Introduction
The blue-green microalga, spirulina (Arthrospira
platensis), has recently been used as a food source due to its
high protein content and nutritional value (1,2).
Spirulina has been extensively studied owing to its nutrient
composition, which has been demonstrated to effectively treat a
number of medical conditions (such as anti-cancer, inhibiting
senescence and enhancing the non-specific cellular immune function)
(3). Spirulina has also been
reported to have an inhibitory effect on ultraviolet B-induced skin
inflammation (4,5). It has an antioxidant defense system
that removes reactive oxygen species that may damage cells
(6-8). Previous studies have reported that
early skin aging can be repaired using an extract from microalgae,
which prevents wrinkle formation and has a tightening effect
(9); thus, such extracts have
been used in many skin care products (10). Despite these benefits, the
mechanisms underlying the protective effects of spirulina crude
protein (SPCP) on skin cells are largely unknown.
The prevention of skin aging has attracted
considerable attention, both scientifically and cosmetically. Owing
to the disruption of its barrier function over time, the aged skin
has a dry appearance and has an increased risk of skin disorders
(11). Deterioration of skin
elasticity and the skin extracellular matrix (ECM) are
characteristics of the aging skin. Reduction of the elastic
properties in dermal layers can lead to the formation of wrinkles
in humans and animals (12-14). Furthermore, three-dimensional
alteration of elastic fibers indicates the marked and continuous
upregulation of an elastin-degrading enzyme in the aging skin. The
ECM of the dermis consists of collagen and is produced by
fibroblasts (15). In particular,
type I collagen is the most prevalent of the fibril-forming
collagens (16,17). Matrix metalloproteinase-8 (MMP-8),
a zinc-dependent endopeptidase, is present at sites of acute
inflammation and potently degrades type I collagen (18,19). Anti-wrinkle strategies therefore
include enhancing skin elasticity and collagen content.
Additionally, the promotion of proliferation and differentiation of
human dermal fibroblasts can also help delay the aging process of
the skin (20-22).
Fibroblast proliferation is regulated by many growth
factors. Epidermal growth factor receptor (EGFR) is one of the most
well-known cell proliferation proteins in the body (23), and it has been reported that EGFR
levels in dermal fibroblasts decline with age (21). A previous study has demonstrated
that EGFR inhibitors may promote human skin aging (24). Green et al (25), reported that EGFR expression
levels are lower in the dorsal skin of old rats (day 23 or day 51)
compared with younger ones (neonatal, day 1). Furthermore, EGFR
expression levels were lower in old human dermal fibroblasts
(Passage 16 or greater) compared with young human fibroblasts
(Passage 9 or less) (26). The
mitogen-activated protein kinase (MAPK) signaling pathway is one of
the EGFR-activated downstream signaling pathways and is an
important regulator of cell proliferation (27,28). Therefore, in the present study,
the effects of SPCP on the EGFR/MAPK signaling pathway in CCD-986sk
cells were investigated. These results shed light on the molecular
mechanism for SPCP increasing the viability of human skin cell and
provide a potential efficient cosmeceutical for protecting human
skin.
Materials and methods
Preparation of SPCP
Spirulina powder [40 g, New Zealand Nutritionals
(2004) Ltd., Burnside Christchurch, New Zealand] was soaked in
distilled water (1l) and mixed for 4 h at room temperature.
Following centrifugation at 2,399 × g at 4°C for 10 min, it was
incubated for 4 h with three volumes of ethanol at 4°C. The
solution was centrifuged at 2,399 × g at 4°C for 10 min. The
supernatant was filtered and concentrated using rotary evaporation
at 40°C (29), and the
concentrated solution was precipitated overnight with 80% saturated
(NH4)2SO4 solution at 4°C. After
standing at 4°C for 10 min, the precipitate was dissolved in and
dialyzed against distilled water. The dialysate was concentrated
(Rotary evaporator, 40°C) and freeze-dried (1000 µg/ml), and
subsequently used as the SPCP preparation in subsequent
experiments.
Assessment of the SPCP protein
profile
The protein profile of the SPCP was analyzed by
subjecting the extract to SDS-PAGE and Coomassie Brilliant Blue
staining. In brief, the SPCP were mixed with 5 × sample loading
buffer [50 mM Tris-HCl, 2% SDS, 10% Glycerol, 0.02% Bromophenol
blue (BPB), 5% 2-mercaptoethanol], and SPCP containing 75 µg
of protein were separated by 15% SDS-PAGE. The gels were stained
with Coomassie Brilliant Blue for 1 h at room temperature and
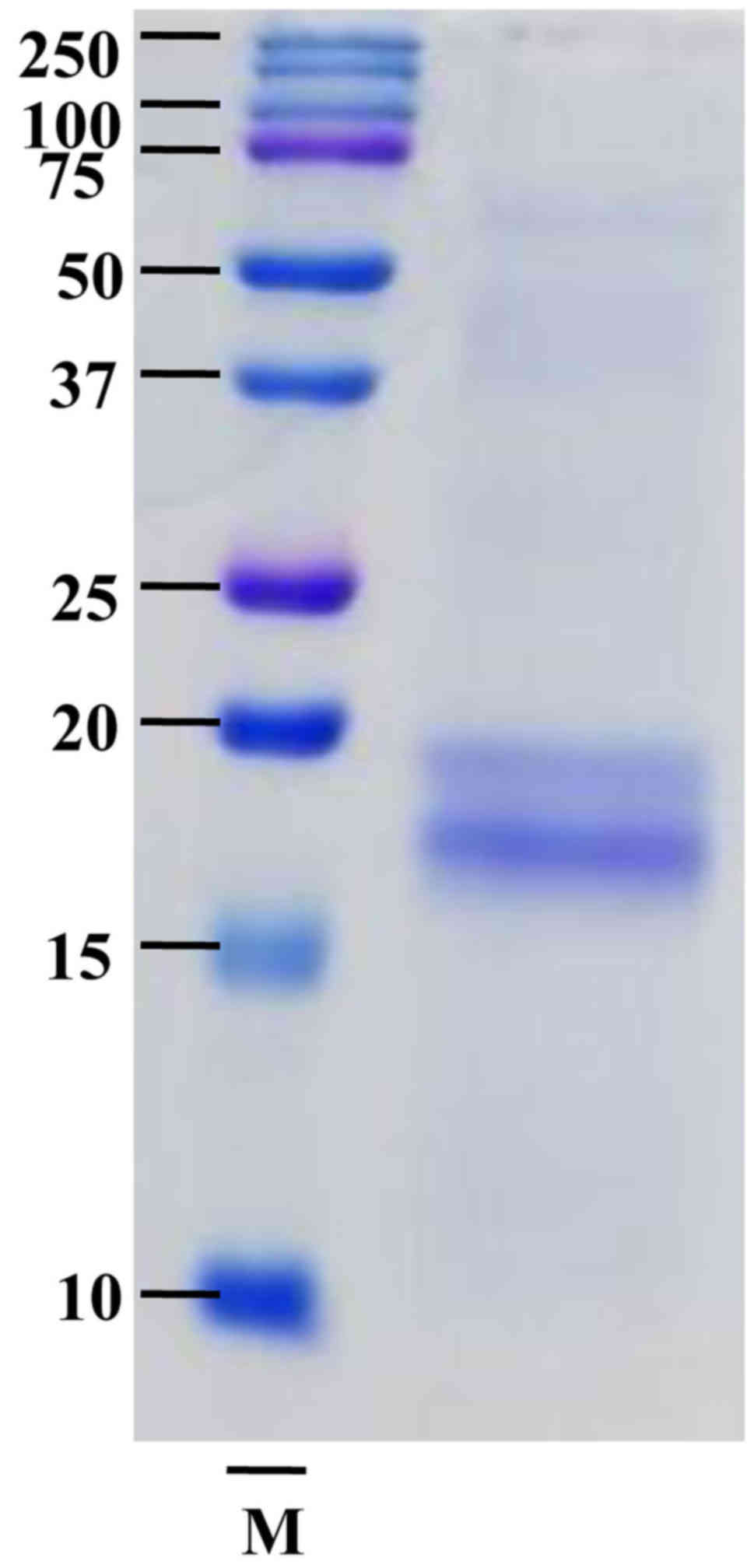
washed with destaining solution until the bands appeared (3). SPCP of 20 kDa and ~16 kDa were
performed a quadrupole time of flight mass spectrometry (Q-TOF
MS/MS) analysis by Peptron, Inc. (Daejeon, Korea).
Cell culture
The CCD-986sk human dermal fibroblast cell line
(CRL-1947; American Type Culture Collection, Manassas, VA, USA),
derived from normal female skin tissue, was cultured in Dulbecco's
Modified Eagle's Medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin/streptomycin at 37°C with 5%
CO2 in a saturated humidified incubator (30). The CCD-986sk cells were cultured
to 60-80% confluency in 100-mm diameter plates, and the medium was
replaced every 2 days.
Cell viability assay
Cell viability was determined using the CellTiter 96
AQueous One Solution Reagent (Promega Corporation, Madison, WI,
USA). CCD-986sk cells were seeded in a 96-well plate at a density
of 0.5×104 cells/well. After 24 h of incubation, the
cells were incubated with serum-free medium (SFM) for 4 h at 37°C.
Serum contains several hormones, which are stimulatory for cell
growth and mask the effects of SPCP. To avoid the complication, SFM
was used in all of the groups tested (control and SPCP-treated
groups) (31). Subsequently,
various concentrations of SPCP (6.25, 12.5 or 25 µg/ml in
SFM) or SFM alone (control) were used to treat the cells. The cells
were further cultured for 24 h. Subsequently, the cells were
exposed to the
3-(4,5-dimethylthiazol-2-yl)-5-(3-caboxyme-thoxy-phenyl)-2-(4-sulfonyl)-2H-tetrazolium
(MTS) assay solution at 37°C for 30 min, and the optical density at
490 nm was measured using a Synergy HTX microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA). Data are expressed as the
percentage of viable SPCP-treated cells compared with viable cells
in the SFM-treated control.
Elastase activity
Elastase activity was measured using
N-succinyl-Ala-Ala-Ala-p-nitroanilide (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). Cells were seeded into 6-well plates at a
density of 5×104 cells/well. After 24 h of culture, the
cells were incubated with SFM for an additional 4 h at 37°C.
Various concentrations of SPCP (6.25, 12.5 or 25 µg/ml in
SFM) or SFM alone (control) were used to treat the cells, and they
were further cultured for 24 h. The cells were collected in
radioimmunoprecipitation assay buffer (iNtRON Biotechnology,
Seongnam, Korea) with 1% protease inhibitor using a cell scraper.
The cell supernatant was collected by centrifugation (1,8341 × g;
4°C; 10 min). Subsequently, the supernatant (98 µl) and 25
mg/ml N-succinyl-Ala-Ala-Ala-p-nitroanilide (2 µl) were
added to each well (32). After
incubation at 37°C for 30 min, the optical density at 410 nm was
measured using a Synergy HTX micro-plate reader (BioTek
Instruments, Inc.). Data are expressed as a percentage of elastase
activity in treated cells compared with the SFM-treated
control.
Procollagen type I C-peptide (PIP) solid
phase enzyme immunoassay
The level of procollagen was measured with the
Takara MK101 kit (Takara Bio, Inc., Otsu, Japan). Cells were seeded
into 6-well plates at a density of 5×104 cells/well.
After 24 h of incubation, the cells were incubated with SFM for 4 h
at 37°C, followed by treatment with various concentrations of SPCP
(6.25, 12.5, or 25 µg/ml in SFM) or SFM (control) for an
additional 24 h. The cell medium was collected and centrifuged
(13,475 × g; 4°C; 10 min). Subsequently, 100 µl of
antibody-peroxidase conjugate solution (included in the Takara
MK101 kit) and 20 µl of supernatant were added to each well.
After incubation for 3 h at 37°C, each well was washed four times
using 400 µl of PBS each time. A total of 100 µl of
3,3',5,5'-tetramethylbenzidine substrate solution was then added to
each well and incubated for 15 min at room temperature (32). The reaction was terminated with
100 µl of stop solution (1 N H2SO4).
The absorbance at 450 nm was measured using a Synergy HTX
microplate reader (BioTek Instruments, Inc.).
Western blotting
CCD-986sk cells were cultured to 50-60% confluency
and then incubated with SFM for 4 h at 37°C. The medium was
replaced with three concentrations of SPCP (6.25, 12.5 or 25
µg/ml in SFM) or SFM (control). The cells were cultured for
another 24 h and proteins were extracted using
radioimmunoprecipitation lysis buffer (iNtRON Biotechnology) with
1% protease inhibitor, followed by centrifugation at 18,341 × g at
4°C for 10 min. Protein concentrations were determined using the
Bicinchoninic assay. The protein samples (30 µg) were
resolved by 7.5-12.5% SDS-PAGE and subsequently transferred
electrophoretically to polyvinylidene difluoride membranes. After
washing with methanol, the membranes were blocked with 1% bovine
serum albumin (MP Biomedicals LLC, USA) in TBS + Tween-20 [10 mM
Tris-HCl, 150 mM NaCl (pH 7.5) and 0.1% Tween-20] and incubated
overnight at 4°C with specific primary antibodies (all 1:1,000)
(33). After washing twice, the
membranes were incubated for 2 h at room temperature with the
secondary antibodies (all 1:10,000). The second antibodies were
horseradish peroxidase (HRP)-conjugated anti-rabbit lg G (cat. no.
7074S; Cell Signaling Technology, Inc., Beverly, MA, USA), donkey
anti-goat lgG (cat. no. A50-101p; Bethyl Laboratories, Inc., MA,
USA) and anti-mouse lgG (cat. no. 7076S; Cell Signaling Technology,
Inc.). The following primary antibodies obtained from Santa Cruz
Biotechnology, Inc., were used: Goat anti-phosphorylated (p)-EGFR
antibody (cat. no. sc-12351), goat anti-EGFR antibody (cat. no.
sc-03), mouse anti-Shc antibody (cat. no. sc-967), rabbit
anti-growth factor receptor bound protein (GRB)2 antibody (cat. no.
sc-255), rabbit anti-Son of sevenless homolog (Sos) antibody (cat.
no. sc-259), rabbit anti-H-Ras antibody (cat. no. sc-520), mouse
anti-p-Mitogen-activated protein kinase kinase (MEK)-1/2 antibody
(cat. no. sc-81503), mouse anti-MEK-1/2 antibody (cat. no.
sc-81504), mouse anti-p-extracellular signal-regulated kinase (ERK)
antibody (cat. no. sc-7383), mouse anti-ERK 1 antibody (cat. no.
sc-271269), rabbit anti-ERK-2 antibody (cat. no. sc-154), and
rabbit anti-GAPDH antibody (cat. no. sc-25778). The signals were
detected using an Enhanced Chemiluminescence western blot kit
(Thermo Fisher Scientific, Inc.) using a bioanalytical imaging
system (Azure Biosystems, Dublin, CA, USA). The densities of the
bands which normal-ized to GAPDH were analyzed using Multi-Gauge
software, v.3.0 (Fujifilm Life Science, Tokyo, Japan).
Statistical analysis
For all assays, at least three independent
experiments were performed. The mean ± standard deviation of the
expression values was calculated using Excel software v.2007
(Microsoft Corporation, Redmond, WA, USA). The differences among
multiple groups were evaluated using one-way analysis of variance
followed by Bonferroni post hoc test using SPSS statistical
software for Windows, v.20.0 (IBM Corp., Armonk, NY, USA).
Results
Positive effects of SPCP on human skin
cell growth
SPCP is a complex mixture of proteins, which were
separated by SDS-PAGE and stained with Coomassie Brilliant Blue
revealing ~20 and ~16 kDa bands (Fig.
1). The two bands were analyzed by Q-TOF MS/MS. The results
revealed that the 20 kDa band didn't correspond to any known
protein while the ~16 kDa protein band was C-phycocyanin α chain,
which has many pharmacological benefits (34). To investigate the effects of SPCP
on the viability of CCD-986sk cells, an MTS assay on cells treated
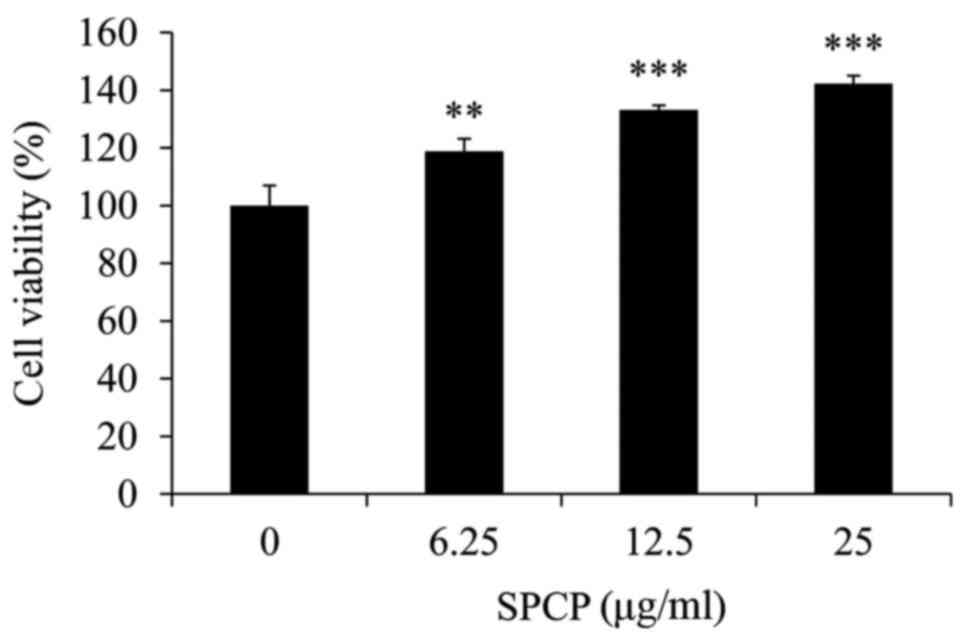
with various doses of SPCP was performed. The viability of cells
treated with 6.25, 12.5 or 25 µg/ml SPCP increased by
18±4.41%, 33±1.62% (P<0.01) and 42±2.82% (P<0.001),
respectively, compared with the control (Fig. 2). Together, the results indicated
that SPCP effectively promoted the growth of CCD-986sk cells at in
a dose-dependent manner.
Inhibitory effect of SPCP on elastase
activity
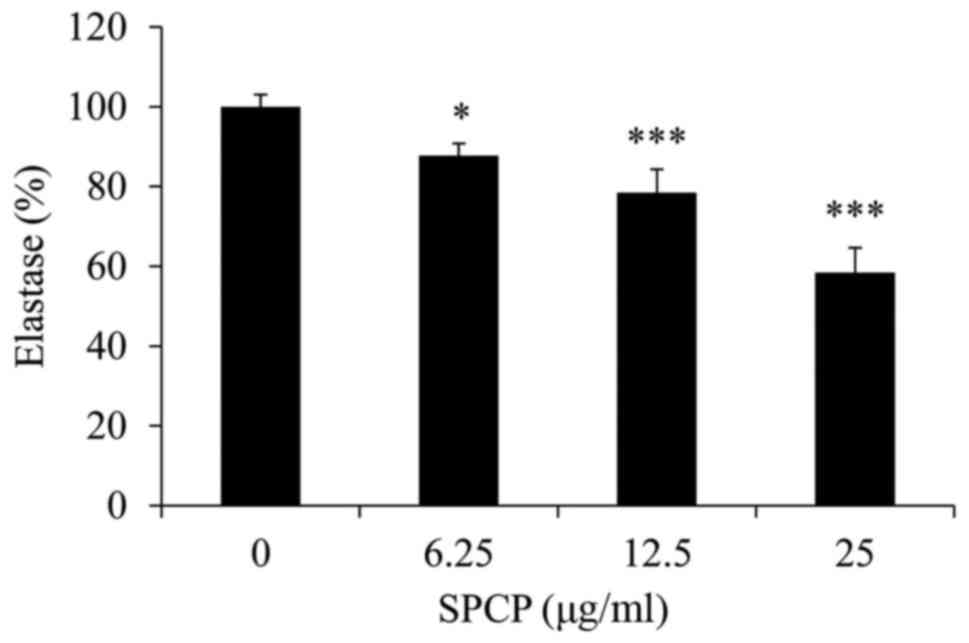
Elastase activity in CCD-986sk cells following
various treatments with SPCP was measured. Compared with the
control, SPCP treatment significantly decreased the elastase
activity in a dose-dependent manner (P<0.05 or P<0.001;
Fig. 3), with a maximum decrease
of 42±6.15% observed in cells treated with 25 µg/ml
SPCP.
Effect of SPCP on PIP levels
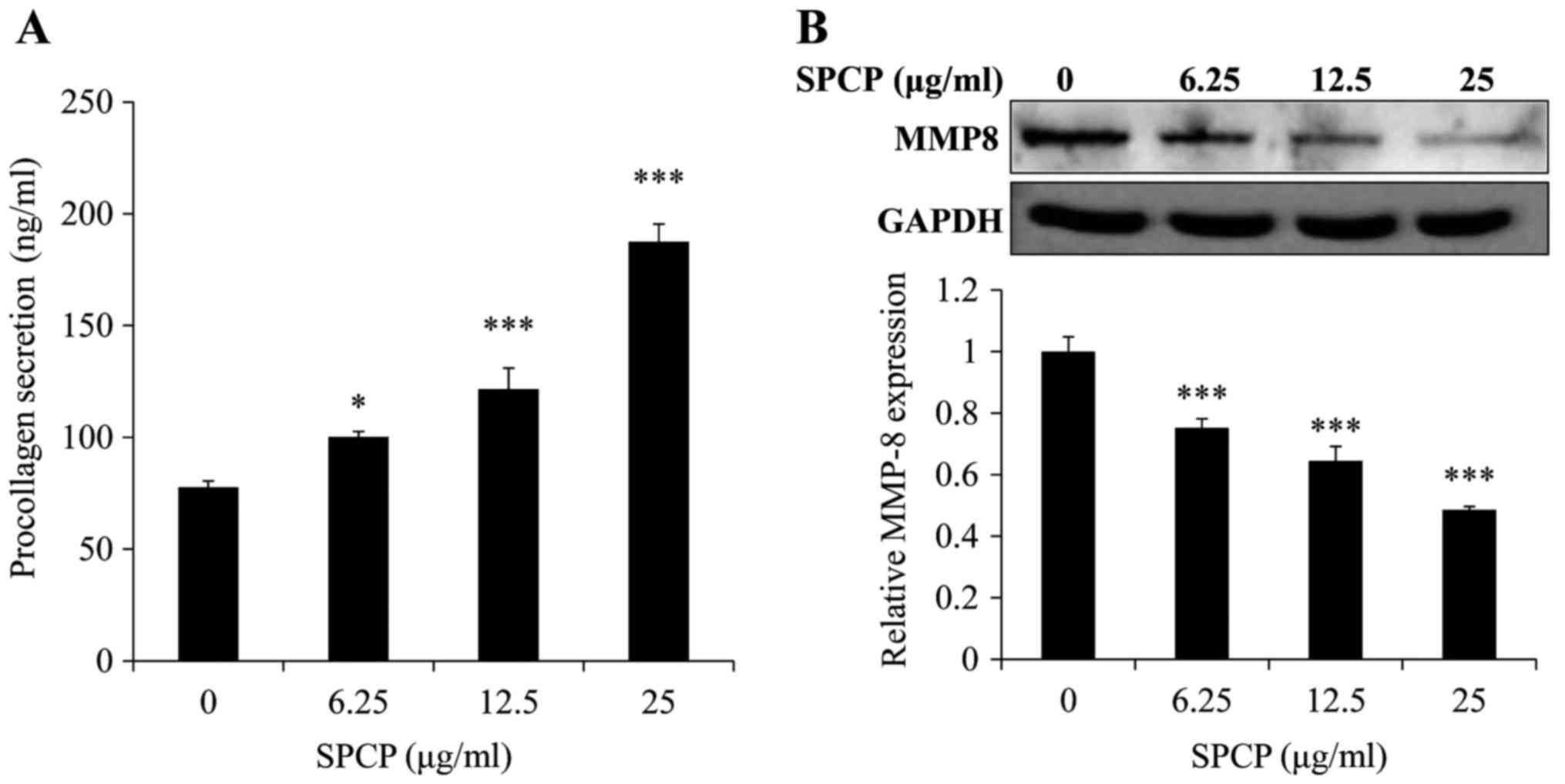
To determine whether SPCP affected the secretion of
PIP, secreted PIP levels were measured using ELISA. SPCP treatments
significantly increased PIP levels in a dose-dependent manner
(P<0.05 or P<0.001; Fig.
4A). Compared with the basal level of 77±2.78 ng/ml, PIP
concentrations of 100±2.36, 121±9.45 and 187±7.92 ng/ml were
induced by 6.25, 12.5, and 25 µg/ml SPCP, respectively. This
result indicated that SPCP promoted the secretion of PIP.
Western blotting was used to determine the effect of
SPCP on MMP-8 expression in CCD-986sk cells. MMP-8 protein
expression levels were significantly decreased by SPCP treatment in
a dose-dependent manner (P<0.001; Fig. 4B). These results suggested that
SPCP promoted the synthesis of collagen and inhibited the
expression of MMP-8.
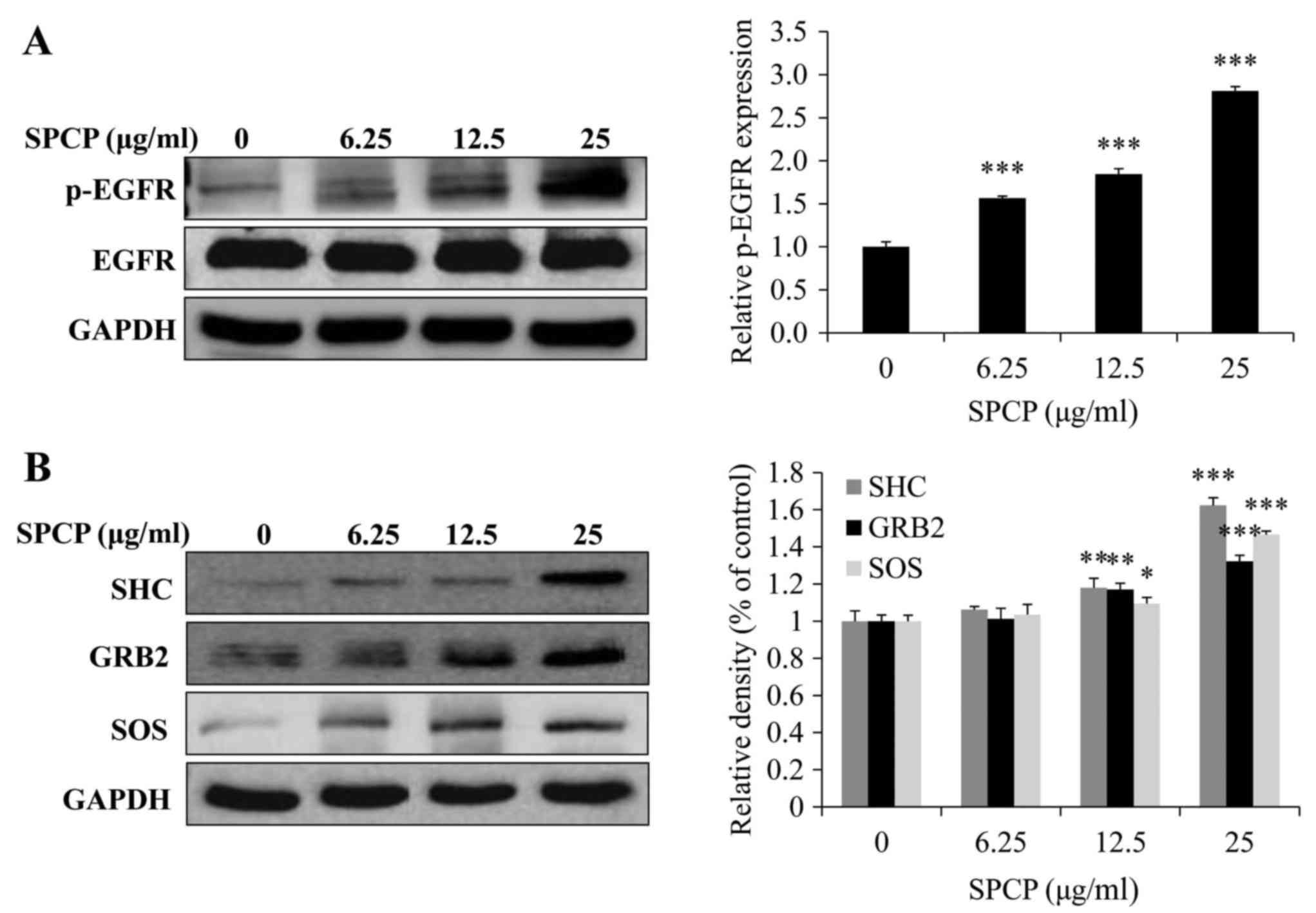
Activation of the EGFR signaling pathway
by SPCP in CCD-986sk cells
As SPCP treatment was revealed to enhance the
viability of CCD-986sk cells (Fig.
2), the potential regulation of the EGFR signaling pathway by
SPCP was investigated. The EGFR signaling pathway was assessed by
analyzing the total and p-EGFR protein expression levels. The
expression levels of p-EGFR significantly increased following
treatment of CCD-986sk cells with SPCP (P<0.001; Fig. 5A). SPCP treatment induced the
expression of essential linkers of epidermal growth factor
receptors to MAP kinase, including the adaptor proteins SHC and
GRB2, and the guanine nucleotide exchange protein, SOS (P<0.01
or P<0.001; Fig. 5B). These
results indicated that SPCP stimulation may activate the EGFR
pathway in a dose-dependent manner.
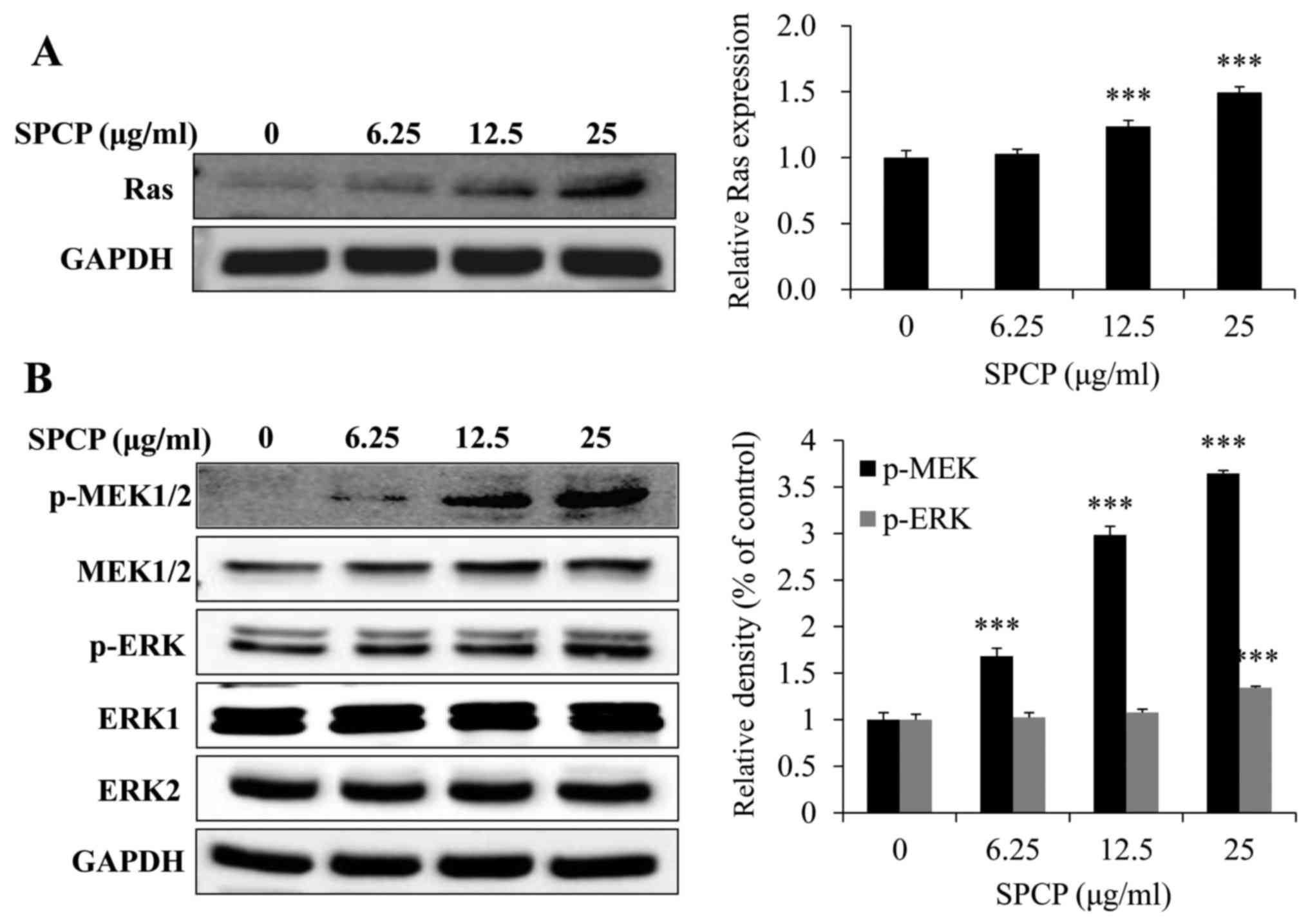
Activation of the MAPK pathway by SPCP in
CCD-986sk cells
To determine whether EGFR activation induced
activation of the MAPK signaling pathway, the expression levels of
Ras were investigated following treatment with various doses of
SPCP for 24 h. The expression levels of Ras significantly increased
after SPCP treatment compared with untreated control cells
(P<0.001; Fig. 6A). Meanwhile,
SPCP also enhanced the phosphorylation and activation of MEK and
ERK in a dose-dependent manner (P<0.001; Fig. 6B). Taken together, these results
indicated that SPCP treatment stimulated CCD-986sk cell viability
by activating the EGFR/MAPK/ERK signaling pathway.
Discussion
Microalgae proteins currently represent one of the
most promising protein sources from food due to their abundant and
balanced amino acid composition (35,36). A. platensis is an edible,
photosynthetic, spiral-shaped, multicellular blue-green alga that
possesses anti-inflammation and antioxidant properties. In the
present study, spirulina was extracted with ethanol and
(NH4)2SO4. This extract (SPCP)
contained proteins as determined by Coomassie Brilliant Blue
staining. Q-TOF MS/MS analysis showed that the ~16 kDa protein band
was C-phycocyanin α chain, which has been reported to have many
pharmacological benefits, including anti-inflammatory, antioxidant
and anticancer (34,37). The MTS assay showed that SPCP
promoted the viability of human fibroblasts in a dose-dependent
manner. Cell viability increased by 18±4.41%, 33±1.62%, and
42±2.82% associated with the control after treatment with 6.25,
12.5 and 25 µg/ml SPCP, respectively. The results were
confirmed by experiments performed in Hs27 cells (data not shown).
The present study results suggested that SPCP treatment may lead to
skin cell growth by enhancing the activation of growth factors in
normal human fibroblasts.
Dermal fibroblasts are the primary cell type
responsible for the production, maintenance and remodeling of the
ECM in human skin (38). Skin
fibrosis and aging are caused by an imbalance between the
generation and degradation of ECM proteins, which results in severe
alterations in the skin connective tissue (39). Skin aging is associated with a
loss of ECM components from the dermis, including collagen,
elastin, fibrillin, and proteoglycan (40). The proportion of type I collagen
in dermis diminishes with intrinsic and extrinsic skin aging. In
the present study, cell collagen production induced by SPCP
treatment was 30-142% higher compared with the control group. The
expression levels of the collagen-degrading protein MMP-8, which
serves as a key enzyme in the degradation of collagen and
stimulates the degradation of other major dermal components (which
subsequently leads to aging) (41), was investigated by western blot
analysis. These were decreased significantly in a dose-dependent
manner in cells treated with SPCP. The increase in collagen might
be due to a decrease in the expression of MMP-8, but this needs to
be investigated further. Treatment of CCD-986sk cells with SPCP
inhibited elastase activity and its activity was reduced in a
dose-dependent manner.
Recent advance in understanding the role of
endogenous growth factors in the aging process provides
opportunities to develop novel anti-aging cosmeceutical products
(24). Growth factors can have a
prominent role in reversing the outcomes of skin aging (22). Topical application of human growth
factors has been shown to reduce the signs and symptoms of skin
aging, as well as increase dermal collagen synthesis, in several
clinical studies (26). The MTS
assay results obtained from the present study, showed that SPCP
increased the viability of CCD-986sk cells. In order to explore the
mechanism, growth factor protein expression in SPCP-treated
CCD-986sk cells were investigated. It is reported that the
activation of EGFR and insulin growth factor (IGF)-1R serve an
important role in cell proliferation (23). Thus, the activation of EGFR and
IGF-1R in CCD-986sk cells was measured. The results showed that
EGFR was activated by SPCP rather than IGF-1R (data not shown).
Furthermore, EGFR associated with skin aging (21). Based on the present data, it was
found that SPCP enhanced the secretion of PIP and inhibited the
activity of elastase. These results are beneficial for delaying
skin aging. In the present study, p-EGFR was increased in
SPCP-treated cells compared with the control cells. Additionally,
the essential linkers from epidermal growth factor receptors to MAP
kinase, including the adaptor proteins GRB2, SHC and SOS, were
induced by SPCP treatment in a dose-dependent manner. These data
indicated that SPCP stimulation induced EGFR pathway activation in
a dose-dependent manner. A previous study reported that one of the
main activated downstream signaling pathways is the MEK-ERK1/2
signaling pathway, which controls cell proliferation and
differentiation (42). There are
three proteins [ERK, c-Jun N terminal kinase (JNK) and p38] in MAPK
signaling pathway (43). Only
ERK1 and ERK2 are activated in response to growth factors, whereas
JNK and p38 are more responsive to environmental stresses and
inflammatory cytokines (44). The
activation of this downstream signaling pathway was investigated
and Ras levels were increased in SPCP treated cells, followed by
phosphorylation and activation of MEK and ERK in a dose-dependent
manner. The phosphorylation of ERK serves an important role in
regulation of cell proliferation (43).
In summary, the expression levels of both MMP-8
protein and elastase were decreased by SPCP, leading to increased
collagen levels. Additionally, SPCP treatment activated the EGFR
and MAPK/ERK signaling pathways. To demonstrate the reproducibility
of these results, other human dermal fibroblasts derived from
different donors, such as different age and gender will be used for
further experiments. Based on these findings, it seems that the
viability of human dermal fibroblasts was activated by SPCP.
Overall, these results suggest that SPCP may be used as a
cosmeceutical for potential applications in protecting human skin;
however, further investigation is required. To further determine
the effects of SPCP, hairless mice should be used to explore the
change of epidermal thickness and collagen fibers with the
application of SPCP.
Funding
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea,
funded by the Ministry of Education (grant no.
2012R1A6A1028677).
Availability of data and materials
The analyzed data sets of the present study are
available from the corresponding author on reasonable request.
Authors' contributions
PL, YHC and TJN made substantial contributions to
the conception and design of this study; PL, MKL and JWC performed
the experiments; PL, MKL and YHC performed the data analysis; PL
and YHC interpreted the experimental results and wrote the
manuscript. All authors read and approved the final manuscript and
agreed to the publication of the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Wu X, Li R, Zhao Y and Liu Y: Separation
of polysaccharides from Spirulina platensis by HSCCC with
ethanol-ammonium sulfate ATPS and their antioxidant activities.
Carbohydr Polym. 173:465–472. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vo TS, Ngo DH, Kang KH, Park SJ and Kim
SK: The role of peptides derived from Spirulina maxima in
downregulation of FcεRI-mediated allergic responses. Mol Nutr Food
Res. 58:2226–2234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang B, Cai T, Liu Q, Whitney JCC, Du M,
Ma Q, Zhang R, Yang L, Cole SPC and Cai Y: Preparation and
evaluation of spirulina polysaccharide nanoemulsions. Int J Mol
Med. 42:1273–1282. 2018.PubMed/NCBI
|
|
4
|
Yogianti F, Kunisada M, Nakano E, Ono R,
Sakumi K, Oka S, Nakabeppu Y and Nishigori C: Inhibitory effects of
dietary Spirulina platensis on UVB-induced skin inflammatory
responses and carcinogenesis. J Invest Dermatol. 134:2610–2619.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu Q, Liu L, Miron A, Klímová B, Wan D and
Kuča K: The antioxidant, immunomodulatory, and anti-inflammatory
activities of Spirulina: An overview. Arch Toxicol. 90:1817–1840.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nawrocka D, Kornicka K, Śmieszek A and
Marycz K: Spirulina platensis improves mitochondrial function
impaired by elevated oxidative stress in adipose-derived
mesenchymal stromal cells (ASCs) and intestinal epithelial cells
(IECs), and enhances insulin sensitivity in equine metabolic
syndrome (EMS) horses. Mar Drugs. 15:2372017. View Article : Google Scholar
|
|
7
|
Vázquez-Velasco M, González-Torres L,
López-Gasco P, Bastida S, Benedí J, Sánchez-Reus MI, González-Muñoz
MJ and Sánchez-Muniz FJ: Liver oxidation and inflammation in Fa/Fa
rats fed glucomannan/spirulina-surimi. Food Chem. 159:215–221.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Al-Dhabi NA and Valan Arasu M:
Quantification of phyto-chemicals from commercial Spirulina
products and their antioxidant activities. Evid Based Complement
Alternat Med. 2016:1–13. 2016. View Article : Google Scholar
|
|
9
|
Spolaore P, Joannis-Cassan C, Duran E and
Isambert A: Commercial applications of microalgae. J Biosci Bioeng.
101:87–96. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim SK, Ravichandran YD, Khan SB and Kim
YT: Prospective of the cosmeceuticals derived from marine
organisms. Biotechnol Bioproc E. 13:511–523. 2008. View Article : Google Scholar
|
|
11
|
Xiong ZM, O'Donovan M, Sun L, Choi JY, Ren
M and Cao K: Anti-aging potentials of methylene blue for human skin
longevity. Sci Rep. 7:24752017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hoseini SM, Kalantari A, Afarideh M,
Noshad S, Behdadnia A, Nakhjavani M and Esteghamati A: Evaluation
of plasma MMP-8, MMP-9 and TIMP-1 identifies candidate
cardiometabolic risk marker in metabolic syndrome: Results from
double-blinded nested case-control study. Metabolism. 64:527–538.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tundis R, Loizzo MR, Bonesi M and
Menichini F: Potential role of natural compounds against skin
aging. Curr Med Chem. 22:1515–1538. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pham QL, Jang HJ and Kim KB: Anti-wrinkle
effect of fermented black ginseng on human fibroblasts. Int J Mol
Med. 39:681–686. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Na J, Bak DH, Im SI, Choi H, Hwang JH,
Kong SY, No YA, Lee Y and Kim BJ: Anti-apoptotic effects of
glycosaminoglycans via inhibition of ERK/AP-1 signaling in
TNF-α-stimulated human dermal fibroblasts. Int J Mol Med.
41:3090–3098. 2018.PubMed/NCBI
|
|
16
|
Meinke MC, Nowbary CK, Schanzer S, Vollert
H, Lademann J and Darvin ME: Influences of orally taken
carotenoid-rich curly kale extract on collagen I/elastin index of
the skin. Nutrients. 9:7752017. View Article : Google Scholar :
|
|
17
|
Rittié L and Fisher GJ: UV-light-induced
signal cascades and skin aging. Ageing Res Rev. 1:705–720. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jadoon S, Karim S, Bin Asad MH, Akram MR,
Khan AK, Malik A, Chen C and Murtaza G: Anti-aging potential of
phytoextract loaded-pharmaceutical creams for human skin cell
longevity. Oxid Med Cell Longev. 2015:7096282015. View Article : Google Scholar
|
|
19
|
Herman MP, Sukhova GK, Libby P, Gerdes N,
Tang N, Horton DB, Kilbride M, Breitbart RE, Chun M and Schönbeck
U: Expression of neutrophil collagenase (matrix
metalloproteinase-8) in human atheroma: A novel collagenolytic
pathway suggested by transcriptional profiling. Circulation.
104:1899–1904. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Burke KE: Mechanisms of aging and
development-A new understanding of environmental damage to the skin
and prevention with topical antioxidants. Mech Ageing Dev.
172:123–130. 2018. View Article : Google Scholar
|
|
21
|
Shiraha H, Gupta K, Drabik K and Wells A:
Aging fibroblasts present reduced epidermal growth factor (EGF)
responsiveness due to preferential loss of EGF receptors. J Biol
Chem. 275:19343–19351. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen J, Li Y, Zhu Q, Li T, Lu H, Wei N,
Huang Y, Shi R, Ma X, Wang X, et al: Anti-skin-aging effect of
epigallocatechin gallate by regulating epidermal growth factor
receptor pathway on aging mouse model induced by d-Galactose. Mech
Ageing Dev. 164:1–7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao M, Zhan YQ, Yu M, Ge CH, Li CY, Zhang
JH, Wang XH, Ge ZQ and Yang XM: Hepassocin activates the EGFR/ERK
cascade and induces proliferation of L02 cells through the
Src-dependent pathway. Cell Signal. 26:2161–2166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gerber PA, Buhren BA, Schrumpf H, Hevezi
P, Bölke E, Sohn D, Jänicke RU, Belum VR, Robert C, Lacouture ME,
et al: Mechanisms of skin aging induced by EGFR inhibitors. Support
Care Cancer. 24:4241–4248. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Green MR, Basketter DA, Couchman JR and
Rees DA: Distribution and number of epidermal growth factor
receptors in skin is related to epithelial cell growth. Dev Biol.
100:506–512. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tran KT, Rusu SD, Satish L and Wells A:
Aging-related attenuation of EGF receptor signaling is mediated in
part by increased protein tyrosine phosphatase activity. Exp Cell
Res. 289:359–367. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang W and Liu HT: MAPK signal pathways
in the regulation of cell proliferation in mammalian cells. Cell
Res. 12:9–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Z, Liu S and Cai Y: EGFR/MAPK signaling
regulates the proliferation of Drosophila renal and nephric stem
cells. J Genet Genomics. 42:9–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shin S, Son D, Kim M, Lee S, Roh KB, Ryu
D, Lee J, Jung E and Park D: Ameliorating effect of akebia quinata
fruit extracts on skin aging induced by advanced glycation end
products. Nutrients. 7:9337–9352. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ryu J, Kwon MJ and Nam TJ: Nrf2 and NF-κB
signaling pathways contribute to porphyra-334-mediated inhibition
of UVA-induced inflammation in skin fibroblasts. Mar Drugs.
13:4721–4732. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Barnes D and Sato G: Methods for growth of
cultured cells in serum-free medium. Anal Biochem. 102:255–270.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim YM, Jung HJ, Choi JS and Nam TJ:
Anti-wrinkle effects of a tuna heart H2O fraction on
Hs27 human fibroblasts. Int J Mol Med. 37:92–98. 2016. View Article : Google Scholar
|
|
33
|
Magadum A, Ding Y, He L, Kim T,
Vasudevarao MD, Long Q, Yang K, Wickramasinghe N, Renikunta HV,
Dubois N, et al: Live cell screening platform identifies PPARδ as a
regulator of cardiomyocyte proliferation and cardiac repair. Cell
Res. 27:1002–1019. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee J, Park A, Kim MJ, Lim HJ, Rha YA and
Kang HG: Spirulina extract enhanced a protective effect in type 1
diabetes by anti-apoptosis and anti-ROS production. Nutrients.
9:13632017. View Article : Google Scholar
|
|
35
|
Lu J, Ren DF, Wang JZ, Sanada H and
Egashira Y: Protection by dietary Spirulina platensis against
D-galactosamine--and acetaminophen-induced liver injuries. Br J
Nutr. 103:1573–1576. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kepekçi RA, Polat S, Çelik A, Bayat N and
Saygideger SD: Protective effect of Spirulina platensis enriched in
phenolic compounds against hepatotoxicity induced by CCl4. Food
Chem. 141:1972–1979. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Romay Ch, González R, Ledón N, Remirez D
and Rimbau V: C-phycocyanin: A biliprotein with antioxidant,
anti-inflammatory and neuroprotective effects. Curr Protein Pept
Sci. 4:207–216. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim MS, Song HJ, Lee SH and Lee CK:
Comparative study of various growth factors and cytokines on type I
collagen and hyaluronan production in human dermal fibroblasts. J
Cosmet Dermatol. 13:44–51. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tanaka M, Koyama Y and Nomura Y: Effects
of collagen peptide ingestion on UV-B-induced skin damage. Biosci
Biotechnol Biochem. 73:930–932. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim MK, Bang CY, Yun GJ, Kim HY, Jang YP
and Choung SY: Anti-wrinkle effects of Seungma-Galgeun-Tang as
evidenced by the inhibition of matrix metalloproteinase-I
production and the promotion of type-1 procollagen synthesis. BMC
Complement Altern Med. 16:1162016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang CY, Li XH, Zhang T, Fu J and Cui XD:
Hydrogen sulfide suppresses the expression of MMP-8, MMP-13, and
TIMP-1 in left ventricles of rats with cardiac volume overload.
Acta Pharmacol Sin. 34:1301–1309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Corcoran RB, Ebi H, Turke AB, Coffee EM,
Nishino M, Cogdill AP, Brown RD, Della Pelle P, Dias-Santagata D,
Hung KE, et al: EGFR-mediated re-activation of MAPK signaling
contributes to insensitivity of BRAF mutant colorectal cancers to
RAF inhibition with vemurafenib. Cancer Discov. 2:227–235. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pearson G, Robinson F, Beers Gibson T, Xu
BE, Karandikar M, Berman K and Cobb MH: Mitogen-activated protein
(MAP) kinase pathways: Regulation and physiological functions.
Endocr Rev. 22:153–183. 2001.PubMed/NCBI
|
|
44
|
Roux PP and Blenis J: ERK and p38
MAPK-activated protein kinases: A family of protein kinases with
diverse biological functions. Microbiol Mol Biol Rev. 68:320–344.
2004. View Article : Google Scholar : PubMed/NCBI
|