Introduction
Cardiovascular disease is currently the leading
cause of human mortality worldwide and represents a major detriment
to human health (1). It is widely
acknowledged that the pathological process of Atherosclerosis (AS)
is one of the main causes of the occurrence, evolution and
deterioration of AS (1). Further
investigation of biomarkers that are directly associated with the
pathogenesis of AS and acute myocardial infarction is required. In
this way, a more accurate basis for the diagnosis of AS, the
evaluation of disease severity and prognostic prediction may be
provided (2).
Oxidative stress is associated with the pathogenesis
of multiple cardiovascular diseases. Oxidative stress serves a key
function in the pathophysiology of diseases including hypertension
and AS by causing endothelial dysfunction (3). Clinical studies have identified that
risk factors leading to AS exert adverse effects on the number and
function of endothe-lial progenitor cells, limiting the capability
for angiogenesis in patients (4).
Oxidative stress has been identified to be associated with the
pathogenesis of AS (4).
Nuclear factor erythroid 2-related factor 2 (Nrf2)
is an important transcription factor of the antioxidant stress
pathway (5). Under normal
physiological conditions, Nrf2 is stably bound to Kelch-like
enoyl-CoA hydratase-associated protein 1 (Keap1) and is stabilized
in the cytoplasm by Keap1 (5).
The bound Nrf2 cannot translocate into the nucleus, and neither is
it able to exert its unique transcriptional activity (6). Under stress, Keap1 can be decoupled
from Nrf2, and unbound Nrf2 is then translocated into the nucleus
and bind to the antioxidant element heme oxygenase 1 (HO-1)
(6). This process, to a certain
extent, enhances the gene expression of its downstream enzymes
(5). Among them, the most typical
include ubiquitin, phase II detoxification enzymes, antioxidant
proteins and proteasomes, expression of which further increases the
ability of cells to resist oxidative stress (7).
Cardiovascular disease is a major group of diseases
that are detrimental to human health. According to its
pathogenesis, cardiovascular disease may be discussed and analyzed
with regard to various aspects, including oxidative stress,
inflammatory reaction, apoptosis and autophagy, myocardial
ischemia-reperfusion and aging, and energy restrictions (8). However, it has been identified that
sirtuin family members are associated with the occurrence and
development of pathogenesis (8).
In addition, sirtuin family members exhibited a marked association
with risk factors of cardiovascular diseases, including glucose and
lipid metabolism (9). The sirtuin
family is a group of regulators of silencing information, which
includes seven members, namely Sirt1-Sirt7 (10). However, it has been demonstrated
that Sirt2 serves a similar or opposite function in cardiovascular
disease and its pathogenesis, and the biological function of Sirt2
in oxidative stress has been reported recently (10). Owing to the increased levels of
hydrogen peroxide, oxidative stress increases the expression of
Sirt2 in NIH3T3 cells (10).
MicroRNAs (miRNAs) are a class of endogenous small
non-coding RNAs widely distributed in eukaryotic organisms, which
specifically recognize target mRNAs (11). miRNAs serve a regulatory function
at the post-transcriptional level by modulating the translation and
degradation of target gene mRNAs (12). There are ~2,000 types of miRNA in
the human genome, which regulate ~30% of human gene expression
(12). miRNAs have been
identified to be markedly stable in the serum, plasma, urine and
other bodily fluids through the protection of endogenous RNases.
Previous studies have identified the existence of various specific
miRNAs in the cardiovascular system (12,13). miRNAs have been identified to
serve an important function in multiple pathophysiological
processes of the cardiovascular system, including regulation of
cardiac development, modulation of cardiac function and involvement
in myocardial cell apoptosis (12,13). In addition, miRNAs have also been
identified to be involved in the major pathological process of
coronary AS (13). These results
indicate that miRNAs may be novel biomarkers for the early
diagnosis, treatment and prognostic prediction of cardiovascular
diseases (14). They may be used
to further evaluate the degree of AS. Therefore, we hypothesize
that, in the future, manipulation of miRNA expression may be used
to suppress the progression of coronary AS, which provides a novel
approach for the treatment of cardiovascular diseases (14). In the present study, the function
of microRNA (miR)-140-5p on hypertension and oxidative stress of AS
was investigated.
Materials and methods
Animals and ethical approval
Wild-type (Wt; male C57BL/6 mice, n=6) and
apolipoprotein E (ApoE)−/− mice (male, n=6) were
purchased from Beijing Vital River Laboratory Animal Technology
Co., Ltd. (Beijing, China). Mice were housed at 22-23°C, 55-60%
humidity, 12-h light/12-h dark cycle, with free access to food and
water. Mice were switched to a high-fat diet (21% fat and 0.15%
cholesterol) for 16 weeks. The study protocols were approved by the
Ethics Committee of The Ninth People's Hospital (Shanghai Jiaotong
University School of Medicine, Shanghai, China). Wt C57BL/6 mice
were the control group and ApoE−/− mice were the AS
group.
Microarray assay
RNA (500 ng) was hybridized to Affymetrix HG-U133
Plus 2.0 GeneChip arrays (containing 54,675 probe sets). Data were
analyzed using Ingenuity Pathway Analysis (Qiagen, Inc., Valencia,
CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
RNA samples were obtained from blood vessel tissue
samples or cells using TransZol® (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocol. cDNA was synthesized from 1 µg
total RNA using a ReverTra Ace® qPCR RT master mix kit
(Toyobo Life Science, Osaka, Japan), according to the
manufacturer's protocol. qPCR was performed using SYBR-Green PCR
master mix-plus (Toyobo Life Sciences) on a 7500 real-time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.).
Thermocycling conditions were: 94°C for 10 min, and 40 cycles of
94°C for 30 sec, 57°C for 30 sec and 72°C for 30 sec. Primers for
miR-140-5p were 5'-CAG UGG UUU UAC CCU AUG GUA G-3' (forward) and
5'-ACC ACA GGG UAG AAC ACG GAC-3' (reverse); and for U6, used as a
reference gene, were 5'-GCT TCG GCA GCA CAT ATA CTA AAA T-3'
(forward) and 5'-CGC TTC ACG AAT TTG CGT GTC AT-3' (reverse). The
relative gene expression was analyzed using the 2−ΔΔCq
method (15).
Hematoxylin and eosin (H&E)
staining
Following induction of the AS model, aorta tissue
was collected and washed with PBS. Tissue samples were fixed with
4% paraformaldehyde for 24 h and embedded in paraffin, and the
5-µm-thick sections were stained with H&E for 15 min at
room temperature. Images of cells were captured under fluorescence
microscopy (magnification, ×100).
Cell culture and transfection
Human umbilical vein endothelial cells (HUVECs) were
obtained from the Shanghai Cell Bank of the Chinese Academy of
Sciences (Shanghai, China) and were maintained in Dulbecco's
modified Eagle's medium supplemented with 10% fetal bovine serum in
a humidified atmosphere containing 5% CO2 at 37°C.
miR-140-5p, anti-miR-140-5p and negative mimic were purchased from
Guangzhou RiboBio Co., Ltd. (Guangzhou, China) and transfected into
cells using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). Following transfection for 24 h, cells
were stimulated with 70 µg/ml oxidized low-density
lipoprotein (ox-LDL) for 24 h. Next, following transfection for 4
h, cells were treated with 10 µM SRT 1720 hydrochloride
(Sirt2 agonist; MedChemExpress, Monmouth Junction, NJ, USA), 1
µM NK-252 (Nrf2 agonist; MedChemExpress) and 5 µM
Troxerutin [reactive oxygen species (ROS) inhibitor;
MedChemExpress] for 20 h, and cells were stimulated with 70
µg/ml ox-LDL for 24 h.
Oxidative stress and ROS measurement
Following induction of the AS model for 16 weeks,
mice were sacrificed by decapitation following anesthesia with 35
mg/kg pentobarbital sodium, and serum was collected at 2,000 × g
for 10 min at 4°C. Serum was used to determine malondialdehyde
(MDA), superoxide dismutase (SOD), glutathione (GSH) and
glutathione peroxidase (GSH-Px) levels using ELISA kits. Next,
cells were collected at 1,000 × g for 10 min at 4°C and ROS (cat.
no. E004), MDA (cat. no. A003-1), SOD (cat. no. A001-1-1), GSH
(cat. no. A006-2) and GSH-Px (cat. no. A005) levels were determined
using ELISA kits (Nanjing Jiancheng Biological Engineering
Institute, Nanjing, China). In addition, ROS levels were determined
in cells using 2',7'-dichlorodihydrofluorescein diacetate (10 mM)
for 30 min at room temperature and cells were observed using
fluorescence microscopy (magnification, ×200).
Dual-luciferase reporter assay
The plasmids containing the wild-type Nrf2,
wild-type Sirt2, mutant Nrf2, mutant Sirt2 and miR-140-5p mimic
were purchased from Guangzhou RiboBio Co., Ltd. These were
co-transfected into HUVECs using Lipofectamine 2000 24 h after
transfection. Luciferase activity was determined using a
Dual-Luciferase Reporter assay kit (Promega Corporation, Madison,
WI, USA).
Western blot analysis
Cells were homogenized using
radioimmunoprecipitation assay lysis buffer and the protein
concentrations of the samples were determined using a bicinchoninic
acid protein assay kit (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The proteins (50 µg/lane) were separated by SDS-PAGE
(10% gel) and transferred onto polyvinylidene difluoride membranes
(EMD Millipore, Billerica, MA, USA). Membranes were blocked with 5%
non-fat milk in Tris-buffered saline for 2 h at room temperature
and were incubated with primary antibodies: Anti-Nrf2 (cat. no.
12721; 1:2,000; Cell Signaling Technology, Inc., Danvers, MA, USA),
anti-Sirt2 (cat. no. 12672; 1:2,000; Cell Signaling Technology,
Inc.) and anti-GAPDH (cat. no. 5174; 1:50,000; Cell Signaling
Technology, Inc.) at 4°C overnight. Membranes were washed with
Tris-buffered saline with 0.1% Tween-20 (TBST) for 20 min and
incubated with horseradish peroxidase-conjugated anti-rabbit
immunoglobulin (GE Healthcare, Chicago, IL, USA) at 37°C for 1 h.
Membranes were developed with enhanced chemiluminescence substrate
solution (GE Healthcare) and analyzed using Image Lab (version 3.0;
Bio-Rad Laboratories, Inc.).
Immunofluorescence assay
Cells were washed with PBS and fixed with 4%
paraformaldehyde for 15 min at room temperature. Cells were blocked
with 5% bovine serum albumin (Beyotime Institute of Biotechnology,
Haimen, China) in TBST and 0.25% Triton X-100 for 1 h at room
temperature. Cells were incubated with anti-Nrf2 and anti-Sirt2
primary antibodies at 4°C overnight. Cells were washed with goat
anti-rabbit immunoglobulin G-Cruz Fluor® 488 or 555
(1:100; cat. nos. sc-362262 and sc-362272; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) at 37°C for 1 h and stained
with DAPI for 30 min in darkness. Images of cells were captured
under fluorescence microscopy.
Statistical analysis
Results are expressed as the mean ± standard
deviation. Student's t-test was performed for statistical
significance between two groups. One-way analysis of variance and
Tukey's post hoc test were performed for parametric multivariable
analysis.
Results
miR-140-5p expression in mice with
AS
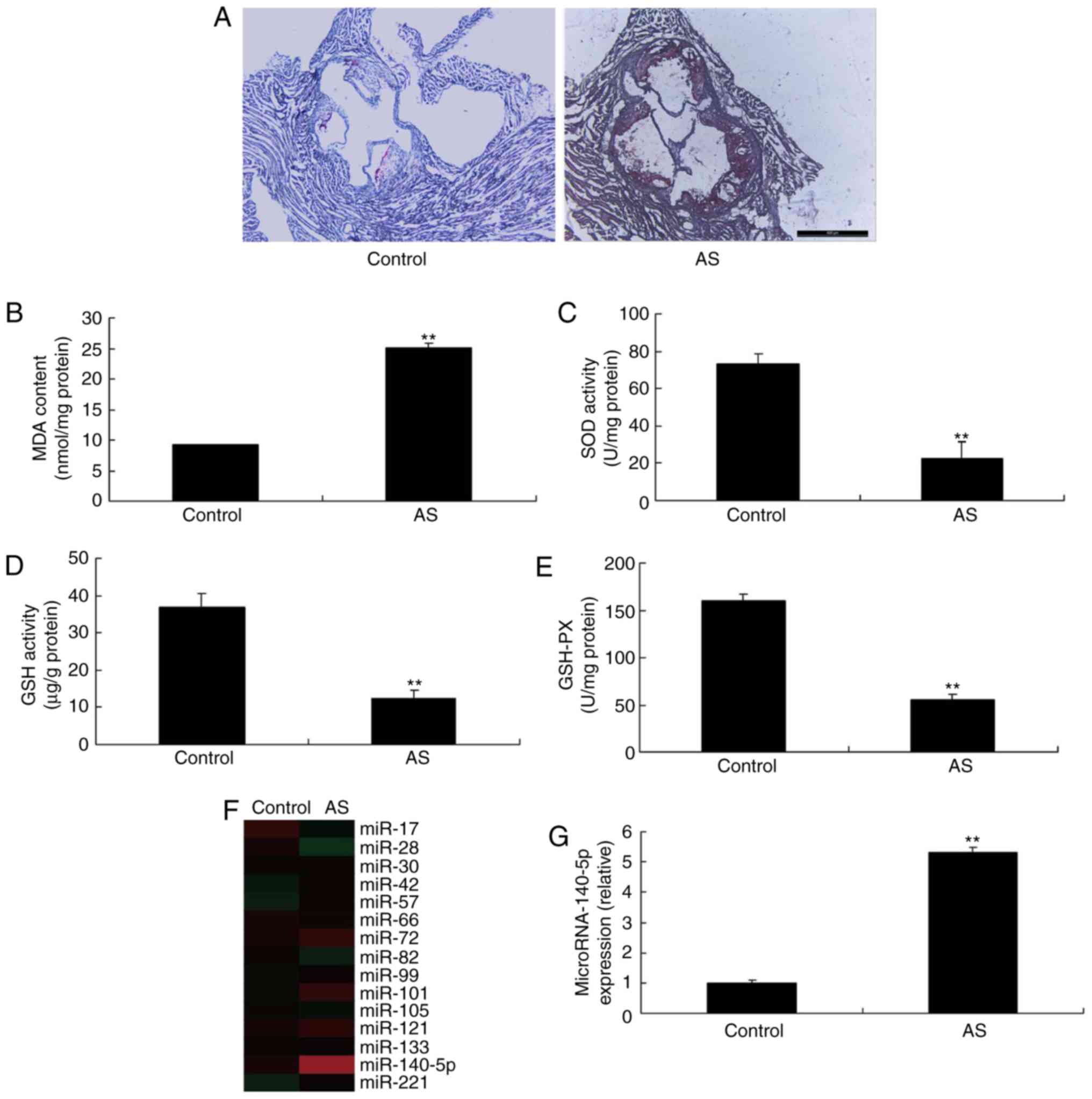
As presented in Fig.
1A, H&E staining indicated plaques in mice with AS,
compared with the control group. Furthermore, the MDA level was
increased, whereas the levels of SOD, GSH and GSH-Px were
decreased, in mice with AS, compared with the control group
(Fig. 1B-E, respectively). A
microarray assay was used to analyze the alterations in miR-140-5p
expression in mice with AS. As presented in Fig. 1F and G, miR-140-5p expression was
increased in mice with AS, compared with the control. Thus,
miR-140-5p expression may serve an important function in AS.
miR-140-5p regulates the expression of
Nrf2 and Sirt2
A microarray assay was utilized to investigate the
underlying molecular mechanism of miR-140-5p in AS. In brief,
miR-140-5p mimic was used to increase the expression of miR-140-5p
in vitro in mice with AS, compared with negative control
group (Fig. 2A). A microarray
assay and qPCR indicated that the expression of Nrf2 and Sirt2 was
inhibited in vitro by overexpression of miR-140-5p, compared
with the negative control group (Fig.
2B-D). A dual-luciferase reporter assay indicated that
miR-140-5p targeted Nrf2 and Sirt2 expression (Fig. 2E-H). In addition, an
immunofluorescence assay indicated that overexpression of
miR-140-5p suppressed the expression of Nrf2 and Sirt2 in the in
vitro model of AS, in comparison with the negative control
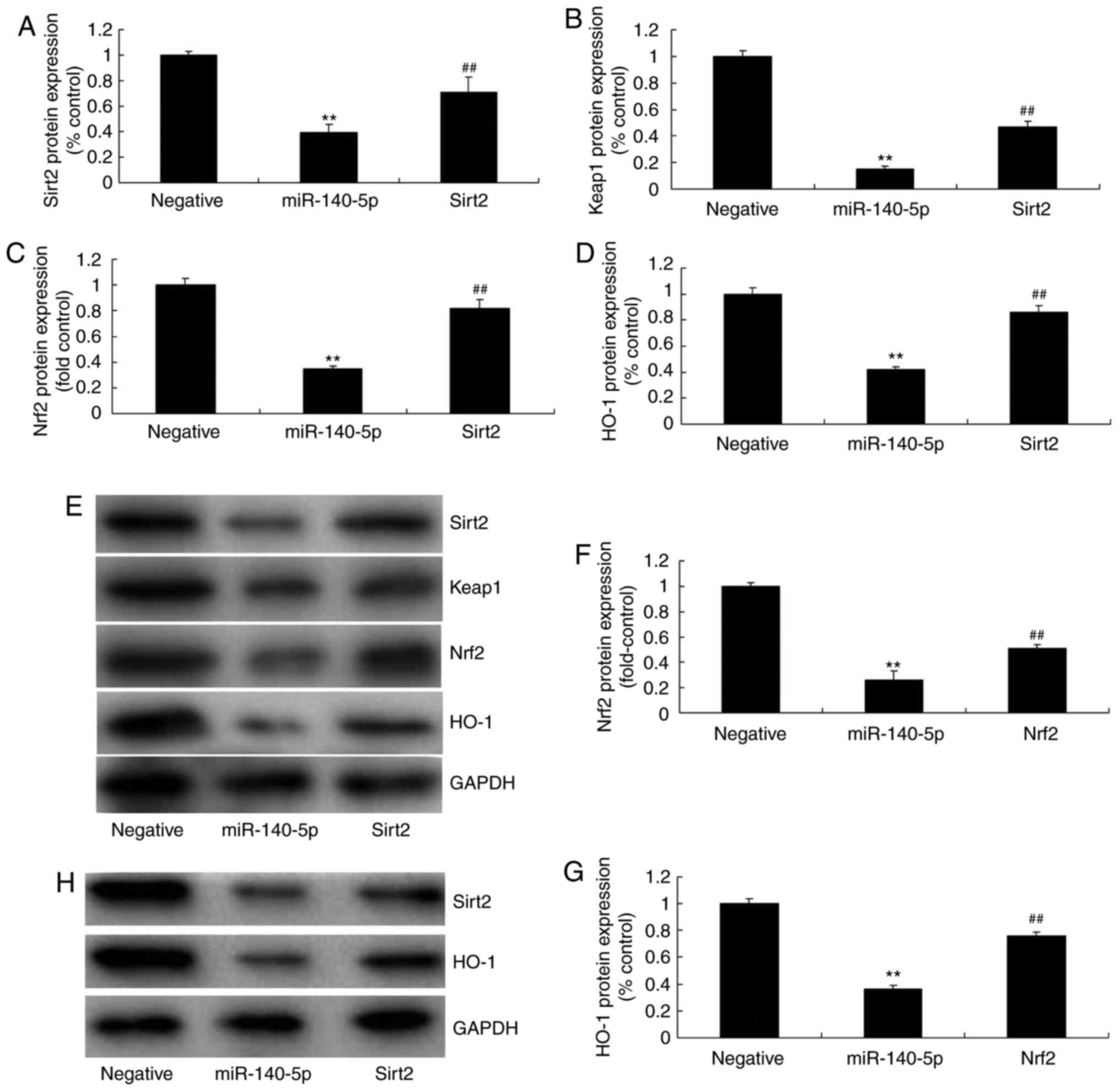
group (Fig. 2I). Western blot
analysis indicated that overexpression of miR-140-5p suppressed the
protein expression of Nrf2, Sirt2, Keap1 and HO-1 in the in
vitro model of AS, compared with the negative control group
(Fig. 3A-E). Furthermore,
anti-miR-140-5p mimic was further employed to decrease the
expression of miR-140-5p in the in vitro model of AS,
compared with the negative control group (Fig. 3F). However, downregulation of
miR-140-5p induced the protein expression of Nrf2, Sirt2, Keap1 and
HO-1 in the in vitro model of AS, compared with the negative
control group (Fig. 3G-K).
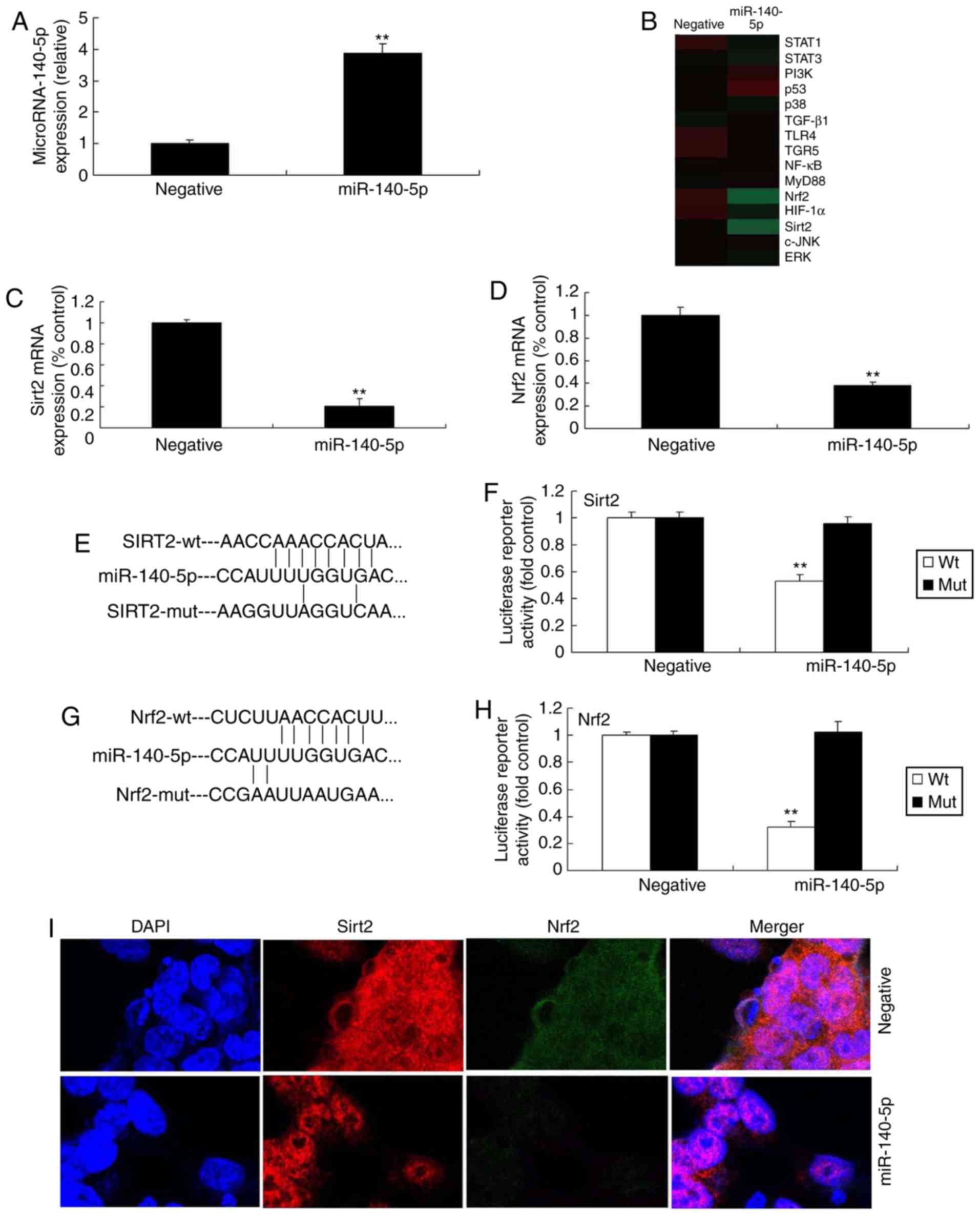 | Figure 2miR-140-5p regulates Nrf2 and Sirt2
expression. (A) qPCR analysis of miR-140-5p. (B) Gene chip assay
for signaling pathway members. qPCR analysis for (C) Nrf2 and (D)
Sirt2 mRNA expression. (E) miR-140-5p targeted Sirt2 sequence. (F)
Dual-luciferase reporter assay for Sirt2. (G) miR-140-5p targeted
Nrf2 sequence. (H) Dual-luciferase reporter assay for Nrf2. (I)
Immunofluorescence analysis of Nrf2 and Sirt2 expression.
**P<0.01 vs. negative control group. miR, microRNA;
Nrf2, nuclear factor erythroid 2-related factor 2; Sirt2, sirtuin
2; wt, wild-type; mut, mutant; STAT, signal transducer and
activator of transcription; PI3K, phosphoinositide 3-kinase;
TGF-β1, transforming growth factor β1; TLR4, Toll-like receptor 4;
TGR5, Takeda G-protein-coupled receptor 5; NF-κB, nuclear factor
κB; MyD88, myeloid differentiation primary response 88; HIF-1α,
hypoxia-inducible factor 1α; c-JNK, c-Jun N-terminal kinase; ERK,
extracellular-signal-regulated kinase. |
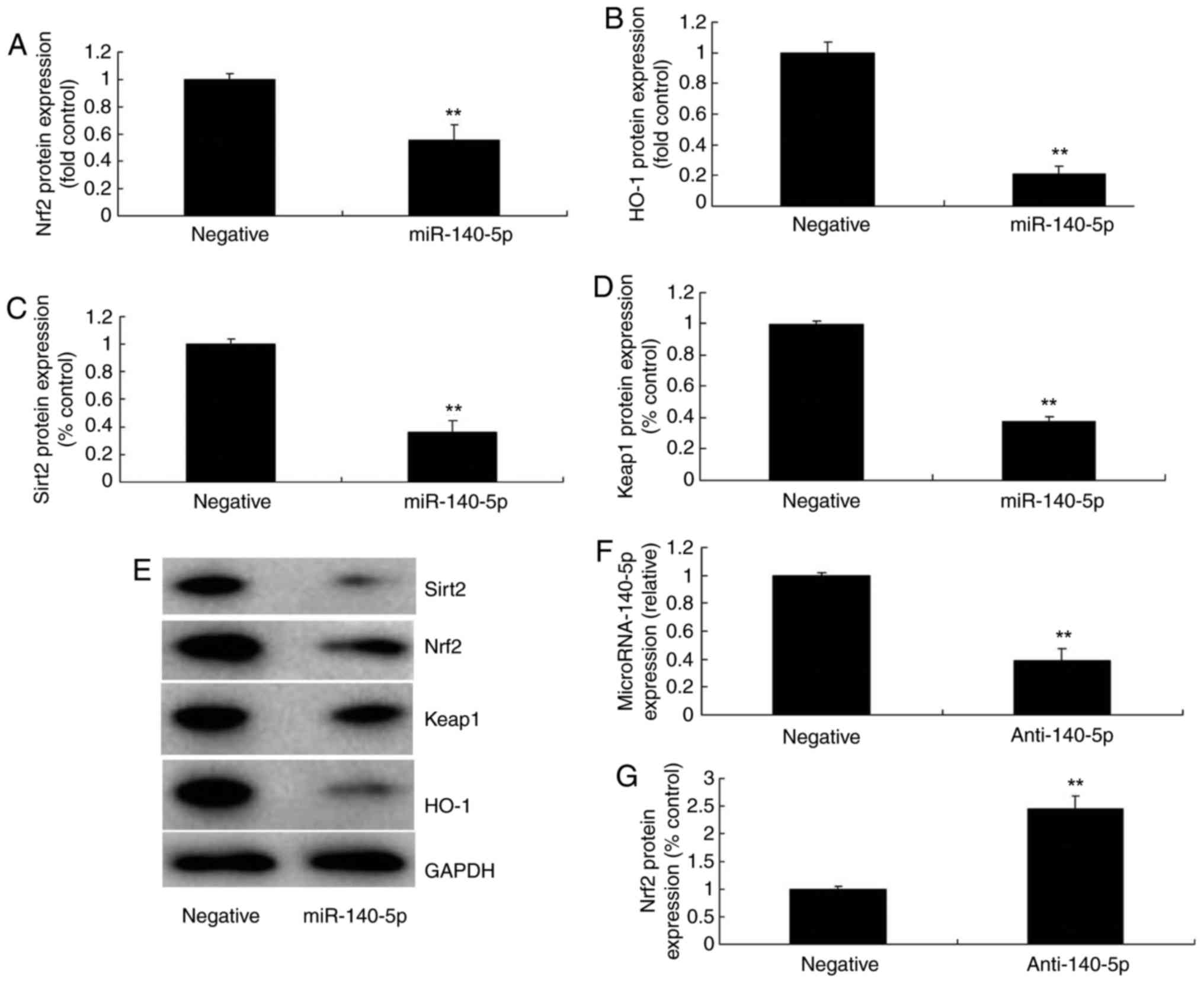 | Figure 3miR-140-5p regulates Nrf2, Sirt2,
Keap1 and HO-1 expression. (A) Nrf2, (B) HO-1, (C) Sirt2 and (D)
Keap1 protein expression as assessed by (E) western blotting
following overexpression of miR-140-5p. (F) Quantitative polymerase
chain reaction analysis of miR-140-5p. (G) Nrf2, (H) Sirt2, (I)
Keap1 and (J) HO-1 protein expression as assessed by (K) western
blotting following downregulation of miR-140-5p.
**P<0.01 vs. negative group. miR, microRNA; Nrf2,
nuclear factor erythroid 2-related factor 2; Sirt2, sirtuin 2;
Keap1, Kelch-like enoyl-CoA hydratase-associated protein 1; HO-1,
heme oxygenase 1. |
miR-140-5p regulates oxidative stress in
vitro
To investigate the function of miR-140-5p in
vitro, the alterations in oxidative stress following miR-140-5p
transfection in HUVECs were investigated. As presented in Fig. 4A-E, overexpression of miR-140-5p
led to increased ROS and MDA levels, whereas it led to decreased
levels of SOD, GSH and GSH-Px in vitro, compared with
negative group. Downregulation of miR-140-5p decreased the levels
of ROS and MDA, and increased those of SOD, GSH and GSH-Px in
vitro, compared with negative group (Fig. 4F-J). These results indicated that
miR-140-5p regulated Nrf2 and Sirt2 expression to affect oxidative
stress in vitro.
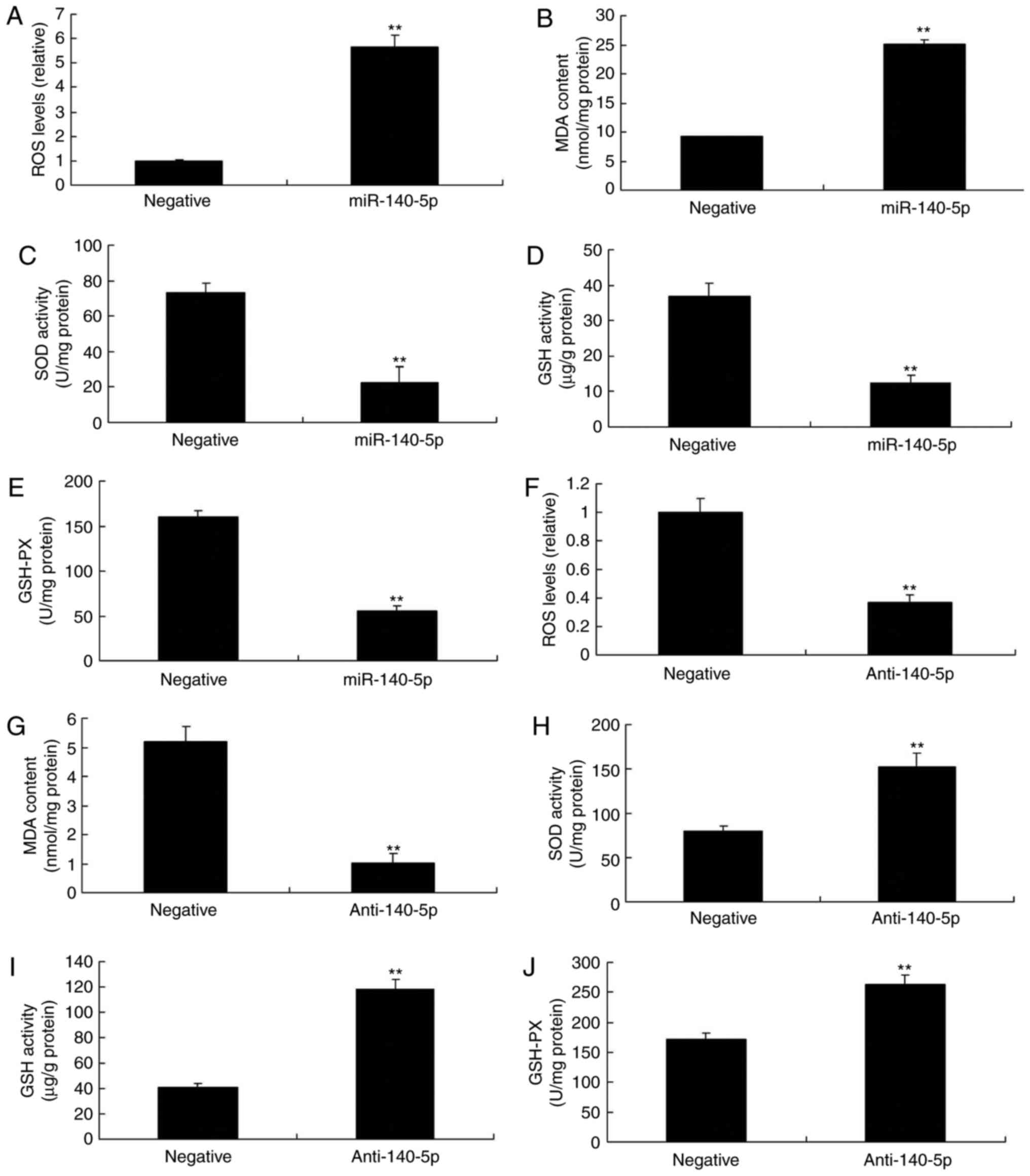 | Figure 4miR-140-5p regulates oxidative stress
in an in vitro model. (A) ROS, (B) MDA, (C) SOD, (D) GSH and
(E) GSH-PX levels following overexpression of miR-140-5p. (F) ROS,
(G) MDA, (H) SOD, (I) GSH and (J) GSH-Px levels following
downregulation of miR-140-5p. **P<0.01 vs. negative
control group. miR, microRNA; ROS, reactive oxygen species; MDA,
malondialdehyde; SOD, superoxide dismutase; GSH, glutathione;
GSH-Px, glutathione peroxidase. |
ROS inhibitor inhibits the effects of
miR-140-5p on oxidative stress in vitro
To investigate the function of ROS on the effects of
miR-140-5p on oxidative stress in vitro, ROS inhibitor was
used to decrease the ROS levels in the in vitro model of
miR-140-5p overexpression, compared with the miR-140-5p
overexpression group in the absence of inhibitor (Fig. 5A and B). ROS inhibitor inhibited
MDA levels, and increased the levels of SOD, GSH and GSH-Px
following overexpression of miR-140-5p, compared with the
miR-140-5p overexpression group in the absence of inhibitor
(Fig. 5C-F).
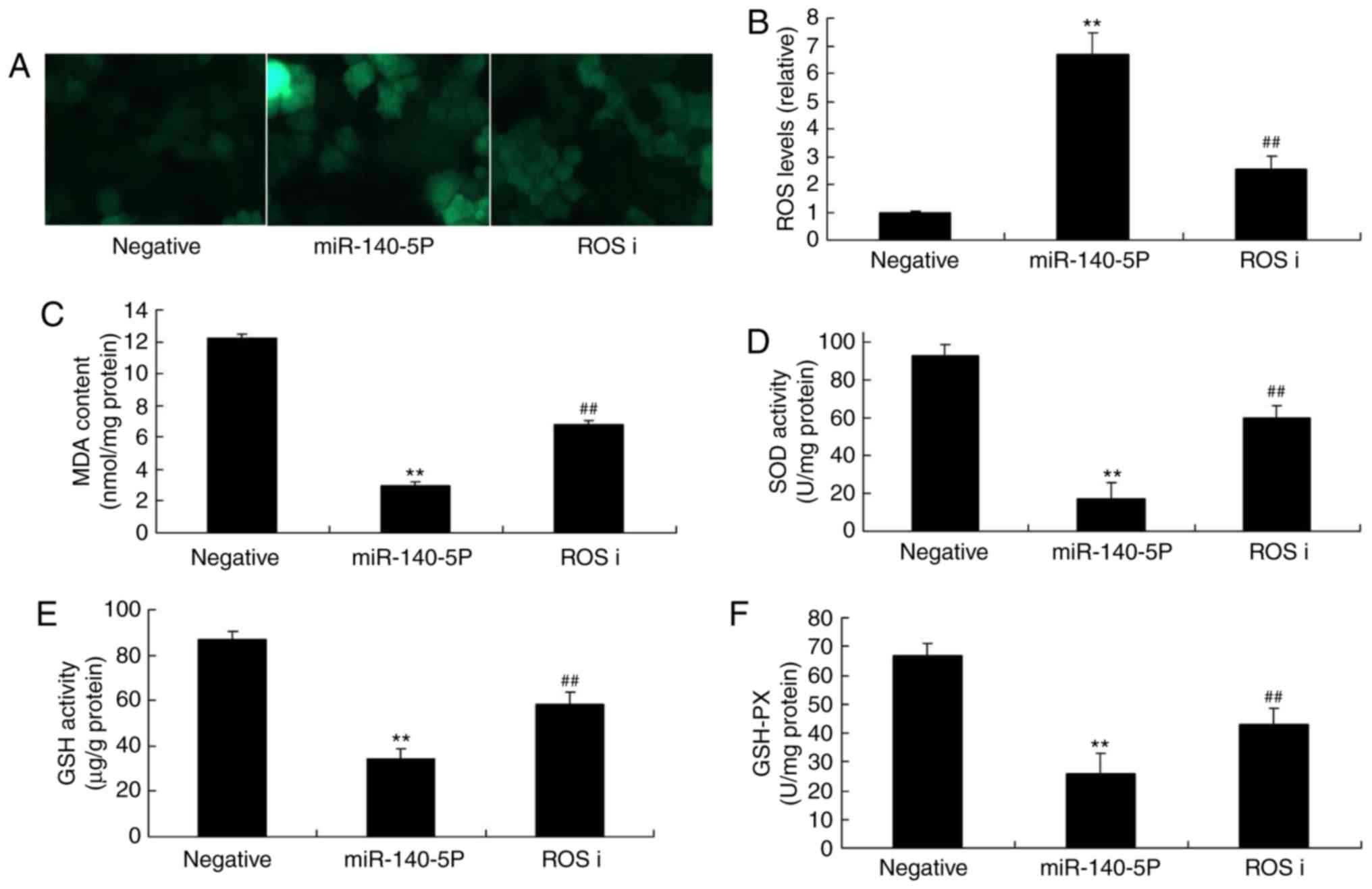 | Figure 5Effect of ROS inhibitor on miR-140-5p
in the oxidative stress in vitro model. (A) ROS
fluorescence. (B) ROS, (C) MDA, (D) SOD, (E) GSH and (F) GSH-PX
levels. ROS inhibitor was added to cells overexpressing miR-140-5p.
ROS, reactive oxygen species; miR, microRNA; MDA, malondialdehyde;
SOD, superoxide dismutase; GSH, glutathione; GSH-PX, glutathione
peroxidase; ROS i, ROS inhibitor. **P<0.01 vs.
negative control group, ##P<0.01 vs. miR-140-5p
overexpression group. |
Sirt2 agonist or Nrf2 agonist inhibits
the effects of miR-140-5p on Nrf2 and Sirt2 expression in
vitro
To further determine the function of Sirt2 or Nrf2
in the effects of miR-140-5p on Nrf2 and Sirt2 expression in
vitro, Sirt2 agonist or Nrf2 agonist was used to increase the
expression of Nrf2 and Sirt2 expression in the in vitro
model of miR-140-5p overexpression, compared with miR-140-5p
overexpression group in the absence of agonist (Fig. 6).
Sirt2 agonist or Nrf2 agonist inhibits
the effects of miR-140-5p on oxidative stress in vitro
Finally, to determine the function of Sirt2 or Nrf2
on the effects of miR-140-5p on oxidative stress in vitro,
the levels of oxidative stress in vitro following treatment
with miR-140-5p and Sirt2 agonist or Nrf2 agonist were determined.
As presented in Fig. 7A-E, Sirt2
agonist inhibited the effects of miR-140-5p on increasing the
levels of MDA and ROS, and on decreasing the levels of SOD, GSH and
GSH-Px in the in vitro model of miR-140-5p overexpression,
compared with the miR-140-5p overexpression group in the absence of
agonist. Sirt2 agonist also decreased the effects of miR-140-5p on
increasing the levels of MDA and ROS, and on decreasing the levels
of SOD, GSH and GSH-Px in the in vitro model of miR-140-5p
overexpression, compared with the miR-140-5p overexpression group
in the absence of agonist (Fig.
7F-J). Together, these results indicated that miR-140-5p
regulated Nrf2 and Sirt2 expression to affect oxidative stress
in vitro.
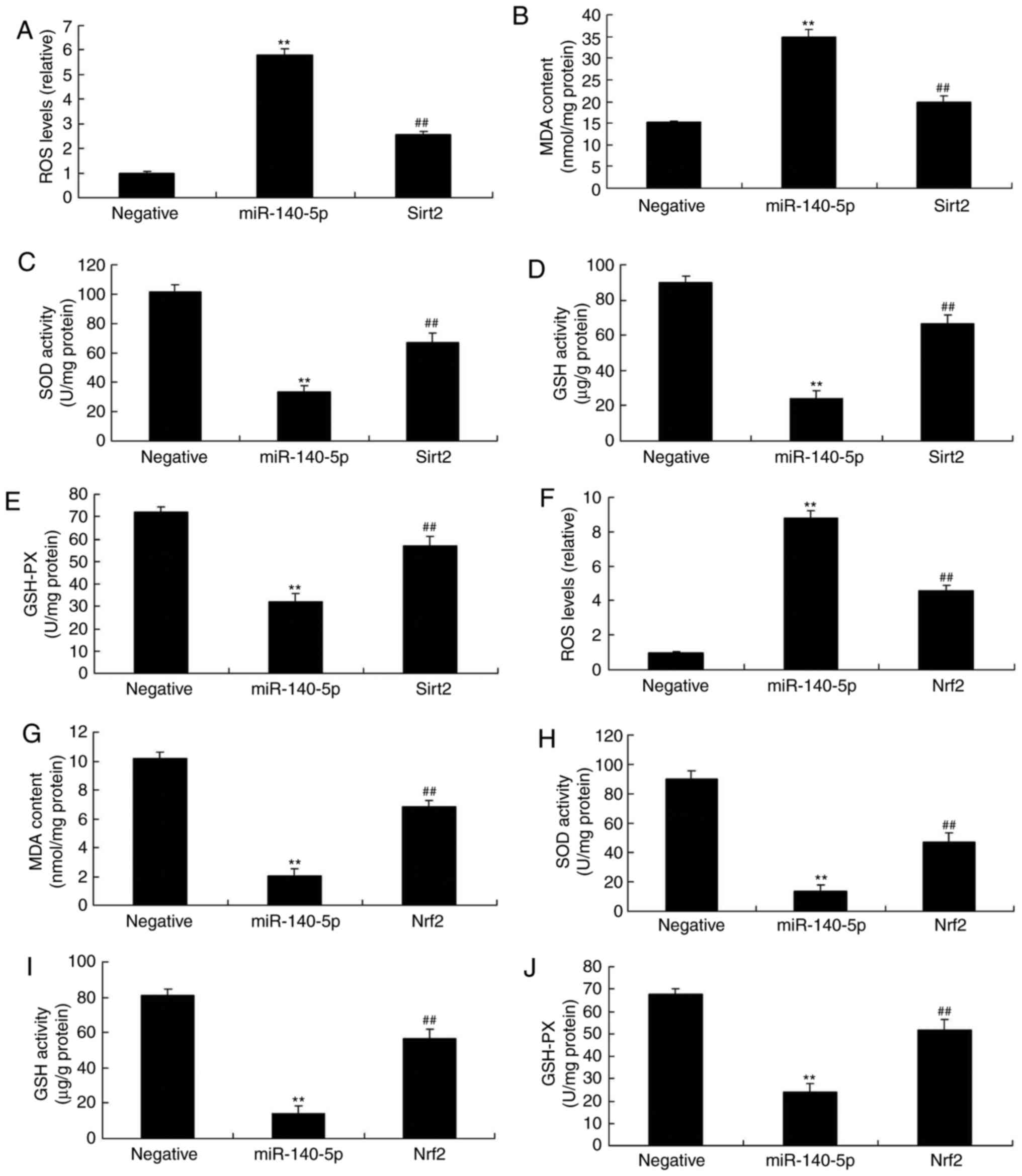 | Figure 7Sirt2 agonist or Nrf2 agonist
inhibits the effects of miR-140-5p in an oxidative stress in
vitro model. (A) ROS, (B) MDA, (C) SOD, (D) GSH and (E) GSH-Px
levels following treatment with a Sirt2 agonist. (F) ROS, (G) MDA,
(H) SOD, (I) GSH and (J) GSH-Px levels following treatment with a
Nrf2 agonist. Agonists were added to cells overexpressing
miR-140-5p.**P<0.01 vs. negative control group.
##P<0.01 vs. miR-140-5p overexpression group. Sirt2,
sirtuin 2; Nrf2, nuclear factor erythroid 2-related factor 2; miR,
microRNA; ROS, reactive oxygen species; MDA, malondialdehyde; SOD,
superoxide dismutase; GSH, glutathione; GSH-Px, glutathione
peroxidase. |
Discussion
AS is considered the pathological basis for the
occurrence and progression of coronary heart disease (16). miRNAs are a group of endogenous
conserved non-coding small-molecule single-stranded RNAs that are
widely distributed in eukaryotes and are involved in the regulation
of gene expression at the post-transcription level (16). Previous studies have identified
that the expression of miRNA is associated with the formation of
atherosclerotic plaques (11,17). miRNA serves a function in the
regulation of cardiac development as well as multiple cardiac
pathophysiological processes such as cardiac hypertrophy,
arrhythmia and heart failure (11,17). Certain biomarkers have been
identified to be associated with AS (17). However, it is necessary to seek
novel biomarkers to provide an earlier, more accurate and sensitive
indication of AS (16,17), leading to early clinical
diagnosis, treatment and prognosis of patients with AS. Previous
studies have demonstrated that the levels of circulating miRNAs
vary markedly among various diseases (16,17). As a result, miRNAs may be used as
biomarkers of diseases or be used to evaluate the severity and
prognosis of disease. In the present study, it was identified that
miR-140-5p expression was also increased in mice with AS. Zhao
et al (18) identified
that miR-140-5p was induced in doxorubicin-induced cardiotoxicity,
therefore miR-140-5p expression may be an important element in
AS.
Oxidative stress levels can be detected by
determining antioxidant enzyme system levels, non-enzymatic
antioxidant levels and oxidative product levels (3). In patients with pre-diabetes
accompanied by a highly oxidative stress state, the generation of
free radicals increases in the body with the aggravation of glucose
metabolism disorders (19). This
will lead to decreased antioxidant capacity, increased levels of
oxidative stress and ultimately imbalance of oxidation and
antioxidation (20). The
occurrence and progression of AS are associated with oxidative
stress (19). Oxidative stress
already exists in patients with AS, accompanied by a weakened
antioxidant capacity. In patients with AS combined with diabetes,
the degree of oxidative damage gradually increases as the disease
progresses (19). It was
identified that overexpression of miR-140-5p increased ROS and MDA
levels, and decreased SOD, GSH and GSH-Px levels, in the in
vitro model of AS. The ROS inhibitor decreased the effects of
miR-140-5p on oxidative stress in the in vitro model of
AS.
The primary physiological characteristic of Nrf2 is
its high sensitivity to oxidative stress and exogenous toxicants in
the body (21). In the process of
body defense, Nrf2 serves a critical function in the oxidative
stress induced by exogenous toxicants. Keap1 is one of the major
receptors of Nrf2; all of its cysteine residues are able to
interact with the HO-1 inducer (5). This inducer interacts with specific
residues on Keap1 and causes conformational changes in Keap1, which
are able to trigger Nrf2 dissociation from Keap1 (21). Thus, alterations in its structure
affect the complete expression of Nrf2, i.e. the nuclear export
sequence in Keap1 is essential to inhibit binding of the
antioxidant-response element (ARE) HO-1 with Nrf2 (21). In the present study, it was
identified that overexpression of miR-140-5p suppressed Nrf2,
Sirt2, Keap1 and HO-1 protein expression in the in vitro
model of AS. Liao et al (22) identified that miR-140-5p
attenuated oxidative stress in cisplatin-induced acute kidney
injury through the Nrf2/ARE signaling pathway.
Sirt2 serves an important function in oxidative
stress and autophagy in endothelial cells (23). The specific regulatory mechanism
of Sirt2 on vascular endothelial cells remains unknown; however, it
has been identified that Sirt2 may be involved in the mediation of
endothelial cell migration induced by angiotensin II by regulating
the acetylation of α-tubulin and microtubule recombination
(23). cDNA microarrays were used
to confirm that Sirt2 was knocked down in primary cultured
endothelial cells, which would lead to changes in general gene
expression during oxidative stress (9). Among them, the majority were not
altered following Sirt1 knockdown (9). Of note, drug inhibition of Sirt2 was
able to decrease oxidative stress-induced endothelial cell injury
(10). In the present study, it
was identified that Sirt2 agonist or Nrf2 agonist inhibited the
effects of miR-140-5p on oxidative stress in an in vitro
model. Zhao et al (18)
identified that miR-140-5p aggravates doxorubicin-induced
cardiotoxicity by targeting Nrf2 and Sirt2. miR-140-5p regulates
ROS levels and the ROS downstream signaling pathway is a focus of
further study, including nuclear factor κB and cryopyrin.
In conclusion, in the present study, the potential
effects and underlying molecular mechanisms of
miR-140-5p-aggravated hypertension and oxidative stress of patients
with AS by targeting Nrf2 and Sirt2 were investigated. It was
identified that miR-140-5p acts as an excellent antioxidant and
induced the Sirt2/Nrf2/HO-1 signaling pathway to suppress oxidative
stress in AS (Fig. 8). Therefore,
the results of the present study suggest that miR-140-5p/Sirt2/Nrf2
have an important function in the prevention and treatment of
AS.
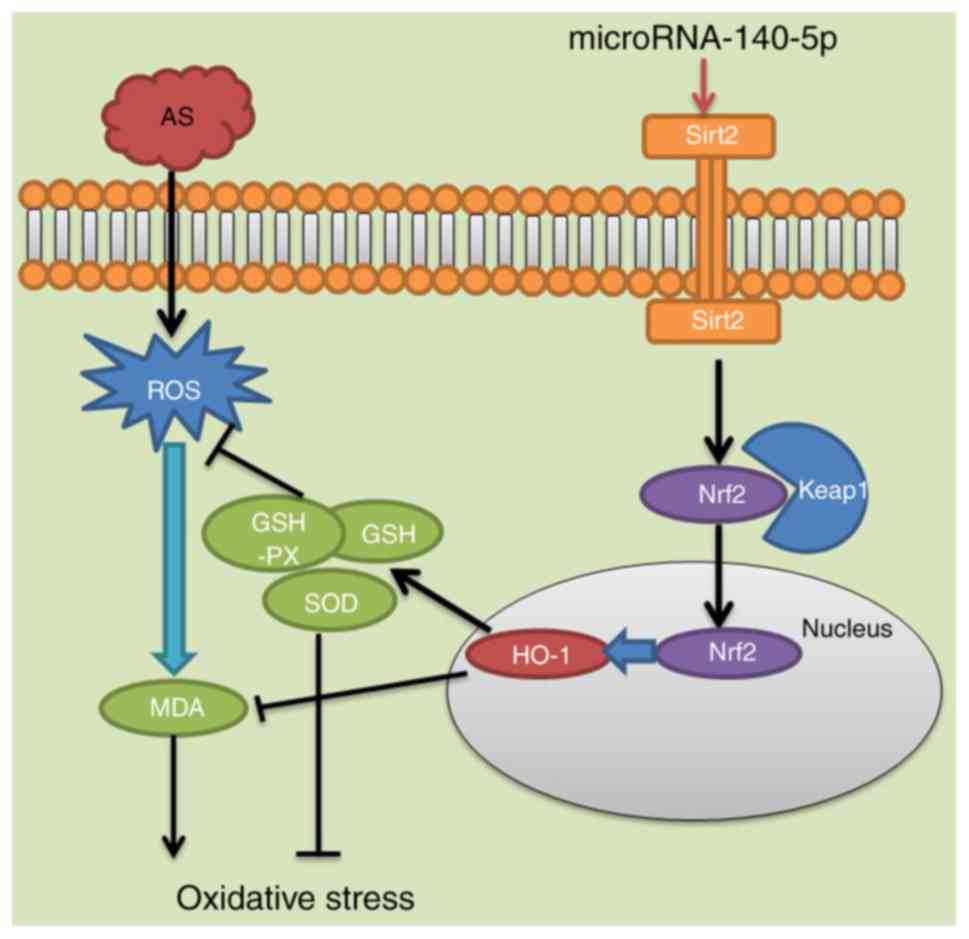 | Figure 8MicroRNA-140-5p aggravates
hypertension and oxidative stress of atherosclerosis via targeting
Nrf2 and Sirt2. Nrf2, nuclear factor erythroid 2-related factor 2;
Sirt2, sirtuin 2; AS, atherosclerosis; ROS, reactive oxygen
species; MDA, malondialdehyde; GSH-PX, glutathione peroxidase; GSH,
glutathione; SOD, superoxide dismutase; HO-1, heme oxygenase 1;
keap1, Kelch-like enoyl-CoA hydratase-associated protein 1 |
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
XHY designed the experiments; QQL, KR, SHL, WML and
CJH performed the experiments; XHY analyzed the data; XHY wrote the
manuscript.
Ethics approval and consent to
participate
Animal experiments were approved by the Ethics
Committee of The Ninth People's Hospital (Shanghai Jiaotong
University School of Medicine, Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Sirotina S, Ponomarenko I, Kharchenko A,
Bykanova M, Bocharova A, Vagaytseva K, Stepanov V, Churnosov M,
Solodilova M and Polonikov A: A Novel polymorphism in the promoter
of the CYP4A11 gene is associated with susceptibility to coronary
artery disease. Dis Markers. 2018:58128022018. View Article : Google Scholar :
|
|
2
|
Kiris T, Avci E and Çelik A: Combined
value of left ventricular ejection fraction and the Model for
End-Stage Liver Disease (MELD) score for predicting mortality in
patients with acute coronary syndrome who were undergoing
percutaneous coronary intervention. BMC Cardiovasc Disord.
18:442018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shahid SU, Shabana and Humphries S: The
SNP rs10911021 is associated with oxidative stress in coronary
heart disease patients from Pakistan. Lipids Health Dis. 17:62018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ishikawa T and Seki K: The association
between oxidative stress and endothelial dysfunction in early
childhood patients with Kawasaki disease. BMC Cardiovasc Disord.
18:302018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu H, Shi L, Zhao S, Sun Y, Gao Y, Sun Y
and Qi G: Triptolide attenuates myocardial ischemia/reperfusion
injuries in rats by inducing the activation of Nrf2/HO-1 defense
pathway. Cardiovasc Toxicol. 16:325–335. 2016. View Article : Google Scholar
|
|
6
|
Donovan EL, McCord JM, Reuland DJ, Miller
BF and Hamilton KL: Phytochemical activation of Nrf2 protects human
coronary artery endothelial cells against an oxidative challenge.
Oxid Med Cell Longev. 2012:1329312012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Siow RC, Ishii T and Mann GE: Modulation
of antioxidant gene expression by 4-hydroxynonenal:
atheroprotective role of the Nrf2/ARE transcription pathway. Redox
Rep. 12:11–15. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tang X, Chen XF, Wang NY, Wang XM, Liang
ST, Zheng W, Lu YB, Zhao X, Hao DL, Zhang ZQ, et al: SIRT2 Acts as
a cardioprotective deacetylase in pathological cardiac hypertrophy.
Circulation. 136:2051–2067. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsushima S and Sadoshima J: The role of
sirtuins in cardiac disease. Am J Physiol Heart Circ Physiol.
309:H1375–H1389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang W, Gao F, Zhang P, Pang S, Cui Y, Liu
L, Wei G and Yan B: Functional genetic variants within the SIRT2
gene promoter in acute myocardial infarction. PLoS One.
12:e01762452017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nanoudis S, Pikilidou M, Yavropoulou M and
Zebekakis P: The role of MicroRNAs in arterial stiffness and
arterial calcification. An update and review of the literature.
Front Genet. 8:2092017. View Article : Google Scholar
|
|
12
|
Li S, Lee C, Song J, Lu C, Liu J, Cui Y,
Liang H, Cao C, Zhang F and Chen H: Circulating microRNAs as
potential biomarkers for coronary plaque rupture. Oncotarget.
8:48145–48156. 2017.PubMed/NCBI
|
|
13
|
Jansen F, Schäfer L, Wang H, Schmitz T,
Flender A, Schueler R, Hammerstingl C, Nickenig G, Sinning JM and
Werner N: Kinetics of circulating MicroRNAs in response to cardiac
stress in patients with coronary artery disease. J Am Heart Assoc.
6:pii: e0052702017. View Article : Google Scholar
|
|
14
|
Li XD, Yang YJ, Wang LY, Qiao SB, Lu XF,
Wu YJ, Xu B, Li HF and Gu DF: Elevated plasma miRNA-122,
-1403p-720, -2861, and -3149 during early period of acute coronary
syndrome are derived from peripheral blood mononuclear cells. PLoS
One. 12:e01842562017. View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Adams A, Bojara W and Schunk K: Early
diagnosis and treatment of coronary heart disease in asymptomatic
subjects with advanced vascular atherosclerosis of the carotid
artery (Type III and IV b Findings Using Ultrasound) and risk
factors. Cardiol Res. 9:22–27. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu R, Shen D, Sohun H, Ge D, Chen X, Wang
X, Chen R, Wu Y, Zeng J, Rong X, et al: miR-186, a serum microRNA,
induces endothelial cell apoptosis by targeting SMAD6 in Kawasaki
disease. Int J Mol Med. 41:1899–1908. 2018.PubMed/NCBI
|
|
18
|
Zhao L, Qi Y, Xu L, Tao X, Han X, Yin L
and Peng J: MicroRNA-140-5p aggravates doxorubicin-induced
cardiotox-icity by promoting myocardial oxidative stress via
targeting Nrf2 and Sirt2. Redox Biol. 15:284–296. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tanabe K, Kawai Y, Kitayama M, Akao H,
Ishida R, Motoyama A, Wakasa M, Saito R, Aoki H, Fujibayashi K, et
al: Increased levels of the oxidative stress marker, nitrotyrosine
in patients with provocation test-induced coronary vasospasm. J
Cardiol. 64:86–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim JY, Lee JW, Youn YJ, Ahn MS, Ahn SG,
Yoo BS, Lee SH, Yoon J and Choe KH: Urinary levels of
8-iso-prostaglandin f2α and 8-hydroxydeoxyguanine as markers of
oxidative stress in patients with coronary artery disease. Korean
Circ J. 42:614–617. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Donnarumma E, Bhushan S, Bradley JM,
Otsuka H, Donnelly EL, Lefer DJ and Islam KN: Nitrite Therapy
ameliorates myocardial dysfunction via H2S and nuclear
factor-erythroid 2-related factor 2 (Nrf2)-dependent signaling in
chronic heart failure. J Am Heart Assoc. 5:pii: e0035512016.
View Article : Google Scholar
|
|
22
|
Liao W, Fu Z, Zou Y, Wen D, Ma H, Zhou F,
Chen Y, Zhang M and Zhang W: MicroRNA-140-5p attenuated oxidative
stress in Cisplatin induced acute kidney injury by activating
Nrf2/ARE athway through a Keap1-independent mechanism. Exp Cell
Res. 360:292–302. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Turdi S, Li Q, Lopez FL and Ren J:
Catalase alleviates cardio-myocyte dysfunction in diabetes: Role of
Akt, Forkhead transcriptional factor and silent information
regulator 2. Life Sci. 81:895–905. 2007. View Article : Google Scholar : PubMed/NCBI
|