Introduction
Growth plate cartilage tissue is avascular, which
restricts its repair capabilities; consequently, once this tissue
is injured or degenerates, cartilage becomes difficult to repair.
Cartilage tissue engineering includes autologous cell-based
cartilage repair, which involves in vitro expansion of a
sufficient number of chondrocytes to be implanted (1). However, chondrocytes are not able to
maintain their phenotypic stability in vitro (2,3)
and lose the expression of sex-determining region-box 9 protein
(SOX9) and cartilage-specific markers, including collagen II chain
and aggrecan (4,5). The development of strategies to
promote the maintenance of the chondrogenic phenotype and induce
re-expression of cartilage-specific markers in order to ensure
regeneration of biochemically and mechanically functional cartilage
tissue remains a difficult challenge. Due to the lack of
vasculature in the growth plate of cartilage tissue, chondrocytes
reside in a hypoxic microenvironment, with an oxygen content from
6% O2 in the superficial zone to 1% O2 in the
deep zone, and accommodate physiological chronic hypoxia (6,7).
Hypoxic conditions have been demonstrated to promote the
maintenance of the chondrogenic phenotype (8) and induce re-expression of
cartilage-specific markers in chondrocytes (9,10).
Hypoxia has also been suggested to promote chondrogenic
differentiation of stem cells through SOX9 mediation (11,12) and inhibit chondrocyte hypertrophy
(13). Hypoxia-inducible factors
(HIFs), which consist of an oxygen-regulatory α subunit and a
constitutively-expressed β subunit, participate in the response of
cells to hypoxia. Under normoxic conditions, the HIF-α subunit
becomes hydroxylated by prolyl hydroxylases, followed by proteasome
degradation; conversely, under hypoxic conditions, HIF-α is
stabilized, forms a heterodimer with the HIF-β subunit in the
nucleus and drives the expression of its targets (14). All of the known HIFs (HIF-1, 2 and
3) are involved in the regulation of the chondrogenic phenotype
under hypoxic conditions (6,15,16). HIF-1α has been indicated to be a
critical survival factor for chondrocytes during development
(6) by inducing expression of
cartilage-specific markers (17,18).
Cartilage tissue is regulated by various endocrine,
paracrine and complex local signaling pathways (19). With the exception of HIF-1α, the
other signaling pathways involved in the maintenance of the
chondrogenic phenotype under hypoxic conditions remain largely
unknown. The present study investigated the role of Yes-associated
protein (YAP) in the maintenance of the chondrogenic phenotype in
chondrocytes under hypoxic conditions. The Hippo-YAP signaling
pathway serves an important role in regulating cell growth and
differentiation (20). Core
components of the Hippo pathway include the kinases mammalian Ste20
(MST) and serine/threonine-protein kinase large tumor suppressor
(LATS). Upon activation of the Hippo pathway, MST phosphorylates
and activates LATS, which subsequently phosphorylates and inhibits
YAP. Phosphorylation of YAP leads to its cytoplasmic retention and
degradation by proteasomes. Conversely, inhibition of the Hippo
pathway results in YAP nuclear retention and activation of
transcriptional activity (21).
In addition to the Hippo signaling pathway, one previous study
indicated that a number of signaling pathways interact with YAP
protein and serve important roles in regulating YAP protein
activation (22). To the best of
our knowledge, the interaction between hypoxia and YAP has not been
examined in growth plate chondrocytes. We hypothesized that hypoxia
may activate YAP in chondrocytes; the possible role of
hypoxia-induced activation of YAP in the maintenance of the
chondrogenic phenotype and re-expression of cartilage-specific
markers was then investigated, including the interaction between
YAP and HIF-1α under hypoxic conditions. The aim of the present
study was to improve the current knowledge on signaling mechanisms
in chondrocytes under hypoxic conditions, in order to improve the
success of cartilage tissue engineering.
Materials and methods
Chondrocytes isolation and culture
Growth plate chondrocytes were obtained from 7-day
old Sprague Dawley (SD) rats (n=20, 12 g). These SD rats were
provided by the Experimental Animal Center of Tongji Hospital
(Wuhan, Hubei, China) and maintained at 25°C, a relative humidity
of 60% and ad libitum access to food and water. The present
study was approved by the Experimental Animal Ethics Committee of
Tongji Hospital, Tongji Medical College of Huazhong University of
Science and Technology (Wuhan, Hubei, China). Briefly, after the SD
rats were sacrificed by cervical dislocation following anesthesia
using sodium pentobarbital, cartilage was obtained from the growth
plate of the distal femur and proximal tibia. Cartilage pieces were
digested with 0.25% trypsin (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) for 30 min at 37°C with gentle shaking, and then
digested with 0.1% collagenase (Invitrogen; Thermo Fisher
Scientific, Inc.) for 8 h at 37°C. Single cells were collected with
a cell strainer, followed by centrifugation (350 x g and 25°C for 8
min) and resuspension in Dulbecco's modified eagle's medium/Ham's
F-12 (DMEM/F-12; HyClone; GE Healthcare Life Sciences, Logan, UT,
USA) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.) and 1% penicillin-streptomycin. A total of
5×105 chondrocytes were seeded into a cell medium and
the medium was changed every 2 days. The chondrocytes were passaged
at 80% confluence up to passage 3.
Hypoxia intervention
Once the chondrocytes had reached 80% confluence,
the cells were cultured under normoxic (21% O2, 74%
N2, 5% CO2, 37°C, saturated humidity) or
hypoxic (1% O2, 94% N2, 5% CO2,
37°C, saturated humidity) conditions.
Drugs treatments and RNA
interference
Chondrocyte culture medium was supplemented with
different concentrations of cobalt chloride (0, 50, 10, 150 and 200
µM; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for the
indicated times. When the growth plate chondrocytes had reached
~50% confluence, the original culture medium was discarded, and
OPTIMEM medium containing YAP small interfering (si)RNA (50 nM),
HIF-1α siRNA (50 nM; Guangzhou Ribobio Co., Ltd., Guangzhou,
Guangdong, China) or negative control (NC) siRNA, and Lipofectamine
2000® (Invitrogen; Thermo Fisher Scientific, Inc.), was
transfected in chondrocytes for 6 h. The sequences of YAP siRNA
were as follows: GCCATGAACCAGAGGATCA. The sequences of HIF-1α siRNA
were as follows: CTGATAACGTGAACAAATA, TCGACAAGCTTAAGAAAGA and
GGACAATATAGACATT. Subsequent experiments were performed after 24 h.
Endogenous YAP and HIF-1a protein knock-out efficiency was examined
by western blot analysis.
Western blot analysis
Following rinsing with pre-cooled PBS, the
chondrocytes were lysed with radioimmunoprecipitation assay lysis
buffer (Boster Biological Technology, Inc., Wuhan, Hubei, China)
containing protease inhibitors and phosphatase inhibitors for 30
min, and the lysate was centrifuged at 12,000 x g at 4°C for 20
min. Subsequent to collection of the supernatant, the protein
concentration was determined by a bicinchoninic acid assay. A total
of 20 μg protein was loaded in 10% Bis-Tris gels. Following
electrophoresis, the separated proteins were transferred onto a
polyvinylidene fluoride membrane. The membranes were blocked with
5% BSA (Biosharp, Inc., Hefei, Anhui, China) for 1 h at room
temperature and incubated with the primary antibodies overnight at
4°C, followed by rinsing with TBS supplemented with 0.1% Tween-20
(TBST) and incubation with horseradish peroxidase-conjugated goat
anti-mouse or goat anti-rabbit secondary antibody (cat no. BA1050
and BA1054, respectively; both 1:5,000 dilution; Boster Biological
Technology) for 1 h at room temperature. Then, following rinsing
again with TBST, exposure was performed using an
electrochemiluminescence (ECL) substrate (Pierce; Thermo Fisher
Scientific, Inc.) with a Bio-Rad exposure machine (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Grayscale analysis was
performed using Quantity One software version 5.1 (Bio-Rad
Laboratories, Inc.) to determine the relative expression of various
target proteins, and β-actin was used as an internal control. The
primary antibodies used were as follows: MST1 (cat. no. 3682;
1:1,000 dilution; Cell Signaling Technology, Inc., Danvers, MA,
USA), phosphorylated (p)-LATS (cat. no. 9157; 1:1,000 dilution;
Cell Signaling Technology, Inc.), LATS (cat. no. 3477; 1:1,000
dilution; Cell Signaling Technology, Inc.), p-YAP (cat. no. 13008;
1:500 dilution; Cell Signaling Technology, Inc.), YAP (cat. no.
14074; 1:500 dilution; Cell Signaling Technology, Inc.), SOX9 (cat.
no. ab3697; 1:5,000 dilution; Abcam, Cambridge, MA, USA), collagen
II chain (cat. no. ab34712; 1:1,000 dilution; Abcam), aggrecan
(cat. no. ab36861; 1:1,000 dilution; Abcam) HIF-1α (cat. no.
ab2185; 1:500 dilution; Abcam), vascular endothelial growth factor
(VEGF; cat. no. ab46154; 1:1,000 dilution; Abcam); connective
tissue growth factor (CTGF; cat. no. ab6992; 1:1,000 dilution;
Abcam), PCNA (cat. no. 13110; 1:1,000 dilution; Cell Signaling
Technology, Inc.), VEGF receptor 1 (cat. no. ab32152, 1:1,000
dilution; Abcam) and β-actin (cat. no. BM5180; 1:400 dilution;
Boster Biological Technology).
Immunofluorescence
The chondrocytes were rinsed three times with PBS,
fixed with 4% paraformaldehyde for 15 min at room temperature,
permeabilized with 0.1% Triton X-100 for 5 min and blocked with
0.1% BSA for 1 h at room temperature. The cells were incubated with
the primary antibodies (YAP, cat no. 14074; 1:200 dilution; Cell
Signaling Technology, Inc.; SOX9, cat. no. ab3697; 1:200 dilution;
Abcam; collagen II chain, cat. no. ab34712; 1:200 dilution; Abcam;
HIF-1α, cat. no. ab2185; 1:200 dilution; Abcam; VEGF, cat. no.
ab46154; 1:00 dilution; Abcam) overnight at 4°C. Following rinsing
with PBS, the CY3-conjugated goat anti-rabbit immunoglobulin G
(cat. no. BA1032; 1:100 dilution; Boster Biological Technology) was
added and the cells were incubated for 1 h at room temperature.
Following rinsing with PBS, DAPI (undiluted; cat. no. AR1176,
Boster Biological Technology) was added to stain the nuclei for 5
min at room temperature. Subsequent to rinsing again, the cells
were imaged using an EVOS FL auto fluorescence microscope
(magnification ×200; Thermo Fisher Scientific, Inc.).
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was used for quantification of gene
expression. Total RNA was isolated with the EZNA Total RNA kit
(Omega Bio-Tek, Inc., Norcross, GA, USA) according to the
manufacturer's protocol, and the A260/280 absorbance ratio of the
samples was measured to determine the RNA quality. RNA was
synthesized using the Reverse Transcription kit (Toyobo Life
Science, Osaka, Japan) and single-stranded cDNA was stored at −20°C
until use. The SYBR Green Real-Time PCR Master Mix (Toyobo Life
Science) was used to amplify the cDNA under the following
conditions: 40 cycles of denaturation at 95°C for 30 sec, 40 cycles
of amplification at 94°C for 5 sec, and at 58°C for 30 sec. β-actin
served as an internal control. There were three duplicate
wells/cDNA sample. The sequences of the primers were as follows:
MST1 forward, 5′-CATGACTGCTGGGTCCTACA-3′; MST1 reverse,
5′-TGCACGACCTTGTTAATCCA-3′; LATS1 forward,
5′-CAGCAACTACTCTGGCAGCA-3′; LATS1 reverse,
5′-TCGGTCGACATCTTGTTCAG-3′; LATS2 forward,
5′-ACAGAAGCAGCACCAGAGGT-3′; LATS2 reverse,
5′-CGACACTCCACCAGTCACAG-3′; CTGF forward,
5′-GAGTCGTCTCTGCATGGTCA-3′; CTGF reverse,
5′-GCAGCCAGAAAGCTCAAACT-3′; YAP forward,
5′-TTTGCCATGAACCAGAGGAT-3′; YAP reverse,
5′-TATCTGCTGCTGCTGGTTTG-3′; SOX9 forward,
5′-TGCAGCACAAGAAAGACCAC-3′; SOX9 backward,
5′-TGGCGTTAGGAGAGATGTGA-3′; collagen α-1(II) chain forward,
5′-CTCAAGTCGCTGAACAAC CA-3′; collagen α-1(II) chain reverse,
5′-CTCAAGTCGCTGAACAAC CA-3′; aggrecan forward,
5′-AACTCAGTGGCCAAACATCC-3′; aggrecan reverse,
5′-AACTCAGTGGCCAAACATCC-3′; HIF-1α forward,
5′-TCAAGTCAGCAACGTGGAAG-3′; HIF-1α reverse,
5′-GGCCAGCTAACTTTCAGCAC-3′; VEGFa forward,
5′-GCTTCCTAGTGGGCTCTGTG-3′; VEGFa reverse,
5′-CACACATACACTCCGGCATC-3′; CTGF forward,
5′-GAGTCGTCTCTGCATGGTCA-3′; CTGF reverse,
5′-GCAGCCAGAAAGCTCAAACT-3′; β-actin forward,
5′-GAGATTACTGCCCTGGCTCCTAGC-3′; β-actin reverse,
5′-CCGGACTCATCGTACTCCTGC TT-3′.
Statistical analysis
All experiments were repeated three times and the
results are presented as mean ± standard deviation. SPSS
statistical software (v.19.0; IMB Corp., Armonk, NY, USA) was used
to analyze statistical differences between groups. An unpaired
Student's t-test was used to assess statistical significance
between two groups. A one-way analysis of variance with Bonferroni
post-hoc test was used for multiple group comparisons. P<0.05
was considered to indicate a statistically significant
difference.
Results
Hypoxia promotes YAP activation in growth
plate chondrocytes via a Hippo-independent signaling pathway
To explore the effect of hypoxia (1% O2)
on YAP activation in growth plate chondrocytes at different time
points, western blot analysis, PCR and immunofluorescence were
used. As indicated in Fig. 1A–C,
hypoxia significantly promoted YAP mRNA and protein expression. As
the total YAP protein level was altered following hypoxia
treatment, the ratio of p-YAP/total YAP was used to analyze YAP
activation. As demonstrated in Fig.
1A, hypoxia promoted YAP dephosphorylation in a time-dependent
manner. Similar results were also obtained by immunofluorescence.
As serum contains lysophosphatidic acid, which is able to activate
YAP protein, chondrocytes were serum-starved for 24 h. As indicated
in Fig. 1D, immunofluorescence
imaging of chondrocytes cultured under normoxia revealed that YAP
was inactivated and primarily located in the cytoplasm of
chondrocytes. Following culture under hypoxic conditions for 4 h,
the YAP protein was activated and trans-located into the nucleus.
These results indicated that hypoxia activated YAP protein.
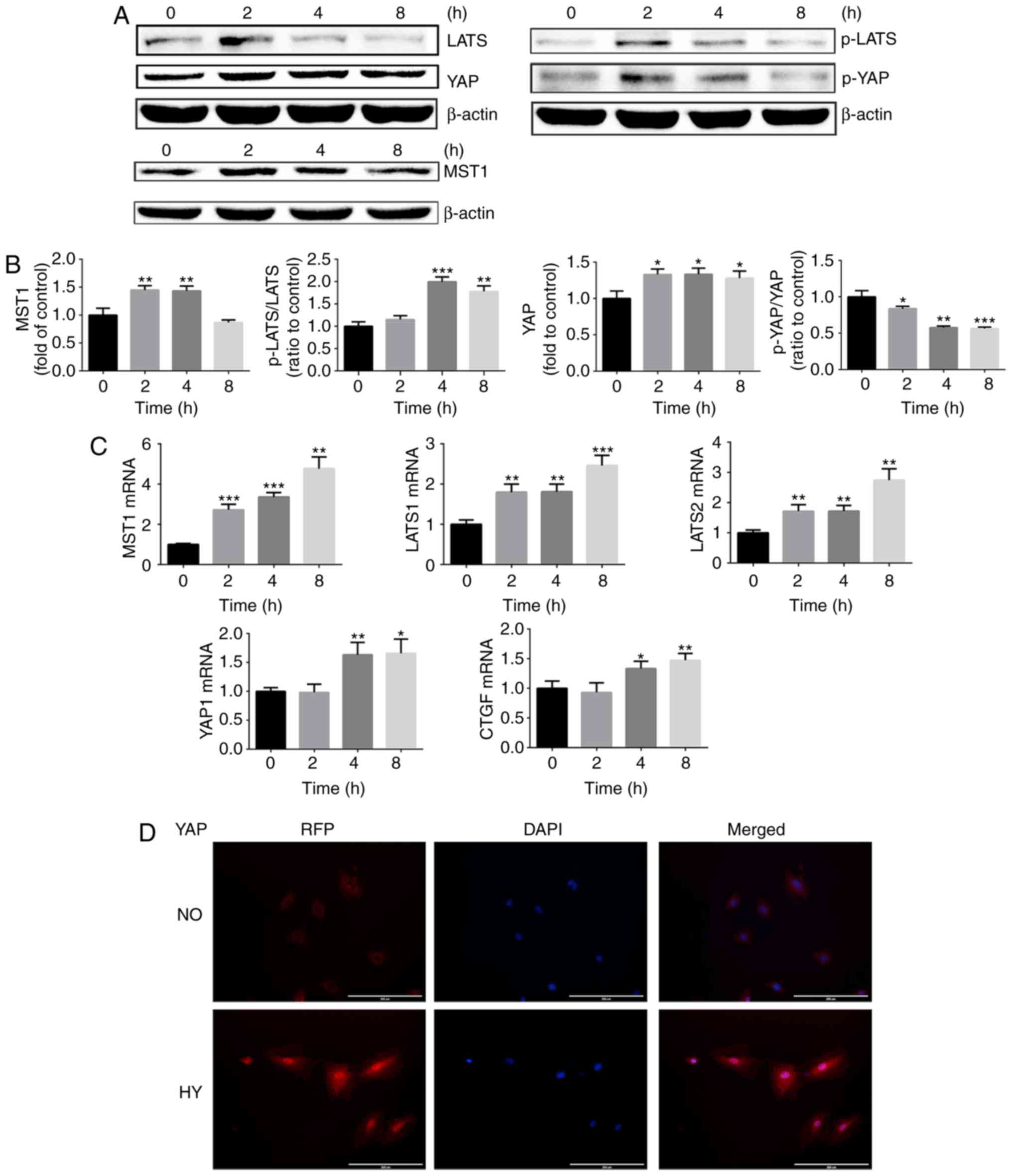 | Figure 1Hypoxia promotes YAP activation in
growth plate chondrocytes via a Hippo-independent signaling
pathway. (A) Protein expression of MST1, p-LATS, LATS, p-YAP and
YAP of growth plate chondrocytes cultured under hypoxia (1%
O2) for 0, 2, 4 and 8 h. β-actin served as an internal
control. (B) The density of the bands were quantified and
normalized to the control group (0 h). (C) mRNA expression of MST1,
LATS1, LATS2, YAP and CTGF in chondrocytes cultured under hypoxia
(1% O2) for 0, 2, 4 and 8 h. (D) Immunofluorescence
imaging of YAP (red) in chondrocytes cultured under normoxia (21%
O2) or hypoxia (1% O2) for 4 h. DAPI (blue)
was used for nuclear staining. Scale bar=200 μm. Data are
presented as mean ± standard deviation (n=3) and normalized to the
control group (0 h). *P<0.05, **P<0.01
and ***P<0.001 vs. the control group (0 h). YAP,
Yes-associated protein; MST1, mammalian Ste20; LATS1/2,
serine/threonine protein kinase large tumor suppressor 1/2; p,
phosphorylated; CTGF, connective tissue growth factor; NO,
normoxia; HY, hypoxia; RFP, red fluorescent protein. |
The Hippo signaling pathway serves an important role
in regulating YAP protein activation. Therefore, whether hypoxia
promoted YAP activation via inhibiting the Hippo signaling pathway
was next investigated. The expression levels of core regulators in
the Hippo-YAP signaling pathway including MST1, p-LATS and LATS
were assessed. The western blot analysis results demonstrated that,
when chondrocytes were cultured under hypoxic conditions, the Hippo
signaling pathway was activated and an increase in MST1 mRNA and
protein expression was observed (Fig.
1A–C). Furthermore, the ratio of p-LATS/LATS was significantly
upregulated under exposure to hypoxic conditions for >4 h
(Fig. 1B). As Hippo signaling
pathway activation may result in YAP protein phosphorylation and
cytoplasm retention, hypoxia may therefore promote YAP activation
in a Hippo-independent manner.
Hypoxia promotes the expression of
cartilage-specific markers at the protein and gene level
The effect of hypoxia on the protein and gene
expression of cartilage-specific markers in chondrocytes under
normoxic (21% O2) or hypoxic (1% O2)
conditions was then investigated. The expression of
cartilage-specific markers, including SOX9, collagen II chain and
aggrecan, was assessed. The protein expression levels of SOX9,
collagen II chain and aggrecan were significantly increased in
response to hypoxia in chondrocytes. Notably, when chondrocytes
remained under hypoxic conditions for 8 h, the protein expression
levels of SOX9, collagen II chain and aggrecan were downregulated
compared with that at 4 h, but the expression level of SOX9 and
collagen II chain remained increased compared with that in the
control group (Fig. 2A and
B).
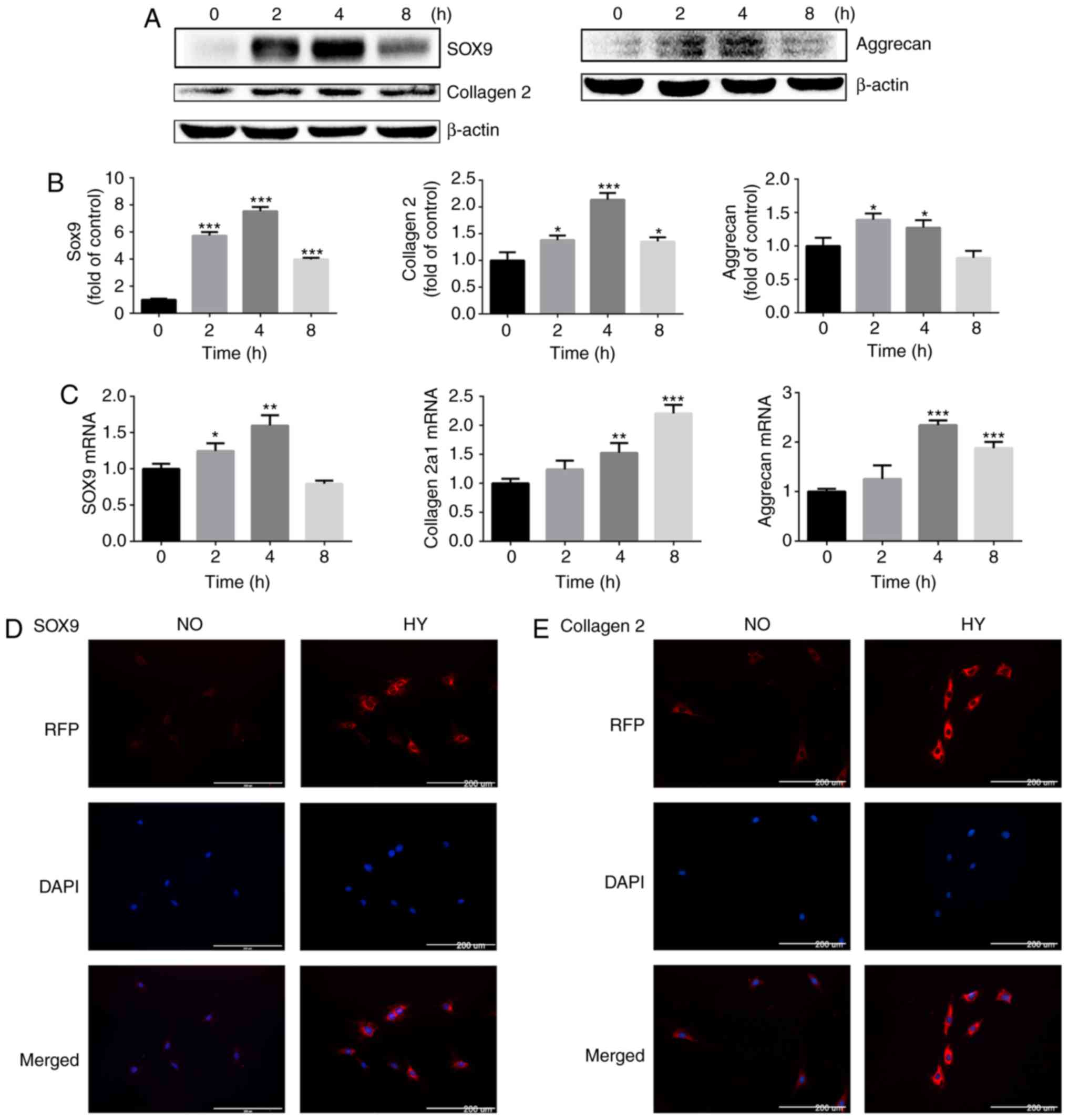 | Figure 2Hypoxia promotes the expression of
cartilage-specific markers at the protein and gene levels. (A)
Protein expression of SOX9, collagen II chain and aggrecan in
growth plate chondrocytes cultured under hypoxia (1% O2)
for 0, 2, 4 and 8 h. β-actin served as an internal control. (B) The
density of the bands was quantified and normalized to the control
group (0 h). (C) mRNA expression of SOX9, collagen α-1(II) chain
and aggrecan in chondrocytes cultured under hypoxia (1%
O2) for 0, 2, 4 and 8 h. (D) Immunofluorescence imaging
of SOX9 (red) of chondrocytes cultured under normoxia (21%
O2) or hypoxia (1% O2) for 4 h. DAPI (blue)
was used for nuclear staining. Scale bar=200 μm. (E)
Immunofluorescence imaging of collagen II chain (red) in
chondrocytes cultured under normoxia (21% O2) or hypoxia
(1% O2) for 4 h. DAPI (blue) was used for nuclear
staining. Scale bar=200 μm. Data are presented as mean ±
standard deviation (n=3) and normalized to the control group (0 h).
*P<0.05, **P<0.01 and
***P<0.001 vs. the control group (0 h). SOX9,
sex-determining region-box 9 protein; NO, normoxia; HY, hypoxia;
RFP, red fluorescent protein. |
The RT-qPCR results demonstrated that hypoxia
promoted SOX9, collagen α-1(II) chain and aggrecan gene expression,
and the mRNA levels of SOX9 and aggrecan peaked at 4 h (Fig. 2C).
The expression levels of SOX9 and collagen II chain
were then investigated by immunofluorescence. Concordant with the
protein and gene expression data, SOX9 and collagen II chain
deposition in chondrocytes was markedly increased by exposure to
hypoxia for 4 h (Fig. 2D and
E).
These results indicate that hypoxia induces the
expression of cartilage-specific markers in chondrocytes, thereby
promoting the maintenance of the chondrogenic phenotype.
Hypoxia promotes YAP activation in a
HIF-1α dependent manner
HIF-1α is key to chondrocyte survival under hypoxic
conditions. Therefore, the contribution of HIF-1α to
hypoxia-induced activation of YAP and maintenance of the
chondrogenic phenotype in chondrocytes was investigated. Firstly,
the expression of HIF-1α and its downstream target gene VEGF was
examined in a hypoxic microenvironment. The protein expression of
HIF-1α in growth plate chondrocytes was significantly increased
under hypoxia, and VEGF protein expression was also significantly
upregulated (Fig. 3A and B). The
RT-qPCR analysis yielded the same results (Fig. 3C). Similar results were also
obtained by immunofluorescence assay (Fig. 3D and E).
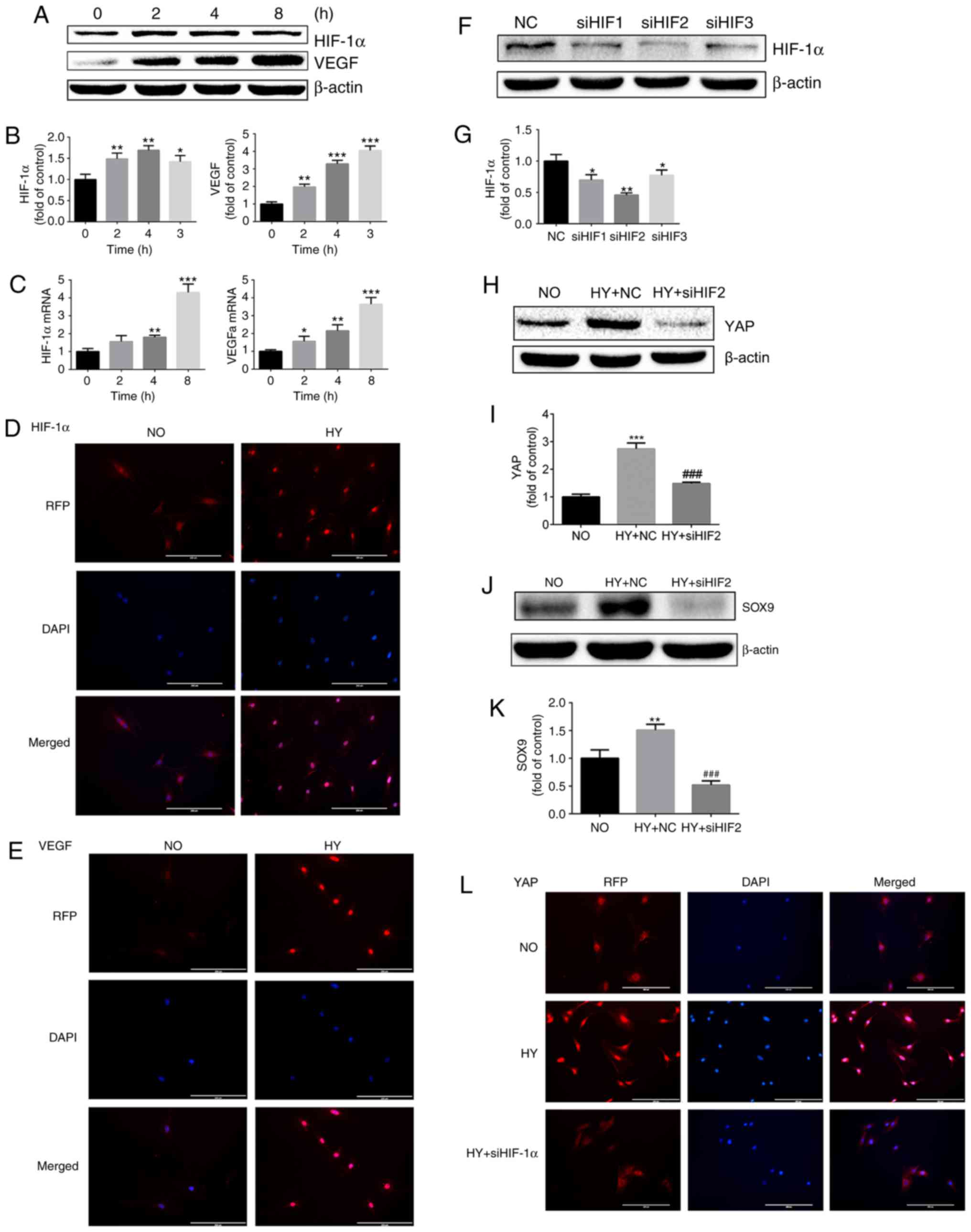 | Figure 3HY promotes YAP activation in a
HIF-1α-dependent manner. (A) Protein expression of HIF-1α and VEGF
in growth plate chondrocytes cultured under HY (1% O2)
for 0, 2, 4 and 8 h. β-actin served as an internal control. (B) The
density of the bands were quantified and normalized to the control
group (0 h). (C) mRNA expression of HIF-1α and VEGF in chondrocytes
cultured under HY (1% O2) for 0, 2, 4 and 8 h. (D)
Immunofluorescence imaging of HIF-1α (red) in chondrocytes cultured
under NO (21% O2) or HY (1% O2) for 4 h. DAPI
(blue) was used for nuclear staining. Scale bar=200 μm. (E)
Immunofluorescence imaging of VEGF (red) in chondrocytes cultured
under NO (21% O2) or HY (1% O2) for 4 h. DAPI
(blue) was used for nuclear staining. Scale bar=200 μm. (F)
Protein expression of HIF-1α in growth plate chondrocytes following
siRNA silencing of HIF-1α and then culture under HY (1%
O2) for 4 h to detect the effect of HIF-1α silencing.
β-actin served as an internal control. (G) The density of the bands
was quantified and normalized to the NC group. Data are presented
as mean ± SD (n=3). *P<0.05 and
**P<0.01 vs. the NC group. (H) Protein expression of
YAP in growth plate chondrocytes cultured under NO or under HY with
siRNA negative control (HY+NC group) or under HY with siHIF2 siRNA
(HY+siHIF2 group) for 4 h. β-actin served as an internal control.
(I) The density of the bands was quantified and normalized to the
NO group. (J) Protein expression of SOX9 in growth plate
chondrocytes cultured under NO (NO group) or under HY with siRNA
negative control (HY+NC group) or under HY with siRNA siHIF2
(HY+siHIF2 group) for 4 h. β-actin served as an internal control.
(K) The density of the bands was quantified and normalized to the
NO group. **P<0.01 vs. the NO group. (L)
Immunofluorescence imaging of YAP (red) in chondrocytes cultured
under NO (21% O2) or HY (1% O2) with or
without siHIF-1α for 4 h. DAPI (blue) was used for nuclear
staining. Scale bar=200 μm. Data are presented as mean ±
standard deviation (n=3), and normalized to the control (0 h)/NC
groups. *P<0.05, **P<0.01 and
***P<0.001 vs. the control (0 h) or NC groups.
###P<0.001 vs. the HY+NC group. YAP, yes-associated
protein; HIF-1α, hypoxia-inducible factor 1α; SOX9, sex-determining
region-box 9 protein; siRNA, small interfering RNA; NO, normoxia;
HY, hypoxia; RFP, red fluorescent protein; VEGF, vascular
endothelial growth factor; NC, negative control. |
Next, siRNA were used to destabilize HIF-1α under
hypoxic conditions and detect the activation of YAP. As indicated
in Fig. 3F, all three siRNA
sequences effectively downregulated the expression of HIF-1α, with
sequence 2 being the most effective (Fig. 3F and G). Following inhibition of
HIF-1α expression by siRNA, it was identified that the activation
of YAP protein induced by hypoxia was significantly inhibited
(Fig. 3H and I). It was also
identified that, following downregulation of HIF-1α expression, the
upregulation of SOX9 caused by hypoxia was also significantly
downregulated (Fig. 3J and K). To
additionally investigate the effect of HIF-1α on YAP localization
and activation, an immunofluorescence assay was conducted. As
demonstrated in Fig. 3L, HIF-1α
siRNA treatment significantly abolished hypoxia-induced YAP nucleus
translocation and activation (Fig.
3L).
These results suggest that HIF-1α serves an
important role in regulating chondrocyte differentiation. Notably,
the results of the present study also indicate that hypoxia-induced
YAP activation is HIF-1α-dependent.
Cobalt chloride upregulates HIF-1α
expression and promotes activation of YAP
To additionally explore the effect of HIF-1α on YAP
activation under normoxia, chondrocytes were treated with cobalt
chloride to stabilize HIF-1α under normoxic conditions. Following
treatment with different concentrations of cobalt chloride (0–200
μM) for 24 h, the gene and protein expression of HIF-1α and
its downstream target, VEGF, were evaluated by western blot
analysis and RT-qPCR. The protein expression of HIF-1α was
significantly upregulated following stimulation of chondrocytes
with different concentrations of cobalt chloride, and VEGF
expression was also upregulated (Fig.
4A and B). At cobalt chloride concentrations of >100
μM, the HIF-1α and VEGFa gene expressions were significantly
increased compared with those in the control group (Fig. 4C).
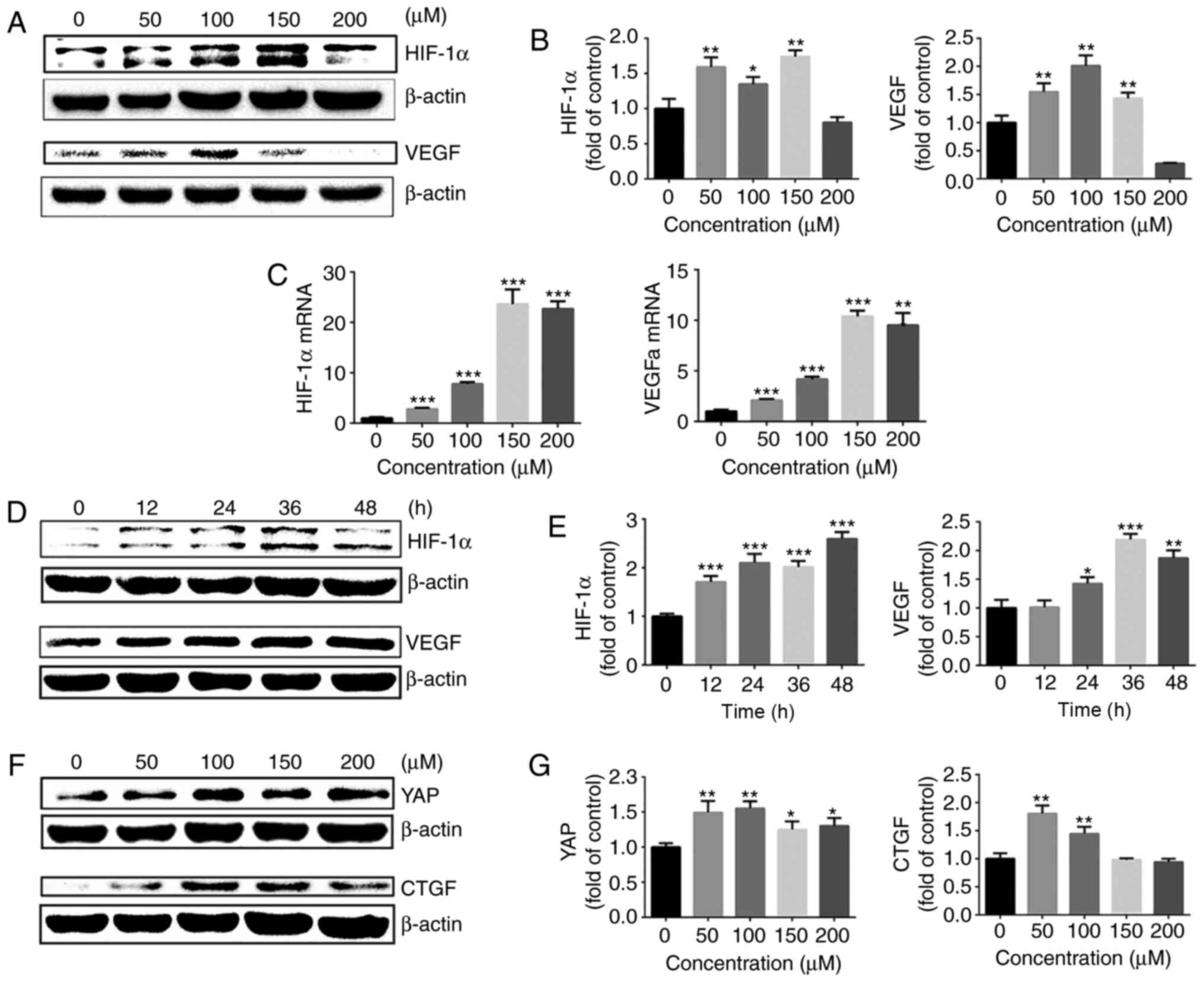 | Figure 4Cobalt chloride upregulates HIF-1α
expression and promotes activation of YAP. (A) Protein expression
of HIF-1α and VEGF in growth plate chondro-cytes cultured with
cobalt chloride at concentrations in 0, 50, 100, 150 or 200
μM under normoxia (21% O2) for 24 h. β-actin
served as an internal control. (B) The density of the bands was
quantified and normalized to the 0 μM group. (C) mRNA
expression of HIF-1α and VEGF in chondrocytes cultured with cobalt
chloride at concentrations of 0, 50, 100, 150 or 200 μM
under normoxia (21% O2) for 24 h. (D) Protein expression
of HIF-1α and VEGF in growth plate chondrocytes cultured with 100
μM cobalt chloride for 0, 12, 24, 36 or 48 h under normoxia
(21% O2). β-actin served as an internal control. (E) The
density of the bands was quantified and normalized to the 0 h
group. (F) Protein expression of YAP and CTGF in chondrocytes
cultured with cobalt chloride at concentrations of 0, 50, 100, 150
or 200 μM under normoxia (21% O2) for 24 h. (G)
The density of the bands was quantified and normalized to the 0
μM group. (H) mRNA expression of YAP and CTGF in growth
plate chondrocytes cultured with cobalt chloride at concentrations
of 0, 50, 100, 150 or 200 μM under normoxia (21%
O2) for 24 h. (I) Protein expression of YAP and CTGF in
growth plate chondrocytes cultured with 100 μM cobalt
chloride for 0, 12, 24, 36 or 48 h under normoxia (21%
O2). β-actin served as an internal control. (J) The
density of the bands was quantified and normalized to the 0 h
group. (K) Immunofluorescence imaging of YAP (red) in chondrocytes
cultured with 100 μM cobalt chloride under normoxia (21%
O2) for 24 h. ‘C’ represents the control. DAPI (blue)
was used for nuclear staining. Scale bar=200 μm. Data are
presented as mean ± standard deviation (n=3) and normalized to the
control (0 μM/0 h) groups. *P<0.05,
**P<0.01 and ***P<0.001 vs. the 0 h/0
μM groups. YAP, yes-associated protein; HIF-1α,
hypoxia-inducible factor 1α; SOX9, sex-determining region-box 9
protein; NO, normoxia; HY, hypoxia; RFP, red fluorescent protein;
VEGF, vascular endothelial growth factor; CTGF, connective tissue
growth factor; NC, negative control. |
Next, 100 μM cobalt chloride was used to
stimulate growth plate chondrocytes for different lengths of time,
and the changes in HIF-1α and VEGF protein levels were assessed.
The protein expression of HIF-1α was significantly up regulated
following stimulation of chondrocytes with 100 μM cobalt
chloride for 12–48 h. The protein expression of VEGF also exhibited
a significant upregulation (Fig. 4D
and E).
Then, the activation of YAP in the presence of
cobalt chloride under normoxia was examined. Different
concentrations of cobalt chloride (0–200 μM) were used to
stimulate chondrocytes for 24 h under normoxia, and the gene and
protein expression levels of YAP and its downstream target gene,
CTGF, were detected. As demonstrated in Fig. 4F–H, cobalt chloride significantly
promoted YAP activation and the expression of CTGF. Cobalt chloride
(100 μM) was used to stimulate growth plate chondrocytes for
different time intervals, and the changes in YAP and CTGF protein
levels were assessed (Fig. 4I and
J). The results of immunofluorescence also revealed that 100
μM cobalt chloride significantly promoted YAP protein
expression and nuclear translocation (Fig. 4K). These results indicated that
the upregulation of HIF-1α with cobalt chloride promoted the
activation of YAP under normoxic conditions.
HIF-1α is useful for maintaining of the
chondrogenic phenotype
Chondrocytes were then treated with cobalt chloride
to stabilize HIF-1α under normoxia and the expression of
cartilage-specific markers was detected. The western blot analysis
results demonstrated that treatment with cobalt chloride at
different concentrations for 24 h upregulated the protein
expression of SOX9 and collagen II chain in chondrocytes, with 100
μM cobalt chloride exerting the most marked effect (Fig. 5A and B). The RT-qPCR results also
revealed that cobalt chloride stimulated the gene expression of
collagen α-1(II) chain, aggrecan and SOX9 (Fig. 5C). Subsequently, the chondrocytes
were stimulated with 100 μM cobalt chloride for different
time intervals. The protein expression levels of collagen II chain
and SOX9 were significantly increased following stimulation with
100 μM cobalt chloride (Fig.
5D and E). The aforementioned results indicated that increasing
the expression of HIF-1α with cobalt chloride in a normoxic
environment effectively promoted the expression of
cartilage-specific markers in chondrocytes, which was beneficial in
promoting the maintenance of the chondrogenic phenotype.
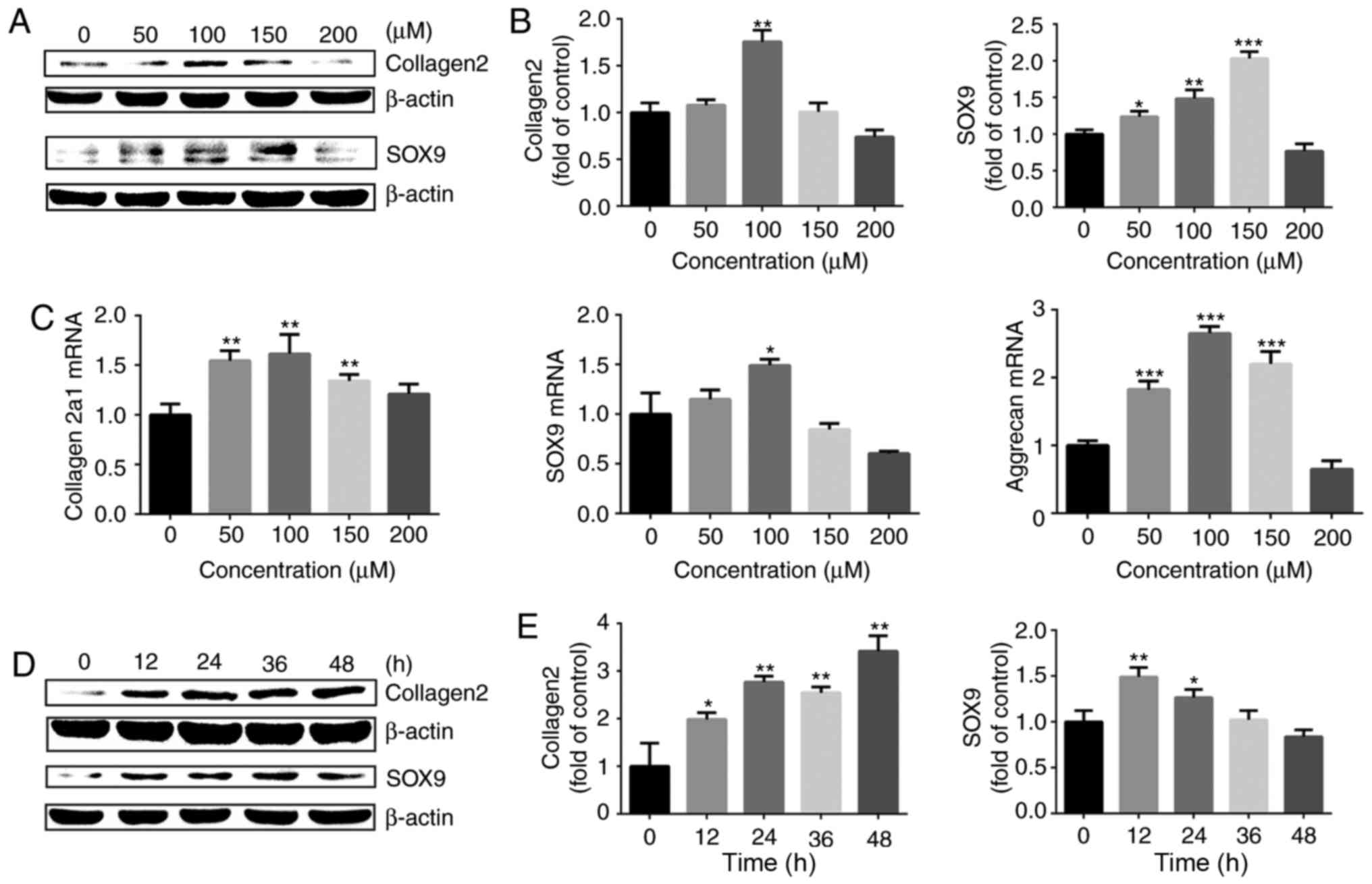 | Figure 5HIF-1α is important for maintaining
of the chondrogenic phenotype. (A) Protein expression of SOX9 and
collagen II chain in growth plate chon-drocytes cultured with
cobalt chloride at concentrations of 0, 50, 100, 150 or 200
μM under normoxia (21% O2) for 24 h. β-actin
served as an internal control. (B) The density of the bands was
quantified and normalized to the 0 μM group. (C) mRNA
expression of collagen α-1(II) chain, SOX9 and aggrecan in
chondrocytes cultured with cobalt chloride at concentrations of 0,
50, 100, 150 or 200 μM under normoxia (21% O2)
for 24 h. (D) Protein expression of SOX9 and collagen II chain in
growth plate chondrocytes cultured with 100 μM cobalt
chloride for 0, 12, 24, 36 or 48 h under normoxia (21%
O2). β-actin served as an internal control. (E) The
density of the bands was quantified and normalized to the 0 h
group. Data are presented as mean ± standard deviation (n=3), and
normalized to the control (0 μM/0 h) groups.
*P<0.05, **P<0.01 and
***P<0.001 vs. the control (0 h/0 μM) group.
HIF-1α, hypoxia-inducible factor 1α; SOX9, sex-determining
region-box 9 protein. |
Effect of YAP on HIF-1α and the
maintenance of the chondrogenic phenotype
To investigate the role of YAP in HIF-1α and the
maintenance of the chondrogenic phenotype in chondrocytes under
hypoxic conditions, siRNA were used to downregulate the expression
of YAP and investigate the expression of SOX9 and HIF-1α in
chondrocytes exposed to hypoxia. SOX9 protein expression was
downregulated following YAP inhibition. However, the levels of
HIF-1α and VEGF did not change significantly (Fig. 6A–C). The results demonstrated that
YAP is implicated in the maintenance of the chondrogenic phenotype
of chondrocytes induced by hypoxia, and the downregulation of YAP
expression did not affect the expression of HIF-1α under
hypoxia.
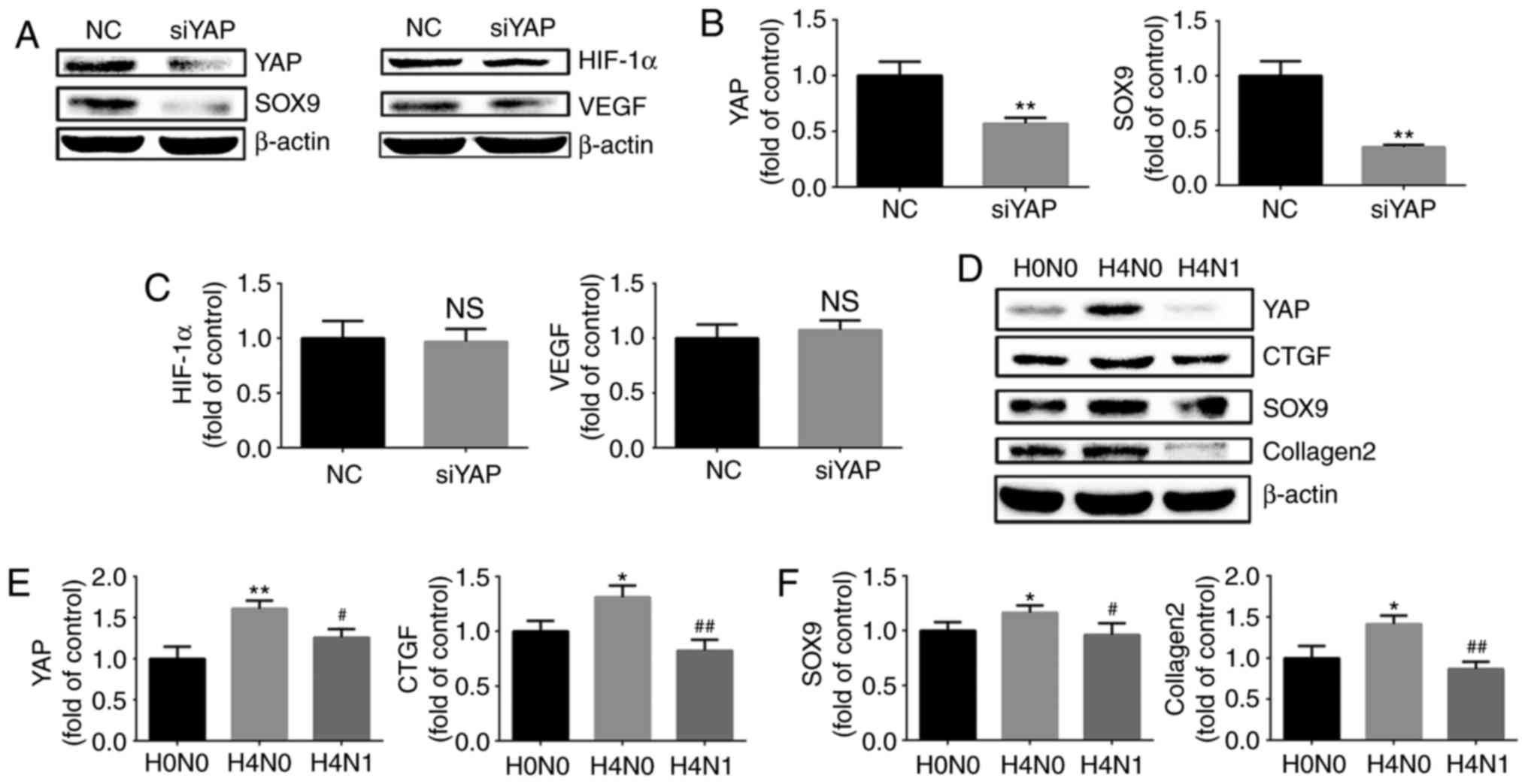 | Figure 6Effect of YAP on HIF-1α and the
maintenance of the chondrogenic phenotype. (A) Protein expression
of YAP, SOX9, HIF-1α and VEGF in growth plate chondrocytes
following siRNA silencing of YAP and culture under hypoxia (1%
O2) for 4 h. β-actin served as an internal control. The
densities of the (B) YAP and SOX9 bands, and (C) HIF-1α and VEGF
bands were quantified and normalized to the NC group. (D) Protein
expression of YAP, CTGF, SOX9 and collagen II chain in growth plate
chondrocytes cultured under normoxia (H0N0), or with (H4N1) or
without reoxygenation for 1 h after hypoxia for 4 h (H4N0). β-actin
served as an internal control. (E) The densities of the YAP and
CTGF bands, and (F) SOX9 and collagen II chain bands were
quantified and normalized to the H0N0 group. Data are presented as
the mean ± standard deviation (n=3). *P<0.05 and
**P<0.01 vs. the NC/H0N0 groups.
#P<0.05 and ##P<0.01 vs. the H4N0
group. YAP, yes-associated protein; HIF-1α, hypoxia-inducible
factor 1α; SOX9, sex-determining region-box 9 protein; CTGF,
connective tissue growth factor; VEGF, vascular endothelial growth
factor; siRNA, small interfering RNA; NC, negative control. |
To investigate whether reoxygenation following
hypoxia may inhibit the maintenance of the chondrogenic phenotype,
the chondrocytes were stimulated by normoxia for 1 h, following
hypoxia for 4 h, to observe the expression of cartilage-specific
markers and YAP. The western blot analysis revealed that the levels
of SOX9 and collagen II chain in chondrocytes were down-regulated
during post-hypoxia reoxygenation. YAP exhibited the same trend.
(Fig. 6D–F). Therefore, it was
concluded that the upregulation of cartilage-specific markers
caused by short-term hypoxia stimulation is reversible, and that
this reversibility may be associated with inhibition of YAP by
reoxygenation.
Discussion
Cartilage tissue is a special type of connective
tissue that lacks vasculature; chondrocytes reside in a hypoxic
microenvironment, and produce and maintain the extracellular matrix
of cartilage, including collagen (primarily collagen II chain) and
proteoglycans (primarily aggrecan). In vitro, chondrocytes
are not able to maintain their phenotypic stability and may lose
the expression of cartilage-specific markers including SOX9,
collagen II chain and aggrecan (4,5)
due to the hyperoxic environment established in vitro
relative to their physiological state. Therefore, understanding the
signaling mechanisms that regulate the maintenance of the
chondrogenic phenotype of chondrocytes is crucial for optimizing
the cultivation of chondrocytes, in order to improve the strategies
of autologous cell-based cartilage repair. In the present study, it
was demonstrated that hypoxia promoted the activation of YAP in
chondrocytes in a HIF-1α-dependent manner.
HIF is a type of transcriptional factor that
mediates adaptation of mammalian cells to hypoxia, and consists of
α and β subunits. HIF-1α is a functional subunit that determines
HIF-1 activity and is regulated by intracellular oxygen
concentration. HIF-1β is a structural subunit that is stably
expressed and not regulated by oxygen concentration (23). Under normoxic conditions, HIF-1α
binds to Von Hippel-Lindau proteins, is recognized by the ubiquitin
ligase E3 receptor and forms an E3 ubiquitin ligase complex,
followed by ubiquitination and degradation. When the oxygen
concentration decreases, HIF-α increases in stability and forms a
heterodimer with HIF-β, which is then translocated to the nucleus
to regulate downstream gene expression (23). It was previously demonstrated that
HIFs may directly regulate the expression and activity of Ras
homolog (Rho) gene family, member A and Rho-associated protein
kinase in hypoxic breast cancer cells, thereby affecting cell
contraction, cell-induced matrix contraction, FA formation, focal
adhesion kinase activation and increased cell motility (24). HIF-1α serves an important role in
energy supply, erythropoiesis, angiogenesis and cell survival under
hypoxia. Pathological hypoxia in vitro promotes the
proliferation of neural stem cells (NSCs), upregulates the
expression of HIF-1α and activates the Wnt signaling pathway.
Silencing HIF-1α decreases the nuclear translocation of β-catenin
and the expression of cyclin D1, and inhibits the proliferation of
NSCs (25). HIF-1 serves an
important role in the normal growth and development of cartilage,
energy metabolism and survival (6,26,27). In the present study, hypoxia
promoted the expression of HIF-1α in chondrocytes in a
time-dependent manner, and cobalt chloride stabilized HIF-1α
activity and induced the expression of downstream genes under
normoxic conditions in a dose- and time-dependent manner. When the
concentration of cobalt chloride was >100 μM, it induced
cytotoxicity, and led to a downregulation of HIF-1α and VEGF. When
the HIF-1α gene of growth plate chondrocytes was knocked out in
vitro, their proliferation capacity was significantly decreased
and the number of apoptotic cells increased (28,29). In chondrocytes, HIF-1α is involved
in anaerobic metabolism and inhibits cell apoptosis under hypoxic
conditions (30); it is also
crucial for the differentiation of mesenchymal stem cells (MSCs)
into chondrocytes (29,31). Hypoxia may promote the expression
of collagen II chain and proteoglycan through HIF-1α and promote
the anabolic metabolism of chondrocytes (16). In the present study, 1%
O2 was used to stimulate the growth plate chondrocytes,
and hypoxia was identified to promote the protein and gene
expression of collagen II chain and aggrecan in a time-dependent
manner. When cobalt chloride was used to upregulate the expression
of HIF-1α under normoxic conditions, the expression of collagen II
chain was also upregulated. Under hypoxic conditions, the
inhibition of HIF-1α by siRNA decreased the upregulation of
collagen II chain induced by hypoxia. This suggested that
hypoxia-induced upregulation of collagen II chain is associated
with activation of HIF-1α. Examination of reoxygenation
post-hypoxia revealed that this hypoxia-induced increase in
collagen II chain expression in chondrocytes was reversible. It was
previously demonstrated that, when human MSCs were stimulated by
hypoxia or HIF-1α in vitro, the expression of collagen II
chain and proteoglycans increased, and collagen α-1(X) chain
synthesis decreased. When the HIF-1α stimulation was removed,
collagen II chain and proteoglycan expression decreased and
collagen α-1(X) chain synthesis was increased (32). In chondritis, HIF-1α was indicated
to protect chondrocytes from inflammatory factors (33).
SOX9 is a major regulator of early chondrogenic
differentiation and may promote chondrocyte differentiation and
interact with transcription factors SOX5 and SOX6 to promote the
proliferation and differentiation of chondrocytes followed by
expression of chondrocyte matrix components, including aggrecan and
collagen II chain. HIF-1α regulates the formation of chondrocytes
by upregulating the expression of SOX9 (34,35). In bone marrow MSCs, hypoxia
increased the nuclear accumulation of HIF-1α and SOX9 gene
transcription (36). The present
study demonstrated that hypoxia upregulated SOX9 expression in
chondrocytes in a time-dependent and a HIF-1α-dependent manner.
The Hippo signaling pathway serves an important role
in controlling the size and tissue regeneration in several organs,
and may regulate cell proliferation and differentiation (35,37). When the classical Hippo signaling
is activated, MST1/2 phosphorylates and interacts with protein
salvador homolog 1 to phosphorylate LATS1/2 kinase; activated
LAS1/2 kinase phosphorylates YAP, resulting in its inactivation;
p-YAP then binds to 14-3-3, followed by proteasomal degradation of
the ubiquitinated protein (38).
Dephosphorylated YAP enters the nucleus and interacts with
Transcriptional enhancer factor TEF family of transcription factors
to induce the expression of the target genes (34,37). An increasing number of studies
have demonstrated that Hippo signaling plays an important role in
stem cell proliferation and differentiation (39). YAP has been indicated to be
involved in the regulation of various cellular processes, including
mechanical conduction (40) and
stem cell differentiation (41).
YAP regulates a series of biological behaviors including
self-renewal and proliferation of stem cells (37,42). However, the role of the Hippo
signaling pathway in chondrocyte differentiation and bone repair
remains unclear. YAP1 may directly regulate the expression of SOX6
to promote the proliferation of chondrocytes (43), and inhibit the expression of
collagen α-1(X) chain by interacting with runt-related
transcription factor 2 (Runx2) to inhibit chondrocyte maturation
(44). In the growth plate of the
mouse embryo, YAP1 is expressed in the resting and proliferating
layers, while p-YAP1 is expressed in the hypertrophic layer, and
the expression of collagen α-1(X) chain and Runx2 in the
hypertrophic layer is marked (43). YAP1 is required for the
proliferation and maintenance of chondrogenic progenitor cells. The
overexpression of YAP1 may increase the proliferation of
chondrocytes, but inhibit their differentiation. In addition, YAP1
may inhibit chondrocyte maturation and endochondral ossification
during bone development and growth (43). YAP1 suppresses chondrogenic
differentiation in MSCs, and the downregulation of YAP expression
contributes to the chondrogenic differentiation of MSCs (22). However, the number of studies on
the interaction between hypoxia and YAP is limited, particularly in
chondrocytes. It has been demonstrated that hypoxia may induce YAP
phosphorylation in ECO cell lines and downregulate total YAP
expression (45). Hypoxia in
prostate cancer increases YAP activity by increasing or decreasing
YAP expression in the nucleus and lowering p-YAP levels (46). In the present study, hypoxia was
used to stimulate growth plate chondrocytes, and it was identified
that hypoxia upregulates the expression of YAP while Hippo
signaling pathway was activated concomitantly, indicating that
hypoxia promoted YAP activation via a Hippo-independent pathway.
The RT-qPCR results also demonstrated that hypoxia may promote the
upregulation of YAP mRNA expression. The results of the
immunofluorescence assay revealed that the expression of YAP was
significantly upregulated under hypoxic conditions, and there was
marked expression in the nucleus. Reoxygenation of the chondrocytes
following treatment with hypoxia demonstrated that the activation
of YAP caused by hypoxia was significantly inhibited. The use of
siRNA to inhibit YAP expression under hypoxic conditions decreased
the expression of SOX9, indicating that SOX9 expression in hypoxia
may be regulated by YAP.
Studies on the interactions between HIF-1α and YAP
under hypoxia are infrequent. It has been suggested that hypoxia
may inactivate Hippo signaling, upregulate YAP expression and
promote the formation of a complex with HIF-1α, thereby stabilizing
the expression of HIF-1α in tumors in vivo (47). In an additional study,
interference with YAP in liver sinusoidal endothelial cells
significantly downregulated HIF-1 protein expression (48). The present study used siRNA to
inhibit the expression of HIF-1α and identified that the
upregulation of YAP expression caused by hypoxia was significantly
inhibited. When cobalt chloride was applied to stabilize HIF-1α
expression under normoxic conditions, the expression of YAP and
CTGF was also significantly upregulated. The treatment of
chondrocytes with cobalt chloride also promoted YAP expression and
nuclear translocation, as demonstrated by immunofluorescence
assays. Therefore, we hypothesized that hypoxia-induced activation
of YAP is HIF-1α-dependent. When siRNAs were used to downregulate
YAP expression, the same inhibition was also observed in the
upregulation of SOX9 expression induced by hypoxia. However, there
was no significant effect on the HIF-1α signaling pathway. Upon
reoxygenation of chondrocytes following exposure to hypoxia, it was
identified that the activation of YAP caused by hypoxia was
significantly inhibited, and the upregulation of SOX9 and collagen
II chain caused by hypoxia was also significantly inhibited.
Therefore, we hypothesized that the activation of YAP serves a key
role in the maintenance of the chondrogenic phenotype in hypoxia,
and is closely associated with the activation of HIF-1α.
Hypoxia may promote the HIF-1α-dependent activation
of YAP; this activation does not depend on Hippo signaling and is
reversible. Hypoxia promotes the maintenance of the chondrogenic
phenotype in a time- and HIF-1α-dependent manner. Inhibiting the
activation of YAP under hypoxia does not affect the HIF-1α
signaling pathway, but inhibits the maintenance of the chondrogenic
phenotype. Notably, the present study demonstrated that hypoxia
ultimately affects the maintenance of the chondrogenic phenotype in
growth plate chondrocytes through the interaction between HIF-1α
and YAP.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 51537004) and (grant
no. 81702155).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HL and XL performed the hypoxia intervention, drugs
treatments, RNA interference, western blot analysis and
immunofluorescence assays, and were major contributors in writing
the manuscript. XJ and JC performed the chondrocytes isolation and
culture. ML and YR performed the reverse transcription quantitative
polymerase chain reaction and analyzed the data. CY analyzed the
data FG and HW designed the experiments, revised the paper and
submitted the final versions. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Experimental
Animal Ethics Committee of Tongji Hospital, Tongji Medical College
of Huazhong University of Science and Technology (Wuhan,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
References
|
1
|
Makris EA, Gomoll AH, Malizos KN, Hu JC
and Athanasiou KA: Repair and tissue engineering techniques for
articular cartilage. Nat Rev Rheumatol. 11:21–34. 2015. View Article : Google Scholar :
|
|
2
|
Caron MM, Emans PJ, Coolsen MME, Voss L,
Surtel DAM, Cremers A, van Rhijn LW and Welting TJ:
Redifferentiation of dedifferentiated human articular chondrocytes:
Comparison of 2D and 3D cultures. Osteoarthr Cartilage.
20:1170–1178. 2012. View Article : Google Scholar
|
|
3
|
Duan L, Ma B, Liang Y, Chen J, Zhu W, Li M
and Wang D: Cytokine networking of chondrocyte dedifferentiation in
vitro and its implications for cell-based cartilage therapy. Am J
Transl Res. 7:194–208. 2015.PubMed/NCBI
|
|
4
|
Darling EM and Athanasiou KA: Rapid
phenotypic changes in passaged articular chondrocyte
subpopulations. J Orthop Res. 23:425–432. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Duval E, Leclercq S, Elissalde JM, Demoor
M, Galéra P and Boumédiene K: Hypoxia-inducible factor 1alpha
inhibits the fibroblast-like markers type I and type III collagen
during hypoxia-induced chondrocyte redifferentiation: Hypoxia not
only induces type II collagen and aggrecan, but it also inhibits
type I and type III collagen in the hypoxia-inducible factor
1alpha-dependent redifferentiation of chondrocytes. Arthritis
Rheum. 60:3038–3048. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schipani E, Ryan HE, Didrickson S,
Kobayashi T, Knight M and Johnson RS: Hypoxia in cartilage:
HIF-1alpha is essential for chondrocyte growth arrest and survival.
Gene Dev. 15:2865–2876. 2001.PubMed/NCBI
|
|
7
|
Brucker PU, Izzo NJ and Chu CR: Tonic
activation of hypoxia-inducible factor 1alpha in avascular
articular cartilage and implications for metabolic homeostasis.
Arthritis Rheum. 52:3181–3191. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schrobback K, Klein TJ, Crawford R, Upton
Z, Malda J and Leavesley DI: Effects of oxygen and culture system
on in vitro propagation and redifferentiation of osteoarthritic
human articular chondrocytes. Cell Tissue Res. 347:649–663. 2012.
View Article : Google Scholar
|
|
9
|
Mhanna R, Öztürk E, Schlink P and
Zenobi-Wong M: Probing the microenvironmental conditions for
induction of superficial zone protein expression. Osteoarthr
Cartilage. 21:1924–1932. 2013. View Article : Google Scholar
|
|
10
|
Mennan C, Garcia J, McCarthy H, Owen S,
Perry J, Wright K, Banerjee R, Richardson JB and Roberts S: Human
articular chondrocytes retain their phenotype in sustained hypoxia
while normoxia promotes their immunomodulatory potential.
Cartilage. Apr 1–2018.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Robins JC, Akeno N, Mukherjee A, Dalal RR,
Aronow BJ, Koopman P and Clemens TL: Hypoxia induces
chondrocyte-specific gene expression in mesenchymal cells in
association with transcriptional activation of Sox9. Bone.
37:313–322. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koay EJ and Athanasiou KA: Hypoxic
chondrogenic differentiation of human embryonic stem cells enhances
cartilage protein synthesis and biomechanical functionality.
Osteoarthr Cartilage. 16:1450–1456. 2008. View Article : Google Scholar
|
|
13
|
Kawato Y, Hirao M, Ebina K, Tamai N, Shi
K, Hashimoto J, Yoshikawa H and Myoui A: Nkx3.2-induced suppression
of Runx2 is a crucial mediator of hypoxia-dependent maintenance of
chondrocyte phenotypes. Biochem Biophys Res Commun. 416:205–210.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maes C, Carmeliet G and Schipani E:
Hypoxia-driven pathways in bone development, regeneration and
disease. Nat Rev Rheumatol. 8:358–366. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thoms BL, Dudek KA, Lafont JE and Murphy
CL: Hypoxia promotes the production and inhibits the destruction of
human articular cartilage. Arthritis Rheum. 65:1302–1312. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Markway BD, Cho H and Johnstone B: Hypoxia
promotes redifferentiation and suppresses markers of hypertrophy
and degeneration in both healthy and osteoarthritic chondrocytes.
Arthritis Res Ther. 15:R92. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Coyle CH, Izzo NJ and Chu CR: Sustained
hypoxia enhances chondrocyte matrix synthesis. J Orthop Res.
27:793–799. 2009. View Article : Google Scholar
|
|
18
|
Pfander D, Cramer T, Schipani E and
Johnson RS: HIF-1alpha controls extracellular matrix synthesis by
epiphyseal chondrocytes. J Cell Sci. 116:1819–1826. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Villemure I and Stokes IA: Growth plate
mechanics and mechanobiology. A survey of present understanding. J
Biomech. 42:1793–1803. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Heallen T, Morikawa Y, Leach J, Tao G,
Willerson JT, Johnson RL and Martin JF: Hippo signaling impedes
adult heart regeneration. Development. 140:4683–4690. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Halder G and Johnson RL: Hippo signaling:
Growth control and beyond. Development. 138:9–22. 2011. View Article : Google Scholar :
|
|
22
|
Yang B, Sun H, Song F, Yu M, Wu Y and Wang
J: YAP1 negatively regulates chondrocyte differentiation partly by
activating the β-catenin signaling pathway. Int J Biochem Cell
Biol. 87:104–113. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Loboda A, Jozkowicz A and Dulak J: HIF-1
versus HIF-2-is one more important than the other. Vasc Pharmacol.
56:245–251. 2012. View Article : Google Scholar
|
|
24
|
Gilkes DM, Xiang L, Lee SJ, Chaturvedi P,
Hubbi ME, Wirtz D and Semenza GL: Hypoxia-inducible factors mediate
coordinated RhoA-ROCK1 expression and signaling in breast cancer
cells. Proc Natl Acad Sci USA. 111:E384–E393. 2014. View Article : Google Scholar
|
|
25
|
Qi C, Zhang J, Chen X, Wan J, Wang J,
Zhang P and Liu Y: Hypoxia stimulates neural stem cell
proliferation by increasing HIF-1α expression and activating
Wnt/β-catenin signaling. Cell Mol Biol (Noisy-le-grand). 63:12–19.
2017. View Article : Google Scholar :
|
|
26
|
Zhang C, Li Y, Cornelia R, Swisher S and
Kim H: Regulation of VEGF expression by HIF-1α in the femoral head
cartilage following ischemia osteonecrosis. Sci Rep. 2:6502012.
View Article : Google Scholar
|
|
27
|
Lefebvre V and Smits P: Transcriptional
control of chondrocyte fate and differentiation. Birth Defects Res
C Embryo Today. 75:200–212. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saito T, Fukai A, Mabuchi A, Ikeda T, Yano
F, Ohba S, Nishida N, Akune T, Yoshimura N, Nakagawa T, et al:
Transcriptional regulation of endochondral ossification by
HIF-2alpha during skeletal growth and osteoarthritis development.
Nat Med. 16:678–686. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Amarilio R, Viukov SV, Sharir A,
Eshkar-Oren I, Johnson RS and Zelzer E: HIF1alpha regulation of
Sox9 is necessary to maintain differentiation of hypoxic
prechondrogenic cells during early skeletogenesis. Development.
134:3917–3928. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kosyna FK, Nagel M, Kluxen L, Kraushaar K
and Depping R: The importin α/β-specific inhibitor Ivermectin
affects HIF-dependent hypoxia response pathways. Biol Chem.
396:1357–1367. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Provot S, Zinyk D, Gunes Y, Kathri R, Le
Q, Kronenberg HM, Johnson RS, Longaker MT, Giaccia AJ and Schipani
E: Hif-1alpha regulates differentiation of limb bud mesenchyme and
joint development. J Cell Biol. 177:451–464. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Duval E, Baugé C, Andriamanalijaona R,
Bénateau H, Leclercq S, Dutoit S, Poulain L, Galéra P and
Boumédiene K: Molecular mechanism of hypoxia-induced chondrogenesis
and its application in in vivo cartilage tissue engineering.
Biomaterials. 33:6042–6051. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Morais-Faria K, Menegussi G, Marta G,
Fernandes PM, Dias RB, Ribeiro AC, Lopes MA, Cernea CR, Brandão TB
and Santos-Silva AR: Dosimetric distribution to the teeth of
patients with head and neck cancer who underwent radiotherapy. Oral
Surg Oral Med Oral Pathol Oral Radiol. 120:416–419. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao B, Tumaneng K and Guan KL: The Hippo
pathway in organ size control, tissue regeneration and stem cell
self-renewal. Nat Cell Biol. 13:877–883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu FX, Zhao B and Guan KL: Hippo pathway
in organ size control, tissue homeostasis, and cancer. Cell.
163:811–828. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Studer L, Csete M, Lee SH, Kabbani N,
Walikonis J, Wold B and McKay R: Enhanced proliferation, survival,
and dopaminergic differentiation of CNS precursors in lowered
oxygen. J Neurosci. 20:7377–7383. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ramos A and Camargo FD: The Hippo
signaling pathway and stem cell biology. Trends Cell Biol.
22:339–346. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim
J, Xie J, Ikenoue T, Yu J, Li L, et al: Inactivation of YAP
oncoprotein by the Hippo pathway is involved in cell contact
inhibition and tissue growth control. Gene Dev. 21:2747–2761. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu H, Jiang D, Chi F and Zhao B: The
Hippo pathway regulates stem cell proliferation, self-renewal, and
differentiation. Protein Cell. 3:291–304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dupont S, Morsut L, Aragona M, Enzo E,
Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M,
Bicciato S, et al: Role of YAP/TAZ in mechanotransduction. Nature.
474:179–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Azzolin L, Zanconato F, Bresolin S,
Forcato M, Basso G, Bicciato S, Cordenonsi M and Piccolo S: Role of
TAZ as mediator of Wnt signaling. Cell. 151:1443–1456. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Byun MR, Jeong H, Bae SJ, Kim AR, Hwang ES
and Hong JH: TAZ is required for the osteogenic and anti-adipogenic
activities of kaempferol. Bone. 50:364–372. 2012. View Article : Google Scholar
|
|
43
|
Deng Y, Wu A, Li P, Li G, Qin L, Song H
and Mak KK: Yap1 Regulates multiple steps of chondrocyte
differentiation during skeletal development and bone repair. Cell
Rep. 14:2224–2237. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ying J, Wang P, Zhang S, Xu T, Zhang L,
Dong R, Xu S, Tong P, Wu C and Jin H: Transforming growth
factor-beta1 promotes articular cartilage repair through canonical
Smad and Hippo pathways in bone mesenchymal stem cells. Life Sci.
192:84–90. 2018. View Article : Google Scholar
|
|
45
|
Chen Ma, Chen YB, Cheng L, Mu H, Li C, Gao
J, Zhou R, Cao C, Liu LJ, et al: Hypoxia regulates Hippo signalling
through the SIAH2 ubiquitin E3 ligase. Nat Cell Biol. 17:95–103.
2015.
|
|
46
|
Chen H, Chen Q and Luo Q: Expression of
netrin-1 by hypoxia contributes to the invasion and migration of
prostate carcinoma cells by regulating YAP activity. Exp Cell Res.
349:302–309. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yan L, Cai Q and Xu Y: Hypoxic conditions
differentially regulate TAZ and YAP in cancer cells. Arch Biochem
Biophys. 562:31–36. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang C, Bian M, Chen X, Jin H, Zhao S,
Yang X, Shao J, Chen A, Guo Q, Zhang F and Zheng S: Oroxylin A
prevents angiogenesis of LSECs in liver fibrosis via inhibition of
YAP/HIF-1α signaling. J Cell Biochem. 119:2258–2268. 2018.
View Article : Google Scholar
|




















