Introduction
Iron homeostasis is tightly regulated under normal
settings, where the hepcidin-ferroportin axis is fundamentally
responsible for the regulation of iron supply, utilization,
recycling and storage. Hepcidin, a peptide hormone, is prominently
synthesized by the liver and secreted into serum followed by tissue
localization through circulation (1). Hepcidin inhibits iron absorption
from the duodenum, and iron export from macrophages and hepatocytes
is reduced by via its hepcidin-induced degradation of iron exporter
ferroportin (FPN) (2). The
expression of hepcidin is regulated by multiple signals directly or
indirectly, including iron levels, anemia, hypoxia, inflammation,
pathological conditions and cytokines, and changes in hepcidin
expression are associated with various diseases (3). Deregulated hepcidin-FPN signaling is
implicated iron-associated diseases, such as hereditary
hemochromatosis and anemia of inflammation (4-6),
and also in atherosclerosis (AS) (7-9).
AS is a chronic vascular disease and it is the
pathological basis of various cardiovascular pathologies. There are
different theories regarding the pathogenesis of AS, including
lipid infiltration theory (10).
With increasing studies on the metabolism of AS, the role of iron
in the progression of AS has been established (11). A previous study reported that high
expression of hepcidin in AS plaques can increase the iron
deposition in macrophages and promote the instability of plaques
(4). Tetramethylpyrazine (TMP), a
natural compound extracted from the Chinese herb Chuanxiong rhizome
(12), has been reported to have
an anti-AS effect by inhibiting aggregation of platelet,
proliferation of smooth muscle cells and reduced lipid peroxidation
and protecting endothelial cell function (12,13). Thus, in the current study, the
potential effect of TMP on hepcidin expression was
investigated.
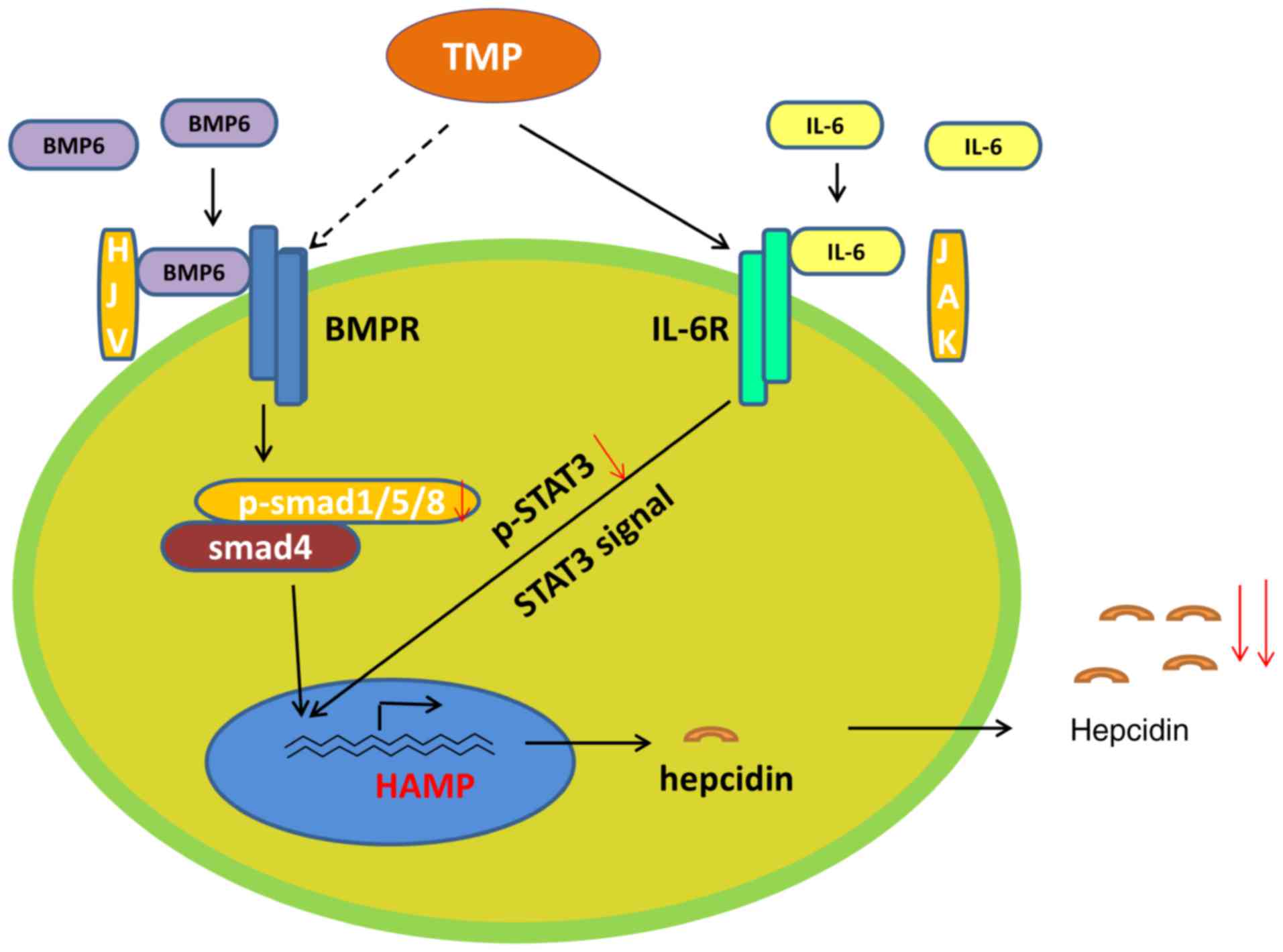
At present, two signaling pathways are recognized to
predominantly control hepcidin expression under normal conditions,
hemojuvelin (HJV)-bone morphogenetic proteins (BMPs)/Smad and Janus
kinase (JAK)/signal transducer and activator of transcription
(Stat) signaling pathways (14,15). BMPs are a class of multifunctional
transforming growth factors, which have an important role in cell
proliferation, differentiation, apoptosis and tissue development
(16). Among them, BMP6 also has
an important role in iron absorption and storage via modulation of
hepcidin expression. HJV, a co-receptor of BMP, promotes the
phosphorylation of Smad1/5/8 and, thus, stimulates the expression
of hepcidin (17). The JAK/Stat
signaling pathway regulates the expression of hepcidin in response
to inflammatory factors, and interleukin 6 (IL-6) is the major
factor that regulates hepcidin expression in inflammatory responses
mediated by Stat3 (18).
Furthermore, it has been reported that JAK/Stat signaling regulates
hepcidin via SMAD4 (19).
Therefore, to fully understand the underlying mechanism of the
potential effects of TMP on hepcidin expression regulation, changes
of the phosphorylation of Stat3 and Smad1/5/8 protein were also
investigated in this study.
Materials and methods
Drug, reagents and diets
TMP (molecular formula
C8H12N2·HCl·2H2O)
extracted from Chinese medicinal plants was purchased from the
National Institutes for Food and Drug Control of China (Beijing,
China). Total cholesterol (TC) reagent and triglyceride (TG)
reagent were purchased from Beijing Wantaiderui Diagnostic
Technology Company (Beijing, China). Serum hepcidin ELISA kit (cat.
no. AE92047Mu) was purchased from AMEKO (Lianshuo Biotechnology
Co., Ltd., Shanghai, China). Ordinary diet was purchased from
Beijing Keao Xieli Feed Co., Ltd. (Beijing, China). High-fat diet
were purchased from Beijing NuoKangYuan Biotechnology Company
(Beijing, China), and the ingredients were as follows: 2%
cholesterol, 0.5% bile salt, 10% lard, 10% egg yolk powder, 5%
sugar and 72.5% basic feed.
Animal experiments
Specific pathogen-free C57BL/6 mice (16 male and 16
female; 4 weeks old; 18-20 g) were purchased from Beijing Weitong
Lihua Experimental Animal Technology Company (Beijing, China), and
housed in the animal breeding room of Xiyuan Hospital, China
Academy of Chinese Medical Sciences (Beijing, China). Mice were
selected randomly for the following experiments. All mice were
divided into the following groups: Control group fed with normal
diets (group N; n=8); high-fat diets (group M; n=8), mice fed with
normal diets plus TMP treatment (group NT; n=8) or high-fat diets
plus TMP treatment (group MT; n=8). The high-fat model was
generated by feeding with high-fat diets for 4 weeks. The control
group mice were administered 2 ml/(kg/day) normal saline via
intraperitoneal injections for 7 days consecutively, and TMP
treatment was performed by daily intraperitoneal injection of 40
mg/kg body weight for 7 days.
Following anesthesia of mice, serum and liver
samples were collected. Liver samples were fixed in 4%
paraformaldehyde or stored at −80°C for further analysis. All
animal feeding and experiments were in compliance with and approved
by the ethics committee of the Gansu University of Traditional
Chinese Medicine (Lanzhou, China).
Assay of serum indices
Serum TG and TC were analyzed using a T300 automatic
biochemical analyzer (DIRUI Industrial Co., Ltd, Changchun, China),
including TG and TC, the methods and procedures were determined
according to the manufacturer’s instructions. The serum levels of
hepcidin were detected using ELISA kits according to the
manufacturer’s instructions.
Western blot analysis
Following treatment with TMP, liver tissues were
snap-frozen in liquid nitrogen and homogenized in the
radioimmunoprecipitation (RIPA) assay lysis buffer (Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China), and
total proteins were then extracted using RIPA supplemented with
protease inhibitor cocktail (Roche Diagnostics, Basel,
Switzerland). The concentration of liver tissue protein was
measured using a bicinchoninic acid protein assay kit according to
the manufacturer’s protocol. Equal amount of protein lysates (30-50
µg/sample) were subjected to SDS-PAGE on 8-12% gels and
transferred onto nitrocellulose membranes, which were blocked with
7% nonfat milk/Tris-buffered saline-Tween or 5% bovine serum
albumin and hybridized with specific primary antibodies overnight
at 4°C, followed by 1 h of hybridization with specific secondary
antibody (anti-mouse antibody, 1:8,000; anti-rabbit antibody
1:8,000) at 37°C as described previously (20,21). The primary antibodies used were
anti-phospho (p)-Stat3 antibody (1:1,000; cat. no. 9145; Cell
Signaling Technology, Inc., Danvers, MA, USA), and anti-Stat3
antibody (1:1,000; cat. no. 9139; Cell Signaling Technology, Inc.),
anti-p-Smad1/5/8 antibody (1:1,000; cat. no. sc-12353; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), anti-Smad1 antibody
(1:1,000; cat. no. sc-7965; Santa Cruz Biotechnology, Inc.), and
anti-GAPDH (1:2,000; cat. no. G8795; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). GAPDH was used as a loading control.
Densitometric measurements of immunoblotted bands were determined
using ImageJ digitalized software (National Institutes of Health,
Bethesda, MA, USA).
Histological examination
The liver tissues were fixed in 10% buffered
formaldehyde at 4°C for 7 days, then embedded in paraffin. Tissue
sections (5-8 µm) were stained with hematoxylin and eosin to
examine the morphology of the liver following a standard protocol
as previously described (21-23).
Statistical analysis
SPSS Statistics 19.0 package (IBM Corp., Armonk, NY,
USA) was used to analyze the experimental data; statistical
differences were analyzed by independent t-test or one-way analysis
of variance (ANOVA) test. Alternatively, post hoc test
(Student-Newman-Keuls) used following ANOVA and non-parametric test
(Kruskal-Wallis H) was employed for non-normal distributions. Data
are presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of high-fat diet and TMP
intervention on lipid metabolism
Initially, the effects of high-fat diet and TMP
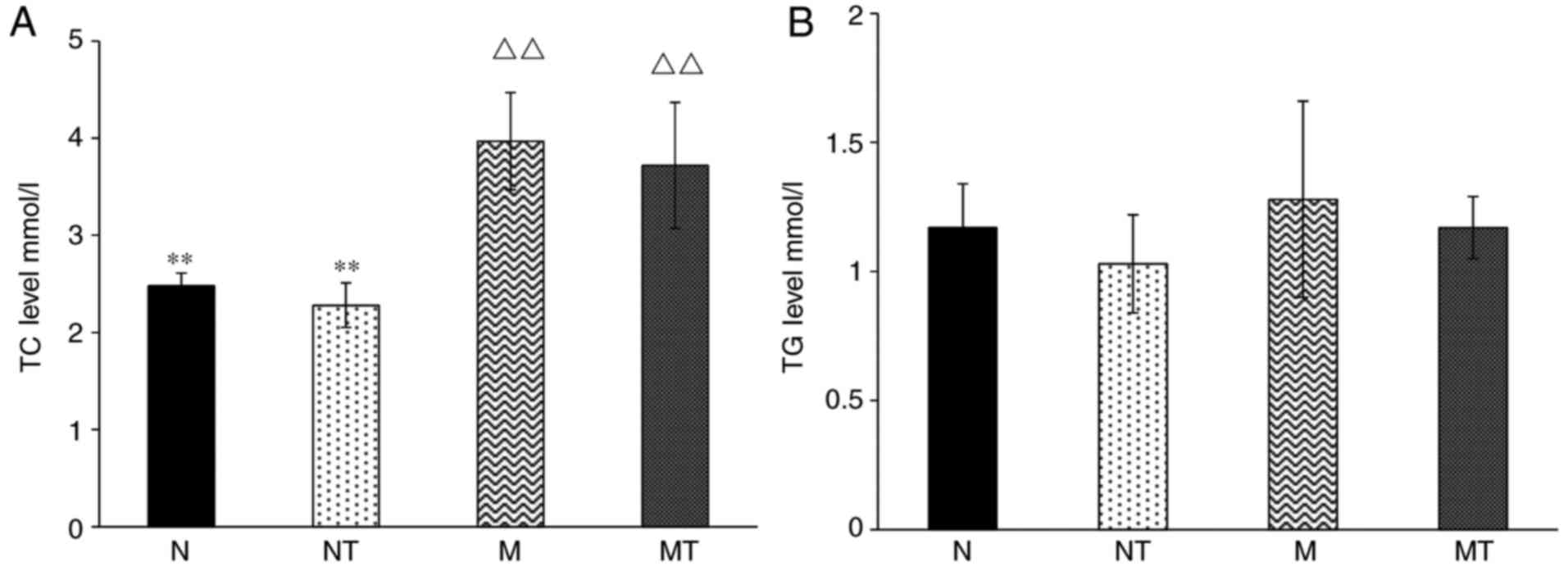
intervention on lipid metabolism were investigated. As shown in
Fig. 1A, the level of serum TC in
groups M and MT was significantly increased compared with group N
(P<0.05), demonstrating the successful establishment of a
hyperlipidemia mouse model. However, there was no significant
change in serum TC among the groups N and FN, and groups M and FM
(P>0.1), demonstrating that TMP intervention does not attenuate
hyperlipidemia. Furthermore, no significant change in serum TG was
observed among all groups (P>0.1; Fig. 1B). These results demonstrated that
TMP intervention had no effect on high-fat diet-induced
hyperlipidemia.
Effect of hyperlipidemia and TMP
intervention on serum hepcidin
As the main regulator of systemic iron homeostasis,
the effects of hyperlipidemia and TMP intervention on hepcidin
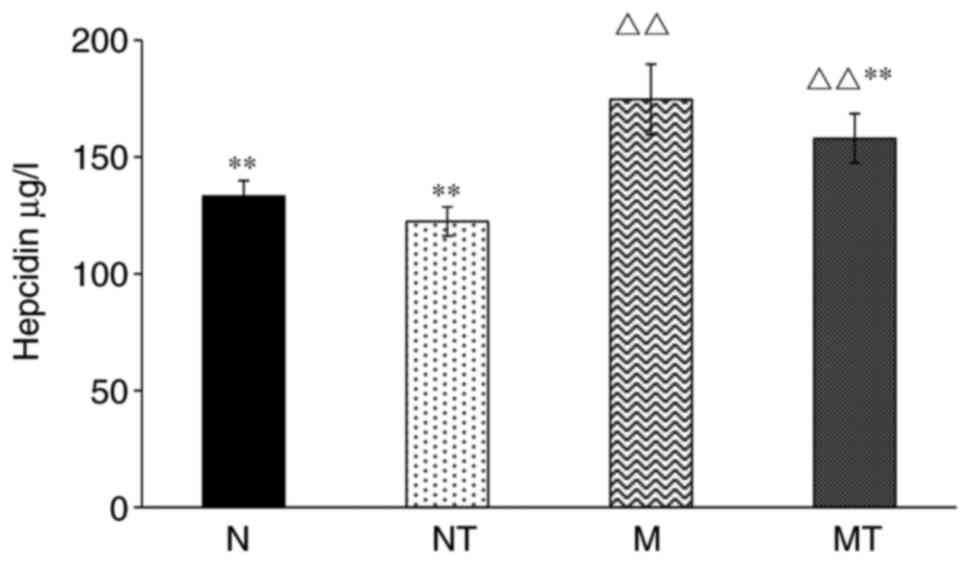
expression was also investigated. As shown in Fig. 2, the levels of serum hepcidin in
groups M and MT were increased dramatically compared with group N
(P<0.01), whereas there was no significant difference between
group NT and group N. Compared with group M, the level of hepcidin
in group MT was significantly decreased (P<0.01). Therefore,
these results demonstrated that the high-fat diet resulted in an
increase of serum hepcidin concentration, which was partially
inhibited by TMP treatment.
Effect of hyperlipidemia and TMP
intervention on the phosphorylation of p-Stat3 protein
In order to understand the molecular mechanism
involved in these effects, the signaling pathways that regulate
hepcidin expression regulation were investigated. Although the
expression of hepcidin is altered by a variety of stimuli, two
signaling pathways are recognized to predominantly control hepcidin
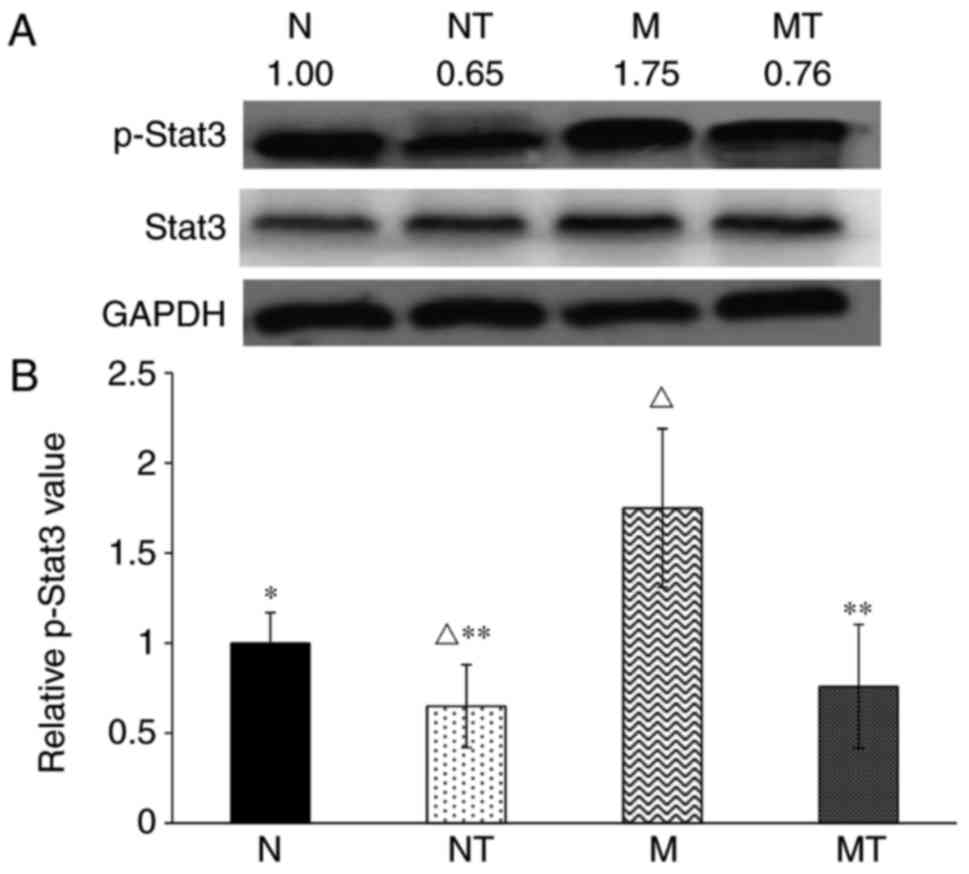
expression: IL-6-JAK-Stat3 and BMP-Smad signaling (14,15). As shown in Fig. 3, the level of p-Stat3 was
significantly increased in group M compared with group N
(P<0.05). Following TMP intervention, p-Stat3 protein contents
the TMP-treated groups were significantly decreased compared with
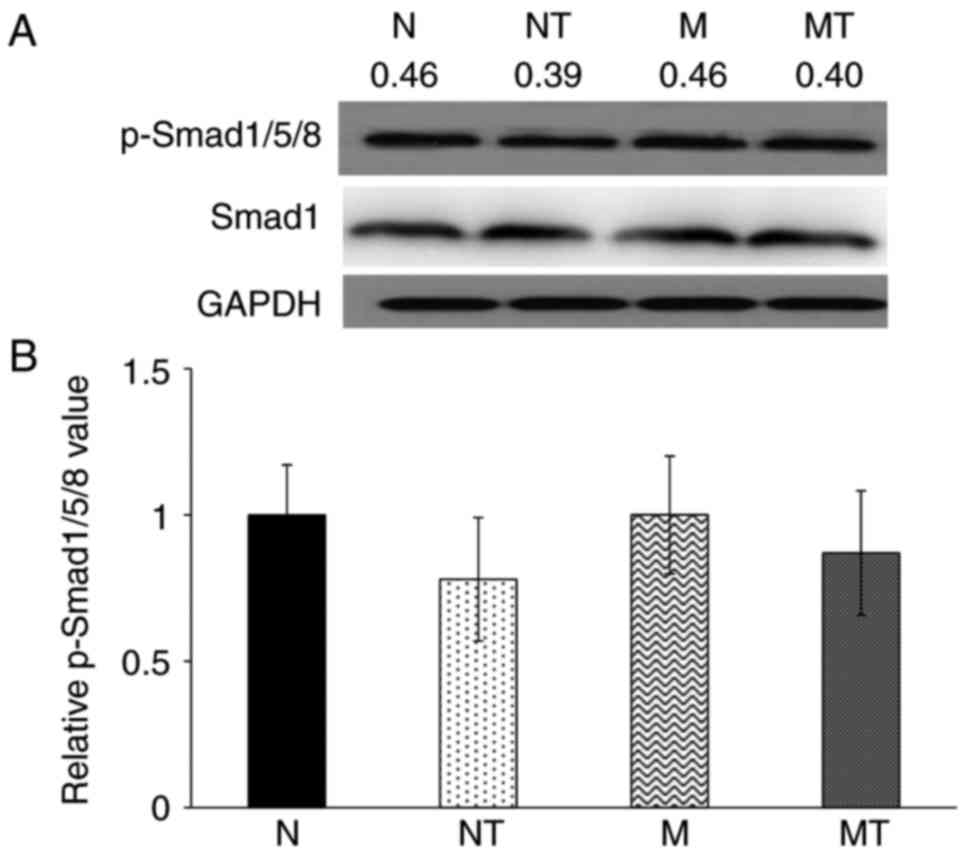
their respective control groups. However, as shown in Fig. 4, there was no significant
difference in the levels of p-Smad1/5/8 in liver samples among all
groups (P>0.1). These results demonstrated that hyperlipidemia
upregulated hepatic hepcidin expression by enhancing the
phosphorylation of Stat3, but not Smad1/5/8, while TMP intervention
inhibited this effect.
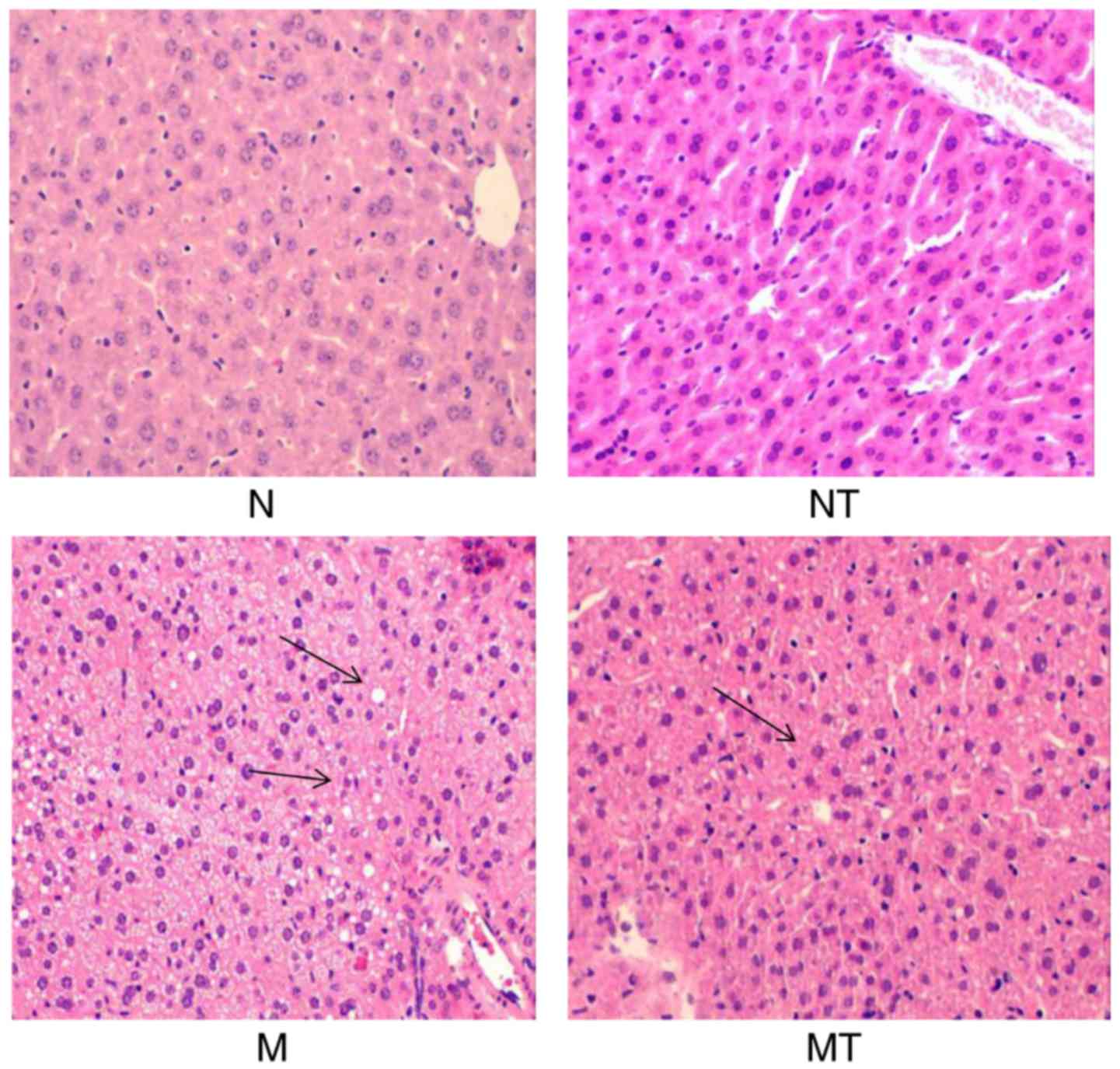
Pathological changes in the liver
As shown in Fig.
5, clear structures of hepatic lobules and hepatic cords were
observed in group N and NT with large and round nuclei in the
center of the cells, which were arranged orderly. However, liver
lobule structures in group M were not clear, as shown by the
circular fat vacuoles with different sizes, and the hepatocytes
with degeneration, inflammatory cell infiltration and apoptosis
were also observed. However, significant improvements in
pathological features were observed in group MT compared with group
M (Fig. 5). No noticeable
alterations were observed in liver sections from group NT compared
with group N. These results demonstrated that TMP intervention
attenuated hyperlipidemia-induced hepatic structural abnormalities,
which may be associated with regulation of hepcidin expression in
the liver.
Discussion
Although iron metabolism has been investigated in
numerous previous studies, the molecular mechanisms of systemic
iron homeostasis have been only been recently identified, with the
characterization of the hepcidin-FPN axis (4,24).
Disorder of iron metabolism is associated with various diseases,
including iron-deficiency anemia and iron overload diseases, and
the deregulation of hepcidin expression is also involved in these
iron disorder diseases (15). It
has been previously reported that hyperlipidemia can induce iron
deposition in the aorta of mice (25). Compared with the use of proteins
and synthesized chemicals as drugs, natural compounds from
traditional Chinese medicinal plants have important properties,
including abundant natural availability, high stability and low
toxicity (26,27). Previous studies have suggested
that certain medicinal plant extracts and natural compounds are
able to regulate hepcidin expression (28,29). Among 16 medicinal plant extracts
tested in a previous study (28),
Caulis Spatholobi exhibited the greatest inhibitory effect on
hepcidin expression. Another study testing 12 natural compounds
reported that icariin enhanced the expression of hepcidin (29). TMP has been reported to have
various therapeutic effects on inflammation and cardiovascular
diseases, and to prevent cells from oxidative damage in
vitro and in experimental animal models (12). TMP also been reported to improve
clinical manifestations and liver function, which may be mediated
by reduced expression of tissue factor via downregulated expression
of the transcription factors, early growth response factor-1 and
nuclear factor-κB p65 (30). In
our previous study, TMP was shown to reverse the effect of iron
deposition on endothelial functions and reduce IL-6 levels in
hyperlipidemic FPN1 knockout mice (25). In the present study, the
association between hyperlipidemia and serum hepcidin, and the
effect of TMP were investigated. The results demonstrated that
hyperlipidemia increased the serum hepcidin level, which was
reversed by TMP treatment.
Additionally, the molecular mechanisms underlying
the stimulating effect of hyperlipidemia and inhibitory effect of
TMP intervention on hepcidin expression were investigated. The
JAK/Stat and HJV-BMP/Smad pathways are two fundamental signaling
pathways that regulate for hepcidin expression (13,14,31). Inflammatory factors, including
IL-1, IL-6 and TNF-α, regulate the expression of hepcidin via the
JAK/STAT pathway, and the role of IL-6 is the most established
(32). IL-6 and IL-6 receptor
binding can activate the JAKs and Stat3; Stat3 is then
phosphorylated, moves into the nucleus and binds the hepcidin gene
promoter to regulate hepcidin expression. HJV is a glycosyl
phosphatidylinositol-linked cell surface protein expressed in
skeletal muscle, liver cells and myocardial cells. HJV acts on BMPs
to stimulate the growth of neurons. BMPs activate BMP receptor
signal transduction, which promotes Smad1/5/8 phosphorylation.
Phosphorylated Smad1/5/8 and SMAD4 form a complex to stimulate
hepcidin expression (33,34). The results of the present study
revealed that the phosphorylation of Stat3 was significantly
enhanced mice with hyperlipidemia, and subsequently inhibited by
TMP intervention. However, phosphorylation of Smad1/5/8 protein was
not significantly altered by hyperlipidemia or TMP. Furthermore,
the disordered liver structure may be associated with the
upregulation of hepcidin expression in mice with hyperlipidemia,
which was also be attenuated by TMP intervention. These results
indicate that TMP may activate IL-6R and then induce Stat3
signaling (Fig. 6).
Notably, TMP had no significant inhibitory effect on
hepcidin expression in normal mice, which may be associated with
the normal expression of inflammatory factors (IL-6 and TNF-α among
others) in these mice. In order to validate a direct causal
relationship, RNA interference/overexpression experiments should be
performed. There are limitations in the present study research will
be undertaken, including setting multiple time points to observe
the differences in the degree of elevated blood lipids, which may
have different effects on the JAK/STAT and HJV-BMP/SMAD pathways,
and effects of different doses of TMP on the expression of hepcidin
and the pathways. In vivo experiments will be designed as
the basis for supporting the in vitro findings.
In conclusion, TMP intervention blocked the
simulation of hepatic hepcidin expression induced by
hyperlipidemia, which decreased serum hepcidin levels, potentially
by inhibiting Stat3 phosphorylation. The results may provide a
promising novel strategy for the treatment of hyperlipidemia and AS
using natural compounds, particularly extracts from traditional
Chinese medicine plants, by targeting hepcidin expression.
Acknowledgments
Not applicable
Funding
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no. 81173584).
All the laboratory members are thanked for their assistance with
experiments and reagents.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
HJY conceived and designed the study. MZ, MYS, CYG
and JSW performed the experiments and analyzed the data. MYS, MZ,
CYG and JSW contributed reagents and materials. MZ, MYS, FQX and
HJY designed the study, wrote and revised the manuscript.
Ethics approval and consent to
participate
All animal feeding and experiments were in
compliance with and approved by the ethics committee of the Gansu
University of Traditional Chinese Medicine (Lanzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
FPN
|
ferroportin
|
|
AS
|
atherosclerosis
|
|
TMP
|
tetramethylpyrazine
|
|
TC
|
total cholesterol
|
|
TG
|
triglycerides
|
|
BMP
|
bone morphogenetic protein
|
|
IL-6
|
interleukin-6
|
|
HJV
|
hemojuvelin
|
|
HH
|
hereditary hemochromatosis
|
|
H&E
|
hematoxylin and eosin
|
References
|
1
|
Park CH, Valore EV, Waring AJ and Ganz T:
Hepcidin, a urinary antimicrobial peptide synthesized in the liver.
J Biol Chem. 276:7806–7810. 2001. View Article : Google Scholar
|
|
2
|
Nemeth E, Tuttle MS, Powelson J, Vaughn
MB, Donovan A, Ward DM, Gnz T and Kaplan J: Hepcidin regulates
cellular iron efflux by binding to ferroportin and inducing its
internalization. Science. 306:2090–2093. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nicolas G, Chauvet C, Viatte L, Danan JL,
Bigard X, Devaux I, Beaumont C, Kahn A and Vaulont S: The gene
encoding the iron regulatory peptide hepcidin is regulated by
anemia, hypoxia, and inflammation. J Clin Invest. 100:1037–1044.
2002. View Article : Google Scholar
|
|
4
|
Ganz T: Hepcidin and iron regulation, 10
years later. Blood. 117:4425–4433. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Drakesmith H and Prentice AM: Hepcidin and
the iron-infection axis. Science. 338:768–772. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fleming RE and Ponka P: Iron overload in
human disease. N Engl J Med. 366:348–59. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ganz T, Olbina G, Girelli D, Nemeth E and
Westerman M: Immunoassay for human serum hepcidin. Blood.
112:4292–4297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maes K, Nemeth E, Roodman GD, Huston A,
Esteve F, Freytes C, Callander N, Katodritou E, Tussing-Humphreys
L, Rivera S, et al: In anemia of multiple myeloma, hepcidin is
induced by increased bone morphogenetic protein 2. Blood.
116:3635–3644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pinnix ZK, Miller LD, Wang W, D’Agostino
R, Kute T, Willingham MC, Hatcher H, Tesfay L, Sui G, Di X, et al:
Ferroportin and iron regulation in breast cancer progression and
prognosis. Sci Transl Med. 2:43–56. 2010. View Article : Google Scholar
|
|
10
|
Babiak J and Rudel LL: Lipoproteins and
atherosclerosis. Baillieres Clin Endocrinol Metab. 1:515–550. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ramos E, Rodriguez L, Rodriguez R, Hansen
M, Gabayan V, Ginzburg Y, Roth MP, Nemeth E and Ganz T: Evidence
for distinct pathways of hepcidin regulation by acute and chronic
iron loading in mice. Hepatology. 53:1333–1341. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Zhang X, Xu C, Zhang G, Zhang Z,
Yu P, Shan L, Sun Y and Wang Y: Synthesis and biological evaluation
of danshensu and tetramethylpyrazine conjugates as cardioprotective
agents. Chem Pharm Bull. 65:381–388. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang YR and Chen KJ: Pharmacological
roles of ligustrazine in cardio-/cerebrovascular systems and its
progress in researches of clinical application. Zhongguo Zhong Xi
Yi Jie He Za Zhi. 33:707–711. 2013.In Chinese. PubMed/NCBI
|
|
14
|
Ganz T: Systemic iron homeostasis. Physiol
Rev. 93:1721–1741. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ganz T and Nemeth E: Hepcidin and iron
homeostasis. Biochimica et Biophysica Acta (BBA) Mol Cell Res.
1823:1434–1443. 2012. View Article : Google Scholar
|
|
16
|
Miyazono K, Maeda S and Imamura T: BMP
receptor signaling: Transcriptional targets, regulation of signals,
and signaling cross-talk. Cytokine Growth Factor Rev. 16:251–263.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Babitt JL, Huang FW, Wrighting DM, Xia Y,
Sidis Y, Samad TA, Campagna JA, Chung RT, Schneyer AL, Woolf CJ, et
al: Bone morphogenetic protein signaling by hemojuvelin regulates
hepcidin expression. Nat Genet. 38:531–539. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Verga Falzacappa MV, Vujic Spasic M,
Kessler R, Stolte J, Hentze MW and Muckenthaler MU: STAT3 mediates
hepatic hepcidin expression and its inflammatory stimulation.
Blood. 109:353–358. 2007. View Article : Google Scholar
|
|
19
|
Yu PB, Hong CC, Sachidanandan C, Babitt
JL, Deng DY, Hoyng SA, Lin HY, Bloch KD and Peterson RT:
Dorsomorphin inhibits BMP signals required for embryogenesis and
iron metabolism. Nat Chem Biol. 4:33–41. 2008. View Article : Google Scholar
|
|
20
|
Chen Y, Wang Z, Xu M, Wang X, Liu R, Liu
Q, Zhang Z, Xia T, Zhao J, Jiang G, et al: Nanosilver incurs an
adaptive shunt of energy metabolism mode to glycolysis in tumor and
nontumor cells. ACS Nano. 8:5813–5825. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang S, Chen Y, Guo W, Yuan L, Zhang D,
Xu Y, Nemeth E, Ganz T and Liu S: Disordered hepcidin-ferroportin
signaling promotes breast cancer growth. Cell Signal. 26:2539–50.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu S, Suragani RN, Han A, Zhao W, Andrews
NC and Chen JJ: Deficiency of heme-regulated eIF2α kinase decreases
hepcidin expression and splenic iron in HFE−/− mice. Haematologica.
93:753–756. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu S, Suragani RN, Wang F, Han A, Zhao W,
Andrews NC and Chen JJ: The function of heme-regulated eIF2alpha
kinase in murine iron homeostasis and macrophage maturation. J Clin
Invest. 117:3296–305. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brissot P, Bardou-Jacquet E, Jouanolle A-M
and Loréal O: Iron disorders of genetic origin: A changing world.
Trends Mol Med. 707–713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun MY, Zhang M, Chen SL, Zhang SP, Guo
CY, Wang JS, Liu X, Miao Y and Yin HJ: The influence of
hyperlipidemia on endothelial function of FPN1 Tek-cre mice and the
intervention effect of tetramethylpyrazine. Cell Physiol Biochemi.
47:119–128. 2018. View Article : Google Scholar
|
|
26
|
Kennedy DO and Wightman EL: Herbal
extracts and phytochemicals: Plant secondary metabolites and the
enhancement of human brain function. Adv Nutr. 2:32–50. 2011.
View Article : Google Scholar :
|
|
27
|
Ziegler G, Ploch M, Miettinen-Baumann A
and Collet W: Efficacy and tolerability of valerian extract LI 156
compared with oxazepam in the treatment of non-organic insomnia-a
randomized, double-blind, comparative clinical study. Eur J Med
Res. 7:480–486. 2002.
|
|
28
|
Guan Y, An P, Zhang Z, Zhang F, Yu Y, Wu
Q, Shi Y, Guo X, Tao Y and Wang F: Screening identifies the Chinese
medicinal plant Caulis Spatholobi as an effective HAMP expression
inhibitor. J Nutr. 143:1061–1066. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang M, Liu J, Guo W, Liu X, Liu S and
Yin H: Icariin regulates systemic iron metabolism by increasing
hepatic hepcidin expression through Stat3 and Smad1/5/8 signaling.
Int J Mol Med. 37:1379–1388. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen Z, Huo JR, Yang L and Zhu HY: Effect
of ligustrazine on mice model of hepatic veno-occlusive disease
induced by Gynura segetum. J Gastroenterol Hepatol. 26:1016–1021.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ganz T and Nemeth E: Hepcidin and
disorders of iron metabolism. Annu Rev Med. 62:347–360. 2011.
View Article : Google Scholar
|
|
32
|
Sen B, Saigal B, Parikh N, Gallick G and
Johnson FM: Sustained Src inhibition results in signal transducer
and activator of transcription 3 (STAT3) activation and cancer cell
survival via altered Janus-activated kinase-STAT3 binding. Cancer
Res. 69:1958–1965. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Derynck R and Zhang YE: Smad-dependent and
Smad independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hentze MW, Muckenthaler MU, Galy B and
Camaschella C: Two to tango: Regulation of Mammalian iron
metabolism. Cell. 142:24–38. 2010. View Article : Google Scholar : PubMed/NCBI
|