Introduction
Stroke is one of the primary diseases threatening
human health. According to the most recent analysis of the global
disease burden, stroke is second among all causes of mortality
world-wide (1). Concurrently,
brain tissue injury is also the leading cause of permanent
disability (1). At present, with
an increase in the size of the aging population in China, China
ranks first in the world in terms of the number of patients
suffering from strokes (2). The
morbidity and mortality in China are increased compared with those
in economically developed countries, including European and the
USA. It remains a serious threat to human health in China.
Concomitantly, the incidence of stroke indicates a younger trend in
China (2): The ages of onset and
mortality of patients suffering strokes in China is decreasing,
compared with the United States of America (3). A previous study have indicated that
the primary subtype of stroke classification is ischemic stroke,
accounting for ~80% of the overall stroke incidence (3). The high morbidity, mortality and
recurrence rates of ischemic stroke lead to heavy financial burdens
on families and society (4).
Therefore, it is an urgent global problem to be solved, of how to
minimize the neurological dysfunction following stroke and to
restore the injured neurological functions as soon as possible
(3).
The pathological process of cerebral
ischemia-reperfusion injury is complicated and affected by multiple
factors (5). The mechanisms of
the occurrence and action of cerebral ischemia-reperfusion injury
involve the interaction of multiple factors (6). The inflammatory process serves a
considerably important role during cerebral ischemia-reperfusion
injury (5). With the increasing
level of understanding of cerebral ischemia-reperfusion injury,
multiple studies have provided novel ideas to prevent and treat
this condition (7). Among them,
blocking the inflammatory cascade following reperfusion is an ideal
strategy to improve cerebral injury following ischemia-reperfusion
events. However, the timing of the inhibition of the inflammatory
reaction is an important problem in the success of this treatment
(7).
Cerebral infarction is a serious vascular
complication of diabetes. Diabetes combined with acute cerebral
infarction accounts for 20-25% of the total number of cases of
cerebral infarction (8). Larger
infarction areas and more severe symptoms of diabetes are more
likely to result in progressive strokes, with poorer prognoses
(9). At present, no effective and
feasible therapeutic approaches have been made available for
diabetic cerebral infarction. MicroRNAs (miRNAs) are a class of
small non-coding RNAs, containing 20-22 nucleotides (10). Previous studies have suggested
that miRNAs regulate gene expression by modulating the
translational process of their targeted mRNAs. They are widely
involved in various biological processes, including cell
differentiation, proliferation and apoptosis (10,11).
In previous years, a study demonstrated that the
activation of NOD-like receptor pyrin domain containing 3 (NLRP3)
inflammasome serves an important role in cerebral
ischemia-reperfusion injury (12). The NLRP3 inflammasome is a
complicated group of multiprotein complexes (12). It belongs to the family of
intracytoplasmic pattern recognition receptors (PRRs) that
recognize exogenous pathogens and intrinsic endogenous danger
signals, including during ischemia-reperfusion injury (13). In addition, the NLRP3 inflammasome
regulates innate immunity and acquired immunity, which is a key
molecular pathway for the inflammatory cascade. A variety of
studies have suggested that the structure, function, expression and
distribution of the NLRP3 inflammasome may affect the
NLRP3-mediated inflammatory reaction (13,14). It is involved in multiple
pathophysiological processes, including immunity,
ischemia-reperfusion injury and cerebral degenerative disease
(13). Ischemia-reperfusion
injury is an inflammatory cascade involving the interaction of
multiple factors. In previous years, a number studies have
demonstrated that the NLRP3 inflammasome, an important PPR in
innate immunity, is involved in the inflammation-associated injury
of ischemia-reperfusion injury (14). Therefore, blocking or inhibiting
the activation of NLRP3 may become a novel therapeutic target in
ischemic cerebrovascular disease (14). Therefore, the present study aimed
to investigate the role of miRNA-20b in inflammation of cerebral
ischemia and the underlying mechanism following cerebral
ischemia.
Materials and methods
Animal model
Sprague-Dawley rats (male, 5-6 week, n=12, 170-200
g) were obtained from Vital River Laboratory Animal Technology Co.,
Ltd. (Beijing, China) and housed at 22-23°C, 55-60% humidity, 12-h
dark cycle light, free access to food and water. Rats were
anesthetized using 35 mg/kg pentobarbital sodium
(intraperitoneally). A nylon filament with its cusp slightly
rounded by heat was advanced into the lumen of the internal carotid
artery to occlude the right middle cerebral artery (MCA) for 1 h,
and the filament was subsequently with-drawn to allow reperfusion.
All experiments were performed in compliance with guidelines for
the ethical use of animals of Hebei General Hospital. The present
study was approved by the Hebei Municipal Committee of Science and
Technology.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from hippocampus tissue samples and HUVECs
cell samples was extracted using a mirVana miRNA Isolation kit
(Ambion; Thermo Fisher Scientific, Inc., Waltham, MA, USA). cDNA
was reverse transcribed using the PrimeScript RT reagent kit at
37°C for 30 min and 84°C for 10 sec. RT-qPCR was performed using
SYBR Premix Ex Taq (Takara Bio., Inc., Otsu, Japan) on an ABI PRISM
7500 Sequence Detection system (Applied Biosystems; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The primers of miR-20b were as
follows: Forward, 5′-TGT CAA CGA TAC GCT ACG A-3′ and
reverse,5′-GCT CAT AGT GCA GGT AGA-3′; U6 forward, 5′-GCT TCG GCA
GCA CAT ATA CTA AAA T-3′ and reverse, 5′-CGC TTC ACG AAT TTG CGT GT
C AT-3′. PCR amplification was performed at 95°C for 10 min prior
to 40 cycles of 95°C for 30 sec, 60°C for 30 sec and 72°C for 30
sec, followed at 72°C for 5 min. miRNA was measured using the
2−ΔΔCq method (15).
miRNA microarray
Total RNA from HUVECs and rat tissues was labeled
and hybridized with the miRCURY™ LNA Array (v.16.0; Exiqon; Qiagen,
Inc., Valencia, CA, USA) and analyzed using Agilent Feature
Extraction Software (version A.10.7.3.1; Agilent Technologies,
Inc., Santa Clara, CA, USA). The statistical significance of the
miRNAs was analyzed by Agilent Feature Extraction Software.
ELISA
Serum samples of rats and cell supernatants samples
were collected at 1,000 × g for 10 min at 4°C. TNF-α (cat. no.
H052), interleukin (IL)-6 (cat. no. H007), IL-18 (cat. no. H015)
and IL-1β (cat. no. H002) levels were measured using ELISA kits
(Nanjing Jiancheng Biology Engineering Institute, Nanjing,
China).
Cell culture and treatment
Human umbilical vein endothelial cells (HUVECs) were
purchased from the Type Culture Collection of the Chinese academy
of sciences (Shanghai, China) and grown in M199 medium supplemented
with 10% heat-inactivated fetal bovine serum (FBS; both HyClone; GE
Healthcare Life Sciences, Logan, UT, USA), 0.1% gelatin and 1%
penicillin-streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.) with 5% CO2 at 37°C. HUVECs were transfected by
100 ng of miRNA-20b (5′-GAA AAG CAG GAA GGA CCC TCG CCC TTC AAA
CCC-3′), 100 ng of anti-miRNA-20b (5′-GGG TTT GAA GGG CGA GGG TCC
TTC CTG CTT TTC-3) or 100 ng of negative mimics (5′-TTC TCC GAA CGT
GTC ACG T-3′) using Lipofectamine 2000® (Invitrogen;
Thermo Fisher Scientific, Inc.) for 4 h. Then, the cells were
cultured in M199 medium without FBS in a hypoxia incubator (Sanyo,
Osaka, Japan) at 37°C, 5% CO2, 94% N2 and 1%
O2 for 12 h. The luciferase assay was performed using
TransMessenger Transfection Reagent (Tiangen Biotech Co., Ltd.,
Beijing, China).
Luciferase reporter assays
The fragment was designated as NLRP3 and miRNA-20b
mimics. The recombinant reporter pGL3M vectors (Promega
Corporation, Madison, WI, USA) with NLRP3 were co-transfected with
miRNA-20b into HUVECs using Lipofectamine 2000®
(Invitrogen; Thermo Fisher Scientific, Inc.) for 4 h. The method of
normalization was used as comparison with Renilla luciferase
activity. After 48 h of transfection, luciferase activity levels
normalized to Renilla activity was measured using an
automatic micro-plate reader with the Dual-Luciferase Reporter
Assay System (Promega Corporation).
ATP and ROS assay
Cell was collected at 1,000 × g for 10 min at 4°C
and used to measured ATP level using ATP kits (cat. no. S0026). ROS
levels were measured using ROS kits (cat. no. S0033; both Beyotime
Institute of Biotechnology).
Hematoxylin and eosin (H&E)
staining
Sections were stained with H&E assay for 10 min
at room temperature. H&E staining was performed on hippocampus
tissues and sections were viewed under confocal microscopy (Leica
SP5, Argon laser; Leica Microsystems, Inc., Buffalo Grove, IL, USA;
magnification ×200).
Western blot analysis
Total protein was extracted from HUVEC samples using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology) from cell samples and protein concentration was
determined by a bicinchoninic acid Protein Assay kit. A total of 30
µg protein was subjected to 10% SDS-PAGE lysis and
transferred to polyvinylidene fluoride membranes. Membranes were
blocked in 5% skimmed milk (Beyotime Institute of Biotechnology)
for 2 h at room temperature and incubated with antibodies against
NLRP3 (cat. no. 13158; 1:1,000), caspase-1 (cat. no. 3866; 1:1,000;
both Cell Signaling Technology, Inc., Danvers, MA, USA), IL-18
(ab191860; 1:1,000; Abcam), IL-1β (cat. no. 12242; 1:1,000) and
GAPDH (5174; 1:5,000; both Cell Signaling Technology, Inc.) at 4°C
overnight. The membranes were then incubated with a horseradish
peroxidase-conjugated anti-rabbit IgG secondary antibody for 1 h at
room temperature (cat. no. 7074; 1:5,000; Cell Signaling
Technology, Inc.) at 37°C for 1 h. Bands were visualized using the
electrochemical luminescence (ECL) western detection reagents and
analyzed using Image-ProPlus 6.0 software (Media Cybernetics, Inc.,
Rockville, MD, USA).
Immunohistochemistry
HUVECs were washed with PBS and fixed with 4%
paraformaldehyde for 15 min at room temperature. Cells were blocked
with 5% bovine serum albumin (Beyotime Institute of Biotechnology)
containing 0.25% Triton-X100 for 1 h at room temperature and
incubated with NLPR3 (1:100; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) at 4°C overnight. Then, cells were washed with
PBST for 20 min and incubated with goat anti-rabbit IgG-CruzFluor™
555 (cat. no. sc-362262; 1:100; Santa Cruz Biotechnology, Inc.) at
37°C for 1 h. Then, cells were washed with PBST for 20 min and
stained with DAPI for 20 min in the dark at room temperature. Cell
was obtained by confocal microscopy (Leica SP5, Argon laser; Leica
Microsystems, Inc.; magnification ×100).
Statistical analysis
Data are presented as the means ± standard
deviation. Differences among two groups were assessed by Student's
t-test for comparison of 2 groups or one-way analysis of variance
followed by Tukey's post hoc test for three groups using SPSS 17.0
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of miRNA-20b in vivo of
cerebral ischemia
In order to investigate the potential implication of
miRNA-20b on cerebral ischemia, brain tissue samples were
collected. H&E staining was performed on the hippocampus, which
revealed increased neurocyte cell death in the cerebral ischemia
group, compared with the control group (Fig. 1A). Serum TNF-α, IL-6, IL-18 and
IL-1β levels were increased in rats with cerebral ischemia compared
with the control group (Fig.
1B-E). Microarray and qPCR was used to analyze the expression
of miRNAs in rats with cerebral ischemia, which demonstrated that
miRNA-20b expression was increased in rats in the cerebral ischemia
group compared with the control group (Fig. 1F-1G). These alterations in cases of
cerebral ischemia suggested that miRNA-20b may be involved in the
molecular pathogenesis of cerebral ischemia.
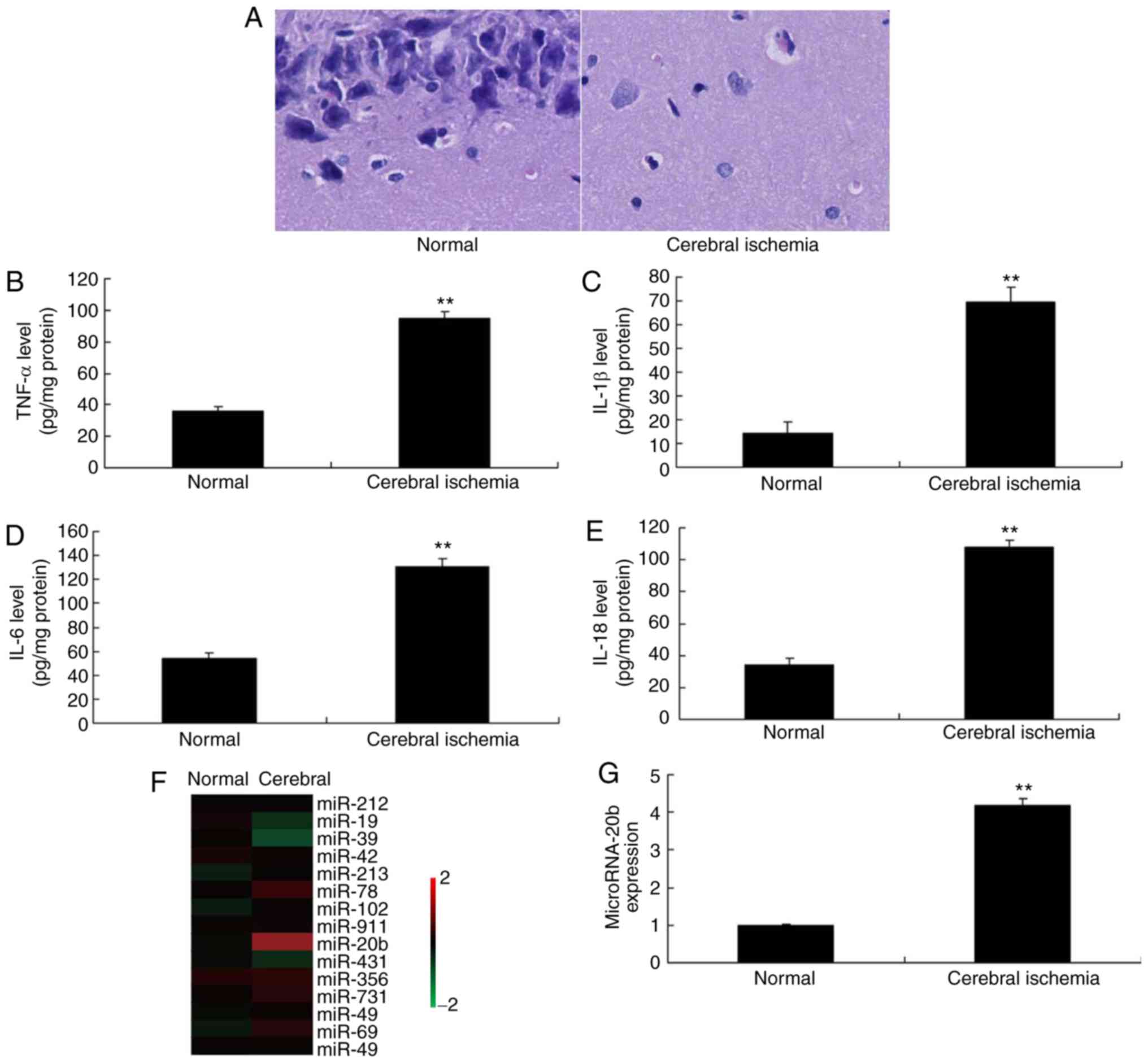 | Figure 1Expression of miRNA-20b in cerebral
ischemia. (A) Hematoxylin and eosin staining of hippocampus
tissues, magnification, ×200. Serum levels of (B) TNF-α, (C) IL-1β,
(D) IL-6 and (E) IL-18. (F) Microarray and (G) quantitative
polymerase chain reaction analysis of miRNA-20b expression.
**P<0.01 vs. normal control group. Normal, normal control group;
Cerebral ischemia, cerebral ischemia group; miRNA, microRNA; TNF-α,
tumor necrosis factor α, IL, interleukin. |
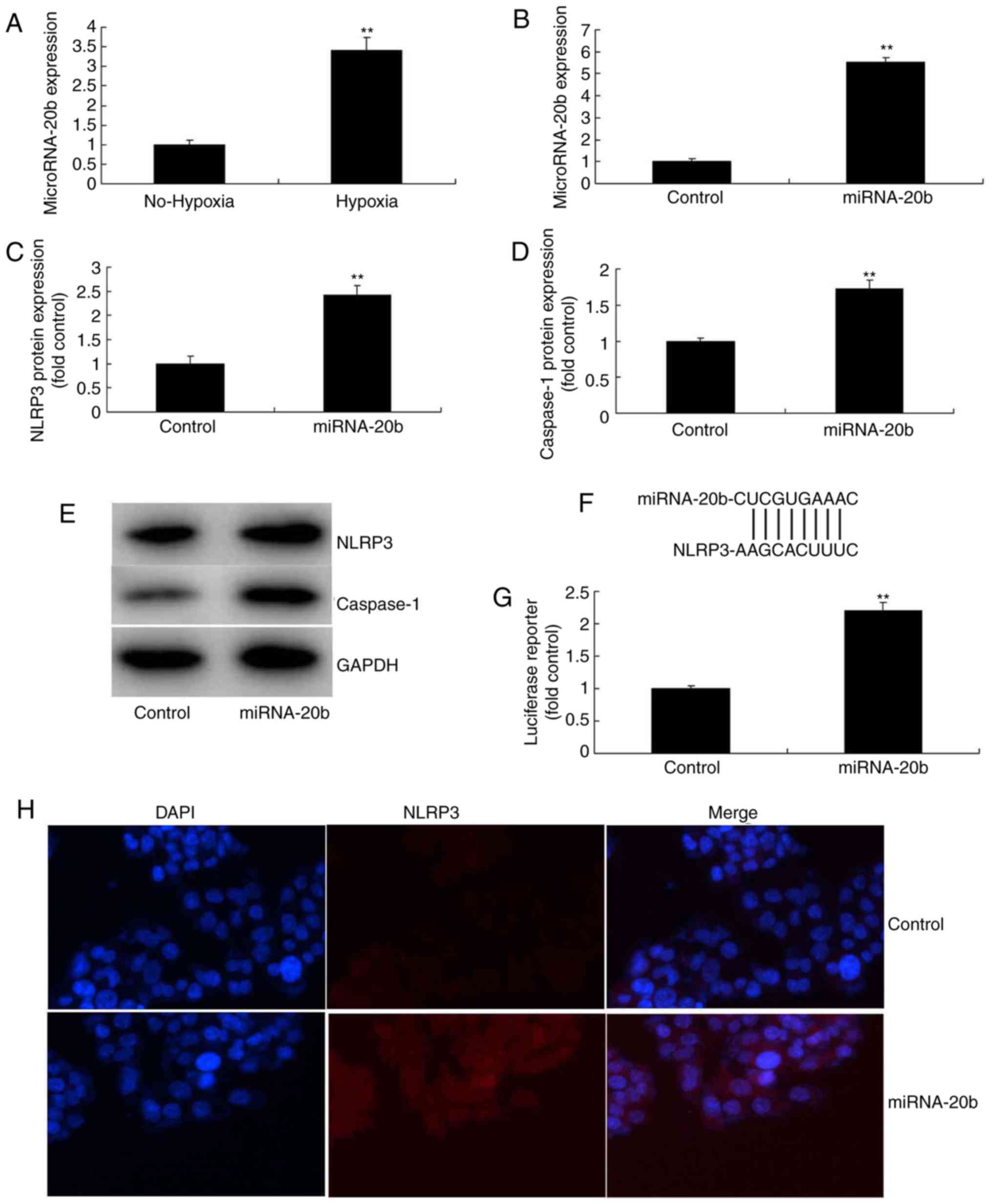
miRNA-20b regulates the NLRP3 signaling
pathway in vitro
To additionally investigate the potential role of
miRNA-20b in regulating the inflammatory response of cerebral
ischemia, microarray analysis was also utilized to analyze the
expression of miRNA-20b in an in vitro model. It was
demonstrated that miRNA-20b expression was increased in the
cerebral ischemia group compared with the control group (Fig. 2A). Then, miRNA-20b mimics were
used to increase the expression of miRNA-20b in the in vitro
model (Fig. 2B), followed by
investigation of the mechanism of action of miRNA-20b on the
inflammatory response during cerebral ischemia. The results of
microarray analysis indicated that the overexpression of miRNA-20b
induced the protein expression of NLPR3 and caspase-1 in cerebral
ischemia compared with the control group (Fig. 2C-E). The luciferase reporter assay
demonstrated that miRNA-20b directly targeted NLPR3, and that the
activity of the luciferase reporter was increased by the
overexpression of miRNA-20b, compared with the control group
(Fig. 2F-G). The
immunofluorescence assay indicated that the overexpression of
miRNA-20b induced NLPR3 protein expression in the cerebral ischemia
group compared with the control group (Fig. 2H).
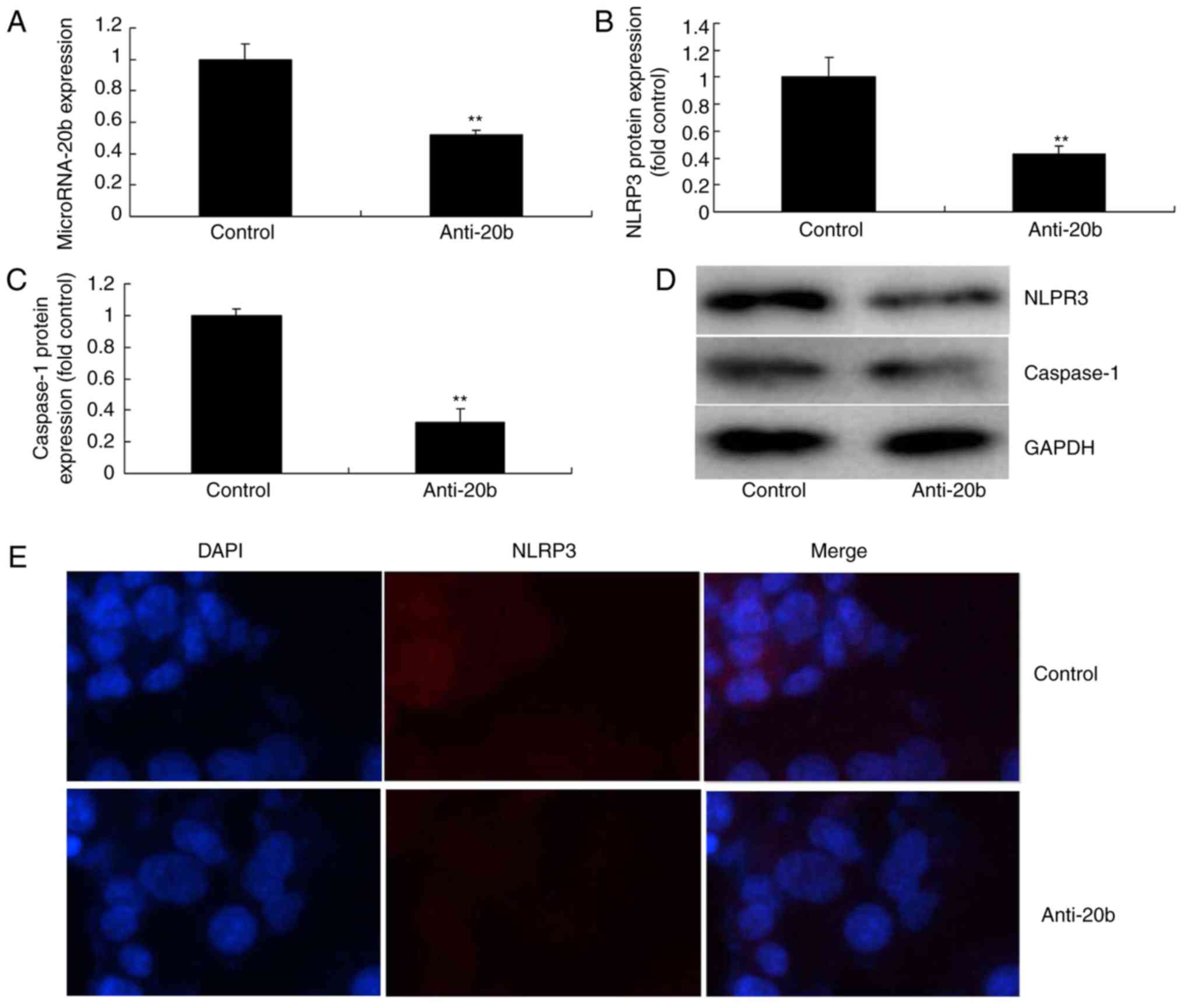
Next, whether miRNA-20b downregulation inhibited the
NLRP3 signaling pathway in cerebral ischemia was analyzed. As
indicated in Fig. 3A,
anti-miRNA-20b mimics inhibited the expression of miRNA-20b in the
cerebral ischemia group compared with the control group. In
addition, the downregulation of miRNA-20b suppressed the protein
expression of NLPR3 and caspase-1 in the cerebral ischemia group
compared with the control group (Fig.
3B-D). Immunofluorescence assays visually demonstrated that the
downregulation of miRNA-20b also suppressed NLPR3 protein
expression in the cerebral ischemia group compared with the
negative group (Fig. 3E).
Together, the aforementioned data indicated that miRNA-20b is a
potential target gene of NLRP3 in cerebral ischemia.
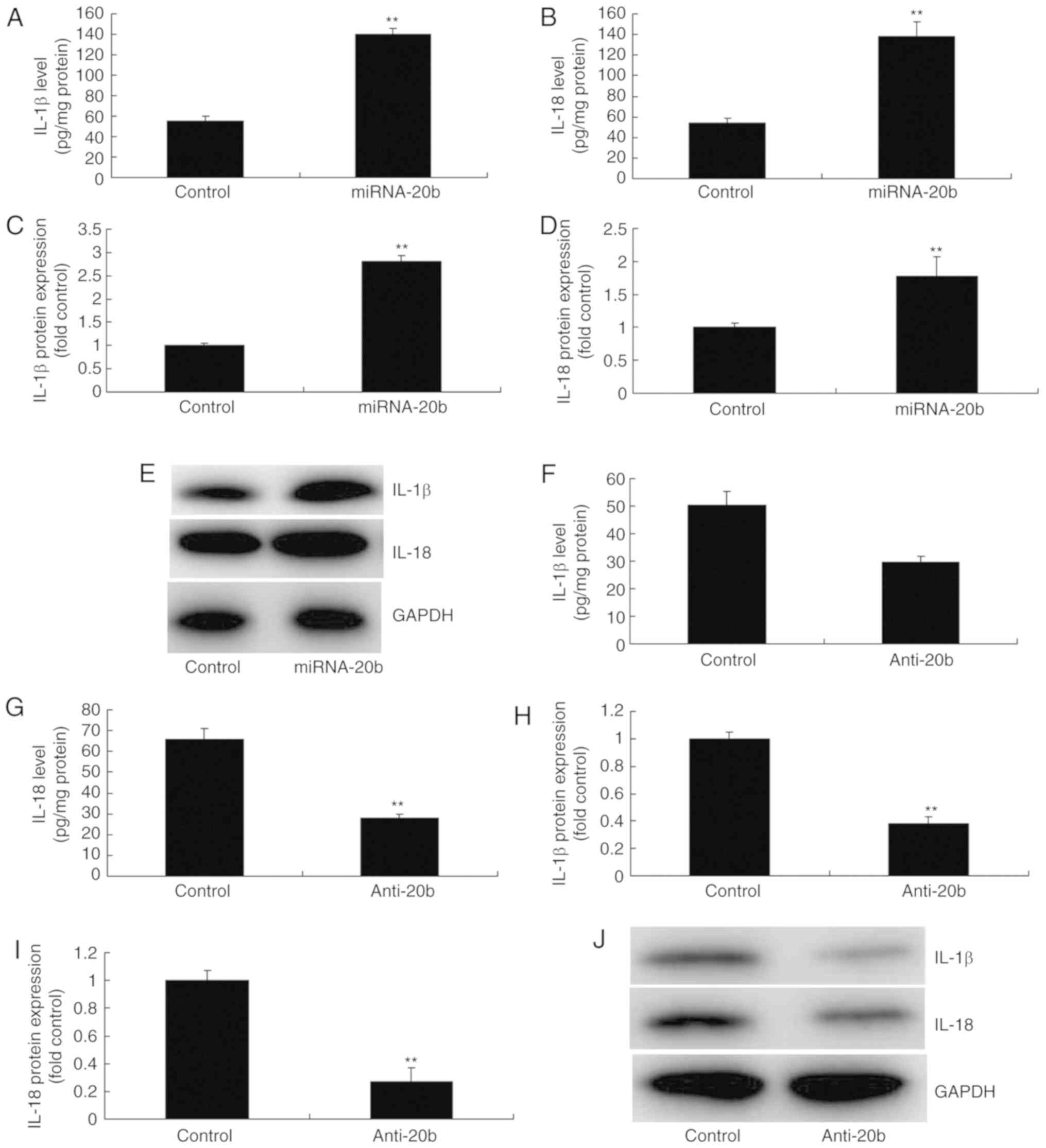
miRNA-20b regulates IL-18 and IL-1β
levels in vitro
NLRP3 has been demonstrated to induce inflammation
by regulating IL-18 and IL-1β to in cerebral ischemia (16). Therefore, whether miRNA-20b served
a role in the expression of IL-18 and IL-1β in vitro was
analyzed. As indicated in Fig.
4A-B, IL-18 and IL-1β levels in the supernatant were increased
in the in vitro model by the overexpression of miRNA-20b
compared with the control group. Western blot analysis data
indicated that the overexpression of miRNA-20b induced the protein
expression of IL-18 and IL-1β in the in vitro model compared
with the control group (Fig.
4C-E). Then, the downregulation of miRNA-20b also decreased
IL-18 and IL-1β levels in the supernatant in the in vitro
model compared with the control group (Fig. 4F-G). In addition, the
downregulation of miRNA-20b also suppressed the protein expression
of IL-18 and IL-1β in vitro compared with the control group
(Fig. 4H-J).
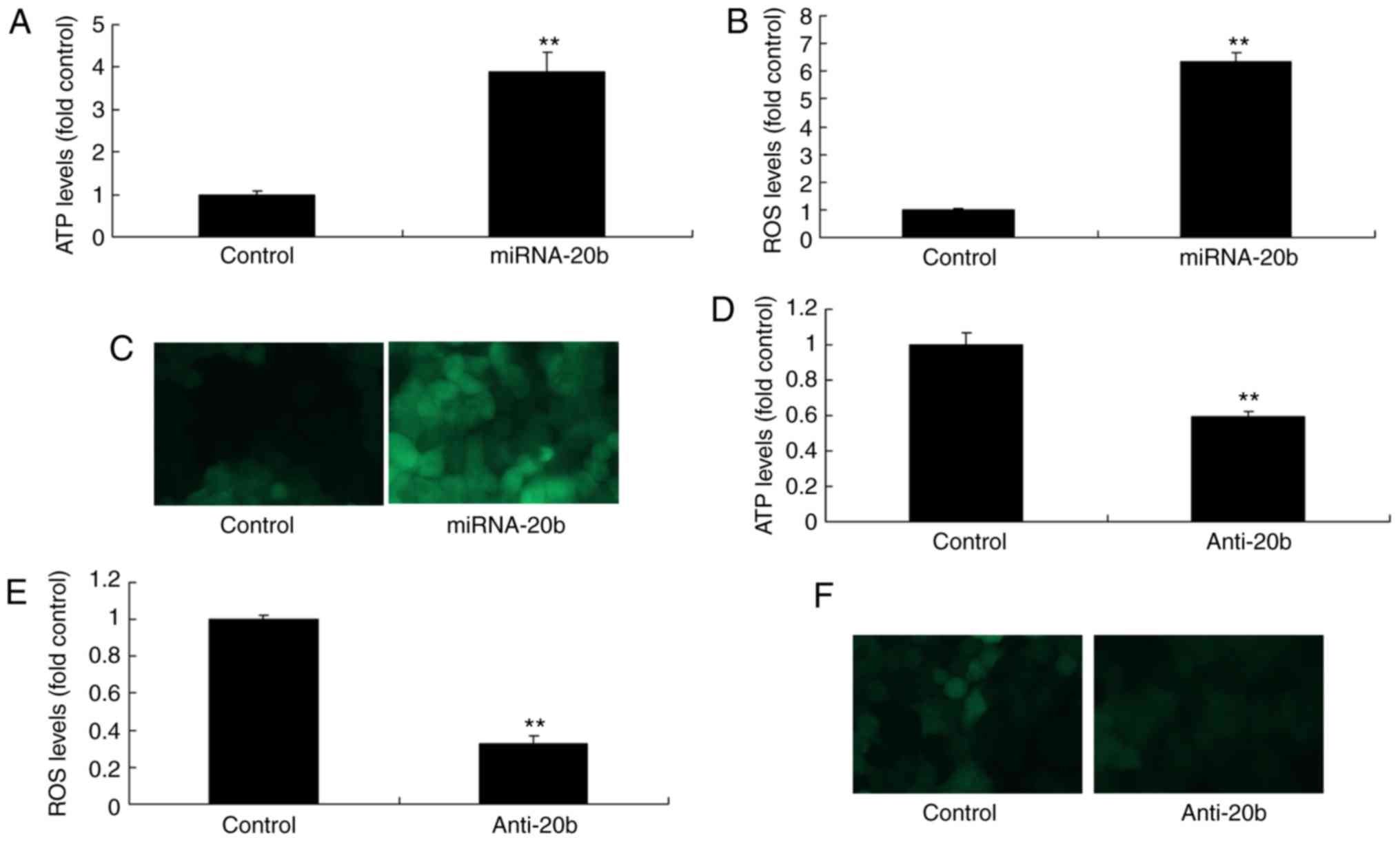
miRNA-20b modulates the levels of ATP and
ROS in cerebral ischemia
In order to verify the association between miRNA-20b
and NLRP3 in cerebral ischemia, ATP and ROS levels were moderating
to NLRP3 signaling pathway (17).
Therefore, whether miRNA-20b affected the levels of ATP and ROS
during cerebral ischemia was assessed. As indicated in Fig. 5A, the overexpression of miRNA-20b
increased the ATP and ROS levels in the cerebral ischemia group
compared with the control group (Fig.
5A-C). On the contrary, the downregulation of miRNA-20b
decreased ATP and ROS levels in the cerebral ischemia group
compared with the control group (Fig.
5D-F). These results suggested that miRNA-20b regulates the
NLRP3 signaling pathway via modulation of ATP and ROS levels during
cerebral ischemia.
Suppression of ATP decreases the
pro-inflammatory effects of miRNA-20b in cerebral ischemia
In order to confirm the role of ATP in the
pro-inflammatory effects of miRNA-20b during cerebral ischemia, an
ATP scavenger (10 µM INF39) was utilized to inhibit ATP
levels, which suppressed the protein expression of NLRP3,
caspase-1, IL-18 and IL-1β in the cerebral ischemia group with
miRNA-20b overexpression, compared with the miRNA-20b
overexpression alone group (Fig.
6A-F). The levels of IL-18 and IL-1β levels in the supernatant
were also decreased in the cerebral ischemia group following
overexpression of miRNA-20b and combined treatment with the ATP
scavenger compared with the miRNA-20b overexpression alone group
(Fig. 6G-H). Taken together,
these results indicated that miRNA-20b regulates the NLRP3
signaling pathway by altering ATP levels during cerebral
ischemia.
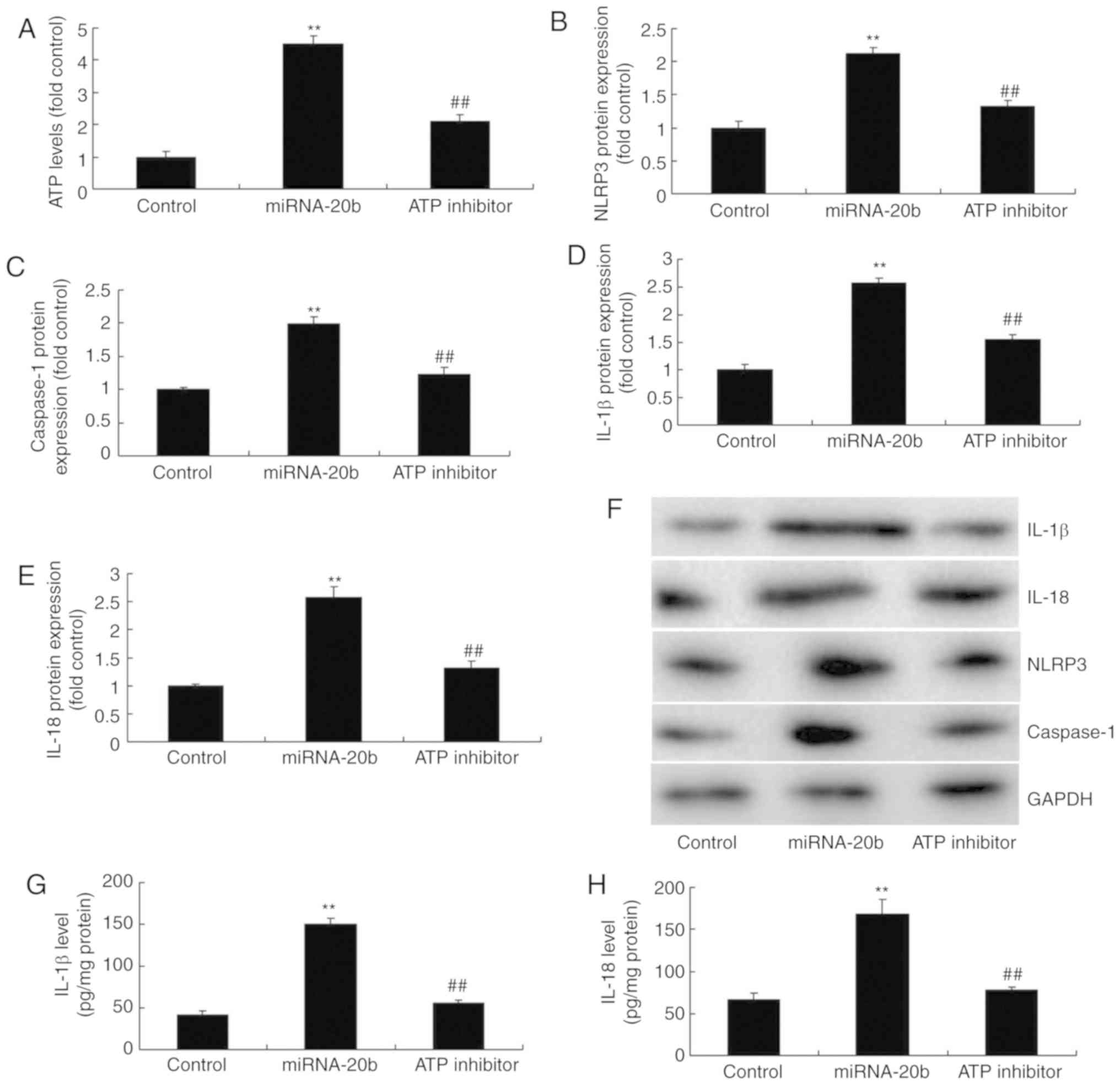 | Figure 6Suppression of ATP decreases the
pro-inflammatory effect of miRNA-20b in cerebral ischemia. (A) ATP
levels were determined using ELISA; densitometric analysis of (B)
NLRP3, (C) caspase-1, (D) IL-18 and (E) IL-1β protein and (F)
western blot analysis of NLRP3, caspase-1, IL-18 and IL-1 protein
expression following miRNA-20b overexpression and ATP inhibition.
Serum levels of (G) IL-1β and (H) IL-18 following miRNA-20b
overexpression and ATP inhibition. **P<0.01 vs.
negative control group. ##P<0.01 vs. miRNA-20b
overexpression group. miRNA, microRNA; ATP, adenosine
5′-triphosphate; NLRP3, NOD-like receptor pyrin domain containing
3; IL, interleukin; control, negative control group; miRNA-20b,
miRNA-20b overexpression group; ATP inhibitor, 10 µM INF39
and miRNA-20b overexpression group. |
Suppression of ROS decreases the
pro-inflammatory effects of miRNA-20b in cerebral ischemia
To validate the role of ROS in the pro-inflammation
of miRNA-20b in cerebral ischemia, a ROS scavenger (1 mM NAC) was
used to decrease ROS levels, which subsequently suppressed the
protein expression of NLRP3, caspase-1, IL-18 and IL-1β in cerebral
ischemia with miRNA-20b overexpression compared with the miRNA-20b
overexpression group (Fig. 7A-F).
The IL-18 and IL-1β levels in the supernatants were also decreased
in the cerebral ischemia group following the overexpression of
miRNA-20b and treatment with the ROS scavenger, compared with the
miRNA-20b overexpression alone group (Fig. 7G-H). These results suggested that
miRNA-20b regulates the NLRP3 signaling pathway by affecting the
levels of ROS during cerebral ischemia.
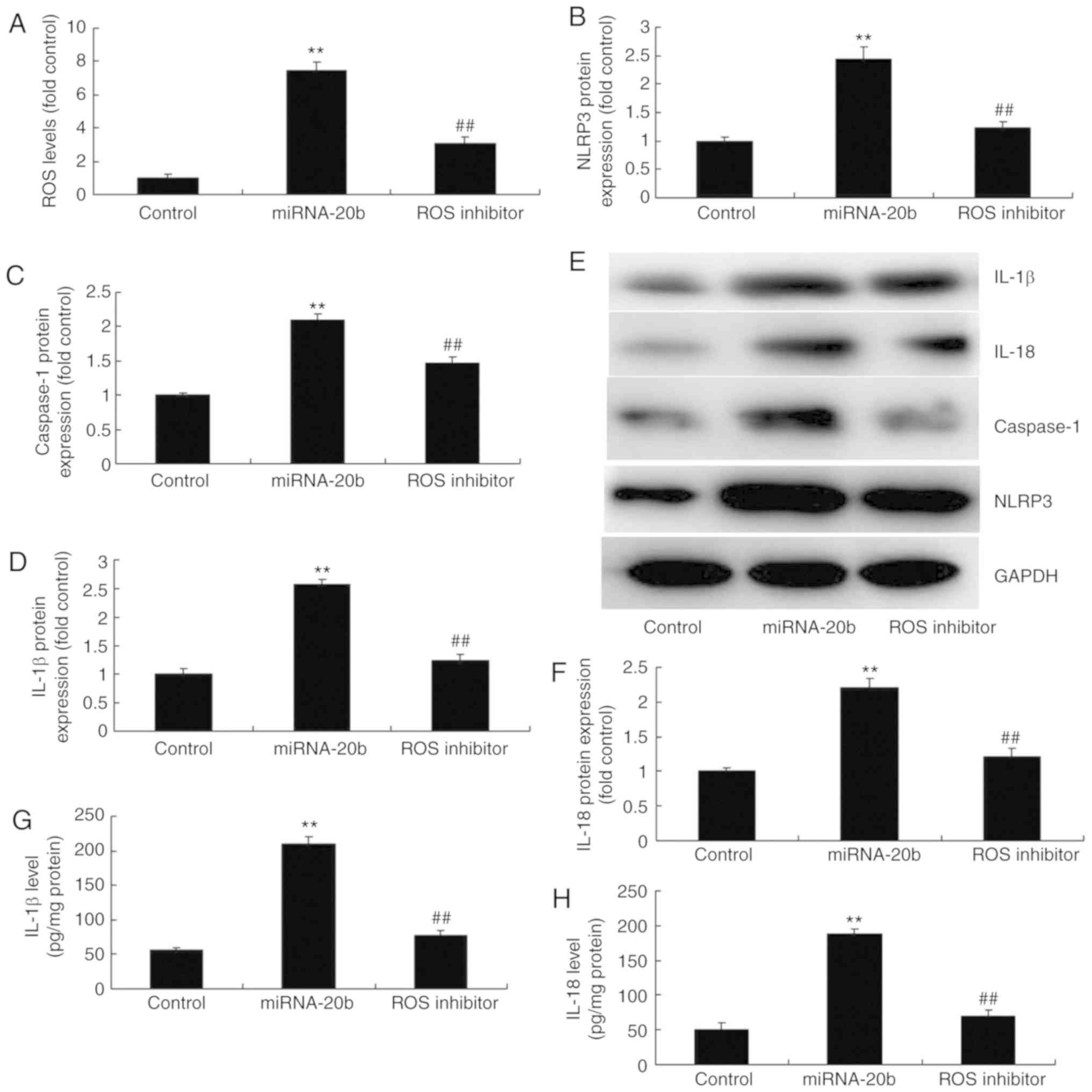 | Figure 7Suppression of ROS decreases the
pro-inflammatory effect of miRNA-20b during cerebral ischemia. (A)
ROS levels were determined using ELISA. Densitometric analysis of
(B) NLRP3, (C) caspase-1, (D) IL-18 and (E) IL-1β protein
expression and (F) western blot analysis of NLRP3, caspase-1, IL-18
and IL-1 protein expression following miRNA-20b overexpression and
ROS inhibition. Serum levels of (G) IL-1β and (H) IL-18 following
miRNA-20b overexpression and ROS inhibition. **P<0.01
vs. negative control group. ##P<0.01 vs. miRNA-20b
overexpression group. miRNA, microRNA; ROS, reactive oxygen
species; NLRP3, NOD-like receptor pyrin domain containing 3;
control, negative control group; miRNA-20b, miRNA-20b
overexpression group; ROS inhibitor, 1 mM NAC and miRNA-20b
overexpression group. |
Suppression of NLRP3 decreases the
pro-inflammatory effects of miRNA-20b in cerebral ischemia
To additionally investigate the function of NLRP3 in
the pro-inflammatory effects of miRNA-20b during cerebral ischemia,
an NLRP3 inhibitor (5 nM MCC950) was used. It was demonstrated that
the inhibition of NLRP3 suppressed the protein expression of NLRP3,
caspase-1, IL-18 and IL-1β in cerebral ischemia compared with the
miRNA-20b overexpression alone group (Fig. 8A-E). The NLRP3 inhibitor decreased
IL-18 and IL-1β levels in the supernatants of the cerebral ischemia
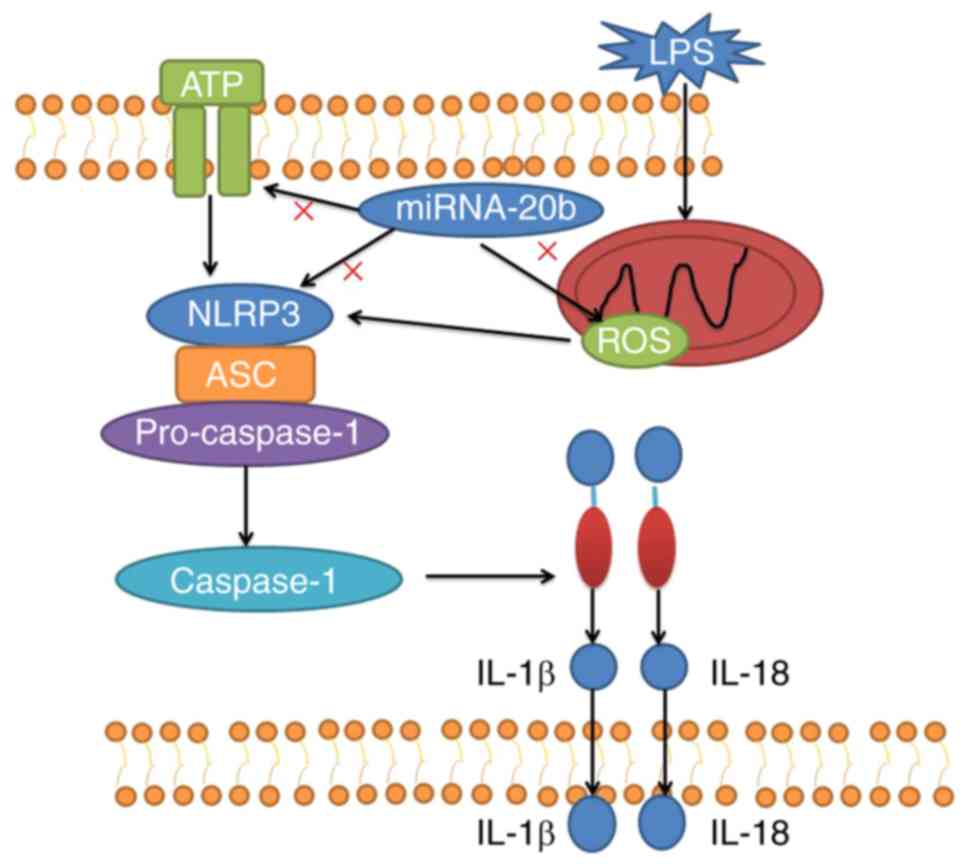
in comparison with the miRNA-20b overexpression group (Fig. 8F-G). The schematic for how
miRNA-20b inhibits cerebral ischemia-induced inflammation through
targeting NLRP3 is demonstrated in Fig. 9.
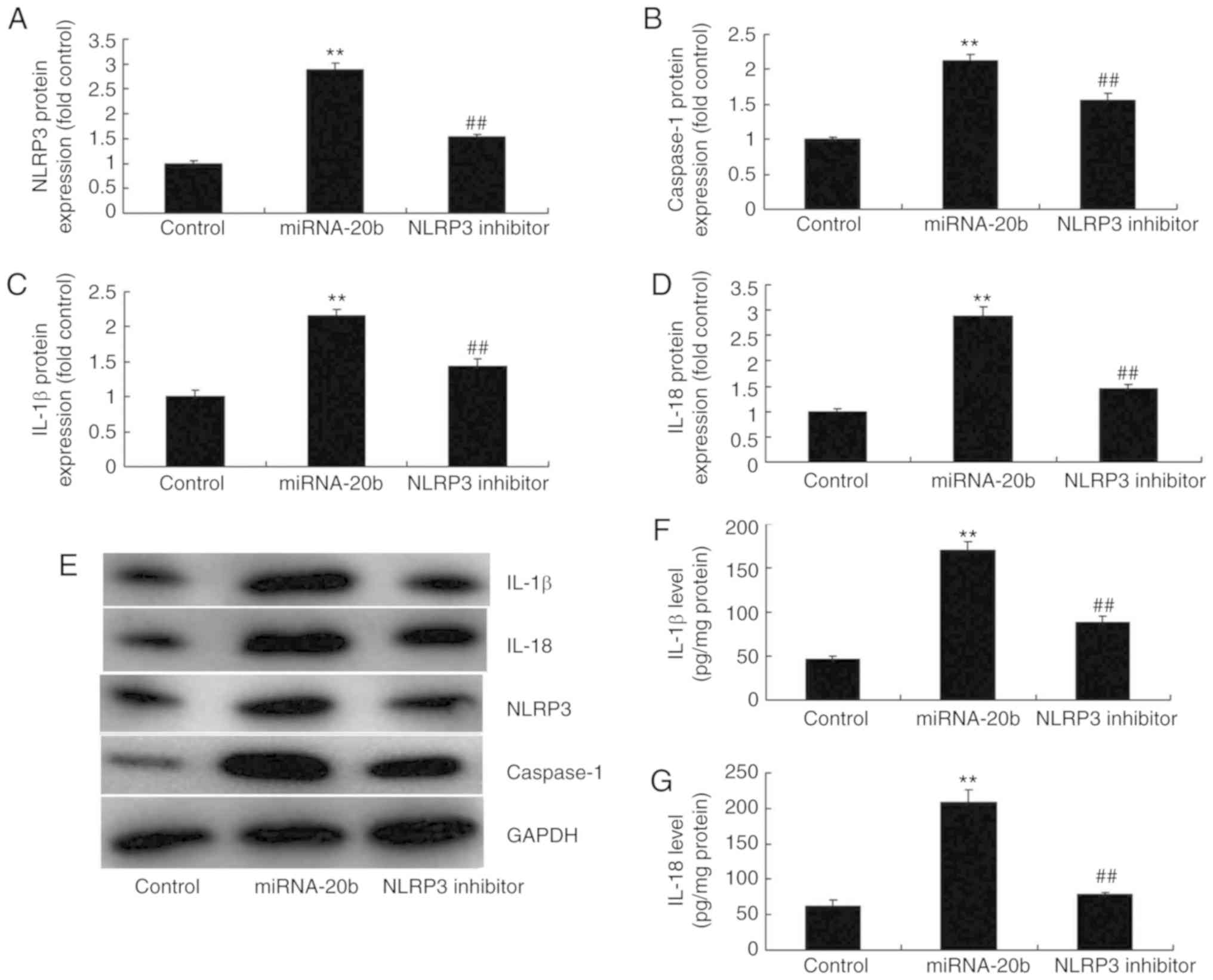 | Figure 8Suppression of NLRP3 decreases the
pro-inflammatory effect of miRNA-20b in cerebral ischemia.
Densitometric analysis of (A) NLRP3, (B) caspase-1, (C) IL-18 and
(D) IL-1β protein and (E) western blot analysis of NLRP3,
caspase-1, IL-18 and IL-1 protein expression. Serum levels of (F)
IL-1β and (G) IL-18 levels. **P<0.01 vs. negative
control group. ##P<0.01 vs. miRNA-20b overexpression
group. miRNA, microRNA; NLRP3, NOD-like receptor pyrin domain
containing 3; IL, interleukin; control, control negative group;
miRNA-20b, miRNA-20b overexpression group; NLRP3 inhibitor, 5 nM
MCC950 and miRNA-20b overexpression group. |
Discussion
Much improvement has been made in the diagnostic and
therapeutic approaches for ischemic stroke in the previous 2
decades. Revascularization or reperfusion therapy remains a key
treatment for patients suffering from ischemic stroke (3). Thrombolysis or endovascular
treatment, delivered within an effective time window, may rescue
cells in the ischemic penumbra (18). Thereby, it may decrease the risk
of neurological deficits, decrease morbidity and mortality and
increase survival. Ultimately, it may improve the outcomes and
future quality of life for patients. However, revascularization may
lead to ischemia-reperfusion injury (4). In the present study, it was
identified that miRNA-20b expression was increased in rats with
cerebral ischemia compared with the control group. Ahmad et
al (19) demonstrated that
miRNA-20b is upregulated in brain metastases from primary breast
cancer.
During cerebral ischemia-reperfusion, there are
numerous inflammatory factors in the ischemic area (20). In addition, the activation and
infiltration of inflammatory cells, and the synthesis and secretion
of adhesion molecules, are considered to be cascade reactions that
reciprocally promote each other (21). Therefore, the inflammatory
response serves an important role in the mechanism of cerebral
ischemia-reperfusion injury. In the present study, it was
identified that the overexpression of miRNA-20b increased IL-18 and
IL-1β levels in an in vitro model of cerebral ischemia. Ma
et al (22) suggested that
miRNA-20b decreased the incidence in asthmatic mice.
NLRP3 is primarily expressed in immune organs and
peripheral immune cells (13). It
has been recently identified to be expressed in the central nervous
and cardiovascular systems, with abundant expression in vascular
endothelial cells (13). A
previous study demonstrated that the expression of NLRP3 was also
detected on the wall of intracranial aneurysms (13). Besides, the expression of NLRP3 on
the wall of ruptured aneurysms was significantly increased compared
with that on the walls of non-ruptured aneurysm (23). These data suggest that NLRP3 may
be involved in the formation and progression of intracranial
aneurysms. By constructing mouse models of MCA occlusion, NLRP3 has
been demonstrated to be primarily expressed in microglia and
vascular endothelial cells, and expressed in neurons and astrocytes
to a lesser degree (23). The
data of the present study demonstrated that the overexpression of
miRNA-20b induced NLPR3 and caspase-1 protein expression in
cerebral ischemia. Coskun et al (24) suggested that miR-20b, miR-98,
miR-125b-1*, and let-7e* are novel potential diagnostic biomarkers
in ulcerative colitis. So, these results demonstrated that
miRNA-20b could be a biomarker for cerebral ischemia.
Numerous studies have demonstrated that NLRP3
expression is upregulated and that the NLRP3 inflammasome is
activated following cerebral ischemia (25). Additionally, the neuronal function
may be protected by regulating the activity of NLRP3 inflammasome,
leading to improved prognoses (25,26). It has been demonstrated that the
protein expression levels of NLRP3, apoptosis-associated speck-like
protein containing a CARD and caspase-1 are increased, the NLRP3
inflammasome is activated and increased levels of IL-1β and IL-18
are secreted following cerebral ischemia and hypoxia by in
vitro and in vivo studies (26). Caspase-1 inhibitors can suppress
neuronal apoptosis and decrease ischemia-reperfusion injury
(26). In addition,
immunoglobulin treatment may result in reduced activities of NLRP1
and NLRP3 inflammasome, and decrease the infarction size and
mortality rate (26). These
results additionally demonstrated that the NLRP3 inflammasome is
involved in the immune inflammatory reaction following cerebral
ischemia-reperfusion injury (26). The present study identified that
the suppression of NLRP3 decreased the pro-inflammatory effect of
miRNA-20b in cerebral ischemia. In agreement with this, Lou et
al (27) indicated that
miRNA-20b may alleviate the inflammatory response in mice with
tuberculosis via targeting the NLRP3/caspase-1/IL-1β pathway.
In conclusion, the present study provided novel
insight into the roles of miRNA-20b upregulation in the promotion
of inflammation following cerebral infarction via the NLRP3
signaling pathway. The identification of the miRNA-20b/NLRP3 axis
may provide novel insight into the potential molecular mechanisms
of cerebral infarction.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
LL designed the experiment. JZ, HW, LD and SS
performed the experiments. LL and JZ analyzed the data. LL wrote
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All experiments were performed in compliance with
guidelines for the ethical use of animals of Hebei General
Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Kan H, Guo W, Huang Y and Liu D:
MicroRNA-520g induces epithelial-mesenchymal transition and
promotes metastasis of hepatocellular carcinoma by targeting SMAD7.
FEBS Lett. 589:102–109. 2015. View Article : Google Scholar
|
|
2
|
Chao Y, Chung YH, Han G, Yoon JH, Yang J,
Wang J, Shao GL, Kim BI and Lee TY: The combination of
transcatheter arterial chemoembolization and sorafenib is well
tolerated and effective in Asian patients with hepatocellular
carcinoma: Final results of the START trial. Int J Cancer.
136:1458–1467. 2015. View Article : Google Scholar
|
|
3
|
Tao ZH, Wan JL, Zeng LY, Xie L, Sun HC,
Qin LX, Wang L, Zhou J, Ren ZG, Li YX, et al: miR-612 suppresses
the invasive-metastatic cascade in hepatocellular carcinoma. J Exp
Med. 210:789–803. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lv N, Kong Y, Mu L, Pan T, Xie Q and Zhao
M: Effect of perioperative parecoxib sodium on postoperative pain
control for transcatheter arterial chemoembolization for inoperable
hepatocellular carcinoma: A prospective randomized trial. Eur
Radiol. 26:3492–3499. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chai R, Fu H, Zheng Z, Liu T, Ji S and Li
G: Resveratrol inhibits proliferation and migration through SIRT1
mediated posttranslational modification of PI3K/AKT signaling in
hepatocellular carcinoma cells. Mol Med Rep. 16:8037–8044. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou SL, Zhou ZJ, Hu ZQ, Li X, Huang XW,
Wang Z, Fan J, Dai Z and Zhou J: CXCR2/CXCL5 axis contributes to
epithelial-mesenchymal transition of HCC cells through activating
PI3K/Akt/GSK-3β/Snail signaling. Cancer Lett. 358:124–135. 2015.
View Article : Google Scholar
|
|
7
|
Ito Y, Miyoshi E, Takeda T, Sakon M, Noda
K, Tsujimoto M, Monden M, Taniguchi N and Matsuura N: Expression
and possible role of ets-1 in hepatocellular carcinoma. Am J Clin
Pathol. 114:719–725. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miskiewicz A, Szparecki G, Durlik M,
Rydzewska G, Ziobrowski I and Górska R: The Q705K and F359L
single-nucleotide polymorphisms of NOD-like receptor signaling
pathway: Association with chronic pancreatitis, pancreatic cancer,
and periodontitis. Arch Immunol Ther Exp (Warsz). 63:485–494. 2015.
View Article : Google Scholar
|
|
9
|
Castaño-Rodríguez N, Kaakoush NO, Goh KL,
Fock KM and Mitchell HM: The NOD-like receptor signalling pathway
in Helicobacter pylori infection and related gastric cancer: A
case-control study and gene expression analyses. PLoS One.
9:e988992014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ungerbäck J, Belenki D, Jawad ul-Hassan A,
Fredrikson M, Fransén K, Elander N, Verma D and Söderkvist P:
Genetic variation and alterations of genes involved in
NFkappaB/TNFAIP3- and NLRP3-inflammasome signaling affect
susceptibility and outcome of colorectal cancer. Carcinogenesis.
33:2126–2134. 2012. View Article : Google Scholar
|
|
11
|
Pontillo A, Oshiro TM, Girardelli M,
Kamada AJ, Crovella S and Duarte AJ: Polymorphisms in inflammasome'
genes and susceptibility to HIV-1 infection. J Acquir Immune Defic
Syndr. 59:121–125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng D, Zhang L, Yang G, Zhao L, Peng F,
Tian Y, Xiao X, Chung RT and Gong G: Hepatitis C virus NS5A drives
a PTEN-PI3K/Akt feedback loop to support cell survival. Liver Int.
35:1682–1691. 2015. View Article : Google Scholar
|
|
13
|
Wang XJ, Feng CW and Li M: ADAM17 mediates
hypoxia-induced drug resistance in hepatocellular carcinoma cells
through activation of EGFR/PI3K/Akt pathway. Mol Cell Biochem.
380:57–66. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong Y, Liang G, Yuan B, Yang C, Gao R and
Zhou X: MALAT1 promotes the proliferation and metastasis of
osteosarcoma cells by activating the PI3K/Akt pathway. Tumour Biol.
36:1477–1486. 2015. View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Chen X, Yan L, Guo Z, Chen Y, Li M, Huang
C, Chen Z and Meng X: Chenodeoxycholic acid attenuates high-fat
diet-induced obesity and hyperglycemia via the G protein-coupled
bile acid receptor 1 and proliferator-activated receptor gamma
pathway. Exp Ther Med. 14:5305–5312. 2017.PubMed/NCBI
|
|
17
|
Bruusgaard A and Andersen RB:
Chenodeoxycholic-acid treatments of rheumatoid arthritis. Lancet.
1:7001976. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao P, Feng F, Dong G, Yu C, Feng S, Song
E, Shi G, Liang Y and Liang G: Estrogen receptor α enhances the
transcriptional activity of ETS-1 and promotes the proliferation,
migration and invasion of neuroblastoma cell in a ligand dependent
manner. BMC Cancer. 15:4912015. View Article : Google Scholar
|
|
19
|
Ahmad A, Ginnebaugh KR, Sethi S, Chen W,
Ali R, Mittal S and Sarkar FH: miR-20b is up-regulated in brain
metastases from primary breast cancers. Oncotarget. 6:12188–12195.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ozaki I, Mizuta T, Zhao G, Zhang H,
Yoshimura T, Kawazoe S, Eguchi Y, Yasutake T, Hisatomi A, Sakai T
and Yamamoto K: Induction of multiple matrix metalloproteinase
genes in human hepatocellular carcinoma by hepatocyte growth factor
via a transcription factor Ets-1. Hepatol Res. 27:289–301. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mamori S and Tajiri H: Ets-1 is increased
in anticancer drug-containing media and hypoxic cultures, similar
to TACE. Scand J Gastroenterol. 44:507–508. 2009. View Article : Google Scholar
|
|
22
|
Ma H, Guo S, Luo Y, Wang Y, Wang H, He J,
Tang J, Shen L and Song C: MicroRNA-20b promotes the accumulation
of CD11b+Ly6G+Ly6Clow myeloid-derived suppressor cells
in asthmatic mice. Cent Eur J Immunol. 42:30–38. 2017. View Article : Google Scholar :
|
|
23
|
Verma D, Bivik C, Farahani E, Synnerstad
I, Fredrikson M, Enerbäck C, Rosdahl I and Söderkvist P:
Inflammasome polymorphisms confer susceptibility to sporadic
malignant melanoma. Pigment Cell Melanoma Res. 25:506–513. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Coskun M, Bjerrum JT, Seidelin JB,
Troelsen JT, Olsen J and Nielsen OH: miR-20b, miR-98, miR-125b-1*,
and let-7e* as new potential diagnostic biomarkers in ulcerative
colitis. World J Gastroenterol. 19:4289–4299. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Girardelli M, Maestri I, Rinaldi RR,
Tognon M, Boldorini R, Bovenzi M, Crovella S and Comar M: NLRP1
polymorphisms in patients with asbestos-associated mesothelioma.
Infect Agent Cancer. 7:252012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pontillo A, Bricher P, Leal VN, Lima S,
Souza PR and Crovella S: Role of inflammasome genetics in
susceptibility to HPV infection and cervical cancer development. J
Med Virol. 88:1646–1651. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lou J, Wang Y, Zhang Z and Qiu W: MiR-20b
inhibits mycobacterium tuberculosis induced inflammation in the
lung of mice through targeting NLRP3. Exp Cell Res. 358:120–128.
2017. View Article : Google Scholar : PubMed/NCBI
|