Introduction
Acute respiratory distress syndrome (ARDS) is a
refractory respiratory failure that represents one of the most
clinically severe acute disorders (1). ARDS is pathophysiologically
characterized by alveolar epithelial cell damage. Currently, the
treatment efficacy of ARDS by mechanical ventilation and medication
is poor, and the mortality rate of ARDS remains high (2). Stem cell-mediated repair of injured
alveolar epithelial cells is promising for improving the outcomes
of ARDS (3). Due to their high
self-renewal and differentiation potential, mesenchymal stem cells
(MSCs) are ideal for cell-based tissue repair strategies (4). Animal studies revealed that
exogenous MSCs can target and repair sites of experimental
ARDS-associated alveolar epithelial injury (5,6).
However, the proportion of MSCs with effective restorative
properties that home to ARDS lung tissue is relatively low due to
low retention time and impaired migration capacity (7,8).
Therefore, determining the mechanisms that promote MSC
differentiation, migration, proliferation, and resistance to injury
may improve the outcomes of ARDS (9,10).
Our laboratory has previously demonstrated that the
inhibition of the Hippo signaling pathway in vitro may
enhance the differentiation of MSCs into alveolar type II
epithelial (ATII) cells, stimulate MSC proliferation and migration
toward ARDS lung tissue, and strengthen MSC resistance to oxidative
stress (11). This approach may
provide a novel method to optimize cell-based ARDS treatments.
However, experimental microenvironmental conditions and regulatory
mechanisms exhibit increased complexity in vivo, and there
remains insufficient evidence of whether the modulation of the
Hippo signaling may enhance the protective role of MSCs in the
lungs of animal models of ARDS.
In a previous study, we successfully constructed a
C57BL/6 mouse bone marrow-derived mesenchymal stem cell (mMSC) line
with Lats1 gene (NCBI Reference Sequence: NM_010690; www.ncbi.nlm.nih.gov/genbank) knockdown and
demonstrated that the downregulation of the Hippo signaling
promoted differentiation, proliferation, and migration of these
mMSCs in vitro (11).
Therefore, the current study aimed to confirm the effect of
manipulating the Hippo signaling on MSC-mediated repair of ARDS
lung injury in vivo by transplanting mMSCs with
shRNA-mediated knockdown of Lats1 into the airways of mouse models
of ARDS.
Materials and methods
Materials and reagents
Lipopolysaccharide (LPS; isolated from
Escherichia coli 0111:B4) and dimethylsulfoxide (DMSO) were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
C57BL/6 mMSCs were obtained from Cyagen Biosciences, Inc. (Santa
Clara, CA, USA). Fetal bovine serum (FBS) and Dulbecco's modified
Eagle's medium/nutrient mixture F-12 (DMEM/F12) were obtained from
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Trypsin was
purchased from Gibco (Thermo Fisher Scientific, Inc.). The
bicinchoninic acid (BCA) protein quantitation reagent kit, nuclear
and cytoplasmic protein extraction kits (cat. no. P0027), and
horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G
(IgG; cat. no. A0216) were purchased from Beyotime Institute of
Biotechnology (Nanjing, China). CellVue NIR815 dye was purchased
from eBioscience (Thermo Fisher Scientific, Inc.). Mouse anti-green
fluorescent protein (GFP) antibody (cat. no. ab1218) was purchased
from Abcam (Cambridge, MA, USA). Rabbit antibodies against Lats1,
surfactant protein C (SPC), occludin, β-actin and GAPDH (cat. nos.
sc-398560, sc-518029, sc-133256, sc-47778 and sc-32233,
respectively) were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Mouse interleukin (IL)-1β, IL-6, IL-4 and IL-10
ELISA detection kits (cat. nos. 559603, 550950, 555232 and 555252,
respectively) were purchased from BD Biosciences (San Jose, CA,
USA). Mouse total protein (TP) and albumin (ALB) ELISA detection
kits (cat. nos. ml001923 and ml022503, respectively) were purchased
from Shanghai Enzyme-Linked Biotechnology Co., Ltd. (Shanghai,
China).
Transfection and culture of mMSCs
Lentiviral transfection of C57BL/6 mMSCs was
performed as previously reported by our laboratory (11). The protein and mRNA expression
levels of Lats1 were measured by western blotting and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) to
confirm stable and effective transfection of mMSCs by lentiviral
vector. Following successful transfection, mMSCs were grown and
passaged in DMEM/F12 culture medium containing 10% FBS and 1%
streptomycin and penicillin at 37°C in an atmosphere with 5%
CO2. Subsequent experiments were performed on passage 6
or 7 cells.
Experimental animals and the induction of
ARDS
A total of 60 male SPF-grade C57BL/6 mice (age, 6–8
weeks; weight, 20–25 g), were obtained from the Research Animal
Center of the Academy of Military Medical Sciences (Beijing,
China). The experimental protocols were approved by the
Institutional Animal Care and Use Committee at Nanjing Medical
University (Wuxi, China), and all animal use met the requirements
of the Guide for the Care and Use of Laboratory Animals (12). The mice were maintained under
specific pathogen-free conditions with a 12-h light/dark cycle
(temperature, 18–23°C; humidity, 40–60%) and fed standard rodent
chow and water ad libitum, and adapted to laboratory
conditions for at least 3 days before experimentation. After the
mice were anesthetized by an intraperitoneal injection of 50 mg/kg
pentobarbital, ARDS was induced by intratracheal administration of
50 µl 2 mg/ml LPS using a micropipette. The mice recovered
in an oxygenated cage until fully awake.
Experimental animal groups
C57BL/6 mice were randomly divided into four groups
of equal size (n=15 mice/group) as follows: Normal mice (control),
mice with ARDS (ARDS), mice with ARDS treated with mMSCs
transfected with empty lentivirus [ARDS + MSC-short hairpin RNA
(sh)control], and mice with ARDS treated with mMSCs transfected
with Lats1-interfering lentivirus (ARDS + MSC-shLats1). Instead of
ARDS induction, the mice in the control group were initially
injected intratracheally with 50 µl of saline and after 4 h,
30 µl of PBS. ARDS was modeled by the intratracheal
injection of 50 µl of LPS solution, and ARDS mice received
an additional 30 µl injection of PBS 4 h after exposure to
LPS. In the ARDS + MSC-shcontrol group, 30 µl PBS containing
mMSCs transfected with empty lentivirus (5×104 cells)
was injected into the airway 4 h after exposure to LPS. In the ARDS
+ MSC-shLats1 group, 30 µl PBS containing mMSCs transfected
with Lats1-interfering lentivirus (5×104 cells) was
injected into the airway 4 h after exposure to LPS. Mice were
sacrificed 3, 7, and 14 days after the establishment of LPS-induced
ARDS, and intact lung tissue was harvested for subsequent
experiments.
Hematoxylin and eosin staining of lung
tissue and lung injury scoring
Lung tissue from the right upper lobe was fixed in
10% neutral formaldehyde at 4°C for 24 h and embedded in formalin.
After sequential staining with hematoxylin for 5 min and eosin for
2 min at 25°C and consecutive transverse slicing into
5-µm-thick sections, 10 high magnification (×400; light
microscopy) visual fields were randomly selected for
semiquantitative evaluation of lung injury based on the method
reported by Smith et al (13), and the average value was
determined.
Modified Masson's staining of lung tissue
and pulmonary fibrosis scoring
Lung tissue from the right lower lobe was fixed in
10% neutral formaldehyde at 4°C for 24 h and embedded in formalin.
After sequential staining with Weigert's Iron haematoxylin for 10
min, Biebrish scarlet-acid fuchsin for 5 min and aniline blue for 5
min (all at 25°C) and consecutive transverse slicing into
4-µm-thinck sections, 10 high magnification (×400; light
microscopy) visual fields were randomly selected for
semiquantitative scoring of pulmonary fibrosis based on the scoring
method reported by Ashcroft et al (14), and the average value was
determined.
mMSC labeling and tracking
The cultured MSC-shcontrol and MSC-shLats1 cells
were harvested and labeled with CellVue NIR815 dye according to the
manufacturer's protocol. Labeled cells (5×105 cells)
were directly injected into the airways of the ARDS + MSC-shcontrol
and ARDS + MSC-shLats1 groups under isoflurane anesthesia. Ex
vivo lungs from three mice/group were imaged using a Maestro II
small animal in vivo imaging system (PerkinElmer, Inc.,
Walham, MA, USA) at 3, 7, and 14 days after cell injection to
observe mMSC retention in the lungs. A filter set with 786 nm
excitation and 814 nm emission was used to detect the fluorescence
signal, and the exposure time was 4,000 msec. The autofluorescence
spectra were then unmixed based on their spectral patterns using
Maestro™ 2.2 software (PerkinElmer, Inc.). The fluorescence
intensity of the lungs was measured by placing fixed size boxes on
the organ of interest to observe the changes in counts in the box
of that organ over time, and the average signals were normalized by
the exposure time and the area of the ROI (scaled counts/sec).
Immunofluorescence staining of lung
tissue
Left lung tissue was embedded in 10% optimal cutting
temperature medium (Thermo Fisher Scientific, Inc.), sectioned at
−20°C, and fixed in 2% acetone at 4°C for 15 min. After air-drying
at 25°C, the samples were hydrated, permeabilized with 0.3% Triton
X-100 (Thermo Fisher Scientific, Inc.) at 25°C for 30 min and
blocked with 3% bovine serum ALB (Thermo Fisher Scientific, Inc.)
at 25°C for 5 min. Primary antibodies against GFP (1:100) and/or
SPC (1:100) were added, incubated at 4°C overnight, and washed with
PBS. A fluorescent fluorescein
isothiocyanate/tetramethylrhodamine-labeled secondary antibody
(cat. no. ab228549; 1:1,000; Abcam) was added, incubated at 25°C
for 1 h, and washed with PBS. Subsequently,
4′,6-diamidino-2-phenylin-dole was added at 25°C for 10 min, and
the slide was mounted and sealed. Five randomly selected high-power
fields for each slide were observed and imaged at high
magnification (×400) using a fluorescence microscope (Olympus
Corporation, Tokyo, Japan). The count of GFP-positive cells
represented the retention of transplanted mMSCs in the lung, while
the ratio of the count of double-positive cells to the count of
GFP-positive cells represented the differentiation of transplanted
mMSCs into ATII cells in the lung (15).
Western blotting
Nuclear protein and cytoplasmic protein extraction
kits were used to isolate the TP and cytoplasmic protein. A BCA
assay was used to measure the protein concentration. Proteins were
denatured and added to the wells in aliquots of 25 µl/well.
After SDS-PAGE (10% gel), the proteins were transferred to a
polyvinylidene fluoride membrane, blocked with Tris-buffered saline
with Tween-20 (TBST) containing 5% skim milk powder at 25°C for 1
h, and incubated at 4°C overnight with primary antibodies against
Lats1, SPC and occludin (all at 1:400 dilution). β-actin (1:400) or
GAPDH (1:400) was used as an internal reference protein. Lats1 and
GAPDH were used with total protein, and SPC, occludin and β-actin
were used with cytoplasmic protein. The membrane was washed three
times with TBST and incubated with the aforementioned horseradish
peroxidase-conjugated secondary antibody (cat. no. A0216; 1:1,000)
at 25°C for 1 h. The membrane was washed four times with TBST,
developed with the Pierce™ Enhanced Chemiluminescence reagent
(Pierce; Thermo Fisher Scientific, Inc.), and placed on an X-ray
film for imaging. Quantity One 4.6.7 software (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) was used for quantitative
analysis of the grayscale values of the bands.
RT-qPCR detection of Lats1 mRNA
expression
Total RNA was extracted from mMSCs in culture plates
using the TRIzol reagent (Thermo Fisher Scientific, Inc.). Total
RNA (2 µg) was subsequently reverse transcribed to yield
single-stranded cDNAs using the High-Capacity cDNA Reverse
Transcription kit (Thermo Fisher Scientific, Inc.). The reverse
transcription is performed accordingly the following protocol: 25°C
for 10 min, 37°C for 120 min and 85°C for 5 min. An ABI Prism 7500
quantitative PCR instrument (Applied Biosystems; Thermo Fisher
Scientific, Inc.) was used for product amplification. Each sample
was analyzed in triplicate with the following thermocycling
conditions: 40 cycles, including a denaturation step at 95°C for 15
sec, an annealing step at 56°C for 20 sec and an extension step at
72°C for 40 sec. SYBR-Green I (Thermo Fisher Scientific, Inc.) was
selected as a fluorophore dye. The 2−ΔΔCq method was
used to calculate the relative expression levels of Mrna (16), and GAPDH was used as the internal
reference gene. The PCR primers used were as follows: Lats1
forward, 5′-CCACCCTACCCAAAACATCTG-3′ and reverse,
5′-CGCTGCTGATGAGATTTGAGTAC-3′; and GAPDH forward,
5′-TATGTCGTGGAGTCTACTGGT-3′ and reverse,
5′-GAGTTGTCATATTTCTCGTGG-3′.
Cytokine and protein detection in
bronchoalveolar lavage fluid (BALF)
Following aesthesia with pentobarbital, the anterior
trachea was exposed and intubated. BALF was collected by flushing 1
ml ice-cold PBS back and forth three times through a tracheal
cannula and subsequent centrifugation at 1,000 x g at 4°C for 10
min, the supernatant was stored at −80°C. To evaluate the
inflammatory responses in lungs and alveolar epithelial
permeability, IL-1β, -6, -4 and -10 concentrations, TP, and ALB
content were measured according to the protocols provided with the
aforementioned ELISA reagent kits.
Detection of pulmonary edema
Whole lung tissue was collected, and residual
bronchioles and connective tissue were carefully cut away.
Absorbent paper was used to completely absorb water from the lung
surface and to remove blood contamination. The lungs were weighed,
and the lung wet weight/body weight ratio (LWW/BW), which reflects
pulmonary edema and the degree of injury, was determined (17).
Statistical analysis
The SPSS statistical software package (version 20.0;
IBM Corp., Armonk, NY, USA) was used for statistical analysis.
Quantitative data are presented as the mean ± standard deviation
and comparison among multiple groups were performed using one-way
analysis of variance followed by Bonferroni's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Successful Lats1 knockdown in mMSCs using
a lentiviral vector
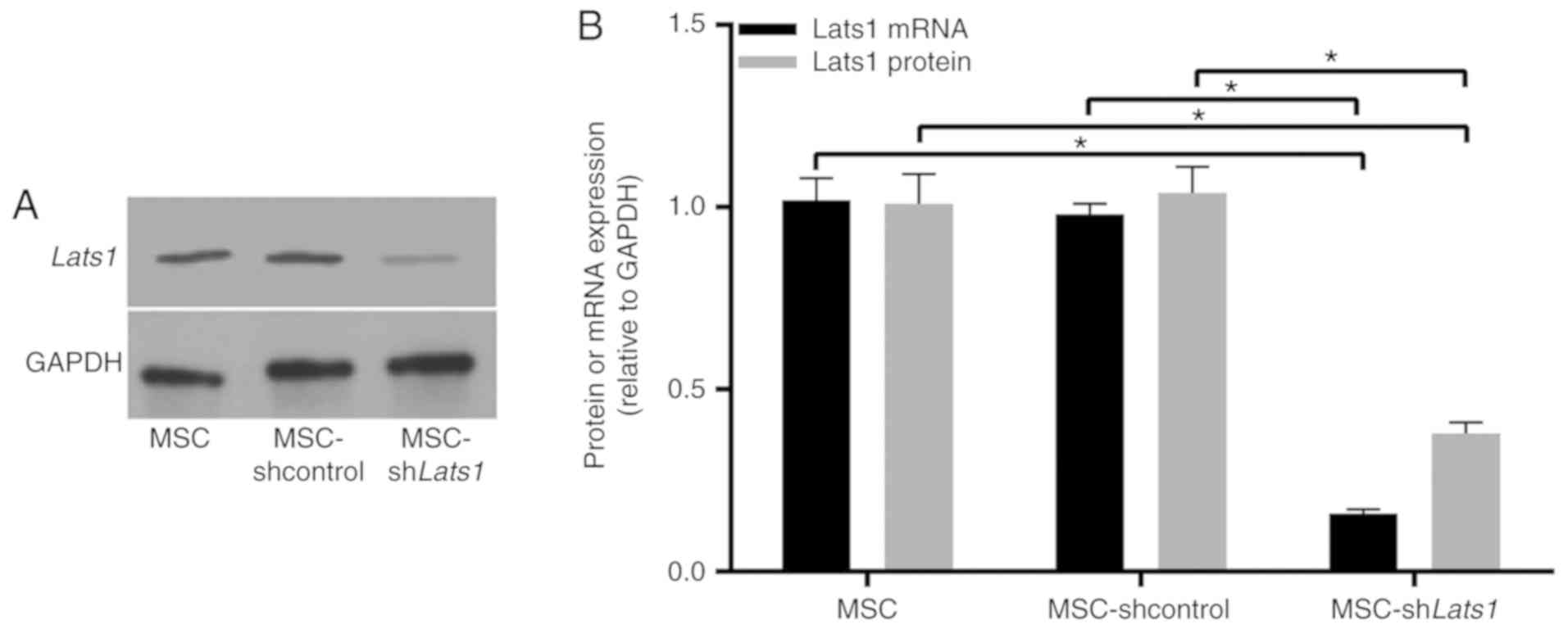
Lats1 protein (Fig.
1A) and mRNA (Fig. 1B;
P<0.05) expression levels in MSC-shLats1 were lower compared
with the MSC and MSC-shcontrol groups, however, there was no
significant difference in Lats1 protein and mRNA expression level
between MSC and MSC-shcontrol. These results suggested that the
lentiviral vector-mediated Lats1 knockdown in mMSCs was
effective.
Lats1 knockdown increases mMSCs retention
in ARDS lung tissue
Analysis of color-coded fluorescence images
indicated that average signals of ex vivo lungs from mice
exposed to shLats1 mMSCs labeled with NIR815 were significantly
greater compared with those in the lungs from ARDS + MSC-shcontrol
animals 3, 7 and 14 days after LPS exposure (all P<0.05);
however, the signal intensities decreased progressively over time
(Fig. 2A). Fluorescence
microscopy revealed that the number of GFP-positive cells in the
lung tissue of the ARDS + MSC-shLats1 group was significantly
greater compared with that in the ARDS + MSC-shcontrol group 3, 7
and 14 days after LPS exposure (all P<0.05); however, the number
of GFP-positive cells decreased progressively over time (Fig. 2B). These results indicate that
Lats1 knockdown increased the retention of mMSCs in ARDS lung
tissue.
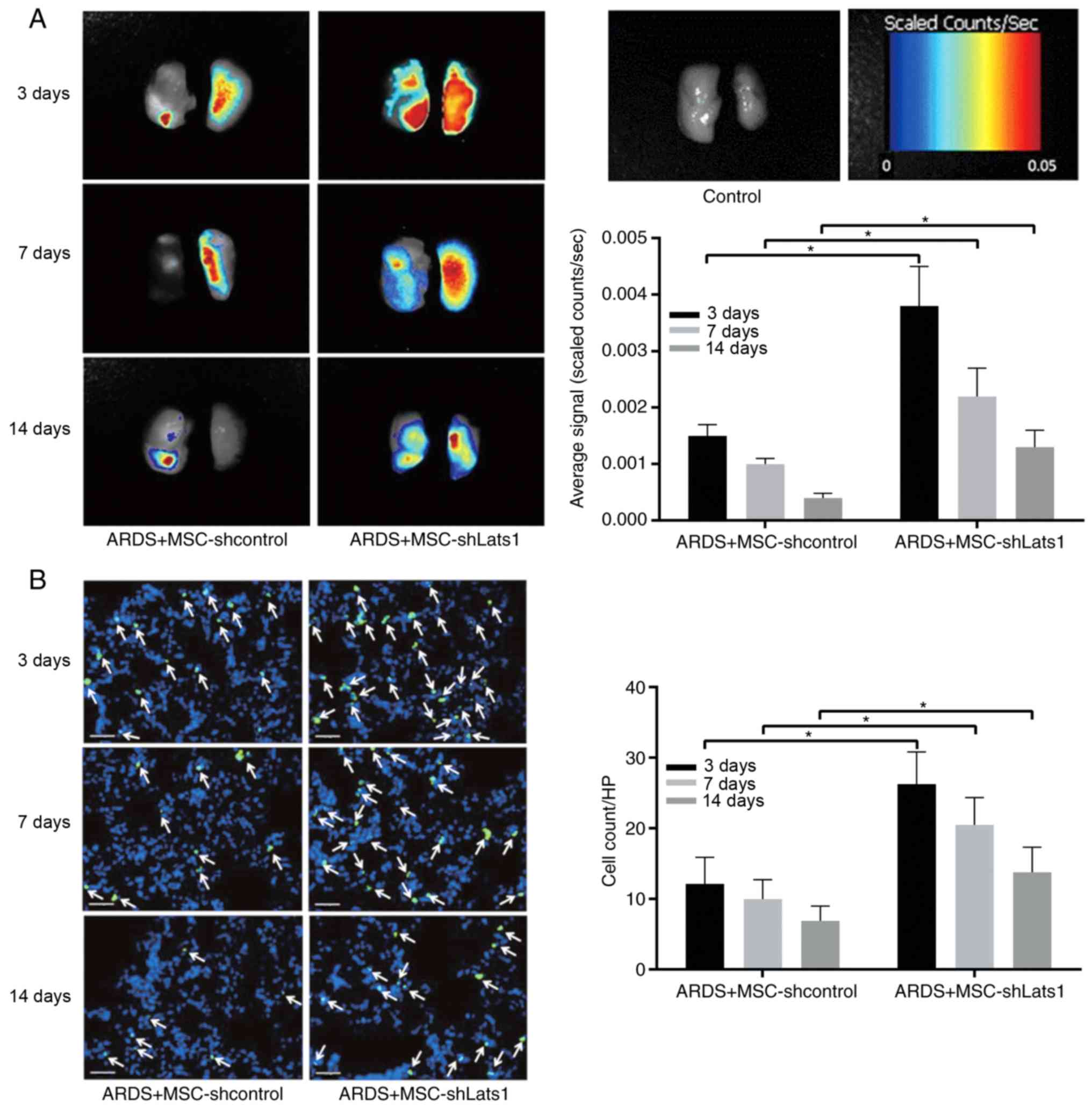 | Figure 2Effect of Lats1 knockdown on mMSCs
retention in ARDS lung tissue. (A) Representative near-infrared
images of ex vivo lungs in the ARDS + MSC-shLats1 and ARDS +
MSC-shcontrol groups are shown from three mouse lungs obtained 3,
7, and 14 days after LPS exposure. (B) Representative
immunofluorescence staining of lung tissue in the ARDS +
MSC-shLats1 and ARDS + MSC-shcontrol groups are presented from
three mouse lungs obtained 3, 7 and 14 days after LPS exposure. The
nuclei were stained with DAPI (blue), and the engrafted mMSCs in
the lung tissue are shown as GFP-positive (green; magnification,
×400; scale bar, 20 µm; white arrows, GFP-positive cells).
The cell count of GFP-positive mMSCs in randomly selected
high-power fields is presented as the mean ± standard deviation
(n=6). *P<0.05. mMSC, bone marrow-derived mesenchymal
stem cell; Lats1, large tumor suppressor kinase; GFP, green
fluorescent protein; sh, short hairpin RNA; HP, high-power
field. |
Lats1 knockdown increases mMSCs
differentiation into ATII cells
To evaluate the differentiation of mMSCs into ATII
cells, the expression of SPC was detected by western blotting and
immunofluorescence staining 14 days after mMSC administration.
Western blot analysis revealed that the protein expression level of
the ATII cell-specific marker SPC was significantly greater in the
ARDS + MSC-shcontrol group compared with the ARDS group
(P<0.05). In the ARDS + MSC-shLats1 group, the SPC protein
expression level was greater compared with the ARDS + MSC-shcontrol
group (P<0.05; Fig. 3A). Cells
positive for GFP (green) or SPC protein expression (red) under
fluorescence microscopy were mMSCs and ATII cells, respectively.
Double-positive cells appeared yellow and indicated mMSCs that
differentiated into ATII cells. There was no difference in the
number of mMSCs and ATII cells in the lung tissue of mice from the
ARDS + MSC-shLats1 and ARDS + MSC-shcontrol groups (data not
shown); however, the proportion of mMSCs that differentiated into
ATII cells was significantly greater in the ARDS + MSC-shLats1
group compared with the ARDS + MSC-shcontrol group (P<0.05;
Fig. 3B). These results suggest
that Lats1 knockdown promoted mMSC differentiation into ATII
cells.
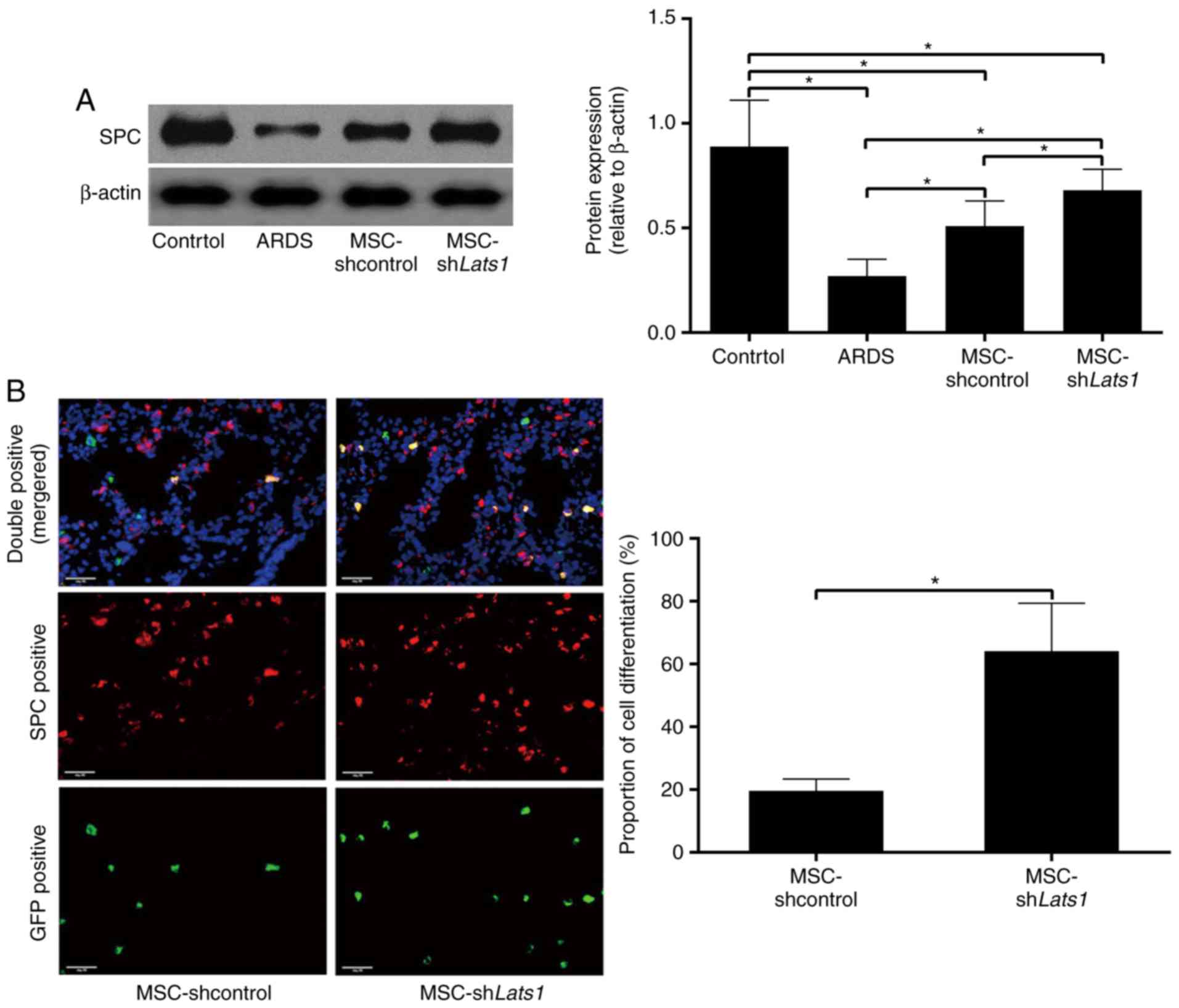 | Figure 3Effect of Lats1 knockdown on the
differentiation of mMSCs into ATII cells. (A) The protein
expression level of SPC in the lung tissue was measured using
western blotting 14 days after mMSC administration. β-actin was
used as an internal reference, and the results are presented as the
mean ± standard deviation (n=6). (B) Differentiation of mMSCs into
ATII cells was detected by immunofluorescence staining in the ARDS
+ MSC-shLats1 and ARDS + MSC-shcontrol groups 14 days after mMSC
administration. Engrafted mMSCs and ATII cells in the lung tissue
are shown as GFP-positive (green) or SPC-positive (red) under
fluorescence microscopy, respectively, while mMSCs that
differentiated into ATII cells are shown as double-positive
(yellow). The nuclei were stained with DAPI (blue; magnification,
×400; scale bar, 20 µm). The ratio of the count of
double-positive cells to the count of GFP-positive cells in
randomly selected high-power fields is presented as the mean ±
standard deviation (n=6). *P<0.05. mMSCs, bone
marrow-derived mesenchymal stem cell; Lats1, large tumor suppressor
kinase 1; SPC, surfactant protein C; ATII, alveolar type II
epithelial; GFP, green fluorescent protein; ARDS, acute respiratory
distress syndrome; sh, short hairpin RNA. |
mMSCs with downregulated Hippo signaling
improve pulmonary edema and permeability of lung epithelium in ARDS
lung tissue
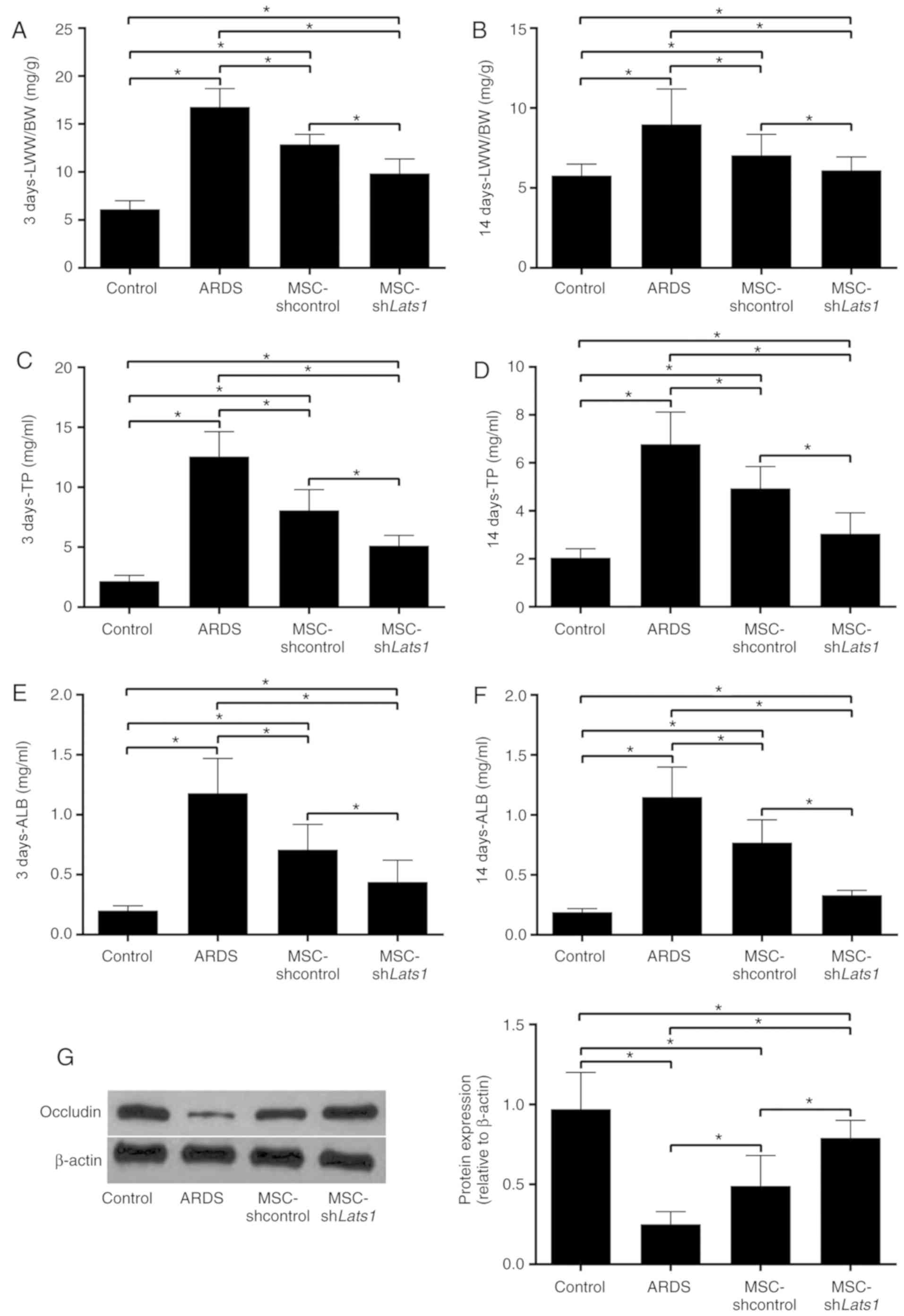
The LWW/BW, which reflects the degree of pulmonary
edema (17), in the ARDS +
MSC-shcontrol group was significantly lower compared with the ARDS
group 3 and 14 days after LPS exposure (both P<0.05).
Furthermore, the LWW/BW in the ARDS + MSC-shLats1 group was lower
compared with the ARDS + MSC-shcontrol group 3 and 14 days after
LPS exposure (both P<0.05; Fig. 4A
and B). TP and ALB concentrations in the BALF reflect the
permeability of the lung epithelium (18). BALF TP and ALB concentrations in
the ARDS + MSC-shcontrol group were significantly lower compared
with the ARDS group 3 and 14 days after LPS exposure (all
P<0.05). Furthermore, the BALF TP and ALB concentrations in the
ARDS + MSC-shLats1 group were lower compared with the ARDS +
MSC-shcontrol group (all P<0.05; Fig. 4C-F). Western blot detection of the
lung tissue epithelial tight junction protein occludin is used for
characterization of permeability of lung epithelium (19). Occludin expression in the lung
tissue of the ARDS + MSC-shcontrol group significantly increased
compared with the ARDS group 14 days after LPS exposure
(P<0.05). Furthermore, occludin protein expression level in the
lung tissue of the ARDS + MSC-shLats1 group increased compared with
the ARDS + MSC-shcontrol group 14 days after LPS exposure
(P<0.05; Fig. 4G). These
results indicate that mMSCs with downregulated Hippo signaling may
improve pulmonary edema and the permeability of lung epithelium in
ARDS lung tissue.
mMSCs with downregulated Hippo signaling
improve inflammation in ARDS lung tissue
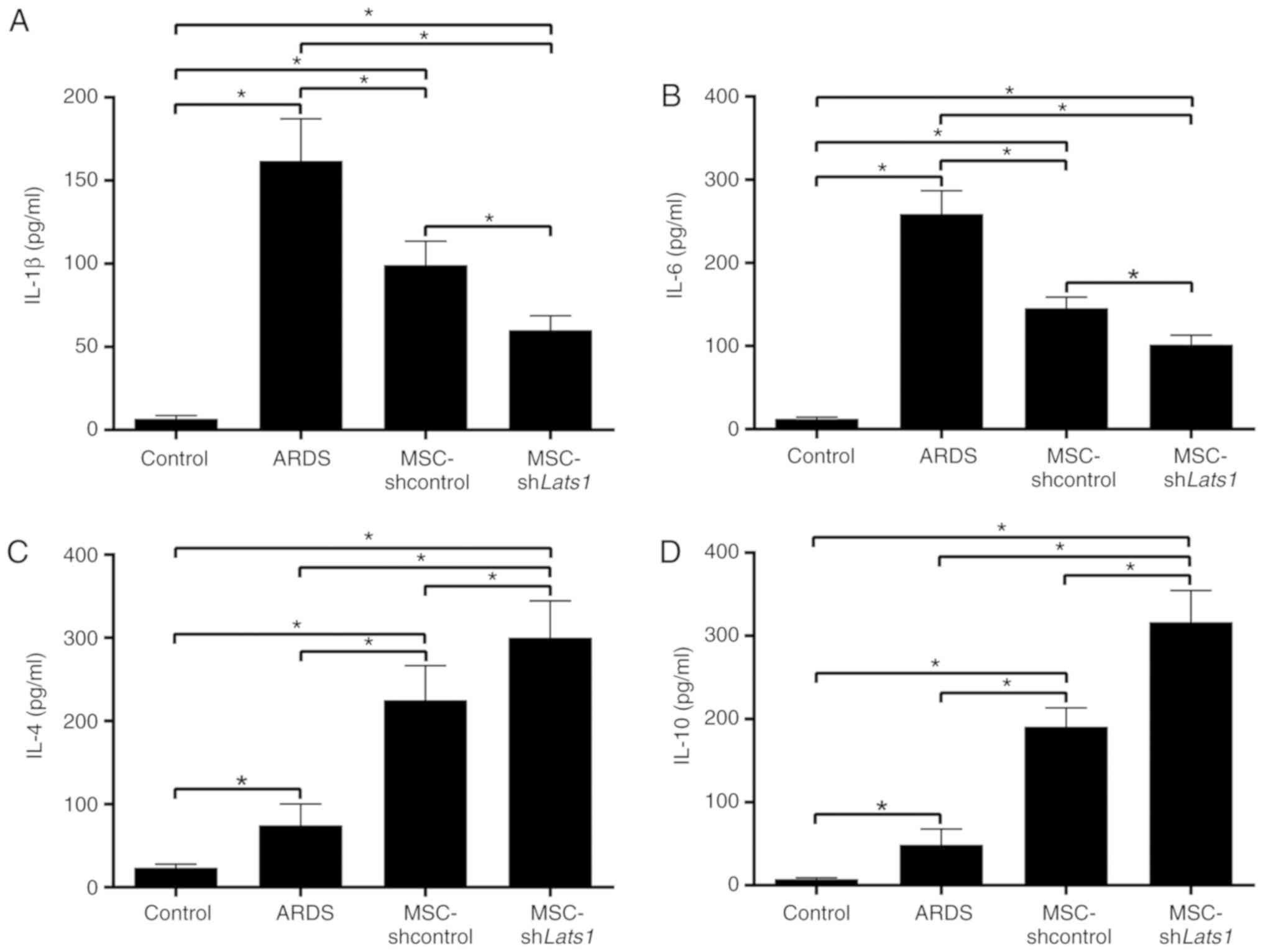
To evaluate the levels of lung inflammation in
different groups, concentrations of the proinflammatory factors
IL-1β and -6, and the anti-inflammatory factors IL-4 and -10 in the
BALF were measured using ELISA kits. IL-1β and -6 concentrations in
the BALF of the ARDS + MSC-shcontrol group were significantly lower
compared with the ARDS group (P<0.05), whereas IL-4 and -10
concentrations were significantly increased compared with the ARDS
group (P<0.05). Furthermore, IL-1β and -6 concentrations in the
BALF of the ARDS + MSC-shLats1 group were lower compared with the
ARDS + MSC-shcontrol group (both P<0.05; Fig. 5A and B), whereas IL-4 and -10
concentrations were greater in the ARDS + MSC-shLats1 group
compared with the ARDS + MSC-shcontrol group (both P<0.05;
Fig. 5C and D). These results
indicate that mMSCs with downregulated Hippo signaling improved
inflammation in ARDS lung tissue by reducing the levels of
proinflammatory factors and increasing the level of
anti-inflammatory factors.
mMSCs with downregulated Hippo signaling
attenuate pathological injuries in ARDS lung tissue
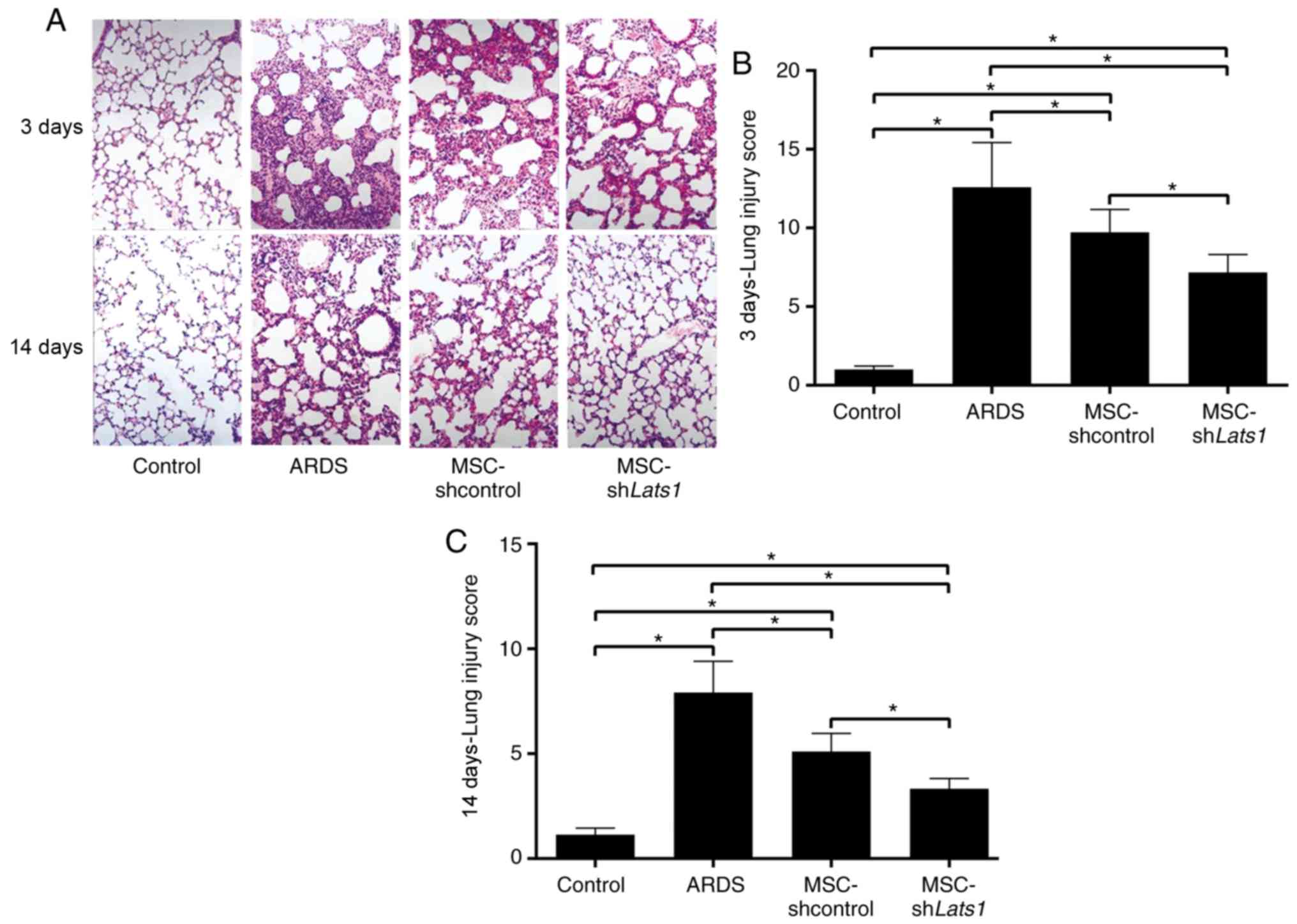
Edema and bleeding were widespread in the alveoli
and interstitium of ARDS lung tissue, whereas no such
manifestations were observed in the control mice (Fig. 6A). There was diffuse inflammatory
cell infiltration, severe alveolar collapse and structural damage
in the lungs of ARDS group animals, and their lung injury score was
the highest among all groups examined (all P<0.05). Pathological
changes and lung injury scores were significantly improved in the
ARDS + MSC-shcontrol group compared with the ARDS group 3 and 14
days after LPS exposure (both P<0.05). Furthermore, pathological
changes and lung injury scores in the ARDS + MSC-shLats1 group were
further improved compared with the ARDS + MSC-shcontrol group 3 and
14 days after LPS exposure (both P<0.05; Fig. 6A-C). These results indicated that
mMSCs with downregulated Hippo signaling may ameliorate the
pathological injuries in ARDS lung tissue.
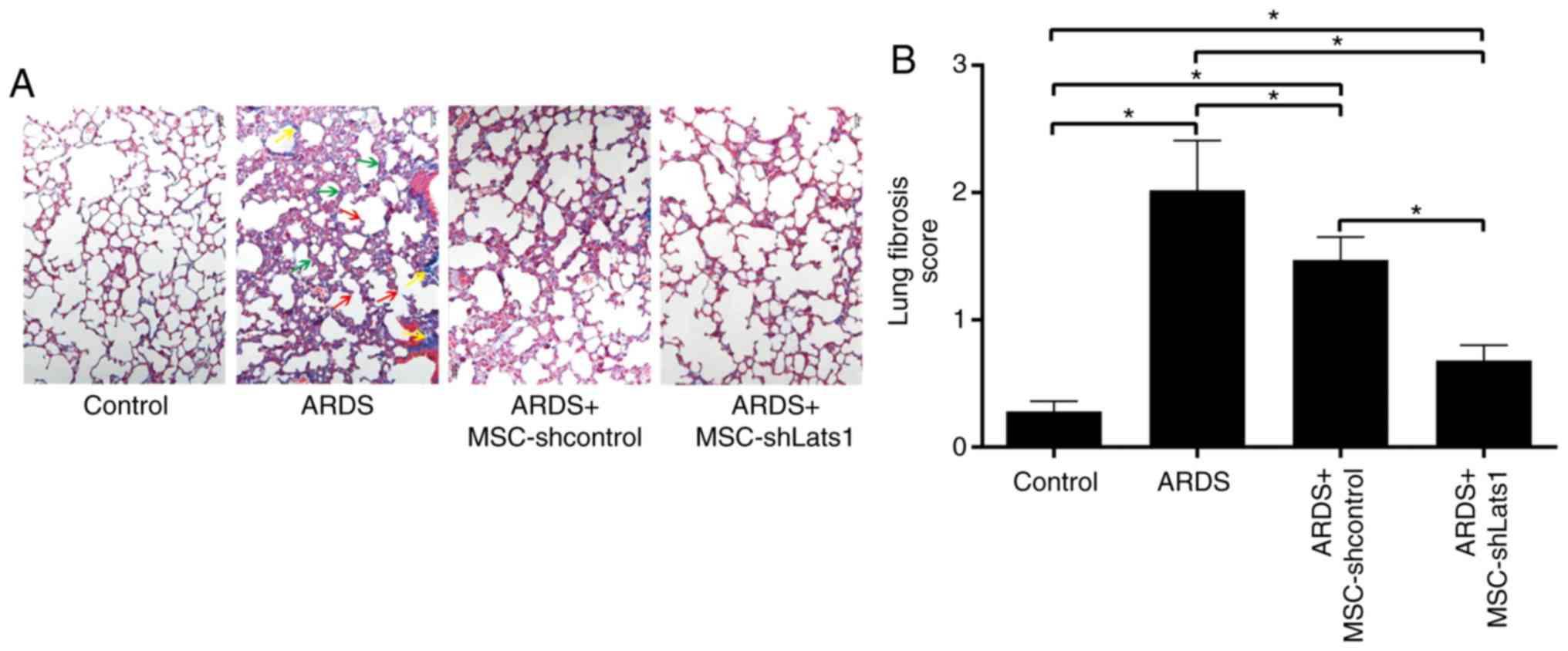
mMSCs with downregulated Hippo signaling
reduce pulmonary fibrosis in ARDS lung tissue
To assess the degree of pulmonary fibrosis in mice
from different experimental groups, modified Masson's staining of
lung tissue samples was conducted, and semiquantitative scoring was
performed, as previously described by Ashcroft et al
(14). This analysis revealed
hyperplasia of collagen fiber tissue in alveolar septa and alveolar
cavities in samples from the ARDS group at 14 days, with clear
thickening of alveolar walls and partial loss of alveolar structure
(Fig. 7A). The lung fibrosis
score in the ARDS group was significantly increased compared with
the control group (P<0.05). A decreased deposition of focal and
filamentous collagen fibers was found in the lung tissue of the
ARDS + MSC-shcontrol group, and the lung fibrosis score was
significantly lower compared with the ARDS group (P<0.05). No
collagen fiber formation was detected in samples from the ARDS +
MSC-shLats1 group, and the lung fibrosis score for these samples
was lower compared with the ARDS + MSC-shcontrol group (P<0.05;
Fig. 7A and B). These results
indicate that mMSCs with downregulated Hippo signaling may reduce
fibrosis in ARDS lung tissue.
Discussion
The present study investigated the effects of mMSCs
on the extent of lung injury and fibrosis in an LPS-induced mouse
model of ARDS lung injury. In addition, prompted by our previously
reported in vitro data (11), the current study aimed to
determine whether the putative beneficial effects of MSC
administration in the model of ARDS could be further potentiated by
the inhibition of the Hippo signaling pathway in these cells.
Alveolar epithelial injury is the main
pathophysiological basis of ARDS (1). ATII cells that remain after the
injury can proliferate and differentiate into ATI epithelial cells
to repair lung damage (4).
However, ATII cells are severely damaged in moderate and severe
ARDS, and the quantity of cells may be insufficient to repair
damaged alveolar epithelium, and supplementation with exogenous
cells for repair is a feasible option (20). mMSCs originate from multipotent
stem cells of the mesoderm, and previous studies have revealed that
exogenous mMSCs delivered to animals with ARDS alleviated the
injured alveolar epithelium and reduced the mortality rate
(5,6). However, the inability of mMSCs to
target the lung, short retention time in the lung and low
differentiation efficiency are issues that remain encountered in
cell-based ARDS treatments (21,22). Previously, in vitro
experiments revealed that low expression of the signaling molecule
Lats1 inhibits the activation of the Hippo signaling pathway and
enhances MSC proliferation, differentiation into ATII cells and
homing to ARDS-affected lung tissue (23). Manipulating Hippo signaling
activity may be an important strategy to maximize the protective
effect of mMSCs in the lungs, and the present study validated this
concept through in vivo experiments.
The Hippo signaling pathway is an essential
biochemical cascade for the regulation of cell proliferation and
differentiation in numerous types of tissues (24). Hippo signaling begins with the
binding of the cell surface ligand dachsous homolog 1 and 2 to the
cell surface receptor FAT tumor suppressor homolog 4 on a
neighboring cell, which activates the serine/threonine-protein
kinase STE20 family mammalian sterile 20-like kinases 1 and 2,
thereby phosphorylating and activating the nuclear dbf2-related
family kinase Lats1 (25). Lats1
kinase phosphorylates the downstream transcriptional regulator
Yes-associated protein (YAP), inhibiting proliferative and
antiapoptotic activities of the Hippo signaling pathway, thus
promoting apoptosis. When Lats1 activity decreases, YAP remains
unphosphorylated, which stimulates proliferative and antiapoptotic
activities of the Hippo signaling pathway, thus promoting cell
proliferation (26). Numerous
ligands, receptors, kinases, transcriptional regulators and
transcription factors of the Hippo signaling pathway are expressed
in mMSCs; therefore, regulating the activity of this pathway may
affect the differentiation, proliferation, migration and other
biological functions of mMSCs (27).
In the current study, a mMSCs cell line with Lats1
expression knockdown was successfully established through cell
transfection with a lentiviral vector carrying the Lats1 shRNA
sequence, thereby inhibiting the Hippo signaling activity in mMSCs.
Ex vivo experiments have confirmed that this manipulation
promoted MSC differentiation, proliferation and migration (11). In the present study, mMSCs with
knockdown of Lats1 were transplanted into ARDS mice via the airway,
and then, these mMSCs were evaluated for their retention in ARDS
lung tissue, differentiation into ATII cells and capacity for
repairing lung injury.
We found that Lats1 knockdown increased the
retention of mMSCs in ARDS lung tissue. Following administration of
cells to mice, the retention of exogenous mMSCs in ARDS lung tissue
provided evidence of their capacity for tissue repair. Xu et
al (28) confirmed that
significantly greater amounts of transplanted MSCs were capable of
homing to ARDS lung tissue compared with the normal lung tissue.
Currently, the molecular mechanisms that regulate MSC migration
toward sites of injury remain unknown. A previous study indicated
that transforming growth factor-β1, IL-1β and tumor necrosis factor
(TNF)-α enhance MSCs homing by inducing the expression of matrix
metalloproteases (29).
Furthermore, it has been reported that stem cell-derived factor-1α
and C-X-C chemokine receptor type 4 may participate in MSCs homing
(30). The present study
confirmed that the Hippo signaling pathway regulates mMSCs
retention in the ARDS lung tissue in vivo, providing a new
perspective for the molecular mechanism of MSC migration and
homing. However, the in vivo efficacy and mechanisms of this
regulation require further investigation.
Lats1 knockdown increased the differentiation of
mMSCs into ATII cells. This result contrasts with data reported in
several previous studies. In a study by An et al (31) the Hippo signaling pathway
positively affected adipogenesis. Furthermore, Tang et al
(32) demonstrated that
activation of the Hippo signaling pathway in mMSCs cultured in a
myogenic environment promoted their differentiation into muscle
fiber cells. However, Chen et al (33) revealed that activation of the
Hippo signaling pathway in mMSCs cultured in an osteogenic
environment inhibited their differentiation into osteocytes. In
addition, He et al (34)
revealed a negative regulatory role of the Hippo signaling pathway
in adipogenesis. Knockdown of YAP in mMSCs cultured in an
adipogenic environment increased the formation of adipocytes
(34). From these studies, it is
evident that the regulatory roles of the Hippo signaling pathway on
mMSCs differ and likely depend on the cell type, environment,
duration and time of treatment (35).
Cell permeability in pulmonary edema resulting from
the damage to tight junction proteins of alveolar epithelial cells
is one of the important factors underlying decreased lung
compliance and intractable hypoxia in ARDS (36). In the present study, mMSCs with
downregulated Hippo signaling differentiated into alveolar
epithelial cells and reinforced alveolar epithelial tight
junctions. This may be a potential mechanism by which mMSCs with
downregulated Hippo signaling can improve pulmonary edema and
permeability in ARDS lung tissue. Previous studies have also
yielded similar results; for example, Pati et al (37) found that the administration of
exogenous MSCs in hemorrhagic shock-associated lung injury improved
endothelial permeability and diminished pulmonary edema through the
protection of endothelial tight junctions of pulmonary blood
vessels. In the present study, the inhibition of the Hippo
signaling pathway further enhanced the protective role of mMSCs on
the alveolar epithelial barrier.
Dysregulation of inflammatory reactions in the lung
is one of the main pathological mechanisms of ARDS, which is
primarily manifested as local proinflammatory reactions in the lung
tissue and reduced anti-inflammatory activity (38). In the present study, mMSCs with
downregulated Hippo signaling reduced the levels of the
proinflammatory factors IL-1β and -6 and increased the levels of
the anti-inflammatory factors IL-4 and -10 in the lung tissue,
thereby ameliorating the imbalance in
proinflammatory/anti-inflammatory reactions in the lungs. These
observations are similar to the results reported by Zhu et
al (39), who revealed that
the administration of exogenous MSCs reduced IL-6 secretion and
augmented IL-10 secretion in the lung tissue of a mouse model of
ARDS. The current study revealed that the inhibition of the Hippo
signaling pathway further reinforced the positive regulatory impact
of mMSCs on lung inflammation in ARDS. In addition, the stimulation
of other type 2 anti-inflammatory cytokines, including IL-13, and
the inhibition of proinflammatory cytokines, such as TNF-α, should
be further investigated (40,41).
The extent of the pathological alterations in the
lung tissue is the key parameter for ARDS diagnosis, treatment
efficacy and determination of prognosis (42). Bleeding, edema, collapse and
inflammatory cell infiltration in the alveoli are typical
pathological alterations associated with ARDS (43). Pulmonary fibrosis resulting from
increased collagen fiber production during the lung injury repair
process may directly affect pulmonary function and the long-term
quality of life of patients with ARDS (44). In addition, a previous study
revealed that pathological changes associated with pulmonary
fibrosis begin to appear 24 h after the development of ARDS
(45). In the present study,
mMSCs with downregulated Hippo signaling improved pathological
injuries and reduced fibrosis in ARDS lung tissue in mice. In
addition, the inhibition of multiple fibrotic pathways may be
involved in the antifibrotic effects of MSC-shLats1, which warrants
further studies (46). Therefore,
similar approaches may potentially reduce the mortality rate of
ARDS and improve the long-term prognosis in human patients. These
results are similar to those reported in several previous studies.
Devaney et al (47)
revealed that the administration of MSCs into the airway improved
pathological injuries in a rat model of ARDS. Furthermore, Shen
et al (48) reported that
the intratracheal administration of MSCs significantly reduced
collagen fiber formation in a mouse model of ARDS. In addition, in
the current study, the inhibition of the Hippo signaling pathway in
administered mMSCs further increased the efficacy of the treatment
of pathological injuries and pulmonary fibrosis in ARDS. Further
studies may be required to investigate the potential use of
MSC-shLats1 for the treatment of other forms of fibrosis, including
liver fibrosis (49).
The present study had certain limitations. First,
ARDS is a clinical disorder with marked heterogeneity in its
etiology and pathophysiology, and LPS-induced ARDS only represents
a direct insult on lung cells (50). Second, the present study did not
investigate the effect of mMSCs with downregulated Hippo signaling
on the LPS-Toll-like receptor 4 signaling pathway, which initiates
inflammation and injury in LPS-induced ARDS (51), and, therefore, further studies are
warranted.
In conclusion, the current study demonstrated that
the transplantation of mMSCs with Lats1 gene knockdown to inhibit
the Hippo signaling pathway in mice with LPS-induced ARDS promoted
mMSCs retention in ARDS lung tissue and their differentiation into
ATII cells. The use of these modified mMSCs improved pulmonary
edema, reduced inflammatory reactions, and attenuated pathological
injuries and fibrotic changes in the lung tissue of animals with
ARDS. The results of the current study indicate that regulation of
the Hippo signaling pathway may enhance the repair efficacy of
mMSCs in ARDS, however, clinical trials are required.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81400054), the
Natural Science Foundation of Jiangsu Province (grant no.
BK20140122), the Talented Youth Program of Jiangsu Province (grant
no. QNRC2017179) and the Fifth '333 High Level Talent Training
Project' of Jiangsu Province (grant no. 2018III-0299).
Availability of data and materials
All data generated or analyzed during this study are
included in this article.
Authors' contributions
LL and LD conceived and designed the study. LL, LD,
JZ, FG, JH and JY performed the experiments, analyzed the data, and
interpreted the results. LD, JH and JY generated the figures and
drafted the manuscript. All authors have read and revised the
manuscript.
Ethics approval and consent to
participate
The experimental protocols were approved by the
Institutional Animal Care and Use Committee at Nanjing Medical
University (approval no. LLSP201505033). All animal experiments
were conducted in accordance with the National Institutes of Health
Guide for the Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors thank Dr Qiuhui Wang (Wuxi People's
Hospital, Wuxi, China) for assistance with modified Masson's
staining and immunofluorescence staining of lung tissues.
References
|
1
|
Thompson BT, Chambers RC and Liu KD: Acute
respiratory distress syndrome. N Engl J Med. 377:562–572. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fan E, Brodie D and Slutsky AS: Acute
respiratory distress syndrome: Advances in diagnosis and treatment.
JAMA. 319:698–710. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lau AN, Goodwin M, Kim CF and Weiss DJ:
Stem cells and regenerative medicine in lung biology and diseases.
Mol Ther. 20:1116–1130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weiss DJ: Stem cells, cell therapies, and
bioengineering in lung biology and diseases. Comprehensive review
of the recent literature 2010–2012. Ann Am Thorac Soc. 10:S45–S97.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee JW, Gupta N, Serikov V and Matthay MA:
Potential application of mesenchymal stem cells in acute lung
injury. Expert Opin Biol Ther. 9:1259–1270. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu F, Hu Y, Zhou J and Wang X: Mesenchymal
stem cells in acute lung injury: Are they ready for translational
medicine? J Cell Mol Med. 17:927–935. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bustos ML, Huleihel L, Kapetanaki MG,
Lino-Cardenas CL, Mroz L, Ellis BM, McVerry BJ, Richards TJ,
Kaminski N, Cerdenes N, et al: Aging mesenchymal stem cells fail to
protect because of impaired migration and antiinflammatory
response. Am J Respir Crit Care Med. 189:787–798. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Laffey JG and Matthay MA: Fifty years of
research in ARDS. Cell-based therapy for acute respiratory distress
syndrome. Biology and potential therapeutic value. Am J Respir Crit
Care Med. 196:266–273. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang SR, Park JR and Kang KS: Reactive
oxygen species in mesenchymal stem cell aging: Implication to lung
diseases. Oxid Med Cell Longev. 2015:4862632015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Espinoza F, Aliaga F and Crawford PL:
Overview and perspectives of mesenchymal stem cell therapy in
intensive care medicine. Rev Med Chil. 144:222–231. 2016.In
Spanish. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li L, Dong L, Hui J, Gao F, Wang Q, Yang
L, Zhang J and Yan J: Under-expression of LATS1 promotes the
differentiation, proliferation and migration of mesenchymal stem
cells by inhibition the Hippo signaling pathway in vitro. Zhonghua
Wei Zhong Bing Ji Jiu Yi Xue. 29:731–737. 2017.In Chinese.
PubMed/NCBI
|
|
12
|
National Research Council (US) Institute
for Laboratory Animal Research: Guide for the Care and Use of
Laboratory Animals. National Academies Press; Washington, DC:
1996
|
|
13
|
Smith KM, Mrozek JD, Simonton SC, Bing DR,
Meyers PA, Connett JE and Mammel MC: Prolonged partial liquid
ventilation using conventional and high-frequency ventilatory
techniques: Gas exchange and lung pathology in an animal model of
respiratory distress syndrome. Crit Care Med. 25:1888–1897. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ashcroft T, Simpson JM and Timbrell V:
Simple method of estimating severity of pulmonary fibrosis on a
numerical scale. J Clin Pathol. 41:467–470. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He H, Liu L, Chen Q, Liu A, Cai S, Yang Y,
Lu X and Qiu H: Mesenchymal stem cells overexpressing
angiotensin-converting enzyme 2 rescue lipopolysaccharide-induced
lung injury. Cell Transplant. 24:1699–1715. 2015. View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Dong L, He HL, Lu XM, Yang Y and Qiu HB:
Modulation of FLT3 signaling targets conventional dendritic cells
to attenuate acute lung injury. APMIS. 120:808–818. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun Y, Liu JG, Zheng YC, Xiao CL, Wan B,
Guo L, Wang XG and Bo W: Research on rat's pulmonary acute injury
induced by lunar soil simulant. J Chin Med Assoc. 81:133–140. 2018.
View Article : Google Scholar
|
|
19
|
Tam A, Wadsworth S, Dorscheid D, Man SF
and Sin DD: The airway epithelium: More than just a structural
barrier. Ther Adv Respir Dis. 5:255–273. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Standiford TJ and Ward PA: Therapeutic
targeting of acute lung injury and acute respiratory distress
syndrome. Transl Res. 167:183–191. 2016. View Article : Google Scholar
|
|
21
|
Moodley Y, Sturm M, Shaw K, Shimbori C,
Tan DB, Kolb M and Graham R: Human mesenchymal stem cells attenuate
early damage in a ventilated pig model of acute lung injury. Stem
Cell Res. 17:25–31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McIntyre LA, Moher D, Fergusson DA,
Sullivan KJ, Mei SH, Lalu M, Marshall J, Mcleod M, Griffin G,
Grimshaw J, et al: Efficacy of mesenchymal stromal cell therapy for
acute lung injury in preclinical animal models: A systematic
review. PLoS One. 11:e01471702016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hayes M, Curley G, Ansari B and Laffey JG:
Clinical review: Stem cell therapies for acute lung injury/acute
respiratory distress syndrome-hope or hype? Crit Care. 16:2052012.
View Article : Google Scholar
|
|
24
|
Sardo FL, Strano S and Blandino G: The
Hippo kinase pathway: A master regulator of proliferation,
development and differentiation. Atlas Genet Cytogenet Oncol
Haematol. 19:65–77. 2015.
|
|
25
|
Johnson R and Halder G: The two faces of
Hippo: Targeting the Hippo pathway for regenerative medicine and
cancer treatment. Nat Rev Drug Discov. 13:63–79. 2014. View Article : Google Scholar :
|
|
26
|
Zhang Q, Meng F, Chen S, Plouffe SW, Wu S,
Liu S, Li X, Zhou R, Wang J, Zhao B, et al: Hippo signalling
governs cytosolic nucleic acid sensing through YAP/TAZ-mediated
TBK1 blockade. Nat Cell Biol. 19:362–374. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao B, Tumaneng K and Guan KL: The Hippo
pathway in organ size control, tissue regeneration and stem cell
self-renewal. Nat Cell Biol. 13:877–883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu J, Woods CR, Mora AL, Joodi R, Brigham
KL, Iyer S and Rojas M: Prevention of endotoxin-induced systemic
response by bone marrow-derived mesenchymal stem cells in mice. Am
J Physiol Lung Cell Mol Physiol. 293:L131–L141. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
De Becker A, Van Hummelen P, Bakkus M,
Vande Broek I, De Wever J, De Waele M and Van Riet I: Migration of
culture-expanded human mesenchymal stem cells through bone marrow
endothelium is regulated by matrix metalloproteinase-2 and tissue
inhibitor of metalloproteinase-3. Haematologica. 92:440–449. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schioppa T, Uranchimeg B, Saccani A,
Biswas SK, Doni A, Rapisarda A, Bernasconi S, Saccani S, Nebuloni
M, Vago L, et al: Regulation of the chemokine receptor CXCR4 by
hypoxia. J Exp Med. 198:1391–1402. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
An Y, Kang Q, Zhao Y, Hu X and Li N: Lats2
modulates adipocyte proliferation and differentiation via hippo
signalling. PLoS One. 8:e720422013. View Article : Google Scholar
|
|
32
|
Tang Y, Feinberg T, Keller ET, Li XY and
Weiss SJ: Snail/Slug binding interactions with YAP/TAZ control
skeletal stem cell self-renewal and differentiation. Nat Cell Biol.
18:917–929. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen Z, Luo Q, Lin C, Kuang D and Song G:
Simulated microgravity inhibits osteogenic differentiation of
mesenchymal stem cells via depolymerizing F-actin to impede TAZ
nuclear translocation. Sci Rep. 6:303222016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
He Q, Huang HY, Zhang YY, Li X and Qian
SW: TAZ is downregulated by dexamethasone during the
differentiation of 3T3-L1 preadipocytes. Biochem Biophys Res
Commun. 419:573–577. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Deel MD, Li JJ, Crose LE and Linardic CM:
A Review: Molecular aberrations within Hippo signaling in bone and
soft-tissue sarcomas. Front Oncol. 5:1902015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin X, Barravecchia M, Kothari P, Young JL
and Dean DA: β1-Na(+), K(+)-ATPase gene therapy upregulates tight
junctions to rescue lipopolysaccharide-induced acute lung injury.
Gene Ther. 23:489–499. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pati S, Gerber MH, Menge TD, Wataha KA,
Zhao Y, Baumgartner JA, Zhao J, Letourneau PA, Huby MP, Baer LA, et
al: Bone marrow derived mesenchymal stem cells inhibit inflammation
and preserve vascular endothelial integrity in the lungs after
hemorrhagic shock. PLoS One. 6:e251712011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Voiriot G, Razazi K, Amsellem V, Tran Van
Nhieu J, Abid S, Adnot S, Mekontso Dessap A and Maitre B:
Interleukin-6 displays lung anti-inflammatory properties and exerts
protective hemodynamic effects in a double-hit murine acute lung
injury. Respir Res. 18:642017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu H, Xiong Y, Xia Y, Zhang R, Tian D,
Wang T, Dai J, Wang L, Yao H, Jiang H, et al: Therapeutic effects
of human umbilical cord-derived mesenchymal stem cells in acute
lung injury mice. Sci Rep. 7:398892017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nicoletti F, Mancuso G, Cusumano V, Di
Marco R, Zaccone P, Bendtzen K and Teti G: Prevention of
endotoxin-induced lethality in neonatal mice by interleukin-13. Eur
J Immunol. 27:1580–1583. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Qi D, Wang D, Zhang C, Tang X, He J, Zhao
Y, Deng W and Deng X: Vaspin protects against LPSinduced ARDS by
inhibiting inflammation, apoptosis and reactive oxygen species
generation in pulmonary endothelial cells via the Akt/GSK3β
pathway. Int J Mol Med. 40:1803–1817. 2017.PubMed/NCBI
|
|
42
|
Kao KC, Hu HC, Chang CH, Hung CY, Chiu LC,
Li SH, Lin SW, Chuang LP, Wang CW, Li LF, et al: Diffuse alveolar
damage associated mortality in selected acute respiratory distress
syndrome patients with open lung biopsy. Crit Care. 19:2282015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Blondonnet R, Constantin JM, Sapin V and
Jabaudon M: A pathophysiologic approach to biomarkers in acute
respiratory distress syndrome. Dis Markers. 2016:35013732016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wilcox ME and Herridge MS: Lung function
and quality of life in survivors of the acute respiratory distress
syndrome (ARDS). Presse Med. 40:e595–603. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Huang K, Wu XM, Wang XY, Kang XW, Xiao JL,
Li ZG and Lu P: The effect of marrow mesenchymal stem cell
transplantation on pulmonary fibrosis in rats. Zhonghua Jie He He
Hu Xi Za Zhi. 35:659–664. 2012.In Chinese. PubMed/NCBI
|
|
46
|
Fagone P, Mangano K, Mammana S, Pesce A,
Pesce A, Caltabiano R, Giorlandino A, Portale TR, Cavalli E,
Lombardo GA, et al: Identification of novel targets for the
diagnosis and treatment of liver fibrosis. Int J Mol Med.
36:747–752. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Devaney J, Horie S, Masterson C, Elliman
S, Barry F, O'Brien T, Curley GF, O'Toole D and Laffey JG: Human
mesenchymal stromal cells decrease the severity of acute lung
injury induced by E. coli in the rat. Thorax. 70:625–635. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shen Q, Chen B, Xiao Z, Zhao L, Xu X, Wan
X, Jin M, Dai J and Dai H: Paracrine factors from mesenchymal stem
cells attenuate epithelial injury and lung fibrosis. Mol Med Rep.
11:2831–2837. 2015. View Article : Google Scholar
|
|
49
|
Fagone P, Mangano K, Pesce A, Portale TR,
Puleo S and Nicoletti F: Emerging therapeutic targets for the
treatment of hepatic fibrosis. Drug Discov Today. 21:369–375. 2016.
View Article : Google Scholar
|
|
50
|
Shaver CM and Bastarache JA: Clinical and
biological heterogeneity in acute respiratory distress syndrome:
Direct versus indirect lung injury. Clin Chest Med. 35:639–653.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu L, Chen L, Jiang C, Guo J, Xie Y, Kang
L and Cheng Z: Berberine inhibits the LPS-induced proliferation and
inflammatory response of stromal cells of adenomyosis tissues
mediated by the LPS/TLR4 signaling pathway. Exp Ther Med.
14:6125–6130. 2017.PubMed/NCBI
|