Introduction
PCOS is a common endocrine and metabolic disease
affecting ~10% of women of reproductive age (1). Clinically, PCOS may manifest a
variety of phenotypes, including chronic anovulation,
hyperandrogenism, insulin resistance (IR) and infertility. Women
with PCOS are also more prone to developing diabetes, coronary
heart disease and metabolic syndrome (2). Therefore, the management of PCOS-IR
remains a significant clinical challenge in gynecology.
Oxidative stress (OS), resulting from an imbalance
between radicals and antioxidant defense, has been found to be a
main pathophysiological mechanism in various human diseases
(3). This occurs due to the
overproduction of specific molecules (4), including reactive oxygen species
(ROS), which are generated from nitric oxide (NO) and
malondialdehyde (MDA) and can damage all components of the cell,
including proteins, lipids and DNA (5). Increasing evidence indicates that OS
may be associated with IR and obesity, and may also contribute to
PCOS and its metabolic associations (6,7).
Mitochondria produce the majority of the body’s
cellular energy via oxidative phosphorylation, releasing ROS as a
toxic byproduct (8). The
increased ROS production may have negative consequences; for
example, enhancing the OS in tissues and organisms, disrupting the
natural genetic code and inducing the mitochondrial-mediated
apoptosis (9). Our previous
studies indicated that mtDNA pathogenic mutations led to
mitochondrial dysfunctions and, together with OS, contributed to
the progression of PCOS-IR (10,11). Considering the importance of
mitochondria in energy production and ROS generation, it was
hypothesized that mitochondria-targeted antioxidants may offer
potential as a novel, potential useful drug to prevent the
pathogenesis of PCOS-IR.
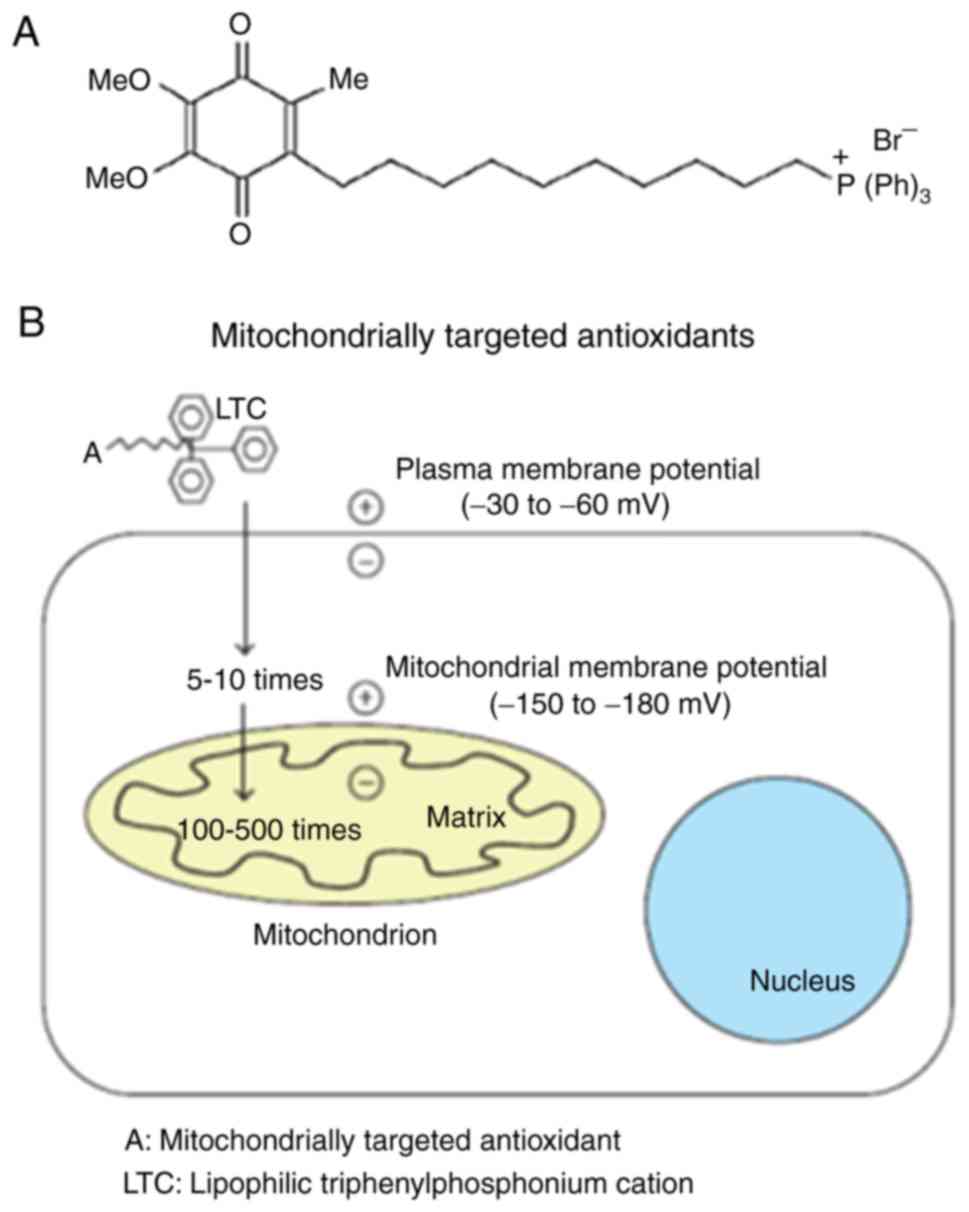
MitoQ10 is one of these
mitochondria-targeted antioxidants, which directly binds to human
mitochondria. At the molecular level, it consists of a
triphenylphosphonium cation (TPP+)2 attached
to a ubiquinone moiety (Fig. 1)
(12). Previous studies have
suggested that MitoQ10 can accelerate the absorbance of
CoQ10 into mitochondria in a mouse model of Alzheimer’s
disease, and this antioxidant is considered to combat ROS
generation in mitochondria (13,14). In vitro and in vivo
experiments have indicated that MitoQ10 has antioxidant
effects (13-15). In addition, MitoQ10 had
been found to be a novel therapeutic intervention in human
autoimmune disease (15) and
cardiovascular disease (16,17). However, whether MitoQ10
has a therapeutic effect on PCOS-IR remains to be elucidated.
In the present study, a rat model manifesting the
features of PCOS-IR was first generated; this was followed by
investigation of the effects of MitoQ10 on the rats and
further discussion of the possible molecular mechanism.
Materials and methods
Animals
A total of 30 female Sprague-Dawley rats (SPF grade,
3 weeks old, 230-250 g) [SCXK (Hu) 2013-0016] were provided by the
Experimental Animal Center, Zhejiang Chinese Medical University
(Hangzhou, China). The rats were housed with four to six animals
per cage under standard laboratory conditions (25°C clean
environment with 50% humidity, 12-h light/dark cycle), all animals
had free access to regular food and tap water. All experiments were
performed in strict accordance with the Animal Care and Use
Committee of Zhejiang Chinese Medical University.
Generation of the PCOS-IR rat model
The 30 rats were divided into three groups for the
following stage of treatment, which were as follows: i) Control
group (n=10): Mice received a normal rodent diet and injection with
olive oil at a volume equal to injections in the experimental
groups; ii) PCOS-IR model group (n=10): 100 µg of
testosterone propionate (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was subcutaneously injected into animals, which was
dissolved in 0.05 ml commercial corn oil. The rats were also fed a
high-fat diet, consisting of 54.2% standard diet, 16.8% lard, 15%
sucrose, 9% casein, 1% minerals, 1% vitamins and 3% malt dextrin.
Following 8 consecutive weeks of treatment, the mice were
sacrificed and the vaginal cytology was analyzed using the
methylene blue staining method by LEICA DM1000 light microscope
(Leica Microsystems GmbH, Wetzlar, Germany); iii)
MitoQ10 treatment group (n=10): Following establishment
of the PCOS-IR animal model, 10 PCOS-IR rats were given clean
drinking water which contained 500 µmol/l MitoQ10
(Sigma-Aldrich; Merck KGaA) for 8 consecutive weeks. The solutions
for MitoQ10 were reformulated fresh every 3 days, and
were stored at 4°C in a dark room.
Detection of endocrine-related
parameters
The orbital venous blood of each animal in the
control, PCOS-IR and MitoQ10 treatment groups were
collected following overnight fasting. The serum levels of fasting
plasma glucose (FPG) and fasting insulin (FINS) were then analyzed.
In addition, the levels of hormones in the rats, including
testosterone (T), lactate dehydrogenase (LH), follicle-stimulating
hormone (FSH), FPG and FINS, were determined using ELISA kits
(Elabscience, Wuhan, China), according to the protocols provided by
the manufacturer. A total of three samples were selected from each
group for analysis. The HOMA-IR was determined as follows: HOMA-IR
= FPG (mmol/l) x FINS (mU/l)/22.5; IR was considered present if the
value of HOMA-IR was >2.8 (18).
Histopathology
All rats were bilaterally ovariectomized following
treatment. The animals were anesthetized with isoflurane, and the
ovaries were removed through dorsolateral incisions. The ovaries
were subsequently underwent 10% formalin-fixed and
paraffin-embedded, incubated overnight at 4°C, stained with
hematoxylin for 5 min and eosin for 3 min under room temperature
(25°C); two pathologists evaluated the morphological changes
between different groups using the LEICA DM1000 light microscope
(Leica Microsystems GmbH).
Measurement of the levels of
OS-associated biomarkers
To examine the expression levels of OS-related
biomarkers, the contents of MDA, total antioxidant capacity
(T-AOC), and the activity of superoxide dismutase (SOD) and
glutathione (GSH) in tissues were analyzed using the kits provided
by Nanjing Jiancheng Bioengineering Institute (Nanjing, China)
according to the manufacturer’s protocol.
ATP analysis
The ATP concentrations in the ovarian tissues were
analyzed using the ATP assay kit, according to the manufacturer’s
protocols. The absorbance was then measured at 636 nm (Nanjing
Jiancheng Bioengineering Institute).
Isolation of mitochondria
The isolation of mitochondria was performed at 4°C
by different centrifugation (700 x g for 10 min; 3,000 x g for 15
min) steps using a mitochondria isolation kit (Baosai Biosciences,
Inc., Beijing, China). The final pellet of mitochondria was
resuspended in buffer, stored on ice, stored for 4°C and used for
experiments within 4 h (19). The
BCA assay was used to determine the final protein
concentration.
Analysis of mitochondrial membrane
potential (MMP) and ROS
MMP was determined using the JC-1 kit (cat. no.
T4069, Sigma-Aldrich; Merck KGaA), according to our previous study
(11). In brief, 50 µg of
the purified rat mitochondria protein was mixed with 5 µg/ml
JC-1 staining for 10 min at 37°C; subsequently, a microscope reader
(Tecan Group, Inc., Ltd., Mannendorf, Switzerland) was used to
record the green and red fluorescence of JC-1 at 530 and 590 nm,
respectively (20). Qualification
of the ROS levels were assessed using 20 µM of ROS-sensitive
dye 2′,7′-dichlo-rodihydrofluorescein diacetate
(H2DCFDA), which cleaves to become DCF (cat. no. D6883;
Sigma-Aldrich; Merck KGaA) (21).
Western blot analysis
For western blot analysis, the total proteins from
the fresh rat ovarian tissues were extracted based on the protocol
originally proposed by Dignam et al (22) with modifications. The 50 µg
protein samples were boiled for 5 min in Laemmli buffer and then
separated by 12% SDS polyacrylamide gel at 180 V. Subsequently, the
separated proteins were transferred onto a polyvinylidene
difluoride membrane at 400 mA for 2 h (EMD Millipore, Billerica,
MA, USA). Following incubation of the PVDF membrane with blocking
solution, the protein bands were mixed with special anti-Bax (cat.
no. 34260; 1:500; Signalway Antibody LLC, College Park, MD, USA),
anti-Bcl-xL (cat. no. 21061; 1:500; Signalway Antibody LLC),
anti-Cyto C (cat. no. ab33484; 1:500; Abcam) and anti-Bcl-2 (cat.
no. ab59348; 1:500; Abcam) antibodies overnight at 4°C. The blots
were then washed and incubated with horseradish peroxidase
(HRP)-conjugated secondary antibodies for 1 h at room temperature,
the second antibodies were as follows: Anti-rabbit IgG, HRP-linked
antibody (cat. no. 7074; 1;500; Signalway Antibody LLC) and
anti-mouse IgG, HRP-linked antibody (cat. no. 7076; 1:500;
Signalway Antibody LLC). The immune complexes were visualized using
an enhanced chemiluminescence kit and analyzed with the
ImageJ® LAS 4000 mini program (National Institutes of
Health, Bethesda, MD, USA).
Statistical analysis
All values are expressed as the mean ± standard
deviation. The statistical analyses were performed using SPSS 19.0
software (IBM SPSS, Armonk, NY, USA). The results were analyzed
with one-way analysis of variance followed by Tukey’s post hoc test
for multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
Reproductive and metabolic features of
the PCOS-IR rat model
Following 8 consecutive weeks under treatment with
testosterone propionate, together with a high fat diet, the PCOS-IR
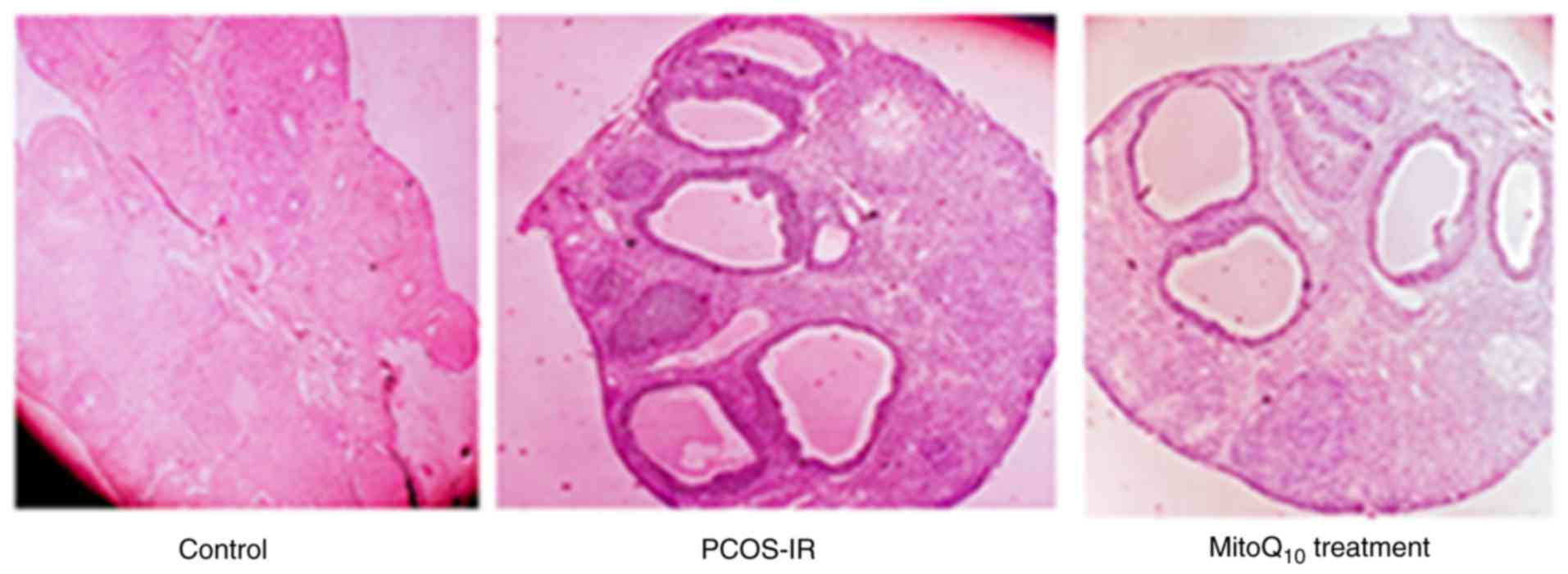
mouse model was successfully established. As presented in Fig. 2, the PCOS-IR animals exhibited
vaginal keratosis and abnormal estrous cycle. In addition, on
measuring endocrine parameters, it was noted that all animals
exhibited IR as the HOMA-IR was >2.8.
Recovery of endocrine status following
MitoQ10 treatment
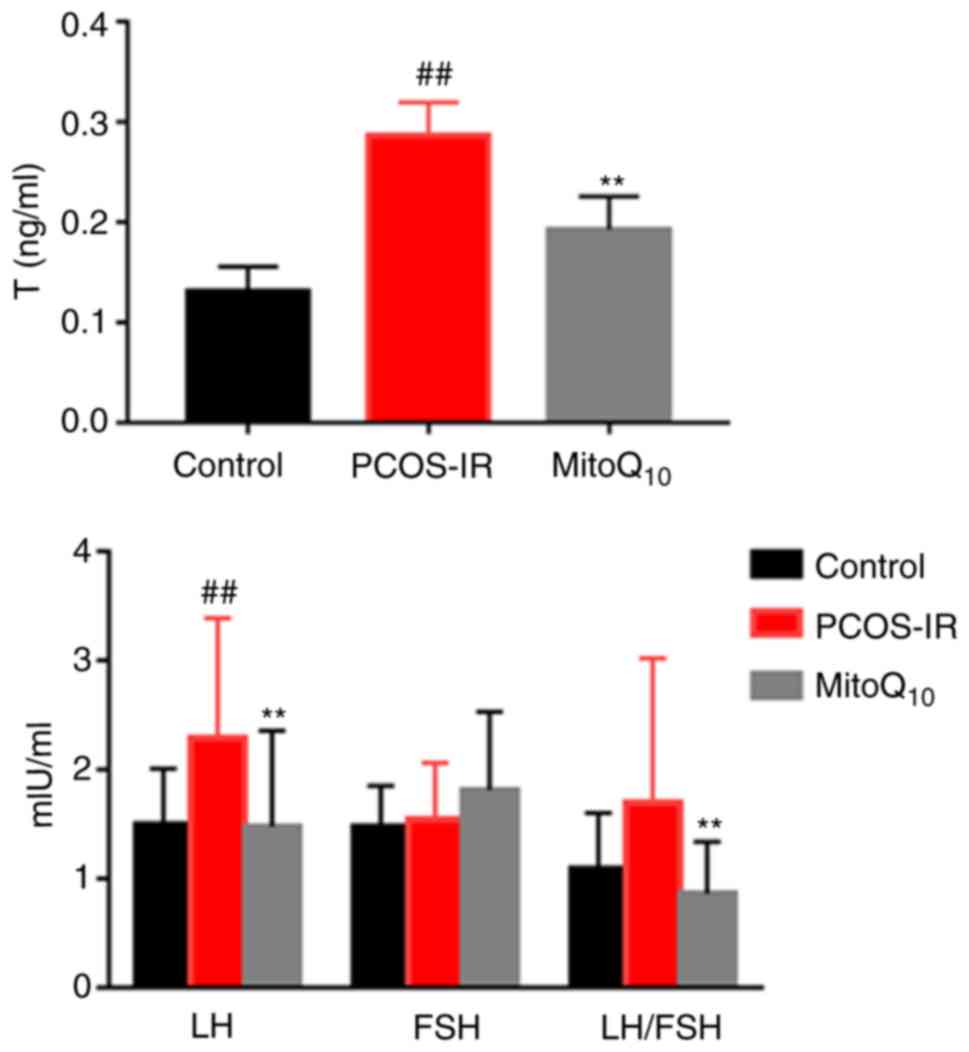
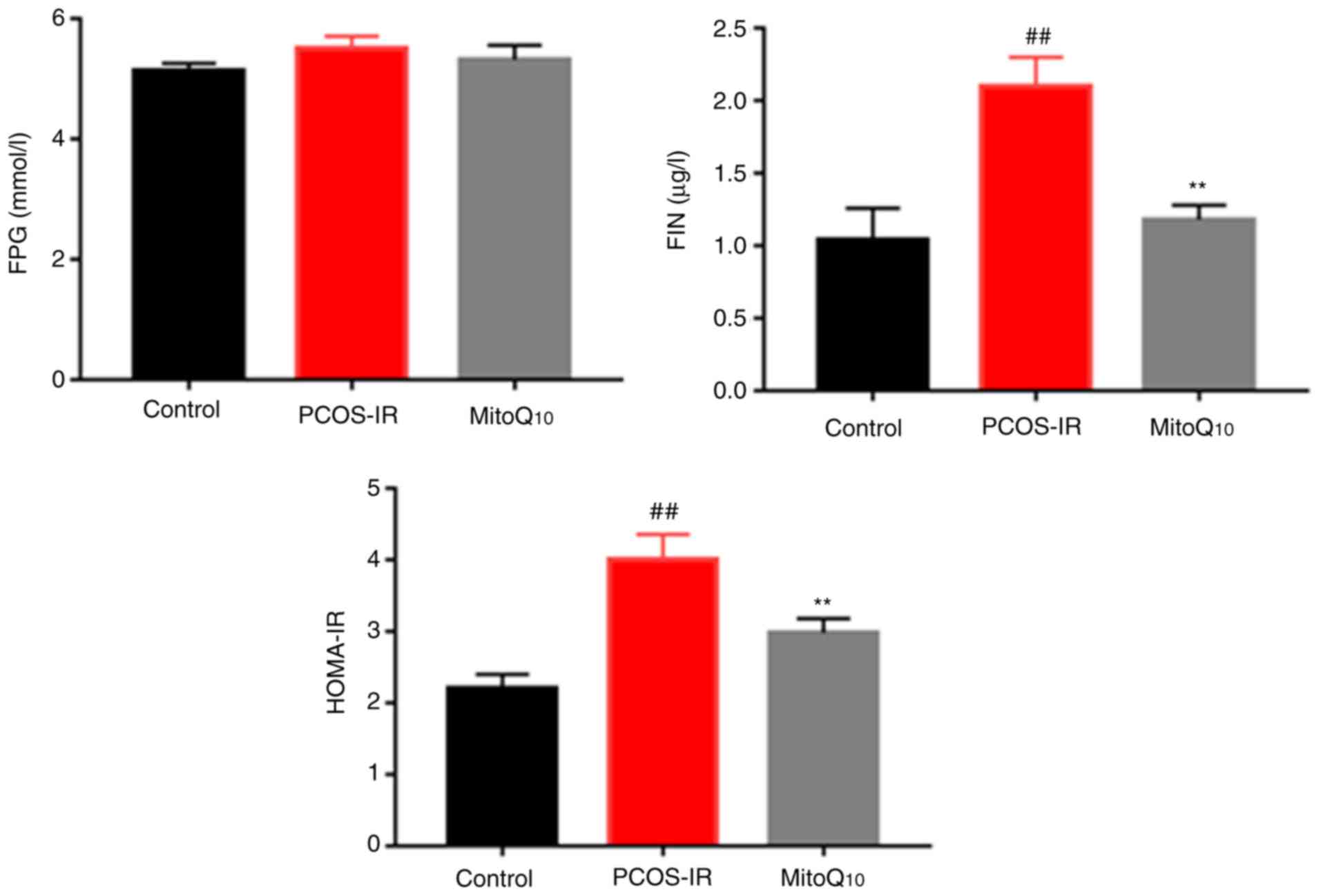
As presented in Figs.
3 and 4, the rats exposed to
testosterone propionate had significantly increased T, LH, LH/FSH,
FIN and HOMA-IR levels, compared with those in the control group
(all P<0.01). Following MitoQ10 treatment, the levels
of these parameters were decreased significantly, compared with
those in the controls (all P<0.01). However, no statistically
significant differences were observed in the levels of FPG or FSH
prior to and following MitoQ10 treatment.
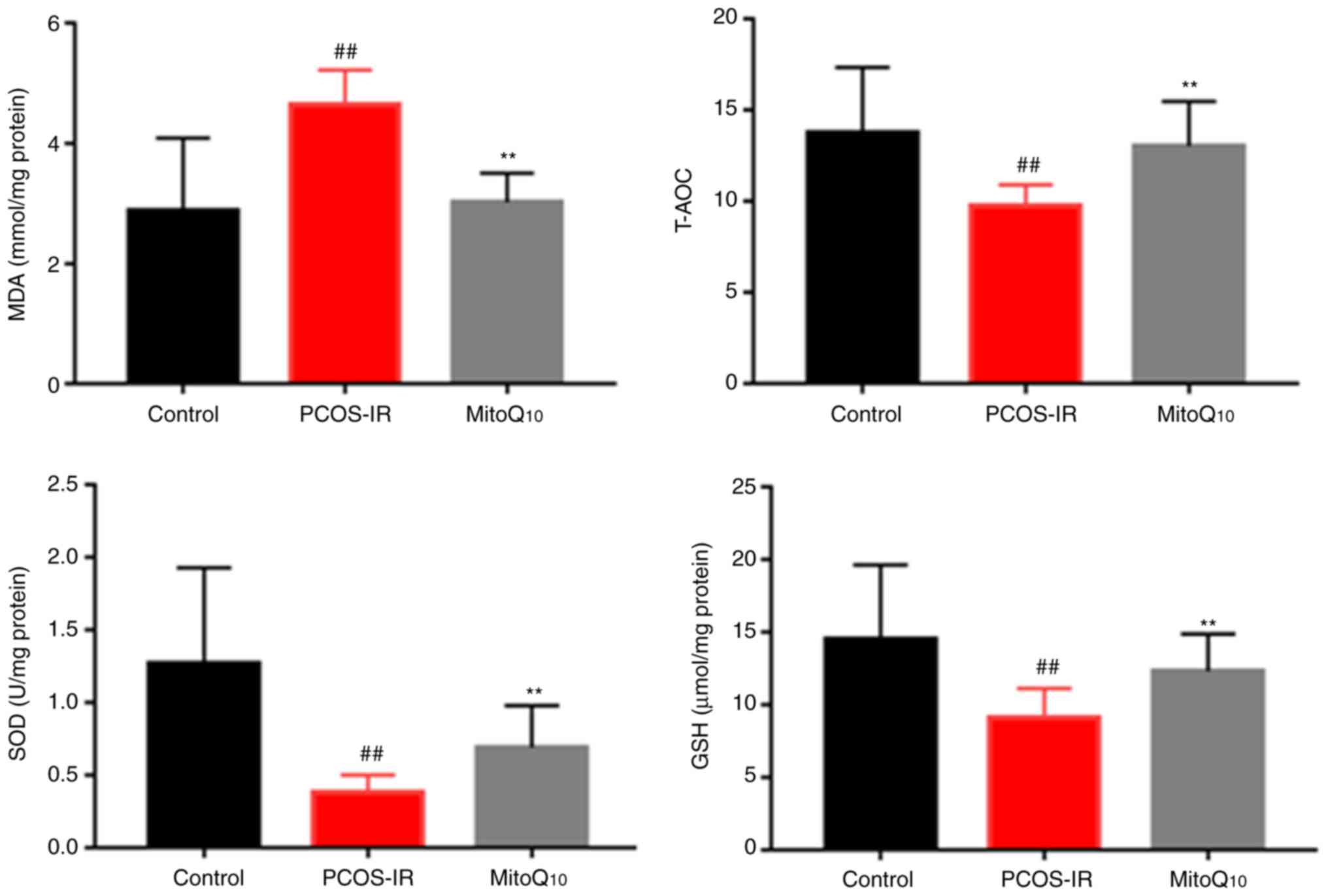
MitoQ10 decreases OS
To determine whether MitoQ10 reversed the
OS of PCOS-IR rats, the expression levels of MDA, T-AOC, SOD and
GSH were analyzed in the rat tissues. As presented in Fig. 5, the levels of T-AOC, SOD and GSH,
which reflected the antioxidant capacity in rats, were enhanced in
the MitoQ10 treatment group, compared with those in the
PCOS-IR rat model (all P<0.01). Consistent with this finding,
the level of MDA, an OS-related biomarker, was significantly
decreased following MitoQ10 administration
(P<0.01).
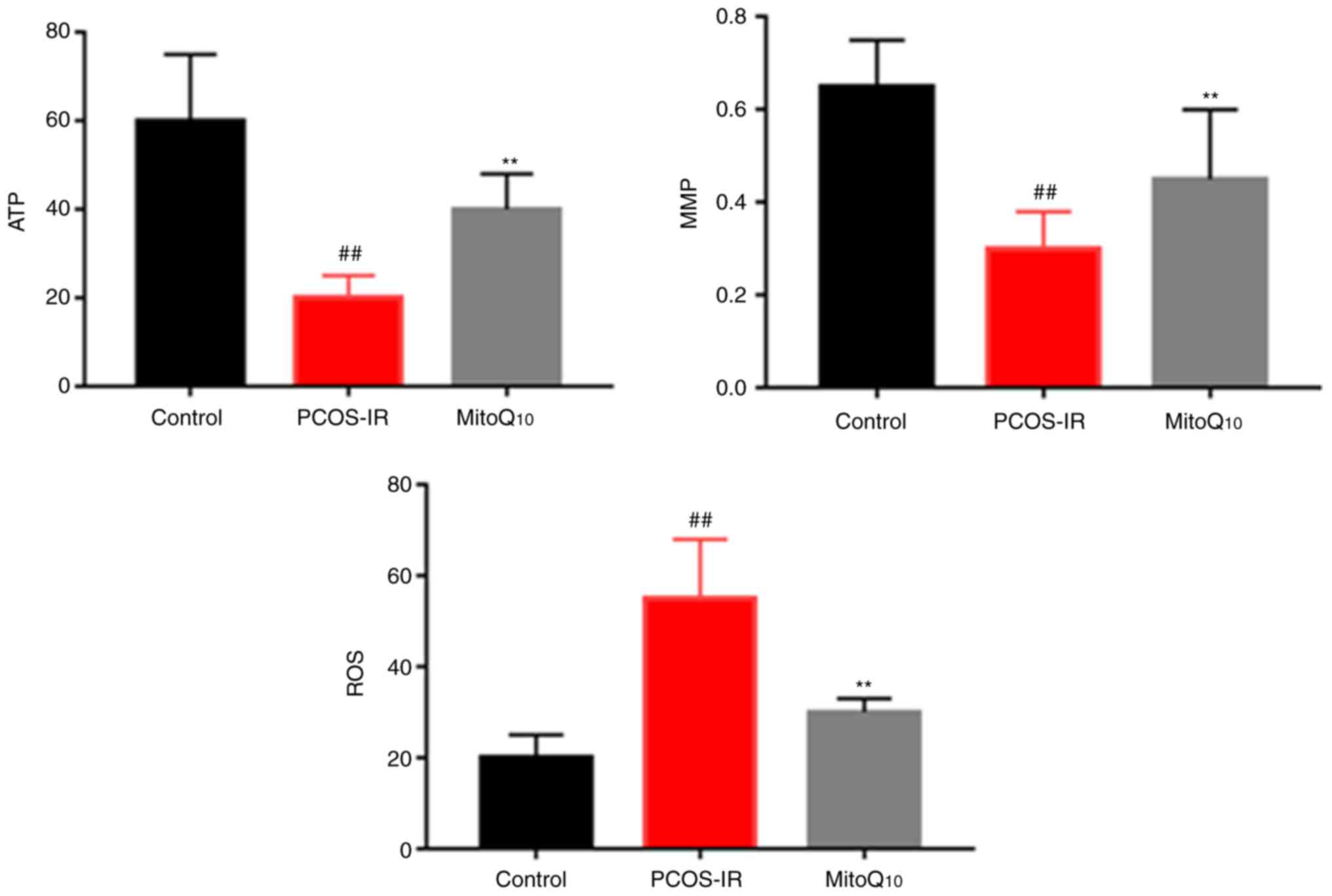
MitoQ10 protects against
mitochondrial damage
To determine whether the administration of
MitoQ10 improved the mitochondrial function, the levels
of ATP, MMP and ROS were analyzed in the Control, PCOS-IR and
MitoQ10 treatment groups. As shown in Fig. 6, it was found that the antioxidant
significantly protected against mitochondrial damage (all
P<0.05).
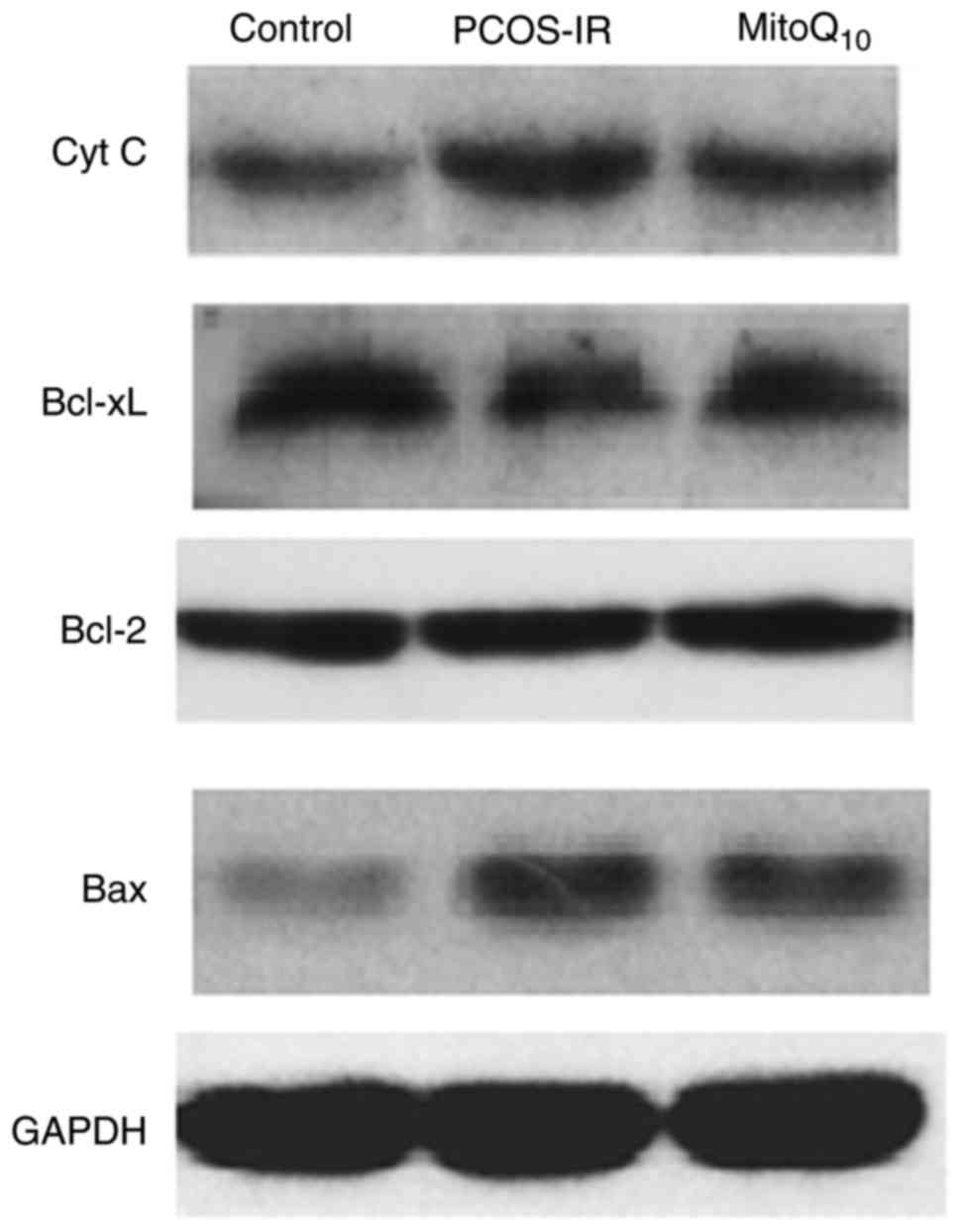
Detection of the expression of Cyto C,
Bcl-xL, Bcl-2 and Bax
The present study further evaluated the expression
levels of the apoptotic and anti-apoptotic proteins, Cyto C,
Bcl-xL, Bcl-2 and Bax, in the different groups to examine whether
the MitoQ10 had a therapeutic effect. As presented in
Figs. 7 and 8, it was found that, compared with the
control group, the PCOS-IR rats exhibited higher levels of Cyto C
and Bax and a higher ratio of Bax to Bcl-2, whereas the level of
Bcl-xL was decreased. However, following MitoQ10
treatment, the levels of Cyto C, Bax and the ratio of Bax to Bcl-2
were decreased and the level of Bcl-xL was increased, compared with
levels in the PCOS-IR group. However, no statistically significant
difference was observed in the level of Bcl-2 between these
groups.
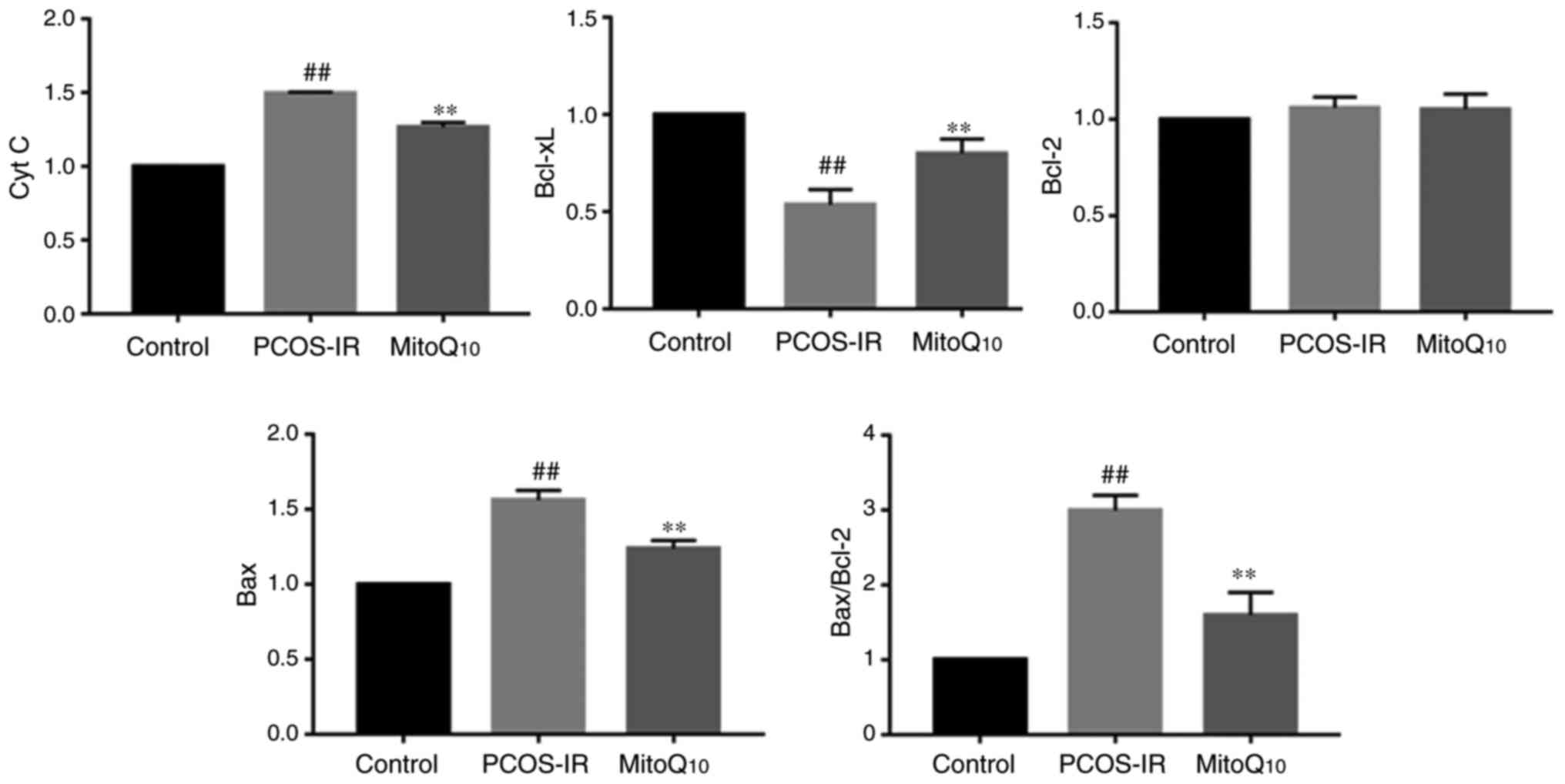 | Figure 8Qualification of the expression
levels of Cyt C, Bcl-xL, Bcl-2 and Bax in the ovaries of the
Control, PCOS-IR and MitoQ10 treatment
groups.##P<0.05, compared with the control;
**P<0.05, compared with the PCOS-IR group. PCOS-IR,
polycystic ovary syndrome and insulin resistance; Cyt C, cytochrome
c; Bcl-2, B-cell lymphoma 2; Bcl-xL, Bcl-extra large; Bax,
Bcl-2-associated X protein. |
Discussion
In the present study, the therapeutic effects of
mitochondria-targeted antioxidant MitoQ10 on an animal
model of PCOS-IR were investigated. Accumulating evidence has
demonstrated that the elevated production of ROS has an active role
in the progression and pathogenesis of PCOS (23,24). Natural antioxidants, including
vitamin C and vitamin E, cannot scavenge sufficient ROS as they
cannot direct bind to mitochondria, highlighting the importance of
developing novel targeted therapies. MitoQ10 can solve
this problem; this antioxidant is composed of ubiquinone moieties
attached to a TPP moiety by a 10-carbon alkyl chain. Lipophilic
cations can readily move through phospholipid bilayers, therefore,
MitoQ10 can be taken up by mitochondria and be effective
in combating ROS (25,26). Once within the mitochondria,
almost all of the accumulated MitoQ10 resides in the
matrix surface of the inner-membrane and prevents OS and
mitochondrial dysfunction (27).
In the present study, to examine the therapeutic
effects of MitoQ10, an animal model of PCOS-IR was
generated via the subcutaneous injection of testosterone
propionate, together with a high-fat diet. In general, this animal
model exhibits the clinical and biochemical characterizations of
women with PCOS, including abnormal estrous cycles, markedly
increased total body weight, polycystic ovaries and variable levels
of T, LH, FSH, FIN and FPG in the serum of rats. Of the 20 rats
injected, 100% exhibited the IR features. Following treatment with
MitoQ10, the morphological characteristic of polycystic
ovaries and the plasma concentrations of FIN in the PCOS group were
improved. These data suggested that MitoQ10 exerted a
therapeutic effect against the development of PCOS-IR.
MDA, T-AOC, SOD and GSH are regarded as common
markers to evaluate OS levels. Of these, MDA is the product of
lipid oxidation, whereas T-AOC indicates the ability of all
antioxidants in different foods to clean harmful free radicals in
blood and cells (28). SOD is an
enzyme which can catalyze superoxide radicals into ordinary
molecular oxygen or hydrogen peroxide, and GSH is another important
antioxidant that can protect cells against ROS. As presented in
Fig. 5, compared with those in
the control group, the rats in the PCOS-IR group had higher MDA
levels and lower T-AOC, SOD and GSH levels, suggesting increased OS
in PCOS-IR. However, the animals treated with MitoQ10
exhibited decreased OS (all P<0.05).
Increasing evidence suggests that the overproduction
of ROS may activate stress signals to the mitochondria and be
important in a number of biological processes; this suggests that
mitochondrial dysfunction, together with the impaired antioxidant
system, may be involved in the pathophysiological mechanism and
progression of PCOS-IR (29,30). There are two general pathways of
apoptosis based on different apoptotic stimuli, termed the death
receptor pathway and the mitochondrial-mediated pathway. In
particular, increased ROS production destroys mitochondrial
function, which may subsequently lead to the loss of MMP and
release of Cyto C (31,32). Therefore, the present study
measured the ROS levels, the level of MMP and the expression of
apoptosis-related proteins to confirm whether MitoQ10
induced apoptosis via the mitochondrial pathway. The data revealed
that the PCOS-IR rats treated with MitoQ10 exhibited
recovery of mitochondrial functions, including increased ATP and
MMP levels; by contrast, the ROS level was significantly decreased.
This supported the hypothesis that MitoQ10 can reduce
the cellular OS resulting from the mitochondrial dysfunction
responsible for PCOS-IR.
Bcl-2 and Bcl-xL are anti-apoptotic members of the
Bcl-2 family, which function as a ‘life/death switch’ that
integrates diverse inter- and intracellular cues to determine
whether or not the stress apoptotic pathway is activated (33,34). In response to apoptotic stimuli,
the balance and interactions of anti-apoptotic and pro-apoptotic
Bcl proteins influence the activation of downstream pro-apoptotic
proteins Bcl-2 homologous antagonist/killer (Bak) and Bax (35). Of note, Bak and Bax are two
nuclear-encoded proteins present in higher eukaryotes that are able
to pierce the mitochondrial outer membrane to mediate cell death by
apoptosis (36). Once the
mitochondrial-mediated cell death pathway is activated, the
conformations of Bax and Bak are altered, MMP is decreased and
pro-apoptotic proteins are released from the intermembrane space
into the cytosol (37,38). Following release into the
cytoplasm, Cyt C stimulates apoptosome formation followed by the
activation of caspase-9. Therefore, the release of Cyt C is an
early event in the pathway of mitochondrial-mediated apoptosis. As
presented in Figs. 7 and 8, the present study found that
MitoQ10 significantly increased the expression of
Bcl-xL, whereas the levels of total Cyt C and Bax decreased
following MitoQ10 treatment. However, no difference was
observed in the expression level of Bcl-2 prior to and following
MitoQ10 administration, suggesting that
MitoQ10 may not affect the downregulation of Bcl-2. A
range of stimuli induce apoptosis by releasing Cyt C from the
mitochondria into the cytoplasm, where it activates caspases; the
mechanisms by which these stimuli cause Cyt C release from
mitochondria remain to be fully elucidated, however, some or all
may involve increased mitochondrial OS (39). MitoQ10 can assist in
elucidating the role of oxidative damage in apoptosis, subsequently
preventing apoptotic cell death.
Although the detection of Cyt C was not based on the
total protein, it was noted that detecting total Cyt C protein has
been reported in several studies; for example, Eleftheriadis et
al (40) demonstrated that
serum Cyt C levels may be a biomarker for mitochondrial and
cellular damage. In addition, Li et al (41) measured Cyt C levels via an optical
microfiber, and a study by Radhakrishnan et al (42) used an ELISA approach to determine
plasma Cyt C levels. Overall, although the qualification of total
Cyt C levels cannot reflect programmed cell death, it is only the
way for reflecting the early event of apoptosis.
Based on the above observations, the present study
hypothesized that the possible molecular mechanism underlying
MitoQ10 in the treatment of PCOS-IR rats may be as
follows: Administration of MitoQ10 may bind to
mitochondria and scavenge ROS, indicating its antioxidant effects.
Subsequently, decreased ROS levels protect the pancreatic cells
from death, most likely from mitochondrial-mediated apoptosis in
the form of increasing the expression levels of Bcl-xL, decreasing
the expression levels of Cyt C and Bax, and recovering
mitochondrial functions, with improved ATP and MMP levels. As a
result, the decreased OS may prevent the pancreatic cells from
death or apoptosis. Finally, the endocrine and reproductive
conditions of PCOS-IR recovered following MitoQ10
treatment. Therefore, this antioxidant may be a potential agent for
patients with PCOS-IR in the future.
Acknowledgments
The authors would like to thank the members of their
lab for discussion. The authors are grateful to Dr Qi Liu from
Shaoxing People’s Hospital, Zhejiang University for critical
reading of this manuscript.
Funding
This study was supported by the grants from the
Hangzhou Bureau of Science and Technology (grant no. 20150633B16),
the Ministry of Public Health from Zhejiang Province (grant nos.
2013KYA158 and 2018ZH019) and the Natural Science Foundation of
Zhejiang Province (grant nos. LY14H270008 and LY15H280007).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors’ contributions
YD designed the experiments, ZJ performed the
histopathological analysis, BX performed the statistical analysis,
LZ performed the animal experiments, CZ and JL analyzed the data.
YD wrote the manuscript. All authors discussed the results and
implications and commented on the manuscript at all stages. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Animal Care and
Use Committee of Zhejiang Chinese Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
McCartney CR and Marshall JC: Clinical
Practice. Clinical practice. Polycystic ovary syndrome. N Engl J
Med. 375:54–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ali AT: Polycystic ovary syndrome and
metabolic syndrome. Ceska Gynekol. 80:279–289. 2015.PubMed/NCBI
|
|
3
|
Kalyanaraman B: Teaching the basics of
redox biology to medical and graduate students: Oxidants,
antioxidants and disease mechanisms. Redox Biol. 1:244–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu X, Zhu Q, Zhang M, Yin T, Xu R, Xiao
W, Wu J, Deng B, Gao X, Gong W, et al: Isoliquiritigenin
ameliorates acute pancreatitis in mice via inhibition of oxidative
stress and modulation of the Nrf2/HO-1 pathway. Oxid Med Cell
Longev. 2018.7161592:2018.
|
|
5
|
Dalle-Donne I, Rossi R, Colombo R,
Giustarini D and Milzani A: Biomarkers of oxidative damage in human
disease. Clin Chem. 52:601–623. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Escobar-Morreale HF, Luque-Ramírez M and
San Millán JL: The molecular-genetic basis of functional
hyperandrogenism and the polycystic ovary syndrome. Endocr Rev.
26:251–282. 2005. View Article : Google Scholar
|
|
7
|
Zuo T, Zhu M and Xu W: Roles of oxidative
stress in polycystic ovary syndrome and cancers. Oxid Med Cell
Longev. 2016.8589318:2016.
|
|
8
|
Picard M, Wallace DC and Burelle Y: The
rise of mitochondria in medicine. Mitochondrion. 30:105–116. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Böttger EC and Schacht J: The
mitochondrion: A perpetrator of acquired hearing loss. Hear Res.
303:12–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ding Y, Xia BH, Zhang CJ and Zhuo GC:
Mitochondrial tRNALeu(UUR) C3275T, tRNAGln T4363C and tRNALys
A8343G mutations may be associated with PCOS and metabolic
syndrome. Gene. 642:299–306. 2018. View Article : Google Scholar
|
|
11
|
Ding Y, Xia BH, Zhang CJ and Zhuo GC:
Mutations in mitochondrial tRNA genes may be related to insulin
resistance in women with polycystic ovary syndrome. Am J Transl
Res. 9:2984–2996. 2017.PubMed/NCBI
|
|
12
|
Kelso GF, Porteous CM, Coulter CV, Hughes
G, Porteous WK, Ledgerwood EC, Smith RA and Murphy MP: Selective
targeting of a redox-active ubiquinone to mitochondria within
cells: antioxidant and antiapoptotic properties. J Biol Chem.
276:4588–4596. 2001. View Article : Google Scholar
|
|
13
|
Smith RA and Murphy MP: Animal and human
studies with the mitochondria-targeted antioxidant MitoQ. Ann NY
Acad Sci. 1201:96–103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Manczak M, Mao P, Calkins MJ, Cornea A,
Reddy AP, Murphy MP, Szeto HH, Park B and Reddy PH:
Mitochondria-targeted antioxidants protect against amyloid-beta
toxicity in Alzheimer’s disease neurons. J Alzheimers Dis. 20(Suppl
2): S609–S631. 2010. View Article : Google Scholar
|
|
15
|
Mao P, Manczak M, Shirendeb UP and Reddy
PH: MitoQ, a mitochondria-targeted antioxidant, delays disease
progression and alleviates pathogenesis in an experimental
autoimmune encephalomyelitis mouse model of multiple sclerosis.
Biochim Biophys Acta. 1832.2322–2331. 2013.
|
|
16
|
Graham D, Huynh NN, Hamilton CA, Beattie
E, Smith RA, Cochemé HM, Murphy MP and Dominiczak AF:
Mitochondria-targeted antioxidant MitoQ10 improves endothelial
function and attenuates cardiac hypertrophy. Hypertension.
54:322–328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McLachlan J, Beattie E, Murphy MP, Koh-Tan
CH, Olson E, Beattie W, Dominiczak AF, Nicklin SA and Graham D:
Combined therapeutic benefit of mitochondria-targeted antioxidant,
MitoQ10, and angiotensin receptor blocker, losartan, on
cardiovascular function. J Hypertens. 32:555–564. 2014. View Article : Google Scholar :
|
|
18
|
Keskin M, Kurtoglu S, Kendirci M, Atabek
ME and Yazici C: Homeostasis model assessment is more reliable than
the fasting glucose/insulin ratio and quantitative insulin
sensitivity check index for assessing insulin resistance among
obese children and adolescents. Pediatrics. 115:e500–e503. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yarian CS, Toroser D and Sohal RS:
Aconitase is the main functional target of aging in the citric acid
cycle of kidney mitochondria from mice. Mech Ageing Dev. 127:79–84.
2006. View Article : Google Scholar
|
|
20
|
Yuan Y, Chen Y, Zhang P, Huang S, Zhu C,
Ding G, Liu B, Yang T and Zhang A: Mitochondrial dysfunction
accounts for aldosterone-induced epithelial-to-mesenchymal
transition of renal proximal tubular epithelial cells. Free Radic
Biol Med. 53:30–43. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Raza H, John A and Howarth FC: Increased
oxidative stress and mitochondrial dysfunction in zucker diabetic
rat liver and brain. Cell Physiol Biochem. 35:1241–1251. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dignam JD, Lebovitz RM and Roeder RG:
Accurate transcription initiation by RNA polymerase II in a soluble
extract from isolated mammalian nuclei. Nucleic Acids Res.
11:1475–1489. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Victor VM, Rovira-Llopis S, Bañuls C,
Diaz-Morales N, Martinez de Marañon A, Rios-Navarro C, Alvarez A,
Gomez M, Rocha M and Hernández-Mijares A: Insulin resistance in
PCOS patients enhances oxidative stress and leukocyte adhesion:
Role of myeloperoxidase. PLoS One. 11:e015–1960. 2016. View Article : Google Scholar
|
|
24
|
Serrano Mujica L, Bridi A, Della Méa R,
Rissi VB, Guarda N, Moresco RN, Premaor MO, Antoniazzi AQ,
Gonçalves PBD and Comim FV: Oxidative stress and metabolic markers
in pre- and postnatal polycystic ovary syndrome rat protocols. J
Inflamm Res. 11:193–202. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ross MF, Kelso GF, Blaikie FH, James AM,
Cochemé HM, Filipovska A, Da Ros T, Hurd TR, Smith RA and Murphy
MP: Lipophilic triphenylphosphonium cations as tools in
mitochondrial bioenergetics and free radical biology. Biochemistry
(Mosc). 70:222–230. 2005. View Article : Google Scholar
|
|
26
|
Firsov AM, Kotova EA, Orlov VN, Antonenko
YN and Skulachev VP: A mitochondria-targeted antioxidant can
inhibit peroxidase activity of cytochrome c by detachment of the
protein from liposomes. FEBS Lett. 590:2836–2843. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
James AM, Cochemé HM and Murphy MP:
Mitochondria-targeted redox probes as tools in the study of
oxidative damage and ageing. Mech Ageing Dev. 126:982–986. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yilmaz N, Inal HA, Gorkem U, Sargin Oruc
A, Yilmaz S and Turkkani A: Follicular fluid total antioxidant
capacity levels in PCOS. J Obstet Gynaecol. 36:654–657. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Abu Bakar MH, Sarmidi MR, Tan JS and
Mohamad Rosdi MN: Celastrol attenuates mitochondrial dysfunction
and inflammation in palmitate-mediated insulin resistance in C3A
hepatocytes. Eur J Pharmacol. 799:73–83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hafizi Abu Bakar M, Kian Kai C, Wan Hassan
WN, Sarmidi MR, Yaakob H and Zaman Huri H: Mitochondrial
dysfunction as a central event for mechanisms underlying insulin
resistance: The roles of long chain fatty acids. Diabetes Metab Res
Rev. 31:453–475. 2015. View Article : Google Scholar
|
|
31
|
Yan Y, Su X, Liang Y, Zhang J, Shi C, Lu
Y, Gu L and Fu L: Emodin azide methyl anthraquinone derivative
triggers mitochondrial-dependent cell apoptosis involving in
caspase-8-mediated Bid cleavage. Mol Cancer Ther. 7:1688–1697.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang XH, Jia DZ, Liang YJ, Yan SL, Ding Y,
Chen LM, Shi Z, Zeng MS, Liu GF and Fu LW: Lgf-YL-9 induces
apoptosis in human epidermoid carcinoma KB cells and multidrug
resistant KBv200 cells via reactive oxygen species-independent
mitochondrial pathway. Cancer Lett. 249:256–270. 2007. View Article : Google Scholar
|
|
33
|
Kluck RM, Bossy-Wetzel E, Green DR and
Newmeyer DD: The release of cytochrome c from mitochondria: a
primary site for Bcl-2 regulation of apoptosis. Science.
275:1132–1136. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang J, Liu X, Bhalla K, Kim CN, Ibrado
AM, Cai J, Peng TI, Jones DP and Wang X: Prevention of apoptosis by
Bcl-2: Release of cytochrome c from mitochondria blocked. Science.
275:1129–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nechushtan A, Smith CL, Lamensdorf I, Yoon
SH and Youle RJ: Bax and Bak coalesce into novel
mitochondria-associated clusters during apoptosis. J Cell Biol.
153:1265–1276. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Susin SA, Lorenzo HK, Zamzami N, Marzo I,
Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler
M, et al: Molecular characterization of mitochondrial
apoptosis-inducing factor. Nature. 397:441–446. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang JY, Yi T, Liu J, Zhao ZZ and Chen
HB: Quercetin induces apoptosis via the mitochondrial pathway in KB
and KBv200 cells. J Agric Food Chem. 61:2188–2195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Westphal D, Dewson G, Czabotar PE and
Kluck RM: Molecular biology of Bax and Bak activation and action.
Biochim Biophys Acta. 1813.521–531. 2011.
|
|
39
|
Hampton MB and Orrenius S: Dual regulation
of caspase activity by hydrogen peroxide: Implications for
apoptosis. FEBS Lett. 414:552–556. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Eleftheriadis T, Pissas G, Liakopoulos V
and Stefanidis I: Cytochrome c as a potentially clinical useful
marker of mitochondrial and cellular damage. Front Immunol.
7:2792016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li H, Huang Y, Chen C, Xiao A, Hou G,
Huang Y, Feng X and Guan BO: Real-time cellular cytochrome c
monitoring through an optical microfiber: Enabled by a
silver-decorated graphene nanointerface. Adv Sci (Weinh).
5:17010742018. View Article : Google Scholar
|
|
42
|
Radhakrishnan J, Origenes R, Littlejohn G,
Nikolich S, Choi E, Smite S, Lamoureux L, Baetiong A, Shah M and
Gazmuri RJ: Plasma cytochrome c detection using a highly sensitive
electrochemiluminescence enzyme-linked immunosorbent assay. Biomark
Insights. 12:11772719177469722017. View Article : Google Scholar : PubMed/NCBI
|