Introduction
Pneumonia is a major global public health concern.
Viral and bacterial pneumonias are major causes of mortality in
children, the elderly, the immunodeficient, and those with
comorbidities worldwide (1). It
is responsible for 120,000,000 episodes and a mortality rate of
1,000,000 each year in children <5 years old (2). Cytomegalovirus (CMV) is one of the
most common causes of lung infection in the host lung. Studies have
reported that CMV infection can cause acute respiratory distress
syndrome and severe lung infection (3). CMV is a species‑specific β-herpes
virus, which establishes life-long latent infections in 70-100% of
the world's population and is capable of causing diseases with high
morbidity and mortality rates in at-risk immunocompromised
populations (4,5). Following primary infection, although
typically asymptomatic during the latency period, CMV persists in
myeloid precursor cells and becomes latent in several organs,
particularly the lungs (6). It
can reactivate when there is a certain degree of immunosuppression.
Once the virus is reactivated, it can modulate the immune response
of the host through the accelerated expression of different genes
(7). Furthermore, under certain
conditions, the virus can affect several organs and systems,
including the lungs, liver, kidneys, salivary glands and mammary
glands, and eventually lead to serious diseases (8). When CMV damages an organ or system
of the host, it can cause several diseases, including CMV pneumonia
(9). Although CMV pneumonia can
generally be controlled by lifelong administration of antiviral
drugs, including ganciclovir, these drugs require frequent dosing
and can potentially cause harmful side-effects. Therefore,
developing novel therapies for pneumonia is urgently required.
Evodiamine (Evo), the structure of which is shown in
Fig. 1A, is an alkaloidal
component extracted from the fruit of Evodia rutaecarpa
(Juss) Benth. Previous studies have shown that Evo can attenuate
chronic high‑glucose challenge inflammatory injury (10), protect septic mice against acute
lung injury (11), restrain tumor
activity in several types of human cancer (12‑14), and improve
acute inflammatory cerebral ischemia (15). However, the effect of Evo on
severe pneumonia remains to be fully elucidated. Therefore, the
present study aimed to evaluate the roles and underlying mechanisms
of Evo on severe pneumonia induced by Methicillin-susceptible
Staphylococcus aureus (MSSA) following CMV reactivation
in vitro and in vivo.
Materials and methods
Reagents and materials
Evo was provided by the National Institute for the
Control of Pharmaceuticals and Biological Products (Beijing,
China). Evo was suspended in 100% dimethylsulfoxide. Enzyme-linked
immunosorbent assay (ELISA) kits were obtained from the Jiancheng
Bioengineering Institute of Nanjing (Nanjing, China. The antibodies
used were as follows: Antibodies against p65 (cat. no. ab16502;
Abcam, Cambridge, UK), Lamin A (cat. no. ab26300; Abcam),
phosphorylated (p)-inhibitor of NF-κBα (p-IκBα; cat. no. 8219; Cell
Signaling Technology, Inc., Danvers, MA, USA), IκBα (cat. no.
9242S; Cell Signaling Technology, Inc.), c-Jun N-terminal kinase
(JNK; cat. no. ab176662; Abcam), p-JNK (cat. no. ab207477; Abcam),
extracellular signal-regulated kinase (Erk; cat. no. ab176641;
Abcam), p-Erk (cat. no. ab192591; Abcam), p-p38 (cat. no. ab178867;
Abcam), p38 (cat. no. ab32142; Abcam)and β-actin (cat. no. ab8226;
Abcam).
Cells and strains
MSSA (ATCC 25923; American Type Culture Collection,
Manassas, VA, USA) strains producing staphylococcal pneumonia were
isolated from clinical samples, grown in Luria Bertani (LB) medium
and conserved at ‑80˚C in 50% LB broth and 50% glycerol. The
BEAS-2B cell line was purchased from the Shanghai Institute of Cell
Biology in the Chinese Academy of Sciences (Shanghai, China) and
cultivated in RPMI‑1640 (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The media were supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) and maintained in a humidified
incubator at 37˚C with 5% CO2. NIH3T3 cells were
purchased from Wuhan University Preservation Center (Wuhan, China).
The Murine CMV (MCMV) Smith strain (ATCC-VR-1399) was ordered from
the American Type culture Collection. The MCMV strain was
propagated on NIH3T3 cells and titrated on the mouse embryo
fibroblasts as described previously (16).
MTT assay
The BEAS-2B cells were cultured in RPMI-1640
supplemented with 10% FBS. The cells were stimulated for 24 h at
37˚C with different concentrations of Evo (20‑100 µM; 20, 40, 60,
80 and 100 µM). Subsequently, 20 µl of 5 mg/ml MTT solution was
added and the plate was incubated at 37˚C for 4 h. Following
aspiration and washing with PBS, 150 µl of DMSO was added to each
well. The absorbance was determined spectrophotometrically at 490
nm on an ELX800 UV universal microplate reader.
Infection of cells with MSSA
The BEAS-2B cells incubated with 1 mL of RPMI-1640
containing the MSSA with the indicated multiplicity of infection
(1×105 CFU) at 37°C in 5% CO2. After 3 h, the
suspension was aspirated, and the cells were washed with PBS and
incubated with Evo (10, 20 and 40 µm) for 24 h.
Animal model
Pathogen-free female BALB/c mice (n=90; 6 weeks old;
22-25 g) were supplied by the Animal Center of Soochow University
(Changzhou, China; certificate no. SCXK 2015-0004). The mice were
housed individually in steel microisolator cages at 22°C with a
12/12‑h light/dark cycle, and fed a standard laboratory diet and
water Animal welfare and experimental procedures and was approved
by the Animal Experimental Ethical Committee of Soochow
University.
The procedures of animal model establishment were
performed as previously described (16). Following anesthetization, the
BALB/c mice were infected by intra-peritoneal injection with
3×104 PFU MCMV Smith strain or saline. At 4 months
post-MCMV primary infection, all mice underwent cecal ligation and
puncture (CLP) to trigger viral reactivation in the MCMV groups.
Briefly, the mice were anesthetized under sodium pentobarbital
following cutaneous asepsis. A 5-mm incision in the lower-right
quadrant of the abdomen was introduced, and the cecum was localized
and ligatured 3 mm above its extremity using an 18G needle. The
abdomen was then sutured, and cutaneous asepsis was performed with
5% alcoholic povidone. After 24 h, the animals were administered
with an intraperitoneal injection of 0.01 mg/g of tramadol. At 14
days post-CLP, the surviving mice in all groups received an
intranasal inoculation with 50 µL 5×108 CFU MSSA
to induce severe pneumonia.
Experimental designs
The mice were randomly divided into five groups:
Control group (saline + CLP + MSSA); model group (MCMV + CLP +
MSSA); and Evo groups (MCMV + CLP + MSSA + 5, 10, or 20 mg/kg Evo).
Each group was divided into two sub-groups: 5 and 15 days.
Following intra-nasal inoculation with MSSA, the mice were
intragastrically administrated with different concentrations of Evo
(5, 10, and 20 mg/kg), which were selected according to the
preliminary experiments, whereas mice in the control and MCMV
groups received an equal volume of distilled water of 0.5%
Tween-80, once per day for a period of 15 days. In the 15-day
cohort, the daily weight was closely monitored every 5 days and
spontaneous mortality of the animals was noted during this period.
On days 5 and 15, all surviving mice were sacrificed with a lethal
dose of thiopental, and samples of blood and the lungs were
subjected to further analysis.
Histological analysis
The animals were sacrificed at 5 and 15 days
post-treatment. The lungs were removed and embedded in paraffin,
cut into 3 mm sections, and stained with hematoxylin and eosin.
Subsequently, pathological changes were evaluated under a light
microscope.
Bacterial counts in lung tissues
The lung tissues were crushed and homogenized in 1
ml of PBS to determine the lung bacterial count. Serial 10-fold
dilutions of the lung homogenates were cultured using a 5% horse
blood (BioMerieux, SA., Marcy-l'Etoile, France) agar plate at 37°C
under anaerobic conditions. Following bacterial culture,
identification of the colonies was performed by culture on Chapman
medium (Bertin Pharma (Montigny le Bretonneux, France) and
confirmed using matrix-assisted laser
desorption/ionisation-time-of-flight mass spectrometry.
ELISA analysis
The concentrations of cytokines in the cell culture
medium and mouse serum collected by cardiac puncture at 5 and 15
days post-infection with MSSA were determined by ELISA for mouse
tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-1β,
interferon (IFN)-α and IFN-γ following the manufacturer's protocol.
Serum was separated by centrifugation at 400 x g for 10 min at 4°C
and stored at −80°C. The 96-well microplates were read using an
ELX800 UV universal microplate reader at 450 nm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
Total RNA of the lung tissue was obtained using
TRIzol reagent. The reverse transcription reactions for mRNA were
performed using PrimeScript™ RT Master mix (Promega Corporation,
Madison, WI, USA). The RT-qPCR analysis was performed using Taq
polymerase (Takara Biotechnology Co., Ltd., Dalian, China)
consisting of a final volume of 20 µl, containing 2
µl cDNA, 10 µl SYBR-Green Mix, 4 µl primer mix
and 4 µl ddH2O (Takara Biotechnology Co., Ltd.). Primers
specific for the TNF-α, IL-6, IL-1β, glyco-protein-B (gB) gene and
β-actin are listed in Table I.
β-actin was selected as an internal control to normalize target
gene expression. Thermocycling conditions were as follows: 95°C for
5 min followed by 40 cycles of 95°C for 10 sec and 60°C for 30 sec,
then a melting curve analysis from 60°C to 95°C every 0.2°C for 1.5
min was obtained. The transcript amount was normalized to U6 and
β-actin and quantified using the 2−ΔΔCq method (17).
 | Table IOligonucleotide primers used for
reverse transcription-quantitative polymerase chain reaction
analysis. |
Table I
Oligonucleotide primers used for
reverse transcription-quantitative polymerase chain reaction
analysis.
| Name | Primer sequence
(5'-3') | Product size
(bp) |
|---|
| TNF-α | Forward:
CTTCTCATTCCTGCTTGTG | |
| Reverse:
ACTTGGTGGTTTGCTACG | 198 |
| IL-1β | Forward:
AGGCTCCGAGATGAACAA | |
| Reverse:
AAGGCATTAGAAACAGTCC | 464 |
| IL-6 | Forward:
TTCTTGGGACTGATGCTG | |
| Reverse:
CTGGCTTTGTCTTTCTTGTT | 380 |
| gB | Forward:
GTCGGCCATCTACGAGAGAC | |
| Reverse:
GACCAGCGGTCTCGAATAAC | 164 |
| β-actin | Forward:
AACAGTCCGCCTAGAAGGAC | |
| Reverse:
CGTTGACTACCGTAAAGACC | 281 |
Western blot analysis
The western blot analysis was performed as described
previously (15). Protein samples
were extracted from the cells or tissues with Protein Extraction
Reagent (Pierce; Thermo Fisher Scientific, Inc.). Subsequently, the
protein concentration was measured using a bicinchoninic acid
protein assay kit (Sangon Biotech Co., Ltd., Shanghai, China).
Total protein (20 µg) in each sample was separated using
SDS-PAGE (10% gel) and transferred onto a polyvinyli-dene
difluoride filter membrane. The membrane was blocked using 5%
non-fat milk at 25°C for 1 h, and then incubated with primary
antibodies overnight at 4°C. Antibodies against IκBα (cat. no.
9242S; 1:1,000; Cell Signaling Technology, Inc.), p-IκBα (cat. no.
8219; 1:1,000; Cell Signaling Technology, Inc.), NF-κB p65 (cat.
no. ab16502; 1:500; Abcam), JNK (cat. no. ab176662; 1:1,000;
Abcam), p-JNK (cat. no. ab207477; 1:1,000; Abcam), Erk (cat. no.
ab176641; 1:1,000; Abcam), p-Erk (cat. no. ab192591; 1:1,000;
Abcam), p38 (cat. no. ab32142; 1:1,000; Abcam), p-p38 (cat. no.
ab178867; 1:1,000; Abcam), β-actin (cat. no. ab8226; 1:3,000;
Abcam) and Lamin A (cat. no. ab26300; 1:3,000; Abcam) were used.
β-actin and Lamin A were used as internal controls. Then, the
membrane was incubated with anti-goat HRP-conjugated antibody (cat.
no. AR1017; 1:5,000; Boster Systems, Inc., Pleasanton, CA, USA) at
25°C for 2 h. Detection was executed with the Odyssey infrared
imaging system (LI-COR Biosciences, Lincoln, NE, USA). The
immunoreactive bands were detected by enhanced chemiluminescence
(Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Statistical analysis
Statistical analysis of data was performed with
GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA,
USA) One-way analysis of variance was used for multiple group
comparison; Student's t-test was performed when only two groups
were compared. Results were expressed as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
Evo inhibits inflammatory cytokines in
vitro
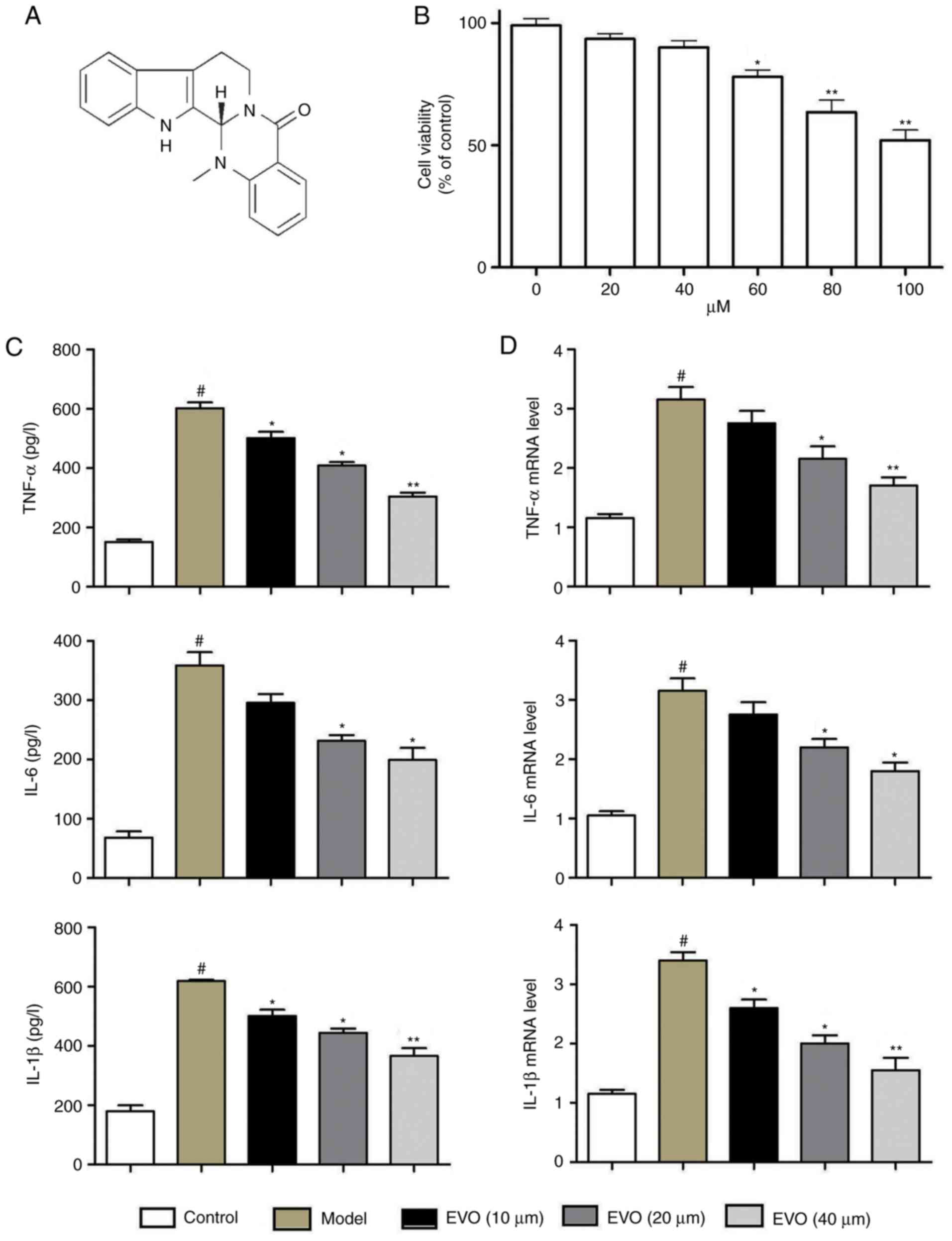
The results of the MTT assay showed that Evo had no
effect on the cell viability of BEAS-2B cells between
concentrations of 0 and 60 µM (Fig. 1B). Evo markedly decreased the
protein levels of TNF-α, IL-6 and IL-1β, whereas MSSA infection
increased these levels (Fig. 1C).
RT-qPCR analysis showed elevated mRNA expression levels of TNF-α,
IL-1β and IL-6 following MSSA infection, whereas Evo (20 and 40
µM) dose-dependently reduced the mRNA expression levels
(Fig. 1D).
Evo inhibits NF-κB and MAPK pathways in
vitro
As shown in Fig.
2A, MSAA infection markedly induced the nuclear translocation
of NF-κB/p65 and p-IκBα, whereas Evo led to marked repression of
these trends in a dose-dependent manner. In addition, Evo reduced
the phosphorylation of JNK, ERK and p38 MAPKs (Fig. 2B).
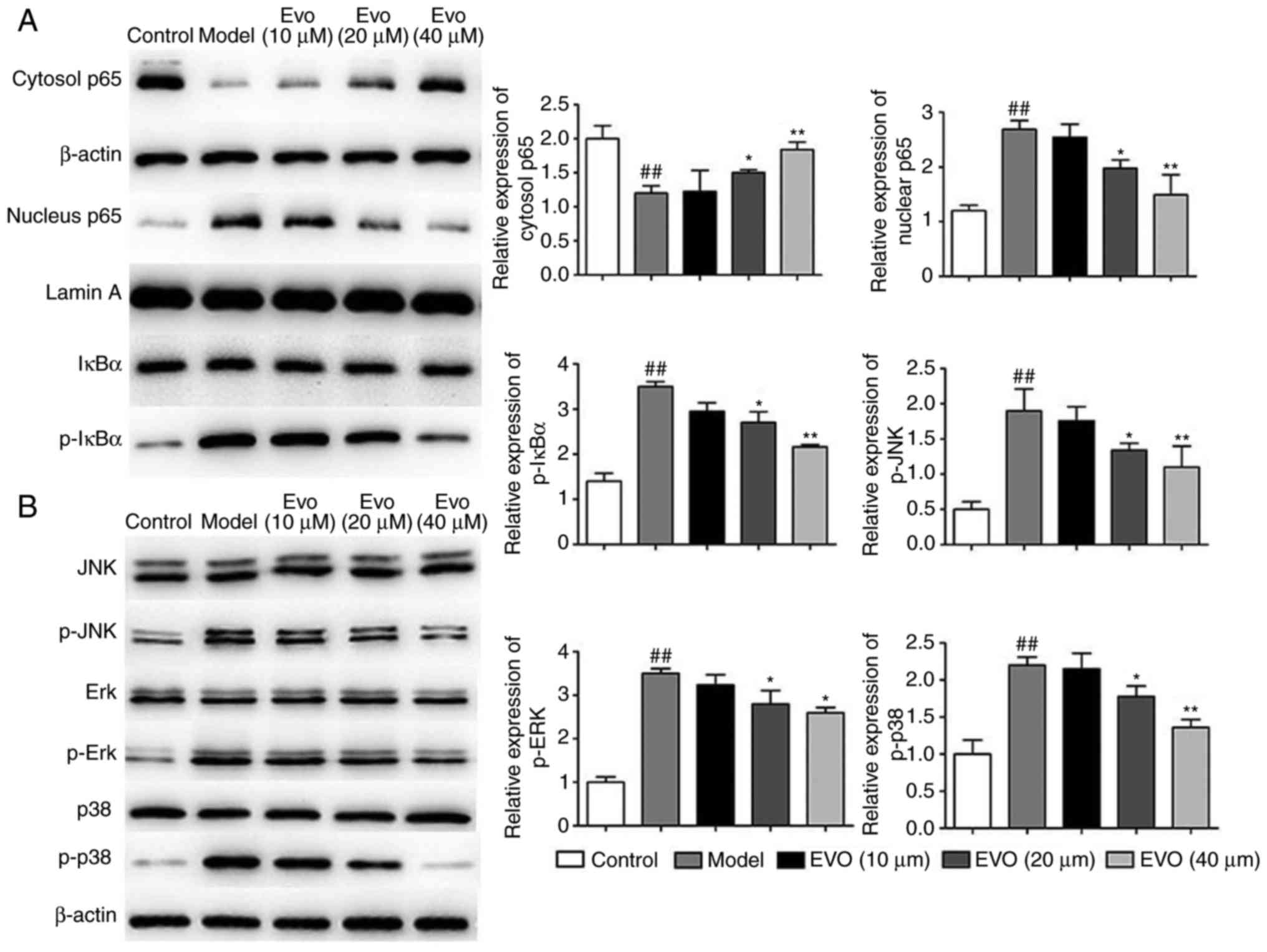 | Figure 2Evo inhibits NF-κB and MAPK pathways
in vitro. Effects of Evo on the expression of proteins of
the (A) NF-κB and (B) MAPK pathways in BEAS-2B cells infected with
MSSA. Data are shown as the mean ± standard deviation.
##P<0.01, vs. control group; *P<0.05
and **P<0.01, vs. MSSA group. Evo, evodiamine; MSSA,
methicillin-susceptible Staphylococcus aureus; NF-κB,
nuclear factor-κB; MAPK, mitogen-activated protein kinase; IκB,
inhibitor of NF-κB; JNK, c-Jun N-terminal kinase; Erk,
extracellular signal-regulated kinase; p-, phosphorylated. |
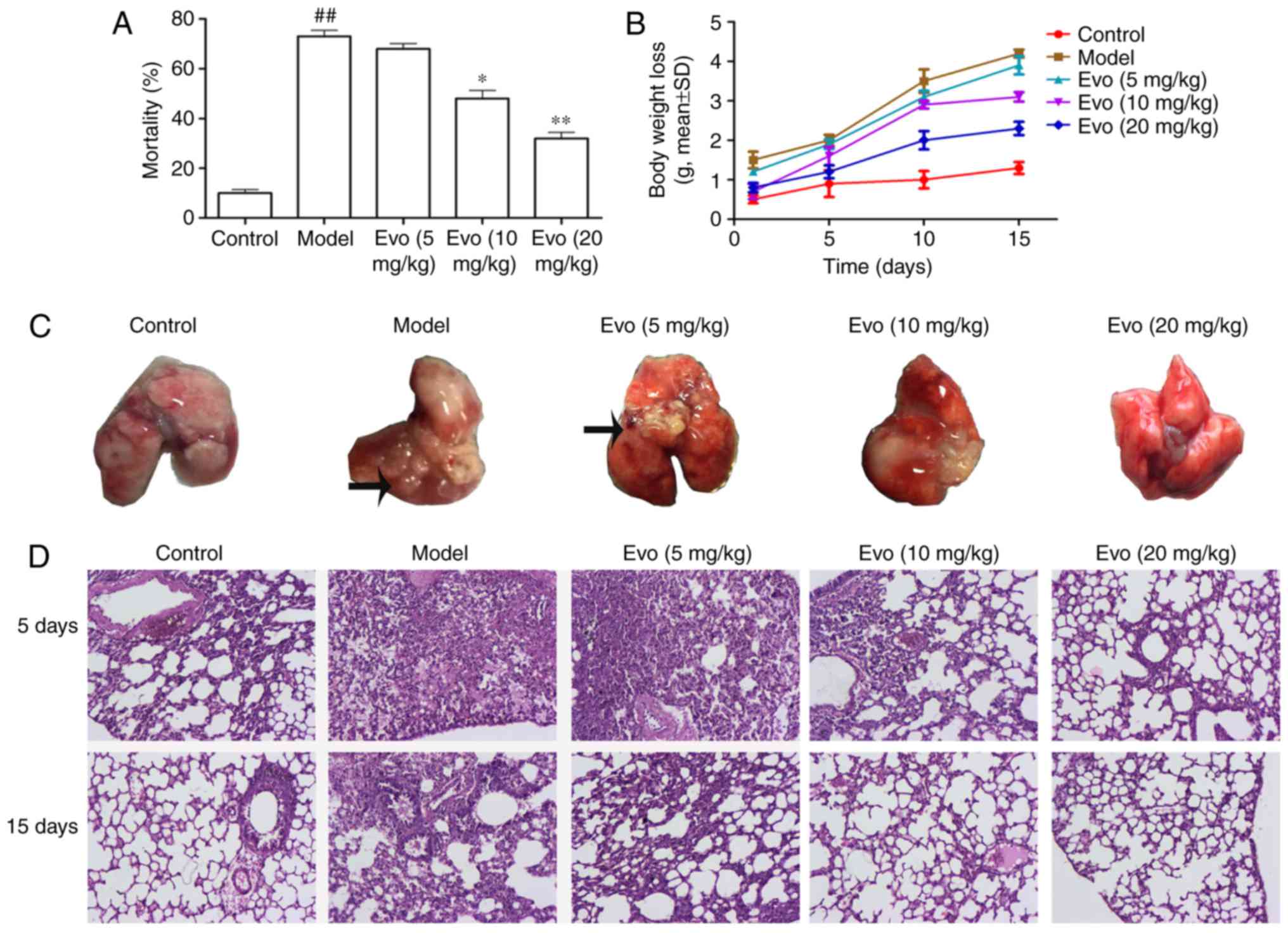
Evo reduces mortality rates
As shown in Fig.
3A, the accumulative mortality rates during 15 days with Evo
doses of 5, 10 and 20 mg/kg were 65, 44 and 29%, respectively,
whereas the mortality rate in the MCMV group was 73%. Evo (10 and
20 mg/kg) dose-dependently improved the survival rate, compared
with that in the MCMV groups.
Evo decreases body weight loss
Compared with the control group, the MCMV mice
showed a trend towards increased weight loss. The weight loss in
the Evo (10 and 20 mg/kg) groups were lower than that in the MCMV
group, whereas the weight loss in the Evo (5 mg/kg) group showed no
statistical significance (Fig.
3B).
Histological assessment
Morphological examination of the lungs from the MCMV
mice sacrificed on days 5 and 15 post-MSSA pneumonia revealed
voluminous right or left lung abscesses (Fig. 3C). Overall, 30.5% mice had
developed abscessing pneumonia in the MCMV group whereas none was
noted in the control group. Following the administration of Evo,
with the exception of the Evo (5 mg/kg) group, the lung abscesses
were largely improved.
As shown in Fig.
3D, no evident histological abnormalities were observed in the
control group. By contrast, the lung tissues of the MCMV group
exhibited severe pathologic changes, including infiltration of
inflammatory cells, alveolar wall thickening and alveolar
hemorrhage. However, these histopathological changes were
significantly reversed following treatment with Evo (10 and 20
mg/kg).
Evo inhibits the secretion of
inflammatory cytokines
As shown in Fig.
4, the levels of TNF-α, IL-1β and IL-6 were significantly
increased in the MCMV mice compared with those in the control
group. By contrast, with the exception of Evo (5 mg/kg),
pretreatment with Evo (10 and 20 mg/kg) efficiently reduced the
production of inflammatory cytokines (Fig. 4A-C). In addition, the levels of
IFN-α and IFN-γ in the Evo (10 and 20 mg/kg)-treated mice were
reduced in comparison with those in the MCMV group (Fig. 4D and E).
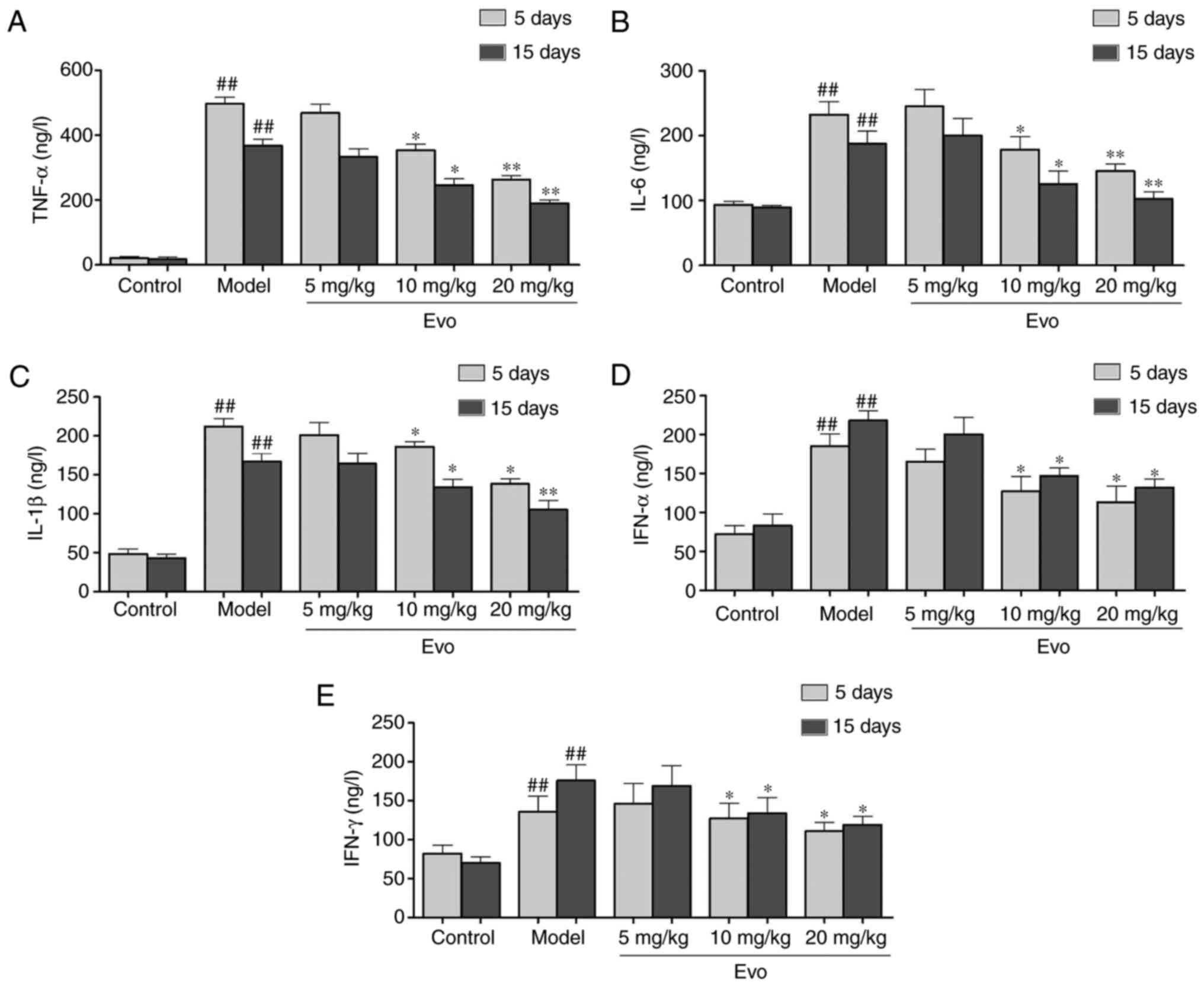 | Figure 4Evo inhibits the secretion of
inflammatory cytokines in mice. Evo decreased the secretion of
inflammatory cytokines in serum of mice with staphylococcal
pneumonia following cytomegalovirus reactivation. The levels of (A)
TNF-α, (B) IL-1β, (C) IL-6, (D) IFN-α and (E) IFN-γ were detected
by enzyme-linked immunosorbent assay. Data are shown as the mean ±
standard deviation. #P<0.05 and
##P<0.01, vs. control group; *P<0.05
and **P<0.01, vs. Model group. Evo, evodiamine; MSSA,
methicillin-susceptible Staphylococcus aureus; TNF-α, tumor
necrosis factor-α; IL, interleukin; IFN, interferon. |
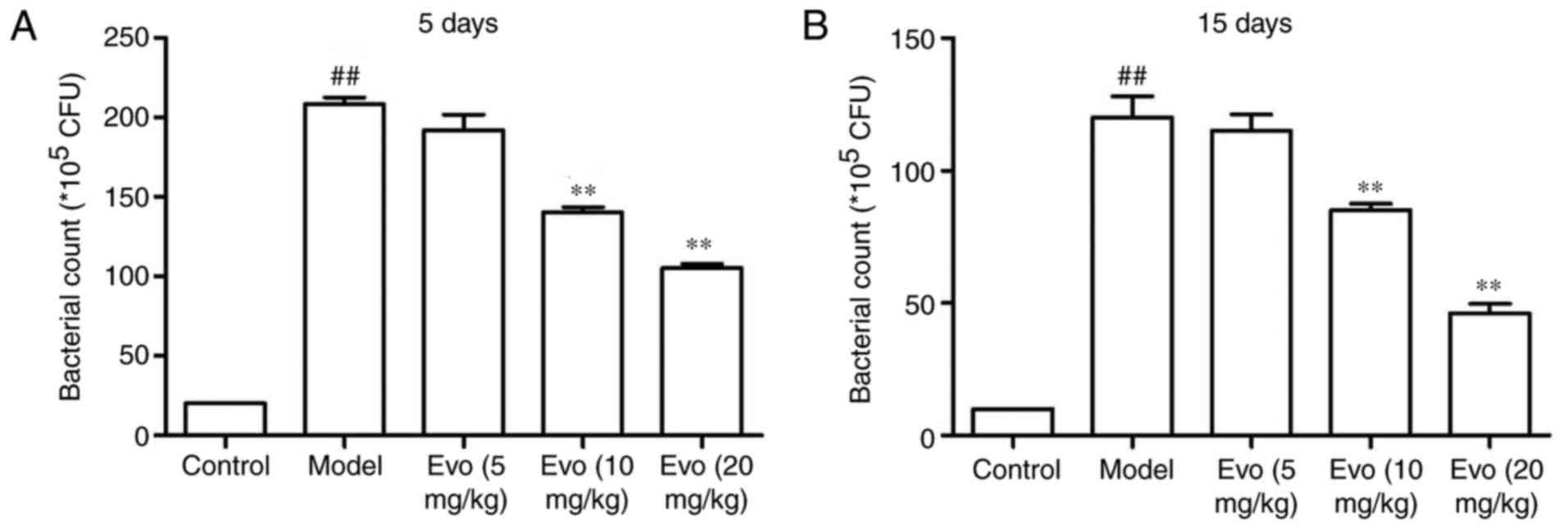
Evo decreases lung bacteria count
As shown in Fig.
5, the lung bacterial count of mice at 5 days post-MSSA
pneumonia was higher in the MCMV group. Treatment with Evo (10 and
20 mg/kg) facilitated bacterial clearance (Fig. 5A). On day 15, the lung bacterial
counts of the Evo groups were also significantly decreased
(Fig. 5B).
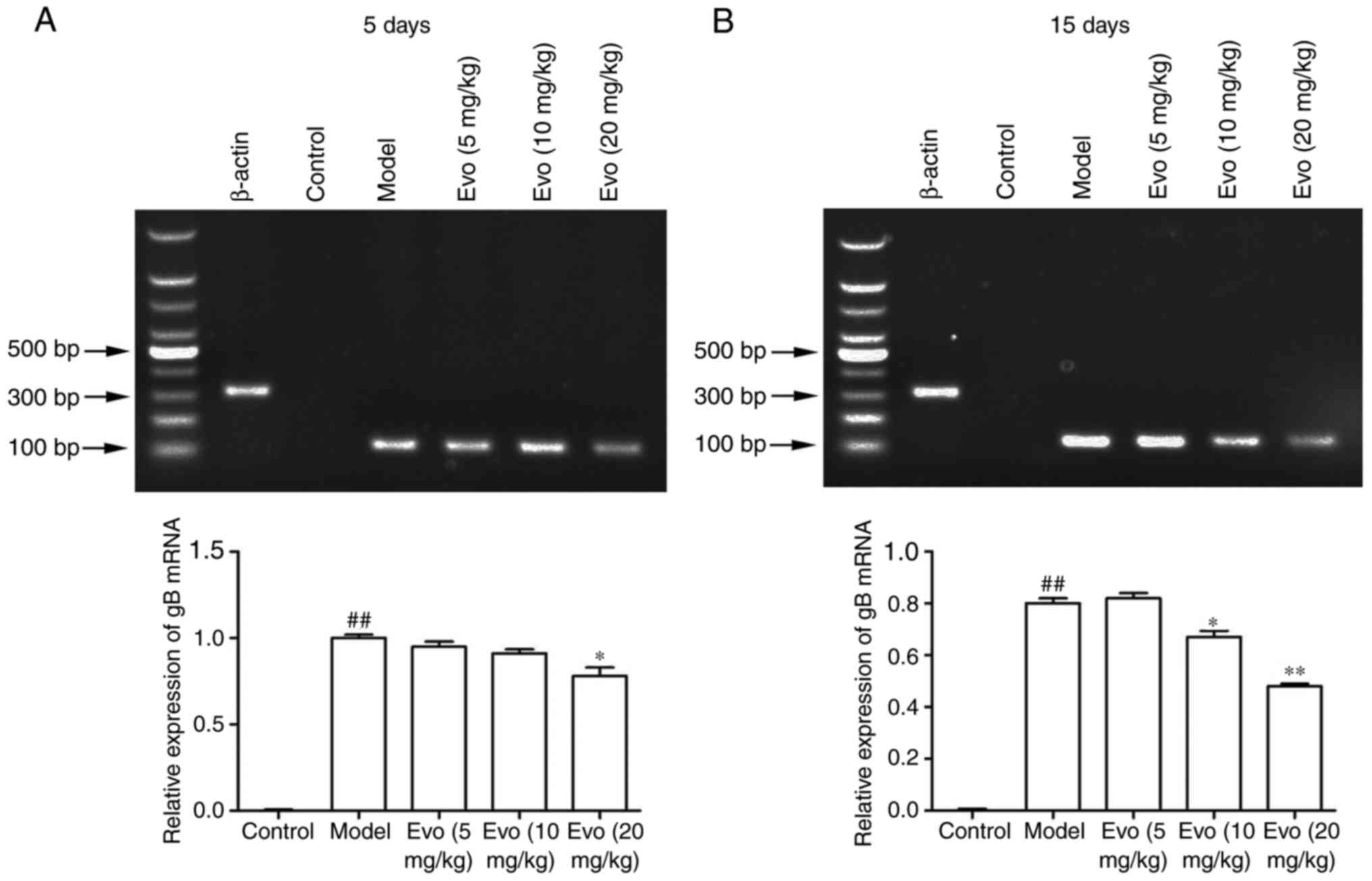
Evo inhibits the mRNA expression of
gB
The mRNA levels of gB in the lung tissues were
determined using RT-qPCR analysis to confirm whether Evo inhibited
staphylococcal pneumonia following CMV reactivation. As shown in
Fig. 6A and B, the mRNA content
of the gB gene in the MCMV group was significantly increased,
whereas no virus was detected in the control group. By contrast,
Evo (10 and 20 mg/kg) at 15 days post-infection had effectively
inhibited the amplification of the gB gene.
Evo suppresses the MAPK/NF-κB
pathways
MCMV stimulation induced the levels of p-IκBα in the
cytoplasm and of p65 in the nucleus. By contrast, Evo (10 and 20
mg/kg) markedly inhibited the p-IκBα and the nuclear translocation
of p65NF-κB (Fig. 7A). Similarly,
with the exception of the 5 mg/kg concentration of Evo, treatment
with Evo (10 and 20 mg/kg) efficiently reduced the levels of p-p38,
p-JNK and p-ERK in a dose-dependent manner (Fig. 7B).
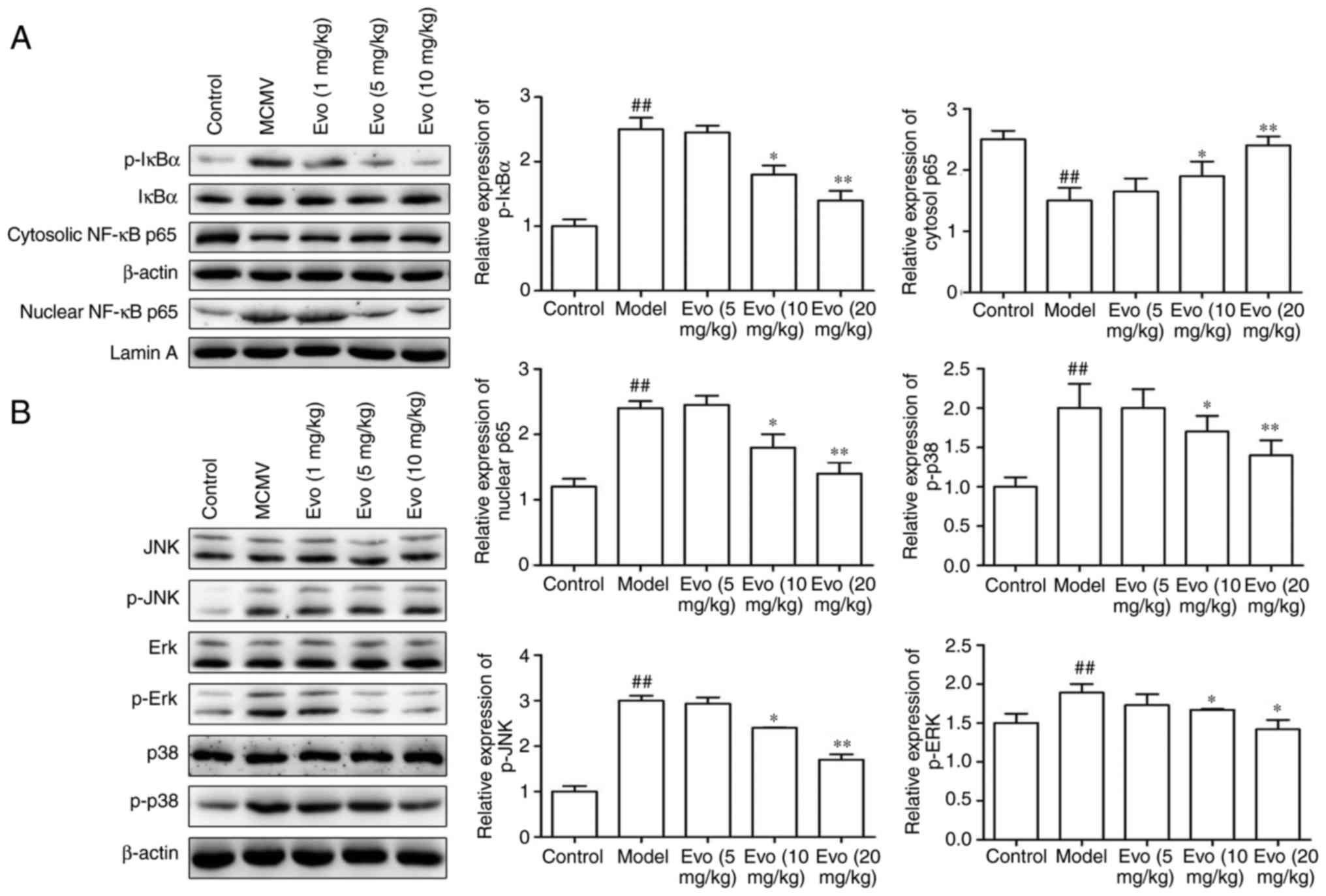 | Figure 7Evo suppresses NF-κB/MAPK pathways.
Evo suppressed the expression of NF-κB and MAPK signaling pathways
in mice with staphylococcal pneumonia following cytomegalovirus
reactivation. (A) Expression levels of p-IκBα, IκBα, and nuclear
and cytosolic p65NF-κB; and the (B) phosphorylation of ERK, JNK and
p38 were detected by western blot analysis. The graph shows the
quantification of results normalized to β-actin or Lamin A levels.
Data are shown as the mean ± standard deviation.
##P<0.01, vs. control group; *P<0.05
and **P<0.01, vs. MCMV/Model group. Evo, evodiamine;
MCMV, mouse cytomegalovirus; MAPK, mitogen-activated protein
kinase; NF-κB, nuclear factor-κB; IκB, inhibitor of NF-κB; JNK,
c-Jun N-terminal kinase; ERK, extracellular signal-regulated
kinase; p-, phosphorylated. |
Discussion
Evo is a botanical alkaloid component extracted from
the traditional Chinese medicinal herb Wu-Zhu-Yu (Evodiae fructus)
and has various pharmacological properties. A patent review
indicated the anticancer, antidiabetic and anti-inflammatory
properties of Evo and also showed that relevant clinical studies of
Evo possess important market potential for the development of based
therapeutics (18). Evo also
effectively suppresses inflammation by repressing inflammatory
cytokine expression and ameliorating the abnormal state in the lung
by inactivating NF-κB and p-IκBα (19). IL-1β often acts synergistically
with TNF-α and IL-6, producing a variety of chemotactic cytokines
which are involved in the inflammatory cytokine cascade (20). The increased levels of IL-1β, IL-6
and TNF-α can facilitate immunosuppression, which will promote
secondary bacterial infection (21). In addition, IFN-α and IFN-γ have
also been characterized in the immune response in post-viral
immunosuppression and susceptibility to bacteria (22). In the present study, Evo
dose-dependently reduced the protein and mRNA expression levels of
TNF-α, IL-6 IL-1β, IFN-α and IFN-γ in vitro and in
vivo. Lung bacterial count was higher in MCMV mice exposed to
S. aureus inhalation, which suggested an impairment of lung
bacterial clearance following reactivation of CMV, whereas Evo
reversed this effect. Therefore the preventive effect of Evo on
tissue destruction in the host may minimize disturbance of the host
defense system. CMV gB encodes the viral envelope glycoprotein,
which is closely associated with virus adsorption and fusion during
the viral proliferation cycle. When the gB gene transcript
disappears in the host body, MCMV can no longer proliferate
(23). The RT-qPCR analysis
showed that Evo alleviated staphylococcal pneumonia by suppressing
amplification of the gB gene. These findings suggested that the
protective effects of Evo on staphylococcal pneumonia were partly
attributed to the suppressed modification of the host's immunity
following MCMV reactivation.
The NF-κB and MAPK pathways have fundamental roles
in controlling the induction and regulation of inflammatory and
immune responses. The activation of NF-κB can regulate the
expression of the specific target gene of pro-inflammatory
cytokines, and are also present in several diseases, including
asthma, septic shock and lung fibrosis (24,25). MAPKs are closely associated with
the expression of inflammatory cytokines and chemokines upon
pathogen challenge. The phosphorylation of ERK, JNK and p38 MAPKs
can also activate NF-Κb (26).
Previous studies have suggested that Evo inhibits the migration and
invasiveness of NPC cells accounting for the inactivation of NF-κB
and MAPKs (27). In the present
study, Evo inhibited p-IκBα, and the nuclear translocation of NF-κB
p65, p-p38, p-JNK and p-ERK MAPKs, which suggested that the
inhibitory effect of Evo on staphylococcal pneumonia may be
associated with inactivation of the NF-κB and MAPK signaling
pathways.
In conclusion, the present study demonstrated that
Evo alleviated severe pneumonia inoculated with S. aureus
following CMV reactivation by inactivating the NF-κB and MAPK
pathways, and these results may facilitate further investigation of
its potential clinical applications for pneumonia.
Funding
No funding was received.
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
HL contributed to the design of the study. XC and SZ
contributed to the acquisition, collection and assembly of the
data. XC contributed to the statistical analysis. XC wrote the main
manuscript text. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Animal welfare and experimental procedures were
approved by the Animal Experimental Ethical Committee of Soochow
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Fraser CS, Jha A and Openshaw PJ: Vaccines
in the prevention of viral pneumonia. Clin Chest Med. 38:155–169.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rudan I, O'Brien KL, Nair H, Liu L,
Theodoratou E, Qazi S, Lukšić I, Fischer Walker CL, Black RE and
Campbell H; Child Health Epidemiology Reference Group (CHERG):
Epidemiology and etiology of childhood pneumonia in 2010: Estimates
of incidence, severe morbidity, mortality, underlying risk factors
and causative pathogens for 192 countries. J Glob Health.
3:0104012013.PubMed/NCBI
|
|
3
|
Choi J, Kim YS, Kim MS, Callaway Z, Youn
UK, Kim HB and Kim CK: Acute respiratory distress syndrome by
cytomegalovirus infection in an immunocompetent infant. Pediatr
Pulmonol. 43:824–827. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanley PJ: Helping the immune system take
the lead. Viruses. 6:2242–2258. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu L, Oza S, Hogan D, Perin J, Rudan I,
Lawn JE, Cousens S, Mathers C and Black RE: Global, regional, and
national causes of child mortality in 2000-13 with projections to
inform post-2015 priorities: An updated systematic analysis.
Lancet. 385:430–440. 2015. View Article : Google Scholar
|
|
6
|
Casiraghi C, Shanina I, Cho S, Freeman ML,
Blackman MA and Horwitz MS: Gammaherpesvirus latency accentuates
EAE pathogenesis: Relevance to Epstein-Barr virus and multiple
sclerosis. PLoS Pathog. 8:e10027152012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sinclair J: Human cytomegalovirus: Latency
and reactivation in the myeloid lineage. J Clin Virol. 41:180–185.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hsiao NY, Zampoli M, Morrow B, Zar HJ and
Hardie D: Cytomegalovirus viraemia in HIV exposed and infected
infants: Prevalence and clinical utility for diagnosing CMV
pneumonia. J Clin Virol. 58:74–78. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ascherio A and Munger KL: Environmental
risk factors for multiple sclerosis. Part I: he role of infection.
Ann Neurol. 61:288–299. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xue Lv, Li YQ, Zou G, Zhang L, Ying X,
Wang M, Guo S, Gao L, Li YG, et al: Beneficial effects of
evodiamine on P2X(4)-mediated inflammatory injury of human
umbilical vein endothelial cells due to high glucose. Int
Immunopharmacol. 28:1044–1049. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ying Z, Xia F, Xue Y, Huaping L and Yan L:
Evodiamine protects septic mice against acute lung injury. J Third
Military Med Univ. 21:2309–2314. 2016.
|
|
12
|
Zhang C, Fan X, Xu X, Yang X, Wang X and
Liang HP: Evodiamine induces caspase-dependent apoptosis and S
phase arrest in human colon lovo cells. Anticancer Drugs.
21:766–776. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang C, Li S and Wang MW:
Evodiamine-induced human melanoma A375-S2 cell death was mediated
by PI3K/Akt/caspase and Fas-L/NF-kappa B signaling pathways and
augmented by ubiquitin-proteasome inhibition. Toxicol In Vitro.
24:898–904. 2010. View Article : Google Scholar
|
|
14
|
Yang J, Cai X, Lu W, Hu C, Xu X, Yu Q and
Cao P: Evodiamine inhibits STAT3 signaling by inducing phosphatase
shatterproof 1 in hepatocellular carcinoma cells. Cancer Lett.
328:243–251. 2013. View Article : Google Scholar
|
|
15
|
Zhao T: Neuroprotection of eodiamine in
the cerebral ischemia: Up-regulated pAkt, p GSK, claudin-5 and
down-regulated NF-κB expression. Hebei Medical University; CLC: pp.
R962013, https://www.dissertationtopic.net/doc/1843193.
|
|
16
|
Hraiech S, Bordes J, Mège JL, de
Lamballerie X, Charrel R, Bechah Y, Pastorino B, Guervilly C and
Forel JM: Cytomegalovirus reactivation enhances the virulence of
Staphylococcus aureus pneumonia in a mouse model. Clin Microbiol
Infect. 23:38–45. 2017. View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔC T method. Methods. 25:402–408.
2001. View Article : Google Scholar
|
|
18
|
Gavaraskar K, Dhulap S and Hirwani RR:
Therapeutic and cosmetic applications of Evodiamine and its
derivatives-A patent review. Fitoterapia. 106:22–35. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fan X, Zhu JY, Sun Y, Luo L, Yan J, Yang
X, Yu J, Tang WQ, Ma W and Liang HP: Evodiamine inhibits
Zymosan-induced inflammation in vitro and in vivo: Inactivation of
NF-κB by inhibiting IκBα phosphorylation. Inflammation.
40:1012–1027. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chi G, Wei M, Xie X, Soromou LW, Liu F and
Zhao S: Suppression of MAPK and NF-κB pathways by limonene
contributes to attenuation of lipopolysaccharide-induced
inflammatory responses in acute lung injury. Inflammation.
36:501–511. 2013. View Article : Google Scholar
|
|
21
|
Hraiech S, Papazian L, Rolain JM and
Bregeon F: Animal models of polymicrobial pneumonia. Drug Des Devel
Ther. 9:3279–3292. 2015.PubMed/NCBI
|
|
22
|
Shahangian A, Chow EK, Tian X, Kang JR,
Ghaffari A, Liu SY, Belperio JA, Cheng G and Deng JC: Type I IFNs
mediate development of postinfluenza bacterial pneumonia in mice. J
Clin Invest. 119:1910–20. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zavala-Vega S, Castro-Escarpulli G,
Hernández-Santos H, Salinas-Lara C, Palma I, Mejía-Aranguré JM,
Gelista-Herrera N, Rembao-Bojorquez D, Ochoa SA, Cruz-Córdova A, et
al: An overview of the infection of CMV, HSV 1/2 and EBV in Mexican
patients with glioblastoma multiforme. Pathol Res Pract.
213:271–276. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim J, Woo J, Lyu JH, Song HH, Jeong HS,
Ha KT, Choi JY, Han CW, Ahn KS, Oh SR, et al: Carthami Flos
suppresses neutrophilic lung inflammation in mice, for which
nuclear factor-erythroid 2-related factor-1 is required.
Phytomedicine. 21:470–478. 2014. View Article : Google Scholar
|
|
25
|
DiDonato JA, Mercurio F and Karin M: NF-κB
as a critical link between inflammation and cancer. Immunol Rev.
246:379–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim HI, Hong SH, Ku JM, Kang S, Kim TY,
Shin YC and Ko SG: Tonggyu-tang, a traditional Korean medicine,
suppresses pro-inflammatory cytokine production through inhibition
of MAPK and NF-κB activation in human mast cells and keratinocytes.
BMC Complement Altern Med. 17:1862017. View Article : Google Scholar
|
|
27
|
Takada Y, Kobayashi Y and Aggarwal BB:
Evodiamine abolishes constitutive and inducible NF-kappaB
activation by inhibiting IkappaBalpha kinase activation, thereby
suppressing NF-kappaB-regulated antiapoptotic and metastatic gene
expression, up-regulating apoptosis, and inhibiting invasion. J
Biol Chem. 280:17203–17212. 2005. View Article : Google Scholar : PubMed/NCBI
|