pathway. Int J
Clin Exp Med. 7:1706–1713. 2014.PubMed/NCBI
|
|
6
|
Zhu G, Wang X, Wu S and Li Q: Involvement
of activation of PI3K/Akt pathway in the protective effects of
puerarin against MPP+-induced human neuroblastoma
SH-SY5Y cell death. Neurochem Int. 60:400–408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hossini AM, Quast AS, Plötz M, Grauel K,
Exner T, Küchler J, Stachelscheid H, Eberle J, Rabien A,
Makrantonaki E and Zouboulis CC: PI3K/AKT signaling pathway is
essential for survival of induced pluripotent stem cells. PLoS One.
11:e01547702016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liao R, Yan F, Zeng Z, Farhan M, Little P,
Quirion R, Srivastava LK and Zheng W: Amiodarone-induced retinal
neuronal cell apoptosis attenuated by IGF-1 via counter regulation
of the PI3k/Akt/FoxO3a pathway. Mol Neurobiol. 54:6931–6943. 2017.
View Article : Google Scholar
|
|
9
|
Chmielarz P, Konovalova J, Najam SS, Alter
H, Piepponen TP, Erfle H, Sonntag KC, Schütz G, Vinnikov IA and
Domanskyi A: Dicer and microRNAs protect adult dopamine neurons.
Cell Death Dis. 8:e28132017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoo HI, Kim BK and Yoon SK: MicroRNA-3305p
negatively regulates ITGA5 expression in human colorectal cancer.
Oncol Rep. 36:3023–3029. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang H, Li XT, Wu C, Wu ZW, Li YY, Yang
TQ, Chen GL, Xie XS, Huang YL, Du ZW and Zhou YX: miR-132 can
inhibit glioma cells invasion and migration by target MMP16 in
vitro. Onco Targets Ther. 8:3211–3218. 2015.PubMed/NCBI
|
|
12
|
Leinders M, Üçeyler N, Pritchard RA,
Sommer C and Sorkin LS: Increased miR-1323p expression is
associated with chronic neuropathic pain. Exp Neurol. 283:276–286.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yao C, Shi X, Zhang Z, Zhou S, Qian T,
Wang Y, Ding F, Gu X and Yu B: Hypoxia-induced upregulation of
miR-132 promotes schwann cell migration after sciatic nerve injury
by targeting PRKAG3. Mol Neurobiol. 53:5129–5139. 2016. View Article : Google Scholar
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
15
|
Zhou ZB, Yang XY, Tang Y, Zhou X, Zhou LH
and Feng X: Subclinical concentrations of sevoflurane reduce
oxidative stress but do not prevent hippocampal apoptosis. Mol Med
Rep. 14:721–727. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo XQ, Cao YL, Hao F, Yan ZR, Wang ML and
Liu XW: Tangeretin alters neuronal apoptosis and ameliorates the
severity of seizures in experimental epilepsy-induced rats by
modulating apoptotic protein expressions, regulating matrix
metalloproteinases, and activating the PI3K/Akt cell survival
pathway. Adv Med Sci. 62:246–253. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Luo T, Liu G, Ma H, Lu B, Xu H, Wang Y, Wu
J, Ge P and Liang J: Inhibition of autophagy via activation of
PI3K/Akt pathway contributes to the protection of ginsenoside Rb1
against neuronal death caused by ischemic insults. Int J Mol Sci.
15:15426–15442. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lian R, Lu B, Jiao L, Li S, Wang H, Miao W
and Yu W: MiR-132 plays an oncogenic role in laryngeal squamous
cell carcinoma by targeting FOXO1 and activating the PI3K/AKT
pathway. Eur J Pharmacol. 792:1–6. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Han B, He Y, Li D, Ma X, Liu Q
and Hao J: MicroRNA-132 attenuates neurobehavioral and
neuropathological changes associated with intracerebral hemorrhage
in mice. Neurochem Int. 107:182–190. 2017. View Article : Google Scholar
|
|
20
|
Meng Y, Wang W, Kang J, Wang X and Sun L:
Role of the PI3K/AKT signalling pathway in apoptotic cell death in
the cerebral cortex of streptozotocin-induced diabetic rats. Exp
Ther Med. 13:2417–2422. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qi L and Zhang Y: The microRNA 132
regulates fluid shear stress-induced differentiation in periodontal
ligament cells through mTOR signaling pathway. Cell Physiol
Biochem. 33:433–445. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zeng Z, Wang X, Bhardwaj SK, Zhou X,
Little PJ, Quirion R, Srivastava LK and Zheng W: The atypical
antipsychotic agent, clozapine, protects against
corticosterone-induced death of PC12 cells by regulating the
Akt/FoxO3a signaling pathway. Mol Neurobiol. 54:3395–3406. 2017.
View Article : Google Scholar
|
|
23
|
Liu MH, Yuan C, He J, Tan TP, Wu SJ, Fu
HY, Liu J, Yu S, Chen YD, Le QF, et al: Resveratrol protects PC12
cells from high glucose-induced neurotoxicity via PI3K/Akt/FoxO3a
pathway. Cell Mol Neurobiol. 35:513–522. 2015. View Article : Google Scholar
|
|
24
|
Zhu W, Bijur GN, Styles NA and Li X:
Regulation of FOXO3a by brain-derived neurotrophic factor in
differentiated human SH-SY5Y neuroblastoma cells. Brain Res Mol
Brain Res. 126:45–56. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim HY, Kwon HY, Ha Thi HT, Lee HJ, Kim
GI, Hahm KB and Hong S: MicroRNA-132 and microRNA-223 control
positive feedback circuit by regulating FOXO3a in inflammatory
bowel disease. J Gastroenterol Hepatol. 31:1727–1735. 2016.
View Article : Google Scholar : PubMed/NCBI
|