Introduction
Cervical cancer, also known as invasive cervical
cancer, is the most common gynecological malignancy, and its
incidence rate is second only to breast cancer (1). Although screening, surgery,
radiotherapy, and other treatments have improved prognoses for
early cervical cancer, metastatic and recurrent cases are difficult
to eradicate (2). Therefore,
elucidating the molecular mechanism of cervical cancer invasion and
metastasis has important scientific significance to improve
prognosis for patients.
Serum response factor (SRF), a widely expressed
transcription factor, belongs to the MADS-box gene family (3,4).
SRF regulates cytoskeleton and cell motility, as well as gene
expression of immediate early genes, muscle-related genes, and
adhesion-related genes (5-8).
SRF overexpression promotes tumor cell invasion and metastasis
(9). It is associated with
downregulation of E-cadherin expression and upregulation of
N-cadherin expression in epithelial-mesenchymal transition (EMT) in
gastric, peritoneal mesothelial, liver, and prostate cancer cells
(10-12). EMT is a crucial process of tumor
cell invasion and metastasis, and the loss of the polarity of
epithelial cells and migration capacity are important features
(13,14). The most important hallmark of EMT
is decreased E-cadherin expression. Additionally, N-cadherin is
upregulated in EMT and may promote tumor cell migration (15). Currently, the role of SRF in the
proliferation and invasion of cervical carcinoma is unclear. The
present study aimed to investigate the molecular mechanism of SRF
in cervical cancer, and this knowledge could be useful in the
future to improve treatment of the disease.
Early growth response-1 (Egr-1), a member of the
zinc finger transcription factor family, acts as an early growth
response gene (16,17). Egr-1 exerts a variety of
biological functions that control synaptic plasticity, wound
healing, cell growth, and apoptosis (18,19). The biological function of Egr-1 is
associated with the development of human cancer. The absence or
increase of abnormally expressed Egr-1 in tumors may be an
important cause of tumorigenesis (20). Egr-1 acts as a tumor suppressor in
lung cancer and liver cancer, and as a cancer-promoting gene in
gastric carcinoma (19,21,22). The role of Egr-1 in EMT of
cervical cancer remains unknown.
SRF reportedly activates Egr-1 expression (23). Therefore, it was hypothesized that
SRF could affect proliferation and invasion in cervical cancer by
regulating Egr-1 and EMT. The present study investigated the
molecular mechanism of SRF in cervical cancer by measuring SRF
expression in cervical cancer cell lines. Cell proliferation and
invasion were examined in cervical cancer cell lines following SRF
knockdown, and the molecular mechanism underlying the effect of SRF
was explored.
Materials and methods
Cells and tissues
The cervical cancer cell lines ME-180 and HeLa
(American Tissue Type Collection, Manassas, VA, USA) were grown in
Dulbecco’s modified Eagle’s medium supplemented with 10% fetal
bovine serum (both from HyClone; GE Healthcare Life Sciences,
Logan, UT, USA), 100 U/ml of penicillin, and 100 μg/ml of
streptomycin. Human cervical epithelial HCerEpic cells (ScienCell
Research Laboratories, Carlsbad, CA, USA) were cultured in cervical
epithelial cell medium (ScienCell Research Laboratories). All cells
were cultured in an atmosphere of 5% CO2 at 37°C.
Cervical tumor samples (n=10) were collected from
cervical cancerous area of patients in the Henan University of
Chinese Medicine (Zhengzhou, China) undergoing hysterectomies
without radiotherapy or chemotherapy. Normal tissues (n=10) were
collected from patients undergoing surgery for myoma or adenomyoma.
Normal tissue samples were nonmalignant and negative for human
papilloma virus and ThinPrep cytological tests. All patients in the
present study were 40-50 years old. Samples were collected under a
protocol approved by the Institutional Review Board of the Henan
University of Chinese Medicine (Zhengzhou, China) between December
2016 and August 2017. Informed consent was obtained from the
patients.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
Total RNA from cells or tissues was extracted by
using TRIzol (Takara Biotechnology Co., Ltd., Dalian, China) and 5
μg RNA was synthesized into cDNA with a Revert Aid First
Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc., Waltham,
MA, USA) according to the manufacturer’s protocol.
The qPCR reaction (20 μl) contained 10
μl SYBR Premix Ex Taq II (Takara Biotechnology Co., Ltd.) as
the enzyme mixture. The reaction program was as follows: 94°C for
30 sec, 40 cycles at 94°C for 10 sec, 60°C for 30 sec, and 72°C for
30 sec. Primers were as follows: SRF, forward, 5′-TTC AAG GTA GAG
AAG ACT GGT TT-3′ and reverse, 5′-CTG ACC CCC ATT CCT GTG TC-3′;
GAPDH, forward, 5′-GGA AGA TGG TGA TGG GAT T-3′ and reverse, 5′-GGA
TTT GGT CGT ATT GGG-3′; and Egr-1, forward, 5′-AGC CCT ACG AGC ACC
TGA C-3′ and reverse, 5′-GGT TTG GCT GGG GTA ACT G-3′. The relative
levels of gene expression were estimated using the
2−ΔΔCq method (24).
Western blot analysis
Protein was extracted with RIPA lysis buffer and was
quantified with a BCA kit (both from Beyotime Institute of
Biotechnology, Haimen, China). Total protein samples (25 μg)
were separated by 12% SDS-PAGE and transferred onto polyvinylidene
fluoride membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
by elec-trophoretic transfer. The membrane was then incubated with
5% skim milk for 2 h, followed by incubation overnight at 4°C with
the following primary antibodies (Cell Signaling Technology, Inc.,
Danvers, MA, USA): anti-SRF (1:500; cat. no. 5147), anti-Egr-1
(1:500; cat. no. 4154), anti-E-cadherin (1:800; cat. no. 3195),
anti-N-cadherin (1:800; cat. no. 13116), and anti-GAPDH (1:500;
cat. no. 5174). Next, the membrane was incubated with secondary
antibody (1:800; cat. no. 7074, Cell Signaling Technology, Inc.)
diluted in the blocking buffer. Finally, the protein was detected
using enhanced chemiluminescence. GAPDH was used as the protein
loading control. Optical density of the bands was measured with the
BandScan imaging analysis system (Glyko Inc., Hayward, CA,
USA).
Construction of the recombinant plasmids
and cell transfection
The full-length cDNA of Egr-1 (accession no.,
NM_001964) was amplified by RT-PCR and incubated with EcoRI
and BamHI enzymes. Subsequently, pcDNA.3.1/myc-His(-)
Avector (Invitrogen; Thermo Fisher Scientific, Inc.) was also
incubated with those two enzymes and the Egr-1 fragment was
inserted at the EcoRI and BamHI restriction sites.
The recombinant plasmid was then amplified in DH5 Escherichia
coli-competent cells (Takara Biotechnology Co., Ltd.), followed
by extraction using a Takara MiniBEST Plasmid Purification kit
version 4.0. Finally, the plasmid was sequenced, and the correct
ones were selected as pcDNA.3.1-Egr-1.
Cell transfection was as follows: ME-180 and HeLa
cells were separately cultured in 96-well plates under a humid
atmosphere with 5% CO2 at 37°C. The transfection program
was based on TurboFect (Thermo Fisher Scientific, Inc.) and
followed the manufacturer protocol. Cell transfection was conducted
when cells are at ~80% confluency. The pcDNA.3.1-Egr-1 (0.3
μg), pcDNA.3.1 (0.3 μg), SRF small interfering (si)
RNA (5′-AAC CAC CCG CCA CTC TTC CT-3′, (0.3 μg), and
non-specific siRNA (5′-ATT CAC CGA CTA TCC AAC AT-3′, 0.3
μg) were transfected separately with 2 μl of
TurboFect. The cells were then cultured in 5% CO2 at
37°C for 24 h. Transfection efficiency was measured by RT-qPCR and
western blot assays.
Cell viability assay
MTT was used to detect cell viability. Cells were
cultured in 96-well plates at 37°C for 24 h. The culture medium was
then changed to PBS containing MTT (20 μl/well) and
incubated for another 4.5 h at 37°C. Afterwards, dimethyl sulfoxide
(150 μl/well) was added to dissolve the formazan. Results
were measured using a microplate reader (Thermo Fisher Scientific,
Inc.) at 490 nm.
Bromodeoxyuridine (BrdU) assay
Cell proliferation was assessed using a BrdU kit
(EMD Biosciences, Inc., Darmstadt, Germany) according to the
specification sheets. Briefly, cells were cultured in a 96-well
plate followed by 1 h of incubation with 10 μl of BrdU
solution per well. After discarding the culture medium, a
denaturing solution (180 μl) was added and incubated for 35
min. Cells were then incubated with an anti-BrdU antibody
conjugated with peroxidase for 30 min. The results were measured at
450 nm using a SpectroFluor Plus multi-well plate reader (Tecan
Group Ltd., Mannedorf, Switzerland).
Cell invasion
Cell invasion ability was tested using Bio-Coat cell
migration chambers (Corning Incorporated, Toledo, NY, USA) coated
with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA).
Transfected cells (1×105 cells) were suspended in 200
μl of serum-free medium and then plated in the upper
chamber. Complete medium (300 μl) was added to the lower
chamber and incubated at 37°C for 48 h. Non-invading cells were
gently removed with a cotton swab from the upper chambers. Invaded
cells were fixed, stained, and observed using a light microscope.
Ten visual fields in each membrane were randomly selected for cell
number counting.
Statistical analysis
Statistical analyses were processed with SPSS
version 22.0 software (IBM Corporation, Armonk, NY, USA).
Differences between multiple groups were evaluated with one-way
analysis of variance followed by a Bonferroni test. Differences
between normal and tumor samples were evaluated with the
Mann-Whitney’s U test. Data are expressed as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
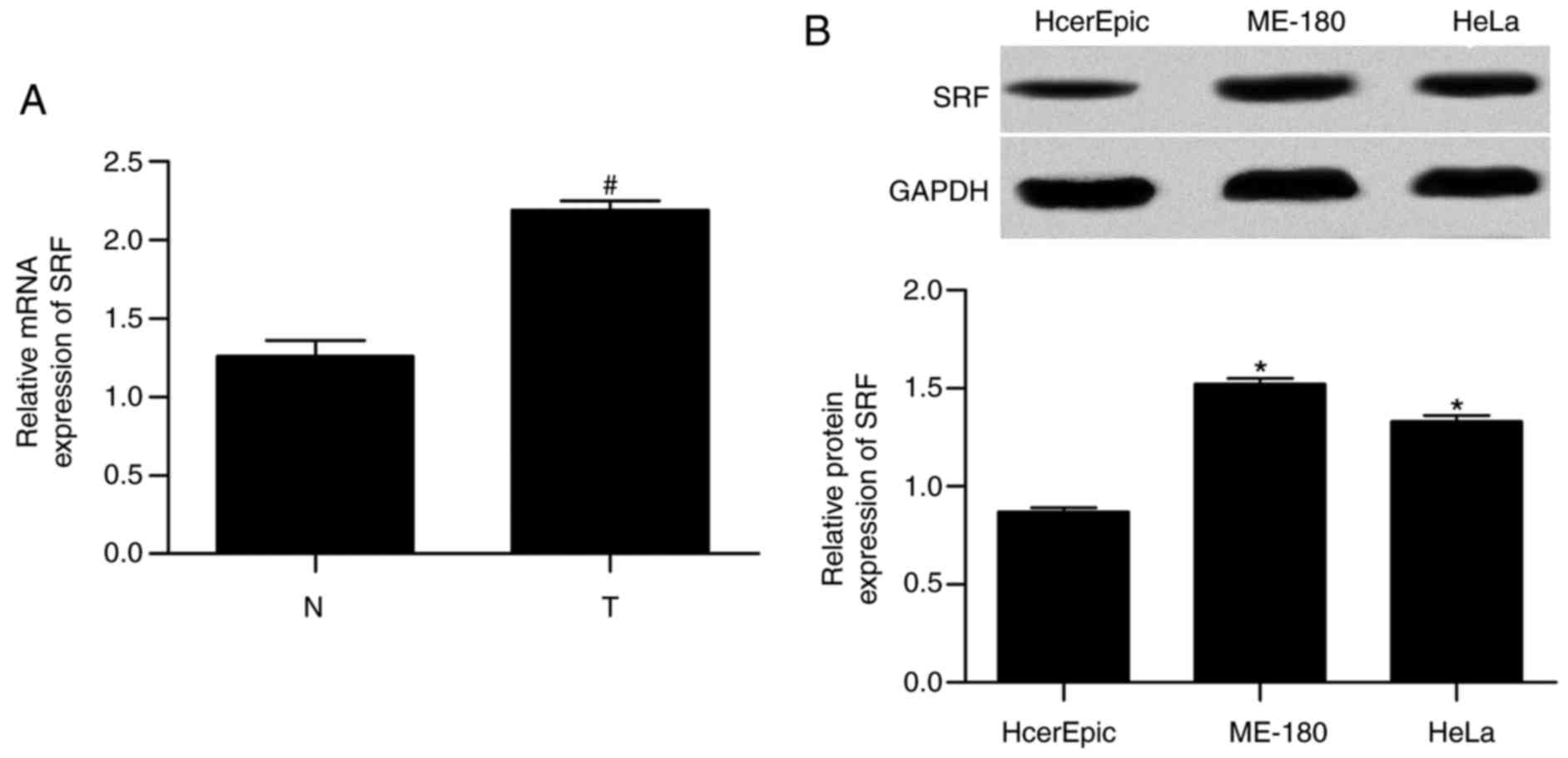
SRF is upregulated in cervical cancer
cell lines and tissues
First, the mRNA expression levels in tissues and the
protein expression levels in cell lines were measured for SRF by
RT-qPCR and western blotting, respectively. The results
demonstrated that mRNA expression (Fig. 1A) in tissues and protein
expression (Fig. 1B) in cell
lines were significantly increased in cervical cancer compared with
normal, implying an important role of SRF in cervical cancer.
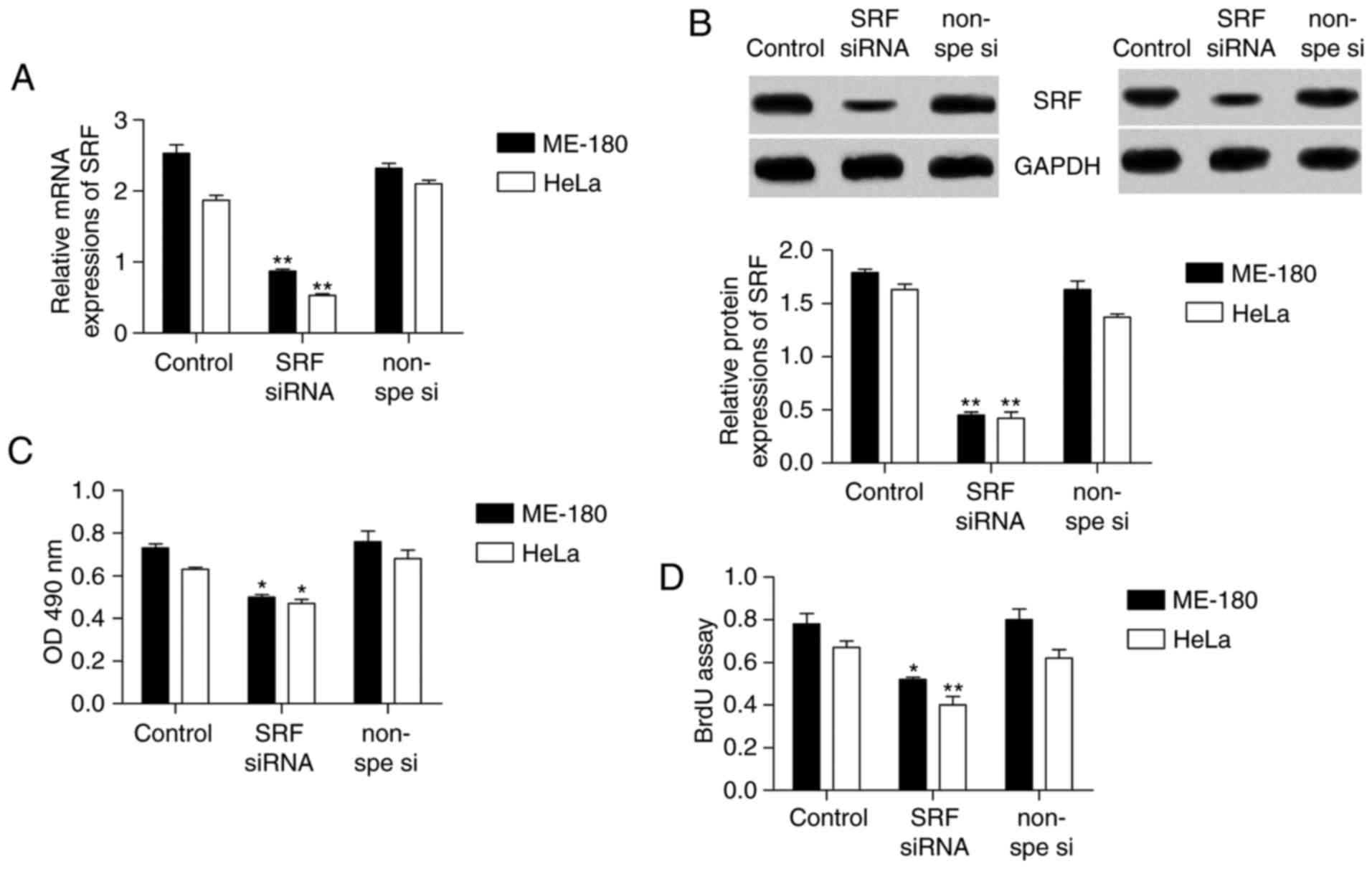
SRF silencing suppresses proliferation in
cervical cancer cells
To investigate the role of SRF in cervical cancer
cell proliferation, MTT and BrdU assays were used to detect
proliferation in th cervical cancer cell lines ME-180 and HeLa,
following SRF knockdown. First, SRF siRNA was transfected into
ME-180 and HeLa cells in order to knockdown SRF expression. The
data demonstrated that both the mRNA (Fig. 2A) and protein (Fig. 2B) levels of SRF were successfully
decreased by >50%. Next, ME-180 and HeLa cell proliferation was
measured. In the MTT assay (Fig.
2C), cell proliferation was significantly decreased in the
cells following SRF knockdown, compared with the control cells.
Similarly, in the BrdU assay (Fig.
2D), cell proliferation also appeared to be markedly
downregulated following SRF knockdown.
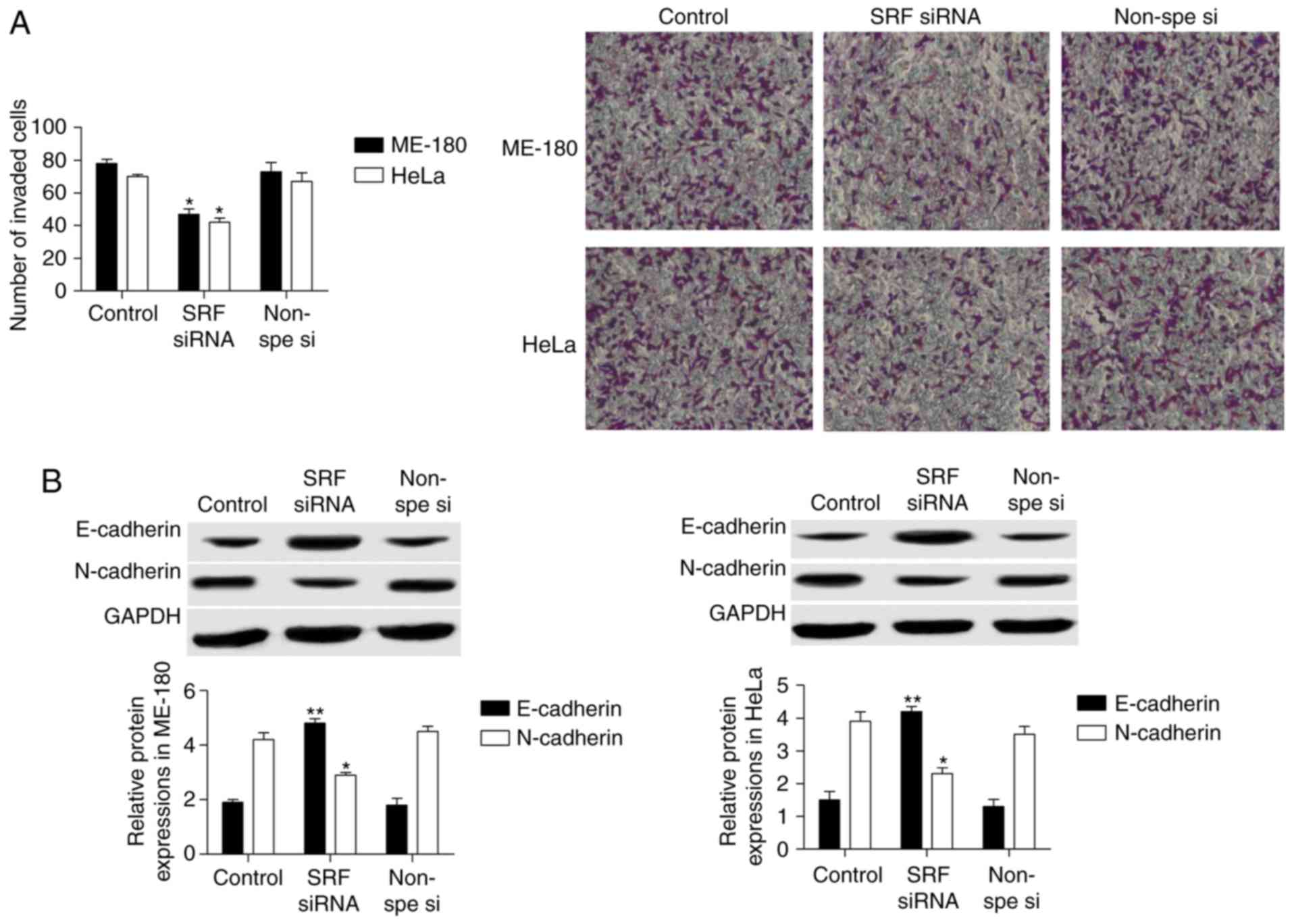
SRF silencing suppresses invasion in
cervical cancer cells
Cell invasion of ME-180 and HeLa cells following SRF
knockdown by siRNA was measured by Transwell assays. Expression of
the established EMT markers E-cadherin and N-cadherin was also
investigated by western blotting. As presented in Fig. 3A, the results from the Transwell
assays revealed that cell invasion was significantly decreased
following SRF knockdown in ME-180 and HeLa cells. The western
blotting results demonstrated that E-cadherin protein expression
was upregulated 2-fold, while N-cadherin was markedly downregulated
in ME-180 and HeLa cells following SRF knockdown (Fig. 3B).
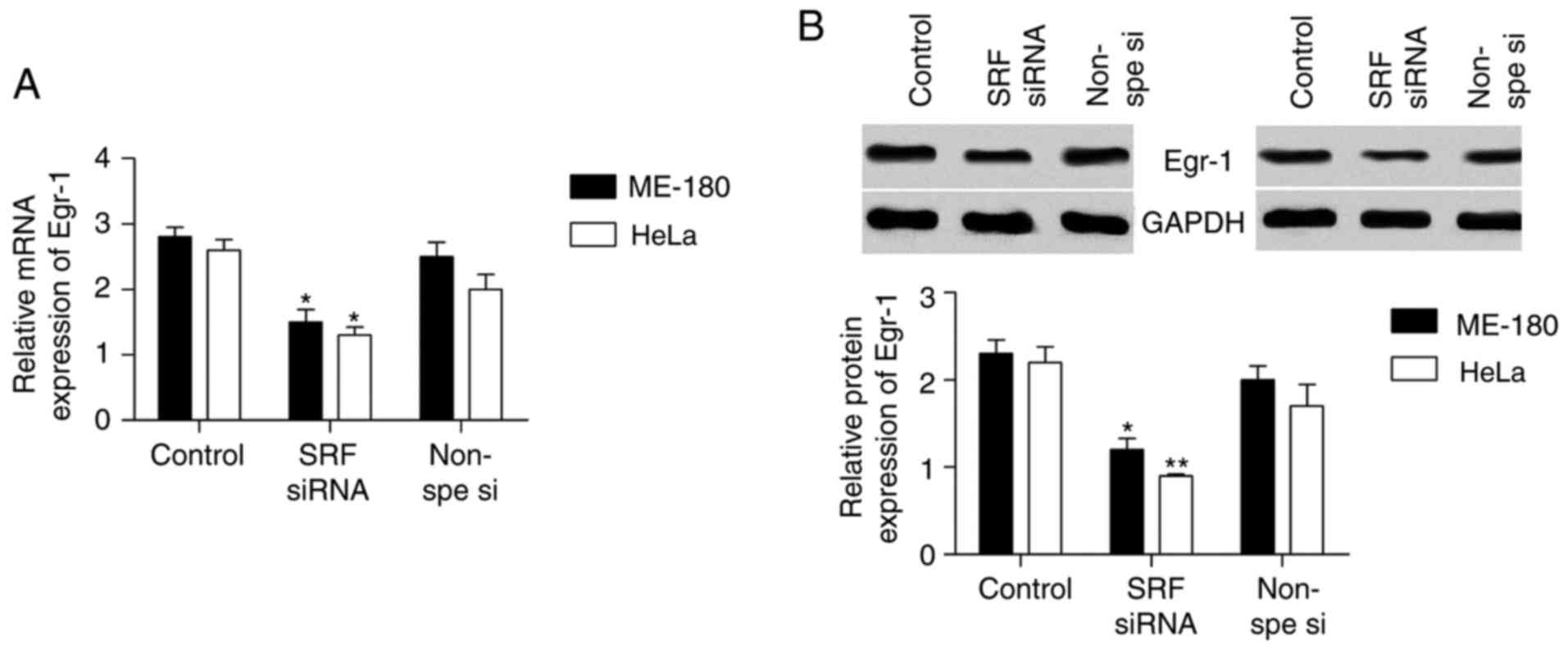
SRF silencing downregulates Egr-1
expression
Egr-1 has been reported to be regulated by SRF and
it affects EMT progression. Thus, it was speculated that SRF may
control Egr-1 expression in cervical cancer cell lines. The mRNA
(Fig. 4A) and protein (Fig. 4B) expression levels of Egr-1 were
detected in ME-180 and HeLa cells following SRF knockdown. Egr-1
expression was significantly decreased in cervical cancer cell
lines following SRF silencing.
SRF silencing controls cervical cancer
cell line proliferation and invasion by regulating Egr-1
To explore the molecular mechanism of SRF in
regulating cervical cancer cell proliferation and invasion, a
gain-of-function experiment was performed for Egr-1 in
SRF-knockdown ME-180 and HeLa cell lines. When the SRF-knockdown
cells were transfected with an Egr-1-overexpressing plasmid, Egr-1
was upregulated by 3-fold compared with cells transfected with an
empty plasmid, suggesting that overexpression was successful
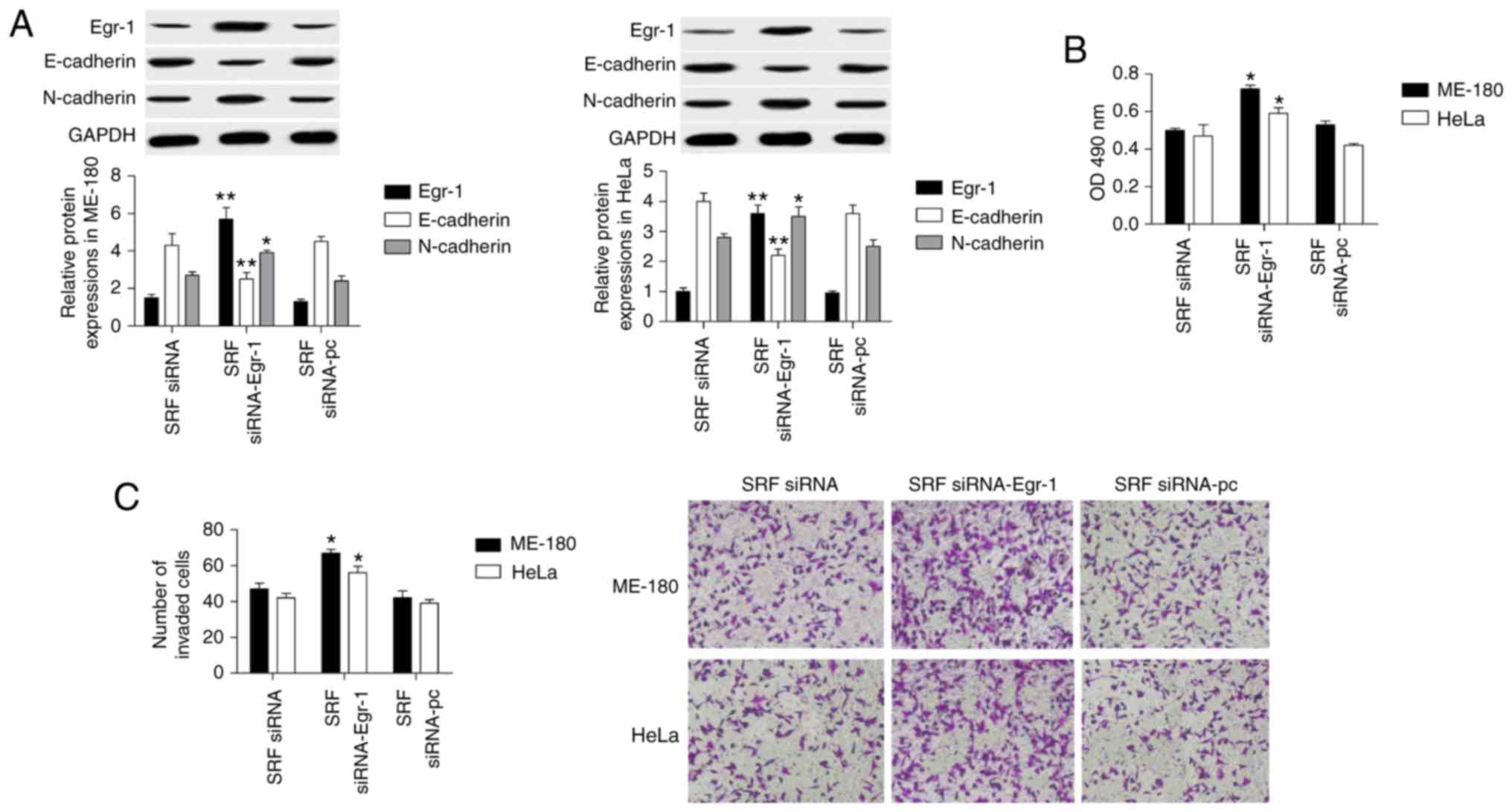
(Fig. 5A). Next, the effect of
Egr-1 overexpression was investigated on cell proliferation and
invasion. The results demonstrated that Egr-1 overexpression in
ME-180 and HeLa cells reversed the inhibitory effect of SRF
knockdown on proliferation (by MTT assay; Fig. 5B) and on invasion (by Transwell
assay; Fig. 5C). Furthermore,
E-cadherin protein expression was significantly decreased, and
N-cadherin increased (Fig. 5A).
Therefore, SRF controlled cervical cancer cell proliferation and
invasion through Egr-1.
Discussion
According to the World Health Organization, 500,000
new cases of cervical cancer occur worldwide each year, ~80% in
developing countries (25).
Cervical cancer rates continue to rise and affect younger women
(26). SRF is closely associated
with cancer metastasis. SRF expression is elevated in prostate and
gastric cancers (27). Zhao et
al (10) reported that SRF
modulates EMT and promotes metastasis in human gastric cancer. Wang
et al (28) reported that
SRF regulates non-small-cell lung cancer invasion and proliferation
via the miR29b/matrix metal-lopeptidase 2 axis. SRF siRNA has been
indicated to reduce the invasion potential of prostate cancer cells
in vitro (12).
SRF is an important transcription factor that
regulates EMT (29). EMT is a
process of cytoskeletal rearrangement that increases cell migration
and invasion abilities (30).
Studies generally agree that EMT is associated with embryonic
development, tissue regeneration, and cancer metastasis (31,32). During tumorigenesis, EMT is
characterized by downregulation of E-cadherin, which causes
differentiated epithelial tumor cells to become tumorigenic cells
with migration and invasion abilities (33). Upregulation of N-cadherin promotes
EMT (34). He et al
(35) demonstrated that SRF
promotes EMT in human peritoneal mesothelial cells. SRF also
provokes EMT in renal tubular epithelial cells of diabetic
nephropathy (36), and it induces
EMT in hepatocellular carcinoma, prostate cancer, and gastric
cancer (37). Therefore, SRF has
a significant role in EMT and cancer metastasis. Nevertheless, its
role in cervical cancer remains unclear. In the present study, it
was demonstrated that SRF expression in cervical cancer tissues and
cell lines was highly increased compared with normal. A
loss-of-function experiment was performed by transfecting SRF siRNA
in cervical cancer cell lines, and SRF silencing significantly
decreased N-cadherin and increased E-cadherin expression in
cervical cancer cell lines. In addition, cell proliferation and
invasion were suppressed following SRF silencing.
Silverman and Collins (23) demonstrated that Egr-1 gene
transcription could be activated by SRF. Egr-1 gene expression can
be regulated by a variety of extracellular signals, subsequently
modulating cell proliferation and invasion (38). Egr-1 also directly affects cell
proliferation in astrocytes, glioma cells, and mesangial cells
(39-41). Additionally, it is closely related
to EMT and cell invasion in nasopharyngeal cancer, human ovarian
cancer, colon cancer, and thyroid cancer cells (20,42,43). Egr-1 serves different roles in
different cancer cells. Previous studies have demonstrated that
Egr-1 decreases the malignancy of human non-small-cell lung
carcinoma by regulating type I intermediate filament chain keratin
18 expression, whereas Egr-1 promotes growth in prostate cancer
cells (44,45).
The effect of Egr-1 on cervical cancer is unclear.
The present study demonstrated that Egr-1 decreased when SRF was
silenced in cervical cancer cell lines. Notably, Egr-1
overexpression abolished the effect of SRF silencing on cell
proliferation, invasion, and E-cadherin and N-cadherin expressions
in cervical cancer cells. Therefore, SRF inhibition may control
cervical cancer metastasis by modulating Egr-1 expression. The data
in the present study demonstrated that SRF was highly expressed in
clinical cervical cancer tissues and cell lines compared with
normal. SRF knockdown restrained Egr-1 expression, resulting in
repression of proliferation, invasion and N-cadherin expression and
induction of E-cadherin expression. Thus, the current study
provides a novel insight into the molecular mechanism of SRF in
cervical cancer and provides a potential target for treatment.
Because the present study was performed mostly in vitro,
further studies with additional tissue samples or in vivo
will be required in the future to fully characterize the role of
SRF in human cervical cancer.
Acknowledgments
Not applicable.
Abbreviations:
|
SRF
|
serum response factor
|
|
EMT
|
epithelial-mesenchymal transition
|
|
Egr-1
|
early growth response-1
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
BrdU
|
bromodeoxyuridine
|
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors’ contributions
LM and YY designed and prepared the experiments. LM
and YY performed the experiments. LM, XQ and YY contributed
reagents/materials/analysis tools. LM and YY wrote the manuscript.
XQ modified and revised the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Patient samples were collected under a protocol
approved by the Institutional Review Board of the Henan University
of Chinese Medicine (Zhengzhou, China), and informed consent was
obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Arnold M, Liu L, Kenter GG, Creutzberg CL,
Coebergh JW and Soerjomataram I: Second primary cancers in
survivors of cervical cancer in the netherlands: Implications for
prevention and surveillance. Radiother Oncol. 111:374–381. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Milosevic MF, Pintilie M, Hedley DW,
Bristow RG, Wouters BG, Oza AM, Laframboise S, Hill RP and Fyles
AW: High tumor interstitial fluid pressure identifies cervical
cancer patients with improved survival from radiotherapy plus
cisplatin versus radiotherapy alone. Int J Cancer. 135:1692–1699.
2014. View Article : Google Scholar
|
|
3
|
Profantova B, Coic YM, Profant V, Štěpánek
J, Kopecký V Jr, Turpin PY, Alpert B and Zentz C: Organization of
the MADS box from human SRF revealed by tyrosine perturbation. J
Phys Chem B. 119:1793–1801. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu LH, Wu J, Zhang ZS, Miao ZQ, Zhao PX,
Wang Z and Xiang CB: Arabidopsis MADS-box transcription factor
AGL21 acts as environmental surveillance of seed germination by
regulating ABI5 expression. Mol Plant. 10:834–845. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stern S and Knoll B: CNS axon regeneration
inhibitors stimulate an immediate early gene response via MAP
kinase-SRF signaling. Mol Brain. 7:862014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ji H, Atchison L, Chen Z, Chakraborty S,
Jung Y, Truskey GA, Christoforou N and Leong KW:
Transdifferentiation of human endothelial progenitors into smooth
muscle cells. Biomaterials. 85:180–194. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Manning CS, Hooper S and Sahai EA:
Intravital imaging of SRF and Notch signalling identifies a key
role for EZH2 in invasive melanoma cells. Oncogene. 34:4320–4332.
2015. View Article : Google Scholar :
|
|
8
|
Sharili AS, Kenny FN, Vartiainen MK and
Connelly JT: Nuclear actin modulates cell motility via
transcriptional regulation of adhesive and cytoskeletal genes. Sci
Rep. 6:338932016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Urbini M, Astolfi A, Indio V, Tarantino G,
Serravalle S, Saponara M, Nannini M, Gronchi A, Fiore M, Maestro R,
et al: Identification of SRF-E2F1 fusion transcript in
EWSR-negative myoepithelioma of the soft tissue. Oncotarget.
8:60036–60045. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao X, He L, Li T, Lu Y, Miao Y, Liang S,
Guo H, Bai M, Xie H, Luo G, et al: SRF expedites metastasis and
modulates the epithelial to mesenchymal transition by regulating
miR-199a-5p expression in human gastric cancer. Cell Death Differ.
21:1900–1913. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li YJ, Dong M, Kong FM and Zhou JP:
Folate-decorated anticancer drug and magnetic nanoparticles
encapsulated polymeric carrier for liver cancer therapeutics. Int J
Pharm. 489:83–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Evans JC, McCarthy J, Torres-Fuentes C,
Cryan JF, Ogier J, Darcy R, Watson RW and O’Driscoll CM:
Cyclodextrin mediated delivery of NF-kappaB and SRF siRNA reduces
the invasion potential of prostate cancer cells in vitro. Gene
Ther. 22:802–810. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Burute M, Prioux M, Blin G, Truchet S,
Letort G, Tseng Q, Bessy T, Lowell S, Young J, Filhol O and Théry
M: Polarity reversal by centrosome repositioning primes cell
scattering during epithelial-to-mesenchymal transition. Dev Cell.
40:168–184. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bhattacharya R, Mitra T, Ray Chaudhuri S
and Roy SS: Mesenchymal splice isoform of CD44 (CD44s) promotes
EMT/invasion and imparts stem-like properties to ovarian cancer
cells. J Cell Biochem. 119:3373–3383. 2018. View Article : Google Scholar
|
|
15
|
Labernadie A, Kato T, Brugues A,
Serra-Picamal X, Derzsi S, Arwert E, Weston A, González-Tarragó V,
Elosegui-Artola A, Albertazzi L, et al: A mechanically active
heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to
drive cancer cell invasion. Nat Cell Biol. 19:224–237. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, McRobb LS and Khachigian LM:
MicroRNA miR-191 targets the zinc finger transcription factor Egr-1
and suppresses intimal thickening after carotid injury. Int J
Cardiol. 212:299–302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khachigian LM: Early growth response-1 in
the pathogenesis of cardiovascular disease. J Mol Med (Berl).
94:747–753. 2016. View Article : Google Scholar
|
|
18
|
Groger N, Bock J, Goehler D, Blume N,
Lisson N, Poeggel G and Braun K: Stress in utero alters neonatal
stress-induced regulation of the synaptic plasticity proteins arc
and egr1 in a sex-specific manner. Brain Struct Funct. 221:679–685.
2016. View Article : Google Scholar
|
|
19
|
Ryu JW, Choe SS, Ryu SH, Park EY, Lee BW,
Kim TK, Ha CH and Lee SW: Paradoxical induction of growth arrest
and apoptosis by EGF via the up-regulation of PTEN by activating
Redox factor-1/Egr-1 in human lung cancer cells. Oncotarget.
8:4181–4195. 2017. View Article : Google Scholar :
|
|
20
|
Cheng JC, Chang HM and Leung PC: Egr-1
mediates epidermal growth factor-induced downregulation of
E-cadherin expression via Slug in human ovarian cancer cells.
Oncogene. 32:1041–1049. 2013. View Article : Google Scholar
|
|
21
|
Peng WX, Wan YY, Gong AH, Ge L, Jin J, Xu
M and Wu CY: Egr-1 regulates irradiation-induced autophagy through
atg4B to promote radioresistance in hepatocellular carcinoma cells.
Oncogenesis. 6:e2922017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park SY, Kim JY, Lee SM, Chung JO, Lee KH,
Jun CH, Park CH, Kim HS, Choi SK, Rew JS, et al: Expression of
early growth response gene-1 in precancerous lesions of gastric
cancer. Oncol Lett. 12:2710–2715. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Silverman ES and Collins T: Pathways of
Egr-1-mediated gene transcription in vascular biology. Am J Pathol.
154:665–670. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Cuzick J, Arbyn M, Sankaranarayanan R, Tsu
V, Ronco G, Mayrand MH, Dillner J and Meijer CJ: Overview of human
papillomavirus-based and other novel options for cervical cancer
screening in developed and developing countries. Vaccine. 10(Suppl
26): K29–K41. 2008. View Article : Google Scholar
|
|
26
|
Fidler MM, Gupta S, Soerjomataram I,
Ferlay J, Steliarova-Foucher E and Bray F: Cancer incidence and
mortality among young adults aged 20-39 years worldwide in 2012: A
population-based study. Lancet Oncol. 18:1579–1589. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qiao J, Liu Z, Yang C, Gu L and Deng D:
SRF promotes gastric cancer metastasis through stromal fibroblasts
in an SDF1-CXCR4-dependent manner. Oncotarget. 7:46088–46099. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang HY, Tu YS, Long J, Zhang HQ, Qi CL,
Xie XB, Li SH and Zhang YJ: SRF-miR29b-MMP2 axis inhibits NSCLC
invasion and metastasis. Int J Oncol. 47:641–649. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schwartz B, Marks M, Wittler L, Werber M,
Währisch S, Nordheim A, Herrmann BG and Grote P: SRF is essential
for mesodermal cell migration during elongation of the embryonic
body axis. Mech Dev. 133:23–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang H, Tao L, Jin F, Gu H, Dai X, Ni T,
Feng J, Ding Y, Xiao W, Qian Y and Liu Y: Cofilin 1 induces the
epithelial-mesenchymal transition of gastric cancer cells by
promoting cytoskeletal rearrangement. Oncotarget. 8:39131–39142.
2017.PubMed/NCBI
|
|
31
|
Nieto MA: Context-specific roles of EMT
programmes in cancer cell dissemination. Nat Cell Biol. 19:416–418.
2017. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pesic M and Greten FR: Inflammation and
cancer: Tissue regeneration gone awry. Curr Opin Cell Biol.
43:55–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rogers CD, Saxena A and Bronner ME: Sip1
mediates an E-cadherin-to-N-cadherin switch during cranial neural
crest EMT. J Cell Biol. 203:835–847. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen L, Munoz-Antonia T and Cress WD:
Trim28 contributes to EMT via regulation of E-cadherin and
N-cadherin in lung cancer cell lines. PLoS One. 9:e1010402014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
He L, Lou W, Ji L, Liang W, Zhou M, Xu G,
Zhao L, Huang C, Li R, Wang H, et al: Serum response factor
accelerates the high glucose-induced epithelial-to-mesenchymal
transition (EMT) via snail signaling in human peritoneal
mesothelial cells. PLoS One. 9:e1085932014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao L, Chi L, Zhao J, Wang X, Chen Z,
Meng L, Liu G, Guan G and Wang F: Serum response factor provokes
epithelial-mesen-chymal transition in renal tubular epithelial
cells of diabetic nephropathy. Physiol Genomics. 48:580–588. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao L, Zhao J, Wang X, Chen Z, Peng K, Lu
X, Meng L, Liu G, Guan G and Wang F: Serum response factor induces
endothelial-mesenchymal transition in glomerular endothelial cells
to aggravate proteinuria in diabetic nephropathy. Physiol Genomics.
48:711–718. 2016. View Article : Google Scholar
|
|
38
|
Sun T, Tian H, Feng YG, Zhu YQ and Zhang
WQ: Egr-1 promotes cell proliferation and invasion by increasing
beta-catenin expression in gastric cancer. Dig Dis Sci. 58:423–430.
2013.
|
|
39
|
Mayer SI, Rossler OG, Endo T, Charnay P
and Thiel G: Epidermal-growth-factor-induced proliferation of
astrocytes requires Egr transcription factors. J Cell Sci.
122:3340–3350. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kaufmann K and Thiel G: Epidermal growth
factor and platelet-derived growth factor induce expression of
Egr-1, a zinc finger transcription factor, in human malignant
glioma cells. J Neurol Sci. 189:83–91. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li Y, Hu F, Xue M, Jia YJ, Zheng ZJ, Wang
L, Guan MP and Xue YM: Klotho down-regulates Egr-1 by inhibiting
TGF-β1/Smad3 signaling in high glucose treated human mesangial
cells. Biochem Biophys Res Commun. 487:216–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee BS, Kang S, Kim KA, Song YJ, Cheong
KH, Cha HY and Kim CH: Met degradation by SAIT301, a met monoclonal
antibody, reduces the invasion and migration of nasopharyngeal
cancer cells via inhibition of EGR-1 expression. Cell Death Dis.
5:e11592014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim J, Kang HS, Lee YJ, Lee HJ, Yun J,
Shin JH, Lee CW, Kwon BM and Hong SH: EGR1-dependent PTEN
upregulation by 2-benzoyloxycinnamaldehyde attenuates cell invasion
and EMT in colon cancer. Cancer Lett. 349:35–44. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Baron V, De Gregorio G, Krones-Herzig A,
Virolle T, Calogero A, Urcis R and Mercola D: Inhibition of Egr-1
expression reverses transformation of prostate cancer cells in
vitro and in vivo. Oncogene. 22:4194–4204. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang H, Chen X, Wang J, Guang W, Han W,
Zhang H, Tan X and Gu Y: EGR1 decreases the malignancy of human
non-small cell lung carcinoma by regulating KRT18 expression. Sci
Rep. 4:54162014. View Article : Google Scholar : PubMed/NCBI
|