Introduction
Parkinson’s disease (PD) is a common neurological
disorder and a typical model of striatal dysfunction (1). It is the second most common
neurodegenerative disorder, following only Alzheimer’s disease in
prevalence (2). One of the
characteristics of PD is the progressive loss of dopaminergic (DA)
neurons in the substantia nigra (3). Patients with PD usually have
symptoms of tremor, rigidity and bradykinesia (4). Genetic and environmental factors
have been considered in the etiology of PD (5). High frequency deep brain stimulation
of the subthalamic nucleus is a popular surgical treatment option;
however, it is not applicable to the early stages of PD, and it is
not accessible to patients in developing countries (6). Current therapies involving
neuroprotective agents, stem cell research, vaccines and various
surgical techniques are reported to have limitations (7-9). A
previous study investigated pharmacological regimens in treating
PD, concluding that further investigations are required to develop
superior regimens (10).
MicroRNAs (miRNAs), have been reported to be key in
neuronal development, plasticity and disease, including PD
(11). The miRNAs are regulators
of post-transcriptional genes, with an important influence on
neuronal diseases (11). miRNA
(miR)-183 belongs to the vertebrate microRNA-183 (miR-183) family
(miR-183, miR-96 and miR-182) located on chromosome 7q32 and is
dysregulated in numerous types of cancer, including non-small cell
lung cancer, breast cancer and colorectal cancer (12-14). The miR-183 family members are
reported to control electroreception, photoreception,
chemosensation and mechanosensation in vertebrate organs by
regulating the majority of ciliated neurosensory epithelial cells
(15). It has been reported that
the oncostatin M receptor (OSMR) gene is located on 5p13.1, the
protein of which, OSMRβ, is able to heterodimerize with the
interleukin (IL)-6 signal transducer to form type II OSMR (16,17). The phosphoinositide 3-kinase
(PI3K) family of lipid kinases have a common biochemical function
to phosphorylate phosphoinositide 3-hydroxyl (18). As a serine/threonine kinase, Akt
is the central regulator of the PI3K pathway, with various
downstream mediators that affect key cellular activities; it has a
potential mechanistic influence on negative signaling in PD
(19). There are previous studies
suggesting that the PI3K-Akt signaling pathway is critical in the
cell survival, proliferation and growth of neurons (20-22). Activation of the PI3K/Akt/ mTOR
pathway correlated with oncogenesis, including glioma, mammary
cancer and omophoria (23-25).
From the findings described above, there may be certain
associations between miR-183, OSMR and the PI3K-Akt signaling
pathway in PD. Therefore, the present study was performed to
examine how miR-183 is involved in the DA neurons within the
substantia nigra pars compacta (SNc) in PD via the PI3K-Akt
signaling pathway.
Materials and methods
Ethical statement
The experiments and the use of all experimental
animals were approved by the Animal Ethics Committee of the Second
Hospital of Dalian Medical University (Dalian, China). All efforts
were made in the experiments to minimize pain in the mice.
Model establishment and behavioral
identification
A total of 60 male C57/BL6 mice of specific-pathogen
free grade (aged 10 months and weighing 20-25 g) were supplied by
the Laboratory Animal Center of Shanxi Medical University [Shanxi,
China; approval no. SCXK (Jin) 20150001]. The mice were housed
under controlled conditions (25±2°C; humidity of 55±10%; noise
<60 dB) and a 12 h light/dark cycle (7 a.m. to 7 p.m.) with free
access to food and water. All mice were confirmed to have no
abnormalities following 5 days of acclimatized feeding. A total of
50 mice subjected to continuous injection of low-dose
1-methyl-4-phenyl-1,2,3,6-tetrahydro-pyridine (MPTP) for 6 weeks
were randomly selected as the PD group. The other 10 mice, which
were subjected to injection of saline, served as the control group.
Behavioral alterations of the mice in the normal and PD groups were
observed 1, 2, 3, 4, 6 and 8 weeks following model establishment.
The mice were placed into a transparent cage 10 min prior to the
assessment to adapt to the environment. The mice were subsequently
induced to rotate by a subcutaneous injection of apomorphine (0.25
mg/kg; cat. no. A14200234234; Tianjin Tianwei Pharmaceutical Co.,
Ltd., Tianjin, China) into the back. The revolving turns of the
mice within 30 min was recorded. When the mice were at a stable
average rotating speed of >5.47 × 10−3 × g, the PD
model was considered successfully established (26). After 8 weeks, the normal group and
PD group (successful model establishment) were ready for subsequent
experiments.
Hematoxylin and eosin (H&E)
staining
Following successful model establishment, the mice
were anesthetized with an intra-peritoneal injection of
pentobarbital sodium (1%; 50 mg/kg). Following sacrifice by rapid
decapitation, their brain tissues were obtained. The substantia
nigra of the midbrain was isolated according to the rat brain
atlas. The tissues of the substantia nigra were fixed at room
temperature in 4% paraformaldehyde overnight, dehydrated with an
ethanol gradient concentration (70, 80, 90, 95 and 100%; 1
min/concentration), cleared twice in xylene (5 min/time), and
finally embedded in paraffin; parts of the sections were prepared
for immunohistochemistry. The tissue blocks were sectioned into 5
µm thick sections and placed in an oven at 80°C for 1 h.
Following cooling, the sections were dehydrated with routine
gradient ethanol, cleared in xylene and washed at room temperature.
The sections were stained using hematoxylin (cat. no. H8070-5g;
Beijing Solarbio Science & Technology, Co., Ltd., Beijing,
China) at room temperature for 4 min and washed with rinsing
buffer. Following differentiation with hydrochloric ethanol for 10
sec, the sections were washed for 5 min, stained blue with ammonia
at room temperature for 10 min, stained with eosin at room
temperature (cat. no. PT001; Shanghai Bogoo Biological Technology
Co., Ltd., Shanghai, China) for 2 min, dehydrated by gradient
ethanol (1 min/concentration), and cleared twice with xylene (1
min/time). The sections were sealed with neutral gum in a draught
cupboard, and were placed under a light microscope (magnification,
×400; cat. no. DMM-300D; Shanghai Cai Kang Optical Instrument Co.,
Ltd., Shanghai, China) to observe their pathological features;
images were captured to observe their coloration.
Immunohistochemistry
The specimens were fixed in 10% formaldehyde for 2
weeks at room temperature; they were subsequently placed in 0.01
mmol l-1 PBS containing 20% sucrose. Following specimen sinking, 4
µm thick continuous paraffin-embedded sections were produced
according to the mouse brain atlas of the SNc. Immunohistochemical
staining was performed using the streptavidin-biotin complex (SABC)
method. The sections were placed in incubators at 60°C for 1 h,
dewaxed with conventional xylene, dehydrated with gradient alcohol,
and placed in 3% H2O2 (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) for incubation at 37°C for 30 min.
Following this, the sections were washed with PBS, placed in
citrate buffer (0.01 M) and boiled for 20 min at 95°C. The sections
were subsequently agitated and rinsed three times with 0.01 mmol/l
PBS (pH 7.4). Following inactivation of the endogenous enzyme by 3%
H2O2, the sections were washed with distilled
water three times, blocked with bovine serum albumin (cat. no.
SW3015; Beijing Solarbio Science & Technology Co., Ltd.) for 20
min at room temperature, cooled to room temperature, and washed
with PBS. Subsequently, rabbit anti-mouse OSMR polyclonal antibody
(cat. no. ab68476, 1:1,500; Abcam, Cambridge, MA, USA) were added
to the sections and incubated at 4°C overnight; biotin-labeled
secondary antibody immunoglobulin G (IgG) serum (cat. no. ab6789;
1:1,000; Abcam) was subsequently added for incubation at room
temperature for 20 min. Subsequent to washing with PBS three times,
the sections were incubated with SABC for 20 min at room
temperature and washed again with PBS four times. The sections were
developed with diaminobenzidine (DAB), stained with hematoxylin at
room temperature for 3-15 min, dehydrated, cleared, sealed at room
temperature and observed under a light microscope. One
interpeduncular nucleus section of each mouse in each group was
selected. Three fields (magnification, ×400) of the selected
immunohistochemistry sections with SNc were randomly selected for
counting positive cells and paragraphing. Cells stained brown in
the cytoplasm and membrane were considered positive cells.
Terminal deoxynucleotidyl transferase
(TdT)-mediated dUTP nick labeling (TUNEL) staining
The sections of each group were dewaxed with water
and blocked with 3% H2O2 for 15 min at room
temperature. Following PBS washing, the sections were digested with
proteinase K (37°C) for 40 min, added to TDT (37°C) for 1 h and
added to conversion liquid (37°C) for 30 min. Following washing,
the sections were developed with DAB for 3 min. The reaction was
terminated when the staining result reached its expected result.
Following staining with TUNEL (cat. no. KGA7022; Nanjing Keygen
Biotech Co., Ltd., Nanjing, China), apoptotic cells were visible as
black or blue/black. The sections were subsequently dried,
dehydrated, cleared by xylene and mounted with neutral gum. A total
of five discontinuous equidistant interval sections of the
substantia nigra nerve tissues were analyzed, and five high-power
(magnification, ×400) visual fields in each section were randomly
selected under a fluorescence microscope to observe the expression
of TUNEL-positive cells in the substantia nigra neurons of the mice
(27).
Cell culture
The PD mice were anesthetized by the
intra-peritoneal injection of pentobarbital sodium (1%, 50 mg/kg).
Following sacrifice via rapid decapitation, substantia nigra
tissues were removed under sterile conditions and washed with
pre-cooled D-Hank’s solution (Beijing Huamaike Biotechnology Co.,
Ltd.) twice with the meninges and blood vessels stripped. The
tissues were cut into smaller segments and added to 5 ml
low-glucose Dulbecco’s modified Eagle’s medium (DMEM; cat. no.
SH30021.01; Beijing Solarbio Science & Technology Co., Ltd.)
containing 0.15% collagenase and transferred into sterile
centrifuge tubes. The tissues were digested using a
constant-temperature electromagnetic stirrer (37°C) for 30 min and
centrifuged at 5.48 × g for 5 min at room temperature with the
supernatant removed. Subsequently, the tissues were added to the
appropriate quantity of low-glucose DMEM containing 20% FBS and
centrifuged again at 5.48 × g and 37°C; the precipitate was
collected and added to the medium to form a cell suspension. The
suspension was percussed, mixed and transferred into a disposable
culture dish to distribute the precipitate evenly. Following
washing with D-Hank’s liquid detergent, the precipitate was added
with 0.25% trypsin (containing 0.02% ethylene diamine tetra acetic
acid) and detached for 7-9 min in the 5% CO2 incubator
at 37°C. The digestion was terminated by adding culture medium
containing 20% FBS. The cells were subsequently centrifuged at
16.10 × g for 5 min at 37°C with the supernatant removed. The cells
were resuspended with DMEM (20% FBS), inoculated in a novel dish,
and cultured in an incubator (37°C; 5% CO2). The medium
was replaced every 2-3 days.
Cell grouping and transfection
Normal cells at the third generation were divided
into the following groups: Normal group (normal cells without
transfection), miR-183 mimic negative control (NC) group (normal
cells transfected with 30 µg/l miR-183 mimic NC sequence)
and miR-183 mimic group (normal cells transfected with 30
µg/l miR-183 mimic). PD cells at the third generation were
grouped into the following groups: Control group (no transfection),
miR-183 inhibitor NC group, miR-183 inhibitor group, OSMR (0.2
µg/l; cells transfected with overexpressed OSMR plasmids)
group, insulin-like growth factor 1 (IGF-1) group (cells treated
with 20 ng/ml IGF-1), miR-183 mimic + IGF-1 group (cells
transfected with miR-183 mimic and 20 ng/ml IGF-1). All plasmids
were purchased from Merck KGaA. IGF-1 (Sigma-Aldrich; Merck KGaA),
is an activator of the PI3K-Akt signaling pathway. The substantia
nigra neurons of the PD and normal mice at the logarithmic growth
phase were inoculated in a 6-well plate. When the cell confluence
reached 30-50%, cell transfection was performed according to the
manufacturer’s protocol using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Serum-free Opti-Minimum Essential Medium (MEM; 250 µl;
Gibco; Thermo Fisher Scientific, Inc.) was used to dilute the
miR-183 mimic, miR-183 inhibitor, OSMR, IGF-1 and miR-183 mimic +
IGF-1 groups. The concentration of each group was 100 pmol, and the
final concentration of the groups was 50 nM. Subsequently, the
cells were gently mixed and incubated at room temperature for 5
min. Serum-free Opti-MEM (250 µl; Gibco; Thermo Fisher
Scientific, Inc.) was used to dilute Lipofectamine® 2000
(5 µl), and was gently mixed and incubated at room
temperature for 5 min. The two diluted mixtures were mixed and
incubated for 20 min at room temperature; finally, 2 ml mixture was
added into each cell culture well at a density of
1-2×105 cells/well and incubated in a 5% CO2
incubator at 37°C for 6-8 h. Following incubation, the original
medium was replaced with a complete medium, and subsequent
experiments were performed following culture for 24-48 h.
Dual luciferase reporter assay
The MicroRNA.org
database (http://www.microrna.org/) was used to
analyze and predict the target gene of miR-183. Target sequences of
OSMR-wild-type (WT) 3′untranslated region (UTR) and OSMR-mutant
(MUT) 3′UTR were constructed manually and inserted into the pmirGLO
reporter plasmid (Promega Corporation, Madison, WI, USA) by double
enzyme digestion of the restriction site
BamHI/HindIII to obtain the WT and MUT plasmids. The
two plasmids were co-transfected with miR-183 mimic and mimic
control into 293T cells (Shanghai Zhong Qiao Xin Zhou Biotechnology
Co., Ltd., Shanghai, China), using Entraster™ reagent (Engreen
Biosystem Co., Ltd., Beijing, China). At 48 h post transfection,
the cells were collected and lysed, and luciferase activity was
detected using a Dual-Luciferase Reporter Assay system (cat. no.
E1910; Promega Corporation). To the cells, 100 µl firefly
luciferase solution was added to detect the activity of firefly
luciferase and 100 µl Renilla luciferase solution was
added to detect the activity of Renilla luciferase. The
relative luciferase activity was calculated. The experiment was
repeated three times.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
An RNA extraction kit (cat. no. 10296010;
Invitrogen; Thermo Fisher Scientific, Inc.) was used to extract the
total RNA from the sample cells. Using an RT kit (cat. no. K1621;
Fermentas; Thermo Fisher Scientific, Inc.), the RNA was reverse
transcribed into cDNA. The RT system was 10 µl and the
reaction conditions were as follows: 42°C for 30-50 min (RT
reaction) and 85°C for 5 sec (reverse transcriptase inactivation).
Primers of miR-183, OSMR, Akt, glycogen synthase kinase-3 (GSK-3β),
B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax),
caspase-9, IGF-1, mTOR, U6 and GAPDH were designed and synthesized
by Takara Biotechnology Co., Ltd. (Dalian, China) (Table I). According to the manufacturer’s
protocol of the PCR kit (cat. no. KR011A1; Tiangen Biotech Co.,
Ltd., Beijing, China), the RT-qPCR reaction was performed under the
following reaction conditions: Pre-denaturation at 95°C for 5 min,
30 cycles of denaturation at 95°C for 40 sec, annealing at 57°C for
40 sec at 72°C for 40 sec, extension at 72°C for 10 min and final
extension following cycles at 4°C for 5 min. The reaction system
included 10 µl SYBR Premix Ex Taq™ II, 0.4 µl PCR
forward primer (10 µM), 0.4 µl PCR reverse primer (10
µM), 2 µl DNA template and 7.2 µl distilled
water. U6 was as an internal reference for the relative expression
of miR-183, and GAPDH was as an internal reference for the relative
expression of OSMR, Akt, GSK-3β, Bax, Bcl-2, caspase-9, IGF-1, and
mTOR. The relative quantitative 2−ΔΔCq method was used
to calculate relative mRNA transcription levels of target genes
miR-183, OSMR, Akt, GSK-3β, Bax, Bcl-2, caspase-9, IGF-1 and mTOR.
The calculation was as follows: ΔΔCq = ΔΔCq PD group −
ΔΔCq normal group, ΔCq = Cq (target genes) −
Cq (internal references). The relative mRNA
transcription level of target genes was determined as
2−ΔΔCq (28). The
experiment was repeated three times and the mRNA expression levels
of genes in each group were compared.
 | Table IPrimer sequences for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I
Primer sequences for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Sequence |
|---|
| miR-183 | F:
5′-TGTAGGACCTCCAGGAGAAGG-3′ |
| R:
5′-TATGGCCCTTCGGTAATTCA-3′ |
| U6 | F:
5′-TCCGACGCCGCCATCTCTA-3′ |
| R:
5′-TATCGCACATTAAGCCTCTA-3′ |
| OSMR | F:
5′-GCATCCCGAAGCGAAGTCTT-3′ |
| R:
5′-GGGCTGGGACAGTCCATTCTA-3′ |
| Akt | F:
5′-ATGAACGACGTAGCCATTGTG-3′ |
| R:
5′-TTGTAGCCAATAAAGGTGCCAT-3′ |
| GSK-3β | F:
5′-ATGGCAGCAAGGTAACCACAG-3′ |
| R:
5′-TCTCGGTTCTTAAATCGCTTGTC-3′ |
| cas-9 | F:
5′-GGCTGTTAAACCCCTAGACCA-3′ |
| R:
5′-TGACGGGTCCAGCTTCACTA-3′ |
| Bcl-2 | F:
5′-GCTACCGTCGTGACTTCGC-3′ |
| R:
5′-CCCCACCGAACTCAAAGAAGG-3′ |
| Bax | F:
5′-AGACAGGGGCCTTTTTGCTAC-3′ |
| R:
5′-AATTCGCCGGAGACACTCG-3′ |
| IGF-1 | F:
5′-CACATCATGTCGTCTTCACACC-3′ |
| R:
5′-GGAAGCAACACTCATCCACAATG-3′ |
| mTOR | F:
5′-CAGTTCGCCAGTGGACTGAAG-3′ |
| R:
5′-GCTGGTCATAGAAGCGAGTAGAC-3′ |
| GAPDH | F:
5′-AGGTCGGTGTGAACGGATTTG-3′ |
| R:
5′-GGGGTCGTTGATGGCAACA-3′ |
Western blot analysis
The substantia nigra nerve tissues of PD mice (10
mg) and normal mice (10 mg), frozen at −80°C, were placed into a
glass grinder; the tissue or cell lysate (500 µl; cat. no.
C1051; Whiga Biosmart Co., Ltd., Guangzhou, China) was added for
grinding into a homogenate in the an ice bath. The homogenate was
combined with protein lysate for dissociation at 4°C for 30 min
(agitation every 10 min) and centrifuged at 16.10 × g for 20 min at
4°C, with the lipid layer removed and the supernatant sub-packed.
According to the bicincho-ninic acid kit (cat. no. 23250; Thermo
Fisher Scientific, Inc.), the total protein concentration was
detected, and the total protein was packed and placed into a
refrigerator at −80°C. The protein (50 µg) in each group was
selected, to which protein denaturants were added (cat. no.
38249090; Sibas Biotechnology Development Co., Ltd., Shanghai,
China); the mixture was boiled for 10 min for degeneration,
followed by separation with SDS-PAGE (10%) and transfer from the
gel onto a polyvinylidene fluoride (PVDF) protein gel membrane via
the electric transfer method (cat. no. HVLP04700; EMD Millipore,
Bedford, MA, USA). The PVDF membrane was blocked in PBST
(containing 10% skim milk powder) at 4°C overnight and rinsed with
PBST three times (each for 5 min). The membrane was subsequently
incubated with the following primary antibodies purchased from
Abcam: Rabbit anti-mouse OSMR (cat. no. ab172254; 1:1,000), rabbit
anti-mouse Akt (cat. no. ab8805; 1:500), rabbit anti-mouse Bax
(cat. no. ab32503; 1:1,000), rabbit anti rat Bcl-2 (cat. no.
ab32124; 1:1,000), rabbit anti-mouse caspase-9 (cat. no. ab32539;
1:1,000), rabbit anti mouse IGF1 (cat. no. ab39398; 1:100), rabbit
anti-mouse mTOR (cat. no. ab2732; 1:2,000) and rabbit anti-mouse
GSK-3β (cat. no. ab68476; 1:500) and rabbit anti-mouse GAPDH (cat.
no. ab9485; 1:2,500). The membrane was incubated with the
antibodies for 2 h at 37°C and subsequently washed with PBST three
times, each time for 10 min. Horseradish peroxidase-labeled goat
anti-rabbit IgG was subsequently added (cat. no. DF109489; 1:1,000;
Yao Yun Biological Technology Co., Ltd., Shanghai, China) and the
membrane was incubated for 2 h at 37°C, and washed fully with PBST
three times (10 min each). An enhanced chemiluminescence kit (cat.
no. 36208ES60; Amersham; GE Healthcare Life Sciences, Chalfont, UK)
was used for coloration, and ImageJ 1.8.0 gray analysis software
(National Institutes of Health, Bethesda, MD, USA) was used for
semi-quantitative analysis of the western blot analysis results.
GAPDH was used as an internal reference, and the relative protein
expression was calculated as the ratio of the gray values of the
target band and the reference band. Three repetitions were
performed for each sample.
Flow cytometry
At 48 h post-transfection, the cells were detached
with trypsin without EDTA, collected, and centrifuged (111.8 × g
for 5 min) at room temperature with the supernatant removed. The
cells were washed twice with pre-cooled PBS, centrifuged at the
same speed for 5 min at room temperature and the supernatant was
removed. An Annexin V-fluorescein isothiocyanate (FITC)/propidium
iodide (PI) kit (cat. no. CA1020; Beijing Solarbio Science &
Technology Co., Ltd.) was used for the detection of cell apoptosis.
The cells were washed with binding buffer. Annexin-V-FITC and
binding buffer were used to prepare intermixtures with a ratio of
1:40 to resuspend cells. Following mixing, the cells were incubated
at room temperature for 30 min. The cells were added to the above
intermixture, mixed and incubated at room temperature for 15 min.
Apoptosis was detected using the Attune NxT flow cytometer (cat.
no. A24864; Thermo Fisher Scientific, Inc.). The experiment was
repeated three times.
Statistical analysis
All data were processed with SPSS v21.0 software
(IBM Corp., Armonk, NY, USA). Measurement data are presented as the
mean ± standard deviation. Comparisons between two groups were made
with an unpaired t-test, and comparisons between groups were
analyzed by one-way analysis of variance. The Student-Newman-Keuls
method was used as a post hoc test. The experiments were repeated
three times. P<0.05 was considered to indicate a statistically
significant difference.
Results
Behavioral changes of mice in the normal
and PD groups
Following model establishment, behavioral changes of
mice in the normal and PD groups were observed at 1, 2, 3, 4, 6 and
8 weeks. In the PD group, abnormal behaviors, including head
deviation, reduced movement, tail stiffness and slow motion were
observed at 7-10 days following surgery, whereas no obvious
abnormal behavior was observed in the normal group. At the
beginning of 2 weeks post-surgery, a number of mice in the
experiment were induced to be directionally rotated via APO
induction. Following the intraperitoneal injection of APO, a number
of mice demonstrated directional rotation within 1-3 min, with a
mean time of 2.2±0.5 min. In the rotation process, the mice mostly
rotated with the left hind limb as the fulcrum, bent left in situ
counterclockwise rotation end to end, and some even made a reverse
circular motion. The number of rotated mice induced by APO
increased significantly, and the rotation rate increased. By 6
weeks post-surgery, the number of mice with rotation induced by APO
and the rotation rate were stabilized. A total of 35 mice kept
rotating, and the mean rotation rate of >7 r/min was considered
as a successful PD model mouse. Therefore, establishment of the PD
model was successful.
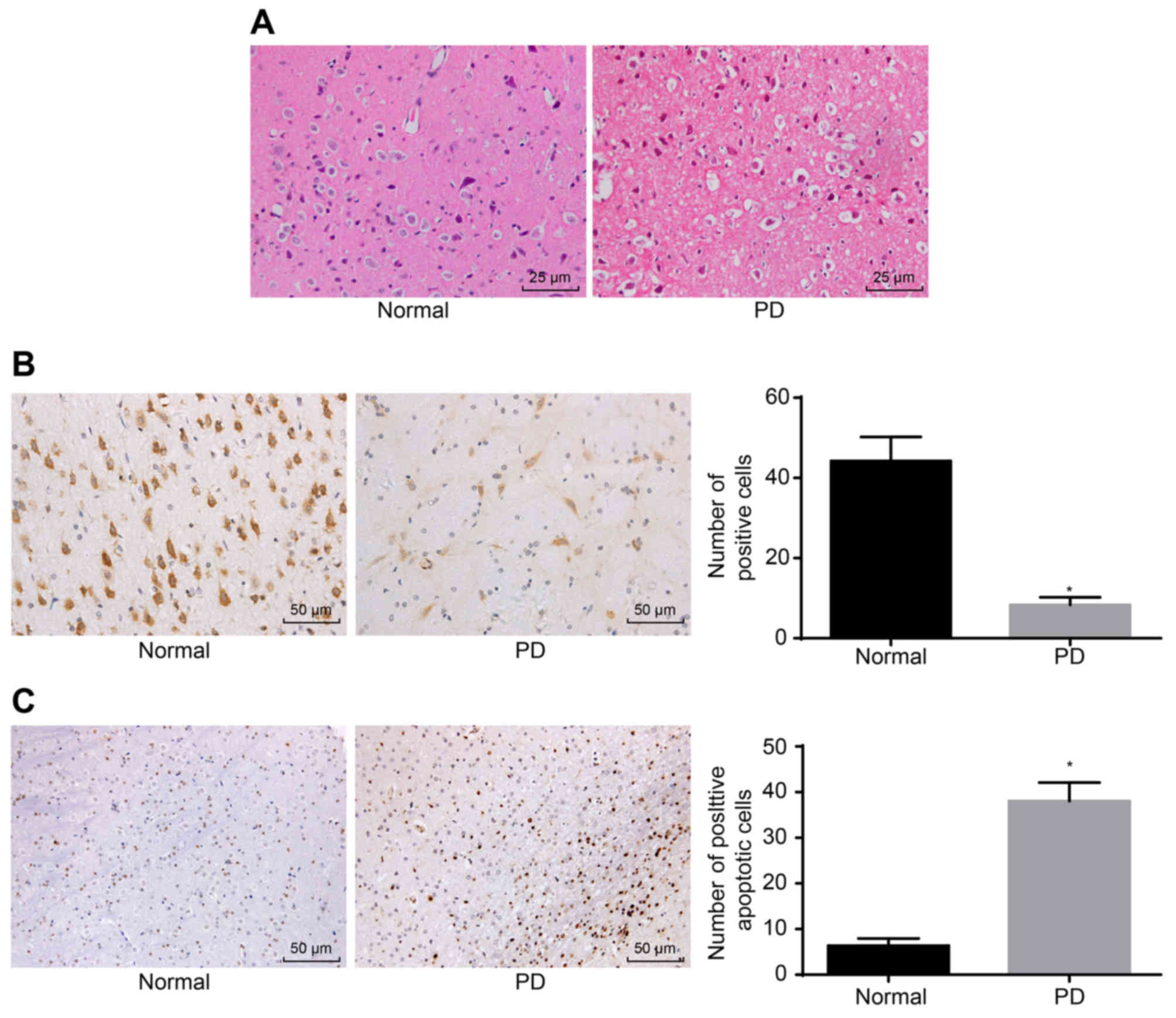
Pathological changes of substantia nigra
neurons
Pathological changes in the substantia nigra neurons
were assessed by H%E staining, immunohistochemistry and TUNEL. The
H&E staining demonstrated that the substantia nigra neurons in
the normal group had a higher density, larger number, and larger
volume, and exhibited elliptical and clear nuclear structures,
whereas the substantia nigra neurons in the PD group had decreased
numbers, pyknosis, condensation, interstitial edema and visible
slender darkly-stained neurons (Fig.
1A). Based on relevant literature, it was identified that OSMR
is associated with neurological diseases. It was reported that the
knockdown of OSMR increased cerebral infarction size and weakened
nerve function, and that OSMR was induced in normal cells (29). Previous studies suggested that
OSMR is a neuroprotective factor that may reduce excitotoxic injury
in vivo and in vitro, functioning in the nervous
system (30,31). Therefore, the present study aimed
to further understand the expression of OSMR in PD mice.
Immunohistochemistry used for detecting the expression of OSMR
revealed that OSMR-positive cells were yellow or brown,
predominantly in the cytoplasm in a conical or elliptical shape. As
presented in Fig. 1B, the number
of OSMR-positive cells (44.23±5.98) in the normal group was higher
compared with the number of OSMR-positive cells in the PD group
(8.32±1.89; P<0.05). The standard for TUNEL-positive cells is
that apoptotic bodies are located in nuclei and appear black or
blue/black. Few apoptotic positive substantia nigra neurons were
observed in the normal group. TUNEL-positive neurons in substantia
nigra were observed in the PD group with a black color, pyknosis,
and a round or irregular shape. Compared with the normal group, the
PD group had a significantly higher number of apoptotic substantia
nigra neurons (P<0.05; Fig.
1C). These results signified that OSMR-positive cells and the
apoptotic rate of substantia nigra neurons were elevated in PD.
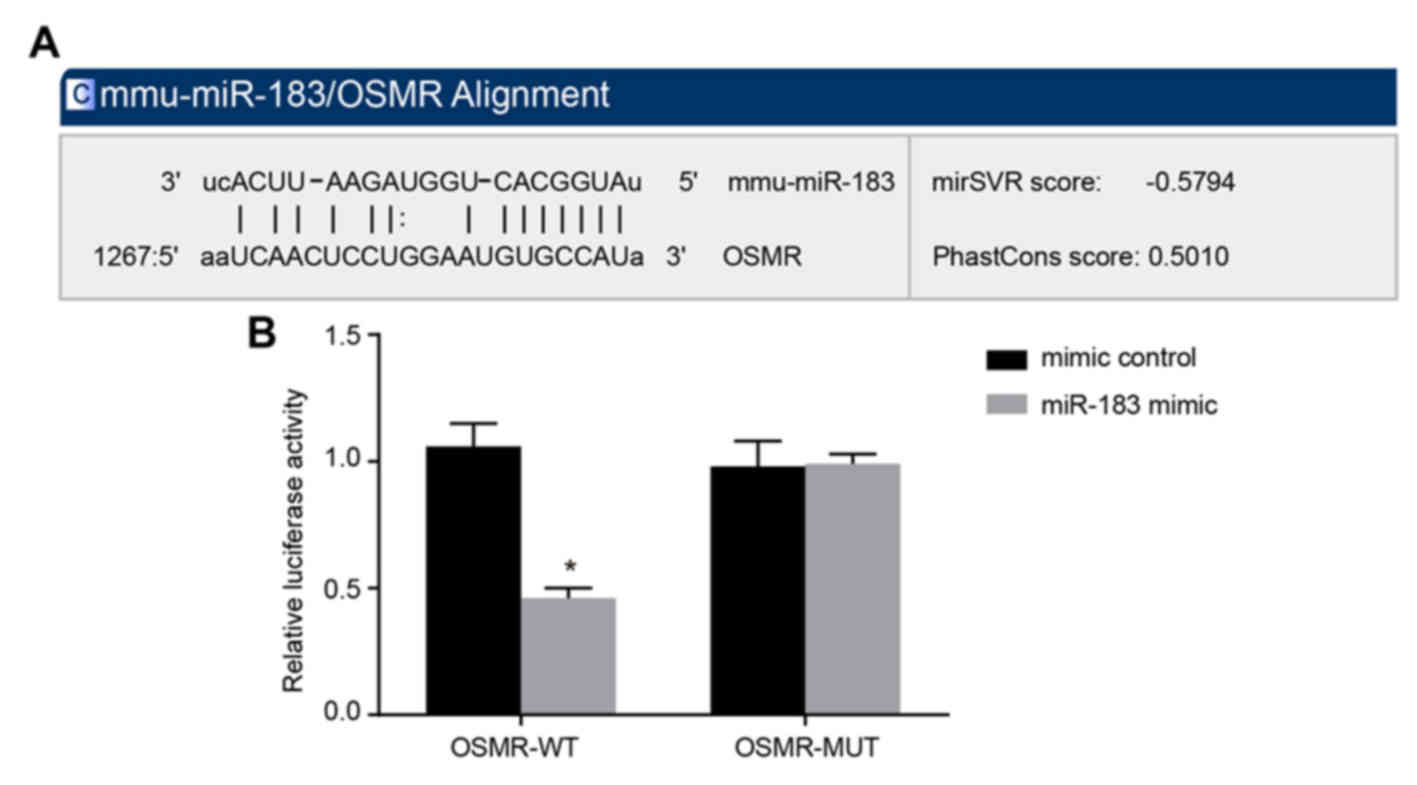
OSMR is the target gene of miR-183
Furthermore, the present study examined whether
miR-183 may directly regulate OSMR by performing online prediction
software analysis and luciferase activity determination. Through
online prediction software analysis, miR-183 and the OSMR 3′UTR had
binding sites (Fig. 2A), and OSMR
was identified as the target gene of miR-183. As presented in
Fig. 2B, compared with the mimic
control group, the luciferase activity intensity of the WT miR-183
mimic group was decreased significantly (P<0.05), whereas the
luciferase activity intensity of the MUT plasmid demonstrated no
significant change (P>0.05). These results suggested that
miR-183 inhibits the gene expression of OSMR. These results
suggested that miR-183 may directly target OSMR.
Cells transfected with miR-183 inhibitor
exhibit decreased expression of miR-183, GSK-3β, Bax and caspase-9,
and elevated OSMR, Akt, Bcl-2, IGF-1 and mTOR
The PI3K/Akt signaling pathway is key in the
development, survival and function of neurons in PD; and Timmons
et al (32) reported that
the expression of Akt was significantly reduced in the dense part
of the substantia nigra of patients with PD. In a PD mouse model
induced by 1-methyl-4-phenyl pyridinium cation, the upregulation of
regulated in development and DNA damage response 1, which may
inhibit mTOR, accelerated cell death by reducing the
mTORC2-dependent phosphorylation of Akt (33,34). One of the pathological hallmarks
of PD is progressive and selective loss of DA neurons in the
substantia nigra (35). Apoptosis
may be the primary reason for the loss of DA neurons in the
substantia nigra. Preventing or slowing the apoptosis of DA neurons
in the substantia nigra has been a focus of interest in the
therapeutics of PD. Matus et al (36), observed that apoptotic neurons in
the substantia nigra of patients with PD were significantly
increased, further supporting the correlation between apoptosis and
the pathogenesis of PD. Based on the above, the present study
hypothesized that miR-183 targeting OSMR may be involved in
apoptosis through the Akt signaling pathway. Therefore, the
expression of miR-183 and the mRNA and protein expression levels of
GSK-3β, Bax, caspase-9, OSMR, Akt, Bcl-2, IGF-1 and mTOR were
determined by RT-qPCR and western blot analysis. No differences in
the expression levels of miR-183, OSMR, Akt, p-Akt, GSK-3β,
p-GSK-3β, Bcl-2, IGF-1, mTOR, p-mTOR, Bax, or caspase-9 were
observed between the normal and miR-183 mimic NC groups
(P>0.05). Compared with the normal group, the miR-183 mimic
group exhibited increased expression levels of miR-183, Bax and
caspase-9, and decreased expression levels of OSMR, Akt, p-Akt,
GSK-3β, p-GSK-3β, Bcl-2, IGF-1, mTOR and p-mTOR (P<0.05;
Fig. 3A-C). Compared with the
control group, the miR-183 inhibitor NC group demonstrated no
significant differences between the above factors (P>0.05),
whereas the expression of miR-183, Bax and caspase-9 were decreased
markedly in the in the miR-183 inhibitor group (P<0.05), and the
expression levels of OSMR, Akt, p-Akt, GSK-3β, p-GSK-3β, Bcl-2,
IGF-1, mTOR and p-mTOR were markedly increased (P<0.05; Fig. 4A-C). Compared with the control
group, the miR-183 mimic + IGF-1 group exhibited increased
expression of miR-183 (P<0.05) and decreased expression of OSMR,
whereas no significant differences were identified in the
expression of Akt, p-Akt, GSK-3β, p-GSK-3β, Bcl-2, IGF-1, mTOR,
p-mTOR, Bax or caspase-9 (P>0.05); the OSMR group demonstrated
no marked differences in the expression of miR-183 (P>0.05);
however, demonstrated reduced expression levels of Bax and
caspase-9, and elevated expression levels of OSMR, Akt, p-Akt,
GSK-3β, p-GSK-3β, Bcl-2, IGF-1, mTOR and p-mTOR (P<0.05). The
expression of miR-183 and OSMR did not differ significantly in the
IGF-1 group (P>0.05), however, the expression levels of Bax and
caspase-9 were decreased and those of Akt, p-Akt, GSK-3β, p-GSK-3β,
Bcl-2, IGF-1, mTOR and p-mTOR were increased in the IGF-1 group
(P<0.05; Fig. 5A-C). Overall,
the obtained results demonstrated that miR-183 inhibited OSMR and
the PI3K-Akt signaling pathway, thus promoting neuronal apoptosis
in the substantia nigra in mice.
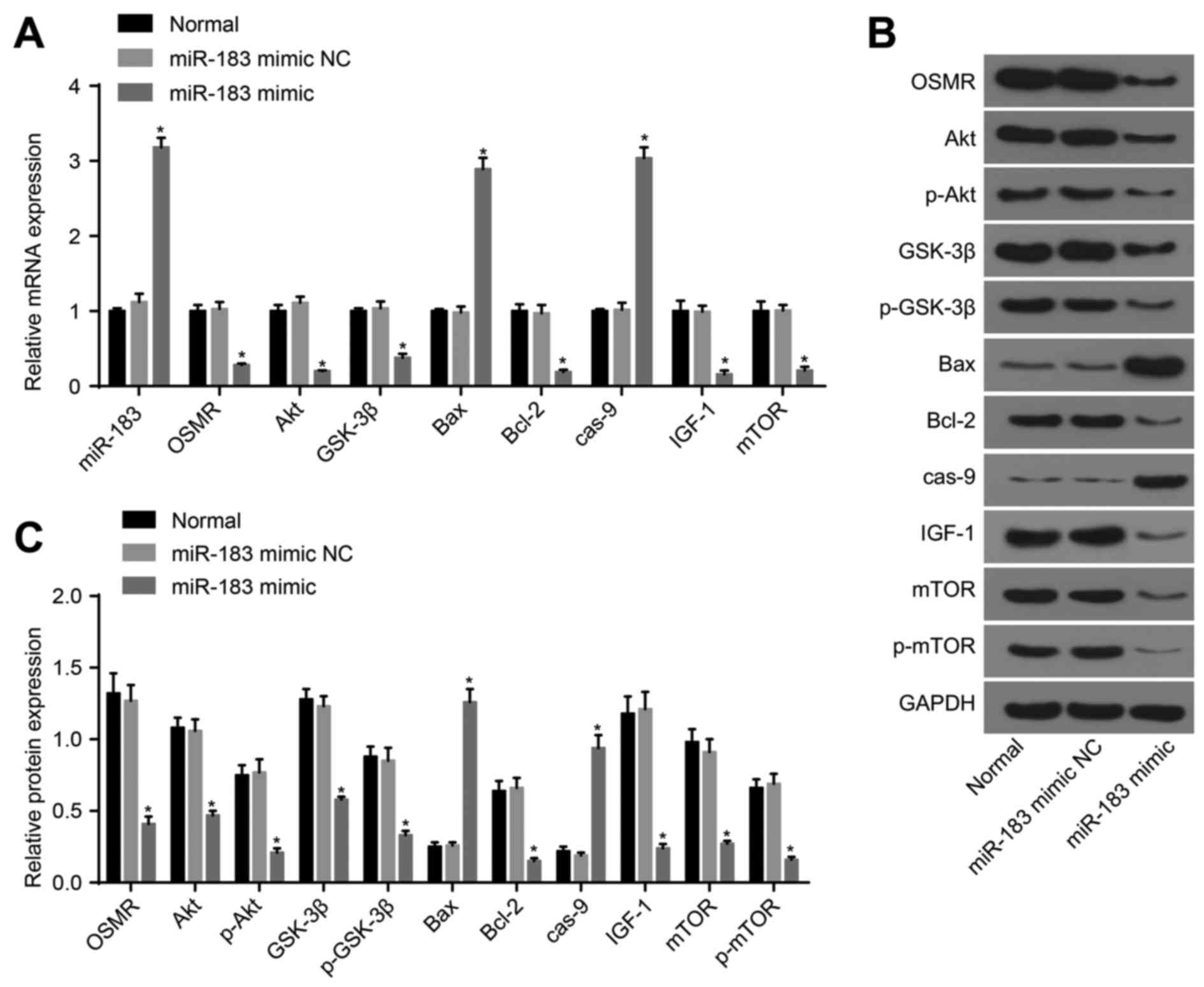 | Figure 3Detection of the expression of
miR-183, OSMR and PI3K-Akt pathway-related genes following
transfection with miR-183 mimic and mimic NC. (A) Expression of
miR-183, OSMR and PI3K-Akt pathway-associated genes in the normal,
miR-183 mimic NC and miR-183 mimic groups; (B) protein expression
of OSMR and PI3K-Akt pathway-related genes among the control,
miR-183 mimic NC and miR-183 mimic groups; (C) histogram of protein
expression; *P<0.05, compared with the normal group.
miR-183, microRNA-183; OSMR, oncostatin M receptor; NC, negative
control; IGF-1, insulin-like growth factor 1; GSK-3β, glycogen
synthase kinase 3β; mTOR, mammalian target of rapamycin; Bcl-2,
B-cell lymphoma 2; Bax, Bcl-2-associated X protein; cas-9,
caspase-9; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; p-,
phosphorylated. |
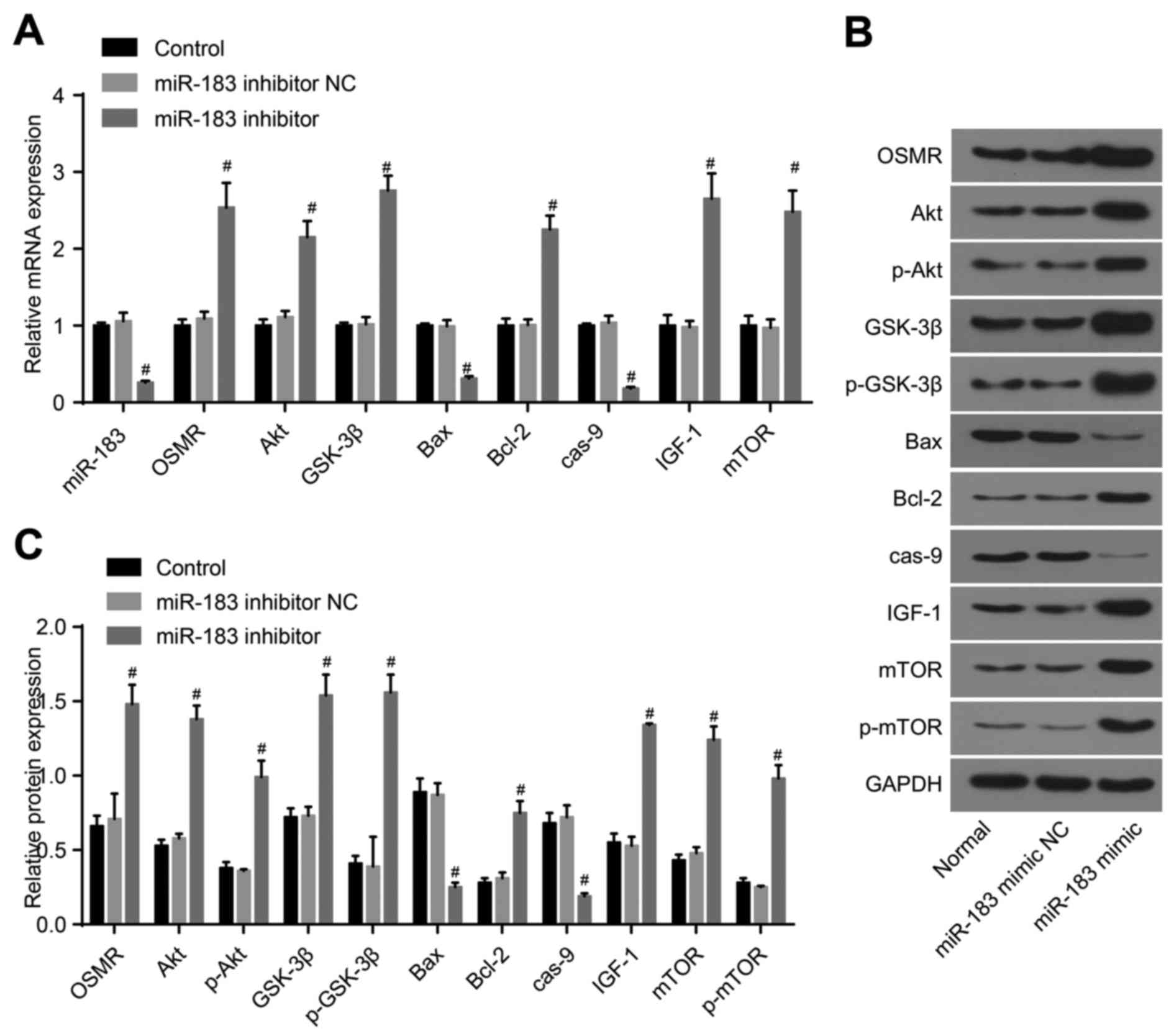 | Figure 4Detection of the expression of
miR-183, OSMR and PI3K-Akt pathway-related genes following
transfection with miR-183 inhibitor and inhibitor NC. (A)
Expression of miR-183, OSMR and PI3K-Akt pathway-related genes in
the control, miR-183 inhibitor NC and miR-183 inhibitor groups; (B)
protein expression of OSMR and PI3K-Akt pathway-related genes among
the control, miR-183 inhibitor NC and miR-183 inhibitor groups; (C)
histogram of protein expression. #P<0.05, compared
with the control group. miR-183, microRNA-183; OSMR, oncostatin M
receptor; NC, negative control; IGF-1, insulin-like growth factor
1; GSK-3β, glycogen synthase kinase 3β; mTOR, mammalian target of
rapamycin; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X
protein; cas-9, caspase-9; GAPDH, glyceraldehyde-3-phosphate
dehydrogenase; p-, phosphorylated. |
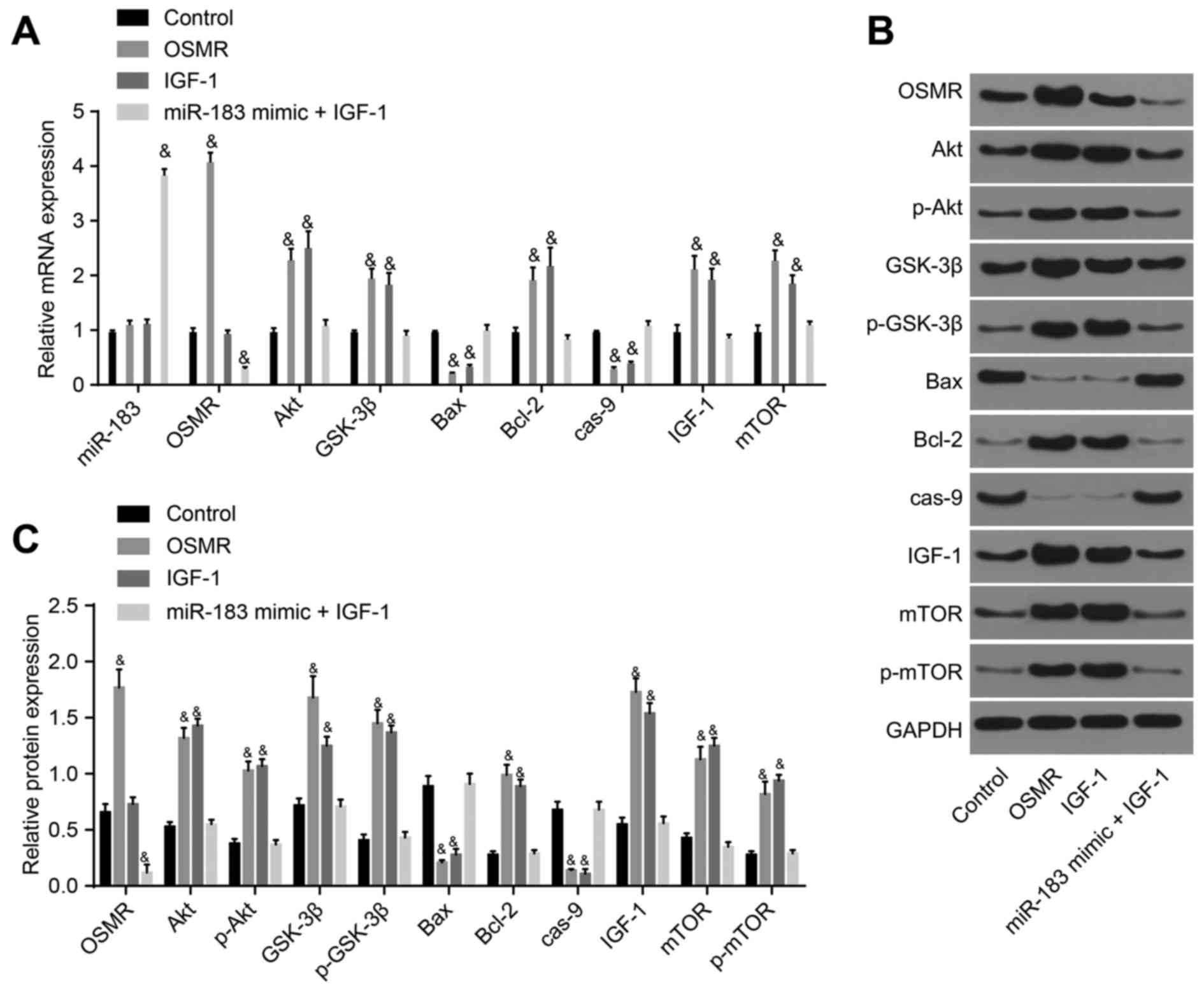 | Figure 5Detection of the expression of
miR-183, OSMR and PI3K-Akt pathway-related genes following
transfection with OSMR, IGF-1, and miR-183 mimic + IGF-1. (A)
Expression of miR-183, OSMR and PI3K-Akt pathway-related genes in
the control, OSMR, IGF-1 and miR-183 mimic + IGF-1 groups; (B)
protein expression of OSMR and PI3K-Akt pathway-related genes among
the control, OSMR, IGF-1 and miR-183 mimic + IGF-1 groups; (C)
histogram of protein expression. &P<0.05,
compared with the control group. miR-183, microRNA-183; OSMR,
oncostatin M receptor; NC, negative control; IGF-1, insulin-like
growth factor 1; GSK-3β, glycogen synthase kinase 3β; mTOR,
mammalian target of rapamycin; Bcl-2, B-cell lymphoma 2; Bax,
Bcl-2-associated X protein; cas-9, caspase-9; GAPDH,
glyceraldehyde-3-phosphate dehydrogenase; p-, phosphorylated. |
Overexpression of miR-183 decreases cell
apoptotic rate
The effects of miR-183 and OSMR on substantia nigra
neuron apop-tosis were assessed via flow cytometry of Annexin
V-FITC/PI double staining. The results (Fig. 6A-C) demonstrated no difference in
the apoptotic rate between the normal and miR-183 mimic NC groups
(P>0.05). Compared with the normal group, the apoptotic rate of
the miR-183 mimic group was increased (P<0.05). No significant
difference in the apoptotic rate was observed between the control
group and the miR-183 inhibitor NC group (P>0.05). Compared with
the control group, the apoptotic rate of the miR-183 inhibitor
group was significantly decreased (P<0.05). No significant
difference in apoptotic rate was observed between the control group
and the miR-183 mimic + IGF-1 group (P>0.05). Compared with the
control group, the apoptotic rates in the OSMR and IGF-1 groups
were decreased significantly (P<0.05). These results suggested
that miR-183 promotes apoptosis by inhibiting the expression of
OSMR and the PI3K-Akt signaling pathway.
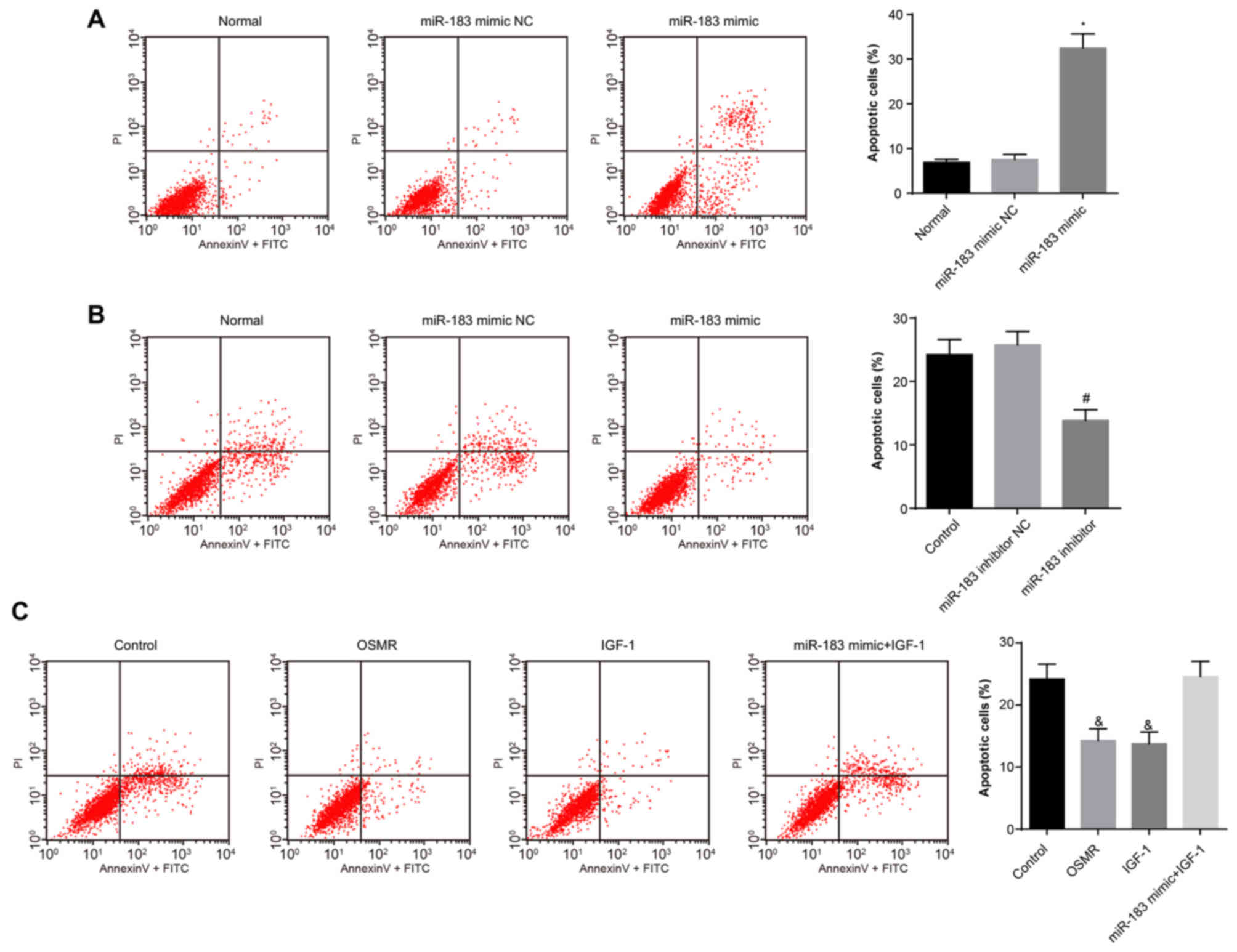 | Figure 6miR-183 promotes apoptosis by
inhibiting the expression of OSMR. (A) Apoptosis in the normal,
miR-183 mimic NC and miR-183 mimic groups (*P<0.05,
compared with the normal group); (B) apoptosis in the control,
miR-183 inhibitor NC and miR-183 inhibitor groups
(#P<0.05, compared with the control group); (C)
apoptosis in the control, OSMR, IGF-1 and miR-183 mimic + IGF-1
groups (&P<0.05, compared with the control
group). miR-183, microRNA-183; OSMR, oncostatin M receptor; NC,
negative control; IGF-1, insulin-like growth factor 1; PI,
propidium iodide; FITC, fluoroscein isothiocyanate. |
Discussion
PD is a progressive neurodegenerative disease
characterized by the loss of DA neurons in the SNc (37). A chronic mechanism of PD is
neuroinflammation, which may be associated with the changes in
glial cells, including astrocytes and microglia (38). Gene treatment has been
demonstrated to be an effective target for treating PD (39). In the present study, it was
verified that OSMR is the target gene of miR-183. In addition, it
was observed that the overexpression of miR-183 may cause increased
apoptotic rates of substantia nigra neurons by inhibiting the
expression of OSMR.
First, the present study revealed that PD mice had
an elevated expression of miR-183 and decreased expression of OSMR.
hsa-miR-183 has been identified to be expressed in PD and has a
higher expression compared with in sporadic amyotrophic lateral
sclerosis (40). Motoyama et
al (41) reported the
overexpression of miR-183 in human colorectal carcinoma. OSMR, a
potent suppressor in tumor cells, is a receptor of OSM, which is a
multifunctional cytokine belonging to the IL-6 family (42,43). OSM has been identified to inhibit
cell differentiation and apoptosis in cancer (44), and OSMR was observed to induce
cell death and apoptosis in adrenocortical Y-1 tumor cells
(45). Furthermore, the present
study performed a dual luciferase reporter assay and verified that
OSMR was the target gene of miR-183.
Second, compared with the blank and NC groups, the
expression of miR-183 was higher, whereas the expression levels of
Akt, GSK-3β, IGF-1 and mTOR were decreased in the substantia nigra
neurons in the miR-183 mimic group. Akt is an important molecule in
the PI3K/Akt pathway, which provides important signaling for
neuroprotection (46).
Furthermore, a previous study confirmed that the PI3K/Akt pathway,
rather than the mitogen-activated protein kinase/extracellular
signal-regulated kinase pathway, significantly contributes to
neuroprotection in PD brains (47). GSK-3β, as one of the substrates of
Akt, is a pleiotropic serine/threonine protein kinase (48). GSK-3β is one of several kinases
associated with the posttranslational modifications of key proteins
known to be causal in PD (49).
IGF-1 is involved in the PI3K/Akt cascades, and its protein
synthesis requires the activation of Akt and mTOR (50). IGF-1 may increase the survival and
maturation of sympathetic nerve cells, can and promote the
development of retinal neurons and the survival of multipolar
neurons in the central nervous system (51,52). miR-96 belongs to the miR-183
family, the overexpression of which may cause the suppressed
expression of IGF-1 (53,54). mTOR deregulation occurs in human
disease (55). The pooled
knockdown of the miR-183 cluster has been shown to induce the
expression of AKT1 (56). As this
was a direct investigation of Akt, follow-up investigations are
required with a focus on the association of miR-183 and Akt1/2 in
PD. OSMR was significantly downregulated in Janus kinase
1-deficient cells, in which the transient expression of Janus
kinase 1 was able to reverse the expression of OSMR (57). Therefore, the present study
suggested that miR-183 may inhibit the expression of OSMR and the
PI3K/Akt signaling pathway in PD.
Third, in the present study, the miR-183 mimic group
exhibited a higher expression of Bax and caspase-9; however, a
lower expression of Bcl-2 and mTOR. In addition, decreased cell
proliferation and increased cell apoptosis were found in the
miR-183 mimic group. The Bcl-2 family members have been identified
to be anti-apoptotic or pro-apoptotic regulators, with wide effects
on cellular activities (58). Bax
contains a non-stabi-lized G8 tract at nucleotides 114-121, which
is a member of the Bcl-2 family and has a mutation in mismatch
repair-deficient tumors (59).
Caspase-9, Bcl-2 and Bax are all closely associated with cell
apoptosis. When the protein expression of Bcl-2 is dominant, cells
may be prevented from apoptosis, whereas increasing the protein
expression of Bax may promote apop-tosis (60). Xu et al (61), demonstrated that ginsenoside Re
protected from MPTP-induced apoptosis in the PD mouse nigral
neurons and may be attributed to increasing the expression of
Bcl-2, downregulating the expression of Bax, and inhibiting the
activation of caspase-3. Elevated immunoreactivity of the
pro-apoptotic protein Bax has been identified in the apoptotic
nigral cells of PD (62).
Caspase-9 belongs to the caspase family of cysteine proteases,
which are involved in apoptosis and cytokine processing (63). A previous study observed that the
overexpression of miR-497 may activate caspase-9/3 (64). In addition, Sangawa et al
(65) investigated the
association between p-caspase-9 and p-Akt in gastric and colorectal
cancer and observed that Akt phosphorylates caspase-9, which may
inhibit cell apoptosis. mTOR regulates autophagy, the failure of
which leads to deficiency in the elimination of abnormal and toxic
protein aggregates, which subsequently triggers cellular stress,
failure, and ultimately death (66). Similar to the Bcl-2 family,
miR-183 is one of the representative apoptosis-associated miRNA
clusters (67). In addition, it
was demonstrated that miR-183 activated the reactive oxygen
species-mediated apoptotic pathway, which weakened the anticancer
effect of temozolomide in treating gliomas (68). Therefore, the overexpression of
miR-183 may promote cell apoptosis in PD mice.
The present study concluded that the upregulation of
miR-183 may inhibit the expression of OSMR to promote the apoptosis
of substantia nigra neurons in PD. In addition, the PI3K/Akt
signaling pathway may be involved in this mechanism. However, due
to the limitations of time and funding, whether miR-183 mediates
other genes associated with apoptosis of substantia nigra neurons
via the PI3K/Akt signaling pathway remain unclear. Oussaief et
al (69), additionally
suggested that the inhibition of Akt upregulated the expression of
miR-183. The regulatory association between miR-183 and Akt
requires further verified by in vivo interference in the
mouse model of PD. The present study may provide clinical reference
by using miR-183 inhibitors to restrain the apoptosis of substantia
nigra neurons in PD.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors’ contributions
JXG and YL designed the study. YL, SNW and XCC
collated the data, designed and developed the database, and
conducted the data analyses. LLL and HZ performed the statistical
analysis. YL, SNW and XCC drafted the paper and contributed
substantially to its revision. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The experiments and the use of all experimental
animals were approved by the Animal Ethics Committee of the Second
Hospital of Dalian Medical University (Dalian, China). All efforts
were made in the experiments to minimize pain in the mice.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chong TT, Bonnelle V, Manohar S, Veromann
KR, Muhammed K, Tofaris GK, Hu M and Husain M: Dopamine enhances
willingness to exert effort for reward in Parkinson’s disease.
Cortex. 69:40–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lenka A, Padmakumar C and Pal PK:
Treatment of older Parkinson’s disease. Int Rev Neurobiol.
132:381–405. 2017. View Article : Google Scholar
|
|
3
|
Farooqui T and Farooqui AA: Lipid-mediated
oxidative stress and inflammation in the pathogenesis of
Parkinson’s disease. Parkinsons Dis. 2011.247467:2011.
|
|
4
|
Karunanayaka PR, Lee EY, Lewis MM, Sen S,
Eslinger PJ, Yang QX and Huang X: Default mode network differences
between rigidity- and tremor-predominant Parkinson’s disease.
Cortex. 81:239–250. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Agim ZS and Cannon JR: Dietary factors in
the etiology of Parkinson’s disease. BioMed Res Int.
2015.672838:2015.
|
|
6
|
Benabid AL, Chabardes S, Mitrofanis J and
Pollak P: Deep brain stimulation of the subthalamic nucleus for the
treatment of Parkinson’s disease. Lancet Neurol. 8:67–81. 2009.
View Article : Google Scholar
|
|
7
|
Gonzales-Portillo GS, Reyes S, Aguirre D,
Pabon MM and Borlongan CV: Stem cell therapy for neonatal
hypoxic-ischemic encephalopathy. Front Neurol. 5:1472014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kahn E, D’Haese PF, Dawant B, Allen L, Kao
C, Charles PD and Konrad P: Deep brain stimulation in early stage
Parkinson’s disease: operative experience from a prospective
randomised clinical trial. J Neurol Neurosurg Psychiatry.
83:164–170. 2012. View Article : Google Scholar
|
|
9
|
Scatena R, Martorana GE, Bottoni P, Botta
G, Pastore P and Giardina B: An update on pharmacological
approaches to neuro-degenerative diseases. Expert Opin Investig
Drugs. 16:59–72. 2007. View Article : Google Scholar
|
|
10
|
Schapira AH, Bezard E, Brotchie J, Calon
F, Collingridge GL, Ferger B, Hengerer B, Hirsch E, Jenner P, Le
Novère N, et al: Novel pharmacological targets for the treatment of
Parkinson’s disease. Nat Rev Drug Discov. 5:845–854. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miñones-Moyano E, Porta S, Escaramís G,
Rabionet R, Iraola S, Kagerbauer B, Espinosa-Parrilla Y, Ferrer I,
Estivill X and Martí E: MicroRNA profiling of Parkinson’s disease
brains identifies early downregulation of miR-34b/c which modulate
mitochondrial function. Hum Mol Genet. 20:3067–3078. 2011.
View Article : Google Scholar
|
|
12
|
Lowery AJ, Miller N, Dwyer RM and Kerin
MJ: Dysregulated miR-183 inhibits migration in breast cancer cells.
BMC Cancer. 10:5022010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu W, Zhou K, Zha Y, Chen D, He J, Ma H,
Liu X, Le H and Zhang Y: Diagnostic Value of Serum miR-182,
miR-183, miR-210, and miR-126 levels in patients with early-stage
non-small cell lung cancer. PLoS One. 11:e01530462016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuan D, Li K, Zhu K, Yan R and Dang C:
Plasma miR-183 predicts recurrence and prognosis in patients with
colorectal cancer. Cancer Biol Ther. 16:268–275. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pierce ML, Weston MD, Fritzsch B, Gabel
HW, Ruvkun G and Soukup GA: MicroRNA-183 family conservation and
ciliated neurosensory organ expression. Evol Dev. 10:106–113. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arita K, South AP, Hans-Filho G, Sakuma
TH, Lai-Cheong J, Clements S, Odashiro M, Odashiro DN, Hans-Neto G,
Hans NR, et al: Oncostatin M receptor-beta mutations underlie
familial primary localized cutaneous amyloidosis. Am J Hum Genet.
82:73–80. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Isozaki O, Tsushima T, Miyakawa M, Emoto
N, Demura H, Arai M and Sato-Nozoe Y: Oncostatin M: a new potent
inhibitor of iodine metabolism inhibits thyroid peroxidase gene
expression but not DNA synthesis in porcine thyroid cells in
culture. Thyroid. 7:71–77. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Markman B, Dienstmann R and Tabernero J:
Targeting the PI3K/Akt/mTOR pathway--beyond rapalogs. Oncotarget.
1:530–543. 2010.
|
|
19
|
Zhang W, He H, Song H, Zhao J, Li T, Wu L,
Zhang X and Chen J: Neuroprotective effects of salidroside in the
MPTP mouse model of Parkinson’s disease: Involvement of the
PI3K/Akt/GSK3β pathway. Parkinsons Dis. 2016.9450137:2016.
|
|
20
|
Shao JL, Wan XH, Chen Y, Bi C, Chen HM,
Zhong Y, Heng XH and Qian JQ: H2S protects hippocampal neurons from
anoxia-reoxygenation through cAMP-mediated PI3K/Akt/p70S6K
cell-survival signaling pathways. Journal of molecular
neuroscience: J Mol Neurosci. 43:453–460. 2011. View Article : Google Scholar
|
|
21
|
Zhang L, Qu Y, Tang J, Chen D, Fu X, Mao M
and Mu D: PI3K/Akt signaling pathway is required for
neuroprotection of thalidomide on hypoxic-ischemic cortical neuron
in vitro. Brain Res. 1357:157–165. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhong Z, Wang Y, Guo H, Sagare A,
Fernández JA, Bell RD, Barrett TM, Griffin JH, Freeman RS and
Zlokovic BV: Protein S protects neurons from excitotoxic injury by
activating the TAM receptor Tyro3-phosphatidylinositol 3-kinase-Akt
pathway through its sex hormone-binding globulin-like region. J
Neurosci. 30:15521–15534. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choe G, Horvath S, Cloughesy TF, Crosby K,
Seligson D, Palotie A, Inge L, Smith BL, Sawyers CL and Mischel PS:
Analysis of the phosphatidylinositol 3′-kinase signaling pathway in
glioblastoma patient in vivo. Cancer Res. 63:2742–2746.
2003.PubMed/NCBI
|
|
24
|
Neve RM, Holbro T and Hynes NE: Distinct
roles for phosphoinositide 3-kinase, mitogen-activated protein
kinase and p38 MAPK in mediating cell cycle progression of breast
cancer cells. Oncogene. 21:4567–4576. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Philp AJ, Campbell IG, Leet C, Vincan E,
Rockman SP, Whitehead RH, Thomas RJ and Phillips WA: The
phosphatidylinositol 3′-kinase p85alpha gene is an oncogene in
human ovarian and colon tumors. Cancer Res. 61:7426–7429.
2001.PubMed/NCBI
|
|
26
|
Lee J, Zhu WM, Stanic D, Finkelstein DI,
Horne MH, Henderson J, Lawrence AJ, O’Connor L, Tomas D, Drago J,
et al: Sprouting of dopamine terminals and altered dopamine release
and uptake in Parkinsonian dyskinaesia. Brain. 131:1574–1587. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee SY, Moon Y, Hee Choi D, Jin Choi H and
Hwang O: Particular vulnerability of rat mesencephalic dopaminergic
neurons to tetrahydrobiopterin: Relevance to Parkinson’s disease.
Neurobiol Dis. 25:112–120. 2007. View Article : Google Scholar
|
|
28
|
Ayuk SM, Abrahamse H and Houreld NN: The
role of photo-biomodulation on gene expression of cell adhesion
molecules in diabetic wounded fibroblast in vitro. J Photochem
Photobiol B. 161:368–374. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li H, Sen G, Lu Y, Zheng A and Li M:
Function and application of type-II oncostatin-M receptor (OSMR) in
cerebral apoplexy diseases. CN Patent 104083754A. Filed July 8,
2014 issued October 8, 2018.
|
|
30
|
Weiss TW, Samson AL, Niego B, Daniel PB
and Medcalf RL: Oncostatin M is a neuroprotective cytokine that
inhibits excitotoxic injury in vitro an in vivo. FASEB J.
20:2369–2371. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pelletier JP and Martel-Pelletier J:
Oncostatin M: Foe or friend. Arthritis Rheum. 48:3301–3303. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Timmons S, Coakley MF, Moloney AM and
O’Neill C: Akt signal transduction dysfunction in Parkinson’s
disease. Neurosci Lett. 467:30–35. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Malagelada C, Ryu EJ, Biswas SC,
Jackson-Lewis V and Greene LA: RTP801 is elevated in Parkinson
brain substantia nigral neurons and mediates death in cellular
models of Parkinson’s disease by a mechanism involving mammalian
target of rapamycin inactivation. J Neurosci. 26:9996–10005. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hsuan SL, Klintworth HM and Xia Z: Basic
fibroblast growth factor protects against rotenone-induced
dopaminergic cell death through activation of extracellular
signal-regulated kinases 1/2 and phosphatidylinositol-3 kinase
pathways. J Neurosci. 26:4481–4491. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kwon DH, Kim JM, Oh SH, Jeong HJ, Park SY,
Oh ES, Chi JG, Kim YB, Jeon BS and Cho ZH: Seven-Tesla magnetic
resonance images of the substantia nigra in Parkinson disease. Ann
Neurol. 71:267–277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Matus S, Castillo K and Hetz C: Hormesis:
Protecting neurons against cellular stress in Parkinson disease.
Autophagy. 8:997–1001. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Harms AS, Barnum CJ, Ruhn KA, Varghese S,
Treviño I, Blesch A and Tansey MG: Delayed dominant-negative TNF
gene therapy halts progressive loss of nigral dopaminergic neurons
in a rat model of Parkinson’s disease. Mol Ther. 19:46–52. 2011.
View Article : Google Scholar
|
|
38
|
Jha SK, Jha NK, Kar R, Ambasta RK and
Kumar P: p38 MAPK and PI3K/AKT Signalling Cascades inParkinson’s
Disease. Int J Mol Cell Med. 4:67–86. 2015.PubMed/NCBI
|
|
39
|
LeWitt PA, Rezai AR, Leehey MA, Ojemann
SG, Flaherty AW, Eskandar EN, Kostyk SK, Thomas K, Sarkar A,
Siddiqui MS, et al: AAV2-GAD gene therapy for advanced Parkinson’s
disease: a double-blind, sham-surgery controlled, randomised trial.
Lancet Neurol. 10:309–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen Y, Wei Q, Chen X, Li C, Cao B, Ou R,
Hadano S and Shang HF: Aberration of miRNAs expression in
leukocytes from sporadic amyotrophic lateral sclerosis. Front Mol
Neurosci. 9:692016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Motoyama K, Inoue H, Takatsuno Y, Tanaka
F, Mimori K, Uetake H, Sugihara K and Mori M: Over- and
under-expressed microRNAs in human colorectal cancer. Int J Oncol.
34:1069–1075. 2009.PubMed/NCBI
|
|
42
|
Tanaka M, Hirabayashi Y, Sekiguchi T,
Inoue T, Katsuki M and Miyajima A: Targeted disruption of
oncostatin M receptor results in altered hematopoiesis. Blood.
102:3154–3162. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hibi K, Goto T, Sakuraba K, Shirahata A,
Saito M, Ishibashi K, Kigawa G, Nemoto H and Sanada Y: Methylation
of OSMR gene is frequently observed in non-invasive colorectal
cancer. Anticancer Res. 31:1293–1295. 2011.PubMed/NCBI
|
|
44
|
Deng G, Kakar S, Okudiara K, Choi E,
Sleisenger MH and Kim YS: Unique methylation pattern of oncostatin
m receptor gene in cancers of colorectum and other digestive
organs. Clin Cancer Res. 15:1519–1526. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Auernhammer CJ, Dorn F, Vlotides G, Hengge
S, Kopp FB, Spoettl G, Cengic N, Engelhardt D and Weber MM: The
oncostatin M receptor/gp130 ligand murine oncostatin M induces
apoptosis in adrenocortical Y-1 tumor cells. J Endocrinol.
180:479–486. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gao Y, Li J, Wu L, Zhou C, Wang Q, Li X,
Zhou M and Wang H: Tetrahydrocurcumin provides neuroprotection in
rats after traumatic brain injury: Autophagy and the PI3K/AKT
pathways as a potential mechanism. J Surg Res. 206:67–76. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Quesada A, Lee BY and Micevych PE: PI3
kinase/Akt activation mediates estrogen and IGF-1 nigral DA
neuronal neuroprotection against a unilateral rat model of
Parkinson’s disease. Dev Neurobiol. 68:632–644. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cross DA, Culbert AA, Chalmers KA, Facci
L, Skaper SD and Reith AD: Selective small-molecule inhibitors of
glycogen synthase kinase-3 activity protect primary neurones from
death. J Neurochem. 77:94–102. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Credle JJ, George JL, Wills J, Duka V,
Shah K, Lee YC, Rodriguez O, Simkins T, Winter M, Moechars D, et
al: GSK-3β dysregulation contributes to parkinson’s-like
pathophysiology with associated region-specific phosphorylation and
accumulation of tau and α-synuclein. Cell Death Differ. 22:838–851.
2015. View Article : Google Scholar
|
|
50
|
Bibollet-Bahena O and Almazan G:
IGF-1-stimulated protein synthesis in oligodendrocyte progenitors
requires PI3K/mTOR/Akt and MEK/ERK pathways. J Neurochem.
109:1440–1451. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kouroupi G, Lavdas AA, Gaitanou M,
Thomaidou D, Stylianopoulou F and Matsas R: Lentivirus-mediated
expression of insulin-like growth factor-I promotes neural
stem/precursor cell proliferation and enhances their potential to
generate neurons. J Neurochem. 115:460–474. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hellerstein MK: Relationship between
precursor enrichment and ratio of excess M2/excess M1 isotopomer
frequencies in a secreted polymer. J Biol Chem. 266:10920–10924.
1991.PubMed/NCBI
|
|
53
|
Li H, Gong Y, Qian H, Chen T, Liu Z, Jiang
Z and Wei S: Brain-derived neurotrophic factor is a novel target
gene of the has-miR-183/96/182 cluster in retinal pigment
epithelial cells following visible light exposure. Mol Med Rep.
12:2793–2799. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Budzinska M, Owczarz M, Pawlik-Pachucka E,
Roszkowska- Gancarz M, Slusarczyk P and Puzianowska-Kuznicka M:
miR-96, miR-145 and miR-9 expression increases, and IGF-1R and
FOXO1 expression decreases in peripheral blood mono-nuclear cells
of aging humans. BMC Geriatr. 16:2002016. View Article : Google Scholar
|
|
55
|
Laplante M and Sabatini DM: mTOR signaling
in growth control and disease. Cell. 149:274–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Weeraratne SD, Amani V, Teider N,
Pierre-Francois J, Winter D, Kye MJ, Sengupta S, Archer T, Remke M,
Bai AH, et al: Pleiotropic effects of miR-183~96~182 converge to
regulate cell survival, proliferation and migration in
medulloblastoma. Acta Neuropathol. 123:539–552. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Radtke S, Hermanns HM, Haan C, De
Schmitz-Van Leur H, Gascan H, Heinrich PC and Behrmann I: Novel
role of Janus kinase 1 in the regulation of oncostatin M receptor
surface expression. J Biol Chem. 277:11297–11305. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yamanaka K, Rocchi P, Miyake H, Fazli L,
Vessella B, Zangemeister-Wittke U and Gleave ME: A novel antisense
oligonucleotide inhibiting several antiapoptotic Bcl-2 family
members induces apoptosis and enhances chemosensitivity in
androgen-independent human prostate cancer PC3 cells. Mol Cancer
Ther. 4:1689–1698. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang L, Yu J, Park BH, Kinzler KW and
Vogelstein B: Role of BAX in the apoptotic response to anticancer
agents. Science. 290:989–992. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lee JS, Jung WK, Jeong MH, Yoon TR and Kim
HK: Sanguinarine induces apoptosis of HT-29 human colon cancer
cells via the regulation of Bax/Bcl-2 ratio and caspase-9-dependent
pathway. Int J Toxicol. 31:70–77. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Xu BB, Liu CQ, Gao X, Zhang WQ, Wang SW
and Cao YL: Possible mechanisms of the protection of ginsenoside Re
against MPTP-induced apoptosis in substantia nigra neurons of
Parkinson’s disease mouse model. J Asian Nat Prod Res. 7:215–224.
2005. View Article : Google Scholar
|
|
62
|
Rekha KR and Selvakumar GP: Gene
expression regulation of Bcl2, Bax and cytochrome-C by geraniol on
chronic MPTP/probenecid induced C57BL/6 mice model of Parkinson’s
disease. Chem Biol Interact. 217:57–66. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kuida K: Caspase-9. Int J Biochem Cell
Biol. 32:121–124. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wu R, Tang S, Wang M, Xu X, Yao C and Wang
S: microRNA-497 induces apoptosis and suppresses proliferation via
the Bcl-2/Bax-caspase9-caspase3 pathway and Cyclin D2 protein in
HUVECs. PLoS One. 11:e01670522016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sangawa A, Shintani M, Yamao N and
Kamoshida S: Phosphorylation status of Akt and caspase-9 in gastric
and colorectal carcinomas. Int J Clin Exp Pathol. 7:3312–3317.
2014.PubMed/NCBI
|
|
66
|
Heras-Sandoval D, Pérez-Rojas JM,
Hernández-Damián J and Pedraza-Chaverri J: The role of
PI3K/AKT/mTOR pathway in the modulation of autophagy and the
clearance of protein aggregates in neurodegeneration. Cell Signal.
26:2694–2701. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Papadopoulos EI, Yousef GM and Scorilas A:
Cytotoxic activity of sunitinib and everolimus in Caki-1 renal
cancer cells is accompanied by modulations in the expression of
apoptosis-related microRNA clusters and BCL2 family genes. Biomed
Pharmacother. 70:33–40. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Tang H, Bian Y, Tu C, Wang Z, Yu Z, Liu Q,
Xu G, Wu M and Li G: The miR-183/96/182 cluster regulates oxidative
apoptosis and sensitizes cells to chemotherapy in gliomas. Curr
Cancer Drug Targets. 13:221–231. 2013. View Article : Google Scholar
|
|
69
|
Oussaief L, Fendri A, Chane-Woon-Ming B,
Poirey R, Delecluse HJ, Joab I and Pfeffer S: Modulation of
microRNA cluster miR-183-96-182 expression by Epstein-Barr virus
latent membrane protein 1. J Virol. 89:12178–12188. 2015.
View Article : Google Scholar : PubMed/NCBI
|