Introduction
Osteoarthritis (OA) is a common degenerative joint
disease characterized by the degradation and destruction of
cartilage (1,2). Although the details of the
pathological mechanism of OA remain unclear, accumulating evidence
had suggested that inflammation serves an important role in its
development. Notably, the inflammation of the synovium is
frequently involved in the occurrence and development of this
disease (3). The synovium is
widely distributed in the cartilage cavity and is in direct contact
with articular cartilage. Synovial fluid provides nutrients for
exchange with articular cartilage. However, synovial inflammation
may secrete certain cytokines, including interleukin-1β (IL-1β),
tumor necrosis factor-α and nitric oxide (4). IL-1β is one of the crucial
inflammatory cytokines, which has been demonstrated to active the
nuclear factor-kB signaling pathway to release inflammatory
mediators, including prostaglandin E2 and matrix metalloproteinases
(MMPs) (5). MMP-3 and MMP-13,
zinc-containing and calcium-dependent proteinases, are frequently
expressed in synoviocytes and chondrocytes in response to
inflammatory cytokines, which collectively degrade all components
of the extracellular matrix (ECM) and serve crucial roles in the
degenerative changes of cartilage matrix in OA (6,7).
Additionally, these mediators may induce the aging and apoptosis of
chondrocytes, and damage to the articular cartilage (8). Therefore, attenuation of the
inflammation response in synoviocytes may have a protective effect
on the metabolism of chondrocytes, thereby providing a promising
treatment for OA.
At present, gene therapy has been regarded as a
promising method of curing diseases. Gene therapy involves the
delivery of a therapeutic gene into target cells to modulate their
function and subsequently treat the disease. However, a key issue
in the therapeutic application of this technology is that vectors
are required for the safe and efficient delivery of plasmid DNA
(pDNA). Although viral gene vectors have high transfection
efficiency, their poor biological safety and highly immunogenic
properties restrict their application (9). By contrast, non-viral gene vectors
have been widely studied due to their clinical safety (10,11).
As a non-viral vector for gene delivery, chitosan
(CS) offers certain advantageous properties, including
non-toxicity, biodegradability and good biocompatibility (12). Nanoparticles composed of CS and a
plasmid encoding the IL-1 receptor antagonist have been
demonstrated to markedly attenuate the severity of histologic
cartilage lesions (13). However,
at present, its low transfection efficiency limits the application
of CS. Previously, it has been suggested that the transfection
efficiency of CS vectors may be improved by combining CS with
cationic or anionic biopolymers, prior to the addition of DNA
(14,15). Hyaluronic acid (HA), a
biocompatible anionic biopolymer, has been used in a wide array of
clinical applications (16). It
has been indicated that HA/CS/pDNA nanoparticles were suitable for
cell transfection via endocytosis and exhibited a higher
transfection efficiency compared with either CS/pDNA nanoparticles
or naked plasmid DNA (17). Lu
et al (18) suggested that
HA/CS/pDNA nanoparticles encoding transforming growth factor β1,
with diameters of 100-300 nm, may promote chondrocyte adhesion,
proliferation and synthesis of the ECM.
In our previous study, HA/CS microspheres were
demonstrated to be a safe carrier for the controlled release of
drugs, due to their good biocompatibility, biodegradabilty and high
stability (19). It was
hypothesized that HA/CS nanoparticles may additionally be suitable
as gene carriers to deliver the therapeutic gene into synoviocytes.
The cytokine response modifier A (CrmA) is a caspase inhibitor that
broadly inhibits the activity of a number of caspases and IL-1β
converting enzyme proteases, and subsequently attenuates IL-1β
induced inflammation and apoptosis in chondrocytes of OA (20,21), which suggested that CrmA pDNA may
be a potential target gene.
In the present study, HA/CS/pCrmA nanoparticles were
constructed as a gene delivery system. The characterization,
safety, transfection efficiency and cytotoxicity of the complex was
additionally measured. It was hypothesized that the HA/CS/pCrmA
nanoparticles may have protective effects against inflammation in
synoviocytes from an in vitro OA model, and the strategy
provides a potential approach to the treatment of OA.
Materials and methods
Preparation of HA/CS/pDNA
nanoparticles
The plasmid DNA (Guangzhou Fulengen Co., Ltd.,
Guangzhou, China) contained the enhanced green fluorescent protein
expression vector (pEGFP) encoding a cytomegalovirus enhancer
inserted upstream and the sequence of CrmA (pEGFP-CrmA). The
plasmid was propagated in Escherichia coli cells, and
subsequently isolated and purified. Following this, the absorption
ratio of the plasmid at λ=260 and 280 nm was measured to ensure the
concentration and purity. The empty plasmid control comprised pEGFP
only (naked pDNA).
The HA/CS nanoparticles were produced as described
previously (20). CA (molecular
weight=5 kDa; deacetylation degree: 90%), and chitosanase were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Sodium hyaluronate (molecular weight=35 kDa) was purchased from
Freda Biochem Co., Ltd. (Jinan, China). HA was dissolved in
ultra-pure water at 1% (w/w, pH 5.5), and CS was dissolved in
acetic acid to obtain a CS solution at 2% (w/w, pH 5.5). Then,
these two solutions were filtered separately through a 0.22
µm membrane, and then combined at a rate of 600 × g for 1 h
at room temperature to obtain a stable HA/CS solution. A well-mixed
solution comprising 100 ml paraffin oil and 1 g Span 80
(Sigma-Aldrich; Merck KGaA) was combined at 300 × g for 1 h at room
temperature. Subsequently, 6 ml HA-CS solution was gradually added
into this paraffin-Span 80 solution at a speed of 1 ml/min. The
reaction system was mixed at the 300 × g for an additional 2 h at
room temperature, following which 10 ml sodium tripolyphosphate
solution (10% w/w) was added. Then, the reaction system was mixed
at the 300 × g for an additional 1 h at room temperature. Following
centrifugation at a speed of 300 × g for 10 min at room temperature
and removal of the supernatant, HA/CS nanoparticles were collected.
The nanoparticles were washed with 100% alcohol and 100% acetone
three times to completely remove the residual paraffin oil and Span
80. Different HA:CS weight ratio (1:1, 1:2, 1:3, 1:4, 1:5, 1:6,
1:7, 1:8) nanoparticles were prepared using the method described
above.
A total of 12.5 µg/ml pDNA was added into the
HA/CS nanoparticles and mixed by the vortex agitator for 5 sec;
subsequently, the mixture was maintained for 1 h at 37°C to form
HA/CS/pDNA nanoparticles completely. The prepared HA/CS/pDNA
nanoparticles were subsequently freeze-dried for subsequent
study.
Characterization of HA/CS/pDNA
nanoparticles
The lyophilized HA/CS/pDNA nanoparticles were
dissolved in distilled water at room temperature to obtain a
homogeneous solution. Subsequently, the solution was dropped onto a
glass slide and dried at 37°C. Subsequent to sputter-coating of the
slide with gold, the morphology of HA/CS/pDNA nanoparticles was
observed by scanning electron microscope (SEM; JSM-6330; JEOL,
Ltd., Tokyo, Japan).
Electrophoresis assay
In order to detect whether pDNA was stably retained
within the HA/CS nanoparticles, the HA/CS/pDNA nanoparticles or
naked pDNA were dissolved in PBS, and then the samples were
analyzed on a 1% agarose gel at 80 V for 45 min. Furthermore, the
nanoparticles were subsequently incubated with 4 µg/ml DNase
I at 37°C for 30 min, following which the complexes were treated
with EDTA for 15 min to stop the reaction. To value the effect of
chitosan on the protection of pDNA, the complexes were additionally
incubated with 2.78 µg/µl chitosanase for an
additional 12 h at 37°C, followed by analysis using an 1% agarose
gel at 80 V for 45 min at room temperature. Finally, the gels were
stained with ethidium bromide for 90 min at room temperature and
imaged visually using a GDS-8000 (UVP, LLC, Phoenix, AZ, USA).
Release assay of pCrmA from HA/CS
nanoparticles in vitro
To obtain an improved understanding of the release
kinetics of the pCrmA from HA/CS nanoparticles, the amount of pCrmA
was evaluated on days 0, 3, 5, 7, 9, 11, 14, 17, 19, 21, 23, 25, 27
and 29. The HA/CS/pCrmA nanoparticles were incubated in PBS
solution (pH 7.4) in a shaker bath at 50 × g at 37°C under sterile
conditions. Following centrifugation of the samples at 4,000 × g at
37°C for 10 min, supernatants were collected and replaced with
equal volumes of fresh sterile PBS solution (pH 7.4) following each
sampling. Finally, the amount of pCrmA released was measured
spectrophotometrically at 260 nm.
Cell culture and transfection
Specific pathogen-free male Sprague-Dawley rats (4
weeks old; weighing 80-100 g) were purchased from the Laboratory
Animal Center of Wuhan University (Wuhan, China). The procedures
involving animals were performed in accordance with the Guidelines
for the Care and Use of Laboratory Animals published by The
National Institutes of Health (Bethesda, MD, USA) and were approved
by the Wuhan University Animal Care and Use Committee (approval no.
2017-0208). The rats were housed under standard conditions (room
temperature: 18-22°C; humidity: 40-60%, 12:12-h dark-light cycle)
and allowed free access to chow and distilled water. Subsequent to
acclimating for 1 week, the rats were sacrificed by anesthesia with
5% isoflurane. The synovial tissue was isolated and sliced into
small pieces, which were digested with 0.25% trypsin for 2 h and
subsequently digested with 0.2% collagenase II at 37°C for 1 h.
Following centrifugation at 300 × g for 5 min at room temperature,
synoviocytes were collected from the supernatants. Subsequently,
the cells were cultured in DMEM/F12 medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.) and 1% antibiotics (100 units/ml
penicillin and 100 µg/ml streptomycin; Sigma-Aldrich; Merck
KGaA) and incubated at 37°C with 5% CO2 in a humidified
atmosphere. After 4 days of culturing, once the cells had reached
80% confluence in the first passage, they were used in the
subsequent experiments.
The synoviocytes were resuspended in 6-wells plates
and the density of the cells was adjusted to 2×105
cells/well. The appropriate HA/CS/pDNA nanoparticles were mixed
throughly in PBS for 20 min at 37°C. The complexes were diluted
with DMEM/F12 medium with 10% FBS and 1% antibiotics. Subsequently,
the cells were washed twice with PBS and 1.5 ml DMEM/F12 medium
with 10% FBS containing 40 µg/ml HA/CS/pEGFP or HA/CS/pCrmA
was added to each well. After incubation at 37°C under 5%
CO2 for 72 h, the nanoparticles were removed and the
cells were subsequently collected. The synoviocytes transfected
with HA/CS/pEGFP were used as a control, and the protein expression
of CrmA was measured by western blot analysis.
Protein extraction was performed using
radioimmunoprecipitation lysis buffer (cat. no. P0013B) and
concentration was measured by a bicinchoninic protein assay kit
(cat. no. P0010; both from Beyotime Institute of Biotechnology,
Haimen, China). A total of 50 µg protein per lane was
separated by 10% SDS-PAGE and transferred to a nitrocellulose
membrane. Following blocking with 5% non-fat dry milk in TBS-0.05%
Tween for 2 h at room temperature, the membrane was incubated at
4°C overnight with primary antibodies, including anti-CrmA (cat.
no. 556427; 1:500; BD Pharmingen, San Diego, CA, USA) and
anti-β-actin (cat. no. BM0627; 1:3,000; Wuhan Boster Biological
Technology, Ltd., Wuhan, China). Subsequently, the membrane was
washed and incubated with a horseradish peroxidase-conjugated
secondary antibody (cat. no. BA1050; 1:5,000; Wuhan Boster
Biological Technology, Ltd.) at 37°C for 2 h. The protein bands
were visualized using an enhanced chemiluminescence system (Thermo
Fisher Scientific, Inc., Waltham, MA, USA).
Cell viability
When the first passage cells reached 80% confluence,
the medium was changed to DMEM/F12 and the cells were incubated at
37°C for an additional 5 h. The synoviocytes were divided into two
groups: One group was cultured with HA/CS/pCrmA nanoparticles at
different concentrations (0, 5, 10, 20, 40, 80 and 160
µg/ml); and the other group was treated with HA/CS/pEGPF
nanoparticles.
Following transfection and incubation for 72 h, cell
viability was valued by colorimetric MTS assay according to the
manufacturer’s protocol. The culture medium was replaced by 1.5 ml
DMEM/F12 containing 10 µl MTS. Following incubation at 37°C
with 5% CO2 for 4 h, the absorbance was measured at 490
nm.
Synoviocytes culture and IL-1β
treatments
Primary synoviocytes were cultured with IL-1β to
generate the OA model, and then divided into four groups: Blank;
control; HA/CS/pEGFP; and HA/CS/pCrmA groups. The blank group was
cultured with medium only, and the control group was treated with
10 ng/ml IL-1β. In the presence of IL-1β, the HA/CS/pEGFP and
HA/CS/pCrmA groups were treated with 40 µg/ml HA/CS/pEGFP
and 40 µg/ml HA/CS/pCrmA nanoparticles, respectively.
Following incubation for 72 h, the synoviocytes were collected and
used in the subsequent experiments.
Effects of HA/CS/pCrmA nanoparticles on
the expression of MMP genes
To investigate the effects of HA/CS/pDNA on the
synoviocytes, the expression levels of MMP-3 and MMP-13 genes were
detected by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR). Following transfection and incubation for 72 h,
total RNA was extracted from synoviocytes using TRIzol®
reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer’s protocol. RNA concentration and
purity were determined by a spectrophotometer, and the RNA was
reverse transcribed into cDNA using the PrimeScript® RT
Master Mix kit (Takara Biotechnology Co., Ltd., Dalian, China) and
amplified by PCR. The qPCR assay was performed using SYBR Prime Ex
Taq II kit (Invitrogen; Thermo Fisher Scientific, Inc.) on an
iCycler iQ Real-Time PCR detection system (Takara Biotechnology
Co., Ltd.) under the following conditions: Initial pre-denaturation
at 95°C for 30 sec, then 40 cycles of denaturation at 95°C for 5
sec, annealing condition at 60°C for 30 sec and final extension at
72°C for 30 sec. The specific PCR products were confirmed by
melting curve analysis and the target genes were normalized to the
expression of GAPDH using the 2−ΔΔCq relative
quantification method (22). The
forward/reverse primers used were: MMP-3 forward,
5′-GGCCATCTCTTCCTTCAG-3′ and reverse, 5′-GTCACTTTCTTTGCATTTGG-3′;
MMP-13 forward, 5′-TTCGGCTTAGAGGTGACAGG-3′ and reverse,
5′-ACTCTTGCCGGTGTAGGTGT-3′; GAPDH forward,
5′-TGTCGTGGAGTCTACTGGTG-3′ and reverse, 5′-GCATTGCTGACAATCTTGAG-3′.
GAPDH was used as a normalization control.
Immunohistochemical (IHC) analysis
IHC staining was used to qualitatively detect
protein expression levels of the MMP-3 and MMP-13 genes. Following
transfection and incubation for 72 h, synovial cells were seeded on
a 24-well plate with a slide in each well at a density of
2×104 cells/well. The synoviocytes were fixed in 4%
paraformaldehyde for 20 min at 25°C, washed with PBS and
subsequently incubated with primary antibodies against MMP-3 (cat.
no. ab52915; 1:200) and MMP-13 (cat. no. ab75606; 1:200; both
Abcam, Cambridge, UK) overnight at 4°C. Following washing with PBS
three times, the slides were incubated with secondary antibody
labeled with horseradish peroxidase (cat. no. ab205718; 1:5,000;
Abcam) at 37°C for 30 min. Finally, diaminobenzidine was used as
the chromogen. The negative control was cells stained without
primary antibodies. The images were visualized and captured using
the light Nikon H550S Photo Imaging System (Nikon Corporation,
Tokyo, Japan; magnifcation, x400). A total of four
positively-stained fields from each group were analyzed using Image
Pro Plus software 6.0 (Media Cybernetics, Inc., Rockville, MD,
USA).
Statistical analysis
All data are expressed as the mean ± standard
deviation and analyzed using SPSS software, version 19.0 (IBM
Corp., Armonk, NY, USA). Comparison of cell viability was analyzed
by unpaired Student’s t-test and the gene and protein expression
levels of MMP3 and MMP13 among groups were analyzed by one-way
analysis of variance followed by a post-hoc Bonferroni test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Characterization of HA/CS/pDNA
nanoparticles
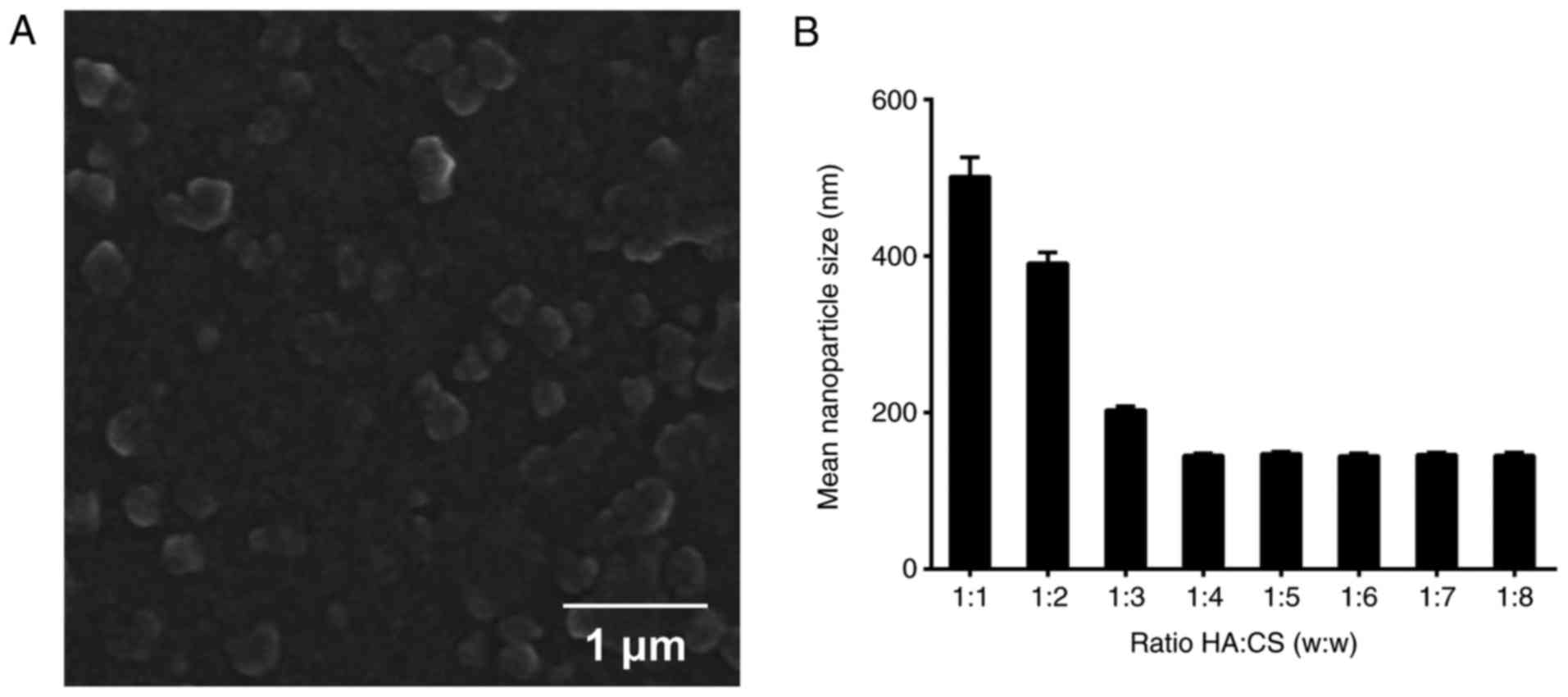
HA/CS/pDNA nanoparticles were prepared and the
morphologies of the HA/CS/pDNA nanoparticles were observed by SEM.
The SEM micrographs indicated that the majority of the HA/CS/pDNA
nanoparticles were spherical, with a diameter between 100-300 nm
(Fig. 1A), which was consistent
with our recent study (23). In
order to identify the association between the weight ratio of HA to
CS and the size of the nanoparticles, nanoparticles with different
weight HA:CS ratios were additionally prepared. As indicated in
Fig. 1B, the nanoparticle size
exhibited a significant decrease with the weight ratios from 1:1 to
1:4; however, no marked alterations between the weight ratios from
1:4 to 1:8 were observed. The smallest size was obtained with the
HA:CS weight ratio of 1:4. Therefore, HA/CS/pDNA particles at the
weight of HA:CS of 1:4 was used in subsequent studies.
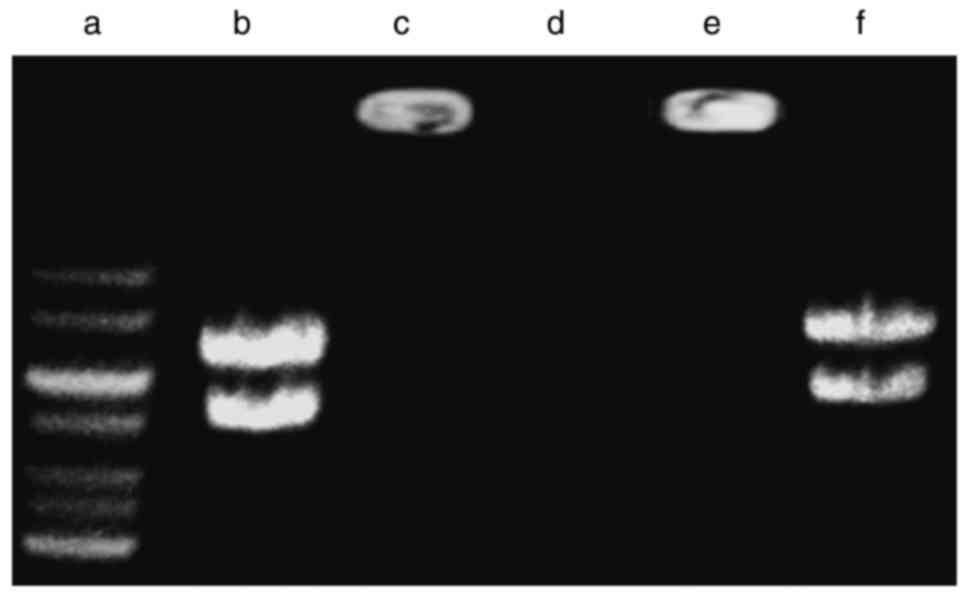
Electrophoresis assay
The capability of pDNA retainment and protection of
the pDNA against degradation by the nanoparticles was examined by
gel electrophoresis. As indicated in Fig. 2, compared with the naked pDNA
(lane b), the migration of pDNA in the agarose gel in the
HA/CS/pDNA samples was inhibited (lane c), which indicated that the
pDNA had completely combined with HA/CS nanoparticles. Following
incubation with DNase I, the naked pDNA was digested (lane d),
while the pDNA from HA/CS nanoparticles remained intact (lane e).
However, following digestion with chitosanase, the pDNA was
released from the nanoparticles and the intensity of the DNA bands
was similar to that of the control (lane f). Therefore, it may be
concluded that HA/CS nanoparticles protected the pDNA from
degradation by DNase I.
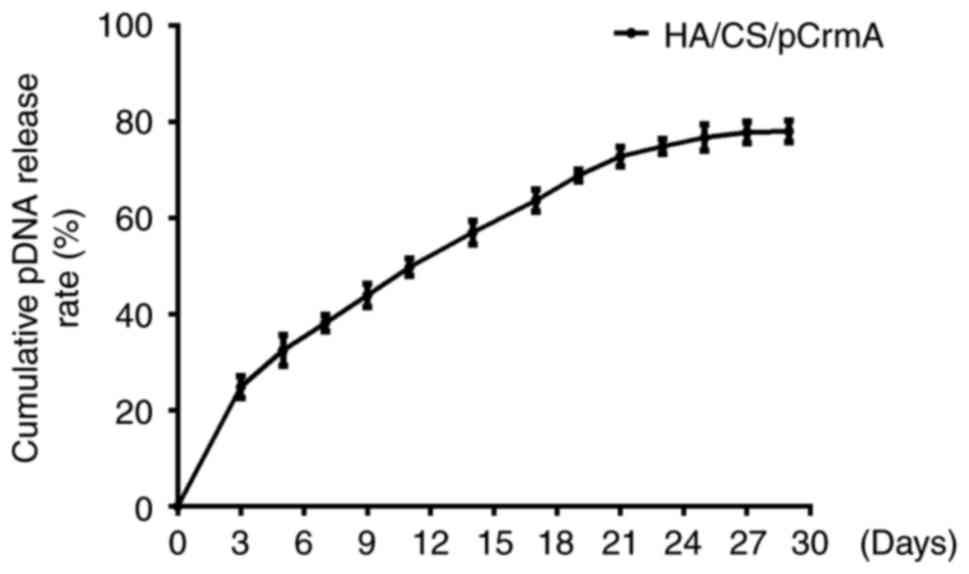
DNA release study in vitro
The in vitro release profiles of pDNA from
the HA/CS nanoparticles in PBS are presented in Fig. 3. The release curve exhibited a
marked increase in the release rate (~25%) in the first 3 days.
Subsequently, a slow release from 37-70% at a constant rate was
observed in the subsequent days. The pDNA release rate slowed and
leveled off on days 25, 27 and 29, at ~77%.
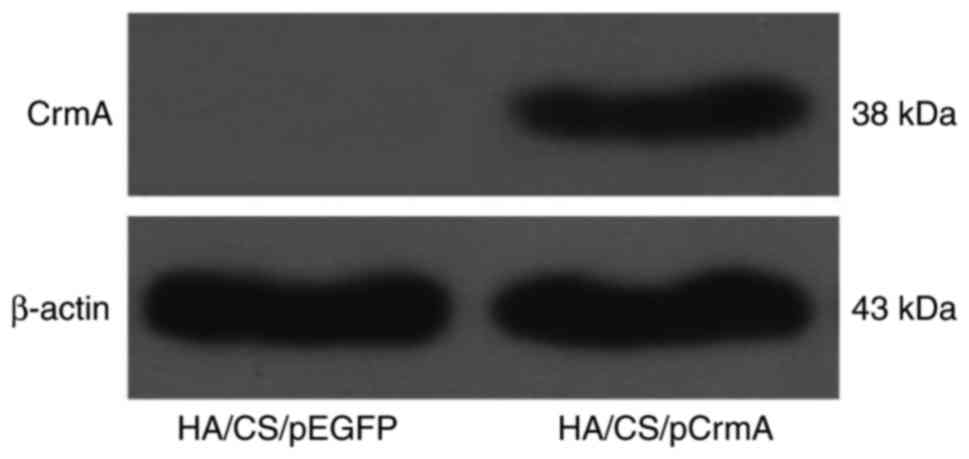
Transfection efficacy of HA/CS/pDNA
nanoparticles in vitro
The synoviocytes were transfected with HA/CS/pDNA
nanoparticles, and then the protein expression levels of the target
genes were visually detected by western blot analysis. The data
presented in Fig. 4 indicated
that CrmA protein was expressed in the HA/CS/pCrmA group. The
expression of CrmA in the HA/CS/pEGFP group was hardly detectable.
These data suggested that the HA/CS nanoparticles were able to
transfect the synoviocytes efficiently.
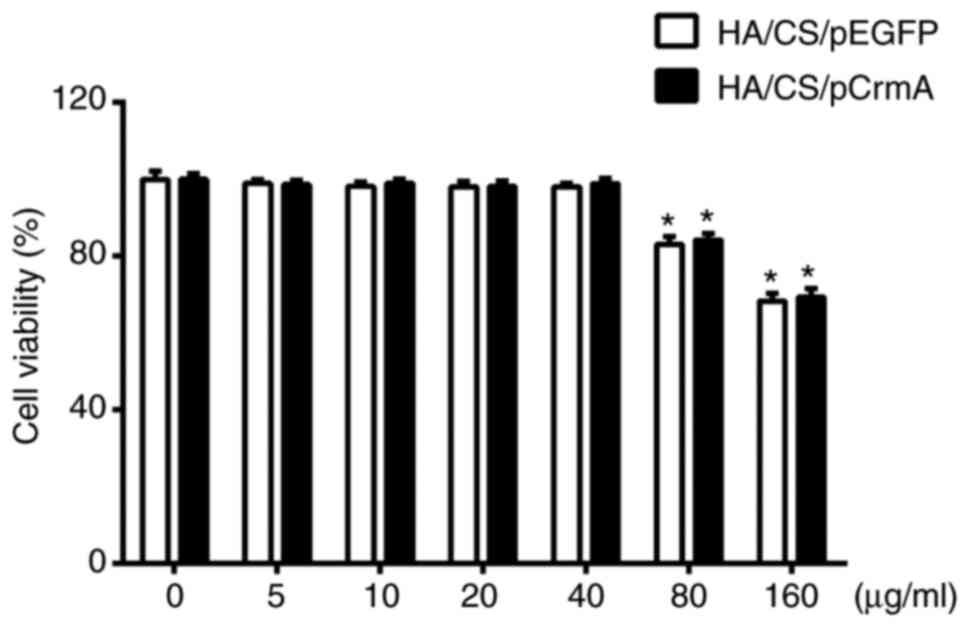
Cell viability
The cytotoxicity of the HA/CS/pDNA nanoparticles was
assessed by MTS assay (Fig. 5).
When the synoviocytes were co-cultured with HA/CS/pDNA
nanoparticles (concentration ≤40 µg/ml), the nanoparticles
exhibited good cytocompatibility in the two experimental groups
(cell viability >95%). There was no difference in the viability
of synoviocytes between the HA/CS/pCrmA and HA/CS/pEGFP groups as
the concentration of HA/CS/pDNA nanoparticles increased to 40
µg/ml. When the concentration of nanoparticles increased to
80 and 160 µg/ml, the cell viability exhibited a significant
decrease in the two groups (P<0.05). Therefore, it was concluded
that the HA/CS nanoparticle was a safe carrier when the
concentration was ≤40 µg/ml. To ensure optimum performance
of the nanoparticles, nanoparticles with a concentration of 40
µg/ml were selected for all subsequent experiments.
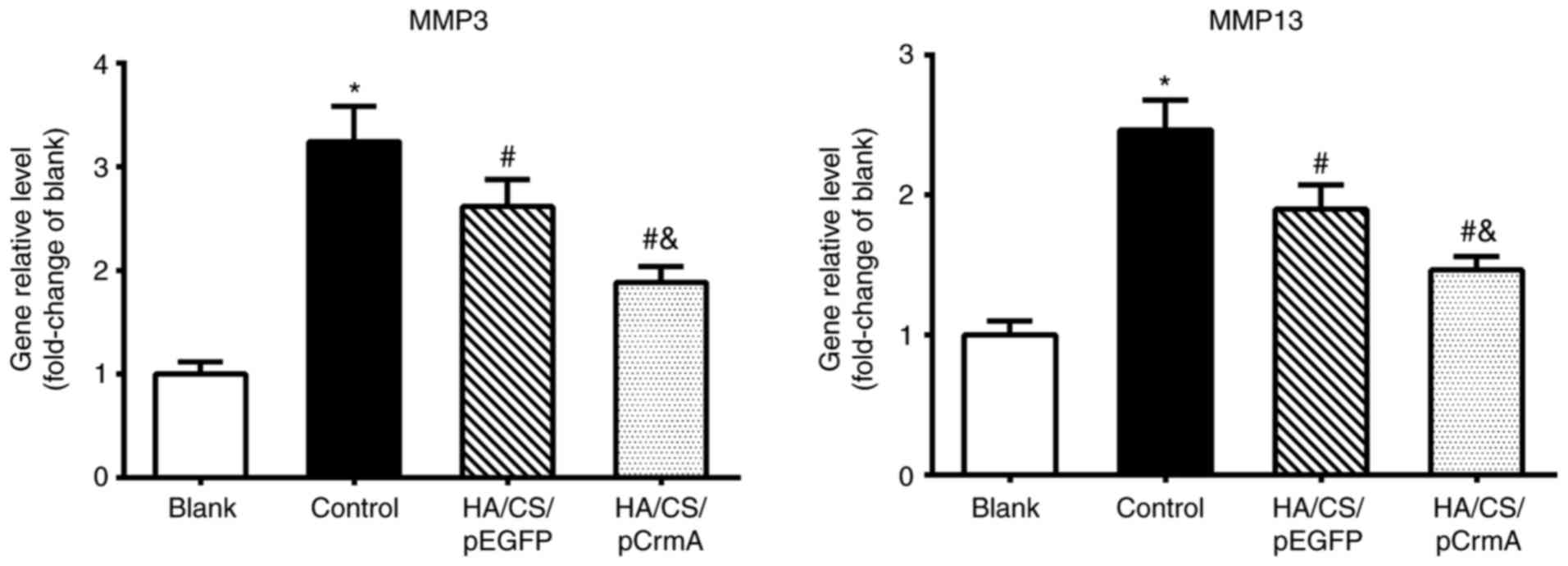
Effects of HA/CS/pCrmA nanoparticles on
the expression of MMPs
To additionally investigate the effects of
HA/CS/pCrmA nanoparticles on synoviocytes of OA, the expression
levels of the MMP-3 and MMP-13 genes at the gene and protein level
were detected. As demonstrated in Fig. 6, the control group exhibited
significantly increased gene expression levels of MMPs compared
with the blank group (P<0.05), indicating that IL-1β may lead to
inflammation and the increased production of MMPs. The gene
expression levels of MMPs in HA/CS/pEGFP and HA/CS/pCrmA group were
decreased compared with the control group (P<0.05). However, the
HA/CS/pCrmA group exhibited a significantly decreased expression
level of MMPs compared with the HA/CS/pEGFP group (P<0.05).
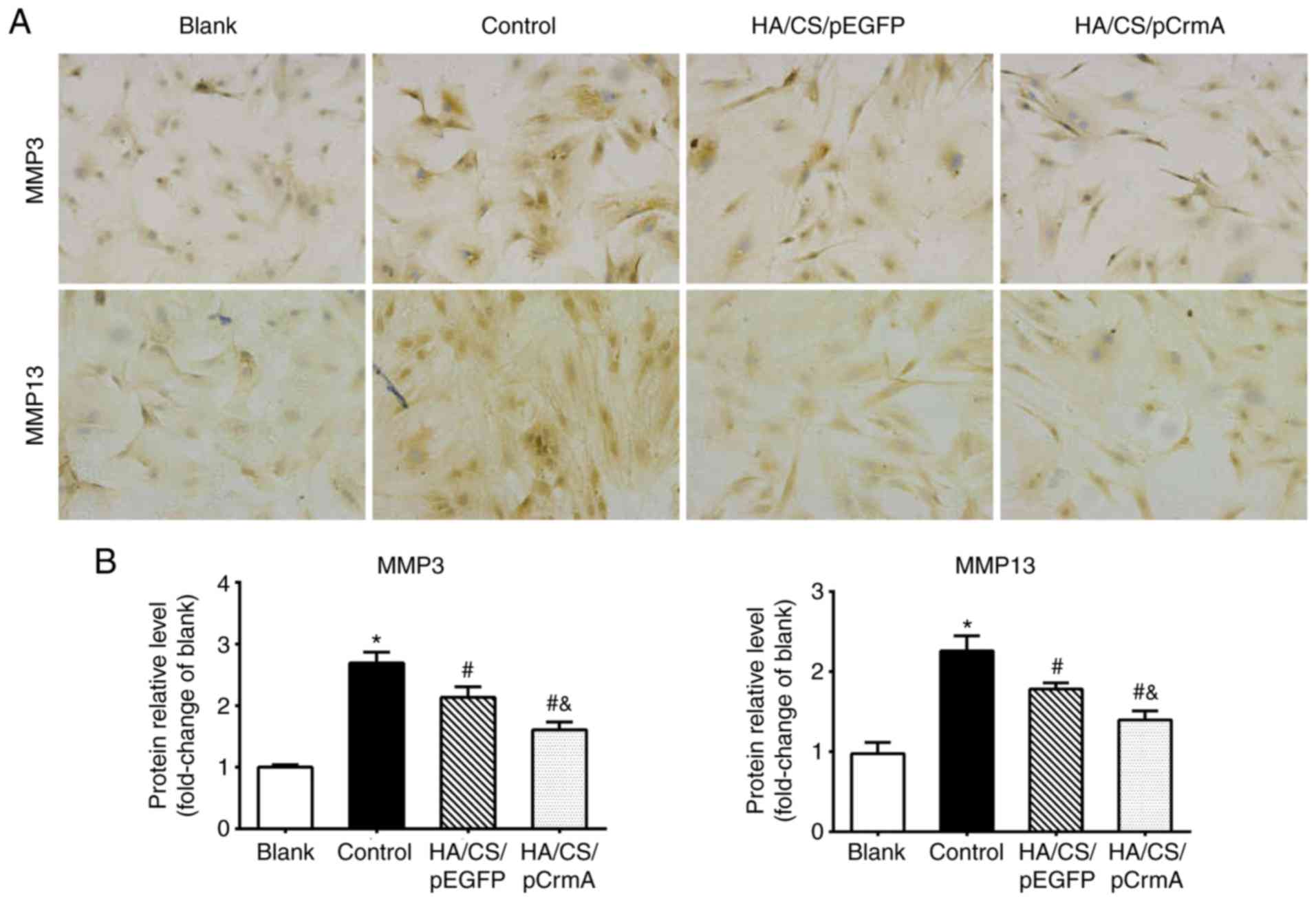
IHC staining was used to assess the protein
expression levels of the MMPs. Fig.
7 indicated that the synoviocytes in the control group were
markedly stained compared with that in the blank group (P<0.05),
suggesting increased expression levels of MMPs in the control
group. In the treatment groups, weakened expression levels of MMPs
were observed compared with that in control group; in particular,
the synoviocytes cultured with HA/CS/pCrmA nanoparticles exhibited
weaker expression of MMPs. These results indicated that HA/CS/CrmA
nanoparticles may attenuate IL-1β mediated inflammation in
synoviocytes.
Discussion
In the present study, HA/CS nanoparticles were used
as vectors to deliver plasmid DNA into synoviocytes in a controlled
manner. HA/CS/pCrmA nanoparticles were prepared and the appropriate
HA:CS weight ratio of 1:4 was identified and selected for improved
transfection efficiency. The synoviocytes were then transfected
with nanoparticles and the results indicated that HA/CS/pCrmA
nanoparticles were a safe carrier and may decrease the expression
level of MMPs genes in synoviocytes.
To ensure the safety and efficiency of gene
delivery, biomaterial is preferred compared with virus vectors for
preparation of transfection systems. CS is widely used to fabricate
carriers and tissue engineering material (24-27). It is a cationic polymer and
interacts with negatively-charged cellular membranes (28). However, the single use of CS, due
to its poor water solubility, has limited its application (24). HA, as an anionic polymer, is an
additional biomaterial with clinical applications (29-31). HA is able to combine with cluster
of differentiation 44 (CD44), which is known as a cell surface
receptor and widely expressed in a variety of cell membranes
(32). In the present study, HA
was associated with CS through electrostatic interaction and the
HA/CS nanoparticles were formed, as previously described (33). The results indicated that there
was a significant decrease in nanoparticle size with the increasing
amount of CS, and the smallest size was obtained with a HA:CS
weight ratio of 1:4. CS has been demonstrated to interact with pDNA
through electrostatic/hydrogen bonding (34). As the amount of CS increased, the
nanoparticle size became smaller and the surface charge became more
positive (35), thereby
contributing to their internalization into synoviocytes and
improvement of transfection efficiency (36). Conversely, the conjunction of HA
may additionally improve the cell adhesion ability and transfection
efficiency (37). HA is able to
bind to the CD44 receptors of synoviocytes to improve
internalization rate and loosen the interaction between CS and pDNA
(38,39), leading to the easy release of the
loaded gene following internalization, thereby facilitating gene
transfection and expression. Therefore, HA/CS/pDNA nanoparticles at
the weight of HA:CS of 1:4 were used in the present study.
Subsequently, the capability of the nanoparticles to retain and
protect the pDNA from degradation was identified using gel
electrophoresis. The results indicated that the migration of pDNA
in the agarose gel was completely inhibited in the HA/CS/pCrmA
group, suggesting that the pDNA had completely combined with HA/CS
nanoparticles. This is consistent with the results of our previous
studies (19,20).
The in vitro release assay demonstrated that
the pDNA release rate leveled off at day 29, and the release rate
measured ~77%, demonstrating the long-term transfection and highly
efficient release capacity of the complex. The nanoparticles
exhibited a sustained release of pDNA over 4 weeks, with a
particularly high initial release rate in the first week. These
release kinetics, in particular the high level of gene expression
in the initial stages, would confer an advantage in the treatment
of OA. The expression level of CrmA is an additional key
characteristic of the nanoparticles. In the present study, western
blot analysis was used to detect the expression level of CrmA in
transfected synoviocytes. As expected, the obvious expression of
CrmA in synoviocytes cultured with HA/CS/pCrmA nanoparticles was
visually observed. This indicates that the HA/CS/pCrmA
nanoparticles had successfully transfected the synoviocytes. The
MTS assay indicated that the HA/CS/pDNA nanoparticles exhibited low
cytotoxicity when cultured with synoviocytes at the concentrations
of 5, 10, 20 and 40 µg/ml, while the nanoparticles at high
concentrations (80 and 160 µg/ml) exhibited significant
levels of cytotoxicity. This result indicated that HA/CS
nanoparticles, at appropriate concentrations, possessed good
cytocompatibility. In summary, HA/CS/pDNA nanoparticles may be used
in a gene delivery system with considerable efficacy and low
toxicity.
IL-1β serves a key role in the pathogenesis and
progression of OA (40). It may
stimulate chondrocytes and synoviocytes to secrete proteinases
leading to cartilage destruction (41), and inhibit the synthesis of
collagen type II (42), which is
the primary component of the ECM in articular cartilage. Therefore,
IL-1β may be a promising target for the treatment of OA. CrmA is a
natural inhibitor of caspase-1 (43), which has great specificity for
cleaving the precursor pro-IL-1β, thereby decreasing the secretion
of IL-1β (44). In the present
study, the IL-1β-induced synoviocytes were considered to represent
an in vitro OA model. To additionally investigate the
therapeutic potential of HA/CS/pCrmA nanoparticles on synoviocytes
of OA, the expression levels of the osteogenic genes MMP-3 and
MMP-13 were detected. As demonstrated in the results, the
HA/CS/pEGFP and HA/CS/pCrmA groups exhibited greater downregulated
expression levels of MMPs compared with the controls. In addition,
the HA/CS/pCrmA nanoparticles decreased the expression level of
MMPs genes significantly compared with the HA/CS/pEGFP
nanoparticles, indicating that the inflammation of synoviocytes was
attenuated by HA/CS/pCrmA nanoparticles. HA and CS have been widely
used in clinical settings. HA injection has been used in the
treatment of OA (45), and CS is
structurally similar to glycosaminoglycans (46), which are important components of
the ECM. It was concluded that the use of HA/CS nanoparticles may
have beneficial effects on synoviocytes; concomitantly, CrmA
exhibited great potential in inhibiting the inflammatory response
in synoviocytes of OA. As aforementioned, the suppression of
inflammatory cytokines activity may be an important mechanism of
attenuating IL-1β-induced inflammation in synoviocytes.
In the present study, HA/CS nanoparticles were
successfully constructed as vectors for the delivery of CrmA into
synoviocytes. As expected, the HA/CS/pCrmA nanoparticles exhibited
good safety and biocompatibility, and conferred protection against
inflammation in synoviocytes induced by IL-1β in vitro.
Furthermore, HA/CS/pCrmA nanoparticles have additionally been
indicated to serve protective effects on the cartilage damage and
synovial inflammation in a rat anterior cruciate ligament
transaction model of OA in vivo in our recent study
(23). Therefore, the HA/CS/
pCrmA nanoparticles may present a potential novel approach for the
gene therapy of OA in a clinical setting.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant nos. 81871816 and
81501921) and Wuhan Science and Technology Project of China (grant
no. 2016060101010045).
Availability of data and materials
The data used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors’ contributions
BQ, X-FX and P-HZ conceived and designed the
experiment. R-HD, G-QX and X-FS performed the experiments. G-QX
acquired the reagents and materials. XS analyzed the data. X-FX and
P-HZ wrote the manuscript. All authors approved the final version
of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee of Wuhan University (approval no.
2017-0208).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Kiener HP, Ackermann J, Redlich K, Radda
I, Steiner CW, Bitzan P, Smolen JS and Drach J: Interphase
fluorescence in situ hybridization analysis of fibroblast-like
synoviocytes of patients with rheumatoid arthritis and
osteoarthritis. Arthritis Res Ther. 3(Suppl 2): P1192001.
View Article : Google Scholar
|
|
2
|
Abramson S, Attur M, Dave M, Leung M,
Patel J, Gomez P and Amin A: Paracrine pathways of cartilage
destruction in osteoarthritis. Arthritis Res Ther. 5(Suppl 3):
S22003. View
Article : Google Scholar
|
|
3
|
Sun AR, Panchal SK, Friis T, Sekar S,
Crawford R, Brown L, Xiao Y and Prasadam I: Obesity-associated
metabolic syndrome spontaneously induces infiltration of
pro-inflammatory macrophage in synovium and promotes
osteoarthritis. PLoS One. 12:e01836932017. View Article : Google Scholar
|
|
4
|
Goldring MB and Otero M: Inflammation in
osteoarthritis. Curr Opin Rheumatol. 23:471–478. 2011. View Article : Google Scholar
|
|
5
|
Wojdasiewicz P, Poniatowski LA and
Szukiewicz D: The role of inflammatory and anti-inflammatory
cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm.
2014:5614592014. View Article : Google Scholar
|
|
6
|
Suzuki M, Hashizume M, Yoshida H, Shiina M
and Mihara M: IL-6 and IL-1 synergistically enhanced the production
of MMPs from synovial cells by up-regulating IL-6 production and
IL-1 receptor I expression. Cytokine. 51:178–183. 2010. View Article : Google Scholar
|
|
7
|
Martel-Pelletier J, Boileau C, Pelletier
JP and Roughley PJ: Cartilage in normal and osteoarthritis
conditions. Best Prac Res Clin Rheumatol. 22:351–384. 2008.
View Article : Google Scholar
|
|
8
|
Liu-Bryan R: Synovium and the innate
inflammatory network in osteoarthritis progression. Curr Rheumatol
Rep. 15:3232013. View Article : Google Scholar
|
|
9
|
Ginn SL, Alexander IE, Edelstein ML, Abedi
MR and Wixon J: Gene therapy clinical trials worldwide to 2012 an
update. J Gene Med. 15:65–77. 2013. View
Article : Google Scholar
|
|
10
|
Zhang Y, Wei H, Xu L, Yan G, Ma C, Yu M,
Wei C and Sun Y: Preparation and evaluation of a non-viral gene
vector for SiRNA: Multifunctional envelope-type nano device. Artif
Cells Nanomed Biotechnol. 44:1259–1265. 2016.
|
|
11
|
Yu B, Chen Y, Ouyang C, Huang H, Ji L and
Chao H: A luminescent tetranuclear ruthenium(II) complex as a
tracking non-viral gene vector. Chem Commun (Camb). 49:810–812.
2013. View Article : Google Scholar
|
|
12
|
Malakooty Poor E, Baghaban Eslaminejad M,
Gheibi N, Bagheri F and Atyabi F: Chitosan-pDNA nanoparticle
characteristics determine the transfection efficacy of gene
delivery to human mesenchymal stem cells. Artif Cells Nanomed
Biotechnol. 42:376–384. 2014. View Article : Google Scholar
|
|
13
|
Zhang X, Yu C, Xushi, Zhang C, Tang T and
Dai K: Direct chitosan-mediated gene delivery to the rabbit knee
joints in vitro and in vivo. Biochem Biophys Res Commun.
341:202–208. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu X, Qiu S, Zhang Y, Yin J and Min S:
PELA microspheres with encapsulated arginine-chitosan/pBMP-2
nanoparticles induce pBMP-2 controlled-release, transfected
osteoblastic progenitor cells, and promoted osteogenic
differentiation. Artif Cells Nanomed Biotechnol. 45:330–339. 2017.
View Article : Google Scholar
|
|
15
|
Mansouri S, Cuie Y, Winnik F, Shi Q,
Lavigne P, Benderdour M, Beaumont E and Fernandes JC:
Characterization of folate-chitosan-DNA nanoparticles for gene
therapy. Biomaterials. 27:2060–2065. 2006. View Article : Google Scholar
|
|
16
|
Almond A: Hyaluronan. Cell Mol Life Sci.
64:1591–1596. 2007. View Article : Google Scholar
|
|
17
|
Lu HD, Zhao HQ, Wang K and Lv LL: Novel
hyaluronic acid-chitosan nanoparticles as non-viral gene delivery
vectors targeting osteoarthritis. Int J Pharm. 420:358–365. 2011.
View Article : Google Scholar
|
|
18
|
Lu H, Lv L, Dai Y, Wu G, Zhao H and Zhang
F: Porous chitosan scaffolds with embedded hyaluronic
acid/chitosan/plasmid-DNA nanoparticles encoding TGF-β1 induce DNA
controlled release, transfected chondrocytes, and promoted cell
proliferation. PLoS One. 8:e699502013. View Article : Google Scholar
|
|
19
|
Qiu B, Gong M, He QT and Zhou PH:
Controlled release of interleukin-1 receptor antagonist from
hyaluronic acid-chitosan microspheres attenuates interleukin-1
β-induced inflammation and apoptosis in chondrocytes. Biomed Res
Int. 2016:62909572016. View Article : Google Scholar
|
|
20
|
Ma BL, Zhou PH, Xie T, Shi L, Qiu B and
Wang Q: Inhibition of interleukin-1beta-stimulated
dedifferentiation of chondrocytes via controlled release of CrmA
from hyaluronic acid-chitosan microspheres. BMC Musculoskelet
Disord. 16:612015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dobo J, Swanson R, Salvesen GS, Olson ST
and Gettins PG: Cytokine response modifier a inhibition of
initiator caspases results in covalent complex formation and
dissociation of the caspase tetramer. J Biol Chem. 281:38781–38790.
2006. View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Zhou PH, Qiu B, Deng RH, Li HJ, Xu XF and
Shang XF: Chondroprotective effects of hyaluronic acid-chitosan
nanoparticles containing plasmid DNA encoding cytokine response
modifier a in a rat knee osteoarthritis model. Cell Physiol
Biochem. 47:1207–1216. 2018. View Article : Google Scholar
|
|
24
|
Luangtana-Anan M, Limmatvapirat S,
Nunthanid J, Chalongsuk R and Yamamoto K: Polyethylene glycol on
stability of chitosan microparticulate carrier for protein. AAPS
Pharmscitech. 11:1376–1382. 2010. View Article : Google Scholar
|
|
25
|
Hou Y, Hu J, Park H and Lee M:
Chitosan-based nanoparticles as a sustained protein release carrier
for tissue engineering applications. J Biomed Mater Res A.
100:939–947. 2012. View Article : Google Scholar :
|
|
26
|
Kang YM, Lee BN, Ko JH, Kim GH, Kang KN,
Kim DY, Kim JH, Park YH, Chun HJ, Kim CH and Kim MS: In vivo
biocompatibility study of electrospun chitosan microfiber for
tissue engineering. Int J Mol Sci. 11:4140–4148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang L and Stegemann JP: Thermogelling
chitosan and collagen composite hydrogels initiated with
beta-glycerophosphate for bone tissue engineering. Biomaterials.
31:3976–3985. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Masarudin MJ, Cutts SM, Pietersz GA,
Evison BJ, Phillips DR and Pigram PJ: Factors determining the
stability, size distribution, and cellular accumulation of small,
monodisperse chitosan nanoparticles as candidate vectors for
anticancer drug delivery: Application to the passive encapsulation
of [(14) C]-doxorubicin. Nanotechnol Sci Appl. 9:47. 2016.
View Article : Google Scholar
|
|
29
|
Huang D, Wang R and Yang S: Cogels of
hyaluronic acid and acellular matrix for cultivation of
adipose-derived stem cells: Potential application for vocal fold
tissue engineering. Biomed Res Int. 2016:65840542016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi J, Ma R, Wang L, Zhang J, Liu R, Li L,
Liu Y, Hou L, Yu X, Gao J and Zhang Z: The application of
hyaluronic acid-derivatized carbon nanotubes in hematoporphyrin
monomethyl ether-based photodynamic therapy for in vivo and in
vitro cancer treatment. Int J Nanomedicine. 8:2361–2373. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huberlant S, Fernandez H, Vieille P,
Khrouf M, Ulrich D, deTayrac R and Letouzey V: Application of a
hyaluronic acid gel after intrauterine surgery may improve
spontaneous fertility: A randomized controlled trial in new zealand
white rabbits. Plos One. 10:e01256102015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim Y and Kumar S: CD44-mediated adhesion
to hyaluronic acid contributes to mechanosensing and invasive
motility. Mol Cancer Res. 12:1416–1429. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Contreras-Ruiz L, de la Fuente M, Parraga
JE, López-García A, Fernández I, Seijo B, Sánchez A, Calonge M and
Diebold Y: Intracellular trafficking of hyaluronic acid-chitosan
oligomer-based nanoparticles in cultured human ocular surface
cells. Mol Vis. 17:279–290. 2011.
|
|
34
|
Centelles MN, Qian C, Campanero MA and
Irache JM: New methodologies to characterize the effectiveness of
the gene transfer mediated by DNA-chitosan nanoparticles. Int J
Nanomedicine. 3:451–460. 2008.
|
|
35
|
Chen Y, Mohanraj VJ, Wang F and Benson HA:
Designing chitosan-dextran sulfate nanoparticles using charge
ratios. AAPS Pharmscitech. 8:E982007. View Article : Google Scholar
|
|
36
|
Oliverira AV, Silva AP, Bitoque DB, Silva
GA and Rosa da Costa AM: Transfection efficiency of chitosan and
thiolated chitosan in retinal pigment epithelium cells: A
comparative study. J Pharm Bioallied Sci. 5:111–118. 2013.
View Article : Google Scholar
|
|
37
|
Granot E, Shouval D and Ashur Y: Cell
adhesion molecules and hyaluronic acid as markers of inflammation,
fibrosis and response to antiviral therapy in chronic hepatitis C
patients. Mediators Inflamm. 10:253–258. 2001. View Article : Google Scholar
|
|
38
|
Khurana SS, Riehl TE, Moore BD, Fassan M,
Rugge M, Romero-Gallo J, Noto J, Peek RM Jr, Stenson WF and Mills
JC: The hyaluronic acid receptor CD44 coordinates normal and
metaplastic gastric epithelial progenitor cell proliferation. J
Biol Chem. 288:16085–16097. 2013. View Article : Google Scholar
|
|
39
|
Wang T, Hou J, Su C, Zhao L and Shi Y:
Hyaluronic acid-coated chitosan nanoparticles induce ROS-mediated
tumor cell apoptosis and enhance antitumor efficiency by targeted
drug delivery via CD44. J Nanobiotechnology. 15:72017. View Article : Google Scholar :
|
|
40
|
Aborehab NM, El Bishbishy MH, Refaiy A and
Waly NE: A putative Chondroprotective role for IL-1 beta and MPO in
herbal treatment of experimental osteoarthritis. BMC Complement
Altern Med. 17:4952017. View Article : Google Scholar
|
|
41
|
Afif H, Benderdour M, Mfuna-Endam L,
Martel-Pelletier J, Pelletier JP, Duval N and Fahmi H: Peroxisome
proliferator-activated receptor gamma 1 expression is diminished in
human osteoarthritis cartilage and is downregulated by IL-1 beta in
articular chondrocytes. Arthritis Res Ther. 9:R312007. View Article : Google Scholar
|
|
42
|
Chia WT, Yeh LT, Chen YW, Lee HS, Chang DM
and Sytwu HK: IL-1 beta in irrigation fluid and mRNA expression in
synovial tissue of the knee joint as therapeutic markers of
inflammation in collagen antibody-induced arthritis. Dis Markers.
26:1–7. 2009. View Article : Google Scholar
|
|
43
|
Ekert PG, Silke J and Vaux DL: Inhibition
of apoptosis and clonogenic survival of cells expressing crmA
variants: Optimal caspase substrates are not necessarily optimal
inhibitors. EMBO J. 18:330–338. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mayer-Barber KD, Barber DL, Shenderov K,
White SD, Wilson MS, Cheever A, Kugler D, Hieny S, Caspar P, Núñez
G, et al: Cutting edge: Caspase-1 independent IL-1 beta production
is critical for host resistance to mycobacterium tuberculosis and
does not require TLR signaling in vivo. J Immunol. 184:3326–3330.
2010. View Article : Google Scholar
|
|
45
|
Wang F and He X: Intra-articular
hyaluronic acid and corticosteroids in the treatment of knee
osteoarthritis: A meta-analysis. Exp Ther Med. 9:493–500. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Georgopoulou A, Kaliva M, Vamvakaki M and
Chatzinikolaidou M: Osteogenic potential of pre-osteoblastic cells
on a chitosan-graft-polycaprolactone copolymer. Materials (Basel).
11:E4902018. View Article : Google Scholar
|