Introduction
Minimally invasive surgery using gas-induced
pneumoperitoneum has become an important surgical approach. The
reduced pain, shorter postoperative hospital stay and better
cosmetic results also make this technique attractive for patients
(1-3). Carbon dioxide (CO2) is
the most commonly used gas for insufflation of the abdominal cavity
to provide better exposure during laparoscopic surgery (4). However, an increasing numbers of
studies have reported that several physiological complications are
closely associated with high intraabdominal pressure caused by
CO2 (5-7).
Kidneys, as important splanchnic organs, are
inevitably affected by intraabdominal pressure (8,9).
Previous clinical and experimental studies have demonstrated that
the laparoscopic procedure with pneumoperitoneum provides a typical
model of ischemia/reperfusion (I/R) injury in the organs (10). Previous animal experiments have
demonstrated that high and erratic elevations of intraabdominal
pressure can decrease venous return, compress the renal vasculature
and cause systemic hormonal changes, which eventually significantly
decrease renal blood flow, urinary output and glomerular filtration
rate (11). Further studies have
observed increases in renal ischemia and oxidative stress response
in the presence of increased intraabdominal pressure (12,13). Although abdominal deflation at the
end of laparoscopic procedures reduces the intraabdominal pressure
and increases renal perfusion, damage from the ischemic injury
remains. Nevertheless, the majority of studies associated with
pneumoperitoneum pressure damage involve normal kidneys (14); however, a number of patients who
undergo laparoscopic surgery also exhibit a degree of kidney
obstruction. To date, only a limited number of studies have
reported how pneumoperitoneum affects kidneys with hydronephrosis
(15).
In our previous study, the effect of
pneumoperitoneum pressure on rabbit kidneys with no hydronephrosis,
and with mild or severe hydronephrosis was investigated (16). The results indicated that severely
obstructed kidneys have reduced cell tolerance to intraabdominal
pressure, and that they are more likely to suffer oxidative damage
and mitochondrial injuries when they were exposed to
pneumoperitoneal pressure. It is generally accepted that oxidative
damage and mitochondrial injuries are common ischemic pathological
changes that can eventually cause apoptosis (17). Considering these facts, it can be
speculated that with the increase of pneumoperitoneal pressure,
apoptosis may occur as a consequence of severe oxidative damage and
mitochondrial injuries in rabbit kidneys with severe
hydronephrosis. Therefore, the present study aimed to examine the
effects of high-pressure pneumoperitoneum with CO2 on
the kidneys of rabbits with severe hydronephrosis and to
investigate the possible mechanism involved.
Materials and methods
Animals and groups
In total, 18 adolescent male New Zealand rabbits
were purchased from the Wuhan Institute of Biotechnology (Wuhan,
China). The average weight of the rabbits was 2.2±0.3 kg, and the
average age was 6±0.2 months. All experiments were performed
according to the guidelines for the Care and Use of Laboratory
Animals (18) and were approved
by the Ethics and Research Committee of the Wuhan University
Medical School (Wuhan, China). The rabbits were housed in standard
cages with free access to tap water and food in a room with a
temperature of 18-25°C and relative humidity of 45-55%. The room
was kept quiet to avoid any stress-inducing factors during the
experimental period. All animals were determined to be healthy on
the basis of clinical examinations, and were allowed to feed and
drink freely 1 week before the experiment to adapt to environmental
conditions.
The rabbits were randomly divided into three groups
consisting of 6 rabbits each, including the 0, 8 and 18 mmHg
groups. All these rabbits underwent surgical procedures to induce
severe hydronephrosis. Following the surgery, the abdomens of the
rabbits in these groups were insufflated with CO2 to
maintain an intraabdominal pressure of 0, 8 or 18 mmHg,
respectively.
Experimental protocol
Following the method described by Wen et al
(19), all the rabbits underwent
surgery to establish a model of kidneys with hydronephrosis.
Briefly, the rabbits were initially anesthetized by auricular vein
injection of sodium pentobarbital at a dose of 30 mg/kg at room
temperature. After 10 min, the left ureter and psoas muscle were
exposed through a midline abdominal incision, and the proximal
ureter was buried in a 2-cm notch within the psoas muscle. After 2
weeks, B-ultrasonography was used to confirm hydronephrosis. Pyelic
distention levels of 1.69±0.34 cm and parenchymal thickness of
0.22±0.05 cm were observed. Next, the rabbits were randomly
assigned to three groups, including the 0, 8 and 18 mmHg groups,
and a second laparotomy was then performed. Following
anesthetization, a Veress needle was introduced in the abdomen, and
the incision was sutured to prevent CO2 leakage from the
abdomen. Intra-abdominal pressure was induced with an insufflator
(Stryker, Kalamazoo, MI, USA) that delivered filtered medical
CO2 at body temperature with a flow rate of 0.5 l/min.
The three groups of rabbits were subjected to intraabdominal
pressures of 0, 8 or 18 mmHg, respectively, for 90 min. Subsequent
to the insufflation, the pneumoperitoneum was released, the psoas
muscle obstruction was relieved, and the abdomen was sutured. The
rabbits were sacrificed 2 days after the pneumoperitoneum with 150
mg/kg sodium pentobarbital (20%) through ear marginal vein
injection, and the left kidneys were collected for biochemical and
histological evaluations.
Tissue processing
Following sacrifice, the abdominal area of the
rabbits was fully exposed, and the experimental kidneys were
removed carefully with sharp scissors. The kidneys were then placed
into cold 0.9% saline solution to wash away most of the blood.
Next, the renal capsule and the adipose tissues around the kidney
were carefully removed with tweezers, avoiding oppression of the
renal tissue during the entire process. Finally, the unwanted renal
pelvis was carefully removed with small scissors, appropriate
sections of renal tissue were cut off with a sharp blade, and the
renal tissue was washed with cold 0.9% saline solution for three
times to clear the residual blood. The tissue samples were treated
differently according to different detection methods.
Detection of reactive oxygen species
(ROS)
All of the rabbits were sacrificed 2 days after
pneumoperitoneum. Kidneys were carefully removed and washed with
cold 0.9% saline solution three times. The kidney tissue samples
were homogenized (T25; IKA-Werke GmbH & Co. KG, Staufen,
Germany) in 100 mmol/l phosphate buffer and centrifuged at 13,000 ×
g for 15 min at 4°C (Heraeus Biofuge Primo R; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), following which the
supernatants were collected. The bicinchoninic acid method was used
to detect protein concentration. Then the homogenized supernatants
were incubated with 1 mmol/l 2,7-dichlorodihydrofluorescein
diacetate (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China) for 30 min at 37°C. Subsequently, an automatic microplate
reader (Multiskan MK3; Thermo Fisher Scientific, Inc.) was used to
detect the absorbance at 500 nm. The results are expressed as the
arbitrary units per mg of protein.
Measurement of mitochondrial membrane
potential (MMP)
An MMP detection kit (Beyotime Institute of
Biotechnology, Haimen, China) was used to determine changes in MMP,
according to the manufacturer’s protocol. Briefly, renal tissue was
cleaned with 0.9% normal saline and digested in a trypsin solution
(Beyotime Institute of Biotechnology) at 37°C for ~20 min, and the
reaction was terminated by addition of 30% fetal bovine serum
(Hangzhou Sijiqing Biological Engineering Materials Co., Ltd.,
Hangzhou, China). Suspended cells were centrifuged at 2,000 × g for
4 min at 4°C and then washed three times with PBS. Subsequent to
this step, JC-1 was added at a final concentration of 0.01 M.
Following incubation for 30 min at 37°C, the cells (approximately
3×105 cells/ml) were washed three times with wash buffer
and immediately analyzed by flow cytometry (FACSCalibur; BD
Biosciences, Franklin Lakes, NJ, USA) and Flowjo software (version
7.6.1, BD Biosciences).
Hematoxylin and eosin (H&E) staining,
and TUNEL detection
Rabbit kidney tissues in all three groups were
harvested and fixed in 10% formalin (pH 7.0) for a time period not
exceeding 24 h. The tissues were processed routinely for paraffin
embedding, and 5-µm paraffin-embedded sections were cut for
detection. The tissue sections were deparaffinized using xylene and
rehydrated using a graded ethanol series prior to staining with
H&E. In order to compare the kidney injury and renal cell
apoptosis in these three groups, sections were screened per
high-power field (HPF) under a microscope (Nikon 80i; Nikon
Corporation, Tokyo, Japan). On each slide, 10 HPFs in the cortex
and outer medulla were randomly selected, and 100 cells were
randomly counted in each of these fields. Kidney injury was graded
on the basis of H&E staining, as follows: 0, absence of
necrosis; 1, mild necrosis; 3, moderate necrosis; and 5, severe
necrosis.
Apoptotic scores were also determined with TUNEL
staining using the In Situ Apoptosis Detection kit (Roche
Applied Sciences, Basel, Switzerland). Apoptotic nuclei were
stained brown, while negative nuclei were stained blue. The
apoptotic index was calculated based on the percentage of positive
nuclei. All quantifications were performed by two pathologists in a
blinded manner.
Kidney ultramicrostructure examination by
electron micros- copy
Renal cortex tissues from the three groups were
fixed for 24 h in 2.5% paraformaldehyde at 4°C and subsequently
fixed at 4°C for 2 h with 2% osmium tetroxide. The kidney samples
were then washed with PBS twice, dehydrated with a series of
ethanol solutions and embedded in Spurr epoxy resin at 45°C for 12
h. Next, tissue specimens were cut into 50-nm sections on an
ultra-microtome using diamond knives, followed by washing and
staining with 2% aqueous uranyl acetate at 25°C for ~1 h. Finally,
a minimum of 10 random fields of view from each section were
visualized under a transmission electron microscope (H-600;
Hitachi, Ltd., Tokyo, Japan).
Western blot analysis
To determine the possible mechanisms underlying the
apoptosis induced by pneumoperitoneum, the expression levels of
B-cell lymphoma 2 (Bcl-2), Bcl-2-associated x protein (Bax),
cytochrome c (Cyt c), caspase-3 and caspase-9 were detected in
rabbit kidney tissues using western blot analysis. Briefly, 6
rabbits from each group were rapidly sacrificed 2 days after
pneumoperitoneum, and the kidneys were removed. Next, tissues were
washed three times with PBS (pH 7.2) and homogenized using an
Ultra-Turrax (T25; IKA-Werke GmbH & Co. kg) in
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology) containing phenylmethylsulfonyl fluoride (Beyotime
Institute of Biotechnology). The samples were then centrifuged at
14,000 × g for 20 min at 4°C. For Cyt c testing, the cytosolic and
mitochondrial extracts were obtained by the mitochondrial
extraction kit (Beyotime Institute of Biotechnology) following the
manufacturer’s protocol. In addition, COX-IV and β-tubulin were
used as controls for the detection of Cyt c in the mitochondria and
cytoplasm, respectively. The concentration of the protein was
detected using the bicinchoninic acid method. Proteins in each
group were subjected to 12% or 15% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred onto a
polyvinylidene difluoride membrane for 1-2 h at 200 mA, according
to their molecular weight. For each group, ~40 µg proteins
were added onto the gels per lane for detection. Subsequent to
blocking with Tris-buffered saline/Tween-20 containing 5% dried
skim milk for 1 h at room temperature (approximately 25°C), the
membranes were incubated with primary antibodies overnight at 4°C.
The antibodies used for western blot analyses were as follows: Bax
(PA5-70418; 1:3,000; Thermo Fisher Scientific, Inc.), Bcl-2
(PA5-68611; 1:2,000; Thermo Fisher Scientific, Inc.), Cyt c
(NB100-56503; 1:5,000; Novus Biologicals, LLC, Littleton, CO, USA),
caspase-3 (ab90437; 1:1,000; Abcam, Cambridge, UK), caspase-9
(ab115161; 1:3,000; Abcam), β-actin (ab28052; 1:5,000; Abcam), Cyt
c oxidase (COX)-IV (ab66739; 1:5,000; Abcam) and β-tubulin
(ab56676; 1:4,000; Abcam). Proteins were subsequently incubated for
1 h at room temperature with anti-mouse/rabbit secondary antibody
(P/N 925-32210 or P/N 925-32211; 1:10,000; LI-COR Biosciences,
Lincoln, NE, USA), which was conjugated to IRDye 800CW. Finally,
the fluorescence signal emitted by the secondary antibody was
quantified by a western blot detection system (Odyssey Infrared
Imaging; LI-COR Biosciences), and semi-quantitative analysis was
conducted to determine the corresponding protein expression levels
(Image Studio version 5.2.5; LI-COR Biosciences).
Statistical analysis
All data are presented as the means ± standard
deviations, and all analyses were performed in duplicate. One-way
analysis of variance and Turkey’s test were performed for
statistical comparison using IBM SPSS software, version 19 (IBM
Corp., Armonk, NY, USA). P-values that were <0.05 were
considered to denote statistically significant differences.
Results
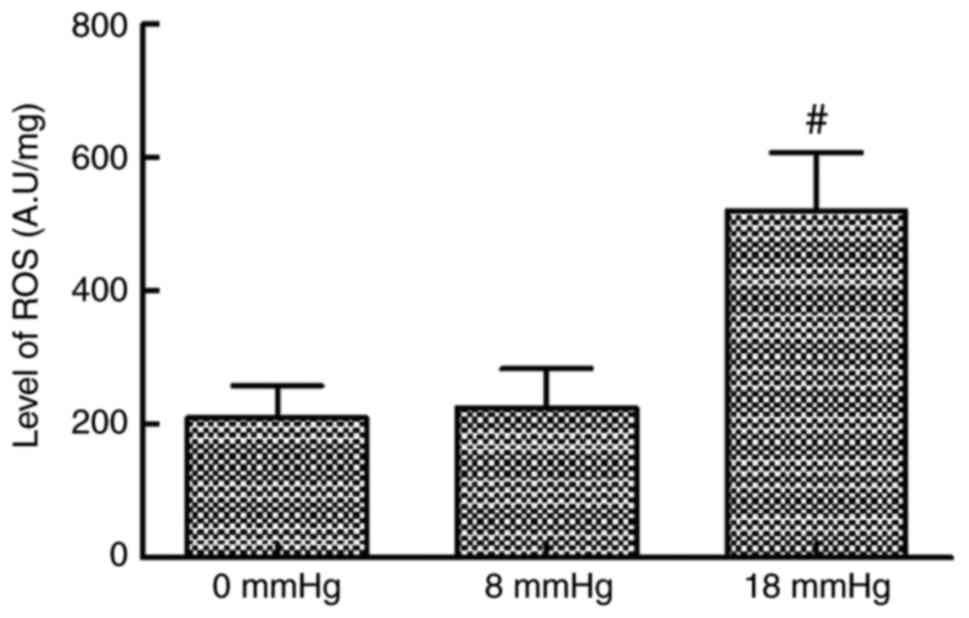
ROS levels
The ROS levels in kidney tissues were comparable
between the 0 and 8 mmHg groups. However, when the intraabdominal
pressure reached 18 mmHg, the ROS level significantly increased
compared with the 0 and 8 mmHg groups (Fig. 1).
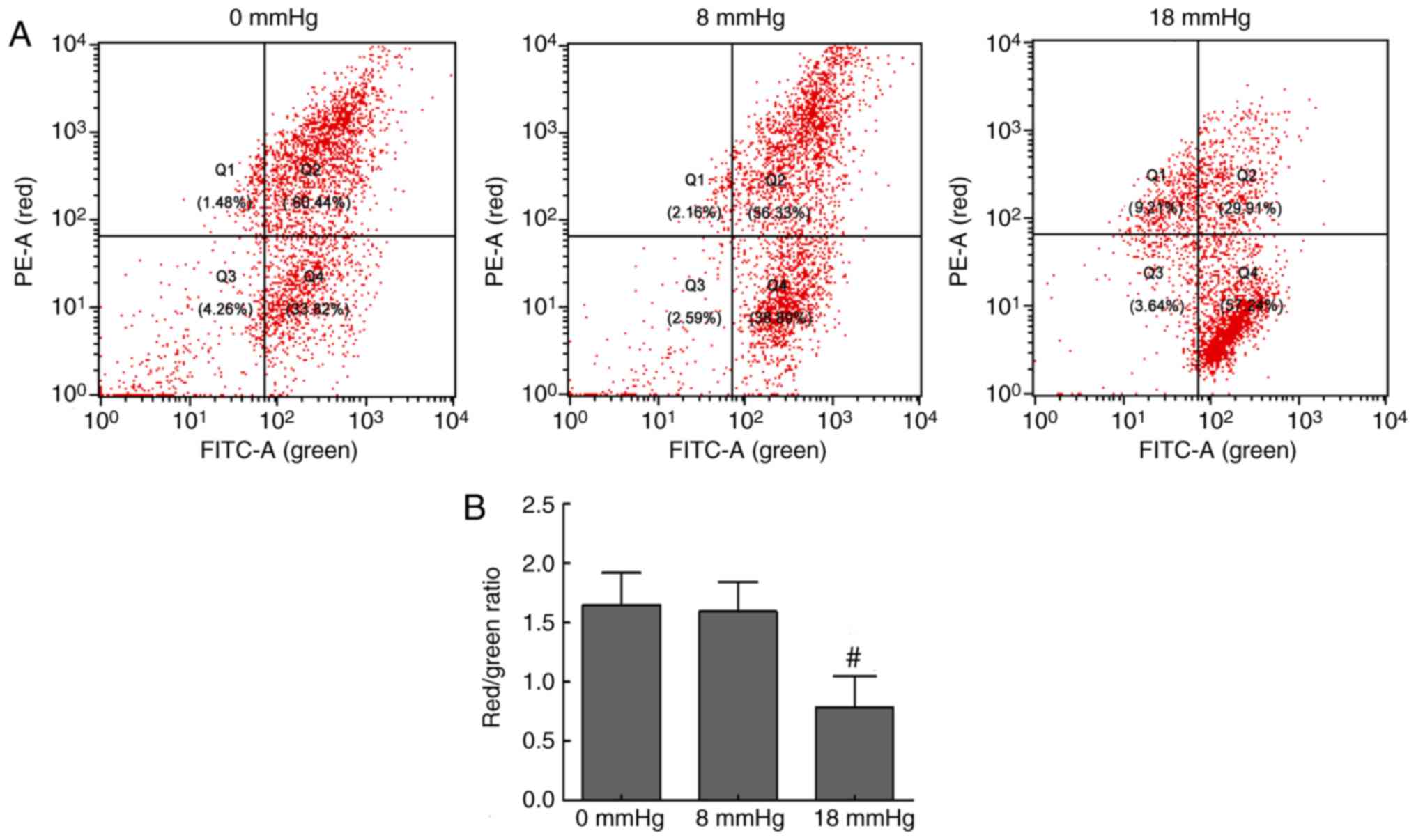
Changes in MMP levels
Disruption of MMP is one of the earliest events in
apoptosis (20). JC-1, as a
fluorescent probe, mainly exists in the mitochondrial matrix as a
polymer, which can emit red fluorescence when MMP levels are high.
However, when the MMP levels are low, JC-1 mainly exists in the
cytoplasm as monomers, which can emit green fluorescence. Thus, a
decrease in the red/green fluorescence intensity ratio indicates
MMP loss (21). In the present
study, the flow cytometry scatter plots and the red/green ratios
were obtained, and are shown in Fig.
2. The renal cells exhibited strong red fluorescence and
relatively weak green fluorescence in the 0 and 8 mmHg groups.
However, when the pneumoperitoneum pressure was increased to 18
mmHg, the red fluorescence was attenuated and green fluorescence
was enhanced, which indicated a loss of MMP levels (Fig. 2). These changes suggested
mitochondrial damage and early cell apoptosis associated with the
mitochondrial pathway in the 18 mmHg group.
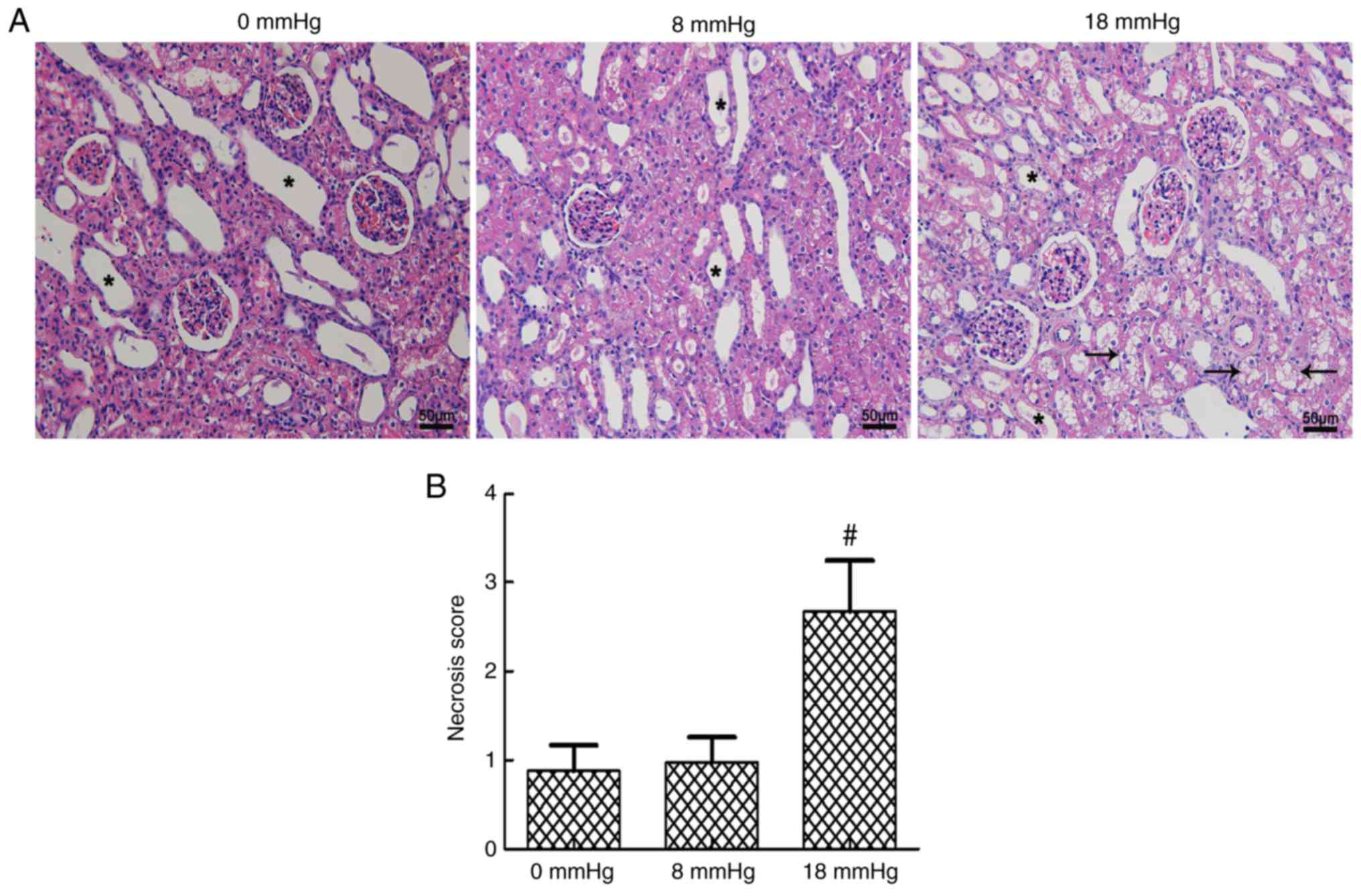
Kidney necrosis
Certain degrees of expansive kidney tubules were
observed in all three groups due to the establishment of rabbit
kidney models with severe hydronephrosis. The tubular necrosis
scores were at a relatively low level when the intraabdominal
pressure was only 0 or 8 mmHg, and no significant difference was
observed between these two groups. However, a sudden significant
increase in the tubular necrosis score was detected when the
pneumoperitoneum pressure reached 18 mmHg (Fig. 3).
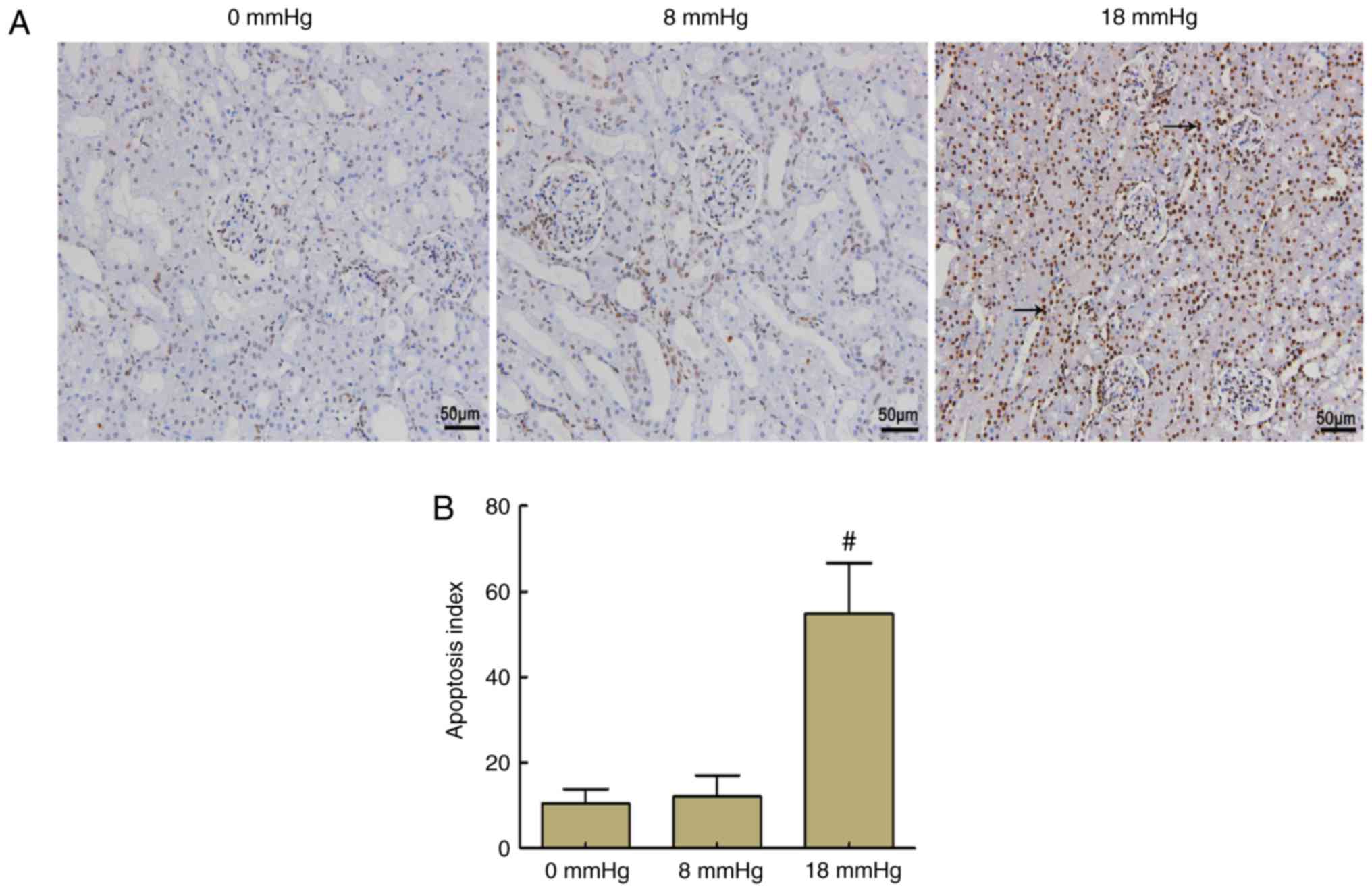
Kidney apoptosis
In the 0 and 8 mmHg groups, only a few nuclei were
stained brown, and no significant difference was observed between
these two groups. By contrast, >50% of the nuclei were stained
brown in the 18 mmHg group, which indicated that the total number
of apoptotic cells was markedly increased. These results indicated
that apoptosis occurred in the kidneys of rabbits with severe
hydronephrosis in the presence of increased pneumoperitoneum
pressure (Fig. 4).
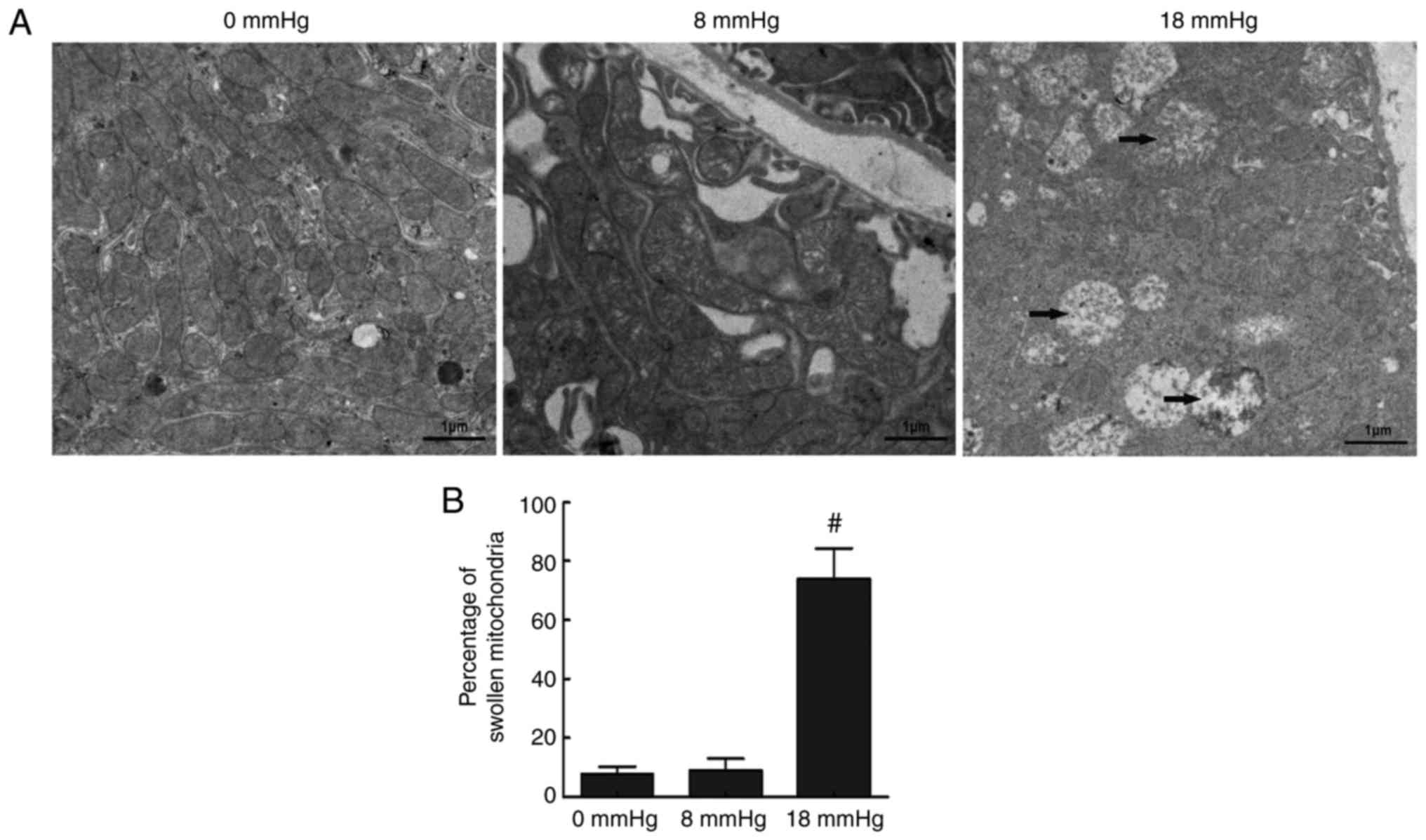
Ultramicrostructure changes of tubular
cells
Mitochondrial-dependent apoptosis is one of the key
apoptosis mechanisms (22). This
mechanism involves ultramicrostructure changes in the cells. In the
current study, transmission electron microscopy was performed to
detect the apoptosis in renal cells by investigating changes to the
mitochondria. In the 0 mmHg group, cells had an overall normal
appearance, with few swollen or vacuolar mitochondria observed. In
the 8 mmHg group, similar results were observed. However, in the 18
mmHg group, the majority of the mitochondria were swollen and
vacuolar (Fig. 5). These findings
revealed that the mitochondria-mediated pathway was involved in
apoptosis induced by high-pressure pneumoperitoneum in obstructed
rabbit kidneys.
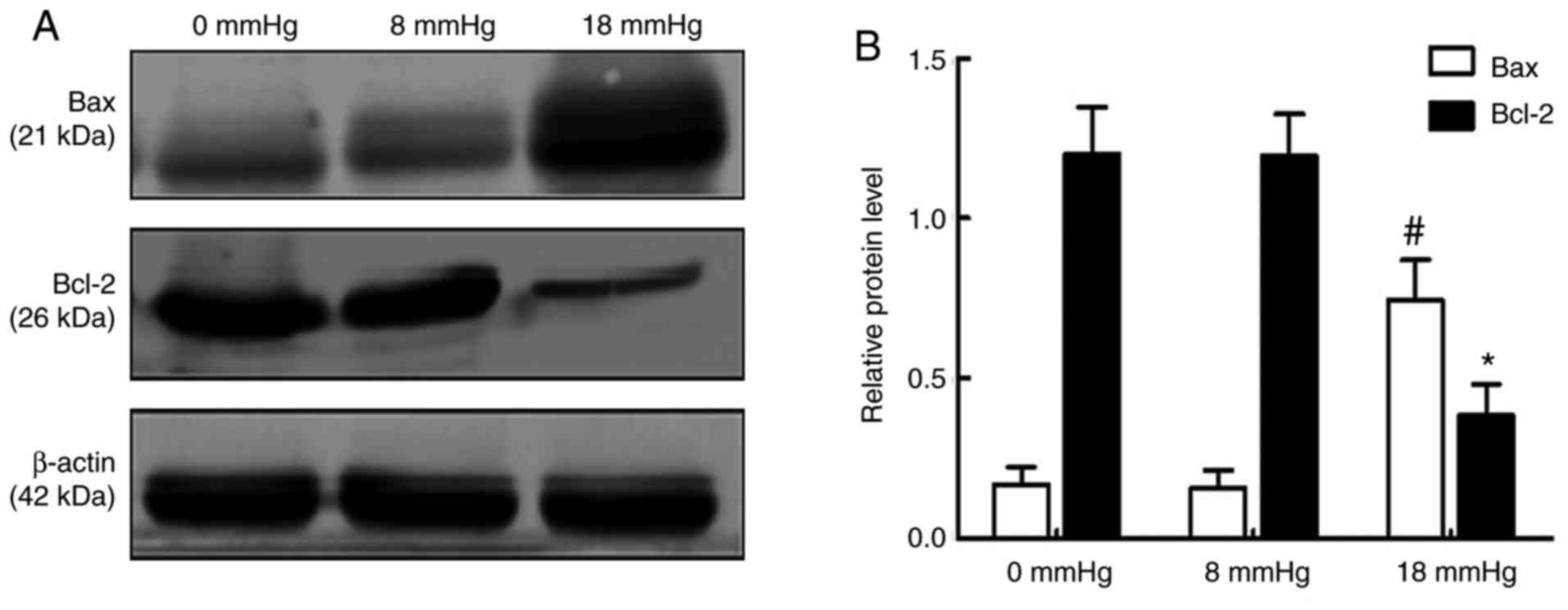
Expression levels of Bax and Bcl-2
An increase in the expression of the pro-apoptotic
protein Bax and reduced expression of the anti-apoptotic protein
Bcl-2 are considered important changes in early apoptosis (23). The results of the present study
demonstrated that the expression of Bax was significantly increased
and the expression of Bcl-2 was markedly decreased when the
pneumoperitoneum pressure reached 18 mmHg. Notably, no significant
changes were observed between the 0 and 8 mmHg groups (Fig. 6).
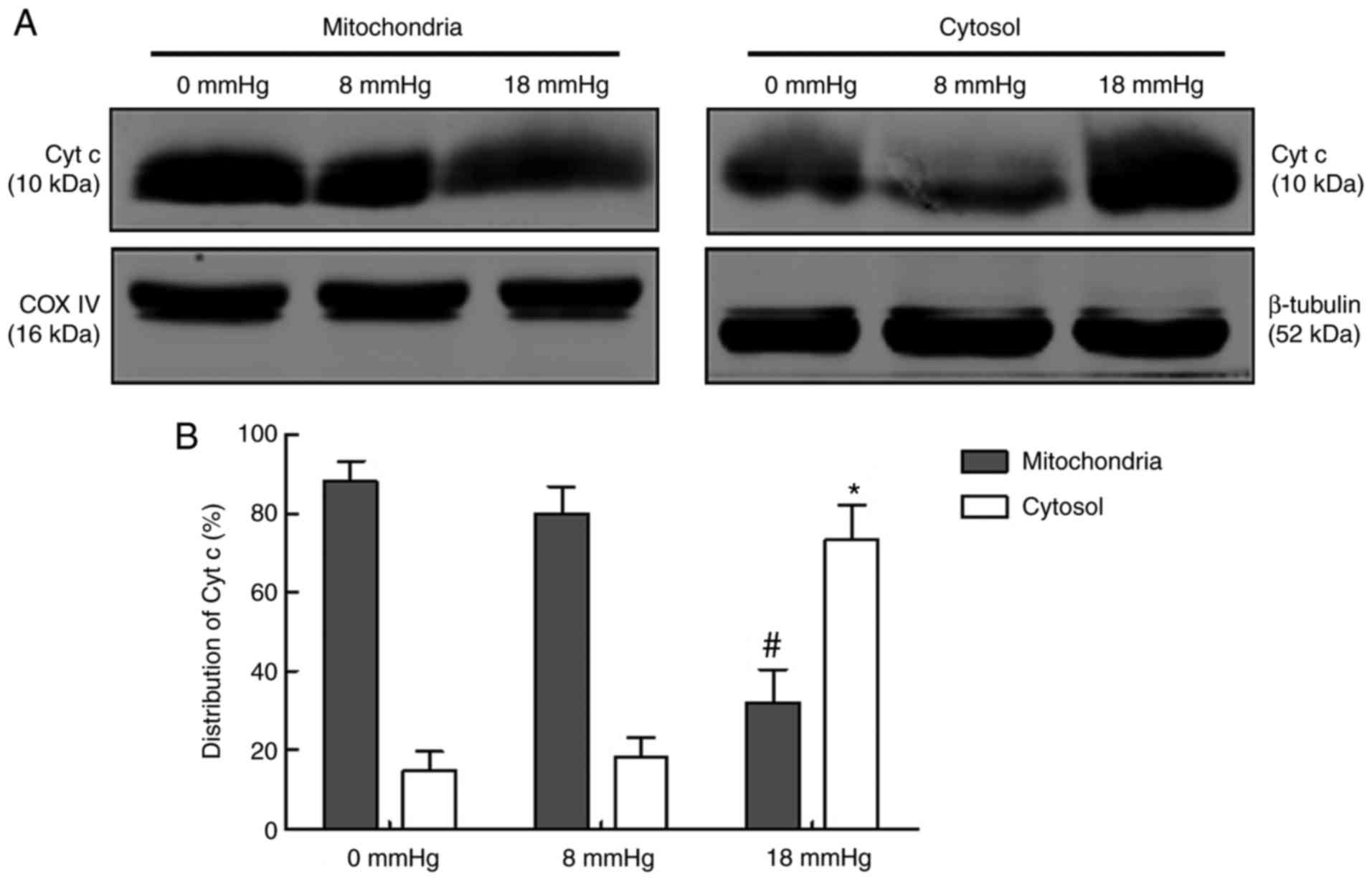
Release of Cyt c
Cyt c is an intermembrane space protein that is
tightly associated with cardiolipin, a key lipid component of the
inner mitochondrial membrane. The release of Cyt c from
mitochondria to the cytosol is considered one of the key steps in
the mitochondria-associated apoptosis (24). In the current study, the
expression of Cyt c in mitochondria and cytosol were separately
detected (Fig. 7). The results
revealed that the majority of Cyt c existed in the mitochondria
rather than the cytosol in the 0 and 8 mmHg groups, indicating that
only limited amount of Cyt c was released. No significant
difference was observed between these two groups. However, in the
18 mmHg group, the majority of the Cyt c existed in the cytosol
rather than the mitochondria, indicating that rabbit kidneys with
hydronephrosis subjected to 18 mmHg pneumoperitoneum were
characterized by Cyt c release from the mitochondria to the cytosol
(Fig. 7).
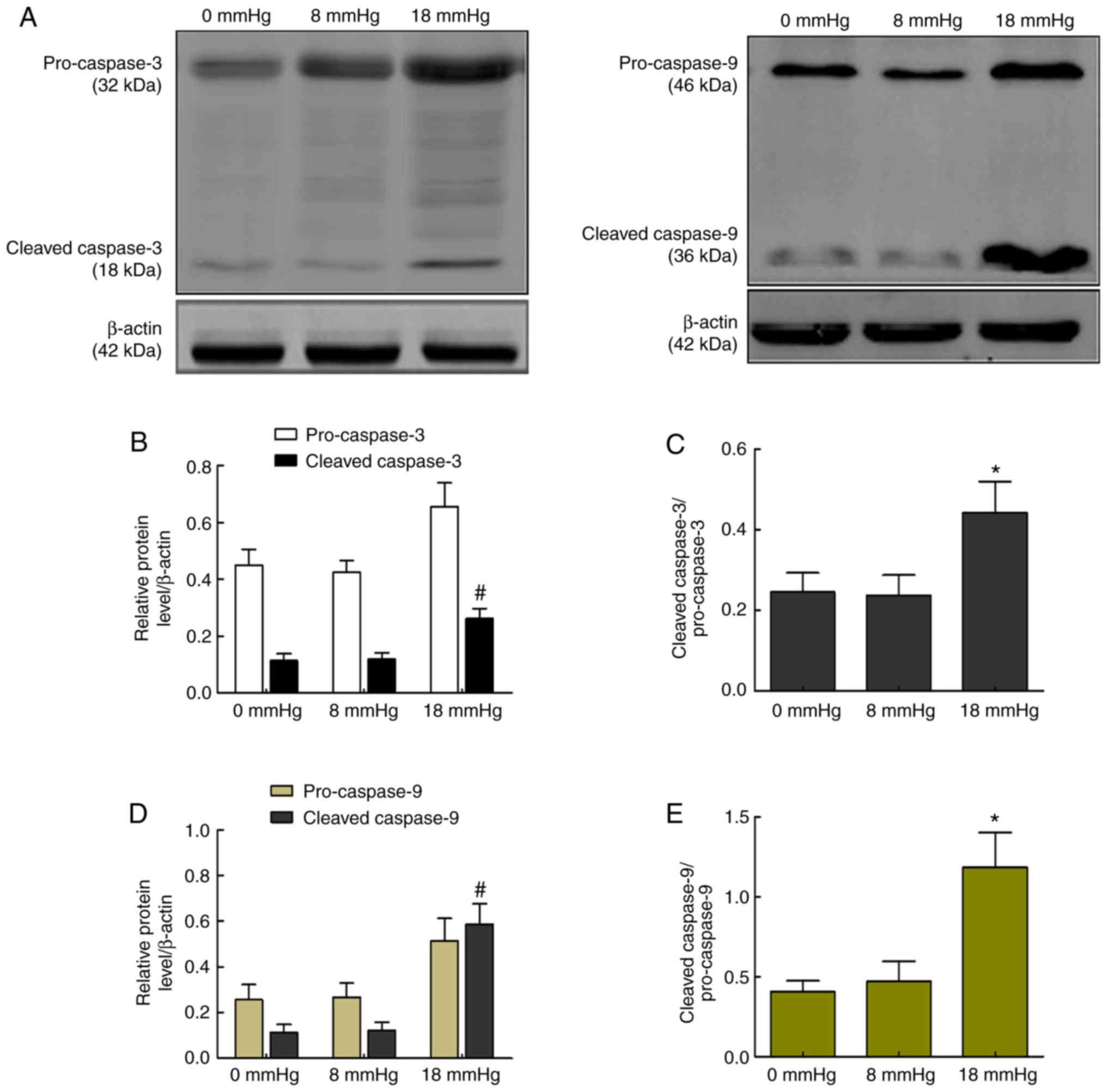
Activity of caspase-3 and caspase-9
The release of Cyt c activates caspase-9, a cysteine
protease, and later cleaved caspase-9 activates caspase-3 and forms
cleaved caspase-3, which is responsible for cell apoptosis
(25). The current study detected
the activities of caspase-3 and caspase-9 to assess whether the
caspase-dependent pathway was involved in rabbit kidneys with
severe hydronephrosis subjected to pneumoperitoneum. As shown in
Fig. 8, cleaved caspase-9 and
cleaved caspase-3, as well as the expression ratio of
cleaved-caspase-3/pro-caspase-3 or cleaved-caspase-9/pro-caspase-9,
were significantly increased when the intraabdominal pressure
reached 18 mmHg. However, no significant changes were observed
between the 0 and 8 mmHg groups (Fig.
8).
Discussion
Pneumoperitoneum, although generally considered to
be essential for adequate exposure in laparoscopic surgery, has
adverse effects on renal physiology (26). A large number of trials have
revealed the side effects of pneumoperitoneum; however, these
trials have mainly focused on renal blood flow changes and renal
function (27,28). A previous study revealed that the
glomerular filtration rate decreased by 14 and 48% in rats when an
intraabdominal pressure of 7 and 14 mmHg was applied, respectively.
In parallel, the renal plasma flow decreased by 28 and 57% under
these conditions (29). Certain
studies have emphasized that high intraabdominal pressure can
noticeably decrease renal blood flow, while other studies reported
that renal blood flow returned to the normal range following
deflation (30,31). During the hypoperfusion and
subsequent reperfusion periods, a typical I/R injury may occur.
Several studies in animals and humans have investigated I/R injury
following laparoscopic surgeries (32-34). Pneumoperitoneum may cause an
increase in the oxidative stress, and active oxygen species (ROS)
may cause damage to lipids and proteins during oxidative stress. A
previous study has also demonstrated that oxidative stress response
decrease and antioxidant defense systems strengthened with the
administration of dexmedetomidine prior to pneumoperitoneum
(35). Furthermore, a typical I/R
injury is closely associated with oxidative damage and
mitochondrial injuries (36),
which can eventually cause cell apoptosis or death.
As mentioned earlier, hydronephrosis is a common
urological disease that can be caused by a kidney stone, tumor or
congenital anomalies. Kidneys with hydronephrosis have a thinner
renal cortex and subnormal blood perfusion, while the
hydronephrosis itself has adverse effects on renal tubule function,
causing hydronephrotic kidneys to be more likely to suffer hypoxia
problems (37). Severe
hydronephrosis leads to prolonged operating time in laparoscopic
surgery, which may consequently lead to increased oxidative stress
(38). Based on these facts and
the observations of our previous study (16), it can be inferred that
high-pressure CO2 pneumoperitoneum may cause severe
oxidative stress, mitochondrial injuries and even cell apoptosis in
rabbit kidneys with severe hydronephrosis. Therefore, the present
study examined the effects of high-pressure CO2
pneumoperitoneum on kidneys with severe hydronephrosis and
investigated the possible underlying mechanism.
In I/R injury, the generation of ROS appears to be
an important source of tissue damage. To a certain extent, ROS
content represents the degree of oxidative damage (39,40). In the experiments of the present
study, the ROS levels, histological changes and apoptosis index
were first detected to evaluate oxidative stress and tissue damage.
As expected, higher oxidative stress, higher apoptosis index and
more severe morphological changes were observed in the 18 mmHg
group as compared with the 0 and 8 mmHg groups. Excessive ROS
induces the peroxidation of mitochondrial membrane lipids, which
promotes extensive release of ROS and spurs a vicious cycle,
eventually leading to a loss of MMP (41,42). Accordingly, the MMP was tested by
JC-1 staining in the current study, and the results identified a
significant decrease in MMP in the 18 mmHg group. To confirm the
changes to the mitochondria, the ultrastructure of kidneys was also
observed by electron microscopy. As expected, large swollen and
vacuolar mitochondria were detected in the 18 mmHg group, while in
the 0 and 8 mmHg groups, few abnormal changes were observed. The
significant reduction of MMP, mitochondrial injuries and apoptosis
largely suggested a mitochondrially mediated phenomenon, which is
associated with the collapse of transmembrane potential, resulting
in the expulsion of apoptogenic molecules.
The mitochondrial pathway is characterized by the
release of apoptosis-promoting factors, such as Cyt c and
apoptosis-inducing factor (AIF), from the mitochondria (43). The release of Cyt c from
mitochondria to cytosol can activate the initiator caspase, namely
caspase-9, and can thus trigger the activation of the executioner
caspase, namely caspase-3, eventually causing caspase-dependent
apoptosis (44). AIF stimulates
caspase-independent apoptosis by moving into the nucleus, where it
binds to DNA and stimulates chromatin condensation and DNA
fragmentation (45).
Additionally, the Bcl-2 family proteins, including anti-apoptotic
(such as Bcl-2) and pro-apoptotic (such as Bax) members, serve an
important role in the mitochondria-mediated pathway (46). The anti-apoptotic proteins inhibit
the release of apoptosis-promoting factors, whereas the
pro-apoptotic proteins promote the apoptotic process by regulating
MMP and the permeability of membranes (47,48). Accordingly, the present study
investigated a number of important proteins associated with the
mitochondria-mediated pathway. The western blot analysis results
revealed significant downregulation of Bcl-2 and upregulation of
Bax, as well as marked release of Cyt c from mitochondria into the
cytosol, in the 18 mmHg group. A significant increase in the
expression of cleaved caspase-3 and cleaved caspase-9 was also
observed in the 18 mmHg group. Taken together, the current results
provide evidence that apoptosis in rabbit kidneys with
hydronephrosis induced by high CO2 pneumoperitoneum is
associated with the mitochondria-dependent pathway. An illustration
of how high-pressure CO2 pneumoperitoneum induces
oxidative stress and mitochondria-dependent apoptosis in rabbit
kidneys with severe hydronephrosis is shown in Fig. 9.
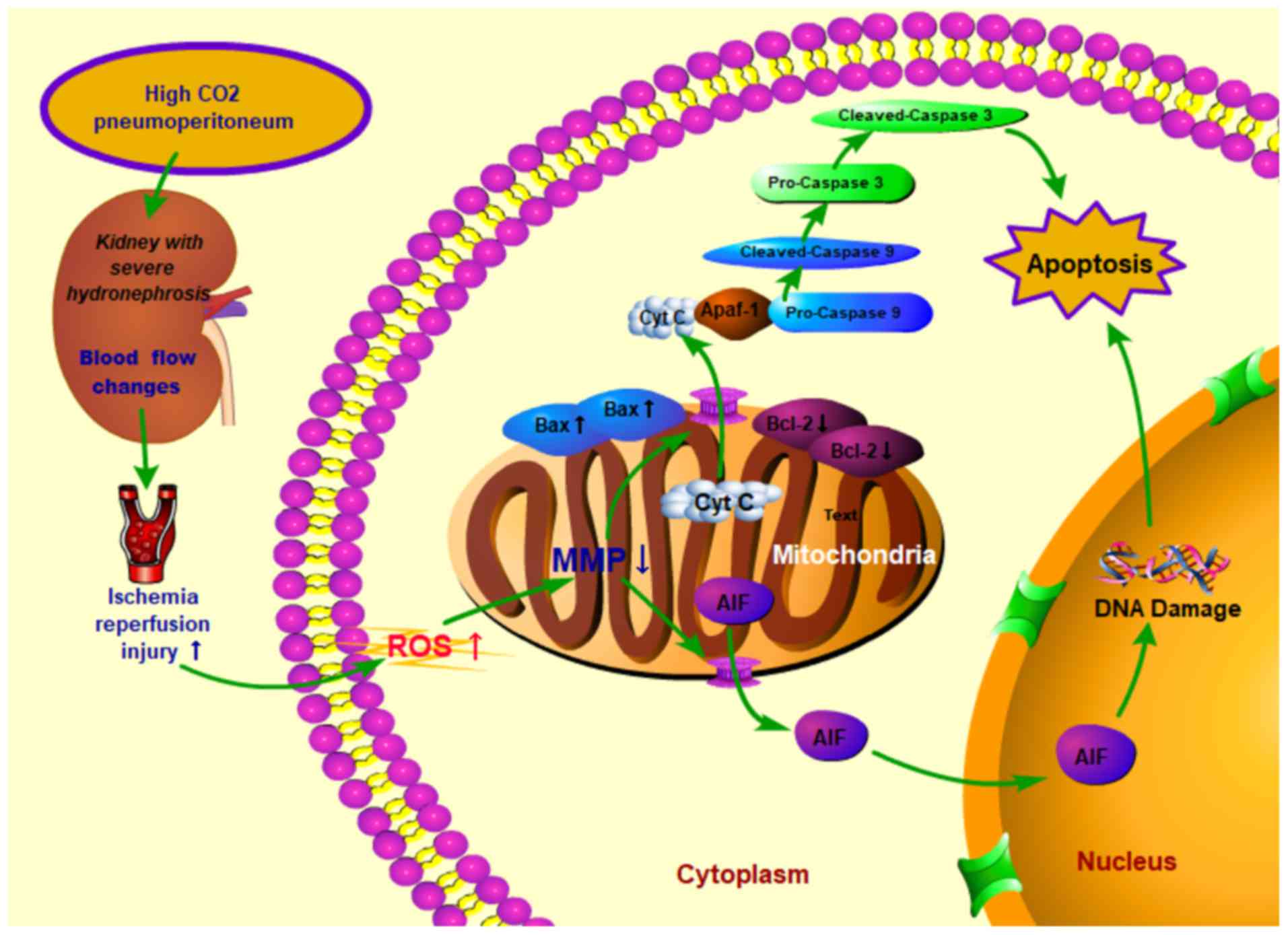 | Figure 9Proposed mechanisms of cell apoptosis
induced by high CO2 pneumoperitoneum pressure in rabbit
kidneys with severe hydronephrosis. High CO2
pneumoperitoneum pressure induced renal blood flow changes and
increased the accumulation of ROS, resulting in upregulated Bax and
downregulated Bcl-2 levels in kidneys. Consequently, the MMP was
reduced, which then accelerated the release of Cyt c into the
cytoplasm, leading to apoptosis via the caspase-3 and
caspase-9-dependent pathway. CO2, carbon dioxide; MMP,
mitochondrial membrane potential; ROS, reactive oxygen species;
Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated x protein; Cyt c,
cytochrome C; AIF, apoptosis inducing factor. |
However, the present study has several important
limitations. While the protocol following the method described by
Wen et al (19) is an
acceptable research model for kidney with hydronephrosis, it
differs from hydronephrosis kidneys in humans. Therefore, how
hydronephrosis affects the renal blood flow may differ between the
species. Another issue to be stressed is the difference in the
effects of pneumoperitoneum in the rabbit abdomen compared with
that in the human abdomen for a given pressure, since the level of
cell tolerance to intraabdominal pressure may differ between humans
and rabbits (49). To the best of
our knowledge, there has been no comparison between the kidney
surface area in the two species; therefore, calculations of force
per unit of surface area are not available. In humans,
pneumoperitoneum pressure is recommended to be controlled to <8
mmHg (8). Considering the
aforementioned important limitations, the experimental grouping was
simplified, and three groups were set up with the pressures of 0
(no pressure), 8 (low pressure) and 18 mmHg (high pressure). We
believed that these three groups were sufficient for to investigate
how high-pressure CO2 pneumoperitoneum affects kidneys
with severe hydronephrosis. Furthermore, the insufflation with
CO2 in the present study was performed at room
temperature (20-25°C) and dry conditions (0-5% relative humidity),
and the intraabdominal pressure lasted for 90 min. According to a
previous meta-analysis, warming and humidifying the gas used for
insufflation has been proposed to reduce the iatrogenic effects of
laparoscopic surgery, including pain, hypothermia and peritoneal
alterations (50). However, a
prospective randomized trial confirmed that heating and
humidification of CO2 during pneumoperitoneum is not
indicated (51). Another
experimental study also confirmed that the use of warming and
humidification of insufflation gas had no effect on oxidative
stress compared with the controls treated with unheated and
non-humidified gas (52).
Furthermore, the findings of Akbulut et al (53) indicated that the operating time
should be limited to <120 min during laparoscopic surgery, since
the prolonged duration of pneumoperitoneal pressure may cause
increased oxidative problems (54). The present study only explored how
high-pressure CO2 pneumoperitoneum affects kidneys with
severe hydronephrosis. However, the properties of the gas and the
duration of intraabdominal pressure should also be studied.
Notably, a number of other important molecules or apoptotic factors
also exist that should be detected in order to clarify the
mechanism of the mitochondria-dependent pathway of apoptosis.
Bcl-2 homology domain 3 (BH3)-only proteins, such as
Bad, Bim, Bid, Puma and Noxa, are known to be important proteins
that initiate and regulate cell apoptosis (55). These proteins are involved in
apoptosis by translocating from the cytoplasm to mitochondria, and
then inhibiting the activity of anti-apoptotic members (such as
Bcl-2) or activating the activity of pro-apoptotic members (such as
Bax/Bak) (56,57). Other apoptosis pathways or certain
other important molecules may also be associated with the
activation of BH3-only proteins. For instance, the phosphorylation
of Bad is associated with the PI3K-Akt pathway, while the cleavage
of Bid is closely associated with the Fas death signaling pathway
(58). P53, another important
apoptotic member in DNA damage apoptosis, regulates the expression
of Noxa and Puma (59). By
contrast, a number of apoptotic molecules, such as Smac, AIF and
Endo G, are also important apoptotic members that are released from
mitochondria subsequent to a decrease of MMP. These molecules play
apoptotic roles by activating the caspase-3 pathway or by causing
DNA damage (60,61). In the present study, the
expression levels of Bcl-2, Bax, Cyt c, caspase-3 and caspase-9
were detected, and the results indicated that the apoptosis in
severe hydronephrosis kidneys induced by high-pressure
CO2 pneumoperitoneum may be associated with the
mitochondria-dependent apoptotic pathway. However, further studies
regarding other aforementioned proteins or potential targets should
be conducted to fully understand the exact mechanism underlying
this type of apoptosis. The application of certain drugs to inhibit
or promote mitochondria-dependent apoptosis in animals can also be
investigated.
In conclusion, the results of the present study
indicate that high-pressure CO2 pneumoperitoneum induces
oxidative stress and causes apoptosis in rabbit kidneys with severe
hydronephrosis through the mitochondrial apoptotic pathway. These
conclusions were supported by the increased generation of ROS, loss
of MMP, increased percentage of swollen or vacuolar mitochondria,
Cyt c release, altered expression of Bcl-2 family proteins and
activation of the caspase-9/3 cascade in the high pressure (18
mmHg) group. Thus, these findings offer theoretical guidance for
the application of pneumoperitoneum in laparoscopic surgery. These
results also provide an insight into the molecular mechanism by
which hydronephrosis protects the kidneys during laparoscopic
surgery.
Acknowledgments
Not applicable.
Funding
The present study was supported by a grant from the
Nation Natural Science Fund Project of China (no. 81400698).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
SZ and WL performed the experiment and were major
contributors in writing the manuscript. FC conceived and designed
the experiments. JN supplied the materials and analyzed the data.
TR, WY and YR collected and analyzed the data. XY and RY supplied
the materials and analyzed the data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical and
Research Committee of Wuhan University Medical School (Wuhan,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siow SL, Mahendran HA, Wong CM, Hardin M
and Luk TL: Laparoscopic versus open repair of perforated peptic
ulcer: Improving outcomes utilizing a standardized technique. Asian
J Surg. 41:136–142. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Seims AD, VanHouwelingen L, Mead J, Mao S,
Loh A, Sandoval JA, Davidoff AM, Wu J and Wang WC: Operative and
immediate postoperative differences between traditional multiport
and reduced port laparoscopic total splenectomy in pediatric
patients. J Laparoendosc Adv Surg Tech A. 27:206–210. 2017.
View Article : Google Scholar
|
|
3
|
Jin B, Chen MT, Fei YT, Du SD and Mao YL:
Safety and efficacy for laparoscopic versus open hepatectomy: A
meta-analysis. Surg Oncol. 27:A26–A34. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Menes T and Spivak H: Laparoscopy:
Searching for the proper insufflation gas. Surg Endosc.
14:1050–1056. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen X, Liu H, Feng L and Liu Y: Effect of
carbon dioxide pneumoperitoneal pressure on the ultrastructure of
implanted endometriotic lesions in a rat model. Eur J Obstet
Gynecol Reprod Biol. 171:319–324. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hejazi M, Pedram MS, Ashegh H, Jafari N,
Ghazisaeedi F and Abdi M: Evaluation of effects of intraperitoneal
CO2 pressure in laparoscopic operations on kidney,
pancreas, liver and spleen in dogs. Iran Red Crescent Med J.
15:809–812. 2013. View Article : Google Scholar
|
|
7
|
Liu Y, Cao W, Liu Y, Wang Y, Lang R, Yue Y
and Wu AS: Changes in duration of action of rocuronium following
decrease in hepatic blood flow during pneumoperitoneum for
laparoscopic gynaecological surgery. Bmc Anesthesiol. 17:452017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sassa N, Hattori R, Yamamoto T, Kato M,
Komatsu T, Matsukawa Y, Funahashi Y and Gotoh M: Direct
visualization of renal hemodynamics affected by carbon
dioxide-induced pneumoperitoneum. Urology. 73:311–315. 2009.
View Article : Google Scholar
|
|
9
|
Lee JY and Choi SH: Results of hepatic and
renal function tests to different CO2 pneumoperitoneum
conditions: An experimental capnoperitoneum study in dogs. Res Vet
Sci. 101:1–5. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akdemir A, Taylan E, Sahin C, Ozgurel B,
Karlitepe A, Zekioglu O and Ercan G: The impact of carbon dioxide
pneumoperitoneum on ovarian ischemia-reperfusion injury during
iaparoscopic surgery: A preliminary study. J Minim Invasive
Gynecol. 25:638–643. 2018. View Article : Google Scholar
|
|
11
|
Demyttenaere S, Feldman LS and Fried GM:
Effect of pneumoperitoneum on renal perfusion and function: A
systematic review. Surg Endosc. 21:152–160. 2007. View Article : Google Scholar
|
|
12
|
Ozmen MM, Zulfikaroglu B, Besler TH, Col
C, Cinel L and Cinel I: The correlation between reactive oxygen
species and histopathology of the liver, gut, and kidneys in
animals with elevated intra-abdominal pressure. J Laparoendosc Adv
Surg Tech A. 19:339–343. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sammour T, Mittal A, Loveday BP, Kahokehr
A, Phillips AR, Windsor JA and Hill AG: Systematic review of
oxidative stress associated with pneumoperitoneum. Br J Surg.
96:836–850. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
de Seigneux S, Klopfenstein CE, Iselin C
and Martin PY: The risk of acute kidney injury following
laparoscopic surgery in a chronic kidney disease patient. NDT Plus.
4:339–341. 2011.PubMed/NCBI
|
|
15
|
Li W, Cao Z, Xia Z, Meng Q, Yu WM, Yao X
and Cheng F: Acute kidney injury induced by various
pneumoperitoneum pressures in a rabbit model of mild and severe
hydronephrosis. Urol Int. 94:225–233. 2015. View Article : Google Scholar
|
|
16
|
Li W, Zhao S, Cheng F, Rao T, Yu W, Ruan
Y, Yuan R and Yao X: Oxidative damage and mitochondrial injuries
differ following pneumoperitoneum pressure in rabbit models of
varying degrees of hydronephrosis. Mol Med Rep. 17:6819–6827.
2018.PubMed/NCBI
|
|
17
|
Chen SD, Yang DI, Lin TK, Shaw FZ, Liou CW
and Chuang YC: Roles of oxidative stress, apoptosis, PGC-1alpha and
mitochondrial biogenesis in cerebral ischemia. Int J Mol Sci.
12:7199–7215. 2011. View Article : Google Scholar :
|
|
18
|
National Research Council: Guide For The
Care and Use of Laboratory Animals. National Acadamies Press;
Washington, DC: 1996
|
|
19
|
Wen JG, Chen Y, F Frøkiaer J, Jørgensen TM
and Djurhuus JC: Experimental partial unilateral ureter
obstruction. I. Pressure flow relationship in a rat model with mild
and severe acute ureter obstruction. J Urol. 160:1567–1571. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ly JD, Grubb DR and Lawen A: The
mitochondrial membrane potential (deltapsi(m)) in apoptosis; an
update. Apoptosis. 8:115–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Perelman A, Wachtel C, Cohen M, Haupt S,
Shapiro H and Tzur A: JC-1: Alternative excitation wavelengths
facilitate mitochondrial membrane potential cytometry. Cell Death
Dis. 3:e4302012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu X, Wang K, Zhang K, Huang B, Zhang J,
Zhang Y, Zhu L, Zhou B and Zhou F: Ziyuglycoside II inhibits the
growth of human breast carcinoma MDA-MB-435 cells via cell cycle
arrest and induction of apoptosis through the mitochondria
dependent pathway. Int J Mol Sci. 14:18041–18055. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen Q, Xu H, Xu A, Ross T, Bowler E, Hu Y
and Lesnefsky EJ: Inhibition of Bcl-2 sensitizes mitochondrial
permeability transition pore (MPTP) opening in ischemia-damaged
mitochondria. PLoS One. 10:e1188342015.
|
|
24
|
Eleftheriadis T, Pissas G, Liakopoulos V
and Stefanidis I: Cytochrome C as a potentially clinical useful
marker of mitochondrial and cellular damage. Front Immunol.
7:2792016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Feinstein-Rotkopf Y and Arama E: Can’t
live without them, can live with them: Roles of caspases during
vital cellular processes. Apoptosis. 14:980–995. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khoury W, Jakowlev K, Fein A, Orenstein H,
Nakache R and Weinbroum AA: Renal apoptosis following carbon
dioxide pneumoperitoneum in a rat model. J Urol. 180:1554–1558.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wiesenthal JD, Fazio LM, Perks AE, Blew
BD, Mazer D, Hare G, Honey RJ and Pace KT: Effect of
pneumoperitoneum on renal tissue oxygenation and blood flow in a
rat model. Urology. 77:1508–1509. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Richards WO, Scovill W, Shin B and Reed W:
Acute renal failure associated with increased intra-abdominal
pressure. Ann Surg. 197:183–187. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bishara B, Ramadan R, Karram T, Awad H,
Abu-Saleh N, Winaver J, Assadi A and Abassi Z: Nitric oxide
synthase inhibition aggravates the adverse renal effects of high
but not low intraabdominal pressure. Surg Endosc. 24:826–833. 2010.
View Article : Google Scholar
|
|
30
|
Hashikura Y, Kawasaki S, Munakata Y,
Hashimoto S, Hayashi K and Makuuchi M: Effects of peritoneal
insufflation on hepatic and renal blood flow. Surg Endosc.
8:759–761. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sodha S, Nazarian S, Adshead JM, Vasdev N
and Mohan-S G: Effect of pneumoperitoneum on renal function and
physiology in patients undergoing robotic renal surgery. Curr Urol.
9:1–4. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nickkholgh A, Barro-Bejarano M, Liang R,
Zorn M, Mehrabi A, Gebhard MM, Büchler MW, Gutt CN and Schemmer P:
Signs of reperfusion injury following CO2
pneumoperitoneum: An in vivo microscopy study. Surg Endosc.
22:122–128. 2008. View Article : Google Scholar
|
|
33
|
Richter S, Olinger A, Hildebrandt U,
Menger MD and Vollmar B: Loss of physiologic hepatic blood flow
(‘hepatic arterial buffer response’) during CO2-pneumoperitoneum in
the rat. Anesth Analg. 93:872–877. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guven S, Muci E, Unsal MA, Yulug E, Alver
A, Kadioglu Duman M and Mentese A: The effects of carbon dioxide
pneumoperitoneum on ovarian blood flow, oxidative stress markers,
and morphology during laparoscopy: A rabbit model. Fertil Steril.
93:1327–1332. 2010. View Article : Google Scholar
|
|
35
|
Oksuz H, Bulbuloglu E, Senoglu N, Ciralik
H, Yuzbasioglu MF, Kilinc M, Dogan Z, Goksu M, Yildiz H, Ozkan OV
and Atli Y: Re-protective effects of pre- and post-laparoscopy
conditioning, zinc, pentoxifylline, and N-acetylcysteine in an
animal model of laparoscopy-induced ischemia/reperfusion injury of
the kidney. Ren Fail. 31:297–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gao Y, Chen T, Lei X, Li Y, Dai X, Cao Y,
Ding Q, Lei X, Li T and Lin X: Neuroprotective effects of polydatin
against mitochondrial-dependent apoptosis in the rat cerebral
cortex following ischemia/reperfusion injury. Mol Med Rep.
14:5481–5488. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chevalier RL, Thornhill BA, Forbes MS and
Kiley SC: Mechanisms of renal injury and progression of renal
disease in congenital obstructive nephropathy. Pediatr Nephrol.
25:687–697. 2010. View Article : Google Scholar
|
|
38
|
Shigeta K, Kikuchi E, Hagiwara M, Hattori
S, Kaneko G, Hasegawa M, Takeda T, Jinzaki M, Akita H, Miyajima A,
et al: Visceral to total obesity ratio and severe hydronephrosis
are independently associated with prolonged pneumoperitoneum
operative time in patients undergoing laparoscopic radical
nephroureterectomy for upper tract urothelial carcinoma.
Springerplus. 4:2902015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bauer V and Bauer F: Reactive oxygen
species as mediators of tissue protection and injury. Gen Physiol
Biophys Spec No. 7–14. 1999.
|
|
40
|
Maslov LN, Naryzhnaia NV, Podoksenov I,
Prokudina ES, Gorbunov AS, Zhang I and Pei Z: Reactive oxygen
species are triggers and mediators of an increase in cardiac
tolerance to impact of ischemia-reperfusion. Ross Fiziol Zh Im I M
Sechenova. 101:3–24. 2015.In Russian. PubMed/NCBI
|
|
41
|
Tajeddine N: How do reactive oxygen
species and calcium trigger mitochondrial membrane
permeabilisation. Biochim Biophys Acta. 1860.1079–1088. 2016.
|
|
42
|
Murphy MP: How mitochondria produce
reactive oxygen species. Biochem J. 417:1–13. 2009. View Article : Google Scholar
|
|
43
|
Zhang M, Zheng J, Nussinov R and Ma B:
Release of cytochrome C from bax pores at the mitochondrial
membrane. Sci Rep. 7:26352017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Huttemann M, Pecina P, Rainbolt M,
Sanderson TH, Kagan VE, Samavati L, Doan JW and Lee I: The multiple
functions of cytochrome c and their regulation in life and death
decisions of the mammalian cell: From respiration to apoptosis.
Mitochondrion. 11:369–381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Boujrad H, Gubkina O, Robert N, Krantic S
and Susin SA: AIF-mediated programmed necrosis: A highly regulated
way to die. Cell Cycle. 6:2612–2619. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Basanez G, Soane L and Hardwick JM: A new
view of the lethal apoptotic pore. PLoS Biol. 10:e10013992012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kushnareva Y, Andreyev AY, Kuwana T and
Newmeyer DD: Bax activation initiates the assembly of a multimeric
catalyst that facilitates Bax pore formation in mitochondrial outer
membranes. PLoS Biol. 10:e10013942012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chipuk JE and Green DR: How do BCL-2
proteins induce mitochondrial outer membrane permeabilization?
Trends Cell Biol. 18:157–164. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Avital S, Itah R, Szomstein S, Rosenthal
R, Inbar R, Sckornik Y and Weinbroum A: Correlation of
CO2 pneumoperitoneal pressures between rodents and
humans. Surg Endosc. 23:50–54. 2009. View Article : Google Scholar
|
|
50
|
Balayssac D, Pereira B, Bazin JE, Le Roy
B, Pezet D and Gagniere J: Warmed and humidified carbon dioxide for
abdominal laparoscopic surgery: Meta-analysis of the current
literature. Surg Endosc. 31:1–12. 2017. View Article : Google Scholar
|
|
51
|
Davis SS, Mikami DJ, Newlin M, Needleman
BJ, Barrett MS, Fries R, Larson T, Dundon J, Goldblatt MI and
Melvin WS: Heating and humidifying of carbon dioxide during
pneumoperitoneum is not indicated: A prospective randomized trial.
Surg Endosc. 20:153–158. 2006. View Article : Google Scholar
|
|
52
|
Sammour T, Mittal A, Delahunt B, Phillips
AR and Hill AG: Warming and humidifcation have no effect on
oxidative stress during pneumoperitoneum in rats. Minim Invasive
Ther Allied Technol. 20:329–337. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Akbulut G, Polat C, Aktepe F, Yilmaz S,
Kahraman A, Serteser M, Gökçe C and Gökçe O: The oxidative effect
of prolonged CO2 pneumoperitoneum on renal tissue of
rats. Surg Endosc. 18:1384–1388. 2004. View Article : Google Scholar
|
|
54
|
Hoekstra LT, Ruys AT, Milstein DM, van
Samkar G, van Berge HM, Heger M, Verheij J and van Gulik TM:
Effects of prolonged pneumoperitoneum on hepatic perfusion during
laparoscopy. Ann Surg. 257:302–307. 2013. View Article : Google Scholar
|
|
55
|
Doerflinger M, Glab JA and Puthalakath H:
BH3-only proteins: A 20-year stock-take. FEBS J. 282:1006–1016.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gerecova G, Kopanicova J, Jaka P, Běhalová
L, Juhásová B, Bhatia-Kiššová I, Forte M, Polčic P and Mentel M:
BH3-only proteins Noxa, Bik, Bmf, and Bid activate Bax and Bak
indirectly when studied in yeast model. Fems Yeast Res. 13:747–754.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Juhasova B, Mentel M, Bhatia-Kissova I,
Zeman I, Kolarov J, Forte M and Polčic P: BH3-only protein Bim
inhibits activity of antiapoptotic members of Bcl-2 family when
expressed in yeast. FEBS Lett. 585:2709–2713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tegla CA, Cudrici C, Patel S, Trippe R,
Rus V, Niculescu F and Rus H: Membrane attack by complement: The
assembly and biology of terminal complement complexes. Immunol Res.
51:45–60. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Schuler M and Green DR: Mechanisms of
p53-dependent apoptosis. Biochem Soc Trans. 29:684–688. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang J, Ye J, Altafaj A, Cardona M, Bahi
N, Llovera M, Cañas X, Cook SA, Comella JX and Sanchis D: EndoG
links Bnip3-induced mitochondrial damage and caspase-independent
DNA fragmentation in ischemic cardiomyocytes. PLoS One.
6:e179982011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kilbride SM and Prehn JH: Central roles of
apoptotic proteins in mitochondrial function. Oncogene.
32:2703–2711. 2013. View Article : Google Scholar
|