1. Introduction
Glaucoma is second leading cause of blindness after
cataract, and the leading cause of irreversible blindness globally
(1-4). Primary open-angle glaucoma (POAG) is
the most prevalent form of glaucoma (5) and is responsible for ~90% of all
cases (6). The pathogenesis of
glaucoma remains unknown; however, accumulating evidence indicates
that genetic factors may play a causative role (7). The myocilin gene (MYOC), which
encodes the secreted protein myocilin, is the first and most
extensively investigated gene associated with familial forms of
POAG (8,9). MYOC is detected in the aqueous humor
and MYOC mutations are the most common type of mutation in patients
with glaucoma (10), accounting
for 10% of juvenile-onset open-angle glaucoma (JOAG) (11) and 2-4% of adult-onset POAG
(12). JOAG, unlike adult-onset
POAG, has a close genotype-phenotype association with regard to
myocilin mutations and glaucoma, and typical signs include high
intra-ocular pressure (IOP) (13,14), severe optic nerve damage and
earlier age at onset (usually <40 years) (15,16), which, if left untreated, results
in severe visual impairment (17).
Myocilin is ubiquitously expressed in normal tissues
and organs; however, myocilin-associated disease only occurs in the
eye (18). Myocilin is widely
expressed in ocular tissues and highly expressed in the trabecular
meshwork (TM) (19). Wild-type
myocilin is secreted in the TM (9), is located in the aqueous humor and
plays an important role in the regulation of IOP (20,21). High IOP is a risk factor for
glaucoma (22,23) and reduction of IOP is an approach
to glaucoma treatment (24).
Mutant myocilin aggregation is closely associated with glaucoma
(25) and it may be involved in
morphological changes in the TM that may result in cell apoptosis
(7). A growing body of evidence
suggests that myocilin, the TM, IOP and glaucoma are closely
associated.
The present review exclusively focused on the
results of the latest studies that provide an insight into the
physiological functions of myocilin and its role in the
pathogenesis of glaucoma in the TM. An overview of the primary
findings and future research topics requiring further investigation
was performed in the present review.
2. Physiological functions and
characteristics of myocilin
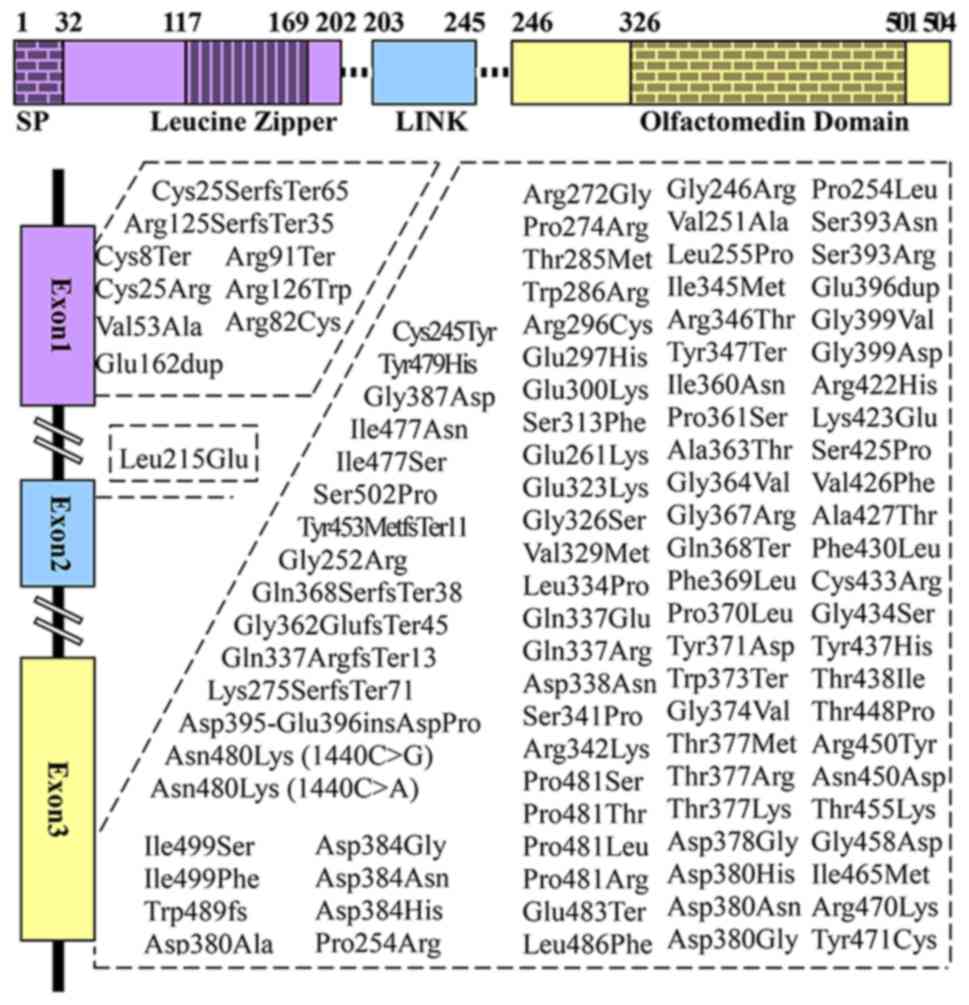
Structure of myocilin
Despite numerous studies over the 20 years since its
discovery as a glaucoma-associated gene in 1997, the physiological
functions and biological activities of myocilin in the TM remain
poorly understood. The first report of identified myocilin
mutations in autosomal dominant POAG came from Stone et al
(26), and myocilin was found to
map to the GLC1A locus at 1q24.3-q25.2 (OMIM: 601652). Myocilin,
which encodes a 504-amino acid glycoprotein and undergoes
glycosylation at amino acid residues 57-59 (27), has three exons and contains two
major homology regions, the N- and C-terminus (Fig. 1) (23,26,28-35). Notably, the majority of myocilin
mutations are localized in exon 3 (Fig. 1). The N-terminus of myocilin
contains leucine zippers (LZ) within two coil-coil domains
(35). Furthermore, the
N-terminus is involved in the initial myocilin oligomerization
through LZ (36), and in the
extracellular interactions of myocilin with other extracellular
proteins through two coil-coil domains (35,37). The C-terminus contains
olfactomedin (OLF), which is important for the structure and
function of myocilin (35),
specifically in the process of intracellular trafficking (36). Notably, N- and C-terminus
functions affect the aqueous humor outflow in the TM.
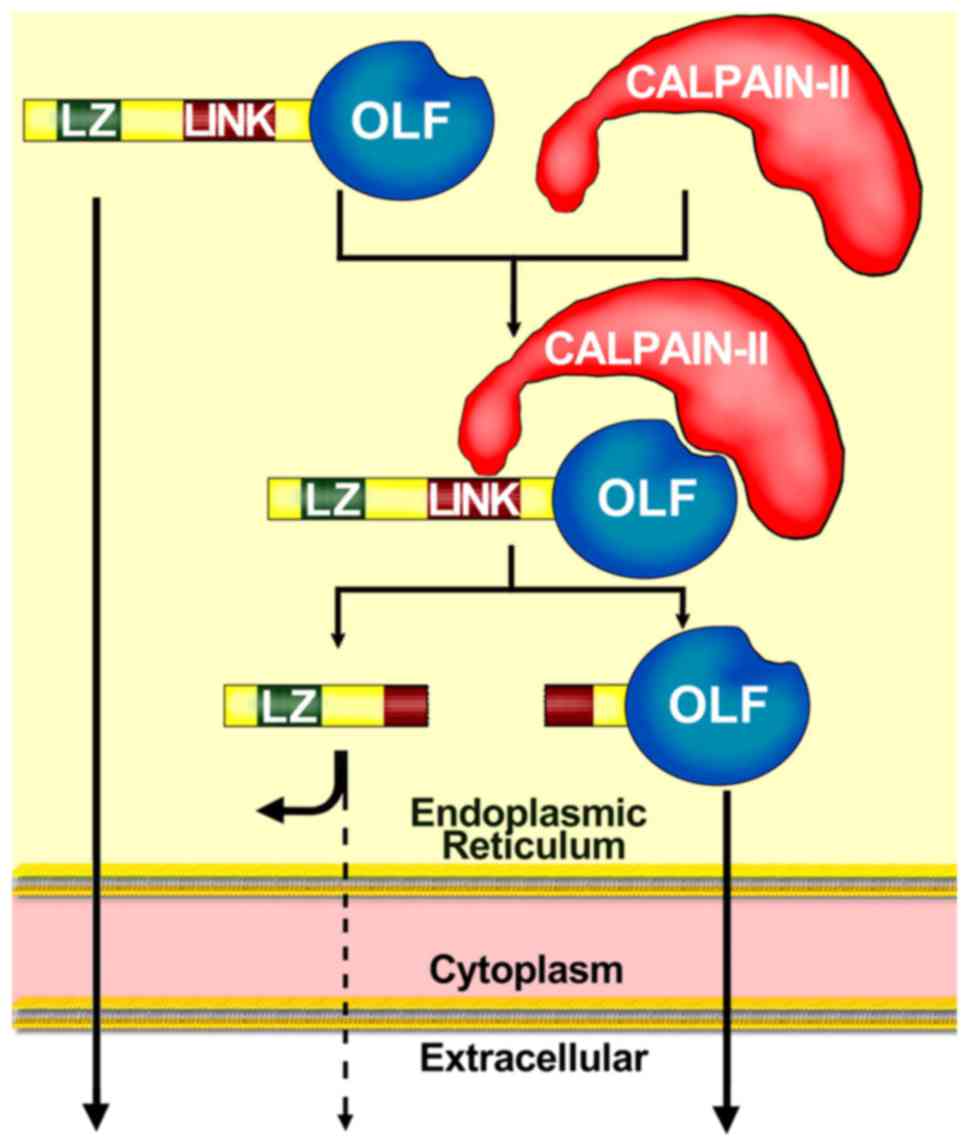
Intracellular proteolytic process
Normally, myocilin is intra-cellularly cleaved
within the endoplasmic reticulum (ER) of TM cells and secreted into
the aqueous humor (33,38). C-terminal myocilin fragments have
been detected in the TM and the aqueous humor (23,33). N-terminal myocilin containing LZ
has also been identified (39);
however, this is intracellularly retained in the ER (23,33,36,40,41). N-terminal fragments can be
intracellularly degraded during proteolytic processing, or they can
interact with other intracellular proteins (40). Previous studies have suggested
that myocilin undergoes proteolytic cleavage, and that the location
of the proteolytic cleavage site is possibly between Glu214-Leu215
(39,42) or between Arg226-Ile227 (23). It was reported that myocilin
fragments containing OLF did not change the outflow capacity of the
aqueous humor, suggesting that both OLF and LZ fragments must
coexist for myocilin to function properly in the intracellular
proteolytic process (39).
Similar to myocilin, calpain II (cysteine protease)
is also present within the lumen of the Golgi apparatus and the ER
(43). Calpain II is required for
the intracellular proteolytic cleavage of myocilin (40). The proteolytic processing of
myocilin does not require the N-terminus, and two different domains
of myocilin participate in the proteolytic processing through
calpain II (40): i) C-terminal
OLF, which likely acts as a substrate binding site recognized by
calpain II; and ii) linker domain (LINK), which acts as the
cleavage site (Fig. 2) (40). These findings are supported by
previous studies (23,42,44), suggesting that myocilin mutations
located at OLF may inhibit the proteolytic processing of myocilin.
Amino acid positions mutated in OLF likely affect the structure of
the myocilin binding site to calpain II (40). Interestingly, Pro370Leu, which
contributes to the most severe glaucoma phenotype (44), produces the most severe inhibition
of proteolytic processing. The inhibition of proteolytic processing
by Glu323Lys and Asp380Ala is less severe, causing less severe
glaucoma (23). However, the
association of the severity of glaucoma with the inhibition of
proteolytic processing remains unclear.
Extracellular proteolytic process
Although the physiological function of the
intracellular proteolytic processing of myocilin remains unknown,
the amount of proteolytic myocilin may be associated with the
regulation of the normal TM structure through extracellular
proteins, including fibrillin-1 (45), secreted protein acidic and rich in
cysteine (SPARC) (34), hevin
(34,46), collagen (45), optimedin (47), decorin (45), fibronectin (48) and laminin (45,49), which contribute to the regulation
of aqueous humor outflow that can affect IOP (23). The identification of proteins
interacting with myocilin is a possible approach to elucidating its
functions, since interacting proteins are typically involved in the
same physiological and pathological processes (50).
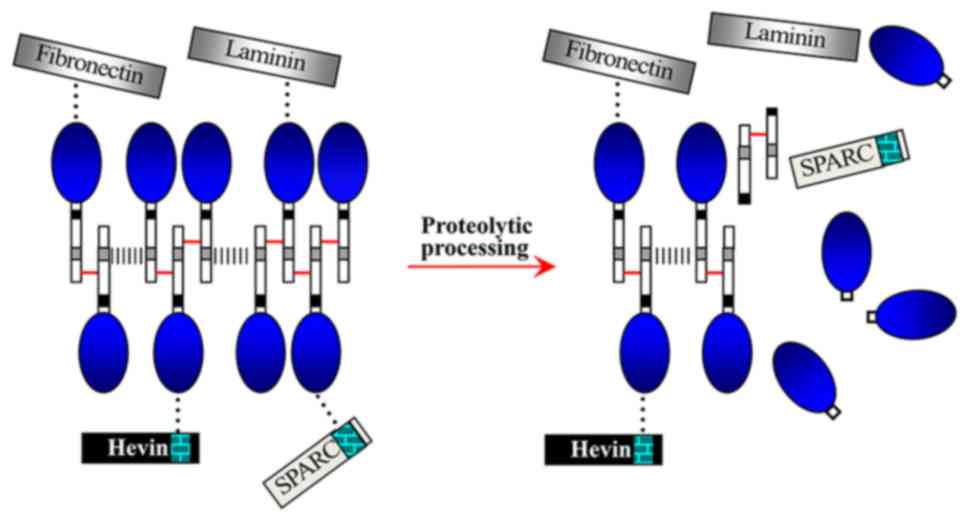
The first report of the possible role of proteolytic
processing in regulating the interactions of myocilin with itself
or other extracellular proteins came from Aroca-Aguilar et al
(33,34), who demonstrated that homoaggregates of myocilin
monomers and myocilin complexes (containing oligomers,
matricellular proteins and extracellular matrix proteins) can form
a dynamic extracellular network (Fig.
3) (34). The proteolytic
processing of myocilin occurs in the relevant elements involved in
IOP homeostasis (such as the aqueous humor and the TM); thus, the
proteolytic cleavage in the dynamic myocilin network possibly
participates in regulating IOP (33) through controlling IOP homeostasis
(aqueous humor production and drainage). Furthermore, the myocilin
network may act as a link between the extracellular matrix and
matricellular proteins (34).
Coincidently, OLFs in the extracellular network are similar to
those resulting from intracellular proteolytic processing of
myocilin through calpain II (Figs.
2 and 3) (23,34,40).
Elevated IOP and overexpression of
myocilin caused by inducers
Myocilin is stimulated by various inducers,
including dexamethasone (DXM) (11,51-55), pentablock copolymer DXM
nanoformulations (56), retinoic
acid (57), transforming growth
factor (TGF)-β1 (11), TGF-β2
(58), optineurin (59,60), mechanical stretch (11), rotenone (61), and hydrogen peroxide-inducible
clone-5 (62). DEX is widely
recognized as a major contributor to the induction of myocilin,
which may cause high IOP.
Elevated IOP is a known primary risk factor for
glaucoma (22,23); however, not all cases of glaucoma
exhibit high IOP (63,64). Elevated IOP is caused by increased
resistance to aqueous humor outflow through the TM (65,66) and accounts for visual field loss;
however, its pathogenesis remains unknown (67). Homeostasis of aqueous humor
drainage through the TM is essential for the maintenance of normal
IOP (68). DXM-induced
overexpression of myocilin in the TM cells (11,54,55) may increase IOP (69). Additionally, overexpression of
myocilin induced by DXM possibly affects focal adhesion, stress
fibers and actin reorganization in TM cells, which subsequently
results in ER aggregation and TM cell stiffness, leading to
increased outflow resistance (62) and elevated IOP. However, the
results regarding the reversal of myocilin (52,70) and increased IOP (70,71) do not support the hypothesis that
myocilin overexpression causes increased IOP or glaucoma; this is
supported by the following data: i) Observation of an equivalent
increase of IOP in MYOC-/- and wild-type mice following
DXM treatment (51); and ii)
observation of increased IOP and absence of myocilin overexpression
following DXM treatment (71).
These studies suggest that increased IOP may not be associated with
myocilin overexpression.
3. Pathogenesis of mutant/misfolded
myocilins
The aforementioned studies suggest that the
biological functions of myocilin remain poorly understood.
Similarly, the role of myocilin in the pathogenesis of glaucoma is
currently unknown, although its mutations have been reported to be
associated with JOAG and adult-onset POAG.
Myocilin mutations
MYOC mutations alter the myocilin protein, which
affects the normal regulation of IOP and may lead to glaucoma
(72). To date, 278 different
myocilin mutations have been reported, among which pathogenic
mutations account for 37.77% (26) (Fig.
1) (23,28-35), 9 of which have been identified in
exon 1, 1 in exon 2, and 95 in exon 3. Myocilin predominantly
displays two types of mutations: Missense mutations (83.8%), which
are associated with JOAG and adult-onset POAG (16); and nonsense mutations (5.7%)
(26). Different myocilin
mutations cause POAG with varying age at onset and glaucoma
phenotypes of varying severity (73). Pro370Leu is one of the most severe
glaucoma phenotypes, which is involved in mitochondrial dysfunction
in TM cells and may lead to apoptosis (74). Gln368Stop is the most common
mutation; however, it exhibits a markedly lower penetrance for
glaucoma (75).
Autosomal dominant disorders resulting from myocilin
(28) may be caused by the
following three pathogenic mechanisms: The dominant negative
effect, gain of function, or haploinsufficiency (7,16).
Some studies have demonstrated that neither haploinsufficiency
(16,19,67,76) nor the dominant negative effect
(77) are involved in
myocilin-associated pathogenicity. However, recent findings
(42,78) have suggested that the dominant
negative effect may be involved in the pathogenic role of myocilin
in glaucoma. Notably, the majority of experimental evidence support
gain-of-function as a pathogenic mechanism involved in myocilin
mutation-associated glaucoma (21,23,36,37,41,42,67,73,76,79-84).
Pathogenesis of ER stress and oxidative
stress (OS) caused by mutant myocilin
Recent studies have indicated that the notable
paucity of normal myocilin (16,67,76,85) or its overexpression (85) are not associated with the
pathogenesis of glaucoma, and that the pathogenesis of glaucoma is
dependent on the expression of mutant/misfolded myocilins (86,87). Notably, mutations may cause
myocilin misfolding (88).
Furthermore, the secretion of wild-type myocilin is inhibited in
the presence of co-expressed mutant myocilin (36,41). The aggregation of
misfolded/wild-type myocilins in the ER may be harmful for TM cells
and lead to apoptosis (19,84). Previous results have suggested
that the TM is several times thicker in patients with glaucoma
harboring mutations compared with that in patients without myocilin
mutations (7). Therefore,
myocilin mutations appear to be involved in the morphological
changes in the TM, which lead to cell apoptosis (7).
Mutant myocilins aggregate intracellularly in
insoluble and soluble aggresomes, interact with ER proteins and
promote ER stress (19,81-84). Mutant myocilins have been
suggested to induce apoptosis and may contribute to TM cell
dysfunction, leading to increased IOP (86,89,90). Subsequently, ER stress may cause
OS (87). During ER stress, the
level of reactive oxygen species (ROS) is increased (91). Excess production and an
accumulation of ROS compromise reduction-oxidation balance and
cause OS (91). Notably, ER
stress and OS are associated events involved in the pathogenesis of
various diseases (91), including
glaucoma-associated TM damage and increased IOP (87,90). However, the molecular pathways
that connect ER stress and OS are poorly understood. OS is caused
by the excessive production and aggregation of ROS (91). The TM, which is the most sensitive
to OS among the tissues in the anterior chamber of the eye
(86), comes into direct contact
with the ROS-containing aqueous humor (87). Furthermore, ROS alone may cause
protein misfolding (86).
Misfolded myocilin can render cells more sensitive to OS (87). It was recently suggested that
mutant myocilin inhibited antioxidant enzymes, such as paraoxonase
2, that efficiently decrease OS and inhibit ER stress-induced
apoptosis (87). Therefore,
decreased antioxidative enzymes in the TM may cause ROS elevation
in the aqueous humor outflow system (87).
It has been reported that OS aggravates ER stress by
inducing the overproduction of misfolded proteins (92). OS has also been considered a major
factor causing damage to the TM (93). To relieve the damage from ER
stress and restore homeostasis, the aggregated misfolded myocilin
activates the unfolded protein response (UPR), which protects the
TM cells (94-96), improves the protein folding
mechanism and degrades misfolded proteins (94,95). However, if ER stress persists, UPR
induces cell apoptosis (19,84). In addition, mutant myocilin cannot
be cleared by ER-associated degradation, which transports degraded
products of misfolded myocilin to the cytosol (19), leading to deleterious aggregation
of amyloid-containing myocilin (97). These studies suggest that myocilin
misfolding, UPR, ROS, OS and ER stress may be related events.
Notably, ER stress and OS are risk factors for glaucoma, and
together with the deleterious effect of misfolded myocilin, may
cause a more severe glaucoma phenotype compared with any of these
factors alone. These associated events disrupt the proteostasis of
myocilin, leading to an imbalance between production and clearance
of misfolded myocilins.
Pathogenesis of the co-aggregation of
glucose-regulated protein (Grp) 94 with mutant myocilin
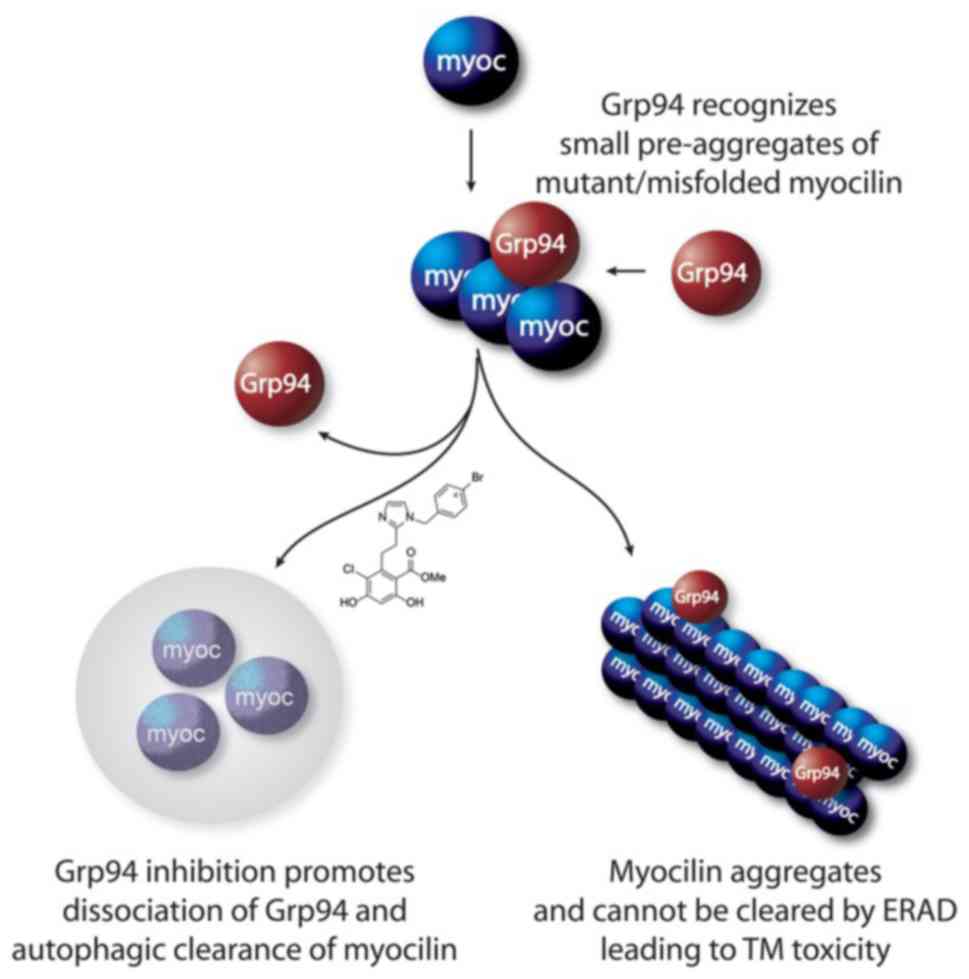
It has been reported that the inhibition of Grp94 is
an effective approach to the treatment of glaucoma (79,98), supporting the co-aggregation of
Grp94 with mutant myocilin and leading to retention within the ER
(Fig. 4) (80). Not only does Grp94 accelerate the
myocilin-OLF aggregation rate, but it also enhances
co-precipitation with OLF (79,80,99). Therefore, Grp94 inhibition
facilitates mutant myocilin clearance as an anti-glaucoma therapy
(79,97,100). 4-Br-BnIm, a Grp94 inhibitor,
significantly clears aggregated myocilin caused by overexpression
mutations (97), and alleviates
mutant myocilin-induced toxicity against TM cells (27,79) via a secondary autophagic pathway
to facilitate clearance (27).
When forced to misfold and aggregate, wild-type myocilin becomes a
client of Grp94 and sensitized to 4-Br-BnIm (79). Recent studies (12,101) reported that myocilin aggregates
were cleared by a ubiquitin-proteasome and autophagy lysosomal
pathways under normal homeostatic conditions. However, when
myocilin is mutated, autophagy is activated due to dysfunction of
the proteasomal degradation pathway (12), and mutant myocilin is preferably
degraded by autophagy (101).
Grp94 inhibitors prevent Grp94 from aggregating with mutant
myocilin, which induces a secondary autophagic pathway to promote
clearance of abnormal myocilin (80). Grp94 is also a regulator of UPR
(79). Therefore, a reduction in
Grp94 may affect UPR, which induces TM cell death under persistent
ER stress due to abnormal aggregations of Grp94 and misfolded
myocilin.
Myocilin and the interleukin
(IL)-1/nuclear factor (NF)-κB inflammatory stress signaling
pathway
Activation of IL-1/NF-κB inflammatory stress is a
defining characteristic of high-tension glaucoma (102). The release of potent
proinflammatory IL-1, including IL-1β, may lead to inflammation
(103) through activation of the
NF-κB pathway, which induces an inflammatory response (104). It has been demonstrated that
chronic low-grade inflammation causes ocular hypertension (105) and is also involved in the
pathogenesis of glaucoma (103).
Mutant myocilins aggregate within the TM cells and activate the
NF-κB signaling pathway, significantly inducing the expression of
IL-1 and IL-1β; however, extracellular mutant myocilin does not
activate the NF-κB signaling pathway (106). Therefore, intracellular
aggregations of mutant myocilins may induce the overexpression of
IL-1 via activating NF-κB, leading to chronic inflammation that can
cause elevated IOP.
Interestingly, IL-1 has additional beneficial
effects on glaucoma, including reducing IOP through stimulation of
matrix metalloproteinase (MMP) expression (106) and inhibiting apoptosis caused by
OS through NF-κB (102). IOP and
OS are associated with the aggregation of misfolded/mutant
myocilins (86). Notably, IL-1
was increased by 10-fold in TM cells harboring Gln368Stop; however,
the increase was only 6-fold in cells harboring Try437His (106). These findings suggest that
abnormal aggregation of myocilin mutations may induce OS and
upregulate IL-1. However, the amount of IL-1 induced by Gln368Stop
is notably higher compared with that induced by Try437His.
Coincidently, Try437His causes a severe glaucoma phenotype;
however, Gln368Stop only causes a moderately severe glaucoma
phenotype. This may be explained by the greater extent of IOP
reduction and inhibition of the apoptotic process caused by OS
through IL-1 via Gln368Stop compared with Try437His in the
short-term. This also explained the finding that Gln368Stop is the
most common mutation; however, it revealed a markedly lower
penetrance for glaucoma caused by high IOP.
4. Imbalance of pathogenic myocilin and
extracellular proteins
Glaucoma-associated TM damage is involved in various
biochemical and morphological changes, including aberrant
extracellular matrix protein aggregation and cell apoptosis
(107). Synthesis and
degradation of the extracellular matrix are in a dynamic balance
that is constantly remodeled by proteolysis and protein deposition
(52). Certain diseases,
including glaucoma, may result from disruption of this dynamic
balance (107). Recently, the
crystal structure of myocilin-OLF was elucidated, and it indicated
that myocilin-OLF belongs to the five-bladed β-propeller family
(108), which is a known hub for
protein-protein interactions. To date, a number of extracellular
proteins interacting with myocilin have been identified, including
tissue inhibitor of matrix metalloproteinase (TIMP3) (8,50),
fibronectin (107), flotillin-1
(109) and hevin (46).
Effect of pathogenic myocilin on MMPs and
MMP inhibitors
MMPs have multiple physiological functions, among
which cell migration and proliferation have been linked to myocilin
(110,111). Changes in MMP activity are
involved in the pathogenesis of glaucoma (112). Notably, MMP2 activity may be
associated with the regulation of IOP, which may be predominantly
associated with the TM, where myocilin and TIMP3 coexist (50). MMP2, which is abundant in the TM
(8), is involved in the breakdown
of extracellular matrix (52),
which facilitates aqueous humor outflow (8). It has also been demonstrated that
myocilin mutations or MYOC null can enhance inhibition of MMP2 by
TIMP3 (8,50). An imbalance between MMPs and TIMP
within the TM can cause POAG (113). Of note, mutant myocilins can
also reduce the active forms of MMP2 (107). Therefore, the effect of mutant
myocilin on MMP2 activation disrupts the balance between MMPs and
TIMPs, leading to glaucoma (96).
In addition, reduction of MMP2 inhibits the decomposition of the
extracellular matrix, disrupting its homeostasis, which may change
the TM morphology and function, leading to increased IOP.
Effect of pathogenic myocilin on
fibronectin, flotillin-1 and hevin
Fibronectin is a key component of the extracellular
matrix of the TM, and is also an important mediator involved in
extracellular matrix formation, which regulates aqueous humor
outflow (114). Aberrant
aggregation of the extracellular matrix in the TM reduces aqueous
humor outflow and increases IOP in POAG (107). Overexpression of fibronectin and
increased ER stress markers c/ebphomologous protein (CHOP) and
Grp78 coexist in mice harboring MYOC-Tyr437His (107). Furthermore, another study
reported that mutant myocilins increased CHOP and Grp78 in the TM
(96). Myocilin, fibronectin,
CHOP and Grp78 expression levels were increased following DXM
treatment (115), which was
similar to the overexpression of myocilin and fibronectin observed
following DXM-Ac treatment (51).
Recent studies have suggested that a reduction of ER stress through
sodium 4-phenylbutyrate decreases the DXM-induced increase of IOP
(115) and fibro-nectin
aggregation caused by mutant myocilin (107) in TM cells. Therefore, myocilin,
fibronectin, CHOP, Grp78 and the extracellular matrix may be
considered as elements implicated in the pathogenesis of glaucoma
in the TM. Additionally, mutant myocilin induces aberrant
aggregation of the extracellular matrix in the ER of TM cells,
which may contribute to decreased aqueous humor outflow and
increased IOP (107).
Furthermore, failure of the TM to reduce ER stress caused by mutant
myocilin accounts for the induction of CHOP, which may also be
associated with apoptosis and increased IOP in the TM cells
(96).
Flotillin-1, a structural protein of lipid rafts,
interacts with myocilin (109).
Myocilin mutations, including Gly364Val, Tyr437His and Lys423Glu,
which are scattered in the OLF, fail to interact with flotillin-1
(109), suggesting that
myocilin-OLF may be required for the interaction. Although the role
of the interaction remains to be elucidated, loss of interaction
with flotillin-1 due to myocilin mutations may be a pathogenic
mechanism underlying the development of POAG (109). Hevin is a matricellular protein
involved in the assembly of the extracellular matrix (116). Mutant myocilin causes
intracellular aggregation of hevin and also affects hevin secretion
(46). Hevin coexpressed with
Pro370Leu, which is also known as a severe glaucoma mutation,
aggravates the impairment of hevin secretion (46). These findings indicate that mutant
myocilins affect the interactions with extracellular proteins,
leading to disruption of extracellular matrix homeostasis, which
may be involved in the pathogenesis of glaucoma.
5. Association of glaucoma pathogenesis with
the crystal structure and stability of myocilin
Crystal structure of myocilin
A better understanding of the conformation of
myocilin can provide a structural basis for investigating myocilin
mis-/unfolding in myocilin-associated glaucoma and, in turn, result
in a better understanding of myocilin-associated glaucoma
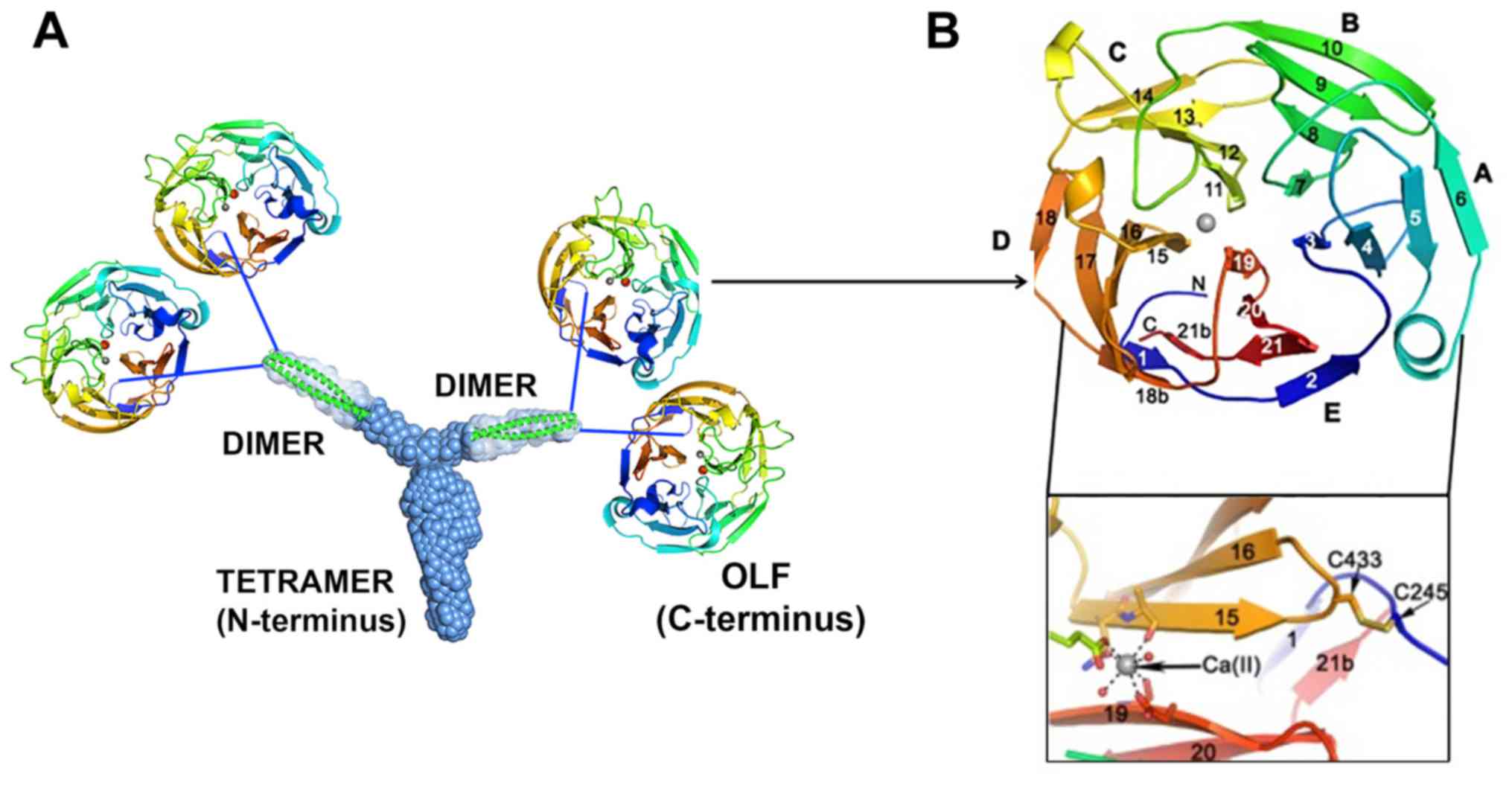
pathogenesis. The myocilin N-terminus has a unique tripartite
architecture, including a Y-shaped parallel dimer-of-dimers with
distinct tetramer and dimer regions. Furthermore, full-length
myocilin should also be branched, with two pairs of C-terminal OLFs
(Fig. 5A) (117). MYOC-Glu396Asp has discontinuous
D-18/D-18b and E-21/ E-21b strands (Fig. 5B) (108,117) that are unlike the continuous
D-18 and E-21 strands in wild-type myocilin. Accumulating evidence
has revealed that pathogenic myocilin mutations are associated with
intracellular aggregation propensity and thermal stability
(19,32,37,41,42,82,108,118,119). Three destabilizing regions are
localized in myocilin-OLF (108): i) Loop B-10/C-11 and cation-π;
ii) hydrophobic β-sheet belt; and iii) Ca2+ site
environs (Fig. 5B).
Structural location and stability of
myocilin mutations
The largest number of mutations are observed in
core β-sheet belts (~40%), loop B-10/C-11 and cation-π (~33%)
(108), particularly the most
destabilized myocilin-OLF mutations, such as Trp286Arg, Tyr437His,
Lys423Glu, Pro370Leu, Ile477Asn and Ile477Ser (118,119). The myocilin-OLF mutations
exhibit changes in the side chains. The most destabilizing
mutations also exhibit other characteristics: i) Lower melting
temperature (Table I) (108); ii) insoluble aggregates
(118); and iii) earlier age at
diagnosis. In contrast to the most destabilizing mutations, the
most stabilizing myocilin-OLF mutations, Ala427Thr and Ala445Val
(118), exhibit similar
stability and structure to those of wild-type myocilin-OLF.
Furthermore, the difference in their melting temperature from that
of wild-type myocilin-OLF (52.7±0.8°C) is only within 2°C, and they
are soluble (118). The trend in
melting temperature follows the general stability of myocilin-OLF
mutations and the severity of their aggregation. Additionally, this
instability may cause structural changes in myocilin-OLF, resulting
in mis-/unfolding. Notably, higher proportion of mis-/unfolded
myocilin has been associated with greater exposure of interior
hydrophobic regions and more severe intracellular aggregation
propensity (118). Intracellular
aggregation of mutant myocilin deforms TM cells (84) and changes the size of pores
between the cells, through which aqueous humor outflows.
Dysfunction of TM cells may contribute to the pathogenesis of
glaucoma (41,84).
 | Table IStructural location and stability of
myocilin mutations. |
Table I
Structural location and stability of
myocilin mutations.
| Mutations | Melting temperature
(°C) | Structural
location | Solubility | Mean age at
diagnosis, years | Secretion | Mean maximum IOP
(mmHg) | (Refs.) |
|---|
| 37°C | 30°C |
|---|
| Wild-type | 52.7±0.8 | NA | Soluble | NA | +++ | +++ | NA | (37,118) |
| Trp286Arg | NA | β-sheet belt | NA | 27.5±13.4 | No | No | 39.5±31.8 | (26,80,97,108,118,121) |
| Pro370Leu | NA | Loop B-10/C-11,
cation-π | Insoluble | 13.3±2.4 | No | Little | 49±8.5 | (15,19,26,82,108,118) |
| Lys423Glu | 34.2±0.4 | Loop B-10/C-11,
cation-π | Insoluble | 28.8±8.1 | No | No | 36±8.5 | (26,77,108,118) |
| Ile477Asn | 37.7±0.8 | β-sheet belt | Insoluble | 20.7±1.3 | No | Little | 44±0 | (26,79,82,97,108,118) |
| Ile477Ser | 39.7±0.2 | β-sheet belt | Insoluble | 33±0 | No | +++ | NA | (26,108,118) |
| Tyr437His | 40.3±0.4 | β-sheet belt | Insoluble | 19.9±0 | No | Little | 43.7±0 | (26,80,82,97,108,118) |
| Cys433Arg | 40.4±0.4 | β-sheet belt | Insoluble | 22.8 ± 8.9 | No | No | 39.1 ± 12.6 | (26,108,118) |
| Arg272Gly | 41.0±0.3 | β-sheet belt | NA | 33±0 | No | ++ | 62±0 | (26,108,118,122) |
| Ser502Pro | 41.0±0.3 | β-sheet belt | Insoluble | 19±0 | No | No | NA | (26,108,118) |
| Val426Phe | 41.5±0.1 | Loop B-10/C-11,
cation-π | Insoluble | 18.6±1.4 | Little | +++ | 43±0 | (26,108,118,122,123) |
| Asn480Lys | 42.4±0.2 | β-sheet belt | Insoluble | 25.4±3.1 | Little | ++ | 37.7±12.4 | (26,80,97,108,118) |
| Gly367Arg | 42.7±0.1 | Loop B-10/C-11,
cation-π | Insoluble | 26.6±5.5 | No | +++ | 51±0 | (26,28,108,118) |
| Ile499Phe | 42.8±0.1 | β-sheet belt | Partially
insoluble | 30.9±7.6 | Little | +++ | 31±6.6 | (26,82,108,118) |
| Gly252Arg | 43.0±0.2 | β-sheet belt | Insoluble | 35±7.3 | No | +++ | 62±0 | (26,108,118,122) |
| Glu323Lys | 44.0±0.5 | Loop B-10/C-11,
cation-π | Insoluble | 19±0 | Little | +++ | 43±0 | (26,108,118,122) |
| Thr377Met | 44.3±0.3 | Loop B-10/C-11,
cation-π | Partially
insoluble | 21.4±5.0 | + | +++ | 44±0 | (26,82,108,118,121,122) |
| Gly364Val | 45.0±0.4 | Loop B-10/C-11,
cation-π | Partially
insoluble | 34±0 | + | +++ | 36±0 | (26,82,108,118,121) |
| Pro481Leu | 45.5±0.4 | Ca2+
site environs | Insoluble | 33±0 | No | ++ | 46±0 | (26,108,118,121) |
| Asp380Ala | 46.6±0.3 | Ca2+
site environs | Partially
insoluble | 11.9±3.5 | Little | +++ | 34.2±17.9 | (26,82,108,118) |
| Ala427Thr | 50.5±0.2 | Ca2+
site environs | Soluble | 61±21.2 | +++ | NA | 31.8±6.1 | (26,108,118,123) |
| Ala445Val | 54.2±0.2 | NA | Soluble | 51.5±16.3 | +++ | NA | 24±0 | (26,108,118,121) |
Myocilin mutations at a moderate level of stability
(Table I) renew the secretion
through shifting temperature from 37 to 30°C (37,82,118), demonstrating their
temperature-sensitive secretion. Trimethylamine N-oxide and
sarcosine also stabilize myocilin mutations through shifting their
melting temperatures to near that of wild-type myocilin (119). Thr377Met, Ile499Phe and
Gly364Val, which are abundantly secreted at 30°C, are associated
with less severe glaucoma (82).
Interestingly, Pro370Leu and Ile477Asn, which are associated with a
more severe glaucoma phenotype (82,118), are not secreted at higher levels
when the temperature changes (82). Thus, temperature-sensitive
secretion may be a notable property of these moderate myocilin
mutations (82). A temperature of
30°C is a condition known to facilitate protein folding (37,42). Therefore, this favorable
temperature promotes the correct folding of myocilin into its
native form. Taken together, these studies demonstrated that the
stability of myocilin mutations may be associated with their
pathogenicity. Notably, the less stable myocilin mutations were
associated with the more severe glaucoma phenotype and were less
sensitive to temperature.
6. Conclusion
Based on the aforementioned evidence, although
myocilin mutations have been reported to be associated with JOAG
and adult-onset POAG, the physiological functions and pathogenicity
of myocilin remain elusive. Thus far, there are several possible
points of view on the pathogenic potential of myocilin: i) Myocilin
misfolding/unfolding; (ii) overexpression of myocilin; iii)
co-aggregation of Grp94; iv) disruption of extracellular matrix
homeostasis caused by mutant myocilin; v) OS, ER stress and
IL-1/NF-κB inflammatory stress; and vi) instability resulting from
conformational disorders caused by mutant myocilin. However,
certain suggestions require further investigation, particularly
those with contradicting conclusions. Furthermore, unsolved or
partially solved problems require further research, as they could
direct future studies on myocilin and contribute to novel
therapeutic approaches to the treatment of myocilin-associated
glaucoma.
The functions of myocilin through interactions of
its binding partners should be further investigated, as interacting
proteins may have similar biological functions. In addition, it is
necessary to gain further insight into the proteolytic processes of
myocilin, as this may contribute to an effective approach to
breaking down abnormal aggregations of myocilin. Furthermore, a
more detailed understanding of the structural basis of myocilin
stability will be valuable for elucidating the roles of
conformational disorders, such as misfolding or unfolding, in
myocilin-associated glaucoma. Notably, this may help elucidate the
role of myocilin mutations in the pathogenesis of glaucoma.
Additionally, the association of glaucoma phenotype severity with
the secretion (associated with temperature, stability and
insolubility) of mutant myocilin should be emphasized, as
unraveling these elusive associations may contribute to
understanding the pathogenesis underlying abnormal aggregations of
mutant myocilin. Future studies regarding myocilin should focus on
the deleterious effect of myocilin misfolding on OS and ER stress
in the TM, which are a series of associated events, each of which
causes the next, leading to further injury to the TM. Furthermore,
these effects are associated with increased IOP, which is a known
primary risk factor for glaucoma.
Funding
The present study was supported by a grant from
Taizhou Science and Technology Support Projects for Social
Development (2016) of Taizhou Science and Technology Bureau
(SSF20160112).
Availability of data and materials
Not applicable.
Authors’ contributions
HW, ML, ZZ, HX, XC and YJ designed the article,
contributed to the conception of the study and critically revised
the article for important intellectual content. HW and YJ drafted
the article. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Xin Wang in the
Library of Qiqihar Medical University for the careful edits and the
knowledge promoted and supported by the Heilongjiang Province
Philosophy and Social Science Research Planning project
(16TQB04).
Abbreviations:
|
JOAG
|
juvenile-onset open-angle
glaucoma
|
|
POAG
|
primary open-angle glaucoma
|
|
UPR
|
unfolded protein response
|
|
ER
|
endoplasmic reticulum
|
|
TGF
|
transforming growth factor
|
|
CHOP
|
c/ebp-homologous protein
|
|
Grp78
|
glucose-regulated protein 78
|
|
IOP
|
intraocular pressure
|
|
OLF
|
olfactomedin
|
|
LINK
|
linker domain
|
|
OS
|
oxidative stress
|
|
IL-1
|
interleukin-l
|
|
ROS
|
reactive oxygen species
|
|
ERAD
|
ER-associated degradation
|
|
Grp94
|
glucose-regulated protein 94
|
|
NF-κB
|
nuclear factor κB
|
|
MMP
|
matrix metalloproteinase
|
|
LZ
|
leucine zippers
|
|
PBA
|
sodium 4-phenylbutyrate
|
|
MYOC
|
myocilin
|
|
TM
|
trabecular meshwork
|
|
SPARC
|
secreted protein acidic and rich in
cysteine
|
|
TIMP3
|
tissue inhibitor of matrix
metalloproteinase 3
|
References
|
1
|
Khawaja AP, Cooke Bailey JN, Wareham NJ,
Scott RA, Simcoe M, Igo RP Jr, Song YE, Wojciechowski R, Cheng CY,
Khaw PT, et al: Genome-wide analyses identify 68 new loci
associated with intraocular pressure and improve risk prediction
for primary open-angle glaucoma. Nat Genet. 50:778–782. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang X, Huai G, Wang H, Liu Y, Qi P, Shi
W, Peng J, Yang H, Deng S and Wang Y: Mutual regulation of the
Hippo/Wnt/LPA/TGF-β signaling pathways and their roles in glaucoma
(Review). Int J Mol Med. 41:1201–1212. 2018.
|
|
3
|
Rangachari K, Bankoti N, Shyamala N,
Michael D, Sameer Ahmed Z, Chandrasekaran P and Sekar K: Glaucoma
Pred: Glaucoma prediction based on myocilin genotype and phenotype
information. Genomics. S0888–S7543. 30087–30089. 2018.PubMed/NCBI
|
|
4
|
Narooie-Nejad M, Rasouli A, Mousavi M and
Rohani MR: Study of MYOC gene mutation in POAG patients in zahedan
iran. Clin Lab. 63:1283–1291. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rasnitsyn A, Doucette L, Seifi M, Footz T,
Raymond V and Walter MA: FOXC1 modulates MYOC secretion through
regulation of the exocytic proteins RAB3GAP1, RAB3GAP2 and SNAP25.
PLoS One. 12:e01785182017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sharma S, Bollinger KE, Kodeboyina SK, Zhi
W, Patton J, Bai S, Edwards B, Ulrich L, Bogorad D and Sharma A:
Proteomic alterations in aqueous humor from patients with primary
open angle glaucoma. Invest Ophthalmol Vis Sci. 59:2635–2643. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hamanaka T, Kimura M, Sakurai T, Ishida N,
Yasuda J, Nagasaki M, Nariai N, Endo A, Homma K, Katsuoka F, et al:
A histologic categorization of aqueous outflow routes in familial
open-angle glaucoma and associations with mutations in the MYOC
gene in japanese patients. Invest Ophthalmol Vis Sci. 58:2818–2831.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fini ME: Another piece of the puzzle: MYOC
and myocilin glaucoma. Invest Ophthalmol Vis Sci. 58:53192017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Donegan RK and Lieberman RL: Discovery of
molecular therapeutics for glaucoma: Challenges successes and
promising directions. J Med Chem. 59:788–809. 2016. View Article : Google Scholar
|
|
10
|
Katoli P, Godbole A, Romanowski MJ, Clark
K, Meredith E, Saenz-Vash V, Wang YK, Lewicki N, Nguyen AA and
Lynch JM: Full-length myocilin protein is purified from mammalian
cells as a dimer. Protein Expr Purif. 147:38–48. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Faralli JA, Clark RW, Filla MS and Peters
DM: NFATc1 activity regulates the expression of myocilin induced by
dexamethasone. Exp Eye Res. 130:9–16. 2015. View Article : Google Scholar
|
|
12
|
Qiu Y, Shen X, Shyam R, Yue BY and Ying H:
Cellular processing of myocilin. PLoS One. 9:928452014. View Article : Google Scholar
|
|
13
|
Gupta V, Somarajan BI, Gupta S, Chaurasia
AK, Kumar S, Dutta P, Gupta V, Sharma A, Tayo BO and Nischal K: The
inheritance of juvenile onset primary open angle glaucoma. Clin
Genet. 92:134–142. 2017. View Article : Google Scholar
|
|
14
|
Mauri L, Uebe S, Sticht H, Vossmerbaeumer
U, Weisschuh N, Manfredini E, Maselli E, Patrosso M, Weinreb RN,
Penco S, et al: Expanding the clinical spectrum of COL1A1 mutations
in different forms of glaucoma. Orphanet J Rare Dis. 11:1082016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang C, Xie L, Wu Z, Cao Y, Zheng Y, Pang
CP and Zhang M: Detection of mutations in MYOC OPTN NTF4 WDR36 and
CYP1B1 in Chinese juvenile onset open-angle glaucoma using exome
sequencing. Sci Rep. 8:4498–4505. 2018. View Article : Google Scholar
|
|
16
|
Wiggs JL and Vollrath D: Molecular and
clinical evaluation of a patient hemizygous for TIGR/MYOC. Arch
Ophthalmol. 119:1674–1678. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gupta V, Ganesan VL, Kumar S, Chaurasia
AK, Malhotra S and Gupta S: Visual disability among juvenile
open-angle glaucoma patients. J Glaucoma. 27:e87-e892018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Borrás T: The effects of myocilin
expression on functionally relevant trabecular meshwork genes: A
mini-review. J Ocul Pharmacol Ther. 30:202–212. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Y and Vollrath D: Reversal of mutant
myocilin non-secretion and cell killing: Implications for glaucoma.
Hum Mol Genet. 13:1193–1204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hernandez H, Millar JC, Curry SM, Clark AF
and McDowell CM: BMP and activin membrane bound inhibitor regulates
the extracellular matrix in the trabecular meshwork. Invest
Ophthalmol Vis Sci. 59:2154–2166. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jain A, Zode G, Kasetti RB, Ran FA, Yan W,
Sharma TP, Bugge K, Searby CC, Fingert JH, Zhang F, et al:
CRISPR-Cas9-based treatment of myocilin-associated glaucoma. Proc
Natl Acad Sci USA. 114:11199–11204. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim JH and Caprioli J: Intraocular
pressure fluctuation: Is it important. J Ophthalmic Vis Res.
13:170–174. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aroca-Aguilar JD, Sánchez-Sánchez F, Ghosh
S, Coca-Prados M and Escribano J: Myocilin mutations causing
glaucoma inhibit the intracellular endoproteolytic cleavage of
myocilin between amino acids Arg226 and Ile227. J Biol Chem.
280:21043–21051. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang L, Eaves S, Dhillon N and Ranjit P:
Postoperative outcomes following trabeculectomy and nonpenetrating
surgical procedures: A 5-year longitudinal study. Clin Ophthalmol.
12:995–1002. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Gao Y, Hill SE, Huard DJE, Tomlin
MO, Lieberman RL, Paravastu AK and Hall CK: Simulations and
experiments delineate amyloid fibrilization by peptides derived
from glaucoma-associated myocilin. J Phys Chem B. 122:5845–5850.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hewitt AW, Mackey DA and Craig JE:
Myocilin myocilin allele-specific glaucoma phenotype database. Hum
Mutat. 29:207–211. 2008. View Article : Google Scholar
|
|
27
|
Stothert AR, Fontaine SN, Sabbagh JJ and
Dickey CA: Targeting the ER-autophagy system in the trabecular
meshwork to treat glaucoma. Exp Eye Res. 144:38–45. 2016.
View Article : Google Scholar :
|
|
28
|
Yao YH, Wang YQ, Fang WF, Zhang L, Yang JH
and Zhu YH: A recurrent G367R mutation in MYOC associated with
juvenile open angle glaucoma in a large chinese family. Int J
Ophthalmol. 11:369–374. 2018.
|
|
29
|
Souzeau E, Burdon KP, Ridge B, Dubowsky A,
Ruddle JB and Craig JE: A novel de novo myocilin variant in a
patient with sporadic juvenile open angle glaucoma. BMC Med Genet.
17:302016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang F, Li Y, Lan L, Li B, Lin L, Lu X and
Li J: Ser341 Pro MYOC gene mutation in a family with primary
open-angle glaucoma. Int J Mol Med. 35:1230–1236. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang Y, Shi Y, Huang X, Li X, Ye Z, Shuai
P, Qu C, Chen R, Xu J, Yang Z, et al: Identification of a novel
MYOC mutation in a Chinese family with primary open-angle glaucoma.
Gene. 571:188–193. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zadoo S, Nguyen A, Zode G and Hulleman JD:
A novel luciferase assay for sensitively monitoring myocilin
variants in cell culture. Invest Ophthalmol Vis Sci. 57:1939–1950.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aroca-Aguilar JD, Martínez-Redondo F,
Sánchez-Sánchez F, Coca-Prados M and Escribano J: Functional role
of proteolytic processing of recombinant myocilin in
self-aggregation. Invest Ophthalmol Vis Sci. 51:72–78. 2010.
View Article : Google Scholar :
|
|
34
|
Aroca-Aguilar JD, Sánchez-Sánchez F, Ghosh
S, Fernández-Navarro A, Coca-Prados M and Escribano J: Interaction
of recombinant myocilin with the matricellular protein SPARC:
Functional implications. Invest Ophthalmol Vis Sci. 52:179–189.
2011. View Article : Google Scholar :
|
|
35
|
Resch ZT and Fautsch MP:
Glaucoma-associated myocilin: A better understanding but much more
to learn. Exp Eye Res. 88:704–712. 2009. View Article : Google Scholar :
|
|
36
|
Caballero M, Rowlette LL and Borras T:
Altered secretion of a TIGR/MYOC mutant lacking the olfactomedin
domain. Biochim Biophys Acta. 1502:447–460. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gobeil S, Letartre L and Raymond V:
Functional analysis of the glaucoma-causing TIGR/myocilin protein:
Integrity of amino-terminal coiled-coil regions and olfactomedin
homology domain is essential for extracellular adhesion and
secretion. Exp Eye Res. 82:1017–1029. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou T, Souzeau E, Sharma S, Landers J,
Mills R, Goldberg I, Healey PR, Graham S, Hewitt AW, Mackey DA, et
al: Whole exome sequencing implicates eye development the unfolded
protein response and plasma membrane homeostasis in primary
open-angle glaucoma. PLoS One. 12:e01724272017. View Article : Google Scholar
|
|
39
|
Goldwich A, Ethier CR, Chan DW and Tamm
ER: Perfusion with the olfactomedin domain of myocilin does not
affect outflow facility. Invest Ophthalmol Vis Sci. 44:1953–1961.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sánchez-Sánchez F, Martínez-Redondo F,
Aroca-Aguilar JD, Coca-Prados M and Escribano J: Characterization
of the intracellular proteolytic cleavage of myocilin and
identification of calpain II as a myocilin-processing protease. J
Biol Chem. 282:27810–27824. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jacobson N, Andrews M, Shepard AR,
Nishimura D, Searby C, Fingert JH, Hageman G, Mullins R, Davidson
BL, Kwon YH, et al: Non-secretion of mutant proteins of the
glaucoma gene myocilin in cultured trabecular meshwork cells and in
aqueous humor. Hum Mol Genet. 10:117–125. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Aroca-Aguilar JD, Sánchez-Sánchez F,
Martínez-Redondo F, Coca-Prados M and Escribano J: Heterozygous
expression of myocilin glaucoma mutants increases secretion of the
mutant forms and reduces extracellular processed myocilin. Mol Vis.
14:2097–2108. 2008.PubMed/NCBI
|
|
43
|
Hood JL, Brooks WH and Roszman TL:
Differential compart-mentalization of the calpain/calpastatin
network with the endoplasmic reticulum and Golgi apparatus. J Biol
Chem. 279:43126–43135. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wei YT, Li YQ, Bai YJ, Wang M, Chen JH, Ge
J and Zhuo YH: Pro370Leu myocilin mutation in a chinese pedigree
with juvenile-onset open angle glaucoma. Mol Vis. 17:1449–1456.
2011.PubMed/NCBI
|
|
45
|
Ueda J, Wentz-Hunter K and Yue BY:
Distribution of myocilin and extracellular matrix components in the
juxtacanalicular tissue of human eyes. Invest Ophthalmol Vis Sci.
43:1068–1076. 2002.PubMed/NCBI
|
|
46
|
Li Y, Aroca-Aguilar JD, Ghosh S,
Sánchez-Sánchez F, Escribano J and Coca-Prados M: Interaction of
myocilin with the C-terminal region of hevin. Biochem Biophys Res
Commun. 339:797–804. 2006. View Article : Google Scholar
|
|
47
|
Torrado M, Trivedi R, Zinovieva R,
Karavanova I and Tomarev SI: Optimedin: A novel
olfactomedin-related protein that interacts with myocilin. Hum Mol
Genet. 11:1291–1301. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Filla MS, Liu X, Nguyen TD, Polansky JR,
Brandt CR, Kaufman PL and Peters DM: In vitro localization of
TIGR/MYOC in trabecular meshwork extracellular matrix and binding
to fibro-nectin. Invest Ophthalmol Vis Sci. 43:151–161.
2002.PubMed/NCBI
|
|
49
|
Fautsch MP, Vrabel AM and Johnson DH: The
identification of myocilin-associated proteins in the human
trabecular meshwork. Exp Eye Res. 82:1046–1052. 2006. View Article : Google Scholar
|
|
50
|
Joe MK, Lieberman RL, Nakaya N and Tomarev
SI: Myocilin regulates metalloprotease 2 activity through
interaction with TIMP3. Invest Ophthalmol Vis Sci. 58:5308–5318.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Patel GC, Phan TN, Maddineni P, Kasetti
RB, Millar JC, Clark AF and Zode GS: Dexamethasone-induced ocular
hypertension in mice: Effects of myocilin and route of
administration. Am J Pathol. 187:713–723. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li G, Cui G, Dismuke WM, Navarro I,
Perkumas K, Woodward DF and Stamer WD: Differential response and
withdrawal profile of glucocorticoid-treated human trabecular
meshwork cells. Exp Eye Res. 155:38–46. 2017. View Article : Google Scholar :
|
|
53
|
Webber HC, Bermudez JY, Sethi A, Clark AF
and Mao W: Crosstalk between TGFβ and Wnt signaling pathways in the
human trabecular meshwork. Exp Eye Res. 148:97–102. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Raghunathan VK, Morgan JT, Park SA, Weber
D, Phinney BS, Murphy CJ and Russell P: Dexamethasone stiffens
trabecular meshwork trabecular meshwork cells and matrix. Invest
Ophthalmol Vis Sci. 56:4447–4459. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nguyen TD, Chen P, Huang WD, Chen H,
Johnson D and Polansky JR: Gene structure and properties of
myocilin an olfactomedin-related glycoprotein cloned from
glucocorticoid-induced trabecular meshwork cells. J Biol Chem.
273:6341–6350. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Agrahari V, Li G, Agrahari V, Navarro I,
Perkumas K, Mandal A, Stamer WD and Mitra AK: Pentablock copolymer
dexamethasone nanoformulations elevate MYOC: In vitro liberation,
activity and safety in human trabecular meshwork cells.
Nanomedicine (Lond). 12:1911–1926. 2017. View Article : Google Scholar
|
|
57
|
Prat C, Belville C, Comptour A, Marceau G,
Clairefond G, Chiambaretta F, Sapin V and Blanchon L: Myocilin
expression is regulated by retinoic acid in the trabecular
meshwork-derived cellular environment. Exp Eye Res. 155:91–98.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wu Y, Chen W, Guo M, He Q and Hu Y:
Effects of transforming growth factor-β2 on myocilin expression and
secretion in human primary cultured trabecular meshwork cells. Int
J Clin Exp Pathol. 7:4827–4836. 2014.
|
|
59
|
Huang X, Li M, Guo X, Li S, Xiao X, Jia X,
Liu X and Zhang Q: Mutation analysis of seven known
glaucoma-associated genes in Chinese patients with glaucoma. Invest
Ophthalmol Vis Sci. 55:3594–3602. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Park J, Kim M, Park CK, Chae H, Lee S, Kim
Y, Jang W, Chi HY, Park HY and Park SH: Molecular analysis of
myocilin and optineurin genes in Korean primary glaucoma patients.
Mol Med Rep. 14:2439–2448. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Maurya N, Agarwal NR and Ghosh I: Low-dose
rotenone exposure induces early senescence leading to late
apoptotic signaling cascade in human trabecular meshwork (HTM) cell
line: An in vitro glaucoma model. Cell Biol Int. 40:107–120. 2016.
View Article : Google Scholar
|
|
62
|
Pattabiraman PP and Rao PV: Hic-5
regulates actin cytoskeletal reorganization and expression of
fibrogenic markers and myocilin in trabecular meshwork cells.
Invest Ophthalmol Vis Sci. 56:5656–5669. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wei X, Cho KS, Thee EF, Jager MJ and Chen
DF: Neuroimmflammation and microglia in glaucoma: Time for a
paradigm shift. J Neurosci Res. 2018.Epub ahead of print.
|
|
64
|
Wareham LK, Buys ES and Sappington RM: The
nitric oxide-guanylate cyclase pathway and glaucoma. Nitric Oxide.
77:75–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Michelessi M, Bicket AK and Lindsley K:
Cyclodestructive procedures for non-refractory glaucoma. Cochrane
Database Syst Rev. 4:CD0093132018.PubMed/NCBI
|
|
66
|
Stamer WD and Acott TS: Current
understanding of conventional outflow dysfunction in glaucoma. Curr
Opin Ophthalmol. 23:135–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kim BS, Savinova OV, Reedy MV, Martin J,
Lun Y, Gan L, Smith RS, Tomarev SI, John SW and Johnson RL:
Targeted disruption of the myocilin gene (Myoc) suggests that human
glaucoma-causing mutations are gain of function. Mol Cell Biol.
21:7707–7713. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Acott TS, Kelley MJ, Keller KE, Vranka JA,
Abu-Hassan DW, Li X, Aga M and Bradley JM: Intraocular pressure
homeostasis: Maintaining balance in a high-pressure environment. J
Ocul Pharmacol Ther. 30:94–101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Fautsch MP, Bahler CK, Jewison DJ and
Johnson DH: Recombinant TIGR/MYOC increases outflow resistance in
the human anterior segment. Invest Ophthalmol Vis Sci.
41:4163–4168. 2000.PubMed/NCBI
|
|
70
|
Patel GC, Liu Y, Millar JC and Clark AF:
Glucocorticoid receptor GRβ regulates glucocorticoid-induced ocular
hypertension in mice. Sci Rep. 8:8622018. View Article : Google Scholar
|
|
71
|
Faralli JA, Dimeo KD, Trane RM and Peters
D: Absence of a secondary glucocorticoid response in C57BL/6J mice
treated with topical dexamethasone. PLoS One. 13:e01926652018.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Nazir S, Mukhtar M, Shahnawaz M, Farooqi
S, Fatima N, Mehmood R and Sheikh N: A novel single nucleotide
polymorphism in exon 3 of MYOC enhances the risk of glaucoma. PLoS
One. 13:e01951572018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Shepard AR, Jacobson N, Millar JC, Pang
IH, Steely HT, Searby CC, Sheffield VC, Stone EM and Clark AF:
Glaucoma-causing myocilin mutants require the Peroxisomal targeting
signal-1 receptor (PTS1R) to elevate intraocular pressure. Hum Mol
Genet. 16:609–617. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Guan Y, Li J, Zhan T, Wang JW, Yu JB and
Yang L: Idebenone maintains survival of mutant myocilin cells by
inhibiting apoptosis. Chin Med J (Engl). 129:2001–2004. 2016.
View Article : Google Scholar
|
|
75
|
Nag A, Lu H, Arno M, Iglesias AI,
Bonnemaijer P, Broer L, Uitterlinden AG, Klaver CC, van Duijn C,
Hysi PG and Hammond CJ: Evaluation of the myocilin mutation
gln368stop demonstrates reduced penetrance for glaucoma in european
populations. Ophthalmology. 124:547–553. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lam DS, Leung YF, Chua JK, Baum L, Fan DS,
Choy KW and Pang CP: Truncations in the TIGR gene in individuals
with and without primary open-angle glaucoma. Invest Ophthalmol Vis
Sci. 41:1386–1391. 2000.PubMed/NCBI
|
|
77
|
Morissette J, Clépet C, Moisan S, Dubois
S, Winstall E, Vermeeren D, Nguyen TD, Polansky JR, Côté G, Anctil
JL, et al: Homozygotes carrying an autosomal dominant TIGR mutation
do not manifest glaucoma. Nat Genet. 19:319–321. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kuchtey J, Chowdhury UR, Uptegraft CC,
Fautsch MP and Kuchtey RW: A de novo MYOC mutation detected in
juvenile open angle glaucoma causes non-secretion of associated
with reduced myocilin protein in aqueous humor. Eur J Med Genet.
56:292–296. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Huard DJE, Crowley VM, Du Y, Cordova RA,
Sun Z, Tomlin MO, Dickey CA, Koren J III, Blair L, Fu H, et al:
Trifunctional high-throughput screen identifies promising scaffold
to inhibit Grp94 and treat myocilin-associated glaucoma. ACS Chem
Biol. 13:933–941. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Stothert AR, Suntharalingam A, Huard DJ,
Fontaine SN, Crowley VM, Mishra S, Blagg BS, Lieberman RL and
Dickey CA: Exploiting the interaction between Grp94 and aggregated
myocilin to treat glaucoma. Hum Mol Genet. 23:6470–6480. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Caballero M and Borras T: Inefficient
processing of an olfactomedindeficient myocilin mutant: Potential
physiological relevance to glaucoma. Biochem Biophys Res Commun.
282:662–670. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Vollrath D and Liu Y: Temperature
sensitive secretion of mutant myocilins. Exp Eye Res. 82:1030–1036.
2006. View Article : Google Scholar
|
|
83
|
Yam GH, Gaplovska-Kysela K, Zuber C and
Roth J: Aggregated myocilin induces russell bodies and causes
apoptosis: Implications for the pathogenesis of myocilin-caused
primary open-angle glaucoma. Am J Pathol. 170:100–109. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Joe MK, Sohn S, Hur W, Moon Y, Choi YR and
Kee C: Accumulation of mutant myocilins in ER leads to ER stress
and potential cytotoxicity in human trabecular meshwork cells.
Biochem Biophys Res Commun. 312:592–600. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Gould DB, Miceli-Libby L, Savinova OV,
Torrado M, Tomarev SI, Smith RS and John SW: Genetically increasing
Myoc expression supports a necessary pathologic role of abnormal
proteins in glaucoma. Mol Cell Biol. 24:9019–9025. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Joe MK, Nakaya N, Abu-Asab M and Tomarev
SI: Mutated myocilin and heterozygous Sod2 deficiency act
synergistically in a mouse model of open-angle glaucoma. Hum Mol
Genet. 24:3322–3334. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Joe MK and Tomarev SI: Expression of
myocilin mutants sensitizes cells to oxidative stress-induced
apoptosis: Implication for glaucoma pathogenesis. Am J Pathol.
176:2880–2890. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Hill SE and Donegan RK: The
glaucoma-associated olfactomedin domain of myocilin forms
polymorphic fibrils that are constrained by partial unfolding and
peptide sequence. J Mol Biol. 426:921–935. 2014. View Article : Google Scholar :
|
|
89
|
Zode GS, Kuehn MH, Nishimura DY, Searby
CC, Mohan K, Grozdanic SD, Bugge K, Anderson MG, Clark AF, Stone EM
and Sheffield VC: Reduction of ER stress via a chemical chaperone
prevents disease phenotypes in a mouse model of primary open angle
glaucoma. J Clin Invest. 121:3542–3553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Maddineni P, Kasetti RB and Zode GS:
Methods for analyzing endoplasmic reticulum stress in the
trabecular meshwork of glaucoma models. Methods Mol Biol.
1695:121–134. 2018. View Article : Google Scholar
|
|
91
|
Chong WC, Shastri MD and Eri R:
Endoplasmic reticulum stress and oxidative stress: A vicious nexus
implicated in bowel disease pathophysiology. Int J Mol Sci.
18:E7712017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Plaisance V, Brajkovic S, Tenenbaum M,
Favre D, Ezanno H, Bonnefond A, Bonner C, Gmyr V, Kerr-Conte J,
Gauthier BR, et al: Endoplasmic reticulum stress links oxidative
stress to impaired pancreatic beta-cell function caused by human
oxidized LDL. PLoS One. 11:e01630462016. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhao J, Wang S, Zhong W, Yang B, Sun L and
Zheng Y: Oxidative stress in the trabecular meshwork (Review). Int
J Mol Med. 38:995–1002. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Grootjans J, Kaser A, Kaufman RJ and
Blumberg RS: The unfolded protein response in immunity and
inflammation. Nat Rev Immunol. 16:469–484. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Luo K and Cao SS: Endoplasmic reticulum
stress in intestinal epithelial cell function and inflammatory
bowel disease. Gastroenterol Res Pract. 2015:3287912015. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Peters JC, Bhattacharya S, Clark AF and
Zode GS: Increased endoplasmic reticulum stress in human
glaucomatous trabecular meshwork cells and tissues. Invest
Ophthalmol Vis Sci. 56:3860–3868. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Huard DJ and Lieberman RL: Progress toward
development of a proteostasis drug for myocilin-associated
glaucoma. Future Med Chem. 10:1391–1393. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Mishra SJ, Ghosh S, Stothert AR, Dickey CA
and Blagg BS: Transformation of the non-selective
aminocyclohexanol-based Hsp90 inhibitor into a Grp94-seletive
scaffold. ACS Chem Biol. 12:244–253. 2017. View Article : Google Scholar :
|
|
99
|
Crowley VM, Khandelwal A, Mishra S,
Stothert AR, Huard DJ, Zhao J, Muth A, Duerfeldt AS, Kizziah JL,
Lieberman RL, et al: Development of glucose regulated protein
94-selective inhibitors based on the BnIm and radamide scaffold. J
Med Chem. 59:3471–3488. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Stothert AR, Suntharalingam A, Tang X,
Crowley VM, Mishra SJ, Webster JM, Nordhues BA, Huard DJE,
Passaglia CL, Lieberman RL, et al: Isoform-selective Hsp90
inhibition rescues model of hereditary open-angle glaucoma. Sci
Rep. 7:179512017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Keller KE and Wirtz MK: Working your SOCS
off: The role of ASB10 and protein degradation pathways in
glaucoma. Exp Eye Res. 158:154–160. 2017. View Article : Google Scholar
|
|
102
|
Wang N, Chintala SK, Fini ME and Schuman
JS: Activation of a tissue-specific stress response in the aqueous
outflow pathway of the eye defines the glaucoma disease phenotype.
Nat Med. 7:304–309. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
103
|
Yerramothu P, Vijay AK and Willcox MDP:
Inflammasomes the eye and anti-inflammasome therapy. Eye (Lond).
32:491–505. 2018. View Article : Google Scholar
|
|
104
|
Meier-Soelch J, Jurida L, Weber A, Newel
D, Kim J, Braun T, Schmitz ML and Kracht M: RNAi-based
identification of gene-specific nuclear cofactor networks
regulating interleukin-1 target genes. Front Immunol. 9:7752018.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Yasuda M, Takayama K, Kanda T, Taguchi M,
Someya H and Takeuchi M: Comparison of intraocular
pressure-lowering effects of ripasudil hydrochloride hydrate for
inflammatory and corticosteroid-induced ocular hypertension. PLoS
One. 12:e01853052017. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Itakura T, Peters DM and Fini ME:
Glaucomatous MYOC mutations activate the IL-1/NF-κB inflammatory
stress response and the glaucoma marker SELE in trabecular meshwork
cells. Mol Vis. 21:1071–1084. 2015.
|
|
107
|
Kasetti RB, Phan TN, Millar JC and Zode
GS: Expression of mutant myocilin induces abnormal intracellular
accumulation of selected extracellular matrix proteins in the
trabecular meshwork. Invest Ophthalmol Vis Sci. 57:6058–6069. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Donegan RK, Hill SE, Freeman DM, Nguyen E,
Orwig SD, Turnage KC and Lieberman RL: Structural basis for
misfolding in myocilin-associated glaucoma. Hum Mol Genet.
24:2111–2124. 2015. View Article : Google Scholar :
|
|
109
|
Joe MK, Sohn S, Choi YR, Park H and Kee C:
Identification of flotillin-1 as a protein interacting with
myocilin: Implications for the pathogenesis of primary open-angle
glaucoma. Biochem Biophys Res Commun. 336:1201–1206. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Joe MK, Kwon HS, Cojocaru R and Tomarev
SI: Myocilin regulates cell proliferation and survival. J Biol
Chem. 289:10155–10167. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Kessenbrock K, Wang CY and Werb Z: Matrix
metalloproteinases in stem cell regulation and cancer. Matrix Biol.
46:184–190. 2015. View Article : Google Scholar
|
|
112
|
De Groef L, Van Hove I, Dekeyster E,
Stalmans I and Moons L: MMPs in the neuroretina and optic nerve:
Modulators of glaucoma pathogenesis and repair. Invest Ophthalmol
Vis Sci. 55:1953–1964. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Ashworth Briggs EL, Toh T, Eri R, Hewitt
AW and Cook AL: TIMP1 TIMP2 and TIMP4 are increased in aqueous
humor from primary open angle glaucoma patients. Mol Vis.
21:1162–1172. 2015.
|
|
114
|
Filla MS, Dimeo KD, Tong T and Peters DM:
Disruption of fibronectin matrix affects type IV collagen fibrillin
and laminin deposition into extracellular matrix of human
trabecular meshwork (HTM) cells. Exp Eye Res. 165:7–19. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Zode GS, Sharma AB, Lin X, Searby CC,
Bugge K, Kim GH, Clark AF and Sheffield VC: Ocular-specific ER
stress reduction rescues glaucoma in murine glucocorticoid-induced
glaucoma. J Clin Invest. 124:1956–1965. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Ho H, Htoon HM, Yam GH, Toh LZ, Lwin NC,
Chu S, Lee YS, Wong TT and Seet LF: Altered anterior segment
biometric parameters in mice deficient in SPARC. Invest Ophthalmol
Vis Sci. 58:386–393. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Hill SE, Nguyen E, Donegan RK,
Patterson-Orazem AC, Hazel A, Gumbart JC and Lieberman RL:
Structure and misfolding of the flexible tripartite coiled-coil
domain of glaucoma-associated myocilin. Structure. 25:1697–1707.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Burns JN, Turnage KC, Walker CA and
Lieberman RL: The stability of myocilin olfactomedin domain
variants provides new insight into glaucoma as a protein misfolding
disorder. Biochemistry. 50:5824–5833. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Burns JN, Orwig SD, Harris JL, Watkins JD,
Vollrath D and Lieberman RL: Rescue of glaucoma-causing mutant
myocilin thermal stability by chemical chaperones. ACS Chem Biol.
5:477–487. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Honda R: Role of the disulfide bond in
prion protein amyloid formation: A thermodynamic and kinetic
analysis. Biophys J. 114:885–892. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Fingert JH, Héon E, Liebmann JM, Yamamoto
T, Craig JE, Rait J, Kawase K, Hoh ST, Buys YM, Dickinson J, et al:
Analysis of myocilin mutations in 1703 glaucoma patients from five
different populations. Hum Mol Genet. 8:899–905. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Shimizu S, Lichter PR, Johnson AT, Zhou Z,
Higashi M, Gottfredsdottir M, Othman M, Moroi SE, Rozsa FW,
Schertzer RM, et al: Age-dependent prevalence of mutations at the
GLC1A locus in primary open-angle glaucoma. Am J Ophthalmol.
130:165–177. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Millá E, Mañé B, Duch S, Hernan I, Borràs
E, Planas E, Dias Mde S, Carballo M and Gamundi MJ; Spanish
Multicenter Glaucoma Group-Estudio Multicéntrico Español de
Investigación Genética del Glaucoma, EMEIGG: Survey of familial
glaucoma shows a high incidence of cytochrome P450 family 1
subfamily B polypeptide 1 (CYP1B1) mutations in non-consanguineous
congenital forms in a Spanish population. Mol Vis. 19:1707–1722.
2013.
|