Introduction
Cyclic variation of hormones leads to monthly
shedding and regrowth of the uterine lining during menstruation.
The ectopic endometrium results in the formation of pelvic cysts
and adhesions (1). Endometriosis
(Ems) is a painful disease that is easily recurrent and refractory
due to its hormone-dependence (2). Therefore, treatment remains
challenging for numerous patients and the clinicians in their
professional field (3).
Consequently, studying the pathogenesis of Ems will provide an
insight into the disease development and create opportunities for
formulating novel prevention strategies (1).
Ems is a common and frequently-occurring disease in
young and middle-aged women, and its morbidity has displayed a
marked increasing trend in recent years (4). It is a refractory disease
characterized by extensive lesion, diversified morphology, invasion
and recurrence (5). Although the
pathogenesis of Ems remains unclear, the leading theory involves
menstrual blood reflux (4). In
addition, the eutopic endometrium determinism hypothesis is
attracting extensive attention from scholars (6). Endometrial cells have to penetrate
three layers, namely the peritoneal fluid, macrophages and
peritoneal extracellular matrix (5), completing the three steps of
adhesion, invasion and angiogenesis. Recently, the immunological
mechanism of Ems has received increasing attention (6). Macrophages are multi-functional
immune cells, and abnormality in their number and function may be
one of the important reasons for Ems pathogenesis (3).
MicroRNAs (miRNAs) are a class of small endogenous
RNA molecules with a length of 22-23 nucleotides and with a
regulatory function, which has been identified in eukaryotes in
recent years (7). miRNAs are
extensively distributed in plants, animals and viruses, and can
negatively regulate gene expression at the post-transcription level
through complementary pairing with their target mRNAs (8). Therefore, they can induce mRNA
degradation or translation inhibition. As important regulatory
molecules, miRNAs participate in a series of important life
processes, including virus defense, hematopoiesis, organ formation,
cell proliferation and apoptosis, fat metabolism and tumorigenesis
(7,8).
Nuclear factor (NF)-κB is an important nuclear
transcription factor and exists in multiple cell types, and is
involved in gene regulation in numerous physiological and
pathological processes, including inflammation, immunity, cell
proliferation, apoptosis and embryogenesis (9,10).
In addition, NF-κB is a universal transcription factor acting on
multiple target genes (11),
which includes the coding genes of cytokines, membrane receptors,
membrane adhesion molecules, acute phase proteins, growth factors
and transcription factors (10).
Therefore, NF-κB activity is associated with biological processes
such as cell growth and differentiation, inflammatory response,
immune response and tumor growth. The NF-κB-mediated signaling
pathway can increase the expression of monocyte and T-cell
selective chemokine RANTES, thus inducing Ems (11).
Vascular endothelial growth factor (VEGF) has also
been reported to be a potential therapeutic target of Ems (12). VEGF, a member of the
platelet-derived growth factor and VEGF family (12), is mainly expressed in the
peripheral meta-nephron, armpit and jugular vein regions at the
embryonic period (13). Research
on Ems has revealed that VEGF serves an important role in this
condition (13). Tang et
al (14) reported that
miR-138 protected against inflammation due to cerebral
ischemia/reperfusion injury in rats. The present study aimed to
investigate the role of miR-138 in Ems and the possible underlying
mechanism.
Materials and methods
Experimental model
The present study was approved by the Institutional
Animal Care and Use Committee of Qilu Hospital of Shandong
University (Jinan, China), and all the procedures were performed
according to the National Institutes of Health Guidelines for the
Care and Use of Laboratory Animals. A total of 16 severe combined
immunodeficiency mice (20±2 g; female; 8-9-weeks-old, n=8/every
group) were purchased from Beijing Vital River Laboratory Animal
Technology Co., Ltd. (Beijing, China) and housed in a light/dark
cycle of 12-h under standard conditions (22-24°C). Under anesthesia
with 30 mg/kg of pentobarbital, endometriotic tissue was collected,
cut into coarse fragments and suspended in PBS. Endometriotic cells
(1×106 cells/l) were then implanted into the peritoneal
cavity of the mice, and the mice were injected with 30
µg/kg/day estradiol benzoate (Shanghai Aladdin Biochemical
Technology Co. Ltd., Shanghai, China) for 2 weeks. In control
group, the mice were implanted with normal saline into the
peritoneal cavity of the mice. Following the induction of
endometriotic cells, mice were sacrificed under 30 mg/kg of
pentobarbital.
Flow cytometry
Subsequent to sacrifice, 5 ml PBS was injected into
the mice by intraperitoneal injection. Following gentle massage for
10 min, peritoneal fluid-containing cells was harvested and
centrifuged at 1,000 × g for 10 min at 4°C, and the cell pellet was
reconstituted in PBS with 2% fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Cells were suspended in 100
µl washing media and incubated with FITC-conjugated rat
anti-mouse CD11b (cat. no. 553310; 1:100; BD Pharmingen; BD
Biosciences, San Jose, CA, USA) at 4°C for 30 min, followed by
analysis using an LSRII flow cytometer (BD Biosciences).
Cell culture and treatments
ESCs were purchased from the Shanghai Cell Bank of
the Chinese Academy of Sciences (Shanghai, China) and cultured in
Dulbecco’s modified Eagle’s medium (DMEM)/F12 (Invitrogen; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.), 20 nM HEPES (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at 37°C and 5% CO2. Next, ESCs were
transfected according to the manufacturer’s protocol with miR-138,
anti-miR-138, NF-κB plasmids and VEGF plasmids and negative mimics
(50 nM) using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) at 37°C and 5% CO2 for 48 h.
Following 4 h of transfection, NF-κB inhibitor (2 µM BAY
11-7085) or VEGF inhibitor (2 µM Tanshinone IIA) was added
into cell for 44 h.
Transwell co-culture
Human leukemia monocytic cell line THP-1 cells
(1×106 cells/well) were purchased from Shanghai cell
bank of Chinese Academy of Sciences and seeded into 6-well plates
and treated with 1 mM phorbol-12-myristate-13-acetate. THP-1 is a
leukemia monocytic cell line and is used to research inflammation.
Co-culture with ESCs and THP-1 cells have been used as an
inflammation model in endometriosis. ESCs transfected for 4 h were
seeded (1×106 cells/well) onto polycarbonate Transwell
inserts in the 6-well plates (pore size, 0.4 µm; Costar;
Corning, Inc., Corning, NY, USA). THP-1 cells were seeded at lower
chambers in DMEM/F12 with 10% FBS. The co-culture of cells was
incubated for 48 h at 37°C and 5% CO2. Then, the cells
from the co-culture were separated and then ESCs were collected by
centrifugation at 500 × g for 10 min for other experiments.
Cell proliferation and lactate
dehydrogenase (LDH) assays
After 48 h of co-culture, MTT solution (5 mg/ml) was
added to each well for 4 h at 37°C. Next, the medium was removed,
and 150 µl DMSO was added to dissolve the purple crystals
for 20 min at 37°C. The absorbance of each well was measured at 490
nm to determine cell proliferation.
In addition, the LDH levels of cells were measured
following 48 h of co-culture using LDH activity kits (cat. no.
C0016; Beyotime Institute of Biotechnology, Nanjing, China)
according to the manufacturer’s protocol. The absorbance of each
well was measured at 490 nm to determine the LDH activity.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was isolated from the endometriosis
tissues after surgery at 2 weeks or cells using 1 ml TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). RNA concentration was
measured using an ELISA instrument. RNA concentration was measured
using corresponding ELISA kits (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China) at optical density (OD) 260/OD280 nm.
Total RNA was used to synthesize cDNA using a PrimeScript RT
Reagent kit (Takara Biotechnology Co., Ltd., Dalian, China). qPCR
was performed using a One Step SYBR PrimeScript PLUS RTPCT kit
(Takara Biotechnology Co., Ltd.) and a Rotor-Gene 3000 Real-Time
DNA analysis system (Corbett; Qiagen GmbH, Hilden, Germany). The
PCR conditions were as follows: 95°C for 90 sec; 40 cycles for 95°C
for 15 sec, 60°C for 30 sec and 72°C for 30 sec. MicroRNA-138
primer 5′-CTC TAT GCG TCT GTA CAA G-3′ and 5′-AGC UGG UGU UGU GAA
UCA GGC CG-3′. miRNA expression was quantified using
2−ΔΔCq method (15).
Gene microarray hybridization
A total of 500 ng total RNA was hybridized using G3
Mouse Whole Genome GE 8 60 K Microarray G4852A platform (Agilent
Technologies, Inc., Santa Clara, CA, USA). Data were quantified
using Agilent Feature Extraction Software (version A.10.7.3.1;
Agilent Technologies, Inc.).
Apoptosis and DAPI assays
In order to determine the apoptosis rate, cells were
washed with PBS and fixed with 4% paraformaldehyde for 15 min.
Next, the cells were stained with the Annexin V-FITC/PI double
staining apoptosis detection kit (BD Biosciences). The apoptosis
rate was analyzed with an LSRII flow cytometer.
In order to stain the cell nucleus, cells were
washed with PBS and fixed with 4% paraformaldehyde for 15 min.
Subsequently, cells were stained with DAPI assay (Beyotime
Institute of Biotechnology) for 15 min in the dark. Cells were
observed and images were captured using an X71 inverted microscope
(Olympus Corporation, Tokyo, Japan).
ELISA assay and western blot analysis for
protein expression determination
The THP-1 and ESCs from the co-culture were
separated and cytokine levels were measured separately. The
supernatant was collected at 48 h following centrifugation at
12,000 × g for 10 min at 4°C. This was then used to measure the
tumor necrosis factor α (TNF-α; cat. no. H052), interleukin (IL)-1β
(cat. no. H002), IL-6 (cat. no. H007) and IL-18 (cat. no. H015)
levels using corresponding ELISA kits (Nanjing Jiancheng Biology
Engineering Institute, Nanjing, China), according to the
manufacturer’s protocol.
For western blot analysis, cells were collected
after centrifugation at 12,000 × g at 4°C and lysed with
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology). The protein concentration was measured using a BCA
kit (Beyotime Institute of Biotechnology). Next, the isolated
protein was subjected to 6-12% SDS-PAGE and then transferred to a
polyvinylidene difluoride membrane. The membrane was blocked with
5% non-fat-milk in Tris-buffered saline/Tween 20 (TBST) for 1 h at
37°C, following by incubation overnight at 4°C with VEGF (cat. no.
sc-81670; 1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), NF-κB (cat. no. sc-71675; 1:1,000; Santa Cruz Biotechnology,
Inc.), B-cell lymphoma 2-associated X protein (Bax; cat. no.
sc-6236; 1:1,000; Santa Cruz Biotechnology, Inc.) and GAPDH (cat.
no. sc-51631; 1:5,000; Santa Cruz Biotechnology, Inc.) primary
antibodies. Subsequently, the membrane was washed with TBST for 30
min and incubated with a horseradish peroxidase-conjugated goat
anti-rabbit IgG secondary antibody (cat. no. sc-2004; 1:5,000;
Santa Cruz Biotechnology, Inc.) for 1 h at 37°C. The protein
signals were detected using a Super ECL Plus kit (KeyGen Biotech
Co., Ltd., Nanjing, China), and GAPDH served as the internal
control. A protein blank was analyzed using Image Lab 3.0 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Immunofluorescence
Cells were washed with PBS and fixed with 4%
paraformaldehyde for 15 min at room temperature. Subsequently,
cells were blocked with 5% bovine serum albumin (Beyotime Institute
of Biotechnology) and 0.1% Tris-X100 in TBST for 1 h at 37°C and
incubated with NF-κB (cat. no. sc-71675; 1:100; Santa Cruz
Biotechnology, Inc.) overnight at 4°C. Cells were washed with PBS
and incubated with goat anti-rabbit IgG-CFL 555 (cat. no.
sc-362272; 1:5,000; Santa Cruz Biotechnology, Inc.) for 1 h at
37°C. Cells were washed with PBS and stained with DAPI assay for 15
min in darkness at room temperature. Cells were observed and images
were captured using fluorescence X71 inverted microscope (Olympus
Corporation).
Statistical analysis
Data are presented as the mean ± standard deviation
using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) and P<0.05 was
considered to indicate a statistically significant difference.
One-way analysis of variance by Tukey’s post hoc test was used to
determine any statistically significant differences among
groups.
Results
miR-138 expression and function in a rat
model of Ems
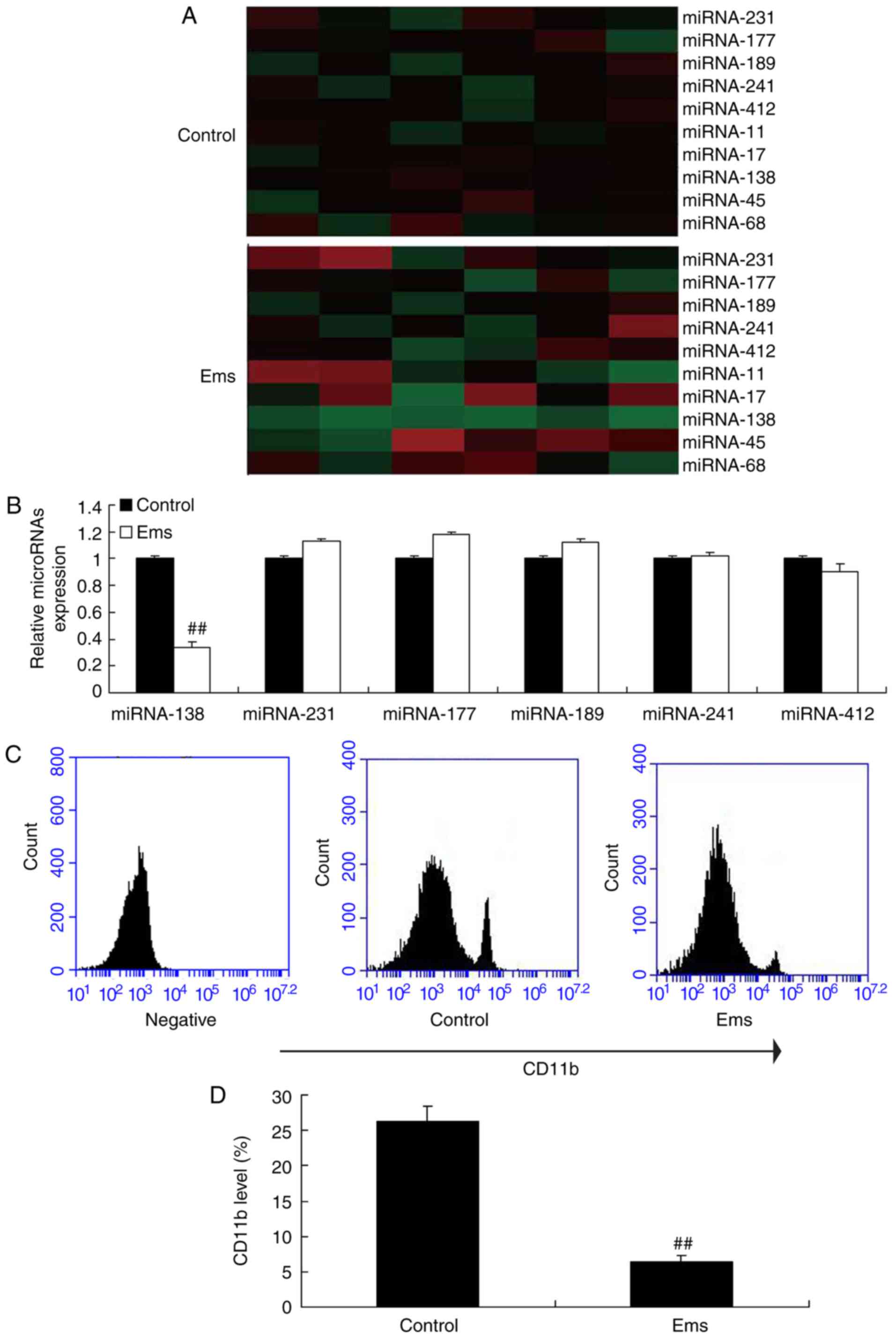
First, a gene chip was used to measure the changes
in different miRNAs in Ems, and it was observed that miR-138
expression was markedly downregulated in Ems rat compared with the
normal control group (Fig. 1A).
Next, RT-qPCR was used to analyzed the expression levels of miRNAs,
and it was also observed that miR-138 expression was significantly
down-regulated in Ems rats as compared with the normal control
group (Fig. 1B). Furthermore, it
was observed that CD11b level was significantly reduced in the
peritoneum of Ems rats, indicating reduced macrophages, compared
with the control group (Fig. 1C and
D).
miR-138 expression affects the growth of
uterine endothelial cells in a co-culture with THP-1 cells
Uterine endothelial and THP-1 cells were co-culture
to establish an in vitro model of Ems in the present study.
miR-138 expression was also upregulated or downregulated in
vitro model of uterine endothelial cells using miR-138 compared
with the control group (Fig. 2A).
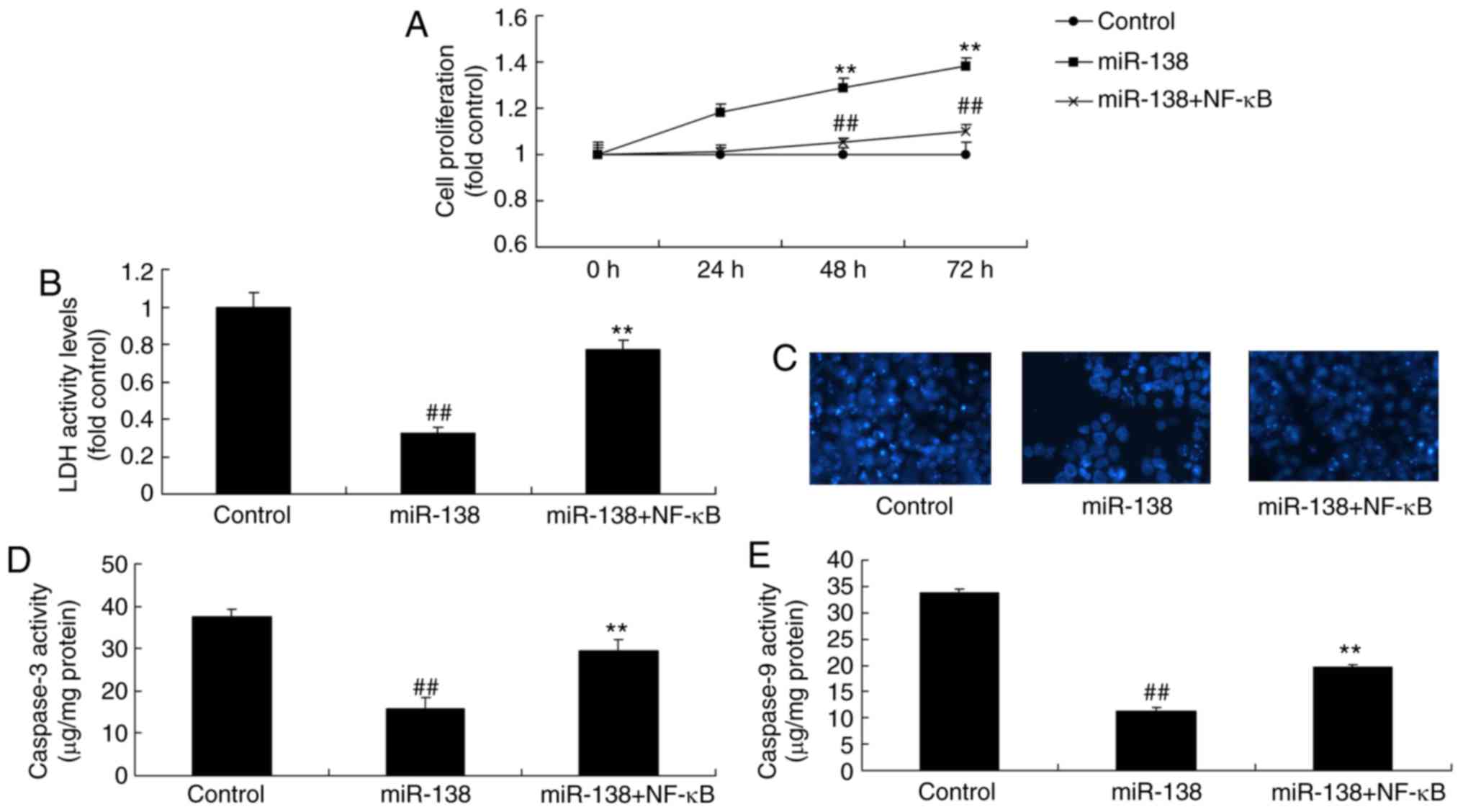
Upregulation of miR-138 promoted the growth and inhibited the LDH
activity of uterine endothelial cells, as well as suppressed
caspase-3/9 levels and cell apoptosis (DAPI assay) in the
co-culture of uterine endothelial and THP-1 cells (Fig. 2B–F). Anti-miR-138 mimics
downregulated miR-138 expression in vitro model of uterine
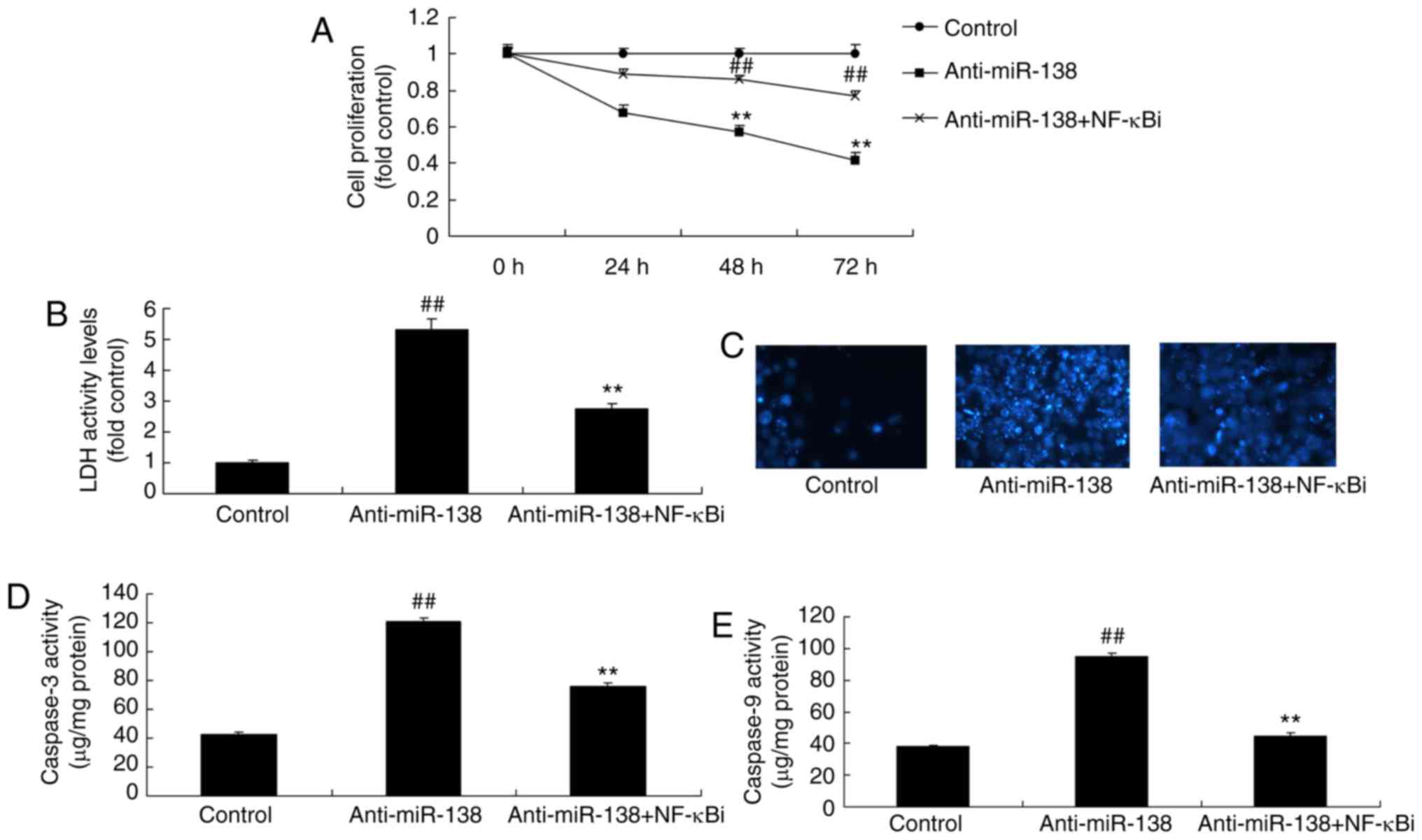
endothelial cells compared with the control group (Fig. 2G). Furthermore, downregulation of
miR-138 reduced the growth and induced the LDH activity of uterine
endothelial cells, while it increased the caspare-3/9 activity and
cell apoptosis (DAPI assay; Fig.
2H–L).
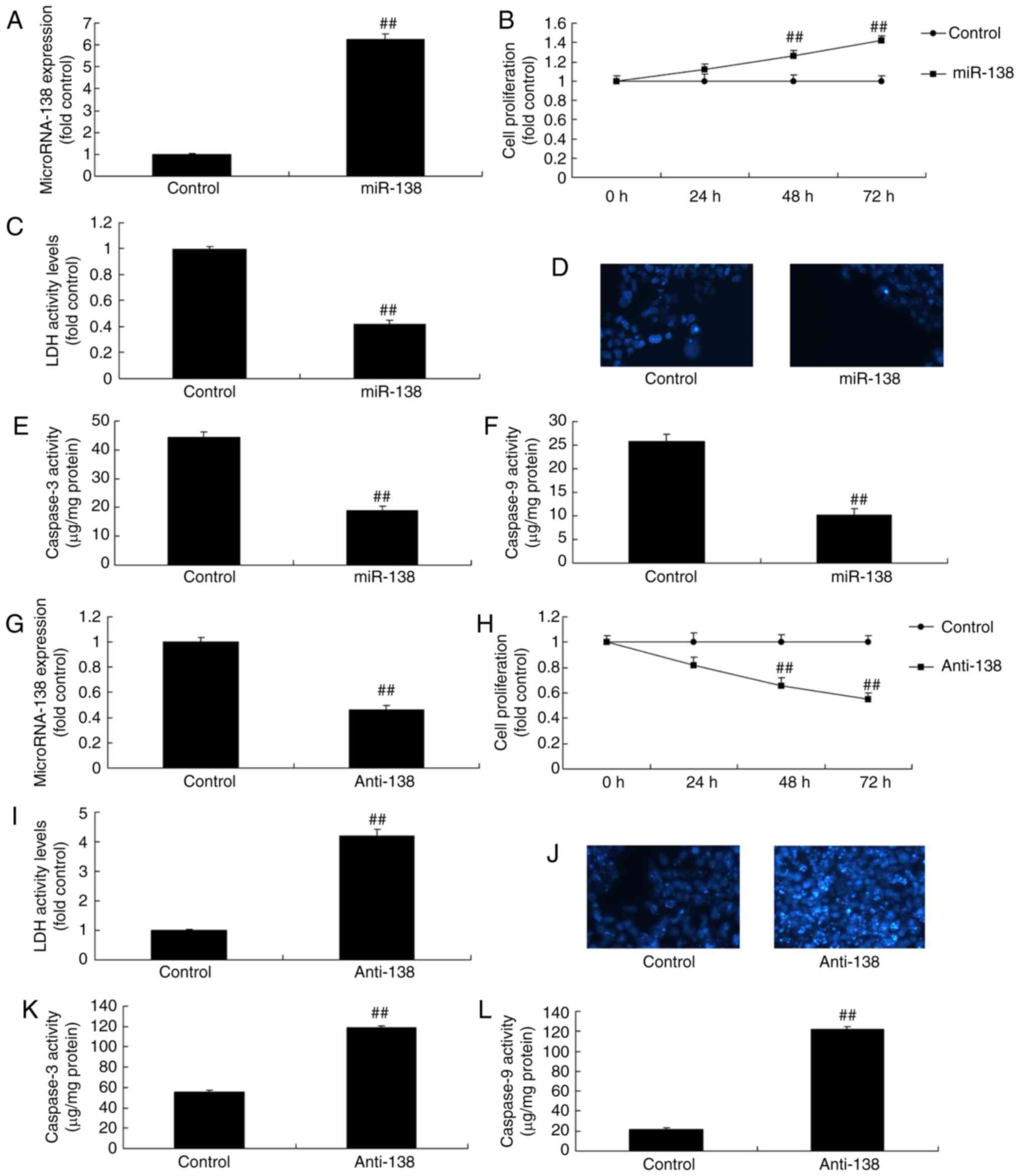 | Figure 2miR-138 expression affects the growth
of uterine endothelial cells in a co-culture with THP-1 cells. (A)
miR-138 expression, (B) cell growth, (C) LDH activity, (D) DAPI
assay, (E) caspase-3 levels and (F) caspase-9 levels were examined
following overexpression of miR-138 by transfection. (G) miR-138
expression, (H) cell growth, (I) LDH activity, (J) DAPI assay, (K)
caspase-3 levels and (L) caspase-9 levels were examined following
down-regulation of miR-138. ##P<0.01 vs. negative
control group. miR, microRNA; LDH, lactate dehydrogenase; miR-138,
overexpression group; anti-138, downregulation group. |
miR-138 expression affects inflammation
in a co-culture of uterine endothelial and THP-1 cells
Next, the study analyzed the changes in inflammation
in the co-culture of uterine endothelial and THP-1 cells.
Upregulation of miR-138 expression inhibited TNF-α, IL-1β, IL-6 and
IL-18 levels compared with the control (Fig. 3A–D). Furthermore, down-regulation
of miR-138 expression also increased TNF-α, IL-1β, IL-6 and IL-18
levels in THP-1 cells, compared with the control group (Fig. 3E–H). Therefore, the results
revealed that upregulation of miR-138 expression reduced in uterine
endothelial cells.
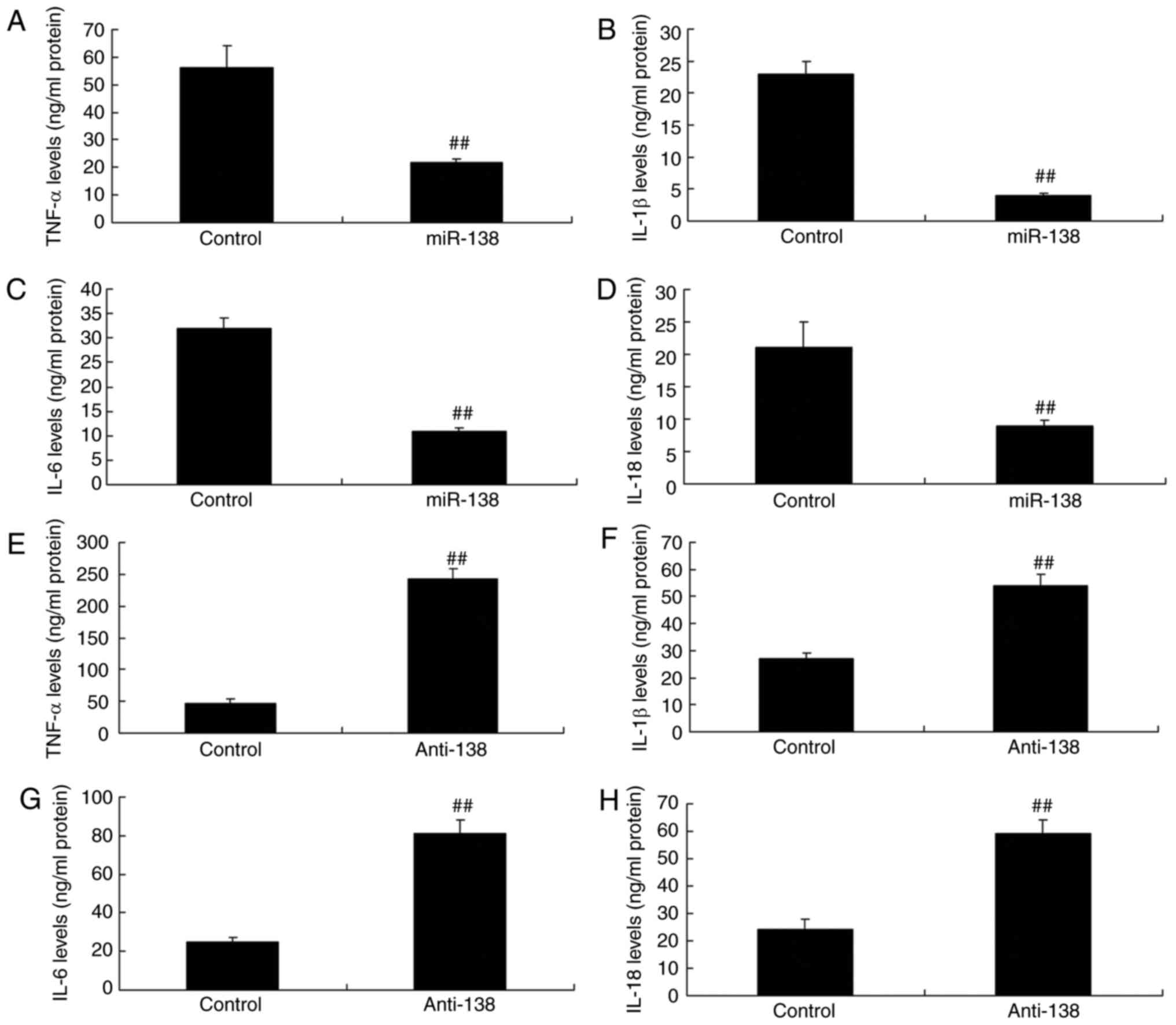 | Figure 3miR-138 expression affects
inflammation in a co-culture of uterine endothelial and THP-1
cells. Overexpression of miR-138 expression inhibited (A) TNF-α,
(B) IL-1β, (C) IL-6 and (D) IL-18 levels, while downregulation of
miR-138 expression enhanced (E) TNF-α, (F) IL-1β, (G) IL-6 and (H)
IL-18 levels. ##P<0.01 vs. negative control group.
miR, microRNA; TNF-α, tumor necrosis factor α; IL, interleukin;
miR-138, overexpression group; anti-138, downregulation group. |
miR-138 expression affects Ems in a
co-culture of uterine endothelial and THP-1 cells through NF-κB and
VEGF protein expression
The mechanism underlying the effects of miR-138 in
Ems was determined. As shown in Fig.
4A and B, miR-138 was identified in the 3′-untranslated region
of p65, and immunofluorescence assay revealed that upregulation of
miR-138 expression suppressed NF-κB protein expression in THP-1
cells compared with the control cells. Subsequently, it was
observed that upregulation of miR-138 expression suppressed Bax,
NF-κB and VEGF protein expression levels in THP-1 cells. By
contrast, downregulation of miR-138 suppressed Bax, NF-κB and VEGF
protein expression levels in THP-1 cells in comparison with those
in the control cells (Fig. 4C–J).
These results demonstrated that miR-138 regulates NF-κB and VEGF
protein expression in endometriosis.
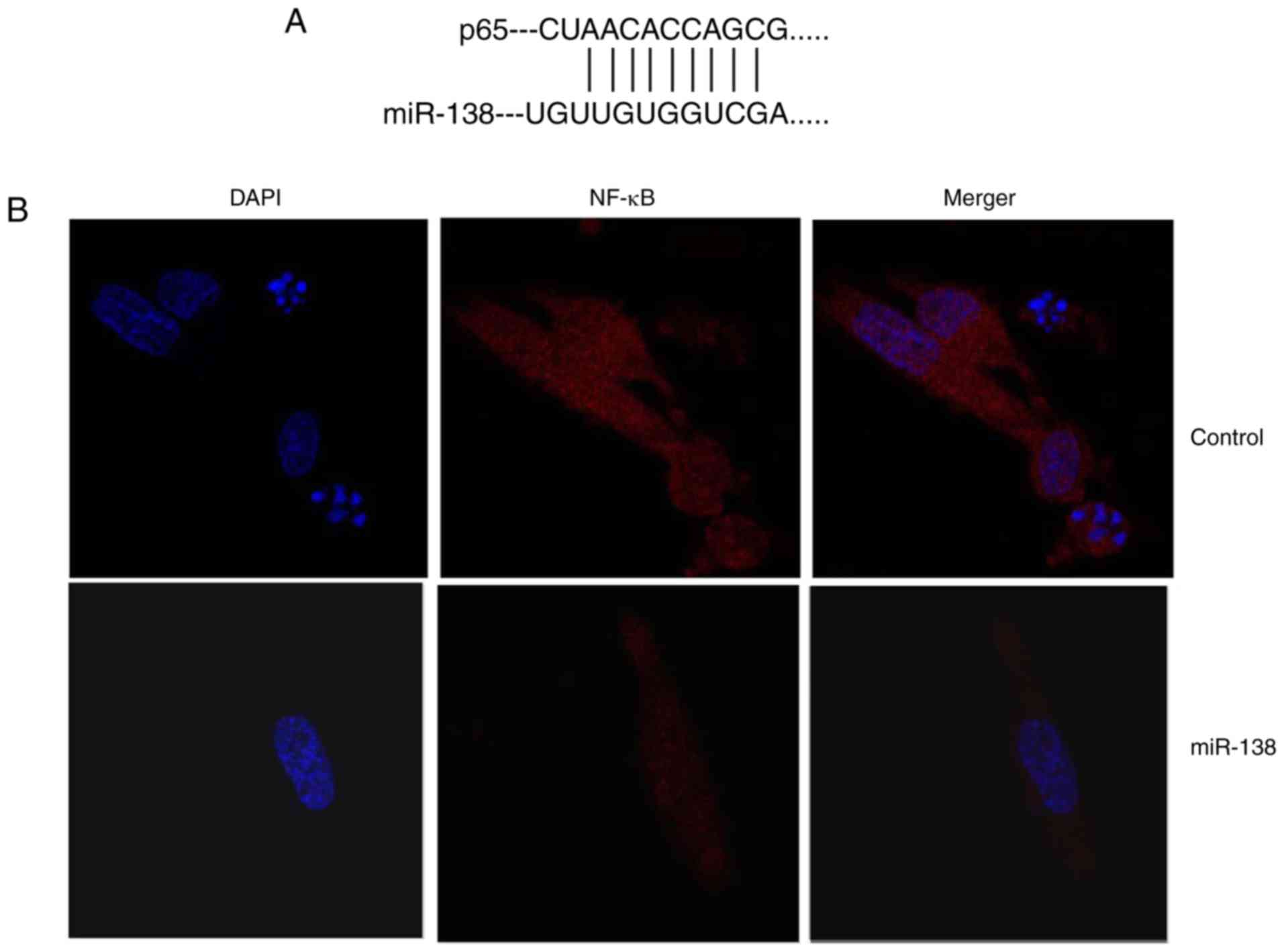 | Figure 4miR-138 expression affects
endometriosis in a co-culture with THP-1 cells and ESCs through
NF-κB expression. (A) miR-138 was identified in the 3′-untranslated
region of p65, and (B) immunofluorescence revealed that NF-κB
expression was suppressed in THP-1 cells (magnification, ×40). (C)
Western blot analysis showing that overexpression of miR-138
affects Bax, NF-κB and VEGF protein levels; quantified protein
expression levels of (D) Bax, (E) VEGF and (F) NF-κB are shown. (G)
Downregulation of miR-138 expression affects Bax, NF-κB and VEGF
protein levels are determined by western blot assay; quantified
results of (H) Bax, (I) VEGF and (J) NF-κB protein expression
levels are shown. ##P<0.01 vs. negative control
group. miR, microRNA; Bax, B-cell lymphoma 2-associated X protein;
NF-κB, nuclear factor-κB; VEGF, vascular endothelial growth factor;
miR-138, overexpression group; anti-138, downregulation group. |
Inhibition of NF-κB inhibits the effects
of miR-138 downregulation on inflammation in co-culture with
uterine endothelial cell and THP-1 cell
The function of NF-κB in the effects of miR-138
downregulation on inflammation and LDH activity of uterine
endothelial cells in the co-culture with THP-1 cells was verified.
As shown in Fig. 5A–D, addition
of an NF-κB inhibitor (2 µM BAY 11-7085) following miR-138
downregulation suppressed NF-κB, VEGF and Bax protein expression
levels in THP-1 cells in the co-culture, as compared with the
levels in the miR-138 downregulation alone group. In addition,
Fig. 5E–H displays that the NF-κB
inhibitor treatment following miR-138 downregulation reduced TNF-α,
IL-1β, IL-6 and IL-18 levels in the cytoplasm and THP-1 cells in
the co-culture in comparison with those in the miR-138
downregulation alone group. Furthermore, NF-κB inhibitor treatment
following miR-138 downregulation increased the growth, and
inhibited the LDH and caspase-3/9 activities of uterine endothelial
cells in the co-culture, when compared with the miR-138
downregulation group (Fig.
6).
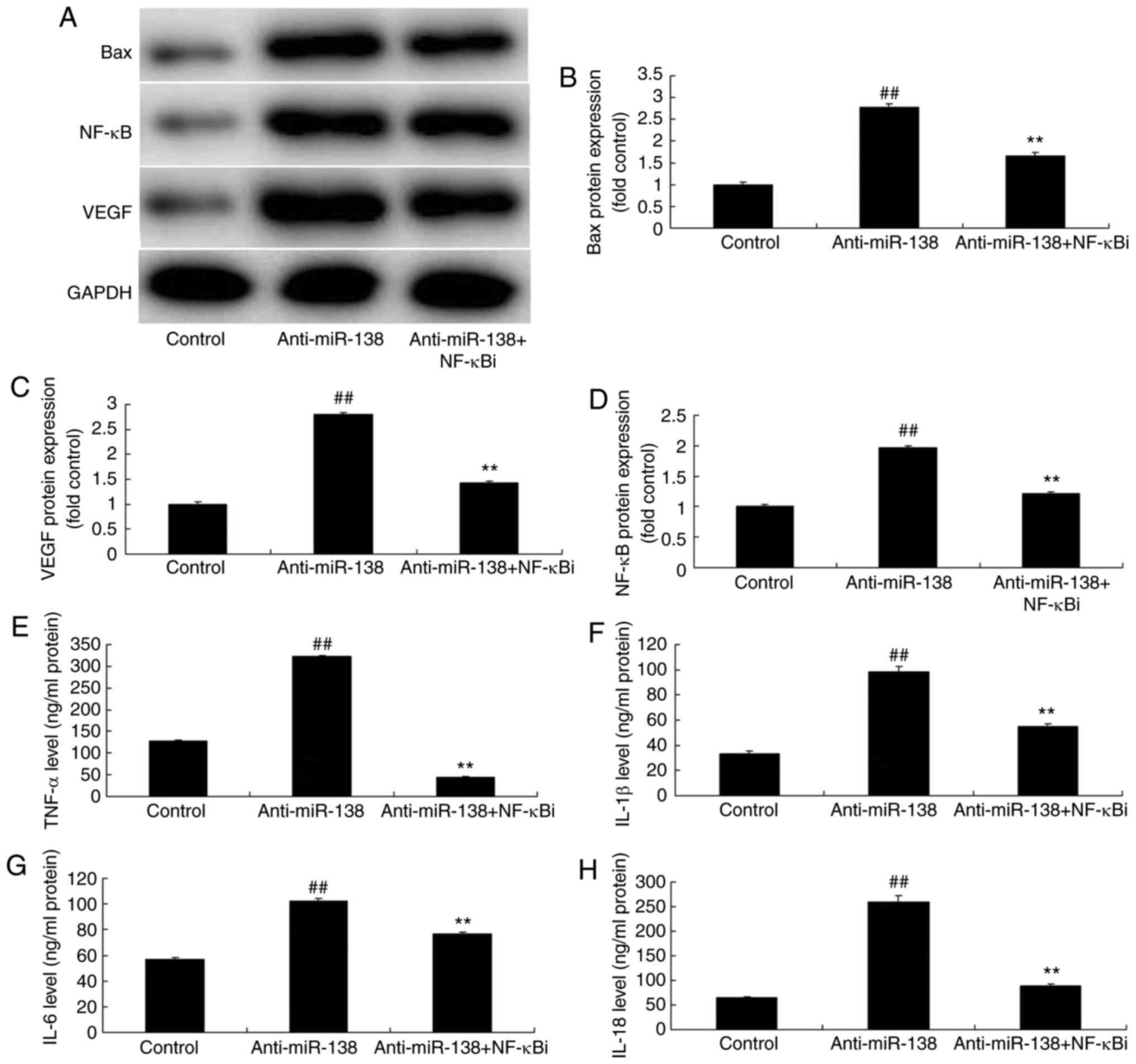 | Figure 5Inhibition of NF-κB inhibited the
effects of miR-138 downregulation on inflammation in a co-culture
of uterine endothelial and THP-1 cells. (A) Western blot assay, and
quantified (B) Bax, (C) VEGF and (D) NF-κB protein expression
levels. (E) TNF-α, (F) IL-1β, (G) IL-6 and (H) IL-18 levels are
shown. ##P<0.01 vs. control group;
**P<0.01 vs. anti-miR-138 group. miR, microRNA; Bax,
B-cell lymphoma 2-associated X protein; NF-κB, nuclear factor-κB;
VEGF, vascular endothelial growth factor; anti-miR-138,
downregulation group; NF-κB i, NF-κB inhibitor. |
Inhibition of VEGF inhibits the effects
of miR-138 downregulation on the LDH activity of uterine
endothelial cells in a co-culture with THP-1 cells
In order to involvement of VEGF in the effect of
miR-138 on the LDH activity of uterine endothelial cells in a
co-culture with THP-1 cells, a VEGF inhibitor (2 µM
Tanshinone IIA) was used. It was observed that VEGF and Bax protein
expression levels of THP-1 cells in the co-culture were
significantly suppressed when treated with VEGF inhibitor following
miR-138 downregulation, as compared with the miR-138 downregulation
alone group (Fig. 7A–C). In
addition, VEGF inhibitor increased the growth, inhibited the LDH
activity and decreased the caspase-3/9 activity of uterine
endothelial cells in the co-culture following miR-138
downregulation, compared with those in cells with miR-138
downregulation alone (Fig.
7D–H).
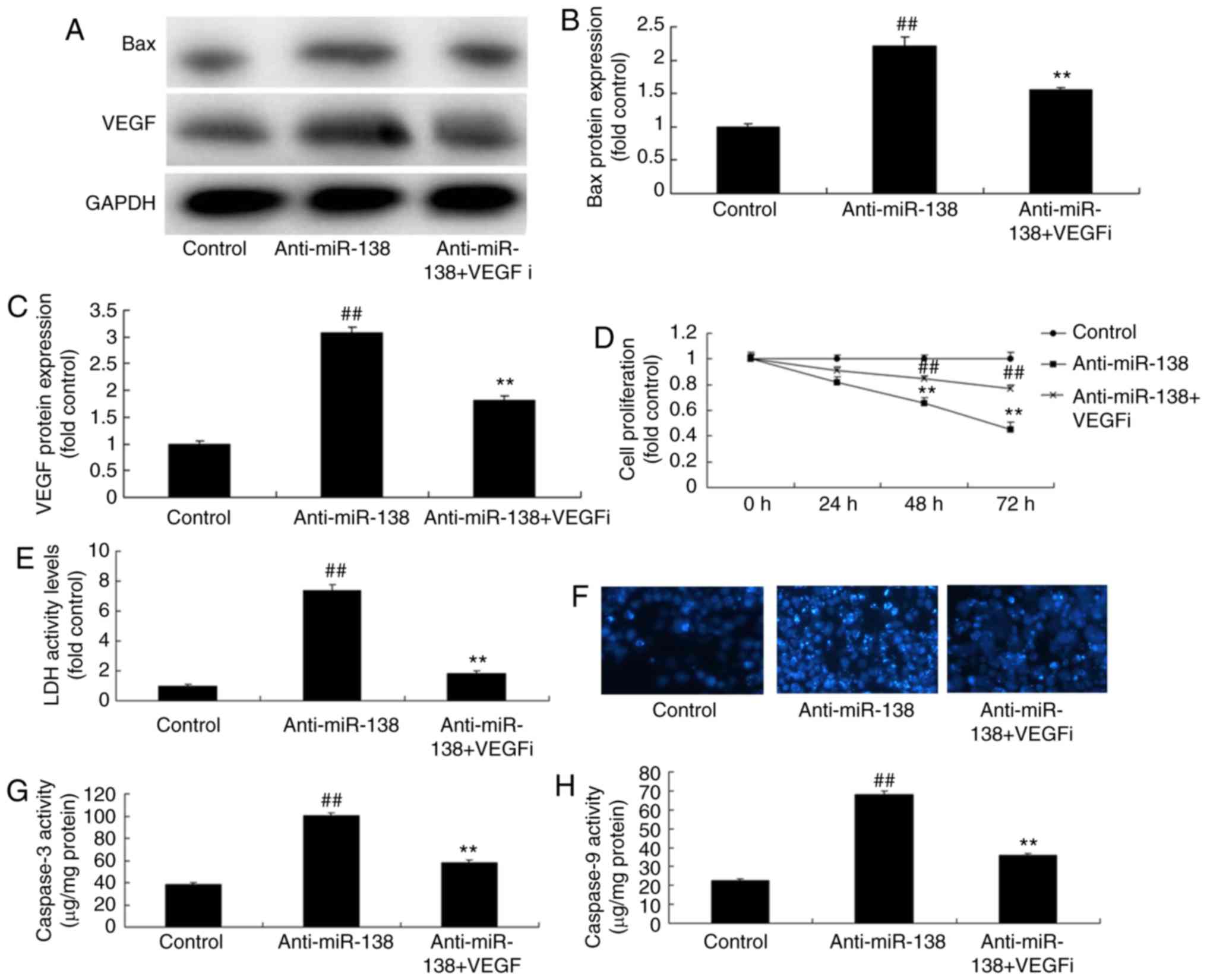 | Figure 7Inhibition of VEGF inhibited the
effects of miR-138 downregulation on uterine endothelial cells in a
co-culture with THP-1 cells. (A) Western blot analysis, and
quantified levels of (B) Bax and (C) VEGF protein expression. (D)
Cell growth, (E) LDH activity, (F) DAPI assay, (G) caspase-3 levels
and (H) caspase-9 levels. ##P<0.01 vs. control group;
**P<0.01 vs. anti-miR-138 group. miR, microRNA; Bax,
B-cell lymphoma 2-associated X protein; VEGF, vascular endothelial
growth factor; LDH, lactate dehydrogenase; anti-miR-138,
downregulation group; VEGFi, VEGF inhibitor. |
Promotion of NF-κB inhibited the effects
of miR-138 upregulation on inflammation in the co-culture of
uterine endothelial and THP-1 cells
The present study further analyzed the promotive
effect of NF-κB on the effects of miR-138 upregulation in
inflammation. As showed in Fig.
8A–D, NF-κB plasmid transfection following miR-138 upregulation
induced NF-κB, VEGF and Bax protein expression in the co-culture of
cells, as compared with the miR-138 upregulation alone group.
Furthermore, NF-κB activation increased TNF-α, IL-1β, IL-6 and
IL-18 levels were increased following miR-138 upregulation in the
co-culture of cells in comparison with the miR-138 upregulation
group cells (Fig. 8E–H). NF-κB
activation following miR-138 upregulation also decreased cell
growth, increased LDH activity and caspase-3/9 activity in uterine
endothelial cells of the co-culture, as compared with the miR-138
upregulation group (Fig. 9).
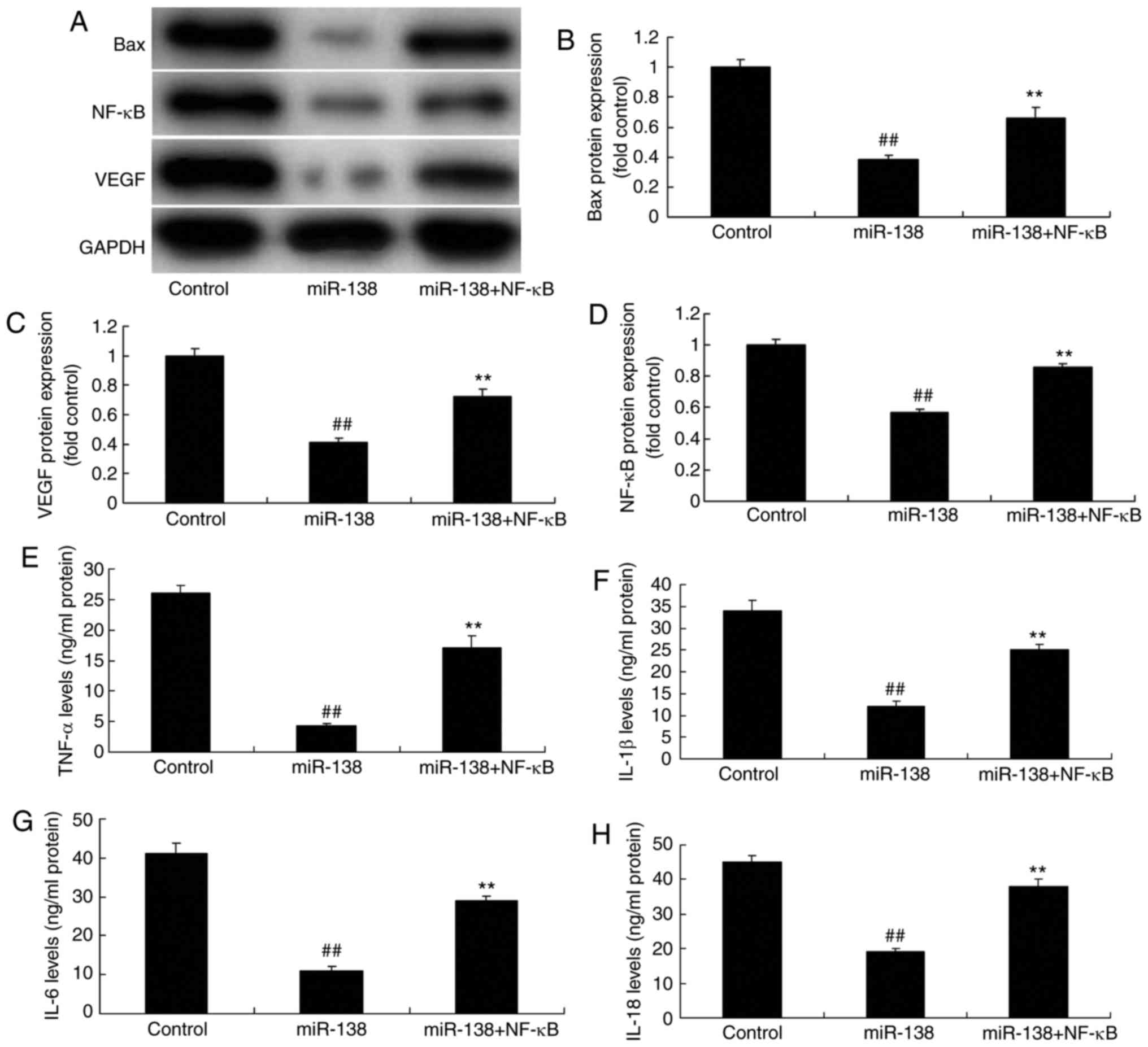 | Figure 8Promotion of NF-κB inhibited the
effects of miR-138 upregulation on inflammation in a co-culture of
uterine endothelial and THP-1 cells. (A) Western blot assay, and
quantified (B) Bax, (C) VEGF and (D) NF-κB protein expression
levels. (E) TNF-α, (F) IL-1β, (G) IL-6 and (H) IL-18 levels are
shown. ##P<0.01 vs. control group;
**P<0.01 vs. miR-138 group. miR, microRNA; Bax,
B-cell lymphoma 2-associated X protein; NF-κB, nuclear factor-κB;
VEGF, vascular endo-thelial growth factor; TNF-α, tumor necrosis
factor α; IL, interleukin; miR-138, overexpression group;
miR-138+NF-κB, overexpression of miR-138 and NF-κB. |
Promotion of VEGF inhibited the effects
of miR-138 upregulation on the LDH activity of uterine endothelial
cells in a co-culture with THP-1 cells
Next, VEGF plasmid transfection following miR-138
upregulation induced VEGF and Bax protein expression levels in the
co-culture of cells, when compared with the miR-138 upregulation
alone group (Fig. 10A–C). VEGF
activation following miR-138 upregulation also increased the
growth, and inhibited the LDH and caspase-3/9 activities of uterine
endothelial cells in the co-culture, as compared with the miR-138
upregulation group (Fig. 10D–H).
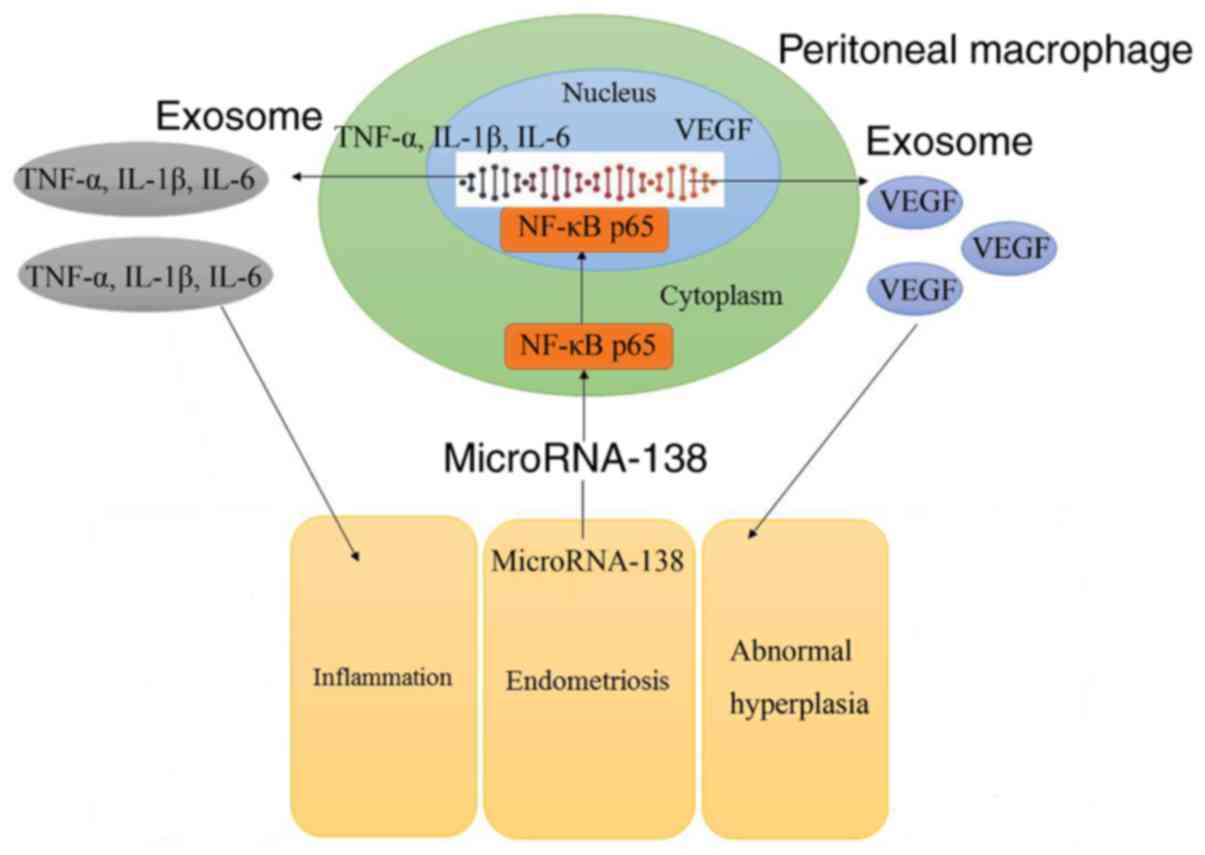
So, these results demonstrated that aberrant miR-138 expression may
be the epigenetic mechanism underlying the actions of VEGF through
the NF-κB signaling pathway (Fig.
11).
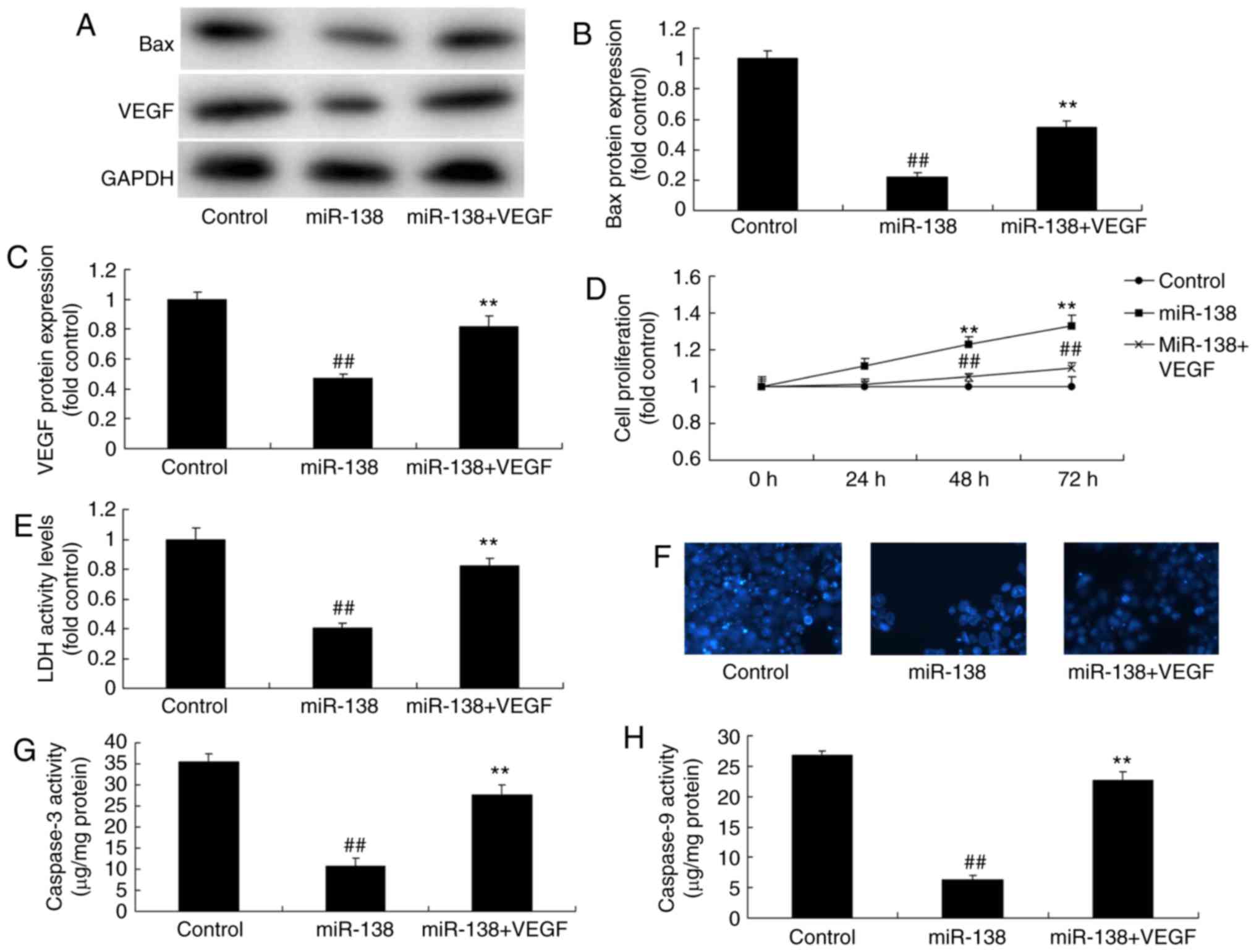 | Figure 10Promotion of VEGF inhibited the
effects of miR-138 upregulation on LDH activity of uterine
endothelial cells in a co-culture with THP-1 cells. (A) Western
blot analysis, and quantified levels of (B) Bax and (C) VEGF
protein expression. (D) Cell growth, (E) LDH activity, (F) DAPI
assay, (G) caspase-3 levels and (H) caspase-9 levels.
##P<0.01 vs. control group; **P<0.01
vs. miR-138 group. miR, microRNA; Bax, B-cell lymphoma 2-associated
X protein; VEGF, vascular endothelial growth factor; LDH, lactate
dehydrogenase; miR-138, overexpression group; miR-138+VEGF,
overexpression of miR-138 and VEGF. |
Discussion
Ems is a common benign disease in gynecology, which
is induced by the growth of endometrial tissue outside the uterine
cavity (16). The ovary is the
most commonly involved organ, and the ectopic endometrial tissue is
constituted by the endometrial gland and stroma, having functional
activity (17). Invasive growth
and repeated hemorrhage is locally observed in this condition. In
addition, corresponding histological changes and clinical symptoms
are observed (17). The condition
generally manifests as chronic pelvic pain and dyspareunia, which
may lead to irregular menstruation and even infertility (17). Cd11b+ cells in the
peritoneum are considered to be markers for the quantification of
macrophages in Ems (18).
The results of the present study demonstrated that
miR-138 expression was significantly downregulated in Ems rats
compared with that in normal control, while the CD11b level was
reduced in the peritoneum of Ems rats, demonstrating reduced number
of macrophages in the peritoneum. In the current study, only an MTT
assay was used to evaluate cell metabolism, which is a limitation
in the evaluation of cellular proliferation. Future studies should
use more methods to evaluate cellular metabolism, such as WST-1 and
other tetrazolium reduction assays.
Macrophages act on multiple target cells through
secreting multiple active factors (19). The mutual promotion or antagonism
of these factors forms a complicated cell-cytokine network
(19). Additionally, mutual
promotion or antagonism of macrophage target can induce
intraperitoneal inflammation and alter the abdominal
microenvironment (20), as well
as promote ectopic endometrial cell proliferation, invasion, growth
and angiogenesis, thus participating in the genesis and development
of Ems (20). Notably,
macrophages are known to secrete ILs. The present study verified
that TNF-α, IL-1β and IL-6 concentrations in Ems peritoneal fluid
were markedly higher in comparison with those in the normal control
group (20). In the present
study, downregulation of miR-138 expression was found to increase
TNF-α, IL-1β, IL-6 and IL-18 levels, decrease cell growth, promoted
LDH activity and caspase-3/9 activity in Ems. Similarly, Tang et
al (14) reported that of
miR-138 protected against inflammation due to cerebral
ischemia/reperfusion injury in rats.
NF-κB is an important transcription factor in cells,
which exists in almost all eukaryotic cells (21). It serves a key role in regulating
the inflammatory, immune stress responses, cell apoptosis and virus
replication (21). In addition,
NF-κB activity is associated with various biological processes,
including cell growth and differentiation, inflammatory and immune
response, and tumor growth. While NF-κB is poorly expressed in
normal endometrium (9), it is
highly expressed in eutopic and ectopic endometrium (10). This indicates that the activation
state of NF-κB is under the continuous regulation in normal
endometrial tissue, whereas its excessive activation in eutopic and
ectopic endometrial tissues is associated with the pathogenesis of
Ems (10). The present study
indicated that downregulation of miR-138 suppressed NF-κB and VEGF
protein expression levels in THP-1 cells. A study by Gong et
al (22) demonstrated that
downregulation of miR-138 sustains NF-κB activation in esophageal
squamous cell carcinoma. Sen et al (23) also reported that miR-138 regulates
hypoxia-induced endothelial cell dysfunction by targeting VEGF.
It has been suggested that VEGF is a potent inducer
of lymphangiogenesis, thus partly increasing the number of
lymphatic vessels (24). As a
result, it may be involved in the contact of invasive cancer cells
with the lymphatic vessels. Furthermore, VEGF promotes malignant
cancer cell invasion of the lymphatic vessel through elevating
lymphatic vessel permeability and tumor stroma pressure, which
further increases the metastatic ability of cancer cells (25). Research has suggested that VEGF
expression in several cancer tissues is associated with the genesis
and development of cancer (24).
VEGF expression in multiple human primary malignant tumor tissues
is also markedly correlated with regional lymph node metastasis,
including in lung, laryngeal, gastric, prostate and esophageal
cancer. The present study demonstrated that treatment with a NF-κB
or VEGF inhibitor promoted cell proliferation, inhibited LDH
activity and reduced inflammation in a co-culture of uterine
endothelial and THP-1 cells following miR-138 downregulation. In a
previous study, Wei et al (26) demonstrated that miR-138 expression
enhanced the destruction of the cartilage tissues among
osteoarthritis patients through p65. Similarly, the present study
suggested that the miR-138/VEGF axis may serve a significant role
in the regulation of Ems.
In conclusion, miR-138 expression was observed to be
significantly downregulated in Ems rats. The findings also
suggested that aberrant miR-138 expression may be the epigenetic
mechanism underlying the actions of VEGF through the NF-κB
signaling pathway. Therefore, these observations may provide novel
candidates that may serve as diagnostic biomarkers and therapeutic
targets in Ems. The data of the present study will be further
validated in our future studies on Ems.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors’ contributions
GW designed the experiment; AZ, LJ, TS and LZ
performed the experiments; GW and AZ analyzed the data; GW wrote
the manuscript. All authors read and approved the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee of Qilu Hospital of Shandong
University (Jinan, China), and all the procedures were performed
according to the National Institutes of Health Guidelines for the
Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schwertner A, Conceicao Dos Santos CC,
Costa GD, Deitos A, de Souza A, de Souza IC, Torres IL, da Cunha
Filho JS and Caumo W: Efficacy of melatonin in the treatment of
endometriosis: A phase II, randomized, double-blind,
placebo-controlled trial. Pain. 154:874–881. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hao Z and Huang S: E3 ubiquitin ligase
Skp2 as an attractive target in cancer therapy. Front Biosci
(Landmark Ed). 20:474–490. 2015. View
Article : Google Scholar
|
|
3
|
Campos Petean C, Ferriani RA, dos Reis RM,
de Moura MD, Jordao AA Jr and Navarro PA: Lipid peroxidation and
vitamin E in serum and follicular fluid of infertile women with
peritoneal endometriosis submitted to controlled ovarian
hyperstimulation: A pilot study. Fertil Steril. 90:2080–2085. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barrueto FF, Audlin KM, Gallicchio L,
Miller C, MacDonald R, Alonsozana E, Johnston M and Helzlsouer KJ:
Sensitivity of narrow band imaging compared with white light
imaging for the detection of endometriosis. J Minim Invasive
Gynecol. 22:846–852. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen JM, Gao HY, Ding Y, Yuan X, Wang Q,
Li Q and Jiang GH: Efficacy and safety investigation of Kuntai
capsule for the add-back therapy of gonadotropin releasing hormone
agonist administration to endometriosis patients: A randomized,
double-blind, blank- and tibolone-controlled study. Chin Med J
(Engl). 128:427–432. 2015. View Article : Google Scholar
|
|
6
|
Ballester M, Santulli P, Bazot M, Coutant
C, Rouzier R and Darai E: Preoperative evaluation of posterior
deep-infiltrating endometriosis demonstrates a relationship with
urinary dysfunction and parametrial involvement. J Minim Invasive
Gynecol. 18:36–42. 2011. View Article : Google Scholar
|
|
7
|
Seifer BJ, Su D and Taylor HS: Circulating
miRNAs in murine experimental endometriosis. Reprod Sci.
24:376–381. 2017. View Article : Google Scholar
|
|
8
|
Wang WT, Sun YM, Huang W, He B, Zhao YN
and Chen YQ: Genome-wide long non-coding RNA analysis identified
circulating LncRNAs as novel Non-invasive diagnostic biomarkers for
gynecological disease. Sci Rep. 6:233432016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu F, Liu M, Pan Y, Wang X and Chen Y:
Small hairpin RNA targeting inhibition of NF-κB gene in
endometriosis therapy of Macaca fascicularis. Zhonghua Fu Chan Ke
Za Zhi. 50:48–53. 2015.In Chinese. PubMed/NCBI
|
|
10
|
Celik O, Ersahin A, Acet M, Celik N,
Baykus Y, Deniz R, Ozerol E and Ozerol I: Disulfiram, as a
candidate NF-κB and proteasome inhibitor, prevents endometriotic
implant growing in a rat model of endometriosis. Eur Rev Med
Pharmacol Sci. 20:4380–4389. 2016.PubMed/NCBI
|
|
11
|
Alvarado-Diaz CP, Núñez MT, Devoto L and
González-Ramos R: Iron overload-modulated nuclear factor kappa-B
activation in human endometrial stromal cells as a mechanism
postulated in endometriosis pathogenesis. Fertil Steril.
103:439–447. 2015. View Article : Google Scholar
|
|
12
|
Fujii EY, Nakayama M and Nakagawa A:
Concentrations of receptor for advanced glycation end products,
VEGF and CML in plasma, follicular fluid, and peritoneal fluid in
women with and without endometriosis. Reprod Sci. 15:1066–1074.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dziunycz P, Milewski L, Radomski D, Barcz
E, Kamiński P, Roszkowski PI and Malejczyk J: Elevated ghrelin
levels in the peritoneal fluid of patients with endometriosis:
Associations with vascular endothelial growth factor (VEGF) and
inflammatory cytokines. Fertil Steril. 92:1844–1849. 2009.
View Article : Google Scholar
|
|
14
|
Tang XJ, Yang MH, Cao G, Lu JT, Luo J, Dai
LJ, Huang KM and Zhang LI: Protective effect of microRNA-138
against cerebral ischemia/reperfusion injury in rats. Exp Ther Med.
11:1045–1050. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Takaesu Y, Nishi H, Kojima J, Sasaki T,
Nagamitsu Y, Kato R and Isaka K: Dienogest compared with
gonadotropin-releasing hormone agonist after conservative surgery
for endometriosis. J Obstet Gynaecol Res. 42:1152–1158. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leone Roberti Maggiore U, Remorgida V,
Scala C, Tafi E, Venturini PL and Ferrero S: Desogestrel-only
contraceptive pill versus sequential contraceptive vaginal ring in
the treatment of rectovaginal endometriosis infiltrating the
rectum: A prospective open-label comparative study. Acta Obstet
Gynecol Scand. 93:239–247. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wieser F, Wu J, Shen Z, Taylor RN and
Sidell N: Retinoic acid suppresses growth of lesions, inhibits
peritoneal cytokine secretion, and promotes macrophage
differentiation in an immunocompetent mouse model of endometriosis.
Fertil Steril. 97:1430–1437. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cakmak H, Seval-Celik Y, Arlier S,
Guzeloglu-Kayisli O, Schatz F, Arici A and Kayisli UA: p38
Mitogen-activated protein kinase is involved in the pathogenesis of
endometriosis by modulating inflammation, but not cell survival.
Reprod Sci. 25:587–597. 2018. View Article : Google Scholar
|
|
20
|
Grandi G, Mueller MD, Bersinger NA,
Facchinetti F and McKinnon BD: The association between progestins,
nuclear receptors expression and inflammation in endometrial
stromal cells from women with endometriosis. Gynecol Endocrinol.
33:712–715. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Celik O, Celik E, Turkcuoglu I, Yilmaz E,
Ulas M, Simsek Y, Karaer A, Celik N, Aydin NE, Ozerol I and Unlu C:
Surgical removal of endometrioma decreases the NF-κB1 (p50/105) and
NF-κB p65 (Rel A) expression in the eutopic endometrium during the
implantation window. Reprod Sci. 20:762–770. 2013. View Article : Google Scholar
|
|
22
|
Gong H, Song L, Lin C, Liu A, Lin X, Wu J,
Li M and Li J: Downregulation of miR-138 sustains NF-κB activation
and promotes lipid raft formation in esophageal squamous cell
carcinoma. Clin Cancer Res. 19:1083–1093. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sen A, Ren S, Lerchenmuller C, Sun J,
Weiss N, Most P and Peppel K: MicroRNA-138 regulates
hypoxia-induced endothelial cell dysfunction by targeting S100A1.
PLoS One. 8:e786842013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Makabe T, Koga K, Miyashita M, Takeuchi A,
Sue F, Taguchi A, Urata Y, Izumi G, Takamura M, Harada M, et al:
Drospirenone reduces inflammatory cytokines, vascular endothelial
growth factor (VEGF) and nerve growth factor (NGF) expression in
human endometriotic stromal cells. J Reprod Immunol. 119:44–48.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rein DT, Schmidt T, Bauerschmitz G, Hampl
M, Beyer IM, Paupoo AA, Curiel DT and Breidenbach M: Treatment of
endometriosis with a VEGF-targeted conditionally replicative
adenovirus. Fertil Steril. 93:2687–2694. 2010. View Article : Google Scholar
|
|
26
|
Wei ZJ, Liu J and Qin J: miR-138
suppressed the progression of osteoarthritis mainly through
targeting p65. Eur Rev Med Pharmacol Sci. 21:2177–2184.
2017.PubMed/NCBI
|